Abstract
Background
Evidence on transitional care for heart failure (HF) in Japan is limited.
Methods and Results
We implemented a transitional HF management program in rural Japan in 2019. This involved collaboration with general practitioners or nursing care facilities and included symptom monitoring by medical/nursing staff using a handbook; standardized discharge care planning and information sharing on self-care and advance care planning using a collaborative sheet; and sharing expertise on HF management via manuals. We compared the outcomes within 1 year of discharge among patients hospitalized with HF in the 2 years before program implementation (2017–2018; historical control, n=198), in the first 2 years after program implementation (2019–2020; Intervention Phase 1, n=205), and in the second 2 years, following program revision and regional dissemination (2021–2022; Intervention Phase 2, n=195). HF readmission rates gradually decreased over Phases 1 and 2 (P<0.05). This association was consistent regardless of physician expertise, follow-up institution, or the use of nursing care services (P>0.1 for interaction). Mortality rates remained unchanged, but significantly more patients received end-of-life care at home in Phase 2 than before (P<0.05).
Conclusions
The implementation of a transitional care program was associated with decreased HF readmissions and increased end-of-life care at home for HF patients in rural Japan.
Key Words: Discharge care planning, General practitioners, Heart failure handbook, Information sharing
Heart failure (HF) is a major health problem in aging societies. A recent Japanese study reported that the number of patients hospitalized for acute HF is expected to reach 250,000 by 2040, with 60% of these patients being aged ≥85 years.1 The management of older patients with HF requires care for their immediate condition, as well as comprehensive assessment and support to meet their physical, psychological, and social needs.2–4 Therefore, interventions from cardiologists alone have their limitations, and collaboration with general practitioners (GPs) and nursing care staff who provide primary care for these patients is essential.5 Various transitional care models for HF have been proposed, mainly in Western countries.6 These models include patient and family education, discharge care planning and coordination, and the use of long-term care services after discharge. These interventions have been shown to reduce mortality and readmission rates, improve quality of life (QOL), reduce caregiver burden, enable patient-centered care, and reduce healthcare costs.6–9 However, Japan has different medical and long-term care systems from Western countries, as well as regional disparities in medical resources between urban and rural areas.10,11 It is therefore important to develop a collaborative system that is tailored to each region. However, there are no established models for transitional HF care in Japan and the development of role models may help promote transitional care for HF in each region.
In response to the Basic Act on Countermeasures against Cardiovascular Diseases enacted in 2019,12 we developed and implemented a transitional HF management program in the western region of Tottori Prefecture,13 a rural area in Japan. The aim of the present study was to investigate whether the implementation of this program was associated with improved outcomes for HF patients in rural Japan.
Methods
Subjects
We conducted a retrospective analysis of 979 consecutive patients who were hospitalized for acute decompensated HF at Tottori University Hospital between January 2017 and December 2022. We diagnosed HF based on Framingham criteria, B-type natriuretic peptide (BNP) concentrations, pulmonary congestion on X-rays, or pulmonary hypertension assessed by Doppler echocardiography, excluding acute coronary syndrome and acute pulmonary embolism.14 Tottori University Hospital established a transitional HF management program in 2019, and has continued it since (Figures 1,2).
Figure 1.
Transitional heart failure (HF) management program in western Tottori Prefecture. GPs, general practitioners. Reproduced with permission from the Western Tottori Medical Association.
Figure 2.
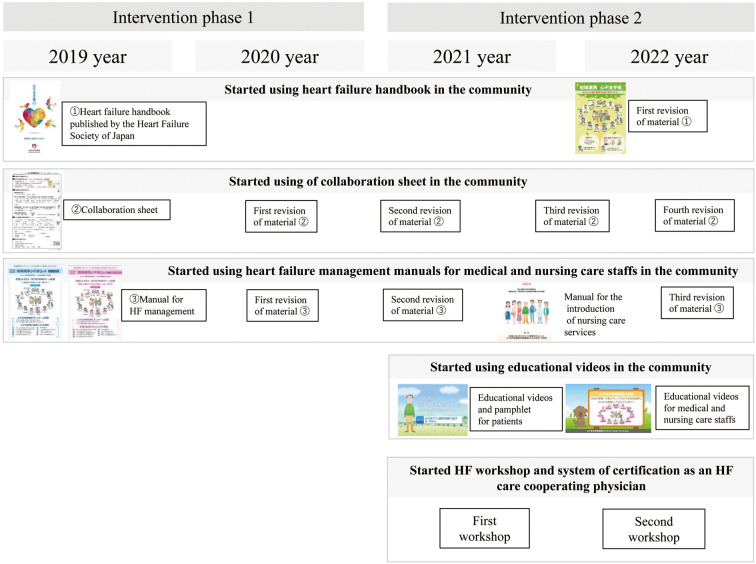
History of development and implementation of the transitional heart failure (HF) management program.
We evaluated the association between program implementation and outcomes over time by comparing 3 groups of patients: those admitted in the 2 years before program implementation (historical control group; n=326), those admitted in the first 2 years of the program in 2019–2020 (Intervention Phase 1; n=344), and those admitted during the second 2 years, after the program’s revision and regional dissemination, in 2021–2022 (Intervention Phase 2; n=309). We excluded in-hospital deaths (n=48), cases transferred to other hospitals or departments (n=206), readmissions of the same patient (n=123), and cases lost to follow-up (n=4). This left 198 patients in the control group, 205 patients in the Phase 1 group and 195 patients in the Phase 2 group for analysis (Supplementary Figure 1).
