This cohort study evaluated the hazards of incident diabetes among women in Canada with gestational diabetes in their first, second, and in both the first and second pregnancies.
Key Points
Question
Across 2 pregnancies, are the order and number of gestational diabetes occurrences linked to maternal diabetes risk in the years after second pregnancy?
Findings
In this cohort study of 431 980 women, those with a first occurrence of gestational diabetes in a second pregnancy had a 76% higher risk for diabetes development than women who had gestational diabetes in the first pregnancy but not in the second, a statistically significant difference. The highest risk was in women with gestational diabetes in both pregnancies.
Meaning
These findings suggest that considering gestational diabetes history in each pregnancy results in more accurate diabetes risk estimation than a simple yes/no dichotomy of past gestational diabetes occurrence.
Abstract
Importance
Gestational diabetes is a type 2 diabetes risk indicator, and recurrence further augments risk. In women with a single occurrence across 2 pregnancies, it is unclear whether first- vs second-pregnancy gestational diabetes differ in terms of risk.
Objective
To compare the hazards of incident diabetes among those with gestational diabetes in the first, in the second, and in both pregnancies with women without gestational diabetes in either.
Design, Setting, and Participants
This was a retrospective cohort study with cohort inception from April 1, 1990, to December 31, 2012. Follow-up was April 1, 1990, to April 1, 2019. Participants were mothers with 2 singleton deliveries between April 1, 1990, and December 31, 2012, without diabetes before or between pregnancies, who were listed in public health care insurance administrative databases and birth, stillbirth, and death registries in Quebec, Canada. Data were analyzed from July to December 2023.
Exposure
Gestational diabetes occurrence(s) across 2 pregnancies.
Main outcomes and measures
Incident diabetes from the second delivery until a third pregnancy, death, or the end of the follow-up period, whichever occurred first.
Results
The 431 980 women with 2 singleton deliveries studied had a mean (SD) age of 30.1 (4.5) years at second delivery, with a mean (SD) of 2.8 (1.5) years elapsed between deliveries; 373 415 (86.4%) were of European background, and 78 770 (18.2%) were at the highest quintile of material deprivation. Overall, 10 920 women (2.5%) had gestational diabetes in their first pregnancy, 16 145 (3.7%) in their second, and 8255 (1.9%) in both (12 205 incident diabetes events; median [IQR] follow-up 11.5 [5.3-19.4] years). First pregnancy–only gestational diabetes increased hazards 4.35-fold (95% CI, 4.06-4.67), second pregnancy–only increased hazards 7.68-fold (95% CI, 7.31-8.07), and gestational diabetes in both pregnancies increased hazards 15.8-fold (95% CI, 15.0-16.6). Compared with first pregnancy–only gestational diabetes, second pregnancy–only gestational diabetes increased hazards by 76% (95% CI, 1.63-1.91), while gestational diabetes in both pregnancies increased it 3.63-fold (95% CI, 3.36-3.93).
Conclusions and relevance
In this retrospective cohort study of nearly half a million women with 2 singleton pregnancies, both the number and ordinal pregnancy of any gestational diabetes occurrence increased diabetes risk. These considerations offer greater nuance than an ever or never gestational diabetes dichotomy.
Introduction
Gestational diabetes (GD) affects 14% of pregnancies globally.1 A recent meta-analysis2 estimates its occurrence is associated with a 10-fold risk increase for type 2 diabetes. Whether risks vary with the order of GD occurrences is not well-studied. We hypothesized that new GD occurrence in a second pregnancy implies transition to a higher risk profile, while a single occurrence in a first pregnancy implies the converse.
One challenge is that GD is conditional on pregnancy (ie, cannot occur without pregnancy) and the number of pregnancies itself is associated with type 2 diabetes risk. The lowest risk occurs in those with 1 pregnancy.3 Two previous studies4,5 tried to improve comparability among participants by requiring that all have at least 2 pregnancies. Both reported a 2.4-fold increase in hazards with GD recurrence compared with its absence in the second pregnancy. They did not include women without any GD or women with a new occurrence of GD in a second pregnancy.
In the longer term, first-pregnancy GD may motivate some to adopt behaviors demonstrated to reduce diabetes risk,6,7 lowering GD recurrence rates and type 2 diabetes development. In contrast, some women without GD in the first pregnancy may enter a higher risk trajectory, related to excess gestational weight gain,8 postpartum weight retention,9 weight gain between pregnancies,10,11 and motherhood demands impeding healthy eating and physical activity.12 The delineation of differences in future incident type 2 diabetes risk between GD occurrence in a first pregnancy compared with new occurrence in a second could allow further personalization of approaches to type 2 diabetes prevention.13 We therefore examined patterns of GD absence, occurrence, and recurrence across 2 pregnancies and their associations with diabetes.
Methods
The McGill University Health Centre’s research ethics board and Quebec Access to Information Commission approved the protocol. These bodies waived informed consent because the study involved deidentified data, analyses at the Quebec Statistical Institute’s secure data centers, and rounded frequencies to multiples of 5. This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Design and Data Sources
We conducted a retrospective cohort study in Quebec, Canada. We examined health administrative databases of the public health insurance plan linked to birth, stillbirth, and death registries by the Quebec Statistical Institute (probabilistic linkage). We obtained mothers’ residential territory and month and year of birth from the public health insurance registry. The Physician Services Claims and Hospitalization Discharge Databases include diagnostic codes (eTable 1 in Supplement 1) and hospitalization dates; we used these to define outcomes, exposures, and other variables alongside data from birth and stillbirth registries (offspring birthdates, gestational age at birth, birthweight, parental country of birth and first language, and years of maternal education). We also had access to the mothers’ Institut national de santé publique du Québec (INSPQ) material and social deprivation index, derived from the 6-digit postal code in the public health registry.14The Institut national de santé publique du Québec (INSPQ) material and social deprivation index is computed from small-area census data. Specifically, the material indices are derived from average income, proportions without high school diploma, and employment to population ratio among those 15 years and older. The social indices are derived from the proportion of the population who are single-parent families, aged 15 years and older living alone, and aged 15 years and older who are separated, divorced, or widowed. To assign the INSPQ index for each woman, we first checked availability of this variable in the index year (year of second delivery).
Study Population
We considered women with 2 or more consecutive singleton deliveries between April 1, 1990, and December 31, 2012, who were alive at 12 weeks following the second delivery (index date) (eFigure 1 in Supplement 1). We excluded mothers with missing offspring gestational age (required to distinguish diabetes from GD),15 and those with diabetes or hypertension before or between pregnancies. We applied the validated Canadian Chronic Disease Surveillance System (CCDSS) diabetes16,17 and hypertension18 definitions of 2 outpatient or 1 hospitalization diagnostic code(s) to (1) the 2-year period before 20 weeks’ gestation in first pregnancy and (2) the period from 12 weeks after the first delivery until 20 weeks’ gestation in the second pregnancy. All required an opportunity to develop GD for both of the pregnancies considered, thus gestation 20 or more weeks was required. In the primary analysis, we required the same partner for each offspring to minimize heterogeneity of within-household factors.19,20,21 This resulted in the removal of stillbirths, for whom paternal data were unavailable; in a sensitivity analysis, we removed the paternal data requirement. Lastly, we excluded those with 2 outpatient visits or 1 hospitalization for cardiovascular disease, the most common diabetes consequence, before the index date.
