Abstract
Radiation therapy (RT) has been a primary treatment modality in cancer for decades. Increasing evidence suggests that RT can induce an immunosuppressive shift via upregulation of cells such as tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). MDSCs inhibit antitumor immunity through potent immunosuppressive mechanisms and have the potential to be crucial tools for cancer prognosis and treatment. MDSCs interact with many different pathways, desensitizing tumor tissue and interacting with tumor cells to promote therapeutic resistance. Vascular damage induced by RT triggers an inflammatory signaling cascade and potentiates hypoxia in the tumor microenvironment (TME). RT can also drastically modify cytokine and chemokine signaling in the TME to promote the accumulation of MDSCs. RT activation of the cGAS-STING cytosolic DNA sensing pathway recruits MDSCs through a CCR2-mediated mechanism, inhibiting the production of type 1 interferons and hampering antitumor activity and immune surveillance in the TME. The upregulation of hypoxia-inducible factor-1 and vascular endothelial growth factor mobilizes MDSCs to the TME. After recruitment, MDSCs promote immunosuppression by releasing reactive oxygen species and upregulating nitric oxide production through inducible nitric oxide synthase expression to inhibit cytotoxic activity. Overexpression of arginase-1 on subsets of MDSCs degrades L-arginine and downregulates CD3z, inhibiting T-cell receptor reactivity. This review explains how radiation promotes tumor resistance through activation of immunosuppressive MDSCs in the TME and discusses current research targeting MDSCs, which could serve as a promising clinical treatment strategy in the future.
Radiation and Systemic Immunity
Radiation therapy (RT) is a highly effective tool that has played a pivotal role in the treatment of cancer for decades.1,2 Enhanced understanding of the effect of RT on the antineoplastic immune response can support design of treatment approaches with improved efficacy. Although RT is regarded primarily as immune stimulating, it can act as a double-edged sword, compromising immune responses by depleting cytotoxic cells and activating immunosuppressive cell activity.2 Thus, RT has the potential to be used as an immunomodulatory treatment when used appropriately.
Lymphocytes play a key role in antitumor immunity.3 Associated with worse outcomes in various types of cancer,4,5 lymphopenia may be present at diagnosis secondary to tumor-associated immunosuppression and can occur consequent to antineoplastic therapies. Grossman et al found that 2 months after starting chemoradiotherapy (CRT), 43% of patients with newly diagnosed tumors, including malignant glioma, pancreatic cancer, and non-small cell lung cancer (NSCLC), developed severe lymphopenia.4 A study of patients with NSCLC highlighted that, contrary to chemotherapy treatment alone, lymphocyte counts decreased sharply after commencement of CRT, providing evidence that RT contributes to the induction and severity of lymphopenia.6 In patients with cervical cancer treated with definitive CRT, the incidence of lymphopenia has been reported to be as high as 95.2% and independently predicted poor survival.5,7 Similar findings have been reported in nasopharyngeal and lung cancer.8,9
Of clinical relevance, high-dose corticosteroids contribute to the development of lymphopenia and may affect the outcomes of RT.10 Nakamura et al proposed that CD4+ and CD8+ lymphocytes are exquisitely radiosensitive and susceptible to cytotoxic degradation in the presence of low-dose RT.11 Interleukin (IL) 7 is a cytokine important for lymphocyte homeostasis, and low IL-7 before RT may be an independent predictor of severe RT-induced lymphopenia.12 Lowered CD4 counts can increase the likelihood of hospitalization among patients, highlighting the importance of evaluating RT-immune interactions in clinical practice.10
Tumor-associated macrophages (TAMs) are a crucial population of myeloid cells that modulate RT-induced immunosuppression.13 Macrophages are generally categorized as classically activated macrophages, M1 macrophages, or alternatively activated (immune suppressive) M2 macrophages.14 TAMs are M2-like and function to promote an immunosuppressive TME.14 TAMs produce inflammatory mediators and growth factors associated with cancer progression, tumor angiogenesis, chemoresistance, and metastasis.15 High densities of TAMs are associated with worse overall survival in gastric, urogenital, and head and neck cancers.16 Importantly, TAMs are more radioresistant than lymphocyte populations, and RT may promote the activation of immunosuppressive TAMs.17
Although Wunderlich et al showed that low doses of radiation (0.01–2 Gy) can increase macrophage chemotaxis into the TME, the extent of TAM activation may depend on many variables unique to the RT protocol.18 Fractionation likely plays a key role in TAM activation and recruitment to irradiated sites. Chen et al determined that 2 distinct RT regimens (multifractionated RT consisting of 60 Gy administered in 15 fractions and single-dose RT administered in one 25 Gy fraction) both promote aggregation of TAMs near hypoxic tumor regions in tumor-bearing mice but that the effect is more pronounced for single-fraction RT.19 In lung epithelial cell lines, RT increases transforming growth factor β (TGF-β)–secreting M2 macrophage migration and promotes the release of tumor-supporting, proinflammatory cytokines.20
Although the effect of RT on lymphocytes and TAMs has been increasingly documented in recent years, less is known about its effect on myeloid-derived suppressor cell (MDSC) populations. MDSCs are critical perpetrators of TME-induced immunosuppression that protect cancer cells from immune system attack.21 Elevated MDSC counts have been associated with poor clinical outcomes, such as shortened overall survival and recurrence-free survival in patients with hepatocellular, rectal, lymphoma, lung, melanoma, esophageal, breast, gastric, brain, and pancreatic cancers, compared with patients with lower MDSC counts.22–24 Murine models suggest that the accumulation of MDSCs in the TME promotes radioresistance through altered enzymatic activity associated with this cell type.25,26 Therapeutic elimination of MDSCs may therefore be an effective future approach to improve radiosensitivity without undue adverse effects. This review discusses the current knowledge of mechanisms underlying RT-induced MDSC accumulation in the TME and strategies that MDSCs use to attenuate immune surveillance and contribute to radioresistance.