Transitional HF Management Program
Tottori University and the Western Tottori Medical Association have been jointly developing and operating a transitional HF program (Figures 1,2).13 Tottori Prefecture has the smallest population in Japan and is a rural area. In the western area of the prefecture (Yonago City and Sakaiminato City), there are 5 hospitals (Tottori University, Sanin Rosai Hospital, Hakuai Hospital, Yonago Medical Center, and Saiseikai Sakaiminato Hospital) with full-time cardiologists who provide inpatient care for HF patients. The aim of the transitional HF program was to improve the quality of HF care in the community by standardizing care during and after hospitalization for patients with worsening HF through a collaboration among these 5 facilities, local GPs, and nursing care facilities. The program comprises 3 elements of regional collaboration: sharing information about a patient’s physical condition, sharing patient information, and sharing HF management practices.
Transitional HF care programs have not been established in Japan, and it is difficult to provide completed programs immediately. Thus, it is necessary to continually update the transitional HF program according to the needs of the medical and care staff. It also takes time for regional care staff to recognize and become familiar with the transitional HF program before treatment outcomes stabilize. Therefore, in order to evaluate the effectiveness of the transitional HF program, it was necessary to evaluate long-term changes over time, such as during program development and implementation (Phase 1) and during program modification and dissemination to the community (Phase 2).
Activities in the Phase 1 of the Program
The following basic elements were developed and implemented during the first 2 years of the transitional HF program (Phase 1).
Use of the HF Handbook in the Community The HF handbook is a resource published by the Heart Failure Society of Japan to support self-care monitoring (https://www.asas.or.jp/jhfs/topics/shinhuzentecho.html). It is designed for use by patients and their families. The Tottori medical association provides the HF handbook free of charge to medical institutions, and patients are advised to bring the handbook when they visit outpatient clinics or nursing care institutions so that medical and nursing staff can check their weight, blood pressure, pulse rate, and HF symptoms. An original flowchart of guidelines for seeking medical attention was pasted on the back of the handbook. Patients were encouraged to consult a healthcare provider if they experienced unexpected weight gain of 2–3 kg, shortness of breath, or swelling. This aspect of the program aimed to facilitate the early diagnosis and treatment of worsening HF in the community.
Use of HF Management Manual by GPs and Nursing Staff Manuals on HF management for GPs and nursing care staff were developed. These manuals explained the purpose of preventing HF from worsening, how to examine and observe HF patients, how to respond to worsening HF, guidelines for consulting a cardiologist, an explanation of guideline-directed medical therapy for HF and drugs that require attention for worsening HF, how to educate patients, and the importance of advance care planning (ACP). The manual was attached to referral letters from the hospital to GPs or nursing care institutions.
Use of a Collaboration Sheet in the Community The collaboration sheet is a resource for standardizing discharge care planning and information sharing (Supplementary Figure 2). During hospitalization, multidisciplinary teams make joint decisions with patients and families on post-discharge self-care, post-discharge support, and ACP, and complete the collaboration sheet. Our program asked families to provide self-care support for patients with self-care problems, such as cognitive impairment. If obtaining family support was difficult, we actively recommended the use of long-term care services. The collaboration sheet is given to patients and their families as a discharge care plan at the time of discharge. The sheet is also shared on the electronic medical record and information is passed to the relevant outpatient setting. The collaboration sheet is also sent with a referral letter to a patient’s GP and/or nursing care institution after discharge, so that all those providing care have seen the post-discharge care plan and ACP, and seamless care can be provided.
Implementation of ACP Four items are completed as an ACP (patient values, treatment preferences, surrogate decision maker, and preferred place of care) using a collaboration sheet (Supplementary Figure 2). After educating the patients about the ACP, nurses reviewed the ACP and completed the sheet. We defined ACP implementation as when any of the 4 items were completed in the collaboration sheet.
Outpatient Follow-up by Nurses Outpatient nurses at Tottori University Hospital interviewed patients on their first outpatient visit after discharge and followed them up after discharge. The introduction of the program strengthened this discharge support. Nurses were required to check patients’ self-care using the HF handbook and collaboration sheet, and to provide additional education when necessary.
Activities in Pahse 2 of the Program
During the second 2 years of program implementation (Phase 2), the basic program was regularly revised. The educational activities of regional staff were also strengthened to disseminate the program, as described below.
Revisions to the Program Each program resource was regularly revised. The HF management manual and collaboration sheet were repeatedly revised in response to requests from medical and care staff. From 2022, we also created and used an original HF handbook with enhanced community collaboration functions. We introduced a manual for the use of nursing care services for HF patients from 2021. This manual provides specific information on how to prevent HF from worsening by using long-term care services, and promotes medical and nursing care cooperation. It was produced by the Ministry of Health, Labour and Welfare’s Scientific Research Group and is available to download free of charge (https://plaza.umin.ac.jp/isobegroup/).
Use of Educational Videos in the Community To standardize education on HF for hospitalized patients and their families, educational videos and pamphlets were introduced in 2021. This material consists of 5 narrative instructional videos, and covers recommendations on self-care, ACP practices, and the use of nursing services. Patients admitted with HF watched the videos while in hospital, and nurses checked their understanding afterwards. The videos and pamphlets were also produced by the Ministry of Health, Labour and Welfare’s Scientific Research Group, and are also available to download free of charge (https://plaza.umin.ac.jp/isobegroup/).
