Key Points
Question
Is frontline ponatinib superior to imatinib when combined with reduced-intensity chemotherapy in adults with newly diagnosed Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL)?
Findings
In this randomized clinical trial, ponatinib demonstrated a significantly higher minimal residual disease–negative complete remission rate at the end of induction (34.4% vs 16.7% with imatinib) and a comparable safety profile vs imatinib when combined with reduced-intensity chemotherapy in adults with newly diagnosed Ph+ ALL.
Meaning
These efficacy and safety results support consideration of ponatinib as a frontline tyrosine kinase inhibitor in combination with chemotherapy for adults with newly diagnosed Ph+ ALL.
Abstract
Importance
In newly diagnosed Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL), disease progression due to acquired resistance to first- or second-generation BCR::ABL1 tyrosine kinase inhibitors is common. Ponatinib inhibits BCR::ABL1 and all single-mutation variants, including T315I.
Objective
To compare frontline ponatinib vs imatinib in adults with newly diagnosed Ph+ ALL.
Design, Setting, and Participants
Global registrational, phase 3, open-label trial in adults aged 18 years or older with newly diagnosed Ph+ ALL. From January 2019 to May 2022, eligible patients at 77 sites were randomized 2:1 to ponatinib (30 mg/d) or imatinib (600 mg/d) with reduced-intensity chemotherapy, followed by single-agent ponatinib or imatinib after the cycle 20 phase of the trial. The last date of follow-up for this analysis was August 12, 2022.
Intervention
Patients received ponatinib, 30 mg/d, or imatinib, 600 mg/d, with reduced-intensity chemotherapy, followed by single-agent ponatinib or imatinib after cycle 20. The ponatinib dose was reduced to 15 mg on achievement of minimal residual disease–(MRD) negative complete remission.
Main Outcomes and Measures
The primary end point of this interim analysis was MRD-negative complete remission (≤0.01% BCR::ABL1 [MR4] centrally assessed by reverse transcriptase–quantitative polymerase chain reaction), with complete remission maintained for at least 4 weeks at the end of cycle 3. The key secondary end point was event-free survival.
Results
Of 245 patients randomized (median age, 54 years; 133 [54.3%] female), 232 (ponatinib, n = 154; imatinib, n = 78) who had p190 or p210 dominant isoforms verified by the central laboratory were analyzed for the primary end point. The MRD-negative complete remission rate (primary end point) was significantly higher with ponatinib (34.4% [53/154]) vs imatinib (16.7% [13/78]) (risk difference, 0.18 [95% CI, 0.06-0.29]; P = .002). At the data cutoff, event-free survival had not met the prespecified number of events. Median event-free survival was not reached in the ponatinib group and was 29 months in the imatinib group. The most common adverse events were similar between treatment groups. Arterial occlusive events were infrequent and comparable between groups (ponatinib, 2.5%; imatinib, 1.2%).
Conclusions and Relevance
Ponatinib demonstrated a superior rate of MRD-negative complete remission at the end of induction vs imatinib when combined with reduced-intensity chemotherapy in adults with newly diagnosed Ph+ ALL. The safety profile of ponatinib was comparable with imatinib.
Trial Registration
ClinicalTrials.gov Identifier: NCT03589326
This randomized trial assesses the effect of ponatinib vs imatinib combined with low-intensity chemotherapy on disease remission in adults with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL).
Introduction
BCR::ABL1 tyrosine kinase inhibitors in combination with chemotherapy and/or steroids have been the standard of care for patients with newly diagnosed Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL), but there is no consensus on the optimal BCR::ABL1 tyrosine kinase inhibitor to use in this setting.1,2,3,4
Treatment with a first-generation (ie, imatinib) or second-generation (eg, dasatinib and nilotinib) tyrosine kinase inhibitor combined with low-intensity chemotherapy (vincristine and dexamethasone during induction) yields complete hematologic remission rates exceeding 94%,5,6,7 with fewer associated toxicities than when combined with intensive chemotherapy such as hyper-CVAD (cyclophosphamide, vincristine, doxorubicin [Adriamycin], and dexamethasone).5,6,7,8,9 Despite favorable initial responses to first- or second-generation BCR::ABL1 tyrosine kinase inhibitor–based regimens, relapse resulting from variant-acquired resistance, particularly T315I, is common.5,6,10 There is an unmet need for a potent frontline BCR::ABL1 tyrosine kinase inhibitor that suppresses all single-mutation variants of BCR::ABL1 associated with resistance and may prevent emergence of compound variants.
Ponatinib is a third-generation tyrosine kinase inhibitor designed to effectively inhibit BCR::ABL1 with or without any single-mutation variants, including T315I.11 Ponatinib has shown robust efficacy and a tolerable safety profile as frontline treatment of Ph+ ALL when combined with chemotherapy or immunotherapy in single-group studies.12,13,14,15,16,17 Minimal residual disease (MRD) is an established indicator of better clinical outcomes in patients with Ph+ ALL,18,19 and achievement of MRD negativity in the first 3 months of tyrosine kinase inhibitor treatment has been correlated with superior long-term survival outcomes.20,21 Indirect comparisons suggest that ponatinib may improve long-term outcomes compared with first- and second-generation BCR::ABL1 tyrosine kinase inhibitors by increasing early, deep MRD rates and by suppressing the emergence of BCR::ABL1 resistance variants.12,20,22
PhALLCON is the first phase 3 randomized clinical trial to compare 2 tyrosine kinase inhibitors for frontline therapy of Ph+ ALL. This trial evaluated the efficacy and safety of ponatinib vs imatinib in combination with low-intensity chemotherapy in newly diagnosed Ph+ ALL.
Methods
Trial Oversight
PhALLCON is a phase 3, open-label, global, randomized clinical trial. The trial protocol, available in Supplement 1, was developed jointly by the sponsor, Takeda Pharmaceutical Co Ltd, and the PhALLCON steering committee (Supplement 2). The trial protocol was approved by the institutional review boards or ethics committees of participating centers. The trial was conducted in compliance with applicable regulatory requirements and International Council for Harmonisation Guideline for Good Clinical Practice. All patients provided written informed consent. Study results are reported according to the CONSORT reporting guideline for randomized clinical trials.23 Data were reviewed by an independent data and safety monitoring committee supported by an independent statistician. Arterial occlusive events and venous thromboembolic events were reviewed by an independent cardiovascular end-point adjudication committee.
