Abstract
Adjuvant is an integral part of all vaccine formulations but only a few adjuvants with limited efficacies or application scopes are available. Thus, developing more robust and diverse adjuvants is necessary. To this end, a new class of adjuvants having α- and β-rhamnose (Rha) attached to the 1- and 6’-positions of monophosphoryl lipid A (MPLA) was designed, synthesized, and immunologically evaluated in mice. The results indicated a synergistic effect of MPLA and Rha, two immunostimulators that function via interacting with toll-liker receptor 4 (TLR4) and recruiting endogenous anti-Rha antibodies, respectively. All the tested MPLA-Rha conjugates exhibited potent adjuvant activities to promote antibody production against both protein and carbohydrate antigens. Overall, MPLA-α-Rha exhibited better activities than MPLA-β-Rha, and 6’-linked conjugates were slightly better than 1-linked ones. Particularly, MPLA-1-α-Rha and MPLA-6’-α-Rha were the most effective adjuvants in promoting IgG antibody responses against protein antigen keyhole limpet hemocyanin and carbohydrate antigen sTn, respectively.
Keywords: Carbohydrates, Glycoconjugates, Lipid A, Rhamnose, Adjuvants, Vaccines
Graphical Abstract

Introduction
Vaccination is the most economical and effective strategy for controlling and preventing infectious diseases ‒ one of the leading causes of human death. In recent years, therapeutic cancer vaccine has become another research frontier.1–5 Nevertheless, for any vaccine to work, it has to be combined with an adjuvant, the medicine that can boost the immune system to attain more robust and persistent immunities. Thus, adjuvant is an integral part of all vaccine formulations.6–8 Adjuvants can also be used independently to achieve certain therapeutic benefits.9–13
Currently, several adjuvants, such as Alum, AS04, MF59 and virosomes, are available for clinical usages.14–16 Among them, Alum, a century-old adjuvant, is still the most widely adopted, but its efficacy is insufficient for many vaccines in development presently, while the recently licensed adjuvants have only been approved for a few vaccines.17 Moreover, each adjuvant has its application scope depending on the properties of both adjuvants and vaccines. For example, the keyhole limpet hemocyanin (KLH) conjugate of globo H, a tumor-associated carbohydrate antigen (TACA), was not functional as a cancer vaccine when formulated with conventional adjuvants, but the same conjugate combined with an experimental adjuvant QS-21 worked well to elicit robust immune responses in cancer patients.18 In addition, most adjuvants are mixtures, making it difficult to study their action mechanisms and improve their efficacy.6–8 Clearly, these adjuvants are constrained by several factors,19 and adjuvants of diverse immunological properties and broad application scopes are demanded by various modern vaccination schemes.
To meet such demands, we explored a new class of adjuvants derived from monophosphoryl lipid A (MPLA) and L-rhamnose (Rha), two potent immunostimulators that have different action mechanisms. We anticipated that conjugating MPLA with Rha would not only harness the immunostimulatory properties of both components but also generate a synergistic effect, thereby leading to potent and widely applicable adjuvants. Accordingly, several MPLA-Rha conjugates were designed, synthesized, and immunologically studied in mice as adjuvants to boost immune responses against both protein and carbohydrate antigens.
Results and Discussion
Design of a new class of conjugate adjuvants
To develop structurally defined adjuvants, we are especially interested in MPLA and Rha that have different immunological properties and action mechanisms. MPLA is a lipid A derivative (Figure 1A) with excellent immunological and safety profiles20 and, thus, has been extensively investigated as both external and internal adjuvants21–25 for antimicrobial26–29 and anticancer vaccines.30–35 MPLA stimulates the immune system via interacting with toll-liker receptor 4 (TLR4)36 to provoke various cytokine and chemokine expression,37–38 which in turn enhances dendritic cell (DC) and other immune cell proliferation, boosts antigen presentation,39–40 activates Th cells, and elicits cytotoxic T cells (CTLs),41 thereby promoting both innate and adaptive immunities.42 Rha is a highly immunogenic hapten, and anti-Rha antibodies are abundant in the human serum.43–44 Rha is attractive as an immunostimulant because in the human body it can recruit endogenous Rha antibodies to interact with Fc receptors on DCs and other immune cells to activate antibody effector functions.45–49 Therefore, Rha and endogenous Rha antibodies have been actively pursued in the development of novel adjuvants and immunotherapies.50–62
Figure 1.

Representative structures of lipid A and MPLA (A), as well as structures of the designed MPLA-Rha conjugate adjuvants 1a, 1b, 2a, and 2b and their retrosynthetic plans (B).
Based on the above discoveries, we designed a new class of adjuvants 1–2 (Figure 1B), which have Rha covalently coupled with MPLA and, thus, are called “conjugate adjuvants”. Our hypothesis is that these conjugates can harness the potent im-munostimulating activities of both adjuvants. In addition, they may exhibit a synergistic effect since MPLA and Rha activate the immune system via different pathways or mechanisms and covalently coupling MPLA and Rha will ensure their co-locali-zation on/in the same immune cells to synchronize their actions to further enhance antigen processing/presentation and other im-munoreactions.63 Moreover, glycolipids can formulate liposomes that can promote the presentation of conjugate adjuvants in a multivalent fashion, providing the opportunity for more efficient clustering of B-cell Ig receptors and enhancement of the immunostimulatory activities.64–67
Specifically, Neisseria meningitidis MPLA was used in the design of 1-2 (Figure 1B) since structure-activity relationship (SAR) studies of lipid A and MPLA in the literature68–72 and by our group29, 73 have revealed that this MPLA is not only potent but also functionally consistent as an immunostimulator. In 1 and 2, Rha is attached to the MPLA 1- and 6’-positions, to which a phosphate group and a polysaccharide are respectively linked in natural lipopolysaccharides (LPSs). Thus, Rha attachment to these positions would not likely interrupt the interaction of 1-2 with TLR4. In addition, 1a/2a and 1b/2b contain α- and β-Rha (Figure 1B),74 respectively. Comparisons of these conjugates will demonstrate related SARs, e.g., influences of the anomeric configurations (α vs β) of Rha and linkage sites to MPLA on the immunological properties of the designed adjuvants.
Synthesis of the designed adjuvants 1a,b and 2a,b
Figure 1B shows our synthetic plan for 1a, 1b, 2a, and 2b. The primary strategy is to prepare properly protected/modified MPLA derivatives 3 and 4 and Rha derivatives 5a and 5b separately and then stitch them together by a regioselective reaction. This synthetic scheme involves a minimum number of intermediates. For example, 5a and 5b can serve as the common intermediates for coupling with 3 and 4 for the assembly of α- and β-linked conjugate adjuvants, respectively.
The synthesis of 5a and 5b started from Rha (Scheme 1). After Rha was converted into 6 in four steps,75 including O-acetylation using Ac2O and pyridine, glycosidation using 4-toluenethiol, deacetylation using MeONa in methanol and benzylation using NaH and benzyl bromide, it was utilized to glycosylate methyl 3-hydroxypropanoate 7, promoted by N-iodosuccinimide (NIS) and trimethylsilyl triflate (TMSOTf), to afford a 1.2:1 mixture of α- and β-anomers 8a and 8b in an excellent yield (93%). The two products were readily separated utilizing silica gel column chromatography, and their anomeric configurations were confirmed by NMR data. The α-anomer 8a had a significantly larger one-bond coupling constant (168.2 Hz) for its anomeric 1H and 13C than β-anomer 8b (153.8 Hz). Eventually, 8a and 8b were individually hydrolysed using aqueous LiOH to provide Rha derivatives 5a and 5b.
Scheme 1.
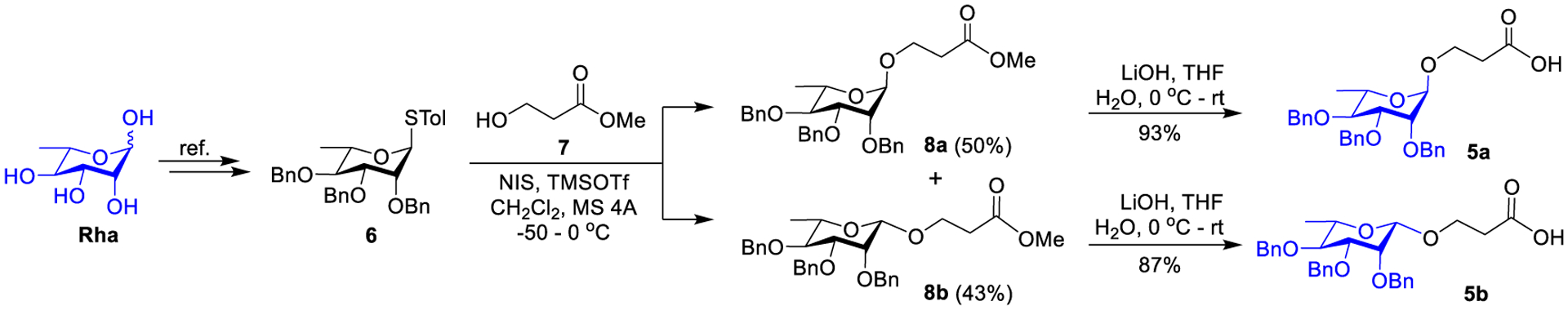
Synthesis of Rha derivatives 5a and 5b.
To access the synthetic targets (Scheme 2), first, we prepared 9 and 10 from D-glucosamine and fatty acids following reported methods.29, 73 Next, the azido group in 9 and 10 was reduced with Zn and acetic acid to give key intermediates 3 and 4, which were directly applied to the next step without purification. The coupling between 3 and 5a or 5b in the presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) was smooth to give conjugates 11a and 11b in good yields (71–73%). Finally, 11a and 11b were individually subjected to global deprotection using H2 and 10% Pd/C in CH2Cl2 and MeOH (1:1) to provide desired conjugate adjuvants 1a and 1b that were purified by column chromatography using silica gel and a mixture of CHCl3 and CH3OH as the eluent. Compound 4 was coupled with 5a and 5b by the same method and protocols, which was followed by global deprotection of the products 12a and 12b to provide conjugate adjuvants 2a and 2b. All final products, as well as the intermediates, were characterized with NMR and MS data.
Scheme 2.

Synthesis of the designed conjugate adjuvants 1a and 1b (A), and 2a and 2b (B).
In the meantime, we also synthesized a free MPLA derivative 13 and L-rhamnoside of a simple lipid 14 (Scheme 3) that were used as controls during immunological studies of 1 and 2. The lipid in 14 could increase its lipophilicity such that it would be incorporated into liposomes when being formulated with 13 and the model vaccine. MPLA 13 was directly obtained via global deprotection of 9 using H2 and 10% Pd/C to reduce the azide and remove all benzyl groups concomitantly (Scheme 3), while 14 was derived from 5a in two separate steps, including EDC-promoted condensation with n-decanamine and global deprotection using H2 and 10% Pd/C. These products were also characterized with NMR and MS data.
Scheme 3.

Synthesis of free MPLA 13 (A) and a simple lipid conjugate 14 of Rha (B) that were used as controls.
