Abstract

This study reports a comparison of the kinetics of electrochemical (EC) versus photoelectrochemical (PEC) water oxidation on bismuth vanadate (BiVO4) photoanodes. Plots of current density versus surface hole density, determined from operando optical absorption analyses under EC and PEC conditions, are found to be indistinguishable. We thus conclude that EC water oxidation is driven by the Zener effect tunneling electrons from the valence to conduction band under strong bias, with the kinetics of both EC and PEC water oxidation being determined by the density of accumulated surface valence band holes. We further demonstrate that our combined optical absorption/current density analyses enable an operando quantification of the BiVO4 photovoltage as a function of light intensity.
Metal oxides are extensively used for both photoelectrochemical (PEC) and electrochemical (EC) water oxidation.1−3 While it is widely recognized that the slow kinetics of water oxidation are a key limitation on the efficiency of both EC and PEC devices for green hydrogen synthesis, direct comparisons of EC versus PEC water oxidation kinetics have been limited to date. Different material classes are typically used in the EC and PEC systems, complicating such comparisons. Wide bandgap semiconducting metal oxides are typically used in PEC water oxidation, while more conductive, metallic metal oxides are typically used in EC systems. EC interfacial reaction kinetics are typically analyzed as a function of applied potential, with the potential modulating the reaction activation energy.4,5 For PEC reactions, a complication in such analyses is the additional photovoltage generated by irradiation, which can be difficult to quantify under operando conditions.6 An additional consideration is that several recent studies, including our own, have highlighted the importance of the chemical rather than potential driven rate-determining steps for both EC and PEC water oxidation catalysis.7,8 In this case, the reaction kinetics are primarily determined not by the potential but by the population density of the reactive intermediates. In the study herein, we focus on operando experimental determination of such population densities for both EC and PEC water oxidation on a widely studied metal oxide, bismuth vanadate (BiVO4), enabling both a direct comparison of EC and PEC reaction kinetics and operando quantification of the photovoltage generated under light irradiation.
BiVO4 is a semiconductor with a bandgap of 2.4 eV and is typically n-doped due to the presence of oxygen vacancies. For single-electron outer-sphere reactions, different reaction mechanisms are typically observed for EC and PEC reactions on semiconducting electrodes. For n-type semiconductors, such EC oxidation reactions are normally driven by electron tunnelling from reactants in the electrolyte into the semiconductor conduction band (CB), without direct involvement of the semiconductor valence band (VB).9−15 In contrast, PEC oxidation reactions are normally considered to be driven by VB holes generated by optical excitation of electrons into the CB.16 However, it is not clear if this difference in reaction mechanism is relevant to multiredox reactions such as water oxidation studied herein. The PEC reaction mechanism can be understood thermodynamically by light irradiation, resulting in a splitting of the electron and hole quasi-Fermi levels (EF,n and EF,p, respectively) by the photovoltage Vphoto,. The simplest assays of Vphoto derive from comparison of light and dark current–voltage (J–V) curves and onset potentials,17 while surface photovoltage measurements provide an alternative assay.18−20 However, such measurements do not provide direct assays of the underlying Fermi level splitting, complicating their interpretation. In the study herein, we address these uncertainties as well as addressing whether a population density model based on a chemical rate-determining step is applicable to both EC and PEC water oxidation on BiVO4.
BiVO4 (photo)anodes were fabricated using a modified metal organic deposition method (details in ESI).21 Cyclic voltammetry was used to confirm that the BiVO4 films were dense and pinhole free (Figures S1 and S15),22 to exclude possible involvement of any exposed fluorine-doped tin oxide substrate during EC water oxidation. To examine the PEC and EC performance, the J–V characteristics of BiVO4 (photo)anodes were measured under chopped simulated AM 1.5 light and in the dark (Figure 1) in a three-electrode PEC cell. It is apparent that the photocurrent onset is shifted cathodically by ca. 1.7 V relative to the dark current, attributed to the photovoltage generated under irradiation.
Figure 1.

Current–voltage characteristics of BiVO4 under chopped light (one sun equivalent) and dark conditions.
