Abstract
Chimeric antigen receptor (CAR) T cell therapies have demonstrated immense clinical success for B cell and plasma cell malignancies. We tested their impact on the viral reservoir in a macaque model of HIV persistence, comparing the functions of CD20 CAR T cells between animals infected with simian/human immunodeficiency virus (SHIV) and uninfected controls. We focused on the potential of this approach to disrupt B cell follicles (BCFs), exposing infected cells for immune clearance. In SHIV-infected animals, CAR T cells were highly functional, with rapid expansion and trafficking to tissue-associated viral sanctuaries, including BCFs and gut-associated lymphoid tissue (GALT). CD20 CAR T cells potently ablated BCFs and depleted lymph-node-associated follicular helper T (TFH) cells, with complete restoration of BCF architecture and TFH cells following CAR T cell contraction. BCF ablation decreased the splenic SHIV reservoir but was insufficient for effective reductions in systemic viral reservoirs. Although associated with moderate hematologic toxicity, CD20 CAR T cells were well tolerated in SHIV-infected and control animals, supporting the feasibility of this therapy in people living with HIV with underlying B cell malignancies. Our findings highlight the unique ability of CD20 CAR T cells to safely and reversibly unmask TFH cells within BCF sanctuaries, informing future combinatorial HIV cure strategies designed to augment antiviral efficacy.
Keywords: CAR T cell, immunotherapy, lymphoid follicle, HIV-1, non-human primate model, B cell leukemia, solid tumor, lymphoma, CD20
Graphical abstract

Peterson and colleagues present a refined non-human primate model of CAR T cell therapy, focusing on the impact of B-cell-depleting CARs during latent viral infections. They observe striking activity in secondary lymphoid tissues, including reversible ablation of B cell follicles and reduction of infected cells in the spleen.
Introduction
HIV is a chronic infection afflicting ∼38 million people worldwide.1 No cure is readily available, and the infection must be managed with lifelong antiretroviral therapy (ART) to halt viral replication and prevent disease progression. When ART is discontinued, the virus rebounds from a dispersed reservoir of long-lived infected cells with resumption of disease progression that eventually drives severe immunodeficiency and infectious complications.2 Lymphoma is a well-described complication of chronic HIV infection, with 11.5- and 7.7-fold increased risk of non-Hodgkin lymphoma and classical Hodgkin lymphoma, respectively, relative to the general population.3 Treatments for lymphoma have evolved to include B-cell-targeting chimeric antigen receptor (CAR) T cells, which have generated considerable successes for treatment of relapsed or refractory lymphoma.4 Although HIV is an established risk factor for lymphoma, only limited data are available for B-cell-targeting CAR T cells in the context of HIV infection.5,6,7
B cell follicles (BCFs) are tissue-associated lymphoid structures involved in B cell education. In this context, BCFs exclude entry of CD8+ T cell effectors, including HIV-specific cells: this provides sanctuary and supports the persistence of latently HIV-infected follicular helper T (TFH) cells within BCFs. In HIV-associated lymphoma, B-cell-targeting CAR T cells show promise of more effectively depleting tissue B cells than other existing therapies.4 Based on this premise, disruption of BCFs could unmask and clear latently infected TFH cells from the body through one of several mechanisms. Infected targets could be (1) directly killed following an influx of cytotoxic, virus-specific T cells; (2) passively depleted by lack of B cell crosstalk during B cell aplasia; or (3) redirected to the periphery and/or other sites that possess a more potent virus-specific immune response. Furthermore, disruption of BCFs may also allow for the penetration of antiretroviral drugs into tissue sites of viral persistence,8,9 while systemic cytokine release associated with CAR T cell expansion may promote killing of infected cells by viral cytopathic effects or immune-mediated clearance.10 Despite these advantages, the effects of B-cell-targeting CAR T cells on the latent HIV reservoir remain uncharacterized. In particular, the ability of BCFs to re-form following CAR-mediated B cell depletion is unknown.
In this study, we modeled the function of B-cell-targeting CAR T cells during ART-suppressed HIV-1 infection in non-human primates (NHPs) and quantified the impact of this approach on BCF structure and virus persistence. Our findings establish that BCF ablation is safe and reversible, informing the treatment of people living with HIV (PLWH) and leukemia/lymphoma.
Results
Validation of NHP CD20 CAR T cell potency and trafficking
We first assessed NHP CD20 CAR T cell function in peripheral blood and key secondary lymphoid tissues at early time points post-infusion in a preliminary cohort of three pigtail macaques (Figures 1A and 1B; Table S1). Manufactured CD20 CAR T cells demonstrated effective ex vivo killing of CD20-expressing LLCMK2 target cells: CD8+ CAR T cells demonstrated more rapid and greater cytotoxicity (Figure S1A), whereas CD4+ CAR T cells secreted higher levels of IL-6 (Figure S1B). We assayed a co-expressed marker (truncated epidermal growth factor receptor or “EGFRt”) as a surrogate for CAR-transduced cells. In this preliminary cohort, CAR T cells were infused at a higher dose than in subsequent cohorts (5 × 107 to 1.1 × 108 CAR+ cells/kg) to match CD8+CAR+ doses from prior NHP experiments (Figures 1C and 1D).11 Infusion product CD4:CD8 ratios ranged from 4 to 10 and featured low expression of activation and exhaustion markers on CAR+ cells, almost universal expression of the proliferation marker Ki67 on CAR+ cells, and a predominantly effector memory phenotype (Figures 1E and S2). Each autologous CD20 CAR T cell product was next evaluated in vivo by infusion into animals, followed by necropsy 1–3 days later to evaluate for CAR T cell distribution (Figure 1A). Within this short time frame, CAR T cells rapidly trafficked to numerous relevant tissue sites for lymphoma and HIV, including axillary, inguinal, and mesenteric lymph nodes; spleen; and lung (Figure S1C).12 Given these promising features of CD20 CAR T cells and relevance to both cancer and HIV persistence, we proceeded to study CD20 CAR T cells in our established NHP model of ART-suppressed HIV infection.
Figure 1.
NHP CD20 CAR T cell study protocol and CAR T cell infusion product characterization
(A) A total of 11 pigtail macaques were infused with CD20 CAR T cells in this study. A preliminary cohort of animals (n = 3) was infused and necropsied 1–3 days later to quantify CAR T cell trafficking to tissues. Next, cohorts of uninfected or SHIV-infected, ART-suppressed animals (n = 4 per cohort) were infused and followed for at least 55 days. (B) Detailed time frame of the CAR T cell infusion study phase, including leukapheresis collections for CAR T cell manufacturing (Leuk), cyclophosphamide conditioning to augment CAR T cell engraftment (Cy), CAR T cell infusion (CAR), tissue surgeries (tissue 1, tissue 2), and necropsy (Nx). (C) Infusion product summary. (D) CD20 CAR modification efficiency compared with non-transduced (“mock”) controls. (E) CD4:CD8 ratios in each infusion product.
Comparable CD20 CAR T cell expansion between uninfected and simian/human immunodeficiency virus (SHIV)/ART NHP
Prior to commencing CAR T cell therapy, HIV replication should be well suppressed with ART to (1) limit hematologic and infectious complications and (2) minimize virus infection of the infused CAR T cell product.5,6,7 We next applied our NHP model to compare the impact of ART-suppressed infection on CD20 CAR T cell function and, conversely, the impact of CD20 CAR T cells on virus persistence during ART. Two groups of four pigtail macaques (“uninfected” and “SHIV/ART” cohorts, Figures 1A and 1B; Table S1) were included in this experiment: SHIV/ART animals were infected with SHIV-1157ipd3N4 via the intravenous route, followed 9–15 weeks later by initiation of suppressive therapy. Animals were treated with ART for at least 16 weeks and had suppressed viral loads (<20 copies/mL) prior to CD20 CAR T cell infusion. To augment CAR T cell engraftment and persistence, we administered a pre-CAR lymphodepletion regimen consisting of two consecutive doses of 40 mg/kg/day cyclophosphamide starting ∼7 days prior to CD20 CAR T cell infusion.13 Prior to cyclophosphamide treatment, SHIV/ART and uninfected cohorts had similar peripheral cell counts (Figure S3A). Following cyclophosphamide conditioning, SHIV/ART animals had significantly lower total leukocytes, total lymphocytes, and lymphocyte subsets, including CD2+ T cells and natural killer (NK) cells, CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD20+ B cells (Figure S3B). These data indicate that SHIV infection is associated with greater sensitivity to lymphodepletion by cyclophosphamide.