Immunological evaluation of target conjugate adjuvants
MPLA-Rha conjugates 1a, 1b, 2a, and 2b were investigated as vaccine adjuvants in vivo employing female C57BL/6J mice. The positive control was Alum, which is currently the most widely used vaccine adjuvant. The negative controls were phosphate-buffered saline (PBS) and an MPLA plus L-rhamnoside mixture (13+14). Comparing the experimental conjugate adjuvants with 13+14 will show whether covalently coupling MPLA with Rha would help generate a synergistic effect of the two components. The model vaccine was sTn-KLH conjugate (with 8.76% sTn loading). sTn is a TACA and its protein conjugate was shown to be poorly immunogenic in the absence of adjuvants.76 In this study, sTn-KLH served as a bifunctional vaccine to examine the impact of experimental adjuvants on antibody responses to both protein antigen KLH and carbohydrate antigen sTn. For vaccine formulation, Alum or PBS was utilized as emulsions prepared according to the manufacturer’s instruction, while experimental adjuvants and 13+14 were formulated as liposomes with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol in a 10:65:50 molar ratio. Liposomes were prepared following a reported procedure.35
For mouse immunization, each vaccine preparation (0.1 mL) of KLH-sTn (containing ~8 μg of sTn antigen) formulated with PBS, 13+14, Alum or an experimental conjugate adjuvant was subcutaneously (s.c.) injected to a group of 6 mice on day 1, 15, 22, and 29. Blood samples were collected from each mouse on day 0 before inoculation (used as blank) and on day 27, 31, and 38 after immunizations. Antisera were prepared from the blood samples following a conventional protocol. Analysis of antigen-specific antibodies in the antisera was conducted using enzyme-linked immunosorbent assay (ELISA). The sTn-human serum album (HSA) conjugate and KLH were used as capture reagents to detect sTn- and KLH-specific antibodies, respectively. Total, IgG, and IgM antibodies were individually detected employing alkaline phosphatase (AP)-linked goat anti-mouse kappa, IgG, and IgM secondary antibodies, with antibody titers determined from the serum dilution number vs optical density (OD) value curves and defined as serum dilution numbers corresponding to an OD value of 0.1.
ELISA results of the day 38 antisera (Figure 2 and Tables S1–S2, SI) reveal that 2a boosted the highest titer of anti-sTn total antibodies (Figure 2A), which included different subclasses of antibodies having varied affinities to the anti-kappa secondary antibody. Analysis of antibody subclasses shows that 2a also helped elicit significantly stronger IgG antibody responses than the positive control (Alum) or other conjugate adjuvants (Figure 2B and Table S1, SI). Nevertheless, 1a, 1b, and 2b also promoted high levels of IgG antibodies. For example, IgG antibody titers in the antisera of 1a and 1b were significantly higher than that in the antiserum of Alum or 13+14, while 2b and Alum were comparable (Figure 2B). Interestingly, 1b group mice produced the highest level of IgM antibodies, and the IgM antibody levels in all MPLA-Rha conjugate groups were higher than that in the 13+14 and Alum groups (Figure 2C and Table S1, SI). The ELISA results further demonstrate that 1a and 1b, rather than 2a, boosted the highest titers of anti-KLH total antibodies (Figure 2D and Table S2, SI). Moreover, the antisera of 1a and 1b also had the highest levels of anti-KLH IgG and IgM antibodies, respectively (Figures 2E and 2F and Table S2, SI). Again, all MPLA-Rha conjugates boosted significantly higher levels of anti-KLH IgG antibodies than 13+14 or Alum, while IgM antibody levels in 1a, 2a, and 2b groups were comparable to that in 13+14 group but slightly higher than that in Alum group. Compared to the negative control PBS, both Alum and 13+14 have promoted evident IgG antibody responses to either KLH or sTn (Figures 2B and 2E and Table S2, SI), validating their adjuvant activity, but these immune responses were not as strong as those observed with some of the new conjugate adjuvants. In addition, Alum and 13+14 showed different properties, as Alum induced stronger anti-sTn antibody responses while 13+14 elicited more robust anti-KLH antibody responses (Figure 2).
Figure 2.

ELISA results of sTn-specific total (A), IgG (B), and IgM (C) and KLH-specific total (D), IgG (E), and IgM (F) antibodies in the day 38 antisera from mice immunized with sTn-KLH plus PBS, 13+14, or an adjuvant including Alum, 1a, 1b, 2a, and 2b. Each column represents the mean antibody titer for a specific group of three parallel experiments, and the error bar indicates the standard error of the mean (SEM). ** p < 0.05, *** p < 0.01, and **** p < 0.001 show the statistically significant level for the difference between two compared groups.
We also examined the dynamics of immune responses to the model vaccine formulated with different adjuvants by analyzing the antisera collected at day 27, 31, and 38 upon immunization 3 and 4 times and over one week after the final immunization, respectively. In this study, we were focused on conjugates 1a and 2a that boosted the highest levels of anti-KLH and anti-sTn IgG antibodies, respectively. The results (Figure 3 and Tables S3 and S4, SI) demonstrate that 1a and 2a consistently boosted higher levels of IgG antibodies in antisera collected at different time points than 13+14 and Alum, which agrees with the above observation. Importantly, in both cases, antibody titers increased with the number of inoculations, suggesting further enhancement of immune responses after each immunization. In addition, the day 38 antisera collected one week after the final immunization exhibited the highest levels of IgG antibodies.
Figure 3.

Anti-sTn (A) and anti-KLH (B) IgG antibody titers of the day 0 (D0, before immunization), day 27, 31, and 38 sera from mice immunized with sTn-KLH in combination with PBS, 13+14, Alum, 1a, and 2a, respectively. Each column represents the mean antibody titer for a specific serum of three parallel experiments, and the error bar indicates SEM. ** p < 0.05, *** p < 0.01, and **** p < 0.001 show the statistically significant level for the difference between two compared groups.
Discussion of the immunological results
Our studies reveal that the tested MPLA-Rha conjugates 1a, 1b, 2a, and 2b are generally more potent adjuvants than Alum to boost immune responses, especially IgG antibody responses, against both sTn and KLH antigens in the model vaccine. The production of high levels of IgG antibodies is an indication of antibody isotype switch and affinity maturation associated with T-cell-dependent immunities and long-term memory of the immune response for effective vaccination. However, despite the relatively small structural differences of these conjugates, i.e., in anomeric configuration and linking site of the Rha unit, they have shown distinct efficacies, suggesting their remarkably different immunological properties. Notably, conjugates 1a and 2a with α-linked Rha assisted the vaccine to provoke the highest levels of IgG antibodies against KLH and sTn, respectively, suggesting that overall MPLA-Rha conjugates with α-Rha are more efficient than those with β-Rha to boost cellular immunity. In addition, the linking site of Rha to MPLA (1- vs 6’-positions) also affects the efficacy of resultant conjugate adjuvants, which is consistent with our previous finding.29 Conjugate adjuvant 1a having α-Rha linked to 1-position of MPLA was more effective than 1b with 6’-linked α-Rha against protein antigen KLH, but for carbohydrate antigen sTn, conjugate adjuvant 2a with 6’-linked α-Rha was more effective than 2b with 1-linked α-Rha. On the other hand, 1b promoted the highest levels of both anti-KLH and anti-sTn IgM antibodies related to innate immunity. Clearly, the structure of conjugates had a significant impact on their immunological properties, and each adjuvant seemed to be most suitable for a specific kind/type of vaccine or antigen and, thus, may have a specific application scope.
Our results also reveal that all the MPLA-Rha conjugates are more potent adjuvants than the MPLA and Rha mixture 13+14 in boosting antibody responses, although 13+14 and Alum are similar and immunoactive. These results suggest a potentially synergistic effect of the two immunostimulators in covalently coupled form. As it is reported that the anti-Rha antibody level in naive mice is rather low,50 we analyzed the pre-immunization sera from our experimental mice and verified that their total and IgG and IgM anti-Rha antibody levels were significant (Figure S3 and Table S5, SI). Furthermore, we found that in most mice, the anti-Rha antibody levels increased during the immunization process (Figure S4 and Table S5, SI) because conjugates 1a, 1b, 1c, and 1c can function as conjugate vaccines.
Although more in-depth studies are necessary to elucidate the immunological mechanisms of this synergistic effect, it is likely that the concerted action of MPLA and Rha in a conjugate on the same immune cells can enhance the interaction of vaccines or antigens with the immune system to boost APC processing and presentation of antigenic epitopes on major histocompatibility complex molecules (MHCs) and improve immune responses.77 For example, both MPLA and Rha can activate DCs through interacting with TLR4 and recruiting endogenous Rha antibody for interaction with Fc receptors, respectively (Figure 4).78–79 This may lead to increased antigen processing/presentation and T-cell engagement, as well as other immune cell activities, thus promoting T-cell-mediated adaptive immunity.79 Other proofs supporting Rha involvement in the immunological activities of these conjugate adjuvants are that Rha is often a pivotal part of the immunodominant epitope for bacterial polysaccharides80 and replacing the Rha-xylose moiety in QS-21 with other disaccharides attenuated its adjuvant activity.81 It is also possible that the formulation of these conjugates into liposomes has facilitated their multivalent interactions with immune cells to further enhance their adjuvant activities.
Figure 4.

Potential mechanisms for the synergistic effect of Rha and MPLA in the covalently linked conjugate adjuvants. MPLA and endogenous Rha antibodies recruited by Rha interact with TLR4 and Fc receptors, respectively, on APCs (such as DCs) to function cooperatively in augmenting antigen processing and presentation and T-cell involvement, thereby promoting T-cell-mediated adaptive immunities against vaccines.79
The results that both anti-KLH and anti-sTn IgG antibody titers increase steadily with repeated vaccination and antisera collected more than a week after the final immunization contain the highest levels of antibodies (Figure 4) indicate that the mice developed robust and memorable immune responses, a pattern consistent with that of T-cell-dependent immunities against the vaccine in the presence of experimental adjuvants, which is desired for both preventative and therapeutic vaccines. These findings further validated the efficacy of new adjuvants 1a and 2a since sTn-KLH has been proved to be poorly immunogenic without adjuvants and elicits relatively weak immune responses when combined with conventional adjuvants (e.g., Alum), as demonstrated in this work.
Conclusion
In the current research, we explored a novel class of vaccine adjuvants, MPLA-Rha conjugates, which are termed conjugate adjuvants. Efficient methods were established for the synthesis of target molecules with α- and β-Rha attached to the 1- and 6’-positions of MPLA, respectively. Immunological evaluations of these conjugates in mice demonstrated that they exhibited a synergistic effect and were more potent adjuvants than Alum to boost robust IgG antibody responses to both protein and carbohydrate antigens. Particularly, conjugates 1a and 2a with α-Rha linked to the MPLA 1- and 6’-positions assisted sTn-KLH to stimulate robust IgG antibody responses to KLH and sTn, respectively. Therefore, 1a and 2a are promising adjuvants worth further research and development. Next, we will investigate whether 1a and 2a are generally applicable to other protein- and carbohydrate-based vaccines. Another future direction of this research will be gaining in-depth understanding of the functional mechanisms of the new adjuvants helpful for their further improvement.
