Graphical abstract
Highlights
-
•
CC16 is an anti-inflammatory, immunomodulatory protein expressed in respiratory club cells.
-
•
Severe abdominal obesity and arterial hypertension robustly decrease serum CC16.
-
•
ACEi/ARBs, uricosurics and chronic heart failure robustly increase serum CC16.
-
•
Effects might be mediated by adipose tissue inflammation as well as RAAS and uric acid disturbance.
-
•
Findings indicate a complex interplay of the metabolic, respiratory and cardiovascular system.
Abstract
Introduction
Clara cell 16-kDa protein (CC16) is an anti-inflammatory, immunomodulatory secreted pulmonary protein with reduced serum concentrations in obesity according to recent data.
Objective
Studies focused solely on bodyweight, which does not properly reflect obesity-associated implications of the metabolic and reno-cardio-vascular system. The purpose of this study was therefore to examine CC16 in a broad physiological context considering cardio-metabolic comorbidities of primary pulmonary diseases.
Methods
CC16 was quantified in serum samples in a subset of the FoCus (N = 497) and two weight loss intervention cohorts (N = 99) using ELISA. Correlation and general linear regression analyses were applied to assess CC16 effects of lifestyle, gut microbiota, disease occurrence and treatment strategies. Importance and intercorrelation of determinants were validated using random forest algorithms.
Results
CC16 A38G gene mutation, smoking and low microbial diversity significantly decreased CC16. Pre-menopausal female displayed lower CC16 compared to post-menopausal female and male participants. Biological age and uricosuric medications increased CC16 (all p < 0.01). Adjusted linear regression revealed CC16 lowering effects of high waist-to-hip ratio (est. −11.19 [−19.4; −2.97], p = 7.99 × 10−3), severe obesity (est. −2.58 [−4.33; −0.82], p = 4.14 × 10−3) and hypertension (est. −4.31 [−7.5; −1.12], p = 8.48 × 10−3). ACEi/ARB medication (p = 2.5 × 10−2) and chronic heart failure (est. 4.69 [1.37; 8.02], p = 5.91 × 10−3) presented increasing effects on CC16. Mild associations of CC16 were observed with blood pressure, HOMA-IR and NT-proBNP, but not manifest hyperlipidemia, type 2 diabetes, diet quality and dietary weight loss intervention.
Conclusion
A role of metabolic and cardiovascular abnormalities in the regulation of CC16 and its modifiability by behavioral and pharmacological interventions is indicated. Alterations by ACEi/ARB and uricosurics could point towards regulatory axes comprising the renin-angiotensin-aldosterone system and purine metabolism. Findings altogether strengthen the importance of interactions among metabolism, heart and lungs.
Introduction
Increasing evidence is pointing towards a pulmonary-metabolic axis of high importance in both communicable infectious diseases, e. g. COVID19 [1], and non-communicable respiratory diseases, e. g. asthma [2] and chronic obstructive pulmonary disease (COPD) [3]. In both acute and chronic scenarios, obesity and type 2 diabetes are recognized as independent risk factors for disease development and prognosis [4], [5] Yet not every person with obesity or type 2 diabetes exhibits pulmonary implications, which indicates an individual complex regulation of this systems interplay.
For an efficient personalized treatment and prevention of respiratory secondary diseases in the context of present metabolic implications, it is necessary to identify valid and applicable risk prediction markers. Recent data indicate high potential of Clara cell 16-kDa protein (CC16) which is a secretory protein expressed in epithelial club cells (former clara cells) in the respiratory tract [6]. Besides being abundant in bronchioalveolar lavage fluid, CC16, among other club cell proteins, can cross the bronchioalveolar blood barrier and systemically circulate in the blood [7]. Of interest, even though CC16 is also expressed in other body sites, circulating CC16 concentrations origin almost exclusively from the lungs, which makes it a valid marker for epithelial integrity and specifically club cell function [8]. Past studies have proposed local and systemic anti-inflammatory and immunomodulatory properties of CC16 that were differentially displayed under acute and chronic pulmonary conditions and by that emphasized the complexity of the underlying regulatory mechanism [6]. A recent study assessed CC16 in a more complex manner which displayed an association of obesity with decreased airway and serum levels that was robust against numerous confounding factors and concluded a contribution of CC16 to the development and severity of asthma in people with overweight and obesity [9].
To date, when assessing CC16 in the context of overweight and obesity, studies focused only on the increased weight, which does not reflect the complex implications of the metabolic and reno-cardio-vascular system that are especially impacted in severe obesity cases. The purpose of the present analysis was therefore the first metabolism-centered evaluation of CC16 spanning from the assessment of lifestyle-associated risk factors to physiological alterations, common cardio-metabolic comorbidities and appropriate intervention strategies using suitable human cohorts.
Patients and methods
Study design and population
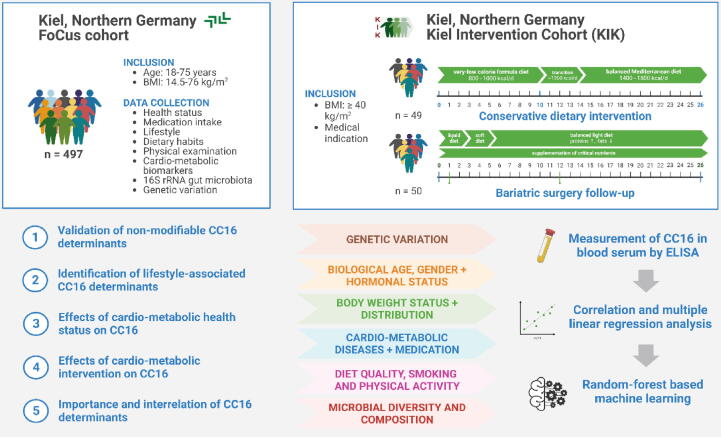
For a precise metabolism-centered assessment of CC16 in human obesity, we used data of a subject subset from the German population-based cross-sectional Food Chain Plus (FoCus) cohort. A detailed cohort description has recently been published by Geisler et al. [10]. In brief, recruitment was performed via local registration offices and the obesity clinic of the University Medical Center Schleswig-Holstein (UKSH) in Kiel. Data collection was performed between 2011 and 2014 including adults aged 18 to 75 years. No further selection criteria were applied in the setup of the cohort. Using a questionnaire specifically set up for metabolic research, extensive health-related data was collected, including e.g. the report of cardio-metabolic diseases and corresponding medication intake were available (for further details see Supplement S1). Furthermore, anthropometric measurements, metabolic and inflammatory parameters, gut microbiota 16S rRNA sequencing data, information about the participants’ diets, physical activities, sleeping duration and smoking status, as well as genetic variation have been obtained and are available for analysis. For this study, participants were selected based on specific metabolic phenotypes of different obesity degrees (0: BMI < 25 kg/m2, I: BMI ≥ 30 and < 35 kg/m2, II: BMI ≥ 35 and < 40 kg/m2, III: BMI ≥ 40 kg/m2) which resulted in a set of N = 497 subjects (female: N = 345; male: N = 152).
Furthermore, two independent strongly obese study populations (BMI ≥ 40 kg/m2) were assessed. These cohorts originate from different weight loss intervention therapies that are offered at the UKSH in Kiel and can be used to extensively assess the effects of weight loss in humans.
