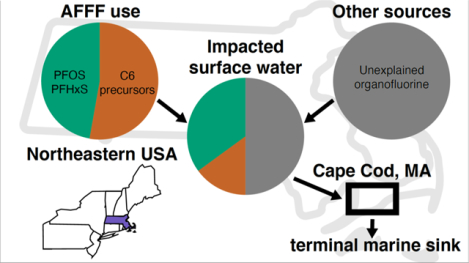
Abstract
Water supplies for millions of U.S. individuals exceed maximum contaminant levels for per- and polyfluoroalkyl substances (PFAS). Contemporary and legacy use of aqueous film forming foams (AFFF) is a major contamination source. However, diverse PFAS sources are present within watersheds, making it difficult to isolate their predominant origins. Here we examine PFAS source signatures among six adjacent coastal watersheds in Cape Cod, MA, USA using multivariate clustering techniques. A distinct signature of AFFF contamination enriched in precursors with six perfluorinated carbons (C6) was identified in watersheds with an AFFF source, while others were enriched in C4 precursors. Principal component analysis of PFAS composition in impacted watersheds showed a decline in precursor composition relative to AFFF stocks and a corresponding increase in terminal perfluoroalkyl sulfonates with <C6 but not those with ≥C6. Prior work shows that in AFFF stocks, all extractable organofluorine (EOF) can be explained by targeted PFAS and precursors inferred using Bayesian inference on the total oxidizable precursor assay. Using the same techniques for the first time in impacted watersheds, we find that only 24%–63% of the EOF can be explained by targeted PFAS and oxidizable precursors. Our work thus indicates the presence of non-AFFF, large organofluorine sources in these coastal watersheds.
Graphical Abstract
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a broad class of persistent anthropogenic chemicals with aliphatic fluorinated chains.1,2 Water supplies for millions of Americans exceed state level maximum contaminant levels (MCLs) for PFAS in drinking water.3,4 At least 600 sites across the United States (USA) have been contaminated by the use of aqueous film forming foam (AFFF) containing PFAS for firefighting and training.5 However, distinguishing AFFF contamination from other sources is challenging because diverse PFAS sources are present in watersheds such as industry, septic systems, wastewater, agriculture, and atmospheric deposition.6
Both legacy (electrochemical fluorination: ECF and fluorotelomer: FT) and contemporary (FT only) AFFF contain large quantities of polyfluoroalkyl precursors,7–10 that may degrade into terminal perfluoroalkyl acids (PFAA) subject to water quality guidelines.7,11,12 PFAS composition in AFFF contaminated surface water and groundwater is expected to change along the hydrological flow path due to preferential sorption13 and degradation of these precursors.7,8,11,12,14 Such processes could potentially confound detection and isolation of the PFAS attributable to AFFF contamination at a given location.
Traditional methods for measuring PFAS (targeted LC-MS/MS) only capture a small fraction of the PFAS present in AFFF and environmental samples.8–10,15,16 The total oxidizable precursor (TOP) assay uses a strong oxidant to transform precursors into terminal PFAA that are detectable using targeted LC-MS/MS. Most prior studies have lumped TOP assay results into a single category of oxidizable precursors.13,17 Ruyle et al.10 developed a new statistical method based on Bayesian inference to infer the original precursor concentrations of a given chain length present in aqueous samples using published laboratory data on oxidative yields of terminal perfluoroalkyl carboxylates (PFCA), and measured concentrations of terminal products in environmental samples following the TOP assay. ECF and FT precursors present in AFFF have unique oxidation yields18–20 that are used in the statistical inference to reconstruct the manufacturing origin of AFFF mixtures.10
Some precursors are resistant to oxidation by the TOP assay or yield ultrashort chain PFCA that are not routinely included in targeted analyte lists.20,21 All of these compounds will be captured by measurements of extractable organofluorine (EOF). Prior work showed virtually all (median 104±19%) of the EOF in ECF and FT AFFF stocks can be explained by targeted PFAS analysis and oxidizable precursors captured by the TOP assay.10 Precursors with six perfluorinated carbons (C6) were most abundant in both ECF and FT AFFF.10 Previous studies in AFFF-impacted environments have reported a large fraction of unexplained organofluorine. However, they only included one or two C6 precursors on the targeted analyte list and could not distinguish AFFF precursors from other sources of unidentified organofluorine.16,22,23
Multivariate clustering techniques and spatial relationships between sampling locations and major PFAS sources can assist in identifying predominant PFAS contamination sources in surface water.24 Prior work has not considered AFFF impacted locations or included C6 precursors because analytical standards needed for the analysis were unavailable at the time. Although several standards for C6 precursors are now available, new standard production can lag decades behind industrial PFAS production and does not automatically result in their inclusion in targeted analyte lists.10 As an intermediate step, routine analysis of the TOP assay with statistical inference to identify the chain length and manufacturing origin of precursors would facilitate their inclusion in source-attribution modeling.
Here, we evaluate whether multivariate clustering techniques using information on targeted PFAS and precursor composition from the TOP assay and statistical inference can distinguish the signature of AFFF contamination from other sources. For this analysis, we use measured PFAS composition in surface water from six coastal watersheds in Cape Cod, MA, USA. Measurements include 27 targeted PFAS, the TOP assay, and EOF. We use these data to characterize the fraction of EOF accounted for by oxidizable precursors and targeted PFAS together for the first time in natural waters and to quantify mass fluxes of unexplained organofluorine in these coastal watersheds.
Materials and Methods
Study region.
We collected surface water from kettle lakes and rivers in the six coastal watersheds in Cape Cod, MA, USA shown in Figure 1. Sites were selected based on well-characterized hydrology as part of past United States Geological Survey (USGS) research efforts.25,26 Three watersheds (Childs, Quashnet, Mill Creek) contain an upgradient AFFF source zone from historical fire-training activity with extensive regional groundwater and surface water contamination, and three (Marston Mills, Mashpee, and Santuit) were used as controls for watersheds without a known AFFF source.17,27,28 Please see the SI for additional site description.
Fig 1. Field sampling locations on western Cape Cod, Massachusetts, USA.
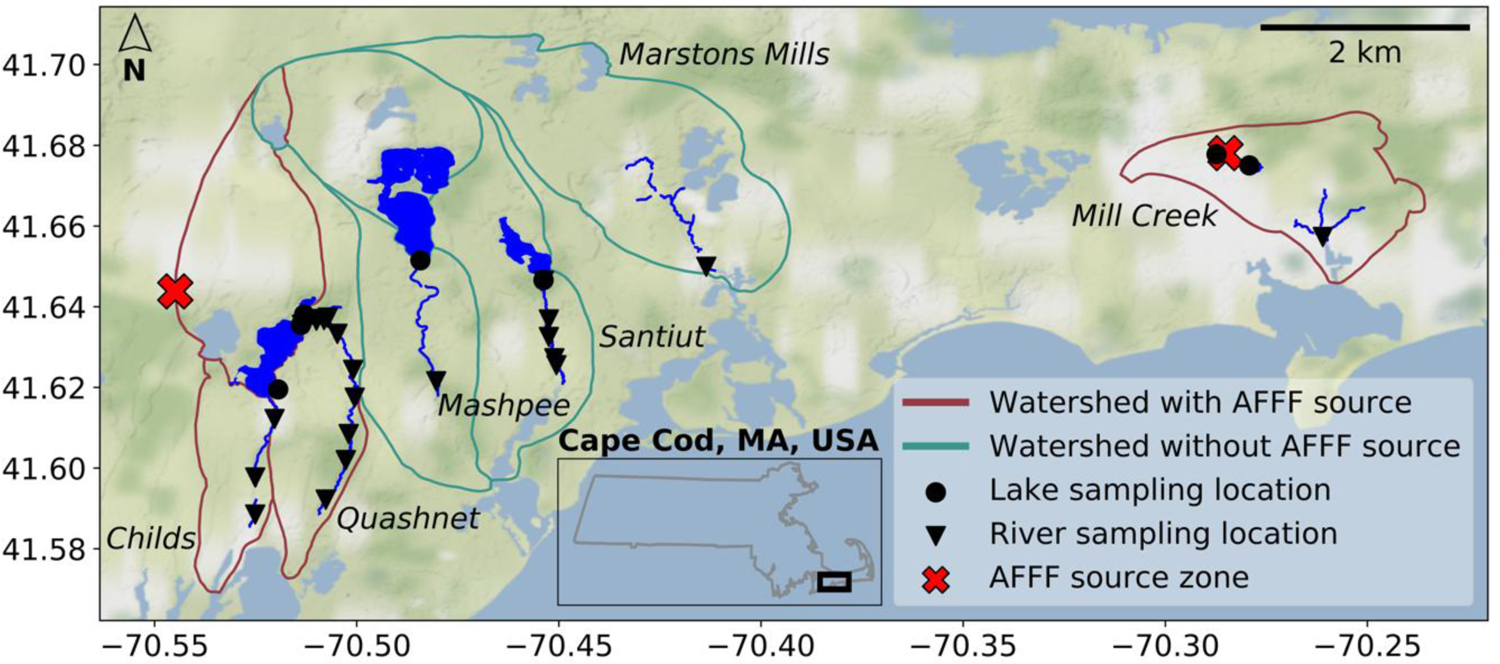
Samples were collected between August 2017 and July 2019. Groundwater-flow models of the sandy glacial Cape Cod aquifer were used to delineate watershed boundaries shown in crimson and green.25,26 Three watersheds outlined in crimson (Childs, Quashnet, Mill Creek) contain a known source zone (marked by red crosses) for PFAS due to use of aqueous film forming foams (AFFF). Three watersheds outlined in green (Marstons Mills, Mashpee, Santuit) represent background locations with no known AFFF source zone.
