Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) stimulates numerous cellular activities capable of contributing to the metastatic phenotype, including growth, motility, invasiveness, and morphogenetic transformation. When inappropriately expressed in vivo, an HGF/SF transgene induces numerous hyperplastic and neoplastic lesions. NK1 and NK2 are natural splice variants of HGF/SF; all interact with a common receptor, Met. Although both agonistic and antagonistic properties have been ascribed to each isoform in vitro, NK1 retains the full spectrum of HGF/SF-like activities when expressed as a transgene in vivo. Here we report that transgenic mice broadly expressing NK2 exhibit none of the phenotypes characteristic of HGF/SF or NK1 transgenic mice. Instead, when coexpressed in NK2-HGF/SF bitransgenic mice, NK2 antagonizes the pathological consequences of HGF/SF and discourages the subcutaneous growth of transplanted Met-containing melanoma cells. Remarkably, the metastatic efficiency of these same melanoma cells is dramatically enhanced in NK2 transgenic host mice relative to wild-type recipients, rivaling levels achieved in HGF/SF and NK1 transgenic hosts. Considered in conjunction with reports that in vitro NK2 induces scatter, but not other activities, these data strongly suggest that cellular motility is a critical determinant of metastasis. Moreover, our results demonstrate how alternatively structured ligands can be exploited in vivo to functionally dissociate Met-mediated activities and their downstream pathways.
Hepatocyte growth factor/scatter factor (HGF/SF) possesses an impressive panoply of biological activities, thereby regulating cellular proliferation and a variety of morphogenetic processes, including cellular migration, extracellular matrix invasion, branching, and tubulogenesis (reviewed in references 15, 21, 29, 51, and 73). Effects of this multifunctional cytokine are all mediated through its cell surface receptor tyrosine kinase (RTK), encoded by the c-MET proto-oncogene (4, 12, 32, 37). Upon HGF/SF binding, MET engages a number of SH2-containing signal transducers, including phosphotidylinositol 3-kinase, phospholipase C-γ, Stat3, Grb2, and the Grb2-associated docking protein Gab1, and indirectly activates the Ras–mitogen-activated protein kinase (MAPK) pathway (39, 40, 69, 70). Typically, HGF/SF is produced in cells of mesenchymal origin, influencing Met-expressing embryonic and adult epithelium through a paracrine mechanism (19, 59, 64). Gene targeting studies have demonstrated that activation of signaling pathways downstream of Met is essential for development of murine skeletal muscle, liver, and placenta (3, 53, 67). In accordance with its various effects on cultured cells, HGF/SF is thought to regulate epithelial-mesenchymal conversion and migration of myogenic precursor cells in vivo.
Chronic MET activation induces the genesis and, more significantly, progression of a multitude of human and murine tumors, including melanomas (for example, see references 2, 13, 14, 22, 33, 41, 43, 44, 46, 47, and 65). MET activation can be achieved through coexpression of HGF/SF, resulting in the creation of an autocrine signaling loop (2, 13, 43, 45, 65). In addition, critical genetic evidence for a role for c-MET in human cancer has come from the discovery that activating c-MET mutations are associated with hereditary papillary renal carcinoma (24, 54, 74). As during embryogenesis, a number of activities ascribed to HGF/SF and Met activation undoubtably contribute to the manifestation of the full metastatic phenotype. These include stimulation of angiogenesis, degradation of local extracellular matrix, production of cell adhesion molecules, migration into vessels and tissues, and colonization at a distant site (reviewed in references 29 and 48).
HGF/SF shows a 38% overall sequence similarity with plasminogen (15) and a 45% identity to HGF-like/macrophage-stimulating protein at the amino acid level (17, 72). The 92-kDa HGF/SF possesses several recognizable structures, which are shared by all family members, including the presence of an enzymatically inactive serine protease domain in the β chain, and an N domain and four kringle domains in the α chain (Fig. 1A). Kringles are highly conserved, three-disulfide, triple-loop polypeptides thought to participate in protein-protein interactions (reviewed in reference 66). HGF/SF mRNA can undergo alternative splicing to create truncated isoforms (Fig. 1A), capable of binding to the HGF/SF receptor with relatively high affinity. Historically, defining the biological activities associated with these variants has been somewhat elusive and a point of contention in the field. One natural variant consisting of the N domain and the first two kringle domains, designated NK2, was originally found to be incapable of stimulating the growth of cultured human mammary epithelial cells but instead antagonized HGF/SF-induced mitogenesis (6, 30). However, NK2 was later reported to act as a partial agonist, able to scatter certain cultured epithelial cells (18, 60). More recently, NK2 was shown to be incapable of triggering induction of tissue inhibitor of metalloproteases 3, urokinase-type plasminogen activator proteolysis, invasion, or tubulogenesis in some cells (5, 23, 31). Interestingly, a unique bivalent monoclonal antibody against a non-binding-site epitope of the extracellular domain of human HGF/SF was, like NK2, found to stimulate cell motility but no other Met-associated activity (42). A second truncated HGF/SF, NK1, was first artificially engineered to consist of the N domain and a single kringle domain but was later found to occur naturally in mouse cells as well (9, 28, 60). NK1 was also originally reported to possess activities antagonistic to HGF/SF in terms of mitogenesis (28) but later found to stimulate mitogenic and motogenic activities (9). Schwall et al. (55) have provided evidence suggesting that the presence of cell surface heparan sulfate proteoglycans can facilitate NK1 mitogenic activity by inducing ligand dimerization. An artificial four-kringle mutant, NK4, was reported to inhibit the mitogenic, motogenic, and morphogenic activities of HGF/SF in vitro (10). Taken together, these data indicate that the in vitro biological activities of these HGF/SF variants are context dependent and greatly influenced by the target cell and the culture conditions in which those cells are grown; moreover, they stand as a testament to the requirement for in vivo models to assess their bona fide activities.
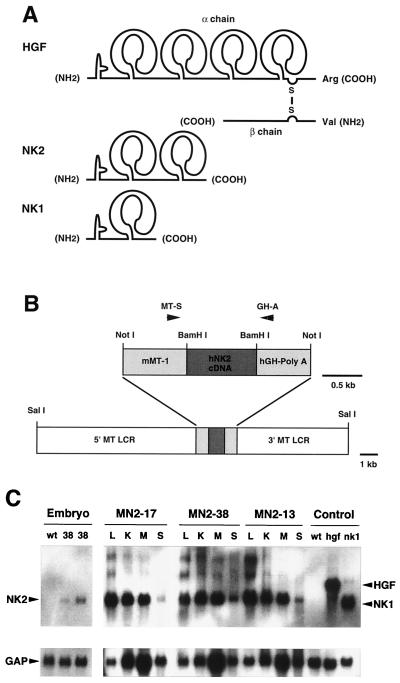
FIG. 1.
Structure and expression of NK2. (A) Schematic comparison of HGF/SF (designated as HGF in this and all other figures) and its natural splice variants NK2 and NK1. Each isoform contains a single so-called N domain at the amino terminus, and either four, two, or one kringle domain, as shown. However, only HGF/SF is processed into two chains, the β chain containing an enzymatically inactive serine protease domain. (B) The SalI-SalI NK2 transgene construct contained the human NK2 cDNA, the mouse MT gene promoter (mMT-1) and 5′ and 3′ flanking sequences (MT LCR), and the hGH poly(A) signal. Mice harboring the NK2 transgene were identified by PCR using primers MT-S and GH-A, as indicated. (C) Analysis of NK2 transgene expression in mouse tissues by Northern blot hybridization. For embryonic expression (three-lane panel at left), wild-type (wt) embryos were harvested at E16.5, and transgenic embryos from line MN2-38 (38) were harvested at E14.5 (middle lane) and E16.5 (right lane). Adult (2-month-old) tissues from three independently generated lines, MN2-17, MN2-38, and MN2-13, were studied. Tissues analyzed included liver (L), kidney (K), skeletal muscle (M), and skin (S). The control lanes at far right show expression of HGF/SF sequences in livers of wild-type and HGF/SF and NK1 transgenic mice. Following hybridization with a human NK2 cDNA probe (top panels), the filter was stripped and rehybridized with a control GAP cDNA probe (bottom panels).
