Abstract
MDM2 is an important regulator of the p53 tumor suppressor protein. MDM2 inhibits p53 by binding to it, physically blocking its ability to transactivate gene expression, and stimulating its degradation. In cultured cells, mdm2 expression can be regulated by p53. Hence, mdm2 and p53 can interact to form an autoregulatory loop in which p53 activates expression of its own inhibitor. The p53/MDM2 autoregulatory loop has been elucidated within cultured cells; however, regulation of mdm2 expression by p53 has not been demonstrated within intact tissues. Here, we examine the role of p53 in regulating mdm2 expression in vivo in order to test the hypothesis that the p53/MDM2 autoregulatory loop is the mechanism by which low levels of p53 are maintained. We demonstrate that basal expression of mdm2 in murine tissues is p53 independent, even in tissues that express functional p53. Transcription of mdm2 is induced in a p53-dependent manner following gamma irradiation, indicating that p53 regulates mdm2 expression in vivo following a stimulus. The requirement for a stimulus to activate p53-dependent regulation of mdm2 expression in vivo appeared to differ from the situation in early-passage mouse embryo fibroblasts, where mdm2 expression is enhanced by the presence of p53. Analysis of mdm2 expression in intact and dispersed embryos revealed that establishment of mouse embryo fibroblasts in culture induces p53-dependent mdm2 expression, suggesting that an unknown stimulus activates p53 function in cultured cells. Together, these results indicate that p53 does not regulate expression of its own inhibitor, except in response to stimuli.
In the majority of human tumors in which the p53 tumor suppressor protein is mutationally inactivated, the responsible mutations are found in the DNA binding domain of this transcriptional activator (reviewed in reference 7). The tumor-suppressive properties of p53 are therefore predicted to be mediated by the transcriptional regulation of a set of genes. A corollary to this prediction is that the transcriptional targets of p53 responsible for tumor suppression should be regulated by p53 in normal tissues. Several genes involved in cell cycle control, apoptosis, and DNA repair have been shown to be regulated by p53 in cultured cells (reviewed in reference 21). However, the existing evidence does not support a central role for p53 in regulating the basal levels of expression of all of these genes in vivo. For example, expression of the p21 gene, which encodes a cyclin-dependent kinase inhibitor, is independent of p53 gene status in most unirradiated adult and embryonic murine tissues (20, 23, 32). Only in the spleen has basal expression of p21 been shown to be increased in the presence of p53 (23). In contrast, p53 regulates basal levels of expression of the proapoptotic Bax protein in several adult murine tissues (27). Bax expression is decreased in spleen, thymus, kidney, small intestine and lung from p53-null mice (27). Therefore, p53 may regulate a specific subset of its target genes in each tissue.
One important outcome of p53's ability to regulate gene expression is an autoregulatory loop in which p53 activates expression of its own inhibitor, MDM2 (2, 40). p53 can specifically stimulate the activity of an internal mdm2 promoter (P2) that directs the synthesis of an RNA lacking exon 1, which is noncoding (11). While RNAs from both the p53-independent (P1) and p53-responsive (P2) promoters can encode full-length MDM2 protein (p90MDM2) (37), RNA from the P2 promoter is approximately eight times more efficiently translated than RNA from the P1 promoter (18). Enhanced p53 activity therefore results in a rapid increase in the amount of p90MDM2 (2). The levels of p90MDM2 are important, because this protein can inhibit p53 function by physically blocking p53's transcriptional activation domain and also by stimulating the degradation of p53 (8, 17, 29). Inhibition of the interaction between p90MDM2 and p53 is thought to be responsible for the stabilization of p53 protein in cultured cells in response to genotoxic stress and oncogene activation (19, 35). The autoregulatory loop model predicts that the p53-dependent induction of the mdm2 P2 promoter in cells exposed to genotoxic agents is the means through which normal levels of p53 protein are recovered (4, 33, 40). While this prediction has not been directly tested, there is some evidence that the ability of p90MDM2 to inhibit p53 function is important biologically. First, overexpression of mdm2 in human tumors appears to be a means of inactivating p53 function, since many tumors overexpressing mdm2 contain wild-type p53 genes (31). Second, a lack of mdm2 expression in murine embryos is lethal unless the mice also lack p53, demonstrating that the negative regulation of p53 by MDM2 is critical for normal development (10, 30). The interaction between p90MDM2 and p53 also appears to be essential for proliferation of cultured cells, since disruption of this interaction results in the cessation of cell division in normal diploid human fibroblasts (3). Together, these observations indicate that MDM2 and p53 may regulate each other constitutively (25, 35).
Because the regulation of mdm2 expression by p53 has been proposed to be the mechanism by which p53 balances its own activity (25, 35), we hypothesized that p53 would constitutively regulate mdm2 expression in vivo. We thought the level of RNA transcribed from the p53-responsive P2 promoter would be highest in those tissues known to express functional p53, including the spleen, thymus, and kidney (23, 27). To investigate whether one aspect of the autoregulatory loop, the regulation of mdm2 expression by p53, is constitutively operational in vivo, we analyzed mdm2 expression by using an S1 nuclease digestion assay to differentiate between mdm2 RNAs transcribed from the upstream, p53-independent (P1) and internal, p53-responsive (P2) promoters (11, 40). We report that the level of expression from the P2 promoter of mdm2 is not influenced by p53 in any of six adult murine tissues, including the spleen, thymus, and kidney, where basal levels of Bax are regulated by p53 (27). Instead, in all tissues, induction of mdm2 by p53 requires a stimulus. The p53-independent expression of mdm2 in vivo contrasts with the constitutive activation of mdm2 expression by p53 in cultured cells (1, 38). We provide evidence that an unknown stimulus activates p53 function in cultured cells. We show that mdm2 expression is not regulated by p53 in 14-day-old embryos, but is induced by p53 during the establishment of mouse embryo fibroblasts (MEFs). This stimulus may increase p53 function by enhancing the specific activity of preexisting p53 or by enhancing expression of p53.
