Abstract
Podocytes are essential to maintaining the integrity of the glomerular filtration barrier, but they are frequently affected in lupus nephritis (LN). Here, we show that the significant upregulation of Drp1S616 phosphorylation in podocytes promotes mitochondrial fission, leading to mitochondrial dysfunction and podocyte injury in LN. Inhibition or knockdown of Drp1 promotes mitochondrial fusion and protects podocytes from injury induced by LN serum. In vivo, pharmacological inhibition of Drp1 reduces the phosphorylation of Drp1S616 in podocytes in lupus-prone mice. Podocyte injury is reversed when Drp1 is inhibited, resulting in the alleviation of proteinuria. Mechanistically, complement component C5a (C5a) upregulates the phosphorylation of Drp1S616 and promotes mitochondrial fission in podocytes. Moreover, the expression of C5a receptor 1 (C5aR1) is notably upregulated in podocytes in LN. C5a-C5aR1 axis-controlled phosphorylation of Drp1S616 and mitochondrial fission are substantially suppressed when C5aR1 is knocked down by siRNA. Moreover, lupus-prone mice treated with C5aR inhibitor show reduced phosphorylation of Drp1S616 in podocytes, resulting in significantly less podocyte damage. Together, this study uncovers a novel mechanism by which the C5a-C5aR1 axis promotes podocyte injury by enhancing Drp1-mediated mitochondrial fission, which could have significant implications for the treatment of LN.
Keywords: lupus nephritis, podocyte injury, drp1, mitochondrial fission, C5a-C5aR1 axis
Graphical abstract

The study by Ye and colleagues unveils how the C5a-C5aR1 axis aggravates podocyte injury via Drp1-mediated mitochondrial fission in lupus nephritis. These findings provide crucial insights into the pathogenesis of lupus nephritis, which could have significant implications for the treatment of the disease.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by a loss of immune tolerance, leading to the production of autoantibodies and organ damage.1 Lupus nephritis (LN) is one of the most common and devastating clinical manifestations in patients with SLE.2 Although the prognosis of LN has improved in recent decades, 5%–20% of patients with LN progress to end-stage renal disease (ESRD) within 10 years,3 underscoring the urgent need to identify novel and effective therapeutic targets for LN.
Podocytes act as a gatekeeper to maintain the integrity of the glomerular filtration barrier (GFB) in the kidneys by anchoring to the basement membrane through foot process extension.4 The integrity and physiological function of GFB rely on the interactions between podocyte-specific proteins, including nephrin, synaptopodin, and the actin cytoskeleton. In patients with SLE, podocytes are the primary target during the process of kidney damage. The deteriorating integrity of the actin cytoskeleton leads to foot process effacement (FPE) and disruption of GFB, resulting in the development of pathological proteinuria in SLE.2,5,6 It has been shown that podocyte injury is closely associated with poor clinical outcomes in patients with LN.7 Therefore, it is important to understand the mechanism of podocyte injury in LN. It has been shown that calcium/calmodulin-dependent protein kinase IV (CaMK4) is upregulated in human LN and lupus-prone mice. Inhibition of CaMK4 ameliorated podocyte injury and reduced proteinuria in lupus-prone mice.8 In our previous study, we found that the NLRP3 inflammasome was involved with podocyte injury in LN.9 In addition, our data revealed that RIP3 accelerated podocyte injury by promoting podocyte necrosis.10 However, specific molecular mediators and the pathophysiological mechanisms underlying LN podocyte injury remain elusive. Further investigation is warranted to fully understand the pathogenesis of podocyte injury in LN.
Mitochondria are dynamically regulated by mitochondrial fission and fusion, which are essential to maintaining cellular metabolism and complex cell signaling events.11 Mitochondrial fission is mediated by dynamin-related protein 1 (Drp1) and mitochondrial fission protein 1, while mitochondrial fusion is mediated by mitofusin 1/2 and optic atrophy protein 1 (OPA1).12 Drp1, a major regulator of mitochondrial fission, translocates to the mitochondrial outer membrane and polymerizes to induce mitochondrial fragmentation. Depending upon the tissue type and disease state, Drp1-induced mitochondrial fragmentation is subsequently associated with increased mitochondrial reactive oxygen species (ROS), cytochrome c release, membrane permeability, mitophagy, and cell apoptosis. This change in mitochondrial dynamics also leads to the depolarization and dysfunction of mitochondria, as shown by downregulated mitochondrial membrane potential and halted ATP synthesis.12,13
Imbalanced mitochondrial dynamics caused by increased mitochondrial fission have been associated with a range of clinical disorders.14,15 Mitochondrial fission is heightened in Alzheimer disease. Inhibition of Drp1 led to reduced amyloid-β-mediated mitochondrial dysfunctions and abrogated neuronal damage in mice with Alzheimer disease.16 In addition, Drp1-mediated mitochondrial fission promoted cardiomyocyte damage in acute ischemia/reperfusion injury.17 Recent data indicated that Drp1-mediated mitochondrial fission also played an important role in kidney disease.18 In diabetic mice, the phosphorylation of Drp1 and mitochondrial fission were increased in podocytes, leading to ROS production and mitochondrial dysfunction, thus causing podocyte damage.19 Inhibition of Drp1 ameliorated epithelial cell death and alleviated kidney damage in mice with acute kidney injury.20 However, the role of Drp1-mediated mitochondrial fission in LN has not been reported. It remains unclear whether Drp1 promotes podocyte injury through mitochondrial fission in LN.
The uncontrolled activation of the complement system induced by the immune complex has been identified as a key feature of LN.21 Complement component 5a (C5a) is generated by the cleavage of C5 during the complement cascade.22 Activation of C5a induces chemotaxis, phagocytosis, and the release of inflammatory cytokines by binding to the G protein-coupled receptor C5a receptor 1 (C5aR1).23 It has been reported that C5aR deficiency reduces glomerular injury in lupus-prone mice by inhibiting the T helper 1 cell response.24 In addition, C5a induces renal injury of tubular epithelial cells by disrupting mitochondrial fatty acid oxidation in diabetic kidney disease.25 However, the direct effect of the C5a-C5aR1 axis on podocytes in LN has not been studied. The underlying mechanism of the C5a-C5aR1 axis in mitochondrial fission is not known, warranting further studies in LN.
In the present study, we aimed to investigate the role of Drp1-mediated mitochondrial fission in the development of LN and the underlying mechanisms. We observed a notable increase in the phosphorylation of Drp1S616 in podocytes from patients with LN and lupus-prone mice. Inhibition or knockdown of Drp1 resulted in the decreased phosphorylation of Drp1S616 and mitochondrial fission, leading to reduced ROS production and podocyte injury. Treatment of lupus-prone mice with a Drp1 inhibitor protected podocytes from injury. Importantly, we identified the C5a-C5aR1 axis as a key factor in promoting podocyte injury by elevating the phosphorylation of Drp1S616 and inducing mitochondrial fission through calcium signaling in LN. This study offers new insights into the pathogenesis of LN and provides a novel approach to treating LN.
Results
Drp1-mediated mitochondrial fission is increased in the podocytes of both human and murine LN
Glomeruli are constantly affected in patients with LN.26 We first analyzed the RNA sequencing (RNA-seq) data from the glomeruli of patients with LN or healthy controls (HCs) to identify potential changes in transcripts in glomerular tissues. A heatmap was generated to illustrate differential expressed genes (DEGs) in the glomeruli of LN and HCs. In addition, upregulated and downregulated DEGs were adopted to evaluate the enriched Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in the glomerular tissue of LN patients. Genes associated with podocyte development and differentiation, including MAGI2, PTPRO, NOTCH2, and MAGED1, were significantly downregulated in the glomerular tissue of LN. Notably, genes related to apoptosis (BAX, BID), mitochondrial fission (DNM1L), C5AR1, calcium ion signaling (CALM1, CALM3), and inflammasome complex assembly pathways (NLRP1, NLRP3) are significantly increased in LN (Figures 1A and 1B).
Figure 1.
Drp1-mediated mitochondrial fission is increased in podocytes from both human and mice with LN
Transcriptomic data of glomerular tissue were obtained from Berthier Lupus Glom Dataset in the public database Nephroseq, which enrolled renal biopsies derived from HC and LN patients. (A) The DEGs were illustrated by heatmap. Genes with p < 0.005 and |log fold change|>1.2 were regarded as DEGs. (B) Up- and downregulated genes were adopted for gene set enrichment analysis. GO terms and KEGG pathways with p < 0.05 were regarded as enriched gene sets. (C and D) Kidney sections from patients with LN or HCs were stained with antibodies against synaptopodin and p-Drp1S616. The number of synaptopodin+p-Drp1S616+ double-positive cells per glomerulus was counted. Scale bar: 50 μm. (E and F) The correlations between the number of synaptopodin+p-Drp1S616+ podocytes and SLEDAI or proteinuria levels. (G and H) Fresh urine samples were collected from patients with LN or HCs. Cellular sediments were enriched and stained with antibodies against synaptopodin and p-Drp1S616. The percentages of synaptopodin+p-Drp1S616+ double-positive cells were summarized from 4 independent samples. Scale bar: 10 μm. (I and J) Kidney sections from MRL/lpr or control mice were stained with antibodies against synaptopodin and p-Drp1S616. The number of synaptopodin+p-Drp1S616+ double-positive cells per glomerular was counted and summarized from 6 independent samples. Scale bar: 20 μm. (K and L) Representative images showed PAS staining of kidney sections. TEM images demonstrated mitochondrial morphology in podocytes. PAS magnification: 400×. Scale bar: 50 μm. Electron microscopy magnification: 5,000×. Scale bar: 1 μm. All of the data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by unpaired Student’s t test.