Patients
Eligible patients were aged 18 years or older with newly diagnosed Ph+ ALL, had an Eastern Cooperative Oncology Group performance status score of 2 or better, and did not have clinically significant or uncontrolled cardiovascular disease. Full eligibility criteria are in the trial protocol (Supplement 1); key criteria are summarized in eTable 1 in Supplement 2.
Trial Procedures
Patients were randomized in a 2:1 ratio to receive oral ponatinib or imatinib therapy with reduced-intensity chemotherapy based on a modified European Working Group on Adult ALL5 low-intensity chemotherapy backbone through induction (cycles 1-3), consolidation (cycles 4-9), and maintenance (cycles 10-20) phases (eFigure 1 in Supplement 2). After cycle 20, patients received single-agent ponatinib or imatinib. Randomization was stratified by age (18-44, 45-59, and ≥60 years). Ponatinib dosing was 30 mg once daily, reduced to 15 mg once daily on achievement of MRD-negative complete remission (defined as ≤0.01% BCR::ABL1IS [MR4] and complete remission for ≥4 weeks) at or after the end of induction and reescalated to 30 mg if patients experienced loss of MRD negativity. Imatinib dosing was 600 mg once daily. Patients continued study treatment until loss of efficacy (failure to achieve complete remission, failure to achieve MRD negativity, or relapse from complete remission), unacceptable toxicity, or proceeding to hematopoietic stem cell transplant (HSCT). Patients who did not achieve MRD-negative complete remission at end of cycle 3 discontinued study treatment. However, patients who achieved complete remission but not MRD negativity or who achieved MRD negativity with incomplete remission (meeting all criteria for complete remission except platelet count and/or absolute neutrophil count) at end of induction could continue study treatment at investigator discretion. Intrathecal therapy (methotrexate, cytarabine, and corticosteroids) was given twice monthly for the first 6 cycles for central nervous system disease prophylaxis. Dose-reduction guidelines for toxicity are provided in eTable 2 and eTable 3 in Supplement 2.
End Points
The primary end point was MRD-negative complete remission at the end of cycle 3 (eTable 4 in Supplement 2). This composite primary end point required investigator-reported complete remission (bone marrow blast response with hematologic recovery as measured by absolute neutrophil and platelet counts) for 4 weeks or longer and central laboratory–reported MRD negativity (defined as ≤0.01% BCR::ABL1IS [MR4]) measured with validated reverse transcriptase–quantitative polymerase chain reaction tests (e1a2/p190, MRDx/p210 BCR::ABL from MolecularMD and run at MolecularMD/ICON or Q2 Beijing) at the end of induction. The key secondary end point was event-free survival (defined as time from randomization until death due to any cause, failure to achieve complete remission by the end of induction, or relapse from complete remission). Secondary and other end points, including duration of complete remission and MRD-negative complete remission, MR4/MR4.5, time to treatment failure, and overall survival, are listed in eTable 4 in Supplement 2. Safety outcomes included treatment-emergent adverse events (TEAEs); arterial occlusive events; dose reductions, interruptions, and discontinuations due to adverse events; and death during treatment.
Statistical Analysis
Sample size determination and the rationale for the interim analysis and stopping guidelines are described in the statistical analysis plan (Supplement 1). The primary end point was analyzed using a Cochran-Mantel-Haenszel χ2 test with stratification according to randomization strata (ages 18-44, 45-59, and ≥60 years). The corresponding P value for absolute treatment difference was calculated; the threshold for statistical significance was P = .05. Risk differences and relative risks are provided with 2-sided 95% CIs. Subgroup analyses for the primary end point and other binary end points were analyzed similarly. Event-free survival and other time-to-event end points were analyzed by Kaplan-Meier methods. An unadjusted stratified Cox model was used to estimate hazard ratios and 95% CIs. Post hoc analyses evaluated progression-free survival, defined as failure to achieve MRD negativity by the end of treatment, loss of MRD negativity, or any of the event-free survival–defined events. Adverse events were summarized descriptively and graded according to Common Terminology Criteria for Adverse Events, version 5.0. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Patients
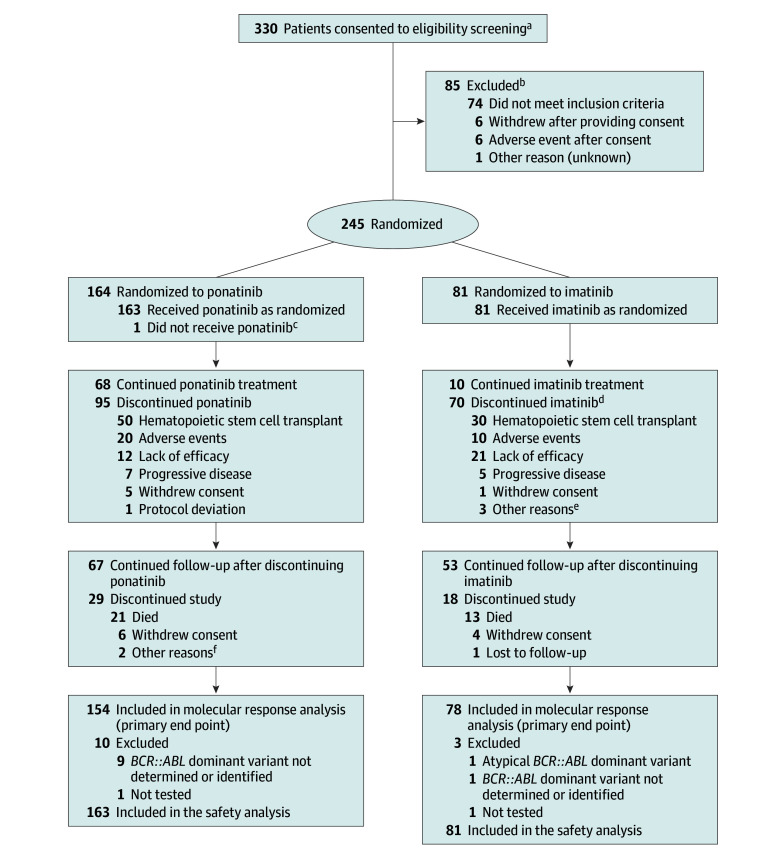
From January 2019 through May 2022, 245 patients were randomized across 77 sites. The primary end point was evaluated in the intention-to-treat population who had p190/p210 confirmed by the central laboratory, which consisted of 232 patients (ponatinib, n = 154; imatinib, n = 78) with baseline BCR::ABL1 p190 (n = 167) or p210 (n = 65) dominant isoforms (Table 1). Thirteen patients had atypical, missing, or unevaluable BCR::ABL1 variant assessments and were not included in the molecular response analysis (Figure 1). In total, 244 patients received at least 1 dose of study drug and were included in the safety analysis.