To this end, we have generated a series of transgenic mice in which either HGF/SF, NK1, or NK2 was broadly expressed using a mouse metallothionein (MT) promoter and associated locus control regions (LCRs) to regulate transcription. Previously, we demonstrated that ectopic expression of HGF/SF had pleiotropic phenotypic consequences, including enhanced liver growth and regeneration, progressive renal disease characterized by glomerulosclerosis, disruption of the olfactory mucosa, aberrant appearance of skeletal muscle in the central nervous system, patterned hyperpigmentation and aberrant localization of melanocytes in the dermis and epidermis, precocious mammary lobuloalveolar development, and susceptibility to diverse tumorigenesis (52, 61–63) (Fig. 2). More recently, we reported that mice expressing an NK1 transgene exhibited a remarkably similar array of phenotypes, albeit with reduced severity, indicating that NK1 is a partial agonist of HGF/SF in vivo (20). In striking contrast, here we demonstrate that NK2 can antagonize most of the phenotypic consequences of HGF/SF expression in mice harboring both transgenes. However, by employing various genetically modified host mice as tumor transplant recipients, we show that NK2 alone retains an impressive ability to facilitate the metastasis of melanoma cells expressing high levels of Met.
FIG. 2.
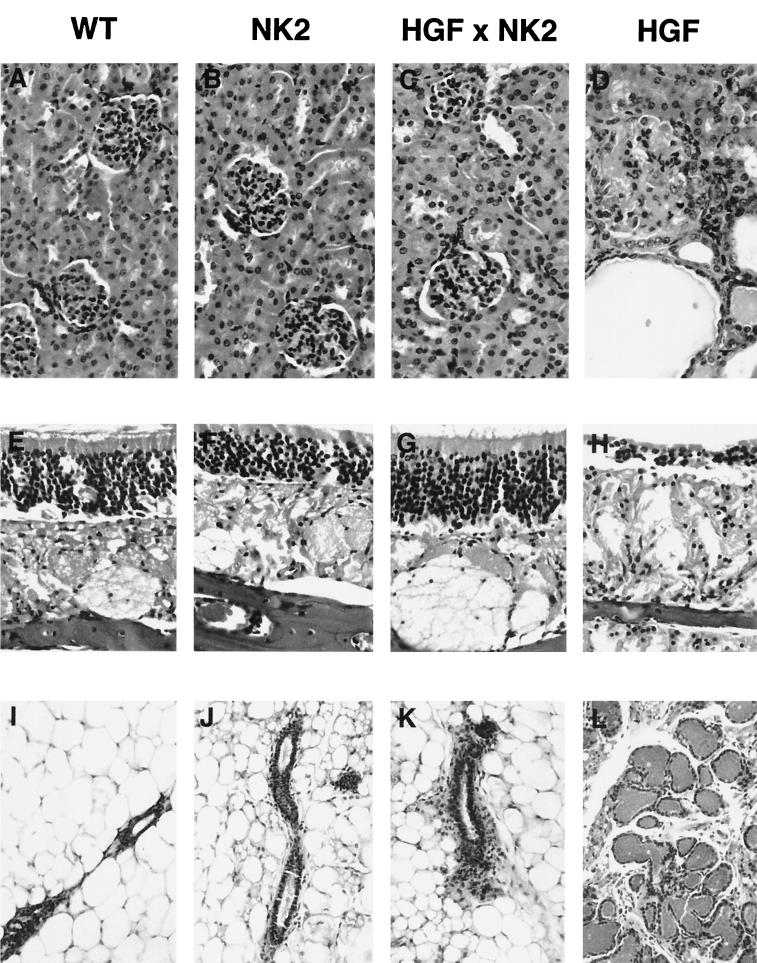
NK2 extinguishes the phenotypic consequences of ectopic HGF/SF expression in bitransgenic mice. Shown are panels of tissues, including kidney (A to D), olfactory mucosa (E to H), and virgin mammary gland (I to L), from wild-type (A, E, and I), NK2 transgenic (B, F, and J), HGF/SF-NK2 bitransgenic (C, G, and K), and HGF/SF transgenic (D, H, and L) mice. Tissues shown are from mice 2.5 months of age. Note that bitransgenic tissues resemble wild-type tissues and do not contain the pathological features characteristically evident in HGF/SF transgenic animals.
MATERIALS AND METHODS
Generation and identification of transgenic mice.
NK2 transgenic mice were generated on an albino FVB/N genetic background employing the expression construct used previously for the HGF/SF and NK1 transgenic mice. Expression of the human NK2 cDNA was placed under the control of the mouse MT-1 promoter. The construct included the human growth hormone (hGH) polyadenylation site [poly(A)] and the 5′ and 3′ flanking regions of mouse MT genes (Fig. 1B). These contain LCRs conferring copy-number-dependent and integration-site-independent transgene expression (36). NK2 transgenic mice were identified by PCR using as template tail genomic DNA and the following primer set: MT-S (5′-ACTCGTCCAACGACTATA-3′), specific to the MT promoter region, and GH-A (5′-AACTTCCAGGGCCAGGAGA-3′), specific to the hGH-poly(A) sequence. HGF/SF transgenic mice were identified by PCR using the following primer set: HGF315 (5′-AGTTATGGTTGTACAATCCCTGAAAAGA-3′), specific to the β chain of mouse HGF/SF sequence, and GH-A. NK1 transgenic mice were identified by PCR using the following primer set: MT-S and HGF292 (5′-CTGAGGAATGTCACAGACTTCGTA-3′), specific to the first kringle domain sequence of mouse HGF/SF cDNA. Diagnostic PCR products for the presence of the NK2, HGF/SF, and NK1 transgenes were 1,019, 451, and 709 bp, respectively. Where noted, mice were maintained on 25 mM ZnSO4 in their drinking water. All mouse work was performed in accordance with the Guide for the Care and Use of Laboratory Animals (33a).
Histopathological assessment and liver growth analysis.
For routine histopathological analysis, mouse tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Metastatic melanomas were visualized using an anti-mouse tyrosinase-related protein 1 (TRP1) antibody, αPEP1 (35), a gift from Vincent Hearing, National Cancer Institute, Bethesda, Md. For comparative analysis of hepatocyte proliferation in vivo, five to seven 1.5-month-old mice of each genotype that had been maintained on ZnSO4 water were given intraperitoneal injections of bromodeoxyuridine (BrdU), according to the manufacturer's instructions (Amersham Life Science; RPN201). After 2 h, all mice were euthanatized and their liver tissues were fixed in 70% ethanol. BrdU incorporation was then detected immunohistochemically (56), and labeled hepatocyte nuclei from between 422 and 589 high-power light microscope fields (400×) for each genotype were scored. For determination of liver growth, between 18 and 27 female mice of each genotype between 1.5 and 3.0 months of age were used. Exposure to ZnSO4 water had no overt effect on liver mass, so data from zinc-treated and non-zinc-treated animals were combined.
Analysis of RNA.
NK2 transgene expression in selected adult (2-month-old) tissues was assessed 6 h after intraperitoneal injection of 5 mg of ZnCl2 per kg of body weight. To compare transgene expression with liver weight/body weight ratios, total RNA was isolated from 1.5-month-old transgenic and bitransgenic female mice maintained on 25 mM ZnSO4 water. For fetal expression, E14.5 and E16.5 mouse embryos were used for RNA isolation. Total RNA was prepared using guanidine thiocyanate, as described previously (25). For Northern blot analysis, 15 μg of total RNA was resolved on a denaturing 1% agarose-formaldehyde gel and transferred to a nitrocellulose membrane (Schleicher & Schuell). The membrane was prehybridized and hybridized at 42°C in a solution whose contents included 50% formamide and 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), washed, and subjected to autoradiography (25). The 2.2-kbp mouse HGF/SF cDNA probe was synthesized by PCR, as described previously (62). The 636-bp mouse NK2 probe, covering only the first and second kringle domain of the HGF/SF cDNA, was synthesized by PCR using the following primer set: HGF313S (5′-GAGTGTGCCAACAGGTGTATCAGG-3′) and HGF291 (5′-AATTGCACAATACTCCCAAGGGGT-3′). For analysis of MT expression, a 355-bp BamHI mouse MT-1 cDNA fragment was used as hybridization probe (generously provided by Richard Palmiter, University of Washington, Seattle). To control for RNA loading and transfer variation, filters were routinely rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAP) cDNA probe (35).
