Abstract
We have recently reported that skeletal muscle of the ob/ob mouse, an animal model of genetic obesity with extreme insulin resistance, exhibits alterations in the expression of multiple genes. Analysis and cloning of a full-length cDNA of one of the overexpressed mRNAs revealed a 300-amino-acid protein that could be identified as the mouse geranylgeranyl diphosphate synthase (GGPP synthase) based on its homology to proteins cloned from yeast and fungus. GGPP synthase catalyzes the synthesis of all-trans-geranylgeranyl diphosphate (GGPP), an isoprenoid used for protein isoprenylation in animal cells, and is a branch point enzyme in the mevalonic acid pathway. Three mRNAs for GGPP synthase of 4.3, 3.2, and 1.7 kb were detected in Northern blot analysis. Western blot analysis of tissue homogenates using specific antipeptide antibodies revealed a single band of 34.8 kDa. Expression level of this protein in different tissues correlated with expression of the 4.3- and 3.2-kb mRNAs. GGPP synthase mRNA expression was increased 5- to 20-fold in skeletal muscle, liver, and fat of ob/ob mice by Northern blot analysis. Western blot analysis also showed a twofold overexpression of the protein in muscle and fat but not in liver, where the dominant isoform is encoded by the 1.7-kb mRNA. Differentiation of 3T3-L1 fibroblasts into adipocytes induced GGPP synthase expression more than 20-fold. Using the immunoprecipitated protein, we found that mammalian GGPP synthase synthesizes not only GGPP but also its metabolic precursor farnesyl diphosphate. Thus, the expression of GGPP synthase is regulated in multiple tissues in obesity and is induced during adipocyte differentiation. Altered regulation in the synthesis of isoprenoids for protein prenylation in obesity might be a factor determining the ability of the cells to respond to hormonal stimulation requiring both Ras-related small GTPases and trimeric G protein-coupled receptors.
Protein prenylation is a posttranslational modification that involves covalent binding of isoprenoid lipids to conserved cysteine residues at or near the C termini of a varied group of proteins (6). Proteins undergoing prenylation include Ras and Ras-related small GTP-binding proteins, such as Rho, Rab, Rac, the γ subunit of the trimeric G proteins, and others. Many of these proteins are involved in signal transduction pathways and play important roles in regulation of cell replication and differentiation, cytoskeletal organization, and vesicular trafficking. Most prenylated proteins require membrane localization for normal activity, and the isoprenoid modification is generally essential for this membrane association. Mutation of the prenylation site or blockade of isoprenoid biosynthesis abolishes both prenylation and membrane association of the protein and usually results in a lost of normal protein function in the cell (14, 39). The isoprenoid moieties used in this modification, farnesyl diphosphate (FPP) (11) and geranylgeranyl diphosphate (GGPP) (10, 29), are isoprenoid diphosphates of 15 and 20 carbons, respectively, synthesized in the initial portion of the mevalonic acid pathway. Both are substrates for branch point reactions that result in a large variety of isoprenoid compounds. In plants and photosynthetic bacteria, GGPP is the precursor of a great number of different compounds, including carotenoids and the phytol moiety of chlorophyll; in animal cells, however, its only known function is to provide the prenyl moiety for protein prenylation. In contrast, FPP, its metabolic precursor, is also the prenyl moiety of heme a and the common precursor of sterol and nonsterol products of the pathway, such as cholesterol, ubiquinone, and dolichol (17). Recent data also have suggested a functional role of FPP and GGPP derivatives as ligands of nuclear receptors involved in gene transcription regulation (12, 13).
The molecular mechanisms of protein prenylation have been extensively studied over the past decade, and the enzymes that transfer these lipids to proteins (protein:prenyl transferases) have been cloned and studied as potential targets for antitumor therapy (14, 21, 37). By contrast, the molecular mechanisms involved in the metabolism of the isoprenoids FPP and GGPP used for this modification and their regulation are still poorly understood (18).
In this paper, we report the cloning and characterization of murine GGPP synthase, based on a clone that was originally identified as an overexpressed gene in the ob/ob mouse, a model of genetic obesity and insulin resistance (36). We demonstrate that mammalian GGPP synthase is able of catalyzing the synthesis of both isoprenoid moieties for protein isoprenylation, GGPP and FPP, and show that its expression is regulated in obesity and adipogenesis.
MATERIALS AND METHODS
Mice.
Male ob/ob mice and their thin littermates (age 6 weeks) were obtained from Jackson Laboratory (Bar Harbor, Maine). Mice were housed at least 4 days after arrival before being used in experiments. All animals received ad libitum diets. Tissues were obtained during the morning from fed animals sacrificed by CO2, immediately frozen in liquid nitrogen, and kept at −80°C until used.
Cloning of the GGPP synthase cDNA.
A lambda Zap mouse brain cDNA library, primed with poly(A) oligonucleotide (Stratagene, La Jolla, Calif.), was screened with a 222-bp DNA probe obtained in an mRNA differential display between skeletal muscles of ob/ob and ob/+ mice (36). A positive clone was isolated, and its cDNA was recovered using the in vivo excision procedure described by the manufacturer. The clone was sequenced in both directions by automatic sequencing using an ABI-373 automated sequencer (Applied Biosystems Inc., Foster City, Calif.) and the T3 and T7 sequencing primers. Searches for DNA and protein homologies and sequences comparisons were performed with the computing facilities of the Molecular Biology Computing Research Resource (Dana Farber Cancer Institute and Harvard School of Public Health, Boston, Mass.).
Northern blot analysis.
mRNA [poly(A) mRNA] was prepared from brain, liver, fat, and skeletal muscle from ob/ob mice and their thin littermates as controls by using a Poly (A) Pure kit (Ambion, Austin, Tex.). Three micrograms of poly(A) RNA was subjected to formaldehyde-agarose gel electrophoresis, transferred to a nylon membrane, UV cross-linked (UV Stratalinker 2400; Stratagene) and hybridized with an [α-32P]dCTP-labeled probe at 42°C for 16 h. The probe was generated by random labeling of a 1-kb DNA fragment containing the coding region of mouse GGPP synthase cDNA. Probes for fatty acid synthase and 36B4 were generously provided by B. M. Spigelman and G. L. King, respectively. The membrane was washed twice in a 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) solution at 50°C for 30 min and exposed to X-Omat film (Eastman Kodak, Rochester, N.Y.). The signals were quantified by densitometry (Molecular Dynamics, Sunnyvale, Calif.).
Cell culture and cell lysate preparation.
