Abstract
Background and Aims
The grass genus Urochloa (Brachiaria) sensu lato includes forage crops that are important for beef and dairy industries in tropical and sub-tropical Africa, South America and Oceania/Australia. Economically important species include U. brizantha, U. decumbens, U. humidicola, U. mutica, U. arrecta, U. trichopus, U. mosambicensis and Megathyrsus maximus, all native to the African continent. Perennial growth habits, large, fast growing palatable leaves, intra- and interspecific morphological variability, apomictic reproductive systems and frequent polyploidy are widely shared within the genus. The combination of these traits probably favoured the selection for forage domestication and weediness, but trait emergence across Urochloa cannot be modelled, as a robust phylogenetic assessment of the genus has not been conducted. We aim to produce a phylogeny for Urochloa that includes all important forage species, and identify their closest wild relatives (crop wild relatives). Finally, we will use our phylogeny and available trait data to infer the ancestral states of important forage traits across Urochloa s.l. and model the evolution of forage syndromes across the genus.
Methods
Using a target enrichment sequencing approach (Angiosperm 353), we inferred a species-level phylogeny for Urochloa s.l., encompassing 54 species (~40 % of the genus) and outgroups. Phylogenies were inferred using a multispecies coalescent model and maximum likelihood method. We determined the phylogenetic placement of agriculturally important species and identified their closest wild relatives, or crop wild relatives, based on well-supported monophyly. Further, we mapped key traits associated with Urochloa forage crops to the species tree and estimated ancestral states for forage traits along branch lengths for continuous traits and at ancestral nodes in discrete traits.
Key Results
Agricultural species belong to five independent clades, including U. brizantha and U. decumbens lying in a previously defined species complex. Crop wild relatives were identified for these clades supporting previous sub-generic groupings in Urochloa based on morphology. Using ancestral trait estimation models, we find that five morphological traits that correlate with forage potential (perennial growth habits, culm height, leaf size, a winged rachis and large seeds) independently evolved in forage clades.
Conclusions
Urochloa s.l. is a highly diverse genus that contains numerous species with agricultural potential, including crop wild relatives that are currently underexploited. All forage species and their crop wild relatives naturally occur on the African continent and their conservation across their native distributions is essential. Genomic and phenotypic diversity in forage clade species and their wild relatives need to be better assessed both to develop conservation strategies and to exploit the diversity in the genus for improved sustainability in Urochloa cultivar production.
Keywords: Urochloa, Brachiaria, crop wild relatives, forage traits, phylogenomics, species tree, tropical forage systems
INTRODUCTION
African grasses have been recognized for their forage potential since the 18th century, and as a result have been transplanted around the globe to upscale beef and dairy production for small-scale and commercial farms (Hartley and Williams, 1956; Parsons, 1972; Cook and Dias, 2006; Visser et al., 2016). Today, arguably the most important of these African grasses belong to the genus Urochloa P. Beauv. (Family: Poaceae, Subfamily: Panicoidaea, Tribe: Paniceae, Subtribe: Melinidinae) (Kellogg, 2015; Soreng et al., 2017) (Fig. 1). This large and diverse genus includes taxa previously placed in Brachiaria (Trin.) Briseb., Chaetium Nees, Eriochloa Kunth, Scutachne Hitchc. & Chase and Megathyrsus (Pilg.) B.K. Simon & S.W.L. Jacobs (Salariato et al., 2010, 2012; Jank et al., 2014; Kellogg, 2015; Namazzi et al., 2020; Ferreira et al., 2021). Urochloa forages are strongly preferred in sub-tropical and tropical regions as they are highly palatable and nutrient dense, are tolerant of low-quality soils, and outcompete alternative forage grasses in terms of biomass productivity such as Pennisetum purpureum Schumach. and Cenchrus ciliaris Fig. & De Not. (Maass et al., 2015; Baptistella et al., 2020; Njarui et al., 2021; Rathore et al., 2022). Since the 1950s these grasses have been adopted in forage systems in Southeast Asia, Australia, and especially Central and South America, with an estimated 99 million hectares of land devoted to Urochloa production in Brazil alone (ANUALPEC, 2008; Jank et al., 2014).
Fig. 1.
(A) Inflorescence with spikelets of Urochloa decumbens. The broadly winged rachises are indicated with red arrows. (B) Field with cultivated Urochloa sp. and grazing cattle in Cali, Colombia.
Livestock rearing for meat and dairy are key areas of economic importance in numerous developing nations, contributing greatly to the livelihoods of rural communities (e.g. Colombia) and commercial farmers (e.g. in Brazil) (Jank et al., 2014; Enciso et al., 2021). Recent breeding projects led by the Centro Internacional de Agricultura Tropical (CIAT; now Alliance Biodiversity & CIAT), Colombia, and Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Brazil, have produced highly productive and globally competitive Urochloa cultivars (do Valle and Savidan, 1996; Miles and do Valle, 1996; do Valle et al., 2013; Worthington and Miles, 2015; Espitia et al., 2020). High demand has resulted in the reintroduction of Urochloa cultivars into African countries including Kenya, Uganda and Madagascar, largely for improving milk production in small-holder farms (Maass et al., 2015; Mutimura et al., 2018).
Cattle rearing contributes greatly to global methane emissions, and the conversion of natural habitats to agricultural forage lands has contributed to global biodiversity loss (Herrero et al., 2013; Peters et al., 2013; Godfray et al., 2018). African Urochloa forage species are often recognized as invasives in Australia and the Americas (Foxcroft et al., 2010; Seabloom et al., 2013; Visser et al., 2016; Overholt and Franck, 2017). African Urochloa species aggressively exclude indigenous vegetation in disturbed environments through a combination of rapid growth and spread, and the production of allelopathic chemicals which hinder the development indigenous flora (Barbosa et al., 2008; Kato-Noguchi et al., 2014; Damasceno et al., 2018). Further, Urochloa forages possess agriculturally undesirable traits, chiefly an inability to survive frost, and are typically grown in monocultures in large-scale systems (do Valle et al., 2013; Krahl and Marocco, 2019). Although recent decades have seen tremendous advances in Urochloa breeding, challenges in understanding the taxonomy and evolutionary history in Urochloa species remain and limit breeders to a relatively small pool of taxa and accessions to choose from, which slows down novel breeding initiatives (do Valle et al., 2013; Sweitzer et al., 2021). As one of the most economically significant forage genera across the tropics, improving the sustainability of Urochloa cultivars while maintaining their high productivity is paramount for achieving development goals.
Urochloa cultivar breeding is difficult due to asexual reproduction via apomixis and diverse ploidy levels present in most genotypes grown as forages (Miles and do Valle, 1996; Miles, 2007; Worthington and Miles, 2015; Worthington et al., 2016; Hanley et al., 2021; Tomaszewska et al., 2021). To overcome the challenges posed by apomixis and polyploidy, forage breeders artificially induce polyploidy in sexually reproducing diploids and cross them with closely related apomictic polyploid species (Ishigaki et al., 2009). For example, tetraploids of the apomictic species U. brizantha and U. decumbens are crossed with artificial tetraploids of the sexual diploid U. ruziziensis, where apomicts act as the pollen donors (Miles and do Valle, 1996). Crosses of these three species have produced the most commercially successful Urochloa cultivars (Pizarro et al., 2013). Exploiting interspecific hybridization is central to modern Urochloa forage breeding, and successful cultivar production requires in-depth knowledge of the taxonomy and ploidy of the species used for crossing. Molecular data have revealed the shared, complex evolutionary history of U. brizantha, U. decumbens and U. ruziziensis. While all three species are clearly closely related, U. brizantha and U. ruziziensis belong to divergent lineages while U. decumbens is probably paraphyletic and split between two lineages based on ploidy; that is, tetraploid U. decumbens populations are more closely related to U. brizantha and diploid U. decumbens populations are more closely related to U. ruziziensis (Triviño et al., 2017; Higgins et al., 2022; Tomaszewska et al., 2023).
Taxonomic uncertainty, mosaics of sexually and asexually reproducing close relatives, and diverse intraspecies ploidy levels are typical of Urochloa forage species, and forage grasses more generally (Sandhu et al., 2015; Ortiz et al., 2020). This is the case for the largely apomictic, hexaploid species U. humidicola, the third most agriculturally significant Urochloa crop species after U. brizantha and U. decumbens (Vigna et al., 2016; Worthington et al., 2019). Urochloa humidicola populations include intermediates morphologically similar to U. dictyoneura, and taxonomists have argued that the two species be lumped (Sosef, 2016). Two additional agricultural species complexes (containing U. mutica with U. arrecta, and U. trichopus with U. mosambicensis respectively) also display overlapping morphologies, and apomictic reproduction (Toutain, 1986; Pereira Filho et al., 2013). A further complication is that the modern taxonomic concept of Urochloa, a monophyletic clade comprising previously separate genera, now includes Megathyrsus maximus [synonyms Panicum maximum Jacq. and U. maxima (Jacq.) R.D. Webster], a globally significant tetraploid forage and weed species indigenous to the African continent (Salariato et al., 2012; Rhodes et al., 2021).
Although belonging to different species complexes, Urochloa forage species share numerous agriculturally relevant traits (Keller-Grein et al., 1996). These important forages display perennial growth habits, a trait directly associated with carbon sequestration (Lal, 2004; Wilson et al., 2018; Ledo et al., 2020). Generally, Urochloa forage grasses are characterized by their tall culms, large and broad leaves, and relatively large seeds (Fisher and Kerridge, 1996; Clayton et al., 2016). Grasses which naturally produce high yields for above-ground biomass are desirable in tropical forage systems, while the production of large and easily harvested seeds is an advantageous trait for seed multiplication (Juntasin et al., 2022). Urochloa cultivars are predominantly sold and distributed through seed, and high yields in vegetative biomass and easy multiplication through seed are a dual aim for forage breeders (Hopkinson et al., 1996; Santos Filho, 1996; Ghimire et al., 2015). The inflorescences of forage species typically consist of simple, unbranched racemes (Reinheimer and Vegetti, 2008; Salariato et al., 2010), and with laterally elongated or ‘winged’ rachises (see Fig. 1A) (Clayton and Renvoize, 1982; Renvoize et al., 1996). These specific traits are not only agriculturally significant, but they are also directly measurable in wild Urochloa species through herbarium collections, or they are recorded in taxonomic treatments and floras (Clayton and Renvoize, 1982). Thus, modelling the evolution of these forage traits across Urochloa sensu lato (s.l.) is possible and can be used to identify wild species/clades with forage potential. To achieve this, a comprehensive and robust phylogeny for Urochloa is required.
Phylogenetic studies of Urochloa and all subsumed genera (hereafter Urochloa s.l.) have been limited to only a select few species, focusing largely on relationships between known economically important species, namely U. brizantha, U. decumbens and U. humidicola (Torres González and Morton, 2005; Pessoa-Filho et al., 2017), or have sampled the genus broadly but with only a handful of chloroplast genes and/or nuclear barcoding markers for tree inference (Salariato et al., 2010; Washburn et al., 2015; Hackel et al., 2018). Understanding the evolution of important forage species in Urochloa s.l. requires a phylogeny inferred across a broad representation of the genus using a large set of independently evolving gene regions. Modelling the evolutionary history of Urochloa with such a dataset will allow us to accurately infer speciation events in the face of population dynamics such an incomplete lineage sorting (Maddison, 1997; Edwards, 2009) and account for gene duplications due to polyploidization events (Wendel, 2015).