MATERIALS AND METHODS
Mice.
FVB/N animals either wild type or nullizygous (9) for the p53 gene were obtained from Paul Lambert (University of Wisconsin). Wild-type C57BL/6 and 129/Sv mice were obtained from Jackson Laboratories, Bar Harbor, Maine. Animals were housed in the American Association for the Accreditation of Laboratory Animal Care-approved McArdle Laboratory Animal Care Facility. The p53 genotype was determined by PCR analysis (9). Following sacrifice of 4- to 6-week-old mice, tissues were removed and frozen immediately in liquid nitrogen. Tissue samples were subsequently stored at −80°C.
Treatment of animals with gamma radiation.
Animals were irradiated at 4 to 6 weeks of age with gamma rays from a 137Cs source at a dose rate of 3.1 Gy/min. Mice were individually subjected to whole-body gamma irradiation for a total dose of 5 Gy and sacrificed 4 h later. Nonirradiated, 4- to 6-week-old animals were used as controls.
Cells and culture conditions.
Matched sets of early-passage wild-type and p53-null MEFs were provided by both A. Levine (Rockefeller University) and S. Jones (University of Massachusetts). All MEFs were derived from mice of a mixed C57BL/6-129/Sv background. The p53-null MEFs from A. Levine lacked p53 protein due to a deletion of exons 2 to 6 (9), while those from S. Jones contained a deletion of part of exon 5 (6). Both deletions have been shown to block production of functional p53 protein (6, 9). To establish MEFs, wild-type embryos of a mixed C57BL/6-129/Sv background were explanted at day 14 of gestation. Immediately upon explantation, two embryos were snap frozen in liquid N2. The remaining embryos were individually minced and trypsinized for 20 min at 37°C. Trypsin was inactivated through the addition of an equal volume of Dulbecco's modification of Eagle's medium with 10% fetal calf serum. Following trypsinization, cells were pelleted for 5 min at 1,000 rpm. At this stage, cell pellets from two embryos were rinsed once in phosphate-buffered saline, pelleted as described above, and snap frozen in liquid N2. The remaining cell pellets were plated and incubated at 37°C. Following overnight incubation, cells from two embryos were washed extensively in phosphate-buffered saline, scraped, pelleted, and snap frozen in liquid N2. Cells from the remaining embryo were split into two populations and passaged every 3 to 4 days when confluent. At the time of passage, a subset of cells was collected as described and stored at −80°C. All cells were cultured in 5% CO2 in Dulbecco's modification of Eagle's medium supplemented with 10% fetal calf serum, penicillin, and streptomycin.
RNA isolation.
Total RNA from tissues or cultured cells was prepared with Trizol reagent (Molecular Research Center, Inc.) according to the manufacturer's instructions. RNA concentrations were determined in triplicate by A260 and averaged.
S1 nuclease protection assays.
RNAs from the mdm2 P1 and P2 promoters were identified as described previously (36) with slight modification. In brief, total RNA was allowed to hybridize overnight at 48°C to a 32P-end-labeled probe containing DNA sequences corresponding to mdm2 exons 1 to 3 and 27 nucleotides of vector sequence at the 5′ end. As a positive control, the probe was allowed to hybridize to RNAs synthesized in vitro (Stratagene) from constructs designed to reflect transcriptional initiation from the mdm2 P1 or P2 promoter (1). Hybridization products were digested with 100 U of S1 nuclease (Gibco-BRL) and electrophoretically separated on a 5% polyacrylamide gel. Products were quantified with a Molecular Dynamics PhosphorImager.
RESULTS
The internal, p53-responsive promoter of mdm2 is active in adult murine tissues.
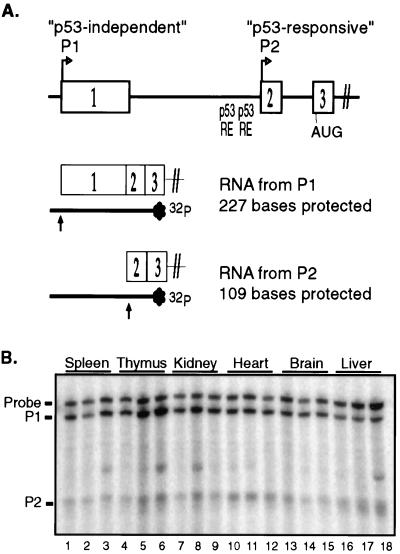
To determine whether both mdm2 promoters are active in adult murine tissues, we used an S1 nuclease protection assay to distinguish RNAs from the p53-independent (P1) and p53-responsive (P2) promoters of mdm2 (Fig. 1A) (36). We measured the amounts of mdm2 RNAs in the spleen, thymus, kidney, heart, brain, and liver. Analysis of RNA from age-matched, wild-type FVB/N mice revealed that RNAs from both mdm2 promoters were present in the spleen, thymus, kidney, heart, brain, and liver (Fig. 1B). RNA initiating from the mdm2 P1 promoter was the most abundant mdm2 RNA in all tissues. RNA initiating at the mdm2 P2 promoter was expressed in all tissues as a relatively minor species of mdm2 RNA, accounting for only about 10 to 30% of the total mdm2 RNA.
FIG. 1.