Table 1. Baseline Participant Demographics and Disease Characteristics.
| Characteristics | Ponatinib (n = 164) | Imatinib (n = 81) |
|---|---|---|
| Age, y | ||
| Median (range) | 54 (19-82) | 52 (19-75) |
| ≥60, No. (%) | 61 (37.2) | 30 (37.0) |
| Sex, No. (%) | ||
| Female | 90 (54.9) | 43 (53.1) |
| Male | 74 (45.1) | 38 (46.9) |
| Eastern Cooperative Oncology Group performance status score, No. (%)a | ||
| 0 (Best) | 72 (43.9) | 33 (40.7) |
| 1 | 85 (51.8) | 43 (53.1) |
| 2 (Worst) | 7 (4.3) | 5 (6.2) |
| Central nervous system disease/extramedullary disease, No. (%)b | 10 (6.1) | 3 (3.7) |
| BCR::ABL1 dominant isoform, No. (%) | ||
| p190 | 114 (69.5) | 53 (65.4) |
| p210 | 40 (24.4) | 25 (30.9) |
| Atypical | 0 | 1 (1.2) |
| Undetermined or not testedc | 10 (6.1) | 2 (2.5) |
| Cardiovascular comorbidities, No. (%)d | ||
| ≥1 | 92 (56.4) | 52 (64.2) |
| ≥2 | 45 (27.6) | 27 (33.3) |
| Hypertensione | 58 (35.6) | 30 (37.0) |
| Diabetes | 39 (23.9) | 24 (29.6) |
| Obesityf | 33 (20.2) | 19 (23.5) |
| Dyslipidemiag | 29 (17.8) | 23 (28.4) |
| History of smokingh | 44 (27.0) | 26 (32.1) |
Eastern Cooperative Oncology Group performance status scores range from 0 to 5, with higher scores indicative of greater disability.
Extramedullary involvement included lymphadenopathy, splenomegaly, skin/gum infiltration, and testicular mass.
Undetermined indicates transcripts were assessed at the central laboratory, but transcript type was not determined. Not tested indicates samples were not received by the central laboratory.
Cardiovascular comorbidities were evaluated in the safety population (ponatinib, n = 163; imatinib, n = 81).
Hypertension was defined as having hypertension, systolic hypertension, or essential hypertension reported as a preferred term in the Standardised MedDRA Queries list for a patient’s medical history.
Obesity was defined as a baseline body mass index ≥30 (calculated as weight in kilograms divided by height in meters squared).
Dyslipidemia was defined as having hypertriglyceridemia, blood cholesterol increased, hypercholesterolemia, hyperlipidemia, dyslipidemia, or low-density lipoprotein increased reported as a preferred term in the Standardized MedDRA Queries list for a patient’s medical history.
History of smoking was not included as a cardiovascular comorbidity when calculating number of patients with multiple comorbidities.
Figure 1. Participant Flow in the PhALLCON Trial.
aThere were 332 screening assessments of 330 patients; 2 patients were rescreened after not meeting eligibility initially. One patient approached for eligibility screening did not consent.
bThe 2 patients rescreened as mentioned in footnote a are included in reasons for exclusion but are not counted in the total number of patients excluded (n = 85).
cThis patient died of COVID-19 after randomization and before receiving any dose of ponatinib.
dReasons for discontinuation derived from electronic case report forms completed by study site personnel. One patient randomized to imatinib was discontinued due to a fatal adverse event; the reason for discontinuation was not reported.
eOther reasons included loss of minimum residual disease negativity and investigator decision to start another treatment (n = 1), loss of cytogenic response (n = 1), and patient not agreeing to study procedure and polymerase chain reaction not at an ideal level (n = 1).
fOther reasons included nonadherence to treatment visits and no longer meeting inclusion criteria (n = 1) and a report of “completed” (additional information not available; n = 1).
Patient demographics and baseline disease characteristics were balanced between groups (Table 1; eTable 5 in Supplement 2). The median age was 54 years (range, 19-82 years), with 37.1% of patients aged 60 years or older. Extramedullary disease was reported in 6.1% and 3.7% of patients treated with ponatinib and imatinib, respectively. Among 244 treated patients, 59.0% had at least 1 cardiovascular comorbidity at baseline and 29.5% had 2 or more comorbidities (Table 1).
As of the data cutoff date (August 12, 2022), the median duration of follow-up was 20.1 months (range, 17.8-23.1 months). A greater proportion of patients were continuing study treatment in the ponatinib group (68/164 [41.5%]) than in the imatinib group (10/81 [12.3%]). The most common reason for study treatment discontinuation in both groups was HSCT (30.5% in the ponatinib group vs 37.0% in the imatinib group) (Figure 1; eTable 6 in Supplement 2). The proportion of patients discontinuing study treatment for lack of efficacy was lower in the ponatinib group (12/164 [7.3%]) than in the imatinib group (21/81 [25.9%]), whereas discontinuations due to adverse events were similar for both groups (12.2% for ponatinib and 12.3% for imatinib).
Efficacy
The MRD-negative complete remission rate (not including incomplete remission) at the end of induction (primary end point) was significantly higher with ponatinib than imatinib, with a doubling of response rate (34.4% vs 16.7%) (relative risk, 2.06 [95% CI, 1.19-3.56]; risk difference, 0.18 [95% CI, 0.06-0.29]; P = .002) (Table 2). Among patients with evaluable samples at the end of induction, MRD negativity (MR4) was significantly higher for ponatinib than imatinib (43.0% vs 22.1%; relative risk, 1.94 [95% CI, 1.19-3.17]; P = .002). Benefit was observed for ponatinib vs imatinib across all subgroups analyzed for MRD-negative complete remission, including patients aged 60 years or older (40.0% vs 10.3%; relative risk, 3.87; risk difference, 0.30 [95% CI, 0.13-0.46]; P < .001) (eFigure 2 in Supplement 2). The median duration of MRD-negative complete remission was not reached (95% CI, 16.6 months to not estimable [NE]) in the ponatinib group and 18.0 months (95% CI, 8.4-27.8 months) in the imatinib group (Table 2; eFigure 3 in Supplement 2).
