Abstract
Background
Chlorpyrifos (CPF) is a widely used pesticide in the production of plant crops. Despite rapid CPF biodegradation, fish were exposed to wastewater containing detectable residues. Recently, medicinal plants and algae were intensively used in aquaculture to replace antibiotics and ameliorate stress impacts.
Methods and results
An indoor experiment was conducted to evaluate the deleterious impacts of CPF pollution on Nile tilapia health and the potential mitigation role of Chlorella vulgaris algae. Firstly, the median lethal concentration LC50 − 72 h of CPF was determined to be 85.8 µg /L in Nile tilapia (35.6 ± 0.5 g body weight) at a water temperature of 27.5 °C. Secondly, fish were exposed to 10% of LC50 − 72 h for six weeks, and tissue samples were collected and examined every two weeks. Also, Nile tilapia were experimentally infected with Streptococcus agalactiae. Exposed fish were immunosuppressed expressed with a decrease in gene expressions of interleukin (IL) 1β, IL-10, and tumor necrosis factor (TNF)-α. Also, a decline was recorded in glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) gene expression in the head kidney tissue. A high mortality rate (MR) of 100% was recorded in fish exposed to CPF for six weeks and challenged with S. agalactiae. Fish that received dietary C. vulgaris could restore gene expression cytokines and antioxidants compared to the control. After six weeks of CPF exposure, fish suffered from anemia as red blood cell count (RBCs), hemoglobin (Hb), and packed cell volume (PCV) significantly declined along with downregulation of serum total protein (TP), globulin (GLO), and albumin (ALB). Liver enzymes were significantly upregulated in fish exposed to CPF pollution, alanine aminotransferase (ALT) (42.5, 53.3, and 61.7 IU/L) and aspartate aminotransferase (AST) (30.1, 31.2, and 22.8) after 2, 4, and 6 weeks, respectively. On S. agalactiae challenge, high MR was recorded in Nile tilapia exposed to CPF (G3) 60%, 60%, and 100% in week 2, week 4, and week 6, and C. vulgaris provided a relative protection level (RPL) of 0, 14.29, and 20%, respectively.
Conclusions
It was concluded that CPF pollution induces immunosuppressed status, oxidative stress, and anemic signs in Nile tilapia. In contrast, C. vulgaris at a 50 g/kg fish feed dose could partially ameliorate such withdrawals, restoring normal physiological parameters.
Keywords: Antioxidant enzymes, Chlorpyrifos, Chlorella vulgaris, Cytokines, Oreochromis niloticus, Streptococcus agalactiae
Introduction
According to the latest FAO report, Nile tilapia (Oreochromis niloticus) is one of the highest-farmed fish species globally; Egypt became among the highest producers, ranking 11th [1].
Pesticides are widely used in plant crop production, and they can reach water streams and aquatic environments [2], allowing increasing bioaccumulation in the tissues of different aquatic animals [3]. Pesticides enter aquatic environments through their extensive agricultural and domestic use, and approximately 64% of agricultural water sheds worldwide are at risk of pesticide pollution [4].
Chlorpyrifos (CPF) [O, O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate] is considered one of the widely used organophosphate pesticides owing to the broad-spectrum of eradicating plant pests and eliminating mosquitoes, the released CPF contaminate the aquatic environment and remain for 8–53 days till decomposition [5]. CPF has highly absorbable properties via the gills, skin, and digestive system of aquatic animals; it bioaccumulates in their tissues (liver and kidney), and its residues have been discovered in farmed and wild fishes, hindering their normal metabolic functions and threatening their life, in addition to causing genotoxicity [6, 7].
Pesticide exposure suppresses fish immunity, adversely impacting cytokine gene expressions such as interleukin (IL)-1β, IL-8, and tumor necrosis factor (TNF)-α [8], making them vulnerable to infectious diseases [9]. Also, Pesticide bioaccumulation could generate reactive oxygen species (ROS), injuring different fish tissues, oxidative stress has recently been hypothesized to be the main mode of CPF toxicity. Antioxidant enzymes are released to detoxify generated ROS, such as glutathione-S transferase (GST), superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) to counteract oxidative damage [10]. The exposure of fish to pesticides is unavoidable, despite legal restrictions on the use of pesticides that can effectively reduce environmental contamination. Also, improving the biological and physiological status of the fish can ameliorate the toxic withdrawals of pesticides [11].
Pollution, antibiotics, and chemotherapy mainly affect the antioxidant status of aquatic animals. In the case of bacterial infection, the mortality rate, clinical signs, and treatment efficacy are impacted by the antioxidant-immune status of diseased fish. So, pollution and bacterial infection could act synergistically to increase the mortality rate and provoke more prominent clinical signs.
Some natural products could mitigate the impacts of several toxicants by increasing antioxidant capacity protecting fish tissues [12–19]. Supplementation with dietary lycopene, chlorella, or citric acid could fully or partially mitigate the impacts of environmental toxicants, which could enhance the antioxidant capacity in African sharp-tooth catfish (Clarias gariepinus) [20].
Chlorella vulgaris is a freshwater green algae that contains different components: 60% protein and 18 amino acids, fiber, vitamins, and minerals, in addition to bioactive substances such as antioxidants and chlorophylls. Recently, C. vulgaris became one of the most frequently used microalgae in aquatic animal diet formulation. Many studies have assessed the ability of C. vulgaris to improve growth, immune responses, and stress amelioration. Also, it combats disease resistance in fish by inhibiting bacterial quorum sensing [21, 22]. It was used as a feed additive for aquatic organisms at a rate of 2.5%, 0.5%, 7%, and 10% of fish feed [23, 24]. Dietary C. vulgaris was used in fish to alleviate the adverse impact of exposure to microplastic [25] and CPF [26], in addition to maintaining the growth performance and biochemical parameters. Inconsistent [27], chlorella algae effectively ameliorates the depressed responses of innate immune and oxidative stress caused by arsenic contamination, suggesting a potential therapeutic role. The antioxidant property of C. vulgaris can counteract sodium nitrite-induced toxicity and prevent oxidative stress [28].
This work is a trial to mitigate Nile tilapia’s immunosuppression and oxidative stress caused by CPF exposure. To counteract Streptococcus agalactiae infection and the adverse impacts of CPF on fish health, fish received C. vulgaris via their diets.
Materials and methods
Fish accommodation and experimental design
Healthy 360 Nile tilapia (Oreochromis niloticus), weighing 35.6 ± 0.4 g, were obtained from the local freshwater fish farm and directly transported to the Kafrelsheikh provincial wet laboratory of Animal Health Research Institute. In the wet laboratory, fish were acclimatized for 14 days in experimental conditions: water temperature, pH, and salinity were 27.5 ± 0.5 °C, 7.9 ± 0.1, and 0.48 ± 0.1 g/L, respectively. Day after day, only one-third of tank water was exchanged with unchlorinated clean water to maintain suitable water parameters. Fish feed was offered twice a day at 0.9.30 a.m. and 03.00 p.m. at a rate of 5% fish of body weight, fish feed composition (Table 1): crude protein 30.2%, digestible energy 3450 kcal/kg as recommended for Nile tilapia [29].
Table 1.