RNAs from both the P1 and P2 promoters of mdm2 are expressed in murine tissues. (A) Schematic of exons 1 to 3 of the mdm2 gene and of the S1 nuclease protection assay used to distinguish mdm2 transcripts from the P1 and P2 promoters. Indicated are the predicted sizes of hybridization products following digestion with S1 nuclease. (B) The amounts of mdm2 RNA from the P1 and P2 promoters were compared in spleen (lanes 1 to 3), thymus (lanes 4 to 6), kidney (lanes 7 to 9), heart (lanes 10 to 12), brain (lanes 13 to 15), and liver (lanes 16 to 18). The indicated tissues were isolated from three age-matched wild-type FVB/N animals. Twenty-five micrograms of total RNA from each tissue was analyzed for mdm2 RNA from the P1 and P2 promoters by S1 nuclease digestion following hybridization to a radiolabeled, denatured DNA probe complementary to mdm2 exons 1 to 3. The band that appears sporadically in some samples is an artifact from the probe.
Basal transcription of mdm2 is not dependent upon p53 in vivo.
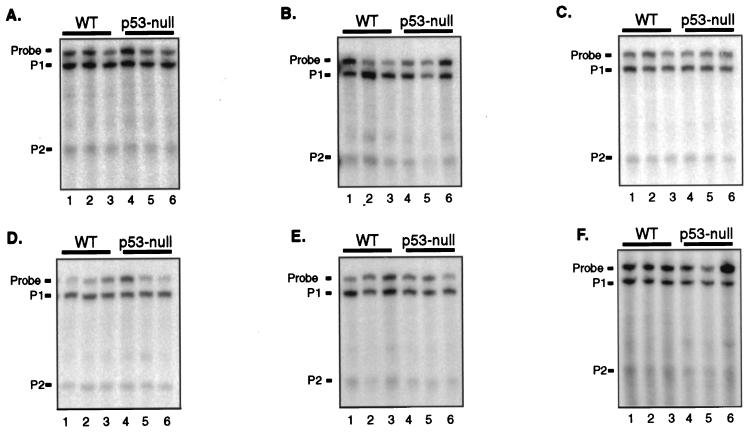
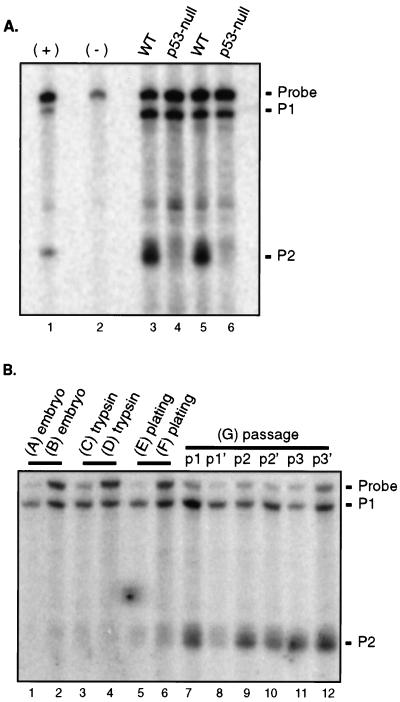
To determine whether basal expression of the mdm2 P2 promoter was a result of p53 function, we used the S1 nuclease protection assay to measure mdm2 RNAs in tissues from wild-type and p53-null FVB/N mice (Fig. 2). RNA from three age-matched animals per genotype was analyzed, and the amount of RNA expressed from each promoter was averaged. The ratio of the amount of RNA expressed in wild-type tissues to that expressed in p53-null tissues revealed that, as expected, transcription from the mdm2 P1 promoter was unchanged in the absence of p53 (Table 1). Similarly, the level of transcription from the mdm2 P2 promoter was not significantly diminished in any p53-null tissue compared to that in the corresponding wild-type tissue. RNA from the mdm2 P2 promoter made up from 10 to 30% of the total mdm2 RNA in tissues from p53-null mice, as it did in tissues from wild-type mice. These results indicate that p53's transactivation function does not contribute to basal transcription from the mdm2 P2 promoter within these adult murine tissues. This is true even in those tissues in which basal levels of p53 protein and activity are detectable e.g., spleen, kidney, brain, and liver (22).
FIG. 2.
Expression from the mdm2 P2 promoter is independent of p53. Three adult FVB/N mice were sacrificed that were either wild type (WT) (lanes 1 to 3) or null for p53 (lanes 4 to 6). Twenty-five micrograms of total RNA from the indicated tissues was analyzed for mdm2 P1 and P2 transcripts by S1 nuclease protection assay. (A) Spleen. (B) Kidney. (C) Brain. (D) Thymus. (E) Heart. (F) Liver.
TABLE 1.
Comparative analysis of levels of mdm2 RNAs from the P1 and P2 promotersa
| Tissue | Mean ± SD fold change in RNAb
|
|||||
|---|---|---|---|---|---|---|
| WT/p53 null
|
WT + IR/ WT − IR
|
WT + IR/ p53 null + IR
|
||||
| P1 | P2 | P1 | P2 | P1 | P2 | |
| Spleen | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 32.0 ± 3.7 | 2.1 ± 0.5 | 23.9 ± 11.7 |
| Thymus | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.3 ± 0.5 | 6.1 ± 2.7 | 1.4 ± 0.2 | 6.6 ± 1.7 |
| Kidney | 2.1 ± 1.2 | 2.9 ± 1.4 | 1.6 ± 0.3 | 5.5 ± 1.8 | 1.8 ± 0.4 | 8.3 ± 2.8 |
| Heart | 1.0 ± 0.1 | 1.3 ± 0.4 | 1.4 ± 0.4 | 7.8 ± 3.2 | 1.9 ± 0.4 | 5.8 ± 1.8 |
| Brain | 1.1 ± 0.2 | 1.5 ± 0.8 | 1.4 ± 0.5 | 5.4 ± 2.9 | 1.2 ± 0.2 | 2.3 ± 0.5 |
| Liver | 1.2 ± 0.2 | 1.6 ± 0.8 | 2.0 ± 0.9 | 4.3 ± 2.2 | 1.0 ± 0.1 | 2.2 ± 0.3 |
mdm2 RNAs were measured by S1 nuclease protection and quantified by PhosphorImager analysis.