Urochloa forage breeders have long recognized the urgent need to increase the pool of genetic diversity used to develop novel cultivars (do Valle et al., 2013; Alves et al., 2014). In an effort to improve disease resistance, abiotic stress tolerance and sustainability of future crops, breeding projects now routinely look to cross crop plants with their nearest wild, non-domesticated relatives, rapidly bringing novel traits from wild populations into crops (Alves et al., 2014; Beloni et al., 2018). These closely related wild species have been termed ‘crop wild relatives’ (CWRs) (Harlan and De Wet, 1971), and their utilization in Urochloa forage breeding is underexploited. Potential CWR species have been identified in Urochloa based on inflorescence morphology (Renvoize et al., 1996), but to our knowledge these relationships have not been tested. A genus-wide phylogeny for Urochloa grasses would help confirm the placement of CWRs, and provide a starting point for expanding upon the species currently used in artificial hybridizations for Urochloa cultivar development.
We aim to infer a genus-wide phylogeny (species tree) for Urochloa s.l. using the Angiosperm 353 target enrichment of nuclear genes (Johnson et al., 2019) and infer the placement of the following agriculturally important species: U. brizantha, U. decumbens, U. ruziziensis, U. humidicola, U. mutica, U. arrecta, U. trichopus, U. mosambicensis, and M. maximus. We aim to define CWRs for all Urochloa forage species based on well-supported recent common ancestry and monophyly. Finally, we infer ancestral trait estimates along a phylogeny for the following agriculturally important traits: perennial vs annual life cycle, culm height, leaf size, rachis wing morphology and seed size. This will allow us to estimate the emergence of species with forage potential across Urochloa s.l.
MATERIALS AND METHODS
Taxon sampling
A total of 64 samples representing 60 species were chosen for phylogenomic analysis using the Angiosperm 353 target capture probe set (Johnson et al., 2019). The complete dataset contained 54 species within Urochloa s.l. (including samples from the genera Brachiaria, Eriochloa, Megathyrsus and Scutachne) and includes six Brachiaria species that have been placed within the subtribe Boivinellinae (Hackel et al., 2018) in the tribe Paniceae. These species are B. antsirabensis A. Camus, B. bemarivensis A. Camus, B. dimorpha A. Camus, B. epacridifolia (Stapf) A. Camus, Brachiaria sp. (MSV_387) and B. tsiafajavonensis A. Camus. Poecilostachys oplismenoides (Hack.) Clayton was also included as a species within the Boivinellinae. To further test the generic limits of Urochloa s.l. we included five additional species within the subtribe Melinidinae namely Melinis repens (Willd.) Zizka, Moorochloa eruciformis (Sm.) Veldkamp, Moorochloa malacodes (Mez & K.Schum.) Veldkamp, Tricholaena monachne (Trin.) Stapf & C.E.Hubb. and Thuarea perrieri A. Camus. Anthephora hermaphrodita (L.) Kuntze was chosen as the outgroup taxon within the tribe Paniceae (see Kellogg, 2015 and; Soreng et al., 2017 for classification within Poaceae).
Of the 64 total samples, 11 were downloaded as raw RNA reads from the European Nucleotide Archive (ENA, https://www.ebi.ac.uk/ena/browser/home). Raw reads for a further 23 samples were obtained from Baker et al. (2022). The remaining samples were selected from herbarium and silica-dried material available from the Royal Botanic Gardens, Kew. Taxa were chosen to meet two objectives: sample species broadly across Urochloa s.l. and to target potential CWRs. The Kew Herbarium index, which orders species within genera based on morphological similarities, as well as Renvoize and Maass (1993) and Renvoize et al. (1996) were used as guides for potential CWR sampling. A summary of all samples used with metadata for downstream analysis and data sources can be found in Supplementary Data Table S1. Leaf material from herbarium and silica-dried specimens was then used for DNA extraction and target enrichment library preparation.
Extraction and target-enrichment sequencing
DNA extraction, library preparation and sequencing.
DNA extraction was performed using a modified CTAB protocol (Doyle and Doyle, 1987). Quantus Fluorometer (Promega, Madison, WI, USA) measurements and gel electrophoresis using a 1 % agarose gel were conducted to estimate extraction quality and DNA fragment size. Samples with average fragment sizes estimated to be larger than 350 bp were sonicated using an M220 Focused ultrasonicator (Covaris, Woburn, MA, USA). Library preparation was then conducted following the NEBNext Ultra II DNA library prep kit protocol, performing half volume reactions, using Primer sets 1 and 2, and NEBNext Multiplex Oligos from Illumina. Following adaptor ligation and size selection, samples were PCR amplified for eight cycles. Library concentrations were assessed using a Quantus Fluorometer and average fragment lengths were measured with the Agilent Technologies 4200 TapeStation (Santa Clara, CA, USA) using High Sensitivity D1000 ScreenTape. Samples with low concentrations were re-amplified for —eight or nine PCR cycles. Libraries were then pooled depending on concentrations and fragment sizes, resulting in four pools of fragment lengths 240–269, 270–330, 340–395 and 400–500 bp. Pooled libraries were normalized for equimolarity and hybridized with the Angiosperm 353 probes (Johnson et al., 2019) for 24 h at 65 °C followed by 12 cycles of PCR. Final products were then assessed for fragment length using the Agilent Technologies 4200 Tapestation with D1000 ScreenTape before being sent to Macrogen Inc. (Seoul, South Korea) for sequencing on an Illumina HiSeqX platform (2 × 150-bp paired-end reads).
Phylogenomic inference
Read processing and loci assembly.
Paired-end and unpaired raw reads for newly sequenced samples and samples obtained from ENA were trimmed of adapters and filtered for low quality using Trimmomatic 0.39 (Bolger et al., 2014). A Phred33 score was specified for all samples. Trimming was performed by assessing the leading and trailing read ends, and a sliding window of 4 bp was used across reads. Base pairs falling below the quality threshold of 30 were removed for leading, trailing and sliding window trimming (LEADING:30; TRAILING:30; SLIDINGWINDOW:4:30). A minimum read length for reads following trimming was set to 36 bp (MINLEN:36).
Trimmed reads were used for loci assembly using HybPiper 1.3.1 (Johnson et al., 2016). An amino acid target fasta file from Baker et al. (2022) was used to capture on-target reads for Angiosperm 353 loci using BLASTX v.2.5.0 (Camacho et al., 2009). This was to ensure synonymous mutations did not bias read mapping to the target files across divergent taxa. Loci were then assembled de novo using SPAdes (Bankevich et al., 2012) and retrieved for each sample using the ‘reads_first.py’ and ‘retrieve_sequences.py’ scripts from HybPiper. Only the exons of assembled genes were used for downstream phylogenomic analysis. This was chosen to standardize alignments in the dataset which contained both target-enrichment and transcriptomic sequences.
Paralogue removal and consensus sequence inference.
In target-enrichment-based phylogenomic inference, paralogous genes are commonly removed from the analysis as they introduce homoplasy and confound estimations of species divergence histories (Nicholls et al., 2015; Andermann et al., 2020; Larridon et al., 2020; Crowl et al., 2022). Further, the presence of paralogues and duplicated genes can confound gene assembly, as reads from different paralogues can be adjoined into contigs (and subsequently genes), leading to chimeric sequence assembly (Kates et al., 2018; Nauheimer et al., 2021). However, removing entire genes with suspected paralogy means that genes with high allelic diversity (i.e. in the case of whole genome duplication and reticulation events) can be purged, resulting in the loss of informative gene sequences (Morales-Briones et al., 2022). Whole genome duplications and interspecific reticulation events are ubiquitous in angiosperms, and specifically prevalent in Poaceae and Urochloa (Van De Peer et al., 2009; McKain et al., 2016; Vigna et al., 2016; Landis et al., 2018).
To ensure that highly paralogous genes were removed while maintaining a large gene dataset, we assessed the heterozygosity of the 353 loci in our dataset based on the distribution of single nucleotide polymorphisms (SNPs) across all genes using HybPhaser (Nauheimer et al., 2021). On-target reads were mapped to loci recovered from HybPiper using BWA v.0.7.17 (Li and Durbin, 2009). Then bcftools v.1.9 (https://samtools.github.io/bcftools/bcftools.html) was used to call SNP variants using the HybPhaser script ‘1_generate_consensus_sequences.sh’ with default parameter settings for minimum allele frequency, minimum coverage and minimum allele count. Across all samples, loci with an SNP diversity that was 1.5 times greater than the third interquartile range for the entire gene dataset were removed using the ‘1a_snp_count.R’ and ‘1b_assessment.R’ scripts. This method allowed us to assess relative heterozygosity across Urochloa loci and flag outlier genes as potential paralogous, while retaining genes with multiple copies as a result of polyploidization events.
Once putative paralogous genes were removed, we phased the remaining gene set in order to infer non-chimeric consensus sequences following a method outlined in Tiley et al. (2021, 2023) and Crowl et al. (2022). Ploidy levels for all samples were required for the pipeline, which were obtained from the literature (Morrone et al., 2006; Tomaszewska et al., 2021) and the Chromosome Count Database (http://ccdb.tau.ac.il/), or estimated from target-enrichment sequencing reads (Tiley et al., 2021, 2023). Ploidy levels were estimated by mapping reads to a reference sequence, in this case loci from the known diploid U. fusca (Morrone et al., 2006). Samples are initially genotyped as diploids using GATK v.3.8.1 (McKenna et al., 2010) and biallelic reads were then mapped to U. fusca loci. The ratio between reads matching the reference sequence and reads carrying the alternate were used to estimate ploidy levels (Tiley et al., 2018). For ploidy estimation, a maximum ploidy level of 6 was chosen to constrain the analysis.
Following ploidy estimation, samples were phased for gene variants with the maximum number of possible haplotypes determined by the estimated ploidy levels. For each sample raw reads were assigned to respective gene sequences from HybPiper using BWA 0.7.17 (Li and Durbin, 2009) and PCR duplicates were flagged using Picard v.2.27.4 (http://broadinstitute.github.io/picard). HaplotypeCaller in GATK3.8.1 was used to assign reads to gene variants with parameter settings left to default (Tiley et al., 2021, 2023; Crowl et al., 2022). Phased gene variants were then assembled using H-PoPG v.0.2.0 (Xie et al., 2016). Phased variants were then collapsed into new consensus sequences where polymorphic sites were coded with ‘N’ to ensure that chimeric assemblies of homoeologous sequences were not present in phylogenetic analysis.
Species tree inference.
Newly inferred consensus sequences were aligned using MAFFT v.7.475 (Katoh and Standley, 2013) with parameters set to L-INS-I (--localpair; --maxiterate 1000) for the highest stringency. Columns in alignments with more than 30 % missing data were removed using Phyutility v.2.2.6. (Smith and Dunn, 2008). Individual maximum-likelihood genes (ML) trees were inferred using IQTREE v.2.1.2 (Minh et al., 2020) with ModelFinder Plus (Kalyaanamoorthy et al., 2017) used to determine the best fit model per gene based on Bayesian Information Criterion (BIC) scores. Ultrafast bootstrapping was implemented to assess branch support using 1000 replicates (Hoang et al., 2018). Gene trees were then concatenated into a single file and nodes with support values of ≤10 were collapsed using Newick Utilities v.1.6 (Junier and Zdobnov, 2010). Outlier taxa with excessively long branch lengths were then removed from each gene tree using TreeShrink v.1.3.9 (Mai and Mirarab, 2018) with the -b parameter kept at the default value of 5. Outlier taxa identified with TreeShrink were then removed from the original gene alignments. Gene trees were inferred from the new alignments using the same IQTREE parameters. A species tree was then inferred using ASTRAL-III v.5.7.7 (Zhang et al., 2018) with the unrooted gene trees used an input. Branch support was assessed using local posterior probabilities (LPP) and quartet scores (QS) using the -t2 flag in ASTRAL-III. Separately, gene alignments were concatenated into a supermatrix which was used to infer a ML phylogeny using IQTREE v.2.1.2. In total, 1000 ultrafast bootstrap replicates were used to assess branch support and a GTR+G+I nucleotide substitution model was chosen due to computational constraints.
Character evolution
Trait data.