Experimental Section
Materials and general methods.
Chemicals and materials were purchased from commercial sources and were used as received without further purification unless otherwise noted. Molecular sieves 4Å (MS 4Å) were flame-dried under high vacuum and used immediately after being cooled to rt under a N2 atmosphere. Analytical TLC was carried out on silica gel 60 Å F254 plates with detection by a UV detector and/or by charring with 10% (v/v) H2SO4 in ethanol. Flash column chromatography was performed on silica gel 60 (230–400 Mesh). NMR spectra were acquired on a Bruker® 600 MHz spectrometer with chemical shifts reported in ppm (δ) referenced to CDCl3 (1H NMR δ 7.26 ppm, 13C NMR δ 77.16 ppm) or CD3OD (1H NMR δ 3.31 ppm, 13C NMR δ 49.0 ppm). Peak and coupling constant assignments are made based on 1H NMR, 1H-1H COSY, 1H-13C HSQC, and 1H-13C HMBC experiments. HR MS data was acquired on a Bruker Daltonics, Impact II QTOF instrument with electro spray ionization (ESI) in a positive or negative mode. Alum, DSPC, and cholesterol were purchased from Sigma-Aldrich. AP-linked goat anti-mouse kappa, IgM, and IgG antibodies (the secondary antibodies) were purchased from Southern Biotechnology. Model vaccine sTn-KLH and sTn-HSA conjugate as coating antigen were previously synthesized. All final products applied to animals were analyzed by HPLC to have purity >95% (SI). Female C57BL/6 mice of 6−8 weeks of age employed for immunological studies were purchased from The Jackson Laboratory. After animals arrived at the animal facility at the University of Florida, they were examined and kept in the cage for 1–2 days to settle in the environment before the start of experiments. The animal use protocol for this study (#201609560) was approved by the IACUC of the University of Florida.
Methyl 3-(2,3,4-tri-O-phenylmethyl-α-L-rhamnopyranosyloxy)propanoate (8a) and methyl 3-(2,3,4-tri-O-phenylmethyl-β-L-rhamnopyranosyl-oxy)propanoate (8b):
The mixture of 6 (560 mg, 5.38 mmol), 7 (3.49 mg, 6.46 mmol), and 4 Å molecular sieves in dry dichloromethane (DCM, 10 mL) was stirred at rt for 30 min and then cooled to −50 °C. NIS (1.82 mg, 8.07 mmol) was added to the mixture followed by adding TfOH (47.5 μL, 0.537 mmol) slowly under N2, and the mixture was stirred at −50 °C to 0 °C for 2 h. After completion of the reaction as indicated by TLC, the reaction was quenched by TEA and was diluted with DCM. The resulting mixture was filtered through Celite, washed with saturated aqueous NaHCO3 solution, and concentrated in a vacuum. The residue was purified by silica gel column chromatography (petrol ether and ethyl acetate = 15:1) to afford 8a (α-anomer, 1.40 g, 50%) and 8b (β-anomer, 1.20 g, 43%) as pale-yellow syrups. Compound 8a: 1H NMR (600 MHz, CDCl3): δ 7.40 – 7.27 (m, 15H, ArH), 4.94 (d, J = 10.9 Hz, 1H, −CH2−Ph), 4.79 (d, J = 1.7 Hz, 1H, H-1), 4.74 (q, J = 12.4 Hz, 2H, −CH2−Ph), 4.64 (d, J = 10.9 Hz, 1H, −CH-Ph), 4.62 (brs, 2H, −CH2−Ph), 3.90 (dt, J = 10.0, 6.3 Hz, 1H, OCH2−), 3.82 (dd, J = 9.2, 3.1 Hz, 1H, H-3), 3.76 (dd, J = 3.0, 2.0 Hz, 1H, H-2), 3.72 −3.58 (m, 6H, H-4, H-5, −OCH3 and −OCH2−), 2.54 (t, J = 6.4 Hz, 2H, −CH2−CO2Me), 1.34 (d, J = 6.2 Hz, 2H, H-6). 13C NMR (151 MHz, CDCl3): δ 171.8, 138.8, 138.7, 138.5, 128.47, 128.46, 128.1, 128.0, 127.76, 127.74, 127.72, 127.62, 98.3, 80.6, 80.2, 75.5, 75.0, 72.9, 72.3, 68.3, 63.0, 51.8, 34.7, 18.1. HR ESI-QTOF-MS m/z: [M + Na]+ calculated for C31H36NaO7 543.2353, found 543.2362. Compound 8b: 1H NMR (600 MHz, CDCl3): δ 7.49 – 7.40 (m, 2H, ArH), 7.35 – 7.25 (m, 13H, ArH), 4.96 (d, J = 11.0 Hz, 1H, −CH2−Ph), 4.94 (d, J = 12.4 Hz, 1H, −CH2−Ph), 4.83 (d, J = 12.6 Hz, 1H, CH2−Ph), 4.64 (d, J = 10.8 Hz, 1H, −C −CH2−Ph), 4.49 (d, J = 11.8 Hz, 1H, −CH2−Ph), 4.42 (d, J = 11.8 Hz, 1H, −CH2−Ph), 4.39 (s, 1H, H-1), 4.15 (dt, J = 9.7, 5.8 Hz, 1H, −OCH2−), 3.88 (d, J = 2.9 Hz, 1H, H-2), 3.81 – 3.74 (m, 1H, −OCH2−), 3.61 (t, J = 9.3 Hz, 1H, H-4), 3.44 (dd, J = 9.4, 3.0 Hz, 1H, H-3), 3.31 (dq, J = 9.3, 6.1 Hz, 1H, H-5), 2.72− 2.67 (m, 1H, −OCH2−), 2.64 (dt, J = 10.5, 5.6 Hz, 1H, −OCH2−), 1.38 (d, J = 6.2 Hz, 1H, H-6). 13C NMR (151 MHz, CDCl3): δ 172.1, 138.8, 138.6, 138.4, 128.6, 128.53, 128.51, 128.47, 128.24, 128.21, 127.8, 127.6, 127.5, 101.8, 82.2, 80.2, 75.6, 74.0, 73.8, 72.1, 71.5, 65.4, 51.9, 35.1, 18.1. HR ESI-QTOF-MS m/z: [M + Na]+ calculated for C31H36O7Na 543.2353, found 543.2361.
3-(2,3,4-tri-O-Phenylmethyl-α-L-rhamnopyranosyloxy)propanoic acid (5a):
To a solution of 8a (200 mg, 0.380 mmol) in THF (4.0 mL) was added a solution of LiOH·H2O (48.3 mg, 1.15 mmol) in H2O (0.50 mL). The resulting mixture was stirred at 0 °C to rt for 12 h until no starting material was detected by TLC. The aqueous layer was acidified to pH 2 with aqueous HCl solution (1.0 mol/L) and was extracted with ethyl acetate (20 mL × 2). The organic layers were washed with brine (20 mL × 2), dried over anhydrous Na2SO4, and concentrated in a vacuum. The crude product was purified by flash chromatography (DCM and MeOH = 10:1) to afford 5a (181 mg, 93%) as colorless syrup. 1H NMR (400 MHz, CDCl3): δ 7.48 – 7.24 (m, 15H, ArH), 4.93 (d, J = 10.8 Hz, 1H, −CH2−Ph), 4.78 (d, J = 1.7 Hz, 1H, H-1), 4.72 (q, J = 12.5 Hz, 1H, −CH2−Ph), 4.63 (d, J = 10.8 Hz, 1H, −CH2−Ph), 4.60 (q, J = 12 Hz, 2H, −CH2−Ph), 3.89 (dt, J = 10.1, 6.2 Hz, 1H, −OCH2−), 3.82 (dd, J = 9.1, 3.1 Hz, 1H, H-3), 3.75 (dd, J = 3.0, 1.9 Hz, 1H, H-2), 3.73 – 3.57 (m, 3H, −OCH2−, H-4 and H-5), 2.57 (t, J = 6.3 Hz, 2H, −OCH2−), 1.33 (d, J = 6.1 Hz, 3H, H-6). 13C NMR (101 MHz, CDCl3): δ 176.7, 138.7, 138.4, 128.5, 128.2, 128.1, 127.8, 127.78, 127.7, 98.3, 80.5, 80.1, 75.5, 75.0, 73.0, 72.3, 68.4, 62.7, 34.5, 18.1. HR ESI-QTOF-MS m/z: [M + Na]+ calculated for C30H34NaO7 529.2197, found 529.2207.
3-(2,3,4-tri-O-Phenylmethyl-β-L-rhamnopyranosyloxy)propanoic acid (5b):
Compound 5b (170 mg, 87%) was synthesized from 8b (200 mg, 0.380 mmol) by the same procedure employed for the synthesis of 5a. 1H NMR (400 MHz, CDCl3): δ 7.45 (d, J = 6.5 Hz, 2H), 7.39 – 7.23 (m, 13H, ArH), 5.03 – 4.91 (m, 2H, −CH2−Ph), 4.85 (d, J = 12.5 Hz, 1H, −CH2−Ph), 4.72 – 4.61 (m, 1H, −CH2−Ph), 4.57 – 4.42 (m, 2H, −CH2−Ph), 4.40 (d, J = 1.7 Hz, 1H, H-1), 4.26 – 4.11 (m, 1H, −OCH2−), 3.91 (s, 1H, H-2), 3.83 – 3.73 (m, 1H, −OCH2−), 3.63 (dt, J = 9.3, 4.7 Hz, 1H, H-4), 3.47 (dd, J = 9.4, 3.0 Hz, 1H, H-3), 3.33 (td, J = 6.2, 3.4 Hz, 1H, H-5), 2.84 – 2.60 (m, 1H, −OCH2−), 1.39 (d, J = 6.0 Hz, 1H, H-6). 13C NMR (101 MHz, CDCl3): δ 177.4, 138.7, 138.5, 138.3, 128.6, 128.5, 128.46, 128.3, 128.2, 127.8, 127.7, 127.6, 101.7, 82.2, 80.2, 75.6, 74.0, 73.8, 72.1, 71.5, 64.9, 35.1, 18.1. HR ESI-QTOF-MS m/z: [M + Na]+ calculated for C30H34NaO7 529.2197, found 529.2200.