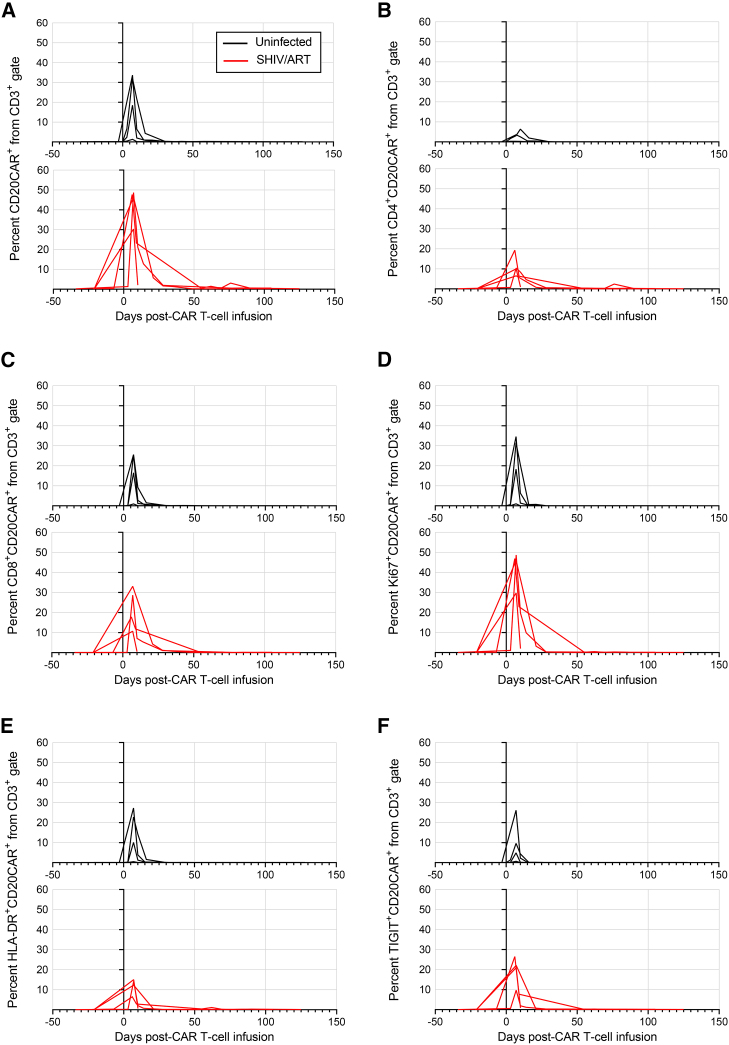
Next, CD20 CAR T cells were manufactured and infused into cyclophosphamide-conditioned animals. Each CAR product was produced from autologous uninfected leukapheresis samples. CAR T cells were infused at 5.7 × 106 to 3.5 × 107 CAR+ cells/kg, with infusion products comprising 6%–84% CAR+ cells (Figures 1C and 1D). Lower doses of CAR T cells were infused compared with preliminary cohorts to limit the potential for cytokine release syndrome (CRS). Infusion products had CD4:CD8 ratios of 0.4–3.3, low expression of activation markers and exhaustion markers on CAR+ cells, high cell cycling of CAR+ cells, and predominantly effector memory CAR+ cells (Figures 1E and S2). Following infusion, CD20 CAR T cell expansion kinetics and immunophenotype were monitored in SHIV/ART and uninfected animals (Figures 2 and S4). CAR T cells rapidly expanded in peripheral blood, peaked at day 7 post-infusion, constituting up to 50% of peripheral T cells, and then contracted with minimal detectable CAR T cells by day 30. At peak CAR expansion in vivo, CAR T cell phenotypes in peripheral blood were predominantly central memory and effector memory, and the absolute number of peripheral CD8+ CAR T cells was greater than that of CD4+ CAR T cells, as previously described (Figure S5).11,14 Peak CAR T cell levels at day 7 coincided with peak CAR T cell proliferation (measured by Ki67 expression) and with maximum expression of the activation marker HLA-DR and the exhaustion marker TIGIT. Markers that peaked at earlier time points on CAR T cells included the naive T cell subset (four of four animals), CD69 (four of four animals), and PD-1 (three of four animals). Early expression of CD69 and PD-1 may be associated with the initial onset of systemic inflammation prior to peak CAR expansion, as discussed below. Despite SHIV-infected animals’ lower lymphocyte counts prior to CAR T cell infusion (Figure S3B), no significant difference in peak CAR expansion was observed between SHIV/ART and uninfected groups (p = 0.4857, Figure 2A). These data confirm that suppressed infection with an HIV-like virus does not have an impact on CD20 CAR T cells’ expansion in vivo.
Figure 2.
Phenotype of peripheral CD20 CAR+ T cells in vivo
Uninfected animals (black lines, n = 4) or animals infected with SHIV-1157ipd3N4 and suppressed by ART (red lines, n = 4) were infused with CD20-specific CAR T cells. At the indicated time points following CAR T cell infusion, peripheral blood samples were quantified by flow cytometry for (A) total CAR+ cells, (B) CD4+CAR+, (C) CD8+CAR+, (D) Ki67+CAR+, (E) HLA-DR+CAR+, and (F) TIGIT+CAR+ subsets.
CD20 CAR T cells traffic to and persist within tissues
We next quantified CD20 CAR T cell trafficking to tissues sampled 2 and 4 weeks post-CAR infusion (Figure 3). Select data from individual animals were unavailable due to limitations in sample collection and/or yield. Consistent with our observations in the preliminary cohort necropsied 1–3 days post-CAR infusion, CAR T cells in SHIV/ART and uninfected animals were detected broadly, including in the lungs, duodenum/jejunum, colon, lymph nodes, spleen, and bone marrow (BM) at 13–16 days post-CAR infusion. By days 27–30, most tissues from both cohorts retained similar levels of CAR expression, except for a significant decline in CAR T cells in BM and CD4+ CAR T cells in peripheral lymph nodes. Overall, no significant differences in CAR T cell distribution in tissues were observed between the uninfected and the SHIV-infected animals. Although expression of activation and exhaustion markers in tissue-associated CAR T cells was likewise consistent between the two tissue time points, expression of specific markers on CAR+ cells was often tissue specific. For example, CAR T cells in the upper gastrointestinal tract (UGI) displayed higher levels of CD69 and HLA-DR than in the lower gastrointestinal tract (LGI), whereas Ki67 and PD-1 expression was relatively comparable between compartments (Figures S6A–S6D). The significant decrease in BM-associated CAR T cells (Figure 3) was accompanied by a modest increase in TIGIT expression that did not reach significance (Figure S6E), which could suggest T cell exhaustion as a driver behind CAR T cell decay, as we have observed previously.15 To address whether activated CAR T cells were affecting unmodified bystander T cells, we also compared the immunophenotype of tissue-associated CAR+ and CAR− subsets in uninfected and SHIV/ART animals. CD69 expression did not significantly differ between CAR+ and CAR− cells, whereas HLA-DR expression was uniformly increased in CAR+ cells across all tissues (Figures S7A and S7B). Other activation and exhaustion markers’ expression was upregulated in CAR+ T cells in a tissue-specific manner, including Ki67 in lymph nodes (Figure S7C); PD-1 in bronchoalveolar lavage, BM, and mesenteric lymph nodes (Figure S7D); and TIGIT in UGI and lymph nodes. Taken together, these data show that activated CD20 CAR T cells persist for up to 4 weeks within diverse secondary lymphoid tissues in vivo, with distinct activation profiles in each site.
Figure 3.
Kinetics of tissue-associated pigtail macaque CD20 CAR+ T cells
At 13–16 days (squares) and 27–30 days (diamonds) post-CD20 CAR T cell infusion, CAR+ cells were quantified from an array of secondary compartments, including lung (bronchoalveolar lavage, BAL), bone marrow (BM), upper (UGI) and lower gastrointestinal tract (LGI), mesenteric (MLN) and peripheral lymph nodes (PLN), and spleen. Samples were collected from uninfected (n = 3, solid symbols) and SHIV-infected, ART-suppressed animals (n = 4, open symbols). Tissues with low cell yield were omitted from analyses. Shown are (A) total CD3+, (B) CD4+, and (C) CD8+ CAR T cells. Statistical comparisons were measured by the Mann-Whitney test (∗p < 0.05).