To elucidate the underlying difference in light and dark J–V curves, photoinduced absorption (PIA) and stepped potential spectroelectrochemistry (SP-SEC) were used to evaluate the operando optical absorption changes during PEC and EC water oxidation, respectively. Both techniques apply a square wave pump signal to perturb steady state water oxidation. In PIA, the pump signal was provided by a variable intensity 365 nm LED light at a fixed potential, in SP-SEC by a variable voltage square wave potential, as shown in Figure 2 inset (see the Supporting Information for details).8,23 In both cases, the resultant optical absorption and current transients were employed to quantify the population densities and reaction kinetics.
Figure 2.

SP-SEC measurements. Optical absorption (550 nm) and current recorded synchronously under each potential step (inset) are shown against applied potential. Right panel: current density and optical absorption trace at a specific applied potential.
We have previously reported PIA studies of water oxidation on similar BiVO4 photoanodes, employing a probe wavelength of 550 nm assigned to the absorbance of photogenerated surface VB holes.21 These measurements were extended herein to dark EC water oxidation, again employing a 550 nm probe (Figure 2, see also Figure S4 for equivalent PIA under light irradiation). It is apparent that the 550 nm absorbance correlates with the EC water oxidation current, indicative of this dark water oxidation also being driven by the VB holes. Full spectral data as a function of applied bias is shown in Figure S2. In addition to a broad absorption from 550 to 800 nm assigned to surface holes, a narrow absorption peak was also observed centered at 460 nm. This 460 nm absorption appeared at much lower applied potentials than the onset of water oxidation (Figure S3) and is therefore assigned, as previously, to intragap states unable to drive water oxidation.24 It is apparent from Figure 2 that the 550 nm optical VB hole signal increases approximately linearly with applied potential, while Jwo increases nonlinearly, as we analyze further below.
We turn now to a comparison of EC and PEC water oxidation kinetics. The hole absorption at 550 nm can be converted to a surface hole density ps using the Beer–Lambert law with a molar extinction coefficient of 420 M–1cm–1.25 Further, the measured current density Jwo divided by ps gives the reaction turnover frequency (TOF) per surface hole. Figure 3 shows plots of Jwo and TOF versus ps for both EC and PEC water oxidation on BiVO4. For both measurements, biphasic behavior is observed. Applying a rate law model, as we have applied previously to PEC water oxidation on BiVO4,25 our EC data demonstrate first-order behavior (α = 0.95 ± 0.11) in the low ps region (most likely resulting from hydroxide oxidation to hydroxyl radicals), explaining the low current <2.4 VRHE.8,26 At higher hole densities, third-order behavior is observed (α = 2.95 ± 0.26), assigned to water oxidation to molecular oxygen.25 Most strikingly, while plots of Jwo versus potential are very different in the dark and light (see Figure 1), our plots of Jwo vs ps are indistinguishable between the EC and PEC data within experimental error (see Figure S9 for comparison of data from a further electrode). Such overlapping kinetic behavior was also found from PEC and EC optical decays (Figure S11). An analogous observation has been reported recently by Cowan et al. for hematite photoanodes.27 An overlap is also observed in our plot of the TOF versus ps (Figure 3b). This indicates that the kinetics of water oxidation in the light and dark, when measured at matched surface hole densities, are indistinguishable. This overlap provides clear evidence that dark EC and light PEC water oxidations on BiVO4 have equivalent reaction mechanisms and that both are driven by the accumulation of surface VB holes.
Figure 3.

(a) The steady state (photo)current densities Jwo and (b) TOF under PEC (purple) and EC (orange) conditions versus ps.
Our conclusion that the kinetics of both EC and PEC water oxidation are determined only by the density of VB holes rather than the applied potential is consistent with several recent reports from other groups.7,28 It confirms that EC water oxidation on BiVO4 is not driven by electron injection into the BiVO4 CB, in contrast to previously studied EC oxidation reactions on n-type semiconductors.9−15 Our observation of VB hole driven EC water oxidation suggests an alternative mechanism, as illustrated in Scheme 1, where the strong electric fields present in the space charge layer (SCL) under EC water oxidation conditions can promote electron tunnelling from the VB at the surface into the bulk CB through the so-called internal field emission, or Zener effect.15,29,30 This Zener effect is enabled by the narrow width of the SCL (∼50 Å at 3.2 VRHE, see Figure S5) and the magnitude of the band bending (the onset dark potential, ca. 2.4 VRHE and the flatband potential, 0.35 VRHE) being comparable to the bandgap of BiVO4.15 Following this model, the main difference between PEC and EC water oxidation is the method of VB hole generation, with this being driven by the high SCL electric fields in the dark and by photoexcitation in the light. This difference from classical theory most likely originates from the chemical rather than potential driven nature of the rate-determining step for water oxidation on BiVO4, which is associated with this being a multiredox, inner-sphere reaction. It furthermore indicates that the underlying mechanism of water oxidation catalysis on BiVO4 is the same for both EC and PEC conditions, independent of the magnitude of the applied potential and potential drops across the Helmholtz layer.