From 2021, short educational videos (3–5 min) for medical and care staff have been created and distributed on YouTube.15 These videos have been played during free time when HF-related lectures are given in the community.
HF Workshop and System of Certification as an HF Care Cooperating Physician The Western Tottori Medical Association has started a unique certification system for GPs called “HF care cooperating physician”. This system aims to improve HF care in the region by holding regular HF workshops and providing credits and certification to those who attend them. Regular HF workshops have been held since 2021 to disseminate the transitional care program. Over a period of 4 years, the intervention program shown in Figures 1 and 2 was developed and established. More information about collaborative programs is available from the Western Tottori Medical Association website (https://www.seibu.tottori.med.or.jp/isikai/path/path.html).
Data Collection
We collected data on demography, medical history, comorbidities, laboratory tests, echocardiograms, and medications at discharge. We calculated the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score, a prognostic indicator based on 13 variables developed in a previous study.16,17 The variables are age, sex, left ventricular ejection fraction, systolic blood pressure, creatinine, body mass index, New York Heart Association functional class, smoking status, diabetes, chronic obstructive disease, history of HF, and medication status.
We conducted a 1-year follow-up of patients using medical records and telephone interviews. The physicians conducting the follow-up were categorized into 2 groups: cardiologists and non-cardiologists. Patients who were followed up primarily by their GPs but also regularly by Tottori University Hospital were defined as receiving collaborative follow-up.18 The outcomes of interest were all-cause mortality, unplanned rehospitalization for HF, death from cardiovascular causes, and the place where end-of-life care was received. Receiving end-of-life care at home was defined as “death at home” or at “skilled nursing home”, and sudden deaths were excluded. We considered death from cardiovascular causes as death due to HF, sudden death, or death from other cardiovascular causes.
This retrospective study followed the principles of the Declaration of Helsinki and the ethical guidelines for epidemiologic studies of the Ministry of Health, Labour and Welfare, Japan. The Research Ethics Committee of Tottori University approved the study. We released research information to the public and obtained and analyzed the data without informed consent from each patient, as allowed by the ethical guidelines.
Statistical Analysis
Continuous variables are expressed as the median with interquartile range (IQR). The significance of differences in continuous variables was determined using the Kruskal-Wallis test. Categorical variables were compared using Fisher’s exact test. The significance of differences between pairs was assessed using the Bonferroni test. We estimated cumulative event curves using the Kaplan-Meier method, and compared them using the log-rank trend test. Cox proportional hazards models were used to assess the association of the program with outcomes over time and its interaction with each subgroup. We created 2 prognostic models. Model 1 is a model in which the historical control group was used as a reference, with the model adjusted for patient background factors that differ between the control, phase 1, and phase 2 groups (P<0.05). Model 2 is a model with well-known prognostic factors, including MAGGIC score, serum sodium, and BNP concentrations, added to Model 1.
P<0.05 (a two-tailed test) was considered statistically significant, and a significant interaction was set at P<0.1. All analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 2.13.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 presents the characteristics of patients enrolled before and after implementation of the program. The implementation rates of the program, defined as the rate of completion of the collaboration sheets, were 89.3% in Phase 1 and 92.3% in Phase 2. Reasons for not fully implementing the program in some patients included no time for intervention due to unscheduled discharge from hospital for delirium and staff’s non-adherence to the program.
Table 1.
Patient Characteristics in the Historical Control, Intervention Phase 1, and Intervention Phase 2 Groups
| Historical control (2017–2018; n=198) |
Phase 1 (2019–2020; n=205) |
Phase 2 (2021–2022; n=195) |
P value | |
|---|---|---|---|---|
| Age (years) | 78 [69–86] | 80 [71–86] | 80 [72–86] | 0.541 |
| Male sex | 109 (55.1) | 120 (58.5) | 113 (57.9) | 0.750 |
| BMI (kg/m2) | 20.7 [18.8–22.6] | 20.8 [18.5–23.4] | 20.8 [18.6–23.1] | 0.913 |
| SBP (mmHg) | 114 [102–129] | 111 [98–125] | 114 [102–130] | 0.141 |
| Prior HF admission | 53 (26.8) | 61 (29.8) | 58 (29.7) | 0.769 |
| NYHA Class III/IV | 23 (11.6) | 29 (14.1) | 35 (17.9) | 0.202 |
| LVEF ≥50% | 89 (44.9) | 106 (51.7) | 87 (44.6) | 0.275 |
| MAGGIC risk score | 25 [21–30] | 26 [22–31] | 26 [22–30] | 0.203 |