Exposure
We adapted a validated health administrative database GD definition22 that applies diabetes and GD diagnostic codes to a pregnancy-specific period. We started this period at 20 weeks’ gestation instead of the 120-day predelivery date used in the validation study, as the information we had on gestational age allowed us to conform with clinical definitions, which considers type 2 diabetes before 20 weeks’ gestation as preexisting.15 We extended the period beyond delivery to 12 weeks post partum, as screening for type 2 diabetes after pregnancy is generally advised by this time.23,24 We required 2 outpatient and/or 1 hospitalization code to maximize specificity (99.5%) and maintain sensitivity (94.1%), as recommended in the validation study.22 Our 4 mutually exclusive exposure categories were absence of GD, its presence in only first pregnancy, in only the second, and in both.
Outcome
Our primary outcome was incident diabetes, using the previously described CCDSS definition.16,17 These diagnostic codes cannot differentiate between type 1 and type 2 diabetes, but because 95% of incident diabetes among adults is type 2 diabetes, they primarily capture incident type 2 diabetes. Follow-up was until the first of incident diabetes, the 120-day time point before a third delivery (we did not have gestational age data for any third pregnancy), death, or the end of the study period (April 1, 2019).
Covariates
For both the first and second pregnancies, we considered other pregnancy and offspring-related factors associated with type 2 diabetes development, specifically, gestational hypertension (with or without preeclampsia), preterm delivery (<37 weeks), and small-for-gestational-age (SGA) and large-for-gestational-age (LGA) status.25,26 For gestational hypertension, we applied the CCDSS hypertension definition to the same pregnancy periods for which we defined GD, and we also considered diagnostic codes for gestational hypertension and preeclampsia.
We considered time between deliveries, comorbid conditions, maternal age at index date, deprivation level (see Table 1 footnotes),14 preexisting paternal diabetes and hypertension (validated CCDSS definitions16,17 applied from 2 years before 20 weeks’ gestation in the first pregnancy to 12 weeks following the second delivery), and ethnocultural background (African/Caribbean [if born in West/South/East/Central Africa or first language was of Caribbean or African descent], Arabic [if born in the Arab league or first language was of Arabic or other North African or South-West Asian descent], Asian [if born in West/East/Central/South/Southeast/Pacific Asia or first language descends from these regions], European [if born in North America, South America, Central America, Mexico, East/South/Southern/West Europe, or Australia and first language was English, French, or other European language], and other [if first language was of Indigenous descent) based on participant-reported place of birth and primary language recorded on the mandatory birth declaration and incorporated into the birth registry. Ethnocultural background was assessed in this study because those with background other than European have a higher baseline risk for diabetes.
Table 1. Baseline Covariates, Stratified by Gestational Diabetes (GD) Occurrence(s) in Each Respective Pregnancy.
| Covariate | Patients, No. (%)a | |||
|---|---|---|---|---|
| No GD (n = 396 660) | GD in first pregnancy (n = 10 920) | GD in second pregnancy (n = 16 145) | GD in both pregnancies (n = 8255) | |
| Prior history of gestational hypertension in either or both pregnanciesb | 29 550 (7.5) | 1275 (11.7) | 2160 (13.4) | 1160 (14.1) |
| Age of mother at second delivery, mean (SD), yc | 30.0 (4.5) | 30.9 (4.6) | 31.6 (4.7) | 32.0 (4.7) |
| Time between deliveries, y | ||||
| <2 | 124 800 (31.5) | 3525 (32.3) | 4055 (25.1) | 2530 (30.6) |
| 2 to <2.5 | 81 610 (20.6) | 2250 (20.6) | 2705 (16.8) | 1545 (18.7) |
| 2.5 to <3.5 | 103 195 (26.0) | 2710 (24.8) | 4010 (24.8) | 2090 (25.3) |
| ≥3.5 | 87 050 (21.9) | 2435 (22.3) | 5380 (33.3) | 2090 (25.3) |
| Material deprivation index, quintilesd | ||||
| 1 | 81 265 (20.5) | 2005 (18.4) | 2900 (18.0) | 1475 (17.9) |
| 2 | 83 975 (21.2) | 2285 (20.9) | 3250 (20.1) | 1630 (19.7) |
| 3 | 78 870 (19.9) | 2135 (19.6) | 3095 (19.2) | 1560 (18.9) |
| 4 | 74 525 (18.8) | 2125 (19.5) | 3155 (19.5) | 1625 (19.7) |
| 5 | 71 280 (18.0) | 2195 (20.1) | 3455 (21.4) | 1840 (22.3) |
| Social deprivation index, quintilesd | ||||
| 1 | 88 545 (22.3) | 2305 (21.1) | 3145 (19.5) | 1760 (21.3) |
| 2 | 85 620 (21.6) | 2250 (20.6) | 3255 (20.2) | 1615 (19.6) |
| 3 | 81 225 (20.5) | 2200 (20.1) | 3325 (20.6) | 1605 (19.4) |
| 4 | 72 980 (18.4) | 2110 (19.3) | 3155 (19.5) | 1685 (20.4) |
| 5 | 61 545 (15.5) | 1885 (17.3) | 2975 (18.4) | 1465 (17.7) |
| Backgrounde | ||||
| America, Australia, or Europe | 346 230 (87.3) | 8865 (81.2) | 12 280 (76.1) | 6040 (73.2) |
| Africa or Caribbean | 7565 (1.9) | 260 (2.4) | 465 (2.9) | 260 (3.2) |
| Arab-speaking regions | 14 840 (3.7) | 600 (5.5) | 1210 (7.5) | 665 (8.1) |
| Asia | 12 040 (3.0) | 640 (5.9) | 1190 (7.4) | 745 (9.0) |
| Other | 15 980 (4.0) | 555 (5.1) | 1005 (6.2) | 545 (6.6) |
| Comorbid conditions | ||||
| Mood disorders and alcohol or drug dependence | 16 315 (4.1) | 440 (4.0) | 850 (5.3) | 405 (4.9) |
| Thyroid disorder | 13 185 (3.3) | 450 (4.1) | 895 (5.5) | 480 (5.8) |
| Arthritis | 8265 (2.1) | 270 (2.5) | 435 (2.7) | 210 (2.5) |
| Asthma or COPD | 7735 (2.0) | 250 (2.3) | 425 (2.6) | 240 (2.9) |
| Small for gestational agef | ||||
| Neither pregnancy | 338 405 (85.3) | 9465 (86.7) | 14 120 (87.5) | 7235 (87.6) |
| First pregnancy only | 24 225 (6.1) | 630 (5.8) | 815 (5.1) | 445 (5.4) |
| Second pregnancy only | 24 240 (6.1) | 580 (5.3) | 890 (5.5) | 435 (5.3) |
| Both pregnancies | 9650 (2.4) | 240 (2.2) | 320 (2.0) | 135 (1.6) |
| Large for gestational agef | ||||
| Neither pregnancy | 339 910 (85.7) | 8735 (80.0) | 12 635 (78.3) | 6355 (77.0) |
| First pregnancy only | 23 290 (5.9) | 845 (7.7) | 1315 (8.1) | 710 (8.6) |
| Second pregnancy only | 23 145 (5.8) | 865 (7.9) | 1370 (8.5) | 690 (8.4) |
| Both pregnancies | 10 175 (2.6) | 470 (4.3) | 825 (5.1) | 495 (6.0) |
| Preterm birth | ||||
| Neither pregnancy | 361 365 (91.1) | 9725 (89.1) | 14 040 (87.0) | 7160 (86.7) |
| First pregnancy only | 18 155 (4.6) | 595 (5.5) | 1050 (6.5) | 530 (6.4) |
| Second pregnancy only | 13 050 (3.3) | 450 (4.1) | 720 (4.5) | 410 (5.0) |
| Both pregnancies | 4085 (1.0) | 150 (1.4) | 340 (2.1) | 155 (1.9) |
| History of paternal diabetesg | 3170 (0.8) | 110 (1.0) | 225 (1.4) | 140 (1.7) |
| History of paternal hypertensiong | 8385 (2.1) | 305 (2.8) | 480 (3.0) | 250 (3.0) |
Abbreviaton: COPD, chronic obstructive pulmonary disease.