The results shown are the mean fold change in the level of each RNA ± standard deviation (n = 3). WT, wild type; IR, ionizing radiation.
The mdm2 P2 promoter is induced in murine tissues following gamma irradiation.
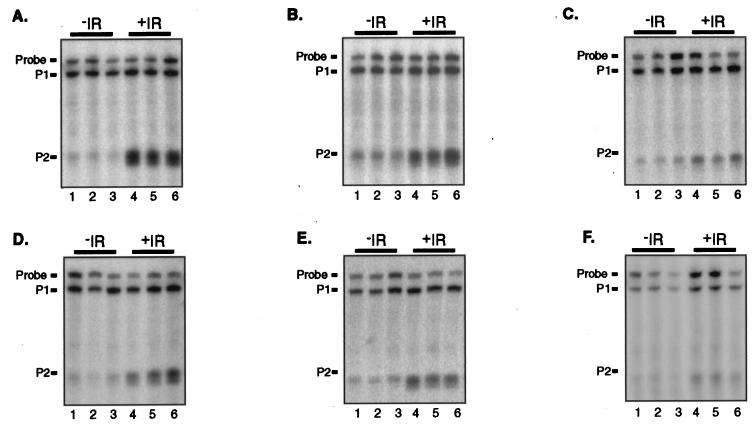
The finding that constitutive expression of the mdm2 P2 promoter was independent of p53 prompted us to determine whether p53 was capable of regulating mdm2 expression in vivo. We therefore determined whether the mdm2 P2 promoter was stimulated in murine tissues in response to whole-body gamma irradiation, a treatment known to induce p53-dependent expression of p21 and Bax (14, 23, 26). The levels of mdm2 RNAs from the P1 and P2 promoters in tissues of wild-type FVB/N animals that had been either untreated or treated 4 h previously with 5 Gy of gamma radiation were measured by S1 nuclease protection (Fig. 3). For each tissue type, three age-matched animals per condition were analyzed, and the average fold change in each mdm2 RNA following gamma irradiation was calculated. Following gamma irradiation, transcription from the mdm2 P2 promoter was specifically induced in all six tissues (Table 1). As expected, no significant change in the level of expression from the mdm2 P1 promoter was observed. The magnitude of the induction of the mdm2 P2 promoter varied among tissues. Most dramatically, transcription from the mdm2 P2 promoter was induced 32-fold within the spleen by 4 h following gamma irradiation. Within the thymus, kidney, and heart, transcription was induced five- to sevenfold. In the brain and liver, transcription from the mdm2 P2 promoter was also induced in response to gamma irradiation, but the induction of mdm2 was slightly diminished from that in other tissues. These data indicate that the P2 promoter of mdm2 can be induced by gamma irradiation in vivo.
FIG. 3.
Induction of the mdm2 P2 transcript following treatment with ionizing radiation (IR). Three adult FVB/N mice were sacrificed that had been either untreated (lanes 1 to 3) or treated 4 h previously with 5 Gy of ionizing radiation (lanes 4 to 6). Twenty-five micrograms of total RNA from the indicated tissues was analyzed for mdm2 P1 and P2 transcripts by S1 nuclease protection assay. (A) Spleen. (B) Kidney. (C) Brain. (D) Thymus. (E) Heart. (F) Liver.
The induction of mdm2 following gamma irradiation of murine tissues is dependent upon p53 function.
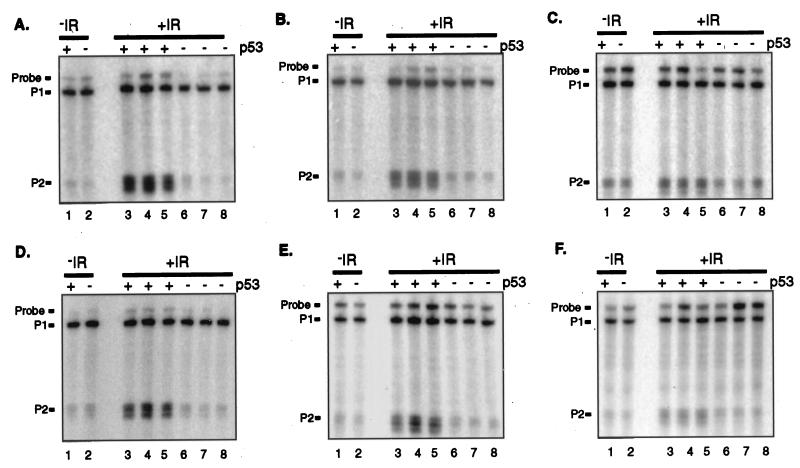
The finding that the mdm2 P2 promoter was induced under conditions known to activate p53 suggested that its activity was a reflection of p53 function in these tissues. To determine whether the induction of the mdm2 P2 promoter following gamma irradiation is attributable to p53 activity, the levels of mdm2 RNA in tissues from wild-type and p53-null FVB/N animals which had been gamma irradiated 4 h previously were measured. For each tissue type, three age-matched animals per genotype were analyzed. The average amount of mdm2 RNA from each of the two mdm2 promoters in irradiated, wild-type tissues was compared to that in irradiated, p53-null tissues (Table 1). As a control, mdm2 RNAs present within wild-type and p53-null tissues prior to gamma irradiation were analyzed by S1 nuclease protection (Fig. 4, lanes 1 and 2 of each panel). Whereas there was no difference in the amount of mdm2 RNA from either promoter in wild-type and p53-null tissues prior to gamma irradiation (Table 1 and Fig. 4), 4 h posttreatment, there was more RNA from the P2 promoter in wild-type, but not p53-null, tissues (Table 1 and Fig. 4). In fact, all of the increase in the amount of mdm2 RNA following gamma irradiation can be attributed to p53 function (Table 1). These results demonstrate that p53 regulates mdm2 expression following gamma irradiation in each of the murine tissues analyzed.
