Abstract
Changes in bone health and strength are common after kidney transplantation and can lead to an increased risk of fracture. This has implications for morbidity, mortality and renal allograft survival. This review will focus on the changes that occur in bone health and fracture risk after kidney transplantation and examine the evidence available to guide diagnostic and therapeutic decisions with the aim of fracture prevention.
Introduction
Kidney transplantation remains the treatment of choice for End Stage Kidney Disease (ESKD) because of improvement in quality of life and mortality [1], [2], [3]. However, there can be a negative impact on bone strength and fracture risk. Fractures are not without consequence in this patient population and are associated with an increased risk of mortality and allograft loss [4]. Salter et al. reported an increase in mortality after spine, hip and extremity fracture with hazard ratios of 2.8, 2.31 and 1.85 respectively in kidney transplant recipients > 55 years of age. There was also an increase in death censored graft loss of 1.34 and 1.3-fold for hip and extremity fractures respectively [4].
Changes in fracture risk and bone health after kidney transplantation
The first 12 to 18 months after transplantation has been associated with a rapid loss in bone mineral density (BMD) as high as 9 % in the spine and hip [5], [6]. Fracture risk is increased with Ball et al. reporting a relative risk of 1.34-fold greater for hip fracture in kidney transplant recipients compared to wait listed patients still on dialysis during the first 1 to 3 years after transplantation [7]. The difference in fracture risk decreased by 1 % per month until the estimated risk became equal for both groups 630 days after transplantation. 3 years after transplantation, bone density stabilized and then increased approximately 6 % between years 6 and 10 posttransplant with an increase of 2 % over the next decade [7].
It is worth noting that this data represents a time when higher doses of corticosteroids were used for maintenance immunosuppression. Practice patterns have changed to now favor lower corticosteroid doses and in select patients, withdrawal of corticosteroids completely. This has impacted patterns of changes in BMD and the change in fracture risk (see Fig. 1, Fig. 2, Fig. 3 and Table 1).
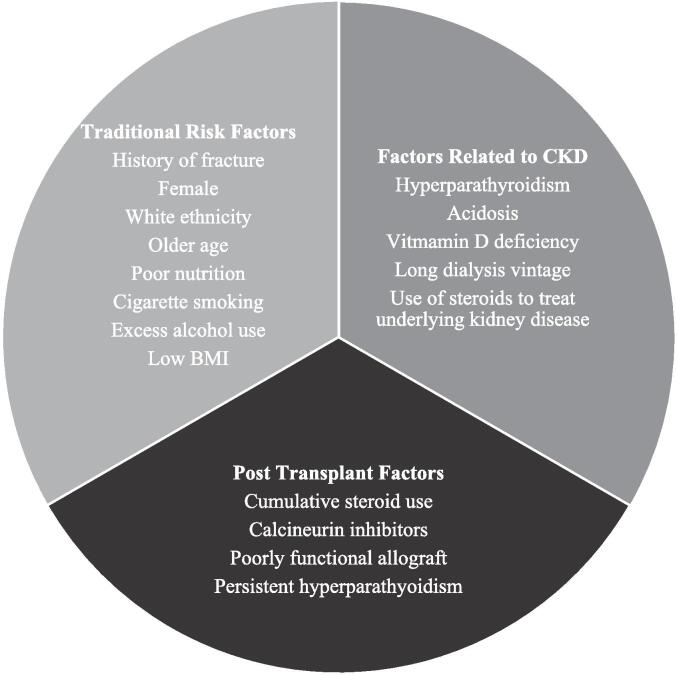
Fig. 1.
Risk factors for osteoporosis and increased fracture risk in kidney transplant recipients.
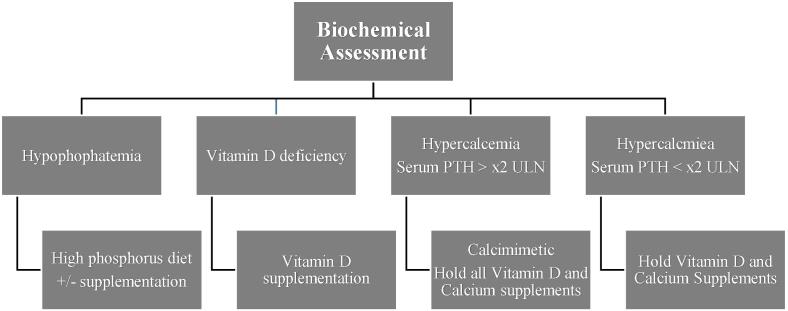
Fig. 2.