| Hospital stay (days) | 21 [16–28] | 22 [17–29] | 21 [15–27] | 0.206 |
| Comorbidity | ||||
| CAD | 59 (29.8) | 62 (30.2) | 63 (32.3) | 0.856 |
| Hypertension | 137 (69.2) | 140 (68.3) | 133 (68.2) | 0.981 |
| Diabetes | 80 (40.4) | 74 (36.1) | 66 (33.8) | 0.392 |
| Dyslipidemia | 85 (42.9) | 88 (42.9) | 65 (33.3) | 0.079 |
| Atrial fibrillation | 85 (42.9) | 96 (46.8) | 81 (41.5) | 0.543 |
| COPD | 10 (5.1) | 19 (9.3) | 18 (9.2) | 0.180 |
| CVD | 34 (17.2) | 33 (16.1) | 39 (20.0) | 0.579 |
| Medications | ||||
| ACE-i/ARB | 133 (67.2) | 109 (53.2)* | 94 (48.2)* | <0.001 |
| ARNI | 0 (0.0) | 1 (0.5) | 32 (16.4)* | <0.001 |
| β-blocker | 146 (73.7) | 149 (72.7) | 148 (75.9) | 0.762 |
| MRA | 68 (34.3) | 80 (39.0) | 87 (44.6) | 0.115 |
| SGLT2 inhibitors | 1 (0.5) | 8 (3.9) | 47 (24.1)*,† | <0.001 |
| Loop diuretics | 183 (92.4) | 192 (93.7) | 172 (88.2) | 0.134 |
| Tolvaptan | 54 (27.3) | 58 (28.3) | 85 (43.6)*,† | 0.001 |
| Device therapy | ||||
| CRT/ICD | 9 (4.5) | 6 (2.9) | 9 (4.6) | 0.590 |
| Laboratory values | ||||
| Hemoglobin (g/dL) | 11.8 [10.4–13.4] | 11.8 [10.2–13.9] | 12.0 [10.5–13.7] | 0.806 |
| Sodium (mEq/L) | 140 [137–141] | 139 [137–141] | 139 [137–141] | 0.079 |
| BUN (mg/dL) | 30.7 [21.3–44.0] | 30.0 [21.0–47.1] | 27.7 [20.1–43.6] | 0.387 |
| eGFR (mL/min/1.73 m2) | 41.8 [28.1–58.6] | 41.6 [26.6–55.5] | 42.7 [28.2–56.6] | 0.721 |
| Albumin (g/dL) | 3.6 [3.3–3.9] | 3.6 [3.3–3.9] | 3.5 [3.1–3.7]† | 0.043 |
| BNP (pg/mL; n=559) | 245.4 [113.8–479.4] | 242.7 [130.5–474.2] | 291.2 [161.1–517.4] | 0.115 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). *P<0.05 compared with the historical control group; †P<0.05 compared with the Phase 1 group. ACE-i, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronized therapy; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter 2.
The mean (±SD) age of enrolled patients was 76±13 years, 57.1% were male, 30.8% had ischemic heart disease, and the mean (±SD) MAGGIC score was 25.4±6.7 points. Patients in the Phase 2 group were more likely to be using angiotensin receptor-neprilysin inhibitor (ARNI), sodium-glucose cotransporter 2 (SGLT2) inhibitors, and tolvaptan than those in the control group (all P<0.05). Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were prescribed less frequently in the Phase 1 and Phase 2 groups than in the historical control group (all P<0.05). The Phase 2 group had lower serum albumin concentrations than the historical control group (P<0.05).
Details of post-discharge follow-up and the use of long-term care services are presented in Table 2. Overall, 62.5% of patients were followed up by other clinics or hospitals after discharge, 37.1% were followed up by non-cardiologists and 13.0% were followed up jointly by Tottori University Hospital and their GP. Outpatient nurses in Tottori University Hospital followed up 35.1% of patients. During the enrolment period, the rate of follow-up in other institutions and the rate of follow-up by non-cardiologists did not change, but the percentage of follow-up by outpatient nurses increased significantly in the Phase 2 group compared with the historical control group (P<0.05). In all, 18.2% of patients were living alone, 34.6% of patients were certified as needing support or nursing care by long-term care insurance, 22.7% used home services such as visiting medical care, visiting nursing, home helper and skilled nursing homes, and 17.4% used daycare services. The use of visiting nursing was significantly higher in the Phase 1 group than in the historical control group (P<0.05).
Table 2.
Cooperation of Medical and Nursing Care, and Clinical Outcomes After Discharge
| Historical control (2017–2018; n=198) |
Phase 1 (2019–2020; n=205) |
Phase 2 (2021–2022; n=195) |
P value | |
|---|---|---|---|---|
| Type of follow-up | ||||
| Other institution | 121 (61.1) | 131 (63.9) | 122 (62.6) | 0.843 |
| Non-cardiologist | 80 (40.4) | 73 (35.6) | 69 (35.4) | 0.516 |
| CollaborativeA | 27 (13.6) | 23 (11.2) | 28 (14.4) | 0.624 |
| Outpatient nurse | 58 (29.3) | 67 (32.7) | 85 (43.6)* | 0.009 |
| Living environment and care services | ||||
| Living alone | 37 (18.7) | 36 (17.6) | 36 (18.5) | 0.964 |
| Support or care required in long-term care insurance |
60 (30.3) | 71 (34.6) | 76 (39.0) | 0.199 |
| In-home service user | 34 (17.2) | 55 (26.8) | 47 (24.1) | 0.056 |
| Visiting medical care | 4 (2.0) | 11 (5.4) | 13 (6.7) | 0.063 |
| Visiting nursing | 16 (8.1) | 37 (18.0)* | 25 (12.8) | 0.011 |
| Home helper | 8 (4.0) | 13 (6.3) | 12 (6.2) | 0.548 |
| Skilled nursing home | 13 (6.6) | 16 (7.8) | 16 (8.2) | 0.822 |
| Daycare service user | 35 (17.7) | 36 (17.6) | 33 (16.9) | 0.981 |
| Clinical outcomes | ||||
| All-cause death | 24 (12.1) | 24 (11.7) | 27 (13.8) | 0.808 |
| Cardiovascular death | 10 (5.1) | 17 (8.3) | 15 (7.7) | 0.389 |
| Sudden death | 2 (1.0) | 5 (2.4) | 5 (2.6) | 0.478 |
| HF hospitalization | 44 (22.2) | 41 (20.0) | 26 (13.3) | 0.057 |
Unless indicated otherwise, data are given as n (%). *P<0.05 compared with the historical control group. ACollaborative follow-up was defined as patient follow up primarily by general practitioners in addition to regular follow up by Tottori University Hospital.