All species were scored for continuous and discrete character traits. Continuous traits assessed were maximum leaf area, maximum culm height and maximum seed size (inferred from maximum fertile lemma length). Discrete traits assessed were growth habit (annual vs perennial growth habit) and rachis wing morphology (wingless, narrowly winged or broadly winged). Trait data were obtained from GrassBase (Clayton et al., 2016). Data were filtered for relevant species and traits using the tidyr (Wickham et al., 2023a) and dplyr (Wickham et al., 2023b) packages in R (R Core Team, 2023). Duplicate taxa and samples not identified to species level were also removed. Updating and reconciling species names between our samples and the GrassBase database was done using the World Checklist of Vascular Plants (WCVP) (Govaerts et al., 2021), accessed through Plants of the World Online (https://powo.science.kew.org/), and Vorontsova (2022).
Ancestral estimation methods.
Estimations for ancestral traits were inferred using the supermatrix ML tree. Taxa with no trait data and duplicate taxa were removed from the tree using the ‘drop.tip’ function in the R package ape (Paradis et al., 2004). For continuous traits (leaf area, culm height and fertile lemma length), values were log transformed and ML of ancestral states were estimated under a Brownian motion model using the fastANC function from the R package phytools (Revell, 2012). To account for uncertainty in trait estimations at nodes, the variance and confidence intervals for every node were also calculated (Losos, 1999). We then tested for phylogenetic signal, the tendency for closely related taxa to share trait values more frequently than by chance (Revell et al., 2008), in continuous traits using Blomberg’s K (Blomberg et al., 2003) and Pagel’s λ (Pagel, 1999).
To determine the best fit model for growth habit we compared ‘Equal Rates’, ‘All Rates Different’, ‘Perennial to Annual’ (but not reversible) and ‘Annual to Perennial’ (but not reversible) models following Revell and Harmon (2022). The model with the best Akaike Information Criterion (AIC) score was chosen for analysis. The same approach was used for rachis wing morphology. The models compared were ‘Equal Rates’, ‘All Rates Different’ and ‘Symmetrical Rates’, following Revell and Harmon (2022). Ancestral state estimations were then inferred using a marginal likelihood ancestral state reconstruction method using the R package corHMM (Beaulieu et al., 2013). Posterior probabilities for ancestral states were then mapped as pie charts to internal nodes of the tree using the R package ggtree v.3.0.2 (Yu et al., 2017).
RESULTS
Sequence recovery, paralogue removal and consensus sequence inference with ploidy
Between 0.84 million and 16 million read pairs were sequenced in this study, with an average of 3.7 million read pairs. Angiosperm 353 gene recovery was high for all samples and ranged between 299 and 346 genes, or 84.7–98 %, and an average of 333 genes were recovered (94.3 %). HybPhaser was used to detect and remove highly paralogous (1.5 times higher than the third interquartile range for SNP percentages across all genes) genes which resulted in the removal of 20 putatively paralogous genes (Supplementary Data Fig. S1). Ploidy levels for all samples are given in Table S1 and the number of diploids and polyploids in the sample set is given in Fig. S2. The final gene alignment lengths ranged from 106 to 3502 bp with a mean alignment length of 791 bp. The final concatenated gene alignment had a sequence length of 257 747 bp.
Phylogeny and origins of forages
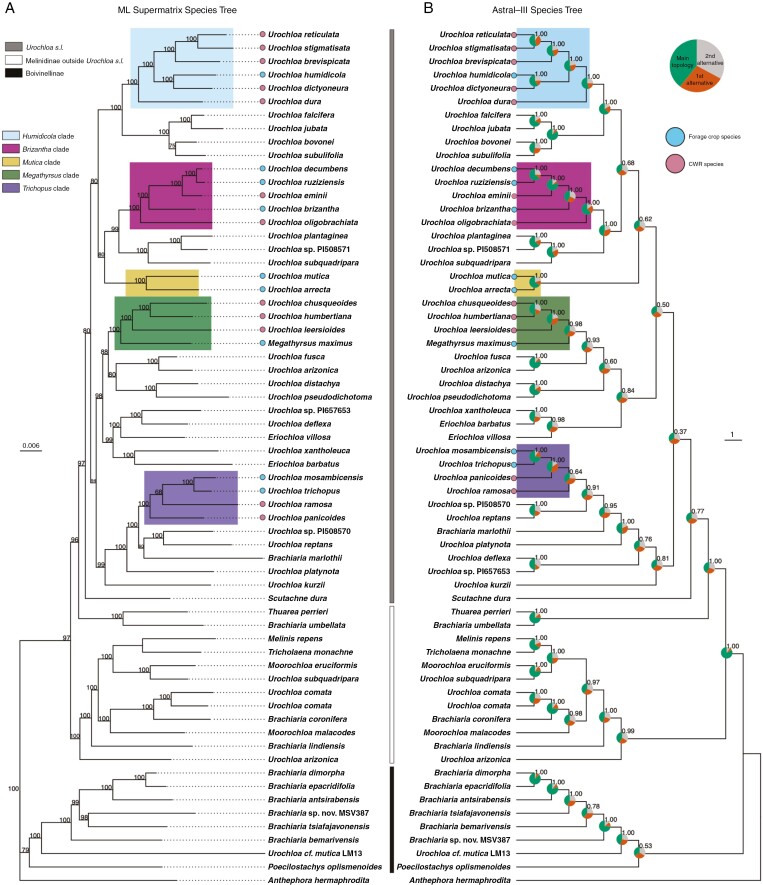
Phylogenetic trees produced using ASTRAL-III and IQTREE (hereafter ASTRAL-III and ML phylogenetic trees respectively) have largely similar topologies, and both phylogenetic trees recovered identical clades containing forage species (Fig. 2). We used the congruence between the ASTRAL-III and the ML phylogenetic trees in recovering these clades to define forage species clades and their CWRs (see Fig. 2, Table 1 for clades and support values). Urochloa humidicola and U. dictyoneura form a clade with the wild species U. brevispicata, U. stigmatisata, U. reticulata and U. dura, hereafter referred to as the ‘Humidicola clade’. The clade is well supported with 100 % bootstrap support (BS) in the ML phylogenetic tree, and 1.00 LPP and moderate gene tree congruence of 54.6 (QS for the main topology) in the ASTRAL-III phylogenetic tree. The three most commercially important Urochloa forages, U. brizantha, U. decumbens and U. ruziziensis, formed a clade with wild species U. eminii and U. oligobrachiata with 100 % BS in the ML phylogenetic tree and 1.00 LPP in the ASTRAL-III phylogeny, forming the ‘Brizantha clade’. The QS score for the Brizantha clade was moderate at 64.8 support for the main topology.
Fig. 2.
Phylogenetic inference of species trees for Urochloa s.l. using a maximum likelihood (ML) method on a concatenated alignment supermatrix using IQTREE2 in (A) and a multispecies coalescent method using ASTRAL-III (B). Numbers above branches in the ML phylogenetic tree (A) represent ultrafast bootstrap support values. For the ASTRAL-III phylogenetic tree (B) branch support is measured as local posterior probability (LPP) plotted above branches, and quartet scores (QS) were calculated and plotted on each node as pie charts. Colours in pie charts represent the main topology (green), the first alternative topology (orange) and the second alternative topology (grey). Forage clades are highlighted in colour in the ML phylogenetic tree (A) and ASTRAL-III phylogenetic tree (B). Scale bar for ML phylogenetic tree (A) indicates nucleotide substitutions per site, and for the ASTRAL-III phylogenetic tree (B) the scale bar indicate coalescent units. Forage species are indicated with blue dots at tips and CWR species are indicated at tips with pink dots. Taxa defined as Urochloa s.l. in the analysis are indicated with a dark grey bar. Taxa defined as sitting outside Urochloa s.l. but within the same subtibe Melinidinae are indicated with a white bar. Taxa within the Boivinellinae, including Brachiaria species endemic to Madagascar previously placed in Boivinellinae (Hackel et al., 2018), are indicated with a black bar.
Table 1.
Urochloa forage crop clades and their crop wild relatives (CWRs) defined here. Comparisons among forage traits between forage species and CWR are summarized.
| Forage species | CWR species | Forage species traits | CWR native to African continent | CWR forage traits | |
|---|---|---|---|---|---|
| Brizantha Clade |
U. brizantha
U. decumbens U. ruziziensis |
U. eminii
U. oligobrachiata |
Perennial growth habit, tall culms, large leaves, broad or narrow winged rachis, large seeds | Yes | Annual growth habits, tall culms, large leaves, broad winged rachis, large seeds |
| Humidicola Clade | U. humidicola |
U. brevispicata
U. dictyoneura U. dura U. reticulata U. stigmatisata |
Perennial growth habit, tall culms, medium leaves, narrow winged rachis, large seeds | Yes | Annual and perennial growth habits, medium culms, small to medium leaves, narrow winged rachis, medium to large seeds |
| Megathyrsus Clade | M. maximus |
U. chusqueoides
U. humbertiana U. leersiodes |
Perennial growth habit, tall culms, large leaves, wingless rachis, large seeds | Yes | Annual and perennial growth habits, short to medium culms, short leaves, wingless rachis, small to medium seeds |
| Mutica Clade |
U. arrecta
U. mutica |
N/A | Perennial growth habit, tall culms, large leaves, broad winged rachis, large seeds | Yes | N/A |
| Trichopus Clade |
U. mosambicensis
U. trichopus |
U. panicoides
U. ramosa |
Perennial or annual growth habit, tall culms, large leaves, narrow winged rachis, large seeds | Yes | Annual growth habits, medium to large leaves, small seeds, medium to tall culms, wingless and narrow winged rachis |
Urochloa arrecta and U. mutica form a well-supported clade in the ML phylogenetic tree with 100 % BS. The same topology was recovered in the ASTRAL-III phylogenetic tree with 1.00 LPP and 65 QS indicating moderate congruence among gene tree topologies. No CWR species were identified from our phylogenetic analyses, meaning U. mutica and U. arrecta are the only species placed in the ‘Mutica’ forage clade. The ASTRAL-III phylogenetic tree places the Mutica clade sister to the Brizantha clade and Humidicola clade, while the ML phylogenetic tree shows the Mutica and Brizantha clades are closely related and the Humidicola clade belongs to a sister lineage. However, branch support for these topologies is moderate in the ML phylogenetic tree with 80 % BS, and low in the ASTRAL-III phylogenetic tree with 0.63 LPP and 36.15 QS.
Both the ML and ASTRAL-III phylogenetic trees confirmed the placement of M. maximus within Urochloa, and M. maximus was placed within a clade containing CWR species U. humbertiana, U. leersioides and U. chusqueoides (Megathyrsus clade). The Megathyrsus clade is well supported (100 % BS in the ML phylogenetic tree and 0.98 LPP in the ASTRAL-III phylogenetic tree). However, ASTRAL-III phylogenetic analysis shows high gene tree incongruence for the clade with a QS of 40.2. Urochloa trichopus and U. mosambicensis formed a clade with CWR species U. panicoides and U. ramosa. This clade is defined as the Trichopus forage clade, and it is highly supported in the ML phylogenetic tree (100 % BS) but poorly supported in the ASTRAL-III phylogenetic tree (0.64 LPP) with a low QS value (37.8). Apart from three forage species within the Brizantha clade, all forage species belonged to independent lineages within the genus Urochloa.
In addition to the genera Scutachne and Megathyrsus, both phylogenetic trees (Fig. 2) support placing Eriochloa within Urochloa and place Moorochloa as a separate but polyphyletic genus. Brachiaria umbellata is closely related to the genus Thuarea (Hackel et al., 2018) and sits outside the Urochloa clade in our analysis. In both phylogenetic trees (Fig. 2), our analysis supports the Boivinellinae clade, containing ‘Brachiaria’ species that are endemic to Madagascar, as sister to the subtribe Melinidinae (Hackel et al., 2018). Both trees show that U. arizonica and U. subquadripara are paraphyletic with an accession from each species falling within the Urochloa clade, and an additional accession for each species placed with the remaining Melinidinae taxa. A morphologically ambiguous accession which appears to be affiliated with U. mutica (Seteraneski s.n., barcode K001102413) is placed outside the Urochloa clade in the subtribe Boivinellinae. This accession is labelled ‘Urochloa cf. mutica LM13’ in Fig. 2.