Values are randomly rounded up or down to a multiple of 5 (for patient confidentiality purposes). Therefore, column sums for each baseline characteristic may not equal the total number of women in each level of the exposure due to this random rounding process.
Gestational hypertension was collapsed as a binary variable (absent or present in either or both pregnancies) as a measure to ensure the proportional hazards assumption was met when tested. When gestational hypertension status was categorized into 4 levels, similar to the primary GD exposure, the assumption was violated. The capture period for this comorbidity was between 20 weeks’ gestation to 12 weeks post partum of each respective pregnancy.
Age was not categorized and instead kept as a continuous variable.
Range from 1 (least deprived) to 5 (most deprived). A total of 7335 women were missing an assigned deprivation score.
Ethnocultural background based on the mother’s region of birth and reported preferred language. Women were categorized as European if born in North America, South America, Central America, Mexico, East/South/Southern/West Europe, or Australia and first language was English, French, or other European language; African or Caribbean if born in West/South/East/Central Africa or first language was of Caribbean or African descent; Arabic if born in the Arab league or first language was of Arabic or other North African or Southwest Asian descent; Asian if born in West/East/Central/South/Southeast/Pacific Asia or first language descends from these regions; or Other (does not fit into any other category), or if first language was of Indigenous descent.
A total of 150 offspring were missing birthweight required to derive offspring size.
Prior history in the father was defined as 1 or more inpatient and/or 2 or more outpatient International Statistical Classification of Diseases and Related Health Problems, Ninth and Tenth Revision codes for any form of diabetes or hypertension, respectively, that occurred during the period from 2 years before 20 weeks’ gestation in their partner’s first pregnancy to 12 weeks post partum in relationship to the second pregnancy.
Statistical Analyses
We computed baseline characteristics (counts and proportions for categorical variables and mean [SD] for continuous variables) and compared them across exposure groups (Pearson χ2 tests for proportions; 1-way analysis of variance for means, as applicable). We calculated type 2 diabetes incidence rates (IR). We assessed for interactions (P < .05 for interaction terms) and multicollinearity (Cramer V > 0.10) among exposures and covariates. We constructed Kaplan-Meier curves and compared these through log-rank testing. We evaluated proportional hazards assumptions (log-minus-log survival plots, Schoenfeld residuals) and performed some transformations to fulfill these (age as a spline variable and binary gestational hypertension category defined as presence in either or both pregnancies vs neither).
We constructed multivariable Cox proportional hazards models to compute hazard ratios (HR) for type 2 diabetes, first with GD absence in either pregnancy as the reference group. We then examined models with GD in only the first pregnancy as the reference group and finally with GD in only the second as the reference group. We retained covariates based on univariate association with type 2 diabetes where P ≤ .25, multivariable association (stepwise selection) where P ≤ .05, and reduced bayesian information criteria values with inclusion (see eMethods in Supplement 1 for omitted variables).
In a sensitivity analysis, we evaluated the change in associations when including calendar years of each pregnancy in our models to account for temporal trends in the screening and diagnosis of GD over the years.27 In another sensitivity analysis, we retained women with stillbirth deliveries and accounted for stillbirths in the model, along with miscarriages between pregnancies. In a third sensitivity analysis, we applied indirect adjustments for obesity and smoking status to our main results, using established methods.28,29 This bias analysis required external estimates for the HRs of obesity and of smoking with incident type 2 diabetes in women, which we respectively estimated as 3.90 (obesity vs no obesity)30 and 1.13 (smoking vs not smoking).31 This method also required external cohort data for obesity and smoking prevalence in groups of women corresponding to our main exposure categories. We used the Canadian Community Health Survey (Cycle 2.2) for this purpose32; 13% were in the obesity category and 24% smoked cigarettes. We applied the following formula for the indirect obesity adjustment:
| HR(corrected for obesity) = HR(from our analysis) / HR(related to obesity, from literature)Poe − Pe × Po |
(see eMethods in Supplement 1, similar formula applied for smoking; Poe = proportion within specific GD category who have obesity; Pe = proportion of those with specific GD category among all women with 2 consecutive singleton pregnancies; Po = proportion with obesity among all women with 2 consecutive singleton pregnancies). We performed analyses with SAS version 9.4 (SAS Institute). Data were analyzed from July 2023 to December 2023.