We searched the Facility Registration Service (FRS) codes for other potential PFAS point sources within the six watersheds, following the same procedure described in Zhang et al.24 Seven airport operations were identified within the watersheds but none were certified for AFFF use.29 Thus, they were unlikely to be important PFAS sources and no other relevant sources were identified.
Most sites were sampled 1–2 times (Childs: Jul-18, Mill Creek: Jul-18, Apr-19; Quashnet: repeat sampling Aug-17 to Jul-19; Marston Mills: Jul-18, Apr-19; Mashpee: Apr-19; Santuit: Jul-18, Jul-19). In total, 54 samples were collected, divided among watersheds with a known AFFF source zone (n = 44) and those without an AFFF source zone (n = 13).
We repeatedly sampled from one location within an AFFF impacted river (Quashnet R.) to assess temporal variability in PFAS concentrations (Aug-17, Jul-18, Oct-18, Feb-19, Apr-19, Jul-19). Data from August 2017 are reported in previous work.30 The Quashnet R. contains groundwater seeps along the flow path and 95% of the riverine flow volume is accounted for by groundwater inputs.30 The Supporting Information (SI) provides additional details of sampling (Table S1) and collection protocols.
PFAS extraction and analysis.
Unfiltered surface water samples (200 mL) were extracted following the methods described in prior work17 and the SI. Samples were analyzed for 27 PFAS with an Agilent (Santa Clara, CA) 6460 triple quadrupole liquid chromatography-tandem mass spectrometer (LC-MS/MS) (Table S2). Targeted analytes included C3–C13 perfluoroalkyl carboxylates (PFCA), C4–C10 perfluoroalkyl sulfonates (PFSA), Cn (n = 4,6,8) perfluoroalkyl sulfonamides, C8 perfluoroalkyl sulfonamide acetates, Cn:2 (n = 4,6,8) fluorotelomer sulfonates, and a polyfluoroalkyl ether carboxylate (DONA) with 5 perfluorinated carbons (Table S3). Method detection limits (MDLs; Table S4) ranged from 0.005 to 2.62 ng L−1. Concentrations are reported in Table S5. Analyte recoveries for targeted analysis ranged from 70% to 130% and are reported in Table S6.
Bayesian inference on TOP assay results.
The total oxidizable precursor (TOP) assay was performed on all aqueous samples. We followed a slightly modified procedure from Houtz and Sedlak18 that is described in a previous publication17 and the SI. Changes in C3–C7 (C3 = PFBA, C7 = PFOA) following the TOP assay are reported in Table S7.
Precursors concentrations grouped by chain length and manufacturing origin (Table S8) are based on TOP assay measurements followed by Bayesian inference (see the SI for details). This inference method is described in Ruyle et al.10 for AFFF stocks. The distributions of C4–C8 ECF and Cn:2 (n = 4,6,8) FT precursors were inferred by multiplying the unique oxidation yields of ECF and n:2 FT precursors and their respective uncertainties18–20 by an iterative simulation sequence of best estimates using Markov chain Monte Carlo (MCMC) analysis implemented by emcee 3.0.231 in Python 3.7.8. The solution minimizes the least-squares of the log difference between the model and measurements of the TOP assay, and the medians are reported here (Table S8). The source code for the Bayesian inference is available for download and use at https://github.com/SunderlandLab/oxidizable-pfas-precursor-inference.
Here we apply the method for the first time to environmental samples with slight modifications. We relaxed the assumptions related to prior probability distributions for precursors in AFFF before performing the inference on samples from watersheds with an AFFF source zone to account for potential biogeochemical changes in precursor composition (Table S9).18 Since the focus of this work was on identifying the signature of AFFF in natural waters, we did not include modifications to the TOP assay to detect <C3 PFCA20 or changes in PFCA > C7 because prior work shows their detection is not needed to complete the PFAS mass balance in ECF or FT AFFF.10 For watersheds that did not contain a known AFFF source, we used a uninformative prior32 because of the lack of quantitative information on precursors in these environments. In an uninformative prior, all precursor concentrations have equal weighting and no information is assumed.
Extractable organofluorine analysis (EOF).
We analyzed extractable organofluorine (EOF) in a subset of samples from the Quashnet watershed with a known AFFF source (n = 7) (Table S10). Extractions followed the method used in prior work10 and extraction volumes ranged between 240–400 mL. Concentrations above the LOD were corrected by subtracting the extraction blank and adjusted by the dilution factor. The MDL (1.95 to 3.26 nM F) was calculated as three times the standard deviation of the extraction blank adjusted by the dilution factor. Additional details are provided in the SI.
Statistical analyses.
13 of the 27 targeted PFAA (C3–C8 PFCA, C4–C8 PFSA), C4–C8 ECF precursors, and n:2 FT (n = 4,6,8) precursors were detected in >70% of samples analyzed (Table 1) and were used in all statistical analyses. A 70% detection frequency was selected because values below the MDL must be imputed (non-zero composition for all measurements is a requirement of the compositional analyses described below) and prior work suggests that 30% censoring is an upper threshold for multivariate analyses such as principal components analysis (PCA) performed here.33,34 Detection frequency and concentration ranges for the 14 targeted PFAS below the MDL in more than 30% of the samples analyzed are reported in Table S11.
Table 1.
Summary statistics for PFAS measured in this study.1
| PFAS | Detection Frequency2 [%] |
Mean pM concentration (±SD) [ng L−1]3 | Mean composition (±SD) [%] | ||||
|---|---|---|---|---|---|---|---|
| AFFF watershed (n = 41) |
non-AFFF watershed (n = 13) |
Mann-Whitney U Test4 [U-value] |
AFFF watershed (n = 41) |
non-AFFF watershed (n = 13) |
Mann-Whitney U Test4 [U-value] |
||
| Σ 13 PFAA 5 | 100 | 600±273 [236] | 35.0±6.0 [11] | *** [0] | 80±5 | 74±16 | [217] |
| PFBA | 83 | 25.8±14.3 [5] | 6.05±1.99 [1] | *** [10] | 4±2 | 13±5 | *** [21] |
| PFPeA | 91 | 61.6±40.8 [16] | 4.60±1.37 [1] | *** [0] | 8±4 | 10±3 | * [149] |
| PFHxA | 98 | 67.6±41.7 [21] | 4.84±2.63 [2] | *** [0] | 9±2 | 10±5 | [254] |
| PFHpA | 87 | 28.6±15.2 [10] | 2.21±0.75 [1] | *** [0] | 4±1 | 5±1 | [177] |
| PFOA | 94 | 40.7±16.6 [17] | 2.95±1.28 [1] | *** [1] | 6±1 | 6±3 | [228] |
| PFNA | 83 | 28.4±22.1 [13] | 0.78±0.39 [0.4] | *** [0] | 4±3 | 2±1 | ** [126] |
| PFBS | 100 | 12.8±3.27 [4] | 6.06±1.69 [2] | *** [20] | 2±1 | 13±3 | *** [2] |
| PFPeS | 81 | 12.3±5.3 [4] | 1.57±0.67 [1] | *** [3] | 2±0 | 4±2 | ** [119] |
| l-PFHxS | 100 | 131±99 [52] | 1.96±0.65 [1] | *** [0] | 17±5 | 4±1 | *** [4] |
| br-PFHxS | 80 | 21.5±15.9 [9] | 2.03±0.96 [1] | *** [7] | 3±1 | 4±2 | * [169] |
| PFHpS | 80 | 4.83±2.83 [2] | 0.47±0.22 [0.2] | *** [6] | 1±0 | 1±1 | [204] |
| l-PFOS | 98 | 117±71 [58] | 0.71±0.21 [0.4] | *** [0] | 15±5 | 2±1 | *** [0] |
| br-PFOS | 96 | 47.5±23.2 [24] | 0.74±0.20 [0.4] | *** [0] | 6±2 | 2±1 | *** [2] |
| ΣPrecursors 5 | 100 | 157±113 | 14.5±11.7 | *** [8] | 20±5 | 26±16 | [217] |
| 4:2 FT | 100 | 5.77±3.01 | 3.50±4.40 | *** [95] | 1±1 | 6±6 | *** [22] |
| 6:2 FT | 100 | 15.6±34.8 | 1.02±1.55 | *** [24] | 2±4 | 2±3 | [266] |
| 8:2 FT | 100 | 8.7±24.4 | 0.75±1.56 | *** [73] | 1±2 | 2±3 | [210] |
| C4 ECF | 100 | 23.1±16.4 | 4.54±4.78 | *** [36] | 3±1 | 8±7 | ** [128] |
| C5 ECF | 100 | 12.6±9.2 | 0.72±0.60 | *** [4] | 2±0 | 1±1 | * [166] |
| C6 ECF | 100 | 86.8±63.6 | 1.35±2.05 | *** [0] | 11±3 | 2±3 | *** [32] |
| C7 ECF | 100 | 1.55±1.01 | 0.70±0.92 | *** [107] | 0±0 | 1±1 | *** [38] |
| C8 ECF | 100 | 2.43±1.76 | 1.88±3.23 | ** [130] | 0±0 | 3±5 | [260] |
Data reported are for PFAS detected in greater than 70% of samples.