Forage trait ancestral state estimations
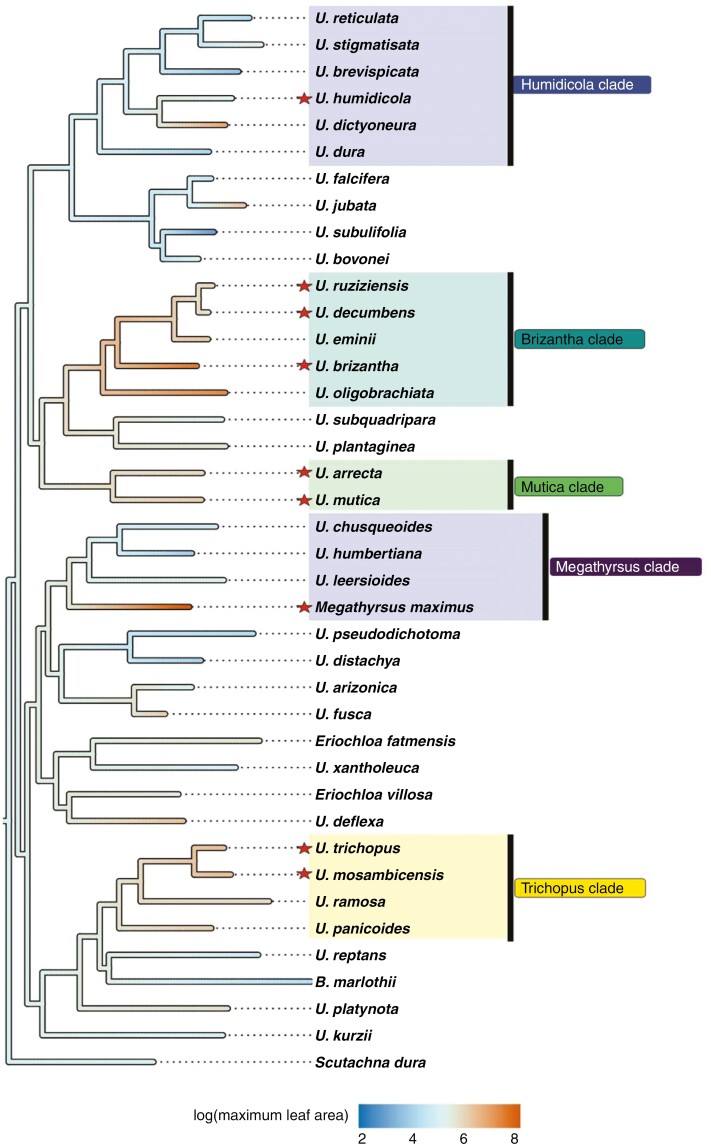
Ancestral states for the chosen continuous traits showed moderate size for leaf area, culm height and seeds (fertile lemma length) across not just Urochloa, but also the subtribe Melinidinae and Malagasy ‘Brachiaria’ species. For the Brizantha and Mutica clades, ancestral state estimations for log leaf area (Fig. 3, and Supplementary Data Fig S3) and log culm height ( Fig. S4) show an increase in size of these traits at the node of their respective most recent ancestors. The Humidicola, Megathyrsus and Trichopus clades show greater variability in these two traits, as agriculturally significant species differ from their closest relatives and their respective estimated ancestors. This is most observably clear for M. maximus, which evolved much larger leaves and culms than its closest living relatives and their most recent shared ancestor.
Fig. 3.
Evolution of log-transformed leaf area (cm2) in the Urochloa s.l. tree excluding Melinidinae, Boivinellinae and outgroup taxa. Forage species are marked with a star and forage clades are highlighted. Ancestral state estimations are inferred along branch lengths.
Estimates for log fertile lemma length (Supplementary Data Fig. S5) show M. maximus, U. trichopus, U. mosambicensis and U. arrecta evolved large seeds from small-seeded ancestors independently. The ancestral state for the Humidicola clade indicates that the common ancestor probably had moderately large seeds, though seed size increased along the U. humidicola/U. dictyoneura lineage and decreased in wild relatives. Long fertile lemma length estimates for the Brizantha clade indicate that this clade evolved from a common ancestor with large seeds. Phylogenetic signal for all three continuous traits was high and statistically significant, indicating that the similarity in trait values across all taxa is the result of a shared phylogenetic history (Table 2).
Table 2.
Phylogenetic signal values (Blomberg’s K and Pagel’s λ) with P-values for natural log values of continuous traits (leaf area, lemma length and culm height).
| Blomberg’s K | P-value | Pagel’s λ | P-value | |
|---|---|---|---|---|
| log Leaf area | 0.775 067 | 0.0016 | 0.800 593 | 9.51344e−5 |
| log Lemma length | 0.68 713 | 0.0052 | 0.999 934 | 0.000952218 |
| log Culm height | 0.636 892 | 0.0254 | 0.891 587 | 0.00495678 |
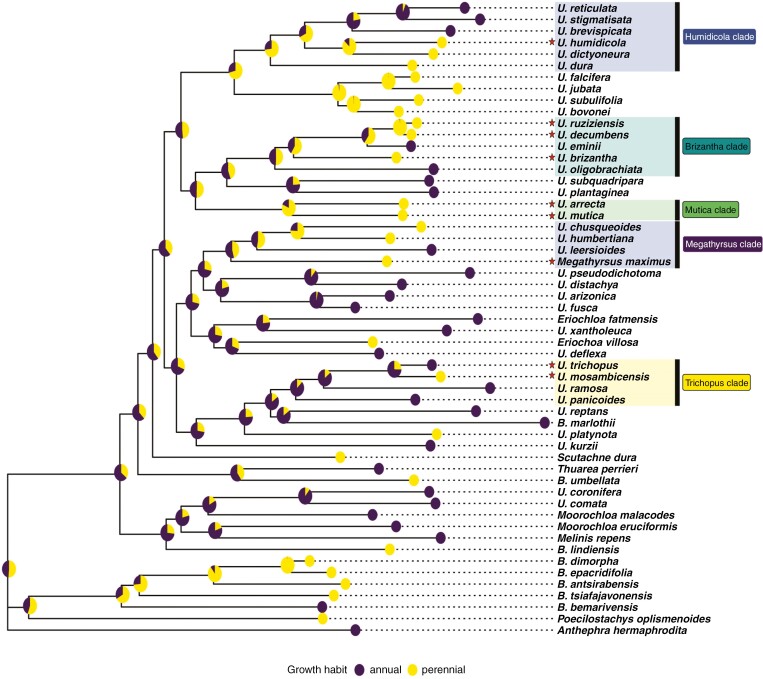
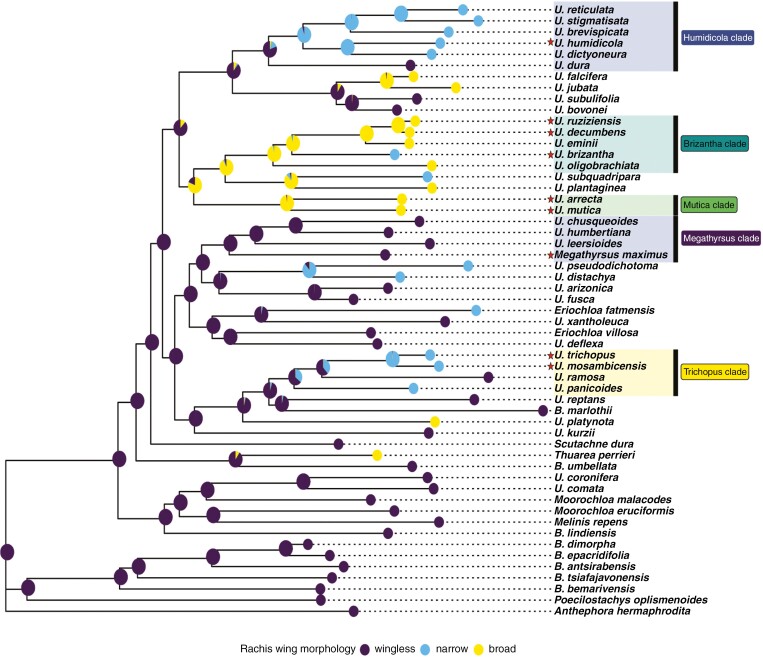
AIC scores determined that an ‘Equal Traits’ model was the best fit for ancestral trait estimation for both discrete traits (i.e. growth habit and rachis wing morphology) (Table 3). Posterior probability scores show that the ancestor of Urochloa grasses was probably an annual and that perennial growth habits probably emerged multiple times at ancestral nodes within the genus (Fig. 4). Estimations of ancestral rachis wing morphology show strongly that Urochloa s.l. evolved from an ancestor with a wingless rachis (Fig. 5). The emergence of a rachis wing (narrow or broad) occurred in parallel across multiple nodes in the phylogeny and is generally associated with forage clades (a notable exception being the Megathyrsus clade where all members are wingless).
Table 3.
Model selection for growth habit (annual vs perennial) and rachis wing morphology with log likelihood, Akaike Information Criterion (AIC), and delta AIC scores.
| Trait | Model | log likelihood | AIC | delta AIC |
|---|---|---|---|---|
| Growth habit | Equal Rates | −43.0951 | 88.19 | 0 |
| All Rates Different | −43.0951 | 90.19 | 2 | |
| Annual to Perennial | −46.12 271 | 94.245 | 6.055209 | |
| Perennial to Annual | −49.5135 | 101.03 | 12.8368 | |
| Rachis morphology | Equal Rates | −51.12 791 | 104.26 | 0 |
| All Rates Different | −47.87 176 | 119.74 | 15.4877 | |
| Symmetrical Rates | −48.81 644 | 109.63 | 5.377 079 |
Fig. 4.
Evolution of growth form (annual vs perennial). Forage species are marked with a star and forage clades are highlighted. Ancestral state estimations for annual versus perennial habits were performed using corHMM in R and posterior probabilities for state estimations were mapped to ancestral nodes.
Fig. 5.
Evolution of rachis morphology (wingless, narrowly winged or broadly winged). Forage species are marked with a star and forage clades are highlighted. Estimates indicate that the common ancestor of extant Urochloa s.l. species did not have a winged rachis (purple), and that the widening of the rachis occurred multiple times within forage clades (blue and yellow for narrow and broad wings respectively). Ancestral state estimations were performed using corHMM in R and posterior probabilities for state estimations were mapped to ancestral nodes.
As the Brizantha and Mutica clades are the most closely related forage clades, posterior probabilities indicate that the shared ancestor of these important forage clades may have already evolved a broad rachis wing. For the Humidicola clade, results indicate that the clade probably evolved from a wingless ancestor, but that a narrowly winged form probably emerged at an early point in the clade’s divergence, as the vast majority of Humidicola clade species possess winged rachises.
DISCUSSION
Crop wild relatives for Urochloa forage breeding
The phylogenetic analyses conducted here provide a platform for interpreting the evolution of Urochloa s.l. A broad definition of Urochloa s.l. (which includes all subsumed genera) is supported in our ASTRAL-III species tree and ML supermatrix tree (Fig. 2), confirming previous results based on chloroplast markers (Salariato et al., 2010, 2012; Hackel et al., 2018; Delfini et al., 2023). Further, both ASTRAL-III and ML trees recovered clades supporting infrageneric groupings in Urochloa based on morphological characters (Renvoize et al., 1996). However, inferring trees using hundreds of nuclear loci allowed us to resolve polytomies previously recovered in Urochloa s.l. phylogenies inferred from chloroplast markers. For example, Salariato et al. (2010, 2012) and Delfini et al. (2023) consistently fail to recover the Humidicola and Mutica clades using chloroplast markers. The recovery of forage clades that reliably agree with morphological subgroups within Urochloa s.l. suggests our phylogenies provide more realistic species relations in the genus compared to previous studies.