List of fish feed ingredients
| Ingredient | % | Ingredient | % |
|---|---|---|---|
| Corn | 24 | MCP | 1 |
| Soya (44%) | 33 | Salt | 0.15 |
| Fish meal | 21 | Methionine | 0.05 |
| DDGs | 4.5 | Choline chloride | 0.05 |
| Corn gluten | 15 | Minerals premix | 0.1 |
| Soya oil | 1 | Vitamins premix | 0.1 |
Note DDGs = Dried Distilled Grains
Fish were reared in water polluted with 10% of median lethal concentration (LC50) chlorpyrifos (CPF), a patent formulation manufactured by El Nasr Chemical Co., Egypt, 48% CPF, O, O-diethyl-O-(3,5,6-trichlor-2-pyridyl) phosphorothioate), for 2, 4, and 6 weeks. A trial to ameliorate CPF toxicity with dietary Chlorella vulgaris, which was provided the Faculty of Agriculture, Kafrelsheikh University. In four groups (G1–4) in triplicate, fish were randomly stocked into 12 glass aquaria (each aquarium measuring 80 × 40 × 40 cm, containing 30 fish). G1–2: Fish were fed on fed on C. vulgaris at a rate of 0 and 50 g/kg fish feed, respectively; the dose of C. vulgaris was recommended by Chen et al. [30] while G3–4 Fish were exposed to CPF (10% of LC50) and fed on C. vulgaris at a rate of 0 and 50 g/kg, respectively.
For sampling, fish was tranquilized by immersion in Tricaine methanesulfonate (MS-222) (SyncaineR, Syndel, Canada) at a dose of 40 mg/L water. Fish was euthanized by immersion in MS-222 solution of 250 mg/L water for 10 min following the methods described by Sherif et al. [31] and Eldessouki et al. [32].
Median lethal concentration
To experiment with CPF stress, its LC50 was detected following the procedure developed by Reed and Muench [33]. Briefly, three hundred and thirty (330) Nile tilapia were equally stocked in 33 glass aquariums (40 × 40 × 50 cm), fish sub-divided into eleven groups (group = 3 aquaria), and every group was subjected to different concentrations of CPF (100, 90, 80, 70, 60, 50, 40, 30, 20, 10, and 0.0 µg/L). Mortalities were recorded for 72 h.
Blood and serum analyses
Blood analyses of experimental Nile tilapia were performed using a hemocytometer and stain (Natt and Herrick) for red blood cell (RBC) and white blood cell (WBC) counts according to Stoskopf [34]. Hemoglobin content (Hb) was determined by the cyanmethemoglobin method [35] and packed cell volume (PCV) was measured using a centrifuge.
The serum of the experimental fish was examined for total protein (TP) [36], albumin (ALB), and globulin (GLO) [37]. Liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [38] were colorimetrically measured using ELISA, and the ELISA-kit reagents were supplied by Diamond Diagnostic Co. (Holliston, USA).
Gene expressions of antioxidant enzymes and immune
Gene expression in the head kidney of experimental Nile tilapia was performed for immunological cytokines interleukin (IL) 1β, IL-10, and tumor necrosis factor (TNF-α), as well as antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). In Table 2, all primer sequences are present based on the National Center for Biotechnology Information (NCBI) database. All primers and kits were supplied by Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Quantitative polymerase chain reaction qPCR was carried out in a thermal cycler (AbiPrism 7300) (Applied Biosystems, USA). The quantitative fold alterations in the examined genes were calculated in relation to β-actin mRNA (household gene) by the 2− DD CT method.
Table 2.
Primers for cytokines, antioxidants enzymes, and household gene
| Target gene | Primer sequence | Amplified segment Length |
Annealing temperature | Accession number |
|---|---|---|---|---|
| β-actin |
F: AGCAAGCAGGAGTACGATGAG R: TGTGTGGTGTGTGGTTGTTTTG |
135 bp | 58.5 °C 30 s | XM_003443127.5 |
| IL-1β |
F: T GCTGAGCACAGAATTCCAG R: GCTGTGGAGAAGAACCAAGC |
172 bp | 60 °C 30 s | XM_019365841.2 |
| IL-10 |
F: CTGCTAGATCAGTCCGTCGAA R: GCAGAACCGTGTCCAGGTAA |
94 bp | 60 °C 30 s | XM_013269189.3 |
| TNF-α |
F: CCAGAAGCACTAAAGGCGAAGA R: CCTTGGCTTTGCTGCTGATC |
82 bp | 59.9 °C 30 s | AY428948.1 |
| SOD |
F: GGTGCCCTGGAGCCCTA R: ATGCGAAGTCTTCCACTGTC |
377 | 56 °C 30 s | JF801727.1 |
| CAT |
F: TCCTGAATGAGGAGGAGCGA R: ATCTTAGATGAGGCGGTGATG |
232 | 56 °C 30 s | JF801726.1 |
| GPx |
F: CCAAGAGAACTGCAAGAACGA R: CAGGACACGTCATTCCTACAC |
237 | 58 °C 30 s | NM_001279711.1 |
Note interleukin (IL), tumor necrosis factor (TNF-α), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT)
Bacterial infection
At the end of each experimental period 2, 4, and 6 weeks, ten fish /group were injected intraperitoneally (IP) with pathogenic S. agalactiae with NCBI accession number (OL471408), and its median lethal dose (LD50) is 0.3 × 105 CFU/ml [39]. In addition, pure saline solution (0.65%) was injected into ten fish as negative controls [40]. The injected fish were observed for 14 days to record the mortality rate (MR).
 |
Whereas the relative protection level (RLP) was verified among the challenged fish following the equation [41]:
 |
Biosafety protocol
The experiment procedure followed the biosafety measures on the pathogen safety data sheets (Infectious substances–S. agalactiae, Pathogen Regulation Directorate [42].
Statistical examination
The impacts of CPF and C. vulgaris algae were statistically assessed on different health parameters of the experimental Nile tilapia. The mean and standard error of the collected values were obtained with the ANOVA test and Duncan’s Multiple Range using SPSS software version 22. Statistical significances were considered at P-values ≤ 0.05.
Results
Median lethal concentration (LC50)
The LC50-72 h was detected to be 85.8 µg /L for experimental Nile tilapia with body weight 35.6 ± 0.5 g at water temperature, pH, and salinity of 27.5 ± 0.5 °C, 7.9 ± 0.1, and 0.48 ± 0.1 g/L, respectively.
Immunological and antioxidants responses
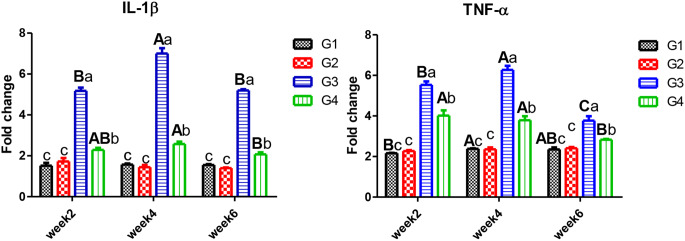
In Figs. 1 and 2, some cytokines IL-1β, TNF-α, and IL-10 gene expression were determined in the head kidney to assess CPF impacts on Nile tilapia’s immunity. Time trend, exposure to CPF (G3) resulted in pro-inflammatory IL-1β and TNF-α upsurge in four weeks after that declined after six weeks. There were no significant alterations in either controls or those who received C. vulgaris. After 2, 4, and 6 weeks of exposure, C. vulgaris supplementation decreased IL-1β, and TNF-α remained significantly higher than the control. The anti-inflammatory IL-10 gene expression had an opposite trend with pro-inflammatory IL-1β and TNF-α.
