Abstract
The steroid hormone progesterone acts via high-affinity nuclear receptors that interact with specific DNA sequences located near the promoter of the hormone-responsive gene. Recent studies suggested that the hormone-occupied progesterone receptor (PR) mediates gene activation by recruiting a cellular coregulatory factor, termed coactivator, to the target promoter. The identity and mechanism of action of the coactivator(s) that regulates transcriptional activity of PR are currently under investigation. Here we provide evidence that the hormone-occupied PR forms a multisubunit receptor-coactivator complex containing two previously described coactivators, CREB-binding protein (CBP) and steroid receptor coactivator 1 (SRC-1, a member of the p160 family of coactivators), in nuclear extracts of human breast tumor T47D cells. The association of CBP and SRC-1/p160 with the receptor complex is entirely hormone dependent. Both CBP and SRC-1/p160 possess intrinsic histone acetyltransferase (HAT) activity, and it has been recently proposed that these coactivators function by modulating chromatin structure at the promoter of the target gene. Interestingly, addition of purified CBP to the nuclear extracts of T47D cells markedly stimulated progesterone- and PR-dependent transcription from a nucleosome-free, progesterone response element (PRE)-linked reporter DNA template. Furthermore, depletion of SRC-1/p160 by immunoprecipitation from these transcriptional extracts also significantly impaired PR-mediated RNA synthesis from a naked PRE-linked DNA template. These results strongly implied that CBP and SRC-1/p160 facilitate receptor-mediated transcription in these cell extracts through mechanisms other than chromatin remodeling. We also observed that the adenoviral oncoprotein E1A, which interacts directly with CBP, repressed PR-mediated transactivation when added to the nuclear extracts of T47D cells. Supplementation with purified CBP overcame this inhibition, indicating that the inhibitory effect of E1A is indeed due to a blockade of CBP function. Most importantly, we noted that binding of E1A to CBP prevented the assembly of a coactivation complex containing PR, CBP, and SRC-1/p160, presumably by disrupting the interaction between CBP and SRC-1/p160. These results strongly suggested that E1A repressed receptor-mediated transcription by blocking the formation or recruitment of coactivation complexes. Collectively, our results support the hypothesis that the assembly of a multisubunit coactivation complex containing PR, CBP, and SRC-1/p160 is a critical regulatory step during hormone-dependent gene activation by PR and that the fully assembled complex has the ability to control transcription through mechanisms that are independent of the histone-modifying activities of its component coactivators.
The steroid hormone progesterone profoundly influences the development and function of tissues such as the uterus, ovary, mammary gland, and brain (6, 13, 35, 49). In the human, the physiological effects of progesterone are mediated through high-affinity progesterone receptor isoforms PR-A and PR-B that are located in the nuclei of target cells (23). PRs belong to the steroid-thyroid receptor superfamily and, like other members of this family, regulate the transcription of specific cellular genes in a hormone-dependent manner (7, 54). Their transcriptional activity, however, is strongly influenced by cell and promoter contexts (16, 55). Whereas PR-B functions as an efficient transactivator of progesterone-responsive genes in all cells tested, the transcriptional activity of PR-A varies widely depending on the cell and promoter types (56). The initial step in the gene regulatory pathway of PR appears to be the interaction of the hormone-occupied receptor with specific DNA sequences located near the target promoter (3). Previous studies using crude transcriptional extracts indicated that PR stimulates mRNA synthesis by facilitating the assembly of a transcription initiation complex containing RNA polymerase II and the basal transcription machinery (4, 27). Recent studies from several laboratories indicated that the DNA-bound, hormone-occupied PR mediates gene activation by recruiting a cellular coregulator, termed coactivator, to the target promoter (see reference 20 and 24 for reviews; 44, 58, 63). The polypeptide components of the coactivator and its mechanism of action in PR-mediated transactivation, however, remain unclear.
During the past 4 years, using yeast two-hybrid assay, far-Western cloning, and biochemical methods based on affinity chromatography, several groups have reported the isolation of novel nuclear receptor-interacting proteins that may serve as coactivators during hormone-induced transactivation (12, 19, 20, 22, 24, 33, 44, 45, 52, 58). A hallmark of these putative coactivators is that they interact with the nuclear receptors in a ligand-dependent manner. Onate et al. initially reported the cloning of steroid receptor coactivator 1 (SRC-1), which functions as a coactivator of the transactivation pathways of several nuclear hormone receptors including PR (44). Additional receptor-interacting proteins, TIF2/GRIP1 and pCIP/ACTR/RAC3/AIB1, which show striking structural similarity to SRC-1, were isolated by other laboratories (1, 12, 22, 33, 44, 52, 58). The pairwise similarities were estimated to be 64% between SRC-1 and TIF-2/GRIP1, 60% between SRC-1 and pCIP/ACTR/RAC3/AIB1, and 65% between TIF-2/GRIP1 and pCIP/ACTR/RAC3/AIB1 (33). The remarkable structural similarity among these proteins, all of which exhibit an approximate molecular size of 160 kDa, indicates the existence of a p160 family of nuclear receptor coactivators. All members of the p160 family are found to interact with the ligand-binding domain (LBD) of PR in an agonist-dependent manner in vitro and significantly enhance progesterone-dependent transactivation of a responsive promoter in transient transfection experiments (33, 44, 58).
In addition to the p160 family of proteins, other potential coactivators have been described (20, 24). Prominent among these is the transcriptional coactivator CREB-binding protein (CBP) (32). It has been reported that CBP and its homologue p300 interact with several nuclear receptors in a hormone-dependent fashion and augment their transcriptional activity (10, 26). Interestingly, CBP/p300 also interacts with p160 proteins, and these two classes of coactivators act together in a synergistic fashion to enhance ligand-dependent transactivation mediated by PR or the estrogen receptor (ER) (47). All p160 proteins as well as CBP/p300 possess intrinsic histone acetyltransferase (HAT) activity and are potential modulators of chromatin structure at the target promoter (5, 12, 43, 48). Microinjection of an anti-SRC-1 or anti-CBP antibody into cultured cells partially blocked ligand-dependent gene activation by PR, ER, other nuclear receptors (10, 52). Most importantly, gene knockout studies show that loss of SRC-1 function partially impaired physiological actions of ER and PR (60). These results indicate that the p160 family of proteins, CBP/p300, and possibly additional cofactors act in unison with the activated nuclear receptors and the basal transcription apparatus to effect steroid-dependent gene activation.
In this study, we investigated the functional contribution of CBP and SRC-1 in PR-mediated gene activation in nuclear extracts of human breast carcinoma T47D cells. Our studies revealed the formation of a multisubunit coactivation complex containing hormone-occupied PR, CBP, and SRC-1/p160. Interestingly, both CBP and SRC-1/p160 are found to be essential for efficient PR-mediated transactivation of nucleosome-free target genes in T47D nuclear extracts. These results suggested that the PR-coactivator complex facilitates transcription of a target gene through alternative mechanisms that are independent of the HAT activities of the coactivators. We also observed that the viral oncoprotein E1A, which binds to CBP, severely impaired PR-mediated transactivation. Our studies indicated that E1A binding to CBP results in the disruption of the PR-CBP-SRC-1/p160 complex. The assembly of the functional coactivation complex therefore emerges as a novel control point for regulation of gene activation by the nuclear receptors.