Scheme 1. Band diagrams of BiVO4.
(a) Dark; (b) dark, under a strong anodic bias; (c) light, under a smaller anodic bias. In the dark, band bending increases with Vapp (panel b). In the light, EF splits into EF,e and EF,p (panel c). Holes (green dots) accumulated in the SCL when strong anodic potentials or light are applied, and electrons (purple dots) drifted away from the SCL.
Having established the equivalent
population driven mechanisms
for both EC and PEC water oxidation, we can employ our PIA and SP-SEC
data to obtain an operando determination of Vphoto, as a function of the light intensity,
ϕ. In our EC measurement, the applied potential Vapp can be taken as a measure of the
BiVO4 surface EF, assuming minimal resistance losses. At matched surface hole
densities, this dark EF(ps, dark) value will
be equivalent to the EF,p(ps, light) present
under irradiation (Scheme 1). As such, Vphoto can be most simply determined from Vphoto(ps) = EF(ps, dark)/e – Vapp(light), where for the studies herein Vapp(light) was held at 1.7
VRHE. In Figure 4 (a), the Vphoto is plotted versus ϕ, illustrating a logarithmic dependence
(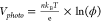 ) (see Figure S13 for further electrodes). We also determined Vphoto using the difference in light and
dark
onset potentials (absence of significant current flow and so not properly
operando) and observed qualitatively similar behavior (Figure S14a and b). For both data sets, “nonideal”
behavior is observed (n > 2), as has also been
reported
for hematite and SrTiO3 photoanodes.17,20 The origin of this nonideal behavior is not clear but most likely
associated with the impact of intragap defect states and/or “photocharging”
effects widely reported for BiVO4.31−35 A full analysis of this nonideal behavior is beyond
the scope of this study. Figure 4 (b) shows a plot of the Jwo of EC and PEC versus EF,p, where we use in the dark EF,p = Vapp(dark) and in the light EF,p = Vapp(light)
+ Vphoto. It is apparent
that these two plots of the current density versus EF,p overlap, strongly supporting the
validity of our determination of Vphoto and emphasizing that the water oxidation current is determined
only by the position of EF,p, and therefore ps, independent of whether the hole population is generated electrochemically
or photoelectrochemically. In contrast to other approaches to determine
the photovoltage,17−20 the experimental approach demonstrated herein provides an operando
determination of hole quasi-Fermi level as a function of light intensity,
opening up the potential for in depth analyses of photovoltage generation.
) (see Figure S13 for further electrodes). We also determined Vphoto using the difference in light and
dark
onset potentials (absence of significant current flow and so not properly
operando) and observed qualitatively similar behavior (Figure S14a and b). For both data sets, “nonideal”
behavior is observed (n > 2), as has also been
reported
for hematite and SrTiO3 photoanodes.17,20 The origin of this nonideal behavior is not clear but most likely
associated with the impact of intragap defect states and/or “photocharging”
effects widely reported for BiVO4.31−35 A full analysis of this nonideal behavior is beyond
the scope of this study. Figure 4 (b) shows a plot of the Jwo of EC and PEC versus EF,p, where we use in the dark EF,p = Vapp(dark) and in the light EF,p = Vapp(light)
+ Vphoto. It is apparent
that these two plots of the current density versus EF,p overlap, strongly supporting the
validity of our determination of Vphoto and emphasizing that the water oxidation current is determined
only by the position of EF,p, and therefore ps, independent of whether the hole population is generated electrochemically
or photoelectrochemically. In contrast to other approaches to determine
the photovoltage,17−20 the experimental approach demonstrated herein provides an operando
determination of hole quasi-Fermi level as a function of light intensity,
opening up the potential for in depth analyses of photovoltage generation.