FIG. 4.
Requirement for p53 for the induction of mdm2 following treatment with (ionizing radiation IR). Adult FVB/N mice that were either wild type (lanes 3 to 5) or null for p53 (lanes 6 to 8) were sacrificed 4 h following 5 Gy of whole-body gamma irradiation. Twenty-five micrograms of total RNA from the indicated tissues was analyzed for RNAs from the mdm2 P1 and P2 promoters by S1 nuclease protection. For comparison, mdm2 RNA in wild-type (lane 1) and p53-null (lane 2) tissues prior to gamma irradiation was also analyzed by S1 nuclease protection. In the gel represented here, multiple start sites at the P2 promoter are distinguishable. (A) Spleen. (B) Kidney. (C) Brain. (D) Thymus. (E) Heart. (F) Liver.
Transcription from the internal promoter of mdm2 reflects p53 activity in MEFs.
Prior studies indicating that p53 regulates expression of mdm2 through an internal promoter (P2) used immortal cell lines in which some component or components of the regulatory pathways affecting p53 function are likely to be abrogated (1, 11, 13, 36). The fact that p53 does not constitutively regulate expression of mdm2 in intact tissues led us to question whether p53 gene status was sufficient to influence the activity of the P2 promoter in cultured cells. We therefore measured mdm2 RNA levels in two matched sets of early-passage wild-type and p53-deficient MEFs. Such strains are isogenic except at the p53 locus and therefore provide a model system in which to assess the contribution of p53 to the regulation of mdm2 expression in cultured cells. We used the S1 nuclease protection assay to discriminate between mdm2 RNAs arising from the p53-independent (P1) and p53-responsive (P2) promoters. The amounts of mdm2 RNA arising from the P1 promoter were similar in MEFs expressing and lacking p53 (Fig. 5A). In contrast, the amount of mdm2 RNA arising from the P2 promoter in wild-type MEFs was increased 5- to-10-fold over the amount of such RNA in MEFs lacking p53. In p53-null MEFs, the fraction of mdm2 RNA that initiated at the P2 promoter was only 10 to 20%, as it was in some wild-type and p53-null tissues (Fig. 1). However, in early-passage wild-type MEFs, the amount of RNA from the P2 promoter was similar to the amount from the P1 promoter. In order to observe a similar level of expression from the mdm2 P2 promoter in intact tissues, a stimulus, gamma irradiation, was required. Therefore, p53 constitutively activates mdm2 expression in cultured MEFs, but not in intact, unirradiated tissues.
FIG. 5.
(A) S1 nuclease protection analysis of mdm2 RNA in MEFs. A comparison of the amounts of mdm2 RNA from the P1 and P2 promoters present in MEFs that were wild type (WT) for p53 (lanes 3 and 5) or null for p53 due to either deletion of p53 exons 2 to 6 (lane 4) or deletion of part of exon 5 (lane 6) is shown. MEFs were all early passage (lanes 3 and 6, passage 2; lanes 4 and 5, passage 1). Ten micrograms of total RNA was protected from S1 nuclease digestion. The negative control (−) was a reaction in which RNA was omitted from the hybridization reaction. As a positive control (+), probe was allowed to hybridize to RNA synthesized in vitro from plasmids designed to reflect transcriptional initiation at the mdm2 P1 or P2 promoter. (B) Levels of mdm2 RNA during establishment of MEFs. Seven wild-type embryos (A to G) were explanted at day 14. Tissue and/or cells were snap frozen in liquid nitrogen at various stages during the establishment of MEFs as follows: embryos A and B, immediately following explantation: C and D, following trypsinization for 15 min at 37°C; E and F, incubated at 37°C overnight following trypsinization; G, treated as embryos E and F, split into two populations (p and p′), and passaged every 3 to 4 days. Twenty-five micrograms of total RNA was analyzed for mdm2 RNAs from the P1 and P2 promoters by S1 nuclease protection.
p53 is functionally activated during the establishment of MEFs.
Our results indicate that p53 does not regulate mdm2 expression in unstressed, adult murine tissues, but does so in cultured MEFs derived from 14-day-old embryos. This apparent paradox could be explained if p53 was functional in embryonic, but not adult, tissues or if p53 was activated during the establishment of MEFs. Although there is some evidence that p53 is transcriptionally active at days 11 to 19 of embryogenesis (15), a previous report indicated that mdm2 expression was not influenced by p53 gene status in intact, 14-day-old embryos (20). Therefore, we tested whether p53's transcriptional activation function was stimulated by disruption of the embryo or by culturing. We isolated 14-day-old murine embryos and measured expression from the P1 and P2 promoters of mdm2 at various stages during the establishment of MEFs in culture. We froze embryos immediately upon explantation or following mincing and trypsinization. We plated minced and trypsinized embryos and froze the attached cells after overnight incubation in a humidified incubator at 37°C. Finally, we serially passaged the plated cells. mdm2 RNA from the P2 promoter was present in 14-day-old embryos, where it made up 10 to 20% of the total mdm2 RNA (Fig. 5B, lanes 1 and 2). The ratio of mdm2 RNA from the P2 promoter to mdm2 RNA from the P1 promoter in wild-type 14-day-old murine embryos (0.15 ± 0.03 [mean ± standard deviation]; n = 4) is similar to the ratio observed in early-passage p53-null MEFs as well as in tissues derived from p53-null animals, suggesting that basal expression of mdm2 is p53 independent in 14-day-old embryos. Levels of RNA from the mdm2 P2 promoter stayed low after embryos had been minced and trypsinized (Fig. 5B, lanes 3 and 4), but rose slightly after plating and overnight incubation of the resulting cells (Fig. 5B, lanes 5 and 6). Upon serial passage of MEFs (Fig. 5B, lanes 7 to 12), the levels of RNA from the mdm2 P2 promoter continued to increase, such that, by passage 3, they exceeded the levels of RNA from the P1 promoter. These results indicate that establishment of MEFs results in a 10- to 40-fold induction of p53-dependent mdm2 expression, presumably through the activation of p53 function.