Across all samples collected in this study.
Mean concentrations for targeted PFAA in ng L−1 are presented in square brackets.
Levels of statistical significance:
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001
PFAA concentrations were quantitated from targeted analysis while precursor concentrations were inferred from the TOP assay.
For the 13 PFAA with > 70% detection in this study (C3–C8 PFCA, C4–C8 PFSA), we imputed values that were below the MDL using robust regression on order statistics (ROS).34 Targeted precursors, which were detected in <50% of samples, were not included in statistical analyses. Instead, precursor concentrations used in statistical analyses were based on results of the Bayesian inference on TOP assay measurements. The targeted precursors in this study oxidize to PFCA measured in the TOP assay and thus contribute to precursor concentrations inferred through Bayesian inference. We performed all statistical analyses in R version 3.4.4 using FactoMineR,35 factoextra,36 and NADA (ros)37 and python version 3.7.8 using SciPy38 and statsmodels.39
We used non-parametric tests throughout this work because the dataset failed tests for normality required for parametric analyses (Shapiro-Wilks test corrected for multiple comparison using false discovery rate [FDR] correction p-value < 0.05). We tested for statistically significant differences in molar concentrations and composition of targeted PFAS from watersheds with and without AFFF sources using the non-parametric Mann-Whitney U test corrected for multiple comparisons using FDR correction (Table 1). We tested for statistically significant temporal differences in PFAS and EOF at the mouth of the Quashnet River using a non-parametric analysis of variance (Kruskal-Wallis corrected for multiple comparisons using FDR correction, Table S12).
Following the approach laid out in prior work,24 we examined clustering patterns for PFAS measured in this work using both principal component analysis (PCA) on surface water samples (n = 54; Table S13) and non-parametric hierarchical clustering using the UPGMA algorithm (Figure S1). We analyzed differences in PFAS composition rather than concentrations to facilitate comparison of PFAS concentrations spanning different orders of magnitude. We tested several data processing methods to see if PCA results were sensitive to the choice of non-detect imputation and transformation. Results were robust across data treatment methods (Figure S2). We performed a second PCA comparing the composition of surface water from watersheds with AFFF sources (n = 41) and ECF (n = 9) and FT (n = 19) AFFF reported in Houtz et al.8 and Ruyle et al.10 (Table S14). Results from the PCA on center-log ratio transformed40 molar compositional data with non-detects imputed using ROS are presented here. The SI contains additional details of the statistical methods and their assumptions.
Results and Discussion
Significant enrichment of PFAS in AFFF impacted watersheds
The average concentration of the 13 targeted PFAS with >70% detection in watersheds with a known AFFF source (600±270 pM) was 17 times larger than those without an AFFF source (35±6 pM). Total concentrations of oxidizable precursors in watersheds with a known AFFF source (160±110 pM) were more than ten times greater than in watersheds without a known AFFF source (15±12 pM). All of the targeted PFAS and oxidizable precursors were statistically significantly enriched in the watersheds with a known AFFF source compared to the other watersheds (Table 1).
All three watersheds with a known AFFF source exceeded the U.S. Environmental Protection Agency (U.S. EPA) Lifetime Health Advisory of 70 ng L−1 for the sum of PFOS and PFOA in drinking water41 and the Massachusetts Department of Environmental Protection (MA DEP) Maximum Contaminant Level of 20 ng L−1 for the sum of six PFAS: PFHpA, PFOA, PFNA, PFDA, PFHxS, and PFOS42 (Table S5). PFOS (max = 533 ng L−1) and PFHxS (max = 576 ng L−1) were detected at the highest levels in the AFFF impacted watersheds (Table 1), consistent with the known abundance of these compounds in legacy ECF AFFF.8,10 PFAS concentrations were below regulatory guidelines in watersheds without a known AFFF source. The most abundant PFAS at these sites, PFBS (max = 9.25 ng L−1) and PFBA (max = 9.41 ng L−1), are not currently subject to regulatory guidelines in the United States, which have largely focused on long chained compounds.
Mean PFAS concentrations measured in the Quashnet R. over a two-year period between August-2017 and July-2019 on six sampling dates did not show statistically significant differences (Table S12). In contrast, the log10 of the flowrate43 fluctuated by more than two standard deviations across sampling periods (Figure S3). Large fluctuations in redox conditions of these watersheds occur seasonally when temperatures increase and biological activity reaches peak levels.44 Concentrations of redox sensitive contaminants such as nitrate show a strong seasonal cycle.44,45 No temporal variability in the observed PFAS concentrations suggests that releases from the source zone rather than geochemical and hydrologic variability dominate observed concentrations in this system.
PFAS composition differed between watersheds with and without AFFF sources. In watersheds without an AFFF source, PFBS and PFBA accounted for a statistically significantly greater molar fraction (26±8%) of PFAS compared to the watersheds with AFFF sources (6±3%) (Table 1). In watersheds with an AFFF source, PFOS and PFHxS together accounted for 40±13% of PFAS and were statistically significantly enriched compared to watersheds without an AFFF source (Table 1). The linear PFOS isomer accounted for 67±10% of total PFOS in watersheds with AFFF sources, closely reflecting the original PFOS composition in ECF products such as LightWater™ AFFF.10 In watersheds without an AFFF source, the linear isomer of PFOS was statistically significantly lower (Mann-Whitney U; p-value<0.001) than the ratio observed in AFFF-contaminated watersheds (50±9% PFOS), consistent with observations in many freshwater systems.46 The linear PFHxS isomer made up 85±2% of total PFHxS in watersheds with AFFF sources, which is similar to reported values for other surface waters (76–93%).46 In watersheds without an AFFF source, linear and branched PFHxS isomers were detected together at only 2/13 sampling sites (77% and 79% linear).
Enrichment of C6 precursors in AFFF impacted watersheds
Seven of the nine targeted polyfluoroalkyl precursors were only detected in watersheds with an AFFF source (Table S11). Such limited detections are common due to the diversity of potential precursors and their transformation intermediates in AFFF7,11,47 and other PFAS-based products.48 Alternatively, the TOP assay can be used to aggregate diverse precursors to overcome methodological detection limits. Oxidizable precursors captured by the TOP assay were detectable at every site (Table S7), including in 11 samples where no targeted precursors were observed (Table S5).
Results of the Bayesian inference on TOP assay results yielded distributions (Figure 2) and median estimates (Table 1, Table S9) of the concentrations of C4–C8 ECF and 4:2, 6:2, and 8:2 FT precursors. Precursor concentrations were statistically significantly enriched in surface waters with AFFF sources (Table 1). The PFAS fraction accounted for by precursors was similar between watersheds with and without an AFFF source (20%−26%), but composition differed significantly (Table 1). In watersheds with a known AFFF source, C6 ECF precursors were most abundant, accounting for 11% of PFAS. In watersheds without a known AFFF source, C4 ECF precursors and 4:2 FT precursors were most abundant, accounting for 14% of PFAS (Table 1). C4 ECF and 4:2 FT precursors were statistically significantly enriched in watersheds without a known AFFF source. C6 ECF precursors were statistically significantly enriched in watersheds with a known AFFF source (Table 1).