Podocytes constitute the epithelial lining that covers the outmost layer of glomerular capillary, forming the functional GFB.27 FPE is the earliest morphological change in podocyte injury in LN, which can lead to massive proteinuria.28 Mitochondrial morphology undergoes constant remodeling and is dynamically regulated by fission and fusion.11 Fine-tuned fission and fusion are essential to maintaining mitochondrial homeostasis. Imbalanced mitochondrial dynamics can lead to mitochondrial structural changes and dysfunction.29 Mitochondrial fission is primarily regulated by Drp1.30 Excessive mitochondrial fission mediated by Drp1 has been linked to mitochondrial dysfunction and cell death.31 We thus speculated that Drp1 may promote mitochondrial fission to induce podocyte damage in LN. To test this hypothesis, we initially assessed the activation of Drp1 in podocytes obtained from patients with LN and lupus-prone mice. Podocytes were labeled using synaptopodin in the staining process. Our data revealed that the phosphorylation of Drp1S616, which promotes mitochondrial fission,32,33 was significantly increased in podocytes from patients with LN (Figures 1C and 1D). Consistent with our hypothesis, the number of p-Drp1S616+ podocytes exhibited a significant correlation with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and the severity of proteinuria (Figures 1E and 1F). Next, we collected urinary sediment from patients with LN and HCs and measured the expression of phosphorylated Drp1S616 in podocytes through immunofluorescent staining. Notably, podocytes from patients with LN displayed elevated levels of phosphorylated Drp1S616, with the percentage of p-Drp1S616+ podocytes being 3.1 times higher than that of HCs (Figures 1G and 1H). Furthermore, we investigated the phosphorylation of Drp1S616 in the podocytes of MRL/lpr lupus-prone mice. Our results showed the phosphorylation of Drp1S616 was significantly increased in the podocytes of MRL/lpr mice compared to control mice (Figures 1I and 1J). Notably, the mitochondria in the podocytes of control mice were more fused and elongated, whereas mitochondria in damaged podocytes from MRL/lpr mice appeared punctate, as demonstrated by transmission electron microscopy (TEM; Figures 1K and 1L). Similar results were observed in another lupus mouse model, NZM2328 mice (Figure S1). These findings collectively suggest that Drp1-mediated mitochondrial fission may contribute to podocyte damage in LN.
Mitochondrial fission impairs mitochondrial function and reduces podocyte viability in patients with LN
The observations above led us to speculate that serum from patients with LN may promote the activation of Drp1 in podocytes. As hypothesized, podocytes cultured with serum from patients with LN exhibited much higher levels of phosphorylated Drp1S616 compared to those cultured with serum from HCs, as measured by western blot (Figures 2A and 2B). Interestingly, proteins such as OPA1 and MFN2, which control mitochondrial fusion, were not altered by exposure to LN serum (Figures 2A and 2B), suggesting that mitochondrial fission in podocytes of LN is primarily regulated by the phosphorylation of Drp1. Mitochondrial division inhibitor 1 (mdivi-1) selectively blocks the function of Drp1 and is widely used to inhibit Drp1-dependent mitochondrial fission.34 Accordingly, the enhanced phosphorylation of Drp1S616 by serum from patients with LN was counteracted by mdivi-1 as measured by flow cytometry (Figures 2C and 2D). Next, we sought to determine whether LN serum promoted mitochondrial fission in podocytes. MitoTracker Green staining revealed that mitochondria in LN serum-treated podocytes appeared small, round-shaped, and fragmented compared to those treated with HC serum. Strikingly, mitochondria in LN serum-treated podocytes exhibited an evenly distributed tubular network when treated with additional mdivi-1 (Figure 2E). Consequently, LN serum treatment led to the significant reduction of mitochondrial mean branch length and mitochondrial footprint in podocytes, respectively, which was fully reversed by mdivi-1 (Figures 2E–2G). These findings suggest that LN serum promotes mitochondrial fission by increasing the phosphorylation of Drp1S616 in podocytes.
Figure 2.
Drp1 impairs mitochondrial function and reduces podocyte viability in LN
Human podocytes were stimulated with serum from patients with LN or HCs in the presence or absence of mdivi-1 (20 μM) for 24 h. (A and B) The expressions of p-Drp1S616, Drp1, OPA1, and MFN2 were measured by western blot. Representative bands were shown, and relative expression was summarized. (C and D) The level of p-Drp1S616 in podocytes was measured by flow cytometry. Representative histograms were shown, and data were summarized from 8 serum samples. (E) Podocytes were stained with Hoechst (blue) and MitoTracker Green (green). Representative images by confocal microscopy showed mitochondrial staining in the cells. Scale bar: 10 μm. (F and G) Mitochondrial branch length and mitochondrial footprint per cell were calculated and summarized from 10 independent serum samples, respectively. (H–J) Cells were stained with MitoSox/DCFH-DA and measured by flow cytometry. Mean fluorescence intensity (MFI) was summarized from 6 serum samples. (K and L) Cells were stained with JC1 probe, and mitochondrial membrane potential was measured by flow cytometry. The ratio of JC1 red to JC1 green MFI was calculated and summarized in (L). (M) ATP production in podocytes was measured by ATP assay kit. The level of ATP was normalized by the amount of protein (mg). (N and O) Cells were stained with 7-AAD and annexin V. The apoptosis rate was measured by flow cytometry. Representative dot plots were shown and data from 8 serum samples were summarized. All of the data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 by unpaired Student’s t test in (B) and 1-way ANOVA with p value adjusted in the remaining panels. ns, not significant.
It has been observed that programmed cell death of podocytes is accelerated in patients with SLE.35 It is unclear whether Drp1-mediated mitochondrial fission is involved with podocyte damage in LN. To link Drp1 activities to podocyte injury in LN, human podocytes were treated with LN serum, and Drp1 was inhibited using mdivi-1. A MitoSox-based flow cytometric assay was used to detect mitochondrial ROS. Our data revealed that mitochondrial ROS production was increased in podocytes exposed to LN serum compared to those of HC serum. In addition, the total intracellular levels of ROS were elevated in podocytes exposed to LN serum, as measured using diacetyldichlorofluorescein (DCFH-DA). Notably, the increased ROS were reduced by blocking Drp1 using mdivi-1 (Figures 2H–2J). The increased ROS production in cells treated with LN serum led to reduced mitochondrial membrane potential, as measured by the JC1 probe (Figures 2K and 2L). The production of ATP was decreased significantly in cells treated with LN serum. Consistent with ROS production, mdivi-1 treatment normalized mitochondrial membrane potential and rescued ATP production in podocytes (Figure 2M). It had been shown that migration ability was increased in podocytes during early injury.36 Our findings revealed that LN serum promoted the motility of podocytes, which was normalized by mdivi-1 (Figures S2A and S2B). The actin cytoskeleton anchors cell-to-cell contact and cell-matrix proteins in podocytes, which are important for podocyte foot process arrangement and adhesion.37 Our findings revealed that the amount of F-actin was dramatically reduced in podocytes treated with LN serum. Mdivi-1 rescued the loss of F-actin in podocytes exposed to LN serum (Figures S2C and S2D). Synaptopodin is an actin-associated protein in podocytes,38 and its expression was reduced by LN serum. Consistently, mdivi-1 reversed the reduction of synaptopodin in podocytes (Figure S2E). Consistent with the previous report,39 we found that LN serum induced podocyte death. Notably, Drp1 inhibition by mdivi-1 reversed the induction of cell death by LN serum (Figures 2N and 2O). These data suggest that LN serum promotes podocyte injury through mitochondrial fission.
Phosphorylation of Drp1S616 dampens mitochondrial function in podocytes by promoting mitochondrial fission in LN
Next, we sought to further investigate the role of Drp1 in LN serum-induced mitochondrial fission. Drp1 expression in podocytes was knocked down using small interfering RNA (siRNA). Knockdown efficiency was confirmed through qPCR and western blot (Figures 3A–3C). Consistently, LN serum promoted mitochondrial fission in podocytes, as evidenced by MitoTracker Green staining. Similarly to mdivi-1 inhibition, knockdown of Drp1 suppressed mitochondrial fission in podocytes significantly. Knockdown of Drp1 led to fused and elongated mitochondria in LN serum-treated podocytes. Mitochondrial morphology was similar in podocytes exposed to LN serum when Drp1 was knocked down, compared to those exposed to HC serum (Figure 3D). Similar results were found when calculating the mean mitochondrial branch length and mitochondrial footprint in podocytes exposed to LN or HC serum. Consequently, mitochondrial branch length and mitochondrial footprint were even increased in LN serum-treated podocytes when Drp1 was knocked down compared to control siRNA-treated cells (Figures 3E and 3F). Mitochondrial and cellular ROS production was notably increased in podocytes treated with LN serum compared to HC serum. LN serum-treated cells also exhibited lower mitochondrial membrane potential (Figures 3G–3K). Knockdown of Drp1 significantly reduced the levels of ROS and recovered mitochondrial membrane potential (Figures 3G–3K). Given the important role of mitochondria in ATP production, a reduction in mitochondrial membrane potential led to decreased ATP production, which was rescued by knocking down Drp1 (Figure 3L). Functionally, knockdown of Drp1 led to an increase in the expression of actin-associated proteins (Figure S3). Cell death induced by LN serum was counteracted when Drp1 was knocked down in podocytes (Figures 3M and 3N). Together, these data suggest that Drp1 is important for mitochondrial fission-induced podocyte damage in LN.
Figure 3.
Knockdown of Drp1 preserves mitochondrial function and protects podocytes from cell death in LN
Human podocytes were treated with Drp1 siRNA (siDrp1) or scramble siRNA (siNC). Cells were then cultured with serum from patients with LN for 24 h. (A–C) Knockdown efficiency of Drp1 was confirmed by qPCR (A) and western blot (B and C). (D) Cells were stained with MitoTracker Green and Hoechst. Mitochondrial staining was visualized by confocal microscopy, and representative images were shown. Scale bar: 10 μm. (E and F) Mitochondrial branch length and mitochondrial footprint per cell were calculated and summarized. (G–I) Cells were stained with MitoSox/DCFH-DA and measured by flow cytometry. MFI was summarized from 8 serum samples. (J and K) Mitochondrial membrane potential in podocytes was measured by flow cytometry using JC1 probe. The ratio of JC1 red to JC1 green MFI was calculated and summarized. (L) ATP production in podocytes was measured by ATP assay kit. The level of ATP was normalized by the amount of protein (mg). (M and N) Apoptosis rate was measured by flow cytometry. Representative dot plots were shown and data from 8 serum samples were summarized. All of the data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗<0.0001 by 1-way ANOVA with p value adjusted.