Suggested Biochemical assessment of bone health after kidney transplantation. PTH – Serum Parathyroid Hormone; ULN – Upper limit of normal.
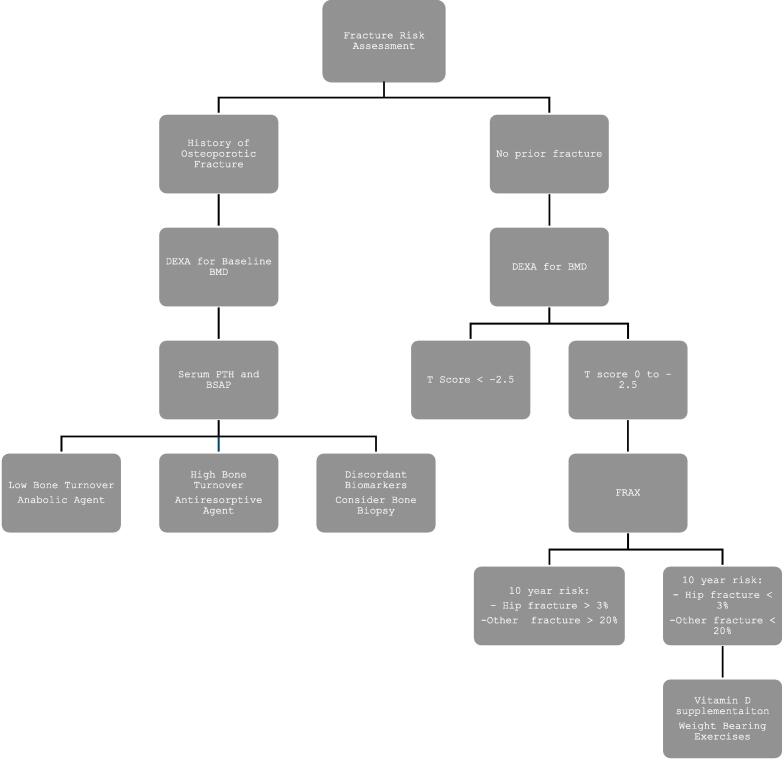
Fig. 3.
Suggested Fracture risk assessment after kidney transplantation. DEXA – Dual X-ray absorptiometry; PTH – Parathyroid hormone; BSAP – Bone specific alkaline phosphatase.
Table 1.
Summary of clinical trials examining the effect of medications on BMD and fracture risk.
| Reference | Intervention | Study Type | Effect on BMD | Effect on Fracture Risk |
|---|---|---|---|---|
| De Sevaux et al. [37] | 1 α hydroxyvitamin D plus Calcium vs placebo | Randomized placebo controlled | Lower decline at lumbar spine and hip | Not reported |
| Torres et al. [38] | Calcitriol vs placebo | Randomized placebo controlled | Lower decline at hip | Not reported |
| Josephson et al. [39] | Calcitriol plus Calcium vs placebo | Randomized placebo controlled | Lower decline at lumbar spine Increase at distal radius |
Not reported |
| Toth-Manikowski et al. [40] | Bisphosphonates vs placebo/Calcium/Vitamin D | Meta-analysis | Increase at lumbar spine and hip | Not reported |
| Palmer et al. [41] | Bisphosphonates vs placebo | Systematic Review | None | Unclear |
| Bonnani et al. [42] | Denosumab vs no treatment | Randomized controlled | Increase at lumbar spine and total hip | Not reported |
| Brunova et al. [43] | Denosumab vs no treatment | Retrospective | Increase at lumbar spine and total hip | Not reported |
| Cejka et al. [45] | Teriparatide vs placebo | Randomized placebo controlled | Lower decline at femoral neck | None |
| Eveneoel et al. [46] | Cinacalcet vs placebo | Randomized placebo controlled | None | Not reported |
| Cruzado et al. [47] | Parathyroidectomy vs Cinacalcet | Randomized controlled trial | Increase at femoral neck | Not reported |
Newer data has examined the effect of low dose or early corticosteroid withdrawal on BMD and fracture risk. Iyer et al. examined the effect of corticosteroid withdrawal on bone mineral density in 39 kidney transplant recipients at 1 year post transplantation. BMD remained stable or increased in the central skeleton but declined in the peripheral skeleton with a decrease of 2.2 % ± 0.56 (P < 0.001) at the distal 1/3 of the radius [8]. There is also data demonstrating a reduction in fracture risk with corticosteroid withdrawal. Nikkel et al. examined United States Renal Data System (USRDS) data looking at 77,430 first time kidney transplant recipients from 2000 to 2006 to determine fracture incidence between patients on a corticosteroid based immunosuppression regimen (CSBI) versus early corticosteroid withdrawal (ECSW). They found that patient is on an ECSW regimen (n = 11,164) experienced a 31 % reduction (HR 0.69; 95 % CI 0.59–0.81) in 5 year cumulative risk for fracture compared to CSBI (n = 62,266) with an annual incident rate of 5.8 and 8 per 1000 patient years respectively [9].