Table 2. Minimum Residual Disease Negativity With Complete Response at End of Induction: Response Rates and Durability of Responsea.
| Outcomes | Ponatinib (n = 154) | Imatinib (n = 78) | Risk difference (95% CI) | Relative risk (95% CI) | P value |
|---|---|---|---|---|---|
| Primary end point: MRD-negative complete remission at end of induction, No. (%)b | 53 (34.4) | 13 (16.7) | 0.18 (0.06-0.29) | 2.06 (1.19-3.56)c | .002c |
| MRD negativity (MR4) at end of induction, No. (%) | 61 (43.0)d | 15 (22.1)d | 0.21 (0.08-0.34) | 1.94 (1.19-3.17)c | .002c |
| Duration of MRD-negative complete remission | n = 53 | n = 13 | |||
| Loss-of-response events, No. (%) | 9 (17.0) | 6 (46.2) | |||
| Duration, median (95% CI), mo | NE (16.6-NE) | 18.0 (8.4-27.8) | NA | NA |
Abbreviations: MRD, minimal residual disease; NA, not available; NE, not estimable.
Outcomes were evaluated in the population of patients in the intention-to-treat population with p190/p210 dominant isoforms confirmed by the central laboratory.
The composite primary end point required central laboratory–reported MRD negativity (defined as ≤0.01% BCR::ABL1IS [MR4]) and investigator-reported complete remission (bone marrow blast response with hematologic recovery as measured by absolute neutrophil and platelet counts) for ≥4 weeks at the end of induction.
Adjusted relative risks, 95% CIs, and P values were calculated based on a Cochran-Mantel-Haenszel χ2 test, with stratification according to randomization strata (ages 18 to <45 years, ≥45 to <60 years, and ≥60 years).
Among patients with evaluable samples at end of trial cycle 3 (n = 142 for ponatinib and n = 68 for imatinib).
Greater proportions of patients achieved a deeper molecular response of MR4.5 (BCR::ABL1IS ≤0.0032%) independent of complete remission with ponatinib (26.8% [95% CI, 19.7%-34.8%]) vs imatinib (14.7% [95% CI, 7.3%-25.4%]) at the end of induction. Cumulative rates of MR4 and MR4.5 consistently increased over time, with MR4 rates reaching 62.1% with ponatinib and 44.9% with imatinib by cycle 9 (eFigure 4 in Supplement 2). Although MRD status was not reported after patients discontinued study treatment, including for HSCT, molecular responses were more durable in the ponatinib group, with the median time to loss of MRD negativity (defined as an increase to BCR::ABL1IS >0.01%) not reached for ponatinib vs 20.9 months for imatinib (eFigure 5 in Supplement 2).
The overall response rate based on cumulative complete remission or incomplete remission by the end of induction was similar between groups (ponatinib, 93.9%; imatinib, 97.5%); median time to relapse from complete remission was not reached in the ponatinib group and was 22.3 months in the imatinib group.
A lower proportion of patients in the ponatinib group experienced treatment failure, defined as discontinuation due to lack of efficacy or to toxicity. The median time to treatment failure was not reached in the ponatinib group and was 21.9 months (95% CI, 12.3-NE) in the imatinib group (eFigure 6 in Supplement 2).
Among patients who discontinued study treatment, the proportion receiving subsequent anticancer therapy was lower in the ponatinib group (57/163 [35.0%]) compared with the imatinib group (46/81 [56.8%]) (eTable 7 in Supplement 2). The median time from randomization to subsequent antineoplastic treatment was longer for ponatinib vs imatinib (32.4 vs 11.0 months, respectively) (eFigure 7 in Supplement 2). In the ponatinib group, 19.0% of patients received a subsequent second- or third-generation tyrosine kinase inhibitor and/or immunotherapy compared with 37.0% of patients in the imatinib group, and a lower percentage of patients in the ponatinib group (56/164 [34.1%]) received HSCT (at any time) compared with patients in the imatinib group (39/81 [48.1%]) (P = .03).
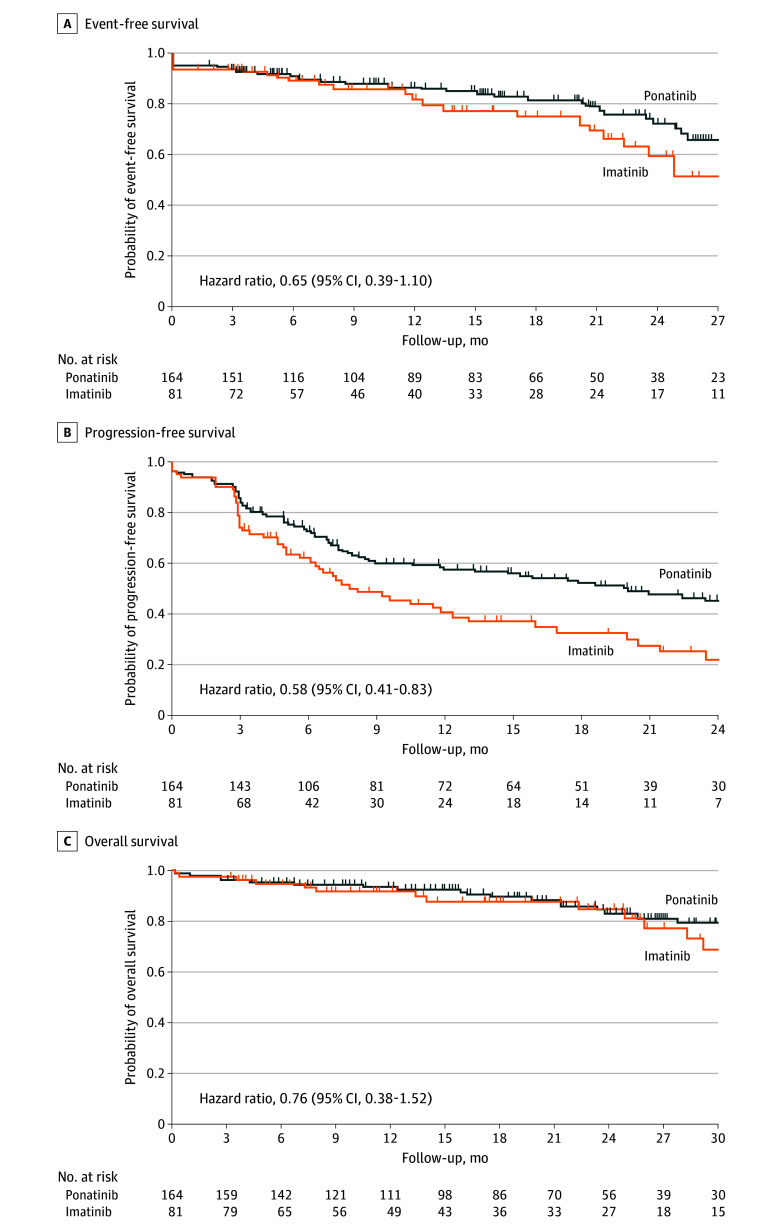
At this interim analysis, the prespecified number of events for statistical analysis of survival end points had not occurred. Median event-free survival was not reached in the ponatinib group and was 29.0 months in the imatinib group (hazard ratio, 0.65 [95% CI, 0.39-1.10]) (Figure 2A). In post hoc analyses, median progression-free survival was 12 months longer in the ponatinib group than in the imatinib group (20.0 months vs 7.9 months; hazard ratio, 0.58 [95% CI, 0.41-0.83]) (Figure 2B). Median overall survival was not reached in either group (hazard ratio, 0.76 [95% CI, 0.38-1.52]) (Figure 2C).