(1) Kiel Intervention Cohort (KIK): At the Day Hospital for Internal Medicine, N = 49 (female: N = 37, male: N = 12) patients completed 12-weeks of fasting (very-low-calorie formula diet, 800 kcal/day) and subsequent 14-week maintenance (balanced low calorie mixed diet, 1200–1600 kcal/day) accompanied by moderate physical activity, medical and psychological care. Anthropometric data and blood parameters were collected at baseline (week 0) and at the end of intervention (week 26).
(2) Bariatric Surgery Intervention Cohort: Patients who received bariatric surgery (sleeve gastrectomy (N = 28, female: N = 19, male: N = 11), gastric bypass (N = 22, female: N = 18, male: N = 4)) were observed. During the first week after surgery, patients receive dietary and behavioral recommendations by trained staff and are expected to consistently follow a high-protein low-calorie diet with additional daily supplementation of critical nutrients and moderate physical activity. During examination before surgery (week 0) and 6 months after surgery as follow up (week 26), weight-related anthropometric data and blood samples were collected.
The setup of study populations as well as data availability and implementation in terms of the study purpose are illustrated in Fig. 1.
Fig. 1.
Cohort set-up and study workflow. This study uses data from two Northern German cohorts constituted for the extensive study of obesity and associated cardio-metabolic implications. From the cross-sectional FoCus cohort, n = 497 participants aged 18–75 years were included. From the longitudinal Kiel Intervention Cohort (KIK), n = 99 severely obese participants (BMI ≥ 40 kg/m2) who either participated in a conservative dietary weight loss program or underwent bariatric surgery with baseline and 6-month weight loss data available for analysis were included. The purpose of the study is a comprehensive metabolism-centered assessment of the pulmonary anti-inflammatory and immunomodulatory marker CC16. This comprises the validation of known non-modifiable CC16 determinants (e.g. biological age, gender and hormonal status), identification of further influencing factors on lifestyle level, the evaluation of cardio-metabolic disease presence, severity and intervention as well as the estimation of variable importance and intercorrelations both with CC16 and among determinants. For this, CC16 has been quantified in blood serum samples using ELISA and assessed in relation to several cardio-metabolic variables using correlation tests, multiparameter linear regression models and random forest-based machine learning as validation.
Ethics statement
Written informed consent was obtained from each included participant/patient. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been priorly approved by the local ethics committee of the Medical Faculty at Kiel University, Germany (FoCus: A156/03, intervention study: D523/18).
Health related questionnaire
Information on the participants’ health status were collected using a questionnaire specifically constituted for nutrition-medical study purposes. Through the questionnaire, we gained information on the presence of various metabolic and cardiovascular diseases as well as any pharmacological intake.
Anthropometric measurements
Patients were weighed on digital scales in light clothing without shoes. Using a stadiometer, height was measured. Waist- and hip-circumferences were obtained using measuring tape. Corresponding body mass index (BMI) and waist-to-hip ratio (WHR) were calculated. Blood pressure was determined using an elastic cuff around the upper arm. All measurements were executed in duplicates using calibrated instruments by trained staff. Analyses were performed using mean values.
Biochemistry
Fasted blood samples were obtained by venipuncture followed by biochemical measurement of metabolic and inflammatory parameters by the central laboratory at the UKSH. The “Homeostatic Model Assessment for Insulin Resistance” (HOMA-IR = fasting glucose [mg/dl] × fasting insulin [µU/l] / 405) and “Homeostatic Model Assessment for beta-cell function” (HOMA-β = 360 × fasting insulin [µU/l] / (fasting glucose [mg/dl] − 63)) were calculated [11]. CC16 was quantified in blood serum samples using a commercial ELISA kit (ab238266, Abcam, Cambridge, MA, USA) according to manufacturer’s instructions. Detectable concentrations of the ELISA kit ranged from 6.3 to 400 pg/ml with a sensitivity of 1.4 pg/ml. Prior to application, thawed samples were diluted 1:200, 1:250, 1:400 or 1:1000, depending on the expected concentrations. Samples were measured in duplicates with a coefficient of variation considered acceptable at < 10 % and mean values were formed prior to statistical analysis.
Nutrition
Dietary intake was assessed using a valid semi-quantitative 12-month retrospective food frequency questionnaire by the European Investigation into Cancer and Nutrition protocol from the German Institute of Human Nutrition in Potsdam (EPIC-FFQ) [12]. The EPIC-FFQ consists of questions regarding frequency of consumption and average portion sizes of 143 foods. Subsequent analysis was done using “EPICsoft” resulting in the daily intake of micro- and macronutrients and food compounds [13]. The EPIC-FFQ also includes questions on the participants’ physical activity level and duration of sleep. Two step validation of the EPIC-FFQ has been performed including an initial validation, which has previously been described by Nöthlings et al. [14] and an additional accuracy verification via unannounced 24 h-dietary recall in 10 % of subjects. For a holistic evaluation the participants’ diet quality, a Healthy Eating Index specifically modified to fit the EPIC-FFQ data (HEI-EPIC). We followed the protocol given by von Rüsten et al. [15] resulting in a scoring system from 0 to 100 with higher values reflecting a better conformation to the German food intake guidelines. In brief, nine food groups that are split in three scoring-categories are considered: 1.) adequation (drinks, vegetables, and fruits), 2.) moderation (sweets/fatty snacks) and 3.) mixture of adequation and moderation (grains/grain products/potatoes, milk/dairy produce, meat/processed meat/fish/eggs, fats and oils). Respectively, scores for are given with either increasing or decreasing consumption or as a combination of both. In the latter case, participants whose consumption did not exceed the recommendation, categories were treated as adequation. If the recommendation was exceeded, categories were treated as moderation. This considers that, although moderate amount of these foods is recommended, excessive consumption, however, makes a significant contribution to daily energy intake [15]. Data were available for 468 FoCus subjects.
Host gut microbiota
DNA extraction, bacterial 16S rRNA sequencing, quality control and taxonomic classification from stool samples were realized by the Institute of Clinical Molecular Biology at Kiel University, Germany. Their procedure has previously been well described elsewhere [16], [17] and will be summarized accordingly in the following.
DNA extraction: DNA extraction from fecal samples was performed with the QIAamp DNA fast stool mini kit automated on the QIAcube (Qiagen). Material was given into Garnet Bead tubes (Dianova) which were prepped with InhibitEx lysis buffer. Bead beating was done using the SpeedMill PLUS (Analytik Jena). Samples were heated to 95 °C for 5 min and procedure was continued according to the manufacturer's protocol. DNA extracts were stored at –20 °C until PCR amplification. During sample extractions, blanks were used as controls.
Bacterial 16S rRNA gene sequencing: 16S rRNA gene variable regions V1 V2 were amplified using dual-barcoding according to Caporaso et al. [18]. DNA was diluted prior to PCR, and PCR products were confirmed using the electrophoresis in agarose gel and normalized with the SequalPrep normalization plate kit (Thermo Fisher Scientific). Afterwards, PCR products were pooled equimolarly and sequenced on the Illumina MiSeq v.3.2 (Illumina Inc.). Demultiplexing after sequencing was based on 0 mismatches in the barcode sequences bcl2fastq.
Quality control and taxonomic classification: Quality control and taxonomic classification workflow are assessable through GitHub (@mruehlemann). In brief, data processing was performed using the DADA2 workflow for big data sets providing abundance tables of amplicon sequence variants (ASV). All sequencing runs were handled separately for error correction, read merging, and combined chimera detection. ASVs underwent taxonomic annotation using the naive Bayesian classifier implemented in DADA2 from the RDP database (v.16) as a taxonomic reference and, if possible, classified sequences at the species level [19], [20].