Preemptive immunosuppression prevents severe CRS while maintaining CAR expansion
CRS is a well-described phenomenon following CAR T cell infusion, whereby immune overactivation can lead to end-organ damage.16 To overcome severe CRS observed in our preliminary cohort, subsequent SHIV/ART and uninfected cohorts were managed with preemptive dexamethasone and intravenous infusion of the anti-IL-6R antibody tocilizumab (Figure 4). This approach allowed for improved management of CRS symptoms17 and cell-based markers of inflammation, including lymphocytosis, monocytosis, neutrophilia, and platelets (Figures S8A and S8D). Notably, ART-suppressed SHIV infection was associated with significantly lower platelet counts following CAR T cell treatment (Figure S8E), with one SHIV/ART animal developing late-onset thrombocytopenia to ∼50 × 103 cells/μL on day 55 post-CAR infusion, leading to early terminal analysis. Detailed comparisons between the timing of peak serum IL-6 and several candidate biomarkers indicated that CD4/CD8 ratio may peak prior to IL-6, a potentially useful early biomarker for CRS (Figure S8F). Collectively, these results show that suppressed SHIV infection does not heighten CRS-dependent CAR toxicity and that preemptive immunosuppression decreases the severity of CRS without influencing CAR T cell expansion.
Figure 4.
Management of cytokine release syndrome during CD20 CAR T cell therapy in pigtail macaques
Following infusion of CD20 CAR T cells in (A–D) uninfected and (E–H) SHIV-infected, ART-suppressed animals, inflammation was quantified via serum concentrations of IL-6, ferritin, and C-reactive protein (CRP). Circles indicate administration of tocilizumab (orange) and dexamethasone (gray for morning dosing, yellow for evening dosing).
CD20 CAR treatment depletes B cells in blood and tissues
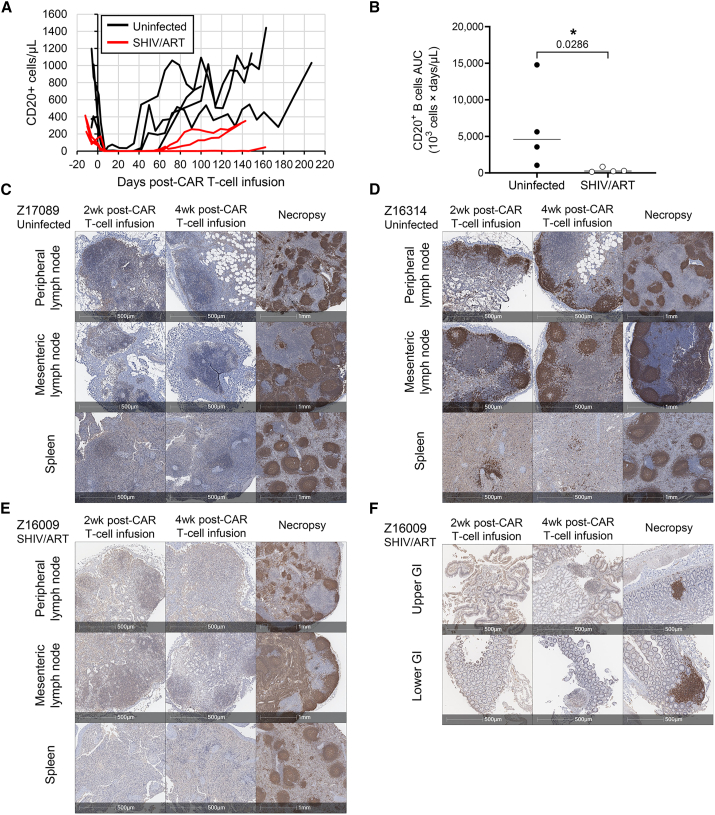
Following infusion of CD20 CAR T cells, all uninfected and SHIV-infected, ART-suppressed animals in our study developed transient B cell depletion in peripheral blood, confirming in vivo potency (Figures 5A and S9). CD20 CAR T-cell-dependent B cell aplasia was associated with marked dysgammaglobulinemia in three animals from the uninfected cohort, relative to six healthy controls (Figure S10), which persisted even after peripheral B cell recovery (Figures 5A and S9). Notably, B cell recovery was prolonged in the SHIV/ART cohort (median 70.0 days B cell aplasia) relative to the uninfected cohort (median 45.5 days B cell aplasia) (Figures 5B and S9). We next quantified the functional impact of B cell depletion in tissues. The goals of these experiments were (1) to quantify the impact of CD20 CAR T cells on the architecture of secondary lymphoid structures such as BCFs where latently infected cells are known to reside, (2) compare BCF depletion across distinct secondary lymphoid compartments, and (3) confirm that BCF targeting was safe and reversible. Immunohistochemistry (IHC) analyses showed dramatic depletion of CD20+ cells and ablation of BCFs in mesenteric lymph nodes, peripheral lymph nodes, and spleen, with BCF architecture recovering by the end of the study (Figures 5C–5E, S11, and S12). Likewise, CD20-expressing B cells in gut-associated lymphoid tissue (GALT) were also ablated post-CD20 CAR treatment and reestablished by the end of the study (Figure 5F). One animal (Z16314) experienced incomplete B cell depletion in blood and tissues, likely due to marginal CAR T cell expansion (Figures 5D, S9, and S11). Overall, these data clearly show that CD20 CAR T cells potently and reversibly disrupt BCFs in diverse tissue sites that are also known to act as sanctuaries for latent HIV-infected cells.
Figure 5.
Depletion of B cells in peripheral blood and B cell follicles following CD20 CAR T cell infusion
(A) Animals from the uninfected (black lines, n = 4) or SHIV/ART cohorts (red lines, n = 4) were infused with CD20-specific CAR T cells, and peripheral blood samples were quantified for CD20+ B cells by flow cytometry. (B) Area under the curve (AUC) calculations from (A) were calculated by the trapezoid method. Statistical comparisons were measured by the Mann-Whitney test (∗p < 0.05). (C–F) Representative immunohistochemistry images of CD20+ B cells (brown) in lymphoid tissues of animals from (C and D) uninfected and (E and F) SHIV/ART cohorts. Tissues were collected 13–16 days (2wk) and 27–30 days (4wk) post-CD20 CAR T cell infusion. Necropsy tissues for IDs Z17089, Z16314, and Z16009 were collected 407, 350, and 143 days post-CD20 CAR T cell infusion, respectively.
CD20 CAR T cells redistribute lymph-node-associated CD4+ cells
The ultimate goal of our CD20 CAR-mediated depletion of BCFs was to unmask TFH cells, a preferred cellular reservoir for HIV/SIV infection.18 Given the essential role of B cells in maintenance of CD4+ TFH cells,19,20,21 we hypothesized that B cell depletion would liberate CD4+ T cells from BCFs and expose latently infected TFH to virus-specific CD8 T cell effectors, leading to reductions in systemic SHIV reservoir size. Following B cell depletion and BCF disruption, we observed a pronounced redistribution of CD4+ T cells within lymph nodes (Figure 6). Rather than the classical compartmentalization in paracortex zones, CD4+ T cells were instead diffusely dispersed throughout the lymph node, including in the peripheral margins, where CD4+ T cells are typically absent (Figure 6A). By flow cytometry, CD4+ TFH cells were severely depleted in whole peripheral and mesenteric lymph nodes collected at 4 weeks post-infusion (Figures 6B and 6C). Although samples were not available at time points prior to BCF depletion, measurements at end-of-study necropsy (Figure 1B) showed significant CD4+ TFH cell recovery (Figure 6C). This finding was consistent with the transient kinetics of BCF disruption that we observed (Figure 5). At this time point, we found that the repleted CD4+ T cells occupied a typical distribution restricted to the paracortex (Figure 6A). These data support a model in which CD20 CAR T cells transiently ablate BCFs and redistribute lymph-node-associated CD4+ cells, which re-form and relocalize following CD20+ B cell recovery.
Figure 6.
Redistribution of CD4+ T cells following CD20 CAR T cell infusion
(A) Representative IHC images of CD4+ T cells (brown) and CD68+/CD163+ macrophages (red) in peripheral lymph nodes collected longitudinally from uninfected and SHIV-infected, ART-suppressed animals. (B) Representative flow cytometry plots showing the gating of TFH cells at early and late post-CAR infusion time points. (C) Frequency of TFH cells in lymph nodes of uninfected and SHIV-infected, ART-suppressed animals. Statistical comparisons were measured by the Mann-Whitney test (∗p < 0.05).