Results
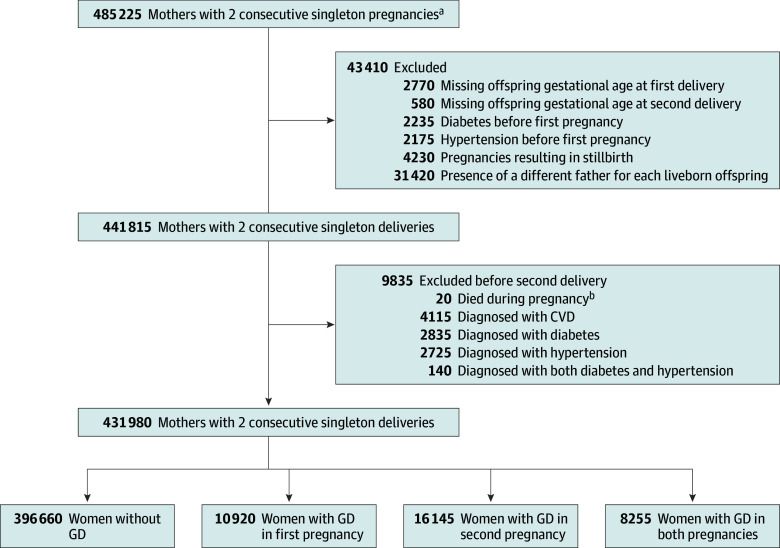
The 431 980 women analyzed (Figure 1) had a mean (SD) age of 30.1 (4.5) years, and a mean (SD) of 2.8 (1.5) years elapsed between deliveries. Overall, 8550 women were African or Caribbean, 17 315 were from Arab-speaking regions, 14 615 were Asian, 373 415 were of European background, 78 770 (18.2%) at the highest material deprivation level (INSPQ deprivation index: quintile 5), 62 605 (14.5%) had 1 or more SGA offspring, 64 195 (14.9%) had 1 or more LGA offspring, 35 290 (8.2%) had 1 or more preterm delivery, and 34 145 (7.9%) had 1 or more gestational hypertension occurrence. In terms of the main exposure, 10 920 (2.5%) had GD in only their first pregnancy, 16 145 (3.7%) had GD in only their second, and 8255 (1.9%) in both (eFigure 2 in Supplement 1). Those without GD in either pregnancy (396 660 participants) (Table 1) were younger with higher proportions of European background and lower proportions with deprivation, comorbid conditions, LGA offspring, preterm births, and partners with diabetes and hypertension.
Figure 1. Cohort Construction.
aValues are rounded either up or down to a multiple of 5 for patient confidentiality purposes.
bFatal events occurring at any point between 20 weeks’ gestation in the second pregnancy and 12 weeks post partum. Five deaths were related to a fatal cardiovascular disease (CVD) event while the remaining 15 fatalities were related to obstetrical complications related to childbirth, major trauma, and suicide.
Associations of Main Exposure Groups With Incident Type 2 Diabetes
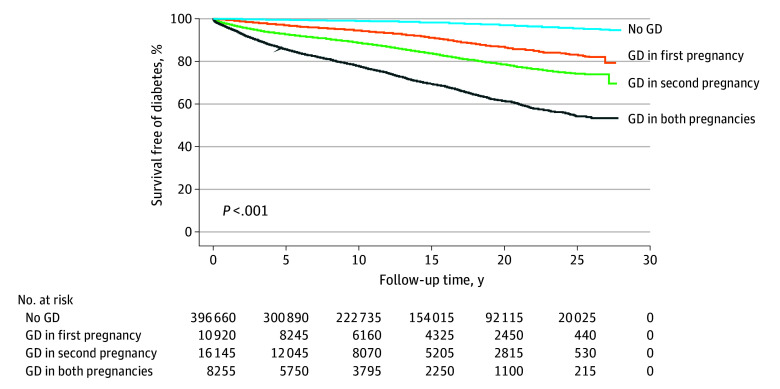
Over a median (IQR) of 11.5 (5.3-19.4) years (5 298 940 total person years), 12 205 mothers developed type 2 diabetes. The IRs per 1000 person-years rose across the no GD (IR, 1.4), GD in first pregnancy only (IR, 6.7), GD in second pregnancy only (IR, 12.4), and GD in both pregnancies (IR, 25.5) categories. Kaplan-Meier curves suggested significant differences in event-free survival across groups (Figure 2). The proportional hazards assumption applied. We did not detect interactions or multicollinearity.
Figure 2. Kaplan-Meier Curves for Diabetes-Free Survival.
The log-rank test suggested significant differences in event-free survival across exposure groups. GD indicates gestational diabetes.
In adjusted models, compared with those without GD, those with GD in first pregnancy had a 4.35-fold higher hazard for type 2 diabetes (95% CI, 4.06-4.67) (Figure 3A), those with GD in the second pregnancy had a 7.68-fold increase (95% CI, 7.31-8.07), and those with GD in both pregnancies demonstrated a 15.80-fold increase (95% CI, 15.00-16.61). Compared with those with GD in the first pregnancy, women with GD in the second had 76% higher hazards (95% CI, 1.63-1.91) (Figure 3B) and those with GD in both pregnancies had a 3.63-fold increase (95% CI, 3.36-3.93). Hazards were 2.06-fold higher among women with GD in both pregnancies (95% CI, 1.94-2.19) (Figure 3C) compared with those with GD in the second pregnancy.
Figure 3. Associations of Incident Diabetes With Gestational Diabetes (GD) in First, Second, or Both Pregnancies, From Adjusted Multivariable Models.
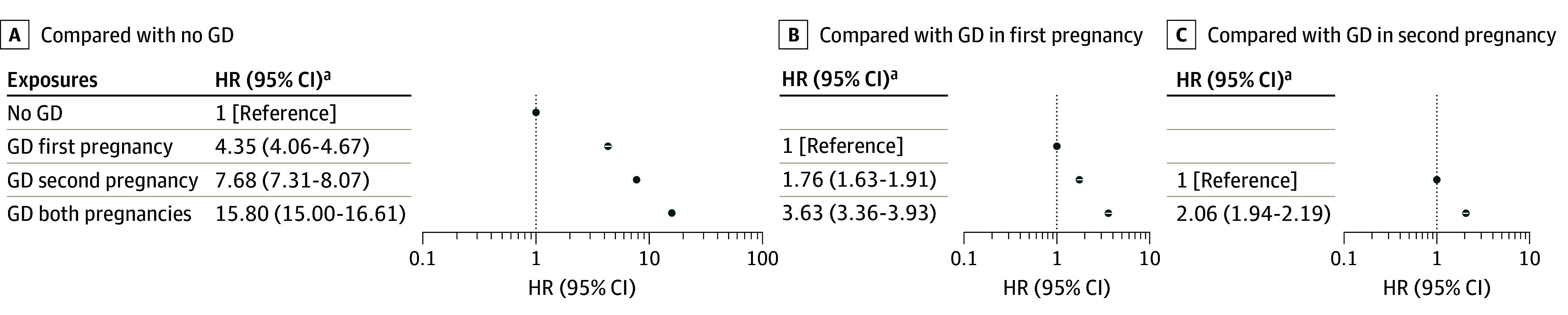
The corresponding unadjusted hazard ratios (HRs) among women with no GD, GD in first pregnancy, GD in second pregnancy and GD in both pregnancies, in Panel A were 4.80 (95% CI, 4.48-5.14), 9.09 (95% CI, 8.67-9.53), and 19.05 (95% CI, 18.10-19.91) for GD in first pregnancy, GD in second pregnancy, and GD in both pregnancies, respectively. The corresponding unadjusted HRs among those in panel B were 1.89 (95% CI, 1.75-2.05) and 3.96 (95% CI, 3.66-4.28) for GD in second pregnancy and GD in both pregnancies, respectively. The corresponding unadjusted HR among those in panel C was 2.09 (95% CI, 1.97-2.22) for GD in both pregnancies.