Fig. 1.
Gene expression of IL-1β and TNF-α in the head kidney of Nile tilapia. Note W2; week2, W4; week4, W6; week6. G1; control, G2; fish fed 50 g C. vulgaris /kg fish feed, G3; fish exposed to CPF (10% of LC50), G4; Fish exposed to CPF (10% of LC50) and fed 50 g C. vulgaris /kg fish feed. Different capital letters indicate significant difference based on the time factor in the same group, different small letters indicate significant difference based on the treatment factor within the same period at P ≤ 0.05
Fig. 2.
Gene expression of IL-10 and SOD in the head kidney of Nile tilapia. Note W2; week2, W4; week4, W6; week6. G1; control, G2; fish fed 50 g C. vulgaris /kg fish feed, G3; fish exposed to CPF (10% of LC50), G4; Fish exposed to CPF (10% of LC50) and fed 50 g C. vulgaris /kg fish feed. Different capital letters indicate significant difference based on the time factor in the same group, different small letters indicate significant difference based on the treatment factor within the same period at P ≤ 0.05
Antioxidants gene expression
Antioxidants enzymes, one of the defense mechanisms to combat the contaminants, SOD (Fig. 2) and GPx (Fig. 3), showed insignificant differences in the CPF and CPF plus C. vulgaris groups. In contrast, those exposed to CPF significantly decreased with time, and the supplementation with C. vulgaris significantly enhanced antioxidant status. With time, the antioxidant responses to CPF exposure remained low compared to the other groups. While gene expression of CAT (Fig. 3) was significantly increased with exposure and with time, it was also noted that C. vulgaris could reduce such an upsurge.
Fig. 3.
Gene expression of CAT and GPx in the head kidney of Nile tilapia. Note W2; week2, W4; week4, W6; week6. G1; control, G2; fish fed 50 g C. vulgaris /kg fish feed, G3; fish exposed to CPF (10% of LC50), G4; Fish exposed to CPF (10% of LC50) and fed 50 g C. vulgaris /kg fish feed. Different capital letters indicate significant difference based on the time factor in the same group, different small letters indicate significant difference based on the treatment factor within the same period at P ≤ 0.05
Blood analyses in Nile tilapia
In Table 3, blood indices confirmed the deleterious impacts of CPF exposure; values of RBCs, Hb, and PCV indicated Nile tilapia was anemic after six weeks of exposure (G3), and fish could restore normal values after six weeks of C. vulgaris supplementation (G4). On the contrary, WBCs were significantly increased on CPF exposure.
Table 3.
Blood and serum analyses of the experimental Nile tilapia
| Item | RBC×106 | WBCs ×103 | Hb g/dl | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| W2 |
2.43 ± 0.05ab |
2.5 ± 0.03a |
2.21 ± 0.12Ab |
2.34 ± 0.02ab |
59.57 ± 0.4c |
58.57 ± 1.47c |
70 ± 1.32a |
64.03 ± 0.87b |
8.23 ± 0.18ab |
8.51 ± 0.11a |
7.51 ± 0.4Ab |
7.94 ± 0.08ab |
| W4 |
2.62 ± 0.02a |
2.7 ± 0.04a |
1.96 ± 0.08Ac |
2.28 ± 0.14b |
59.7 ± 1.06b |
58.4 ± 1.25b |
68.1 ± 1.35a |
63 ± 1.71b |
8.89 ± 0.08a |
9.17 ± 0.15a |
6.65 ± 0.27Ac |
7.76 ± 0.46b |
| W6 |
2.45 ± 0.13a |
2.57 ± 0.14a |
1.61 ± 0.02Bb |
2.27 ± 0.04a |
57.7 ± 1.2c |
56 ± 1.2c |
66.7 ± 0.5a |
62.7 ± 1.24b |
8.32 ± 0.46a |
8.74 ± 0.47a |
5.46 ± 0.08Bb |
7.71 ± 0.12a |
| PCV % | TP g/dL | GLO g/dL | ||||||||||
| W2 |
25.87 ± 0.54ab |
26.65 ± 0.38a |
23.55 ± 1.23Ab |
24.9 ± 0.26ab |
5.93 ± 0.27 |
5.67 ± 0.19B |
5 ± 0.42A |
5.2 ± 0.3A |
2.78 ± 0.25 |
2.51 ± 0.16B |
1.99 ± 0.4 |
2.05 ± 0.33A |
| W4 |
27.88 ± 0.25a |
28.75 ± 0.47a |
20.86 ± 0.87Ac |
24.3 ± 1.45b |
5.55 ± 0.18b |
6.26 ± 0.03Aa |
4.33 ± 0.07ABd |
4.83 ± 0.04Ac |
2.45 ± 0.16b |
3.17 ± 0.07Aa |
1.21 ± 0.06d |
1.73 ± 0.02ABc |
| W6 |
26.12 ± 1.43a |
27.4a ± 1.44 |
17.11 ± 0.26Bb |
24.2 ± 0.4a |
5.9 ± 0.06b |
6.38 ± 0.08Aa |
3.6 ± 0.15Bd |
4.18 ± 0.09Bc |
2.83 ± 0.09b |
3.33 ± 0.08Aa |
1.32 ± 0.07c |
1.08 ± 0.06Bc |
| ALB g/dL | ALT IU/L | AST IU/L | ||||||||||
| W2 |
3.15 ± 0.03 |
3.16 ± 0.12 |
3.01 ± 0.03A |
3.15 ± 0.03 |
27.4 ± 0.47c |
28.23 ± 0.5Ac |
42.5 ± 0.76Ba |
39.2 ± 0.6Ab |
31.5 ± 0.03 |
31.6 ± 0.12 |
30.1 ± 0.03A |
31.5 ± 0.03 |
| W4 |
3.1 ± 0.05 |
3.1 ± 0.04 |
3.12 ± 0.1A |
3.1 ± 0.03 |
28.3 ± 0.4c |
26.6 ± 0.66ABc |
53.3 ± 5.05Aa |
39 ± 1.1Ab |
31 ± 0.05 |
31. ± 0.04 |
31.2 ± 0.1A |
31 ± 0.03 |
| W6 |
3.07 ± 0.04a |
3.05 ± 0.04a |
2.28 ± 0.1Bb |
3.09 ± 0.06a |
27.8 ± 0.54c |
25.1 ± 0.32Bd |
61.7 ± 1Aa |
34.3 ± 0.67Bb |
30.7 ± 0.04a |
30.5 ± 0.04a |
22.8 ± 0.1Bb |
30.9 ± 0.06a |
Note W2; week2, W4; week4, W6; week6. G1; control, G2; fish fed 50 g C. vulgaris /kg fish feed, G3; fish exposed to CPF (10% of LC50), G4; Fish exposed to CPF (10% of LC50) and fed 50 g C. vulgaris /kg fish feed. Different capital letters indicate significant difference based on the time factor in the same group, different small letters indicate significant difference based on the treatment factor within the same period at P ≤ 0.05
In Table 3, after weeks of CPF exposure, serum TP, GLO, and ALB significantly decreased with time trend. After four weeks, fish exposed to CPF suffered from low TP and GLO with an insignificant decrease of ALB, whereas, after six weeks, all serum proteins declined, including ALB value.