Changes in practice with respect to corticosteroid use post transplantation has also resulted in changes in bone quality as assessed by bone biopsy. Monier-Faugere et al. reported on 57 kidney transplant recipients who underwent bone biopsy with time elapsed after transplantation 5.4 ± 0.8 years and range 6 months to 27 years. Average prednisone dose was 8.8 ± 0.85 mg/day at the time of bone biopsy. Cumulative and maintenance dose corticosteroids and time since transplantation correlated in negatively with bone volume and turnover [10]. Low trabecular bone volume was present in 56 % of patients and 45.6 % of patient had low bone turnover. In contrast, Keronen etl al reported on 27 recipients who underwent bone biopsy prior to transplantation, while on dialysis, and then 2 years after kidney transplantation [11]. Corticosteroids were withdrawn after 1 year in all patients. Trabecular bone volume was unchanged suggesting that minimization of corticosteroid exposure after kidney transplantation reduces adverse effects on the quality of trabecular bone.
Risk factors for increase in fracture risk after kidney transplantation
Kidney transplant recipients have multiple reasons to have abnormal bone health with osteoporosis and an increased risk of fracture. There are factors related to chronic kidney disease prior to transplantation, electrolyte and hormone abnormalities after transplantation and effects of immunosuppression medications on bone.
Patients with chronic kidney disease prior to transplantation, have high rates of osteopenia and osteoporosis with estimates as high as 32 % and 15 % respectively [12], [13]. There is also a significantly higher rate of fracture for these patients which is 2 to 14 times greater than the general population and this risk increases with a decline in GFR [14], [15], [16]. Therefore, patients come to kidney transplant with decreased bone strength and a higher risk of fracture. This is due to a complex interplay of electrolyte abnormalities involving calcium and phosphorus, hormone abnormalities involving parathyroid hormone (PTH), fibroblast growth factor –23 (FGF-23), Klotho and Vitamin D. There are also changes to bone structure with respect to turn over, mineralization, volume and linear growth [17].
Hypophosphatemia, hypovitaminosis D and abnormalities of PTH are common after kidney transplantation [18], [19], [20]. Hypophosphatemia develops in up to 90 % of post kidney transplant recipients, typically within the first 3 months and is thought to be secondary to renal phosphorus wasting induced primarily by elevated levels of FGF-23 [20]. It improves or resolves in approximately 86 % of recipients by 1 year post transplantation [21], [22] but can persist well beyond this and a small subset of patients. Prolonged phosphate depletion can potentially lead to osteomalacia. Vitamin-D deficiency is also very common and is estimated to be present in up to 80 % of recipients by 3 months with risk factors being lower allograft function and elevated FGR-23 levels [18]. Vitamin-D deficiency can result in hypocalcemia and subsequent bone loss [23].
Hyperparathyroidism resolves completely in 30 % and 57 % of recipients by the end of the first and second years posttransplant respectively [19]. In some studies, persistent hyperparathyroidism with elevated PTH levels after kidney transplantation was associated with cortical and trabecular bone loss [8], [23] and with an increased risk of fracture [24]. However, there is conflicting evidence in the literature where PTH levels did not correlate with bone turnover [10] or decrease in BMD [25], [26].
