Main text
Ischemic stroke is a deadly vascular central nervous system disease that causes disability and death worldwide.1 Enzymatic thrombolysis and mechanical thrombectomy are the main treatment strategies for ischemic stroke treatment at present.2 However, neuroprotection against cerebral ischemia-reperfusion injury is critical to improve the quality of life and lifespan of patients. Unfortunately, no neuroprotectants have been successful in clinical trials due to insufficient drug delivery, off-target effects, and intolerable side effects.3 The blood-brain barrier (BBB) also poses a problem by limiting the amount of drugs that reach the ischemic area.4 Although damage to the BBB can lead to increased permeability and drug penetration in the acute phase of ischemic stroke, this permeability is transient and resolves during the recovery period after ischemic stroke.5 In addition, it has remained difficult to target delivery of candidate neuroprotectants to cells within the ischemic penumbra. Multiple cell types, including neurons, endothelial cells, microglia, astrocytes, and neutrophils, are involved in the pathological processes of ischemic stroke and require targeted drug delivery to prevent off-target effects. Therefore, new platforms that can deliver drugs to the ischemic penumbra, target specific cells, selectively release drugs, and improve drug bioavailability are necessary for the treatment of ischemic stroke.
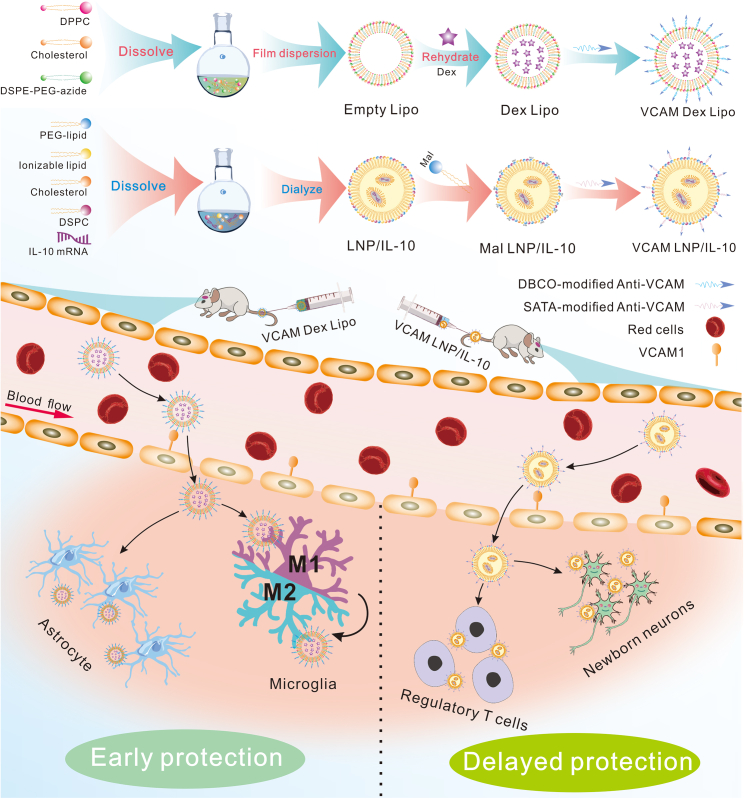
In this issue of Molecular Therapy, Nong and colleagues developed a novel vascular cellular adhesion molecule-1 (VCAM)-targeted lipid nanocarrier (NC) that demonstrates proof of concept for in vivo targeting of the penumbra in acute ischemic stroke (AIS) via specific affinity between antigens and antibodies.6 This approach achieved specific enrichment of mRNA and dexamethasone-21-phosphate (Dex) in ischemic brain (Figure 1). The authors utilized VCAM antibody-modified liposomes to deliver Dex and VCAM antibody-modified lipid nanoparticles (LNPs) to deliver interleukin-10 (IL-10) mRNA and validated their targeting and therapeutic effects on stroke in vivo. In addition, the authors found that VCAM-targeted NCs could deliver therapeutic agents to endothelial cells and leukocytes, key cell types involved in post-stroke inflammation.
Figure 1.
Overview of VCAM-targeting lipid platform for the treatment of ischemic stroke
Schematic diagram of the VCAM-targeting lipid platform structure (top) highlighting the assembly process. To conjugate targeting ligands, azide-functionalized liposomes were incubated with dibenzocyclooctyne (DBCO)-modified antibodies at 4°C. For targeted LNPs, N-succinimidyl S-acetylthioacetate (SATA)-treated antibodies were conjugated to LNP particles via SATA-maleimide conjugation chemistry at room temperature. After intravenous injection, VCAM-targeted NCs (mRNA LNPs and liposomes) showed superior targeting and specificity to the injured brain and were taken up by endothelial cells. VCAM-targeted liposomes immediately provided “early protection” due to the strong anti-inflammatory effect of Dex. Alternatively, for “delayed protection,” transcription and protein synthesis after mRNA delivery takes hours, but the effect can last from 24 h to days. Overall, the VCAM-targeted lipid platform achieves full protection of neurons in the ischemic brain.
Both AIS in humans and transient middle cerebral artery occlusion (tMCAO) in mice cause severe acute inflammation in the ischemic penumbra, which upregulates inducible CAMs.7 Thus, the authors first assessed the vascular accessibility of CAMs and targeted CAM delivery in tMCAO and AIS. At 24 h post-reperfusion, Nong et al. injected radiolabeled monoclonal antibodies (mAbs) against platelet endothelial CAM (PECAM), intercellular adhesion molecule-1 (ICAM), and VCAM. Compared to PECAM and ICAM mAbs, VCAM mAbs exhibited higher brain uptake, especially in ischemic brain. To further evaluate the potential of targeted delivery of VCAM for stroke, the authors used LNPs and liposomes as model NCs. Anti-VCAM liposomes and LNPs showed the best targeted delivery in tMCAO brains, exceeding untargeted NCs by 1–2 orders of magnitude. This finding demonstrated the successful targeted delivery of VCAM NCs to the tMCAO brain.