Genetic variation
CC16 A38G polymorphism (rs3741240) is known for its impact on the expression of CC16 from epithelial cells in the respiratory system and has been displayed to negatively promote the development of chronic pulmonary diseases. This polymorphism is also associated with decreased serum levels [21], [22] which is why we included it into our analyses. Genotyping was executed by the Institute of Clinical Molecular Biology at Kiel University, Germany. Depending on the availability of whole blood samples and obtained DNA extracts, genotyping was performed using the Infinium® Global Screening Array (GSA) v1.0 and Illumina platform. SNPCHIPDB was used to provide genetic information in standard PLINK format. Genetic information was available for N = 445 participants of which 190 displayed the wildtype, 200 the homozygous and 55 the heterozygous polymorphism.
Statistical analysis
Statistical analysis and visualization were performed using R (R Core Team, 2022) and RStudio Version 4.1.2 (Rstudio., Inc., Boston, Massachusetts, USA). Five subjects with CC16 concentrations higher than mean + 3 standard deviations were excluded from analysis. Using Shapiro-Wilk test, variables were tested for normal distribution. Normally distributed data are presented as mean ± standard deviation, non-normally data as median (interquartile range), and categorical variables as number of subjects and percentages. Correlation analysis between CC16 and continuous variables was performed using Spearman rank correlation (rs). For the comparison of CC16 between groups, Mann-Whitney U (two groups) or Kruskal-Wallis (>two groups) test was applied.
Evaluation of the gut microbiota was performed in R using the packages “phyloseq”, “microbiome” and “vegan”. A core measurable microbiota (CMM) was extracted including 58 Amplicon Sequencing Variants (ASV) present in > 40 % of participants with a relative abundance of > 0.05 %. Overall inter-individual variation in the gut microbial composition was evaluated by Bray Curtis dissimilarity index [23], and, using the CMM, binary Jaccard index [24]. Corresponding p-values were calculated between CC16 terciles (low: 0.2 – 7.61 ng/ml, medium: 7.61 – 13.3 ng/ml, and high: 13.3 – 44.0 ng/ml) using permutational multivariate analysis of variance (PERMANOVA). Intra-individual microbial diversity was determined as the microbial species richness indicating the number of distinct species represented per individual and the Shannon index as an additional measure for evenness of microbial distribution. Index significance in relation to CC16 levels was assessed using Spearman’s rank correlation test. To evaluate taxonomical alterations of the microbial composition, relative abundances of core genera were compared between CC16 terciles using adjusted linear regression models.
The initial univariate analysis revealed the A38G polymorphism (chr11:3741240), biological sex, age, smoking habit, gut microbial species richness and uricosuric medication intake as strong effectors (p < 0.01) on CC16 serum levels. Consequently, the effect of metabolic and cardiovascular diseases and corresponding treatment was assessed using general linear regression model that were adjusted for the identified confounders. For all disease analyses, the absence of disease was set as the reference group.
For the assessment of weight loss effects, differences of BMI and CC16 serum concentrations between week 0 and week 26 were tested using paired Wilcoxon test.
Results
Characterization of the FoCus study population
Using cross-sectional cohort data from our German population-based FoCus cohort, we aim to precisely investigate the effect of obesity and other metabolic and cardiovascular diseases on serum levels of the respiratory marker CC16 using data on the metabolic profile, disease occurrence and treatment, lifestyle, gut microbial diversity and genetic variation of N = 497 human subjects. Clinical characteristics of this study population and initial correlation of variables with CC16 serum concentrations are displayed in Table 1.
Table 1.
Characterization of metabolic profile in the FoCus study population.
| non-obese | obese | rS | p | |
|---|---|---|---|---|
| number of subjects, n (%) | 205 (41.25) | 292 (48.75) | – | – |
| female sex, n (%)a | 149 (72.68) | 196 (67.12) | – | 0.22 |
| age, yearsb | 51 (42; 60)*** | 53 (46; 61.25)*** | 0.269 | 1.11 × 10−9 |
| anthropometric measurements | ||||
| height, cmb | 171 (167; 178) | 170 (164; 179)** | 0.15 | 7.78 × 10–4 |
| weight, kgb | 63.05 (56.4; 69.95) | 117.08 (98.83; 138.9) | −0.044 | 0.33 |
| BMI, kg/m2,b | 21.59 (19.76; 23.35) | 40.51 (33.76; 46.74)** | −0.083 | 0.07 |
| waist-to-hip-ratiob | 0.81 (0.75; 0.88) | 0.95 (0.9; 1.01) | 0.036 | 0.46 |
| SBP, mmHg | 120 (110; 130) | 135 (130; 140) | 0.013 | 0.78 |
| DBP, mmHg | 80 (70; 80) | 80 (80; 90) | −0.024 | 0.59 |
| metabolic markers | ||||
| glucose, mg/dlb | 91 (87; 98) | 104 (94; 113) | −0.014 | 0.78 |
| insulin, µU/lb | 6.2 (4.5; 8.7) | 17.9 (11.7; 26.7) | −0.068 | 0.17 |
| HOMA-IRb | 1.43 (0.99; 2.04) | 4.49 (2.97; 7.38) | −0.068 | 0.18 |
| HOMA-βb | 84 (61.41; 114.55) | 157.97 (110.92; 230.05) | −0.073 | 0.14 |
| triglycerides, mmol/lb | 77 (60; 101) | 136.5 (104.75; 193.5) | −0.07 | 0.14 |
| inflammatory markers | ||||
| CRP, ng/lb | 2 (1.3; 3.6)* | 5.6 (2.95; 10.3) | −0.137 | 1.45 × 10–2 |
| IL-6, ng/lb | 3.2 (2.2; 4.97) | 4.6 (3.4; 6.25) | 0.01 | 0.85 |
| wnt5a, ng/mlb | 0.39 (0.24; 0.54) | 0.37 (0.26; 0.56)** | −0.071 | 0.23 |
| SFRP5, ng/mlb | 6.1 (2.08; 13.61) | 8.86 (3.98; 19.39)** | 0.102 | 0.1 |
| CC16, ng/ml | 10.7 (6.47; 16.17) | 9.86 (5.99; 15.26) | – | – |
| cardiac marker | ||||
| NT-proBNP, ng/l | 8.56 (8.48; 152.75) | 8.48 (8.48; 109.76) | 0.082 | 0.11 |
| cardio-metabolic disease prevalence | ||||
| arterial hypertension, n (%)a | 37 (18.23) | 220 (76.39) | – | 0.61 |
| hyperlipidemia, n (%)a | 45 (22.5) | 125 (43.71) | – | 0.09 |
| type 2 diabetes, n (%)a | 8 (3.9) | 145 (49.66) | – | 0.51 |
| chronic heart failure, n (%)a | 4 (1.96) | 24 (8.45) | – | 5.7 × 10−4 |
| coronary artery disease, n (%)a | 6 (2.99) | 20 (7.12) | – | 3.65 × 10−2 |
| myocardial infarction, n (%)a | 4 (1.95) | 16 (5.54) | – | 1.9 × 10−2 |
Using Shapiro-Wilk test, variables were tested for normal distribution. Normally distributed data are presented as mean ± standard deviation, non-normally data as median (interquartile range), and categorical variables as number of subjects and percentages. a Statistical significance was tested using Mann-Whitney-U test. b Statistical significance was tested using Spearman’s rank correlation test. Significant associations with CC16 identified in analyses stratified for metabolic status are indicated in each respective box as * p < 0.05, ** p < 0.01 or *** p < 0.001. Abbreviations: BMI = Body Mass Index; SBP = systolic blood pressure; DBP = diastolic blood pressure; HOMA-IR = Homeostatic Model Assessment of Insulin Resistance; HOMA-β = Homeostatic Model Assessment of beta-cell function; CRP = C-reactive Protein; IL-6 = interleukin 6; wnt5a = wingless-type MMTV integration site family, member 5A; SFRP5 = Secreted Frizzled Related Protein 5; CC16 = Clara cell 16–kDa protein; NT-proBNP = N-terminal prohormone of brain natriuretic peptide.