Liver enzymes
In Table 3, liver enzymes ALT (42.5, 53.3, and 61.7 IU/L) and AST (30.1, 31.2, and 22.8) significantly increased after CPF exposure and increased with time, while the C. vulgaris supplementation could partially restore normal status as it was still higher than un-exposed fish ALT (39.2, 39, and 34.3 IU/L) and AST (31.5, 31, and 30.9 IU/L) in week 2, week 4, and week 6, respectively.
Bacterial infection with S. agalactiae
In Fig. 4, a low MR was recorded in Nile tilapia challenged with LD50 of S. agalactiae fed on a C. vulgaris supplemented diet 50, 50, and 40, compared to the control fish; 60, 50, and 60% in week 2, week 4, and week 6, respectively, giving an RPL of 16.67% (week2), 0 (week4), and 33.33% (week6). Nile tilapia exposed to CPF and fed on dietary C. vulgaris (G4) showed a decrease in MR 60%, 60, and 80%compared with those exposed to CPF (G3) 60%, 60%, 100% in week 2, week 4, and week 6, respectively. Dietary C. vulgaris provided a RPL of 0, 14.29, and 20% against CPF pollution for week 2, week 4, and week 6, respectively, and challenged with S. agalactiae.
Fig. 4.
Mortality rate (MR) and relative level of protection (RPL) of Nile tilapia challenged withS. agalactiae.(n = 10). Note: W2; week2, W4; week4, W6; week6. G1; control, G2; fish fed 50 g C. vulgaris /kg fish feed, G3; fish exposed to CPF (10% of LC50), G4; Fish exposed to CPF (10% of LC50) and fed 50 g C. vulgaris /kg fish feed. Different capital letters indicate significant difference based on the time factor in the same group, different small letters indicate significant difference based on the treatment factor within the same period at P ≤ 0.05
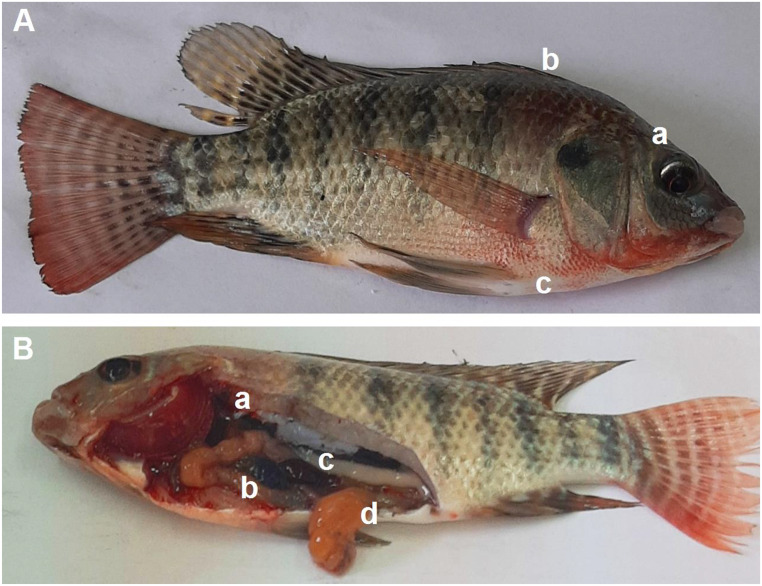
In Fig. 5 (A), Nile tilapia (G1) of the control group challenged with S. agalactiae, showed slight exophthalmia and skin hemorrhages (b, c). Post-mortem changes (B) were an empty intestinal tract, dark liver (a), distended gallbladder (b), splenomegaly (c), and partially empty intestine (d).
Fig. 5.
Nile tilapia (control group) infected with S. agalactiae.(A) Fish showed exophthalmia (a), skin hemorrhages dorsal (b) and ventral abdomen extended to mouth (c). (B) Fish showed dark liver (a), distended gall bladder with greenish content (b), splenomegaly (c), and partially empty intestine (d)
In Fig. 6 (A), CPF-exposed fish (G3) suffered from exophthalmia (a), friable liver (d), distended gallbladder with clear content (f), and splenomegaly (c), and empty intestinal tract (e).
Fig. 6.
Nile tilapia infected with S. agalactiae. (A) Fish exposed to CPF, exophthalmia (a), turbid air bladder (b), splenomegaly (c), friable liver (d), empty intestinal tract (e), distended gall bladder with light yellow-greenish content (f). (B) Fish exposed to CPF and fed on dietary C. vulgaris, exophthalmia (a), dark brownish liver (b), distended gall bladder with dark greenish content (c), empty clumped intestinal tract (d), and splenomegaly (e)
In Fig. 6 (B), fish exposed to CPF and received dietary C. vulgaris (G4) showed exophthalmia, dark-brownish liver, distended gallbladder with dark greenish content, splenomegaly, and empty clumped intestinal tract.
Discussion
In this study, the LC50-72 h of CPF was detected to be 85.8 µg /L for experimental Nile tilapia. Near to our findings, LC50-48 h was 90 µg/L of CPF for Nile tilapia [43] and LC50-96 h 105.3 µg/L ten spotted live-bearer (Cnesterodon decemmaculatus) [44]. The higher values recorded for the rohu (Labeo rohita) LC50-96 h were 442.8 µg CPF /L [45]. Whereas the lower values of LC50-48 h were 2.26 µg CPF /L for Pejerrey (Odontesthes bonariensis) [46] and 5.47 µg /L for the larvae of banded gourami (Trichogaster fasciata) [47]. These differences in LC50 value could be attributed to fish spp. as weight and physiological status or water conditions as temperature, salinity, and pH.
Sub-lethal concentrations of some contaminants drastically impact the immunity of aquatic animals, as many researchers found that gene expression of cytokines (IL-1β, IL-8, and TNF-α) was significantly altered [8, 48, 49].
In this study, exposure to 10% of CPF LC50 resulted in pro-inflammatory IL-1β and TNF-α upsurge in four weeks, while they declined after six weeks. The anti-inflammatory IL-10 gene expression had an opposite trend with pro-inflammatory. Similarly, gene expression of IL-1β was upsurged in the head kidney of Chinook salmon reared in water polluted with CPF at 7.3 µg/L [50]. In addition, common carp (Cyprinus carpio) exhibited similar responses of IL-1β expression at 1.16 and 11.6 µg/L of CPF for 40 days with declining after 20 days recovery period [51], with the same concentration and period TNF- α, IL-6, and IL-8 in both head kidney and spleen of common carp showed similar findings [52]. From our results, dietary C. vulgaris enhanced fish health by decreasing the exaggerated immune responses of IL-1β to CPF stress, whereas TNF-α remained significantly higher than the control. In consistence, CPF-induced toxicity in common carp resulted in high IL-10 and TNF-α expression genes that could be mitigated by using both dietary C. vulgaris [52]. Different findings, 10% dietary C. vulgaris to fish diets significantly increased the expression of splenic and hepatic IL-1β and TNF-α in Nile tilapia exposed to deltamethrin toxicity [53]. This difference could be due to the high concentration of the organophosphorus compound.