Compound 11a:
To a solution of 9 (36.0 mg, 0.016 mmol) in AcOH/THF (1/3, 2.0 mL) was added activated Zn (52.4 mg, 0.800 mmol). After the mixture was stirred at rt for 8 h, it was filtered through a Celite pad, and the filtrate was concentrated under reduced pressure. The crude product was washed with dry toluene (3×3 mL) and then dissolved in 1 mL of dry CH2Cl2. To this solution were added 5a (19.2 mg, 0.037 mmol), EDC (14.5 mg, 0.075 mmol), and DMAP (1.10 mg, 0.009 mmol) in 2 mL of dry CH2Cl2 at 0 °C. The mixture was stirred at rt overnight and diluted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3 solution, water, and brine, and dried over Na2SO4. The solution was concentrated under a vacuum, and the residue was purified by silica gel column chromatography to offer 11a (24.8 mg, 71%) as colorless syrup. 1H NMR (600 MHz, CDCl3): δ 7.45 – 7.14 (m, 45H, ArH), 6.50 (brs, 1H, −C(O)NH), 5.96 (d, J = 7.4 Hz, 1H, −C(O)NH), 5.78 (d, J = 8.6 Hz, 1H, , −C(O)NH), 5.65 – 5.48 (dd, J = 9.2 Hz, 1H, H-3’), 5.17 – 5.02 (m, 3H, lipid-CH2−CH(O)−, H-3, H-1’), 5.01 – 4.96 (m, 1H, lipid-CH2−CH(O)−), 4.93 (d, 1H, J = 10.9 Hz, PhCH2O−), 4.90 – 4.82 (m, 5H, PhCH2O−, H-1”), 4.75 (q, J = 12.2 Hz, 2H, PhCH2O−), 4.64 (d, J = 10.9 Hz, 1H, PhCH2O−), 4.62 (brs, 1H, PhCH2O−), 4.54 – 4.35 (m, 10H, PhCH2O−, 2×lipid-CH2−CH(OBn), −OCH2CH2CO−, H-4’, H-1, H-2”), 4.00 (d, J = 10.9 Hz, 1H, −OCH2CH2N−), 3.93 − 3.80 (m, 5H, , −OCH2CH2N−, H-2, H-3” and lipid-H), 3.78 – 3.71 (m, 3H, H-6, H-6’, lipid-H), 3.72 – 3.64 (m, 4H, H-5”, lipid-H), 3.64 – 3.58 (m, 3H, −CH2N−), 3.54 − 3.45 (m, 3H), 3.37 (m, 3H, H-2’,), 2.63 – 2.39 (m, 6H, −CH2−CO−), 2.38 − 2.15 (m, 8H, −CH2−CO−), 2.00 (dd, J = 15.0, 5.6 Hz, 1H, −CH2−CO−), 1.55 – 1.43 (m, 7H, 3×−CH2−), 1.33 (d, J = 6.2 Hz, 3H, H-6”), 1.32 – 1.14 (m, 100H, 50×−CH2−), 0.96 − 0.80 (m, 18H, 6×CH3). 13C NMR (151 MHz, CDCl3): δ 173.7, 173.6, 172.0, 171.1, 170.1, 138.83, 138.82, 138.7, 138.65, 138.6, 138.3, 137.6, 135.8, 135.7, 128.7, 128.65, 128.6, 128.58, 128.53, 128.51, 128.45, 128.43, 128.3, 128.2, 128.12, 128.11, 128.1, 128.0, 127.9, 127.83, 127.78, 127.71, 127.69, 127.68, 127., 101.1, 100.0, 98.0, 80.8, 80.6, 80.3, 79.7, 76.1, 75.8, 75.7, 75.5, 75.4, 75.2, 75.1, 74.8, 74.6, 74.4, 74.4, 74.2, 73.4, 72.9, 72.1, 71.4, 71.13, 71.1, 70.6, 69.8, 69.7, 69.6, 69.59, 68.9, 68.3, 68.2, 68.17, 67.8, 63.9, 63.6, 61.4, 56.0, 54.5, 42.0, 41.9, 41.2, 39.7, 39.4, 38.9, 37.4, 36.5, 34.6, 34.6, 34.5, 34.3, 34.3, 32.1, 32.07, 29.9, 29.88, 29.84, 29.83, 29.81, 29.79, 29.76, 29.75, 29.70, 29.63, 29.59, 29.53, 29.52, 29.50, 29.44, 29.39, 25.5, 25.4, 25.35, 25.32, 25.2, 22.8, 18.2, 14.3. 31P NMR (243 MHz, CDCl3): δ −2.06. HR ESI-QTOF-MS m/z: [M + 2NH4]2+ calculated for C162H246N3O28P, 1370.8870 found 1370.8843.
Compound 1a:
To a solution of 11a (24.0 mg, 8.87 μmol) in CH2Cl2 (3.0 mL) and MeOH (1.0 mL) was added Pd/C (10%, 5.20 mg) under an N2 atmosphere. The reaction mixture was stirred under an H2 atmosphere at rt for 2 d, and then filtered. The filtrate was concentrated in vacuum. The residue was purified by column chromatography with CHCl3 and CH3OH (3:1) as eluents to give 1a as a white solid (10.6 mg, 63%). 1H NMR (600 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ 5.20 (t, J = 10.1 Hz, 1H, H-3’), 5.13 – 5.05 (m, 3H, 2×lipid-H), 4.99 (dd, J = 10.4, 8.9 Hz, 1H, H-3), 4.72 (s, 1H, H-1”), 4.59 (d, J = 8.4 Hz, 1H, H-1’), 4.50 (d, J = 10.8 Hz, 1H, H-1), 4.22 (q, J = 9.3 Hz, 1H, H-4’), 4.09 (d, J = 10.3 Hz, 1H), 4.04 (dq, J = 8.8, 4.5 Hz, 1H), 3.97 (dq, J = 10.7, 3.8 Hz, 1H), 3.94 – 3.85 (m, 3H), 3.85 – 3.83 (m, 1H), 3.82 – 3.74 (m, 4H), 3.71 – 3.64 (m, 2H), 3.62 – 3.56 (m, 2H), 3.55 − 3.47 (m, 2H), 3.47 − 3.34 (m, 4H, −CH2N−), 3.25 − 3.20 (m, 1H, −CH2N−), 2.58 − 2.21 (m, 14H, −CH2−CO−), 1.71 − 1.05 (m, 115H, 56×lipid-CH2−, H-6”), 0.86 (t, J = 6.9 Hz, 18H, 6×−CH3). 13C NMR (151 MHz, CDCl3:MeOD:D2O = 1:1:0.2): δ 174.9, 174.8, 174.6, 174.4, 173.3, 173.05, 172.2, 102.2, 101.7, 100.6, 77.7, 76.1, 75.9, 75.3, 74.2, 74.0, 73.2, 71.8, 71.6, 71.2, 69.2, 69.1, 69.0, 68.9, 68.8, 64.1, 60.8, 54.5, 54.3, 42.8, 42.4, 41.7, 41.6, 40.1, 40.0, 37.9, 37.0, 35.2, 35.1, 34.5, 32.6, 30.33, 30.30, 30.2, 30.1, 30.0, 29.9, 29.8, 26.3, 26.2, 26.1, 25.9, 25.8, 25.7, 23.3, 17.8, 14.5. 31P NMR (243 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ −1.47. HR ESI-QTOF-MS m/z: [M - H]− calculated for C99H183N3O28P 1893.2720, found 1893.2684.
Compound 11b:
It (24.8 mg, 71%) was synthesized from 9 (36.0 mg, 0.016 mmol) by the same procedure employed for the synthesis of 11a. 1H NMR (600 MHz, CDCl3): δ 7.42 (d, J = 7.1 Hz, 2H, ArH), 7.32 – 7.24 (m, 33H, ArH), 7.23 – 7.20 (m, 8H, ArH), 7.18 – 7.14 (m, 2H, ArH), 6.73 (brs, 1H, −C(O)NH), 6.07 (d, J = 7.8 Hz, 1H, −C(O)NH), 5.80 (d, J = 8.7 Hz, 1H, −C(O)NH), 5.48 (dd, J = 9.1 Hz 1H, H-3’), 5.10 – 5.01 (m, 3H, 2×lipid-H, H-3), 5.00 (d, J = 8.2 Hz, 1H, H-1’), 4.94 (d, J = 10.8 Hz, 1H, PhCH2−O), 4.92 – 4.84 (m, 5H, PhCH2−O), 4.82 (d, J = 12.4 Hz, 1H, PhCH2−O), 4.62 (d, J = 10.8 Hz, 1H, PhCH2−O), 4.53 – 4.33 (m, 15H, 5×PhCH2−O, H-4’, H-1” and H-1, H-4), 4.13 – 4.05 (m, 1H, −OCH2CH2N−), 3.97 (d, J = 10.0 Hz, 1H, H-3”), 3.92 (d, J = 2.9 Hz, 1H, H-2”), 3.89 – 3.85 (m, 1H, H-2), 3.83 – 3.79 (m, 2H, −OCH2CH2CO−), H-5), 3.78 – 3.71 (m, 3H, H-6, −OCH2CH2C(O)−), 3.68 – 3.55 (m, 6H, H-5’, H-5”, H-6’, H-6, H-4”), 3.54 – 3.49 (m, 1H, −OCH2CH2N−), 3.45 (dd, J = 9.5, 2.9 Hz, 2H, −CH2−N−), 3.44 – 3.39 (m, 1H, H-2’), 3.36 (dt, J = 8.5, 4.2 Hz, 1H, −CH2−N−), 3.34 – 3.27 (m, 1H, H-6”), 2.59 – 2.41 (m, 6H, −CO-CH2−), 2.35 (dd, J = 14.6, 6.9 Hz, 1H, −CO-CH2−), 2.31 – 2.22 (m, 6H, −CO-CH2−), 2.05 (dd, J = 15.1, 5.6 Hz, 1H, −CO-CH2−), 1.61 – 1.44 (m, 7H, 3×−CH2−), 1.36 (d, J = 6.1 Hz, 1H, H-6”), 1.33 – 1.16 (m, 100H, 50×CH2−), 0.89 – 0.86 (m, 18H, 6×CH3). 13C NMR (151 MHz, CDCl3): δ 173.7, 173.6, 171.9, 171.6, 171.1, 170.03, 169.98, 138.9, 138.74, 138.69, 138.62, 138.4, 138.3, 137.6, 135.8, 135.77, 135.74, 135.72, 128.68, 128.64, 128.58, 128.50, 128.47, 128.44, 128.42, 128.3, 128.2, 128.1, 128.05, 127.9, 127.77, 127.75, 127.71, 127.69, 127.68, 127.66, 127.63, 101.7, 101.0, 100.2, 82.2, 80.3, 76.2, 75.7, 75.5, 75.2, 74.9, 74.5, 74.4, 74.21, 74.19, 74.16, 73.4, 72.4, 72.1, 71.54, 71.47, 71.1, 70.7, 69.74, 69.70, 69.62, 69.58, 68.8, 68.2, 67.7, 65.9, 55.7, 54.4, 41.9, 41.2, 39.7, 39.6, 38.9, 37.0, 34.7, 34.4, 34.33, 34.28, 32.08, 32.07, 29.89, 29.88, 29.86, 29.84, 29.83, 29.81, 29.79, 29.76, 29.75, 29.71, 29.67, 29.64, 29.61, 29.58, 29.52, 29.50, 29.43, 29.39, 25.5, 25.4, 25.3, 25.2, 22.8, 18.2, 14.3. 31P NMR (243 MHz, CDCl3): δ −2.05. HR ESI-QTOF-MS m/z: [M + 2NH4]2+ calculated for C162H246N3O28P, 1370.8870 found 1370.8850.
