Abstract
Background and Aims
The staghorn fern genus Platycerium is one of the most commonly grown ornamental ferns, and it evolved to occupy a typical pantropical intercontinental disjunction. However, species-level relationships in the genus have not been well resolved, and the spatiotemporal evolutionary history of the genus also needs to be explored.
Methods
Plastomes of all the 18 Platycerium species were newly sequenced. Using plastome data, we reconstructed the phylogenetic relationships among Polypodiaceae members with a focus on Platycerium species, and further conducted molecular dating and biogeographical analyses of the genus.
Key Results
The present analyses yielded a robustly supported phylogenetic hypothesis of Platycerium. Molecular dating results showed that Platycerium split from its sister genus Hovenkampia ~35.2 million years ago (Ma) near the Eocene–Oligocene boundary and began to diverge ~26.3 Ma during the late Oligocene, while multiple speciation events within Platycerium occurred during the middle to late Miocene. Biogeographical analysis suggested that Platycerium originated in tropical Africa and then dispersed eastward to southeast Asia–Australasia and westward to neotropical areas.
Conclusions
Our analyses using a plastid phylogenomic approach improved our understanding of the species-level relationships within Platycerium. The global climate changes of both the Late Oligocene Warming and the cooling following the mid-Miocene Climate Optimum may have promoted the speciation of Platycerium, and transoceanic long-distance dispersal is the most plausible explanation for the pantropical distribution of the genus today. Our study investigating the biogeographical history of Platycerium provides a case study not only for the formation of the pantropical intercontinental disjunction of this fern genus but also the ‘out of Africa’ origin of plant lineages.
Keywords: Epiphytic ferns, global climate change, long-distance dispersal, pantropical disjunction, Platycerium, Polypodiaceae, species diversification
INTRODUCTION
Biogeographical analysis is an important method in reconstructing the evolutionary history of groups of organisms (Sanmartín, 2012; Thomas et al., 2015), and in clarifying the mechanisms and processes for the formation of disjunct distributions of plant lineages among different continents, such as the pantropical intercontinental disjunction (Scheben et al., 2016; Rahaingoson et al., 2022) and the East Asian–North American disjunction (Zhou et al., 2019; Ye et al., 2022), and these have greatly attracted the attention of biogeographers. Over 330 genera of seed plants have been documented with a pantropical disjunction distribution pattern among tropical regions of the Americas, Africa and southeast Asia–Australia (Thorne, 1972), and thus this intercontinental disjunction is considered to be a major biogeographical pattern in plants (Thorne, 1972; Liu et al., 2013;Thomas et al., 2015).
At least three different mechanisms may be responsible for the pantropical disjunction: (1) dispersal via high-latitude boreotropical flora (Wolfe, 1975; Davis et al., 2002), (2) transoceanic long-distance dispersal (LDD) (Thorne, 2004; Yuan et al., 2005) and (3) the movement of tectonic plates (Thorne, 2004; de Queiroz, 2005). Additionally, recent overland migration has also been used to illustrate the disjunction between tropical Africa and southeast Asia–Australia (Yu et al., 2014), which is a component of the pantropical disjunction and has also been the focus of considerable biogeographical research (e.g. Zhou et al., 2012; Yu et al., 2014; Yao et al., 2016). Results from previous studies have greatly advanced our knowledge of the biogeographical process leading to the formation of a pantropical disjunction in plants, but these studies focused mostly on seed plants, and fern lineages were rarely involved. However, there are several limitations that may have hindered a better understanding of the biogeographical history of plant genera that have a pantropical disjunction. First, tropical genera are usually species rich and it is not easy to achive adequate species sampling (Nie et al., 2013). Second, species-level relationships within these genera are usually not well resolved or are weakly supported at many phylogenetic nodes, especially in studies based on analysis of limited DNA markers (e.g. Liu et al., 2013; Appelhans et al., 2018; Oliveira et al., 2021).
Platycerium, the staghorn fern genus (Fig. 1), is one of the most commonly grown ornamental ferns and belongs to the subfamily Platycerioideae in the family Polypodiaceae (Kreier and Schneider, 2006; Wei and Zhang, 2022). There are 18 species currently accepted in Platycerium and they are grow predominantly as epiphytes or sometimes on rocks in lowland forests within subtropical to tropical regions (Kreier and Schneider, 2006). The genus evolved to occupy a typical pantropical intercontinental disjunction, with six species endemic to tropical Africa, 11 species endemic to southeast Asia–Australasia and one species endemic to the neotropics (Kreier and Schneider, 2006). As a small genus with a typical pantropical disjunction, Platycerium thus represents an ideal opportunity to better understand the establishment of this distribution pattern in ferns.
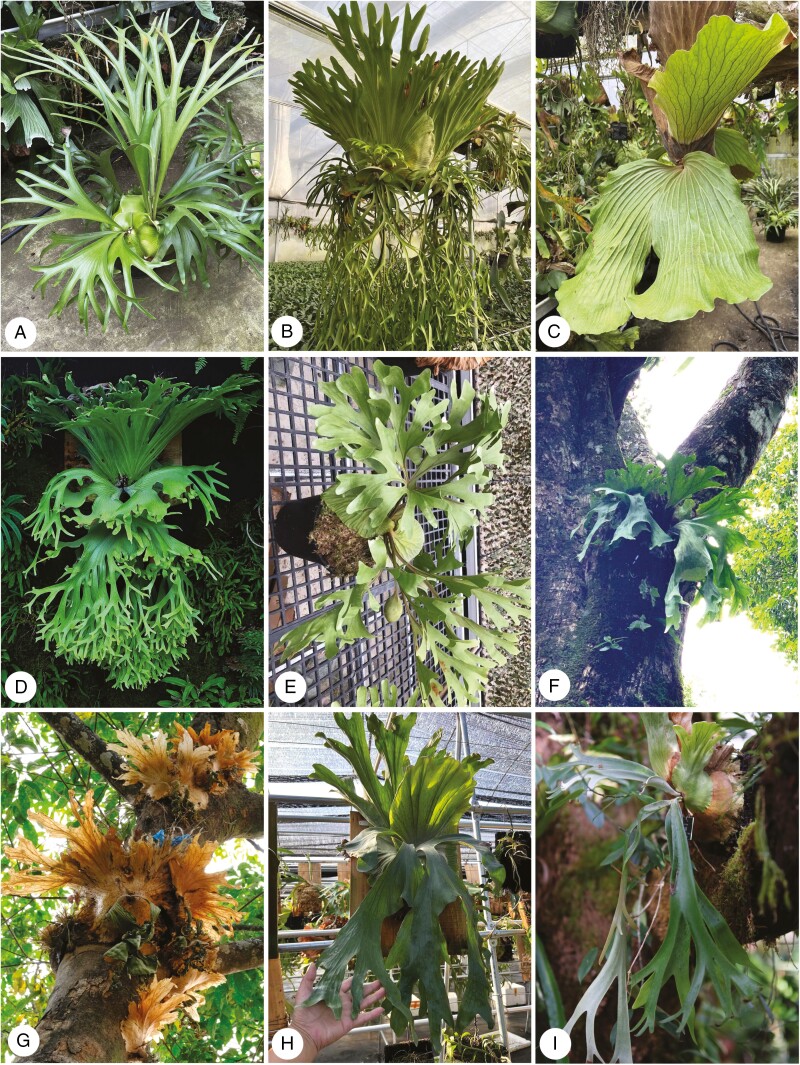
Fig. 1.
Photographs of some Platycerium taxa. (A) P. alcicorne; (B) P. coronarium; (C) P. angolense; (D) P. holttumii; (E) P. ridleyi; (F & G) P. wallichii, field images from Yunnan Province, China; (H) P. wandae; (I) P. willinckii. Photographed by Erfeng Huang.
Previous phylogenetic studies have improved our understanding of the evolutionary history among Platycerium members. Kreier and Schneider (2006) reconstructed the phylogenetic relationships among all 18 species of Platycerium based on analysis of four plastid markers (rbcL, rps4, rps4-trnS, trnL-F), and revealed three well-supported clades within the genus, i.e. the Malayan–Asian (MA) clade, the Javan–Australian (JA) clade and the Afro-American (AA) clade, which are highly congruent with those circumscribed by Hoshizaki (1972) on the basis of morphological data. Relationships among the three major clades were all resolved with high support, but some species-level relationships within these clades were not resolved or were only weakly supported, especially the phylogenetic position of the neotropical species Platycerium andinum Baker within the AA clade (Kreier and Schneider, 2006). Recently, 14 Platycerium species were included in a phylogenetic analysis focused on the genus Pyrrosia Mirb. on the basis of five plastid markers (rbcL, rps4, rps4-trnS, trnL, trnL-F) (Zhou et al., 2017), but the monophyly of both the AA clade and the MA clade revealed in Kreier and Schneider (2006) was not supported, because the African species Platycerium stemaria (P. Beauv.) Desv. was nested deeply within the MA clade, and species-level relationships within Platycerium were also mostly not strongly supported. Based on results from biogeographical analysis, Kreier and Schneider (2006) speculated that LDD events may have played an important role in shaping the current distribution pattern of Platycerium, but whether the genus originated from tropical Africa or southeast Asia–Australasia was unclear, because the ancestral range distributions for some basal nodes within the genus were not well resolved. Additionally, the temporal evolutionary history of the genus has not been explored to date. Thus, a well-resolved phylogenetic framework of Platycerium is needed for downstream analyses, such as biogeographical analysis and diversification analysis.