Figure 2. Kaplan-Meier Estimates of Survival Outcomes in the Intention-to-Treat Population.
A, Median event-free survival was not estimable (NE) (95% CI, NE-NE) with ponatinib and was 29.0 months (95% CI, 22.3 months–NE) with imatinib, with median follow-up of 17.3 (IQR, 7.3-26.3) months and 15.9 (IQR, 7.0-28.9) months, respectively. Event-free survival occurred in 34 patients in the ponatinib group and 24 patients in the imatinib group. B, Median progression-free survival was 20.0 months (95% CI, 11.8 months–NE) with ponatinib and 7.9 (95% CI, 6.2-12.4) months with imatinib, with median follow-up of 20.7 (IQR, 12.5-29.5) months and 19.2 (IQR, 10.8-35.0) months, respectively. Progression-free survival occurred in 77 patients in the ponatinib group and 54 patients in the imatinib group. C, Median overall survival was NE (95% CI, NE-NE) in the ponatinib group and NE (95% CI, 29.0 months–NE) in the imatinib group, with median follow-up of 20.4 (IQR, 10.4-29.5) months and 18.1 (IQR, 9.8-30.5) months, respectively. Overall survival occurred in 21 patients in the ponatinib group and 13 patients in the imatinib group. Patients who received hematopoietic stem cell transplant were censored (event-free survival: 47/164 [28.7%] for ponatinib and 28/81 [34.6%] for imatinib; progression-free survival: 25/164 [15.2%] for ponatinib and 15/81 [18.5%] for imatinib; overall survival: 48/164 [29.3%] for ponatinib and 34/81 [42.0%] for imatinib).
Safety
Adverse event rates were comparable between the ponatinib and imatinib groups, including grade 3 or 4 TEAEs (85.3% and 87.7%, respectively) and treatment-related adverse events (65.6% and 59.3%, respectively) (Table 3). The most common grade 3 or 4 hematologic TEAEs were decreased platelet count (ponatinib, 63.2%; imatinib, 58.0%), white blood cell count (53.4% and 49.4%, respectively), and neutrophil count (49.1% and 45.7%, respectively) (eFigure 8A and eTable 8 in Supplement 2). The most common grade 3 or 4 nonhematologic TEAEs were increased alanine aminotransferase (ponatinib, 19.0%; imatinib, 8.6%), hypertension (12.3% and 6.2%, respectively), increased lipase (12.9% and 18.5%, respectively), and hypokalemia (6.1% and 18.5%, respectively) (eFigure 8B and eTable 8 in Supplement 2). Pancreatitis occurred in 31.3% of patients in the ponatinib group (2.5% serious events) and 37.0% of patients in the imatinib group (no serious events). Serious TEAEs occurred in 59.5% of patients in the ponatinib group and 55.6% of patients in the imatinib group (eTable 9 in Supplement 2).
Table 3. Adverse Events.
| Adverse events | No. (%) | |||
|---|---|---|---|---|
| Ponatinib (n = 163) | Imatinib (n = 81) | |||
| Treatment-emergent adverse eventsa | ||||
| Any | 162 (99.4) | 80 (98.8) | ||
| Serious | 97 (59.5) | 45 (55.6) | ||
| Grade 3-4 | 139 (85.3) | 71 (87.7) | ||
| Grade 5 | 8 (4.9) | 4 (4.9) | ||
| Treatment-related adverse eventsa | ||||
| Any | 141 (86.5) | 67 (82.7) | ||
| Serious | 34 (20.9) | 16 (19.8) | ||
| Grade 3-4 | 107 (65.6) | 48 (59.3) | ||
| Grade 5 | 0 | 1 (1.2) | ||
| Adverse events of special interest | Any grade | Grade 3-4 | Any grade | Grade 3-4 |
| Adjudicated arterial occlusive eventsb | ||||
| Any | 4 (2.5) | 2 (1.2) | 1 (1.2) | 0 |
| Cardiovascular events | 2 (1.2) | 2 (1.2) | 0 | 0 |
| Cerebrovascular events | 1 (0.6) | 0 | 1 (1.2) | 0 |
| Peripheral vascular events | 1 (0.6) | 0 | 0 | 0 |
| Adjudicated venous thromboembolic eventsb | ||||
| Any | 19 (11.7) | 6 (3.7) | 10 (12.3) | 1 (1.2) |
| Peripherally inserted central catheter line or central venous catheter related | 8 (4.9) | NA | 6 (7.4) | NA |
| Deep vein thrombosis | 13 (8.0) | 5 (3.1) | 9 (11.1) | 0 |
| Superficial vein thrombosis | 5 (3.1) | 0 | 0 | 0 |
| Pulmonary embolism | 2 (1.2) | 1 (0.6) | 1 (1.2) | 1 (1.2) |
Abbreviation: NA, not available.