For all forage species, we were able to identify CWRs based on monophyletic clades recovered in both ASTRAL-III and ML supermatrix analyses. CWRs are important taxa for agricultural purposes as they provide the comparative context for in-depth analysis for phenotypic developmental and molecular studies in crops (Harlan and DeWet, 1971; Pironon et al., 2020; Viruel et al., 2021). Additionally, crosses between crop species and their wild relatives have produced hybrid progeny with highly desirable agricultural traits, such as fungal and nematode disease resistance in peanuts (Bertioli et al., 2021), or increase heat and drought tolerance in wheat (Molero et al., 2023). Interspecific crossing is routine in Urochloa cultivar production as the most popular commercially available lines, Mulato and Mulato II, were developed from U. brizantha × U. decumbens × U. ruziziensis hybrids (Argel et al., 2007; Pizarro et al., 2013).
Urochloa brizantha, U. decumbens and U. ruziziensis share a most recent common ancestor with CWR species U. eminii and U. oligobrachiata, confirming the observations of Renvoize et al. (1996) based on morphological data. Further, alpha taxonomists have long argued that intermediate specimens are common between U. ruziziensis and U. decumbens, and U. decumbens and U. brizantha respectively (Renvoize and Maass, 1993; Renvoize et al., 1996; Sosef, 2016), demonstrating that detailed morphological studies still provide insight into evolutionary dynamics present in even highly reticulate species complexes. Sosef (2016) argued that U. decumbens and U. ruziziensis be lumped together along with U. eminii, and our analysis confirms that the three taxa are the most closely related species within the Brizantha complex. To our knowledge, U. eminii and U. oligobrachiata are not used in forage grass breeding programmes, but it is likely that these CWRs can be hybridized with U. brizantha, U. decumbens and U. ruziziensis to produce cultivars with novel phenotypes (though this will need to be tested empirically).
Cultivar development for U. humidicola forages lags behind the Brizantha clade at a commercial scale (Boldrini et al., 2011). While apomictic reproduction in the complex has been overcome by the chance discovery of sexually reproductive accessions (Jungmann et al., 2009), cultivars are developed by crossing sexual and apomictic U. humidicola accessions only (Jungmann et al., 2009; de Figueiredo et al., 2019; Berchembrock et al., 2020). High ploidy levels (hexaploidy and heptaploidy typically) in U. dictyoneura and U. humidicola and reduced genetic diversity in seed bank collections creates a stumbling block for breeders (Miles and do Valle, 1996; Vigna et al., 2016; Higgins et al., 2022). Introducing CWRs into breeding systems remains a viable option for overcoming these limitations, particularly for increasing genetic diversity. Renvoize et al. (1996) identified U. stigmatisata, U. reticulata and U. brevispicata as close relatives of U. humidicola and U. dictyoneura, which is supported by our phylogenetic analyses (Fig. 2). However, Renvoize et al. (1996) grouped U. dura with Brizantha clade species, whereas our analyses show that U. dura belongs to the Humidicola clade. Further, Renvoize et al. (1996) placed U. platynota as a close relative to the Humidicola clade, but our analysis shows it is a distantly related species. Finally, Renvoize et al. (1996) grouped U. falcifera, U. jubata, U. subulifolia and U. bovonei with Humidicola clade species in their analysis. Our results show that these taxa form a well-supported clade sister to the Humidicola clade (Fig. 2).
Broadly, a suite of CWRs and sister taxa to the U. humidicola/U. dictyoneura complex have been inferred in the literature and are strongly supported by our phylogenetic results (Fig. 2). Introducing CWRs and interspecific breeding into U. humidicola/U. dictyoneura cultivars could result in new forage lines with novel agricultural traits, as has already successfully been demonstrated in hybrid Brizantha clade cultivars (Argel et al., 2007; Pizarro et al., 2013). Additionally, cytological results and fluorescence in situ hybridization have provided evidence for the inferred allopolyploid origins of U. humidicola and, crucially, potential subgenome identification in the species (Vigna et al., 2016; Tomaszewska et al., 2023). CWRs provide a sensible starting point for investigating the putative donors of U. humidicola subgenomes, as has been demonstrated across numerous allopolyploid crop species, such as bread wheat, strawberries and Brassica crops (He et al., 2017; Edger et al., 2019; Yim et al., 2022).
Trichopus and Mutica clade species are less commercially important in a global context, though their importance as livestock feed at small scales has been noted (Fischer and Kerridge, 1996; Pereira Filho et al., 2013). Urochloa trichopus and U. mosambicensis have been shown to be a nutrient-dense food source for goats in low-precipitation regions of Brazil such as the Caatinga (do Santos Pessoa et al., 2022). While the Trichopus clade is distantly related to other forage clades, the Mutica clade shares a recent common ancestor with the Brizantha and Humidicola clade. The placement of Megathyrsus within Urochloa is strongly supported, and its placement with species with more broadly spaced and pedicelled spikelets (i.e. U. chusqueoides and U. humbertiana) (Renvoize et al., 1996) provides a sensible framework for comparative analysis of inflorescence diversity in Urochloa s.l. See Supplementary Data Table S2 for a summary of Urochloa CWR species and their traits.
Agricultural trait evolution
Modelling character evolution is challenging in groups where data availability is limited for both taxa and appropriate traits. Herbarium accessions play a pivotal role in bolstering taxon representation in phylogenies, especially for groups with geographical ranges spanning multiple countries and continents such as Urochloa (Besnard et al., 2007; Baker et al., 2021; Larson et al., 2023). Comprehensive phylogenies are commonly utilized for evolutionary and ecological trait comparisons in numerous biological disciplines (Revell and Harmon, 2022), but the application of these methods in assessing agricultural potential across plant species (and clades) is underexplored. Forage grasses present a unique opportunity to apply phylogenetic comparative methods for agricultural purposes as traits of interest for breeders, taxonomists and ecologists share considerable overlap (leaf size, plant height, growth habit, etc.) and are probably present in floras and databases (Clayton and Renvoize, 1982; Clayton et al., 2016). For select Urochloa species, the domestication process is well within the initial phases (Dusi et al., 2010; Jank et al., 2014).
The three continuous traits assessed in this study (leaf area, culm height and seed size) had moderate to high values for Blomberg’s K and Pagel’s λ, and all values were statistically significant (Table 2). Values approaching 1 for both Blomberg’s K and Pagel’s λ are interpreted as high phylogenetic signal for the trait in question (Revell et al., 2008). This is evidence that shared values for the continuous traits assessed are due to shared ancestry in Urochloa grasses. Ancestral trait estimates for continuous traits generally show size increases along branches from ancestral clade nodes for important forage species and their close relatives. Our discrete character state estimations show a similar trend. A winged rachis morphology emerged independently in all forage clades except for the Megathyrsus clade where all species (including M. maximus) have wingless rachises and lax inflorescences. A winged rachis has been noted to impose greater rigidity on spikelet ordering (Renvoize et al., 1996), and is partly associated with a ‘homogenized’ inflorescence morphology as outlined by Salariato et al. (2010) and Reinheirmer and Vegetti (2008). Further investigation of how inflorescence structure influences seed retention (non-shattering phenotype) in Urochloa is essential, as non-shattering is among the first selected traits in plant domestication for grain production (Konishi et al., 2006; Yu et al., 2020). Estimating ancestral growth habit shows more state uncertainty, particularly at deeper nodes in the phylogeny. However, there remains evidence that perennial growth habits have evolved multiple times across Urochloa in forage clades. Improving certainty for node state estimates can be achieved by more dense sampling of Urochloa species in future.
Palatable perennial grasses are common across the African continent (Ezenwa et al., 2006; Bond, 2008), though the independent emergence of species with forage potential across Urochloa is notable. The goal of forage grass breeding is to develop cultivars with unique phenotypes to suit specific geographical and climatic regions, while not sacrificing nutritional content and biomass production (do Valle et al., 2013; Nguku, 2015). Achieving this goal sustainably will require selecting material from genetically diverse accessions and taxa (Ferreira et al., 2021). Based on our results, the independent evolution of forage syndromes across African grasses implies a high amount of taxonomic and genetic diversity that forage breeders can draw from for future cultivar development.
A viable strategy for exploiting Urochloa s.l. diversity for agricultural gain is to introduce CWR species into forage cultivar breeding programmes. It is important to emphasize that CWR species are naturally endemic to Africa and are distributed across numerous African countries. For example, U. eminii (Brizantha clade CWR) has a range spanning west, east and central Africa (POWO, https://powo.science.kew.org/). Within-species variation for CWRs would be highly informative to breeders, but little is known about the CWR species identified in this study. To best utilize the agricultural potential of Urochloa CWRs, efforts must be made to understand their trait and genetic diversity in the wild. This implies that natural populations of Urochloa CWRs must be identified and, crucially, conserved. Here we highlight a clear overlap between agriculture and conservation interests: the genetic diversity in Urochloa forage species and their CWR exists in African savannah grasslands for breeders to utilize, and so the conservation of African grasslands is vital for the future of sustainable forage grass breeding both in Africa and across the tropics.
Future considerations
Despite containing the world’s most important tropical forage grass species, Urochloa s.l. still contains an enormous amount of agricultural potential that has not been explored. Introducing CWRs into future breeding programmes is a stepping stone towards improving commercially available grasses. Interspecific Urochloa crosses are only successful if ploidy levels between parental species match and at least one parent is sexually reproductive. Addressing these knowledge gaps will require high-quality, chromosome-scale genome assemblies for important forage species and CWRs (Risso-Pascotto et al., 2005; Simeão et al., 2021). Within the forage clades identified in this study, only chromosome-scale genome assemblies exist for U. ruziziensis (Pessoa-Filho et al., 2019, available online but analysis unpublished; Worthington et al., 2021). Additional high-quality genome assemblies will be valuable and can be used to determine ancestral genome origins in polyploids and chromosome rearrangements in the various species with distinct chromosome numbers, and to provide reference genomes for alignment of polymorphic markers from reduced-representation sequencing.
Genome assemblies could reveal the genetic pathways associated with Urochloa invasiveness into non-agricultural land, an unfortunate trend seen in African grasses globally (Visser et al., 2016). For example, Mutica clade species have been introduced from African countries to various parts of the world with the putative aim of improving pastures for livestock rearing (Williams and Baruch, 2000). While these species clearly have good forage characteristics, as demonstrated in this study and elsewhere (Fischer and Kerridge, 1996; Veldkamp, 1996), little scientifically informed breeding has been attempted in U. mutica and U. arrecta, and the two species are commonly classified as invasive weeds outside the African continent (Boyden et al., 2019). Even in the commercially important Brizantha clade, U. decumbens is an aggressive invasive in the Cerrado, a dry savannah region in Brazil (Pivello et al., 1999). This is probably a consequence of the species’ early introduction to South America as a forage grass prior to the establishment of genetic breeding programmes (Pivello et al., 1999; Barbosa et al., 2008). There exists a substantive link between forage potential and aggressive invasiveness in African grasses, and genomic resources could help mitigate this undesirable attribute (Daehler and Carino, 1998; Williams and Baruch, 2000; Cook and Dias, 2006; Barbosa et al., 2008; Barbosa, 2016).