MATERIALS AND METHODS
Materials.
Affinity matrix containing an immobilized monoclonal antibody that recognizes the influenza virus hemagglutinin (HA) epitope, YPYDVPDYA, was purchased from Covance, Denver, Pa. The free HA peptide was also obtained from the same source. Protein A-Sepharose and glutathione-Sepharose resins were purchased from Amersham-Pharmacia Biotech, Piscataway, N.J.
Antibodies.
A truncated human SRC-1 cDNA encoding amino acids 379 to 1440 was incorporated in a pET15b vector (Novagen, Madison, Wis.) and expressed in Escherichia coli according to previously published procedures (51). Several hexahistidine-tagged proteolytic fragments of SRC-1 were isolated by Ni affinity chromatography, and a mixture of these peptides was used as an antigen to raise a polyclonal antibody in the rabbit. Rabbit polyclonal antibodies against CBP and the polyhistidine domain were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif.
Culture of T47D cells and preparation of transcriptional extracts.
T47D human breast cancer cells were cultured in a growth medium containing 5% charcoal-stripped fetal calf serum, and nuclear extracts were prepared from these cells as described previously (3, 63).
Cell-free transcription assay.
The conditions for progesterone-dependent, cell-free transcription in T47D nuclear extracts and isolation of 32P-labeled transcripts have been described previously (3, 63).
Baculovirus expression and purification of PR-B and CBP.
Sf9 insect cells were grown in spinner vessels in Grace's insect medium/TNM-FH (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, Utah). An N-terminal HA-tagged recombinant CBP (a gift of P. Lieberman) was expressed in Sf9 cells. Cells were infected with the recombinant virus at a multiplicity of infection of 5 for 40 h at 27°C. Cells were harvested, and nuclear extracts were prepared by Dounce homogenization in buffer C containing 20 mM HEPES, 20% glycerol, 0.2 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, and 400 mM KCl. Nuclear extracts were then incubated with resin linked to a monoclonal antibody against HA (Covance) for 2 h at 4°C with end-over-end rotation. The resin was washed three times with buffer D (20 mM HEPES [pH 7.9], 20% glycerol, 0.2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, 100 mM KCl). The bound proteins were eluted at 30°C with buffer D containing 1 mg of HA peptide per ml. After elution, dithiothreitol was added to each fraction to a final concentration of 1 mM. Protein expression was confirmed by Western blotting using a CBP antibody. The purified HA-tagged CBP was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining and was typically 70 to 80% pure.
Baculovirus containing recombinant hexahistidine-tagged hPR-B (hPRO) and its mutants hPRΔCore and hPRABC were constructed as described previously (28). These proteins were expressed in Sf9 cells as described above. The cells were harvested 48 h postinfection. Nuclear extracts were prepared by Dounce homogenization in buffer C containing 350 mM NaCl; 100 μl of a 1:1 suspension of Ni-nitrilotriacetic acid (NTA)-agarose (Qiagen, Valencia, Calif.) was added to each nuclear extract, and the mixtures were incubated at 4°C for 1 h with constant end-over-end rotation. The resin was washed three times with buffer C containing 15 mM imidazole and a mixture of protease inhibitors. The resin-bound PR and its mutants were then used in in vitro protein interaction experiments.
Isolation of PR-coactivator complexes.
Immobilized hexahistidine-tagged PR-B and its mutants were treated with or without progesterone (1 μM) at room temperature for 15 min and then incubated with approximately 1 mg of T47D nuclear extracts for an additional 2 h at 4°C with mild agitation. The protein-bound resin was washed three times with phosphate buffered saline. For purification of the PR-CBP-SRC-1 complex, buffer C containing 160 mM imidazole was used as the elution buffer. For other experiments, the bound proteins were eluted by boiling with SDS-gel sample buffer and then subjected to Western blotting using antibodies against hexahistidine tag (PR-B), CBP, and SRC-1.
Expression and purification of E1A.
A full-length cDNA encoding the 13S form of E1A with 5′ NdeI and 3′ BamHI ends was introduced between NdeI and BamHI sites of the bacterial expression vector pET15b (Novagen). The expression of E1A as a hexahistidine (linked to the amino terminus of the protein) fusion protein in E. coli and its purification by nickel affinity chromatography were carried out as described previously (51). The Ni affinity-purified E1A preparations were typically greater than 90% pure as estimated by SDS-PAGE. To generate glutathione S-transferase (GST)-tagged E1A, we incorporated E1A with 5′ NdeI and 3′ BamHI ends between NdeI and BamHI sites of the bacterial expression vector GSTpET15b. Crude bacterial lysates containing GST-tagged E1A were prepared following a previously published procedure (51); the fusion protein was immobilized on glutathione-Sepharose and used in certain reactions.
Immunodepletion of SRC-1 from T47D nuclear extracts.
Anti-SRC-1 polyclonal antibody or control immunoglobulin G (10 μg) was immobilized on protein A-agarose (25 μl) by incubation at 4°C for 2 h. The resin-bound antibody was then incubated with T47D nuclear extracts (100 to 200 μg) at 4°C for 2 h with mild agitation. The resin was separated from the extract by brief centrifugation. The pellet and the supernatant were collected and tested for the presence of SRC-1 by Western blotting. The supernatant was then assayed for its ability to support progesterone-induced transactivation as described in Results.
RESULTS
Hormone-dependent interactions of PR-B with CBP and SRC-1/p160.
Our previous studies showed that PR mediates progesterone-induced transactivation in nuclear extracts of T47D cells (3, 63). We also demonstrated that depletion of putative coactivator(s) from these extracts by a competing ligand-bound nuclear receptor impairs PR-dependent transactivation (63). The coactivator(s) that plays a role in the transcriptional response of PR, however, remains unknown. Recent studies indicated that SRC-1 and CBP are potential coactivators of PR and other nuclear receptors (10, 26, 44, 47). We therefore investigated whether PR interacts with endogenous CBP and SRC-1 in T47D nuclear extracts in a hormone-dependent fashion.