Higher grades indicate more serious adverse events. The sum of values for the serious and grade 3, 4, or 5 adverse event categories does not sum to the value in the “any” category because individual patients could have had events in multiple categories.
Arterial occlusive events and venous thromboembolic events were reviewed based on an adjudication charter written before the start of the study by an independent cardiovascular end point adjudication committee comprising experts with experience and training appropriate for reviews of the cardiovascular end points. The committee adjudicated all arterial occlusive and venous thromboembolic events reported by the study sites (ie, initial diagnoses, laboratory values, results of procedures, and hospital discharge summaries).
Dose reductions owing to TEAEs occurred at similar rates in the ponatinib group (20.2%) and the imatinib group (22.2%) (eTable 10 in Supplement 2). Dose interruptions because of TEAEs were more frequent with ponatinib (68.1%) than with imatinib (39.5%). Rates of discontinuation due to TEAEs were similar in both groups (ponatinib, 10.4%; imatinib, 8.6%).
Arterial occlusive events were reported in 2.5% of patients receiving ponatinib and in 1.2% receiving imatinib; grade 3 or 4 arterial occlusive events were reported in 1.2% of patients receiving ponatinib (Table 3). Rates of venous thromboembolic events were comparable between groups (ponatinib, 11.7%; imatinib, 12.3%), including events associated with peripheral catheterization (4.9% and 7.4%, respectively).
There were no deaths related to arterial occlusive events or venous thromboembolic events in either group. Deaths during the study (reported up to 30 days after the last dose of study drug) occurred in 4.9% of patients in both groups (ponatinib, 8 patients; imatinib, 4 patients) (eTable 11 in Supplement 2). One treatment-related death was reported in the imatinib group (sudden death; depressed level of consciousness); all other deaths were due to infections or respiratory events unrelated to treatment.
Discussion
The primary end point of PhALLCON was met, demonstrating a significantly higher MRD-negative complete remission rate with ponatinib (34.4%) compared with imatinib (16.7%) (P = .002) when combined with reduced-intensity chemotherapy. This composite end point had more stringent criteria than typically applied in clinical practice, requiring 4-week durable complete remission (excluding incomplete remission) and MRD negativity as determined by the central laboratory at the end of induction, and has not been reported in previous studies of tyrosine kinase inhibitors in Ph+ ALL.5,6,7,14,15,24 Ponatinib demonstrated significantly higher rates of MRD negativity regardless of complete remission outcome (MR4: 43.0% vs 22.1%; P = .002), and deeper molecular response (MR4.5: 26.8% vs 14.7%) at the end of induction compared with imatinib. These rates are comparable with complete molecular response (MR4) rates previously observed at the end of induction with ponatinib (47%-48%),14,15 imatinib (29%),6 and second-generation tyrosine kinase inhibitors dasatinib (18%)24 and nilotinib (14%),7 in combination with lower-intensity chemotherapy or steroids.6,14,15 Although the primary end point was evaluated early (approximately 3 months), the cumulative rate of MRD negativity continued to increase over time, reaching 62% by cycle 9 in the ponatinib group.
Ponatinib showed durability of response and longer median time to loss of MRD negativity compared with imatinib (not reached vs 21 months) despite dose reduction from 30 mg/d to 15 mg/d on attainment of MRD negativity. This is consistent with the findings of a retrospective chart review of 204 patients with newly diagnosed Ph+ ALL in which ponatinib demonstrated superiority to imatinib and dasatinib for inducing and maintaining a 3-month complete molecular response, regardless of subsequent HSCT.21
Demonstrating improved long-term survival is important for comparing treatment outcomes in ALL. However, adequate statistical evaluation of overall survival requires lengthy follow-up in Ph+ ALL, wherein 5-year overall survival rates can exceed 70% with ponatinib.13,21 A surrogate outcome measure such as early absence of MRD can have prognostic power for long-term survival outcomes in patients with ALL.20,25,26 The prognostic value of MRD-negative complete remission at the end of induction (the primary end point of this trial) in newly diagnosed Ph+ ALL was suggested in a meta-analysis demonstrating significantly longer overall survival in patients achieving MRD-negative complete remission at the end of induction compared with those who were MRD-positive or had complete remission alone.26 Therefore, the observed superiority of ponatinib over imatinib for achieving the primary end point at this first interim analysis may translate to improved long-term survival with additional follow-up. This is further supported by emerging clinically meaningful trends for improved median event-free survival (ponatinib, not reached; imatinib, 29.0 months) and progression-free survival (20.0 months vs 7.9 months) in this analysis, despite insufficient event numbers for formal statistical testing.
While the low-intensity chemotherapy regimen in this study was inspired by the EWALL trial,5 we elected to include three 28-day cycles of induction in alignment with the GIMEMA LAL1509 study, which also assessed MRD negativity at the end of a 3-month induction period.24 This approach allowed for assessment of MRD negativity at 3 months, which, as noted above, is correlated with better long-term outcomes in patients with Ph+ ALL.21,26
Ponatinib’s benefit over imatinib for reaching MRD-negative complete remission was consistent across all subgroups evaluated, including in patients aged 60 years or older. These data are encouraging because older patients represent a challenging-to-treat population with suboptimal response to limited treatment options, including HSCT and aggressive therapies like hyper-CVAD.6,27,28,29,30 The potency of ponatinib in combination with low-dose chemotherapy or no chemotherapy may render HSCT unnecessary for older or frail patients who achieve a 3-month MRD-negative complete remission.16 Overall, the proportion of patients proceeding to HSCT was lower with ponatinib than imatinib, indicating that treating physicians were more likely to maintain treatment with ponatinib than with imatinib, possibly owing to durability of response and acceptable tolerability.