DISCUSSION
The p53 tumor suppressor protein regulates the expression of its own inhibitor, MDM2, in cultured cells (2, 40). p53 binds to the mdm2 gene and stimulates the activity of an internal promoter, P2, without influencing the activity of an upstream promoter, P1 (11). P90MDM2 binds to p53, inactivates p53's transcriptional activation function, and stimulates the degradation of p53 (8, 17, 29). Therefore, p53 and MDM2 can form an autoregulatory loop in which p53 regulates the levels of its own inhibitor. Regulation of mdm2 expression by p53 and the subsequent inhibition of p53 function by MDM2 have been put forth as the normal mechanism by which p53 function is kept in check to prevent widespread apoptosis (19, 28, 35). While there is evidence that MDM2 regulates p53 during murine embryogenesis (10, 30), it was not known whether p53 regulates mdm2 expression in vivo.
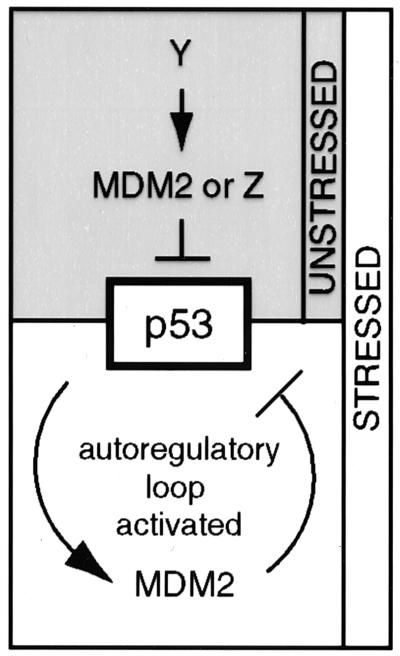
Here, we assessed one aspect of the p53/MDM2 autoregulatory loop, the regulation of mdm2 expression by p53, in vivo. Our results show that the levels of mdm2 RNAs are not detectably influenced by p53 in 14-day-old embryos or in any of six adult murine tissues. While both the P1 and P2 promoters of mdm2 are active in vivo, constitutive levels of RNAs from both promoters are independent of p53 in the absence of genotoxic stress, even in tissues such as the spleen, where p53 constitutively regulates Bax and p21 expression (23, 27). Thus, in the absence of a signal, it appears that factors other than p53 regulate mdm2 expression in intact tissues, and only after a stimulus is mdm2 expression regulated by p53 (Fig. 6). The factors regulating basal activity of the mdm2 P1 and P2 promoters are not known. The p53-related p63 and p73 proteins may contribute to basal activity of the P2 promoter, because they are capable of activating some p53-responsive genes (12, 41). However, neither p63 nor p73 activates the P2 promoter in response to gamma irradiation, because our results demonstrate that induction of this promoter is abrogated in the absence of p53.
FIG. 6.
Model illustrating the regulation of p53 function and mdm2 expression under unstressed and stressed conditions. Under unstressed conditions, expression of mdm2 is regulated by an unknown factor, Y. The function of p53 is regulated by MDM2 or by unknown factor Z. Under stressed conditions, p53 activates mdm2 expression and MDM2 inhibits p53.
Levels of the p53-responsive p21 gene are not influenced by p53 gene status in most embryonic and adult murine tissues (20, 23, 32); however, p53 does regulate transcription of p21 in the spleen (23). Bax expression is also enhanced by p53 in this tissue, as well as in the thymus, kidney, and choroid plexus (27, 42). These observations suggest that p53 selectively regulates a subset of genes in a tissue-specific manner. If the p53/MDM2 autoregulatory loop is in fact the mechanism by which p53 levels are kept under control, then it is predicted that, in tissues in which the expression of genes such as p21 and bax is clearly being influenced by p53, p53 would necessarily influence mdm2 expression. However, we did not detect an influence of p53 on mdm2 expression in any of the tissues tested, including those in which p53 has a demonstrable function. Therefore, the full p53/MDM2 autoregulatory loop does not appear to be the mechanism by which p53 levels are kept under control in these tissues. These results do not eliminate the possibility that MDM2 may still regulate p53; however, basal levels of mdm2 expression appear to be determined by a factor other than p53. It is possible that p53 does regulate mdm2 expression and that the balance between MDM2 and p53 is exquisitely regulated such that an influence of p53 on mdm2 expression is undetectable. In such a scenario, MDM2 would regulate a population of p53 distinct from that which regulates p21 and bax, because expression of these genes is influenced by p53.
Following whole-body gamma irradiation, RNA from the mdm2 P2 promoter is specifically induced in all six tissues in a p53-dependent manner. The magnitude of this induction varies between tissues and is greatest in the spleen, which is very sensitive to p53-mediated apoptosis following gamma irradiation (26). However, there is not a strict correlation between the magnitude of the increase in mdm2 expression and the sensitivity of the tissues to p53-mediated apoptosis. The induction of mdm2 differs from that of Bax, which is induced in radiosensitive tissues, such as the thymus and spleen, but not in radioresistant tissues, such as the liver (14). The pattern of induction of mdm2 is more similar to that of p21, which is induced in every tissue tested, including the radiosensitive thymus and spleen and the radioresistant brain and kidney (23).