For this purpose, we incubated nuclear extracts of T47D cells, which were grown in charcoal-stripped serum, with purified hexahistidine-tagged PR-B immobilized on Ni affinity resin in the presence or in the absence of progesterone. Following extensive washing of the resin-bound receptor, the bound proteins were eluted and analyzed by Western blotting using antibodies against SRC-1, CBP, and the hexahistidine moiety of PR (Fig. 1). As expected, both hormone-free and hormone-bound PR-B were equally retained on the Ni affinity column (lanes 1 and 2). No SRC-1 or CBP signal was observed in the protein fraction containing hormone-free PR-B (lanes 3 and 5). In contrast, SRC-1 and CBP were present in the protein fraction containing hormone-occupied PR-B only (lanes 4 and 6). These results showed that SRC-1/p160 and CBP associate with PR in a progesterone-dependent manner. Both of these proteins therefore fulfill a major criterion to serve as the coactivator of PR in T47D nuclear extracts. It is important to mention here that although we used a polyclonal antibody that was raised against SRC-1, we cannot rule out the possibility that the antibody cross-reacts with other members of the p160 family and the observed signal may include contributions from these proteins. We therefore refer to the SRC-1 signal as SRC-1/p160 throughout the text.
FIG. 1.
Ligand-dependent interaction of PR with CBP and SRC-1. Nuclear extracts (1 mg) of Sf9 cells containing baculovirus-expressed hexahistidine-tagged PR-B were incubated with 50 μl of Ni-NTA-agarose in the presence or absence of progesterone (1 μM). The resin-bound PR was washed extensively and then incubated with T47D nuclear extracts (1 mg) for an additional 2 h at 4°C. The column was washed three times with phosphate-buffered saline and the bound proteins were eluted by SDS-gel sample buffer. The eluted proteins were subjected to Western blotting using antibodies against the hexahistidine tag (lanes 1 and 2), CBP (lanes 3 and 4), and SRC-1 (lanes 5 and 6). The arrows indicate the full-length polypeptides.
Evidence for the generation of a hormone-dependent PR-CBP-SRC-1/p160 complex.
The hormone-dependent binding of SRC-1/p160 and CBP to PR-B raised the possibility that these coactivators are components of a multiprotein receptor-coactivator complex. Alternatively, SRC-1/p160 and CBP could exist in distinct receptor-coactivator complexes. To distinguish between these possibilities and to further analyze the nature of the association of SRC-1/p160 and CBP with ligand-bound PR-B, we performed the experiment described in Fig. 2. We initially incubated nuclear extracts of T47D cells with immobilized hexahistidine-tagged PR-B in the presence or in the absence of progesterone as described in the legend to Fig. 1. We expected that under these conditions, all possible receptor-coactivator complexes such as PR-SRC-1/p160, PR-CBP, and PR-CBP-SRC-1/p160, will be retained by the affinity resin. The proteins that bound to hormone-free or hormone-bound PR-B were eluted under native conditions and then subjected to immunoprecipitation with an anti-CBP antibody. One would expect that of all the possible populations of PR-coactivator complexes, only those containing CBP, such as PR-CBP and PR-CBP-SRC-1/p160, will be immunoprecipitated by the CBP antibody. For the sake of convenience, we will refer to the immunoprecipitates obtained from the proteins eluted from hormone-free and hormone-occupied PR-B as minus-hormone IP and plus-hormone IP, respectively. Western analyses of both types of IP were performed using antibodies against PR, CBP, and SRC-1.
FIG. 2.
Isolation of a hormone-dependent PR-CBP-SRC-1/p160-complex. Baculovirus-expressed hexahistidine-tagged PR-B was immobilized on Ni-NTA-agarose and incubated with T47D nuclear extracts in the presence or in the absence of progesterone (1 μM) as described in the legend to Fig. 1. The bound proteins were eluted with buffer containing 160 mM imidazole. The eluted proteins were incubated for 2 h at 4°C with a CBP antibody immobilized on protein A-agarose. The protein A beads were washed extensively, and the bound proteins were eluted by SDS-gel sample buffer. The eluted proteins were subjected to Western blotting using antibodies against the hexahistidine tag (lanes 1 and 2), CBP (lanes 3 and 4), and SRC-1 (lanes 5 and 6). Arrows indicate the full-length polypeptides.
As shown in Fig. 2, PR-B was detected only in the plus-hormone IP (lanes 1 and 2). Whereas only a trace amount of CBP was detected in the minus-hormone IP (lane 3), a robust CBP signal was observed in the plus-hormone IP (lane 4). These results are entirely consistent with the results of Fig. 1, indicating that PR interacts with CBP in a hormone-dependent manner. As expected, no SRC-1/p160 signal was detected in the minus-hormone IP (lane 5). Surprisingly, an intense signal was detected in the plus-hormone IP upon Western blotting with the SRC-1 antibody. The most likely explanation for the appearance of the SRC-1/p160 signal in plus-hormone IP is the generation of a hormone-dependent multisubunit receptor-coactivator complex containing that PR, CBP, and SRC-1/p160 in the T47D cell extracts.
PR LBD is required for the formation of a coactivation complex.
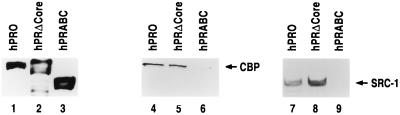
PR-B harbors two major transactivation functions, AF-1 and AF-2 (9, 38). The core of the AF-1 activity is located within the amino acids 457 to 538 (38). The AF-2 function, on the other hand, is located within the carboxy-terminal amino acids 642 to 933 containing the LBD of PR (14). A third transactivation function, AF-3, is located within the N-terminal 164 amino acids of PR-B, and this appears to function only in restricted cell and promoter contexts (46). Previous studies indicated that SRC-1 and CBP could individually interact with the LBD of PR and other nuclear receptors (10, 26, 44). Recent reports, however, suggested that the N-terminal region containing AF-1 of certain nuclear receptors can also interact with SRC-1 (53, 59). We therefore investigated the region of PR-B that is involved in interactions with the coactivator complex. For this purpose, we tested two PR mutants for interaction with the coactivator complex: (i) hPRΔCore, lacking the AF1 core function, and (ii) hPRABC, lacking the LBD and the AF-2 function. These PR mutants, as well as the full-length PR-B, were immobilized on an affinity resin and incubated with T47D nuclear extracts treated with or without progesterone. The receptor-bound proteins were analyzed for the presence of CBP and SRC-1/p160. We found that, like the wild-type PR, hPRΔCore bound to SRC-1/p160 and CBP in a hormone-dependent manner (Fig. 3, lanes 5 and 8). In contrast, hPRABC did not exhibit any significant binding to either SRC-1/p160 or CBP irrespective of hormone (Fig. 3, lanes 6 and 9). Our results clearly indicated that the LBD of PR is critical for binding to the CBP-SRC-1/p160 coactivator complex.
FIG. 3.
PR LBD is necessary for interaction with the coactivator complex. Nuclear extracts (1 mg) of Sf9 cells containing baculovirus-expressed hexahistidine-tagged wild-type PR (hPRO) and its mutants hPRΔCore and hPRABC were incubated with 50 μl of Ni-NTA-agarose in the presence of progesterone (1 μM). The resin-bound wild-type and mutant PRs were washed extensively and incubated with T47D nuclear extracts as described in the legends to Fig. 1 and 2. The column-bound proteins were eluted by SDS-gel sample buffer and subjected to Western blotting using antibodies against the hexahistidine tag (lanes 1 to 3), CBP (lanes 4 to 6), and SRC-1 (lanes 7 to 9).