MDSCs Act as Immunosuppressive Agents in Cancer
MDSCs are a heterogeneous population of immature myeloid cells that acquire immunosuppressive qualities when exposed to host-derived factors.27,28 Myeloid cells develop from maturation of multipotent hematopoietic stem cells.29 Myeloid progenitor cells can differentiate into specialized cell types such as suppressive macrophages and immune-stimulating dendritic cells (DCs) that play unique roles in maintaining immune homeostasis.30,31 Cancer skews the differentiation of myeloid cells toward cancer-supportive phenotypes, such as MDSCs.29 MDSC counts are elevated in patients with cancer compared with healthy individuals and correlate with cancer stage and disease recurrence status.32 Diverse tumor cell lines, including those derived from cervical, ovarian, colorectal, renal cell, and head and neck carcinomas, induce MDSC production in coculture with peripheral blood mononuclear cells.33
MDSCs are traditionally distinguished from classical monocytes and neutrophils by their ability to inhibit T-cell activity, but both TAMs and tumor-associated neutrophils (TANs) can also exert immunosuppressive activity.34–37 MDSCs are neutrophils and monocytes that have been programmed to support cancers.35 Classical activation of neutrophils and monocytes is short lived and occurs in response to invading pathogens and tissue injury.38 This activation is characterized by high phagocytosis, respiratory burst, and secretion of proinflammatory (anticancer) cytokines.35
In cancer, MDSC pathologic activation occurs when tumor-secreted growth factors and inflammatory signals, such as macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), IL-6, and IL-1β, stimulate myelopoiesis in the bone marrow and spleen, generating a population of immature myeloid cells.30,35,39 Under healthy conditions, immature myeloid cells migrate to peripheral tissue and differentiate into mature granulocytes, macrophages, and dendritic cells. Pathologic stimulation, however, prevents this maturation, resulting in the accumulation of immature myeloid cells at the tumor site.30 T cells and tumor stroma cells then activate immature myeloid cells into MDSCs through factors such as interferon γ (IFN-γ), IL-4, IL-13, and TGF-β.30,39 This pathologic activation produces myeloid cells with poor phagocytic activity that secrete high levels of reactive oxygen species (ROS), nitric oxide (NO), and anti-inflammatory (procancer) cytokines.35
In humans, MDSCs are generally defined as CD11b+CD33+HLA-DR− and subdivided into CD11b+ CD33+HLA-DR−CD15+ granulocytic/polymorphonuclear MDSCs (G-MDSCs), CD11b+CD33+HLA-DR−CD14+ monocytic MDSCs (M-MDSCs), and CD11b+CD33+HLA-DR−CD14−CD15− early MDSCs (eMDSCs).28 Although M-MDSCs have a monocytic origin, they exhibit less MHC class II expression compared with monocytes.39 It is hypothesized that monocytes, M-MDSCs, and TAMs exist in different differentiation states, driven by tumor-secreted factors.35 During myelopoiesis, expression of retinoic acid–related orphan receptor (RORC1/RORg) is required for M-MDSC and TAM expansion and induced by IL-1β, G-CSF, GM-CSF, and M-CSF.40 G-MDSCs and neutrophils share characteristic cell surface markers and morphologic similarities, making them difficult to distinguish. However, key differences in G-MDSC and neutrophil maturity, surface and gene expression, and secretion aid in their distinction. G-MDSCs resemble an immature neutrophil phenotype in breast cancer, displaying downregulated expression of the neutrophil maturity markers CD10, CD13, and CD45.41 In peripheral blood of patients with NSCLC, lectin-type oxidized LDL receptor 1 (LOX-1) encoded by the OLR1 gene is overexpressed in G-MDSCs but undetectable in neutrophils, illustrating their distinct gene profiles related to endoplasmic reticulum (ER) stress.42 Specifically in renal cell carcinoma, arginase mRNA is elevated in G-MDSCs compared with neutrophils.43
Clinical Evidence of the Effects of RT on MDSCs
Conventional RT regimens generally consist of 1.5 to 3 Gy administered daily for 3 to 7 weeks.44 Hypofractionated regimens deliver RT at a higher dose per fraction and can be employed to shorten the total duration of treatment.44 Stereotactic body radiation therapy (SBRT) or SABR is carried out in 1 to 5 sessions and uses specialized planning techniques to deliver focused RT to the tumor site with limited dose to the surrounding tissue.44,45 Emerging research suggests that non-conventional ablative RT promotes different biological responses than conventionally fractionated RT.45,46 In murine pancreatic cancer models, 40 Gy administered in 4 fractions induces greater MDSC infiltration to the TME than a single fraction of 25 Gy.46 Furthermore, ablative RT promotes a greater increase in CD8+ T-cell infiltration, suggesting fractionation may be an important predictor of tumor response to therapy.46 In murine colon tumors, MDSC quantity in tumors increases a mean of 5% 35 days after a single dose of 30 Gy compared with 20% 10 days after 30 Gy in 10 fractions.47 Transient but marked temporal MDSC changes after RT are an important consideration, as demonstrated by a study that observed an initial 50% MDSC increase into the TME 3 days post-RT before decreasing to 5% at 35 days.47 These findings highlight the need for further studies to evaluate the effects of dose, timing, and fractionation on TME remodeling that include consideration of MDSC influx and signaling.
Clinical studies of RT-MDSC interactions in humans remain limited and have yielded varying results (Table 1). However, several have reported increases in peripheral MDSC counts after RT. Studies of patients with NSCLC and with head and neck squamous cell carcinoma have reported elevated general MDSC counts after RT,48,49 whereas other studies have reported MDSC subtype-specific increases after RT. In one study of head and neck squamous cell carcinoma, G-MDSC counts increased during RT and had detectable signal transducer and activator of transcription 3 (STAT3) and programmed death ligand 1 (PD-L1) activity, underscoring their suppressive activation,50 but M-MDSC levels did not change in response to RT.50
Table 1.
Clinical outcomes of radiation therapy on MDSC counts in peripheral blood
| Total dose | Fractionation | Disease | Clinical outcome | PBMC preparation | Reference number |
|---|---|---|---|---|---|
| 2 Gy | 2 Gy | Non-small cell lung cancer | RT resulted in an increase in peripheral MDSC counts (CD33+CD11b+HLA-DR−). During RT, MDSC counts increased in patients with non-small cell lung cancer adenocarcinoma. |
Frozen | 48 |
| 70 Gy | <3 Gy/fraction over 5–7 wk | Head and neck squamous cell carcinoma | MDSC (CD14−APC+, HLA-DR PC7−) counts increased in most patients after RT. | Frozen | 49 |
| 66–70 Gy | 2- to 2.2-Gy fractions | Stage III-IV head and neck squamous cell carcinoma | RT was correlated with accumulation of G-MDSCs (CD14−CD15+CD33+) with high STAT3 and PD-L1 activity. | Frozen | 50 |
| 70 Gy | 35 fractions of 2 Gy over 7 wk | Stage III-IV oropharyngeal squamous cell cancer | M-MDSC (CD33+CD11b+ HLA-DR−CD14+) counts increased by nearly 3-fold 3 wk after RT. Absolute CD8+ T-cell and CD8+ counts and MDSC effector/suppressor ratios decreased for 6 mo after RT. |
Frozen | 51 |
| 34–70 Gy | Unspecified | Lung or head and neck cancer | M-MDSC (HLA-DRlow/−CD33+CD14+CD11b+) counts increased significantly in 8 of 20 patients. | Frozen | 52 |
| 46–50 Gy | 23 fractions of 2 Gy or an equivalent dose of 1.8 Gy/fraction | Stage 1B1-IV cervical cancer | M-MDSC (CD11b+CD3−CD19−CD1a−HLA-DR−CD14+CD15−) counts increased after RT in 9 of 10 patients. | Frozen | 53 |
| 66 Gy | 24 fractions of 2.75 Gy for 5 wk | Non-small cell lung cancer | CD14+CD33+HLA-DRlow M-MDSC counts increased after RT. | Frozen | 54 |
| 20 Gy | Single-fraction IORT | Low-risk breast cancer | No statistically significant change in MDSC (CD45+CD33+CD11b+) counts was found. | Fresh | 56 |
| 25 Gy | 5 fractions of 5 Gy | Locally advanced rectal cancer | M-MDSC (CD14+HLA-DR−/lowCD11b+CD33+) counts decreased 5 wk from RT and then remained stable. G-MDSC (LIN−HLA-DR−CD11b+CD14−CD15+CD33+) counts decreased 5 wk from RT and continued to decrease before surgery at week 8. Poor responders had lower M-MDSC counts. |
Fresh | 58 |
| 50–60 Gy | 4 patients received 8 fractions of 7.5 Gy and 3 received 4 fractions of 12.5 Gy | Lung cancer | M-MDSC (CD33+CD11b+CD14+HLA-DR−/low) counts decreased throughout the study. G-MDSC (CD33+CD11b+CD14−) counts increased 72 h after RT and then progressively declined 6 mo after RT. |
Fresh | 59 |
| 60 Gy | 2 Gy/fraction and 5 fractions/wk | Hepatocellular carcinoma | M-MDSC (CD14+HLA-DR−/low) frequency decreased after RT. Higher frequencies of M-MDSC correlated with shorter overall survival. Pre- and post-RT MDSC (CD14+HLA-DR−/low) frequencies >14.6% were associated with a worse prognosis. |
Fresh | 60 |
| 40–66 Gy | Unspecified | Esophageal squamous cell carcinoma | MDSC (CD11b+CD33+HLA-DR−) counts were elevated after RT in patients who developed disease failure and had a higher risk of death. | Frozen | 61 |
| 50–64 Gy | 1.8–2 Gy per fraction for 25–32 fractions | Esophageal squamous cell carcinoma | A high lymphocyte-to-monocyte ratio pre-RT was a predictor of good clinical tumor response to RT, a lower risk of recurrence, and a decreased risk of death. | Fresh | 62 |
Abbreviations: G-MDSC = granulocytic/polymorphonuclear myeloid-derived suppressor cell; IORT = intraoperative radiation therapy; MDSC = myeloid-derived suppressor cell; M-MDSC = monocytic myeloid-derived suppressor cell; PBMC = peripheral blood mononuclear cell; RT = radiation therapy.