Beyond this study, there is still a need for more in-depth knowledge of the basic biology and diversity in Urochloa s.l., and greater emphasis must be placed on conserving and collecting wild Urochloa grasses, particularly in African countries. While commercial cultivars are predominantly utilized at large scale in South America (Jank et al., 2014; Maass et al., 2015), African nations have begun reintroducing cultivars in beef, dairy and push–pull pest control systems with notable successes (Mutimura, 2012; Khan et al., 2014; Clémence-Aggy et al., 2021). As the centre of Urochloa s.l. diversity, natural populations of African species probably contain the genes and traits needed to tailor new cultivars for the specific and varying needs of farmers, livestock and ecosystems across African nations. Conservation of African grasses is a global sustainability imperative as African grasslands form the basis of ancient habitats (Bond, 2016; Solofondranohatra et al., 2020; Buisson et al., 2022), perform natural carbon sequestration (Vågen et al., 2005; Dobson et al., 2022), and support the livelihoods of millions of people and animals (Bengtsson et al., 2019). African grassland conservation will safeguard the biodiversity needed to address issues of economic development and food security, and Urochloa is a genus of primary consideration in this regard.
CONCLUSION
We have reconstructed a nuclear phylogeny for the grass genus Urochloa s.l. that is both comprehensively sampled and data rich, focusing on forages and their relatives. Our phylogenomic analysis allowed us to infer the placement of key agricultural species within the genus and identify their closest wild relatives. Additionally, we were able to estimate the ancestral state of numerous agriculturally important traits and demonstrate their convergent emergence in agriculturally important lineages. Urochloa s.l. is a highly morphologically diverse genus replete with polyploidization events and a natural distribution spanning the near entirety of the southern hemisphere. These attributes make Urochloa a prime example of how African grasses should serve as model systems for studying complicated evolutionary events, how a strong taxonomic and phylogenetic foundation can aid these studies, and how this knowledge can facilitate more sustainable agricultural practices in countries where it is most required.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: metadata for all samples used in this study including estimated ploidy levels, trait data and accession data where available. Table S2: forage clades, CWR and forage traits obtained from GrassBase (Clayton et al., 2016). Figure S1: histogram and boxplot of putative paralogues in the Angiosperm 353 locus sequences for samples used in this study. Figure S2: bar graph of ploidy levels (estimated or taken from the literature) for all samples used in this study. Figure S3: ancestral trait estimation for leaf area. Figure S4: ancestral trait estimation for culm height. Figure S5: ancestral trait estimation for fertile lemma length.
ACKNOWLEDGEMENTS
The authors would like to acknowledge RBG, Kew Herbarium curator, Martin Xanthos, for assistance with specimen sampling for this study. We would also like to thank RBG, Kew Jodrell Laboratory staff Laszlo Csiba, Robyn S. Cowan and Niroshini Epitawalage for assistance with DNA extraction and library preparation. We thank staff at CIAT, Juan Jose Gonzalez and Rosa Jauregui, for insightful comments on Urochloa forages they provided throughout this study. We also thank the Plant and Fungal trees of life project (PAFTOL), funded by the Calleva foundation, for sharing unpublished data used in this work. Finally, we thank the James Hutton Institute and NIAB for proving computational resources through the ‘UK’s Crop Diversity Bioinformatics HPC’ (BBSRC grant BB/S019669/1) which contributed to the results obtained in this paper.
Contributor Information
Lizo E Masters, Department of Genetics and Genome Biology, Institute for Environmental Futures, University of Leicester, Leicester LE17RH, UK; Accelerated Taxonomy/Trait Diversity and Function, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, UK.
Paulina Tomaszewska, Department of Genetics and Genome Biology, Institute for Environmental Futures, University of Leicester, Leicester LE17RH, UK; Department of Genetics and Cell Physiology, University of Wroclaw, 50-328 Wroclaw, Poland.
Trude Schwarzacher, Department of Genetics and Genome Biology, Institute for Environmental Futures, University of Leicester, Leicester LE17RH, UK; Key Laboratory of Plant Resources Conservation and Sustainable Utilization/Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China.
Jan Hackel, Accelerated Taxonomy/Trait Diversity and Function, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, UK; Department of Biology, University of Marburg, Karl-von-Frisch-Straße 8, 35043 Marburg, Germany.
Alexandre R Zuntini, Accelerated Taxonomy/Trait Diversity and Function, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, UK.
Pat Heslop-Harrison, Department of Genetics and Genome Biology, Institute for Environmental Futures, University of Leicester, Leicester LE17RH, UK; Key Laboratory of Plant Resources Conservation and Sustainable Utilization/Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China.
Maria S Vorontsova, Accelerated Taxonomy/Trait Diversity and Function, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, UK.
FUNDING
L.M. was funded through the Central England NERC Training Alliance 2 (CENTA2) research and training grant NE/S007350/1. J.H. was funded through the Future Leaders in Plant and Fundal Science fellowship and a pilot grant from RBG, Kew. P.T. has received support from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 844564 and no. 101006417 for the analysis of polyploid chromosomal evolution.
LITERATURE CITED
- Alves GF, de Figueiredo UJ, Filho ADP, Barrios SCL, do Valle CB. 2014. Breeding strategies for Brachiaria spp. to improve productivity - An ongoing project. Tropical Grasslands-Forrajes Tropicales 2: 1–3. [Google Scholar]
- Andermann T, Torres Jiménez MF, Matos-Maraví P, et al. 2020. A guide to carrying out a phylogenomic target sequence capture project. Frontiers in Genetics 10: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANUALPEC. 2008. Anuário da pecuária brasileira. São Paulo: Informa Economics FNP. [Google Scholar]
- Argel PJ, Miles JW, Guidot JD, Cuadrado H, Lascano CE.. 2007. Cultivar Mulato II (Brachiaria hybrid CIAT 36087) A high quality forage grass, resilient to spittlebug and adapted to well-drained acid tropical soils. Cali: CIAT. [Google Scholar]
- Baker WJ, Dodsworth S, Forest F, et al. 2021. Exploring Angiosperms353: An open, community toolkit for collaborative phylogenomic research on flowering plants. American Journal of Botany 108: 1059–1065. [DOI] [PubMed] [Google Scholar]
- Baker WJ, Bailey P, Barber V, et al. 2022. A comprehensive phylogenomic platform for exploring the angiosperm tree of life. Systematic Biology 71: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, et al. 2012. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19: 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptistella JLC, de Andrade SAL, Favarin JL, Mazzafera P.. 2020. Urochloa in tropical agroecosystems. Frontiers in Sustainable Food Systems 4: 1–17. [Google Scholar]
- Barbosa FG. 2016. The future of invasive African grasses in South America under climate change. Ecological Informatics 36: 114–117. [Google Scholar]
- Barbosa EG, Pivello VR, Meirelles ST.. 2008. Allelopathic evidence in Brachiaria decumbens and its potential to invade the Brazilian cerrados. Brazilian Archives of Biology and Technology 51: 625–631. [Google Scholar]
- Beaulieu JM, O’Meara BC, Donoghue MJ.. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: The evolution of plant habit in campanulid angiosperms. Systematic Biology 62: 725–737. [DOI] [PubMed] [Google Scholar]
- Beloni T, Santos PM, Rovadoscki GA, Balachowski J, Volaire F.. 2018. Large variability in drought survival among Urochloa spp. cultivars. Grass and Forage Science 73: 947–957. [Google Scholar]
- Bengtsson J, Bullock JM, Egoh B, et al. 2019. Grasslands—more important for ecosystem services than you might think. Ecosphere 10: e02582. [Google Scholar]
- Berchembrock YV, de Figueiredo UJ, Nunes JAR, do Valle CB, Barrios SCL.. 2020. Comparison of selection methods among and within full-sibling progenies in Urochloa humidicola. Grass and Forage Science 75: 145–152. [Google Scholar]
- Bertioli DJ, Gao D, Ballen-Taborda C, et al. 2021. Registration of GA-BatSten1 and GA-MagSten1, two induced allotetraploids derived from peanut wild relatives with superior resistance to leaf spots, rust, and root-knot nematode. Journal of Plant Registrations 15: 372–378. [Google Scholar]
- Besnard G, Rubio De Casas R, Vargas P.. 2007. Plastid and nuclear DNA polymorphism reveals historical processes of isolation and reticulation in the olive tree complex (Olea europaea). Journal of Biogeography 34: 736–752. [Google Scholar]
- Blomberg SP, Garland T, Ives AR.. 2003. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Boldrini KR, Adamowski EV, Silva N, Pagliarini MS, Valle CB.. 2011. Meiotic behavior in nonaploid accessions of Brachiaria humidicola (Poaceae) and implications for breeding. Genetics and Molecular Research 10: 169–176. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond WJ. 2008. What limits trees in C4 grasslands and savannas? Annual Review of Ecology, Evolution, and Systematics 39: 641–659. [Google Scholar]
- Bond WJ. 2016. Ancient grasslands at risk. Science 351: 120–122. [DOI] [PubMed] [Google Scholar]
- Boyden J, Wurm P, Joyce KE, Boggs G.. 2019. Spatial dynamics of invasive para grass on a monsoonal floodplain, Kakadu National Park, Northern Australia. Remote Sensing 11: 20190901. [Google Scholar]
- Buisson E, Archibald S, Fidelis A, Suding KN.. 2022. Ancient grasslands guide ambitious goals in grassland restoration. Science 377: 594–598. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, et al. 2009. BLAST+: Architecture and applications. BMC Bioinformatics 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton WD, Renvoize SA.. 1982. Gramineae (Part 3). In: Polhill RM. ed. Flora of tropical East Africa, Vol. 3. Rotterdam: Balkema, 451–898. [Google Scholar]
- Clayton W, Vorontsova M, Harman T, Williamson H.. 2016. GrassBase - the online World Grass Flora. https://www.kew.org/data/grasses-db.html (1 February 2023, date last accessed). [Google Scholar]
- Clémence-Aggy N, Fidèle N, Raphael KJ, Agbor EK, Ghimire SR.. 2021. Quality assessment of Urochloa (syn. Brachiaria) seeds produced in Cameroon. Scientific Reports 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook GD, Dias L.. 2006. Turner Review No. 12. It was no accident: Deliberate plant introductions by Australian government agencies during the 20th century. Australian Journal of Botany 54: 601–625. [Google Scholar]
- Crowl AA, Fritsch PW, Tiley GP, et al. 2022. A first complete phylogenomic hypothesis for diploid blueberries (Vaccinium section Cyanococcus). American Journal of Botany 109: 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daehler CC, Carino DA.. 1998. Recent replacement of native pili grass (Heteropogon contortus) by invasive African grasses in the Hawaiian Islands. Pacific Science 52: 220–227. [Google Scholar]
- Damasceno G, Souza L, Pivello VR, Gorgone-Barbosa E, Giroldo PZ, Fidelis A.. 2018. Impact of invasive grasses on Cerrado under natural regeneration. Biological Invasions 20: 3621–3629. [Google Scholar]
- de Figueiredo UJ, Lima Barrios SC, do Valle CB, Nunes JAR.. 2019. Combining ability among apomictic and sexual parents of Urochloa humidicola. Grass and Forage Science 74: 678–686. [Google Scholar]
- Delfini C, Salariato DL, Aliscioni SS, Zuloaga FO.. 2023. Systematics and phylogenetic placement of Panicum L. species within the Melinidinae based on morphological, anatomical, and molecular data (Poaceae, Panicoideae, Paniceae). Plants 12: 12020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A, Hopcraft G, Mduma S, et al. 2022. Savannas are vital but overlooked carbon sinks. Science 375: 392. [DOI] [PubMed] [Google Scholar]
- dos Santos Pessoa RM, da Silva DS, Pereira Filho JM, de Azevedo Silva AM, de Sousa Ferreira JM, do Nascimento GV.. 2022. Forage availability and weight gain of goats on caatinga enriched with Urochloa trichopus (Hochst.) Stapf subjected to fallowing and fertilized with phosphate. Ciencia Animal Brasileira 23: 20220311. [Google Scholar]
- do Valle CD, Savidan YH.. 1996. Genetics, cytogenetics, and reproductive biology of Brachiaria. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 147–163. [Google Scholar]
- do Valle CB, Euclides VPB, Montagner DB, et al. 2013. BRS Paiaguás: a new Brachiaria (Urochloa) cultivar for tropical pastures in Brazil. Tropical Grasslands-Forrajes Tropicales 1: 121–122. [Google Scholar]
- Doyle JJ, Doyle JL.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, Botanical Society of America 19: 11–15. [Google Scholar]
- Dusi DMA, Alves ER, Willemse MTM, Falcão R, do Valle CB, Carneiro VTC.. 2010. Toward in vitro fertilization in Brachiaria spp. Sexual Plant Reproduction 23: 187–197. [DOI] [PubMed] [Google Scholar]
- Edger PP, Poorten TJ, VanBuren R, et al. 2019. Origin and evolution of the octoploid strawberry genome. Nature Genetics 51: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SV. 2009. Is a new and general theory of molecular systematics emerging? Evolution 63: 1–19. [DOI] [PubMed] [Google Scholar]
- Enciso K, Charry A, Castillo AR, Burkart S.. 2021. Ex-ante evaluation of economic impacts of adopting improved forages in the Colombian Orinoquía. Frontiers in Environmental Science 9: 1–17. [Google Scholar]
- Espitia P, Hernández LM, Velasco J, Castiblanco V.. 2020. Report on evaluation of promising Brachiaria hybrid populations for resistance against spittlebug. Alliance of Biodiversity and CIAT: 6. [Google Scholar]
- Ezenwa IV, Kalmbacher RS, Arthington JD, Pate FM.. 2006. Creeping signal grass versus bahia grass for cow and calf grazing. Agronomy Journal 98: 1582–1588. [Google Scholar]
- Ferreira RCU, Costa Lima Moraes A, Chiari L, Simeão RM, Vigna BBZ, de Souza AP.. 2021. An overview of the genetics and genomics of the Urochloa species most commonly used in pastures. Frontiers in Plant Science 12: 770461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MJ, Kerridge PC.. 1996. The agronomy and physiology of Brachiaria species. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 43–45. [Google Scholar]
- Foxcroft LC, Richardson DM, Rejmánek M, Pyšek P.. 2010. Alien plant invasions in tropical and sub-tropical savannas: patterns, processes and prospects. Biological Invasions 12: 3913–3933. [Google Scholar]
- Ghimire S, Njarui D, Mutimura M, et al. 2015. Climate-smart Brachiaria for improving livestock production in East Africa: emerging opportunities. In: Proceedings of 23rd International Grassland Congress 2015-Keynote Lectures.