Over the past decade, enormous progress in phylogenomics has been made in clarifying many recalcitrant relationships among seed plants (e.g. Gitzendanner et al., 2018; OTPTI, 2019; Zhao et al., 2021a; Baker et al., 2022; Yang et al., 2022; Yao et al., 2023), and phylogenomic studies on the basis of large numbers of gene sequences from both plastid and nuclear genomes have also been used to disentangle relationships among fern lineages (e.g. Qi et al., 2018; Du et al., 2021; Fawcett et al., 2021; Wei et al., 2021; Lima et al., 2023). In a recent plastid phylogenomic analysis, the subfamily-level framework of Polypodiaceae was provided (Wei et al., 2021). Thus, in the present study, we reconstruct the phylogenetic relationships among Polypodiaceae members with a focus on the genus Platycerium using plastid phylogenomic methods, and combining divergence time estimation and historical biogeography analysis, in order to (1) clarify the species-level relationships of Platycerium, (2) explore the spatiotemporal evolution of the genus, and (3) elucidate the mechanism(s) and process(es) responsible for the pantropical intercontinental disjunction of the genus.
MATERIALS AND METHODS
Taxa sampling
All 18 species of the genus Platycerium were sampled and newly sequenced in the present study. The other two genera currently circumscribed within the subfamily Platycerioideae, namely Hovenkampia Li Bing Zhang & X.M. Zhou and Pyrrosia, were also sampled according to the phylogenetic results of Zhou et al. (2017) and Wei et al. (2021). Representatives of all other subfamilies circumscribed within Polypodiaceae were sampled as outgroups, as referred from Wei and Zhang (2022). The total taxa sampling consisted of 53 species in 21 genera, and detailed information about taxon sampling is provided in Supplementary Data Table S1.
DNA sequences and phylogenetic analyses
Total genomic DNA of the 18 species of Platycerium was extracted using the modified CTAB method (Doyle and Doyle, 1987). The method of genome skimming sequencing following Yao et al. (2023) was adopted to obtain the whole plastid genome of the species sampled. Procedures involved in library construction and sequencing followed the study of Yao et al. (2023). About 3 G of data were obtained for each sample. Plastid sequence reads were assembled using the software GetOrganelle (Jin et al., 2020), with the reference plastid genomes of Pyrrosia subfurfuracea (Hook.) Ching (GenBank accession number: NC_047436) and Hovenkampia schimperiana (Mett. ex Kuhn) Li Bing Zhang & X.M. Zhou (MW876325). The genome sequences obtained were then annotated by PGA (Qu et al., 2019). Plastome sequences newly obtained here have been submitted to the NCBI database (www.ncbi.nlm.nih.gov). The plastid genomes of all the other species sampled were directly downloaded from NCBI. Eighty-eight coding regions (including 84 protein-coding genes and four rRNA genes; Supplementary Data Table S2) were extracted from the plastid genomes of all the species sampled, and then concatenated. Sequence names and lengths for each of the coding regions used here are given in Table S2. GenBank accession numbers of all the plastomes newly sequenced here as well as those obtained from NCBI are listed in Table S1.
Phylogenetic analyses were conducted using Bayesian inference (BI) in MrBayes v.3.2.7 (Ronquist and Huelsenbeck, 2003) and maximum likelihood (ML) in RAxML-HPC2 (8.1.2) (Stamatakis, 2006) on the CIPRES cluster (Miller et al., 2010). Before the BI analysis, the model of nucleotide substitution for the combined matrix was selected under the corrected Akaike Information Criterion (AICc) in jModelTest v.3.7 (Posada, 2008): GTR+I+Γ. In BI analysis, the Markov chain Monte Carlo (MCMC) analysis was run for 10 000 000 generations and sampled every 200 generations. The number of generations for this analysis was sufficient, because the effective sample size (ESS) of all parameters was over 200 as evaluated in Tracer v.1.6 (Rambaut et al., 2014), and the average standard deviation (SD) of split frequencies was below 0.01. The first 50 % of trees obtained in BI analysis were discarded as burn-in and then posterior probability (PP) values were determined from the posterior distribution. ML analysis was run under the GTR+Γ model, with remaining parameters left at default values. A rapid bootstrap (BS) analysis using the same model with 1000 pseudoreplicates was conducted to obtain support values. In the present study, we defined high or strong support as BS ≥ 80 % or PP ≥ 0.95, moderate support as 70 % ≤ BS < 80 % or 0.90 ≤ PP < 0.95, and low or weak support as BS < 70 % or PP < 0.90, following the definition suggested by Zhao et al. (2021a).
Divergence time estimation
Penalized likelihood (PL) dating analysis was conducted using treePL (Smith and O’Meara, 2012), because this method usually performs well for large genomic datasets (Zhang et al., 2017). Due to the lack of fossil records of Platycerium, calibration constraints for phylogenetic nodes outside the genus were adopted. Results from the BEAST (Bayesian evolutionary analysis by sampling trees; Drummond et al., 2012) and PL dating analyses of Polypodiales in Du et al. (2021) revealed that the crown of Polypodiaceae began to diversify ~71.17 million years ago (Ma) and 79.38 Ma, respectively. Thus, we used the two ages as the minimum-age and maximum-age calibrations for the crown of Polypodiaceae, respectively. In addition, three fossil calibrations (scheme I) were used here: (1) a leaf fossil of Polypodium radonii Z.Kvaček (Kvaček, 2001) reported from the Oligocene of the Czech Republic was used recently in dating analyses (Testo and Sundue, 2016; Chen et al., 2022), and thus it was used here as the minimum-age calibration for the stem node of the genus Polypodium, and the age 23.03 Ma was adopted; (2) a leaf fossil of Aglaomorpha heraclea (Kunze) Copel. [=Drynaria heraclea (Kunze) T.Moore] reported from the upper Miocene of Sumatra (Van Uffelen, 1991) was also used recently in dating analyses (Testo and Sundue, 2016; Chen et al., 2022), and it was used here as the minimum-age calibration for the stem node of Drynaria heraclea, and the age 5.33 Ma was adopted; and (3) a leaf fossil of Protodrynaria takhtajanii Vikulin & A. Bobr, which belongs to the subfamily Crypsinoideae and reported from the Eocene–Oligocene boundary of Russia (Vikulin and Bobrov, 1987), was used widely in molecular dating analyses (e.g. Schuettpelz and Pryer, 2009; Sundue et al., 2015; Le Péchon et al., 2016; Sessa et al., 2017; Du et al., 2021), so was used here as the minimum-age calibration for the stem node of Crypsinoideae and the age 33.9 Ma was adopted. However, the confident assignment of the third fossil to Crypsinoideae was questioned in recent studies due to its poor preservation of critical characters (Testo and Sundue, 2016; Chen et al., 2022). To explore the impact of the last fossil in the present dating analysis, another calibration scheme (scheme II) was adopted with the exclusion of this fossil in analysis. One thousand BS pseudoreplicates with branch lengths were generated using RAxML, and confidence age intervals for the internal nodes were then calculated from dating 1000 BS trees by using treePL and TreeAnnotator v.1.8.4 (Drummond et al., 2012).
Biogeographical analysis
The model-based Bayesian binary MCMC (BBM) implemented in the program RASP (Reconstruct Ancestral-States-in-Phylogenies) v.4.2 (Yu et al., 2015) was applied to reconstruct the biogeographical history of Platycerium. The maximum clade credibility (MCC) tree derived from the results of PL analysis and summarized with TreeAnnotator in BEAST was used for BBM analysis, and the taxa sampled outside Platycerium and its close relative Hovenkampia were removed in this analysis. According to the distribution data of both Hovenkampia and Platycerium (Kreier and Schneider, 2006; Zhou et al., 2017), four geographical areas were revealed as regions of intercontinental disjunction: Afro-Madagascar (defined here as area A), southeast Asia (area B), Australasia (area C) and America (area D). Lydekker’s Line (Lydekker, 1896), which could be determined from the plate tectonic history within the southeast Aisa and southwestern Pacific regions (Hall, 2002), was adopted previously in the definition of biogeographical regions (Labiak et al., 2014; Peng et al., 2021), and was also used here as the boundary between area B and area C. In RASP, two independent runs with ten chains (including nine hot Markov chains and one cold chain) were conducted for 5 000 000 generations and sampled every 100 generations. Additionally, the discard samples and temperature parameters were set as 100 and 0.1, respectively. State frequency was set as ‘Estimated (F81)’ and among-site rate variation was set as ‘GAMMA (+Γ)’.