CBP enhances PR-mediated transactivation of a nucleosome-free PRE-linked DNA template.
Previous studies indicated that overexpression of a coactivator often leads to enhanced transcriptional activity of a nuclear receptor (10, 12, 26, 44, 47). Since CBP is a component of the hormone-dependent PR-coactivator complex in T47D extracts, we sought to examine whether addition of this cofactor influences the hormone-dependent transcriptional activity of the receptor. For this purpose, we used a previously described cell-free transcription system in which progesterone induced RNA synthesis from a progesterone response element (PRE)-driven promoter in nuclear extracts of T47D cells (3). The transcriptional activation was triggered by the hormone-induced binding of endogenous PRs to PREs. Correct initiation of transcription from the PRE-containing test template resulted in the synthesis of a 360-nucleotide transcript (Fig. 4B, solid arrowhead). A slightly longer transcript for the test template represents basal transcripts initiated randomly on the template upstream of the G-free region and processed by T1 RNase present in the transcription reaction to generate a full-length G-free cassette product. As shown in Fig. 4B, lane 1, in the absence of progesterone, only basal transcription was observed from the test template. In the presence of progesterone, activated PR bound to the PREs and directed significant (about 10-fold) induction in the synthesis of accurately initiated transcripts from the test promoter (Fig. 4B, lane 2, solid arrowhead).
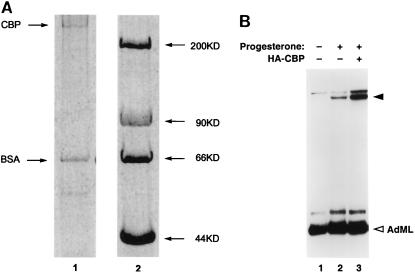
FIG. 4.
CBP stimulates PR-dependent transcription from a chromatin-free DNA template. (A) SDS-PAGE analysis of purified baculovirus-produced CBP. An N-terminal HA-tagged recombinant CBP was expressed in Sf9 cells and purified by HA immunoaffinity chromatography as described in Materials and Methods. An aliquot of the purified CBP preparation was analyzed by SDS-PAGE, and its polypeptide components were visualized by silver staining (lane 1). Bovine serum albumin (BSA) was added in the elution buffer as a carrier protein. Lane 2 indicates the molecular weight markers. (B) T47D nuclear extracts were preincubated at 25°C with or without 1 μM progesterone for 15 min. The nuclear extracts were assayed in cell-free transcription reactions containing the test DNA template PRE2TATA and control DNA template pAdML200. Purified HA-tagged CBP (10 pmol) was added as indicated to transcription reactions. The transcription reactions (volume, 30 μl each) were carried out, and the transcripts were analyzed as described previously (3, 63). The solid and open arrowheads indicate the correctly initiated transcripts generated from the PRE-linked promoter and the internal control AdML promoter, respectively. The CBP preparation was typically greater than 80% pure. The concentration of CBP was determined by Coomassie staining followed by comparison of the intensity of the CBP band with that of BSA of known concentration.
Recombinant HA epitope-tagged CBP was produced in baculovirus-infected insect cells and was purified by anti-HA immunoaffinity chromatography (Fig. 4A). Addition of highly purified CBP to nuclear extracts of T47D cells did not significantly affect transcription from a control adenovirus major late (AdML) promoter (Fig. 4B). The addition of CBP, however, led to a marked stimulation of progesterone- and PR-dependent transactivation from a PRE-linked reporter template (compare lane 3 with lane 2). By our estimation, the magnitude of this stimulation was at least four- to fivefold greater than that induced by hormone-bound PR in these extracts. Previous studies reported that CBP harbors a HAT activity, which is thought to regulate chromatin structure (5, 43). However, in the cell-free transcription system, the DNA templates are mostly devoid of functional nucleosomes. Our results therefore suggested that CBP, which is recruited in a PR-coactivator complex in a hormone-dependent manner, is a rate-limiting coactivator capable of significantly enhancing receptor-induced RNA synthesis even from a chromatinless target DNA template.
SRC-1/p160 is essential for PR-mediated transactivation.
Since SRC-1 is a component of the multiprotein complex containing hormone-bound PR and CBP, we next investigated whether it also functions as a coactivator of PR in T47D nuclear extracts. To test this possibility, our approach was to remove endogenous SRC-1 from these extracts by immunoprecipitation and analyze the effects of its depletion on PR-mediated transactivation. Incubation of T47D nuclear extracts with an immobilized SRC-1 antibody led to a significant (>80%) depletion of this protein from these extracts (Fig. 5A, lanes 3 and 4). We then assessed the hormone-dependent transcriptional activity of PR in these depleted extracts by using a naked DNA template containing PREs. As shown in Fig. 5B, immunodepletion of SRC-1 markedly inhibited progesterone-induced transactivation by PR (compare lanes 2 and 4). By our estimate, the PR-dependent transactivation was inhibited 65 to 70% upon removal of the SRC-1 protein. As mentioned above, we cannot rule out the possibility that the SRC-1 antibody may cross-react with and deplete other members of the p160 family from these extracts. Nevertheless, our results demonstrated that SRC-1, and perhaps other members of the p160 family, play an essential role in PR-dependent transcription in T47D nuclear extracts. Most importantly, the results of Fig. 4 and 5 strongly indicated that the coactivator functions of SRC-1/p160 and CBP are not limited to chromatin templates. It is therefore reasonable to conclude that these cofactors enhance PR-mediated transactivation via mechanisms that are independent of their HAT activities. Recently a similar conclusion about the role of SRC-1 in receptor-dependent transcription was reached by Liu et al. (34).
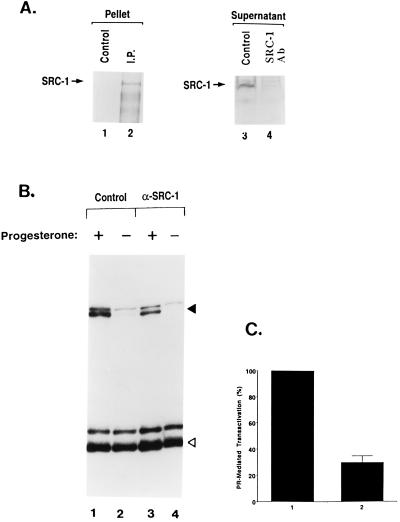
FIG. 5.