Comparatively, other studies of head and neck cancer have reported elevations in M-MDSC levels after CRT.51,52 Among these, Parikh et al51 notably found that M-MDSC frequency increased nearly 3-fold within weeks of receiving RT for oropharyngeal cancer, and Boustani et al52 reported significantly increased M-MDSC counts in 40% of patients with lung or head and neck cancer, but the overall mean change of M-MDSC levels in the latter study was not significant. In a study of patients with cervical cancer, M-MDSC counts increased significantly and displayed clear temporal changes in 9 out of 10 patients.53 Peripheral MDSC counts were highest 3 weeks post-RT, and MDSC counts remained elevated for 9 weeks and prominently affected M-MDSC populations.53 Significant increases in M-MDSC frequencies after RT have also been observed in lung cancer.54
In contrast, several studies have reported no change in peripheral or tumor MDSC counts during or after RT. In a cohort of patients with rectal cancer who received conventionally fractionated neoadjuvant RT, Teng et al found that pre-RT MDSC counts in biopsy specimens did not differ from post-RT values.55 A study evaluating the effect of 20 Gy delivered via intraoperative RT in low-risk breast cancer at the time of surgical resection found no significant changes from baseline in MDSC percentage.56 This finding in breast carcinoma accords with a prior study that demonstrated MDSC production is not induced in healthy human donor peripheral blood mononuclear cells when cocultured with several breast carcinoma cell lines.33 One study evaluating MDSC changes in patients with glioblastoma reported no significant changes in MDSC composition in pre- and post-RT blood samples.57 Of note, this study quantified MDSCs in a distinct way, integrating percentage frequency from flow cytometry data and clinical complete blood counts of monocyte populations. The fact that monocytes do not include all MDSCs may account in part for discrepancies with similar investigations.
Other studies have reported decreases in MDSC frequencies after RT. In a pilot study of patients with locally advanced rectal cancer receiving 25-Gy short-course RT, both G-MDSC and M-MDSC counts decreased after RT.58 In a study of patients with lung cancer receiving 50- to 60-Gy SBRT, M-MDSC counts steadily decreased for months while G-MDSC counts transiently increased 72 hours after RT but declined thereafter for 6 months.59 Finally, M-MDSC counts have been observed to decrease in patients with hepatocellular carcinoma after RT.60 Interestingly, the average RT dose fractionation was higher than that of conventional RT, ranging from 5 to 20 Gy in most studies that reported no change or decrease in MDSC levels after RT.56,58,59 This supports that conventional fractionation elicits a greater suppressive response when quantified by MDSC frequency alterations. However, other factors, such as RT volume, can play a role in MDSC accumulation.48 Collectively, these clinical findings suggest that MDSC subtype concentration changes are likely secondary to multiple underlying factors, including histology, RT dose and fractionation, and timing after completion of treatment.
Because MDSC counts change with treatment, MDSC concentrations may act as predictive and prognostic indicators of response. In a study of patients with locally advanced rectal cancer, a decrease in M-MDSC concentration was associated with poor response to multimodality treatment.58 Relative changes in peripheral MDSC concentrations to baseline values may therefore offer a noninvasive, indirect measure of clinical response.58 In patients with esophageal squamous cell carcinoma, increased peripheral MDSC concentration has been correlated with increased recurrence and reduced overall survival.61 Similarly, Wang et al found that high pretreatment and posttreatment MDSC frequency were associated with a worse prognosis.60 As a predictive measure, higher pre-RT lymphocyte-to-monocyte ratios in patients with esophageal cancer have been associated with increased progression-free survival and overall survival.62 These findings are based on the assumption that many MDSCs are included in the monocyte portion.
Radiation Alters the Tumor Microenvironment
The TME is a complex, heterogeneous environment that includes proliferating tumor cells, stromal cells, blood vessels, infiltrating inflammatory cells, and tumor-induced interactions.63 TME composition is linked to the progression, survival, local invasion, and metastatic dissemination of cancer cells.64 RT promotes direct and indirect cell damage, inflammation, vascular depletion or endothelial cell death, fibrotic changes through increased TGF-β1 signaling, and vascular changes that alter vessel architecture to promote hypoxia in irradiated tissues.65 RT can drastically alter the cellular composition and signaling of the TME.17 In one study, tumors from patients with rectal cancer receiving 25 to 28 fractions of 40- to 45-Gy neoadjuvant CRT had increased CD8+ and CD4+ T cells after treatment compared with baseline tumor sample evaluation.55 However, this proliferation was insufficient to combat the immune tolerance established by immunosuppressive factors such as MDSC, FOX3+ tumor-infiltrating lymphocytes, cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), and PD-L1.55
After RT, dying tumor cells secrete cytokines, chemokines, tumor-associated antigens, and general inflammatory mediators attracting immune cells to the TME.66 RT damage to radiosensitive endothelial cells triggers an inflammatory cascade in which several proinflammatory molecules, including IL-1, IL-6, tumor necrosis factor α (TNF-α), IFN-γ, and GM-CSF, promote the recruitment of MDSCs.17,67 RT induces the secretion of CC chemokines such as CCL2, CCL3, CCL5, CCL8, CCL11, CCL20, and CCL22,66 of which CCL2 and CCL5 have been implicated in M-MDSC and G-MDSC mobilization, respectively.68 In murine breast cancer models, CCL2 promotes CD14+CD16− inflammatory monocyte and CD14lowCD16+ resident monocyte mobilization, promoting tumor extravasation.69 In mouse melanoma models, CCR5 overexpression on MDSCs promotes migration to the primary tumor and potentiates their suppressive behavior.70 In vitro murine studies have demonstrated that the migration of suppressive myeloid cells to the tumor can be prevented by neutralizing CCL2 and blocking CCR2.71 Ultimately, the post-RT TME favors MDSC production through soluble factor concentration, cellular composition, and other cellular factors.
cGAS-STING Pathway Mediates Antitumor Immunity
The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway is involved in cytosolic DNA sensing and is a critical mediator of inflammatory responses (Fig. 1).72 It has emerged as an important target in cancer immunotherapy due to its role in T-cell priming.72,73 In tumor cells, the cGAS-STING pathway is suppressed by the inhibition of IL-6 and downstream JAK2/STAT3 signaling, preventing the induction of CD8+ T-cell function.74,75 Although STING activity is traditionally underactive in tumor cells, RT-mediated DNA damage acts as a danger signal, catalyzing cGAS-STING pathway activation and subsequent inflammatory gene production.76 RT-induced STING activation is a powerful adjuvant tool, promoting the release of type I IFNs and subsequent CD8+ effector T-cell activity.77,78
Fig. 1.