Relation of non-modifiable factors and CC16 serum concentrations
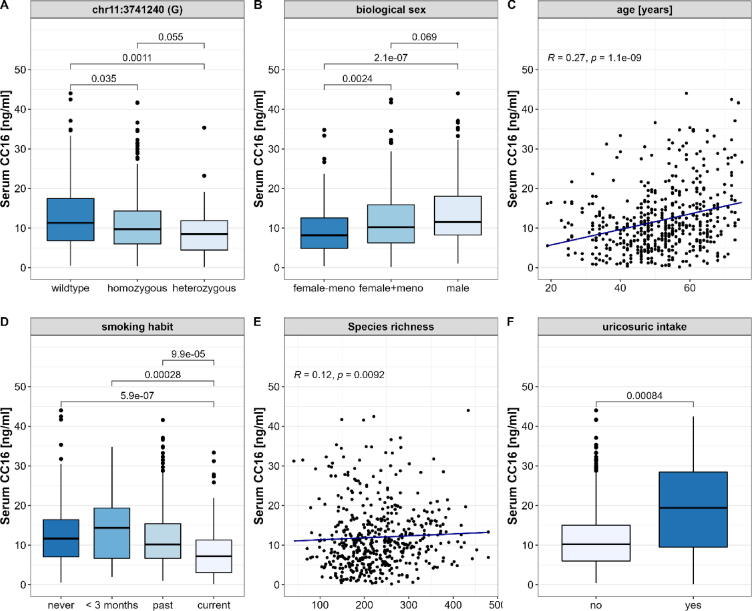
In previous studies, several determinants that contributed to alterations of CC16 serum concentrations were identified including non-modifiable factors. Hence, in this first analysis, we assessed the relation of a genetic variation, the participants biological sex, age and menopause with circulating CC16. A38G polymorphism (chr11:3741240) is recognized to decrease CC16 expression from epithelial cells resulting in decreased serum levels. Homozygous variation at this genetic locus was present in 200 and heterozygous in 55 of the assessed subjects. Compared to the wildtype, both types of genetic variation displayed significantly decreased CC16 (homozygous: p = 3.5 × 10–2; heterozygous: p = 1.1 × 10–3, Fig. 2A). Biological females displayed significantly lower CC16 serum levels than males (p = 2.1 × 10−7). Of interest, this difference diminished after the onset of menopause (p = 0.69, Fig. 2B). Independent of sex and menopause, CC16 serum concentrations strongly correlated with the participants’ age (p = 1.1 × 10−9, Fig. 2C).
Fig. 2.
Relation of CC16 serum concentrations and selected influencing factors. We assessed data from 497 human subjects for an initial identification of non-modifiable and common modifiable metabolic risk factors on CC16 serum concentrations. Here, we were able to display significant contribution of (A) the variation at the genetic loci chr11:3741240 whereby both homozygous and heterozygous mutations were associated to decreased CC16, (B) the participants’ biological sex with decreased CC16 in women prior to menopause as well as (C) a strong positive correlation with the participants’ age (D). Lower CC16 was also seen with active smoking, (E) decreased gut microbial species richness (F). In return, the intake of uricosuric medication, which is a treatment of hyperuricemia, significantly increased serum CC16. Statistical significance was tested using univariate pairwise Mann-Whitney-U tests for categorical and Spearman’s rank correlation test for continuous variables. Based on the displayed strongly significant influences (p < 0.01), these factors were defined as confounders for the following analyses.
Relation of modifiable lifestyle-related risk factors and CC16 serum concentrations
Beside these non-modifiable determinants, the lifestyle is also strongly influencing the development and course of chronic diseases which is the case for all - pulmonary, metabolic and cardiovascular diseases [25]. In this next set of analyses, we aimed to determine the relation of CC16 serum levels and the participants’ lifestyle by assessing the diet, physical activity and duration of sleep, as well as smoking habits and the gut microbial diversity. Detailed results are displayed in Table2.
Table 2.
Characterization of lifestyle factors in the FoCus study population.
| non-obese | obese | rS | p | |
|---|---|---|---|---|
| number of subjects, n (%)a | 205 (41.25) | 292 (48.75) | – | – |
| diet | ||||
| HEI-EPIC, 0-100b | 52.15 ± 9.5 | 51.37 ± 9.73 | 0.043 | 0.35 |
| total energy intake, kj/dayb | 8617.95 (7492.25; 10649) | 8463.5 (7037.3; 10724.8) | 0.016 | 0.73 |
| water, l/dayb | 2.95 (2.48; 3.58)* | 3.34 (2.78; 4.06) | –0.087 | 0.06 |
| alcohol, g/dayb | 2.64 (0.96; 4.53) | 0.93 (0.4; 2.54) | 0.013 | 0.78 |
| physical activity | ||||
| moderate activity, h/weekb | 15.12 (9.12; 26.41) | 14.75 (10; 24.25)* | –0.057 | 0.22 |
| sports, h/weekb | 4 (2; 7.09) | 1.5 (0; 4) | 0.016 | 0.73 |
| TV watching, h/weekb | 2 (1.5; 3) | 3 (2; 5) | –0.046 | 0.32 |
| sleep | ||||
| sleep, h/24 hb | 7.25 (7; 8) | 7 (6.5; 8.5) | –0.016 | 0.73 |
| smoking habit | ||||
| current smoker, n (%)c | 38 (19.29) | 47 (16.61) | – | 6.83 × 10–7 |
| intensity, cigarettes/dayb | 10 (7; 20) | 20 (10; 25) | –0.053 | 0.38 |
| gut microbial diversity | ||||
| species richness, n speciesb | 228 (178.75; 277.75) | 214 (164.5; 273.5)* | 0.117 | 9.24 × 10–3 |
| Shannon index | 4.08 (3.71; 4.4) | 3.88 ± 0.51 | 0.003 | 0.94 |
Due to missing values, number of subjects can vary. b Statistical significance was tested using univariate Spearman’s rank correlation tests. c Statistical significance was tested using univariate Mann-Whitney U tests. Significant associations with CC16 identified in analyses stratified for metabolic status are indicated in each respective box as * p < 0.05, ** p < 0.01 or *** p < 0.001Abbreviation: HEI-EPIC = Healthy Eating Index calculated with EPIC-Food Frequency Questionnaire data.
Diet, physical activity and sleep: In brief, we assessed the participants diet using the Healthy Eating Index which we calculated from the available EPIC-FFQ data (HEI-EPIC). Values which range from 0 to 100 and reflect the participants’ adherence to a balanced and generally healthy diet, did not correlate with circulating CC16. Daily total caloric or water intake also showed no effect on the serum levels. Above that, neither the duration of physical activity nor sleep showed effects on CC16 (Table2).