Immunodepletion of SRC-1 impairs PR-dependent transcription. Aliquots (50 μl each) of nuclear extracts (protein concentration, 5 mg/ml) of hormone-free T47D cells were preincubated with SRC-1 antibody or control antibody immobilized on protein A-linked agarose as described in Materials and Methods. (A) The immunoprecipitate and supernatant were analyzed by SDS-PAGE followed by Western blotting with SRC-1 antibody. (B) Aliquots (15 μl each) of control (lanes 1 and 2) and SRC-1-depleted (lanes 3 and 4) nuclear extracts were assayed for progesterone-induced RNA synthesis in cell-free transcription reactions as described previously (3, 63). The solid and open arrowheads indicate the correctly initiated transcripts generated from the PRE-linked promoter and the internal control AdML promoter, respectively. Six independent sets of the same experiment were performed, and the results of a representative experiment are shown. (C) Intensities of the RNA transcripts (360 nucleotides) generated from the test template were quantified by densitometry and normalized with respect to the AdML transcripts. The value of the progesterone-dependent transcription supported by 15 μl of control nuclear extract was adjusted to 100% (lane 1). The standard error of the mean value of the SRC-1-depleted extract is shown (lane 2).
Oncoprotein E1A blocks PR-mediated transactivation.
Since CBP appears to play a crucial role in PR-mediated transactivation, we reasoned that inhibition of its function would also impair this process. It has been observed previously that the oncoprotein E1A physically interacts with CBP and inhibits its HAT activity and transactivation function (11, 61). Indeed, we observed that E1A strongly interacts with endogenous CBP in T47D nuclear extracts. In the experiment described in Fig. 6A, we incubated GST-E1A or GST (control) immobilized on glutathione resin with T47D nuclear extracts. Analysis of the resin-bound polypeptides by Western blotting revealed that GST-E1A, but not GST, efficiently retained CBP present in the nuclear extracts (compare lane 1 with lane 2 or 3).
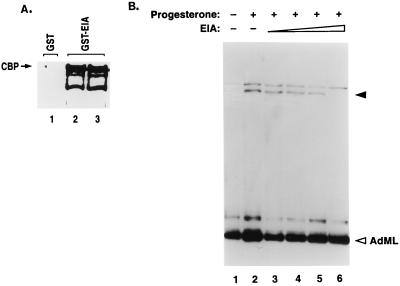
FIG. 6.
Repression of PR-dependent transcription by E1A. (A) E1A interacts with CBP in nuclear extracts of T47D cells. Bacterially expressed GST-E1A or GST was immobilized on glutathione-linked Sepharose as described in Materials and Methods. The resin was then incubated with T47D nuclear extracts for 2 h at 4°C with gentle agitation. The bound proteins were eluted by SDS-gel sample buffer and subjected to Western blotting using an antibody against CBP. Lane 1, 1 μg of GST; lanes 2 and 3, 1 and 2 μg of GST-E1A, respectively. (B) Transcriptional inhibition by E1A. Increasing amounts of purified bacterially expressed recombinant E1A (7, 15, 22, and 30 pmol) were added to the cell-free transcription reactions containing progesterone-treated T47D nuclear extracts. The E1A protein was preincubated with T47D nuclear extracts for 15 min on ice before transcription reaction was performed as described previously (3, 63). The solid and open arrowheads indicate the correctly initiated transcripts generated from the PRE-linked promoter and the internal control AdML promoter, respectively. The concentration of E1A was determined by comparing the intensity of the stained E1A band with that of BSA in SDS-PAGE. Two independent sets of the same experiment were performed, and the results of one representative experiment are shown.
We next analyzed the functional effects of the binding of E1A to CBP on PR-dependent transactivation in T47D nuclear extracts. For this purpose, we added increasing amounts of purified recombinant E1A to such extracts and monitored the effects on PR-induced RNA synthesis. As shown in Fig. 6B, addition of up to 30 pmol of E1A did not have any significant effect on transcription from a control AdML promoter. E1A addition, on the other hand, progressively inhibited PR-mediated transactivation. These results strongly implied that binding of E1A to CBP impaired its function as a coactivator in PR-mediated gene activation.
Supplementation of CBP reverses E1A-mediated repression of PR-dependent transcription.
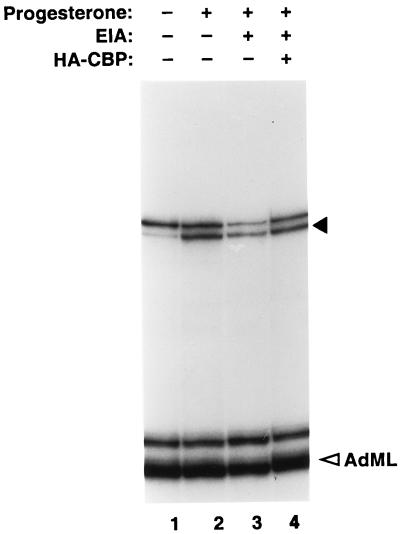
By definition, if E1A represses hormone-dependent transactivation by PR by directly interacting with and inhibiting the function of endogenous CBP in T47D nuclear extracts, one would expect that this repression would be relieved by adding back excess CBP to these extracts. To test this hypothesis, we performed the experiment described in Fig. 7. As observed above, addition of E1A specifically inhibited progesterone-induced transactivation by PR (compare lanes 2 and 3). This E1A-mediated repression was efficiently reversed upon addition of purified recombinant CBP to these extracts (lane 4). These results are therefore consistent with our view that CBP, an essential coactivator of PR, is a major target of E1A in T47D nuclear extracts, and a functional consequence of this interaction is the inhibition of receptor-mediated transactivation.
FIG. 7.
Supplementation of CBP alleviates E1A-mediated repression of PR-dependent transcription. E1A (30 pmol) was preincubated with or without purified CBP (30 pmol) for 30 min on ice before addition to the cell-free transcription reactions containing progesterone-treated T47D nuclear extracts. The transcription reactions (volume, 30 μl each) were carried out, and the transcripts were analyzed as described previously (3, 63). The solid and open arrowheads indicate the correctly initiated transcripts generated from the PRE-linked promoter and the internal control AdML promoter, respectively. Lane 3, E1A without CBP; lane 4, E1A with CBP.
Binding of E1A to CBP inhibits assembly of the coactivation complex.