The experimental Nile tilapia exposed to CPF showed a decline in SOD and GPx gene expression in the head kidney, while CAT increased after six weeks of exposure. Similarly, SOD activity decreased in the freshwater gastropod (Bellamya bengalensis) on the 20th day, with a significant increase in CAT under the interactions of elevated water temperature and CPF contamination [54]. In addition, SOD activity was reduced in common carp exposed to CPF for 40 days in the spleen and head kidney at 1.16 and 11.6 µg/L, followed by an increase after 20 days of recovery [55]. In consistency with our findings, exposure of zebrafish (Danio rerio) to CPF resulted in the downregulation of SOD and GPx activities but with a decrease in CAT activity [56, 57]. These findings could be explained by the fact that the interaction of GPx with electrophilic compounds can inhibit its activity [58], such as the metabolite of CPF in zebrafish [59].
Antioxidant activities of SOD and CAT enzymes in living organisms could eliminate and neutralize the reactive oxygen species (ROS) generated in response to exposure to toxic compounds [60, 61]. In this work, C. vulgaris significantly enhances SOD and GPx gene expression and could reduce such an upsurge of CAT gene expression, counteracting the CPF impacts. These findings may be due to the composition of C. vulgaris, which contains flavonoids, tocopherols, chlorophyll, carotenoids, and polyphenols, which could combat generated oxidative stress resulting from exposure to streptozotocin [62] and penoxsulam herbicide [23] also, it contains β-glucan, which can induce growth and antioxidant enzymes in several aquatic animals [63].
In our findings, blood indices such as RBCs, Hb, and PCV indicated an anemic status of Nile tilapia after only two weeks of CPF exposure. On CPF exposure, a significant reduction in RBCs, Hb, and PCV values in Nile tilapia [64], Caspian brown trout (Salmo trutta caspius) at a concentration of 26 µg/L for 20 days [65]. Different explanations for previous findings include the increases in the rate of RBC damage or inhibition of its formation, which could be due to a decline of serum iron concentrations, thereby lowering Hb synthesis in common carp exposed to CPF [66], Mozambique tilapia (Oreochromis mossambicus) exposed to cadmium and CPF [67]. Another explanation is that RBC became more fragile on CPF exposure because of the generated oxidative stress that impacted erythrocyte membranes [68]. In contrast, Jaffer et al. [69] claimed that common carp exposed to CPF (52, 79, and 158 µg/L) did not alter after three successive weeks of exposure. Blood analyses of the experimental Nile tilapia revealed a significant increase in WBCs after CPF exposure. Similarly, WBCs, neutrophils, and lymphocytes were increased in fish exposed to different contaminants, such as pesticides, to cope with the generated immunosuppression status [70].
In this work, CPF exposure resulted in a decline of serum TP, GLO, and ALB. Similarly, exposure to CPF decreased the TP and GLO values in common carp [5]. Other reports supported these findings, and they mentioned that CPF could stimulate significant alterations in blood biochemical indices that could result in immune-compromised status in fish [5, 43]. Fish could restore normal blood indices values after six weeks of receiving dietary C. vulgaris. Similarly, Sayed et al. [21] reported that dietary C. vulgaris at a rate of 5% of fish feed for 15 days could enhance the serum total protein, globulin, and albumin of s in African sharp-tooth catfish impacted by the toxicity microplastics (500 mg/kg fish feed). Also, Galal et al. [23] stated that C. vulgaris could protect fish health by maintaining normal blood parameters of Nile tilapia exposed to sub-lethal concentrations of penoxsulam (herbicide).
The liver is the primary organ for detoxification and processing of toxicants, becoming the site of bioaccumulation, for example, CPF toxicity in Indian carp (Catla catla), Labeo rohita, and Cirrhinus mrigala [71]. In this work, liver enzymes ALT and AST significantly increased after CPF exposure. Similarly, an upsurge in serum AST and ALT values was found in Nile tilapia exposed to CPF acute toxicity [72], golden mahseer (Tor putitora) [73], and freshwater crayfish (Pontastacus leptodactylus) [74]. However, dietary C. vulgaris could partially decrease the serum liver enzymes of the experimental Nile tilapia; they were still higher than in the control ones (unexposed to CPF). Similar results were obtained with Nile tilapia exposed to diazinon-toxicity and received dietary Spirulina, Chlorella, or their mixture [75].
In this work, after the S. agalactiae challenge, Nile tilapia exposed to CPF exhibited higher MR than other groups and reached 100% after six weeks of exposure. Similarly, common carp exposed to pesticides (CPF) were vulnerable to infectious pathogens as they were stressed and immunosuppressed [6, 76]. Meanwhile, dietary C. vulgaris could lower MR% and raise the RPL%. Similarly, C. vulgaris has antibacterial properties against many Gram-positive and G-negative bacteria [77]. The decline of MR and high RLP could be anticipated after the previously mentioned results concerning the enhancements of immune-oxidative gene expression and blood analyses of Nile tilapia received dietary C. vulgaris. These findings could be due to bioactive compounds secreted by microalgae preventing microbial growth [20–23].
Experimental Nile tilapia, challenged against S. agalactiae, harbored signs of septicemic diseases such as skin hemorrhages and exophthalmia, whereas post-mortem lesions were empty intestinal tract, distended gallbladder, and splenomegaly. Similarly, Nile tilapia infected with S. agalactiae had signs of bacterial septicemia such as exophthalmia, splenomegaly, hepatomegaly, and distended gall bladder [37, 78].
Conclusion
CPF stress could be evaluated in Nile tilapia by measuring gene expression of cytokines and antioxidant enzymes. Even C. vulgaris at a dose of 50 g/kg fish feed could ameliorate immunosuppression and oxidative impacts, but it could not fully help fish restore normal health parameters. The anemic status of Nile tilapia was the most prominent sign of stressed fish. Nile tilapia exposed to CPF and challenged against S. agalactiae had the highest mortalities compared to the control.
Author contributions
Walaa S. Tawfeek: Writing original draft, Formal analysis, Data curation. Amina S Kassab: Writing review & editing, Methodology. Eman T. Al-Sokary: Writing – review & editing. Mona E. Abass: Methodology, Formal analysis, Data curation. Ahmed H. Sherif: Writing original draft, Methodology.
Funding
Funding Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data is available on request from the corresponding author.
Code availability
Code availability Not applicable.
Declarations
Ethical approval
The above-described methodology was approved by the Ethics Committee at the Animal Health Research Institute and European Union directive 2010/63UE, and all methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). This paper does not contain any studies with human participants by any of the authors. No specific permissions were required for access to the artificial pond in wet laboratory Animal Health Research Institute, Kafrelsheikh, Egypt. The field studies did not involve endangered or protected species.