3T3-L1 fibroblasts were grown to confluence in 100-mm-diameter plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, Utah) in a 10% CO2 atmosphere. Two days after confluence, the cells were differentiated into adipocytes by incubating for 48 h in DMEM supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis, Mo.) containing dexamethasone (5 μM), 3-isobutyl-1-methylxanthine (500 μM), and insulin (5 μg/ml) (Boehringer Mannheim, Indianapolis, Ind.) and then for 3 additional days in DMEM supplemented with 10% FBS containing insulin (5 μg/ml). After cells were shifted to DMEM with 10% FBS, the medium was changed every day. The adipocytes were used 10 days after the initiation of differentiation, by which time >90% of the cells had an adipocyte morphology. For preparation of cell lysates, the medium was removed, and cells were washed in cold phosphate-buffered saline (PBS) and collected by scraping in 1 ml of lysis buffer composed of 50 mM HEPES, 1% Triton X-100, 2 mM Na3VO4, 1 mM EDTA, 10 μg of aprotinin per ml, and 2 mM phenylmethylsulfonyl fluoride. The lysates were incubated on ice for 30 min and then centrifuged at 15,000 × g for 15 min at 4°C, the supernatant was recovered, and the protein concentration was measured by the Bradford method using bovine serum albumin (BSA) as a standard (Bio-Rad Laboratories, Hercules, Calif.). Total protein concentrations were adjusted with Laemmli sample buffer (5 mM sodium phosphate [pH 7.0], 10% [vol/vol] glycerin, 2% [wt/vol] SDS, 0.002% [wt/vol] bromophenol blue, 100 mM dithiothreitol), and 50 μg of protein was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed by Western blotting using specific anti-GGPP synthase polyclonal antibodies.
Immunoprecipitation of GGPP synthase from animal tissues.
Mouse tissues were homogenized in the same buffer used for cell lysate using a Polytron homogenizer for 30 s at maximum speed on ice. The homogenate was centrifuged at 105,000 × g for 1 h at 4°C, and the supernatant was recovered. Protein concentration of the samples were adjusted with lysate buffer as required. Specific anti-GGPP synthase polyclonal antibodies were raised in rabbits against peptides corresponding to amino acids 234 to 247 (JD223) and 288 to 300 (JD220) synthesized by the Peptide Biochemistry Core (Joslin Diabetes Center) and coupled to Imject maleimide-activated keyhole limpet hemocyanin (Pierce, Rockford, Ill.) as a carrier. Immunization of rabbits was carried out by Hazelton Research Products, Inc. (Denver, Pa.). Anti-GGPP synthase antiserum was added to the homogenate at a dilution of 1:100 and incubated in a 1-ml volume for 1 h at 4°C. Protein A-Sepharose 6 MB beads (Pharmacia) were added in a 20-μl volume and incubated with constant rotation for 1 h at 4°C. The immunocomplexes bound to the beads were collected by centrifugation at 15,000 × g for 5 min at 4°C, the supernatant was discarded, and the beads were washed three times in lysate buffer. GGPP synthase purified in this way was used for Western blot analysis and enzymatic assays.
Subcellular fractionation.
For subcellular fractionation experiments, tissues were homogenized in HES buffer (20 mM HEPES [pH 7.4], 1 mM EDTA, 255 mM sucrose, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride). Homogenates were centrifuged at 350 × g for 10 min at 4°C to eliminate debris and unbroken cells, pellets were discarded, and supernatants were centrifuged again at 105,000 × g for 1 h to separate cytosolic and membrane fractions. Supernatants were subjected to a second centrifugation at 105,000 × g and the middle region of this supernatant was used for immunoprecipitation. The pellet was suspended in 150 mM Tris-HCl (pH 8.0) and centrifuged again at 105,000 × g for 1 h to eliminate adsorbed cytosolic proteins. This pellet was considered a total-membrane fraction and was used for immunoprecipitation after being resuspended in HES buffer.
Western blots.
Total proteins from cell lysates or immunoprecipitated from tissue homogenates were subjected to SDS-PAGE and electrotransferred to nitrocellulose membranes for 1 h at 100 V and 4°C in Bio-Rad electrotransfer cells. Membranes were blocked in blocking solution (3% BSA, 10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.02% Tween 20, 0.02% sodium azide) for 45 min and incubated first in a 1:100 dilution of anti-GGPP synthase antiserum in blocking solution for 2 h, washed three times for 10 min in wash solution (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.02% Tween 20, 0.02% sodium azide), and then incubated with 125I-protein A (32 μCi/mg; Pharmacia) at a concentration of 0.1 μCi/ml in blocking solution for 45 min. After removal of the antibody solution, membranes were washed three times for 10 min in wash solution and exposed to a Kodak X-Omat AR film, and the radioactive signals were visualized and quantified by densitometry. All incubations were carried out at room temperature.
Enzymatic activity assay and HPLC purification of the reaction products.
The catalytic activity of mouse GGPP synthase was tested using the immunoprecipitated protein as source of purified enzyme with the method of Sagami et al. (31). The immunocomplexes bound to the beads were washed three times with 1 ml of assay washing buffer (50 mM sodium phosphate buffer [pH 7.0], 2 mM MgCl), and the supernatant was aspirated after the last wash. The reaction was initiated by adding 25 μl of the reaction mixture to the pellet containing the immunocomplexes. The reaction mixture was composed of 20 mM of FPP, geranyl diphosphate (GPP), or dimethylallyl diphosphate (DMAPP) (Sigma) and 25 mM 14C-isopentenyl diphosphate (14C-IPP; 80 mCi/mmol; Pharmacia) as substrates in a 50 mM phosphate buffer (pH 7.0) containing 2 mM MgCl, 1% BSA, 100 mM dithiothreitol, and 1% octyl glucoside. After 30 min of incubation at 37°C, the reaction was stop by adding 300 μl of an ethanol-HCl (4:1) mixture. The radioactive organic compounds synthesized were extracted from the reaction mixture by vortexing in the presence of 300 μl of hexane. Organic and aqueous phases were separated by 5 min of centrifugation at maximum speed in a microcentrifuge, and the radioactivity of an aliquot from the organic phase was measured in a scintillation counter (Beckman LS 6500).
The reaction products were subjected to reverse-phase high-pressure liquid chromatography (HPLC) as described by Zhang and Poulter (38), with some modifications. The 25-μl volume of the reaction was increased to 200 μl with assay buffer, and 150 μl was prepurified in a C18 Sepack cartridge (Pharmacia) preconditioned with acetonitrile. The cartridge was washed twice with 5 ml of 25 mM ammonium carbonate buffer (pH 8.0) and then eluted with 1 ml of acetonitrile. The volume was reduced in a Speed Vac (Savant, Holbrook, N.Y.) and adjusted to 100 μl of 20% acetonitrile–25 mM ammonium carbonate. HPLC was carried out on an LKB system. Samples were injected in a 25- by 1.8-cm Bio-Sil C18 HL 90-3S column (Bio-Rad) and eluted with a 20 to 100% acetonitrile-ammonium carbonate gradient at a flow of 1 ml/min. The eluent was collected in 0.5-ml fractions, and the radioactivity was counted in a liquid scintillation counter. To determine the retention volume of both isoprenoids on the column, FPP (25 μg) and GGPP (30 μg) were separated under the same conditions and detected by monitoring the absorbance of the effluent at 214 nm using a UV detector (LKB programmable detector module 166).