Cell culture and transplantation.
Both the mouse cell lines, 37-32 and 37-7, were derived from neoplasms arising in HGF/SF transgenic mouse line MH-37 (35, 62). Both lines were maintained in Dulbecco modified Eagle medium (DMEM) (Gibco) supplemented with 15% fetal bovine serum (Gibco), 100 IU of penicillin (Gibco) per ml, 100 μg of streptomycin (Gibco) per ml, 2 mM l-glutamine (Gibco), 5 μg of insulin (Upstate Biotechnology Inc.) per ml, and 5 ng of epidermal growth factor (Upstate Biotechnology Inc.) per ml, and incubated in 5% CO2 at 37°C. Subcutaneous tumors were produced by injection of 106 cells in 0.3 ml of DMEM under the back skin of 2- to 3-month-old wild-type, HGF/SF transgenic, NK2 transgenic, or HGF/SF-NK2 bitransgenic male and female mice. Tumor diameters were measured every 3 days using a caliper, and tumor volumes were calculated according to the formula V = a × b2/2. Rates of tumor growth were determined based on 3-day intervals. For the experimental metastasis assay, 105 or 106 cells in 0.3 ml of DMEM, as indicated, were intravenously injected via the tail vein into 2- to 5.5-month-old male and female HGF/SF transgenic, NK1 transgenic, NK2 transgenic, and wild-type mice. The conclusions from the metastasis assay were essentially the same whether 105 or 106 melanoma cells were injected. Gross tumor numbers were obtained by visual inspection of liver, spleen, kidneys, lungs, diaphragm, and pleural cavity in mice euthanatized 18 to 25 days posttransplantation. Microscopic quantification of metastasis was performed on representative formalin-fixed, H&E-stained sections of all liver lobes from two to eight representative animals between 1.5 and 2.5 months of age; all mice in this study were euthanatized and analyzed 21 days posttransplantation. For tumor size determination, 725, 98, and 65 tumors were measured from representative sections of each liver lobe from NK2, NK1, and HGF/SF mice, respectively. Statistical analysis was performed using the Student t test.
Analysis of Met and Met activity.
Quantification of Met and Met tyrosine phosphorylation was performed as described previously (35). Lysates were prepared from 37-32 cells treated for 10 min at 37°C with the factors indicated (HGF/SF, 100 ng/ml; NK2, 300 ng/ml). Cultured cells were solubilized in RIPA buffer (50 mM Tris [pH 7.4], 50 mM NaCl, 1% Triton X-100, 5 mM EDTA, 10 mM sodium pyrophosphate [Sigma], 50 mM sodium fluoride [Sigma], 1 mM sodium orthovanadate [Sigma], 1 mM phenylmethylsulfonyl fluoride [Boehringer Mannheim], 10 μg of leupeptin [Boehringer Mannheim] per ml, 10 μg of pepstatin [Boehringer Mannheim] per ml, and 10 μg of aprotinin [Boehringer Mannheim] per ml). Equivalent amounts of the resulting lysates were incubated with anti-Met antibody (Santa Cruz Biotechnology) for 2 h. Following addition of GammaBind G Sepharose (Pharmacia Biotech) and washing in RIPA buffer, samples were fractionated by reducing sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel electrophoresis (PAGE). After electrophoretic transfer to Immobilon-P membranes (Millipore), filters were blocked and incubated with anti-Met antibody (Santa Cruz Biotechnology) overnight. Met was visualized by incubation with anti-rabbit antibody conjugated to horseradish peroxidase, followed by enhanced chemiluminescence (ECL; Amersham). After stripping, filters were reblocked and incubated overnight with a phosphotyrosine monoclonal antibody (Upstate Biotechnology).
To determine the comparative effects of HGF/SF and NK2 on Met-induced MAPK activity, Western blotting for anti-active MAPK was performed as described previously (11). Lysates, prepared after exposure of 37-32 cells to either HGF/SF or NK2 (as above), were fractionated by SDS–12% PAGE, transferred to Immobilon-P membrane, and probed with anti-phospho-MAPK antibody (New England Biolabs) as per the manufacturer's instructions. Positive staining was detected by ECL (Amersham).
Analysis of HGF/SF and NK2.
Mouse liver lysates (25 μg of total protein per sample per lane) were fractionated by SDS–10% PAGE and electrophoretically transferred to Immobilon-P membrane. Membranes were blocked with bovine serum albumin, probed with anti-human HGF/SF (N-17; Santa Cruz Biotech) in 0.1% bovine serum albumin–0.05% NP-40–phosphate-buffered saline, and detected by ECL (Amersham). A series of standards of purified mouse HGF/SF and human NK2 run in the same gel as the liver samples allowed a quantitative assessment of HGF/SF and NK2 levels. Purified mouse HGF/SF was a generous gift from Ermanno Gherardi, MRC Center, Cambridge, United Kingdom; human NK2 was prepared as described previously (60). These standards revealed that the anti-human HGF/SF antiserum used for immunoblot analysis recognized human HGF/SF with greater overall sensitivity than it recognized mouse HGF/SF, although both were readily detectable. NK2 levels in sera were quantified using an enzyme-linked immunosorbent assay, as described previously (55).
RESULTS
Mice expressing NK2 are healthy and exhibit no overt hyperproliferative lesions.
To determine the in vivo biological activities of the natural HGF/SF splice variant, NK2, the human cDNA was placed under the transcriptional control of the mouse MT gene promoter and LCRs (Fig. 1B), and the resulting expression vector was used to make four lines of NK2 transgenic mice. An identical MT expression construct was used in our previously described HGF/SF and NK1 transgenic mice (20, 61), so as to allow a direct comparison of phenotypic consequences of expression of the various HGF/SF isoforms. Northern blot hybridization (Fig. 1C) was used to demonstrate that the MT-NK2 transgene, like the MT-HGF/SF and MT-NK1 transgenes, was highly and broadly expressed in three lines, two of which (MN2-17 and MN2-38) were chosen for further analysis. In all experiments presented here, results obtained using these two lines were indistinguishable. Transgene expression was also assessed during development in line MN2-38. As with the MT-HGF/SF transgene (61), the MT-NK2 transgene was clearly active in E14.5 and E16.5 embryos (Fig. 1C). An enzyme-linked immunosorbent assay showed that NK2 levels in the serum of MN2-38 mice averaged 27 ng/ml; wild-type control serum had an NK2 level less than the 7.8-ng/ml limit of detection. Previously, HGF/SF transgenic mouse serum was found to contain an average of 16.4 ng of HGF/SF per ml compared to wild-type levels of 3.9 ng/ml (61).
In contrast to mice bearing either the MT-HGF/SF or MT-NK1 transgene, NK2 transgenic mice failed to exhibit overt abnormal phenotypes. No hyperplastic lesions were observed in the kidney or olfactory mucosa (Fig. 2B and F), and no anomalies were associated with the mammary gland (Fig. 2J) or skeletal muscle (data not shown). NK2 mice did not experience gastrointestinal obstruction or progressive renal disease, which is highly characteristic of HGF/SF mice (Fig. 2D). The liver was not enlarged; in fact, when expression of the MT-NK2 transgene was stimulated by exposing juvenile mice to zinc-containing water, the weight of the liver relative to the body appeared to be slightly reduced. When crossed with the pigmented strain C57BL/6, first-generation MN2-38 and MN2-17 transgenic mice were found to exhibit no overt hyperpigmentation. However, histopathological analysis of their skin revealed the occasional presence of pigment cells outside the normal confines of the hair shaft, in the dermis and epidermis (data not shown). This ectopic pigment cell localization was more obvious in line MN2-38, which was characterized by higher transgene expression in the skin (Fig. 1C). Such aberrant pigment cell localization was not observed in wild-type animals.