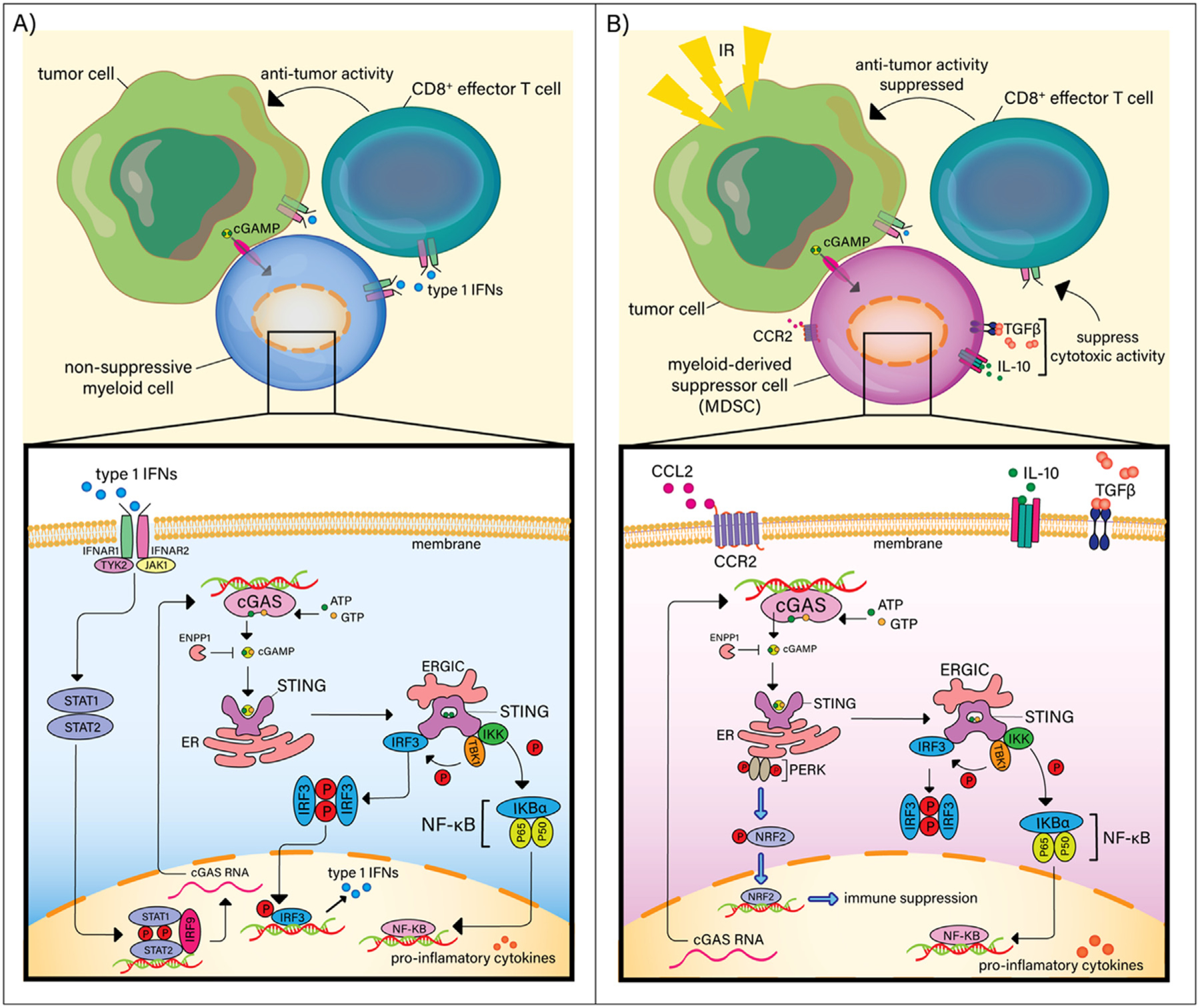
Radiation promotes myeloid-derived suppressor cell (MDSC) accumulation and immunosuppression via STING. (A) In non-suppressive myeloid cells, STING activation results in the recruitment and phosphorylation of tank binding kinase 1 (TBK1) and IκB kinase (IKK), which recruit and phosphorylate interferon regulatory factor 3 (IRF3) and nuclear factor kappa B (NF-κB), resulting in IRF3 and NF-κB translocation into the nucleus and inducing type 1 interferons (IFNs) and immune-stimulating cytokines. (B) Radiation-induced activation of the STING/IFN pathway in MDSCs enhances tumor suppressive activity due to the subsequent CCL2/CCR2-mediated recruitment of MDSCs. In MDSCs, type 1 IFN production is inhibited by PERK-dependent IFNAR1 downregulation and production of NRF-2, which negatively regulate STING, inhibiting subsequent production of type 1 IFNs.
Interestingly, Liang et al found that RT-induced activation of the STING/IFN pathway in tumor cells enhanced suppressive activity in tumors secondary to MDSC recruitment via CCR2, a chemokine overexpressed on the surface of subsets of M-MDSCs.79,80 Their study demonstrated that the recruitment of MDSCs through STING activation within tumor cells increases future radioresistance.79 In classical monocytes, STING activates inflammasomes, triggering the release inflammatory cytokines IL-1β and IL-18 and activating the cell death program.81,82 Unlike other myeloid cells, MDSCs possess unique, intrinsic resistance mechanisms to STING activation (Fig. 1). Traditionally, type 1 IFNs, including IFNα and IFNβ, bind the IFNa receptor (IFNAR), activating JAK1 and tyrosine kinase 2 (TYK2) and phosphorylating the STAT1/STAT2 heterodimer.83 IFNAR1 is downregulated on subsets of MDSCs, resulting in significant inhibition of the IFN 1 pathway and reduced production of type 1 IFNs.84
In MDSCs, upregulated PKR-like endoplasmic reticulum (ER) kinase (PERK) signaling promotes the downregulation IFNAR1.85,86 When RT induces STING activation, a direct physical association between STING and PERK induces phosphorylation of eukaryotic initiation factor 2α (eIF2α), decreasing the rate of protein synthesis, and subsequent production of type 1 IFNs in MDSCs.87–89 Deletion of PERK in mice increases MDSC differentiation into myeloid cells that prime antitumor CD8+ T-cell immunity.86 These previous findings highlight the usefulness of targeting STING-down-regulation in MDSCs to increase radiosensitivity.
MDSC Recruitment by the Hypoxic Tumor Environment
In response to RT damage, tumors secrete hypoxia-inducible factors, potentiating tumor revascularization, promoting MDSC accumulation, and upregulating MDSC immunosuppressive activity (Fig. 2).90–92 Hypoxia is a major barrier to the efficacy of RT and immunotherapy and is linked to MDSC accumulation, infiltration, and maintenance.91,93 Hypoxic stress in tumors triggers endothelial cell signaling and secretion of soluble molecules such as vascular endothelial growth factor (VEGF), which mobilizes MDSCs to the TME.92,94
Fig. 2.