Trends for improvement in progression-free survival with ponatinib over imatinib were observed despite 37.0% (30/81) of the patients in the imatinib group discontinuing study drug to receive a second- or third-generation tyrosine kinase inhibitor and/or immunotherapy, including 16.0% (13/81) of patients who received ponatinib-based subsequent therapy. This therapy switching may have positively impacted Kaplan-Meier estimates of event-free survival and progression-free survival for the imatinib group. The improved efficacy outcomes observed with ponatinib over imatinib may derive from ponatinib’s higher potency against BCR::ABL1 and/or its ability to prevent single-mutation variants such as T315I.11 To this end, samples collected at the end of treatment were analyzed for emerging variants, but limited data are available to date, as many patients discontinued treatment for reasons such as HSCT or failure to achieve MRD negativity before resistance mechanisms had developed or could be detected. There also is a possibility that outcome improvements could be related to ponatinib’s known activity against a broader range of tyrosine kinases, which may affect targets of Ph+ ALL biology inaccessible to imatinib.31
The tolerability of ponatinib treatment was comparable with that of imatinib when combined with reduced-intensity chemotherapy in patients with newly diagnosed Ph+ ALL and without serious cardiovascular comorbidities (per study protocol). Arterial occlusive events were infrequent and similar between treatment groups despite the prevalence of baseline cardiovascular comorbidities (diabetes, hypertension, and dyslipidemia) and the longer median follow-up in the ponatinib group. These event rates are lower than previously reported.32 The lower arterial occlusive event rate may be driven by multiple factors, including reduced starting dose, prospective dose-reduction strategies (thereby resulting in shorter duration at the 30-mg/d dose), and more stringent exclusion criteria. Furthermore, the patient population enrolled in PhALLCON was not as heavily pretreated as patients in prior ponatinib trials.33,34
Limitations
This study has multiple limitations. First, the short duration of follow-up at this analysis did not allow for statistical analysis of event-free survival and overall survival; additional analyses are forthcoming after sufficient events have occurred. Second, the comparator tyrosine kinase inhibitor in this study, imatinib, has largely been replaced by second-generation tyrosine kinase inhibitors (eg, dasatinib) in current practice in the US. However, second-generation tyrosine kinase inhibitors are not widely accessible or approved for Ph+ ALL in all countries, and imatinib remains standard of care in many countries.35 Therefore, imatinib was selected as the most appropriate comparator for PhALLCON, in which approximately two-thirds of patients were enrolled outside of the US. While it is unclear how the current findings compare with second-generation tyrosine kinase inhibitors in the absence of a head-to-head randomized trial, the MRD negativity rate at the end of induction in the imatinib group in PhALLCON (22%) was comparable with previous reports for second-generation tyrosine kinase inhibitors (14%-18%).7,24 In addition, a propensity score matching–adjusted indirect comparison analysis demonstrated prolonged overall survival with ponatinib vs imatinib when combined with hyper-CVAD.22 Third, we acknowledge that ponatinib in combination with low-intensity chemotherapy may not be the treatment of choice given recent alternatives (eg, ponatinib plus blinatumomab),36 but results from this study may inform tyrosine kinase inhibitor selection in future treatment combinations.
Conclusions
In the PhALLCON trial, laboratory evidence showed that the rate of MRD-negative complete remission was significantly higher with ponatinib than imatinib. Ponatinib demonstrated a manageable safety profile comparable with imatinib. These results suggest suitability of ponatinib as a frontline tyrosine kinase inhibitor in combination with chemotherapy for newly diagnosed Ph+ ALL.
Trial Protocol and Statistical Analysis Plan
eAppendix. PhALLCON Investigators, PhALLCON Steering Committee, and Supplemental Methods
eTable 1. Key Eligibility Criteria
eTable 2. Ponatinib Dose Modification Guidelines for Drug-Related Adverse Events
eTable 3. Ponatinib Dose Modification Guidelines for Arterial Occlusive Events and Venous Thromboembolic Events
eTable 4. Endpoint Definitions and Response Criteria
eTable 5. Additional Patient Demographics and Baseline Disease Characteristics
eTable 6. Patient Disposition
eTable 7. Subsequent Anticancer Therapy
eTable 8. Treatment-Emergent Adverse Events
eTable 9. Serious Treatment-Emergent Adverse Events Reported in More Than Two Patients
eTable 10. Dose Modification for Treatment-Emergent Adverse Events
eTable 11. Treatment-Emergent Adverse Events Leading to Death
eFigure 1. PhALLCON Study Design and Reduced-Intensity Chemotherapy Regimens
eFigure 2. MRD-Negative CR Rate at End of Induction by Patient Subgroup
eFigure 3. Duration of MRD-Negative CR
eFigure 4. Cumulative Molecular Response Rates by Treatment Cycle
eFigure 5. Duration of MRD Negativity
eFigure 6. Time to Treatment Failure
eFigure 7. Time to Subsequent Antineoplastic Treatment
eFigure 8. Treatment-Emergent Adverse Events
Data Sharing Statement
References
- 1.Park HS. Current treatment strategies for Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Blood Res. 2020;55(S1):S32-S36. doi: 10.5045/br.2020.S006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh K, Fernandez A, Pasquier F. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults. Cancers (Basel). 2022;14(7):1805. doi: 10.3390/cancers14071805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C; ESMO Guidelines Committee . Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69-v82. doi: 10.1093/annonc/mdw025 [DOI] [PubMed] [Google Scholar]
- 4.Jabbour E, Haddad FG, Short NJ, Kantarjian H. Treatment of adults with Philadelphia chromosome–positive acute lymphoblastic leukemia—from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 2022;8(9):1340-1348. doi: 10.1001/jamaoncol.2022.2398 [DOI] [PubMed] [Google Scholar]
- 5.Rousselot P, Coudé MM, Gokbuget N, et al. ; European Working Group on Adult ALL (EWALL) . Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-782. doi: 10.1182/blood-2016-02-700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalandon Y, Thomas X, Hayette S, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) . Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719. doi: 10.1182/blood-2015-02-627935 [DOI] [PubMed] [Google Scholar]
- 7.Ottmann OG, Pfeifer H, Cayuela JM, et al. Nilotinib (Tasigna) and low intensity chemotherapy for first-line treatment of elderly patients with BCR-ABL1-positive acute lymphoblastic leukemia: final results of a prospective multicenter trial (EWALL-PH02). Blood. 2018;132(suppl 1):31. doi: 10.1182/blood-2018-99-11455229752258 [DOI] [Google Scholar]