NK2 antagonizes HGF/SF-induced pathology in bitransgenic mice.
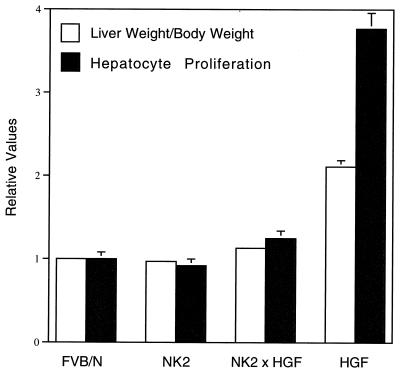
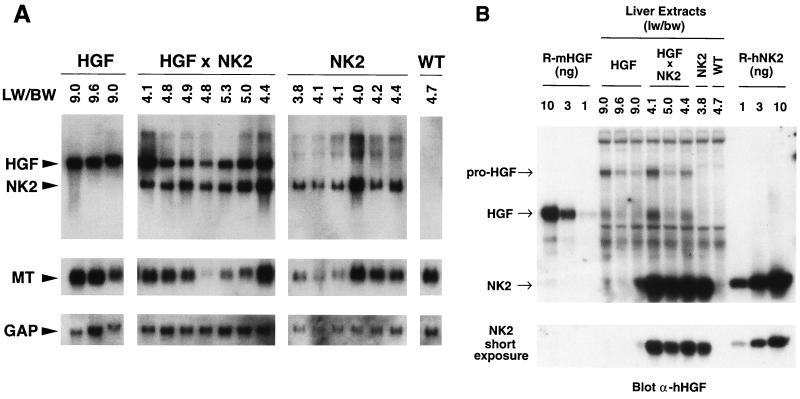
The small reduction in liver size observed in zinc-treated NK2 transgenic mice raised the possibility that NK2 was capable of inhibiting HGF/SF-mediated hepatocyte proliferation in vivo. To further test this hypothesis, bitransgenic mice harboring both the MT-HGF/SF and MT-NK2 transgenes were generated. Figure 3 shows that NK2 expression in all bitransgenic mice reduced to nearly normal levels the anomalous liver growth associated with the constitutive activation of Met in HGF/SF transgenic hepatocytes (52). Moreover, analysis of BrdU incorporation revealed that the labeling index of bitransgenic hepatocytes was significantly decreased relative to HGF/SF transgenic hepatocytes (Fig. 3). The presence of the MT-NK2 transgene could be inhibiting hepatocyte proliferation in bitransgenic mice by specifically antagonizing HGF/SF activity or by squelching expression of the MT-HGF/SF transgene through competition for MT-specific transcription factors. To distinguish between these two possibilities, the structure and activity of these two transgenes were compared. Southern blot analysis revealed that the two MT-driven transgenes were equivalently represented in terms of copy number (data not shown). Northern blot analysis demonstrated that at the level of RNA, expression of the two transgenes and the endogenous MT gene was coordinately regulated in bitransgenic hepatocytes (Fig. 4A), indicating that there was no shortage of MT-specific transcription factors for which the two transgenes would have to compete. The same result was also seen in livers from bitransgenic juvenile mice that were exposed to water containing zinc (data not shown). Western blotting was then utilized to quantify the relative levels of HGF/SF and NK2 protein in transgenic and bitransgenic liver extracts. Based on comparisons with recombinant standards, it can be estimated that, in 25 μg of transgenic liver extract, HGF/SF and NK2 are represented at levels between 2 and 4 ng and 5 and 10 ng, respectively (Fig. 4B). Thus, the 33-kDa NK2 appears to be present in the bitransgenic livers in about a sevenfold molar excess relative to the 92-kDa HGF/SF. Figure 4B also demonstrates that NK2 is not expressed at the expense of HGF/SF and confirms that the normal liver weight/body weight ratios characteristic of bitransgenic mice are not caused by diminution of HGF/SF levels. These data support the contention that NK2 can antagonize certain HGF/SF-mediated activities at the level of ligand-receptor interaction in vivo, as has been shown in vitro (6, 11, 31).
FIG. 3.
NK2 inhibits the proliferative effects of HGF/SF on hepatocytes. Shown are mean liver weight/body weight ratios (white bars) and hepatocyte labeling indices (black bars) from wild-type (FVB/N), NK2 transgenic, NK2-HGF/SF bitransgenic, and HGF/SF transgenic livers. All values were normalized to wild type (set at 1.0), and error bars represent standard errors of the means. For both liver size and hepatocyte proliferation, the P value is <0.0001 in NK2-HGF/SF bitransgenic versus HGF/SF transgenic mice.
FIG. 4.
Comparative transgene expression in livers of HGF/SF and NK2 transgenic and HGF/SF-NK2 bitransgenic mice. (A) Northern blot analysis of total liver RNA (15 μg/sample) using as probe mouse sequences equivalent to NK2 (top panels), mouse MT cDNA (middle panels), or mouse GAP cDNA (bottom panels). Numbers at the top indicate liver weight/body weight ratios (LW/BW). All mice were 1.5 months of age. (B) Quantitative Western blot analysis of mouse HGF/SF and human NK2 transgenic mouse liver extracts (25 μg/sample) using an anti-human HGF/SF antibody. Arrows mark positions of unprocessed pro-HGF/SF, processed HGF/SF, and NK2. Liver weight/body weight ratios (lw/bw) are shown at top. Recombinant mouse HGF/SF (R-mHGF) and human NK2 (R-hNK2) standards of known amounts (in nanograms) are displayed on the extreme left and right, respectively. A short exposure shown at bottom permits quantification of NK2 levels in liver extracts. In general, RNA and protein results in panels A and B, respectively, are in accord.
Prompted by these liver results, we analyzed other tissues from HGF/SF-NK2 bitransgenic mice up to 6 months of age. In contrast to HGF/SF transgenic mice (Fig. 2D, H, and L), in HGF/SF-NK2 bitransgenic mice the kidney exhibited little or no glomerulosclerosis or tubular hyperplasia; the olfactory mucosa was overtly normal, with no sign of olfactory gland hyperplasia or nervous depletion; and virgin mammary epithelium demonstrated no obvious precocious alveolar development (Fig. 2C, G, and K). These results indicate that the pathological consequences of chronic, HGF/SF-mediated Met activation associated with anomalous cellular proliferation can be effectively antagonized in vivo by the splice variant NK2.
NK2 facilitates metastasis but not growth of Met-overexpressing malignant melanoma cells in vivo.
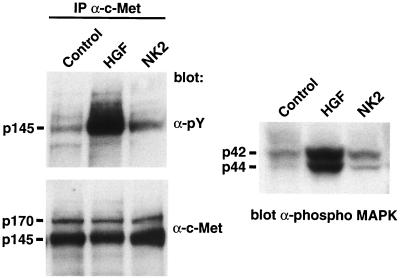
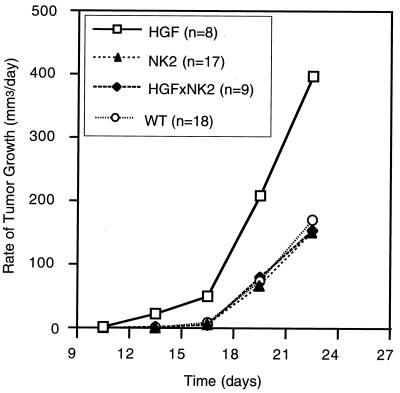
Previously, we determined that cultured 37-32 cells, a melanoma line established from HGF/SF transgenic mice and overexpressing both the transgene and endogenous c-met, could be growth inhibited up to, but not more than, 60% in minimal medium supplemented with recombinant NK2 to levels in 100-fold excess (0.3 to 1.0 μg/ml) of those that can be achieved in serum, in vivo (35). Unlike HGF/SF, NK2 is incapable of inducing robust Met autophosphorylation or MAPK in these melanoma cells (Fig. 5). To ascertain the relative effect of NK2 on growth and metastasis of 37-32 cells in vivo, transgenic lines of mice overexpressing various HGF/SF isoforms were exploited as genetically modified hosts for transplantation challenge. Initially, 106 melanoma cells were injected subcutaneously into syngeneic wild-type FVB/N, transgenic HGF/SF, transgenic NK2, or HGF/SF-NK2 bitransgenic host mice. Figure 6 shows that melanomas grew in the NK2 transgenic hosts nearly as well as in the wild-type mice. However, when transplanted subcutaneously into HGF/SF mice, melanomas became palpable much earlier and grew at an accelerated rate relative to those in either wild-type or NK2 mice. Significantly, the time of appearance and rate of growth of tumors transplanted into bitransgenic host animals expressing both NK2 and HGF/SF were no different than those for either the wild-type or NK2 single transgenic hosts. Together, these data indicate that, while NK2 could not effectively disrupt the HGF/SF-Met autocrine signaling loop driving baseline 37-32 melanoma growth, this variant was able to completely antagonize the paracrine-enhanced in vivo growth associated with overexpression of the HGF/SF transgene originating from genetically modified host mouse tissues.