RESULTS
Phylogenetic analysis
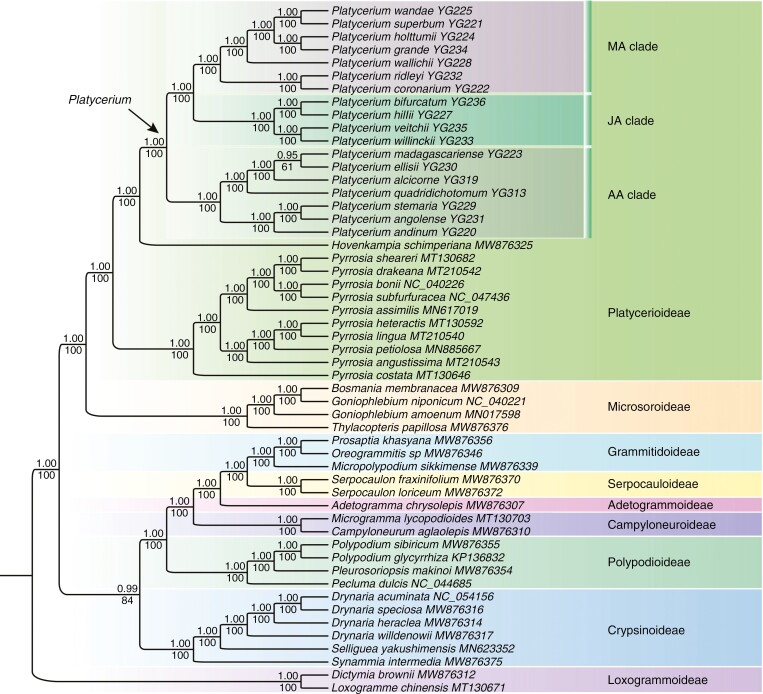
Plastomes of all 18 Platycerium species were newly sequenced in the present study. The average depth of sequencing coverage of the newly assembled plastomes ranged from 280.8 × (P. grande J. Sm.) to 604.8 × [P. quadridichotomum (Bonap.) Tardieu], and the plastome length ranged from 149 640 bp (P. angolense Welw. ex Hook.) to 163 542 bp (P. ellisii Baker) (Supplementary Data Table S1). The total length of the aligned matrix that including 88 coding regions contained 77 534 bp. Phylogenetic relationships among all the nine subfamilies of Polypodiaceae were well resolved with high support (BS ≥ 84 %, PP ≥ 0.99; Fig. 2). Within Platycerioideae, the genus Platycerium was well supported as monophyletic (BS = 100 %, PP = 1.00; Fig. 2) and was sister to the continental African endemic genus Hovenkampia with strong support (BS = 100 %, PP = 1.00; Fig. 2). Species of Platycerium clustered into three major clades (Fig. 2): the AA clade, the JA clade and the MA clade, with the latter two clades sister to each other with high support (BS = 100 %, PP = 1.00). The species relationships within Platycerium were well resolved with strong support (BS = 100 %, PP = 1.00), except that the sister relationship between the two Madagascar endemic species P. ellisii and P. madagascariense Baker within the AA clade was weakly supported in ML analysis (BS = 61 %), but this sister relationship was highly supported in BI analysis (PP = 0.95). The Australian species P. superbum de Jonch. & Hennipman and New Guinea species P. wandae Racib. were sister to each other with strong support (BS = 100 %, PP = 1.00), and deeply nested within the MA clade. The neotropical species P. andinum clustered within a clade comprising African species and was sister to P. angolense–P. stemaria (P. Beauv.) Desv., which is endemic to continental Africa, with strong support (BS = 100 %, PP = 1.00).
Fig. 2.
Plastome-based phylogeny of Polypodiaceae reconstructed by analysis of 88 plastid genes using the maximum-likelihood (ML) method. Posterior probability values in Bayesian inference and bootstrap values in ML analysis are indicated above and below the stem branch of each phylogenetic node, respectively. The arrow indicates the crown of Platycerium. AA: Afro-American; JA: Javan–Australian; MA: Malayan–Asian.
Divergence time estimation
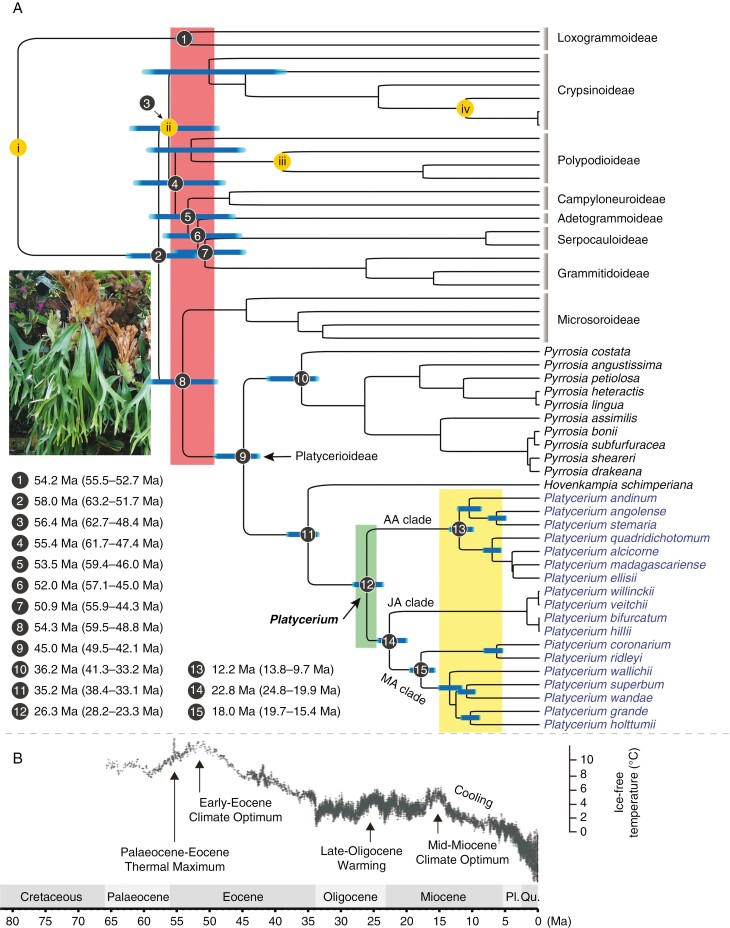
Similar results were recovered from PL analyses on the basis of two different calibration schemes (Fig. 3; Supplementary Data Table S3); for example, the ages estimated for the crown of the subfamily Platycerioideae (node 9 in Fig. 3) was ~45.0 Ma (age interval 49.5–42.1 Ma) under scheme I vs. ~45.1 Ma (49.8–42.1 Ma) under scheme II, the stem of Platycerium (node 11 in Fig. 3) was ~35.2 Ma (38.4–33.1 Ma) vs. ~35.2 Ma (38.7–33.3 Ma), and the crown of Platycerium (node 12 in Fig. 3) was ~26.3 Ma (28.2–23.3 Ma) vs. ~26.3 Ma (28.3 Ma–23.3 Ma). Comparison of ages estimated at other nodes can be found in Table S3. Here, we mainly discuss the dating results derived from analysis on the basis of calibration scheme I (Fig. 3).
Fig. 3.
(A) Chronogram using the matrix including 88 plastid genes and four calibration points (i–iv), from the penalized likelihood analysis on the basis of calibration scheme I: (i) crown node of the family Polypodiaceae; (ii) stem node of the subfamily Crypsinoideae; (iii) stem node of Polypodium; and (iv) stem node of Drynaria heraclea. Blue bars represent the time intervals of phylogenetic nodes; the red region indicates the time interval from the Palaeocene–Eocene Thermal Maximum (PETM) period (ca. 56 Ma; Huurdeman et al., 2021) to the Early Eocene Climate Optimum (EECO) period (53.3‒49.1 Ma; Elson et al., 2022); the green region indicates the Late Oligocene Warming (LOW) period (27.8‒24.5 Ma; Hauptvogel et al., 2017); and the yellow region indicates the period of Miocene cooling that occurred following the mid-Miocene Climate Optimum (MMCO; 17–14.7 Ma; Holbourn et al., 2015). Field image is of Platycerium willinckii, photographed by Gang Yao. Divergence ages for some key nodes marked on the tree (numbers 1–15) are provided at the lower left. (B) Global climate curve since the Palaeocene. Modified from Zachos et al. (2008). Major climate events are indicated following Zhou et al. (2022) and Zhang et al. (2023). Pl.: Pliocene; Qu.: Quaternary; Ma: million years ago.