Smoking: Smoking habits were stratified using four categories - never smoked, smoked in the past, smoked for <3 months and current smoker. When comparing these groups, current smokers show significantly decreased CC16 serum concentrations in comparison to currently non-smoking participants of all other groups (Fig. 2D). In turn, severity of smoking behavior, which we assessed by correlating the number of daily smoked cigarettes with the serum CC16 levels was not significantly associated (Table2).
Gut microbial diversity: A decreased gut microbial diversity is associated with several unfavorable health outcomes and is assumed to be a strong contributor to the development of metabolic implications in obesity and related comorbidities [26]. Using available 16S rRNA sequencing data from 493 FoCus subjects, we calculated the microbial inter-individual variance between CC16 concentration terciles which by failing to reach statistical significance does not point towards alterations in the microbial composition as a cause of varying CC16 levels (Bray Curtis: p = 0.06; Jaccard: p = 0.14). The intra-individual species richness, which was assessed as the number of microbial strains present in the microbiota per individual was positively associated to circulating CC16 (rs = 0.117, p = 9.24 × 10–3, Fig. 2E), whereas the Shannon index, which aside from the microbiota’s richness also accounts for the distribution of strains does not significantly correlate with CC16 serum levels (rs = 0.003, p = 0.94, Table2).
Effects of medication intake on CC16 serum concentrations
When evaluating the serum profile in relation to the presence of disease, the use of medications should be considered as a possible source of influence. Information on daily intake of medication was provided through a health questionnaire and subsequently stratified into 31 categories. The analysis of these categories revealed mild decrease in CC16 serum concentration under the intake of specific psychotropic drugs and contraceptives and, in turn, an increase under the intake of opioids and uricosurics (n = 28, p = 8.37 × 10–4, Fig. 2F). Complete medication analysis is provided in Supplement S2.
Effects of metabolic and cardiovascular disease profiles on CC16 serum concentrations
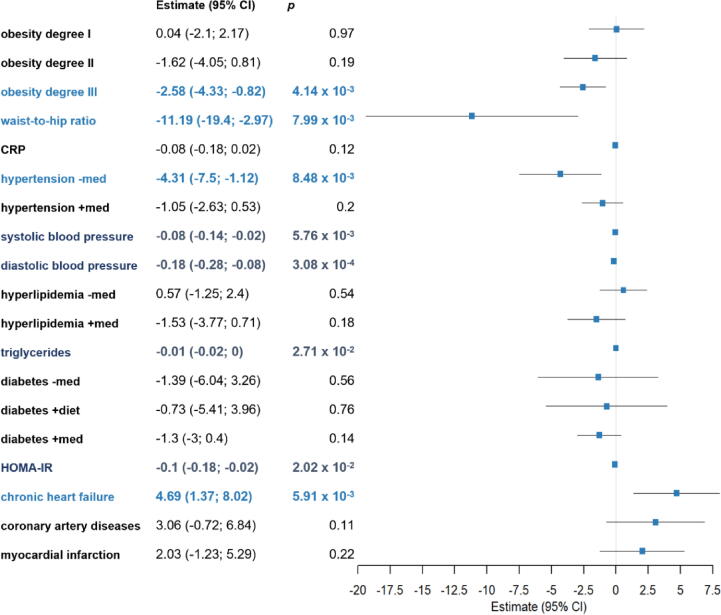
In a next set of analyses, we evaluated the effects of the presence and, where appropriate, treatment effects of metabolic and cardiovascular diseases and associated phenotypic profiles on CC16 serum levels using general linear regression models adjusted for biological age, sex, smoking habit, presence of CC16 A38G polymorphism (rs3741240) and uricosuric intake. Detailed results are displayed in Fig. 3. For all disease analyses, the absence of disease was set as the reference group.
Fig. 3.
Effect of metabolic and cardiovascular implications on CC16 serum concentrations. We assessed data from 497 human subjects on the presence and, where appropriate, treatment of metabolic and cardiovascular diseases in relation to CC16 serum levels using general linear regression models adjusted for biological age, sex, smoking habit, the presence of CC16 A38G polymorphism (rs3741240) and uricosuric intake. These analyses indicate an inverse relation of severe obesity, and especially abdominal body fat distribution reflected by higher waist-to-hip ratio, and CC16 serum concentrations. Moreover, untreated arterial hypertension is associated with decreased circulating CC16 which can be counteracted by antihypertensive medication treatment. Atherosclerotic cardiovascular diseases (myocardial infarction, coronary artery diseases and chronic heart failure) display increased CC16 serum levels that, in case of chronic heart failure, are even robust against confounding factors and remain statistically significant. Conforming with these findings, mild associations with small effect sizes were seen for cardio-metabolic measures, like blood pressure, triglyceride levels, as well as HOMA index indicative for insulin resistance. For all disease analyses, the absence was set as reference group. Results are displayed as estimate (95% confidence interval) and corresponding p-values. Abbreviations: CRP = C-reactive Protein, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, CI = confidence interval.
Obesity: To gain information about obesity in relation to CC16 serum concentrations, we stratified participants into the WHO’s severity degrees. Subsequent general linear regression reveals a decrease of CC16 with higher disease severity degree that reaches statistical significance in obesity degree III (est. = –2.58 (–4.33; –0.82), p = 4.14 × 10–3)). High waist-to-hip ratio, as indicator of a metabolically unfavorable fat distribution, exerts even stronger lowering effects on CC16 serum levels (est. = –11.19 (–19.4; –2.97), p = 7.99 × 10–3). In return, microbial dysbiosis, as important implication associated with obesity, only minorly affected serum CC16, with adjusted linear regression of 21 identified core genera only revealing a statistically significant lower percentage of Dialister with higher CC16 (est. = –0.01 (-0.002; −0.02), p = 1.3 × 10−2).
To further evaluate the effects of obesity–induced metabolic implications on CC16 serum levels, we performed an analysis only including subjects with obesity (BMI ≥ 30 kg/m2) which revealed a negative association of wnt5a, a pro–inflammatory cytokine important in the so called metabolic inflammation with serum CC16 levels (est. = –5.58 (–10.85; –0.3), p = 4 × 10–2) using adjusted linear regression. Of interest, wnt5a levels were also increased in obese participants with simultaneous presence of a chronic respiratory disease (p = 3.5 × 10–2).
Arterial hypertension: Within our study population, hypertension was present in 52.34 % of the subjects. These participants were stratified by the presence or absence of antihypertensive medication. Adjusted linear regression analysis revealed significantly decreased levels of CC16 in subjects with, in comparison participants without reported arterial hypertension (est. = –4.31 (–7.5; –1.12), p = 8.48 × 10–3)). Independent of hypertension diagnosis and treatment, CC16 serum levels also correlated with the blood pressure. However, this relation displays only small effect sizes (systolic: est. = –0.08 (–0.14; –0.02), p = 5.76 × 10–3; diastolic: est. = –0.18 (–0.28; –0.08), p = 3.08 × 10−4).
Hyperlipidemia: Next, we examined dyslipidemia as a common comorbidity seen in obesity and a major risk factor for atherosclerosis. But, neither the presence of hyperlipidemia nor the treatment (e. g. by statins) was related to changes in CC16.