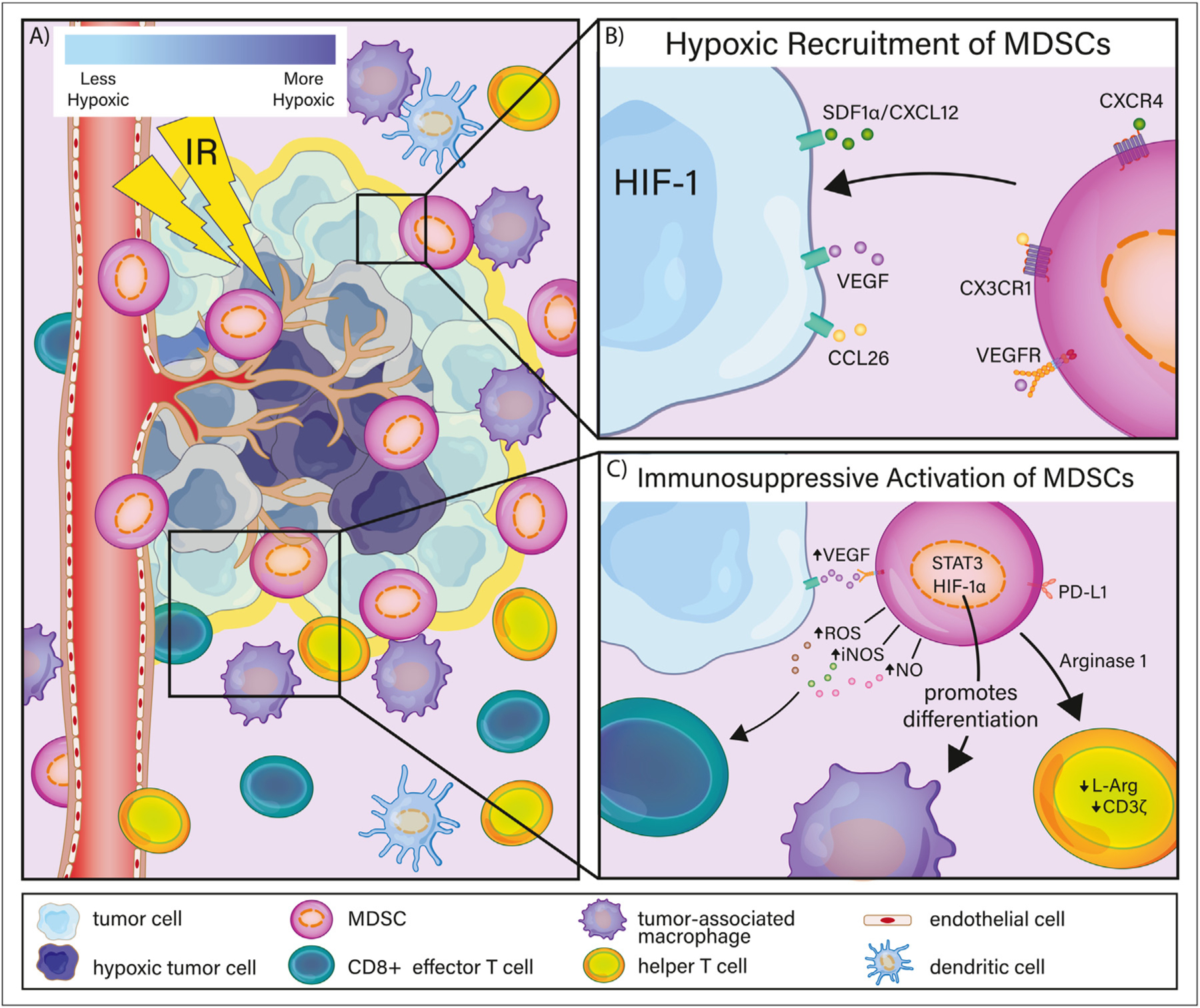
Hypoxic recruitment and activation of myeloid-derived suppressor cells (MDSCs). (A) Radiation therapy induces vascular damage, potentiating tumor hypoxia and releasing hypoxia-inducible factor-1 (HIF-1). (B) After radiation therapy, hypoxic tumor cells secrete soluble factors such as CCL26, SDF1α/CXCL12, and vascular endothelial growth factor (VEGF), mobilizing MDSCs to the tumor microenvironment. (C) After recruitment to the tumor microenvironment, MDSCs interact with several cell types to facilitate immunosuppressive activity. Although hypoxic conditions prevent MDSC differentiation into immune-stimulating dendritic cells, MDSCs are preferentially differentiated into tumor-associated macrophages. The upregulation of arginase-1 on subsets of MDSCs degrades T-cell receptor activity, whereas MDSC production of reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), and nitric oxide (NO) hamper the antitumor cytotoxic activity of CD8+ T cells.
Hypoxia inducible factor 1 (HIF-1) is a heterodimeric protein responsible for activating the transcription of genes associated with cancerous progression.95 In response to RT, tumors protect endothelial cells by secreting cytokines capable of inhibiting apoptosis through a HIF-1–mediated mechanism.90 Moeller et al reported that radiation-induced tumor reoxygenation increased HIF-1 levels after two 5-Gy fractions in mice, suggesting that the upregulation of ROS after RT contributes to HIF-1 accumulation.90 In hepatocellular carcinoma mouse models, HIF-1 induces large amounts of ectoenzyme and ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2/CD39L1), which promote the accumulation of MDSCs in the TME.96
HIF-1 promotes MDSC accumulation through several factors, including VEGF, CCL26, and stromal-derived factor 1a (SDF1α/CXCL12).91,97,98 HIF-1 activation stimulates VEGF production, promoting tumor angiogenesis and tumor immune escape.92,99 RT has been shown to increase VEGF expression.100,101 In vivo and in vitro analyses have shown that VEGF stimulates the mobilization of MDSCs from bone marrow to peripheral blood, and in high-grade ovarian cancer models VEGF expression has been positively correlated with MDSC mobilization and infiltration at the tumor site.102,103 HIF-1 transactivates the enzymatic attachment of phenyl groups to matrix metalloproteinase (MMP)-9, promoting the soluble release of cytokines that bind to VEGFR on MDSCs, such as VEGF.104,105
MMPs promote the invasion and spread of tumor cells by actively degrading surrounding basement membranes and extracellular matrices.104,106 The absence of HIF-1 and subsequent reduction of MMP-9 expression has been observed to abrogate angiogenesis and tumor invasion in humans with glioblastoma, and MMP-9 expressing MDSCs have been shown to decrease in the absence of HIF-1α in glioblastoma mouse models.98 In a mouse model of head and neck squamous cell carcinoma, inhibition of JAK2/STAT3 decreased VEGF and HIF-1α, which effectively prevented angiogenesis and reduced MDSC accumulation in the TME through reduced VEGF and casein kinase 2 (CK2).107 Noman et al deduced that hypoxia induces STAT3 phosphorylation in NSCLC cell lines, promoting the secretion of VEGF and impairing the cytotoxic T lymphocyte–mediated killing of tumor cells.108 These findings indicate that hypoxia is a prominent driver of MDSCs into the TME.
Hypoxic Activation of MDSC Suppressive Activity
As RT modulates the hypoxic tumor milieu, HIF-1α enables MDSC immunosuppression through a variety of mechanisms.92 Induction of HIF-1 after RT may play an important role in maintaining the suppressive phenotype of MDSCs. In one study, MDSCs isolated from spleens of tumor-bearing mice cultured in hypoxic conditions directed their differentiation toward TAMs, a mechanism mediated by HIF-1α.109 Another study found that HIF-1 promotes the conversion of extracellular ATP to 5’AMP, preventing MDSC differentiation into immune-stimulating DCs.96
HIF-1α is also implicated in the release of arginase-1 (Arg-1) from MDSCs.110,111 High Arg-1 expression from subsets of MDSC degrades L-arginine (L-Arg).43,112 When L-Arg is degraded, CD3z is downregulated and the responsiveness and proliferation of T-cell receptor decreases, as well as the production of IFN-γ, IL-5, and IL-10.113,114 Arg-1-expressing MDSCs also promote the upregulation of ROS, inducible nitric oxide synthase (iNOS), and NO.30,115 Murine models suggest that HIF-1α is bound to a hypoxia-response element in the miR-20 promoter, resulting in increased Arg-1 and NO production.110
Other factors, such as TGF-β, IL-6, IL-3, platelet-derived growth factor (PDGF), and GM-CSF, can stimulate MDSC production of ROS.30 Corzo et al reported that tumor MDSCs from tumor-bearing mice expressed high levels of Arg-1 and iNOS and were prone to differentiate into TAMs.109 Nagaraj et al found that the reaction of peroxynitrite, a product of NO and ROS, with MDSC-derived ROS prevents CD8+ T cells from binding phosphorylated MHC and inducing antigen-specific tolerance, suggesting that it is the upregulation of ROS rather than NO that is responsible for suppressing CD8+ T-cell activity.116
MDSC-mediated T-cell suppression also occurs via upregulated PD-L1 signaling.117 In tumor-bearing mice, hypoxia prompts the rapid upregulation of PD-L1 on MDSCs resulting from the direct binding of HIF-1α to the hypoxia-response element in the PD-L1 proximal promoter. In contrast, PD-L1 blockade enhances T-cell activation and down-regulates IL-10 and IL-6.117 PD-L1 is highly expressed in activated tumor-infiltrating MDSCs.118 One study found that PD-L1 blockade significantly enhanced immune function in patients with NSCLC, melanoma, and renal cell cancer.119 Essentially, hypoxia not only recruits more MDSCs to the TME but also enhances their activity.