Fig 2. Inferred concentrations of oxidizable precursors and their perfluorinated chain length using Bayesian inference on results of the TOP assay.
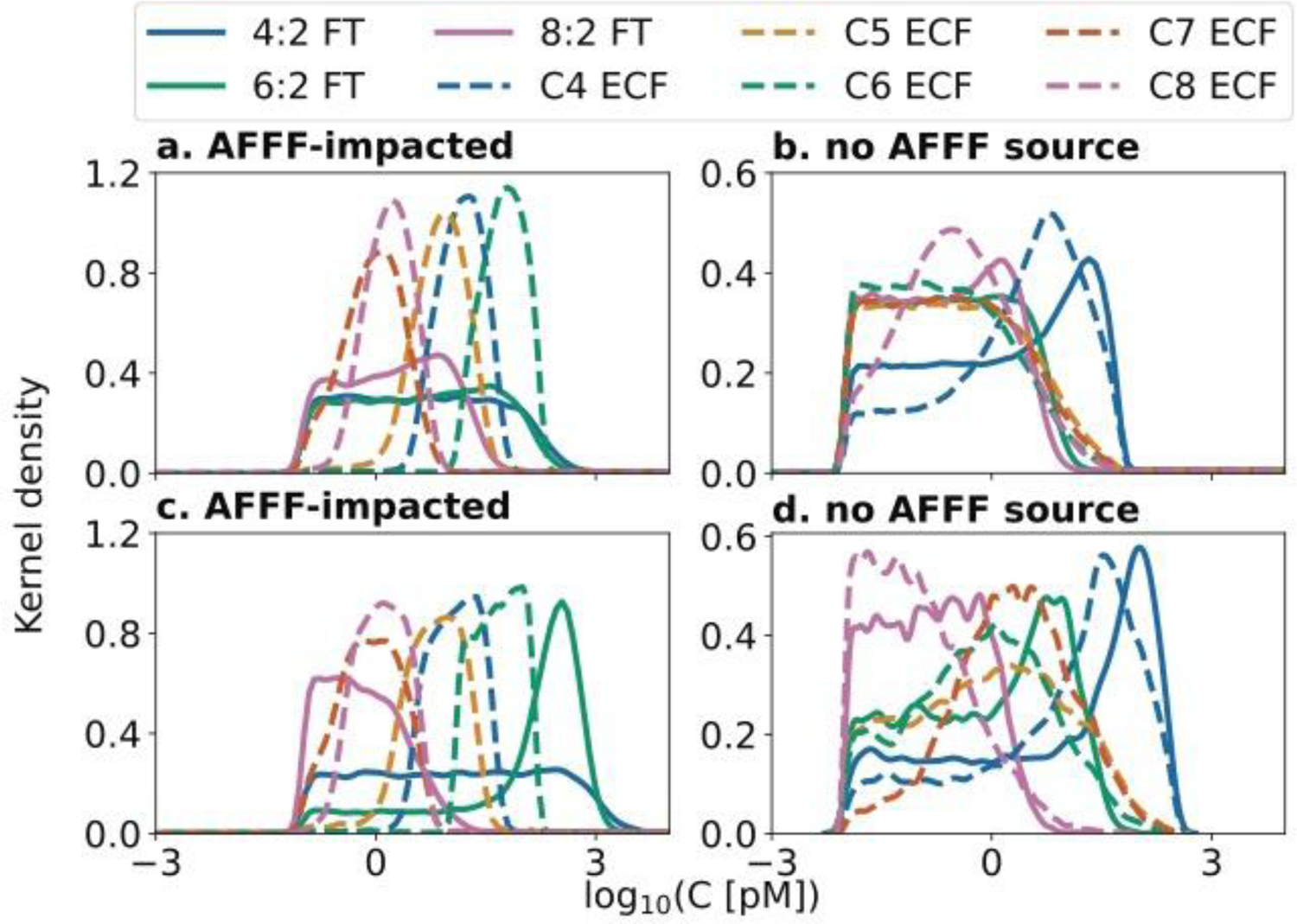
Panels show probability density functions estimated by the non-parametric kernel density of the concentrations of oxidizable precursors. A high kernel density indicates greater probability of the estimate. Precursors are grouped by perfluorinated chain length and manufacturing source. Electrochemical fluorination (ECF) precursors range from 4–8 perfluorinated carbons (C4–C8) while fluorotelomer (FT) precursors have n perfluorinated carbons followed by two aliphatic hydrocarbons (n:2, n=4,6,8).2 Samples downstream of AFFF source zones are shown in panel (a) the Quashnet R., and panel (c) Mill Creek. Samples from background sites are shown in panel (b) the Santuit R., and (d) Marstons Mills R.
Figure 2 shows the modeled distributions of oxidizable precursors across watersheds. The kernel density (y-axis) indicates the probability of occurrence of a given precursor, while the x-axis shows expected concentrations given constraints from terminal PFCA produced following the TOP assay. In the AFFF impacted watershed downstream of the Joint Base Cape Cod (Fig 2a; Quashnet R. sample), C6 ECF precursors occur at the highest probabilities and concentrations. Much lower probabilities (kernel densities) are observed for the FT precursors. By contrast, the kernel density and concentrations of 6:2 FT precursors are much higher in the downgradient environment of a municipal airport fire-training area in Mill Creek. Targeted C6 precursors (perfluorohexane sulfonamide and 6:2 fluorotelomer sulfonate) accounted for up to 66% of oxidizable C6 precursors at these sample locations (Table S9).
ECF and FT AFFF were often used at the same sites. Poor recordkeeping has confounded the true history of AFFF use.2 The Bayesian inference performed on TOP assay results helps to estimate the relative amount of ECF and FT AFFF used at each site. For example, if ECF AFFF (approximately 50% precursors) and FT AFFF (>99% precursors) (Table S14) were used in equal amounts, the percentage of ECF precursors in downstream environments would be approximately 33%, assuming that the biogeochemical influences on precursor composition affects both kinds of precursors at similar rates. ECF precursors accounted for 72±11% of precursors in the Quashnet R. watershed downstream of the military fire training area, suggesting ECF AFFF was used at a greater than 2:1 ratio on the Joint Base Cape Cod. This is consistent with estimates of the US military’s historical AFFF stock which consisted of 70–75% ECF AFFF.49 In Mill Creek, ECF precursors contributed 45±26% of precursors, suggesting that both ECF and FT AFFF were used at that site in similar proportions.
Mixed precursors were present at background sites without a known AFFF source zone (Fig 2b,d). 4:2 FT precursors occurred at the highest concentrations, followed by C4 ECF precursors. At the Santuit site (Fig 2b), 8:2 FT and C8 ECF precursors occurred at higher concentrations compared to the watershed that did not contain a known AFFF source (Marstons Mills, Fig 2d).
Distinct signature of AFFF contamination
Principal component analysis (PCA) using the molar composition of PFAS in all samples reveals distinct PFAS clustering along Dimension 1 (Figure 3) based on the presence or absence of AFFF sources within the watershed. PCA results were corroborated using hierarchical clustering (Figure S1) and were robust to all data preprocessing methods tested (Figure S2).
Figure 3. Scores and loading vectors for the first (Dimension 1) and second (Dimension 2) principal components in surface water and AFFF.