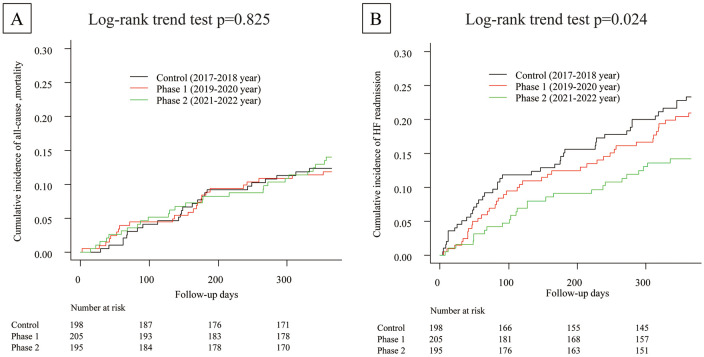
Figure 3 shows Kaplan-Meier curves for all-cause mortality and HF readmission. The mortality rate remained unchanged and the cause of death did not differ between the control, Phase 1, and Phase 2 groups (Table 2). However, the HF readmission rate decreased gradually from Phase 1 to Phase 2 (log-rank Ptrend<0.05). Table 3 shows the multivariate Cox hazard models for predicting all-cause mortality and HF readmissions. Even after adjustment for differences in patient characteristics (Model 1) or well-known prognostic factors (Model 2), the rate of readmission was significantly lower in the Phase 2 group than in the historical control group (P<0.05), whereas mortality rates remained unchanged.
Figure 3.
Clinical outcomes for the historical control, Phase 1, and Phase 2 groups: (A) all-cause mortality; (B) heart failure (HF) rehospitalization.
Table 3.
Prognostic Models for All-Cause Mortality and HF Rehospitalization
| Predictors | All-cause mortality | HF rehospitalization | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1 | ||||
| Control | Reference | – | Reference | – |
| Phase 1 | 0.963 (0.544–1.706) | 0.898 | 0.793 (0.516–1.221) | 0.292 |
| Phase 2 | 0.990 (0.556–1.764) | 0.973 | 0.444 (0.261–0.753) | 0.003 |
| ACE-i/ARB | 0.663 (0.416–1.056) | 0.084 | 0.546 (0.371–0.804) | 0.002 |
| ARNI | 0.354 (0.080–1.566) | 0.171 | 0.828 (0.271–2.532) | 0.740 |
| SGLT2 inhibitors | 0.615 (0.182–2.076) | 0.433 | 0.504 (0.171–1.483) | 0.213 |
| Tolvaptan | 3.489 (2.157–5.645) | <0.001 | 3.390 (2.309–4.977) | <0.001 |
| Albumin | 0.339 (0.2193–0.523) | <0.001 | 0.823 (0.569–1.191) | 0.302 |
| Model 2 | ||||
| Control | Reference | – | Reference | – |
| Phase 1 | 0.931 (0.501–1.730) | 0.820 | 0.886 (0.568–1.382) | 0.593 |
| Phase 2 | 1.163 (0.628–2.155) | 0.631 | 0.515 (0.296–0.894) | 0.018 |
| MAGGIC risk score | 1.060 (1.014–1.109) | 0.011 | 1.054 (1.018–1.090) | 0.003 |
| ARNI | 0.360 (0.076–1.698) | 0.197 | 1.138 (0.363–3.562) | 0.825 |
| SGLT2 inhibitors | 0.388 (0.089–1.683) | 0.206 | 0.461 (0.154–1.378) | 0.166 |
| Tolvaptan | 3.137 (1.856–5.302) | <0.001 | 2.791 (1.857–4.194) | <0.001 |
| Albumin | 0.439 (0.266–0.724) | <0.001 | 0.988 (0.657–1.486) | 0.953 |
| Sodium | 0.966 (0.904–1.032) | 0.309 | 0.965 (0.916–1.018) | 0.193 |
| BNP | 1.000 (1.000–1.000) | 0.109 | 1.000 (0.999–1.000) | 0.563 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
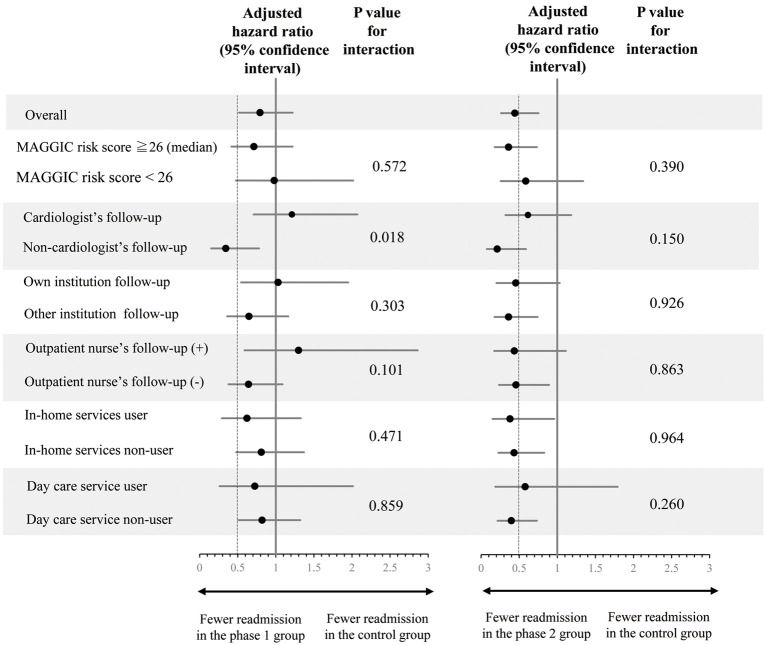
Figure 4 shows the association of program implementation with HF readmission for each subgroup. In the Phase 1 group, there was a significant interaction between program implementation and physician expertise. The decrease in the rate of readmissions for HF after program introduction was greater for non-cardiologists than cardiologists (P<0.05 for interaction). However, the decreased rate of readmissions for HF in Phase 2 was similar across all subgroups, regardless of the risk of worsening HF (MAGGIC risk score), physician expertise, follow-up institution, or use of nursing care services (P>0.1 for interaction).