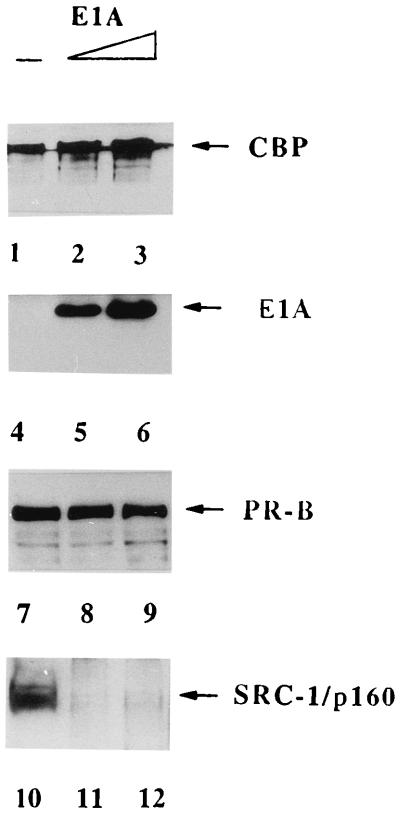
It has been reported that E1A inhibits the HAT activity of CBP/p300 (11). However, it is unlikely that such a mechanism is operative during the E1A-mediated repression of PR-dependent transcription from a nucleosome-free template in our in vitro system. To address the mechanism of E1A-induced inhibition, we therefore considered the possibility that the binding of E1A to CBP alters the molecular interactions within the receptor-coactivator complex. To investigate this, we initially incubated hexahistidine-tagged PR-B with hormone-treated T47D nuclear extracts in the presence or in the absence of E1A. The resulting receptor-coactivator complexes were isolated by binding to nickel affinity resin and elution by imidazole. The eluted proteins, a mixture of all potential receptor coactivator complexes, were then immunoprecipitated with an anti-CBP antibody. The immunoprecipitate, which would include complexes containing CBP, was analyzed by Western blotting to check for the presence PR-B, CBP, SRC-1, and E1A. As described before, in the absence of E1A, immunoprecipitation of CBP coprecipitated PR-B and SRC-1/p160, indicating the formation of a PR-CBP-SRC-1/p160 complex (Fig. 8, lanes 1, 7, and 10). Addition of increasing amounts of E1A led to an increased binding of this protein to the immunoprecipitated complex (lanes 5 and 6). Binding of E1A to CBP resulted in a partial reduction in the PR-B signal associated with CBP (compare lane 7 with lanes 8 and 9). Strikingly, E1A binding to CBP led to a complete disappearance of the SRC-1/p160 signal in the CBP immunoprecipitate (compare lane 10 with lanes 11 and 12). These results strongly suggested that binding of E1A to CBP alters the interactions between the components of the PR-CBP-SRC-1/p160 complex in such a way that it prevents the recruitment of a key component, SRC-1/p160, to the coactivation complex. We propose that this disruption of the assembly of a functional coactivation complex contributes to the E1A-mediated repression of PR-induced transactivation as seen in Fig. 6.
FIG. 8.
E1A binding to CBP inhibits the assembly of a coactivation complex. Progesterone-treated hexahistidine-tagged PR-B was immobilized on Ni-NTA-agarose and incubated with T47D nuclear extracts as described in the legend to Fig. 1. Purified hexahistidine-tagged E1A (none, 1 μg, and 2 μg) was added during the incubation as indicated. The bound proteins were eluted with buffer containing 160 mM imidazole. The eluted proteins were immunoprecipitated with a CBP antibody immobilized on protein A-agarose. The protein A beads were washed extensively, and the bound proteins were eluted by SDS-gel sample buffer. The eluted proteins were subjected to Western blotting using antibodies against the hexahistidine tag, CBP, and SRC-1. The arrows indicate the full-length CBP (lanes 1 to 3), E1A (lanes 4 to 6), PR-B (lanes 7 to 9), and SRC-1/p160 (lanes 10 to 12).
DISCUSSION
Recent studies from many laboratories indicated that most members of the steroid-thyroid-retinoid receptor superfamily require coactivators in addition to RNA polymerase II transcription machinery to activate target gene transcription. The coactivators are thought to be dispensable for basal transcription but are essential to mediate the transcriptional response by the activated hormone receptors (20, 23). A coactivator for a nuclear receptor is expected to interact with the receptor in a ligand-dependent manner. Furthermore, receptor-mediated transactivation but not the basal transcription should be enhanced upon addition of the coactivator protein to a receptor-regulated gene expression system. In the present study, we observed that SRC-1/p160 and CBP interact with PR in a ligand-dependent manner. Addition of purified CBP significantly enhanced PR-mediated transactivation in a cell-free transcription system. Moreover, immunodepletion of SRC-1/p160 markedly repressed PR-mediated transactivation in such a system. Therefore, according to the criteria described above, both CBP and SRC-1/p160 qualified as coactivators of PR in human T47D breast carcinoma cells.
Previous studies by Kamei et al. indicated that a receptor-coactivator complex may contain both SRC-1/p160 and CBP (26). These workers used recombinant thyroid hormone receptor bound to an amino-terminal (positions 1 to 450) fragment of CBP to pull down SRC-1 from whole cell extracts of CV1 cells. The formation of a ternary complex between SRC-1/p160, CBP, and a liganded nuclear receptor was not surprising, as binary interactions between each pair of these components had been shown to occur (10, 26). Later McKenna et al. performed fractionation on a gel filtration column in an attempt to isolate coactivator complexes bound to liganded PR in T47D nuclear extracts (37). Although they were successful in isolating complexes containing PR and members of p160 family of proteins, they failed to isolate any receptor complex containing CBP. These studies indicated that a binary PR-CBP or a ternary PR-CBP-SRC-1/p160 complex, if it exists, is less stable than the PR-SRC-1/p160 complexes under the fractionation conditions employed by McKenna et al. (37). Using a combination of affinity chromatography and immunoprecipitation, we now demonstrate that a stable coactivation complex containing PR, CBP, and SRC-1/p160 can indeed be isolated. In this complex SRC-1/p160 and CBP simultaneously participate in a multiprotein receptor-coactivator complex that is formed on the LBD of the hormone-bound PR. The functional requirements for both CBP and SRC-1/p160 in PR-dependent transactivation is consistent with the presence of these cofactors in the coactivation complex. Although we focused on only these two classes of coactivators in this study, it is conceivable that the coactivation complex contains an additional cofactor(s). A possible candidate is PCAF, a protein that was previously reported to interact with CBP/p300 as well as members of p160 family and function as a coactivator for nuclear receptors and other transcription factors (8, 12, 29, 42, 48).
The details of the molecular interactions between PR, CBP, and SRC-1/p160 within the receptor-coactivator complex remain to be worked out. A number of plausible scenarios should be considered. Each member of the p160 family contains several hydrophobic LXXLL signature motifs that are thought to be involved in receptor-coactivator interactions (21, 52). Crystal structure analysis suggested that certain of these motifs fit into a shallow hydrophobic groove created by the folding of helices within the LBD of hormone-occupied nuclear receptors (15, 36). In one possible scenario, SRC-1 or another p160 family member is in direct contact with the receptor, and CBP/p300 is recruited to this complex primarily via its interactions with the p160 protein. In support of this model, it has been reported that a C-terminal region of CBP/p300 binds to SRC-1 in a receptor-independent manner (26, 62). In another scenario, CBP/p300 may interact directly with the nuclear receptor LBD and SRC-1/p160 is recruited through interaction with the C terminus of CBP. This concept is consistent with the observation that the N-terminal domain (1 to 450) of CBP/p300 directly interacts with several nuclear receptors, including retinoic acid, thyroid, retinoid X, and glucocorticoid receptors, in a ligand-dependent manner (10, 26). This domain of CBP/p300 contains two LXXLL motifs, either one or both of which may potentially interact with the receptor (52). Finally, simultaneous contacts of SRC-1/p160 and CBP/p300 with the receptor LBD should also be considered. It is interesting that the LBD of PR appears to be sufficient for the assembly of a coactivation complex. In our experiments, the N-terminal region of PR containing the AF-1 and AF-3 functions displayed no detectable interaction with SRC-1 and only very weak interaction with CBP (Fig. 3). These findings, however, do not rule out the possibility that this domain may interact with the coactivators in response to agents that induce chemical modifications such as phosphorylation (53).