Consent to participate
not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO (2022) World fisheries and aquaculture food and Agriculture Organization. Rome 1–244. https://www.fao.org/documents/card/en/c/cc0461en
- 2.Soliman NF, Yacout DM (2016) Aquaculture in Egypt: status, constraints and potentials. Aquacult Int 24:1201–1227 [Google Scholar]
- 3.Storelli MM (2008) Potential human health risks from metals (hg, cd, and pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46:2782–2788 [DOI] [PubMed] [Google Scholar]
- 4.Tang FHM, Lenzen M, McBratney A, Maggi F (2021) Risk of pesticide pollution at the global scale. Nat Geosci 14:206–210 [Google Scholar]
- 5.Lu J, Wu L, Newman J, Faber B, Merhaut DJ, Gan J (2006) Sorption and degradation of pesticides in nursery recycling ponds. J Environ Qual 35(5):1795–1802. 10.2134/jeq2006.0123 [DOI] [PubMed] [Google Scholar]
- 6.Hatami M, Banaee M, Nematdoost Haghi B (2019) Sub-lethal toxicity of chlorpyrifos alone and in combination with polyethylene glycol to common carp (Cyprinus carpio). Chemosphere 219:981–988. 10.1016/j.chemosphere.2018.12.077 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Liu L, Zhang Y, Fang Q, Li Y, Li Y (2013) Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio). Chemosphere 93(2):428–433 [DOI] [PubMed] [Google Scholar]
- 8.Deb N, Das S (2013) Chlorpyrifos toxicity in fish: a review. Curr World Environ 8:77–84 [Google Scholar]
- 9.Secombes C (2002) Cloning and bioactivity of fish cytokines and their relevance to disease resistance. Bull -Eur Assoc Fish Pathol 22:110–116 [Google Scholar]
- 10.Kumar N, Prabhu PAJ, Pal A, Remya S, Aklakur M, Rana R, Gupta S, Raman R, Jadhao S (2011) Anti-oxidative and immuno-hematological status of Tilapia (Oreochromis mossambicus) during acute toxicity test of endosulfan. Pestic Biochem Physiol 99:45–52 [Google Scholar]
- 11.Yalsuyi AM, Vajargah MF, Hajimoradloo A, Galangash MM, Proki´c MD, Faggio C (2021) Evaluation of behavioral changes and tissue damages in common carp (Cyprinus carpio) after exposure to the herbicide glyphosate. Vet Sci 8(10):218. 10.3390/vetsci8100218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherif AH, Mahfouz ME (2019) Immune status of Oreochromis niloticus experimentally infected with Aeromonas hydrophila following feeding with 1, 3 β-glucan and levamisole immunostimulants. Aquaculture 509:40–46. 10.1016/j.aquaculture.2019.05.016 [Google Scholar]
- 13.Abdallah AA, El-Deen N, El-Aziz NA, Heba A, Neamat-Allah I, A. N (2020) Effect of the aqueous root extract of Curcuma longa L. (turmeric) against thermally oxidized oil-induced hematological, biochemical and histopathological alterations. Comp Clin Path 29(4):837–845 [Google Scholar]
- 14.Sherif AH, Al-Sokary ET, Rizk WF, Mahfouz ME (2020) Immune status of Oreochromis niloticus subjected to long-term lead nitrate exposure and a Arthrospira platensis treatment trial. Environ Toxicol Pharmacol 76:103352. 10.1016/j.etap.2020.103352 [DOI] [PubMed] [Google Scholar]
- 15.Arunachalam KD, Kuruva JK, Pradhoshini KP, Musthafa MS, Faggio C (2021) Antioxidant and antigenotoxic potential of Morinda Tinctoria Roxb. Leaf extract succeeding cadmium exposure in Asian catfish, Pangasius sutchi. Comp Biochem Physiol Part C Toxicol Pharmacol 249:109149. 10.1016/j.cbpc.2021.109149 [DOI] [PubMed] [Google Scholar]
- 16.Sharma R, Jindal R, Faggio C (2021) Cassia fistula ameliorates chronic toxicity of cypermethrin in Catla catla. Comp Biochem Physiol Part C Toxicol Pharmacol 248:109113. 10.1016/j.cbpc.2021.109113 [DOI] [PubMed] [Google Scholar]
- 17.Sherif AH, Gouda MY, Al-Sokary ET, Elseify MM (2021) Lactobacillus plantarum enhances immunity of Nile tilapia Oreochromis Niloticus challenged with Edwardsiella tarda. Aquac Res 52(3):1001–1012. 10.1111/are.14955 [Google Scholar]
- 18.Sinha R, Jindal R, Faggio C (2021) Protective effect of Emblica officinalis in Cyprinus carpio against hepatotoxicity induced by malachite green: ultrastructural and molecular analysis. Appl Sci 11(8):3507. 10.3390/app11083507 [Google Scholar]
- 19.Sherif AH, Prince A, Adel Seida A, Saad Sharaf M, Eldessouki EA, Harfoush MA (2022) Moringa oleifera mitigates oxytetracycline stress in Oreochromis niloticus. Aquac Res 53(5):1790–1799. 10.1111/are.15707 [Google Scholar]
- 20.Sayed AEDH, Hana MN, Hamed M, Abdel-Latif HM, Lee JS, Soliman HA (2023) Protective efficacy of dietary natural antioxidants on microplastic particles-induced histopathological lesions in African catfish (Clarias gariepinus). Environ Sci Pollut Res 30(9):24424–24440. 10.1007/s11356-022-23789-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicula M, Pacala N, Stef L, Pet I, Dronca D, Ahmadi M, Gherbon A (2018) Garlic and chlorella biomodulate lead toxicity on manganese homeostasis in Carassius gibelio Bloch. Rev Chim 69:986–989 [Google Scholar]
- 22.Sayed AEDH, Hamed M, Badrey AE, Soliman HA (2021) Bioremediation of hemotoxic and oxidative stress induced by polyethylene microplastic in Clarias gariepinus using lycopene, citric acid, and chlorella. Comp Biochem Physiol Part - C: Toxicol Pharmacol 250:109189. 10.1016/j.cbpc.2021.10919 [DOI] [PubMed] [Google Scholar]
- 23.Sergejevová M, Masojídek J (2012) Chlorella biomass as feed supplement for freshwater fish: sterlet, a cipenser ruthenus. Aquac Res 44(1):157–159 [Google Scholar]
- 24.Galal AA, Reda RM, Mohamed AAR (2018) Influences of Chlorella vulgaris dietary supplementation on growth performance, hematology, immune response and disease resistance in Oreochromis niloticus exposed to sub-lethal concentrations of penoxsulam herbicide. Fish Shellfish Immunol 77:445–456 [DOI] [PubMed] [Google Scholar]
- 25.Sayed EDH, Hamed M, Ismail RF (2022) Natural antioxidants can improve microplastics-induced male reproductive impairment in the African catfish (Clarias gariepinus). Front Environ Sci 9:811466 [Google Scholar]
- 26.Abu-Srea H, Risha E, Zahran E, Abdalla O (2018) Protective effects of Chlorella Vulgaris Dietary supplementation on growth performance, hematological and biochemical parameters in Nile tilapia (Oreochromis niloticus) exposed to Chlorpyrifos. Ann Vet Anim Sci 5:1–12 [Google Scholar]