CD20 CAR T-cell-mediated B cell depletion does not affect latent SHIV reservoirs
To test whether transient BCF disruption and TFH redistribution decreased the size of the latent SHIV reservoir,18,22 we applied multiple assays, including intracellular cytokine staining (ICS) for SHIV-specific T cells (Figure S13), cell-associated SHIV RNA and DNA (Figure 7), plasma viral loads (Figure 8A), and SHIV RNAscope (Figures 8B and 8C). We first investigated whether cyclophosphamide conditioning and CAR-dependent depletion of CD20+ B cells led to changes in peripheral SHIV-specific T cells. ICS was used to assay four time points from each of the four animals in the SHIV/ART group, including SHIV infection weeks 8–10, ART weeks 13–16, and CAR T cell time points 6–7 days and 17–21 weeks post-infusion (Figure S13A). We observed a range of SHIV Gag and Env peptide-specific responses in CD4+ (Figure S13B) and CD8+ cells (Figure S13C). Induction of CD107a, granzyme B, IFNγ, TNF-α, IL-2, and MIP-1β did not follow consistent trends. For example, CD4+TNF-α+ responses were substantially ablated at the early post-CAR time point, when B cells were also depleted, and partially recovered 17–21 weeks later when B cells rebounded (Figure S13D). In contrast, CD4+IL-2+ (Figure S13E) and CD4+IFNγ+ responses (Figure S13F) were variably depleted at the early post-CAR time point. In the CD8 compartment, CD8+CD107a+ and CD8+granzyme B+ responses were depleted at the early post-CAR time point and remained undetectable 17–21 weeks later, even when B cells had rebounded (Figures S13G and S13H), whereas CD8+IFNγ+ responses were variably depleted at the early post-CAR time point and more uniformly depleted 17–21 weeks later (Figure S13I). Collectively, our ICS data suggest that cyclophosphamide conditioning and CAR-dependent B cell depletion do not uniformly affect SHIV-specific T cells, but likely play a role in sustaining selected subsets with varying kinetics. Next, we analyzed virological endpoints from the same tissue time points 2 and 4 weeks post-CAR infusion, along with an exhaustive set of 25 distinct tissues at end-of-study necropsy. Our control group for this experiment was a previously published cohort of four SHIV/ART pigtail macaques that did not receive cyclophosphamide conditioning or CD20 CAR T cells.23 We did not observe a significant change in levels of peripheral blood mononuclear cell (PBMC)-associated SHIV DNA in the CD20 CAR-treated SHIV/ART cohort over time (Figure 7A). Spleen samples from the same time points revealed a statistically significant reduction in spleen-associated SHIV DNA (15.8-fold reduction, p = 0.029, Figure 7B), which persisted through the end-of-study necropsy time point (6.4-fold reduction, p = 0.029, Figure 7C).23 Unexpectedly, the end-of-study time point also demonstrated a significant increase in SHIV RNA in the jejunum (p = 0.029) and a trend toward increased SHIV RNA in the ileum (p = 0.057) (Figure 7D). No other differences in SHIV DNA or SHIV RNA levels were observed across tissue sites. Immunosuppression with tocilizumab and dexamethasone did not have an impact on ART suppression of SHIV in peripheral blood (Figure 8A). RNAscope assays quantified SHIV-infected cells with active transcription of viral RNA (vRNA) in lymph node and spleen (Figures 8B and 8C). Similar to the total quantification of SHIV RNA, the frequency of vRNA+ cells did not change longitudinally following CD20 CAR T cell therapy. The low frequency of vRNA+ cells (0–15 vRNA+ cells per 105 cells) limited the ability to conduct spatial comparisons of vRNA+ cells over time. Collectively, our reservoir assays showed a lack of systemic impact on SHIV reservoir size following BCF depletion and TFH redistribution, suggesting that these transient perturbations are insufficient to effectively deplete latently infected cells.
Figure 7.
Cell-associated SHIV DNA persists during CD20 CAR T cell treatment
(A) PBMC-associated SHIV DNA was quantified longitudinally at the indicated time points and normalized to a housekeeping gene, MRPP30. (B) Tissue-associated SHIV DNA was quantified longitudinally from the indicated tissues at the indicated time points. (C and D) At necropsy while on suppressive ART, (C) SHIV DNA and (D) SHIV RNA copies per million cell equivalents were measured from the indicated tissue sources. Tissue #1 and tissue #2 correspond to collection of blood and tissues at 13–16 and 27–30 days post-CAR infusion, respectively. Three of four necropsies were conducted 139–163 days post-CAR infusion, while one animal (Z15338) ended the study at 55 days post-CAR infusion due to persistent thrombocytopenia. MLN, mesenteric lymph node; PLN, peripheral lymph node; LGI, lower GI (colon); UGI, upper GI (duodenum/jejunum); BM, bone marrow; BAL, bronchoalveolar lavage. Control (n = 4) represents a historical SHIV-infected, ART-suppressed cohort that did not receive cyclophosphamide or CAR T cells.23 Statistical comparisons were measured by the Mann-Whitney test (∗p < 0.05).
Figure 8.
Impact of CD20 CAR therapy on SHIV viral load in blood and tissues
(A) Plasma SHIV viral load was monitored from the time of SHIV-1157ipd3N4 infection through ART initiation and CD20 CAR T infusion. (B) The frequency of SHIV viral RNA+ (vRNA+) cells in mesenteric lymph nodes (MLN), peripheral lymph nodes (PLN), and spleen was measured by RNAscope at 13–16 (tissue #1) and 27–30 days (tissue #2) post-CAR infusion as well as at the end of the study post-CAR T cell infusion (necropsy, Nx). (C) Representative RNAscope images of the spleen with yellow arrows identifying SHIV RNA+ cells (red).
Discussion
In our unique NHP model of CAR T cell trafficking, function, and persistence, we show that CD20 CAR T cells potently and reversibly deplete peripheral CD20+ B cells, as well as B-cell-enriched tissue structures, including BCFs. We further describe an effective protocol to manage CAR T cell activity and associated toxicities, namely CRS, in the setting of ART-suppressed SHIV infection. Although CD20 CAR T cell potency in SHIV-infected, ART-suppressed animals is comparable to or greater than that in uninfected controls, we conclude that B cell depletion, namely in tissue-associated BCFs, is insufficient to have an impact on latent SHIV reservoirs. Our model offers highly valuable insights not only on the role of B cells during persistent HIV-1 infection but also on mechanisms governing T cell function in key tissue sites relevant to a myriad of infectious diseases and solid tumors.
CAR T cell therapy is increasingly used to treat relapsed/refractory lymphoma. Although HIV infection is an established risk factor for lymphoma,3 little is known about CAR T cell function in PLWH5,6,7 or, critically, whether B-cell-targeting CAR T cells in the course of lymphoma treatment can concomitantly reduce the size of the HIV reservoir. To address these questions, we applied our established macaque model of suppressed HIV-1 infection that recapitulates key characteristics of PLWH, such as immunodeficiency, including chronic infection; seeding of tissue reservoirs; viral suppression with ART; and viral rebound following ART interruption.24 Our CD20 CAR molecule was previously validated in a rhesus macaque model11 and shows promise as a therapeutic in humans25,26,27,28,29; the comparable functional profile that we observe in pigtail macaques serves as further validation of our model. In SHIV-infected, ART-suppressed animals, CD20 CAR T cells were functional and rapidly expanded with distribution to broad tissue sites, including well-characterized SHIV reservoirs. ART-suppressed infection did not amplify CRS but exacerbated CAR T cell hematologic toxicity, including greater sensitivity to cyclophosphamide conditioning, prolonged B cell depletion, and lower platelet counts. We show for the first time that CD20 CAR T cells potently yet transiently disrupt BCFs and redistribute HIV-infected cells in lymphoid tissues. Despite efficient BCF ablation and a reduction in splenic viral reservoirs, we did not observe substantial depletion of systemic viral reservoirs, suggesting that unmasking of this canonical site of HIV/SIV/SHIV persistence is insufficient alone for an HIV cure.