Figure 4.

(a) Relationship between Vphoto and ϕ under PEC conditions. (b) The overlap of dark and light current density versus EF,p.
To summarize, we have demonstrated that water oxidation on BiVO4 proceeds via the same kinetics and mechanism under PEC and EC conditions. The accumulation of a sufficient density of surface VB holes is shown to be the key step for both PEC and EC water oxidation, consistent with a chemical rather than potential driven rate determining step. Moreover, the optical absorption-based analyses enable the operando determination of Vphoto as a function of light irradiation. While further study is needed to determine the wider applicability of this methodology to determine Vphoto, such as the potential impact of Fermi level pinning, it offers a new approach to investigating operando photovoltage generation in photoelectrodes.
Acknowledgments
We thank the European Union Sun2Chem Project 884444 for funding. L.I.O. thanks the Imperial College London’s Faculty of Natural Sciences Schrödinger Scholarship for Ph.D. funding. L.T. acknowledges the V. R. International Postdoc Fellowship (No. 2020-06511) from the Swedish Research Council and is sincerely grateful for the supports from his host supervisor, Prof. Xiaodong Zou, Stockholm University. We also acknowledge the efforts made by Ms. Dikshita Bhattacharyya and Mr. Tianhao He in the beginning of this project as well as Ms. Sirui Li and Ms. Salma AlQahtani for synthesizing one of the samples measured in this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c03178.
Additional information including BiVO4 preparations, experiment procedures (LSV, CV and operando PIA and SP-SEC), and detailed explanation mentioned in main text (PDF)
Author Present Address
§ Cavendish Laboratory, University of Cambridge, Cambridge, CB3 0HE, U.K
Author Present Address
∥ Walter Schottky Institute, Technical University of Munich, Am Coulombwall 4, 85748 Garching, Germany
Author Present Address
⊥ Inorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QR, U.K.
The authors declare no competing financial interest.
Supplementary Material
References
- Hisatomi T.; Kubota J.; Domen K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43 (22), 7520–7535. 10.1039/C3CS60378D. [DOI] [PubMed] [Google Scholar]
- Sivula K.; van de Krol R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1 (2), 15010. 10.1038/natrevmats.2015.10. [DOI] [Google Scholar]
- Doyle R. L.; Godwin I. J.; Brandon M. P.; Lyons M. E. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15 (33), 13737–13783. 10.1039/c3cp51213d. [DOI] [PubMed] [Google Scholar]
- Zhang L. H.; Mathew S.; Hessels J.; Reek J. N.; Yu F. Homogeneous Catalysts Based on First-Row Transition-Metals for Electrochemical Water Oxidation. ChemSusChem 2021, 14 (1), 234–250. 10.1002/cssc.202001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken J. B.; McAlpin J. G.; Chen J. Y.; Rigsby M. L.; Casey W. H.; Britt R. D.; Stahl S. S. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 2011, 133 (36), 14431–14442. 10.1021/ja205647m. [DOI] [PubMed] [Google Scholar]
- Laskowski F. A.; Oener S. Z.; Nellist M. R.; Gordon A. M.; Bain D. C.; Fehrs J. L.; Boettcher S. W. Nanoscale semiconductor/catalyst interfaces in photoelectrochemistry. Nat. Mater. 2020, 19 (1), 69–76. 10.1038/s41563-019-0488-z. [DOI] [PubMed] [Google Scholar]