Sensitivity Analyses
Inclusion of calendar years for each pregnancy did not importantly alter HRs (eTable 2 in Supplement 1). In another sensitivity analysis including women with stillbirth pregnancies in our study cohort (435 685 participants; 12 415 events), stillbirths were associated with 19% increased hazards (HR, 1.19; 95% CI, 1.04-1.38), compared with women without stillbirth deliveries (eTable 3 in Supplement 1). Miscarriages between pregnancies were not conclusively associated with incident diabetes (HR, 1.03; 95% CI, 0.97-1.09). Furthermore, retaining stillbirths among the 2 pregnancies examined and considering miscarriages between pregnancies did not importantly alter the association between GD occurrences and incident diabetes.
Indirect adjustments for obesity (no GD: reference; GD in first pregnancy HR, 2.72; 95% CI, 2.46-2.83; GD in second pregnancy HR, 5.48; 95% CI, 5.22-5.76; GD in both pregnancies HR, 9.62; 95% CI, 9.15-10.10) (see eMethods in Supplement 1 for other comparisons) somewhat attenuated the HRs for the incident type 2 diabetes outcome. Indirect adjustments for smoking (no GD: reference; GD in first pregnancy HR, 4.23; 95% CI, 3.94-4.53; GD in second pregnancy HR, 7.44; 95% CI, 7.09-7.83; GD in both pregnancies HR, 15.50; 95% CI, 14.70-16.21) did not significantly alter the HRs.
Other Associations Observed
Gestational hypertension in either or both pregnancies was associated with a 65% increase in hazards for diabetes development (Table 2). Compared with appropriate size for gestational age of both offspring, LGA was consistently associated with diabetes development, with a 60% increase in hazards whether it occurred in the first or second pregnancies, and a doubling when it occurred in both pregnancies or when LGA occurred in 1 pregnancy and SGA in the other. Preterm delivery in 1 or both pregnancies was associated with a 10% to 20% increase in hazards of diabetes compared with full-term delivery in both pregnancies. Deprivation levels were associated with a stepwise increase in hazards. All ethnocultural groups other than European had higher diabetes hazards compared with European women, and all comorbid conditions considered were associated with increased hazards. Paternal diabetes was associated with a 43% increase in hazards for maternal diabetes development.
Table 2. Association of Incident Diabetes With Covariates Included in the Final Model.
| Covariatea | HR (95% CI) | |
|---|---|---|
| Unadjusted | Adjusted | |
| Gestational hypertension affecting either pregnancy or both pregnanciesb | 2.14 (2.04-2.25) | 1.65 (1.57-1.73) |
| Offspring indicators | ||
| Offspring size | ||
| AGA: both offspring | 1 [Reference] | 1 [Reference] |
| SGA: first offspring only | 0.97 (0.90-1.05) | 0.94 (0.87-1.02) |
| SGA: second offspring only | 0.96 (0.89-1.04) | 0.91 (0.84-1.00) |
| SGA: both offspring | 1.00 (0.89-1.13) | 0.97 (0.86-1.10) |
| LGA: first offspring only | 1.82 (1.71-1.94) | 1.60 (1.50-1.70) |
| LGA: second offspring only | 1.86 (1.75-1.98) | 1.60 (1.50-1.70) |
| LGA: both offspring | 2.77 (2.57-2.98) | 2.01 (1.86-2.17) |
| SGA: first offspring, LGA: second offspring | 2.85 (2.02-4.01) | 2.14 (1.51-3.02) |
| LGA: first offspring, SGA: second offspring | 2.75 (1.95-3.87) | 1.94 (1.38-2.73) |
| Gestational age of offspring at birth | ||
| Term birth: both offspring | 1 [Reference] | 1 [Reference] |
| Preterm birth | ||
| First offspring only | 1.33 (1.23-1.44) | 1.11 (1.03-1.20) |
| Second offspring only | 1.50 (1.38-1.63) | 1.19 (1.09-1.29) |
| Both offspring | 1.71 (1.49-1.95) | 1.21 (1.06-1.39) |
| Paternal indicators | ||
| Prior history of paternal diabetes | 2.09 (1.80-2.42) | 1.43 (1.24-1.66) |
| Prior history of paternal hypertension | 1.34 (1.20-1.49) | 1.12 (1.00-1.25) |
| Maternal indicators | ||
| Time between deliveries, y | ||
| <2 | 1 [Reference] | 1 [Reference] |
| 2 to 2.5 | 0.85 (0.81-0.90) | 0.90 (0.86-0.95) |
| 2.5 to 3.5 | 0.82 (0.78-0.86) | 0.84 (0.80-0.88) |
| ≥3.5 | 1.06 (1.01-1.11) | 0.94 (0.89-0.98) |
| Material deprivation index, quintilesc | ||
| 1 (Least deprived) | 1 [Reference] | 1 [Reference] |
| 2 | 1.25 (1.17-1.32) | 1.24 (1.17-1.32) |
| 3 | 1.38 (1.30-1.47) | 1.35 (1.27-1.43) |
| 4 | 1.56 (1.47-1.66) | 1.44 (1.36-1.53) |
| 5 (Most deprived) | 1.99 (1.87-2.10) | 1.67 (1.58-1.78) |
| Social deprivation index, quintilesc | ||
| 1 (Least deprived) | 1 [Reference] | 1 [Reference] |
| 2 | 1.08 (1.02-1.14) | 1.08 (1.02-1.14) |
| 3 | 1.14 (1.08-1.21) | 1.10 (1.04-1.17) |
| 4 | 1.32 (1.25-1.40) | 1.16 (1.10-1.23) |
| 5 (Most deprived) | 1.53 (1.45-1.62) | 1.26 (1.19-1.34) |
| Backgroundd | ||
| America, Australia, or Europe | 1 [Reference] | 1 [Reference] |
| Africa or Caribbean | 2.68 (2.44-2.95) | 1.90 (1.72-2.10) |
| Arab-speaking regions | 2.24 (2.07-2.42) | 1.60 (1.48-1.74) |
| Asia | 2.50 (2.33-2.69) | 1.62 (1.50-1.74) |
| Other | 2.00 (1.86-2.15) | 1.46 (1.35-1.58) |
| Comorbid conditionse | ||
| Mood disorders and alcohol or drug dependence | 1.50 (1.39-1.62) | 1.40 (1.29-1.51) |
| Thyroid disorder | 1.82 (1.68-1.98) | 1.40 (1.29-1.53) |
| Arthritis | 1.49 (1.35-1.65) | 1.26 (1.14-1.40) |
| Asthma or COPD | 1.95 (1.77-2.15) | 1.67 (1.52-1.84) |
Abbreviations: AGA, appropriate for gestational age; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LGA, large for gestational age; SGA, small for gestational age.