Figure 4.
Subgroup analysis of the effects of the transitional program on heart failure (HF) readmission showing historical control vs. Phase 1 (Left) and Phase 2 (Right). Cox hazard models were adjusted for albumin concentrations and the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, angiotensin receptor-neprilysin inhibitor, sodium-glucose cotransporter 2 inhibitors, and tolvaptan. Dashed lines indicate a hazard ratio of 0.5. MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure.
Supplementary Figure 3 shows ACP implementation during hospitalization. If ACP is defined as the completion of 1 of the 4 items (patient values, treatment preferences, surrogate decision-maker, and preferred place of care) on the collaboration sheet, ACP was implemented for 72.0% of patients in Phase 1 and 81.0% of patients in Phase 2, with the rate of implementation increasing in Phase 2 compared with Phase 1 (P<0.05).
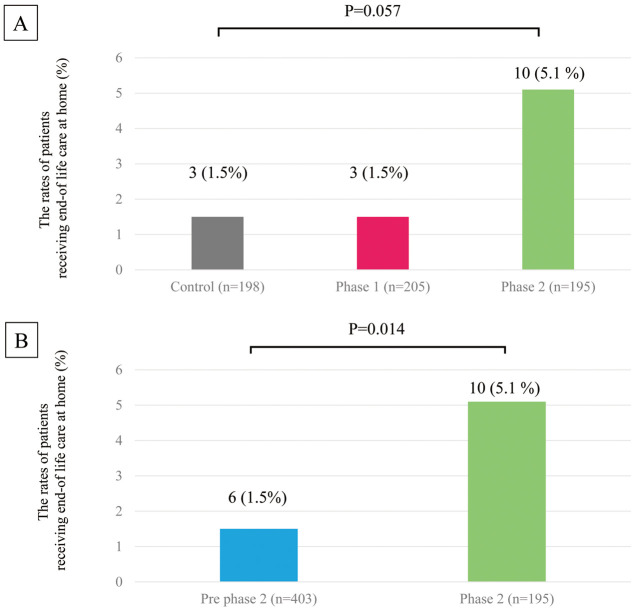
Figure 5 shows the rates of end-of-life care at home for the historical control, Phase 1, and Phase 2 groups. Comparing the 3 groups shows that the rate of care tended to be higher in Phase 2, but the number of events in the control and Phase 1 groups was small and did not reach statistical significance (Figure 5A; P=0.057). We therefore combined the control and Phase 1 groups into a new group (“pre-Phase 2 group”) and found that the rate of end-of-life care at home was significantly higher in the Phase 2 than pre-Phase 2 group (Figure 5B; P<0.05).
Figure 5.
Rates of patients receiving end-of-life care at home in the (A) historical control, Phase 1, and Phase 2 groups and (B) pre-Phase 2 (historical control plus Phase 1) and Phase 2 groups.
Discussion
We implemented a transitional HF care program involving collaboration with GPs and nursing care facilities in rural Japan. HF readmissions gradually decreased after the introduction of this transitional care program (Figure 3). We also found that program implementation was associated with decreased HF readmissions regardless of the risk of HF worsening, physician expertise, follow-up institution, or the use of nursing care services (Figure 4). Mortality did not improve, but the rate of end-of-life care at home increased (Figure 5). Our program therefore appears to have reduced HF readmissions and provided patient-centered care by promoting community collaboration between hospitals, GPs, and nursing care facilities.
The transitional care model for HF has shown positive outcomes in Western countries.6 A recent meta-analysis found that visiting nursing and multidisciplinary outpatient management can improve prognosis and prevent rehospitalization for HF patients.9 In Japan, small randomized controlled trials have also reported effects of visiting nursing and nurse case management on HF rehospitalization and QOL.19,20 We have expanded previous findings by testing the usefulness of broader collaboration involving GPs and nursing care facilities in routine clinical practice. Our program includes regional collaboration using the HF handbook. The HF handbook helps patients to monitor their weight, blood pressure, pulse rate, and HF symptoms (shortness of breath, swelling) and to seek early treatment if they worsen. A previous study reported that the use of the HF handbook can reduce the risk of HF rehospitalization by providing standardized monitoring criteria and encouraging consultation with a healthcare provider.21 In Phase 2 of our program, we created an original HF handbook (Figure 2). The handbook was designed to encourage patients, family members, and healthcare providers to consult in the event of worsening HF and to help build consensus on the process of consultation in our region. The revision of the HF handbook may be one reason for the decrease in HF readmissions in the Phase 2 group.