The magnitude of the increase in mdm2 expression following gamma irradiation is underestimated in assays that do not discriminate between RNAs from the two promoters, because the increase in mdm2 RNA from the p53-responsive P2 promoter is masked by the basal amount of mdm2 RNA from the p53-independent P1 promoter (S.M.M., unpublished observations). This observation may account for the fact that mdm2 was not previously identified, by cDNA array hybridization, as one of the p53-dependent genes whose expression was induced by whole-body gamma irradiation of adult mice (16). Our results clearly demonstrate that p53 induces expression of mdm2 in all tissues tested in response to gamma irradiation.
In cultured cells, expression of mdm2 is highly dependent on p53 (1, 38). We show here that the mdm2 P2 promoter is activated by the establishment of MEFs in culture. At the first passage, there is a 10-fold increase in RNA from the P2 promoter of mdm2. This result suggests that even very-early-passage MEFs have received a stimulus that activates p53. It follows that the activity of p53 is higher in primary MEFs than in 14-day-old embryos. This interpretation may explain the observation that expression of the p53-responsive p21 gene is independent of p53 in 14-day-old embryos, but dependent on p53 in MEFs (20, 24, 32). We hypothesize that an unknown stimulus activates p53 during the establishment of MEFs in culture, resulting in the induction of both p21 and mdm2. This stimulus may require increased levels of p19ARF to activate p53 (34, 43). The level of p19ARF rises as MEFs are cultured, and p19ARF inhibits MDM2's ability to stimulate the degradation of p53 (34, 43, 44). Thus, the increased levels of p19ARF may mediate the activation of p53 seen here. However, recent evidence suggests that p19ARF may inhibit MDM2 in late-passage MEFs, but not during establishment (39). It will be important to determine whether p19ARF plays a role in the activation of p53 during the establishment of MEFs in culture.
In contrast to its minimal role in unstressed cells, p53 plays a central role in the response of cells to stresses such as DNA damage, hypoxia, exposure to teratogenic agents, ribonucleotide depletion, and oncogene expression (reviewed in reference 21). Our data demonstrate that p53-mediated regulation of mdm2 is apparent in all tissues after a stimulus. We propose that the activation of mdm2 expression by p53 would be detectable only under conditions that activate the function of p53 in response to stress. The activation may be mediated through changes in the levels or specific activity of p53.
Our data have important implications for the interpretation of experiments designed to reveal the mechanisms of regulation of p53 function. In particular, they prompt us to reevaluate the regulatory relationships between p53 and MDM2. These results indicate that unknown transcription factors indirectly determine p53's basal activity by regulating basal levels of expression of mdm2. However, this prediction is true only if MDM2 is the critical regulator of p53 levels and activity in vivo in unstressed tissues.
We would like to raise the intriguing possibility that MDM2 may not regulate p53 in the absence of a stimulus. In light of our results, it appears plausible that all of the experiments indicating that MDM2 regulates p53 function involved conditions of stress and are therefore situations in which p53 activates mdm2 expression, resulting in activation of the full p53/MDM2 autoregulatory loop. To date, there are three clear examples of regulation of p53 function by MDM2 in cells. First, MDM2 has been shown to regulate the level and activity of endogenous p53 in normal, diploid human fibroblasts (3). We have shown previously that p53 stimulates the mdm2 P2 promoter in such cells (38), so the full autoregulatory loop is intact under these circumstances. These results can be explained if, as seen here, culture conditions activate p53. A second example is during murine embryogenesis. The death of 6-day-old embryos lacking mdm2 and their rescue in the absence of p53 implies that mdm2 is required to regulate p53 function for at least a portion of development (10, 30). It is not known whether p53 regulates mdm2 expression in 6-day-old embryos; however, based on our model of the p53/MDM2 autoregulatory loop, we predict that mdm2 expression would be elevated in 6-day-old embryos in response to a stimulus that activates p53. This stimulus could be the hyperproliferative signal proposed by Jones et al. (10) to activate p53. The third example is in tumor cell lines, where inhibition of mdm2 expression by antisense oligonucleotides results in enhanced p53 levels and activity (5). Again, we suggest that culturing conditions activate the full autoregulatory loop such that p53 regulates mdm2 expression and MDM2 regulates p53 function (Fig. 6). Thus, MDM2 may not constitutively regulate p53 function, but may do so only under conditions of stress, when the autoregulatory loop is initiated by activation of p53. The generation of mice carrying conditional-null alleles of mdm2 would allow us to determine whether MDM2 regulates p53 in adult tissues, in the absence of stress.
Our results indicate that the full p53/MDM2 autoregulatory loop is not constitutively active in adult tissues. Instead, regulation of mdm2 expression appears to be independent of p53, except in response to a stimulus, such as genotoxic stress, that activates p53. It may be that MDM2 is required to inhibit p53 only under conditions of stress. Recently, much effort has been given to disturbing the interaction between MDM2 and p53 in tumor cells overexpressing mdm2; disruption of this interaction in cultured tumor lines sensitizes them to chemotherapeutic agents (5). If MDM2 does not regulate p53 function in unstressed cells, such therapies may be specific for preneoplastic and tumor cells.
ACKNOWLEDGMENTS
We thank Arnie Levine and Steve Jones for MEFs and Amy Liem and Paul Lambert for FVB/N mice. We are grateful for critical insights from Bill Dove, Norman Drinkwater, Paul Lambert, and John Petrini.
This work was supported by developmental funds from Cancer Center Support grant CA-07175 to the McArdle Laboratory for Cancer Research and by NIH grant CA-70718 to M.E.P. S.M.M. was supported by NCI Predoctoral Training grant CA-09135 from the National Institutes of Health.