The Cox proportional hazards model adjusted for gestational diabetes occurrences across pregnancies, as well as each of the variables listed. Maternal age at second delivery was also included as a spline variable in the adjusted model; the corresponding unadjusted hazard ratio for each additional year in age was 1.03 (95% CI, 1.02-1.03).
Gestational hypertension was collapsed as a binary variable (absent or present in either or both pregnancies) as a measure to ensure the proportional hazards assumption was met when tested. When gestational hypertension status was categorized into 4 levels (similar to the primary GD exposure), the assumption was violated. The capture period for this comorbidity was between 20 weeks’ gestation to 12 weeks post partum of each respective pregnancy.
A total of 7335 women were missing a value for the material deprivation index.
Compared with women of European descent, those from other ethnic origins demonstrated increased hazards of developing type 2 diabetes during the follow-up period.
The reference group are women with the absence of each condition. Comorbid conditions were defined in accordance with the Chronic Disease Surveillance System’s definition of chronic disease, requiring 1 or more inpatient or 2 or more outpatient International Classification of Diseases, Ninth and Tenth Revision codes to be present within 2 years before the index date.
Discussion
Among nearly half a million mothers with 2 consecutive singleton pregnancies, our analyses demonstrate that GD in only the second pregnancy was associated with higher hazards for type 2 diabetes development than GD in only the first pregnancy. The highest hazards are with GD occurrence in both pregnancies. Compared with women without GD in either pregnancy, there were 4.35-fold, 7.68-fold, and 15.80-fold greater hazards for type 2 diabetes with GD in the first, in the second, and in both pregnancies, respectively. Indirect adjustments for obesity somewhat attenuated these values to 2.72-fold, 5.48-fold, and 9.62-fold, respectively, but the magnitude remained high, and the differences persisted. Direct comparisons between GD groups were also conclusive. For example, compared with first pregnancy–only GD, second pregnancy–only GD increased hazards by 76%, while GD in both pregnancies increased hazards 3.63-fold.
We did not identify any prior study that compared GD in a first pregnancy with GD in a second among women with 2 pregnancies. Our specific estimate for the increase in hazards associated with GD recurrence (HR, 3.63; HR, 2.21 with indirect adjustment for obesity) compared with women with a GD occurrence in only a first pregnancy was similar to the greater than 2-fold increase in hazards reported in 2 previous studies4,5 that restricted analyses to women with at least 2 pregnancies. Other studies that examined GD recurrence had a higher degree of variability in numbers of pregnancies. One reported a 16% increase in hazards with GD recurrence33 while the other estimated a 2-fold increase.34 We identified a single study that examined hazards of type 2 diabetes after pregnancy in relationship to numbers of prior GD pregnancies (Sister Study).35 It differed in several other respects from ours. As such, its interpretation applies to an older group of women, several years beyond pregnancy. In the Sister Study, women with a history of 2 GD pregnancies experienced 6.2-fold higher hazards for type 2 diabetes in middle age compared with women with no GD pregnancies. The reference group included women without pregnancies. The investigators accounted for time since last GD pregnancy and self-reported weight. In our study, women with 2 pregnancies and GD in both had a 15.8-fold increase in hazards for type 2 diabetes development between their 30s and 40s, starting soon after their second pregnancy, compared with women without GD in either pregnancy. We indirectly adjusted for obesity and demonstrated that the association was attenuated to a 10-fold increase in hazards.
Our key discovery is that a single GD occurrence in a first pregnancy is associated with lower hazards for type 2 diabetes than a single GD occurrence in a second pregnancy. A subgroup analysis of the American Diabetes Prevention Program among women with a GD history showed that healthy diet-induced weight loss and higher physical activity levels could reduce type 2 diabetes risk.6,7 Women with a first GD pregnancy may be motivated to adopt behavioral changes that both prevent GD in a second pregnancy and lower hazards of incident type 2 diabetes development thereafter. Supporting this, a recent cohort study11 determined that among women without GD in their first pregnancy, weight loss between pregnancies was associated with reduced risk for new occurrence of GD in a subsequent pregnancy. In another study10 among women with excess weight and GD in a first pregnancy, weight loss between pregnancies lowered the risk for GD recurrence in a second pregnancy.
In our analyses, the women without GD in the first pregnancy who developed GD in the second may have gained excess weight in the first pregnancy and had difficulty losing it9 or gained weight between pregnancies.10,11 In an Australian investigation11 among women without GD in their first pregnancy, higher levels of weight gain between pregnancies were associated with stepwise increases in the risk of new occurrence of GD in second pregnancy. For many women, the additional responsibilities of motherhood12 may challenge efforts to engage in behaviors to enhance personal health. Furthermore, the metabolic stresses inherent to pregnancy may impair their β-cell function,36 making them more susceptible to developing GD in the second pregnancy, and ultimately to type 2 diabetes development.
Alongside health behaviors and physiological changes, our analyses reinforce the importance of social factors, including material and social deprivation and non-European background. The underpinnings of such associations likely stem from other related upstream characteristics, such as food insecurity,37 local environments not conducive to physical activity,38,39 and structural inequity.40 Partner diabetes was another risk indicator for maternal type 2 diabetes development in our analyses, with a 43% increase in hazards. This is consistent with our prior studies41,42 demonstrating increases in hazards for the development of diabetes in fathers whose partners had GD compared with those whose partners did not. Shared partner type 2 diabetes risk may be related to shared health behaviors, resources, social factors, and household environments.20,43,44,45,46 Assortative mating (similar demographics, attitudes, behaviors, and traits at the outset) may also play a role.46,47,48,49
Strengths and Limitations
Our large sample size of nearly half a million women was possible through linkage of Quebec’s health administrative and vital statistics databases. Limitations to these data include lack of information on GD management, prepartum weight status, gestational weight gain, smoking status, and laboratory values. GD and weight excess are intimately associated. However, all of our models accounted for LGA in both the first and second pregnancies, a strong correlate of prepregnancy and gestational weight gain.50,51 Furthermore, we performed indirect adjustments for obesity using established methods.28,29 We could not corroborate International Statistical Classification of Diseases and Related Health Problems, Ninth and Tenth Revision–coded diagnoses of diabetes with laboratory data, but to mitigate for misclassification, we applied validated definitions of GD and diabetes.16,17 The diagnostic codes do not reliably distinguish between type 1 and type 2 diabetes, but given that 95% of diabetes onset among adults is type 2 diabetes, the majority of events captured with our codes are type 2 diabetes. We acknowledge that women with more than 1 GD occurrence may already be undergoing more frequent screening for diabetes than women with only 1 occurrence, perhaps partly accounting for their higher diabetes hazards. We also acknowledge potential misclassification of ethnocultural background, as second- and third-generation women and/or Indigenous women would have been classified as European if their first language was English, French, or another European language. Lastly, we did not examine women without pregnancies or women with a single pregnancy, but our focus was women with 2 consecutive deliveries; restriction to women with 2 or more deliveries overcame some methodological challenges, as discussed.