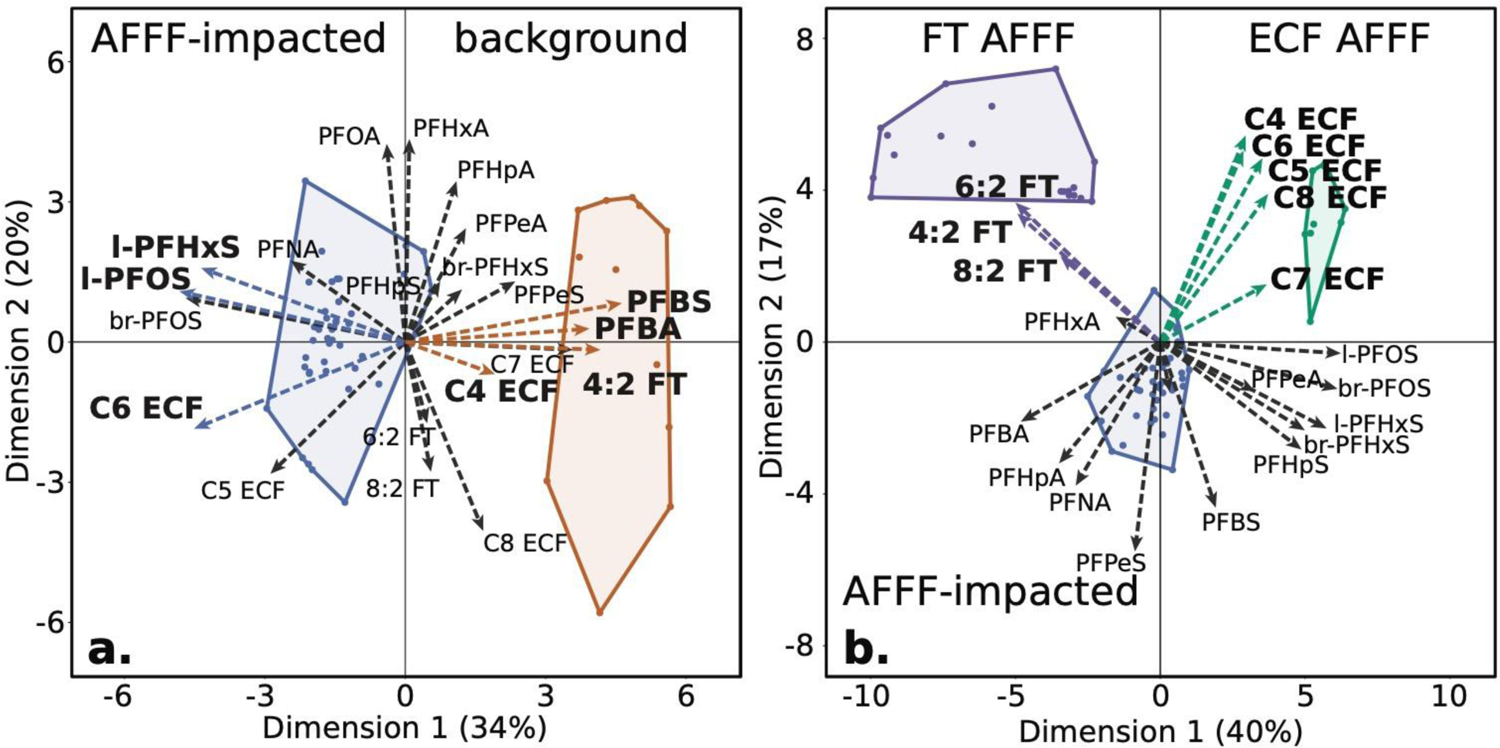
Panel (a): surface water from watersheds with an AFFF source zone (blue, n = 41) and without an AFFF source (orange, n = 13). Panel (b): Literature data on fluorotelomer (FT) AFFF (purple, n = 19, manufactured between 1986–2017), electrochemical fluorination (ECF) AFFF (green, n = 9, manufactured between 1988–2001), and AFFF impacted surface water from this study (blue, n = 41). Circles in panels (a) and (b) represent the minimum convex hull that encircles all data within the group.
Watersheds with a known AFFF source score negatively along Dimension 1 and were enriched in PFOS, PFHxS, and C5 and C6 ECF precursors (Fig 3a). These compounds constitute 82±2% PFAS in ECF AFFF (Table S14). This result reinforces that legacy ECF AFFF use was the dominant source of targeted and oxidizable PFAS in AFFF impacted watersheds in this study, overwhelming potential contributions from diffuse sources that are reflected in the background sites and a wastewater infiltration system.17 The AFFF source signature is preserved in the downstream environment, which agrees with prior work that found associations between the presence of military fire-training areas and increased probability of detecting PFHxS and PFOS in proximate drinking water.3
Surface waters in watersheds without an AFFF source score positively on Dimension 1 and are enriched in short chain PFAA and C4 precursors. Prior work in the same region showed PFBS was the dominant PFAS in private wells contaminated by septic systems.50 It is therefore plausible that the observed enrichment in C4 PFAS in this study in watersheds without a known AFFF source reflects contamination from septic systems that integrate PFAS present in modern consumer products.51
We performed a second PCA to distinguish ECF and FT AFFF from the PFAS composition observed in watersheds with a known AFFF source (Figure 3b). Clusters of legacy ECF AFFF, legacy and contemporary FT AFFF, and impacted surface water are shown in Figure 3b. AFFF products were weighted 0.69 for ECF products and 0.31 for FT products based on the average inferred ratio of AFFF manufacturing origin across all sites in watersheds with an AFFF source. We see clear separation and clustering of PFAS in ECF AFFF, FT AFFF and impacted surface water samples. Dimension 1 of the PCA (40% of variance) separates ECF- and FT-based AFFF. Dimension 2 (17% of variance) distinguishes the commercial AFFF products and impacted surface water samples.
Differences in the PCA (Figure 3b) along Dimension 2 reflect a decline in the relative abundance of all precursors in surface waters from AFFF-impacted watersheds compared to FT AFFF and ECF AFFF. One possibility is that this reflects dilution by other PFAS sources containing more terminal PFAS. However, watersheds without an AFFF source in this region contained a similar proportion of precursor compounds (Table 1), suggesting such a scenario is unlikely.
The decline in C6 ECF precursors in AFFF impacted ecosystems relative to ECF AFFF was not accompanied by a corresponding increase in the terminal PFSA as they were for the C4 and C5 ECF precursors (Figure 3b). PCA results show a chain length dependence between the magnitude of the loading vector for ECF precursors and their terminal PFSA (Fig 3b, Table S13). Along Dimension 2, we observe the greatest loading vector delta between C4 and C5 ECF precursors and their terminal PFSA. For ≥C6 ECF precursors, we see diminished loading vector deltas corresponding to an increase in the terminal PFSA with declines in precursor composition relative to AFFF.
Prior work has shown ECF precursors degrade into terminal PFSA over a variety of timescales.12,52 We hypothesize that the chain length dependent relationship between loading vectors of precursors shown in Figure 3b and their terminal PFSA may reflect greater bioavailability of short chain precursors. This could be due to increased preference for the aqueous phase of shorter chain PFAS, as predicted by perfluorinated chain length-sorption relationships in the literature.53,54 These studies have focused on establishing such relationships for terminal PFAS, thus further laboratory investigations using precursors with different chain lengths would be highly informative.
High concentrations of unexplained extractable organofluorine (EOF)
Concentrations of EOF detected downgradient from the AFFF source zone in the Quashnet R. ranged from below the LOD to 29.0 nM F (Table S10). Figure 4 compares EOF measured at AFFF impacted sites to targeted PFAS concentrations (panel a) and the sum of oxidizable precursors from the TOP assay and targeted PFAAs (panel b). Both metrics fail to account for all the EOF detected in the same samples. Targeted PFAS account for 18–46% of the EOF across samples (Figure 4a). The inclusion of oxidizable precursors captured an additional 15±6% (min=5%, max=20%) of EOF across the samples (Fig 4b, Table S10).
Figure 4. Organofluorine measurements (n = 5) in AFFF-impacted surface water.
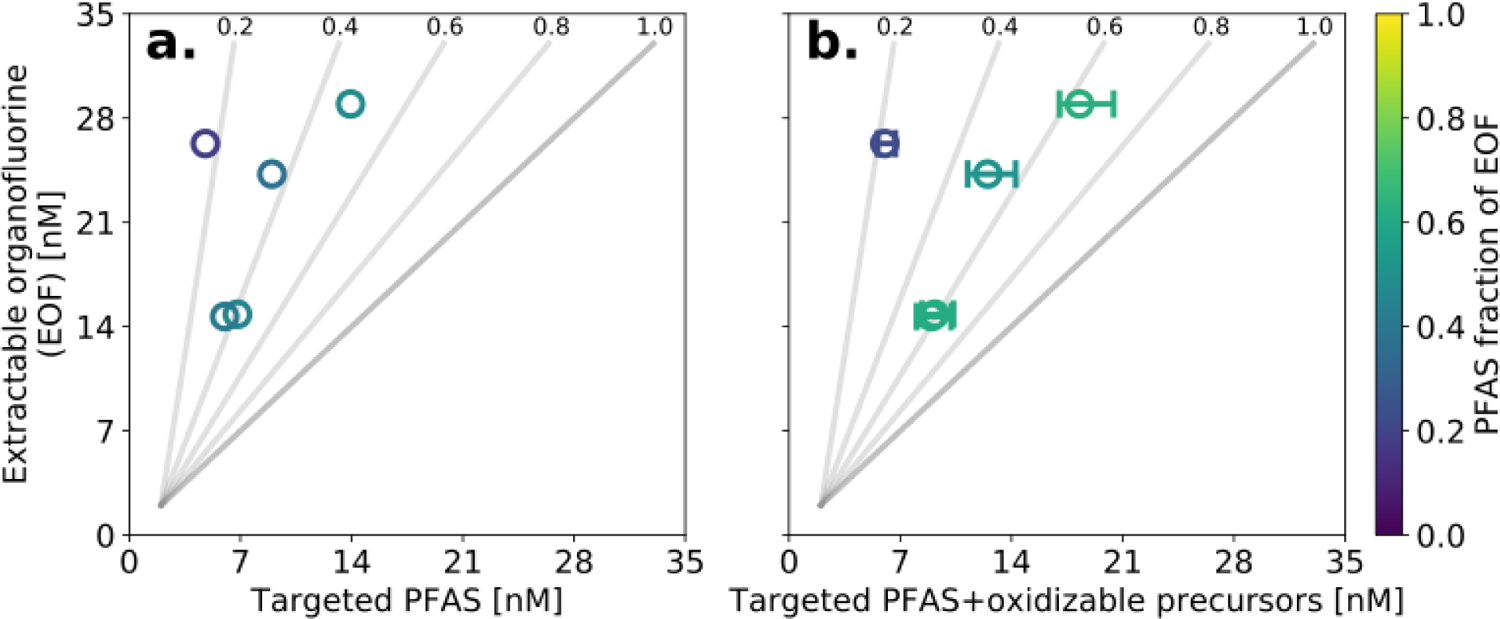
Panel (a) compares the sum of targeted PFAS to EOF in six samples downgradient of an AFFF source zone, including five samples from the Quashnet R. and one sample from Moody Pond. Panel (b) compares the sum of targeted PFAS and oxidizable precursors identified using the TOP assay and EOF in the same samples. Error bars represent the 25% and 75% of inferred unknown oxidizable precursors using Bayesian inference (see methods and SI). The observations are shaded by the fraction of EOF explained by PFAS analysis and are compared to the 1:1 line (gray). Two samples below the LOD for EOF are not shown.