After implementation of the program, the use of outpatient nurse follow-up and visiting nursing increased significantly (Table 2). This type of multidisciplinary outpatient follow-up has been reported to be an effective transitional care strategy.9 In the present study, when adjusted for factors associated with readmission (MAGGIC score and tolvaptan use), patients who were followed up by a nurse had an approximately 30% lower risk of readmission than those who were not, although the difference was not statistically significant (hazard ratio [HR] 0.701; 95% confidence interval [CI] 0.468–1.049; P=0.084). However, there were no significant differences in the risk of readmission between patients who received home-visit nursing and those who did not (HR 1.357; 95% CI 0.848–2.172; P=0.203). There may be confounding factors, such as self-care issues and cognitive function, that were not assessed in the present study that differ between patients who required home-visit nursing and those who did not, and the effects of home-visit nursing may not have been fully examined; thus, further studies are needed.
We developed and repeatedly revised HF management manuals and held regular workshops for local medical and nursing care staff (Figure 2). A survey in Japan found that GPs who were not cardiologists were less knowledgeable about HF management than cardiologists.5 A survey of nursing care staff in Japan also found that nursing care staff had limited knowledge of HF and few opportunities to learn about it.15,22 Nursing care staff who had less exposure to HF education were also less likely to participate in community collaborations on HF.15 It is therefore important to share expertise on HF management with GPs and community nursing care staff. The effect of the program implementation on reducing HF readmission appears to be faster among non-cardiologists than cardiologists (Figure 4 Left). The intervention may therefore have more potential to result in improvements among non-specialist professions and is an effective strategy in rural areas where access to specialized care is limited. Phase 2 of the program included regular HF workshops. We also provided educational materials, such as patient education videos and manuals on how to use nursing care services, and started community education activities using YouTube videos (Figure 2). These educational activities may have developed the care teams’ knowledge and gradually improved outcomes after implementation of the program.
Mortality did not improve with the implementation of the program (Figure 3). This may be partly because older HF patients often have comorbidity and frailty.23 However, the use of end-of-life care at home increased in Phase 2 of the program (Figure 5). This may be because our program included the implementation of ACPs (Supplementary Figure 3) and their sharing of these plans with the community team. Allowing patients to receive care in their preferred place may have contributed to patient-centered care and improved QOL. End-of-life care at home may also have contributed to fewer hospital readmissions, resulting in cost savings and better use of medical resources.24
This study has some limitations. First, it was conducted during the COVID-19 pandemic, which may have affected the healthcare system and outcomes. However, Tottori Prefecture had a lower incidence of infection than the rest of Japan, so the impact was probably minimal. Second, after 2021, new therapeutic agents, such as SGLT2 inhibitors and ARNI, were prescribed more frequently as Japanese guidelines were updated,25 which may have reduced the rate of HF rehospitalizations in the Phase 2 group. However, the results after adjustment for the use of these medications suggest that the program remained effective (Table 3). Third, patient-reported outcomes and self-care scores were not assessed in the present study. In addition, the frequency of unscheduled medical visits could not be assessed. Finally, this study was a retrospective single-center observational study. Prospective studies are needed to confirm the effectiveness of the program in other regions.
In conclusion, the implementation of a transitional care program was associated with decreased HF readmissions and increased use of end-of-life care at home for HF patients in rural Japan. Continuous program improvement and community outreach may be the keys to improved outcomes.
Sources of Funding
This study was supported by a grant from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant no. 20K08403).
Disclosures
Y.K. has received lecturer fees from AstraZeneca Co. Ltd and Boehringer Ingelhem Co., Ltd. K.Y. has received research grants from Abbott Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Biotronik Japan Inc., Japan Lifeline Co., Ltd., Fukuda Denshi; Medtronic Japan Co., Ltd., and Boston Scientific Co., Ltd., as well as lecturer fees from Otsuka Pharmaceutical Co., Ltd., Novartis, Daiichi-Sankyo Co. Ltd., and Bayer Yakuhin Ltd. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Ethics Committee of Tottori University (No. 2418).
Supplementary Files
Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Acknowledgment
The authors thank Melissa Leffler, MBA, from Edanz (https://www.jp.edanz.com/ac) for editing a draft of this manuscript.
Data Availability
The deidentified participant data will not be shared.