Results showed that early diversifications within Polypodiaceae mostly occurred from the Palaeocene–Eocene boundary to the early Eocene (Fig. 3), especially near the Palaeocene–Eocene Thermal Maximum (PETM; ~56 Ma; Huurdeman et al., 2021); for example, the crown age of subfamily Loxogrammoideae (node 1 in Fig. 3) was estimated as ~54.2 Ma (55.5‒52.7 Ma), the stem ages of Crypsinoideae (node 3 in Fig. 3) and Polypodioideae (node 4 in Fig. 3) were ~56.4 Ma (62.7‒48.4 Ma) and ~55.4 Ma (61.7‒47.4 Ma) respectively, and the split between Platycerioideae and Microsoroideae (node 8 in Fig. 3) was at ~54.3 Ma (59.5‒48.8 Ma). Splits among other subfamilies and the crown diversifications of some subfamilies also occurred mainly during the early Eocene (Fig. 3), especially during the Early Eocene Climate Optimum (EECO, 53.3–49.1 Ma; Elson et al., 2022). The crown age of the subfamily Platycerioideae (node 9 in Fig. 3) was estimated as ~45.0 Ma (49.5‒42.1 Ma) during the mid-Eocene. The genus Platycerium split from its sister (node 11 in Fig. 3), the genus Hovenkampia, at ~35.2 Ma (38.4‒33.1 Ma) near the Eocene–Oligocene boundary, and it started to diversify (node 12 in Fig. 3) at ~26.3 Ma (~28.2‒23.3 Ma) during the late Oligocene. Within Platycerium, the split between the JA clade and MA clade (node 14 in Fig. 3) occurred at ~22.8 Ma (~24.8‒19.9 Ma) near the Oligocene–Miocene boundary, and most speciation events occurred after the mid-Miocene, especially those within the AA and MA clades, including the splits between the neotropical species P. andinum and its African sister lineage P. angolense–P. stemaria that occurred at ~10.6 Ma (~12.2‒8.3 Ma), and between the Javan–Australian lineage P. wandae–P. superbum and their Malayan–Asian sister lineage P. grande J. Sm.–P. holttumii T. Moore that occurred at ~12.7 Ma (~14.2‒10.7 Ma).
Biogeographical analysis
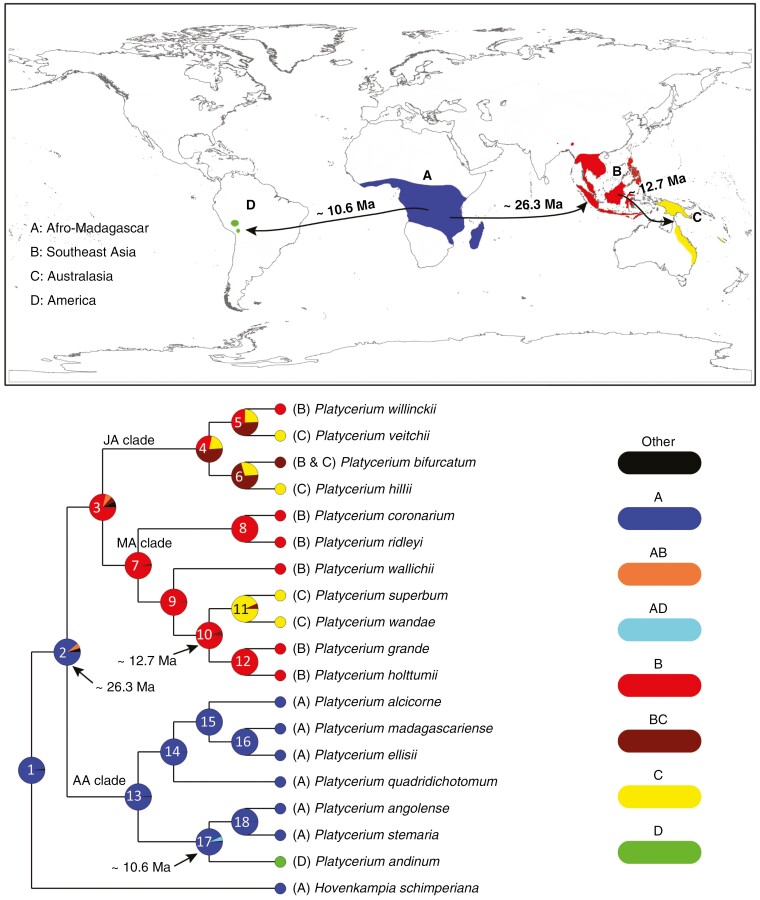
The ancestral range reconstructions inferred from BBM analysis showed strong support (relative probability ~0.98) for the split between Platycerium and its sister genus Hovenkampia in area A (node 1 in Fig. 4). The most recent common ancestor (MRCA) of Platycerium was also probably distributed in the same area with a probability of ~0.89 (node 2 in Fig. 4). The probable ancestral range of the MRCA of the MA clade and JA clade (node 3 in Fig. 4) is in area B with a probability of 0.79 (node 3 in Fig. 4). The probable ancestral range of the MRCA of the JA clade was estimated as the combined area BC (probability ~0.51; node 4 in Fig. 4), and the two areas B and C also had a probability of ~0.28 and ~0.21, respectively. Results from BBM analysis also suggested that seven dispersal events (nodes 2, 3, 4, 6, 10 and 17 in Fig. 4) occurred in Platycerium after its origin in tropical Africa, among which two dispersal events may have occurred around node 4, but with a negligible probability (~0.15). Three dispersal events recovered here seem to be definitive, including a dispersal from area A to area B (node 2; with a probability ~0.69), a dispersal from area B to area C (node 10; 0.88), and a dispersal from area A to area D (node 17; 0.93).
Fig. 4.
Biogeographical reconstruction for Platycerium using the Bayesian binary MCMC (BBM) method implemented in the program RASP (Reconstruct Ancestral-States-in-Phylogenies). The distribution map was modified from Kreier and Schneider (2006). Phylogenetic nodes are marked with numbers from 1 to 18. Pie charts show the relative probabilities of alternative ancestral distributions. Three dispersal events between areas A and B, between areas A and D, and between areas B and C are indicated on the map.
DISCUSSION
Improved plastid phylogenetic resolution of Platycerium
The interspecific relationships within Platycerium are robustly resolved here by plastid phylogenomic analysis (Fig. 2). The three major clades of Platycerium recovered here are highly consistent with those reported by Kreier and Schneider (2006), and also the results of Hoshizaki (1972) from analysis of morphological data. These clades are morphologically distinguishable from each other by the number(s) and position(s) of their soral patches per frond (Hoshizak, 1972; Kreier and Schneider, 2006). Interspecific relationships within these major clades were better resolved and more highly supported in the present study compared with those reported previously (Kreier and Schneider, 2006; Zhou et al., 2017). The neotropical endemic species P. andinum was clustered as the sister of a lineage comprising mainly species from Madagascar with weak support (BS = 57 %) by Kreier and Schneider (2006), but it was recovered here to be the sister of P. angolense–P. stemaria endemic to continental Africa with strong support (BS = 100 %, PP = 1.00; Fig. 2). The synthesis of a series of morphological traits observed in P. andinum, such as the high density of stomates and high numbers of hair cells per mm2 on leaves, may also indicate the close relationship between the neotropical species and the lineage endemic to continental Africa, in contrast to the Madagascan lineage (including P. alcicorne Desv., P. ellisii, P. madagascariense and P. quadridichotomum) (Hoshizak, 1972). Additionally, P. andinum has long slender rhizome scales with partially developed stellate hairs on leaves, which was also observed from the continental African species P. angolense (Hoshizak, 1972). Furthermore, it is of note that the sequence lengths of the plastomes obtained here also seem to be informative phylogenetically to some extent, because the length of the plastome of P. andinum (150 884 bp) is similar to those of the two species P. angolense (149 640 bp) and P. stemaria (149 703 bp) endemic to continental Africa, but it is much shorter than those of the Madagascan species (near or longer than 160 000 bp) (Fig. 2; Supplementary Data Table S1). The sister relationships between the Indo-China species P. holttumii and the Philippines species P. grande, and between the Eastern Australian species P. superbum and the New Guinea species P. wandae, which were moderately and weakly supported respectively in Kreier and Schneider (2006) and all not supported in Zhou et al. (2017), were all highly supported in the present study (all BS = 100 %, PP = 1.00; Fig. 2). Moreover, the sister relationship between P. grande–P. holttumii and P. superbum–P. wandae, which was weakly supported in Kreier and Schneider (2006) and not supported in Zhou et al. (2017), was also highly supported here (BS = 100 %, PP = 1.00; Fig. 2). The robust phylogenetic framework of Platycerium obtained here provides a good basis for further diversification and historical biogeographical analyses in this genus.