RESULTS
Cloning of murine GGPP synthase.
The original clone obtained by differential mRNA display from ob/ob mice, OB-10, consisted on 222 bases with homology to 67 amino acids from the C terminus of yeast and fungal GGPP synthase plus 21 bases of 3′ untranslated sequence (36). To obtain a full-length cDNA for the murine GGPP synthase, we screened a mouse brain cDNA library with this cDNA probe and obtained a single positive clone of 2.2 kb, which was sequenced using an ABI automatic sequencer. The 300-amino-acid polypeptide encoded had a predicted molecular weight of 34,747. A BLAST search of DNA databases with the 900 bases of the coding region found no homologous sequence. By contrast, the encoded protein was 43% identical to the protein encoded by the yeast GGPP synthase (BTS1 gene) (19) and 59% to the fungus GGPP synthase (albino-3 gene) (25). The sequence also shows the five sequence domains that are conserved among isoprenyl synthases (4). Based on these homologies, we tentatively identified this clone as mouse GGPP synthase.
Amino acid sequence comparison of eukaryotic GGPP synthases.
Isoprenyl synthases are a family of enzymes that catalyze the elongation of linear isoprenoids by sequential condensations of the C5 isoprenoid IPP with allylic substrates (26). The sequence of reactions begins with the C5 isoprenoid DMAPP as an allylic substrate, resulting in the C10 isoprenoid GPP, from which C15 (FPP), C20 (GGPP), and longer linear isoprenoids are synthesized by sequential additions of the monoallylic substrate IPP. The members of the family differ in their selectivity for the chain length and double bond stereochemistry of both the allylic substrate and the final product.
All isoprenyl synthases contain two conserved aspartate-rich motifs, DDXX(XX)D, where X can be any amino acid. These aspartate residues are involved in binding of the diphosphate moieties and are essential for enzymatic activity (2). Sequence alignments of isoprenyl diphosphate synthases from bacteria, fungus, yeast, and animal organisms have shown that the primary structures of these enzymes are similar, with five conserved domains, and a secondary structure dominated by α helices (4). Based on these similarities, it has been suggested that these enzymes are phylogenetically related and originate from a common ancestor.
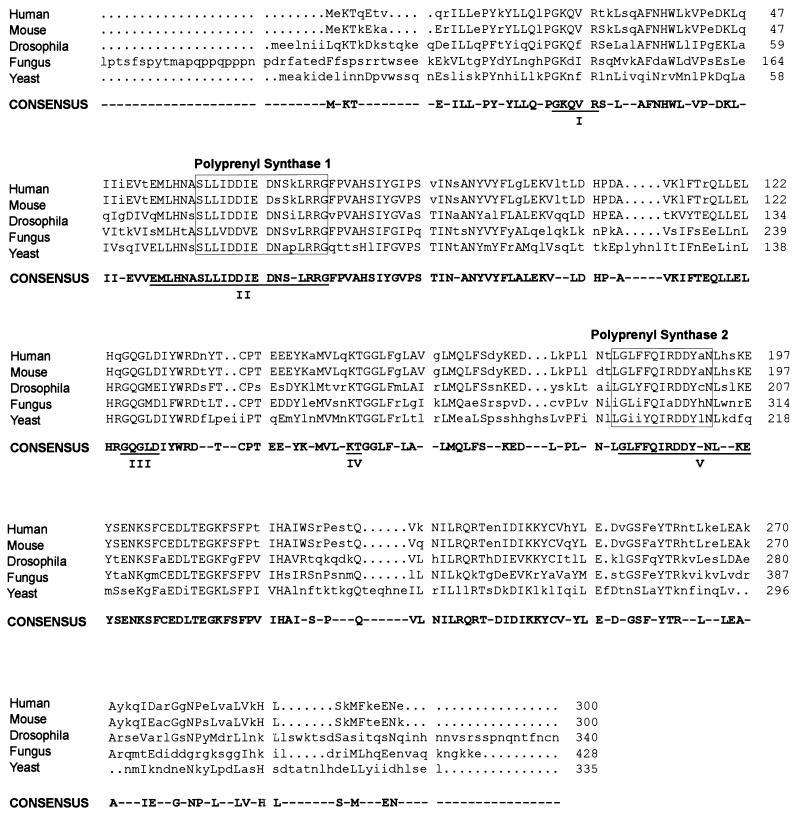
Using the previously reported sequences for fungus (Neurospora crassa) (25) and yeast (Saccharomyces cerevisiae) (19), the recently reported amino acids sequences for human (7, 22) and Drosophila melanogaster (23) GGPP synthases, and the mouse sequence that we have cloned, we constructed an alignment that outlines a consensus sequence for eukaryotic GGPP synthase covering 230 amino acids (Fig. 1). The five proteins show the five conserved domains including the two aspartate-rich motifs. The amino acid sequence identity between human GGPP synthase and other eukaryotic GGPP synthases varies between 93% for the mouse protein and 43% with that of S. cerevisiae. The homology rests on the central part of the protein, whereas the amino- and carboxy-terminal regions are poorly conserved or not conserved at all. At the DNA level there is no significant homology between the mammalian GGPP synthases and the GGPP synthases from fungus and yeast by BLAST alignment. Interestingly, in the case of the sequence from D. melanogaster a stretch of 42 bases that corresponds to a functionally relevant region of the protein exhibits homology with the mammalian sequence. The amino acid sequence (MLHNSSLLIDDIED) encoded by these 42 bases corresponds to the second domain (domain II) containing the first aspartate-rich region. From the three-dimensional structure of avian FPP synthase, the only isoprenyl diphosphate synthase that has been crystallized, is known that the lateral chains of the amino acids located at -4 and -5 before the first DDXX(XX)D motif form the floor of a hydrophobic depression in the active site of the enzyme that binds the isoprenoid tail of the allylic substrate (35). Site-directed mutagenesis experiments have shown that those amino acids determine the length of the final product of the reaction (27, 34).
FIG. 1.
Alignment of human, mouse, drosophila, fungus, and yeast GGPP synthases, created using progressive, pairwise alignments by PileUp. The consensus sequence was calculated by Pretty with a plurality level of 3. The five sequence domains conserved in isoprenyl diphosphate synthases are underlined and labeled I to V, and the polyprenyl synthases signatures I and II are shown in labeled boxes. The first 84 amino acids of the N. crassa sequence have been omitted for clarity.