At the sampling site closest to the source zone (Moody Pond), 37% of the EOF was unexplained (Table S10), and near the estuarine mouth of the Quashnet R., 39–76% of EOF was unexplained. Molar concentrations of these unidentified compounds are extremely high since targeted PFAS and oxidizable precursors together account for an average of 8 nM F (Table S10) and individual PFAS exceed water quality guidelines. Because all of the EOF in ECF and FT AFFF source material can be explained by targeted PFAS and oxidizable precursors,10 high concentrations of unexplained EOF in AFFF impacted watersheds likely did not originate directly from AFFF use.
Moody et al.22 similarly observed large fractions of PFAS that could not be explained by PFOS, PFOA, and PFHxS in surface water after an accidental release of ECF AFFF. Assuming that PFOS accounted for 30% to 50% of PFAS, consistent with the composition in ECF AFFF (Table S14), we estimate the fraction of unexplained PFAS in that work between 25±18% and 56±11% (Table S15). This is comparable to our results (39–76%). Together, these results suggest large fractions of unidentified organofluorine compounds from other PFAS sources in some North American surface waters.
Previous work hypothesized that fluorinated agrochemicals and pharmaceuticals, which are not considered PFAS, account for some of the unexplained EOF16.2 Several measurements of these compounds are available from municipal and private wells from the region (equivalent to 0.23 to 0.48 nM F; Table S16; adapted from Schaider et al.50,55). Using these data we estimate that the most commonly prescribed fluorinated pharmaceuticals could at most account for 3% of the unexplained EOF in the Quashnet R. Fluorinated agrochemicals may have been used historically.56 However, most chemicals in the class contain at most one perfluorinated carbon and would have to be present at extremely high concentrations >3±1 nM (approximately six times greater than PFAS) to account for all unexplained EOF. We therefore speculate that abundant private septic systems in the region are the most likely source of unexplained EOF.20,21,51 Septic systems could contain PFAS not on our analyte list (including ultrashort chain PFAS) and/or PFAS not amenable to detection by the TOP assay. The magnitude of this unexplained EOF signature is alarming and warrants additional investigation.
Implications for PFAS inputs from rivers to marine ecosystems
Groundwater fed rivers within the sandy Cape Cod aquifer flow into downstream marine ecosystems and the ocean, which represents the terminal sink for global PFAS discharges. Previous modeling work57–60 estimated that continental discharges of legacy PFAS would be negligible after 2014 due to their phase-out in consumer products and a relatively small lag time (less than one year) between releases to rivers and inputs to the marine environment. Measurements performed here indicate that legacy PFAS in slowly moving groundwater constitute a large source to the downstream coastal environment, representing a substantial lag between environmental PFAS releases and inputs to marine ecosystems. For example, the major use of AFFF at the Joint Base Cape Cod occurred in the 1980s,17 yet legacy PFAS that exceed regulatory advisories are still being observed almost 40 years later near the mouth of the Quashnet R. that flows into a local estuary (Waquoit Bay).
We estimated the flux of PFOS (2.9±0.8 kmol yr−1) to Waquoit Bay from the Quashnet R. based on the average PFAS concentration measured at the most downstream location and freshwater discharges to the estuary (Table S12).61 The measured flux of PFOS to Waquoit Bay reported here is 1.9 orders of magnitude larger than predicted by the population-based PFOS discharge relationship developed in previous work.58 Documented AFFF-use occurred at more than 70 coastal military bases and airports across the United States.5 Our results thus suggest riverine discharges in these watersheds may be a substantial and ongoing source of legacy PFAS to the marine environment.
For Waquoit Bay, the reported fluxes may represent a lower bound for PFAS loading because submarine groundwater discharge accounts for between 2% and 17% of freshwater inputs to the estuary.62,63 Submarine groundwater discharge to estuaries is known to represent a substantial source of nutrients to the marine environment,64,65 and similarly may result in delayed transport of legacy PFAS. Such a process could explain the prevalence of legacy PFAS in seawater along the northwestern Atlantic coast and shelf obtained in 2014 and 2016.66
Previous studies have assumed that precursors mainly enter the ocean through wet deposition following atmospheric oxidation of volatile precursors.57,67,68 The measured precursor flux from the Quashnet R. to the downstream estuary (Waquoit Bay) in this study (Table S12) demonstrates direct riverine discharge is also an important transport pathway to the marine environment. The fate of these compounds in marine ecosystems is not well understood and warrants further investigation. For example, Zhang et al.66 suggested precursors to C5 and C6 PFCA may preferentially accumulate in marine food webs relative to legacy PFAS.
Quantitative detection methods developed for LC-MS/MS (targeted analysis and TOP) fail to explain substantial fractions of EOF in surface water downstream of AFFF sources. Unexplained EOF indicates the presence of additional, large non-AFFF sources,10 which may lead to biological exposures. In a study of marine mammals from Nantucket Sound and Massachusetts Bay, Spaan et al.69 found unexplained EOF accounted for 30–75% of the organofluorine in their livers.
High levels of unexplained EOF discharged to coastal ecosystems are not accounted for in previously published estimates of continental PFAS discharges to the ocean.57,58,60,70 If a similar ratio of PFOS to EOF (approximately 10%, Table S10) was observed across all sources, cumulative continental discharges of PFAS to the ocean could be 2,850–50,800 Mg. This estimate is likely conservative because AFFF-impacted surface water is relatively enriched in PFOS compared to diffuse sources (Table 1). Such discharges could be greater than cumulative estimated global emissions of PFCA, PFOS, and PFOS precursors,59,60 indicating large potential releases of previously unidentified PFAS to the marine environment that may enhance PFAS exposures of aquatic life and seafood consumers.
Supplementary Material
Acknowledgements
We acknowledge financial support for this work from the National Institute for Environmental Health Sciences (NIEHS) Superfund Research Program (P42ES027706), the Strategic Environmental Research and Development Program (SERDP ER18-1280), and the U.S. Geological Survey (USGS) Toxic Substance Hydrology Program. We thank Jeffrey Barbaro, Curtis Yaeger, and Gordon McQuaid (USGS) for assistance in the field.
Footnotes
The authors declare no competing financial interest.
Supporting Information. Supplementary site information, Sampling protocol, PFAS extraction and analysis, Extractable organofluorine analysis, Bayesian inference method, Supplementary statistical methods, Composition of AFFF in the principal component analysis, PFAS fluxes from the Quashnet River to Waquoit Bay.
References
- (1).OECD. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs); Series on Risk Management; 39; OECD: Paris, 2018; p 24. [Google Scholar]
- (2).The Interstate Technology & Regulatory Council (ITRC); Per- and Polyfluoroalkyl Substances (PFAS) Team. PFAS Technical and Regulatory Guidance Document and Fact Sheets; PFAS-1; The Interstate Technology & Regulatory Council: Washington, D.C, 2020. [Google Scholar]
- (3).Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett 2016, 3 (10), 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Andrews DQ; Naidenko OV Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environ. Sci. Technol. Lett 2020, 7, 931–936. 10.1021/acs.estlett.0c00713. [DOI] [Google Scholar]
- (5).Northeastern University Social Science Environmental Health Research Institute (SSEHRI). PFAS Contamination Site Tracker https://pfasproject.com/pfas-contamination-site-tracker/ (accessed Dec 16, 2019).