Compound 1b:
It (11.3 mg, 67%) was synthesized from 11b (24.0 mg, 8.87 μmol) by the same procedure employed for the synthesis of 1a. 1H NMR (600 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ 5.17 (t, J = 9.9 Hz, 1H, H-3’), 5.11 – 5.04 (m, 2H, 2×lipid-H), 4.95 (t, J = 9.6 Hz, 1H, H-3), 4.55 (d, J = 8.5 Hz, 1H, H-1’), 4.52 (s, 1H, H-1”), 4.47 (d, J = 8.4 Hz, 1H, H-1), 4.25 – 4.17 (m, 1H, H-4’), 4.14 – 4.00 (m, 3H), 3.98 – 3.89 (m, 4H), 3.84 – 3.71 (m, 5H), 3.55 (dd, J = 12.3, 6.6 Hz, 2H), 3.51 – 3.44 (m, 3H), 3.41 (d, J = 9.9 Hz, 3H), 3.39 – 3.33 (m, 3H), 3.26 (ddd, J = 15.0, 7.9, 4.8 Hz, 3H, −CH2N−), 3.21 – 3.15 (m, 1H, −CH2N−), 2.57 – 2.40 (m, 7H, −CH2−CO−), 2.40 – 2.21 (m, 7H, −CH2−CO−), 1.63 – 1.08 (m, 115H, 56×lipid-CH2−, H-6”), 0.85 (t, J = 6.9 Hz, 18H, 6×-CH3). 13C NMR (151 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ 174.9, 174.7, 173.4, 173.3, 173.0, 172.1, 171.9, 102.2, 101.9, 100.5, 77.4, 76.2, 76.0, 75.3, 74.2, 74.1, 73.1, 72.0, 71.8, 71.7, 71.5, 70.5, 69.1, 68.9, 68.8, 68.6, 66.1, 65.4, 64.0, 61.8, 60.8, 54.3, 53.7, 42.8, 42.4, 41.7, 41.5, 41.4, 40.2, 39.7, 39.4, 38.7, 37.9, 37.1, 37.01, 36.99, 35.1, 34.7, 34.5, 32.5, 31.0, 30.3,30.0, 29.9, 29.8, 26.24, 26.17, 26.0, 25.9, 25.8, 25.7, 23.3, 17.8, 14.4. 31P NMR (243 MHz, CDCl3:MeOD:D2O = 1:1:0.2): δ −0.34. HR ESI-QTOF-MS m/z: [M - H]− Calculated for C99H183N3O28P 1893.2720, found 1893.2785.
Compound 12a:
It (17.9 mg, 72%) was synthesized from 10 (21.0 mg, 0.098 mmol) by the same procedure employed for the synthesis of 11a. 1H NMR (600 MHz, CDCl3): δ 7.53 – 7.07 (m, 40H, ArH), 6.55 (s, 1H, −C(O)-NH−), 5.93 (d, J = 5.8 Hz, 1H, −C(O)-NH−), 5.54 (d, J = 7.7 Hz, 1H, −C(O)-NH−), 5.40 (t, J = 9.2 Hz, 1H, H-3’), 5.19 (t, J = 9.2 Hz, 1H, H-3), 5.10 – 5.03 (m, 1H, lipid-H), 5.01 – 4.95 (m, 2H, lipid-H, H-1’), 4.94 – 4.84 (m, 4H, PhCH2O−) 4.81 (d, J = 1.31 Hz, 1H, H-1”), 4.71 (d, J = 12.3 Hz, 2H, PhCH2O−), 4.63 (d, J = 10.9 Hz, 1H, PhCH2O−), 4.59 (brs, 2H, PhCH2O−), 4.53 (d, J = 11.1 Hz, 1H, PhCH2O-), 4.50 (d, J = 11.6 Hz, 1H, PhCH2O−), 4.45 (d, J = 11.7 Hz, 1H, PhCH2O−), 4.43 (d, J = 11.1 Hz, 1H, PhCH2O−), 4.42 (d, J = 8.2 Hz, 1H, H-1), 4.41 (d, J = 11.1 Hz, 1H, PhCH2O−), 4.37 (d, J = 11.3 Hz, 1H, PhCH2O−), 4.24 (q, J = 9.3 Hz, 1H, H-4’), 4.03 – 3.77 (m, 7H, H-6’, H-2, −OCH2CH2CO−, H-4”), 3.77 – 3.63 (m, 4H, −OCH2CH2CO−, H-5”, H-6), 3.63 – 3.54 (m, 2H), 3.54 – 3.45 (m, 2H, H-5’), 3.42 (s, 3H, −OCH3), 3.39 – 3.32 (m, 1H, H-2’), 3.28 −3.20 (m, 1H, H-6’), 2.56 (dd, J = 15.9, 7.1 Hz, 1H, −CH2−CO−), 2.46 (dd, J = 15.9, 5.1 Hz, 1H, −CH2−CO−), 2.40 − 2.20 (m, 10H, −CH2−CO−), 2.17 (dd, J = 15.3, 7.0 Hz, 1H, −CH2CO−), 1.99 (dd, J = 15.3, 5.3 Hz, 1H, −CH2−CO−), 1.61 – 1.11 (m, 112H, 54×lipid-CH2−, H-6”), 0.96 – 0.80 (m, 18H, 6×-CH3). 13C NMR (151 MHz, CDCl3): δ 173.8, 173.6, 171.8, 171.0, 170.7, 170.0, 169.8, 140.2, 140.0, 139.9, 138.8, 138.7, 138.6, 137.7, 135.5, 128.8, 128.7, 128.6, 128.57, 128.5, 128.3, 128.2, 128.15, 128.1, 128.0, 127.8, 127.7, 101.8, 100.4, 98.2, 80.6, 80.3, 76.4, 76.0, 75.7, 75.6, 75.5, 75.4, 75.3, 75.1, 74.9, 74.5, 72.9, 72.2, 71.4, 71.2, 70.0, 68.9, 68.3, 64.7, 64.0, 63.6, 56.5, 56.1, 54.1, 42.4, 41.9, 41.4, 39.8, 39.7, 38.7, 36.8, 34.6, 34.4, 34.3, 34.1, 32.1, 29.8, 29.6, 29.5, 29.4, 25.5, 25.4, 25.3, 25.2, 22.8, 18.2, 14.3. 31P NMR (243 MHz, CDCl3): δ −1.23. HR ESI-QTOF-MS m/z: [M + 2NH4]2+ calculated for C154H238N5O28P 1310.8582, found 1310.8584.
Compound 2a:
It (4.7 mg, 51%) was synthesized from 12a (11.0 mg, 4.25 μmol) by the same procedure employed for the synthesis of 1a. 1H NMR (600 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ 5.15 (t, J = 9.9 Hz, 1H, H-3’), 5.14 – 5.04 (m, 2H, 2×lipid-H), 4.95 (dd, J = 10.6, 8.9 Hz, 1H, H-2), 4.74 (s, 1H, H-1”), 4.58 (d, J = 8.4 Hz, 1H, H-1’), 4.39 (d, J = 8.4 Hz, 1H, H-1), 4.12 – 4.04 (m, 1H,-H4’), 4.05 – 3.98 (m, 2H), 3.97 – 3.90 (m, 2H), 3.85 – 3.76 (m, 3H), 3.74 – 3.62 (m, 4H), 3.60 – 3.48 (m, 3H), 3.47 – 3.43 (m, 1H), 3.40 (s, 3H, −OCH3), 3.14 (q, J = 7.4 Hz, 1H), 2.51 – 2.22 (m, 14H, −CH2−CO−), 1.68 – 1.11 (m, 112H, lipid-CH2−), 0.85 (t, J = 7.0 Hz, 18H, 6×-CH3). 13C NMR (126 MHz, CDCl3:MeOD:D2O, 2:1:0.2; derived from HSQC NMR spectrum): δ 100.0, 101.8, 102.1, 76.2, 75.4, 74.4, 74.3, 74.2, 73.1, 73.0, 71.5, 72.1, 71.2, 71.0, 70.4, 69.0, 69.7, 68.7, 66.0, 63.7, 63.5, 56.9, 54.0, 53.9, 49.6, 49.2, 46.9, 42.6, 42.4, 41.5, 41.4, 37.7, 37.6, 37.1, 37.0, 36.9, 36.88, 36.7, 36.3, 35.8, 34.9, 34.4, 32.5, 32.36, 30.1, 29.9, 29.6, 28.2, 25.9, 25.7, 25.5, 23.1, 22.9, 17.7, 14.3. 31P NMR (243 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ −0.57. HR ESI-QTOF-MS m/z: [M + NH4]+ calculated for C98H186N4O27P 1883.3070, found 1883.3154.
Compound 12b:
It (17.5 mg, 71%) was synthesized from 10 (21.0 mg, 0.098 mmol) by the same procedure employed for the synthesis of 11a. 1H NMR (600 MHz, CDCl3): δ 7.43 (d, J = 6.9 Hz, 2H, ArH), 7.34 – 7.12 (m, 38H, ArH), 6.64 (brs, 1H, −C(O)NH−), 5.99 (brs, 1H, −C(O)NH−), 5.54 (d, J = 7.7 Hz, 1H, -C(O)NH−), 5.40 (t, J = 9.3 Hz 1H, H-3’), 5.19 (dd, J = 9.2, 9.4 Hz, 1H, H-3), 5.08 (dt, J = 12.5, 6.6 Hz, 1H, lipid-H), 5.01 – 4.85 (m, 7H, lipid-H, PhCH2O−, H-1’), 4.81 (d, J = 12.5 Hz, 1H, PhCH2O−), 4.61 (d, J = 10.9 Hz, 1H, PhCH2O−), 4.52 (d, J = 11.1 Hz, 1H, PhCH2O−), 4.50 (d, J = 11.6 Hz, 1H, PhCH2O−), 4.48 – 4.34 (m, 7H, PhCH2O−, H-1”, H-1), 4.24 (q, J = 9.2 Hz, 1H, H-4), 4.07 (dt, J = 9.7, 5.9 Hz, 1H, −OCH2CH2CO−), 3.96 − 3.88 (m, 3H, H-2”, H-6,), 3.87 – 3.76 (m, 4H, −OCH2CH2CO−, H-6’, H-4’, H-4”), 3.75 – 3.68 (m, 2H, H-6), 3.60 – 3.54 (m, 2H, H-5’, H-5”), 3.52 – 3.44 (m, 1H, H-5, H-2), 3.44 – 3.41 (m, 4H, −OCH3), 3.38 − 3.31 (m, 2H, H-6’), 3.29 (ddd, J = 12.3, 7.7, 4.6 Hz, 1H, H-2’), 2.56 (dd, J = 16.0, 7.2 Hz, 1H, −CH2−CO−), 2.54 – 2.21 (m, 11H, −CH2−CO−), 2.18 (dd, J = 15.2, 7.0 Hz, 1H, −CH2−CO−), 2.00 (dd, J = 15.2, 5.4 Hz, 1H, −CH2−CO−), 1.67 – 1.38 (m, 13H, lipid-CH2), 1.36 (d, J = 6.1 Hz, 3H, H-6”), 1.35 – 1.06 (m, 98H, lipid-CH2−), 0.91 – 0.85 (m, 18H, 6×-CH3). 13C NMR (151 MHz, CDCl3): δ 173.8, 173.6, 171.8, 171.1, 170.1, 169.9, 169.8, 139.0, 138.7, 138.68, 138.63, 138.4, 137.7, 128.81, 128.78, 128.75, 128.73, 128.62, 128.57, 128.47, 128.44, 128.42, 128.29, 128.27, 128.23, 128.19, 128.09, 127.88, 127.83, 127.82, 127.77, 127.66, 127.63, 127.57, 101.8, 100.5, 82.2, 80.3, 76.3, 75.7, 75.6, 75.5, 75.28, 75.25, 74.9, 74.5, 74.1, 73.0, 72.1, 72.0, 71.9, 71.4, 71.4, 71.2, 71.1, 70.6, 70.04, 70.01, 70.0, 69.0, 65.8, 56.5, 56.1, 54.1, 41.9, 41.5, 39.9, 39.7, 38.8, 37.11, 37.09, 34.6, 34.4, 34.33, 34.29, 34.1, 32.1, 29.9, 29.83, 29.81, 29.79, 29.78, 29.76, 29.74, 29.70, 29.67, 29.63, 29.60, 29.56, 29.52, 29.50, 29.42, 29.39, 25.5, 25.4, 25.33, 25.30, 25.2, 22.8, 18.2, 14.3. 31P NMR (243 MHz, CDCl3): δ −1.34. HR ESI-QTOF-MS m/z: [M + 2NH4]2+ calculated for C154H238N5O28P 1310.8582, found 1310.8596.