Pharmacological inhibition of Drp1 reduces podocyte injury and preserves renal function in lupus-prone mice
For further investigation into the role of Drp1-mediated mitochondrial fission in podocyte injury in vivo, MRL/lpr mice were treated with the Drp1 inhibitor mdivi-1 for 6 weeks (Figure 4A). Remarkably, treatment with mdivi-1 resulted in a reduction in the phosphorylation of Drp1S616 in podocytes, as demonstrated by immunofluorescence staining (Figure 4B). The phosphorylation of Drp1S616 in podocytes from mice treated with mdivi-1 or vehicle was further assessed by flow cytometry. Consistent with the immunofluorescence data, flow cytometry analysis revealed a 51.6% decrease in the phosphorylation of Drp1S616 in podocytes from mdivi-1-treated mice (Figures 4C–4E). Moreover, mitochondrial and cellular ROS in podocytes from mdivi-1-treated mice were both decreased, as determined by MitoSox- and DCFH-DA-based flow cytometric assays (Figures 4F and 4G). Next, we aimed to assess the integrity of podocytes in mdivi-1 or vehicle-treated mice using TEM. Podocyte injury in LN is characterized by FPE, downregulation of podocyte marker expression, and cell detachment.7 Our findings revealed significant FPE in podocytes from vehicle-treated mice. Remarkably, Mdivi-1 treatment effectively alleviated FPE (Figure 4H). Nephrin is an important component in the formation and maintenance of the slit diaphragm, which connects the podocyte foot processes.40,41 To assess podocyte damage and the integrity of the filtration barrier, we immunostained nephrin in the glomeruli. Our data revealed a notable increase in nephrin+ podocytes in the glomeruli of mdivi-1-treated mice (Figure 4H), indicating preserved glomerular slit diaphragms. This increase in nephrin+ podocytes also suggested reduced podocyte loss in mdivi-1-treated mice. Due to reduced podocyte injury, disease severity markedly decreased in mdivi-1-treated mice histologically and functionally, as evidenced by periodic acid-Schiff (PAS) staining and reduced proteinuria (Figures 4I–4K). Furthermore, mdivi-1 treatment improved renal function, as indicated by decreased concentrations of creatinine and blood urea nitrogen (BUN; Figures 4L and 4M).
Figure 4.
Pharmacological inhibition of Drp1 reduces podocyte injury and preserves renal function in lupus-prone mice
(A) Scheme of the mouse experiment. Female MRL/lpr mice (12 weeks old) were treated with Drp1 inhibitor mdivi-1 (30 mg/kg) or vehicle intraperitoneally every 2 days for 6 weeks. (B) The level of p-Drp1S616 in podocytes from mice treated with mdivi-1 or vehicle was measured by immunofluorescent staining. Representative confocal microscopy images were shown. Scale bar: 20 μm. (C–G) Single-cell suspension was prepared from kidneys treated with mdivi-1 or vehicle. (C) Cells were stained with antibodies against CD45, CD26, and nephrin and measured by flow cytometry. Gating strategy: CD45−CD26+nephrin+ cells were identified as podocytes. (D and E) The levels of p-Drp1S616 in podocytes were measured by flow cytometry. (F and G) The levels of MitoSOX/DCFH-DA in podocytes were measured by flow cytometry. (H) Podocyte integrity were visualized by electron microscopy. Representative images were shown. Magnification 5,000×. Scale bar: 10 μm (left); 2 μm (right). Fluorescent images showed nephrin staining in the glomeruli from mice treated with mdivi-1 or vehicle. Scale bar: 100 μm. (I and J) Representative images of PAS staining were shown. Magnification 400×. Scale bar: 50 μm. Renal pathology score was calculated and summarized. (K) Proteinuria was scored, and cumulative incidence of severe proteinuria was recorded over the course of treatment. (L and M) The levels of creatinine and BUN in the serum of mice treated with mdivi-1 or vehicle. n = 7. All of the data are presented as mean ± SEM. ∗p < 0.05 and ∗∗∗p < 0.001 by unpaired Student’s t test.
To further confirm the pharmacologic effects of mdivi-1 in alleviating disease severity in lupus-prone mice, another lupus model, the NZM2328 mouse,42 was adopted and treated with mdivi-1 for 6 weeks (Figure S4A). Consistent with the findings in MRL/lpr mice, mdivi-1 treatment led to a significant reduction in p-Drp1S616 levels in the podocytes of NZM2328 mice (Figure S4B). Moreover, the FPE of podocytes was significantly reduced in mice treated with mdivi-1 (Figure S4C). Similarly, the number of nephrin+ podocytes was notably increased in mice treated with mdivi-1 (Figure S4C). Importantly, mdivi-1 treatment led to decreased disease severity as demonstrated by amelioration in pathology score, reduced proteinuria, and improved kidney function (Figures S4D–S4H). These findings suggest that Drp1 promotes podocyte damage in LN.
Inhibition of C5aR1 reduces LN serum-induced mitochondrial dysfunction and podocyte injury
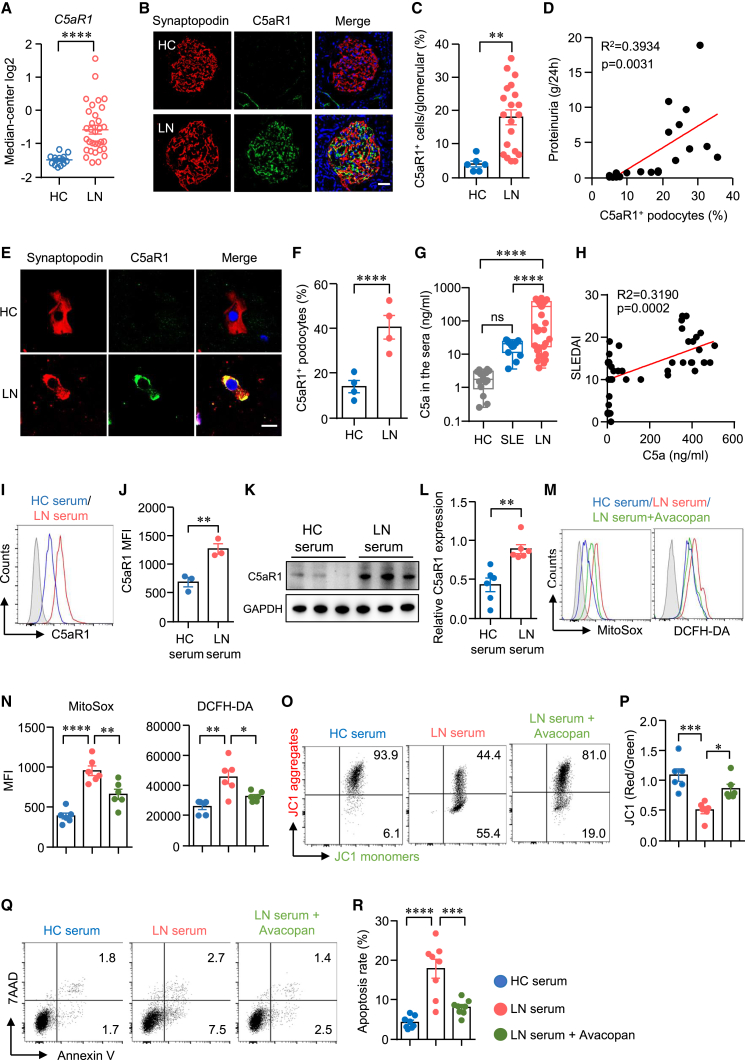
The serum contains multiple factors that could affect mitochondrial homeostasis and podocyte viability in LN. The specific factor that dampens podocytes in LN needs further investigation. We hypothesized that the serum factor may promote podocyte damage by interacting with cell membrane receptors. The RNA-seq data showed that genes associated with C5AR1 and calcium ion signaling (CALM1, CALM3) pathways were significantly increased in LN (Figures 1A and 1B), suggesting the potential involvement of C5aR1 signaling in LN pathogenesis. The transcript of C5AR1 was notably upregulated in glomerular tissues from LN (Figure 5A), inconsistent with the recent report.43 The complement fragment C5a, a significant effector component in the complement system, exerts various functions by binding to its primary receptor, C5aR1.44 It has been shown that C5a promotes the development of experimental LN in mice.24,45 Subsequently, we conducted immunofluorescence staining to evaluate C5aR1 expression in kidney tissues. Our analysis demonstrated a marked increase in the number of C5aR1+ cells within the glomeruli of patients with LN compared to HCs (Figures 5B and 5C). Moreover, we observed a close correlation between the percentages of C5aR1+ podocytes and the severity of proteinuria (Figure 5D). Urinary sediments were collected, and C5aR1 expression in detached podocytes was assessed. Our analysis revealed that the percentage of C5aR1+ podocytes in patients with LN was 2.9 times higher than that in HCs (Figures 5E and 5F). Similar results were observed in lupus-prone mice: the number of C5aR1+ cells in the glomeruli of MRL/lpr mice was 3.3 times higher than that of normal mice (Figure S5). Next, we sought to determine whether C5a levels were upregulated in LN serum. The ELISA data showed that the concentration of C5a in the serum was notably higher in LN patients compared to HC or SLE patients without kidney involvement (Figure 5G). Importantly, C5a concentration positively correlated with disease activities in LN (Figure 5H). Notably, exposure of podocytes to LN serum led to a significant increase in C5aR1 expression, as confirmed by both flow cytometry and western blot analysis (Figures 5I–5L). These findings suggest a role for C5aR1 in podocyte injury in LN. However, it remains unclear whether the C5a-C5aR1 axis directly contributes to podocyte injury in LN. To further investigate the role of the C5a-C5aR1 axis in LN serum-induced podocyte damage, we used the C5aR-specific inhibitor avacopan46 to block the C5a-C5aR1 interaction. Our data revealed that LN serum-induced mitochondrial and cellular ROS production in podocytes were normalized when avacopan was included (Figures 5M and 5N). Consistent with the aforementioned findings, exposure of podocytes to LN serum resulted in a reduction in mitochondrial membrane potential. However, it is noteworthy that mitochondrial membrane potential was completely preserved in LN serum-treated podocytes when the C5a-C5aR1 axis was blocked by avacopan (Figures 5O and 5P). Crucially, the increased cell death induced by LN serum was effectively counteracted by avacopan (Figures 5Q and 5R). Taken together, these findings suggest that the C5a-C5aR1 axis promotes mitochondrial dysfunction and podocyte damage in LN.
Figure 5.
C5a-C5aR1 modulates podocyte viability in LN
(A) RNA-seq data were acquired from Nephroseq. C5aR1 expression level in the kidney of patients with LN or HCs was summarized. (B and C) Kidney sections were stained with antibodies against synaptopodin and C5aR1. The expression of C5aR1 in synaptopodin+ podocytes were measured by immunofluorescence. The percentages of C5aR1+ podocytes were calculated and summarized, respectively. Scale bar: 50 μm. (D) Correlation between the percentages of C5aR1+ podocytes and proteinuria was examined. (E and F) Urine cellular sediments from LN or HCs were stained with antibodies against synaptopodin and C5aR1. The percentages of C5aR1+ podocytes were calculated and summarized from 4 independent samples. Scale bar: 10 μm. (G and H) C5a concentration in the serum was measured by ELISA. The correlation of C5a concentration with SLEDAI was assessed. (I–L) Podocytes were stimulated with serum from patients with LN or HCs. C5aR1 expression was measured by flow cytometry and western blot, respectively. Representative plots were shown, and C5aR1 expression was summarized. (M–R) Podocytes were cultured with LN serum or HC serum. C5aR1 inhibitor avacopan (1 μM) was included in some of the experiments. (M and N) MitoSOX and DCFH-DA were used to detect ROS in podocytes and measured by flow cytometry. Representative histograms were shown, and MFI was summarized, respectively. (O and P) Podocytes were stained with JC1 probe and measured by flow cytometry. The ratio of JC1 red to JC1 green MFI was calculated and summarized. (Q and R) Apoptosis rate was quantified by flow cytometry. Representative dot plots were shown. Data were derived from 6 independent serum samples. All of the data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗<0.0001 by unpaired Student’s t test in (A), (C), (F), (J), and (L) and by 1-way ANOVA with p value adjusted in (G), (N), (P), and (R).