- (6).Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol 2019, 29 (2), 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Weiner B; Yeung LWY; Marchington EB; D’Agostino LA; Mabury SA Organic Fluorine Content in Aqueous Film Forming Foams (AFFFs) and Biodegradation of the Foam Component 6 : 2 Fluorotelomermercaptoalkylamido Sulfonate (6 : 2 FTSAS). Environ. Chem 2013, 10 (6), 486. 10.1071/EN13128. [DOI] [Google Scholar]
- (8).Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ. Sci. Technol 2013, 47 (15), 8187–8195. 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- (9).Dubocq F; Wang T; Yeung LWY; Sjöberg V; Kärrman A Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol 2019, 54, 245–254. 10.1021/acs.est.9b05440. [DOI] [PubMed] [Google Scholar]
- (10).Ruyle BJ; Thackray CP; McCord JP; Strynar MJ; Mauge-Lewis KA; Fenton SE; Sunderland EM Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett 2021, 8 (1), 59–65. 10.1021/acs.estlett.0c00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Harding-Marjanovic KC; Houtz EF; Yi S; Field JA; Sedlak DL; Alvarez-Cohen L Aerobic Biotransformation of Fluorotelomer Thioether Amido Sulfonate (Lodyne) in AFFF-Amended Microcosms. Environ. Sci. Technol 2015, 49 (13), 7666–7674. 10.1021/acs.est.5b01219. [DOI] [PubMed] [Google Scholar]
- (12).Mejia-Avendaño S; Vo Duy S; Sauvé S; Liu J Generation of Perfluoroalkyl Acids from Aerobic Biotransformation of Quaternary Ammonium Polyfluoroalkyl Surfactants. Environ. Sci. Technol 2016, 50 (18), 9923–9932. 10.1021/acs.est.6b00140. [DOI] [PubMed] [Google Scholar]
- (13).McGuire ME; Schaefer C; Richards T; Backe WJ; Field JA; Houtz E; Sedlak DL; Guelfo JL; Wunsch A; Higgins CP Evidence of Remediation-Induced Alteration of Subsurface Poly- and Perfluoroalkyl Substance Distribution at a Former Firefighter Training Area. Environ. Sci. Technol 2014, 48 (12), 6644–6652. 10.1021/es5006187. [DOI] [PubMed] [Google Scholar]
- (14).Moe MK; Huber S; Svenson J; Hagenaars A; Pabon M; Trümper M; Berger U; Knapen D; Herzke D The Structure of the Fire Fighting Foam Surfactant Forafac®1157 and Its Biological and Photolytic Transformation Products. Chemosphere 2012, 89 (7), 869–875. 10.1016/j.chemosphere.2012.05.012. [DOI] [PubMed] [Google Scholar]
- (15).Wang Z; DeWitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol 2017, 51 (5), 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- (16).Koch A; Kärrman A; Yeung LWY; Jonsson M; Ahrens L; Wang T Point Source Characterization of Per- and Polyfluoroalkyl Substances (PFASs) and Extractable Organofluorine (EOF) in Freshwater and Aquatic Invertebrates. Environ. Sci. Process. Impacts 2019, 21 (11), 1887–1898. 10.1039/C9EM00281B. [DOI] [PubMed] [Google Scholar]
- (17).Weber AK; Barber LB; LeBlanc DR; Sunderland EM; Vecitis CD Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol 2017, 51 (8), 4269–4279. 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- (18).Houtz EF; Sedlak DL Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol 2012, 46 (17), 9342–9349. 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- (19).Martin D; Munoz G; Mejia-Avendaño S; Duy SV; Yao Y; Volchek K; Brown CE; Liu J; Sauvé S Zwitterionic, Cationic, and Anionic Perfluoroalkyl and Polyfluoroalkyl Substances Integrated into Total Oxidizable Precursor Assay of Contaminated Groundwater. Talanta 2019, 195, 533–542. 10.1016/j.talanta.2018.11.093. [DOI] [PubMed] [Google Scholar]
- (20).Janda J; Nödler K; Scheurer M; Happel O; Nürenberg G; Zwiener C; Lange FT Closing the Gap – Inclusion of Ultrashort-Chain Perfluoroalkyl Carboxylic Acids in the Total Oxidizable Precursor (TOP) Assay Protocol. Environ. Sci. Process. Impacts 2019, 21 (11), 1926–1935. 10.1039/C9EM00169G. [DOI] [PubMed] [Google Scholar]
- (21).Zhang C; Hopkins ZR; McCord J; Strynar MJ; Knappe DRU Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett 2019, 6 (11), 662–668. 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Moody CA; Kwan WC; Martin JW; Muir DCG; Mabury SA Determination of Perfluorinated Surfactants in Surface Water Samples by Two Independent Analytical Techniques: Liquid Chromatography/Tandem Mass Spectrometry and 19F NMR. Anal. Chem 2001, 73 (10), 2200–2206. 10.1021/ac0100648. [DOI] [PubMed] [Google Scholar]
- (23).D’Agostino LA; Mabury SA Certain Perfluoroalkyl and Polyfluoroalkyl Substances Associated with Aqueous Film Forming Foam Are Widespread in Canadian Surface Waters. Environ. Sci. Technol 2017, 51 (23), 13603–13613. 10.1021/acs.est.7b03994. [DOI] [PubMed] [Google Scholar]
- (24).Zhang X; Lohmann R; Dassuncao C; Hu XC; Weber AK; Vecitis CD; Sunderland EM Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett 2016, 3 (9), 316–321. 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Masterson JP; Hess KM Ground-Water Recharge Areas and Travel Times to Pumped Wells, Ponds, Streams, and Coastal Water Bodies, Cape Cod, Massachusetts; Scientific Investigations Map I-2857; U.S. Geological Survey: 2004. [Google Scholar]
- (26).Carlson CS; Masterson JP; Walter DA; Barbaro JR Development of Simulated Groundwater-Contributing Areas to Selected Streams, Ponds, Coastal Water Bodies, and Production Wells in the Plymouth-Carver Region and Cape Cod, Massachusetts; Data Series; Data Series 1074; U.S. Geological Survey: Reston, Virginia, 2017. [Google Scholar]
- (27).Final Supplemental Remedial Investigation Data Gap Work Plan for 1,4-Dioxane and Perfluorinated Compounds at Ashumet Valley, Joint Base Cape Cod, MA; 658003-EC-AV-QAPP-005; Prepared by CH2M HILL for AFCEC/MMR, Installation Restoration Program, Otis Air National Guard Base, MA: Otis Air National Guard Base, MA, 2018. [Google Scholar]
- (28).Alger M; Thibault R Phase I Initial Site Investigation & Tier I Classification Submittal: Barnstable County Fire and Rescue Training Academy; 4–26179; Nover-Armstrong Associates, Inc.: Barnstable, MA, 2018. [Google Scholar]
- (29).U.S. Department of Transportation Federal Aviation Administration. Programs for Training of Aircraft Rescue and Firefighting Personnel; 2015; Vol. AC No: 150/5210–17C.