Compound 2b:
It (6.10 mg, 60%) was synthesized from 12b (14.0 mg, 5.41 μmol) by the same procedure employed for the synthesis of 1a. 1H NMR (600 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ 5.18 – 5.05 (m, 3H, 2×lipid-H, H-3’), 4.97 (dd, J = 12.5, 7.0 Hz, 1H, H-3), 4.39 (d, J = 8.3 Hz, 1H, H-1), 4.15 − 4.05 (m, 1H, H-4’), 4.04 − 3.98 (m, 3H), 3.98 − 3.92 (m, 2H), 3.91 − 3.85 (m, 2H), 3.82 − 3.76 (m, 4H), 3.74 − 3.68 (m, 2H), 3.57 − 3.51 (m, 1H), 3.51 − 3.44 (m, 2H), 3.44 – 3.41 (m, 1H), 3.40 (s, 3H, −OCH3), 3.26 − 3.21 (m, 1H), 3.16 − 3.11 (m, 1H), 2.51 – 2.22 (m, 14H, −CH2−CO−), 1.63 − 1.11 (m, 112H, lipid-CH2−), 0.85 (t, J = 7.0 Hz, 18H, 6×-CH3). 13C NMR (126 MHz, CDCl3:MeOD:D2O, 2:1:0.2; derived from HSQC NMR spectrum): δ 101.6 (2C), 100.1, 76.1, 75.7, 75.0, 74.1, 73.7, 73.5, 73.3, 72.9, 72.3, 70.7, 69.3, 68.1, 65.5, 65.3, 61.9, 59.2, 56.2, 53.7, 53.3, 52.1, 48.7, 48.5, 48.4, 48.2, 47.4, 45.8, 42.09, 41.95, 41.4, 41.0, 39.2, 36.3, 36.2, 35.4, 35.5, 35.1, 34.3, 34.3, 33.9, 33.5, 33.2, 33.1, 27.5, 25.7, 25.3, 24.5, 24.9, 24.2, 22.5, 22.0, 14.5, 13.7. 31P NMR (243 MHz, CDCl3:MeOD:D2O = 2:1:0.2): δ −0.56. HR ESI-QTOF-MS m/z: [M + NH4]+ calculated for C98H186N4O27P 1883.3070, found 1883.3163.
Compound 13:
It (7.10 mg, 66%) was synthesized from 9 (14.5 mg, 6.46 μmol) by the same procedure and reaction conditions employed for the synthesis of 1a. 1H NMR (600 MHz, CDCl3:MeOD:D2O, 3:2:0.1): δ 5.25 − 5.07 (m, 2H), 5.08 – 4.94 (m, 1H), 4.55 (d, J = 8.1 Hz, 1H), 4.50 (d, J = 9.5 Hz, 1H), 4.30 – 4.18 (m, 1H), 4.14 − 3.97 (m, 5H), 3.96 − 3.79 (m, 4H), 3.76 − 3.66 (m, 2H), 3.51 − 3.46 (m, 1H), 3.46 − 3.41 (m, 1H), 3.29 – 3.15 (m, 1H), 3.16 – 3.02 (m, 1H), 2.63 – 2.14 (m, 12H), 1.76 − 1.20 (m, 108H), 0.89 (t, J = 6.9 Hz, 18H). 13C NMR (151 MHz, CDCl3:MeOD:D2O, 3:2:0.1): δ 174.6, 174.4, 173.1, 172.9, 172.2, 171.9, 102.4, 102.1, 75.9, 75.33, 75.1, 74.9, 73.7, 71.71, 71.68, 71.64, 70.9, 70.6, 69.7, 69.6, 69.3, 69.2, 69.1, 68.89, 68.87, 68.4, 67.8, 54.6, 54.4, 53.6, 49.9, 49.7, 42.7, 42.3, 41.8, 41.7, 41.0, 38.0, 37.8, 37.71, 37.66, 37.62, 35.02, 34.99, 34.7, 34.59, 34.55, 32.4, 30.22, 30.20, 30.16, 30.14, 30.07, 30.04, 30.00, 29.98, 29.86, 29.70, 29.69, 26.2, 26.1, 25.9, 25.81, 25.74, 25.61, 25.57, 23.2, 14.4. 31P NMR (243 MHz, CDCl3:MeOD:D2O, 3:2:0.1): δ 0.63. HR ESI-QTOF-MS m/z: [M + H]+ calculated for C90H171N3O22P 1678.2112, found 1678.2199.
N-Decyl-3-(α-L-rhamnopyranosyloxy)propanamide (14).
To a solution of 5a (55 mg, 0.106 mmol) in DCM were added EDC (31.2 mg, 0.162 mmol) and DMAP (2.60 mg, 0.022 mmol) at 0 °C.After stirring for 15 min, n-dodecyl amine (20.5 mg. 0.130 mmol) was added. The temperature was allowed to raise to rt, and the mixture was stirred overnight and then diluted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3 solution, water, and brine and then dried over Na2SO4. The solution was concentrated under a vacuum, and the residue was purified by silica gel column chromatography to give N-decyl-3-(2,3,4-tri-O-phenylmethyl-α-L-rhamnopyranosyl) oxypropanamide (intermediate 14a, 64.0 mg, 91%) as yellowish syrup. Rf: 0.6 (ethyl acetate); 1H NMR (600 MHz, CDCl3): δ 7.40 – 7.27 (m, 15H), 5.58 (t, J = 5.1 Hz, 1H), 4.93 (d, J = 10.9 Hz, 1H, −OCH2−Ph), 4.78 (d, J = 1.6 Hz, 1H, anomeric), 4.73 (q, J = 12.4 Hz, 2H, −OCH2−Ph), 4.64 (d, J = 10.9 Hz, 1H, −OCH2−Ph), 4.60 (s, 2H, −OCH2−Ph), 3.88 (dt, J = 10.1, 5.9 Hz, 1H), 3.78 (dd, J = 8.8, 3.1 Hz, 1H), 3.76 – 3.73 (m, 1H), 3.69 – 3.57 (m, 3H), 3.18 (qd, J = 7.5, 4.0 Hz, 2H), 2.49 – 2.30 (m, 2H), 1.42 (h, J = 6.1, 5.5 Hz, 2H), 1.33 (d, J = 5.9 Hz, 3H, −CH3), 1.31 – 1.16 (m, 14H), 0.87 (t, J = 7.1 Hz, 3H, −CH3). 13C NMR (151 MHz, CDCl3): δ 170.7, 138.7, 138.6, 138.4, 128.51, 128.49, 128.1, 128.0, 127.84, 127.77, 127.71, 127.70, 98.2, 80.6, 80.1, 75.5, 74.9, 72.9, 72.3, 68.5, 63.8, 39.7, 37.1, 32.0, 29.8, 29.73, 29.70, 29.44, 29.43, 27.1, 22.8, 18.2, 14.3. HR ESI-QTOF-MS m/z: [M - H]− calculated for C40H55NO6 645.4029, found 644.3964. Then, this compound (28.0 mg, 43.4 μmol) was dissolved in CH2Cl2 (1.0 mL) and MeOH (3.0 mL), and to the solution was added Pd/C (10%, 5.10 mg, 0.004 μmol) under an N2 atmosphere. The mixture was stirred under an H2 atmosphere at rt for 2 d and filtered, and the filtrate was concentrated. The residue was purified by column chromatography with CHCl3 and CH3OH (3:1) as eluents to give 14 as a white solid (14.1 mg, 87%). 1H NMR (600 MHz, MeOD:CDCl3 = 3:1): δ 4.71 (d, J = 0.9 Hz, 1H), 3.93 (dt, J = 9.8, 6.2 Hz, 1H), 3.81 (dd, J = 3.2, 1.5 Hz, 1H), 3.68 (dt, J = 9.8, 6.3 Hz, 1H), 3.62 (dd, J = 9.5, 3.4 Hz, 1H), 3.58 (dq, J = 9.5, 6.3 Hz, 1H), 3.37 (t, J = 9.5 Hz, 1H), 3.18 (td, J = 7.1, 2.9 Hz, 2H), 2.45 (t, J = 6.2 Hz, 2H), 1.51 (h, J = 7.0, 5.6 Hz, 2H), 1.35 – 1.23 (m, 17H,), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, MeOD:CDCl3 = 3:1): δ 172.0, 100.0, 72.7, 71.2, 70.6, 68.4, 63.4, 39.4, 36.3, 31.8, 29.4, 29.2, 29.1, 26.8, 22.5, 17.1, 13.6. HR ESI-QTOF-MS m/z: [M + Na]+ calculated for C19H37NO6 375.2621, found 398.2506.
General procedure for the preparation of liposomes of the conjugate adjuvants:
The mixture of an MPLA-Rha conjugate (0.477 μmol) or 13+14 (0.477 μmol each), DSPC (2.45 mg, 3.10 μmol), and cholesterol (0.922 mg, 2.38 μmol) (10:65:50 molar ratio) was dissolved in a mixture of CH2Cl2 and MeOH (1:1, v/v, 2 mL) in a 10 mL round-bottomed flask. Then, the solvents were removed in vacuum with a rotary evaporator to generate a thin lipid film on the flask wall, which was followed by adding 2.0 mL of HEPES buffer (20 mM, pH 7.5) containing NaCl (150 mM) for lipid hydration. The mixture was shaked under an argon atmosphere at 40 °C for 1 h. The milky suspension was sonicated for 1 min to obtain the desired liposomes. The liposomes were sealed and preserved at 4 °C before used as adjuvants for immunizations.