C5a impairs the mitochondrial function of podocytes
The data above revealed that C5aR1 expression in podocytes was increased upon exposure to LN serum, implying the potential of C5a to activate podocytes through binding to C5aR1. To validate the significance of the C5a-C5aR1 axis in podocytes, podocytes were stimulated with recombinant C5a protein. Upon C5a stimulation, we observed an increase in podocyte motility (Figure S6), suggesting early podocyte injury. As expected, the production of mitochondrial ROS and cellular ROS were both significantly increased in podocytes cultured with C5a (Figures S7A–S7C), implying that C5a stimulation could cause mitochondrial dysfunction in podocytes. Mitochondria are the primary consumers of oxygen, and mitochondrial dysfunction leads to reduced oxygen consumption by the cells.47,48 Subsequently, we assessed oxygen consumption using the Seahorse assay (Seahorse Bioscience, China). Our findings demonstrated a significant reduction in oxygen consumption at the basal levels in C5a-treated podocytes. In addition, ATP-linked oxygen consumption rate (OCR) and maximal OCR in podocytes were consistently decreased by C5a, whereas spared mitochondrial respiration was not changed by C5a (Figures S7D and S7E). Furthermore, mitochondrial membrane potential was significantly lower in C5a-treated podocytes (Figures S7F and S7G). These findings suggest that C5a dampens mitochondrial function directly. Moreover, impaired mitochondrial function was associated with increased cell death upon C5a stimulation (Figures S7H and S7I).
The C5a-C5aR1 axis promotes mitochondrial fission of podocytes through calcium signaling
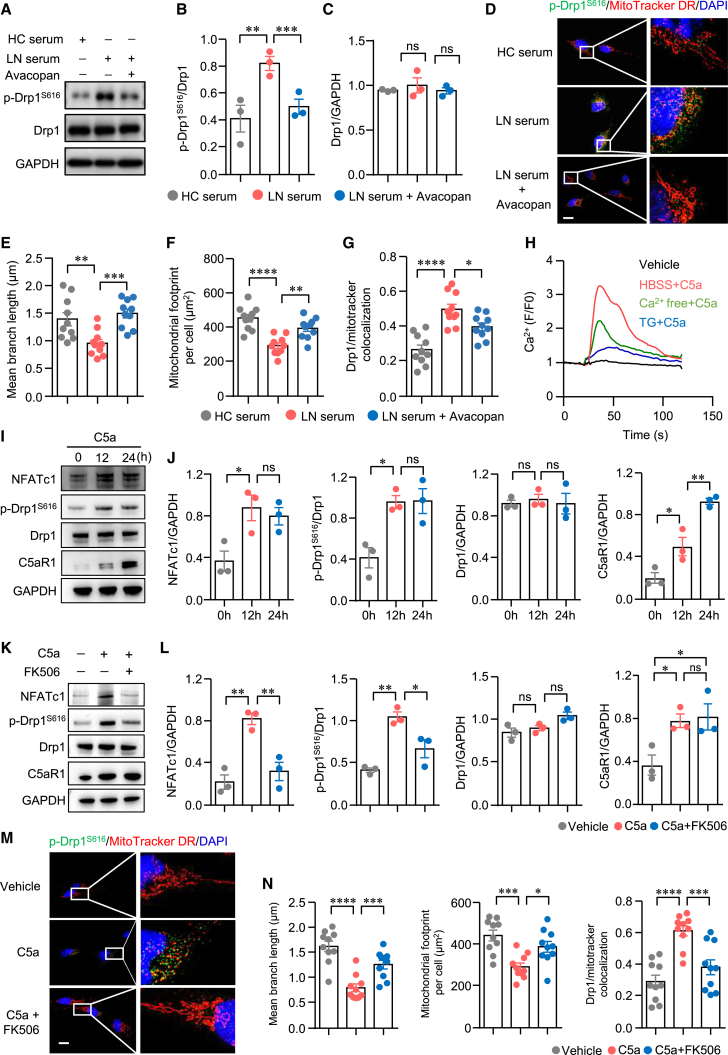
The preceding data have linked the C5a-C5aR1 axis to mitochondrial dysfunction in podocytes. It remains uncertain whether the C5a-C5aR1 axis promotes mitochondrial dysfunction through Drp1. Initially, we stimulated podocytes with LN serum or HC serum and quantified the phosphorylation of Drp1S616 via western blot analysis. Our findings revealed that the phosphorylation of Drp1S616 was notably increased in podocytes cultured with LN serum. However, the total protein levels of Drp1 remained unchanged. Of note, the C5aR inhibitor avacopan significantly attenuated the phosphorylation of Drp1S616 induced by LN serum (Figures 6A–6C). It has been shown that the localization of p-Drp1S616 to mitochondria induces mitochondrial fission.49 Subsequently, we aimed to study the localization of p-Drp1S616 to mitochondria through immunofluorescence analysis. Confocal microscopy data depicted mitochondria as small, round-shaped, and fragmented in podocytes treated with LN serum. Furthermore, there was a significant enhancement in the localization of phosphorylated Drp1S616 to mitochondria in podocytes treated with LN serum. Inhibition of the C5a-C5aR1 axis with avacopan dramatically reduced the localization of phosphorylated Drp1S616 to mitochondria, leading to a fine-tuned mitochondrial network (Figures 6D–6G). These findings suggest that the C5a-C5aR1 axis promotes mitochondrial fission by enhancing the phosphorylation of Drp1S616, necessitating further investigation into the underlying mechanism. C5aR1 is a prototypic G protein-coupled receptor, and its activation by C5a induces calcium mobilization and influx.23,50 Similarly, we observed enhanced calcium influx in podocytes stimulated with C5a. Moreover, C5a increased cytoplasmic calcium levels in calcium-free buffer (Figure 6H), suggesting the involvement of the store-operated calcium entry mechanism in C5a-C5aR1 axis-mediated calcium mobilization. Subsequently, we investigated whether the C5a-C5aR1 axis directly controls the activation of Drp1. Our findings demonstrated that C5a stimulation led to the increased expression of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), a downstream target of calcium signaling. Simultaneously, C5a significantly upregulated the phosphorylation of Drp1S616, as determined by western blot analysis (Figures 6I and 6J). In addition, C5a stimulation induced the expression of C5aR1 in podocytes (Figures 6I and 6J). Of note, the elevated phosphorylation of Drp1S616 induced by C5a was attenuated upon the inhibition of calcium signaling with FK50651 (Figures 6K and 6L), indicating the necessity of calcium signaling in C5a-C5aR1 axis-mediated mitochondrial fission. To further confirm the involvement of the C5a-C5aR1 axis in mitochondrial fission, podocytes were stimulated with C5a, and the localization of phosphorylated Drp1S616 to mitochondria was assessed using confocal microscopy. C5a stimulation caused increased phosphorylation of Drp1S616 in podocytes. Notably, the colocalization of phosphorylated Drp1S616 with mitochondria was augmented, suggesting that the C5a-C5aR1 axis promotes mitochondrial fission in podocytes. Mean mitochondrial length and footprint were significantly reduced in C5a-treated podocytes (Figures 6M and 6N). Surprisingly, the decreased mitochondrial length was reversed by FK506. Moreover, the colocalization of p-Drp1S616 with mitochondria, induced by C5a stimulation, was inhibited by FK506 (Figures 6M and 6N). Functionally, actin-associated proteins were effectively preserved in podocytes when the C5a-C5aR1 axis was blocked by avacopan (Figure S8), consistent with a previous finding that the inhibition of calcineurin by cyclosporine A stabilized the actin cytoskeleton in kidney podocytes.52 CaMK, a downstream component of calcium signaling, has been implicated in podocyte injury in LN.3 However, our data revealed that CaMK4 inhibitor KN93 had no effect on the phosphorylation of Drp1S616 or total Drp1 expression induced by C5a (Figures S9A–S9C). In addition, KN93 did not reverse mitochondrial fission in podocytes stimulated by C5a (Figures S9D–S9F). Together, these findings suggest that the C5a-C5aR1 axis promotes Drp1-mediated mitochondrial fission through calcium signaling.
Figure 6.
C5a-C5aR1 controls mitochondrial fission through calcium signaling in podocytes in LN
(A–G) Human podocytes were cultured with HC serum or LN serum. Avacopan (1 μM) was included in some of the experiments. (A–C) The levels of p-Drp1S616 and total Drp1 in podocytes was measured by western blot. Representative bands of 3 repeated experiments were shown. (D–G) Podocytes were first labeled with MitoTracker Deep Red (DR) and then stained with antibodies against p-Drp1S616. Images were generated using fluorescent confocal microscopy. Representative images were shown. Scale bar: 10 μm. Mitochondrial branch length (E), mitochondrial footprint per cell (F), and p-Drp1S616/MitoTracker colocalization (Manders’ coefficient) (G) were summarized for each group. (H) Podocytes were labeled with calcium probe Fluo-3. Calcium mobilization in podocytes stimulated by C5a were recorded by fluorescent microscopy. (I and J) Podocytes were cultured in the presence of C5a for the indicated time. The levels of NFATc1, p-Drp1S616, total Drp1, and C5aR1 in podocytes were measured by western blot. The experiment was repeated 3 times, and representative bands were shown. (K–N) Podocytes were cultured in the presence of C5a for 24 h. FK506 was included in some of the experiments. (K and L) The levels of NFATc1, p-Drp1S616, total Drp1, and C5aR1 in podocytes were measured by western blot. Representative bands of 3 repeated experiments were shown. (M and N) Podocytes were labeled with MitoTracker DR and then stained with antibodies against p-Drp1S616. Images were generated using fluorescent microscopy. Representative images are shown. Scale bar: 10 μm. Mitochondrial branch length, mitochondrial footprint per cell, and p-Drp1S616/MitoTracker colocalization (Manders’ coefficient) were summarized for each group. All of the data are presented as mean ± SEM. ∗∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 by 1-way ANOVA with p value adjusted in (B)–(L) and unpaired Student’s t test in (N). ns, not significant.