- Godfray HCJ, Aveyard P, Garnett T, et al. 2018. Meat consumption, health, and the environment. Science (New York, N.Y.) 361: eaam5324. [DOI] [PubMed] [Google Scholar]
- Govaerts R, Nic Lughadha E, Black N, Turner R, Paton A.. 2021. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Scientific Data 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel J, Vorontsova MS, Nanjarisoa OP, et al. 2018. Grass diversification in Madagascar: In situ radiation of two large C3 shade clades and support for a Miocene to Pliocene origin of C4 grassy biomes. Journal of Biogeography 45: 750–761. [Google Scholar]
- Hanley SJ, Pellny TK, De Vega JJ, et al. 2021. Allele mining in diverse accessions of tropical grasses to improve forage quality and reduce environmental impact. Annals of Botany 128: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JR, Wet JMJ.. 1971. Toward a rational classification of cultivated plants. Taxon 20: 509–517. [Google Scholar]
- Hartley W, Williams RJ.. 1956. Centres of distribution of cultivated pasture grasses and their significance for plant introduction. Proceedings of the 7th International Grassland Congress, 190–201.
- He Z, Wang L, Harper AL, et al. 2017. Extensive homoeologous genome exchanges in allopolyploid crops revealed by mRNAseq-based visualization. Plant Biotechnology Journal 15: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Havlík P, Valin H, et al. 2013. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proceedings of the National Academy of Sciences of the United States of America 110: 20888–20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Tomaszewska P, Pellny TK, et al. 2022. Diverged subpopulations in tropical Urochloa (Brachiaria) forage species indicate a role for facultative apomixis and varying ploidy in their population structure and evolution. Annals of Botany 130: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS.. 2018. UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JM, de Souza FHD, Diulgheroff S, Ortiz A, Sanchez M.. 1996. Reproductive physiology, seed Production, and seed quality of Brachiaria. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 124–140. [Google Scholar]
- Ishigaki G, Gondo T, Suenaga K, Akashi R.. 2009. Induction of tetraploid ruzigrass (Brachiaria ruziziensis) plants by colchicine treatment of in vitro multiple-shoot clumps and seedlings. Grassland Science 55: 164–170. [Google Scholar]
- Jank L, Barrios SC, Do Valle CB, Simeão RM, Alves GF.. 2014. The value of improved pastures to Brazilian beef production. Crop and Pasture Science 65: 1132–1137. [Google Scholar]
- Johnson MG, Gardner EM, Liu Y, et al. 2016. HybPiper: Extracting coding sequence and introns for phylogenetics from high‐throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: 1600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MG, Pokorny L, Dodsworth S, et al. 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann L, Vigna BBZ, Paiva J, et al. 2009. Development of microsatellite markers for Brachiaria humidicola (Rendle) Schweick. Conservation Genetics Resources 1: 475–479. [Google Scholar]
- Junier T, Zdobnov EM.. 2010. The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics 26: 1669–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntasin W, Imura Y, Thaikua S, Poungkaew R, Kawamoto Y.. 2022. Effects of plant spacing on seed yield and seed quality in new Urochloa cultivars. Grassland Science 68: 88–98. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS.. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates HR, Johnson MG, Gardner EM, Zerega NJC, Wickett NJ.. 2018. Allele phasing has minimal impact on phylogenetic reconstruction from targeted nuclear gene sequences in a case study of Artocarpus. American Journal of Botany 105: 404–416. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H, Kobayashi A, Ohno O, Kimura F, Fujii Y, Suenaga K.. 2014. Phytotoxic substances with allelopathic activity may be central to the strong invasive potential of Brachiaria brizantha. Journal of Plant Physiology 171: 525–530. [DOI] [PubMed] [Google Scholar]
- Keller-Grein G, Maass BL, Hanson J.. 1996. Natural variation in Brachiaria and existing germplasm collections. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 16–42. [Google Scholar]
- Kellogg EA. 2015. Flowering plants. Monocots: Poaceae. Berlin: Springer. [Google Scholar]
- Khan ZR, Midega CAO, Pittchar JO, et al. 2014. Achieving food security for one million sub-Saharan African poor through push-pull innovation by. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Izawa T, Lin SY, et al. 2006. An SNP caused loss of seed shattering during rice domestication. Science 312: 1392–1396. [DOI] [PubMed] [Google Scholar]
- Krahl G, Marocco DH.. 2019. Manejo para a recuperação de forrageiras perenes estivais a danos por geadas. Revista Brasileira de Agropecuária Sustentável 9: 78–86. [Google Scholar]
- Lal R. 2004. Soil carbon sequestration to mitigate climate change. Geoderma 123: 1–22. [Google Scholar]
- Landis JB, Soltis DE, Li Z, et al. 2018. Impact of whole-genome duplication events on diversification rates in angiosperms. American Journal of Botany 105: 348–363. [DOI] [PubMed] [Google Scholar]
- Larridon I, Villaverde T, Zuntini AR, et al. 2020. Tackling rapid radiations with targeted sequencing. Frontiers in Plant Science 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DA, Chanderbali AS, Maurin O, et al. 2023. The phylogeny and global biogeography of Primulaceae based on high-throughput DNA sequence data. Molecular Phylogenetics and Evolution 182: 107702. [DOI] [PubMed] [Google Scholar]
- Ledo A, Smith P, Zerihun A, et al. 2020. Changes in soil organic carbon under perennial crops. Global Change Biology 26: 4158–4168. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB. 1999. Uncertainty in the reconstruction of ancestral character states and limitations on the use of phylogenetic comparative methods. Animal Behaviour 58: 1319–1324. [DOI] [PubMed] [Google Scholar]
- Maass BL, Midega CAO, Mutimura M, et al. 2015. Homecoming of Brachiaria: Improved hybrids prove useful for African animal agriculture. East African Agricultural and Forestry Journal 81: 71–78. [Google Scholar]
- Maddison WP. 1997. Gene trees in species trees. Systematic Biology 46: 523–536. [Google Scholar]
- Mai U, Mirarab S.. 2018. TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKain MR, Tang H, McNeal JR, et al. 2016. A phylogenomic assessment of ancient polyploidy and genome evolution across the Poales. Genome Biology and Evolution 8: 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, et al. 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JW. 2007. Apomixis for cultivar development in tropical forage grasses. Crop Science 47: S239–S249. [Google Scholar]
- Miles JW, do Valle CB.. 1996. Manipulation of apomixis in Brachiaria. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 164–177. [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, et al. 2020. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero G, Coombes B, Joynson R, et al. 2023. Exotic alleles contribute to heat tolerance in wheat under field conditions. Communications Biology 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Briones DF, Gehrke B, Huang CH, et al. 2022. Analysis of paralogs in target enrichment data pinpoints multiple ancient polyploidy events in Alchemilla s.l. (Rosaceae). Systematic Biology 71: 190–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone O, Escobar A, Zuloaga F.. 2006. Chromosome studies in American Panicoideae (Poaceae). Annals of the Missouri Botanical Garden 93: 647–657. [Google Scholar]
- Mutimura M. 2012. On-farm evaluation of improved Brachiaria grasses in low rainfall and aluminium toxicity prone areas of Rwanda. International Journal of Biodiversity and Conservation 4: 137–154. [Google Scholar]
- Mutimura M, Ebong C, Rao IM, Nsahlai IV.. 2018. Effects of supplementation of Brachiaria brizantha cv. Piatá and Napier grass with Desmodium distortum on feed intake, digesta kinetics and milk production in crossbred dairy cows. Animal nutrition (Zhongguo xu mu shou yi xue hui) 4: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazzi C, Sserumaga JP, Mugerwa S, et al. 2020. Genetic diversity and population structure of Brachiaria (syn. Urochloa) ecotypes from Uganda. Agronomy 10: 1193. [Google Scholar]
- Nauheimer L, Weigner N, Joyce E, Crayn D, Clarke C, Nargar K.. 2021. HybPhaser: a workflow for the detection and phasing of hybrids in target capture data sets. Applications in Plant Sciences 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguku SA. 2015. An evaluation of Brachiaria grass cultivars productivity in semi arid Kenya. MSc Thesis.
- Nicholls JA, Pennington RT, Koenen EJM, et al. 2015. Using targeted enrichment of nuclear genes to increase phylogenetic resolution in the neotropical rain forest genus Inga (Leguminosae: Mimosoideae). Frontiers in Plant Science 6: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njarui DMG, Gatheru M, Ndubi JM, Gichangi AW, Murage AW.. 2021. Forage diversity and fertiliser adoption in napier grass production among smallholder dairy farmers in Kenya. Journal of Agriculture and Rural Development in the Tropics and Subtropics 122: 245–256. [Google Scholar]
- Ortiz JPA, Pupilli F, Acuña CA, Leblanc O, Pessino SC.. 2020. How to become an apomixis model: The multifaceted case of Paspalum. Genes 11: 974–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt WA, Franck AR.. 2017. The invasive legacy of forage grass introductions into Florida. Natural Areas Journal 37: 254–264. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K.. 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Parsons JJ. 1972. Spread of African pasture grasses to the American tropics. Journal of Range Management 25: 12. [Google Scholar]
- Pereira Filho JM, Silva AM de A, Cézar MF.. 2013. Manejo da caatinga para produção de caprinos e ovinos. Revista Brasileira de Saude e Producao Animal 14: 77–90. [Google Scholar]
- Pessoa-Filho M, Martins AM, Ferreira ME.. 2017. Molecular dating of phylogenetic divergence between Urochloa species based on complete chloroplast genomes. BMC Genomics 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa-Filho M, Sobrinho FS, Fragoso RR, Silva-Junior OB, Ferreira ME.. 2019. A draft genome assembly for the forage grass Urochloa ruziziensis based on single-molecule real-time sequencing.