Mediation of MDSC Expansion in the TME by Several Factors
IL-10 signaling pathways mediate immunosuppressive responses such as the reduction of effector CD4+ and CD8+ T cells and have been strongly associated with cancer progression.120,121 After RT, IL-10 production is upregulated and antagonizes IFN-γ and toll-like receptor signaling pathways, suppressing macrophage and DC-mediated proliferation of antigen-specific CD4+ T cells.122–124 IL-10 also inhibits DC maturation, promoting the maintenance of immature MDSCs by inhibiting IFN-γ-induced production of IL-6, IL-1, IL-4, and TNF-α.125,126 IL-10 inhibits the production of several proinflammatory or Th2 cytokines, leading to the suppression of immune surveillance in the TME.127 IL-10 also activates STAT3 signaling, upregulating DNA methyltransferase 3b expression, leading to the hypermethylation of the IRF8 promoter, effectively silencing IRF8 expression in epithelial cells.128 This IL-10+ MDSC-induced IRF8 abrogation decreases CD8+ T-cell infiltration and effective therapeutic responses.129 In a murine viral infection model, researchers demonstrated a crucial role for IRF8 in the induction of type 1 IFNs, finding that IRF8−/− DCs inhibited type 1 IFN production, while the reintroduction of IRF8 reinstated type 1 IFN induction.129 Furthermore, in mouse colon cancer models, IRF8 silencing has been associated with increased tumorigenesis.128
TGF-β1 has been extensively implicated in tumor progression, invasion, and resistance to traditional modalities of treatment.130 TGF-β1 derives antiproliferative abilities through cyclin kinase activities, such as by inducing inhibitors of p15, p21, and p27 cyclin-dependent kinases and preventing the formation of active cyclin E-cdk2 complexes.131–133 TGF-β1 is bound to a latency-associated peptide, forming a small latent TGF-β complex that can then bind to several latent binding proteins and form a large latent complex.134 TGF-β1 and MDSC interplay creates a procancerous partnership and contributes to widespread tumor progression.135
TGF-β1 mediates the accumulation and induction of MDSCs within the stroma and impairs natural killer cells and regulatory B lymphocyte education/recognition of MDSCs, propagating immunosuppression.136 Hematopoietic progenitors and monocytes cultured with GM-CSF and TGF-β1 induce M-MDSC accumulation.137 Furthermore, TGF-β1-enriched tumor exosomes influence myeloid cells toward an MDSC-like phenotype, contributing to the development and accumulation of protumor MDSCs in the TME.138 MDSCs generated in the presence of TGF-β1 are more effective at suppressing T-cell proliferation and promoting Treg accumulation.139 Murine models have demonstrated that the accumulation and induction of MDSCs are mediated by TGF-β1-induced microRNA (miR) expression.136
TGF-β1 is the primary mediator of miR-494, which aids in recruitment of MDSCs to the TME and contributes to the functional enhancement of CXCR4-mediated MDSC chemotaxis.140 TGF-β also promotes the upregulation of miR-155 and miR-21 during MDSC induction.141 Upon depletion of miR-155 and miR-21, the frequency of cytokine-induced MDSCs decreases.141 Together, these findings elucidate important mechanisms by which RT- and tumor-induced inflammatory signaling stimulate an environment suitable for MDSC induction and recruitment, effectively dampening antitumor immune responses.
Current Clinical Interventions and Future Directions
There is tremendous rationale to combine RT with immunotherapy to minimize RT-induced immunosuppressive effects. Indeed, emerging immunotherapies have shown promise in improving treatment response rates in primary tumors and metastases when combined with RT.142 Yang et al found that both blocking tumor production of lactate and deleting HIF-1α in MDSCs inhibited tumor growth and decreased tumor resistance to RT in mice.143 Oweida et al found that neither radiation nor STAT3 inhibition alone delayed tumor growth in mice but that STAT3 inhibition in combination with a single fraction of 10 Gy decreased quantities of MDSCs, Tregs, and suppressive macrophages and enhanced effector T-cell function.144 Other preclinical animal studies have successfully used agonists such as liver-X nuclear receptors to target MDSC accumulation, in turn decreasing acquired radioresistance.145
Despite these promising results in murine models, clinical studies combining immunotherapy and RT in human populations remain limited and have yielded ambiguous results. In patients with oligometastases, Chen et al found that sunitinib, a tyrosine kinase inhibitor of VEGFR1, VEGFR2, and VEGFR3, in combination with 50 Gy SBRT in 1 fraction decreased M-MDSC accumulation, STAT3 phosphorylation, and arginase expression in M-MDSC.146 Importantly, MDSC response to treatment is a predictor of progression-free survival and treatment-related death, highlighting the potential relevance of MDSC quantification in clinical prognosis.146 In a study using bevacizumab, a monoclonal antibody, to target VEGF in combination with RT and temozolomide, Treg counts decreased but MDSC counts were unaffected.147 Finkelstein et al found that intra-tumorally injected DCs in combination with fractionated RT to a total dose of 50.4 Gy in patients with soft tissue sarcoma induced a tumor-specific immune response in individuals with low MDSC counts but no change in the absolute number of MDSCs.148 These clinical data further highlight that the systemic immune composition can affect the efficacy of treatment and is a target for future manipulation to optimize radio-response. Additional research efforts focusing on clinical applications are needed to target RT-induced immunosuppression.
Conclusion: Tackling Procancerous MDSCs With RT
RT is an effective but complex tool for treating cancers.1 Increasing evidence suggests that RT can promote immunosuppression by depleting radiosensitive lymphocytes and enhance the tumor supportive activity of TAMs and MDSCs.2 MDSCs reprogram the TME toward a suppressive environment by hampering T-cell response and decreasing immune surveillance.30,149 The secretion of soluble factors such as Arg-1, ROS, IL-10, and TGF-β from MDSCs promotes the development of a procancerous niche in the TME.113,116,124,130 Clinically, MDSCs are important as biological response factors to RT, as they provide crucial prognostic indicators of treatment outcomes. Several studies have identified notable increases in MDSC counts after RT.48–54 Several other studies have observed that high MDSC frequency is associated with worse treatment outcomes.58,60–62
Although dose fractionation may play a role in MDSC response, more research is needed to understand the nuances between RT dose and MDSC induction. Given the accumulating evidence that RT increases MDSC accumulation and activation, targeting RT-mediated MDSC expansion is important to maximize the therapeutic effect of RT. There is additional opportunity to advance our understanding of how MDSCs respond to currently available clinical interventions and to identify avenues for future targeted immunotherapies that maximize RT efficacy.
Acknowledgments—
We thank Geezer R. Nelson, recipient of the TEMPRRS2:ERG gene fusion rearrangement at the University of Minnesota, for his generous philanthropic support that made this review possible. We also thank graphic illustrator, Julia Line, from the University of Minnesota, who was compensated for artistic contributions included in this review.
Footnotes
Disclosures: L.R.K. has contracts or grants with Bristol Myers Squibb, Novartis, Novocure, and Incyte. L.S. acknowledges funding from the Department of Radiation Oncology, Masonic Cancer Center, and the Medical School at the University of Minnesota and from the National Institutes of Health under grant numbers P01 CA254849 and U54 CA268069. D.J.O. acknowledges funding from the National Institutes of Health under grant numbers U54 CA210190, P01 CA254849, and U54 CA268069. Geezer R. Nelson supported an undergraduate student summer internship within the Sloan Laboratory at the University of Minnesota. The medical artwork in this publication was funded by startup funding to the Sloan Laboratory at the University of Minnesota Medical School, Minneapolis, MN.