C5a promotes Drp1-mediated mitochondrial fission through C5aR1
We first knocked down C5aR1 in podocytes, and the knockdown efficiency was confirmed by western blot (Figures 7A and 7B). The cells were then stimulated with LN or HC serum. We found that knockdown of C5aR1 reduced calcium mobilization as stimulated by LN serum (Figures 7C and 7D). In addition, knockdown of C5aR1 resulted in a notably lower level of p-Drp1S616 in podocytes when stimulated by LN serum (Figures 7E–7H). Subsequently, we sought to confirm whether C5a directly regulate mitochondrial fission in podocytes through C5aR1. Our findings demonstrated a reduction in calcium levels upon C5a stimulation when C5aR1 was knocked down. NFATc1 in podocytes was almost undetected by western blot with C5aR1 knockdown. In addition, C5a failed to induce the phosphorylation of Drp1S616 when C5aR1 was knocked down in podocytes (Figures 7I–7M). Consequently, the shortening of mitochondrial branch length induced by C5a stimulation was reversed upon C5aR1 knockdown. This was further evidenced by the restoration of the reduced mitochondrial footprint when C5aR1 was knocked down in podocytes (Figures 7N–7P). Notably, pretreatment with siC5aR1 normalized mitochondrial ROS production and mitigated cell death induced by C5a in podocytes (Figures 7Q–7T). Taken together, these results suggest that C5a promotes Drp1-mediated mitochondrial fission and podocyte injury through C5aR1.
Figure 7.
Blocking C5a-C5aR1 interaction inhibits mitochondrial fission through calcium signaling in podocytes
(A–H) Human podocytes were treated with C5aR1 siRNA (siC5aR1) or scramble siRNA (siNC). Cells were then cultured with serum from LN or HCs for 24 h. (A and B) Knockdown efficiency of C5aR1 was confirmed by western blot. (C and D) Podocytes were labeled with calcium probe Fluo-3 measured by fluorescent microscopy. Representative images were shown and Fluo-3 MFI was summarized, respectively. (E–H) The levels of p-Drp1S616, total Drp1, and C5aR1 in podocytes were measured by western blot. Representative bands of 3 repeated experiments were shown, and relative expressions were summarized, respectively. (I–T) Human podocytes were treated with siC5aR1 or scramble siNC. Cells were then cultured in the presence of C5a or vehicle for 24 h. (I) Calcium probe Fluo-3 was used to detect calcium mobilization, and Fluo-3 MFI was summarized from 3 samples. (J–M) Western blot was used to measure the expression of NFATc1, p-Drp1S616, and total Drp1, respectively. Representative bands of western blot are shown. The levels of NFATc1 (K), p-Drp1S616 (L), and total Drp1 (M) were summarized from 3 samples. (N–P) Podocytes were harvested and stained with MitoTracker Green and Hoechst. Mitochondrial staining was visualized by confocal microscopy. Representative images were shown. Scale bar: 10 μm. Mitochondrial branch length (O) and mitochondrial footprint per cells (P) were calculated and summarized. (Q and R) MitoSOX in podocytes was measured by flow cytometry. Representative histograms are shown, and MFI were summarized. (S and T) Apoptosis rate of podocytes was measured by flow cytometry. Representative dot plots were shown. Data were derived from 8 independent serum samples. All of the data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 by 1-way ANOVA with p value adjusted.
The C5a-C5aR1 axis promotes podocyte injury through Drp1 in lupus-prone mice
The role of the C5a-C5aR1 axis in podocyte injury in LN was further investigated in vivo. MRL/lpr mice were treated with the C5aR1 antagonist PMX-53 for 6 weeks (Figure 8A). Treatment with PMX-53 reduced the number of p-DrpS616+ podocytes in MRL/lpr mice, notably measured by immunofluorescence (Figure 8B). Subsequently, single cells were isolated from the kidneys, and the phosphorylation of Drp1S616 in podocytes was assessed using flow cytometry, with podocytes gated as in Figure 4. PMX-53 treatment significantly suppressed the phosphorylation of Drp1S616 (Figures 8C and 8D). Furthermore, inhibition of C5a-C5aR1 interaction by PMX-53 led to a reduction in ROS production in podocytes (Figures 8E–8H). Podocyte injury was further evaluated by TEM. FPE was dramatically reduced in mice treated with PMX-53 (Figures 8I and 8J). In addition, both pathological and functional changes were ameliorated by PMX-53, as evidenced by PAS staining and proteinuria measurement (Figures 8K–8M). Renal function was improved by PMX-53, with reduced concentrations of creatinine and BUN observed following C5aR1 inhibition (Figures 8N and 8O). Collectively, these findings suggest that the interruption of the C5a-C5aR1 axis protects podocytes from the injurious effects observed in LN.
Figure 8.
Inhibition of C5a-C5aR1 axis reduces Drp1-mediated podocyte injury in lupus-prone mice
(A) Scheme of the mouse experiment. MRL/lpr mice (12 weeks old) were injected subcutaneously with C5aR1 antagonist PMX-53 (1 mg/kg) or vehicle for 6 weeks. Kidneys were harvested for further experimentation. (B) The expression of p-Drp1S616 in podocytes was measured by immunofluorescent staining. Representative confocal microscopy images are shown. Scale bar: 20 μm. (C–H) Single-cell suspension was prepared from kidneys treated with PMX-53 or vehicle. The levels of p-Drp1S616, mitochondrial ROS, and cellular ROS in podocytes were measured by flow cytometry. Representative histograms were shown, and MFI of p-Drp1S616 (D), mitochondrial ROS (F), and cellular ROS (H) were summarized, respectively. (I) Podocyte integrity was visualized by electron microscopy. Representative images are shown. Foot process width was measured and summarized for 10 samples. Magnification 5,000×. Scale bar: 1 μm. (K and L) Representative images of PAS staining are shown. Magnification 400×. Scale bar: 50 μm. Renal pathology score was calculated and summarized. (M) Cumulative incidence of severe proteinuria was calculated. (N and O) The levels of creatinine and BUN in the serum of mice treated with PMX-53 or vehicle. All of the data are presented as mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01 by unpaired Student’s t test.
Discussion
LN is the most common cause of morbidity and mortality in patients with SLE. Over the years, the prognosis of patients with LN has improved greatly due to therapeutic advancements. However, with a low response rate to the current regimen, 5%–20% of patients with LN develop ESRD within 10 years of diagnosis.3 The pathogenesis of kidney damage in LN is not fully understood. In our study, we showed that phosphorylation of Drp1S616 was significantly upregulated in podocytes from patients with LN. Drp1-mediated mitochondrial fission promoted mitochondrial dysfunction and podocyte injury. Pharmacologically, inhibition of Drp1 alleviated podocyte injury in lupus-prone mice. Our study elucidated that the C5a-C5aR1 axis controlled mitochondrial fission and podocyte injury in LN via a Drp1-dependent mechanism. Our study linked mitochondrial fission to the pathogenesis of podocyte injury in LN.
Mitochondrial morphology is continuously and dynamically controlled by fission and fusion. Given the importance of mitochondria in ATP generation, the balance of mitochondrial dynamics is essential to maintaining normal cellular function.53 Dysfunction of mitochondrial dynamics has been linked to many clinical disorders.29,54 Recently published data showed that increased mitochondrial fission was associated with kidney diseases, including renal fibrosis, acute kidney injury, and diabetic kidney disease.20,55,56 Mitochondrial fission is tightly regulated by the dynamin superfamily GTPase Drp1.57 Phosphorylation of Drp1 at serine 616 promotes the translocation of Drp1 from the cytosol to the outer membrane of mitochondria. In contrast, phosphorylation of Drp1 at serine 637 inhibits Drp1 activities.14 In our study, we observed that the phosphorylation of Drp1S616 was significantly upregulated in podocytes from patients with LN. Podocytes exposed to LN serum exhibited small, round-shaped mitochondria, representing the morphologic feature of mitochondrial fission, suggesting disease-featured mitochondria. The previous study has demonstrated that heightened mitochondrial fission causes excessive ROS production and loss of mitochondrial membrane potential, both of which are indicative of mitochondrial dysfunction.58 Mitochondrial fission was increased in diabetic nephropathy,59 and podocyte-specific Drp1-deficient mice were protected from cell damage in diabetic kidney disease.60 In accordance with these previous findings, our study demonstrated that mitochondrial fission in podocytes driven by LN serum caused mitochondrial dysfunction and, subsequently, cell death. Podocyte injury in LN is characterized by FPE, loss of podocyte marker, and cell detachment.61 Our data revealed that pharmacological inhibition of Drp1 led to reduced FPE and loss of podocytes in lupus mouse models, highlighting the critical role of mitochondrial fission in promoting podocyte injury in LN.
Cleavage of C5 by C5 convertases results in the production of the anaphylatoxin C5a, which binds to GPCR C5aR1 to mediate leukocyte migration and activation.62 C5aR1 is widely expressed on immune cells, epithelial cells, and endothelial cells.63 In addition to its role in immune regulation, the C5a-C5aR1 axis has been shown to regulate type 2 diabetic kidney disease25,64,65 and renal fibrosis.66 A study showed that podocytes express low levels of C5aR1.67 Consistent with this observation, we also observed low levels of C5aR1 expression in podocytes from HCs or control mice. Strikingly, our data revealed a profound upregulation of C5aR1 expression in podocytes from patients with LN or from lupus-prone mice. A previous study showed that C5a induced pyroptosis in membranous nephropathy by mediating mitochondrial dysfunction.68 In human LN, serum levels of C5a were significantly higher in LN and neuropsychiatric SLE than in SLE alone.69 Consistently, C5a was notably increased in the serum of individuals with LN in this study. Furthermore, we found that C5a in lupus serum induced mitochondrial dysfunction and podocyte damage. In addition, podocytes stimulated with C5a exhibited significantly reduced mitochondrial oxygen consumption. The upregulation of the C5a-C5aR1 axis may promote disease progression by disrupting mitochondrial function in podocytes in LN.