Conclusions
Our retrospective cohort study suggests that among women with 2 consecutive singleton pregnancies, without diabetes before or between pregnancies, the absence of GD in a second pregnancy following GD in the first suggests that the mother is taking effective diabetes prevention measures. If confirmed, she should be encouraged to continue. New onset GD or recurrent GD in a second pregnancy, however, should inspire urgent action for prevention or adjustments to ongoing efforts. We also confirmed the importance of material deprivation and ethnocultural background in type 2 diabetes risk estimation, and we identified paternal diabetes as a factor associated with risk for type 2 diabetes development in mothers. Our results provide a personalized medicine–oriented pathway to diabetes risk estimation in women. This should be coupled with tailored prevention programs and equitable referral pathways to reduce the burden of type 2 diabetes and its complications.
eTable 1. ICD Codes
eTable 2. Comparing Effect Estimates of the Diabetes Outcome, With and Without Inclusion of Years of Pregnancy, with Account for Temporal Trends in the Screening and Diagnosis of Gestational Diabetes
eTable 3. Associations of Incident Diabetes With Gestational Diabetes Occurrences When Including Women With Stillbirth Pregnancies in Cohort
eMethods.
eFigure 1. Timeline of Applied Exclusions and Exposure Ascertainment Windows
eFigure 2. Distribution of Gestational Diabetes Exposure Categories
Data Sharing Statement
References
- 1.Wang H, Li N, Chivese T, et al. ; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group . IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s criteria. Diabetes Res Clin Pract. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050 [DOI] [PubMed] [Google Scholar]
- 2.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters SAE, Yang L, Guo Y, et al. ; China Kadoorie Biobank Collaboration Group . Pregnancy, pregnancy loss and the risk of diabetes in Chinese women: findings from the China Kadoorie Biobank. Eur J Epidemiol. 2020;35(3):295-303. doi: 10.1007/s10654-019-00582-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yefet E, Schwartz N, Nachum Z. Characteristics of pregnancy with gestational diabetes mellitus and the consecutive pregnancy as predictors for future diabetes mellitus type 2. Diabetes Res Clin Pract. 2022;186:109826. doi: 10.1016/j.diabres.2022.109826 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein J, Lee-Parritz A, Quinn E, et al. After gestational diabetes: impact of pregnancy interval on recurrence and type 2 diabetes. Biores Open Access. 2019;8(1):59-64. doi: 10.1089/biores.2018.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroda VR, Christophi CA, Edelstein SL, et al. ; Diabetes Prevention Program Research Group . The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100(4):1646-1653. doi: 10.1210/jc.2014-3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratner RE, Christophi CA, Metzger BE, et al. ; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774-4779. doi: 10.1210/jc.2008-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benham JL, Booth JE, Donovan LE, Leung AA, Sigal RJ, Rabi DM. Prevalence of and risk factors for excess weight gain in pregnancy: a cross-sectional study using survey data. CMAJ Open. 2021;9(4):E1168-E1174. doi: 10.9778/cmajo.20200276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rong K, Yu K, Han X, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr. 2015;18(12):2172-2182. doi: 10.1017/S1368980014002523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorbye LM, Cnattingius S, Skjaerven R, et al. Interpregnancy weight change and recurrence of gestational diabetes mellitus: a population-based cohort study. BJOG. 2020;127(13):1608-1616. doi: 10.1111/1471-0528.16364 [DOI] [PubMed] [Google Scholar]
- 11.Black KI, Schneuer F, Gordon A, Ross GP, Mackie A, Nassar N. Estimating the impact of change in pre-pregnancy body mass index on development of Gestational Diabetes Mellitus: an Australian population-based cohort. Women Birth. 2022;35(6):563-569. doi: 10.1016/j.wombi.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 12.Neven ACH, Lake AJ, Williams A, et al. ; ME-MaGDA Study Group . Barriers to and enablers of postpartum health behaviours among women from diverse cultural backgrounds with prior gestational diabetes: a systematic review and qualitative synthesis applying the theoretical domains framework. Diabet Med. 2022;39(11):e14945. doi: 10.1111/dme.14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron NA, Yee LM, Dolan BM, O’Brien MJ, Greenland P, Khan SS. Trends in cardiovascular health counseling among postpartum individuals. JAMA. 2023;330(4):359-367. doi: 10.1001/jama.2023.11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29(4):178-191. doi: 10.24095/hpcdp.29.4.05 [DOI] [PubMed] [Google Scholar]
- 15.Feig DS, Berger H, Donovan L, et al. ; Diabetes Canada Clinical Practice Guidelines Expert Committee . Diabetes and pregnancy. Can J Diabetes. 2018;42(suppl 1):S255-S282. doi: 10.1016/j.jcjd.2017.10.038 [DOI] [PubMed] [Google Scholar]
- 16.Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina-Zubieta A, Rahme E. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PLoS One. 2013;8(10):e75256. doi: 10.1371/journal.pone.0075256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong A, Dasgupta K, Chiasson JL, Rahme E. Estimating the population prevalence of diagnosed and undiagnosed diabetes. Diabetes Care. 2013;36(10):3002-3008. doi: 10.2337/dc12-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pace R, Peters T, Rahme E, Dasgupta K. Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol. 2017;33(8):1052-1059. doi: 10.1016/j.cjca.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 19.Jurj AL, Wen W, Li HL, et al. Spousal correlations for lifestyle factors and selected diseases in Chinese couples. Ann Epidemiol. 2006;16(4):285-291. doi: 10.1016/j.annepidem.2005.07.060 [DOI] [PubMed] [Google Scholar]
- 20.Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007;64(11):2297-2310. doi: 10.1016/j.socscimed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Cobb LK, Godino JG, Selvin E, Kucharska-Newton A, Coresh J, Koton S. Spousal influence on physical activity in middle-aged and older adults: the ARIC study. Am J Epidemiol. 2016;183(5):444-451. doi: 10.1093/aje/kwv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah BR, Booth GL, Feig DS, Lipscombe LL. Validation of algorithms to identify gestational diabetes from population-level health-care administrative data. Can J Diabetes. 2023;47(1):25-30. doi: 10.1016/j.jcjd.2022.06.010 [DOI] [PubMed] [Google Scholar]