Diversification of Polypodiaceae and Platycerium
Our study provides a comprehensive subfamily-level dating analysis of Polypodiaceae and the first species-level dating analysis of Platycerium (Fig. 3). The ages estimated here for the split between Microsoroideae and Platycerioideae (~54.3 Ma), and between Grammitidoideae and Polypodioideae (~55.4 Ma) are largely congruent with those reported in Du et al. (2021) (~51.9 and ~55.1 Ma, respectively). The crown ages estimated here for the subfamily Polypodioideae and the genus Pyrrosia, as well as the stem of Platycerium are ~45.0 Ma (~49.5‒42.1 Ma), 36.2 Ma (~41.3‒33.2 Ma) and 35.2 Ma (~38.4‒33.1 Ma), respectively, and are also largely consistent with those reported in Wei et al. (2017), as ~38.0 Ma (~46.7‒25.8 Ma), 33.7 Ma (~42.4‒22.6 Ma) and 26.3 Ma (~39.4‒16.8 Ma), respectively. The crown age of Polypodioideae estimated here is also largely congruent with that reported recently in Chen et al. (2022; ~40.1 Ma, 49.4‒31.4 Ma).
Global climatic changes have been considered to have great impacts on the evolutionary history of different biological lineages (Sage et al., 2012; Hua and Wiens, 2013), because they could accelerate the extinction risk of species (Urban, 2015) and also might trigger the evolutionary adaptation of populations (Hoffmann and Sgrò, 2011). Previous studies have suggested that epiphytic ferns might be more susceptible to climate changes in contrast to terrestrial ferns (Anderson, 2021; Hernandez-Rojas et al., 2021), because the epiphytic habit means they are directly exposed to climatic factors and depend strongly on water availability to survive in the canopy habitat (Zotz, 2016). Results from our study revealed that, as one of the most diversified epiphytic fern lineages (Sundue et al., 2015), Polypodiaceae have undergone rapid radiations during the PETM–EECO period, with most of the subfamilies originated during this period (Fig. 3). The PETM–EECO period was characterized by the highest global temperatures in the Cenozoic era (Fig. 3; Zachos et al., 2008; Elson et al., 2022), and the excellent hydrothermal conditions that appeared during this period were suggested to have produced extensive diversification for major lineages in different plant families, such as Asteraceae (Huang et al., 2016), Caryophyllaceae (Xue et al., 2023) and Fagaceae (Zhou et al., 2022), and also to have triggered the rapid expansion of canopy forests (Jaramillo et al., 2010; Huurdeman et al., 2021; Srivastava et al., 2023), which could thus provide abundant new canopy habitats for epiphytes. A similar evolutionary history during the PETM–EECO period was also reported recently for epiphytic orchids (Zhang et al., 2023). As summarized in Zhang et al. (2023), the habitats within the newly developed canopy forests could have provided different levels of sunlight and water along the tree trunks and branches, and thus may have provided vertically diverse niches for the development of epiphytes. Furthermore, the high concentration of organic matter held by the forest canopies, and the reduced competition within the newly appeared niches in contrast to the terrestrial niches, also may have benefited the rapid diversification of epiphytes (Zhang et al., 2023). Sundue et al. (2015) have suggested that the repeated successful exploration of novel habitat types along elevation gradients may have driven the radiation of Polypodiaceae. Here, in light of our results, we suggest that the particular climatic conditions during the PETM–EECO period also may have contributed to the early evolutionary radiation of Polypodiaceae (Fig. 3).
Additionally, the large number of speciation events within Platycerium seems to be contemporaneous with global climate changes in different geological eras (Fig. 3), such as the warmer climate of the Late Oligocene Warming (LOW) period (27.8‒24.5 Ma; Hauptvogel et al., 2017) and the Miocene cooling that occurred following the mid-Miocene Climate Optimum (MMCO, ~17–14.7 Ma; Holbourn et al., 2015). Multiple analyses focused on angiosperm families that predominantly inhabited pantropical forests all have revealed a significantly accelerated speciation rate during the LOW period (e.g. Couvreur et al., 2011; Xue et al., 2020; Zhao et al., 2021b), indicating that the pantropical forests may have undergone another episode of development during this period. Thus, new niches in pantropical canopy forests during the LOW period may have provided an important ecological opportunity for the diversification of the crown node of Platycerium (~26.3 Ma; Fig. 3). Schneider et al. (2010) also have suggested that the global warming of the late Oligocene may have offered tropical plants the opportunity to expand their ranges and perhaps to develop rapid adaptive radiations. A similar temporal evolutionary history occurred during the late Oligocene for the tropical epiphytic fern genus Microgramma C. Presl (~26.9 Ma; Almeida et al., 2021) and other epiphytic plant genera, such as the liverwort genus Ceratolejeunea (Spruce) J. B. Jack & Steph. (~25.5 Ma; Scheben et al., 2016), the orchid genus Dendrobium Sw. (~25.2 Ma; Xiang et al., 2016) and the aroid genus Philodendron Schott (~25 Ma; Canal et al., 2019).
The cooling climatic conditions following the MMCO were suggested to be important in the ‘making of the modern world’, because many extant biological groups and biomes were established or underwent expansion during this period, such as the C4 plants (Sage et al., 2012), dry land flora (Wu et al., 2018), the African savanna (Charles-Dominique et al., 2016) and the East Asian subtropical evergreen broadleaved forests (Yu et al., 2017). However, the particular climate conditions since the MMCO were suggested to have led to the reduction of tropical rainforest areas (Kissling et al., 2012), which might, to some extent, have provided a potential mechanism in strengthening the biogeographical isolation among populations, and then facilitated the process of speciation within Platycerium (especially within the AA and MA clades; Fig. 3), although the genus has a low species diversity. Sundue et al. (2015) also revealed that extensive speciation events within the family Polydodiaceae occurred during the period 15‒10 Ma. The globally cooler and drier climate following the MMCO may also have promoted the rapid speciation events seen within multiple fern genera, such as the middle to late Miocene speciation events revealed in Ctenitis (C. Chr.) C. Chr. (Hennequin et al., 2017), Diplazium Sw. (Wei et al., 2015), Microgramma (Almeida et al., 2021), Polystichum Roth (Le Péchon et al., 2016) and Pteris L. (Chao et al., 2014). Thus, we suggest that the particular climate conditions of both the late Oligocene and middle to late Miocene may have triggered at least two episodes for the development of the staghorn fern genus Platycerium (Fig. 3), and also may have promoted the species diversification of different plant lineages.
On the other hand, large body size and humus-collecting leaves may have been the key innovations in triggering the radiation of some fern families (Janssen and Schneider, 2005; Ramírez-Barahona et al., 2016). However, Sundue et al. (2015) and Testo and Sundue (2018) revealed that both the changes to having a larger body size and humus-collecting leaves, which are characteristic of Platycerium and some other members of the same family, were not positively correlated with diversification rates in Polypodiaceae. Sundue et al. (2015) even suggested a lower diversification rate of Platycerium compared with that of its relatives in Polypodiaceae, which seems reasonable given that the genus actually has a low species diversity, although it has undergone a long evolutionary history at least since the late Oligocene. Which factors may have advanced the diversification of Platycerium needs further studies involving detailed investigation on both morphology and physiology.
The biogeographical origin of Platycerium
The biodiversity of ferns in Africa is much lower than that in both South America and southeast Asia–Australia (Janssen et al., 2007), and it was even suggested that the biodiversity of ferns in Africa may be associated with multiple immigrants from America and/or Asia–Australia (Janssen et al., 2007; Kessler, 2010), which was confirmed recently in multiple case studies of fern genera, such as Ctenitis (Hennequin et al., 2017), Deparia Hook. & Grev. (Kuo et al., 2016), Diplazium (Wei et al., 2015), Dryopteris Adans. (Sessa et al., 2017) and Polystichum Roth (Le Péchon et al., 2016). However, a recent biogeographical analysis of the pantropical fern genus Bolbitis Schott showed that this large genus originated from Africa during the early Eocene and then dispersed to Asia–Australia and America, although it has a very low species diversity in Africa (Nie et al., 2023). In the present study, with the inclusion of all three genera of Platycerioideae in taxon sampling, our results explicitly show that Platycerium originated from tropical Africa with its crown node diversifying there during the late Oligocene, and then dispersed eastward to southeast Asia–Australasia and westward to neotropical areas (Fig. 4). Therefore, our study provides another example of an ‘out of Africa’ origin in fern lineages at the generic level.
Biogeographical analysis further revealed that seven dispersal events may have occurred within Platycerium (Fig. 4), among which a late Oligocene dispersal from tropical Africa to southeast Asia (node 2 in Fig. 4), a middle Miocene dispersal from southeast Asia to Australasia (node 10 in Fig. 4) and a late Miocene dispersal from tropical Africa to neotropical area (node 17 in Fig.4) are clear. However, other dispersal events between southeast Asia and Australasia in the JA clade seem to be ambigouous due to the low probabilities as revealed in the biogeographical analysis.