REFERENCES
- 1.Barak Y, Gottlieb E, Juven-Gershon T, Oren M. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994;8:1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- 2.Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaydes J P, Wynford-Thomas D. The proliferation of normal human fibroblasts is dependent upon negative regulation of p53 function by mdm2. Oncogene. 1998;16:3317–3322. doi: 10.1038/sj.onc.1201880. [DOI] [PubMed] [Google Scholar]
- 4.Chen C Y, Oliner J D, Zhan Q, Fornace A J, Jr, Vogelstein B, Kastan M B. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Lu W, Agarwal S, Zhou W, Zhang R, Chen J. Ubiquitous induction of p53 in tumor cells by antisense inhibition of MDM2 expression. Mol Med. 1999;5:21–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 7.Hainaut P, Hernandez T, Robinson A, Rodriguez-Tome P, Flores T, Hollstein M, Harris C C, Montesano R. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–213. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 9.Jacks T, Remington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 10.Jones S N, Roe A E, Donehower L A, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 11.Juven T, Barak Y, Zauberman A, George D L, Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993;8:3411–3416. [PubMed] [Google Scholar]
- 12.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan J C, Valent A, Minty A, Chalon P, Lelias J M, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 13.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 14.Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed J C. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene. 1996;12:187–192. [PubMed] [Google Scholar]
- 15.Komarova E A, Chernov M V, Franks R, Wang K, Armin G, Zelnick C R, Chin D M, Bacus S S, Stark G R, Gudkov A V. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 1997;16:1391–1400. doi: 10.1093/emboj/16.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komarova E A, Diatchenko L, Rokhlin O W, Hill J E, Wang Z J, Krivokrysenko V I, Feinstein E, Gudkov A V. Stress-induced secretion of growth inhibitors: a novel tumor suppressor function of p53. Oncogene. 1998;17:1089–1096. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- 17.Kubbutat M H, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 18.Landers J E, Cassel S L, George D L. Translational enhancement of mdm2 oncogene expression in human tumor cells containing a stabilized wild-type p53 protein. Cancer Res. 1997;57:3562–3568. [PubMed] [Google Scholar]
- 19.Lane D P, Hall P A. MDM2—arbiter of p53's destruction. Trends Biochem Sci. 1997;22:372–374. doi: 10.1016/s0968-0004(97)01119-5. [DOI] [PubMed] [Google Scholar]
- 20.Leveillard T, Gorry P, Niederreither K, Wasylyk B. MDM2 expression during mouse embryogenesis and the requirement of p53. Mech Dev. 1998;74:189–193. doi: 10.1016/s0925-4773(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 21.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 22.MacCallum D E, Hupp T R, Midgley C A, Stuart D, Campbell S J, Harper A, Walsh F S, Wright E G, Balmain A, Lane D P, Hall P A. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene. 1996;13:2575–2587. [PubMed] [Google Scholar]
- 23.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 24.Michieli P, Chedid M, Lin D, Pierce J H, Mercer W E, Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 25.Midgley C A, Lane D P. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene. 1997;15:1179–1189. doi: 10.1038/sj.onc.1201459. [DOI] [PubMed] [Google Scholar]
- 26.Midgley C A, Owens B, Briscoe C V, Thomas D B, Lane D P, Hall P A. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci. 1995;108:1843–1848. doi: 10.1242/jcs.108.5.1843. [DOI] [PubMed] [Google Scholar]
- 27.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 28.Momand J, Zambetti G P. Mdm-2: “big brother” of p53. J Cell Biochem. 1997;64:343–352. [PubMed] [Google Scholar]
- 29.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 30.Montes de Oca Luna R, Wagner D S, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 31.Oliner J D, Kinzler K W, Meltzer P S, George D L, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 32.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 33.Perry M E, Piette J, Zawadzki J A, Harvey D, Levine A J. The mdm-2 gene is induced in response to UV light in a p53-dependent manner. Proc Natl Acad Sci USA. 1993;90:11623–11627. doi: 10.1073/pnas.90.24.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomerantz J, Schreiber-Agus N, Liegeois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H W, Cordon-Cardo C, DePinho R A. The Ink4a tumor suppressor gene product, p19ARF, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 35.Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 36.Saucedo L J, Carstens B P, Seavey S E, Albee L D, Perry M E. Regulation of transcriptional activation of mdm2 gene by p53 in response to UV radiation. Cell Growth Differ. 1998;9:119–130. [PubMed] [Google Scholar]
- 37.Saucedo L J, Myers C D, Perry M E. Multiple murine double minute gene 2 (MDM2) proteins are induced by ultraviolet light. J Biol Chem. 1999;274:8161–8168. doi: 10.1074/jbc.274.12.8161. [DOI] [PubMed] [Google Scholar]
- 38.Seavey S E, Holubar M, Saucedo L, Perry M E. The E7 oncoprotein of human papillomavirus type 16 stabilizes p53 through a mechanism independent of p19ARF. J Virol. 1999;73:7590–7598. doi: 10.1128/jvi.73.9.7590-7598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber J D, Taylor L J, Roussel M F, Sherr C J, Bar-Sagi D. Nucleolar Arf sequences Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 41.Yang A, Kaghad M, Wang Y, Gillett E, Fleming M D, Dotsch V, Andrews N C, Caput D, McKeon F. p63, p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 42.Yin C, Knudson C M, Korsmeyer S J, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Xiong Y, Yarbrough W G. ARF promotes MDM2 degradation and stabilizes p53: ARF INK4a locus deletion impairs both the Rb and p53 tumor suppressor pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 44.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Scherr C J, Roussel M F. Myc signalling via the ARF tumor suppressor regulates p53-dependent apoptosis. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]