We first validated our model by showing that CD20 CAR T cell therapy effectively depletes CD20-expressing targets in the background of suppressed SHIV infection. We characterized an initial 7-day expansion of CAR T cells in peripheral blood, followed by a contraction phase with a slower decay rate in tissues compared with blood. Our CAR T cells were manufactured using a balanced CD4:CD8 approach that enhances CAR T cell proliferation and potency.14,30 We observed heightened IL-6 production from CD4+ CAR T cells compared with their CD8+ counterparts, suggesting a potential role for the CD4+ subset in CRS. Several effective strategies have been developed to mitigate CRS risk without compromising efficacy,14 and variations in CD4:CD8 ratio should likewise be studied for impacts on CRS and therapeutic effectiveness. SHIV infection did not have an impact on CD20 CAR T cell expansion or persistence and did not predispose to worsened CRS, which we effectively managed with preemptive immunosuppression. Systemic cytokine release in the context of routine vaccine regimens can promote latency reversal, which led us to hypothesize that CAR-dependent CRS may similarly reactivate the latent reservoir.10,31 Likewise, humoral immune responses also play an important role in control of HIV replication,32 raising the possibility that CAR-dependent depletion of B cells may possess further latency-reversing properties. However, we did not observe SHIV viral blips during ongoing ART. SHIV RNA levels were elevated in the small intestine of CAR-treated animals relative to controls, which may be related to the persistence of CAR T cells in the GI tract. Overall, the majority of our data suggest that B cell depletion by CAR T cells does not impair viral control, consistent with studies of the CD20-depleting antibody rituximab in an NHP natural host model.33
HIV infection is associated with various cytopenias that may be only partially rescued with ART.34,35,36,37 Using our in vivo model, we found that CD20 CAR T cell therapy during suppressed SHIV infection is associated with a moderately increased risk of hematologic toxicity: SHIV-infected, ART-suppressed animals exhibited greater sensitivity to lymphodepletion by cyclophosphamide across all lymphocyte subsets, including CD2+ NK cells, T cells, and B cells. Following contraction of CD20 CAR T cells, SHIV-infected animals also displayed slower recovery of B cells and platelets relative to CAR-treated, uninfected animals. Hematologic toxicity may be further exacerbated in advanced HIV disease due to shared risk factors for CAR T cell hematologic toxicity.38,39,40 Based on the expanding application of CAR T cells for B cell malignancies, our findings warrant additional clinical studies for HIV-associated malignancies to better understand CAR-dependent toxicities that may be amplified in PLWH.
CD20 CAR T cells trafficked to primary and secondary lymphoid tissues, where lymphomas may reside, and persisted in an activated state for at least 4 weeks, suggesting that CAR T cells can be effective in PLWH with underlying B cell malignancies. BCFs are known sanctuaries for HIV-infected TFH cells,18,22 a major reservoir of infected cells in lymphoid tissues during chronic infection.41 After confirming that CAR T cells trafficked to HIV reservoir sites, we studied whether these engineered effector cells could have an impact on the viral reservoir in our NHP model. We hypothesized that B-cell-targeting CAR T cells would disrupt BCFs, exposing infected cells to elimination by SHIV-specific immune cells.33 We observed potent and transient B cell aplasia and ablated BCFs in established HIV reservoir sites, including lymph nodes, spleen, and GALT, with an associated reduction in total IgG, IgM, and IgA levels in plasma. BCF ablation led to considerable depletion of lymph-node-associated TFH cells post-CAR treatment, which were replenished in parallel with BCF re-formation. Transient BCF disruption and redistribution of TFH cells was concurrent with a decrease in the splenic SHIV reservoir following CD20 CAR T cell infusion, but an effect on other tissue reservoirs was not observed. Importantly, we refer to “redistribution” of TFH because our study cannot distinguish between (1) killing of TFH cells following increased exposure to virus-specific CD8+ T cells and other subsets; (2) TFH cell death following loss of B cell support; (3) persistence of lymph-node-associated TFH, but with transient downregulation of defined phenotypic markers; and/or (4) retrafficking of TFH to other sites of virus persistence and subsequent repopulation of re-formed BCFs. We favor a model in which TFH cells are liberated from BCFs but, in the absence of a broad and potent adaptive immune response, are not cleared, as suggested by a lack of consistent impact on SHIV-specific T cells and the absence of significant changes to the viral reservoir. Combination therapies with immune effectors may be a promising strategy to clear liberated TFH cells. The lack of notable reservoir reductions could also be attributed to TFH cells representing a minor population within lymphoid tissues (∼12% of CD4+ memory cells as measured in ART-suppressed SIV infection)18 and inherent limitations of total DNA measurements of the viral reservoir. While TFH cells have a similar frequency of viral DNA to non-TFH cells in ART-suppressed SIV infection,18 TFH cells harbor a disproportionate fraction of replication-competent virus during chronic suppressed HIV infection, suggesting that total DNA measurements of the reservoir may overlook crucial changes in the replication-competent reservoir.22,41 Importantly, our data are the first to demonstrate the impact of CAR T cells on the depletion of BCFs and associated TFH cells and highlight their full recovery following CAR T cell contraction. Future studies should be conducted to further define the fates and phenotypes of replication-competent HIV-infected cells within lymphoid tissues following CAR T cell therapy.
Although we observed that B-cell-targeting CAR T cells modestly reduced the splenic viral reservoir, the limited impact on systemic reservoirs suggests that CD20 CAR T cells alone are insufficient to induce sustained viral remission. Disruption of BCFs may also contribute to a combinatorial HIV reservoir-reducing approach. Whereas B-cell-specific CAR T cells target an abundant antigen that supports massive effector proliferation, a major barrier to HIV-specific CAR T cells is a paucity of viral antigen.15 Based on our critical observation that B-cell-specific CAR T cells obliterate BCF sanctuaries that can expose persistently infected cells residing in BCFs, one potential combination strategy would merge the expression of CD20 CARs and HIV-specific CARs in the same T cell to prolong expansion and augment clearance of rare HIV-infected cells.15 Our data emphasize that BCF ablation is safe, transient, and reasonable to include in a combinatorial reservoir-targeting strategy. Notably, while data from our group and others confirm that SHIV-1157ipd3N4 recapitulates HIV disease progression and persistence, its low pathogenicity relative to other HIV-like viruses may lead to lower-level reservoir seeding, i.e., decreasing the dynamic range in which we were able to measure changes in our study.24,42,43 CD20 CAR T cells could hence display a measurable impact on the viral reservoir in other models, namely using SIVmac239. Due to sampling constraints, we were unable to compare the impact of CAR-dependent B cell depletion with that of the lymphodepleting cyclophosphamide conditioning regimen used to augment CAR T cell expansion. We speculate that this mild level of conditioning played a necessary but insufficient role in driving B cell depletion. Likewise, we did not observe any evidence that cyclophosphamide affected the SHIV reservoir, which remained highly stable between pre-CAR and CAR infusion time points. This alkylating chemotherapy likely acts non-specifically on uninfected and infected T cells, as suggested by a clinical trial demonstrating that cyclophosphamide did not reduce PBMC-associated total HIV DNA.44 The lack of impact on total PBMC-associated viral DNA levels in our NHP model and in a clinical trial suggests that cyclophosphamide conditioning transiently depletes T cells with no preference for infected vs. uninfected T cells.
In summary, we have developed an NHP model of B-cell-targeting CAR T cells during persistent HIV infection. CD20 CAR T cells were well tolerated and potent in healthy animals as well as those with suppressed SHIV infection, supporting the application and study of CAR T cells in PLWH with lymphoma. We show that CD20 CAR T cells transiently ablate lymphoid follicles and expose SHIV-infected targets, which leads to a decline in the splenic SHIV reservoir, but were unable to affect the systemic viral reservoir. These findings provide the critical foundational knowledge for the evaluation of combination strategies incorporating both CD20-directed CAR T cells and anti-HIV effectors such as HIV-targeting CAR T cells.15 Our NHP platform is the ideal setting to combine and test enhanced virus-specific immune functions with novel approaches to unmask viral reservoirs, such as CD20 CAR T cells.
Materials and methods
CD20 CAR T cell manufacturing
CD20 CAR-expressing, self-inactivating lentivirus (vector plasmids kindly provided by Dr. Michael Jensen) was produced by the Fred Hutch Preclinical Vector Production Core using a third-generation packaging system pseudotyped with Cocal envelope.15 Vectors were titered by the transduction of HT1080 cells and measurement of lentiviral integration by qPCR.45,46 CAR T cells were manufactured ex vivo using an optimized protocol as described.15 Briefly, PBMCs were collected from each NHP by leukapheresis; for SHIV-infected animals, leukapheresis was performed prior to SHIV infection. CD4+ and CD8+ T cells were isolated from cryopreserved PBMCs by bead-based selection, followed by stimulation with artificial antigen-presenting cells (aAPCs; generous gift from Dr. James Riley). Three days later, the CD4+ and CD8+ T cells were transduced separately with CD20 CAR lentiviral vector at a multiplicity of infection of 1–12. Transduction was carried out by co-incubating lentivirus with cells (4 × 106 cells/mL) and protamine sulfate (4 μg/mL) and rotating the transduction reaction for 4 h at 37°C. The next day, the transduced CD4+ and CD8+ T cells were pooled at a 1:1 ratio and seeded into G-REX100 flasks (Wilson Wolf, Saint Paul, MN) for 7–8 days of expansion.