Eukaryotic GGPP synthase exhibits the five sequence domains conserved in isoprenyl diphosphate synthases. All of the residues conserved in FPP and other GGPP synthases are present in eukaryotic GGPP synthase, with two exceptions in domain V. Both of these occur in the second aspartate-rich region, where the third aspartate residue and the next conserved residue, a glycine, are replaced by two asparagine residues. In spite of these similarities with other isoprenyl synthases, the homology between human FPP and GGPP synthases determined by BLAST alignment is restricted to the longest domains (II, III, and V), without significant homology in other parts of the proteins. Human and murine GGPP synthases are almost identical, with only 19 changes between the amino acid sequences, of which 10 are conservative. Most of the changes occur in the amino and carboxy termini.
Expression and regulation of GGPP synthase.
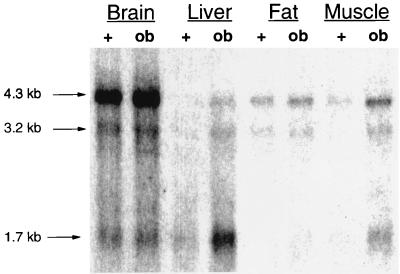
As noted above, GGPP synthase was initially identified by differential display as an mRNA (OB-10) that was overexpressed in skeletal muscle of ob/ob mice. To determine the range of tissue expression, we prepared a Northern blot containing 3 μg of poly(A) RNA from brain, liver, fat, and skeletal muscle of lean and ob/ob mice and hybridized it with a DNA probe specific for the coding region of the cloned cDNA (Fig. 2). Three mRNA species of 4.3, 3.2, and 1.7 kb were detected. The 4.3-kb message was the major message in brain, fat, and muscle, whereas the 1.7-kb band was dominant in liver. All three mRNAs were overexpressed in the skeletal muscle of the ob/ob mouse between five- and eightfold. The 4.3- and 3.2-kb GGPP synthase mRNAs were also increased in the other tissues, whereas in liver the major change of expression was in the 1.7-kb message.
FIG. 2.
Northern blot analysis of GGPP synthase mRNA expression in brain, liver, fat, and skeletal muscle of lean and ob/ob mice. Aliquots (3 μg) of poly(A) RNA from brain, liver, fat, and skeletal muscle of lean (+) and ob/ob mice (ob) were hybridized with a DNA probe containing the coding region of GGPP synthase. Three mRNA species of 4.3, 3.2, and 1.7 kb were detected in all tissues.
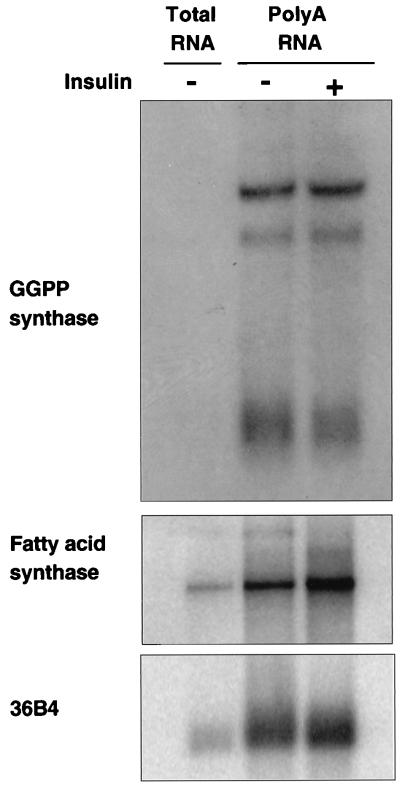
To examine the ability of insulin to regulate GGPP synthase expression, we incubated 3T3-L1 adipocytes in the presence and absence of 1 μM insulin for 24 h and determined its mRNA expression level by Northern blot analysis (Fig. 3). Insulin failed to increase GGPP synthase mRNA expression, while the expression of an insulin-responsive gene, fatty acid synthase (28), increased by 80%. This result suggests that the overexpression of GGPP synthase in the ob/ob mouse is not a direct effect of the hyperinsulinemia that distinguishes this animal.
FIG. 3.
Regulation of GGPP synthase mRNA expression by insulin in 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were starved from serum for 24 h and then incubated in the absence (−) or presence (+) of 1 μM insulin for another 24 h. Poly(A) mRNA was purified from 150 μg of total RNA and analyzed by Northern blotting together with 10 μg of total RNA to show the relative level of expression of the different messages tested. The membrane was probed for GGPP synthase (top), stripped, and reprobed for fatty acid synthase as a positive control for insulin regulation of gene expression (middle) and for 36B4 as control for RNA loading (1) (bottom).
Two different polyclonal antibodies were raised in rabbits to GGPP synthase as indicated in Materials and Methods. Both detected a protein of 34.8 kDa on Western blots of immunoprecipitated mouse brain homogenate corresponding to the predicated size. There was also a good correlation between the amount of protein loaded on to the gel and the intensity of the signal in the Western blot. Competition experiments with the peptides used to raise the antibodies showed that the 34.8-kDa band was specific for both antibodies (data not shown).
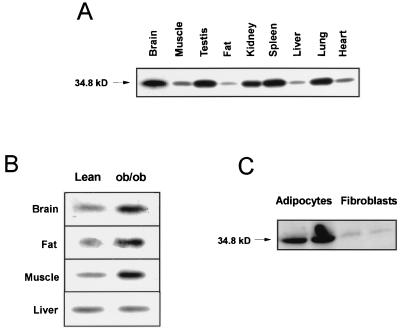
Western blot analysis of cytosolic fractions of multiple tissues showed that the immunoprecipitated GGPP synthase was ubiquitously expressed and of the same size in all tissues (Fig. 4A). Expression was highest in brain, spleen, lung, testis, and kidney, intermediate in skeletal muscle and heart, and low in fat and liver, demonstrating a good correlation with the expression of the 4.3-kb band detected by Northern blot analysis. Also as predicted by the Northern blot analysis, the expression of GGPP synthase in brain, fat, and skeletal muscle was about two times higher in the ob/ob mouse that in its thin littermate. In liver, no difference in the expression of GGPP synthase protein could be detected (Fig. 4B). This suggests that the putative protein encoded by the 1.7-kb mRNA detected with the cDNA probe has different immunological determinants and is not detected by Western blots of the immunoprecipitated protein with this antibody. Western blot analysis of cells lysates showed that expression of GGPP synthase was induced 20-fold during differentiation of 3T3-L1 fibroblasts into adipocytes (Fig. 4C).
FIG. 4.
GGPP synthase protein expression. (A) Tissue distribution of GGPP synthase protein in mouse. Aliquots (5 mg) of total protein from different tissues were immunoprecipitated using a specific C-terminal antibody (JD220), separated by SDS-PAGE, electrotransferred to a nitrocellulose membrane, and immunoblotted with an antibody directed against a different peptide of the protein (JD223) and with 125I-protein A. A single band of the predicted size for the cloned protein, 34.8 kDa, was detected in all tissues. (B) GGPP synthase expression in brain, fat, muscle, and liver in ob/ob mice and their lean littermates. Western blot analysis of the immunoprecipitated protein was conducted as for panel A. The exposure time of the autoradiography was adjusted to the expression level. (C) Induction of GGPP synthase protein expression during adipocyte differentiation. Cell lysates from 3T3-L1 cell before (fibroblasts) and after (adipocytes) induction of adipocyte differentiation were subjected to Western blot analysis using JD223 antiserum.