Immunization of mouse:
The vaccine formulations were prepared by integrating KLH-sTn conjugate (containing 8.0 μg of sTn antigen) that was dissolved in 50 μL of PBS and 50 μL of PBS, Alum or a conjugate adjuvant (liposomes of 1a, 1b, 2a, 2b or 13+14) per injection. This mixture was mixed vigorously on a vortex mixer. Each experimental group is composed of five female C57BL/6 mice of 6−8 weeks of age. The mice were immunized on day 1 by s.c. injection of a vaccine formulation (0.1 mL) using a 26G 1/2 (0.45 mm 13 mm) needle. Following the initial immunization, mice were boosted 3 times on day 15, 22, and 29 through s.c. injection of the same vaccine/adjuvant formulation by the same immunization protocol. Blood samples were collected from each mouse through the saphenous vein on day 0 prior to initial immunization and on day 27, 31, and 38 after boost immunizations, respectively. The blood samples were clotted to prepare antisera by standard protocols, and antisera were stored at −80 °C before use.
ELISA Protocols:
A solution of the sTn-HSA conjugate or KLH (2.00 μg/mL, 100 μL) dissolved in coating buffer (0.1 M bicarbonate, pH 9.6) was added to each well of ELISA plates, and the plates were incubated at 37 °C for 1 h. After the plates were washed with PBS buffer containing 0.05% Tween 20 (PBST) 3 times, a blocking solution (1% BSA in PBST, 100 μL) was added to each well to block the plates. The plates were washed with PBST 3 times, and then a pooled day 0 serum or an individual mouse antiserum with serial half-log dilutions from 1:300 to 1:218700 in PBS was added to the coated ELISA plates (100 μL/well), which was followed by incubation at 37 °C for 2 h. The plates were washed with PBS and then incubated with a 1:1000 diluted solution of AP-linked goat anti-mouse kappa, IgM, or IgG antibody (100 μL/well), respectively, at rt for 1 h. Finally, the plates were washed with PBS and developed with a solution of p-nitrophenylphosphate (PNPP) in PBS (1.67 mg/mL, 100 μL) at rt for 30 min, followed by colorimetric readout using a BioTek plate reader at 405 nm wavelength. For antibody titer analysis, obtained OD values were plotted against antiserum dilution values, and a best-fit line was obtained. The equation of this line was applied to calculating the dilution value at which an OD of 0.1 was achieved, and the antibody titer was determined at the inverse of the dilution value.
Statistical analysis:
All data were analyzed by one-way analysis of variance (for data of more than 2 groups) or independent t-test (for data between 2 groups), using GraphPad software. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENT
This work is supported by a research grant (R21 AI170129) from NIH/NIAID. The MS instrument was supported by NIH grants S10 OD021758 and S10 OD030250. Z. Guo is grateful to Drs. Steven and Rebecca Scott for their endowment to support our research.
ABBREVIATIONS USED
- AP
alkaline phosphatase
- CTL
cytotoxic T cell
- DC
dendritic cell
- DCM
dichloromethane
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- EDC
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide
- ELISA
enzyme-linked immunosorbent assay
- ESI
electrospray ionization
- HSA
human serum album
- KLH
keyhole limpet hemocyanin
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex molecules
- MPLA
monophosphoryl lipid A
- MS 4Å
molecular sieves 4Å
- NIS
N-iodosuccinimide
- OD
optical density
- PBS
phosphate-buffered saline
- PBST
PBS buffer containing 0.05% Tween 20
- PNPP
p-nitrophenylphosphate
- Rha
rhamnose
- s.c.
subcutaneous
- SAR
structure-activity relationship
- SEM
standard error of mean
- TACA
tumor-associated carbohydrate antigen
- TLR4
toll-liker receptor 4
- TMSOTf
trimethylsilyl triflate
Footnotes
Supporting Information
Additional procedures and results of immunological studies and NMR and MS spectra of new compounds in a PDF file, as well as molecular formula strings in a CSV file. This material is available free of charge via the Internet at http://pubs.acs.org.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information
REFERENCES
- 1.Hollingsworth RE; Jansen K, Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonarelli G; Corti C; Tarantino P; Ascione L; Cortes J; Romero P; Mittendorf EA; Disis ML; Curigliano G, Therapeutic cancer vaccines revamping: Technology advancements and pitfalls. Ann. Oncol 2021, 32, 1537–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczmarek M; Poznańska J; Fechner F; Michalska N; Paszkowska S; Napierała A; Mackiewicz A, Cancer vaccine therapeutics: Limitations and effectiveness-a literature review. Cells 2023, 12, 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J; Fu M; Wang M; Wan D; Wei Y; Wei X, Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol 2022, 15, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin MJ; Svensson-Arvelund J; Lubitz GS; Marabelle A; Melero I; Brown BD; Brody JD, Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [DOI] [PubMed] [Google Scholar]
- 6.Awate S; Babiuk LA; Mutwiri G, Mechanisms of action of adjuvants. Front. Immunol 2013, 4, e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulendran B; Ahmed R, Immunological mechanisms of vaccination. Nat. Immunol 2011, 12, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S; Zhu H; Xia X; Liang Z; Ma X; Sun B, Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [DOI] [PubMed] [Google Scholar]
- 9.O’Hagan DT; Fox CB, New generation adjuvants--from empiricism to rational design. Vaccine 2015, 33, B14–20. [DOI] [PubMed] [Google Scholar]
- 10.Wilson-Welder JH; Torres MP; Kipper MJ; Mallapragada SK; Wannemuehler MJ; Narasimhan B, Vaccine adjuvants: current challenges and future approaches. J. Pharm. Sci 2009, 98, 1278–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovsky N; Aguilar JC, Vaccine adjuvants: Current state and future trends. Immunol. Cell. Biol 2004, 82, 488–496. [DOI] [PubMed] [Google Scholar]
- 12.Kaisho T; Akira S, Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 2002, 1589, 1–13. [DOI] [PubMed] [Google Scholar]
- 13.De Gregorio E; Caproni E; Ulmer JB, Vaccine adjuvants: Mode of action. Front. Immunol 2013, 4, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RR; Hassett KJ; Brito LA, Overview of vaccine adjuvants: Introduction, history, and current status. In Vaccine Adjuvants: Methods and Protocols, Fox CB, Ed. Humana Press: New York, 2017; pp 1–14. [DOI] [PubMed] [Google Scholar]
- 15.Guimarães LE; Baker B; Perricone C; Shoenfeld Y, Vaccines, adjuvants and autoimmunity. Pharmacol. Res 2015, 100, 190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mount A; Koernig S; Silva A; Drane D; Maraskovsky E; Morelli AB, Combination of adjuvants: the future of vaccine design. Expert Rev. Vaccines 2013, 12, 733–746. [DOI] [PubMed] [Google Scholar]
- 17.Del Giudice G; Rappuoli R; Didierlaurent AM, Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol 2018, 39 14–21. [DOI] [PubMed] [Google Scholar]
- 18.Danishefsky SJ; Shue Y-K; Chang MN; Wong C-H, Development of Globo‑H Cancer Vaccine. Acc. Chem. Res 2015, 48, 643–652. [DOI] [PubMed] [Google Scholar]
- 19.Batista-Duharte A; Martínez DT; Carlos IZ, Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother 2018, 105, 616–624. [DOI] [PubMed] [Google Scholar]
- 20.Persing DH; Coler RN; Lacy MJ; Johnson DA; Baldridge JR; Hershberg RM; Reed SG, Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002, 10, S32–S37. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich JT; Cantrell JL; Gustafson GL; Myers KR; Rudbach JA; Hiernaux JR, The adjuvant activity of monophosphoryl lipid A. Top. Vaccine Adjuvant Res 1991, 133–143. [Google Scholar]
- 22.De Becker G; Moulin V; Pajak B; Bruck C; Francotte M; Thiriart C; Urbain J; Moser M, The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol 2000, 12, 807–815. [DOI] [PubMed] [Google Scholar]
- 23.Baldridge J; Myers K; Johnson D; Persing D; Cluff C; Hershberg R, Monophosphoryl lipid A and synthetic lipid A mimetics as TLR4-based adjuvants and immunomodulators. In Vaccine Adjuvants, Hackett CJ; Harn DAJ, Eds. Humana Press Inc.: Totowa, N.J., 2006; pp 235–255. [Google Scholar]
- 24.Zhang Y; Gaekwad J; Wolfert MA; Boons G-J, Modulation of innate immune responses with synthetic lipid A derivatives. J. Am. Chem. Soc 2007, 129, 5200–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingale S; Wolfert MA; Buskas T; Boons GJ, Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. Chembiochem 2009, 10, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bojang KA; Milligan PJ; Pinder M; Vigneron L; Alloueche A; Kester KE; Ballou WR; Conway DJ; Reece WH; Gothard P; Yamuah L; Delchambre M; Voss G; Greenwood BM; Hill A; McAdam KP; Tornieporth N; Cohen JD; Doherty T, Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: A randomised trial. Lancet 2001, 358, 1927–1934. [DOI] [PubMed] [Google Scholar]
- 27.Boland G; Beran J; Lievens M; Sasadeusz J; Dentico P; Nothdurft H; Zuckerman JN; Genton B; Steffen R; Loutan L; Van Hattum J; Stoffel M, Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 2004, 23, 316–320. [DOI] [PubMed] [Google Scholar]
- 28.Goepfert PA; Tomaras GD; Horton H; Montefiori D; Ferrari G; Deers M; Voss G; Koutsoukos M; Pedneault L; Vandepapeliere P; McElrath MJ; Spearman P; Fuchs JD; Koblin BA; Blattner WA; Frey S; Baden LR; Harro C; Evans T, Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 2007, 25, 510–518. [DOI] [PubMed] [Google Scholar]
- 29.Wang L; Feng S; Wang S; Li H; Guo Z; Gu G, Synthesis and immunological evaluation of lipoarabinomannan oligosaccharide-monophosphoryl lipid A conjugates as novel antituberculosis vaccines. J. Org. Chem 2017, 82, 12085–12906 [DOI] [PubMed] [Google Scholar]
- 30.Schultz N; Oratz R; Chen D; Zeleniuch-Jacquotte A; Abeles G; Bystryn J-C, Effect of DETOX as an adjuvant for melanoma vaccine. Vaccine 1995, 13, 503–508. [DOI] [PubMed] [Google Scholar]
- 31.Sondak VK; Liu PY; Tuthill RJ; Kempf RA; Unger JM; Sosman JA; Thompson JA; Weiss GR; Redman BG; Jakowatz JG; Noyes RD; Flaherty LE, Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J. Clin. Oncol 2002, 20, 2058–2066. [DOI] [PubMed] [Google Scholar]
- 32.Butts C; Murray N; Maksymiuk A; Goss G; Marshall E; Soulières D; Cormier Y; Ellis P; Price A; Sawhney R; Davis M; Mansi J; Smith C; Vergidis D; Ellis P; MacNeil M; Palmer M, Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol 2005, 23, 6674–6681. [DOI] [PubMed] [Google Scholar]