After verifying the ischemic brain-targeting ability of VCAM, the authors sought to determine which cell types in the brain facilitated VCAM delivery. To address this question, the authors intravenously injected fluorescent-labeled VCAM-targeted liposomes into tMCAO mice. Flow cytometry showed greater accumulation of VCAM-targeted liposomes in endothelial cells and leukocytes in the ipsilateral hemisphere than in those in the contralateral hemisphere. Next, to characterize the distribution of the cargo after VCAM-targeted NC delivery, the authors used VCAM-targeted liposomes encapsulated with Dex, an anti-inflammatory drug, and VCAM-targeted LNPs loaded with mRNA encoding for Cre recombinase. As described by the authors, the delivery of Dex and mRNA by the VCAM-targeted platform resulted in a higher concentration in the ischemic hemisphere. Through a series of elegant experiments, the authors demonstrated the mechanism of VCAM targeting and verified the successful delivery of VCAM-targeted NCs to the ischemic brain.
Subsequently, the authors evaluated the potential of VCAM-targeted liposome delivery of Dex as a therapeutic agent for ischemic stroke. Dex can reduce inflammatory brain injury but lacks clinical efficacy in AIS, perhaps due to the low concentration of Dex that reaches the ischemic region. Thus, the authors loaded Dex into VCAM-targeted liposomes and intravenously injected them into tMCAO mice. No significant effects on infarct size were detected for free Dex or empty anti-VCAM liposomes, but anti-VCAM liposomes loaded with Dex reduced infarct volume by 35.0% ± 4.8%. Mice receiving free Dex had 50% survival vs. 85% for mice receiving anti-VCAM Dex liposomes. These results demonstrated the great potential of VCAM-targeted liposome delivery of small-molecule drugs for ischemic stroke treatment.
Next, the authors sought to verify the ability of VCAM-targeted LNPs to deliver mRNA. They selected IL-10 mRNA due to its anti-inflammatory and neuroprotective effects. Treatment with VCAM-targeted mRNA LNPs resulted in a 62% reduction in infarct volume and a 100% survival rate with no bleeding or lung damage in tMCAO mice. The authors emphasized that VCAM-targeted mRNA LNPs accumulated in the injury site, thereby ameliorating neuronal death in the inflammatory penumbra and reducing off-target toxicity. Therefore, VCAM-targeted LNPs provide a convenient platform for delivering mRNA encoding therapeutic proteins for the treatment of ischemic stroke.
At present, tissue type plasminogen activator is the only US Food and Drug Administration-approved drug for restoring blood flow in AIS.8 However, due to the short therapeutic window and risk of reperfusion injury and intracerebral bleeding, most patients are unable to receive timely treatment.9,10 Herein, the findings reported by Nong and colleagues represent proof of concept for Dex and even IL-10 mRNA delivery to the penumbra in AIS by a novel VCAM-targeted lipid platform. This will undoubtedly pave the way for the targeted delivery of therapeutic small molecule drugs or mRNA for the treatment of ischemic stroke in the future. In addition, these lipid NCs can provide both early and delayed protection for damaged neurons (Figure 1). Although the safety, efficacy, and large-scale production of this novel drug delivery system need to be further assessed before clinical translation can be considered, this study represents an exciting first step toward targeted therapeutic LNPs for the treatment of AIS.
Acknowledgments
Declaration of interests
The authors declare no competing interests.
Contributor Information
Feifei Li, Email: lifeifei@njmu.edu.cn.
Hongliang Xin, Email: xhl@njmu.edu.cn.
References
- 1.Anna R., Rolf R., Mark C. Update of the organoprotective properties of xenon and argon: from bench to beside. Intensive Care Med. Exp. 2020;8:11. doi: 10.1186/s40635-020-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthels D., Das H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jinatongthai P., Kongwatcharapong J., Foo C.Y., Phrommintikul A., Nathisuwan S., Thakkinstian A., Reid C.M., Chaiyakunapruk N. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. Lancet. 2017;390:747–759. doi: 10.1016/S0140-6736(17)31441-1. [DOI] [PubMed] [Google Scholar]
- 5.Kadry H., Noorani B., Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nong J., Glassman P.M., Shuvaev V.V., Reyes-Esteves S., Descamps H.C., Kiseleva R.Y., Papp T.E., Alameh M.G., Tam Y.K., Mui B.L., et al. Targeting lipid nanoparticles to the blood brain barrier to ameliorate acute ischemic stroke. Mol. Ther. 2024 doi: 10.1016/j.ymthe.2024.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y.M., Zhang C.L., Chen A.Q., Wang H.L., Zhou Y.F., Li Y.N., Hu B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.678744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao T., Liu M., Chen M., Luo Y., Wang C., Xu T., Jiang Y., Guo Y., Zhang J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020;216 doi: 10.1016/j.pharmthera.2020.107695. [DOI] [PubMed] [Google Scholar]
- 9.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z., Zhou W., Dong H., Ma X., He Z. Dexmedetomidine pretreatment inhibits cerebral ischemia/reperfusion-induced neuroinflammation via activation of AMPK. Mol. Med. Rep. 2018;18:3957–3964. doi: 10.3892/mmr.2018.9349. [DOI] [PubMed] [Google Scholar]