The mechanism for the pantropical disjunction of Platycerium
The timing of the dispersal events within Platycerium estimated here is long after the period of both the break-up of Gondwana before the Tertiary (McLoughlin, 2001) and the boreotropical migration that occurred during the early Tertiary (Wolfe, 1975; Nie et al., 2013; Dick and Pennington, 2019), and thus the pantropical intercontinental disjunction of this genus could not be the outcome of either the movement of tectonic plates or dispersal through high-latitude boreotropical flora, which agrees with the conclusions of Kreier and Schneider (2006). On the other hand, overland migration between Africa and southeast Asia has been used to explain the disjunction between tropical regions of the two continents in previous studies (e.g. Zhou et al., 2012; Yu et al., 2014), but the genus Platycerium has not been recorded in large areas that lie to the east of the Arabian Peninsula (Hoshizaki, 1972; Kreier and Schneider, 2006). Although several populations of Platycerium wallichii Hook. were recorded in east India and Yunnan Province of China, most wild populations of this species are distributed in tropical lowland rain forests from Thailand to Malaysia, and this species is also nested deeply within the MA clade in Platycerium as recovered in phylogenetic tree (Fig. 2). Therefore, the hypothesis of overland migration also does not appear to be a good candidate in explaining the disjunction of Platycerium between tropical Africa and southeast Asia–Australasia. Compared with the above-mentioned hypotheses, transoceanic LDD thus might be the best explanation for the pantropical disjunction of Platycerium. Except for the transoceanic LDD events suggested in Platycerium as mentioned above, the disjunction between continental Africa and Madagascar within the AA clade as well as the geographical distribution of P. alcicorne in along the coasts of East Africa and Madagascar also indicate that transoceanic dispersal events between continental Africa and Madagascar may have occurred in different geological eras (Fig. 3). Some isolated islands and shallow marine areas that existed between continental Africa and Madagascar since the late Miocene (Masters et al., 2021) may have facilitated the transoceanic dispersal of Platycerium between the two regions.
Transoceanic LDD is suggested to be frequent among fern lineages due to their effective dispersal via spores (Kessler, 2010), and it was confirmed recently in a series of case studies of fern lineages, including the genera Deparia (Kuo et al., 2016), Diplazium (Wei et al., 2015), Lastreopsis Ching (Labiak et al., 2014), Microgramma C. Presl (Almeida et al., 2021), Rumohra Raddi (Bauret et al., 2017b), as well as the grammitid ferns (Sundue et al., 2014; Bauret et al., 2017a). A single fern individual may produce millions of spores that might have the potential to be widely dispersed, possibly over thousands of kilometres via air currents (Smith, 1993; Kessler, 2010), and experiments have also clearly demonstrated that fern spores are able to survive in the extreme environmental conditions of the high atmosphere (Gradstein and van Zanten, 2001). Miocene transoceanic LDD also been invoked to explain intercontinental disjunctions in angiosperm genera, such as in Bridelia Willd. (Li et al., 2009), Dalbergia L.f. (Rahaingoson et al., 2022) and Pogostemon Desf. (Yao et al., 2016).
CONCLUSIONS AND FUTHER DIRECTIONS
This study provides a comprehensive plastome-based phylogenomic analysis of the staghorn fern genus Platycerium. The inclusion of complete species sampling coupled with complete plastid genomes strengthened our understanding of the plastid-based relationships among Platycerium species, and the present study provides a robustly supported phylogenetic hypothesis of the genus. In addition, the temporal origin of the genus was explored, and results from molecular dating analysis provided insights into the impact of global climate changes during different geological eras on the evolutionary histories of both the family Polypodiaceae and the genus Platycerium. Furthermore, results from biogeographical analysis provided an example of the formation of the pantropical intercontinental disjunction of a fern genus and also the ‘out of Africa’ origin of plant lineages. Transoceanic LDD may have played an important role in the formation of the pantropical intercontinental disjunction of Platycerium, and combined with subsequent speciation events in its area of distribution for the its diversification, especially within palaeotropical areas. Nevertheless, nuclear phylogenomic study of Platycerium is still needed to better understand the evolutionary history of the genus, such as to demonstrate whether the genus has undergone some special evolutionary events such as hybridization, incomplete lineage sorting and/or polyploidization.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1. List of taxa sampled in this study, with information on voucher numbers, plastid genome size and average depth of sequencing coverage of the Platycerium species newly sequenced in this study, and GenBank accession numbers of all the species sampled. Table S2. Sequence names and lengths for each of the 88 coding regions used in the present study. Table S3. Ages estimated with calibration schemes I and II.
ACKNOWLEDGEMENTS
We thank Mr Yu-Ling Xiong for providing DNA materials of Platycerium madagascariense, P. stemaria and P. wandae, and Mr Yu-Heng Wang for providing DNA material of P. quadridichotomum.
Contributor Information
Bine Xue, College of Horticulture and Landscape Architecture, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, China.
Erfeng Huang, Guangxi Nanning Roy Garden Co., Ltd, Nanning 530227, China.
Guohua Zhao, Shenzhen Key Laboratory of Southern Subtropical Plant Diversity, Fairy Lake Botanical Garden, Shenzhen & Chinese Academy of Sciences, Shenzhen 518004, Guangdong, China.
Ran Wei, State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, 100093, China.
Zhuqiu Song, Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China.
Xianchun Zhang, State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, 100093, China.
Gang Yao, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou 510642, China.
FUNDING
This research was supported by the National Natural Science Foundation of China (Grant No. 31872651) and Guangdong Basic and Applied Basic Research Foundation (2019A1515011695).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Almeida TE, Salino A, Dubuisson JY, Hennequin S.. 2021. Insights into long-distance dispersal and ecological and morphological evolution in the fern genus Microgramma from phylogenetic inference. Botanical Journal of the Linnean Society 196: 294–312. [Google Scholar]
- Anderson OR. 2021. Physiological ecology of ferns: Biodiversity and conservation perspective. International Journal of Biodiversity and Conservation 13: 49–63. [Google Scholar]
- Appelhans MS, Reichelt N, Groppo M, Paetzold C, Wen J.. 2018. Phylogeny and biogeography of the pantropical genus Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). Molecular Phylogenetics and Evolution 126: 31–44. [DOI] [PubMed] [Google Scholar]
- Baker WJ, Bailey P, Barber V, et al. 2022. A comprehensive phylogenomic platform for exploring the angiosperm tree of life. Systematic Biology 71: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauret L, Gaudeul M, Sundue MA, et al. 2017a. Madagascar sheds new light on the molecular systematics and biogeography of grammitid ferns: New unexpected lineages and numerous long-distance dispersal events. Molecular Phylogenetics and Evolution 111: 1–17. [DOI] [PubMed] [Google Scholar]
- Bauret L, Rouhan G, Hirai RY, et al. 2017b. Molecular data, based on an exhaustive species sampling of the fern genus Rumohra (Dryopteridaceae), reveal a biogeographical history mostly shaped by dispersal and several cryptic species in the widely distributed Rumohra adiantiformis. Botanical Journal of the Linnean Society 185: 463–481. [Google Scholar]
- Canal D, Köster N, Celis M, Croat TB, Borsch T, Jones KE.. 2019. Out of Amazonia and back again: Historical biogeography of the species-rich neotropical genus Philodendron (Araceae). Annals of the Missouri Botanical Garden 104: 49–68. [Google Scholar]
- Chao YS, Rouhan G, Amoroso VB, Chiou WL.. 2014. Molecular phylogeny and biogeography of the fern genus Pteris (Pteridaceae). Annals of Botany 114: 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles-Doninique T, Davies TJ, Hempson GP, et al. 2016. Spiny plants, mammal browsers, and the origin of African savannas. Proceedings of the National Academy of Sciences of the United States of America 113: E5572–E5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Hyvönen J, Schneider H.. 2022. Re‐terrestrialization in the phylogeny of epiphytic plant lineages: Microsoroid ferns as a case study. Journal of Systematics and Evolution 61: 613–626. [Google Scholar]
- Couvreur TLP, Forest F, Baker WJ.. 2011. Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biology 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CC, Bell CD, Mathews S, Donoghue MJ.. 2002. Laurasian migration explains Gondwanan disjunctions: evidence from Malpighiaceae. Proceedings of the National Academy of Sciences of the United States of America 99: 6833–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Queiroz A. 2005. The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology and Evolution 20: 68–73. [DOI] [PubMed] [Google Scholar]
- Dick CW, Pennington RT.. 2019. History and geography of neotropical tree diversity. Annual Review of Ecology, Evolution, and Systematics 50: 279–301. [Google Scholar]
- Drummond AJ, Suchard MA, Dong X, Rambaut A.. 2012. Bayesian phylogenetics with BEAUTi and the BEAST 17. Molecular Biology and Evolution 29: 1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15. [Google Scholar]
- Du XY, Lu JM, Zhang LB, et al. 2021. Simultaneous diversification of Polypodiales and angiosperms in the Mesozoic. Cladistics 37: 518–539. [DOI] [PubMed] [Google Scholar]
- Elson AL, Rohrssen M, Marshall J, Inglis GN, Whiteside JH.. 2022. Hydroclimate variability in the United States continental interior during the early Eocene Climatic Optimum. Palaeogeography, Palaeoclimatology, Palaeoecology 595: 110959. [Google Scholar]
- Fawcett S, Smith AR, Sundue M, et al. 2021. A global phylogenomic study of the Thelypteridaceae. Systematic Botany 46: 891–915. [Google Scholar]
- Gitzendanner MA, Soltis PS, Wong GKS, Ruhfel BR, Soltis DE.. 2018. Plastid phylogenomic analysis of green plants: A billion years of evolutionary history. American Journal of Botany 105: 291–301. [DOI] [PubMed] [Google Scholar]
- Gradstein SR, van Zanten BO.. 2001. High altitude dispersal of spores, an experimental approach. XVI International Botanical Congress, St. Louis. Abstract Number 15.14.13.