The effects of glucocorticoids on the skeleton are well established [27]. Glucocorticoids inhibit bone formation by reducing osteoblast proliferation, function, and life span [28]. They also promote osteoclastogenesis by increasing levels of RANK Ligand and decreasing levels of osteoprotegerin which is a decoy receptor for the RANK Ligand [29]. The negative effects of glucocorticoids on bone turnover and volume are related to the cumulative dose of glucocorticoids in renal transplant recipients [10].
Calcineurin inhibitors have been linked to osteoporosis in kidney transplant recipients [30], [31]. There is also animal model evidence to support this [32]. However, the isolated effects of calcineurin inhibitors on bone health and fracture risk is difficult to evaluate in human studies as they are frequently used together with glucocorticoids. Little to no data exists for other commonly used immunosuppressants such as mycophenolate mofetil, sirolimus and azathioprine other than rat models with these drugs did not affect bone volume [33], [34], [35].
Clinical assessment of fracture risk and bone health after kidney transplantation
Evidence to guide the assessment of bone disease and fracture risk after kidney transplantation is scarce. The 2017 KDIGO guidelines are the most widely used in clinical practice but acknowledges that guidance is limited to the first 12 months post transplantation as there is insufficient data to guide long term recommendations [26]. Assessment of bone health is done using biochemical parameters, dual X-ray absorptiometry scan for bone mineral density and consideration to bone biopsy. The recommendations do not mandate bone biopsy but state it is reasonable to consider to guide treatment.
Recommended biochemical assessment is limited to markers routinely used in clinical practice. These are serum calcium, phosphorus, parathyroid hormone (PTH) and bone specific alkaline phosphatase (BSAP). Using these markers, the clinician attempts to classify bone disease as high or low turnover. Elevated levels of serum PTH and BSAP would indicate high turnover bone disease and levels below the lower limit of normal for both would indicate low turnover bone disease. If there is discordance with respect to these results, a bone biopsy can be done to help guide treatment.
Bone density is determined by DEXA and World Health Organization T score thresholds are used to define osteopenia and osteoporosis. In addition, a history of prior fractures is taken into consideration. Biopsy can be considered to guide treatment if there is discordance between suggested treatment based on biochemical assessment versus DEXA moving forward.
For most clinical practices, these guidelines are extrapolated past the first-year post transplantation and there is tremendous variability in practice patterns from one institution to another.
Management of low bone mineral density and reduction in fracture risk after kidney transplantation
KDIGO suggests that kidney transplant recipients with an eGFR of > 30 ml/min/1.73 m2 and low BMD receive Vitamin D, calcitriol/alfacalcidol and/or and antiresorptive agent [36]. There is no other recommendation to guide the choice of antiresorptive agent or other strategies but there are several classes of pharmacologic agents used to treat osteoporosis and prevent fractures which are being used clinically in kidney transplant recipients.
Vitamin-D and calcium Supplementation
No study has examined the effect of vitamin-D and calcium supplementation on fracture risk after transplantation. There is data from small studies which examine the effect of this supplementation on BMD but they were all limited to the first year after transplant.
De Sevaux et al. examined the effect of supplementation with 0.25 µg of 1α hydroxyvitamin D and 1000 mg of elemental calcium compared to no treatment in a randomized non blinded study involving 111 kidney transplant recipient [37]. Sixty five (65) patients received supplementation, and 46 did not. BMD was assessed at 0, 3 and 6 months after transplant at the lumbar spine and hip. In both groups, lumbar BMD decreased after 6 months but patients randomized to the treatment arm had smaller losses in bone density in the lumbar (total loss −2.6 ± 5 % vs −5 ± 5 %; p < 0.002) and femoral BMD (total loss −0.22 ± 6 % vs −4 ± 6 %; p < 0.001). Similar findings were reported by Torres et al. who conducted a double blinded randomized study of calcitriol therapy compared to placebo in 86 kidney transplant recipients [38]. Both arms (45 treatment; 41 placebo) received supplemental oral calcium at a dose of 500 mg/day. BMD was assessed at 0, 3 and 12 months. Patients in the treatment arm had a significantly lower decline in BMD at total hip compared to the placebo arm (3 months: 0.04 ± 3.3 % vs −1.93 ± 3.2 %; p = 0.01; 12 months: 0.32 ± 4.8 % vs −2.17 ± 4.4 %; p = 0.03).