It is well established that C5a binds to C5aR1, resulting in the activation of downstream signals.62 Given the significance of the C5a-C5aR1 axis in mitochondria, it is intriguing to investigate whether the C5a-C5aR1 axis plays a role in regulating mitochondrial dynamics in podocytes during the progression of LN. Our mechanistic study revealed that phosphorylation of Drp1S616 was notably increased in podocytes stimulated by C5a. Furthermore, C5a stimulation led to enhanced p-Drp1S616 localization to mitochondria, thereby promoting mitochondrial fission in podocytes. Clinically, LN serum induced Drp1 phosphorylation and mitochondrial fission, which were reversed upon blocking or knocking down C5aR. Inhibition of C5aR in lupus-prone mice also led to reduced phosphorylation of Drp1S616 in podocytes and ameliorated proteinuria.
The engagement of C5a to C5aR1 leads to the influx of calcium into the cells.70,71 Accordingly, knockdown of C5aR1 resulted in reduced calcium levels and downstream calcium signaling in podocytes. Calcium serves as an important second messenger essential for cellular functions.72 It has been shown that Drp1 deficiency results in an increase in mitochondrial calcium uptake.73 Moreover, patient cells lacking the Drp1 adaptor protein MiD49 fail to undergo injury-triggered mitochondrial fission and mitochondrial calcium increase,74 highlighting the link between mitochondrial fission and intracellular calcium mobilization. Reciprocally, our data revealed that phosphorylation of Drp1S616 is regulated by calcium signaling. Specifically, the phosphorylation of Drp1S616 induced by C5a was notably reduced when calcineurin was inhibited by FK506, suggesting that calcium is required for C5a-C5aR1 axis-controlled mitochondrial fission. A previous study showed that calcineurin interacted with Drp1 and dephosphorylated Drp1 at serine 637.75 Since phosphorylation of Drp1 at serine 637 inhibits mitochondrial fission, the dephosphorylation of Drp1S637 by calcium signaling would lead to enhanced Drp1 activities in promoting mitochondrial fission. Accordingly, our data showed that calcineurin is required for the phosphorylation of Drp1S616 in podocytes. Together, these findings suggest that the C5a-C5aR1 axis controls Drp1-mediated mitochondrial fission through calcium signaling.
In conclusion, we identified mitochondrial fission as a potent driver of mitochondrial dysfunction and podocyte injury in LN. Importantly, our study uncovers a novel mechanism whereby the elevated phosphorylation of Drp1S616 by the C5a-C5aR1 axis promotes mitochondrial fission, thereby contributing to podocyte injury in LN.
Materials and methods
Patient samples
Kidney specimens were obtained from LN patients who underwent diagnostic kidney biopsies. Normal renal samples were derived from patients undergoing nephrectomy for therapeutic purposes. Patients who fulfilled the American College of Rheumatology criteria for the classification of SLE with renal involvement were recruited.76 In addition, age- and gender-matched HCs were also recruited. Serum samples were obtained from LN and HCs. Written informed consent was obtained from all of the individuals involved. The study was approved by the Human Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Clinical parameters in association with kidney disease activities in LN were collected, and demographics of the recruited patients or HC are summarized in Table S1.
Cell culture
The human podocyte cell line, originally established by M. Saleem (University of Bristol, Bristol, UK), was cultured as previously described.77 The cells were initially cultured on plates in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin (complete medium) and 1× insulin-transferrin-selenium (Sigma-Aldrich, USA) at 33°C in an atmosphere of 5% CO2. The cells proliferated at 33°C and grew to 70%–80% confluence, followed by passaging to 37°C for 10–14 days for differentiation.
Podocyte experiments
Well-differentiated podocytes were stimulated with 10% HC serum or LN serum for 24 h. To investigate the involvement of mitochondrial fission in podocyte damage, podocytes were pretreated with mdivi-1 (20 μM, Selleck Chemicals, USA) for 4 h and then stimulated with LN serum. In addition, Drp1 was knocked down using siRNA (RiboBio, China). Briefly, podocytes were transfected with Drp1 specific siRNA (siDrp1) or control siRNA (siNC) using a Lipo8000 Transfection system (Beyotime, China) according to the manufacturer’s instruction. Cells were treated with LN serum subsequently. To explore the impact of the C5a-C5aR1 axis in mitochondrial fission and podocyte injury, cells were stimulated with LN serum or recombinant human C5a protein (100 nM, MedChemExpress, USA) in the presence of C5aR1 antagonist avacopan (1 μM, Selleck Chemicals). In addition, C5aR1 in podocytes was silenced by C5aR1 siRNA (siC5aR1, RiboBio). Moreover, C5a recombinant protein (100 nM, MedChemExpress) was added into the podocyte culture system in the presence of calcineurin inhibitor FK506 (1 μm, Selleck Chemicals) or CaMK4 inhibitor KN93 (4 μM, MedChemExpress) to elucidate the involvement of downstream pathways of the C5a-C5aR1 axis.
Cytoskeleton staining
Human podocytes were washed with prewarmed PBS 3 times. Cells were then fixed with 4% paraformaldehyde at room temperature (RT) for 30 min. Briefly, cells were washed with PBS 3 times and stained with anti-human Alexa Fluor 488 conjugated-phalloidin (F-actin) (1:1,000, AAT Bioquest, USA) at 37°C for 30 min to detect F-actin. Cells were stained with Hoechst 33342 (1:200, Thermo Fisher, USA) and imaged under fluorescence microscope (Olympus, USA).
Cell migration assay
Podocytes were cultured on 6-well plates, and a sterile plastic pipette tip was used to scrape 1 line when the cells reached 95% confluence. Images were taken at different time points (0, 12, or 24 h). Cell migration properties were assessed by the numbers of podocytes migrated into wound closure area using ImageJ software.
Flow cytometry analysis
For the phosphorylation of Drp1S616 (p-Drp1S616) staining, cells were fixed and permeabilized with fixation/permeabilization solution and washed with Perm/Wash Buffer (BD Biosciences, USA). Cells were first stained with primary antibody against human p-Drp1S616 at 4°C, followed by incubation with Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibody. For mitochondrial ROS measurement, podocytes were incubated with MitoSox Red (5 μM, Invitrogen, USA) at 37°C for 20 min. Similarly, JC1 (10 μM, MedChemExpress) and DCFH-DA (10 μM, Sigma-Aldrich) was used to stain podocytes (37°C, 20 min) to measure the mitochondrial membrane potential and intracellular ROS, respectively. For apoptosis analysis, podocytes were incubated with Accutase cell detachment solution (BioLegend, USA) for 10 min and dissociated from the culture plate. The digestion process was stopped by addition of RPMI 1640 containing 10% fetal bovine serum. The dissociated podocytes were stained with allophycocyanin (APC)-anti-annexin V (1:100) and 7-aminoactinomycin D (7-AAD) (1:50) (BioLegend) at RT for 10 min. For C5aR1 staining, podocytes were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human/mouse C5aR1 (1:200, clone R63, Santa Cruz, USA) at 4°C for 30 min. Data were acquired by a fluorescence-activated cell sorting (FACS) analyzer (Thermo Fisher).
Western blot analysis
Cells were processed for western blot analysis as described previously.78 Podocytes were detached from cell culture plates after incubation with trypsin. Cells were then lysed with radioimmunoprecipitation assay. After separated by 10% SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane (Millipore). The membranes were then blocked with 5% BSA and incubated with the following primary antibodies at 4°C overnight: mouse anti-Drp1 (1:500, clone C-5, Santa Cruz), rabbit anti-p-Drp1S616 (1:1,000, clone D9A1, Cell Signaling Technology, USA), rabbit anti-OPA1 (1:1,000, clone D6U6N, Cell Signaling Technology), rabbit anti-mitofusin-2 (1:1,000, clone D2D10, Cell Signaling Technology), mouse anti-NFATc1 (1:2,000, clone 1E1B10, Proteintech, USA), mouse anti-C5aR1 (1:500, clone R63, Santa Cruz), mouse anti-synaptopodin (1:500, clone D-9, Santa Cruz), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1,000, clone D16H11, Cell Signaling Technology), rabbit anti-β-actin (1:1,000, catalog no. ab8227, Abcam, UK). Blots were incubated with secondary antibodies correspondingly. Signals on the blots were detected using a chemiluminescence analysis kit (Millipore).
qPCR
The procedure of qPCR was conducted as described previously.78 Total RNA was isolated using the TRIzol reagent and converted to cDNA using Evo M-MLV RT Premix (Accurate Biology, China). SYBR was used for qPCR reaction. Real-time PCR amplification was conducted by denaturing at 95°C for 30 s, then amplification for 35 cycles at 95°C for 5 s and annealing and elongation at 60°C for 30 s, respectively. Reactions were run on a qPCR system (Applied Biosystems, USA). Gapdh was used as a housekeeping gene, and relative gene mRNA expression was calculated. The primer sequences for Dnm1l (forward: TGCAGGACGTCTTCAACACA, reverse: GACCACACCAGTTCCTCTGG) and Gapdh (forward: GGAGCGAGATCCCTCCAAAAT, reverse: GGCTGTTGTCATACTTCTCATGG) were used in this study.
ELISA
Serum C5a concentrations were measured using a commercially available ELISA kit for human C5a according to the manufacturer’s instruction (Solarbio Life Sciences, China).
Mito Stress assay
Mitochondrial OCR was measured using the XF96 Extracellular Flux Analyzer (Seahorse Bioscience). Following the manufacturer’s protocol, the Seahorse XF Cell Mito Stress Test Kit was used to measure OCR. In brief, podocytes were plated on the wells of Seahorse XF96 cell culture microplates and incubated at 37°C for 1 h without CO2 at one standard atmosphere. Subsequently, 1.5 mM oligomycin, 1.5 mM carbonyl cyanide p-trifluoromethoxy phenylhydrazone, and 1 mM rotenone/antimycin A were added during measurement. The output data were collected for analysis by Wave software version 2.6.1 (Agilent Technologies, USA).
ATP measurement
Cellular ATP production of podocytes was tested using a Beyotime ATP Assay Kit following the manufacturer’s instructions. The result was further quantified using the Thermo Scientific Varioskan LUX.
Ca2+ measurement
Podocytes were stained with Fluo-3 AM (2.5 μM, Sigma-Aldrich) in Ca2+-containing buffer (Hank’s balanced salt solution) or Ca2+-free buffer at 37°C for 20 min. After washing with PBS, podocytes labeled with Fluo-3 were immobilized on 96-well plates coated with poly-D-lysine. C5a supplemented with Ca2+-free buffer was added to stimulate Ca2+ influx during measurement. In addition, thapsigargin (Sigma-Aldrich) was used to deplete Ca2+ in endoplasmic reticulum. The microscopic calcium image was conducted to record the influx of Ca2+.