- (30).Briggs MA; Tokranov AK; Hull RB; LeBlanc DR; Haynes AB; Lane JW Hillslope Groundwater Discharges Provide Localized Stream Ecosystem Buffers from Regional Per- and Polyfluoroalkyl Substances Contamination. Hydrol. Process 2020, 34 (10), 2281–2291. 10.1002/hyp.13752. [DOI] [Google Scholar]
- (31).Foreman-Mackey D; Hogg DW; Lang D; Goodman J Emcee: The MCMC Hammer. ArXiv12023665 Astro-Ph Physicsphysics Stat 2013. 10.1086/670067. [DOI] [Google Scholar]
- (32).Gelman A; Carlin JB; Stern HS; Rubin DB Bayesian Data Analysis, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, 2004. [Google Scholar]
- (33).Farnham I; Singh A; Stetzenbach K; Johannesson K Treatment of Nondetects in Multivariate Analysis of Groundwater Geochemistry Data. Chemom. Intell. Labortory Syst. 2002, 60 (1–2), 265–281. [Google Scholar]
- (34).Helsel DR Statistics for Censored Environmental Data Using Minitab and R, Second.; Wiley: Denver, CO, 2012. [Google Scholar]
- (35).Husson F; Josse J; Le S; Mazet J FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. R package version 2.3. https://cran.r-project.org/web/packages/FactoMineR/index.html (accessed Oct 27, 2020). [Google Scholar]
- (36).Kassambara A; Mundt F factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. https://cran.r-project.org/web/packages/factoextra/index.html (accessed Oct 27, 2020). [Google Scholar]
- (37).Lee L NADA: Nondetects and Data Analysis for Environmental Data; 2020. R package version 1.6–1.1. https://cran.r-project.org/web/packages/NADA/index.html (accessed Oct 27, 2020). [Google Scholar]
- (38).SciPy 1.0 Contributors; Virtanen P; Gommers R; Oliphant TE; Haberland M; Reddy T; Cournapeau D; Burovski E; Peterson P; Weckesser W; Bright J; van der Walt SJ; Brett M; Wilson J; Millman KJ; Mayorov N; Nelson ARJ; Jones E; Kern R; Larson E; Carey CJ; Polat İ; Feng Y; Moore EW; VanderPlas J; Laxalde D; Perktold J; Cimrman R; Henriksen I; Quintero EA; Harris CR; Archibald AM; Ribeiro AH; Pedregosa F; van Mulbregt P SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Seabold S; Perktold J Statsmodels: Econometric and Statistical Modeling with Python; Austin, Texas, 2010; pp 92–96. 10.25080/Majora-92bf1922-011. [DOI] [Google Scholar]
- (40).Aitchison J The Statistical Analysis of Compositional Data; The Blackburn Press: Caldwell, New Jersey, 1986. [Google Scholar]
- (41).U.S. EPA. Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate; 2016; Vol. FRL-9946–91-OW, pp 33250–33251. [Google Scholar]
- (42).Massachusetts Department of Environmental Protection. Per- and Polyfluoroalkyl Substances (PFAS) Monitoring and Analytical Requirements; 2019; Vol. 22.07G. [Google Scholar]
- (43).Blum AG; Archfield SA; Vogel RM On the Probability Distribution of Daily Streamflow in the United States. Hydrol. Earth Syst. Sci 2017, 21 (6), 3093–3103. 10.5194/hess-21-3093-2017. [DOI] [Google Scholar]
- (44).Woodwell Climate Research Center. Cape Cod Rivers Observatory Data http://www.caperivers.org/data/ (accessed Jun 17, 2019).
- (45).Colman JA; LeBlank DR; Böhlke JK; McCobb TD; Kroeger KD; Belaval M; Cambareri TC; Pirolli GF; Brooks TW; Garren ME; Stover TB; Keeley A Geochemical Conditions and Nitrogen Transport in Nearshore Groundwater and the Subterranean Estuary at a Cape Cod Embayment, East Falmouth, Massachusetts, 2013–14; Scientific Investigations Report; Scientific Investigations Report 5095; U.S. Geological Survey: Reston, Virginia, 2018. [Google Scholar]
- (46).Schulz K; Silva MR; Klaper R Distribution and Effects of Branched versus Linear Isomers of PFOA, PFOS, and PFHxS: A Review of Recent Literature. Sci. Total Environ 2020, 733, 139186. 10.1016/j.scitotenv.2020.139186. [DOI] [PubMed] [Google Scholar]
- (47).Barzen-Hanson KA; Roberts SC; Choyke S; Oetjen K; McAlees A; Riddell N; McCrindle R; Ferguson PL; Higgins CP; Field JA Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol 2017, 51 (4), 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- (48).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag 2011, 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Darwin R Estimated Quantities of Aqueous Film Forming Foam (AFFF) in the United States; Stockholm Convention: Baltimore, MD, 2004. [Google Scholar]
- (50).Schaider LA; Ackerman JM; Rudel RA Septic Systems as Sources of Organic Wastewater Compounds in Domestic Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ 2016, 547, 470–481. 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- (51).Wang Z; Cousins IT; Scheringer M; Hungerbühler K Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environ. Int 2013, 60, 242–248. 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- (52).Plumlee MH; McNeill K; Reinhard M Indirect Photolysis of Perfluorochemicals: Hydroxyl Radical-Initiated Oxidation of N -Ethyl Perfluorooctane Sulfonamido Acetate (N -EtFOSAA) and Other Perfluoroalkanesulfonamides. Environ. Sci. Technol 2009, 43 (10), 3662–3668. 10.1021/es803411w. [DOI] [PubMed] [Google Scholar]
- (53).Higgins CP; Luthy RG Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol 2006, 40 (23), 7251–7256. 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
- (54).Guelfo JL; Higgins CP Subsurface Transport Potential of Perfluoroalkyl Acids at Aqueous Film-Forming Foam (AFFF)-Impacted Sites. Environ. Sci. Technol 2013, 47 (9), 4164–4171. 10.1021/es3048043. [DOI] [PubMed] [Google Scholar]
- (55).Schaider LA; Rudel RA; Ackerman JM; Dunagan SC; Brody JG Pharmaceuticals, Perfluorosurfactants, and Other Organic Wastewater Compounds in Public Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ 2014, 468–469, 384–393. 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- (56).Sandler H Weed Management 2005; University of Massachusetts Amherst Cranberry Station: East Wareham, MA, 2005. [Google Scholar]
- (57).Armitage JM; Schenker U; Scheringer M; Martin JW; MacLeod M; Cousins IT Modeling the Global Fate and Transport of Perfluorooctane Sulfonate (PFOS) and Precursor Compounds in Relation to Temporal Trends in Wildlife Exposure. Environ. Sci. Technol 2009, 43 (24), 9274–9280. 10.1021/es901448p. [DOI] [PubMed] [Google Scholar]
- (58).Zhang X; Zhang Y; Dassuncao C; Lohmann R; Sunderland EM North Atlantic Deep Water Formation Inhibits High Arctic Contamination by Continental Perfluorooctane Sulfonate Discharges. Glob. Biogeochem. Cycles 2017, 31 (8), 1332–1343. 10.1002/2017GB005624. [DOI] [Google Scholar]
- (59).Wang Z; Cousins IT; Scheringer M; Buck RC; Hungerbühler K Global Emission Inventories for C4–C14 Perfluoroalkyl Carboxylic Acid (PFCA) Homologues from 1951 to 2030, Part I: Production and Emissions from Quantifiable Sources. Environ. Int 2014, 70, 62–75. 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- (60).Wang Z; Boucher JM; Scheringer M; Cousins IT; Hungerbühler K Toward a Comprehensive Global Emission Inventory of C4 –C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C8 -Based Products and Ongoing Industrial Transition. Environ. Sci. Technol 2017, 51 (8), 4482–4493. 10.1021/acs.est.6b06191. [DOI] [PubMed] [Google Scholar]
- (61).U.S. Geological Survey. USGS Current Conditions for USGS 011058837 Quashnet River at Waquoit Village, MA http://waterdata.usgs.gov/nwis/ (accessed Jan 20, 2020).
- (62).Cambareri TC; Eichner EM Watershed Delineation and Ground Water Discharge to a Coastal Embayment. Ground Water 1998, 36 (4), 626–634. [Google Scholar]
- (63).Michael HA; Lubetsky JS; Harvey CF Characterizing Submarine Groundwater Discharge: A Seepage Meter Study in Waquoit Bay, Massachusetts. Geophys. Res. Lett 2003, 30 (6). 10.1029/2002GL016000. [DOI] [Google Scholar]
- (64).Johannes RE The Ecological Significance of the Submarine Discharge of Groundwater. Mar. Ecol. Prog. Ser 1980, 3, 365–373. [Google Scholar]
- (65).Moore WS Large Groundwater Inputs to Coastal Waters Revealed by 226Ra Enrichments. Nature 1996, 380, 612–614. [Google Scholar]
- (66).Zhang X; Lohmann R; Sunderland EM Poly- and Perfluoroalkyl Substances in Seawater and Plankton from the Northwestern Atlantic Margin. Environ. Sci. Technol 2019, 53 (21), 12348–12356. 10.1021/acs.est.9b03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Wania F A Global Mass Balance Analysis of the Source of Perfluorocarboxylic Acids in the Arctic Ocean. Environ. Sci. Technol 2007, 41 (13), 4529–4535. 10.1021/es070124c. [DOI] [PubMed] [Google Scholar]
- (68).Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol 2006, 40 (1), 32–44. 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- (69).Spaan KM; van Noordenburg C; Plassmann MM; Schultes L; Shaw S; Berger M; Heide-Jørgensen MP; Rosing-Asvid A; Granquist SM; Dietz R; Sonne C; Rigét F; Roos A; Benskin JP Fluorine Mass Balance and Suspect Screening in Marine Mammals from the Northern Hemisphere. Environ. Sci. Technol 2020, 54, 4046–4058. 10.1021/acs.est.9b06773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Paul AG; Jones KC; Sweetman AJ A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environ. Sci. Technol 2009, 43 (2), 386–392. 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