Another prospective, randomized, double blinded study by Josephson et al. in 64 kidney transplant recipients randomized to calcium supplementation, calcium plus calcitriol or placebo [39]. BMD was assessed at 0, 6 and 12 months. There was no evidence of a beneficial effect of calcium alone at any time. At 1 year, patients in the calcium plus calcitriol group had a 0.1 % decline in BMD at the lumbar spine with a 2.9 % increase at the distal radius compared to a 2 % decline in both areas for the placebo group.
This data has led to routine supplementation with calcium and vitamin-D after kidney transplantation. While improvements in BMD at various sites is encouraging, change to fracture risk is unclear.
Bisphosphonates
Bisphosphonates increase BMD by inhibiting osteoclast function and suppressing bone turnover. However, their ability to reduce fracture risk after kidney transplantation is unclear. Furthermore, even data to support increase in BMD consistently in this population is conflicting and limited to the first 12 months after transplantation.
A 2016 meta-analysis by Toth-Manikowski et al. of 12 studies including a total of 621 kidney transplant recipients concluded that bisphosphonates were associated with improvements in BMD at the femoral neck (mean difference 0.055 g/cm2; 95 % CI 0.012 to 0.099) and lumbar spine (mean difference 0.053 g/cm2; 95 % CI 0.032 to 0.074) compared to controls [40]. This corresponded to an unweighted improvement in BMD of 6 % and 7.4 % respectively. However, there was no difference in the incidents of fracture between patients treated with bisphosphonates and those who were not. A variety of bisphosphonates were used in the studies included in this analysis such as pamidronate, alendronate, clodronate, ibandronate, zoledronic acid and risedronate. Controlled groups were given calcium, vitamin-D, normal saline, calcitriol or placebo either singularly or in combination.
In contrast, a 2019 Cochrane review by Palmer et al. using data from RCTs and quasi RTCs where bisphosphonates were administered for a median of 12 months concluded there was not a significant increase in BMD at the spine or hip compared to placebo [41]. In addition, there was only low certainty evidence that they prevented fracture (RR 0.62; 95 % CI 0.38–1.01).
In spite of this uncertainty, bisphosphonates remain commonly used for treatment of osteoporosis after kidney transplantation.
Denosumab
Denosumab is a monoclonal antibody against the receptor activator of the NF-κB ligand. It inhibits osteoclast development and proliferation and functions as an anti-resorptive agent. It is being used to treat osteoporosis in kidney transplant recipients but, as with other agents, there is limited data in this patient population for an increase in BMD and fracture prevention.
There is only one trial by Bonnani et al. which examined changes in BMD in new kidney transplant recipients using denosumab [42]. Ninety (90) kidney transplant recipients were randomized in a 1:1 fashion 2 weeks after transplantation to receive denosumab 60 mg at baseline and a second dose 6 months later or no treatment. At 1 year, BMD in the denosumab group increased significantly at the lumbar spine and total hip compared to the placebo group (5 %; p < 0.001 and 2 %; p = 0.04, respectively). The effect on fracture risk during this time, if any, was not reported.
Data regarding the effect of denosumab after the first year of transplantation is also lacking. A retrospective study by Brunova et al. examined the effect of denosumab on BMD in 63 solid organ transplant recipients, 49 of which had kidney transplants [43]. The time after transplantation varied widely in the cohort with mean 6.4 ± 6.3 years and the mean duration of therapy was 1.65 ± 0.7 years. Denosumab decreased the prevalence of osteoporosis in the lumbar spine from 75 % to 27 % (p < 0.001) and proximal femur from 54 % to 36 % (p < 0.05).
This data although limited, does support the ability of denosumab to increase BMD. Because of this, it is being used as an alternative to bisphosphonates for patients with osteoporosis after kidney transplantation. However, data on effect on fracture risk is lacking.
Teriparatide
Teriparatide is a recombinant peptide of the first 34 amino acids of the N terminal of PTH. This peptide interacts with the type 1 PTH receptor expressed on osteoblasts resulting in an increase in the activation and maturation of osteoblasts and a decrease in osteoblast apoptosis [44].