- Nong H. N.; Falling L. J.; Bergmann A.; Klingenhof M.; Tran H. P.; Spöri C.; Mom R.; Timoshenko J.; Zichittella G.; Knop-Gericke A.; et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 2020, 587 (7834), 408–413. 10.1038/s41586-020-2908-2. [DOI] [PubMed] [Google Scholar]
- Le Formal F.; Pastor E.; Tilley S. D.; Mesa C. A.; Pendlebury S. R.; Grätzel M.; Durrant J. R. Rate law analysis of water oxidation on a hematite surface. J. Am. Chem. Soc. 2015, 137 (20), 6629–6637. 10.1021/jacs.5b02576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake T.; Hijii S.; Sonoyama N.; Sakata T. Electrochemical luminescence of n-type ZnO semiconductor electrodes doped with rare earth metals under the anodic polarization. Appl. Surf. Sci. 2006, 253 (4), 1753–1757. 10.1016/j.apsusc.2006.03.008. [DOI] [Google Scholar]
- Rajeshwar K. Fundamentals of semiconductor electrochemistry and photoelectrochemistry. Encyclopedia of Electrochemistry; Bard, A. J., Ed.; Wiley 2002, 6, 1–53. 10.1002/9783527610426.bard060001. [DOI] [Google Scholar]
- Dutoit E.; Cardon F.; Gomes W. Electrochemical properties of the semiconducting TiO2 (rutile) single crystal electrode. Ber. Bunsenges. Phys. Chem. 1976, 80 (6), 475–481. 10.1002/bbpc.19760800604. [DOI] [Google Scholar]
- Möllers F.; Memming R. Electrochemical studies of semiconducting SnO2-electrodes. Ber. Bunsenges. Phys. Chem. 1972, 76 (6), 469–475. 10.1002/bbpc.19720760603. [DOI] [Google Scholar]
- Nozik A. J.; Memming R. Physical chemistry of semiconductor-liquid interfaces. J. Phys. Chem. 1996, 100 (31), 13061–13078. 10.1021/jp953720e. [DOI] [Google Scholar]
- Noufi R. N.; Kohl P. A.; Frank S. N.; Bard A. J. Semiconductor Electrodes: XIV. Electrochemistry and Electroluminescence at n-Type in Aqueous Solutions. J. Electrochem. Soc. 1978, 125 (2), 246. 10.1149/1.2131422. [DOI] [Google Scholar]
- Pettinger B.; Schöppel H. R.; Yokoyama T.; Gerischer H. Tunnelling processes at highly doped ZnO electrodes in contact with aqueous electrolytes. Part II: Electron Exchange with the Valence Band. Ber. Bunsenges. Phys. Chem. 1974, 78 (10), 1024–1030. 10.1002/bbpc.19740781012. [DOI] [Google Scholar]
- Hegner F. S.; Herraiz-Cardona I.; Cardenas-Morcoso D.; López N. r.; Galán-Mascarós J.-R. n.; Gimenez S. Cobalt hexacyanoferrate on BiVO4 photoanodes for robust water splitting. ACS Appl. Mater. Interfaces 2017, 9 (43), 37671–37681. 10.1021/acsami.7b09449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Lyu M.; Chen P.; Wang S.; Wang L. Energy loss analysis in photoelectrochemical water splitting: a case study of hematite photoanodes. Phys. Chem. Chem. Phys. 2018, 20 (35), 22629–22635. 10.1039/C8CP04021D. [DOI] [PubMed] [Google Scholar]
- Daemi S.; Kundmann A.; Becker K.; Cendula P.; Osterloh F. E. Contactless measurement of the photovoltage in BiVO4 photoelectrodes. Energy Environ. Sci. 2023, 16 (10), 4530–4538. 10.1039/D3EE02087H. [DOI] [Google Scholar]
- Daemi S.; Kaushik S.; Das S.; Hamann T. W.; Osterloh F. E. BiVO4-Liquid Junction Photovoltaic Cell with 0.2 % Solar Energy Conversion Efficiency. J. Am. Chem. Soc. 2023, 145 (47), 25797–25805. 10.1021/jacs.3c09546. [DOI] [PubMed] [Google Scholar]
- Chen R.; Zhang D.; Wang Z.; Li D.; Zhang L.; Wang X.; Fan F.; Li C. Linking the Photoinduced Surface Potential Difference to Interfacial Charge Transfer in Photoelectrocatalytic Water Oxidation. J. Am. Chem. Soc. 2023, 145 (8), 4667–4674. 10.1021/jacs.2c12704. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Pendlebury S. R.; Reynal A.; Le Formal F.; Durrant J. R. Dynamics of photogenerated holes in undoped BiVO4 photoanodes for solar water oxidation. Chem. Sci. 2014, 5 (8), 2964–2973. 10.1039/C4SC00469H. [DOI] [Google Scholar]
