Abstract
The aim of this study was to review the current literature regarding the effects of intra-articularly applied, fat-derived orthobiologics (FDO) in the treatment of primary knee osteoarthritis over a mid-term follow-up period. A systematic literature search was conducted on the online databases of Scopus, PubMed, Ovid MEDLINE, and Cochrane Library. Studies investigating intra-articularly applied FDO with a minimum number of 10 knee osteoarthritis patients, a follow-up period of at least 2 years, and at least 1 reported functional parameter (pain level or Patient-Reported Outcome Measures) were included. Exclusion criteria encompassed focal chondral defects and techniques including additional arthroscopic bone marrow stimulation. In 28 of 29 studies, FDO showed a subjective improvement in symptoms (pain and Patient-Reported Outcome Measures) up to a maximum follow-up of 7.2 years. Radiographic cartilage regeneration up to 3 years postoperatively, as well as macroscopic cartilage regeneration investigated via second-look arthroscopy, may corroborate the favorable clinical findings in patients with knee osteoarthritis. The methodological heterogeneity in FDO treatments leads to variations in cell composition and represents a limitation in the current state of knowledge. However, this systematic review suggests that FDO injection leads to beneficial mid-term results including symptom reduction and preservation of the affected joint in knee osteoarthritis patients.
Keywords: adipose tissue-derived stromal cells, cartilage regeneration, knee osteoarthritis, mesenchymal stem cells, orthobiologics, stromal vascular fraction
1. Introduction
Total joint arthroplasty is an established and beneficial therapy for advanced stages of knee osteoarthritis (OA) with satisfaction rates of around 80%. However, revision rates of up to 12% after 10 years of follow-up have been reported causing substantial loss of function. Additionally, it was already shown by event simulation, that 21% of all total knee replacements could be avoided, by state-of-the-art cartilage repair procedures [1]. Therefore, researchers worldwide are striving to promote joint regeneration and cartilage healing. Attempts to study cartilage healing date back to Pridie, who detected “a method of resurfacing osteoarthritic knee joints” via drilling knee cartilage lesions in 1959 [2] and Steadman et al. who refined this concept of facilitating the differentiation of bone marrow mesenchymal stem cells (MSCs) to functional fibrocartilage using arthroscopic microfracture [3].
In 1999, Pittenger et al. managed to isolate MSCs from bone marrow for the first time, proposing their potential to differentiate into adipocytes, osteoblasts, and chondrocytes [4]. MSCs have since been extensively studied and can be isolated from various sources, including the umbilical cord, adipose tissue, synovial membrane, placenta, cartilage, and skeletal muscle [5]. In 2001, Zuk et al. were the first to characterize adipose-derived MSCs from autologous subcutaneous fat tissue which are commonly referred to as adipose-derived stromal/stem cells (ASC) [6]. In 2011, Pak et al. published the first-in-human case series of knee and hip OA patients treated with ASC following encouraging animal experiments [7]. The clinical use of adipose-tissue-based injections for cartilage regeneration can be summarized by the term “fat-derived orthobiologics” (FDO). However, the procession of lipoaspirate after liposuction for the purpose of intra-articular injection varies widely in the current literature. Due to terminological inhomogeneity in the medical literature, the following terminology was proposed: Enzymatic separation of lipoaspirate, which is widely performed as described by Zuc et. al., was called cellular SVF (cSVF) [8,9]. If the cell population of cSVF is further incubated in a culture medium for in vitro expansion of ASC, the subsequent injection is called ASC therapy [10]. To distinguish the cell populations of cSVF and ASC, they must contain a minimum of 70% and 90% viable cells, respectively, as well as a minimum frequency of 1% or 5% of fibroblastoid colony-forming units, respectively [11]. By contrast, mechanically processed lipoaspirate is called tissue SVF (tSVF) [8,9].
In the past decade, several favorable outcomes resulting from intra-articularly applied, autologous FDO have been reported and summarized in systematic reviews [9,12,13,14]. While the majority of reviews concentrate on certain subcharacteritics of FDO, the literature lacks an investigation into the sustainability or functional outcome of an FDO treatment after a mid-term follow-up period.
To fill this literature gap, this systematic review aims to screen the current literature for studies with a minimum of 2 years of follow-up investigating intra-articularly applied, autologous FDO as a standalone procedure on functional parameters in treating primary knee OA. Studies combining FDO with arthroscopic bone marrow stimulation were excluded from this review.
2. Materials and Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered in the Inplay Register [15].
2.1. Eligibility Criteria
To assess the current state of knowledge on intra-articular injection of FDO, studies on patients suffering from primary knee OA were identified. Because FDO is a regenerative approach to a progressive disease, a minimal follow-up period of 2 years was deemed reasonable to monitor sustainable treatment outcomes.
The inclusion criteria for this review were as follows:
Patients with primary knee OA;
Autologous, processed or non-processed, intra-articular fat tissue injection including cSVF, tSVF, and ASC;
Mean or median follow-up period of more than 2 years with minimum one clinical parameter (visual analogue scale (VAS) for pain or Patient-Reported Outcome Measures (PROMs)) available;
Minimal number of 10 patients.
The following exclusion criteria were defined:
Patients with focal chondral defects;
Additional arthroscopic bone marrow stimulation (microfracture or drilling).