- 33.North SA; Graham K; Bodnar D; Venner P, A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J. Urol 2006, 176, 91–95. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z; Mandal S; Liao G; Guo Z, GM2-monophosphoryl lipid A conjugate as a fully synthetic self-adjuvant cancer vaccine. Sci. Rep 2017, 7, e6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q; Zhou Z; Tang S; Guo Z, Carbohydrate-monophosphoryl lipid A conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol 2012, 7, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeshima N; Fernandez RC, Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front. Cell. Infect. Microbiol 2013, 3, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobrovolskaia MA; Vogel SN, Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [DOI] [PubMed] [Google Scholar]
- 38.Martin M; Michalek SM; Katz J, Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun 2003, 71, 2498–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonuleit H; Knop J; Enk AH, Cytokines and their effects on maturation, differentiation and migration of dendritic cells. Arch. Dermatol. Res 1996, 289, 1–8. [DOI] [PubMed] [Google Scholar]
- 40.Masihi KN; Lange W; Johnson AG; Ribi E, Enhancement of chemiluminescence and phagocytic activities by nontoxic and toxic forms of lipid A. J. Biol. Response Mod 1986, 5, 462–469. [PubMed] [Google Scholar]
- 41.Reisser D; Pance A; Jeannin J-F, Mechanisms of the antitumoral effect of lipid A. BioEssays 2002, 24, 284–289. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki A; Medzhitov R, Toll-like receptor control of the adaptive immune responses. Nat. Immunol 2004, 5, 987–995. [DOI] [PubMed] [Google Scholar]
- 43.Oyelaran O; McShane LM; Dodd L; Gildersleeve JC, Profiling human serum antibodies with a carbohydrate antigen microarray. J. Proteom. Res 2009, 8, 4301–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheridan RT; Hudon J; Hank JA; Sondel PM; Kiessling LL, Rhamnose glycoconjugates for the recruitment of endogenous anti-carbohydrate antibodies to tumor cells. Chembiochem 2014, 15, 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bournazos S; Wang TT; Dahan R; Maamary J; Ravetch JV, Signaling by antibodies: Recent progress. Annu. Rev. Immunol 2017, 35, 285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakobsche CE; Parker CG; Tao RN; Kolesnikova MD; Douglass EFJ; Spiegel DA, Exploring binding and effector functions of natural human antibodies using synthetic immunomodulators. ACS Chem. Biol 2013, 8, 2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hossain MK; Vartak A; Sucheck SJ; Wall KA, Liposomal Fc domain conjugated to a cancer vaccine enhances both humoral and cellular immunity. ACS Omega 2019, 4, 5204–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hossain MK; Vartak A; Karmakar P; Sucheck SJ; Wall KA, Augmenting vaccine immunogenicity through the use of natural human anti-rhamnose antibodies. ACS Chem. Biol 2018, 13, 2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou K; Hong H; Lin H; Gong L; Li D; Shi J; Zhou Z; Xu F; Wu Z, Chemical synthesis of antibody-hapten conjugates capable of recruiting the endogenous antibody to magnify the Fc effector immunity of antibody for cancer immunotherapy. J. Med. Chem 2022, 65, 323–332. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar S; Lombardo SA; Herner DN; Talan RS; Wall KA; Sucheck SJ, Synthesis of a single-molecule L-rhamnose-containing three-component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies. J. Am. Chem. Soc 2010, 132, 17236–17246. [DOI] [PubMed] [Google Scholar]
- 51.Chen W; Gu L; Zhang W; Motari E; Cai L; Styslinger TJ; Wang PG, L-rhamnose antigen: A promising alternative to α-gal for cancer immunotherapies. ACS Chem. Biol 2011, 6, 185–191. [DOI] [PubMed] [Google Scholar]
- 52.Parashar S; Gupta V; Bhatnagar R; Kausar A, A clickable folic acid-rhamnose conjugate for selective binding to cancer cells. Results Chem. 2022, 4, 100409. [Google Scholar]
- 53.Sarkar S; Salyer AC; Wall KA; Sucheck SJ, Synthesis and immunological evaluation of a MUC1 glycopeptide incorporated into l-rhamnose displaying liposomes. Bioconjug Chem. 2013, 24, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H; Wang B; Ma Z; Wei M; Liu J; Li D; Zhang H; Wang PG; Chen M, L-Rhamnose enhances the immunogenicity of melanoma-associated antigen A3 for stimulating antitumor immune responses. Bioconjug. Chem 2016, 27, 1112–1118. [DOI] [PubMed] [Google Scholar]
- 55.Li X; Rao X; Cai L; Liu X; Wang H; Wu W; Zhu C; Chen M; Wang PG; Yi W, Targeting tumor cells by natural anti-carbohydrate antibodies using rhamnose-functionalized liposomes. ACS Chem. Biol 2016, 11, 1205–1209. [DOI] [PubMed] [Google Scholar]
- 56.Liet B; Laigre E; Goyard D; Todaro B; Tiertant C; Boturyn D; Berthet N; Renaudet O, Multifunctional glycoconjugates for recruiting natural antibodies against cancer cells. Chemistry 2019, 25, 15508–15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hossain MK; Vartak A; Sucheck SJ; Wall KA, Synthesis and immunological evaluation of a single molecular construct MUC1 vaccine containing l-rhamnose repeating units. Molecules 2020, 25, 3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ou C; Prabhu SK; Zhang X; Zong G; Yang Q; Wang LX, Synthetic antibody-rhamnose cluster conjugates show potent complement-dependent cell killing by recruiting natural antibodies. Chemistry 2022, 28, e202200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong H; Lin H; Li D; Gong L; Zhou K; Li Y; Yu H; Zhao K; Shi J; Zhou Z; Huang Z; Wu Z, Chemoenzymatic synthesis of a rhamnose-functionalized bispecific nanobody as a bispecific antibody mimic for cancer immunotherapy. Angew. Chem. Int. Ed 2022, 61, e202208773. [DOI] [PubMed] [Google Scholar]
- 60.Lin H; Hong H; Wang J; Li C; Zhou Z; Wu Z, Rhamnose modified bovine serum albumin as a carrier protein promotes the immune response against sTn antigen. Chem. Commun 2020, 56, 13959–13962. [DOI] [PubMed] [Google Scholar]
- 61.Karmakar P; Lee K; Sarkar S; Wall KA; Sucheck SJ, Synthesis of a liposomal MUC1 glycopeptide-based immunotherapeutic and evaluation of the effect of l-rhamnose targeting on cellular immune responses. Bioconjug. Chem 2016, 27, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z; Li X; Lu Z; Qin X; Hong H; Zhou Z; Pieters RJ; Shi J; Wu Z, Repurposing the pentameric B-subunit of shiga toxin for Gb3-targeted immunotherapy of colorectal cancer by rhamnose conjugation. J. Pharm. Sci 2022, 111, 2719–2729. [DOI] [PubMed] [Google Scholar]
- 63.Xu Z; Moyle PM, Bioconjugation approaches to producing subunit vaccines composed of protein or peptide antigens and covalently attached Toll-like receptor ligands. Bioconjug. Chem 2018, 29, 572–586. [DOI] [PubMed] [Google Scholar]
- 64.Babu JS; Nair S; Kanda P; Rouse BT, Priming for virus-specific CD8+ but not CD4+ cytotoxic T lymphocytes with synthetic lipopeptide is influenced by acylation units and liposome encapsulation. Vaccine 1995, 13, 1669–1676. [DOI] [PubMed] [Google Scholar]
- 65.Gupta RK; Varanelli CL; Griffin P; Wallach DF; Siber GR, Adjuvant properties of non-phospholipid liposomes (Novasomes) in experimental animals for human vaccine antigens. Vaccine 1996, 14, 219–225. [DOI] [PubMed] [Google Scholar]
- 66.Estevez F; Carr A; Solorzano L; Valiente O; Mesa C; Barroso O; Sierra GV; Fernandez LE, Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP). Vaccine 1999, 18, 190–197. [DOI] [PubMed] [Google Scholar]
- 67.Alving CA; Rao M, Lipid A and liposomes containing lipid A as antigens and adjuvants. Vaccine 2008, 26, 3036–3045. [DOI] [PubMed] [Google Scholar]
- 68.Qureshi N; Kutuzova G; Takayama K; Rice PA; Golenbock DT, Structure of lipid A and cell activation. J. Endotoxin Res 1999, 5, 147–150. [Google Scholar]
- 69.Morrison DC; Silverstein R; Luchi M; Shnyra A, Structure-function relationships of bacterial endotoxins. Contribution to microbial sepsis. Infect. Dis. Clin. N. Am 1999, 13, 313–340. [DOI] [PubMed] [Google Scholar]
- 70.Darveau RP, Lipid A diversity and the innate host response to bacterial infection. Curr. Opin. Microbiol 1998, 1, 36–42. [DOI] [PubMed] [Google Scholar]
- 71.Rietschel ET; Kirikae T; Schade FU; Mamat U; Schmidt G; Loppnow H; Ulmer AJ; Zahringer U; Seydel U; Di Padova F; Schreier M; Brade H, Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [DOI] [PubMed] [Google Scholar]
- 72.Stover AG; Correia JDS; Evans JT; Cluff CW; Elliott MW; Jeffery EW; Johnson DA; Lacy MJ; Baldridge JR; Probst P; Ulevitch RJ; Persing DH; Hershberg RM, Structure-activity relationship of synthetic toll-like receptor 4 agonists. J. Biol. Chem 2004, 279, 4440–4449. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Z; Mondal M; Liao G; Guo Z, Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org. Biomol. Chem 2014, 12, 3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Long DE; Karmakar P; Wall KA; Sucheck SJ, Synthesis of α-L-rhamnosyl ceramide and evaluation of its binding with anti-rhamnose antibodies. Bioorg. Med. Chem 2014, 22, 5279–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu C-S; Wang H-Y; Chiang L-W; Pei K, Synthesis of the rhamnosyl trisaccharide repeating unit to mimic the antigen determinant of Pseudomonas syringae lipopolysaccharide. Synthesis 2007, 1412–1420. [Google Scholar]
- 76.Wang Q; Ekanayaka SA; Wu J; Zhang J; Guo Z, Synthetic and immunological studies of 5’-N-phenylacetyl sTn to develop carbohydrate-based cancer vaccines and to explore the impacts of linkage between carbohydrate antigens and carrier proteins. Bioconjugate Chem. 2008, 19, 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu HG; Li YM, Emerging adjuvants for cancer immunotherapy. Front. Chem 2020, 8, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuzzubbo S; Mangsbo S; Nagarajan D; Habra K; Pockley AG; McArdle SEB, Cancer vaccines: Adjuvant potency, importance of age, lifestyle, and treatments. Front. Immunol 2020, 11, 615240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pacheco R; Contreras F; Prado C, Cells, molecules and mechanisms involved in the neuro-immune interaction. In Cell Interaction, Gowder S, Ed. IntechOpen: London, UK, 2012; pp 139–166. [Google Scholar]
- 80.Park S; Nahm MH, L-rhamnose is often an important part of immunodominant epitope for pneumococcal serotype 23F polysaccharide antibodies in human sera immunized with PPV23. PLoS One 2013, 8, e83810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuentes R; Ruiz-de-Angulo A; Sacristán N; Navo CD; Jiménez-Osés G; Anguita J; Fernández-Tejada A, Replacing the rhamnose-xylose moiety of QS-21 with simpler terminal disaccharide units attenuates adjuvant activity in truncated saponin variants. Chemistry 2021, 27, 4731–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information