There has been a single randomized, double-blind, placebo controlled trial which has examined the efficacy of teriparatide in bone disease after kidney transplantation [45]. Twenty four (24) patients were randomized in a 1:1 ratio to receive daily subcutaneous injections of 20 µg of teriparatide or placebo within 1 month of transplantation. BMD was performed at 0 and 6 months. Femoral neck BMD remained stable in the teriparatide group (mean 0.68 g/cm2; 0.54–0.96 to mean 0.69 g/cm2; 0.52–0.94) but decreased in the placebo group (main 0.7 g/cm2; 0.5–0.94 to 0.66 g/cm2; 0.46–0.86). BMD at the lumbar spine and distal forearm remain unchanged in both groups. There was no report of fracture in either group for the study duration. Patient enrollment fell short of the predicted 55 patients in each arm to power the study to adequately established statistical differences between them.
Given the extremely limited data on teriparatide kidney transplant recipients, it’s routine use for treatment of osteoporosis or reduction of fracture risk cannot be recommended.
Calcimimetics and Parathyroidectomy
Calcimimetics increase the sensitivity of the parathyroid calcium sensing receptors (CaS-R) to calcium resulting in suppression of PTH secretion and decreased serum calcium concentrations. Cinacalcet is the only oral calcimimetic use clinically to treat posttransplant hyperparathyroidism.
There is data to show that cinacalcet is effective in reducing serum PTH levels in kidney transplant recipients. A placebo controlled RTC by Evenepoel et al. evaluated the effect of cinacalcet on serum PTH and calcium levels in 114 kidney transplant recipients who were > 8 weeks but ≤ 24 months after transplant with hyperparathyroidism and hypercalcemia [46]. Participants were randomized to cinacalcet or placebo in a 1:1 ratio and followed for 56 weeks. The results were significant for decrease in serum PTH levels (mean 128 pg/ml vs 10.6 pg/ml; p = 0.002). In addition, serum calcium levels were normalized in 70 % of the patient's in the treatment on compared to 4 % in the placebo arm.
Despite improvements in biochemical parameters, evidence for the ability of cinacalcet to increase BMD or prevent fracture is either disappointing or absent. The same study by Evenepoel et al. failed to demonstrate any difference in BMD at the spine, hip and radius between the treatment and placebo groups [46]. In addition, no published study has evaluated the effect of cinacalcet on fracture risk in kidney transplant recipients.
One published RCT compared subtotal parathyroidectomy to cinacalcet in kidney transplant recipients ≥ 6 months after transplant with secondary hyperparathyroidism, hypercalcemia and hypophosphatemia [47]. Fifteen (15) patients underwent parathyroidectomy and 15 patients received cinacalcet. At 12 months, parathyroidectomy was superior to cinacalcet in normalizing serum calcium (p = 0.04). It was also superior for normalization of serum PTH (10 patients vs 0 patients; p = 0.002) and increasing BMD at the femoral neck (+3.8 ± 6.1 % vs −3 ± 5.1 %; p = 0.01). Although these results are encouraging, it is difficult to draw firm conclusions from a single small study. The role of parathyroidectomy is unclear and clinically, has been reserved for patients with persistent hyperparathyroidism with hypercalcemia refractory to medical therapy. Whether this reduces the fracture risk in these patients is not known.
Conclusion
Changes in bone health and increase in fracture risk are common after kidney transplantation and has implications for patient morbidity and mortality as well as allograft survival. Despite this knowledge, there is a paucity of evidence to guide strong recommendations with respect to evaluation of bone health and fractures and strategies for fracture prevention. There is need for further research in these areas to guide clinical practice.