- 23.Carter EB, Martin S, Temming LA, Colditz GA, Macones GA, Tuuli MG. Early versus 6-12 week postpartum glucose tolerance testing for women with gestational diabetes. J Perinatol. 2018;38(2):118-121. doi: 10.1038/jp.2017.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner EF, Has P, Rouse D, Clark MA; Society for Maternal-Fetal Medicine (SMFM) . Two-day postpartum compared with 4- to 12-week postpartum glucose tolerance testing for women with gestational diabetes. Am J Obstet Gynecol. 2020;223(3):439.e1-439.e7. doi: 10.1016/j.ajog.2020.05.036 [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh KK, Grobman WA, Wu J, et al. Association of a large-for-gestational-age infant and maternal prediabetes mellitus and diabetes mellitus 10 to 14 years after delivery in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Am J Obstet Gynecol. 2023;228(6):756-758.e3. doi: 10.1016/j.ajog.2023.02.017 [DOI] [PubMed] [Google Scholar]
- 26.Parikh NI, Gonzalez JM, Anderson CAM, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council . Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902-e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 27.Mussa J, Meltzer S, Bond R, Garfield N, Dasgupta K. Trends in National Canadian Guideline recommendations for the screening and diagnosis of gestational diabetes mellitus over the years: a scoping review. Int J Environ Res Public Health. 2021;18(4):1454. doi: 10.3390/ijerph18041454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin HH, Cakmak S, Brion O, et al. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ Res. 2014;134:482-487. doi: 10.1016/j.envres.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 29.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol. 2014;43(6):1969-1985. doi: 10.1093/ije/dyu149 [DOI] [PubMed] [Google Scholar]
- 30.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36(9):551-559. doi: 10.1093/eurheartj/ehu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spijkerman AM, van der A DL, Nilsson PM, et al. ; InterAct Consortium . Smoking and long-term risk of type 2 diabetes: the EPIC-InterAct study in European populations. Diabetes Care. 2014;37(12):3164-3171. doi: 10.2337/dc14-1020 [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta K, Mussa J, Brazeau AS, et al. Associations of free sugars from solid and liquid sources with cardiovascular disease: a retrospective cohort analysis. BMC Public Health. 2023;23(1):756. doi: 10.1186/s12889-023-15600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retnakaran R, Austin PC, Shah BR. Effect of subsequent pregnancies on the risk of developing diabetes following a first pregnancy complicated by gestational diabetes: a population-based study. Diabet Med. 2011;28(3):287-292. doi: 10.1111/j.1464-5491.2010.03179.x [DOI] [PubMed] [Google Scholar]
- 34.Russell C, Dodds L, Armson BA, Kephart G, Joseph KS. Diabetes mellitus following gestational diabetes: role of subsequent pregnancy. BJOG. 2008;115(2):253-259. doi: 10.1111/j.1471-0528.2007.01459.x [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Santana MV, O’Brien KM, Park YM, Sandler DP, Weinberg CR. Persistence of risk for type 2 diabetes after gestational diabetes mellitus. Diabetes Care. 2022;45(4):864-870. doi: 10.2337/dc21-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347(8996):227-230. doi: 10.1016/S0140-6736(96)90405-5 [DOI] [PubMed] [Google Scholar]
- 37.Tait CA, L’Abbé MR, Smith PM, Rosella LC. The association between food insecurity and incident type 2 diabetes in Canada: a population-based cohort study. PLoS One. 2018;13(5):e0195962. doi: 10.1371/journal.pone.0195962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajna S, Ross NA, Brazeau AS, Bélisle P, Joseph L, Dasgupta K. Associations between neighbourhood walkability and daily steps in adults: a systematic review and meta-analysis. BMC Public Health. 2015;15:768. doi: 10.1186/s12889-015-2082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booth GL, Creatore MI, Luo J, et al. Neighbourhood walkability and the incidence of diabetes: an inverse probability of treatment weighting analysis. J Epidemiol Community Health. 2019;73(4):287-294. doi: 10.1136/jech-2018-210510 [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Wade AN, Mbanya JC, et al. The role of structural racism and geographical inequity in diabetes outcomes. Lancet. 2023;402(10397):235-249. doi: 10.1016/S0140-6736(23)00909-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pace R, Brazeau AS, Meltzer S, Rahme E, Dasgupta K. Conjoint associations of gestational diabetes and hypertension with diabetes, hypertension, and cardiovascular disease in parents: a retrospective cohort study. Am J Epidemiol. 2017;186(10):1115-1124. doi: 10.1093/aje/kwx263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta K, Ross N, Meltzer S, et al. Gestational diabetes mellitus in mothers as a diabetes predictor in fathers: a retrospective cohort analysis. Diabetes Care. 2015;38(9):e130-e131. doi: 10.2337/dc15-0855 [DOI] [PubMed] [Google Scholar]
- 43.Jackson SE, Steptoe A, Wardle J. The influence of partner’s behavior on health behavior change: the English Longitudinal Study of Ageing. JAMA Intern Med. 2015;175(3):385-392. doi: 10.1001/jamainternmed.2014.7554 [DOI] [PubMed] [Google Scholar]
- 44.Pettee KK, Brach JS, Kriska AM, et al. Influence of marital status on physical activity levels among older adults. Med Sci Sports Exerc. 2006;38(3):541-546. doi: 10.1249/01.mss.0000191346.95244.f7 [DOI] [PubMed] [Google Scholar]
- 45.Di Castelnuovo A, Quacquaruccio G, Arnout J, et al. ; European Collaborative Group of IMMIDIET Project . Cardiovascular risk factors and global risk of fatal cardiovascular disease are positively correlated between partners of 802 married couples from different European countries. Report from the IMMIDIET project. Thromb Haemost. 2007;98(3):648-655. doi: 10.1160/TH07-01-0024 [DOI] [PubMed] [Google Scholar]
- 46.Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta-analysis. BMC Med. 2014;12:12. doi: 10.1186/1741-7015-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lillard LA, Panis CW. Marital status and mortality: the role of health. Demography. 1996;33(3):313-327. doi: 10.2307/2061764 [DOI] [PubMed] [Google Scholar]
- 48.Speakman JR, Djafarian K, Stewart J, Jackson DM. Assortative mating for obesity. Am J Clin Nutr. 2007;86(2):316-323. doi: 10.1093/ajcn/86.2.316 [DOI] [PubMed] [Google Scholar]
- 49.Salces I, Rebato E, Susanne C. Evidence of phenotypic and social assortative mating for anthropometric and physiological traits in couples from the Basque country (Spain). J Biosoc Sci. 2004;36(2):235-250. doi: 10.1017/S0021932003006187 [DOI] [PubMed] [Google Scholar]
- 50.Li C, Liu Y, Zhang W. Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS One. 2015;10(8):e0136850. doi: 10.1371/journal.pone.0136850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207-2225. doi: 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD Codes
eTable 2. Comparing Effect Estimates of the Diabetes Outcome, With and Without Inclusion of Years of Pregnancy, with Account for Temporal Trends in the Screening and Diagnosis of Gestational Diabetes
eTable 3. Associations of Incident Diabetes With Gestational Diabetes Occurrences When Including Women With Stillbirth Pregnancies in Cohort
eMethods.
eFigure 1. Timeline of Applied Exclusions and Exposure Ascertainment Windows
eFigure 2. Distribution of Gestational Diabetes Exposure Categories
Data Sharing Statement