Insulin resistance and type 2 diabetes: The assessment of disturbances in the glucose metabolism was assessed using the HOMA for insulin resistance and beta-cell function, as well as the presence of type 2 diabetes and respective treatment form. Results only show a mild association of CC16 serum levels with the degree of insulin resistance (est. –0.1 (–0.18, –0.02), p = 2.02 × 10−2), but no alterations due to decreased beta-cell function, manifest type 2 diabetes or any form of treatment.
Structural cardiovascular disease: Evaluation of effects by cardiovascular diseases included the presence of coronary artery disease, chronic heart failure, and the history of myocardial infarction. All three disease types revealed increased CC16 using univariate Mann-Whitney U tests. However, after the adjustment for confounders using linear regression, this relation only remained statistically significant for chronic heart failure (est. = 4.69 (1.37; 8.02), p = 5.91 × 10–3). Complementary, increased CC16 serum levels were seen in participants with pathological N-terminal fragment of proBNP (NT–proBNP) levels, a serum marker for cardiac function, when stratifying participants according to age- and sex-specific reference ranges (p = 2.19−4). Yet, this association was dampened after the correction for confounders using linear regression (p > 0.05).
Effect of established metabolic and cardiovascular treatments on CC16 serum concentrations
With our previous analyses, we were able to present a strong influence of metabolic and cardiovascular implications on the respiratory marker CC16 pointing towards a joint regulation path of the metabolic and respiratory system. To counteract the resulting health risk, it is essential to control associated modifiable contributors using adequate interventions, like behavioral changes and medication. The aim of the following analyses was to assess whether these intervention strategies effect circulating CC16.
Smoking: Nicotine abstinence is the most effective way to reduce health damages made by chronic cigarette smoking. Our data indicated significantly reduced CC16 serum concentrations in current smokers. However, participants who have smoked in the past do not show this alteration (Fig. 2D) which implies the reversibility of this implication.
Hypertension: Antihypertensive treatment agents are the most effective intervention to normalize derailed chronically high blood pressure. Our analysis revealed significantly decreased CC16 serum concentration in participants with untreated arterial hypertension (Fig. 3). In turn, participants who stated the intake antihypertensive medication displayed levels similar to those of unaffected participants which indicates that this form of treatment can initiate an effective normalization of CC16 serum concentrations. Of interest, we filtered participants who reported only one type of antihypertensive (ACEi/ARB, Calcium-channel blocker, diuretic or beta-blocker) which revealed that CC16 effects were initiated by ACEi/ARB but not the other types of anti-hypertensive medications (for details, see Supplement S2).
Weight reduction: To counteract the health impairments associated to overweight and obesity, weight reduction is a highly effective form of treatment. To assess the effect of different therapy forms, we included two independent cohorts that either participated in a dietary intervention program (n = 49, 75.5 % female) or underwent bariatric surgery (sleeve gastrectomy: n = 28, 67.9 % female; gastric bypass: n = 22, 81.8 % female). Over 26 weeks, all subjects achieved significant reduction of the BMI (Fig. 4A-C) and expected metabolic improvements (data not shown). After surgical intervention, alterations are accompanied by a mild decrease in serum CC16 (sleeve gastrectomy: p = 3.58 × 10–2, gastric bypass: p = 3.29 × 10–2, Fig. 4E-F), while there were no differences in concentrations at the end of the dietary intervention program (p = 0.72, Fig. 4D).
Fig. 4.
Effect of weight loss on serum CC16 concentrations. We assessed the effect of weight loss in the time course of 26 week achieved by three different intervention types: non-surgical conservative obesity complex therapy (Dietary Intervention, n = 48), surgical sleeve gastrectomy (n = 22) and surgical gastric bypass (n = 28). Data indicate that the patients’ BMI drastically decreased (A-C) and metabolic profiles improved (data not shown) due to either dietary or surgical intervention. After surgical intervention, alterations are overall accompanied by a mild decrease in serum CC16 (E + F), while there were no differences in concentrations at the end of the dietary intervention program (D). Statistical significance was tested using univariate paired Wilcoxon-tests.
Analysis of variable importance and relations by random forest
Through the present primary analysis, we identified 23 variables related or possibly related to CC16 serum concentrations comprising anthropometric measures, metabolic biomarkers, the presence of metabolic and cardiovascular diseases, as well as the adjusted Healthy Eating Index as an overall measure of nutritional status and several measures of gut microbial alpha- and beta-diversity. To validate the above described findings, with an additional analysis of these variables using random forest-based machine learning, we assessed their relationships, both regarding their influence on CC16 levels and among potential confounders. A detailed description is provided in Supplement S3. In brief, by this machine learning analysis, main findings regarding the association of CC16 and age, biological sex, menopausal status in women, uricosuric medication and the presence of structural heart disease could be confirmed whereby this analysis also enabled deeper insights into the underlying interactions of assessed features.
Discussion
With our study, we provide evidence that CC16, a protein secreted almost exclusively from the lungs, is not only affected by pulmonary pathologies but also by metabolic and cardiovascular conditions. We would like to point out that the effects of obesity and A38G polymorphism found in the present study were similar to the results of a recent report which used 4 independent cohorts [9]. This indicates that our FoCus cohort data are valid and suitable for a broader CC16 metabolic characterization. In the present study, we showed that beyond obesity, high waist-to-hip ratio, representative of an unfavorable body fat distribution, had the strongest decreasing effect on circulating CC16 levels that was robust against important modifiable and non-modifiable confounders. On the contrary, chronic heart failure robustly increased serum CC16. With these findings we strengthen the presence of complex interactions among metabolism, heart and lungs on a systems biology level that possibly contribute to the worse outcome of subjects with chronic and acute pulmonary diseases when additionally suffering from obesity [4] and/or structural cardiovascular diseases [27].
Previous studies already showed that the CC16 serum concentration depends on different non-modifiable determinants, including the biological age, gender and onset of menopause [28], [29]. In this context, Nomori et al. proposed a role of sex steroid hormones on CC16 synthesis and secretion by pulmonary club cells. They found CC16 in females to decrease from puberty and to increase after menopause compared to males [30]. These findings are in line with the present FoCus data further validating our results. We identified an overall positive correlation of CC16 with age, which, when accounted for the onset of menopause in women, showed that previously lower levels were upregulated in the post-menopausal state reaching those seen in men.
Due to the well-established impact of cigarette smoking on lung health, the effect of this behavior on CC16 levels has already been assessed in numerous studies that consistently displayed reduced CC16 in smokers compared to non-smokers [7], [28], [31]. This association was explained by the reduction of CC16-expressing club cells in the respiratory tract [32]. Guerra et al. further indicated the ability of regeneration of this implication after smoking cessation since former smokers and subjects who had never smoked did not show differences in CC16 serum levels [29]. Of interest, we were able to reproduce these findings within our study.
While smoking behavior is regarded a key trigger of pulmonary diseases, the diet and an altered gut microbiota have to date not been seen as such and, to the best of our knowledge, have not yet been assessed in relation to CC16. To initially evaluate the potential of the diet as a determining factor of CC16 serum levels, we calculated the Healthy Eating Index, which is a tool to measure a person’s adherence to an overall balanced and health-retaining diet [15], [33]. A low index has been linked to a variety of chronic diseases [34], but in our analysis does not affect circulating CC16. Further dietary evaluation along with the lack of change in CC16 during 6-month diet-based weight loss intervention, our data imply that the diet does not majorly contribute to alterations in CC16 serum concentrations. In contrast, the evaluation of gut microbial diversity revealed an association of low species richness and low CC16 serum concentrations. Like low diet quality, low diversity in the gut microbiota is linked to diverse health implications, including metabolic, cardiovascular and respiratory diseases [35]. Regarding the latter, emerging evidence indicates a gut-lung-axis associated to disease development via mutual alterations of immune responses [36] which agrees with our findings.