- 8.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653-661. doi: 10.3324/haematol.2014.118588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070-2077. doi: 10.1182/blood-2009-12-261586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soverini S, De Benedittis C, Papayannidis C, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome–positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era. Cancer. 2014;120(7):1002-1009. doi: 10.1002/cncr.28522 [DOI] [PubMed] [Google Scholar]
- 11.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401-412. doi: 10.1016/j.ccr.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome–positive acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2016;122(23):3650-3656. doi: 10.1002/cncr.30231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618-e627. doi: 10.1016/S2352-3026(18)30176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribera JM, García-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. doi: 10.1182/bloodadvances.2022007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinelli G, Papayannidis C, Piciocchi A, et al. INCB84344-201: ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022;6(6):1742-1753. doi: 10.1182/bloodadvances.2021004821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabbour E, Short NJ, Jain N, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. 2023;10(1):e24-e34. doi: 10.1016/S2352-3026(22)00319-2 [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Short NJ, Jain N, et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am J Hematol. 2023;98(3):493-501. doi: 10.1002/ajh.26816 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Jang E. Association of minimal residual disease with clinical outcomes in Philadelphia chromosome positive acute lymphoblastic leukemia in the tyrosine kinase inhibitor era: a systemic literature review and meta-analysis. PLoS One. 2021;16(8):e0256801. doi: 10.1371/journal.pone.0256801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hein K, Short N, Jabbour E, Yilmaz M. Clinical value of measurable residual disease in acute lymphoblastic leukemia. Blood Lymphat Cancer. 2022;12:7-16. doi: 10.2147/BLCTT.S270134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504-507. doi: 10.1182/blood-2016-03-707562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki K, Kantarjian HM, Short NJ, et al. Prognostic factors for progression in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia in complete molecular response within 3 months of therapy with tyrosine kinase inhibitors. Cancer. 2021;127(15):2648-2656. doi: 10.1002/cncr.33529 [DOI] [PubMed] [Google Scholar]
- 22.Ribera JM, Prawitz T, Freitag A, et al. Ponatinib vs. imatinib as frontline treatment for Philadelphia chromosome-positive acute lymphoblastic leukemia: a matching adjusted indirect comparison. Adv Ther. 2023;40(7):3087-3103. doi: 10.1007/s12325-023-02497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. doi: 10.1371/journal.pmed.1000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiaretti S, Ansuinelli M, Vitale A, et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica. 2021;106(7):1828-1838. doi: 10.3324/haematol.2020.260935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi: 10.1001/jamaoncol.2017.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashaye A, Chalandon Y, Dombret H, et al. Minimal residual disease-negative complete remission at the end of induction is a prognostic indicator of long-term survival in adult patients with Ph+ acute lymphoblastic leukemia receiving first-line therapy. Blood. 2023;142(suppl 1):6075. doi: 10.1182/blood-2023-179645 [DOI] [Google Scholar]
- 27.Sawalha Y, Advani AS. Management of older adults with acute lymphoblastic leukemia: challenges & current approaches. Int J Hematol Oncol. 2018;7(1):IJH02. doi: 10.2217/ijh-2017-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieduwilt MJ. How should we treat older adults with Ph+ adult ALL and what novel approaches are being investigated? Best Pract Res Clin Haematol. 2017;30(3):201-211. doi: 10.1016/j.beha.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 29.Ottmann OG, Wassmann B, Pfeifer H, et al. ; GMALL Study Group . Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer. 2007;109(10):2068-2076. doi: 10.1002/cncr.22631 [DOI] [PubMed] [Google Scholar]
- 30.Thyagu S, Minden MD, Gupta V, et al. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia with imatinib combined with a paediatric-based protocol. Br J Haematol. 2012;158(4):506-514. doi: 10.1111/j.1365-2141.2012.09182.x [DOI] [PubMed] [Google Scholar]
- 31.Cuellar S, Vozniak M, Rhodes J, Forcello N, Olszta D. BCR-ABL1 tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract. 2018;24(6):433-452. doi: 10.1177/1078155217710553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarjian HM, Jabbour E, Deininger M, et al. Ponatinib after failure of second-generation tyrosine kinase inhibitor in resistant chronic-phase chronic myeloid leukemia. Am J Hematol. 2022;97(11):1419-1426. doi: 10.1002/ajh.26686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404. doi: 10.1182/blood-2016-09-739086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):2042-2050. doi: 10.1182/blood.2021012082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva W, Rego E. How to manage Philadelphia-positive acute lymphoblastic leukemia in resource-constrained settings. Cancers (Basel). 2023;15(24):5783. doi: 10.3390/cancers15245783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiaretti S, Leoncin M, Elia L, et al. Comparison between dasatinib-blinatumomab vs ponatinib-blinatumomab chemo-free strategy for newly diagnosed Ph+ acute lymphoblastic leukemia patients: preliminary results of the GIMEMA ALL2820 trial. Blood. 2023;142(suppl 1):4249. doi: 10.1182/blood-2023-189632 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. PhALLCON Investigators, PhALLCON Steering Committee, and Supplemental Methods
eTable 1. Key Eligibility Criteria
eTable 2. Ponatinib Dose Modification Guidelines for Drug-Related Adverse Events
eTable 3. Ponatinib Dose Modification Guidelines for Arterial Occlusive Events and Venous Thromboembolic Events
eTable 4. Endpoint Definitions and Response Criteria
eTable 5. Additional Patient Demographics and Baseline Disease Characteristics
eTable 6. Patient Disposition
eTable 7. Subsequent Anticancer Therapy
eTable 8. Treatment-Emergent Adverse Events
eTable 9. Serious Treatment-Emergent Adverse Events Reported in More Than Two Patients
eTable 10. Dose Modification for Treatment-Emergent Adverse Events
eTable 11. Treatment-Emergent Adverse Events Leading to Death
eFigure 1. PhALLCON Study Design and Reduced-Intensity Chemotherapy Regimens
eFigure 2. MRD-Negative CR Rate at End of Induction by Patient Subgroup
eFigure 3. Duration of MRD-Negative CR
eFigure 4. Cumulative Molecular Response Rates by Treatment Cycle
eFigure 5. Duration of MRD Negativity
eFigure 6. Time to Treatment Failure
eFigure 7. Time to Subsequent Antineoplastic Treatment
eFigure 8. Treatment-Emergent Adverse Events
Data Sharing Statement