FIG. 5.
Quantification of Met and Met activity in melanoma cells. Extracts prepared from 37-32 melanoma cells treated with either nothing (control), HGF/SF, or NK2 were immunoprecipitated (IP) with anti-Met antibody and subsequently probed with either anti-phosphotyrosine (α-pY, top left) or anti-Met (α-c-Met, bottom left) antibodies. Extracts were also directly probed with anti-phospho-MAPK antibody (right). Molecular masses in kilodaltons are shown. Note that, relative to NK2, HGF/SF induces phosphorylation of Met and MAPK without altering the levels of Met.
FIG. 6.
NK2 antagonizes paracrine, but not autocrine, HGF/SF-induced subcutaneous melanoma growth. One million 37-32 melanoma cells were injected under the back skin of 2- to 3-month-old wild-type (WT), HGF/SF transgenic, NK2 transgenic, and HGF/SF-NK2 bitransgenic mice; tumor sizes were measured; and growth rates were calculated.
The 37-32 melanoma cells, derived on an FVB/N inbred genetic background, were shown to be highly metastatic to a number of nude mouse tissues but with a clear partiality for liver (35). To determine the specific effect of the host-generated HGF/SF isoforms on metastasis, 37-32 melanoma cells were introduced intravenously into syngeneic wild-type and HGF/SF, NK1, and NK2 transgenic mice. Gross metastatic colonization by 37-32 cells of the liver and other organs was elevated in HGF/SF transgenic mice relative to that in wild-type controls (Table 1; Fig. 7A), reminiscent of the heightened growth response of these same melanoma cells when transplanted subcutaneously into HGF/SF transgenic mice (Fig. 6). Gross metastatic efficiency of 37-32 cells was enhanced in NK1 transgenic hosts as well (data not shown). When this experimental metastasis assay was repeated with another cell line containing barely detectable levels of HGF/SF and Met (HGF/SF transgenic mouse-derived line 37-7 [35]), the incidence of gross metastasis to a variety of target organs in both HGF/SF and NK2 transgenic host mice was not significantly different from that for wild-type mice (Table 1). This result suggests that host-generated HGF/SF isoforms were acting directly through responsive melanoma cells and not by creating a more permissive host.
TABLE 1.
Incidence of metastasis and organ site selection of malignant cells in host transgenic mice expressing HGF/SF or its splice variantsa
| Host genotype | Analysis | High-Met 37-32 cells
|
Low-Met 37-7 cells
|
||||
|---|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Lung | Chest | Diaphragm | ||
| WT | Gross | 5.1 ± 0.8 | 5.6 ± 1.3 | 0.2 ± 0.1 | 3.8 ± 0.4 | 0.4 ± 0.2 | 0.4 ± 0.3 |
| Microscopic | 12 ± 1.5bc | 6.7 ± 0.6 | 0.0 ± 0.0bc | 25 ± 1.9d | ND | ND | |
| NK2 | Gross | >100 | 11 ± 1.5 | 1.0 ± 0.3 | 4.3 ± 0.4 | 0.6 ± 0.5 | 0.1 ± 0.1 |
| Microscopic | 107 ± 8.1b | 9.7 ± 0.8 | 0.8 ± 0.2b | 29 ± 2.8d | ND | ND | |
| HGF/SF | Gross | >50 | 21 ± 5.6 | 29 ± 12.5 | 3.0 ± 0.8 | 1.8 ± 1.3 | 0.8 ± 0.7 |
| Microscopic | 39 ± 10c | ND | 11 ± 3.9c | 54 ± 14d | ND | ND | |
Shown are the mean numbers of metastatic tumors per organ (± standard errors of the means) observed either grossly or microscopically in H&E-stained sections at their most prevalent sites following tail vein injections of either 105 (37-32) or 106 (37-7) cultured cells. All host mice (wild type [WT], NK2, and HGF/SF transgenics) were on the FVB/N genetic background, were between 2.5 and 3 months of age, and were sacrificed either at 21 days (37-32) or at 18 days (37-7) following intravenous injection. The cell line 37-32 robustly expressed c-Met (High-Met), while 37-7 expressed c-Met poorly (Low-Met); see the work of Otsuka et al. (35) for details on cell lines. ND, not done; in the case of the HGF/SF spleens, the 37-32 tumors had begun to fuse, preventing accurate quantification. For wild-type hosts, 59 and 22 mice were used for injection of 37-32 and 37-7 cells, respectively; for NK2 hosts, 29 and 14 mice were used for 37-32 and 37-7 cells, respectively; for HGF/SF hosts, 7 and 6 mice were used for 37-32 and 37-7 cells, respectively.
P < 0.0005 comparing individual wild-type and transgenic organs.
P < 0.05 comparing individual wild-type and transgenic organs.
Not statistically different.
FIG. 7.
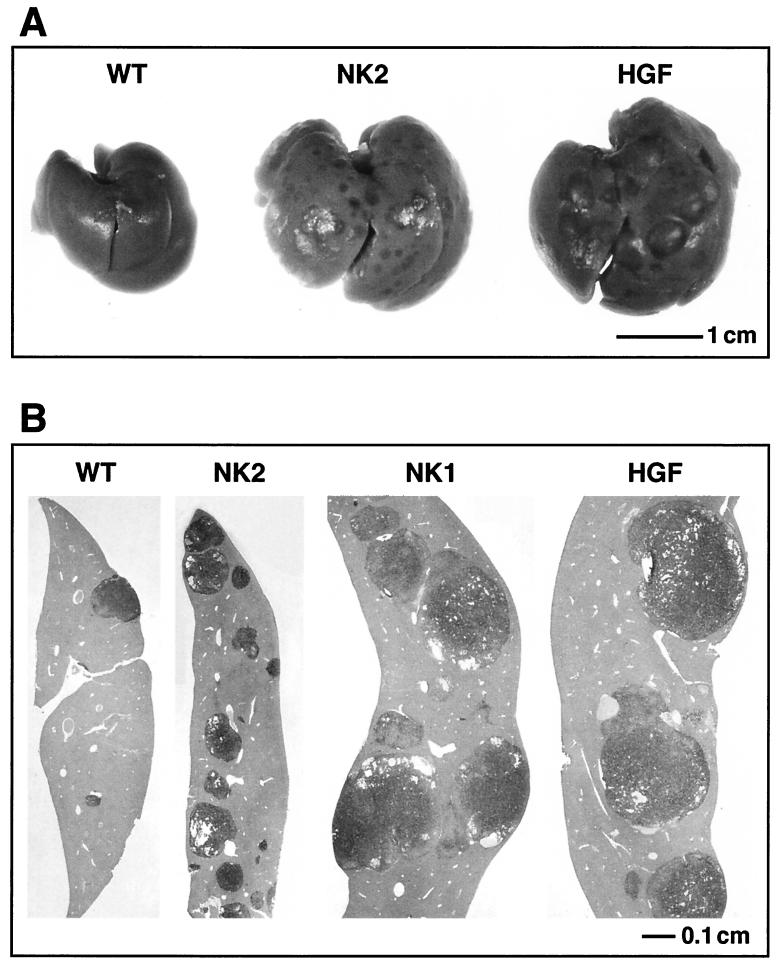
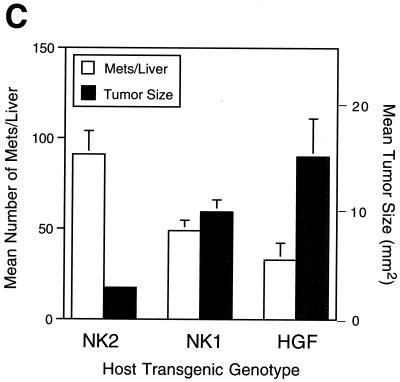
NK2 enhances metastatic efficiency, but not growth, of high-Met-expressing melanoma cells. The figure shows results of analysis of liver metastasis of melanoma cells in genetically modified host mice. One million 37-32 melanoma cells were injected intravenously into the tail vein of wild-type (WT), NK2 transgenic, NK1 transgenic, and HGF/SF transgenic mice. (A and B) After 3 weeks, livers were examined grossly (A) and histopathologically (B) for the presence of metastatic tumors. Melanomas were immunohistochemically visualized (brown staining) using an anti-mouse TRP1 antibody. (C) Liver preparations from the genetically modified host mice were used to quantify both mean numbers of 37-32 melanoma cell metastases (white bars) and mean tumor sizes (black bars). Error bars indicate standard errors of the means. There was no statistically significant difference in the numbers of metastases per liver in the three transgenic lines. For mean tumor size, P value was <0.001 for NK2 versus either NK1 or HGF/SF; P value was 0.2 for NK1 versus HGF/SF.