The intake of uricosuric agents, a medication administered to eliminate uric acid from the circulation was strongly associated to increased CC16 concentrations. Interestingly, high serum and pulmonary uric acid concentrations are currently being discussed in the pathophysiology of respiratory diseases, including pulmonary hypertension, COPD and lung cancer [37], [38], [39], [40]. This finding might point towards the impairment of epithelial club cells and therefore CC16 expression via the interaction with uric acid.
To account for these diverse impacts of both internal and external factors on CC16 serum levels, we evaluated the effects of metabolic and cardiovascular diseases using adjusted linear regression models. By that, we displayed a decrease in CC16 with increasing obesity severity, which was even more pronounced with visceral body fat deposition indicated by high waist-to-hip ratios. These findings are in line with those of Goudarzi et al. [9]. In their analysis, the authors also describe a reduced number of CC16-expressing cells in obese mice and humans, suggesting a potential mechanism [9]. In agreement with our results, previous studies showed that the body fat distribution pattern had a stronger impact on pulmonary function (measured by FEV(1) and FVC) than weight or BMI [41], [42]. In accordance with this finding, a recent study by Kashtanova et al. demonstrated that CC16 was, among other markers for lower respiratory tract damage, reduced in chronic bronchitis patients with additional abdominal obesity. Authors conclude that this effect could be due to the presence of another chronic inflammation source from adipose tissue [43]. In support, we revealed an association of CC16 to the pro-inflammatory adipokine wnt5a, a key regulator of metabolic inflammation associated to abdominal obesity [44].
Arterial hypertension is a very common comorbidity of obesity and was also associated with decreased CC16 concentrations. This effect diminished with the intake of anti-hypertensive ACEi/ARB medication which could point towards an interaction of CC16 and the renin‐angiotensin‐aldosterone system (RAAS). Of interest, we found strongly increased CC16 concentrations in structural cardiovascular diseases that were most pronounced in chronic heart failure - a disease characterized by an impaired RAAS with pathophysiological contribution through the initiation of pro-inflammatory, pro-fibrotic and pro-hypertrophic effects [45]. This could indicate a compensatory upregulation of CC16 expression in response to RAAS impairment. Though, it should be noted that a significant increase of CC16 in the circulation is not only seen as a representation of lung expression levels but rather an indicator of bronchoalveolar-blood barrier impairment [6].
Besides inflammation and cardiovascular implications, the induction of insulin resistance and manifestation of type 2 diabetes are also commonly seen in obesity. Yet, no alteration of CC16 appeared under the presence of type 2 diabetes proposing a differential immunological regulation in these metabolic diseases.
Individual CC16 serum levels may have clinical implications as multi-system disease risk marker. A recent study showed a correlation of CC16 with mortality in early phases of COVID-19 [46], which may give an example of the applicability of CC16 as biomarker in the future. CC16 would have to be correlated with solid disease specific and overall clinical endpoints such as exacerbation of COPD, myocardial infarction, and/or overall mortality. It would be useful to observe CC16 levels in large clinical trials, potentially from biobanked samples, to derive its performance as prognostic marker. High serum levels of CC16 may then drive individual drug therapy changes or intensification of therapy in general.
While we provide the first extensive cardio-metabolic assessment of CC16 using cohort data that include over 500 human subjects, some limitations must be named: Apart from CC16 serum concentrations, no further data on the pulmonary function that could strengthen our findings was available. Furthermore, characterizing data from patients undergoing weight loss intervention was limited and did not allow for the adjustment of confounders, which impacts the significance of observed CC16 alterations.
Conclusions
In conclusion, we clearly present complex effects of metabolic and cardiovascular health implications on circulating levels of the anti-inflammatory, immunomodulatory marker CC16. We also demonstrate the modifiability of CC16 by selected behavioral and pharmacological interventions, whereby the alteration through ACEi/ARB and uricosuric agents could pave the way for the identification of novel regulatory axes of clinical relevance. Altogether, since circulating CC16 origins almost exclusively from pulmonary club cells, our findings strengthen the presence of interactions among metabolism, heart and lungs that should be considered in disease prevention and management. Further studies and especially the extension to functional testing are needed.
Funding information
This study was supported by the Bundesministerium für Bildung und Forschung (FoCus – Food Chain Plus (0315539A), the Bundesministerium für Ernährung und Landwirtschaft, the JPI HDHL (BIONUGUT: 2816ERA14E; INTIMIC: 01EA1906A) and by the Else Kröner-Fresenius-Stiftung (EKFS, Number 2018_Kolleg.01 – Clinician Scientist).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.06.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes/Metabolism Res Rev 2021;37:e3377. 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed]
- 2.Sharma V, Cowan DC. Obesity, Inflammation, and Severe Asthma. An Update. Current allergy and asthma reports 21, 46; 10.1007/s11882-021-01024-9 (2021). [DOI] [PMC free article] [PubMed]
- 3.Hanson C, Rutten EP, Wouters EFM, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chronic Obstructive Pulmonary Disease 2014;9:723–733; 10.2147/COPD.S50111 (2014). [DOI] [PMC free article] [PubMed]
- 4.Brock JM, Billeter A, Müller-Stich BP, Herth F. Obesity and the Lung. What We Know Today. Respiration; international review of thoracic diseases 99, 856–866; 10.1159/000509735 (2020). [DOI] [PubMed]
- 5.Khateeb J, Fuchs E, Khamaisi M, Diabetes and Lung Disease. A Neglected Relationship. The review of diabetic studies: RDS 15, 1–15; 10.1900/RDS.2019.15.1 (2019). [DOI] [PMC free article] [PubMed]
- 6.Almuntashiri S., Zhu Y., Han Y., Wang X., Somanath P.R., Zhang D. Club cell secreted protein CC16. Potential applications in prognosis and therapy for pulmonary diseases. JCM. 2020;9(4039) doi: 10.3390/jcm9124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shijubo N, Itoh Y, Yamaguchi T, Shibuya Y, Morita Y, Hirasawa M et al. Serum and BAL Clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respirat J 1997;10:1108–1114; 10.1183/09031936.97.10051108 (1997). [DOI] [PubMed]
- 8.Wang X.-Y., Keefe K.M., Jensen-Taubman S.M., Yang D., Yan K., Linnoila R.I., et al. Novel method for isolation of murine clara cell secretory protein-expressing cells with traces of stemness. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goudarzi H., Kimura H., Kimura H., Makita H., Matsumoto M., Takei N., et al. Effects of obesity on CC16 and their potential role in overweight/obese asthma. Respir Res. 2022;23(1) doi: 10.1186/s12931-022-02038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisler C., Schlicht K., Knappe C., Rohmann N., Hartmann K., Türk K., et al. Cohort profile. The food chain plus (FoCus) cohort. Eur J Epidemiol. 2022;37(10):1087–1105. doi: 10.1007/s10654-022-00924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment. Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(412–419) doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Boeing H., Wahrendorf J., Becker N. EPIC-Germany–A source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Ann Nutr Metab. 1999;43(195–204) doi: 10.1159/000012786. [DOI] [PubMed] [Google Scholar]