CRediT authorship contribution statement
Vishal Jaikaransingh: Writing – original draft, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Schnuelle P., Lorenz D., Trede M., Van Der Woude F.J. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 2.Suthanthiran M., Strom T.B. Renal transplantation. N Engl J Med. 1994;331(6):365–376. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 3.Port F.K., Wolfe R.A., Mauger E.A., Berling D.P., Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270(11):1339–1343. [PubMed] [Google Scholar]
- 4.Salter M.L., Liu X., Bae S., et al. Fractures and subsequent graft loss and mortality among older kidney transplant recipients. J Am Geriatr Soc. 2019;67(8):1680–1688. doi: 10.1111/jgs.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horber F.F., Casez J.P., Steiger U., Czerniak A., Montandon A., Jaeger P. Changes in bone mass early after kidney transplantation. J Bone Miner Res. 1994;9(1):1–9. doi: 10.1002/jbmr.5650090102. [DOI] [PubMed] [Google Scholar]
- 6.Julian B.A., Laskow D.A., Dubovsky J., Dubovsky E.V., Curtis J.J., Quarles L.D. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544–550. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 7.Ball A.M., Gillen D.L., Sherrard D., et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288(23):3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 8.Iyer S.P., Nikkel L.E., Nishiyama K.K., et al. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol. 2014;25(6):1331–1341. doi: 10.1681/ASN.2013080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikkel L.E., Mohan S., Zhang A., et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant. 2012;12(3):649–659. doi: 10.1111/j.1600-6143.2011.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monier-Faugere M.C., Mawad H., Qi Q., Friedler R.M., Malluche H.H. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol. 2000;11(6):1093–1099. doi: 10.1681/ASN.V1161093. [DOI] [PubMed] [Google Scholar]
- 11.Keronen S., Martola L., Finne P., Burton I.S., Kröger H., Honkanen E. Changes in bone histomorphometry after kidney transplantation. Clin J Am Soc Nephrol. 2019;14(6):894–903. doi: 10.2215/CJN.09950818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregorini M., Sileno G., Pattonieri E.F., et al. Understanding bone damage after kidney transplantation: a retrospective monocentric cross sectional analysis. Transplant Proc. 2017;49(4):650–657. doi: 10.1016/j.transproceed.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Dolgos S., Hartmann A., Bønsnes S., et al. Determinants of bone mass in end-stage renal failure patients at the time of kidney transplantation. Clin Transplant. 2008;22(4):462–468. doi: 10.1111/j.1399-0012.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 14.Nickolas T.L., McMahon D.J., Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 15.Alem A.M., Sherrard D.J., Gillen D.L., et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 16.Dooley A.C., Weiss N.S., Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51(1):38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Moe S.M., Drüeke T., Lameire N., Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14(1):3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Evenepoel P., Naesens M., Claes K., Kuypers D., Vanrenterghem Y. Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7(5):1193–1200. doi: 10.1111/j.1600-6143.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 19.Lou I., Foley D., Odorico S.K., et al. How well does renal transplantation cure hyperparathyroidism? Ann Surg. 2015;262(4):653–659. doi: 10.1097/SLA.0000000000001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhan I., Shah A., Holmes J., et al. Post-transplant hypophosphatemia: Tertiary 'Hyper-Phosphatoninism'? Kidney Int. 2006;70(8):1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 21.Baia L.C., Heilberg I.P., Navis G., de Borst M.H. NIGRAM investigators. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol. 2015;11(11):656–666. doi: 10.1038/nrneph.2015.153. [DOI] [PubMed] [Google Scholar]
- 22.Evenepoel P., Meijers B.K., de Jonge H., et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3(6):1829–1836. doi: 10.2215/CJN.01310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGregor R., Li G., Penny H., Lombardi G., Afzali B., Goldsmith D.J. Vitamin D in renal transplantation - from biological mechanisms to clinical benefits. Am J Transplant. 2014;14(6):1259–1270. doi: 10.1111/ajt.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrin P., Caillard S., Javier R.M., et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13(10):2653–2663. doi: 10.1111/ajt.12425. [DOI] [PubMed] [Google Scholar]