Having established the positive impact of host-generated HGF/SF on metastasis of high-Met melanoma cells, the effects of host-generated NK2 were next considered. Remarkably, rather than antagonizing metastasis, as it did growth, host-generated NK2 dramatically stimulated the incidence of gross hepatic metastasis of 37-32 cells, achieving levels that were at least as high as those found in HGF/SF transgenic hosts (Table 1; Fig. 7A). This gross observation was confirmed microscopically and quantified by subjecting several experimental livers to histopathological analysis (Fig. 7B). The efficiency of microscopic metastasis to liver was approximately ninefold greater in NK2 transgenic hosts than in their wild-type counterparts (P < 0.0005) and equal to or greater than that in either HGF/SF or NK1 mice (Table 1 and data not shown). In contrast, the average size of the metastatic tumors in the NK2 mouse livers appeared to be equivalent to that in wild type and reduced compared to either NK1 or HGF/SF mice (Fig. 7A and B). Morphometric quantification confirmed that, although the number of liver metastases was enhanced by NK2 as for the other isoforms, growth at the colonization site relative to that for HGF/SF and NK1 was significantly reduced (Fig. 7C). Kidney metastases were also smaller and therefore less conspicuous at both the gross and the microscopic level in NK2 transgenic hosts compared to those in HGF/SF mice (data not shown); however, kidney metastases were present in significantly greater numbers relative to wild-type hosts as well (Table 1).
DISCUSSION
The ability of the HGF/SF alternative splice variants, NK1 and NK2, to function in vitro as HGF/SF agonists or antagonists appears to be contextual, depending on the cell type and the conditions under which it is cultured (6, 9, 18, 23, 28, 30, 31, 34, 55, 58, 60). These isoforms have been detected in mice and humans, and yet their in vivo function is unknown. Recently, we demonstrated that NK1, when broadly expressed in mice, induces all phenotypes observed in HGF/SF transgenic mice, although with reduced severity (20). From this result, we concluded that NK1 acts in vivo as a partial agonist of HGF/SF. In the present study, we show that the in vivo behavior of NK2 is distinct from that of NK1, and more complex. With the exception of relatively mild ectopic localization of melanocytes outside the hair follicles, NK2 transgenic mice exhibited no overt phenotypic abnormalities. However, NK2 effectively mitigated the constellation of HGF/SF-mediated lesions in HGF/SF-NK2 bitransgenic mice, including liver enlargement and elevated hepatocyte proliferation, olfactory gland hyperplasia and mucosal disorganization, renal tubular hyperplasia and subsequent glomerulosclerosis, alveolar mammary hyperplasia, and hyperpigmentation. These lesions are generally attributable to dysregulated cellular growth. The observed coordinate regulation of expression of the two MT-driven transgenes, at the level of both RNA and protein, in bitransgenic livers indicated that amelioration of the phenotypes associated with HGF/SF overexpression in these animals was not the simple consequence of transcription factor competition, or squelching. Instead, NK2 protein itself appeared to effectively antagonize HGF/SF-induced, Met-mediated mitogenic signaling in vivo.
Remarkably, however, when introduced intravenously into the tail vein of NK2 transgenic mice, 37-32 melanoma cells exhibited a ninefold enhancement in efficiency of metastasis to the liver, their preferred site, relative to wild-type host mice of the same age, sex, and genetic background. Moreover, the incidence of metastasis in NK2 animals was at least as high as that observed in either HGF/SF or NK1 transgenic hosts, indicating that NK2 functions in vivo as a potent agonist of Met-driven metastatic dissemination. Notably, however, the average mass of liver metastases from these NK2 transgenic hosts was reduced approximately five- or threefold relative to those arising over the same time in HGF/SF or NK1 animals, respectively, indicating that, in this experimental model of metastasis, NK2 was incapable of stimulating melanoma growth in the manner demonstrated by host-generated HGF/SF. This metastatic behavior was mirrored in other organs as well. Together, these findings clearly show that the activities associated with HGF/SF, and mediated by a single RTK, Met, can be functionally dissociated in vivo. Furthermore, the same isoform can apparently serve in vivo as both HGF/SF agonist and antagonist.
How can NK2 simultaneously induce agonistic and antagonistic activities, and why do NK1 and NK2 evoke such different in vivo responses? Despite the availability of a relatively detailed blueprint of Met signaling pathways and their integral components, the mechanistic basis by which various HGF/SF isoforms differentially elicit Met-mediated cellular responses is not yet understood. It is assumed that ligand-induced dimerization triggers the activation-autophosphorylation of generic RTKs, including Met (reviewed in reference 71), and that processed, two-chain HGF/SF binds Met, either as a monomer or as a dimer, inducing a conformational shift toward a stabilized active receptor configuration (reviewed in reference 7). The recently resolved crystal structure of NK1 suggests assemblage as a homodimer capable of simultaneously engaging two Met receptors and provides a rationale for the agonism demonstrated by NK1 (8, 68). Interaction with endogenous glycosaminoglycans may be exceedingly important in realizing the agonistic behavior of this variant in vivo (8, 9, 52, 55, 68). If it is a pure antagonist, the behavior of NK2 could be explained through its ability to compete with HGF/SF for receptor binding, without inducing the appropriate activating conformational change in Met. However, we show in this report that NK2 does not behave as a pure antagonist in vivo. Although all HGF/SF isoforms appear to engage and activate Met (18, 23, 31, 51, 60), we demonstrate here that Met autophosphorylation and MAPK activation are quantitatively different in 37-32 melanoma cells treated with NK2 than in those treated with HGF/SF. NK2 activities could also be explained by qualitative differences in Met tyrosine phosphorylation, which could in turn affect transducer recruitment and/or modify substrate target selection. Alternatively, differential signal persistence and/or intensity in individual pathways may account for the behavior of NK2. For example, the Met pathway(s) engaged in the induction of metastasis-associated behavior in vivo might require a weaker signal relative to other activities and still be triggered by NK2 despite its reduced ability to activate Met. Our in vitro data suggest that MAPK does not regulate this pathway. However, a credible candidate is the pathway(s) mediated by phosphotidylinositol 3-kinase, which can be activated by NK2 in some cultured cell lines (11) and in association with p110 can bind directly to the C-terminal Y1349/Y1356 multifunctional docking site of Met (38, 39) and mediate cellular activities capable of contributing to metastatic spread (1, 16, 26, 27, 49, 57).