References
- 1. Fujimoto W, Toh R, Takegami M, Hayashi T, Kuroda K, Hatani Y, et al.. Estimating incidence of acute heart failure syndromes in Japan: An analysis from the KUNIUMI Registry. Circ J 2021; 85: 1860–1868, doi:10.1253/circj.CJ-20-1154. [DOI] [PubMed] [Google Scholar]
- 2. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, et al.. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur J Heart Fail 2020; 22: 2112–2119, doi:10.1002/ejhf.1926. [DOI] [PubMed] [Google Scholar]
- 3. Kato N, Kinugawa K, Yao A, Hatano M, Shiga T, Kazuma K.. Relationship of depressive symptoms with hospitalization and death in Japanese patients with heart failure. J Card Fail 2009; 15: 912–919, doi:10.1016/j.cardfail.2009.06.442. [DOI] [PubMed] [Google Scholar]
- 4. White-Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, et al.. Addressing social determinants of health in the care of patients with heart failure: A scientific statement from the American Heart Association. Circulation 2020; 141: e841–e863, doi:10.1161/CIR.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 5. Kinugasa Y, Saitoh M, Ikegame T, Ikarashi A, Kadota K, Kamiya K, et al.. Differences in priorities for heart failure management between cardiologists and general practitioners in Japan. Circ J 2021; 85: 1565–1574, doi:10.1253/circj.CJ-21-0335. [DOI] [PubMed] [Google Scholar]
- 6. Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, et al.. Transitions of care in heart failure: A scientific statement from the American Heart Association. Circ Heart Fail 2015; 8: 384–409, doi:10.1161/HHF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Fu MR, Fang J, Zheng H, Luo B.. The effectiveness of transitional care interventions for adult people with heart failure on patient-centered health outcomes: A systematic review and meta-analysis including dose-response relationship. Int J Nurs Stud 2021; 117: 103902, doi:10.1016/j.ijnurstu.2021.103902. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Fu MR, Luo B, Li M, Zheng H, Fang J.. The effectiveness of transitional care interventions on health care utilization in patients discharged from the hospital with heart failure: A systematic review and meta-analysis. J Am Med Dir Assoc 2021; 22: 621–629, doi:10.1016/j.jamda.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 9. Van Spall HGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, et al.. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur J Heart Fail 2017; 19: 1427–1443, doi:10.1002/ejhf.765. [DOI] [PubMed] [Google Scholar]
- 10. Ito T, Kameda I, Fujimoto N, Momosaki R.. Regional disparities in cardiac rehabilitation volume throughout Japan based on open data from a national database of health insurance claims. J Rural Med 2022; 17: 221–227, doi:10.2185/jrm.2022-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsuo Y, Yoshimine F, Fuse K, Suzuki K, Sakamoto T, Iijima K, et al.. Regional disparities in adherence to guidelines for the treatment of chronic heart failure. Intern Med 2021; 60: 525–532, doi:10.2169/internalmedicine.4660-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuwabara M, Mori M, Komoto S.. Japanese national plan for promotion of measures against cerebrovascular and cardiovascular disease. Circulation 2021; 143: 1929–1931, doi:10.1161/CIRCULATIONAHA.120.052737. [DOI] [PubMed] [Google Scholar]
- 13. Kinugasa Y, Fukuki M, Hirota Y, Ishiga N, Kato M, Mizuta E, et al.. Differences in needs for community collaboration for heart failure between medical and nursing care staff. Heart Vessels 2022; 37: 969–975, doi:10.1007/s00380-021-01988-8. [DOI] [PubMed] [Google Scholar]
- 14. Kinugasa Y, Kato M, Sugihara S, Yanagihara K, Yamada K, Hirai M, et al.. Multidisciplinary intensive education in the hospital improves outcomes for hospitalized heart failure patients in a Japanese rural setting. BMC Health Serv Res 2014; 14: 351, doi:10.1186/1472-6963-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinugasa Y, Adachi T, Fukuki M, Hirota Y, Ishiga N, Kato M, et al.. Factors affecting the willingness of nursing care staffs for cooperation with heart failure care and the role of internet video education. J Gen Fam Med 2023; 25: 19–27, doi:10.1002/jgf2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, et al.. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413, doi:10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 17. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, et al.. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail 2018; 5: 610–619, doi:10.1002/ehf2.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Washida K, Kato T, Ozasa N, Morimoto T, Yaku H, Inuzuka Y, et al.. A comparison between hospital follow-up and collaborative follow-up in patients with acute heart failure. ESC Heart Fail 2023; 10: 353–365, doi:10.1002/ehf2.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuchihashi-Makaya M, Matsuo H, Kakinoki S, Takechi S, Kinugawa S, Tsutsui H, et al.. Home-based disease management program to improve psychological status in patients with heart failure in Japan. Circ J 2013; 77: 926–933, doi:10.1253/circj.CJ-13-0115. [DOI] [PubMed] [Google Scholar]
- 20. Mizukawa M, Moriyama M, Yamamoto H, Rahman MM, Naka M, Kitagawa T, et al.. Nurse-led collaborative management using telemonitoring improves quality of life and prevention of rehospitalization in patients with heart failure. Int Heart J 2019; 60: 1293–1302, doi:10.1536/ihj.19-313. [DOI] [PubMed] [Google Scholar]
- 21. Nakane E, Kato T, Tanaka N, Kuriyama T, Kimura K, Nishiwaki S, et al.. Association of the induction of a self-care management system with 1-year outcomes in patients hospitalized for heart failure. J Cardiol 2021; 77: 48–56, doi:10.1016/j.jjcc.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 22. Shimomoto R, Kubo T, Yamanaka F, Tsuchihashi-Makaya M, Suganuma N, Kitaoka H.. Current state of knowledge and information sharing among home healthcare professionals involved in heart failure management. J Cardiol 2023; 81: 292–296, doi:10.1016/j.jjcc.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 23. Nishino M, Egami Y, Kawanami S, Abe M, Ohsuga M, Nohara H, et al.. Prognostic comparison of octogenarian vs. non-octogenarian with acute decompensated heart failure: AURORA study. Circ J 2024; 88: 103–109, doi:10.1253/circj.CJ-23-0470. [DOI] [PubMed] [Google Scholar]
- 24. Roberts B, Robertson M, Ojukwu EI, Wu DS.. Home based palliative care: Known benefits and future directions. Curr Geriatr Rep 2021; 10: 141–147, doi:10.1007/s13670-021-00372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al.. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ J 2021; 85: 2252–2291, doi:10.1253/circj.CJ-21-0431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Data Availability Statement
The deidentified participant data will not be shared.