Murine GGPP synthase possesses geranyltransferase and farnesyltransferase activities but no dimethylallyltransferase activity.
The reaction catalyzed by GGPP synthases are sequential condensations in the trans configuration that terminate at C20, all-trans-GGPP, as the final product. The preferred allylic substrate in most of the GGPP synthases that have been characterized from bacteria and plants is DMAPP, and the consecutive reactions yielding C10 (GPP), C15 (FPP), and C20 (GGPP) take place without accumulation of intermediary products. The animal GGPP synthase isolated by Sagami et al. (31) uses GPP or FPP as an allylic substrates; when GPP is used, the reaction accumulates FPP.
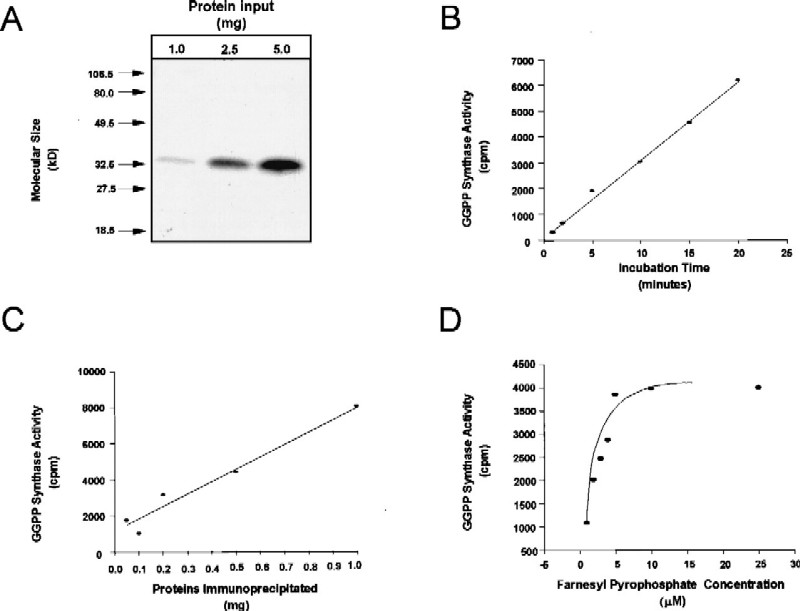
To determine the enzymatic activity of this putative GGPP synthase protein, we prepared immunoprecipitated complexes from mouse brain extracts using the antipeptide antibodies described above and assessed GGPP synthase activity by incubating appropriate substrates, FPP and 14C-IPP, and measuring the amount of radioactivity incorporated into hexane-extractable material (31). As shown in Fig. 5A and C, the amount of immunoprecipitated GGPP synthase correlated with the amount of protein used in the immunoprecipitation reaction. Furthermore, when a single concentration of protein was used, accumulation of GGPP was linear with time (Fig. 5B). Using different concentrations of the FPP substrate, the enzyme showed typical Michaelis-Menten saturation kinetics, with an estimated KM of 2.0 μM (Fig. 5D).
FIG. 5.
Enzymatic activity of the immunoprecipitated GGPP synthase. (A) Western blot analysis of GGPP synthase immunoprecipitated from 1 to 5 mg of proteins prepared from brain tissue. Arrows and numbers on the left indicate molecular size marker positions. (B) Time-dependent accumulation of radioactive compounds synthesized by GGPP synthase immunoprecipitated from 1 mg of brain proteins for up to 20 min. (C) GGPP synthase activity is dependent on the amount of immunoprecipitated protein in the assay. (D) GGPP synthase activity dependence on substrate (FPP) concentration. For this experiment, 0.5-mg aliquots of brain proteins were used.
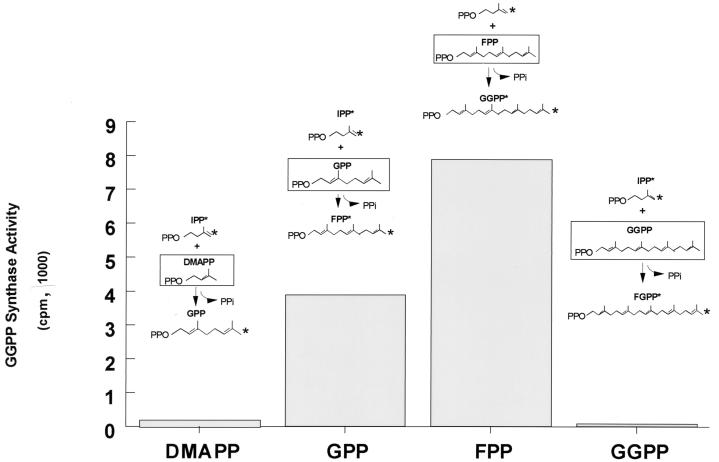
To determine the substrate specificity of the enzyme, we assessed the ability of the immunocomplexes to catalyze the condensation between isoprenyl diphosphates of different lengths and 14C-IPP (Fig. 6). The maximum amount of radioactivity incorporated by the immunoprecipitated GGPP synthase corresponded to the condensation of 14C-IPP with FPP to form GGPP. The activity of this enzyme was also significant, albeit about 50% less, in the condensation of 14C-IPP with GPP to form FPP. The condensation of 14C-IPP with DMAPP was only 3% of the activity shown for FPP; in the case of higher chain length, on the other hand (i.e., for GGPP), the activity was not above the background assay controls. Thus, murine GGPP synthase was capable of using GPP and FPP as allylic donors to form FPP and GGPP but was not able to use DMAPP or GGPP to form shorter or longer products.
FIG. 6.
Substrate specificity of GGPP synthase, assessed by determining the ability of the GGPP synthase immunoprecipitated from 1 mg of brain proteins to catalyze the condensation between IPP and isoprenyl pyrophosphates of different lengths. The enzymatic activity was measured by the amount of 14C-IPP incorporated into hexane-extractable compounds in the reaction.