- 27.Abbas N, El-shafei R, Zahran E, Amer M (2020) Some pharmacological studies on Chlorella vulgaris in tilapia fish. Kafrelsheikh Vet Med J 18:6 [Google Scholar]
- 28.Eissa MM, Ahmed MM, Abd Eldaim MA, Orabi SH, Elbaz HT, Mohamed MA et al (2020) Methanolic Extract of Chlorella Vulgaris protects against Sodium Nitrite-Induced Reproductive toxicity in male rats. Andrologia 52:e13811. 10.1111/and.13811 [DOI] [PubMed] [Google Scholar]
- 29.NRC (2011) Nutrient requirements of fish and shrimp. National Academy, Washington, DC [Google Scholar]
- 30.Chen W, Gao S, Huang Y, Chang K, Zhao X (2022) Addition of Chlorella sorokiniana meal in the diet of juvenile rainbow trout (Oncorhynchus mykiss): Influence on fish growth, gut histology, oxidative stress, immune response, and disease resistance against Aeromonas salmonicida Fish Shellfish Immunol. 129:243–250 [DOI] [PubMed]
- 31.Sherif AH, Eldessouki EA, Sabry NM, Ali NG (2023) The protective role of iodine and MS-222 against stress response and bacterial infections during Nile tilapia (Oreochromis niloticus) transportation. Aquac Int 1–16. 10.1007/s10499-022-00984-7
- 32.Eldessouki EA, Salama SSA, Mohamed R, Sherif AH (2023) Using Nutraceutical to alleviate transportation stress in the Nile tilapia. Egypt J Aquat Biol Fish 27(1):413–429. 10.21608/ejabf.2023.287741 [Google Scholar]
- 33.Reed LJ, Muench H (1938) Simple method of estimating 50% endpoint. Am J Hyg 27:493–497 [Google Scholar]
- 34.Stoskopf MK (1993) Fish Medicine. W.B. Saunders Company, London [Google Scholar]
- 35.Drubkin D (1964) Spectrophotometeric methods XIV. The crystographic and optical properties of the haemoglobin of man in comparison with those of other species. J Biol Chem 164:703–723 [PubMed] [Google Scholar]
- 36.Weichsellbaum TE (1946) An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol 16(3):40–49. 10.1093/ajcp/16.3_ts.40 [PubMed] [Google Scholar]
- 37.Doumas BT, Waston WA, Biggs HG (1971) Albumin standards and the measurements of serum albumin with bro-mocresol green. Clin Chim Acta 31:87–96. 10.1016/0009-8981(71)90365-2 [DOI] [PubMed] [Google Scholar]
- 38.Reitman S, Frankel S (1957) Determination of AST and ALT in serum. Am J Clin Pathol 28:56–56 [DOI] [PubMed] [Google Scholar]
- 39.Sherif AH, Abdellatif JI, Elsiefy MM, Gouda MY, Mahmoud AE (2022) Occurrence of infectious Streptococcus agalactiae in the farmed Nile tilapia. Egypt J Aquat Biol Fish 26(3):403–432. 10.21608/ejabf.2022.243162 [Google Scholar]
- 40.Boijink CL, Brandao DA, Vargas AC, Costa MM, Renosto AV (2001) Inoculac¸a˜o de suspensa˜o bacteriana de Plesiomonas shigelloides em jundia´, Rhamdia quelen (teleostei: pimelodidae). Cienc Rural 31(3):497–501. 10.1590/S0103-84782001000300023 [Google Scholar]
- 41.Ruangroupan L, Kitao T, Yoshida T (1986) Protective efficacy of Aeromonas hydrophila vaccines in Nile tilapia. Vet Immunol Immunopathol 12(1–4):345–350. 10.1016/0165-2427(86)90139-X [DOI] [PubMed] [Google Scholar]
- 42.Public Health Agency of Canada (2010) The honourable Leona Aglukkaq. P.C., M.P. Minister of Health
- 43.Majumder R, Kaviraj A (2018) Acute and sublethal effects of organophosphate insecticide chlorpyrifos on freshwater fish Oreochromis niloticus. Drug Chem Toxicol 26:1–9. 10.1080/01480545.2018.1425425 [DOI] [PubMed] [Google Scholar]
- 44.Paracampo A, Solis M, Bonetto C, Mugni H (2015) Acute toxicity of chlorpyrifos to the non-target organism Cnesterodon decemmaculatus. Int J Environ Health Res 25(1):96–103. 10.1080/09603123.2014.903903 [DOI] [PubMed] [Google Scholar]
- 45.Ismail M, Ali R, Shahid M, Khan MA, Zubair M, Ali T, Mahmood Khan Q (2018) Genotoxic and hematological effects of chlorpyrifos exposure on freshwater fish Labeo rohita. Drug Chem Toxicol 41(1):22–26. 10.1080/01480545.2017.1280047 [DOI] [PubMed] [Google Scholar]
- 46.L´opez Aca V, Gonzalez PV, Carriquiriborde P (2018) Lethal and sublethal responses in the fish, Odontesthes bonariensis, exposed to chlorpyrifos alone or under mixtures with endosulf´an and lambda-cyhalothrin. Ecotoxicology 27(7):968–979. 10.1007/s10646-018-1941-5 [DOI] [PubMed] [Google Scholar]
- 47.Sumon KA, Saha SV, Peeters ET, Bosma RH, Rashid H (2017) Acute toxicity of chlorpyrifos to embryo and larvae of banded gourami Trichogaster fasciata. J Environ Sci Health Part B 52(2):92–98. 10.1080/03601234.2016.1239979 [DOI] [PubMed] [Google Scholar]
- 48.Sherif AH, Alsokary ET, Esam HA (2019) Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila infection in Oreochromis niloticus. J Hellenic Vet Med Soc 70(3):1697–1706. 10.12681/jhvms.21796 [Google Scholar]
- 49.Sherif AH, Elshenawy AM, Attia AA, Salama SAA (2021) Effect of aflatoxin B1 on farmed Cyprinus carpio in conjunction with bacterial infection. J Aquat Biol Fish 25(2):465–485. 10.21608/EJABF.2021.164686 [Google Scholar]
- 50.Eder KJ, Leutenegger CM, Köhler H-R, Werner I (2009) Effects of neurotoxic insecticides on heat-shock proteins and cytokine transcription in Chinook salmon (Oncorhynchus tshawytscha). Ecotoxicol Environ Saf 72:182–190 [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Xing H, Li X, Xu S, Wang X (2011) Effects of atrazine and chlorpyrifos on the mRNA levels of IL-1 and IFN-(2b in immune organs of common carp. Fish Shellfish Immunol 31:126–133 [DOI] [PubMed] [Google Scholar]
- 52.Chen D, Zhang Z, Yao H, Cao Y, Xing H, Xu S (2014) Pro-and anti-inflammatory cytokine expression in immune organs of the common carp exposed to atrazine and chlorpyrifos. Pestic Biochem Physiol 114:8–15 [DOI] [PubMed] [Google Scholar]
- 53.Mahmoud EA, El-Sayed BM, Mahsoub YH, El-Murr AI, Neamat-Allah ANF (2020) Effect of Chlorella vulgaris enriched diet on growth performance, hematoimmunological responses, antioxidant and transcriptomics profile disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 102:422–429 [DOI] [PubMed] [Google Scholar]