In vivo NHP CAR T cell studies
This study was approved by the Institutional Animal Care and Use Committees of the Fred Hutchinson Cancer Center/University of Washington (protocol no. 3235-04). Pigtail macaques (Macaca nemestrina) were housed and cared for under conditions that meet NIH standards as stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996), ILAR recommendations, and AAALAC accreditation standards, as described previously.15 The lymphodepletion conditioning regimen consisted of cyclophosphamide (40 mg/kg per dose) on two consecutive days starting on day −7 prior to CAR T cell infusion. Exceptions include animal Z17318, which received cyclophosphamide starting on day −4, and animal Z15382, which received cyclophosphamide starting on day −8. CAR T cells were infused at doses ranging from 5.7 × 106 to 1.1 × 108 CAR+ cells/kg. Blood was collected longitudinally until necropsy. Biopsies of the duodenum/jejunum (UGI), colon (LGI), spleen, and lymph nodes, along with bronchoalveolar lavage, were collected at 2 and 4 weeks following infusion of CAR T cells, as previously described.15 Tissues were dissociated for downstream analysis using previously described protocols.15
SHIV infection of NHPs
Pigtail macaques were intravenously challenged with SHIV-1157ipd3N4, as described previously.15 Briefly, SHIV-1157ipd3N4 virus stocks (1.9 × 104 TCID50/mL) were prepared by in vitro infection of rhesus PBMCs that were subsequently stored at −80°C. Virus stocks were thawed for intravenous infection using 500 μL of virus stock per inoculation. ART was initiated 9–15 weeks post-infection with a first-line HIV treatment regimen as recommended by the World Health Organization (WHO)47: tenofovir disoproxil fumarate (5.1 mg/kg), emtricitabine (40 mg/kg) (kindly provided by Gilead Sciences, Foster City, CA), and dolutegravir (2.5 mg/kg) (donated by Viiv Healthcare, Research Triangle, NC). ART was continued for 16–18 weeks, including during cyclophosphamide conditioning and subsequent CAR T cell infusion, as described above. These animals were compared with an SHIV-infected control group (n = 4) from a previous study in which animals were similarly infected with SHIV-1157ipd3N4 and initiated on ART 12–24 weeks post-infection but did not receive cyclophosphamide or CAR T cells.23 Plasma SHIV viral load and tissue-associated SHIV DNA and RNA were quantified using previously described protocols.48,49
CRS management and serum cytokine assays
Animals in the SHIV-infected and uninfected groups were given tocilizumab (8 mg/kg per dose) and dexamethasone (4 mg/kg per dose) under clinician direction to prevent and treat CRS. Blood samples were analyzed by the University of Washington Clinical Laboratory for C-reactive protein (CRP), ferritin, IL-6, IgG, IgM, and IgA.
Flow cytometry and real-time cell analysis
Immunophenotyping of CAR T cell infusion products, PBMCs, and tissues was performed using the following fluorophore-conjugated antibodies: CD3 (BD, Franklin Lakes, NJ; clone SP34-2), CD45 (BD; clone D058-1283), CD4 (BioLegend, San Diego, CA; clone OKT4), CD8 (BD; clone SK1), PD-1 (eBioscience, San Diego, CA; clone J105), TIGIT (eBioscience; clone MBSA43), CD69 (BioLegend; clone FN50), HLA-DR (BD; clone G46-6), Ki67 (BD; clone B56), CD45RA (BD; clone 5H9), CCR5 (BD; clone 3A9), CCR7 (BD; clone 3D12), CD28 (BD; clone CD28.2), and CD95 (BD; clone DX2). EGFRt was used as a surrogate marker of CAR-transduced cells and was detected using fluorophore-conjugated cetuximab (Eli Lilly, Indianapolis, IN). T cells were identified by expression of CD3 and CD45. Immunophenotyping of TFH cells was performed using the following fluorophore-conjugated antibodies: CD3 (BD; clone SP34-2), CD4 (BD; clone L200), CD8 (BD; clone SK1), CCR7 (BD; clone 15053), CD95 (BD; clone DX2), CCR5 (BD; clone 3A9), CD28 (Beckman Coulter; clone CD28.2), CXCR5 (eBioscience; clone MU5UBEE), CD200 (BioLegend; clone OX-104), and PD-1 (BioLegend; clone EH12.2H7). TFH cells were identified as CD4+CD95+CD200hiPD-1hi T cells. All panels included a live/dead stain. CAR T cell cytotoxicity was evaluated ex vivo using the real-time cell analysis (RTCA) assay on the xCELLigence platform,15 in which effector CAR T cells were co-cultured with CD20-expressing LLCMK2 target cells at varying effector-to-target ratios. Cell death of targets was measured as a “normalized cell index.”
Histopathology and immunohistochemistry
Tissues were fixed in freshly prepared 4% paraformaldehyde for 24 h, transferred to 80% ethanol, and embedded in paraffin within 7–10 days, and blocks were sectioned at 5 μm. Slides were deparaffinized in xylene and rehydrated through a series of graded ethanol to distilled water solutions. Heat-induced epitope retrieval (HIER) was performed with the antigen retrieval citrate buffer (pH 6.0) in a NxGen decloaking chamber (Biocare Medical, Pacheco, CA) at 110°C for 15 min, cooled for 20 min, and then rinsed twice in ddH2O and 1× TBS with 0.05% Tween 20 (TBS-T). Slides were incubated with blocking buffer (TBS-T with 0.25% casein) for 30 min at room temperature, rinsed with TBS-T, and incubated at room temperature with antibody against CD20 (Invitrogen, Waltham, MA; cat. no. PA1-9024; at 1:800 for 1 h), which was diluted in blocking buffer. Endogenous peroxidases were blocked with 1.5% H2O2 in TBS-T for 5 min. Slides were then incubated with goat Polink-1 HRP (GBI Labs, Origene, Rockville, MD; cat. no. D33-110). Slides were developed using Impact DAB (3,3′-diaminobenzidine; Vector Laboratories, Newark, CA), washed in ddH2O, counterstained with hematoxylin (Biocare Medical), mounted in Permount (Fisher Scientific, Waltham, MA), and scanned at 20× magnification.
RNAscope in situ hybridization
RNAscope in situ hybridization was performed as previously described.50 In brief, after slides were deparaffinized in xylene and rehydrated through a series of graded ethanol to distilled water, slides were incubated in 3% H2O2 in PBS for 10 min at room temperature. Retrieval was performed for 15 min in RNAscope 1X Target Retrieval Reagent (ACD cat. no. 322000) at 94°C–96°C, followed by treatment with Protease Plus (ACD cat. no. 322331) for 15 min at 40°C. Prior to hybridization, probe stocks were centrifuged at 13,000 rpm using a microcentrifuge for 10 min and then diluted 1:2 in probe diluent (ACD cat. no. 300041) to reduce probe aggregation tissue artifacts. The slides were incubated for 2 h at 40°C with the V-SHIV-C1157 specific probe (ACD cat. no. 481261). Slides were developed using the RNAscope 2.5 HD Detection Reagents-RED (ACD cat. no.322360), and amplification steps were performed according to the ACD manufacturer’s protocol.
Quantitative image analysis
Quantitative image analysis was performed using HALO software (v.3.4.2986; Indica Labs, Albuquerque, NM) on lymph node or splenic sections from each animal. For CD20 quantification, the Multiplex IHC module was used to detect CD20+ cells, and quantification is presented as a proportion of total tissue (CD20+ cells/mm2). For vRNA quantification, the ISH module was used to determine the total number of SHIV vRNA+ cells/105 total cells. In all instances, manual curation was performed to confirm accurate annotations.