- 13.Noethlings U., Hoffmann K., Bergmann M.M., Boeing H. Portion size adds limited information on variance in food intake of participants in the EPIC-Potsdam study. J Nutr. 2003;133(2):510–515. doi: 10.1093/jn/133.2.510. [DOI] [PubMed] [Google Scholar]
- 14.Nöthlings U., Hoffmann K., Bergmann M.M., Boeing H. Fitting portion sizes in a self-administered food frequency questionnaire. J Nutr. 2007;137(12):2781–2786. doi: 10.1093/jn/137.12.2781. [DOI] [PubMed] [Google Scholar]
- 15.von Rüsten A., Boeing H., Flothkötter M. [The evaluation of food intake using a “Healthy Eating Index”]. German: Die Bewertung der Lebensmittelaufnahme mittels eine “Healthy Eating Index”. Ernährungsumschau. 2009:450–456. [Google Scholar]
- 16.Pisani A., Rausch P., Bang C., Ellul S., Tabone T., Marantidis Cordina C., et al. Dysbiosis in the gut microbiota in patients with inflammatory bowel disease during remission. Microbiology spectrum. 2022;10(3) doi: 10.1128/spectrum.00616-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautmann T., Bang C., Franke A., Vincent D., Reinshagen K., Boettcher M. The impact of oral sodium chloride supplementation on thrive and the intestinal microbiome in neonates with small bowel ostomies a prospective cohort study. Front. Immunol. 2020;11(1421) doi: 10.3389/fimmu.2020.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N., et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schloss P.D., Quackenbush J. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS One. 2009;4(12) doi: 10.1371/journal.pone.0008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(5261–5267) doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laing Ingrid A., Hermans Cedric, Bernard Alfred, Burton Paul R., Goldblatt Jack, Lesouëf Peter N. Association between plasma CC16 levels, the A38G polymorphism, and asthma. Am J Respir Crit Care Med. 2000;161(1):124–127. doi: 10.1164/ajrccm.161.1.9904073. [DOI] [PubMed] [Google Scholar]
- 22.Ku M-S, Sun H-L, Lu K-H, Sheu J-N, Lee H-S, Yang S-F et al. The CC16 A38G polymorphism is associated with the development of asthma in children with allergic rhinitis. Clin Exp Allergy: J Brit Soc Allergy Clin Immunol 41, 794–800; 10.1111/j.1365-2222.2010.03679.x (2011). [DOI] [PubMed]
- 23.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27, 325–349; 10.2307/1942268 (1957).
- 24.Jaccard P. The distribution of the flora in the alpine zone.1. New Phytol. 1912;11(2):37–50. [Google Scholar]
- 25.Tremblay M.S., Colley R.C., Saunders T.J., Healy G.N., Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35(6):725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 26.Gomes A.C., Hoffmann C., Mota J.F. The human gut microbiota. Metabolism and perspective in obesity. Gut Microbes. 2018;9(308–325) doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramalho S.H.R., Shah A.M. Lung function and cardiovascular disease. A link. Trends Cardiovasc Med. 2021;31(2):93–98. doi: 10.1016/j.tcm.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomas D.A., Silverman E.K., Edwards L.D., Miller B.E., Coxson H.O., Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63(12):1058–1063. doi: 10.1136/thx.2008.102574. [DOI] [PubMed] [Google Scholar]
- 29.Guerra S., Halonen M., Vasquez M.M., Spangenberg A., Stern D.A., Morgan W.J., et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3(8):613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomori H., Horio H., Fuyuno G., Kobayashi R., Morinaga S., Hirabayashi Y. Protein 1 (Clara cell protein) serum levels in healthy subjects and patients with bacterial pneumonia. Am J Respir Crit Care Med. 1995;152(2):746–750. doi: 10.1164/ajrccm.152.2.7633737. [DOI] [PubMed] [Google Scholar]
- 31.Hermans C., Aly O., Nyberg B.-I., Peterson C., Bernard A. Determinants of Clara cell protein (CC16) concentration in serum: a reassessment with two different immunoassays. Clin Chim Acta. 1998;272(2):101–110. doi: 10.1016/s0009-8981(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 32.Broeckaert F., Clippe A., Knoops B., Hermans C., Bernard A. Clara Cell secretory protein (CC16). Features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923(68–77) doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 33.Krebs-Smith S.M., Pannucci T.E., Subar A.F., Kirkpatrick S.I., Lerman J.L., Tooze J.A., et al. Update of the healthy eating index. HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morze J., Danielewicz A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes. A second update of a systematic review and meta-analysis of cohort studies. J Acade Nutr Diet. 2020;120(12):1998–2031.e15. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 35.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases. A review. Antonie Van Leeuwenhoek. 2020;113(12):2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 36.Enaud R., Prevel R., Ciarlo E., Beaufils F., Wieërs G., Guery B., et al. The gut-lung axis in health and respiratory diseases a place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect, Microbiol. 2020;10(9) doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savale L., Akagi S., Tu L.y., Cumont A., Thuillet R., Phan C., et al. Serum and pulmonary uric acid in pulmonary arterial hypertension. Eur Respir J. 2021;58(2):2000332. doi: 10.1183/13993003.00332-2020. [DOI] [PubMed] [Google Scholar]
- 38.Kahnert K., Alter P., Welte T., Huber R.M., Behr J., Biertz F., et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD. A multi-dimensional approach. Respir Res. 2018;19(1) doi: 10.1186/s12931-018-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanwar Y., Singh C., Chakrabarty S. Comparison of serum uric acid levels in patients with stable chronic obstructive pulmonary disease and patients with acute exacerbation. J Assoc Physicians India. 2022;70:11–12. [Google Scholar]
- 40.Horsfall L.J., Hall I.P., Nazareth I. Serum urate and lung cancer. A cohort study and Mendelian randomization using UK Biobank. Respir Res. 2021.1093/aje/kwx246.;22(179):10. doi: 10.1186/s12931-021-01768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leone N., Courbon D., Thomas F., Bean K., Jégo B., Leynaert B., et al. Lung function impairment and metabolic syndrome. The critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 42.Ochs-Balcom H.M., Grant B.J.B., Muti P., Sempos C.T., Freudenheim J.L., Trevisan M., et al. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129(4):853–862. doi: 10.1378/chest.129.4.853. [DOI] [PubMed] [Google Scholar]
- 43.Kashtanova EV, Polonskaya YV, Striukova EV, Shcherbakova LV, Kurtukov EA, Shramko VS et al. Blood levels of indicators of lower respiratory tract damage in chronic bronchitis in patients with abdominal obesity. Diagnostics (Basel, Switzerland) 12; 10.1038/s41598-018-25321-y (2022). [DOI] [PMC free article] [PubMed]
- 44.Relling I., Akcay G., Fangmann D., Knappe C., Schulte D.M., Hartmann K., et al. Role of wnt5a in Metabolic Inflammation in Humans. J Clin Endocrinol Metab. 2018;103(11):4253–4264. doi: 10.1210/jc.2018-01007. [DOI] [PubMed] [Google Scholar]
- 45.Ames M.K., Atkins C.E., Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33(2):363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiezzi M, Morra S, Seminerio J, van Muylem A, Godefroid A, Law-Weng-Sam N et al. SP-D and CC-16 Pneumoproteins' kinetics and their predictive role during SARS-CoV-2 infection. Front Med 8, 761299; 10.3389/fmed.2021.761299 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.