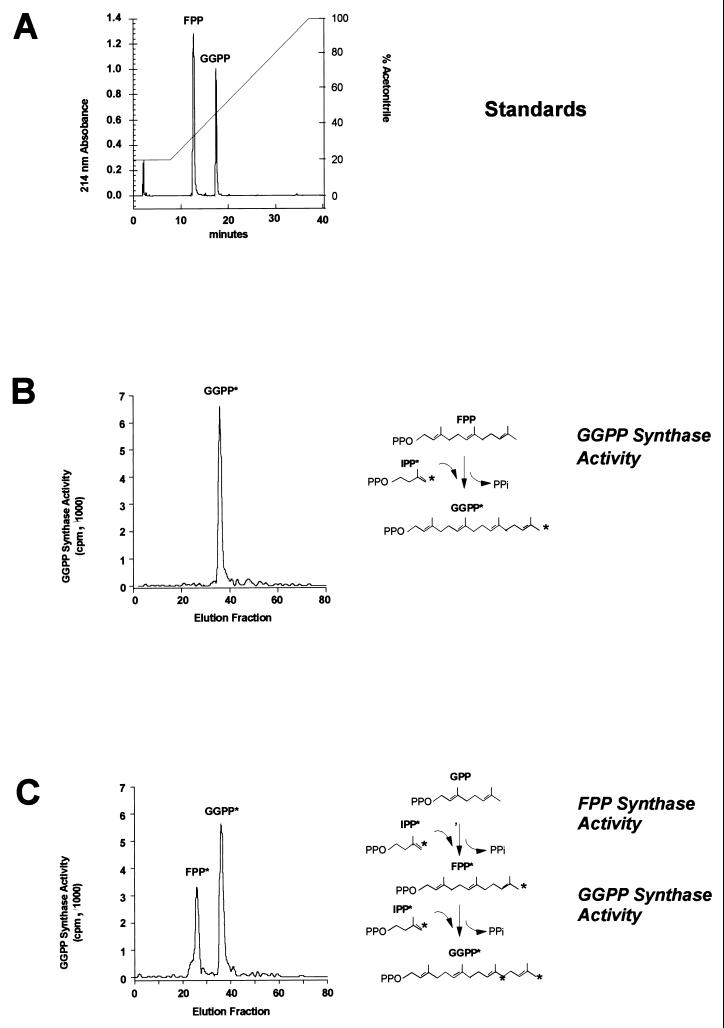
To confirm the exact nature of the products of these reactions, the hexane-extractable lipids were further separated by reverse-phase HPLC on a Bio-Sil C18 HL 90-3S column (Bio-Rad) (38). The retention times of FPP and GGPP in the column were determined using cold standards detected by their absorbance at 214 nm (Fig. 7A), and the radioactive products of the reaction catalyzed by the immunocomplexes were identified by comparing their elution profiles with those of the cold standards. When the allylic compound was FPP, only GGPP was detectable as a product of the reaction (Fig. 7B). When the substrates of the reaction were IPP and GPP, both FPP and GGPP were generated (Fig. 7C). Therefore, the mammalian GGPP synthase displayed FPP and GGPP synthase activities, the two isoprenoids implicated in protein isoprenylation.
FIG. 7.
Products of the reaction catalyzed by GGPP synthase. The hexane-extractable lipids product of the reaction catalyzed by GGPP synthase were separated by reverse-phase HPLC. The enzymatic assay was performed with radioactive labeling of the formed compounds as for Fig. 6. (A) Retention time of unlabeled FPP (25 μg) and GGPP (30 μg) detected by their absorbance at 214 nm. (B) A single radioactive peak that corresponds to GGPP was detected when 14C-IPP was incubated with FPP. (C) Two radioactive peaks identified as FPP and GGPP were detected when 14C-IPP was incubated with GPP.
Subcellular distribution of GGPP synthase.
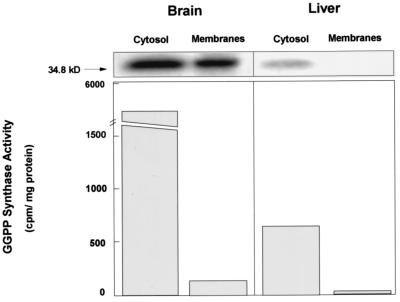
All steps necessary for the synthesis of isoprenoids for protein isoprenylation from mevalonic acid and their transfer to the acceptor proteins take place in the cytosol (24). Using cell fractionation methods, Ericsson et al. (9) found two different GGPP synthase activities in mammalian tissues: one, in the cytosol, produces all-trans-GGPP and is used for protein isoprenylation; the other (cis-prenyltransferase) is bound to membranes and produces trans-trans-cis-GGPP as the first step in the synthesis of dolichol. Figure 8 shows the amount of immunoprecipitable protein and the associated enzymatic activity from cytosolic and whole-membrane fractions from brain and liver homogenates. In brain tissue, the amounts of immunoreactive GGPP synthase immunoprecipitated per milligram of protein from the fractions were comparable, but the GGPP synthase enzymatic activity was almost completely restricted to the cytosolic fraction. In the case of the liver, both protein and activity were present only in the cytosolic fraction. This result shows that besides the cytosolic GGPP synthase involved in prenylation, there is a noncytosolic isoform in the brain with undetectable enzymatic activity under the conditions assayed. This protein might represent a posttranslational modification of the enzyme affecting its activity.
FIG. 8.
Subcellular distribution of GGPP synthase. Mouse brain and liver were homogenized and cytosolic and membrane fractions were prepared as described in Materials and Methods. GGPP synthase was immunoprecipitated from 1 mg of cytosolic proteins and 5 mg of membrane protein for both tissues. Enzymatic activity was determined as in the previous experiments and is expressed as counts per minute per milligram of protein immunoprecipitated.
DISCUSSION
Beginning with a PCR fragment that was overexpressed in the ob/ob mouse by mRNA differential display (36), we have cloned and characterized mouse GGPP synthase. This is the branch point enzyme in the mevalonate pathway for the synthesis of GGPP, the major isoprenoid involved in modification of proteins.
Metabolic flux in the mevalonic acid pathway depends on the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which regulates the first part of the pathway and the overall synthesis of cholesterol (17). Experiments with inhibitors of this enzyme have shown that protein prenylation is preserved under conditions that drastically reduce the biosynthesis of cholesterol, suggesting that there are regulatory mechanisms acting downstream HMG-CoA reductase to preserve the supply of FPP and GGPP, the two isoprenoids used in protein isoprenylation (32). Good candidates for such a regulatory role are the enzymes that synthesize these isoprenoids: FPP and GGPP synthases.
As mentioned before, FPP is the common precursor for sterol and nonsterol products of the pathway, including GGPP and prenylated proteins. Our current understanding of how specific regulation in the synthesis of these functionally different end products is achieved involves the compartmentalization of the synthesis and utilization of FPP in the cell. GGPP synthase is restricted to the cytosol, where all components of the enzymatic system required for protein isoprenylation from mevalonate are present. Total synthesis of FPP and GGPP in the cytosolic fraction of different organs varies according with their functional requirements. The synthesis of FPP is especially active in the liver, relative to cholesterol synthesis, while the highest GGPP synthase activity corresponds to the brain, presumably because the rich supply of geranylgeranylated proteins in this organ. The distribution of cytosolic all-trans-GGPP synthase activity in rat tissues reported by Ericsson et al. (9) correlates with the mRNA and protein tissue distribution that we have found in the mouse, indicating that the protein described in this paper is responsible for the GGPP synthase activity present in the cytosolic fraction of tissues that supplies GGPP for protein geranylgeranylation. The presence of a membrane-bound GGPP synthase in brain which is enzymatically inactive suggests a posttranslation processing of the protein in this organ that affects the localization and activity of the protein and thus regulates its function.