- 25.Mikuls T.R., Julian B.A., Bartolucci A., Saag K.G. Bone mineral density changes within six months of renal transplantation. Transplantation. 2003;75(1):49–54. doi: 10.1097/00007890-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Grotz W.H., Mundinger F.A., Gugel B., Exner V.M., Kirste G., Schollmeyer P.J. Bone mineral density after kidney transplantation. A cross-sectional study in 190 graft recipients up to 20 years after transplantation. Transplantation. 1995;59(7):982–986. [PubMed] [Google Scholar]
- 27.Buckley L., Humphrey M.B. Glucocorticoid-Induced Osteoporosis. N Engl J Med. 2018;379(26):2547–2556. doi: 10.1056/NEJMcp1800214. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canalis E., Delany A.M. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 30.Ugur A., Guvener N., Işiklar I., Turan M., Erdal R., Haberal M. Osteoporosis after renal transplantation: single center experience. Transplantation. 2001;71(5):645–649. doi: 10.1097/00007890-200103150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Marcén R., Caballero C., Pascual J., et al. Lumbar bone mineral density in renal transplant patients on neoral and tacrolimus: a four-year prospective study. Transplantation. 2006;81(6):826–831. doi: 10.1097/01.tp.0000203557.36884.e3. [DOI] [PubMed] [Google Scholar]
- 32.Kirino S., Fukunaga J., Ikegami S., et al. Regulation of bone metabolism in immunosuppressant (FK506)-treated rats. J Bone Miner Metab. 2004;22(6):554–560. doi: 10.1007/s00774-004-0523-1. [DOI] [PubMed] [Google Scholar]
- 33.Joffe I., Katz I., Sehgal S., et al. Lack of change of cancellous bone volume with short-term use of the new immunosuppressant rapamycin in rats. Calcif Tissue Int. 1993;53(1):45–52. doi: 10.1007/BF01352014. [DOI] [PubMed] [Google Scholar]
- 34.Bryer H.P., Isserow J.A., Armstrong E.C., et al. Azathioprine alone is bone sparing and does not alter cyclosporin A-induced osteopenia in the rat. J Bone Miner Res. 1995;10(1):132–138. doi: 10.1002/jbmr.5650100119. [DOI] [PubMed] [Google Scholar]
- 35.Dissanayake I.R., Goodman G.R., Bowman A.R., et al. Mycophenolate mofetil: a promising new immunosuppressant that does not cause bone loss in the rat. Transplantation. 1998;65(2):275–278. doi: 10.1097/00007890-199801270-00025. [DOI] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group: KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Available at: https://kdigo.org/guidelines/ckd-mbd/. Accessed January 3, 2024. [DOI] [PMC free article] [PubMed]
- 37.De Sévaux R.G., Hoitsma A.J., Corstens F.H., Wetzels J.F. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. J Am Soc Nephrol. 2002;13(6):1608–1614. doi: 10.1097/01.asn.0000016082.70875.36. [DOI] [PubMed] [Google Scholar]
- 38.Torres A., García S., Gómez A., et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int. 2004;65(2):705–712. doi: 10.1111/j.1523-1755.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 39.Josephson M.A., Schumm L.P., Chiu M.Y., Marshall C., Thistlethwaite J.R., Sprague S.M. Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation. 2004;78(8):1233–1236. doi: 10.1097/01.tp.0000137937.44703.42. [DOI] [PubMed] [Google Scholar]
- 40.Toth-Manikowski S.M., Francis J.M., Gautam A., Gordon C.E. Outcomes of bisphosphonate therapy in kidney transplant recipients: a systematic review and meta-analysis. Clin Transplant. 2016;30(9):1090–1096. doi: 10.1111/ctr.12792. [DOI] [PubMed] [Google Scholar]
- 41.Palmer SC, Chung EY, McGregor DO, Bachmann F, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2019;10(10):CD005015. Published 2019 Oct 22. 10.1002/14651858.CD005015.pub4. [DOI] [PMC free article] [PubMed]
- 42.Bonani M., Frey D., Brockmann J., et al. Effect of Twice-Yearly Denosumab on Prevention of Bone Mineral Density Loss in De Novo Kidney Transplant Recipients: A Randomized Controlled Trial. Am J Transplant. 2016;16(6):1882–1891. doi: 10.1111/ajt.13692. [DOI] [PubMed] [Google Scholar]
- 43.Brunova J., Kratochvilova S., Stepankova J. Osteoporosis therapy with denosumab in organ transplant recipients. Front Endocrinol (Lausanne) 2018;9:162. doi: 10.3389/fendo.2018.00162. Published 2018 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torregrosa J.V., Ferreira A.C., Cucchiari D., Ferreira A. Bone mineral disease after kidney transplantation. Calcif Tissue Int. 2021;108(4):551–560. doi: 10.1007/s00223-021-00837-0. [DOI] [PubMed] [Google Scholar]
- 45.Cejka D., Benesch T., Krestan C., et al. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant. 2008;8(9):1864–1870. doi: 10.1111/j.1600-6143.2008.02327.x. [DOI] [PubMed] [Google Scholar]
- 46.Evenepoel P., Cooper K., Holdaas H., et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am J Transplant. 2014;14(11):2545–2555. doi: 10.1111/ajt.12911. [DOI] [PubMed] [Google Scholar]
- 47.Cruzado J.M., Moreno P., Torregrosa J.V., et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol. 2016;27(8):2487–2494. doi: 10.1681/ASN.2015060622. [DOI] [PMC free article] [PubMed] [Google Scholar]