Peptide stimulation assay
SHIV peptide stimulation was performed as described previously.51 Briefly, cryopreserved PBMCs were thawed and rested overnight at 37°C. Cells were stimulated with a pool of peptides spanning SHIV Env and Gag (15-mers overlapping by 11 amino acids) at a final concentration of 2.5 μg/peptide/106 cells in the presence of anti-CD28 (BD; clone CD28.2), anti-CD107a (eBioscience; clone H4A3), brefeldin A (Sigma-Aldrich; 10 μg/mL), and GolgiStop (BD; 0.7 μL/mL) for 6 h at 37°C. After stimulation, the cells were washed in PBS, stained for surface markers, fixed/permeabilized with Cytofix/Cytoperm (BD), and stained for intracellular markers. Antibodies against the following antigens were used for staining at predetermined concentrations: perforin (Mabtech), granzyme B (BD; clone GB11), MIP-1β (BD; clone D21-1351), IFNγ (BioLegend; 4S.B3), TNF-α (BioLegend; MAb11), IL-2 (BD; clone MQ1-17H12), Ki67 (BD; clone B56), CD3 (BD; clone SP34-2), CD4 (BioLegend; OKT4), CD8 (BD; clone SK1), and CD95 (BD; clone DX2). CD4+ and CD8+ memory T cells were defined as CD95+ singlet, live, CD3+ lymphocytes and by positive/negative gating based on clearly grouped populations, historical-determined expression, and the use of internal controls, including staphylococcal enterotoxin B (1 μg/mL).
Statistical analyses
Statistical comparisons were conducted using the two-tailed non-parametric Mann-Whitney test. Correlations between continuous variables were measured by simple linear regression and the Spearman rank correlation coefficient.
Data and code availability
The raw data required to reproduce the above findings are available from the corresponding authors upon request.
Acknowledgments
The authors thank Veronica Nelson, Erica Wilson, Kelvin Sze, Sarah Herrin, Michelle Hoffman, Megan Brown, and Alan Ung for outstanding support for the NHP studies. All NHP work was performed at the Washington National Primate Research Center (WaNPRC), including support from Dr. Robert Murnane (veterinary pathology) and Solomon Wangari, Britni Curtis, Joel Ahrens, and Naoto Iwayama for tissue collections and laparoscopies. The authors thank Katherine Brandenstein for T cell manufacturing efforts; the Fred Hutchinson Cancer Center Vector Production core, including support from Andres Alvarez, Kenson Jean, Mark Mendoza, and Dr. Megha Gupta, for lentiviral vector manufacturing; Teresa Einhaus for DNA and RNA extractions; the WaNPRC Virology and Immunology core (WaNPRC V&IC), led by Dr. Sandra Dross, for cell subset quantification; the University of Washington Laboratory Medicine Research Testing Service, including Kimberly Van Leuven, for immunoglobulin quantification; Dr. James Riley for artificial antigen presenting cells; and Gilead Sciences and Viiv Healthcare for generously supplying ART drugs. We are grateful to Dr. Leslie Kean, for helpful advice in our CD20 CAR NHP studies, and Dr. Michael Jensen for providing CD20 CAR vector plasmids. We also thank Drs. Afam Okoye, Joshua Hill, Megan O’Connor, Scott Kitchen, and Anjie Zhen for helpful discussions. Finally, we wish to thank Helen Crawford for her assistance with manuscript and figure preparation and submission. This study was supported by grants from the NIH/NIAID (T32 AI7140 and AI118690 to J.K.B., UM1 AI126623 and U19 AI117941 to H.-P.K., R01 AI167004 and R01 AI170214 to C.W.P.), NIH/NHLBI (U19 HL156247 to H.-P.K.), and NIH/ORIP (P51 OD010425 and U42 OD011123 to the Washington National Primate Research Center). This research was supported by the following Fred Hutch/University of Washington/Seattle Children’s Cancer Consortium Core Facilities (P30 CA015704): Flow Cytometry Core RRID: SCR_022613 and Experimental Histopathology RRID: SCR_022612. Production of CD20 CAR vectors was supported by the Fred Hutchinson Cancer Center Cell Manipulation Tools Core-Vector Production (NIDDK Cooperative Center of Excellence in Hematology U54 DK106829). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
C.W.P. and H.-P.K. designed the experiments; N.H.P. manufactured CAR T cell products; B.J.R. performed RTCA and Luminex assays; N.H.P. processed longitudinal samples and performed flow cytometry assays; K.R.J. performed virologic quantification assays; C.E.S. performed immunohistochemistry, RNAscope, and TFH assays; J.K.B., C.E.S., and C.W.P. performed analyses; J.K.B., C.E.S., C.W.P., and H.-P.K. wrote the manuscript.
Declaration of interests
The authors declare the following competing interests: H.-P.K. is or was a consultant to and has or had ownership interests in Rocket Pharmaceuticals, Homology Medicines, Vor Biopharma, and Ensoma, Inc.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2024.02.030.
Contributor Information
Hans-Peter Kiem, Email: hkiem@fredhutch.org.
Christopher W. Peterson, Email: cwpeters@fredhutch.org.
Supplemental information
References
- 1.UNAIDS AIDSinfo: Global Data on HIV Epidemiology and Response. 2022. https://AIDSinfo.unaids.org
- 2.Mellors J.W., Rinaldo C.R., Jr., Gupta P., White R.M., Todd J.A., Kingsley L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Ramirez R.U., Shiels M.S., Dubrow R., Engels E.A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4:e495–e504. doi: 10.1016/S2352-3018(17)30125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby E., Avigdor A. CAR T cells for the long run in aggressive B-cell lymphoma. Lancet Oncol. 2021;22:1347–1348. doi: 10.1016/S1470-2045(21)00412-5. [DOI] [PubMed] [Google Scholar]
- 5.Allred J., Bharucha K., Özütemiz C., He F., Janakiram M., Maakaron J., Carrier C., Grzywacz B., Bachanova V. Chimeric antigen receptor T-cell therapy for HIV-associated diffuse large B-cell lymphoma: case report and management recommendations. Bone Marrow Transpl. 2021;56:679–682. doi: 10.1038/s41409-020-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson J.S., Irwin K.E., Frigault M.J., Dietrich J., McGree B., Jordan J.T., Yee A.J., Chen Y.B., Raje N.S., Barnes J.A., Davis B. Successful anti-CD19 CAR T-cell therapy in HIV-infected patients with refractory high-grade B-cell lymphoma. Cancer. 2019;125:3692–3698. doi: 10.1002/cncr.32411. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi A., Peeke S., Shah N., Mustafa J., Khatun F., Lombardo A., Abreu M., Elkind R., Fehn K., de Castro A., et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J. Hematol. Oncol. 2020;13:1. doi: 10.1186/s13045-019-0838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher C.V., Staskus K., Wietgrefe S.W., Rothenberger M., Reilly C., Chipman J.G., Beilman G.J., Khoruts A., Thorkelson A., Schmidt T.E., et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagarapu A., Piovoso M.J., Zurakowski R. An Integrated Spatial Dynamics-Pharmacokinetic Model Explaining Poor Penetration of Anti-retroviral Drugs in Lymph Nodes. Front Bioeng. Biotechnol. 2020;8:667. doi: 10.3389/fbioe.2020.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siliciano J.D., Siliciano R.F. Low Inducibility of Latent Human Immunodeficiency Virus Type 1 Proviruses as a Major Barrier to Cure. J. Infect Dis. 2021;223:13–21. doi: 10.1093/infdis/jiaa649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taraseviciute A., Tkachev V., Ponce R., Turtle C.J., Snyder J.M., Liggitt H.D., Myerson D., Gonzalez-Cuyar L., Baldessari A., English C., et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8:750–763. doi: 10.1158/2159-8290.CD-17-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong J.K., Yukl S.A. Tissue reservoirs of HIV. Curr. Opin. HIV AIDS. 2016;11:362–370. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M., et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rust B.J., Kean L.S., Colonna L., Brandenstein K.E., Poole N.H., Obenza W., Enstrom M.R., Maldini C.R., Ellis G.I., Fennessey C.M., et al. Robust expansion of HIV CAR T cells following antigen boosting in ART-suppressed nonhuman primates. Blood. 2020;136:1722–1734. doi: 10.1182/blood.2020006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris E.C., Neelapu S.S., Giavridis T., Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022;22:85–96. doi: 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caimi P.F., Pacheco Sanchez G., Sharma A., Otegbeye F., Ahmed N., Rojas P., Patel S., Kleinsorge Block S., Schiavone J., Zamborsky K., et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol. 2021;12:745320. doi: 10.3389/fimmu.2021.745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukazawa Y., Lum R., Okoye A.A., Park H., Matsuda K., Bae J.Y., Hagen S.I., Shoemaker R., Deleage C., Lucero C., et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva N.S., Klein U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ise W., Fujii K., Shiroguchi K., Ito A., Kometani K., Takeda K., Kawakami E., Yamashita K., Suzuki K., Okada T., Kurosaki T. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. 2018;48:702–715.e4. doi: 10.1016/j.immuni.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Perreau M., Savoye A.L., De Crignis E., Corpataux J.M., Cubas R., Haddad E.K., De Leval L., Graziosi C., Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson C.W., Wang J., Deleage C., Reddy S., Kaur J., Polacino P., Reik A., Huang M.L., Jerome K.R., Hu S.L., et al. Differential impact of transplantation on peripheral and tissue-associated viral reservoirs: Implications for HIV gene therapy. Plos Pathog. 2018;14:e1006956. doi: 10.1371/journal.ppat.1006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson C.W., Younan P., Polacino P.S., Maurice N.J., Miller H.W., Prlic M., Jerome K.R., Woolfrey A.E., Hu S.L., Kiem H.P. Robust suppression of env-SHIV viremia in Macaca nemestrina by 3-drug ART is independent of timing of initiation during chronic infection. J. Med. Primatol. 2013;42:237–246. doi: 10.1111/jmp.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Q., Tan J., Liu R., Kang L., Zhang Y., Wang E., Li Y., Zhang J., Xiao H., Xu N., et al. CD20-specific chimeric antigen receptor-expressing T cells as salvage therapy in rituximab-refractory/relapsed B-cell non-Hodgkin lymphoma. Cytotherapy. 2022;24:1026–1034. doi: 10.1016/j.jcyt.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J., et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Till B.G., Jensen M.C., Wang J., Qian X., Gopal A.K., Maloney D.G., Lindgren C.G., Lin Y., Pagel J.M., Budde L.E., et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rufener G.A., Press O.W., Olsen P., Lee S.Y., Jensen M.C., Gopal A.K., Pender B., Budde L.E., Rossow J.K., Green D.J., et al. Preserved activity of CD20-specific chimeric antigen receptor-expressing T cells in the presence of rituximab. Cancer Immunol. Res. 2016;4:509–519. doi: 10.1158/2326-6066.CIR-15-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen M.C., Popplewell L., Cooper L.J., DiGiusto D., Kalos M., Ostberg J.R., Forman S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transpl. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J., Riddell S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yek C., Gianella S., Plana M., Castro P., Scheffler K., García F., Massanella M., Smith D.M. Standard vaccines increase HIV-1 transcription during antiretroviral therapy. AIDS. 2016;30:2289–2298. doi: 10.1097/QAD.0000000000001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhlmann A.S., Haworth K.G., Barber-Axthelm I.M., Ironside C., Giese M.A., Peterson C.W., Kiem H.P. Long-term persistence of anti-HIV broadly neutralizing antibody-secreting hematopoietic cells in humanized mice. Mol. Ther. 2019;27:164–177. doi: 10.1016/j.ymthe.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaufin T., Pattison M., Gautam R., Stoulig C., Dufour J., MacFarland J., Mandell D., Tatum C., Marx M.H., Ribeiro R.M., et al. Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host. J. Virol. 2009;83:10347–10357. doi: 10.1128/JVI.00880-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir S., Fauci A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Firnhaber C., Smeaton L., Saukila N., Flanigan T., Gangakhedkar R., Kumwenda J., La Rosa A., Kumarasamy N., De Gruttola V., Hakim J.G., Campbell T.B. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int. J. Infect Dis. 2010;14:e1088–e1092. doi: 10.1016/j.ijid.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi S.Y., Kim I., Kim N.J., Lee S.A., Choi Y.A., Bae J.Y., Kwon J.H., Choe P.G., Park W.B., Yoon S.S., et al. Hematological manifestations of human immunodeficiency virus infection and the effect of highly active anti-retroviral therapy on cytopenia. Korean J. Hematol. 2011;46:253–257. doi: 10.5045/kjh.2011.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Eekeren L.E., Matzaraki V., Zhang Z., van de Wijer L., Blaauw M.J.T., de Jonge M.I., Vandekerckhove L., Trypsteen W., Joosten L.A.B., Netea M.G., et al. People with HIV have higher percentages of circulating CCR5+ CD8+ T cells and lower percentages of CCR5+ regulatory T cells. Sci. Rep. 2022;12:11425. doi: 10.1038/s41598-022-15646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay K.A., Hanafi L.A., Li D., Gust J., Liles W.C., Wurfel M.M., Lopez J.A., Chen J., Chung D., Harju-Baker S., et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rejeski K., Perez A., Sesques P., Hoster E., Berger C., Jentzsch L., Mougiakakos D., Frolich L., Ackermann J., Bucklein V., et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juluri K.R., Wu Q.V., Voutsinas J., Hou J., Hirayama A.V., Mullane E., Miles N., Maloney D.G., Turtle C.J., Bar M., Gauthier J. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022;6:2055–2068. doi: 10.1182/bloodadvances.2020004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banga R., Procopio F.A., Noto A., Pollakis G., Cavassini M., Ohmiti K., Corpataux J.M., de Leval L., Pantaleo G., Perreau M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 2016;22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- 42.Hsu D.C., Sunyakumthorn P., Wegner M., Schuetz A., Silsorn D., Estes J.D., Deleage C., Tomusange K., Lakhashe S.K., Ruprecht R.M., et al. Central Nervous System Inflammation and Infection during Early, Nonaccelerated Simian-Human Immunodeficiency Virus Infection in Rhesus Macaques. J. Virol. 2018;92 doi: 10.1128/JVI.00222-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humbert M., Rasmussen R.A., Song R., Ong H., Sharma P., Chenine A.L., Kramer V.G., Siddappa N.B., Xu W., Else J.G., et al. SHIV-1157i and passaged progeny viruses encoding R5 HIV-1 clade C env cause AIDS in rhesus monkeys. Retrovirology. 2008;5:94. doi: 10.1186/1742-4690-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett J.A., Miralles G.D., Sevin A.D., Silberman M., Pruitt S.K., Ottinger J., Gryszowska V., Fiscus S.A., Bucy R.P., ACTG 380 Study Team Addition of cyclophosphamide to antiretroviral therapy does not diminish the cellular reservoir in HIV-infected persons. AIDS Res. Hum. Retroviruses. 2002;18:535–543. doi: 10.1089/088922202753747888. [DOI] [PubMed] [Google Scholar]
- 45.Adair J.E., Waters T., Haworth K.G., Kubek S.P., Trobridge G.D., Hocum J.D., Heimfeld S., Kiem H.P. Semi-automated closed system manufacturing of lentivirus gene-modified haematopoietic stem cells for gene therapy. Nat. Commun. 2016;7:13173. doi: 10.1038/ncomms13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radtke S., Perez A.M., Venkataraman R., Reddy S., Haworth K.G., Humbert O., Kiem H.P., Peterson C.W. Preparation and gene modification of nonhuman primate hematopoietic stem and progenitor cells. J. Vis. Exp. 2019 doi: 10.3791/58933. [DOI] [PubMed] [Google Scholar]
- 47.WHO TEAM . Interim guidance World Health Organization; 2018. World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. [Google Scholar]
- 48.Peterson C.W., Benne C., Polacino P., Kaur J., McAllister C.E., Filali-Mouhim A., Obenza W., Pecor T.A., Huang M.L., Baldessari A., et al. Loss of immune homeostasis dictates SHIV rebound after stem-cell transplantation. JCI Insight. 2017;2:e91230. doi: 10.1172/jci.insight.91230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhen A., Peterson C.W., Carrillo M.A., Reddy S.S., Youn C.S., Lam B.B., Chang N.Y., Martin H.A., Rick J.W., Kim J., et al. Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. Plos Pathog. 2017;13:e1006753. doi: 10.1371/journal.ppat.1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deleage C., Wietgrefe S.W., Del Prete G., Morcock D.R., Hao X.P., Piatak M., Jr., Bess J., Anderson J.L., Perkey K.E., Reilly C., et al. Defining HIV and SIV Reservoirs in Lymphoid Tissues. Pathog. Immun. 2016;1:68–106. doi: 10.20411/pai.v1i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamoreaux L., Roederer M., Koup R. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data required to reproduce the above findings are available from the corresponding authors upon request.