As mammalian GGPP synthase has the capability of catalyzing the formation of FPP and GGPP, this enzyme might be the branch point of the mevalonic acid pathway not only for geranylgeranylation but also for both farnesylation and geranylgeranylation. In support of this hypothesis is the fact that FPP and GGPP synthase activities in vivo are regulated differently, as is transcription of their genes. Synthesis of FPP in the liver cytosolic fraction is regulated by conditions that affect cholesterol biosynthesis (9). The gene encoding FPP synthase was cloned as a cholesterol-regulated gene (5), and its transcription has been shown to be regulated by sterol regulatory element binding proteins (8), a family of transcription factors that regulate multiple genes involved in cholesterol synthesis and uptake in response to changes in the level of cholesterol in the cell (3). Synthesis of GGPP, on the contrary, does not change or even decreases after treatment with inhibitors of HMG-CoA reductase (9). Recently Ericsson et al. (7) have shown that its mRNA expression in HeLa cells is not regulated under conditions that increase FPP synthase and other sterol-regulated genes expression.
The regulation of GGPP expression during conversion of fibroblasts to adipocytes and the wide variation at mRNA and protein levels among different tissues indicates that GGPP synthase expression is regulated by differentiation programs. The altered expression present in the ob/ob mouse suggests that GGPP synthase expression is responsive to regulatory mechanisms operating in differentiated mature cells, perhaps under hormonal control.
Although a number of previous studies have characterized the catalytic activity responsible for the synthesis of GGPP in some animal tissues (9, 30, 31) and the cDNAs for the human and mouse proteins have been recently reported (7, 20, 22), our knowledge about the exact molecular species involved in GGPP synthase in animal cells is still in an early stage. GGPP synthase expression is ubiquitous, but the level of expression among tissues varies remarkably at both mRNA and protein levels. Three mRNAs of 4.3, 3.2, and 1.7 kb were detected in Northern blot analysis. Expression of the 4.3- and 3.2-kb messages correlates best with expression of the protein by Western blotting using an anti-carboxy-terminal peptide antibody, whereas expression of the 1.7-kb message does not. Thus, the 1.7-kb message probably encodes a different isoform of the protein that is not recognized for the antibodies. Interestingly, this isoform is the main component in the liver, which plays a prevalent role in cholesterol metabolism, and this mRNA is also highly overexpressed in the ob/ob mouse. Based on the lack of immunoreactivity with the C-terminal antibody, we can predict that this second isoform has a different C terminus. Modifications of the C terminus of FPP synthase, either by addition of an epitope tag (33) or by site-directed mutations of conserved residues (2), alters the affinity of the enzyme for IPP which results in changes in the distribution of the products of the reaction. If a similar situation occurs for GGPP synthase, differences in the C terminus of the liver isoform could result in a change in specificity and in the relative amounts of FPP and GGPP synthesized by the enzyme.
As noted above, we initially found mammalian GGPP synthase as an overexpressed gene in multiple tissues of the ob/ob mouse. GGPP synthase expression is also increased during adipocyte differentiation in culture. Adipocyte differentiation is associated with increased insulin sensitivity, whereas obesity is associated with insulin resistance. Whether the change in expression of GGPP synthase contributes to either of these processes is unknown, but it is possible that this would have consequences on the prenylation and function of several small G proteins. Goalstone et al. have demonstrated that hyperinsulinemia stimulates the activity of the enzymes that transfer FPP and GGPP to Ras and Rab proteins, respectively, increasing the cellular pool of the prenylated form of these proteins and making the cells more responsive to growth factors (15, 16). Our data indicate that insulin does not regulate GGPP synthase mRNA expression in 3T3-L1 cells, suggesting that the overexpression of GGPP synthase in obesity is related to some aspects other than the presence of hyperinsulinemia.
In conclusion, mammalian GGPP synthase catalyzes the synthesis of FPP and GGPP, the two isoprenoids that take part in protein prenylation. This suggests that this enzyme, by itself or in combination with FPP synthase, serves as a favored point for regulation of prenyl groups production for protein prenylation. This protein appears highly regulated during adipogenesis and is overexpressed in an animal model of obesity and insulin resistance.
ACKNOWLEDGMENTS
This work was supported by NIH grant DK45935 (C.R.K.), an ADA research grant (E.M.-F.), and Joslin's DERC grant (P30 PK36836).
We thank Toshihiko Nishiyama for helpful suggestions during the course of this project, Jongsoon Lee for help in the HPLC experiments, the Joslin Diabetes Center's DNA Core Facility and Animal Facility, and Terri-Lyn Azar for excellent secretarial assistance.