- Hall R. 2002. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. Journal of Asian Earth Sciences 20: 353–431. [Google Scholar]
- Hauptvogel DW, Pekar SF, Pincay V.. 2017. Evidence for a heavily glaciated Antarctica during the late Oligocene ‘warming’ (278–245 Ma): stable isotope records from ODP Site 690. Paleoceanography 32: 384–396. [Google Scholar]
- Hennequin S, Rouhan G, Salino A, et al. 2017. Global phylogeny and biogeography of the fern genus Ctenitis (Dryopteridaceae), with a focus on the Indian Ocean region. Molecular Phylogenetics and Evolution 112: 277–289. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rojas AC, Jürgen K, Noben S, et al. 2021. Phylogenetic diversity of ferns reveals different patterns of niche conservatism and habitat filtering between epiphytic and terrestrial assemblages. Frontiers of Biogeography 13: e50023. [Google Scholar]
- Hoffmann AA, Sgrò CM.. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- Holbourn A, Kuhnt W, Kochhann KGD, Andersen N, Meier KJS.. 2015. Global perturbation of the carbon cycle at the onset of the Miocene Climatic Optimum. Geology 43: 123–126. [Google Scholar]
- Hoshizaki BJ. 1972. Morphology and phylogeny of Platycerium. Biotropica 4: 93–117. [Google Scholar]
- Hua X, Wiens JJ.. 2013. How does climate influence speciation? The American Naturalist 182: 1–12. [DOI] [PubMed] [Google Scholar]
- Huang CH, Zhang CF, Liu M, et al. 2016. Multiple polyploidization events across Asteraceae with tow nested events in the early history revealed by nuclear phylogenomics. Molecular Biology and Evolution 33: 2820–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurdeman EP, Frieling J, Reichgelt T, et al. 2021. Rapid expansion of meso‐megathermal rain forests into the southern high latitudes at the onset of the Paleocene‐Eocene Thermal Maximum. Geology 49: 40–44. [Google Scholar]
- Jaramillo C, Ochoa D, Conteras L, et al. 2010. Effects of rapid global warming at the Paleocene-Eocene boundary on Neotropical vegetation. Science 330: 957–961. [DOI] [PubMed] [Google Scholar]
- Janssen T, Kreier HP, Schneider H.. 2007. Origin and diversification of African ferns with special emphasis on Polypodiaceae. Brittonia 59: 159–181. [Google Scholar]
- Janssen T, Schneider H.. 2005. Exploring the evolution of humus collecting leaves in drynarioid ferns (Polypodiaceae, Polypodiidae) based on phylogenetic evidence. Plant Systematics and Evolution 252: 175–197. [Google Scholar]
- Jin JJ, Yu WB, Yang JB, et al. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biology 21: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaček Z. 2001. A new fossil species of Polypodium (Polypodiaceae) from the Oligocene of northern Bohemia (Czech Republic). Feddes Repertorium 112: 159–177. [Google Scholar]
- Kessler M. 2010. Biogeography of ferns. In: Mehltreter K, Walker LR, Sharpe JM, eds. Fern ecology. Cambridge: Cambridge University Press, 22–46. [Google Scholar]
- Kissling WD, Eiserhardt WL, Baker WJ, et al. 2012. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proceedings of the National Academy of Sciences of the United States of America 109: 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreier HP, Schneider H.. 2006. Phylogeny and biogeography of the staghorn fern genus Platycerium (Polypodiaceae, Polypodiidae). American Journal of Botany 93: 217–225. [DOI] [PubMed] [Google Scholar]
- Kuo LY, Ebihara A, Shinohara W, et al. 2016. Historical biogeography of the fern genus Deparia (Athyriaceae) and its relation with polyploidy. Molecular Phylogenetics and Evolution 104: 123–134. [DOI] [PubMed] [Google Scholar]
- Labiak PH, Sundue M, Rouhan G, Hanks JG, Mickel JT, Moran RC.. 2014. Phylogeny and historical biogeography of the lastreopsid ferns (Dryopteridaceae). American Journal of Botany 101: 1207–1228. [DOI] [PubMed] [Google Scholar]
- Le Péchon T, Zhang L, He H, et al. 2016. A well-sampled phylogenetic analysis of the polystichoid ferns (Dryopteridaceae) suggests a complex biogeographical history involving both boreotropical migrations and recent transoceanic dispersals. Molecular Phylogenetics and Evolution 98: 324–336. [DOI] [PubMed] [Google Scholar]
- Li YQ, Dressler S, Zhang DX, Renner SS.. 2009. More Miocene dispersal between Africa and Asia – the case of Bridelia (Phyllanthaceae). Systematic Botany 34: 521–529. [Google Scholar]
- Lima LV, Salino A, Kessler M.. 2023. Phylogenomic evolutionary insights in the fern family Gleicheniaceae. Molecular Phylogenetics and Evolution 184: 107782. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Ickert-Bond SM, Chen LQ, Wen J.. 2013. Molecular phylogeny of Cissus L of Vitaceae (the grape family) and evolution of its pantropical intercontinental disjunctions. Molecular Phylogenetics and Evolution 66: 43–53. [DOI] [PubMed] [Google Scholar]
- Lydekker R. 1896. A geographical history of mammals. Cambridge: Cambridge University Press. [Google Scholar]
- Masters JC, Génin F, Zhang Y, et al. 2021. Biogeographic mechanisms involved in the colonization of Madagascar by African vertebrates: Rifting, rafting and runways. Journal of Biogeography 48: 492–510. [Google Scholar]
- McLoughlin S. 2001. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany 49: 271–300. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T.. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetics trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, LA, 1–8.
- Nie ZL, Deng T, Meng Y, Sun H, Wen J.. 2013. Post-Boreotropical dispersals explain the pantropical disjunction in Paederia (Rubiaceae). Annals of Botany 111: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie LY, Zhang L, Liang ZL, et al. 2023. Phylogeny, character evolution, and biogeography of the fern genus Bolbitis (Dryopteridaceae). Molecular Phylogenetics and Evolution 178: 107633. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Rodrigues DP, Hopkins HCF, Lewis GP, Hopkins MJG.. 2021. Phylogeny and historical biogeography of the pantropical genus Parkia (Leguminosae, Caesalpinioideae, mimosoid clade). Molecular Phylogenetics and Evolution 163: 107219. [DOI] [PubMed] [Google Scholar]
- One Thousand Plant Transcriptomes Initiative (OTPTI). 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng DX, Dang VC, Habib S, et al. 2021. Historical biogeography of Tetrastigma (Vitaceae): Insights into floristic exchange patterns between Asia and Australia. Cladistics 37: 803–815. [DOI] [PubMed] [Google Scholar]
- Posada D. 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Qi X, Kuo LY, Huo C, et al. 2018. A well-resolved fern nuclear phylogeny reveals the evolution history of numerous transcription factor families. Molecular Phylogenetics and Evolution 127: 961–977. [DOI] [PubMed] [Google Scholar]
- Qu XJ, Moore MJ, Li DZ, Yi TS.. 2019. PGA: a software package for rapid, accurate, and fexible batch annotation of plastomes. Plant Methods 15: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaingoson FR, Oyebanji O, Stull GW, Zhang R, Yi TS.. 2022. A dated phylogeny of the pantropical genus Dalbergia Lf (Leguminosae: Papilionoideae) and its implications for historical biogeography. Agronomy 12: 1612. [Google Scholar]
- Rambaut A, Suchard MA, Drummond AJ.. 2014. Tracer version 1.6. http://beast.bio.ed.ac.uk/Tracer (10 February 2023, date last accessed).