- Kavan L.; Steier L.; Grätzel M. Ultrathin buffer layers of SnO2 by atomic layer deposition: perfect blocking function and thermal stability. J. Phys. Chem. C 2017, 121 (1), 342–350. 10.1021/acs.jpcc.6b09965. [DOI] [Google Scholar]
- Bozal-Ginesta C.; Rao R. R.; Mesa C. A.; Liu X.; Hillman S. A.; Stephens I. E.; Durrant J. R. Redox-State Kinetics in Water-Oxidation IrOx Electrocatalysts Measured by Operando Spectroelectrochemistry. ACS Catal. 2021, 11 (24), 15013–15025. 10.1021/acscatal.1c03290. [DOI] [Google Scholar]
- Selim S.; Pastor E.; Garcia-Tecedor M.; Morris M. R.; Francas L.; Sachs M.; Moss B.; Corby S.; Mesa C. A.; Gimenez S.; et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 2019, 141 (47), 18791–18798. 10.1021/jacs.9b09056. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Mesa C. A.; Pastor E.; Kafizas A.; Francas L.; Le Formal F.; Pendlebury S. R.; Durrant J. R. Rate law analysis of water oxidation and hole scavenging on a BiVO4 photoanode. ACS Energy Lett. 2016, 1 (3), 618–623. 10.1021/acsenergylett.6b00263. [DOI] [Google Scholar]
- Lemasson P.; Gautron J.; Dalbéra J. P. On the Use of Electroreflectance in the Interpretation of Anodic Electrochemical Behavior of the ZnSe Semiconductor Electrode. Ber. Bunsenges. Phys. Chem. 1980, 84 (8), 796–800. 10.1002/bbpc.19800840820. [DOI] [Google Scholar]
- Saeed K. H.; Garcia Osorio D.-A.; Li C.; Banerji L.; Gardner A. M.; Cowan A. J. Monitoring interfacial electric fields at a hematite electrode during water oxidation. Chem. Sci. 2023, 14 (12), 3182–3189. 10.1039/D2SC05628C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi G.; Plescher J.; Schmidt F.-P.; Campen R. K.; Fabris S.; Knop-Gericke A.; Schlögl R.; Jones T. E.; Teschner D.; Piccinin S. On the origin of multihole oxygen evolution in haematite photoanodes. Nat. Catal. 2022, 5 (10), 888–899. 10.1038/s41929-022-00845-9. [DOI] [Google Scholar]
- Zener C. A theory of the electrical breakdown of solid dielectrics. Proc. R. Soc. London, Ser. A 1934, 145 (855), 523–529. [Google Scholar]
- Boddy P. Oxygen evolution on semiconducting TiO2. J. Electrochem. Soc. 1968, 115 (2), 199. 10.1149/1.2411080. [DOI] [Google Scholar]
- Trześniewski B. J.; Smith W. A. Photocharged BiVO4 photoanodes for improved solar water splitting. J. Mater. Chem. A 2016, 4 (8), 2919–2926. 10.1039/C5TA04716A. [DOI] [Google Scholar]
- Liu E. Y.; Thorne J. E.; He Y.; Wang D. Understanding photocharging effects on bismuth vanadate. ACS Appl. Mater. Interfaces 2017, 9 (27), 22083–22087. 10.1021/acsami.7b06528. [DOI] [PubMed] [Google Scholar]
- Arcas R.; Cardenas-Morcoso D.; Spadaro M. C.; García-Tecedor M.; Mesa C. A.; Arbiol J.; Fabregat-Santiago F.; Giménez S.; Mas-Marzá E. Direct Observation of the Chemical Transformations in BiVO4 Photoanodes upon Prolonged Light-Aging Treatments. Sol. RRL 2022, 6 (7), 2200132. 10.1002/solr.202270074. [DOI] [Google Scholar]
- Fernández-Climent R.; Giménez S.; García-Tecedor M. The role of oxygen vacancies in water splitting photoanodes. Sustain. Energy Fuels 2020, 4 (12), 5916–5926. 10.1039/D0SE01305F. [DOI] [Google Scholar]
- Su S.; Siretanu I.; Van den Ende D.; Mei B.; Mul G.; Mugele F. Nanometer-Resolved Operando Photo-Response of Faceted BiVO4 Semiconductor Nanoparticles. J. Am. Chem. Soc. 2024, 146 (3), 2248–2256. 10.1021/jacs.3c12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