REFERENCES
- 1.Aiello L P, Robinson G S, Lin Y W, Nishio Y, King G L. Identification of multiple genes in bovine retinal pericytes altered by exposure to elevated levels of glucose by using mRNA differential display. Proc Natl Acad Sci USA. 1994;91:6231–6235. doi: 10.1073/pnas.91.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashby M N, Edwards P A. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene. J Biol Chem. 1990;265:13157–13164. [PubMed] [Google Scholar]
- 3.Brown M S, Goldstein J L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Kroon P A, Poulter C D. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 1994;3:600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke C F, Tanaka R D, Svenson K, Wamsley M, Fogelman A M, Edwards P A. Molecular cloning and sequence of a cholesterol-repressible enzyme related to prenyltransferase in the isoprene biosynthetic pathway. Mol Cell Biol. 1987;7:3138–3146. doi: 10.1128/mcb.7.9.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 7.Ericsson J, Greene J M, Carter K C, Shell B K, Duan D R, Florence C, Edwards P A. Human geranylgeranyl diphosphate synthase: isolation of the cDNA, chromosomal mapping and tissue expression. J Lipid Res. 1998;39:1731–1739. [PubMed] [Google Scholar]
- 8.Ericsson J, Jackson S M, Lee B C, Edwards P A. Sterol regulatory element binding protein binds to a cis element in the promoter of the farnesyl diphosphate synthase gene. Proc Natl Acad Sci USA. 1996;93:945–950. doi: 10.1073/pnas.93.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ericsson J, Runquist M, Thelin A, Andersson M, Chojnacki T, Dallner G. Distribution of prenyltransferases in rat tissues: evidence for a cytosolic all-trans-geranylgeranyl diphosphate synthase. J Biol Chem. 1993;268:832–838. [PubMed] [Google Scholar]
- 10.Farnsworth C C, Gelb M H, Glomset J A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990;247:320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farnsworth C C, Wolda S L, Gelb M H, Glomset J A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989;264:20422–20429. [PMC free article] [PubMed] [Google Scholar]
- 12.Forman B M, Goode E, Chen J, Oro A E, Bradley D J, Perlmann T, Noonan D J, Burka L T, McMorris T, Lamph W W, Evans R M, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 13.Forman B M, Ruan B, Chen J, Schroepfer G J, Jr, Evans R M. The orphan nuclear receptor LXRα is positively and negatively regulated by distinct products of mevalonate metabolism. Proc Natl Acad Sci USA. 1997;94:10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glomset J A, Farnsworth C C. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- 15.Goalstone M L, Leitner J W, Golovchenko I, Stjernholm M R, Cormont M, LeMarchand-Brustel Y, Draznin B. Insulin promotes phosphorylation and activation of geranylgeranyltransferase II. Studies with geranylgeranylation of rab-3 and rab-4. J Biol Chem. 1999;274:2880–2884. doi: 10.1074/jbc.274.5.2880. [DOI] [PubMed] [Google Scholar]
- 16.Goalstone M L, Leitner J W, Wall K, Dolgonos L, Rother K I, Accili D, Draznin B. Effect of insulin on farnesyltransferase. Specificity of insulin action and potentiation of nuclear effects of insulin-like growth factor-1, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1998;273:23892–23896. doi: 10.1074/jbc.273.37.23892. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 18.Grunler J, Ericsson J, Dallner G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim Biophys Acta. 1994;1212:259–277. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Proteau P, Poulter D, Ferro-Novick S. BTS1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J Biol Chem. 1995;270:21793–21799. doi: 10.1074/jbc.270.37.21793. [DOI] [PubMed] [Google Scholar]
- 20.Kainou T, Kawamura K, Tanaka K, Matsuda H, Kawamukai M. Identification of the GGPS1 genes encoding geranylgeranyl diphosphate synthases from mouse and human. Biochim Biophys Acta. 1999;1437:333–340. doi: 10.1016/s1388-1981(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 21.Koblan K S, Kohl N E, Omer C A, Anthony N J, Conner M W, deSolms S J, Williams T M, Graham S L, Hartman G D, Oliff A, Gibbs J B. Farnesyltransferase inhibitors: a new class of cancer chemotherapeutics. Biochem Soc Trans. 1996;24:688–692. doi: 10.1042/bst0240688. [DOI] [PubMed] [Google Scholar]
- 22.Kuzuguchi T, Morita Y, Sagami I, Sagami H, Ogura K. Human geranylgeranyl diphosphate synthase. cDNA cloning and expression. J Biol Chem. 1999;274:5888–5894. doi: 10.1074/jbc.274.9.5888. [DOI] [PubMed] [Google Scholar]
- 23.Lai C, McMahon R, Young C, Mackay T F, Langley C H. quemao, a Drosophila bristle locus, encodes geranylgeranyl pyrophosphate synthase. Genetics. 1998;149:1051–1061. doi: 10.1093/genetics/149.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz R J, McLain T M, Sinensky M J. Feedback inhibition of polyisoprenyl pyrophosphate synthesis from mevalone in vitro. Implications for protein prenylation. J Biol Chem. 1992;267:7983–7986. [PubMed] [Google Scholar]
- 25.Nelson M A, Morelli G, Carattoli A, Romano N, Macino G. Molecular cloning of a Neurospora crassa carotenoid biosynthetic gene (albino-3) regulated by blue light and the products of the white collar genes. Mol Cell Biol. 1989;9:1271–1276. doi: 10.1128/mcb.9.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogura K, Koyama T, Sagami H. Polyprenyl diphosphate synthase. Sub-Cell Biochem. 1997;28:57–87. doi: 10.1007/978-1-4615-5901-6_3. [DOI] [PubMed] [Google Scholar]
- 27.Ohnuma S I, Hirooka K, Tsuruoka N, Yano M, Ohto C, Nakane H, Nishino T. A pathway where polyprenyl diphosphate elongates in prenyltransferase. Insight into a common mechanism of chain length determination of prenyltransferases. J Biol Chem. 1998;273:26705–26713. doi: 10.1074/jbc.273.41.26705. [DOI] [PubMed] [Google Scholar]
- 28.Paullauskis J D, Sul H S. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J Biol Chem. 1988;263:7049–7054. [PubMed] [Google Scholar]
- 29.Rilling H C, Breunger E, Epstein W W, Crain P F. Prenylated proteins: the structure of the isoprenoid group. Science. 1990;247:318–320. doi: 10.1126/science.2296720. [DOI] [PubMed] [Google Scholar]
- 30.Sagami H, Korenaga T, Ogura K. Geranylgeranyl diphosphate synthase catalyzing the single condensation between isopentenyl diphosphate and farnesyl diphosphate. J Biochem (Tokyo) 1993;114:118–121. doi: 10.1093/oxfordjournals.jbchem.a124125. [DOI] [PubMed] [Google Scholar]
- 31.Sagami H, Morita Y, Ogura K. Purification and properties of geranylgeranyl-diphosphate synthase from bovine brain. J Biol Chem. 1994;269:20561–20566. [PubMed] [Google Scholar]
- 32.Sinensky M, Beck L A, Leonard S, Evans R. Differential inhibitory effects of lovastatin on protein isoprenylation and sterol synthesis. J Biol Chem. 1990;265:19937–19941. [PubMed] [Google Scholar]
- 33.Song L, Poulter C D. Yeast farnesyl-diphosphate synthase: site-directed mutagenesis of residues in highly conserved prenyltransferase domains I and II. Proc Natl Acad Sci USA. 1994;91:3044–3048. doi: 10.1073/pnas.91.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarshis L C, Proteau P J, Kellogg B A, Sacchettini J C, Poulter C D. Regulation of product chain length by isoprenyl diphosphate synthases. Proc Natl Acad Sci USA. 1996;93:15018–15023. doi: 10.1073/pnas.93.26.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarshis L C, Yan M, Poulter C D, Sacchettini J C. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-A resolution. Biochemistry. 1994;33:10871–10877. doi: 10.1021/bi00202a004. [DOI] [PubMed] [Google Scholar]
- 36.Vicent D, Piper M, Gammeltoft S, Maratos-Flier E, Kahn C R. Alterations in skeletal muscle gene expression of ob/ob mice by mRNA differential display. Diabetes. 1998;47:1451–1458. doi: 10.2337/diabetes.47.9.1451. [DOI] [PubMed] [Google Scholar]
- 37.Vogt A, Sun J, Qian Y, Hamilton A D, Sebti S M. The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces 21(WAF1/CIP1/SDI2) in a p53-independent manner. J Biol Chem. 1997;272:27224–27229. doi: 10.1074/jbc.272.43.27224. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, Poulter C D. Analysis and purification of phosphorylated isoprenoids by reversed-phase HPLC. Anal Biochem. 1993;213:356–361. doi: 10.1006/abio.1993.1432. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]