- Ramírez-Barahona S, Barrera-Redondo J, Eguiarte LE.. 2016. Rates of ecological divergence and body size evolution are correlated with species diversification in scaly tree ferns. Proceedings Biological Sciences 283: 20161098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP.. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F.. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63: 19–47. [DOI] [PubMed] [Google Scholar]
- Sanmartín I. 2012. Historical biogeography: evolution in time and space. Evolution: Education and Outreach 5: 555–568. [Google Scholar]
- Scheben A, Bechteler J, Lee GE, Pócs T, Schäfer-Verwimp A, Heinrichs J.. 2016. Multiple transoceanic dispersals and geographical structure in the pantropical leafy liverwort Ceratolejeunea (Lejeuneaceae, Porellales). Journal of Biogeography 43: 1739–1749. [Google Scholar]
- Schneider H, Kreier HP, Janssen T, Otto E, Muth H, Heinrichs J.. 2010. Key innovations versus key opportunities: identifying causes of rapid radiations in derived ferns. In: Glaubrecht M, ed. Evolution in action. Berlin: Springer, 61–75. doi: 10.1007/978-3-642-12425-9_4. [DOI] [Google Scholar]
- Schuettpelz E, Pryer KM.. 2009. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proceedings of the National Academy of Sciences of the United States of America 106: 11200–11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa EB, Juslén A, Väre H, Chambers SM.. 2017. Into Africa: molecular phylogenetics and historical biogeography of sub-Saharan African woodferns (Dryopteris). American Journal of Botany 104: 477–486. [DOI] [PubMed] [Google Scholar]
- Smith AR. 1993. Phytogeographic principles and their use in understanding fern relationships. Journal of Biogeography 20: 255–264. [Google Scholar]
- Smith SA, O’Meara BC.. 2012. treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28: 2689–2690. [DOI] [PubMed] [Google Scholar]
- Srivastava G, Bhatia H, Verma P, Singh Y, Utescher T, Mehrotra RC.. 2023. High rainfall afforded resilience to tropical rainforests during Early Eocene Climate Optimum. Palaeogeography, Palaeoclimatology, Palaeoecology 628: 111762. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sundue MA, Parris BS, Ranker TA, et al. 2014. Global phylogeny and biogeography of grammatid ferns (Polypodiaceae). Molecular Phylogenetics and Evolution 81: 195–206. [DOI] [PubMed] [Google Scholar]
- Sundue MA, Testo WL, Ranke TA.. 2015. Morphological innovation, ecological opportunity, and the radiation of a major vascular epiphyte lineage. Evolution 69: 2482–2495. [DOI] [PubMed] [Google Scholar]
- Testo W, Sundue M.. 2016. A 4000-species dataset provides new insight into the evolution of ferns. Molecular Phylogenetics and Evolution 105: 200–211. [DOI] [PubMed] [Google Scholar]
- Testo WL, Sundue MA.. 2018. Are rates of species diversification and body size evolution coupled in the ferns? American Journal of Botany 105: 525–535. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Chatrou LW, Stull GW, et al. 2015. The historical origins of palaeotropical intercontinental disjunctions in the pantropical flowering plant family Annonaceae. Perspectives in Plant Ecology, Evolution and Systematics 17: 1–16. [Google Scholar]
- Thorne RF. 1972. Major disjunctions in the geographic ranges of seed plants. The Quarterly Review of Biology 47: 365–411. [Google Scholar]
- Thorne R. 2004. Tropical plant disjunctions: a personal reflection. International Journal of Plant Sciences 165: S137–S138. [Google Scholar]
- Urban MC. 2015. Accelerating extinction risk from climate change. Science 348: 571–573. [DOI] [PubMed] [Google Scholar]
- Van Uffelen G. 1991. Fossil Polypodiaceae and their spores. Blumea 36: 253–272. [Google Scholar]
- Vikulin S, Bobrov A.. 1987. A new fossil genus Protodrynaria (Polypodiaceae) from the Paleogene flora of Tim (the south of the middle Russian upland). Botanicheskii Zhurnal 72: 95–98. [Google Scholar]
- Wei X, Qi Y, Zhang X, et al. 2017. Phylogeny, historical biogeography and characters evolution of the drought resistant fern Pyrrosia Mirbel (Polypodiaceae) inferred from plastid and nuclear markers. Scientific Reports 7: 12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Xiang Q, Schneider H, et al. 2015. Eurasian origin, boreotropical migration and transoceanic dispersal in the pantropical fern genus Diplazium (Athyriaceae). Journal of Biogeography 42: 1809–1819. [Google Scholar]
- Wei R, Yang J, He LJ, et al. 2021. Plastid phylogenomics provides insights into the infrafamilial relationships of Polypodiaceae. Cladistics 37: 717–727. [DOI] [PubMed] [Google Scholar]
- Wei R, Zhang XC.. 2022. A revised subfamilial classification of Polypodiaceae based on plastome, nuclear ribosomal, and morphological evidence. Taxon 71: 288–306. [Google Scholar]
- Wolfe JA. 1975. Some aspects of plant geography of the Northern Hemisphere during the late Cretaceous and Tertiary. Annals of the Missouri Botanical Garden 62: 264–279. [Google Scholar]
- Wu SD, Zhang LJ, Lin L, Yu SX, Chen ZD, Wang W.. 2018. Insights into the historical assembly of global dryland floras: the diversification of Zygophyllaceae. BMC Evolutionary Biology 18: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang XG, Mi XC, Zhou HL, et al. 2016. Biogeographical diversification of mainland Asian Dendrobium (Orchidaceae) and its implications for the historical dynamics of evergreen broad-leaved forests. Journal of Biogeography 43: 1310–1323. [Google Scholar]
- Xue B, Guo X, Landis JB, et al. 2020. Accelerated diversification correlated with functional traits shapes extant diversity of the early divergent angiosperm family Annonaceae. Molecular Phylogenetics and Evolution 142: 106659. [DOI] [PubMed] [Google Scholar]
- Xue B, Song Z, Cai J, et al. 2023. Phylogenetic analysis and temporal diversification of the tribe Alsineae (Caryophyllaceae) with the description of three new genera, Hesperostellaria, Reniostellaria and Torreyostellaria. Frontiers in Plant Science 14: 1127443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ferguson DK, Liu B, et al. 2022. Recent advances on phylogenomics of gymnosperms and a new classification. Plant Diversity 44: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G, Drew BT, Yi TS, Yan HF, Yuan YM, Ge XJ.. 2016. Phylogenetic relationships, character evolution and biogeographic diversification of Pogostemon sl (Lamiaceae). Molecular Phylogenetics and Evolution 98: 184–200. [DOI] [PubMed] [Google Scholar]
- Yao G, Zhang YQ, Barrett C, et al. 2023. A plastid phylogenomic framework for the palm family (Arecaceae). BMC Biology 21: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye WQ, Zhu SS, Comes HP, et al. 2022. Phylogenomics and diversification drivers of the Eastern Asian-Eastern North American disjunct Podophylloideae. Molecular Phylogenetics and Evolution 169: 107427. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Gao LM, Soltis DE, et al. 2017. Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. The New Phytologist 215: 1235–1248. [DOI] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, Blair C, He X.. 2015. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Molecular Phylogenetics and Evolution 87: 46–49. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Maki M, Drew BT, et al. 2014. Phylogeny and historical biogeography of Isodon (Lamiaceae): Rapid radiation in south-west China and Miocene overland dispersal into Africa. Molecular Phylogenetics and Evolution 77: 183–194. [DOI] [PubMed] [Google Scholar]
- Yuan YM, Wohlhauser S, Möller M, Klackenberg J, Callmander MW, Küpfer P.. 2005. Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the India Ocean Basin resulted from long distance dispersal and extensive radiation. Systematic Biology 54: 21–34. [DOI] [PubMed] [Google Scholar]
- Zachos JC, Dickens GR, Zeebe RE.. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu Y, Huang MZ, et al. 2023. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. Journal of Integrative Plant Biology 65: 1204–1225. [DOI] [PubMed] [Google Scholar]
- Zhang SD, Jin JJ, Chen SY, et al. 2017. Diversification of Rosaceae since the Late Cretaceous based on plastid phylogenomics. The New Phytologist 214: 1355–1367. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chen YP, Salmaki Y, et al. 2021a. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biology 19: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Yu XQ, Kress WJ, Wang YL, Xia YM, Li QJ.. 2021b. Historical biogeography of the gingers and its implications for shifts in tropical rain forest habitats. Journal of Biogeography 49: 1339–1351. [Google Scholar]
- Zhou Z, Hu JJ, Wen J, Sun H.. 2019. Morphometric, phylogenetic and biogeographic analyses of Pyrularia (Santalales), a parasitic disjunct lineage between eastern Asia and eastern North America. Taxon 68: 47–71. [Google Scholar]
- Zhou L, Su YCF, Thomas DC, Saunders RMK.. 2012. ‘Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). Journal of Biogeography 39: 322–335. [Google Scholar]
- Zhou BF, Yuan S, Crowl AA, et al. 2022. Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the North Hemisphere. Nature Communications 13: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XM, Zhang L, Chen CW, et al. 2017. A plastid phylogeny and character evolution of the Old World fern genus Pyrrosia (Polypodiaceae) with the description of a new genus: Hovenkampia (Polypodiaceae). Molecular Phylogenetics and Evolution 114: 271–294. [DOI] [PubMed] [Google Scholar]
- Zotz G. 2016. Plants on plants: the biology of vascular epiphytes. Berlin: Springer. doi: 10.1007/978-3-319-39237-0 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.