Mouse experiment
NZM2328 mice were kindly provided by Dr. S.M. Fu from the University of Virginia. MRL/lpr mice were purchased from SLAC Laboratory Animal Company (Shanghai, China). Age- and gender-matched C57B6/L mice were obtained from Guangdong Province Experimental Animal Center (Guangzhou, China). All of the mice were maintained in the specific pathogen-free colony at the Experiment Animal Center of Sun Yat-sen University. The study protocol was approved by the Ethics Committee of Sun Yat-sen University and all of the experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Female NZM2328 (20 weeks old) and MRL/lpr (12 weeks old) mice were treated with either specific Drp1 antagonist mdivi-1 (30 mg/kg, Selleck Chemicals) or an equal volume of vehicle intraperitoneally every other day for 6 weeks. To study the impact of the inhibition of C5a-C5aR1 axis in vivo, MRL/lpr (12 weeks old) mice were injected with 1 mg/kg PMX-53 (Selleck Chemicals) or vehicle subcutaneously for 6 weeks. Murine urine samples were collected weekly for proteinuria measurement. After treatment, murine kidneys were obtained for flow cytometry and microscopic analysis.
Kidney samples were minced and subsequently incubated with digestion solution containing Liberase (1 mg/mL, Roche, USA) and DNase-I (0.1 mg/mL, Roche) to prepare single-cell suspensions. Cells were then stained with APC-cyanine 7-conjugated anti-mouse CD45 (1:100, clone 30-F11, BioLegend, USA) for immune cells, and anti-human/mouse nephrin (1:100, Bioss, USA), phycoerythrin-conjugated anti-mouse CD26(1:100, clone H194-112, BioLegend) for podocytes. Cells were further stained with anti-p-Drp1S616 after fixation and permeabilization. Samples were analyzed using a BD FACS LSR Fortessa (BD Biosciences). FlowJo (Tree Star, USA) software was used for the data analysis.
Measurement of proteinuria, blood creatinine, and BUN
Mouse urine samples were collected and tested with proteinuria analysis strips (Albustix, Bayer, USA). Concentrations of serum creatinine and BUN were determined using a commercial autoanalyzer (Beckman Coulter, USA).
Immunofluorescence
Human and murine renal acetone-fixed cryostat tissue sections (4 μm) were blocked with 5% BSA in PBS for 1 h at RT. Then, sections were incubated with primary antibodies against p-Drp1S616 (rabbit anti-human/mouse IgG, 1:2,000, clone D9A1; Cell Signaling Technology) at 4°C overnight. After being washed with PBS 3 times, sections were incubated with secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit IgG) at RT for 1 h. Then, sections were washed with PBS and incubated with Alexa Fluor 594-conjugated anti-human/mouse synaptopodin at 4°C overnight. For C5aR1 staining, the sections were incubated with FITC-conjugated anti-human/mouse C5aR1 (1:200, clone R63, Santa Cruz). Sections were counterstained with DAPI and imaged using fluorescence microscope (Olympus). In addition, urine samples derived from LN patients and HCs were freshly collected, centrifuged, washed with PBS, and then spun onto slides. The slides were subsequently fixed with cold acetone and washed with PBS. Subsequently p-Drp1S616, C5aR1, and synaptopodin staining were applied as described above.
Histology of mouse kidney
Murine kidney was fixed in formalin, embedded in paraffin, and stained with PAS. Glomerular lesion was graded on a scale of 0 (normal)–3 (severe pathological lesion with immune cell infiltration/cell proliferation and crescent bodies) by two independent researchers as previously reported.9
TEM
Murine kidney tissues were processed as previously described.9 In brief, kidney tissues were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M PBS overnight at 4°C. Then, the fixed tissues were processed for TEM and imaged with a G2 Spirit Twin electron microscope (Tecnai, USA).
Nephroseq database
Transcriptomic data of glomerular tissue were obtained from Berthier Lupus Glom Dataset in the public database Nephroseq, which enrolled renal biopsies derived from HC and LN patients. Correspondingly, the RNA from glomerular compartments was extracted and processed for microarrays analysis (https://www.nephroseq.org). By using data derived from this Berthier Lupus Glom Dataset cohort, DNM1L or C5AR1 expression from healthy living donors was correspondingly compared to those derived from LN.79 Over- and underexpressed gene lists were also obtained from Nephroseq. Genes with p < 0.005 and |log2 fold change|>1.2 were regarded as DEGs. A heatmap was used to illustrate DEGs between renal tissues of LN patients and HCs. Subsequently, upregulated and downregulated DEGs were adopted to evaluate the enriched signaling pathways using R package clusterProfiler.80 GO terms and KEGG pathways with p < 0.05 were regarded as enriched gene sets.
Statistical analyses
All of the data are expressed as mean ± SEM. GraphPad Prism 8.0 was used to perform statistical analyses. The differences were assessed by t test or one-way ANOVA as appropriate. Pearson’s correlation analysis was applied as appropriate for correlation analysis. It was considered statistically significant if two-tailed p < 0.05.
Data and code availability
The transcriptomic data in glomerular tissue from Berthier Lupus Glom Dataset can be obtained in the public database Nephroseq (https://www.nephroseq.org). Other original data of the study can be found in the article/supplemental information, and further inquiries can be directed to the corresponding authors.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (82071819) and the Fundamental Research Funds for the Central Universities (22hytd11) to H.Z., the National Natural Science Foundation of China (82171770) to N.Y., and the National Natural Science Foundation of China (82001712) to C.G. The graphical abstract was created with BioRender.com.
Author contributions
H.Z. and N.Y. conceived and designed the study. Y.L., Shuang Wang, J.L., and N.X. recruited patients and processed blood and urine samples. B.Y., B.C.,Shuyi Wang, C.G., M.L., and M.Z., performed the in vitro experiments. B.Y., N.X., and S.W. performed the tissue staining. B.Y., B.C., and C.G. performed the mouse experiments. B.Y., Y.H., Shuyi Wang, and H.Z. analyzed and interpreted the data. H.Z., B.C., and N.Y. wrote the manuscript, with all of the authors providing feedback.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2024.03.003.
Contributor Information
Niansheng Yang, Email: yangnsh@mail.sysu.edu.cn.
Hui Zhang, Email: zhangh656@mail.sysu.edu.cn.
Supplemental information
References
- 1.Kiriakidou M., Ching C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020;172:ITC81–ITC96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 2.Mohan C., Zhang T., Putterman C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat. Rev. Nephrol. 2023;19:491–508. doi: 10.1038/s41581-023-00722-z. [DOI] [PubMed] [Google Scholar]
- 3.Anders H.J., Saxena R., Zhao M.H., Parodis I., Salmon J.E., Mohan C. Lupus nephritis. Nat. Rev. Dis. Primers. 2020;6:7. doi: 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89:1221–1230. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Perico L., Conti S., Benigni A., Remuzzi G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 2016;12:692–710. doi: 10.1038/nrneph.2016.127. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava R., Li H., Tsokos G.C. Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr. Opin. Rheumatol. 2023;35:107–116. doi: 10.1097/BOR.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 7.Yu F., Haas M., Glassock R., Zhao M.H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017;13:483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 8.Maeda K., Otomo K., Yoshida N., Abu-Asab M.S., Ichinose K., Nishino T., Kono M., Ferretti A., Bhargava R., Maruyama S., et al. CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. J. Clin. Invest. 2018;128:3445–3459. doi: 10.1172/JCI99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu R., Guo C., Wang S., Huang Y., Jin O., Hu H., Chen J., Xu B., Zhou M., Zhao J., et al. Podocyte Activation of NLRP3 Inflammasomes Contributes to the Development of Proteinuria in Lupus Nephritis. Arthritis Rheumatol. 2017;69:1636–1646. doi: 10.1002/art.40155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo C., Fu R., Zhou M., Wang S., Huang Y., Hu H., Zhao J., Gaskin F., Yang N., Fu S.M. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 2019;103 doi: 10.1016/j.jaut.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cel Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 12.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cel Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 13.Chen H., Chan D.C. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 15.Quiles J.M., Gustafsson Å.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022;19:723–736. doi: 10.1038/s41569-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek S.H., Park S.J., Jeong J.I., Kim S.H., Han J., Kyung J.W., Baik S.H., Choi Y., Choi B.Y., Park J.S., et al. Inhibition of Drp1 Ameliorates Synaptic Depression, Abeta Deposition, and Cognitive Impairment in an Alzheimer's Disease Model. J. Neurosci. 2017;37:5099–5110. doi: 10.1523/JNEUROSCI.2385-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong S.B., Subrayan S., Lim S.Y., Yellon D.M., Davidson S.M., Hausenloy D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 18.Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin X., Zhao Y., Gong J., Huang W., Su H., Yuan F., Fang K., Wang D., Li J., Zou X., et al. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction. Theranostics. 2019;9:1698–1713. doi: 10.7150/thno.30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry H.M., Huang L., Wilson R.J., Bajwa A., Sesaki H., Yan Z., Rosin D.L., Kashatus D.F., Okusa M.D. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2018;29:194–206. doi: 10.1681/ASN.2017060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyam A., Hisada R., Bhargava R., Tsokos M.G., Tsokos G.C. Intertwined pathways of complement activation command the pathogenesis of lupus nephritis. Transl. Res. 2022;245:18–29. doi: 10.1016/j.trsl.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holers V.M. Complement therapeutics are coming of age in rheumatology. Nat. Rev. Rheumatol. 2023;19:470–485. doi: 10.1038/s41584-023-00981-x. [DOI] [PubMed] [Google Scholar]
- 23.Pandey S., Maharana J., Li X.X., Woodruff T.M., Shukla A.K. Emerging Insights into the Structure and Function of Complement C5a Receptors. Trends Biochem. Sci. 2020;45:693–705. doi: 10.1016/j.tibs.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Wenderfer S.E., Ke B., Hollmann T.J., Wetsel R.A., Lan H.Y., Braun M.C. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J. Am. Soc. Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- 25.Tan S.M., Ziemann M., Thallas-Bonke V., Snelson M., Kumar V., Laskowski A., Nguyen T.V., Huynh K., Clarke M.V., Libianto R., et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes. 2020;69:83–98. doi: 10.2337/db19-0043. [DOI] [PubMed] [Google Scholar]