Data Sharing Statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci 2014;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Andrade Carvalho H, Villar RC. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018;73: e557s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell–mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia 2011;13:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw 2015;13:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Xu Z, Ma L, et al. Early onset of severe lymphopenia during definitive radiotherapy correlates with mean body dose and predicts poor survival in cervical cancer. CBM 2022;34:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013;31:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taguchi A, Furusawa A, Ito K, et al. Postradiotherapy persistent lymphopenia as a poor prognostic factor in patients with cervical cancer receiving radiotherapy: A single-center, retrospective study. Int J Clin Oncol 2020;25:955–962. [DOI] [PubMed] [Google Scholar]
- 8.Xie X, Gong S, Jin H, et al. Radiation-induced lymphopenia correlates with survival in nasopharyngeal carcinoma: Impact of treatment modality and the baseline lymphocyte count. Radiat Oncol 2020;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia WY, Zhu XR, Feng W, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio associations with heart and body dose and their effects on patient outcomes in locally advanced non-small cell lung cancer treated with definitive radiotherapy. Transl Lung Cancer Res 2020;9:1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: Low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 2005;62:1423–1426. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123:224–227. [PubMed] [Google Scholar]
- 12.Byun HK, Chung SY, Kim KJ, Seong J. Role of interleukin-7 in the development of and recovery from radiation-induced lymphopenia: A post-hoc analysis of a prospective cohort. Cancer Res Treat 2021;53:962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemo- and immunotherapies. Front Immunol 2017;8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol 2020;11 583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhysh-kowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. OncoImmunology 2019;8 e1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012;7:e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wunderlich R, Ernst A, Rödel F, et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol 2014;179:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen FH, Chiang CS, Wang CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res 2009;15:1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HR, Jo SK, Jung U. Ionizing radiation promotes epithelial–to–mesenchymal transition in lung epithelial cells by TGF-β-producing M2 macrophages. In Vivo 2019;33:1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tesi RJ. MDSC; the most important cell you have never heard of. Trends Pharmacol Sci 2019;40:4–7. [DOI] [PubMed] [Google Scholar]
- 22.Ai L, Mu S, Wang Y, et al. Prognostic role of myeloid-derived suppressor cells in cancers: A systematic review and meta-analysis. BMC Cancer 2018;18:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan L, Sen R, Doucet M, et al. The immunodynamics of myeloid-derived suppressor cell and monocyte populations in the peripheral blood in patients with newly diagnosed glioblastoma undergoing adjuvant temozolomide and radiation therapy. Int J Radiat Oncol Biol Phys 2019;105:E650–E651. [Google Scholar]
- 24.Alban TJ, Bayik D, Otvos B, et al. Glioblastoma myeloid-derived suppressor cell subsets express differential macrophage migration inhibitory factor receptor profiles that can be targeted to reduce immune suppression. Front Immunol 2020;11:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrand-Rosenberg S, Horn LA, Ciavattone NG. Radiotherapy both promotes and inhibits myeloid-derived suppressor cell function: Novel strategies for preventing the tumor-protective effects of radiotherapy. Front Oncol 2019;9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard W, Dufait I, Schwarze JK, et al. Myeloid-derived suppressor cells reveal radioprotective properties through arginase-induced l-arginine depletion. Radiother Oncol 2016;119:291–299. [DOI] [PubMed] [Google Scholar]
- 27.Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-Derived suppressor cells: Paradoxical roles in infection and immunity. J Innate Immun 2015;7:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandau S, Dorhoi A, eds. Myeloid-Derived Suppressor Cells. Vol. 2236. Springer US; 2021. [Google Scholar]
- 29.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res 2009;30:7–15. [DOI] [PubMed] [Google Scholar]
- 32.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009;58:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechner MG, Megiel C, Russell SM, et al. Functional characterization of human Cd33+ And Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 2011;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Z, Zhang S. Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Front Immunol 2021;12 741305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan M, Zheng M, Niu R, et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol 2022;10 938289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021;54:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 2021;21:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss L, Sangaletti S, Consonni FM, et al. RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell 2015;28:253–269. [DOI] [PubMed] [Google Scholar]
- 41.Mehmeti-Ajradini M, Bergenfelz C, Larsson AM, et al. Human G-MDSCs are neutrophils at distinct maturation stages promoting tumor growth in breast cancer. Life Sci Alliance 2020;3 e202000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condamine T, Dominguez GA, Youn JI, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonu-clear myeloid-derived suppressor cells in cancer patients. Sci Immunol 2016;1:aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I–producing myeloid-derived suppressor cells in renal cell carcinoma are a sub-population of activated granulocytes. Cancer Res 2009;69:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papiez L, Timmerman R. Hypofractionation in radiation therapy and its impact: Radiation therapy hypofractionation. Med Phys 2007;35:112–118. [DOI] [PubMed] [Google Scholar]
- 45.Yang JF, Lo CH, Lee MS, et al. Stereotactic ablative radiotherapy versus conventionally fractionated radiotherapy in the treatment of hepatocellular carcinoma with portal vein invasion: A retrospective analysis. Radiat Oncol 2019;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YH, Yu CF, Yang YC, Hong JH, Chiang CS. Ablative radiotherapy reprograms the tumor microenvironment of a pancreatic tumor in favoring the immune checkpoint blockade therapy. IJMS 2021;22:2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015;21:3727–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv M, Zhuang X, Shao S, et al. Myeloid-derived suppressor cells and CD68+CD163+M2-like macrophages as therapeutic response biomarkers are associated with plasma inflammatory cytokines: A preliminary study for non-small cell lung cancer patients in radiotherapy. J Immunol Res 2022;2022:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sridharan V, Margalit DN, Lynch SA, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer 2016;115:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampath S, Won H, Massarelli E, et al. Combined modality radiation therapy promotes tolerogenic myeloid cell populations and STAT3-related gene expression in head and neck cancer patients. Oncotarget 2018;9:11279–11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parikh F, Duluc D, Imai N, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res 2014;74:7205–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boustani J, Joseph ELM, Martin E, et al. Cisplatin-based chemoradiation decreases telomerase-specific CD4 TH1 response but increases immune suppressive cells in peripheral blood. BMC Immunol 2021;22:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Meir H, Nout RA, Welters MJP, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. OncoImmunology 2017;6 e1267095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talebian Yazdi M, Schinkelshoek MS, Loof NM, et al. Standard radiotherapy but not chemotherapy impairs systemic immunity in non-small cell lung cancer. OncoImmunology 2016;5 e1255393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, fork-head box P3, programmed death ligand-1, and cytotoxic T lymphocyte–associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res 2015;166:721–732.e1. [DOI] [PubMed] [Google Scholar]
- 56.Linares-Galiana I, Berenguer-Frances MA, Cañas-Cortés R, et al. Changes in peripheral immune cells after intraoperative radiation therapy in low-risk breast cancer. J Radiat Res 2021;62:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol 2011;13:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Napolitano M, D’Alterio C, Cardone E, et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 2015;6:8261–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navarro-Martín A, Galiana I, Berenguer Frances M, et al. Preliminary study of the effect of stereotactic body radiotherapy (SBRT) on the immune system in lung cancer patients unfit for surgery: Immuno-phenotyping analysis. IJMS 2018;19:3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14+HLA-DR−/low myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumor Biol 2016;37:10427–10433. [DOI] [PubMed] [Google Scholar]
- 61.Chen MF, Chen PT, Kuan FC, Chen WC. The predictive value of pre-treatment neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. Ann Surg Oncol 2019;26:190–199. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. OTT 2017;10:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson NM, Simon MC. The tumor microenvironment. Curr Biol 2020;30:R921–R925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as a “game changer” in cancer radiotherapy. IJMS 2019;20:3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Jiang J, Chen Y, Jia Q, Chu Q. The roles of CC chemokines in response to radiation. Radiat Oncol 2022;17:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through toll-like receptor 4. J Leukoc Biol 2009;85:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li R, Mukherjee MB, Lin J. Coordinated regulation of myeloid-derived suppressor cells by cytokines and chemokines. Cancers 2022;14:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Umansky V, Blattner C, Gebhardt C, Utikal J. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother 2017;66:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 2007;252:86–92. [DOI] [PubMed] [Google Scholar]