- Peters M, Rao I, Fisher M, et al. 2013. Tropical forage -based systems to mitigate greenhouse gas emissions. In: Hershey CH. eds. CIAT. Eco-efficient: From vision to reality – issues in tropical agriculture. Cali: Colombia, 1–20. [Google Scholar]
- Pironon S, Borrell JS, Ondo I, et al. 2020. Towards unifying global hotspots of wild and domesticated biodiversity. MDPI Plants 9: 9091128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivello VR, Shida CN, Meirelles ST.. 1999. Alien grasses in Brazilian savannas: A threat to the biodiversity. Biodiversity and Conservation 8: 1281–1294. [Google Scholar]
- Pizarro EA, Hare MD, Mutimura M, Changjun B.. 2013. Brachiaria hybrids: potential, forage use and seed yield. Tropical Grasslands-Forrajes Tropicales 1: 31–35. [Google Scholar]
- R Core Team. 2023. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/ (1 Feb 2023, date last accessed). [Google Scholar]
- Rathore P, Schwarzacher T, Heslop-Harrison JS, Bhat V, Tomaszewska P.. 2022. The repetitive DNA sequence landscape and DNA methylation in chromosomes of an apomictic tropical forage grass, Cenchrus ciliaris. Frontiers in Plant Science 13: 952968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheimer R, Vegetti AC.. 2008. Inflorescence diversity and evolution in the PCK Clade (Poaceae: Panicoideae: Paniceae). Plant Systematics and Evolution 275: 133–167. [Google Scholar]
- Renvoize SA, Maass B.. 1993. Brachiaria. A Report to CIAT, Colombia, on the species and specimens held in the germplasm collection. Kew: Royal Botanic Gardens. [Google Scholar]
- Renvoize SA, Clayton WD, Kabuye CHS.. 1996. Morphology, taxonomy, and natural distribution of Brachiaria (Trin.) Griseb. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 1–15. [Google Scholar]
- Revell LJ. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Revell LJ, Harmon LJ.. 2022. Phylogenetic comparative methods in R. Princeton: Princeton University Press. [Google Scholar]
- Revell LJ, Harmon LJ, Collar DC.. 2008. Phylogenetic signal, evolutionary process, and rate. Systematic Biology 57: 591–601. [DOI] [PubMed] [Google Scholar]
- Rhodes AC, Plowes RM, Goolsby JA, et al. 2021. The dilemma of Guinea grass (Megathyrsus maximus): a valued pasture grass and a highly invasive species. Biological Invasions 23: 3653–3669. [Google Scholar]
- Risso-Pascotto C, Pagliarini MS, Do Valle CB.. 2005. Meiotic behavior in interspecific hybrids between Brachiaria ruziziensis and Brachiaria brizantha (Poaceae). Euphytica 145: 155–159. [Google Scholar]
- Salariato DL, Zuloaga FO, Giussani LM, Morrone O.. 2010. Molecular phylogeny of the subtribe Melinidinae (Poaceae: Panicoideae: Paniceae) and evolutionary trends in the homogenization of inflorescences. Molecular Phylogenetics and Evolution 56: 355–369. [DOI] [PubMed] [Google Scholar]
- Salariato DL, Morrone O, Zuloaga FO.. 2012. Mayariochloa, a new monotypic genus segregated from Scutachne (Poaceae, Panicoideae, Paniceae). Systematic Botany 37: 105–116. [Google Scholar]
- Sandhu JS, Kumar D, Yadav VK, Singh T, Sah RP, Radhakrishna A.. 2015. Recent trends in breeding of tropical grass and forage species. In: International Grassland Congress Proceedings. 23rd International Grassland Congress, 337–348.
- Santos Filho LF. 1996. Seed production: perspectives from the Brazilian private sector. In: Miles JW, Maass BL, do Valle CB. eds. Brachiaria: biology, agronomy, and improvement. Cali: CIAT, 141–146. [Google Scholar]
- Seabloom EW, Borer ET, Buckley Y, et al. 2013. Predicting invasion in grassland ecosystems: Is exotic dominance the real embarrassment of richness? Global Change Biology 19: 3677–3687. [DOI] [PubMed] [Google Scholar]
- Simeão RM, Resende MDV, Alves RS, et al. 2021. Genomic selection in tropical forage grasses: current status and future applications. Frontiers in Plant Science 12: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Dunn CW.. 2008. Phyutility: a phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 24: 715–716. [DOI] [PubMed] [Google Scholar]
- Solofondranohatra CL, Vorontsova MS, Hempson GP, et al. 2020. Fire and grazing determined grasslands of central Madagascar represent ancient assemblages: Grasslands are shaped by disturbance. Proceedings of the Royal Society B: Biological Sciences 287: 20200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreng RJ, Peterson PM, Romaschenko K, et al. 2017. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of two 2015 classifications. Journal of Systematics and Evolution 55: 259–290. [Google Scholar]
- Sosef MSM. 2016. Taxonomic novelties in central African grasses (Poaceae), Paniceae 1. Plant Ecology and Evolution 149: 356–365. [Google Scholar]
- Sweitzer E, Hernandez L, Florian D, Notenbaert A, Burkart S.. 2021. Review of Urochloa breeder’s toolbox with the theory of change and stage gate system approach. International Grassland Congress Proceedings, 3.
- Tiley GP, Barker MS, Gordon Burleigh J.. 2018. Assessing the performance of Ks plots for detecting ancient whole genome duplications. Genome Biology and Evolution 10: 2882–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley GP, Crowl AA, Manos PS, et al. 2021. Phasing alleles improves network inference with allopolyploids 2. bioRxiv preprint. [Google Scholar]
- Tiley GP, Flouri T, Jiao X, et al. 2023. Estimation of species divergence times in presence of cross-species gene flow. Systematic Biology 72: 820–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewska P, Pellny TK, Hernández LM, et al. 2021. Flow cytometry-based determination of ploidy from dried leaf specimens in genomically complex collections of the tropical forage grass Urochloa s. l. Genes 12: 12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewska P, Vorontsova MS, Renvoize SA, et al. 2023. Complex polyploid and hybrid species in an apomictic and sexual tropical forage grass group: genomic composition and evolution in Urochloa (Brachiaria) species. Annals of Botany 131: 87–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres González AM, Morton CM.. 2005. Molecular and morphological phylogenetic analysis of Brachiaria and Urochloa (Poaceae). Molecular Phylogenetics and Evolution 37: 36–44. [DOI] [PubMed] [Google Scholar]
- Toutain B. 1986. Fodder grasses of the genera Urochloa and Brachiaria for the Pacific. Revue d’Élevage et do Médecine Vétérinaire de Nouvelle Calédonie 7: 47–56. [Google Scholar]
- Triviño NJ, Perez JG, Recio ME, et al. 2017. Genetic diversity and population structure of Brachiaria species and breeding populations. Crop Science 57: 2633–2644. [Google Scholar]
- Vågen TG, Lal R, Singh BR.. 2005. Soil carbon sequestration in sub-Saharan Africa: a review. Land Degradation and Development 16: 53–71. [Google Scholar]
- Van De Peer Y, Maere S, Meyer A.. 2009. The evolutionary significance of ancient genome duplications. Nature Reviews Genetics 10: 725–732. [DOI] [PubMed] [Google Scholar]
- Veldkamp, JF. 1996. Brachiaria, Urochloa (Gramineae-Paniceae) in Malesia. Blumea 41: 413–437. [Google Scholar]
- Vigna BBZ, Santos JCS, Jungmann L, et al. 2016. Evidence of allopolyploidy in Urochloa humidicola based on cytological analysis and genetic linkage mapping. PLoS One 11: e0153764–e0153723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viruel J, Kantar MB, Gargiulo R, et al. 2021. Crop wild phylorelatives (CWPs): phylogenetic distance, cytogenetic compatibility and breeding system data enable estimation of crop wild relative gene pool classification. Botanical Journal of the Linnean Society 195: 1–33. [Google Scholar]
- Visser V, Wilson JRU, Fish L, Brown C, Cook GD, Richardson DM.. 2016. Much more give than take: South Africa as a major donor but infrequent recipient of invasive non-native grasses. Global Ecology and Biogeography 25: 679–692. [Google Scholar]
- Vorontsova MS. 2022. Revision of some Malagasy forage grasses and their relatives within Brachiaria, Echinochloa, Moorochloa, and Urochloa. Candollea 77: 199–236. [Google Scholar]
- Washburn JD, Schnable JC, Davidse G, Pires JC.. 2015. Phylogeny and photosynthesis of the grass tribe Paniceae. American Journal of Botany 102: 1493–1505. [DOI] [PubMed] [Google Scholar]
- Wendel JF. 2015. The wondrous cycles of polyploidy in plants. American Journal of Botany 102: 1753–1756. [DOI] [PubMed] [Google Scholar]
- Wickham H, Vaughan D, Girlich M.. 2023a. tidyr. Tidy Messy Data. R Package version 1.3.0.https://github.com/tidyverse/tidyr, https://tidyr.tidyverse.org (7 February 2023, date last accessed).
- Wickham H, Francois R, Henry L, Muller K, Vaughn D.. 2023b. dplyr. A grammar of data manipulation. R Package Version 1.1.4.https://github.com/tidyverse/dplyr, https://dplyr.tidyverse.org (7 February 2023, date last accessed).
- Williams DG, Baruch Z.. 2000. African grass invasion in the Americas: ecosystem consequences and the role of ecophysiology. Biological Invasions 2: 123–140. [Google Scholar]
- Wilson CH, Strickland MS, Hutchings JA, Bianchi TS, Flory SL.. 2018. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Global Change Biology 24: 2997–3009. [DOI] [PubMed] [Google Scholar]
- Worthington ML, Miles JW.. 2015. Reciprocal Full-sib recurrent selection and tools for accelerating genetic gain in apomictic Brachiaria. In: Budak H, Spangenberg G. eds. Molecular breeding for forage and turf. Cham: Springer, 19–30. [Google Scholar]
- Worthington M, Heffelfinger C, Bernal D, et al. 2016. A parthenogenesis gene candidate and evidence for segmental allopolyploidy in apomictic Brachiaria decumbens. Genetics 203: 1117–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington M, Ebina M, Yamanaka N, et al. 2019. Translocation of a parthenogenesis gene candidate to an alternate carrier chromosome in apomictic Brachiaria humidicola. BMC Genomics 20: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington M, Perez JG, Mussurova S, et al. 2021. A new genome allows the identification of genes associated with natural variation in aluminium tolerance in Brachiaria grasses. Journal of Experimental Botany 72: 302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Wu Q, Wang J, Jiang T.. 2016. H-PoP and H-PoPG: Heuristic partitioning algorithms for single individual haplotyping of polyploids. Bioinformatics 32: 3735–3744. [DOI] [PubMed] [Google Scholar]
- Yim WC, Swain ML, Ma D, et al. 2022. The final piece of the Triangle of U: evolution of the tetraploid Brassica carinata genome. Plant Cell 34: 4143–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Smith DK, Zhu H, Guan Y, Lam TTY.. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution 8: 28–36. [Google Scholar]
- Yu Y, Hu H, Doust AN, Kellogg EA.. 2020. Divergent gene expression networks underlie morphological diversity of abscission zones in grasses. The New Phytologist 225: 1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S.. 2018. ASTRAL-III: Polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.