- 26.Davidson A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 2016;12:143–153. doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp J.B., Anders H.J., Susztak K., Podestà M.A., Remuzzi G., Hildebrandt F., Romagnani P. Podocytopathies. Nat. Rev. Dis. Primers. 2020;6:68. doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greka A., Mundel P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W., Zhao H., Li Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target. Ther. 2023;8:333. doi: 10.1038/s41392-023-01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.E., Westrate L.M., Wu H., Page C., Voeltz G.K. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youle R.J., Karbowski M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cel Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 32.Han H., Tan J., Wang R., Wan H., He Y., Yan X., Guo J., Gao Q., Li J., Shang S., et al. PINK1 phosphorylates Drp1(S616) to regulate mitophagy-independent mitochondrial dynamics. EMBO Rep. 2020;21 doi: 10.15252/embr.201948686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashatus J.A., Nascimento A., Myers L.J., Sher A., Byrne F.L., Hoehn K.L., Counter C.M., Kashatus D.F. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol. Cel. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassidy-Stone A., Chipuk J.E., Ingerman E., Song C., Yoo C., Kuwana T., Kurth M.J., Shaw J.T., Hinshaw J.E., Green D.R., Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cel. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y., Li P., Li K., Li N., Liu H., Zhang X., Liu W., Liu Y. Pathological mechanisms and crosstalk among different forms of cell death in systemic lupus erythematosus. J. Autoimmun. 2022;132 doi: 10.1016/j.jaut.2022.102890. [DOI] [PubMed] [Google Scholar]
- 36.Zhong Y., Lee K., Deng Y., Ma Y., Chen Y., Li X., Wei C., Yang S., Wang T., Wong N.J., et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 2019;10:4523. doi: 10.1038/s41467-019-12433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endlich N., Endlich K. Stretch, tension and adhesion - adaptive mechanisms of the actin cytoskeleton in podocytes. Eur. J. Cel Biol. 2006;85:229–234. doi: 10.1016/j.ejcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Mundel P., Heid H.W., Mundel T.M., Krüger M., Reiser J., Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cel Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi Y.Y., Zhou X.J., Cheng F.J., Hou P., Ren Y.L., Wang S.X., Zhao M.H., Yang L., Martinez J., Zhang H. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann. Rheum. Dis. 2018;77:1799–1809. doi: 10.1136/annrheumdis-2018-213028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestilä M., Jalanko H., Holmberg C., Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Chuang P.Y., D'Agati V.D., Dai Y., Yacoub R., Fu J., Xu J., Taku O., Premsrirut P.K., Holzman L.B., He J.C. Nephrin Preserves Podocyte Viability and Glomerular Structure and Function in Adult Kidneys. J. Am. Soc. Nephrol. 2015;26:2361–2377. doi: 10.1681/ASN.2014040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Q., Wang S., Li M., Wang S., Guo C., Ruan X., Watanabe R., Lai Y., Huang Y., Yin X., et al. Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab. 2023;35:837–854.e8. doi: 10.1016/j.cmet.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Tampe D., Hakroush S., Tampe B. Molecular signatures of intrarenal complement receptors C3AR1 and C5AR1 correlate with renal outcome in human lupus nephritis. Lupus Sci. Med. 2022;9 doi: 10.1136/lupus-2022-000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y., Zhao C., Deng Y., Wang H., Ma L., Liu S., Tian X., Wang B., Bin Y., Chen P., et al. Mechanism of activation and biased signaling in complement receptor C5aR1. Cell Res. 2023;33:312–324. doi: 10.1038/s41422-023-00779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao L., Osawe I., Puri T., Lambris J.D., Haas M., Quigg R.J. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 2005;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo Y., Miyabe Y. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2021;384:e81. doi: 10.1056/NEJMc2104672. [DOI] [PubMed] [Google Scholar]
- 47.Perry C.G.R., Kane D.A., Lanza I.R., Neufer P.D. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62:1041–1053. doi: 10.2337/db12-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Divakaruni A.S., Jastroch M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat. Metab. 2022;4:978–994. doi: 10.1038/s42255-022-00619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang C.R., Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klos A., Wende E., Wareham K.J., Monk P.N. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 2013;65:500–543. doi: 10.1124/pr.111.005223. [DOI] [PubMed] [Google Scholar]
- 51.Dumont F.J. FK506, an immunosuppressant targeting calcineurin function. Curr. Med. Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 52.Faul C., Donnelly M., Merscher-Gomez S., Chang Y.H., Franz S., Delfgaauw J., Chang J.M., Choi H.Y., Campbell K.N., Kim K., et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan D.C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Quan Y., Park W., Jin J., Kim W., Park S.K., Kang K.P. Sirtuin 3 Activation by Honokiol Decreases Unilateral Ureteral Obstruction-Induced Renal Inflammation and Fibrosis via Regulation of Mitochondrial Dynamics and the Renal NF-kappaBTGF-beta1/Smad Signaling Pathway. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayanga B.A., Badal S.S., Wang Y., Galvan D.L., Chang B.H., Schumacker P.T., Danesh F.R. Dynamin-Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016;27:2733–2747. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalia R., Wang R.Y.R., Yusuf A., Thomas P.V., Agard D.A., Shaw J.M., Frost A. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 2018;558:401–405. doi: 10.1038/s41586-018-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willems P.H.G.M., Rossignol R., Dieteren C.E.J., Murphy M.P., Koopman W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cel Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Ma Y., Chen Z., Tao Y., Zhu J., Yang H., Liang W., Ding G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr. Connect. 2019;8:1206–1212. doi: 10.1530/EC-19-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tagaya M., Kume S., Yasuda-Yamahara M., Kuwagata S., Yamahara K., Takeda N., Tanaka Y., Chin-Kanasaki M., Nakae Y., Yokoi H., et al. Inhibition of mitochondrial fission protects podocytes from albumin-induced cell damage in diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2022.166368. [DOI] [PubMed] [Google Scholar]
- 61.Sakhi H., Moktefi A., Bouachi K., Audard V., Hénique C., Remy P., Ollero M., El Karoui K. Podocyte Injury in Lupus Nephritis. J. Clin. Med. 2019;8 doi: 10.3390/jcm8091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh M., Rana S. The anaphylatoxin C5a: Structure, function, signaling, physiology, disease, and therapeutics. Int. Immunopharmacol. 2023;118 doi: 10.1016/j.intimp.2023.110081.lo. [DOI] [PubMed] [Google Scholar]
- 63.Verschoor A., Karsten C.M., Broadley S.P., Laumonnier Y., Köhl J. Old dogs-new tricks: immunoregulatory properties of C3 and C5 cleavage fragments. Immunol. Rev. 2016;274:112–126. doi: 10.1111/imr.12473. [DOI] [PubMed] [Google Scholar]
- 64.Li L., Wei T., Liu S., Wang C., Zhao M., Feng Y., Ma L., Lu Y., Fu P., Liu J. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut-kidney axis. J. Cell. Mol. Med. 2021;25:960–974. doi: 10.1111/jcmm.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yiu W.H., Li R.X., Wong D.W.L., Wu H.J., Chan K.W., Chan L.Y.Y., Leung J.C.K., Lai K.N., Sacks S.H., Zhou W., Tang S.C.W. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol. Dial. Transpl. 2018;33:1323–1332. doi: 10.1093/ndt/gfx336. [DOI] [PubMed] [Google Scholar]
- 66.Peng Q., Wu W., Wu K.Y., Cao B., Qiang C., Li K., Sacks S.H., Zhou W. The C5a/C5aR1 axis promotes progression of renal tubulointerstitial fibrosis in a mouse model of renal ischemia/reperfusion injury. Kidney Int. 2019;96:117–128. doi: 10.1016/j.kint.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 67.Weiss S., Rosendahl A., Czesla D., Meyer-Schwesinger C., Stahl R.A.K., Ehmke H., Kurts C., Zipfel P.F., Köhl J., Wenzel U.O. The complement receptor C5aR1 contributes to renal damage but protects the heart in angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2016;310:F1356–F1365. doi: 10.1152/ajprenal.00040.2016. [DOI] [PubMed] [Google Scholar]
- 68.Wang H., Lv D., Jiang S., Hou Q., Zhang L., Li S., Zhu X., Xu X., Wen J., Zeng C., et al. Complement induces podocyte pyroptosis in membranous nephropathy by mediating mitochondrial dysfunction. Cell Death Dis. 2022;13:281. doi: 10.1038/s41419-022-04737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakuma Y., Nagai T., Yoshio T., Hirohata S. Differential activation mechanisms of serum C5a in lupus nephritis and neuropsychiatric systemic lupus erythematosus. Mod. Rheumatol. 2017;27:292–297. doi: 10.1080/14397595.2016.1193965. [DOI] [PubMed] [Google Scholar]
- 70.Mackerness K.J., Jenkins G.R., Bush A., Jose P.J. Characterisation of the range of neutrophil stimulating mediators in cystic fibrosis sputum. Thorax. 2008;63:614–620. doi: 10.1136/thx.2007.089359. [DOI] [PubMed] [Google Scholar]
- 71.Hwang J.I., Fraser I.D.C., Choi S., Qin X.F., Simon M.I. Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc. Natl. Acad. Sci. USA. 2004;101:488–493. doi: 10.1073/pnas.0307549100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cel Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 73.Favaro G., Romanello V., Varanita T., Andrea Desbats M., Morbidoni V., Tezze C., Albiero M., Canato M., Gherardi G., De Stefani D., et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 2019;10:2576. doi: 10.1038/s41467-019-10226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horn A., Raavicharla S., Shah S., Cox D., Jaiswal J.K. Mitochondrial fragmentation enables localized signaling required for cell repair. J. Cel Biol. 2020;219 doi: 10.1083/jcb.201909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cereghetti G.M., Stangherlin A., Martins de Brito O., Chang C.R., Blackstone C., Bernardi P., Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 77.Wu J., Zheng C., Wang X., Yun S., Zhao Y., Liu L., Lu Y., Ye Y., Zhu X., Zhang C., et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. J. Clin. Invest. 2015;125:4091–4106. doi: 10.1172/JCI81061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li M., Lai Y., Chen B., Guo C., Zhou M., Zhao S., Wang S., Li J., Yang N., Zhang H. NAMPT is a metabolic checkpoint of IFNgamma-producing CD4(+) T cells in lupus nephritis. Mol. Ther. 2023;31:193–210. doi: 10.1016/j.ymthe.2022.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berthier C.C., Bethunaickan R., Gonzalez-Rivera T., Nair V., Ramanujam M., Zhang W., Bottinger E.P., Segerer S., Lindenmeyer M., Cohen C.D., et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J. Immunol. 2012;189:988–1001. doi: 10.4049/jimmunol.1103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptomic data in glomerular tissue from Berthier Lupus Glom Dataset can be obtained in the public database Nephroseq (https://www.nephroseq.org). Other original data of the study can be found in the article/supplemental information, and further inquiries can be directed to the corresponding authors.