- 72.Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol 2021;21:548–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang M, Chen P, Wang L, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol 2020;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suter MA, Tan NY, Thiam CH, et al. cGAS–STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells. Sci Rep 2021;11:7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demaria O, De Gassart A, Coso S, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA 2015;112:15408–15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storozynsky Q, Hitt MM. The impact of radiation-induced DNA damage on cGAS-STING-mediated immune responses to cancer. IJMS 2020;21:8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon–dependent innate and adaptive immunity. Cancer Res 2011;71:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim JYH, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol Immunother 2014;63:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang H, Deng L, Hou Y, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun 2017;8:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu HX, Arumugam TV, Gelderblom M, Magnus T, Drummond GR, Sobey CG. Role of CCR2 in inflammatory conditions of the central nervous system. J Cereb Blood Flow Metab 2014;34:1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaidt MM, Ebert TS, Chauhan D, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 2017;171:1110–1124.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol 2021;18:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014;14:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alicea-Torres K, Sanseviero E, Gui J, et al. Immune suppressive activity of myeloid-derived suppressor cells in cancer requires inactivation of the type I interferon pathway. Nat Commun 2021;12:1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J, HuangFu WC, Kumar KGS, et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe 2009;5:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohamed E, Sierra RA, Trillo-Tinoco J, et al. The unfolded protein response mediator perk governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 2020;52:668–682.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhattacharya S, Qian J, Tzimas C, et al. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J Biol Chem 2011;286:22069–22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teske BF, Wek SA, Bunpo P, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell 2011;22:4390–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olagnier D, Brandtoft AM, Gunderstofte C, et al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat Commun 2018;9:3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors. Cancer Cell 2004;5:429–441. [DOI] [PubMed] [Google Scholar]
- 91.Chiu DKC, Xu IMJ, Lai RKH, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016;64:797–813. [DOI] [PubMed] [Google Scholar]
- 92.Chouaib S, Umansky V, Kieda C. The role of hypoxia in shaping the recruitment of proangiogenic and immunosuppressive cells in the tumor microenvironment. 2018;2018:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. IJN 2018;13:6049–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. In: Sen CK, ed. Methods in Enzymology. Vol. 381. Elsevier; 2004:335–354. [DOI] [PubMed] [Google Scholar]
- 95.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–732. [DOI] [PubMed] [Google Scholar]
- 96.Chiu DKC, Tse APW, Xu IMJ, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun 2017;8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med Oncol 2020;37:2. [DOI] [PubMed] [Google Scholar]
- 98.Du R, Lu KV, Petritsch C, et al. HIF1a induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008;13:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takenaga K Angiogenic signaling aberrantly induced by tumor hypoxia. Front Biosci 2011;16:31. [DOI] [PubMed] [Google Scholar]
- 100.Kil WJ, Tofilon PJ, Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol 2012;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park JS, Qiao L, Su ZZ, et al. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 2001;20:3266–3280. [DOI] [PubMed] [Google Scholar]
- 102.Koinis F, Vetsika EK, Aggouraki D, et al. Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non–small cell lung cancer. J Thorac Oncology 2016;11:1263–1272. [DOI] [PubMed] [Google Scholar]
- 103.Horikawa N, Abiko K, Matsumura N, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res 2017;23:587–599. [DOI] [PubMed] [Google Scholar]
- 104.Choi JY, Jang YS, Min SY, Song JY. Overexpression of MMP-9 and HIF-1α in breast cancer cells under hypoxic conditions. J Breast Cancer 2011;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002;109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pritchard SC, Nicolson MC, Lloret C, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: Implications for MMP inhibition therapy. Oncol Rep 2001;8:421–424. [PubMed] [Google Scholar]
- 107.Liu JF, Deng WW, Chen L, et al. Inhibition of JAK2/STAT3 reduces tumor-induced angiogenesis and myeloid-derived suppressor cells in head and neck cancer. Mol Carcinog 2018;57:429–439. [DOI] [PubMed] [Google Scholar]
- 108.Noman MZ, Buart S, Van Pelt J, et al. The cooperative induction of hypoxia-inducible factor-1α and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 2009;182:3510–3521. [DOI] [PubMed] [Google Scholar]
- 109.Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010;207:2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noman MZ, Janji B, Hu S, et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res 2015;75:3771–3787. [DOI] [PubMed] [Google Scholar]
- 111.Maenhout SK, Van Lint S, Emeagi PU, Thielemans K, Aerts JL. Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared with their peripheral counterparts. Int J Cancer 2014;134:1077–1090. [DOI] [PubMed] [Google Scholar]
- 112.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004;64:5839–5849. [DOI] [PubMed] [Google Scholar]
- 113.Zea AH, Rodriguez PC, Culotta KS, et al. L-arginine modulates CD3z expression and T cell function in activated human T lymphocytes. Cell Immunol 2004;232:21–31. [DOI] [PubMed] [Google Scholar]
- 114.Raber P, Ochoa AC, Rodríguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012;41:614–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol 2018;9:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007;13:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014;211:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. OncoImmunology 2016;5 e1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alhakeem SS, McKenna MK, Oben KZ, et al. Chronic lymphocytic leukemia-derived IL-10 suppresses antitumor immunity. JI 2018;200:4180–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bornstein S, Schmidt M, Choonoo G, et al. IL-10 and integrin signaling pathways are associated with head and neck cancer progression. BMC Genomics 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol 2001;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meziani L, Robert C, Classe M, et al. Low doses of radiation increase the immunosuppressive profile of lung macrophages during viral infection and pneumonia. Int J Radiat Oncol Biol Phys 2021;110: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (IL-10)-mediated immunosuppression. J Biol Chem 2015;290:27158–27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology 2002;107:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–3822. [PubMed] [Google Scholar]
- 127.Armstrong L, Jordan N, Millar A. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax 1996;51:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ibrahim ML, Klement JD, Lu C, et al. Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell Reports 2018;25:3036–3046.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tailor P, Tamura T, Kong HJ, et al. The feedback phase of type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity 2007;27:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117–129. [DOI] [PubMed] [Google Scholar]
- 131.Koff A, Ohtsuki M, Polyak K, Roberts JM, Massagué J. Negative regulation of G1 in mammalian cells: Inhibition of cyclin e-dependent kinase by TGF-β. Science 1993;260:536–539. [DOI] [PubMed] [Google Scholar]
- 132.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 1995;92:5545–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin M, Lefaix JL, Delanian S. TGF-β1 and radiation fibrosis: A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000;47:277–290. [DOI] [PubMed] [Google Scholar]
- 134.Anscher MS. Targeting the TGF-β1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 2010;15:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mojsilovic S, Mojsilovic SS, Bjelica S, Santibanez JF. Transforming growth factor-beta1 and myeloid-derived suppressor cells: A cancerous partnership. Dev Dynam 2022;251:85–104. [DOI] [PubMed] [Google Scholar]
- 136.Santibanez JF, Bjelica S. Transforming growth factor-beta1 and myeloid-derived suppressor cells interplay in cancer. TOCIJ 2017;6:1–14. [DOI] [PubMed] [Google Scholar]
- 137.Casacuberta-Serra S, Parés M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol 2017;95:538–548. [DOI] [PubMed] [Google Scholar]
- 138.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009;124:2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gonzalez-Junca A, Driscoll KE, Pellicciotta I, et al. Autocrine TGFb is a survival factor for monocytes and drives immunosuppressive lineage commitment. Cancer Immunol Res 2019;7:306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. JI 2012;188:5500–5510. [DOI] [PubMed] [Google Scholar]
- 141.Li L, Zhang J, Diao W, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. JI 2014;192:1034–1043. [DOI] [PubMed] [Google Scholar]
- 142.Shevtsov M, Sato H, Multhoff G, Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front Oncol 2019;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang X, Lu Y, Hang J, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res 2020;8:1440–1451. [DOI] [PubMed] [Google Scholar]
- 144.Oweida AJ, Darragh L, Phan A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst 2019;111:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang H, Shen X. LXR activation radiosensitizes non-small cell lung cancer by restricting myeloid-derived suppressor cells. Biochem Biophys Res Commun 2020;528:330–335. [DOI] [PubMed] [Google Scholar]
- 146.Chen HM, Ma G, Gildener-Leapman N, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res 2015;21:4073–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomas AA, Fisher JL, Hampton TH, et al. Immune modulation associated with vascular endothelial growth factor (VEGF) blockade in patients with glioblastoma. Cancer Immunol Immunother 2017;66: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Finkelstein SE, Fishman M, Conley AP, Gabrilovich D, Antonia S, Chiappori A. Cellular immunotherapy for soft tissue sarcomas. Immunotherapy 2012;4:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zalfa C, Paust S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front Immunol 2021;12 633205. [DOI] [PMC free article] [PubMed] [Google Scholar]