An important question then arises concerning the identity of the specific cellular activity, or activities, induced by NK2 that so efficiently facilitate metastasis in vivo. A number of studies on NK2 behavior in vitro proffer some insight. As originally described, NK2 was unable to induce, or could actually block, mitogenesis of cultured cells (6, 18, 30, 34). More recent in vitro studies showed that NK2 alone was incapable of inducing many other activities associated with metastasis, including in vitro invasion or urokinase-type plasminogen activator proteolysis (23), branching morphogenesis (23, 31), or angiogenesis (58). In contrast, NK2 was able to scatter canine MDCK cells in vitro (18) and stimulate cellular migration in a modified Boyden chamber assay system (60). Relying on these in vitro studies as a backdrop, our results present a strong case for enhanced cellular motility being critically influential in promoting metastasis in a bona fide animal model. However, enhanced NK2-induced scattering is almost certainly not sufficient for the acquisition of a full metastatic phenotype (16). In our melanoma model system, other requisite Met-mediated activities are likely being provided via the autocrine stimulation from the transgenic HGF/SF produced by the 37-32 cells themselves. Indeed, the fact that the subcutaneous growth of 37-32 tumor transplants is not effectively inhibited by host-generated NK2 suggests that the autocrine HGF/SF-Met signaling pathway is resistant to the antimitogenic effects of exogenous NK2. Such a conclusion raises serious doubts about the therapeutic efficacy of RTK antagonists whose malignant targets arise as a consequence of the creation of such autocrine RTK signaling loops.
Conclusions from the above discussion depend on the assumption that the metastatic enhancement induced by either NK2, NK1, or HGF/SF is a direct consequence of ligand binding to Met robustly expressed by the 37-32 malignant melanoma cells. However, an intriguing alternative possibility is that genetically modified host animals ectopically expressing these ligands become broadly permissive for metastasis, irrespective of the ability of the transplanted tumor cell to respond directly to Met ligands. HGF/SF and its variants could, for example, interact with host cells to induce subtle alterations in extracellular matrix or angiogenic networking, making these animals more susceptible to metastatic colonization in general. Although we cannot rule out this possibility at this time, the fact that low-Met 37-7 cells fail to demonstrate significantly enhanced metastatic behavior in these same transgenic hosts argues against it. This important issue will continue to be investigated; either way, these studies demonstrate the great experimental opportunity offered by an approach exploiting genetically modified host mice as tumor transplant recipients.
ACKNOWLEDGMENTS
We thank Ralph Schwall for quantification of NK2 levels in mouse sera, Miriam Anver for histopathologic consultation and morphometric measurements, Richard Palmiter for the mouse MT-1 cDNA probe, Ermanno Gherardi for the purified mouse HGF/SF standard, Vincent Hearing for the TRP1 antibody, Ricardo Dreyfuss and Steve Neal for photography, and Nelson Ellmore and Barbara Kasprzak for technical assistance with cell culture and immunohistochemistry, respectively. We are indebted to Andrew Chan and Stuart Aaronson for the NK2 cDNA and for highly useful discussions.
REFERENCES
- 1.Bardelli A, Basile M L, Audero E, Giordano S, Wennstrom S, Menard S, Comoglio P M, Ponzetto C. Concomitant activation of pathways downstream of Grb2 and PI 3-kinase is required for MET-mediated metastasis. Oncogene. 1999;18:1139–1146. doi: 10.1038/sj.onc.1202607. [DOI] [PubMed] [Google Scholar]
- 2.Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery J-P, Jouanneau J. Creation of a hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- 3.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 4.Bottaro D P, Rubin J S, Faletto D L, Chan A M, Kmiecik T E, Vande Woude G F, Aaronson S A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 5.Castagnino P, Soriano J V, Montesano R, Bottaro D P. Induction of tissue inhibitor of metalloproteinases-3 is a delayed early cellular response to hepatocyte growth factor. Oncogene. 1998;17:481–492. doi: 10.1038/sj.onc.1201957. [DOI] [PubMed] [Google Scholar]
- 6.Chan A M-L, Rubin J S, Bottaro D P, Hirschfield D W, Chedid M, Aaronson S A. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991;254:1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- 7.Chirgadze D Y, Hepple J, Byrd R A, Sowdhamini R, Blundell T L, Gherardi E. Insights into the structure of hepatocyte growth factor/scatter factor (HGF/SF) and implications for receptor activation. FEBS Lett. 1998;430:126–129. doi: 10.1016/s0014-5793(98)00558-4. [DOI] [PubMed] [Google Scholar]
- 8.Chirgadze D Y, Hepple J P, Zhou H, Byrd A, Blundell T L, Gherardi E. Crystal structure of the NK1 fragment of HGF/SF suggests a novel mode for growth factor dimerization and receptor binding. Nat Struct Biol. 1999;6:72–79. doi: 10.1038/4947. [DOI] [PubMed] [Google Scholar]
- 9.Cioce V, Csaky K G, Chan A M-L, Bottaro D P, Taylor W G, Jensen R, Aaronson S A, Rubin J S. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J Biol Chem. 1996;271:13110–13115. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- 10.Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1–6. doi: 10.1016/s0014-5793(97)01475-0. [DOI] [PubMed] [Google Scholar]
- 11.Day R M, Cioce V, Breckenridge D, Castagnino P, Bottaro D P. Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene. 1999;18:3399–3406. doi: 10.1038/sj.onc.1202683. [DOI] [PubMed] [Google Scholar]
- 12.Dean M, Park M, Vande Woude G F. Characterization of the rearranged tpr-met oncogene breakpoint. Mol Cell Biol. 1987;7:921–924. doi: 10.1128/mcb.7.2.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferracini R, Di Renzo M F, Scotlandi K, Baldini N, Olivero M, Lollini P, Cremona O, Campanacci M, Comoglio P M. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene. 1995;10:739–749. [PubMed] [Google Scholar]
- 14.Ferracini R, Olivero M, Di Renzo M F, Martano M, De Giovanni C, Nanni P, Basso G, Scotlandi K, Lollini P-L, Comoglio P M. Retroviral expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene. 1996;11:1697–1705. [PubMed] [Google Scholar]
- 15.Gherardi E, Stoker M. Hepatocyte growth factor-scatter factor: mitogen, motogen, and Met. Cancer Cells. 1991;3:227–232. [PubMed] [Google Scholar]
- 16.Giordano S, Bardelli A, Zhen Z, Menard S, Ponzetto C, Comoglio P M. A point mutation in the MET oncogene abrogates metastasis without affecting transformation. Proc Natl Acad Sci USA. 1997;94:13868–13872. doi: 10.1073/pnas.94.25.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Stuart L A, Degen S J. Characterization of the DNF15S2 locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry. 1991;30:9768–9780. doi: 10.1021/bi00104a029. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann G, Naldini L, Weidner K M, Sachs M, Vigna E, Comoglio P M, Birchmeier W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but no mitogenesis. Proc Natl Acad Sci USA. 1992;89:11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer A, Kmiecik T E, Park M, Daar I, Blair D, Dunn K J, Sutrave P, Ihle J N, Bodescot M, Vande Woude G F. Structure, tissue-specific expression, and transforming activity of the mouse met protooncogene. Cell Growth Differ. 1990;1:87–95. [PubMed] [Google Scholar]
- 20.Jakubczak J, LaRochelle W J, Merlino G. NK1, a natural splice variant of hepatocyte growth factor/scatter factor, is a partial agonist in vivo. Mol Cell Biol. 1998;18:1275–1283. doi: 10.1128/mcb.18.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffers M, Rong S, Vande Woude G F. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med. 1996;74:505–513. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- 22.Jeffers M, Rong S, Anver M, Vande Woude G F. Autocrine hepatocyte growth factor/scatter factor-Met signaling induces transformation and the invasive/metastatic phenotype in C127 cells. Oncogene. 1996;13:853–861. [PubMed] [Google Scholar]