Recent studies in a number of laboratories indicated that a coactivator might function by regulating chromatin structure at the target promoter via histone acetylation (50). While hypoacetylation of histones is thought to create a repressive chromatin conformation leading to gene repression, hyperacetylation may destabilize nucleosomes on a chromatin DNA. This, in turn, is likely to allow the binding of a RNA polymerase II transcription initiation complex at the core promoter, leading to gene activation. It is therefore postulated that a promoter-bound transcription factor such as a steroid hormone receptor may facilitate transcriptional activation by recruiting one or more HATs to the target promoter (12, 29, 48). Consistent with this hypothesis, many of the candidate nuclear receptor coactivators such as CBP, PCAF, and SRC-1/p160 are known to possess intrinsic HAT activity (5, 12, 43, 48). Interestingly, recent reports suggested that CBP/p300 or SRC-1 enhanced steroid receptor-dependent transactivation from a chromatinized hormone-responsive template (30, 34). Although it is possible that this effect may involve modulation of chromatin, it has not been determined whether HAT activity of CBP/p300 or SRC-1 plays any role in this process.
A growing body of evidence, however, suggests that chromatin remodeling may not be the only mechanism by which a coactivator mediates its transcriptional response. It was observed that the loss or inhibition of histone deacetylase function in yeast, Drosophila, or mammalian cells does not always correlate with enhanced transcription but produces rather complex, mixed effects involving defects in both repression as well as activation (57). Moreover, several nuclear receptors function as activators or repressors in cell-free transcription experiments where the majority of the reporter DNA templates are not packaged into chromatin (3, 4, 19, 51). Our present studies showed that addition of exogenous CBP to T47D nuclear extracts stimulated PR-dependent transcription from a reporter DNA template that is devoid of any functional nucleosomal structure. These results suggest that beyond its possible involvement in chromatin structure modulation, a coactivator(s) may activate transcription by an alternative or additional mechanism that may not involve acetylation of core histones. One of the possible mechanisms of PR-mediated activation may involve the coactivator(s) providing a functional link between the hormone-bound receptor and the basal transcription machinery. In support of such a scenario, it has been demonstrated that CBP/p300 can interact with certain components of the RNA polymerase II initiation complex (32, 40, 41). While such interactions may promote gene activation by stabilizing the transcription initiation complex through protein-protein interactions, recent studies compel us to also consider the possible involvement of targeted acetylation of certain basal initiation factors (25).
Our studies with the adenoviral E1A oncoprotein also support the concept that the coactivators may regulate nuclear receptor function by mechanisms independent of any chromatin modulatory activity. E1A is known to inhibit cellular differentiation by repressing a number of cellular enhancers and promoters (2, 17, 39). Such inhibitory effects of E1A correlate well with its ability to interact directly with CBP/p300 and retinoblastoma proteins (18, 61). E1A binding to CBP/p300 was reported to disrupt its interaction with other coactivators such as PCAF and p/CIP (31, 61). Another important consequence of E1A binding to CBP/p300 is the repression of the HAT activity of this coactivator (11). In this study, we observed that E1A repressed PR-dependent transactivation in cell-free transcriptional extracts (Fig. 6). We clearly showed that CBP is the target of E1A-mediated repression since this inhibition is alleviated by addition of excess CBP. However, as our in vitro transcription system contained only chromatin-free DNA templates, it is highly unlikely that the observed repression by E1A is due to an inhibition of HAT activity of CBP/p300 or PCAF. We therefore turned our attention to test whether E1A had any effect on the stability of the receptor-coactivator complex. We found that addition of E1A inhibited the assembly of a PR-CBP-SRC-1/p160 complex. Based on these results, we favor the hypothesis that E1A inhibits the PR-dependent transcription by disrupting the assembly of a coactivation complex rather than inhibiting the chromatin modulatory function of a component coactivator(s).
It is conceivable that one or more of the potential receptor-coactivator complexes, such as PR-CBP, PR-SRC-1/p160, and PR-CBP-SRC-1/p160, may contribute to the PR-mediated transactivation observed in our cell-free transcription system. CBP, which markedly enhanced PR-mediated transcription, is likely to act by boosting the levels of rate-limiting amounts of PR-CBP or PR-CBP-SRC-1/p160. Since E1A targets CBP to exert its repressive effects, it is reasonable to assume that it inhibits the function of either PR-CBP or PR-CBP-SRC-1/p160. While E1A binding to CBP completely abolished its interaction with SRC-1/p160, the level of CBP-associated PR decreased only marginally even in the presence of excess E1A (Fig. 8). We will therefore argue that the inhibition of assembly of a PR-CBP-SRC-1/p160 coactivation complex is a major contributory factor in the strong repression displayed by E1A. We speculate that the CBP-SRC-1/p160 interaction is crucial to hold together the functional coactivation complex. E1A binding to CBP may sterically block the recruitment of SRC-1/p160 to the complex. Alternatively, E1A binding may induce a conformational change in CBP that triggers the dissociation of SRC-1/p160 from the coactivation complex. Finally, one should also consider the possibility that E1A binding to CBP also leads to the dissociation of additional cofactors such as PCAF, that might contribute to the stability and function of such a complex.
The pathway of assembly of a multisubunit coactivator complex is currently unknown. Multiple interactions between coactivators of nuclear receptors are known to generate a variety of subcomplexes. It has been documented that binary complexes form in vitro between SRC-1 and CBP/p300, SRC-1 and TIF2, CBP/p300 and PCAF, and SRC-1 and PCAF in a receptor-independent manner (26, 37, 48, 52). These findings raise the intriguing possibility that such smaller subcomplexes may exist in the cell and the generation of a fully functional higher-order coactivation complex would require sequential assembly of these subcomplexes. The specific subcomplex(es) recruited by a promoter-bound nuclear receptor may be dependent on the relative abundance and stability of this complex in the cell as well as its affinity for that particular receptor. In this way, combinatorial diversity in the nature and function of the fully assembled coactivation complex may be achieved. It is therefore conceivable that transcriptional response of the receptor will be regulated by factors that affect the formation and steady-state levels of these subcomplexes. This concept is strengthened by our observation that binding of E1A to CBP disrupts its interaction with SRC-1/p160. A decline in the steady-state level of CBP-SRC-1/p160 may inhibit efficient assembly of the coactivation complex. Further studies are clearly necessary to gain insights into the precise composition of the coactivation complex and the mechanisms by which its assembly is regulated in the cell.