- 54.Baag S, Mahapatra S, Mandal S (2021) An integrated and multibiomarker approach to delineate oxidative stress status of Bellamya bengalensis under the interactions of elevated temperature and chlorpyrifos contamination. Chemosphere 262(2):128512. 10.1016/j.chemosphere.2020.128512 [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Xing H, Jiang Y, Wu H, Sun G, Xu Q, Xu S (2013) Accumulation, histopathological effects and response of biochemical markers in the spleens and head kidneys of common carp exposed to atrazine and chlorpyrifos. Food Chem Toxicol 62:148–158 [DOI] [PubMed] [Google Scholar]
- 56.Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pestic Biochem Physiol 103:74–80 [Google Scholar]
- 57.Sutha J, Anila PA, Gayathri M, Ramesh M (2022) Long term exposure to tris (2- chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): in vivo and computational analysis. Comp Biochem Physiol Part C Toxicol Pharmacol 254:109263. 10.1016/j.cbpc.2021.109263 [DOI] [PubMed] [Google Scholar]
- 58.Ma X, Deng D, Chen W (2017) Inhibitors and activators of SOD, GSH-Px, and CAT. In: Senturk, M. (Ed.), Enzyme Inhibitors and Activators. IntechOpen, pp. 207–224. 10.5772/65936)
- 59.Jin Y, Liu Z, Peng T, Fu Z (2015) The toxicity of chlorpyrifos on the early life stage of zebrafish: a survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish Immunol 43(2):405–414. 10.1016/j.fsi.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 60.Tellez-Banuelos A, Santerre J, Casas-Solis A, Bravo-Cuellar G, Zaitseva (2009) Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol 27(2):105–111 [DOI] [PubMed] [Google Scholar]
- 61.Neamat-Allah AN, Hakim AE, Mahmoud Y, E.A (2020) Alleviating effects of β-glucan in oreochromis niloticus on growth performance, immune reactions, antioxidant, transcriptomics disorders, and resistance to aeromonas sobria caused by atrazine. Aquac Res 51(5):1801–1812 [Google Scholar]
- 62.Shibata S, Natori Y, Nishihara T, Tomisaka K, Matsumoto K, Sansawa H, Nguyen VC (2003) Antioxidant and anti-cataract effects of Chlorella on rats with streptozotocin-induced diabetes. J Nutr Sci Vitaminol 49(5):334–339 [DOI] [PubMed] [Google Scholar]
- 63.Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Seedevi P, Rajan DK (2019) Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: a review. Aquaculture 500:250–263 [DOI] [PubMed] [Google Scholar]
- 64.Ibrahim RE, El-Houseiny W, Behairy A, Mansour MF, Abd-Elhakim YM (2019) Ameliorative effects of Moringa oleifera seeds and leaves on chlorpyrifos-induced growth retardation, immune suppression, oxidative stress, and DNA damage in Oreochromis niloticus. Aquaculture 505:225–234 [Google Scholar]
- 65.Adel M, Dadar M, Khajavi SH, Pourgholam R, Karimí B, Velisek J (2017) Hematological, biochemical and histopathological changes in Caspian brown trout (Salmo trutta caspius Kessler, 1877) following exposure to sublethal concentrations of chlorpyrifos. Toxin Rev 36:73–79 [Google Scholar]
- 66.Ramesh M, Saravanan M (2008) Haematological and biochemical responses in a freshwater fish Cyprinus carpio exposed to chlorpyrifos. Int J Integr Biol 3:80–83 [Google Scholar]
- 67.Muttappa K (2015) Haematological and histological changes in Tilapia (Oreochromis mossambicus) exposed to Cadmium and Chlorpyrifos. Karnataka Veterinary, Animal and Fisheries Sciences University, Bidar [Google Scholar]
- 68.Deeba F, Raza I, Muhammad N, Rahman H, Ur Rehman Z, Azizullah A, Khattak B, Ullah F, Daud MK (2017) Chlorpyrifos and lambda cyhalothrin-induced oxidative stress in human erythrocytes. Toxicol Ind Health 33:297–307 [DOI] [PubMed] [Google Scholar]
- 69.Jaffer NS, Rabee AM, Al-Chalabi SM (2017) Biochemical and hematological parameters and histological alterations in fish Cyprinus carpio L. as biomarkers for water pollution with chlorpyrifos. Hum Ecol Risk Assess: Int J 23:605–616 [Google Scholar]
- 70.Singh NN, Srivastava AK (2010) Haematological parameters as bioindicators of insecticide exposure in teleosts. Ecotoxicol 19:838–854 [DOI] [PubMed] [Google Scholar]
- 71.Tilak K, Veeraiah K, Rao DK (2004) Toxicity and bioaccumulation of chlorpyrifos in Indian carp Catla catla (Hamilton), Labeo rohita (Hamilton), and Cirrhinus mrigala (Hamilton). Bull Environ Contam Toxicol 73:933–941 [DOI] [PubMed] [Google Scholar]
- 72.Fırat ¨O, Tutus R (2020) Comparative acute toxicity assessment of organophosphate and avermectin insecticides on a freshwater fish Oreochromis niloticus. Bull Environ Contam Toxicol 105(4):582–587. 10.1007/s00128-020-02990-y [DOI] [PubMed] [Google Scholar]
- 73.Kunwar PS, Sinha AK, De Boeck G, Sapkota K (2022) Modulations of blood biochemical parameters of golden mahseer, Tor putitora following exposures to single and mixed organophosphate. Comp Biochem Physiol Part C Toxicol Pharmacol 251:109207. 10.1016/j.cbpc.2021.109207 [DOI] [PubMed] [Google Scholar]
- 74.Banaee M, Akhlaghi M, Soltanian S, Sureda A, Gholamhosseini A, Rakhshaninejad M (2020) Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus leptodactylus. Ecotoxicol 29(9):1500–1515. 10.1007/s10646-020-02233-0 [DOI] [PubMed] [Google Scholar]
- 75.Fadl SE, ElGohary MS, Elsadany AY, Gad DM, Hanaa FF, El-Habashi NM (2017) Contribution of microalgae-enriched fodder for the Nile tilapia to growth and resistance to infection with Aeromonas hydrophila. Algal Res 27:82–88 [Google Scholar]
- 76.Jiao W, Han Q, Xu Y, Jiang H, Xing H, Teng X (2019) Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: through oxidative stress and apoptosis. Fish Shellfish Immunol 86:239–245. 10.1016/j.fsi.2018.08.060 [DOI] [PubMed] [Google Scholar]
- 77.Ibrahim K, Ramli R, Rashid A, Mohd Yusof AH, Y.A (2015) Antimicrobial property of water and ethanol extract Chlorella vulgaris: a value-added advantage for a New Wound Dressing Material. Int Med J 22:399–401 [Google Scholar]
- 78.Delannoy CM, Samai H, Labrie L (2021) Streptococcus agalactiae serotype IV in farmed tilapia. Aquaculture 737033. 10.1016/j.aquaculture.2021.737033
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request from the corresponding author.
Code availability Not applicable.