- 23.Jeffers M, Rong S, Vande Woude G F. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-Met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol. 1996;16:1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffers M, Schmidt L, Nakaigawa N, Webb C P, Weirich G, Kishida T, Zbar B, Vande Woude G F. Activating mutations for the MET tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jhappan C, Stahle C, Harkins R N, Fausto N, Smith G H, Merlino G T. TGFα overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 26.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 27.Khwaja A, Lehmann K, Marte B M, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 28.Lokker N A, Godowski P J. Generation and characterization of a competitive antagonist of human hepatocyte growth factor, HGF/NK1. J Biol Chem. 1993;268:17145–17150. [PubMed] [Google Scholar]
- 29.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 30.Miyazawa K, Kitamura A, Naka D, Kitamura N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur J Biochem. 1991;197:15–22. doi: 10.1111/j.1432-1033.1991.tb15876.x. [DOI] [PubMed] [Google Scholar]
- 31.Montesano R, Soriano J V, Malinda K M, Ponce M L, Bafico A, Kleinman H K, Bottaro D P, Aaronson S A. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ. 1998;9:355–365. [PubMed] [Google Scholar]
- 32.Naldini L, Vigna E, Narsimhan R P, Gaudino G, Zarnegar R, Michalopoulos G K, Comoglio P M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 33.Natali P G, Nicotra M R, Di Renzo M R, Prat M, Bigotti A, Cavaliere R, Comoglio P M. Expression of the c-MET/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993;68:746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.National Academy of Sciences. Guide for the care and use of laboratory animals. Washington, D.C.: Institute for Laboratory Animal Resources, National Research Council, National Academy of Sciences; 1996. [Google Scholar]
- 34.Okigaki M, Komada M, Uehara Y, Miyazawa K, Kitamura N. Functional characterization of human hepatocyte growth factor mutants obtained by deletion of structural domains. Biochemistry. 1992;31:9555–9561. doi: 10.1021/bi00155a007. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle W J, Bottaro D, Ellmore N, Vieira W, Owens J W, Anver M, Merlino G. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- 36.Palmiter R D, Sandgren E P, Koeller D M, Brinster R L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993;13:5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park M, Dean M, Cooper C S, Schmidt M, O'Brien S J, Blair D G, Vande Woude G F. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- 38.Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield M D, Comoglio P M. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 40.Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile M L, Giordano S, Narsimhan R, Comoglio P M. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J Biol Chem. 1996;271:14119–14123. doi: 10.1074/jbc.271.24.14119. [DOI] [PubMed] [Google Scholar]
- 41.Prat M, Narsimhan R P, Crepaldi T, Nicotra M R, Natali P G, Comoglio P M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- 42.Prat M, Crepaldi T, Pennacchietti S, Bussolino F, Comoglio P M. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J Cell Sci. 1998;111:237–247. doi: 10.1242/jcs.111.2.237. [DOI] [PubMed] [Google Scholar]
- 43.Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude G F. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rong S, Jeffers M, Resau J H, Tsarfaty I, Oskarsson M, Vande Woude G F. Met expression and sarcoma tumorigenicity. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- 45.Rong S, Oskarsson M, Faletto D L, Tsarfaty I, Resau J, Nakamura T, Rosen E, Hopkins R, Vande Woude G F. Tumorigenesis induced by coexpression of human hepatocyte growth factor and the human MET protooncogene leads to high levels of expression of the ligand and receptor. Cell Growth Differ. 1993;4:563–569. [PubMed] [Google Scholar]
- 46.Rong S, Segal S, Anver M, Resau J H, Vande Woude G F. Invasiveness and metastasis of NIH/3T3 cells induced by Met-HGF/SF autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong S, Donehower L A, Hansen M F, Strong L, Tainsky M, Jeffers M, Resau J H, Hudson E, Tsarfaty I, Vande Woude G F. Met proto-oncogene product is overexpressed in tumors of p53-deficient mice and tumors of Li-Fraumeni patients. Cancer Res. 1995;55:1963–1970. [PubMed] [Google Scholar]
- 48.Rosen E M, Lamszus K, Laterra J, Polverini P J, Rubin J S, Goldberg I D. HGF/SF in angiogenesis. Ciba Found Symp. 1997;212:215–226. doi: 10.1002/9780470515457.ch14. [DOI] [PubMed] [Google Scholar]
- 49.Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- 50.Rubin J S, Chan A M, Bottaro D P, Burgess W H, Taylor W G, Cech A C, Hirschfield D W, Wong J, Miki T, Finch P W, Aaronson S A. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin J S, Bottaro D P, Aaronson S A. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155:357–371. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 52.Sakata H, Takayama H, Sharp R, Rubin J S, Merlino G, LaRochelle W J. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- 53.Schmidt C, Bladt F, Goedecke S, Brinkman V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt L, Duh F M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J, Walther M M, Tsui L C, Geil L, Orcutt M L, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson M D, Moch H, Storkel S, Lerman M I, Linehan W M, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 55.Schwall R H, Chang L Y, Godowski P J, Kahn D W, Hillan K J, Bauer K D, Zioncheck T F. Heparin induces dimerization and confers proliferative activity onto the hepatocyte growth factor antagonists NK1 and NK2. J Cell Biol. 1996;133:709–718. doi: 10.1083/jcb.133.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp R, Babyatsky M W, Takagi H, Tagerud S, Wang T C, Bockman D E, Brand S J, Merlino G. Transforming growth factor-α disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development. 1995;121:149–161. doi: 10.1242/dev.121.1.149. [DOI] [PubMed] [Google Scholar]
- 57.Shaw L M, Rabinovitz I, Wang H H, Toker A, Mercurio A M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 58.Silvagno F, Follenzi A, Arese M, Prat M, Giraudo E, Gaudino G, Camussi G, Comoglio P M, Bussolino F. In vivo activation of met tyrosine kinase by heterodimeric hepatocyte growth factor molecule promotes angiogenesis. Arterioscler Thromb Vasc Biol. 1995;15:1857–1865. doi: 10.1161/01.atv.15.11.1857. [DOI] [PubMed] [Google Scholar]
- 59.Sonnenberg E, Meyer D, Weidner K M, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-Met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stahl S J, Wingfield P T, Kaufman J D, Pannell L K, Cioce V, Sakata H, Taylor W G, Rubin J S, Bottaro D P. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem J. 1997;326:763–772. doi: 10.1042/bj3260763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takayama H, LaRochelle W J, Anver M, Bockman D E, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takayama H, LaRochelle W J, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson S A, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takayama H, LaRochelle W J, Sabnis S G, Otsuka T, Merlino G. Renal tubular hyperplasia, polycystic disease and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth factor/scatter factor. Lab Investig. 1997;77:131–138. [PubMed] [Google Scholar]
- 64.Tsarfaty I, Rong S, Resau J H, Rulong S, Pinto da Silva P, Vande Woude G F. Met mediated signaling in mesenchymal to epithelial cell conversion. Science. 1994;263:98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 65.Tuck A B, Park M, Sterns E E, Boag A, Elliott B E. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- 66.Tulinsky A. The structures of domains of blood proteins. Thromb Haemostasis. 1991;66:16–31. [PubMed] [Google Scholar]
- 67.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 68.Ultsch M, Lokker N A, Godowski P J, de Vos A M. Crystal structure of the NK1 fragment of human hepatocyte growth factor at 2.0 A resolution. Structure. 1998;6:1383–1393. doi: 10.1016/s0969-2126(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 69.Weidner K M, Sachs M, Riethmacher D, Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc Natl Acad Sci USA. 1995;92:2597–2601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 71.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura T, Yuhki N, Wang M H, Skeel A, Leonard E J. Cloning, sequencing and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993;268:15461–15468. [PubMed] [Google Scholar]
- 73.Zarnegar R, Michalopoulos G K. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhuang Z, Park W S, Pack S, Schmidt L, Vortmeyer A O, Pak E, Pham T, Weil R J, Candidus S, Lubensky I A, Linehan W M, Zbar B, Weirich G. Trisomy 7-harbouring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Genet. 1998;20:66–69. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]