ACKNOWLEDGMENTS
We thank Raquel Marin-Cruzado for initiating the studies of PR-coactivator complexes. We thank David Savitsky for raising the polyclonal antibody against human SRC-1 and Ronald Baker for the culture of T47D cells. We are grateful to Paul Lieberman, Wistar Institute, Philadelphia, Pa., for the gift of the baculovirus expressing HA-tagged CBP. We also acknowledge Srilata Bagchi, University of Illinois, Chicago, for the gift of E1A 13S cDNA. We thank Jean Schweis for carefully reading the manuscript and Evan Read for preparing the artwork.
This work was supported by the NIH grants R01 DK 50257-05 and U54 HD13541-18 (SCCPRR), the New York State Breast Cancer Research and Education Fund, and a grant from the Gustavus and Louise Pfeiffer Foundation.
REFERENCES
- 1.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X-Y, Sauter G, Kallioniemi O-P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi M K, Tsai S Y, Tsai M-J, O'Malley B W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990;345:547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- 4.Bagchi M K, Tsai S Y, Weigel N L, Tsai M-J, O'Malley B W. Regulation of in vitro transcription by progesterone receptor: characterization and kinetic studies. J Biol Chem. 1990;265:5129–5134. [PubMed] [Google Scholar]
- 5.Bannister A J, Kouzarides T. The CBP coactivator is a histoneacetyl transferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 6.Baulieu E E. Contragestion and other clinical applications of RU486, an antiprogesterone at the receptor. Science. 1989;245:1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- 7.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 8.Blanco J, Minucci S, Lu J, Yang X-J, Walker K K, Chen H, Evans R M, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocquel M T, Kumar V, Stricker C, Chambon P, Gronemeyer H. The contribution of N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989;17:2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarti D, Ogryzko V, Kao H-Y, Nash A, Chen H, Nakatani Y, Evans R M. A viral mechanism for inhibition of p300 and PCAF acetyl transferase activity. Cell. 1999;96:393–404. doi: 10.1016/s0092-8674(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Lin R J, Schiltz R L, Chakravarty D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 13.Clarke C L, Sutherland R L. Progestin regulation of cellular proliferation. Endocrine Rev. 1990;11:266–300. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 14.Danielian P S, White R, Lees J A, Parker M G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darimont B D, Wagner R L, Apriletti J W, Stallcup M R, Kushner P J, Baxter J D, Fletterick R J, Yamamoto K R. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12:3343–3356. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denner L A, Weigel N L, Maxwell B L, Schrader W T, O'Malley B W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 17.Dyson N, Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- 18.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 19.Fondell J D, Ge H, Roeder R G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93:8323–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman L P. Increasing the complexity of coactivation in nuclear receptor signaling. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 21.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional coactivators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 22.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, A novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz K B, Alexander P S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983;113:2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 25.Imhof A, Yang X-J, Ogryzko V, Nakatani Y, Wolff A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 26.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 27.Klein-Hitpass L, Tsai S Y, Weigel N L, Allan G A, Riley D, Rodriguez R, Schrader W T, Tsai M-J, O'Malley B W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable pre-initiation complex. Cell. 1990;60:247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- 28.Klotzbucher M, Schwerk C, Holewa B, Klein-Hitpass L. Activation of transcription by progesterone receptor involves derepression of activation functions by a cofactor. Mol Endocrinol. 1997;11:768–778. doi: 10.1210/mend.11.6.0016. [DOI] [PubMed] [Google Scholar]
- 29.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T-M, Glass C K, Rosenfeld M G. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 30.Kraus W L, Kadonaga J T. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurokawa R, Kalafus D, Ogliastro M-H, Kioussi C, Xu L, Torchia J, Rosenfeld M G, Glass C K. Differential use of CREB binding protein-coactivator complexes. Science. 1998;279:700–703. doi: 10.1126/science.279.5351.700. [DOI] [PubMed] [Google Scholar]
- 32.Kwok R P S, Lundland J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Gomes P J, Chen J D. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Wong J, Tsai S Y, Tsai M-J, O'Malley B W. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc Natl Acad Sci USA. 1999;96:9485–9490. doi: 10.1073/pnas.96.17.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Shyamala G, Conneely O M, O'Malley B W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 36.McInerney E M, Rose D W, Flynn S E, Westin S, Mullen T-M, Krones A, Inostroza J, Nolte R T, Assa-Munt N, Milburn M V, Glass C K, Rosenfeld M G. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–3368. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna N J, Nawaz Z, Tsai S Y, Tsai M-J, O'Malley B W. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer M E, Pornon A, Ji J, Bocquel M T, Chambon P, Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990;9:3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran E. DNA tumor virus transforming proteins and the cell cycle. Curr Opin Genet Dev. 1993;3:63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T, Uchida C, Anderson S F, Lee C-G, Hurwitz J, Parvin J D, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M. Analysis of a cAMP-responsive activator reveals a two component mechanism for transcriptional induction via signal dependent factors. Genes Dev. 1997;11:738–743. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 42.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X-J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetyl transferase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 43.Ogryzko V V, Schlitz R L, Russanova V, Howard B H, Nakatani Y. The transcription coactivators p300 and CBP are histone acetyltransferase. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 44.Onate S A, Tsai S Y, Tsai M-J, O'Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 45.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Ligand-dependent transactivation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 46.Sartorius C A, Melville M Y, Hovland A R, Tung L, Takimoto G S, Horwitz K B. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B isoform. Mol Endocrinol. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- 47.Smith C L, Nawaz Z, O'Malley B W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 48.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M-J, O'Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 49.Strauss J F, Gurpide E. The endometrium: regulation and dysfunction. In: Yen S S C, Jaffe R B, editors. Reproductive endocrinology: physiology, pathophysiology, and clinical management. W. B. Philadelphia, Pa: Saunders Co.; 1991. pp. 309–357. [Google Scholar]
- 50.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 51.Tong G-X, Jeyakumar M, Tanen M R, Bagchi M K. Transcriptional silencing by unliganded thyroid hormone receptor β requires a soluble corepressor that interacts with the ligand-binding domain of the receptor. Mol Cell Biol. 1996;16:1909–1920. doi: 10.1128/mcb.16.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional coactivator p/CIP binds CBP and mediates nuclear receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 53.Tremblay A, Tremblay G B, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Cell. 1999;3:513–520. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 54.Tsai M-J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 55.Tzukerman M T, Esty A, Santiso-Mere D, Danielian P, Parker M G, Stein R B, Pike J W, McDonnell D P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994;8:21–29. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 56.Vegeto E, Shahbaz M M, Wen D X, Goldman M E, O'Malley B W, McDonnell D P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 57.Vidal M, Gaber R F. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 59.Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen M P, Chen D, Huang S M, Subramanian S, McKinerney E, Katzenellenbogen B S, Stallcup M R, Kushner P J. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M-J, O'Malley B W. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 61.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y A. p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 62.Yao T-P, Ku G, Zhou N, Scully R, Livingston D M. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Jeyakumar M, Bagchi M K. Ligand-dependent cross-talk between steroid and thyroid hormone receptors. J Biol Chem. 1996;271:14825–14833. [PubMed] [Google Scholar]