Randomized controlled trials (RCTs), prospective or retrospective cohort studies, or case series as study types were included. In contrast, conference abstracts, clinical trial entries, editorials, and commentaries were excluded, because they lack of required details or parameters assessed in this review.
2.2. Literature Search Strategy
To identify relevant studies, a systematic literature search was implemented until the 1st of November 2023. The search was conducted on the online databases of Scopus, PubMed, Ovid MEDLINE, and Cochrane Library. All studies in English and German language were included. The search algorithm (in the title and abstract) is presented in Table 1. The term “knee” was not incorporated in the search algorithm to detect studies encompassing various types of knee OA (uni-, bi- or tricompartimental).
Table 1.
Search algorithm.
| First Search Term | Boolean Operator | Second Search Term |
|---|---|---|
| “osteoarthritis” OR “arthritis” |
AND | “adipose-derived stem cells” OR “fat grafting” OR “fat injection” OR “fat transfer” OR “fat transplantation” OR “mesenchymal stem cells” OR “mesenchymal stromal cells” OR “microfragmented adipose tissue” OR “stromal vascular fraction” |
2.3. Identification and Selection of Eligible Studies
First, duplicates were manually consolidated. The first author screened the titles and abstracts of all references. Studies not meeting the inclusion criteria were excluded. Following this, full texts of the included references were obtained. The first and last authors screened the full texts and re-assessed the above-mentioned inclusion and exclusion criteria.
2.4. Data Extraction Process and Data Items
The first and last authors extracted the following parameters from the full texts: study design, study population, follow-up period, and details regarding the technique including procession of the fat tissue (mechanically or enzymatically with or without culture; days between harvest and implantation were given in brackets in case they were not performed during the same procedure) were noted. Moreover, the injection of additional substances was documented and articles were screened whether the FDO was administered intra-articularly or implanted focally into the arthritically degenerated cartilage. If available, visual analogue scale (VAS) values for pain and PROMs were noted. If these parameters were presented using diagrams, the approximate median or mean value was given using a “≈” symbol. If the included studies report a radiological follow-up examination, the imaging modality (MRI or X-ray) used and the number of patients at the longest follow-up appointment were recorded. Furthermore, the articles were screened for a second-look arthroscopy.
All included studies pertaining to FDO injection were extracted in duplicate by the two reviewers, while a third reviewer resolved any discrepancies. The data were descriptively presented using a table.
2.5. Quality Assessment
The methodology of all included studies was assessed according to the recommendations of the Oxford Centre for Evidence-Based Medicine concerning the level of evidence (LoE) [16]. The methodological quality (MQ) of the studies was evaluated based on the respective study type. RCTs were rated using the modified Jadad scale, which ranges from 0 to 8 points [17]. Non-randomized studies were assessed using the Methodological Index for Nonrandomized Studies (MINORS) score [18]. For non-comparative studies, this score ranges from 0 and 16 points. In the case of comparative studies, this score incorporates an additional domain, resulting in a range between 0 and 24 points. The maximum score indicates the ideal assessment for each assessment. Both the first and the last author assessed the level of evidence and the quality of the studies, while any discrepancies were also resolved by a third reviewer.
2.6. Data Synthesis
Due to the methodological inhomogeneity of various FDO techniques, the above-mentioned parameters were summarized and tabulated primarily in a descriptive manner. Even a comparison of outcome parameters between the categories cSVF, ASC, and tSVF is associated with a high risk of bias. Thus, the number of patients treated with cSVF, ASC, or tSVT was calculated. Moreover, a geographic analysis was conducted aiming to indicate how many patients were included in FDO studies on every continent.
3. Results
In total, 4123 records in Scopus, 4038 records in PubMed, 3051 in Ovid MEDLINE and 314 records in the Cochrane Library were identified, comprising a total of 5247 articles screened in this review after duplicates were removed. Ultimately, the study selection process resulted in 29 papers assessed in this review (see Figure 1).
Figure 1.
PRISMA flowchart indicating the review process.
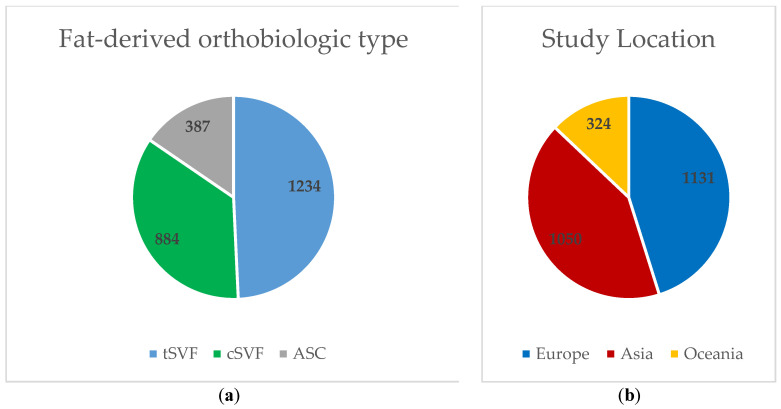
Figure 2 depicts the overall number of patients included in this review by FDO type and continent of the study’s location. All studies from the continent of Oceania included patients with ASC therapy. With the exception of one study comparing tSVF to ASC [19], all studies conducted in Asia used either ASC or cSVF.
Figure 2.
Patients categorized by (a) fat-derived orthobiologic type and (b) continent. Legends: ASC (adipose tissue-derived stromal/stem cells); cSVF (cellular stromal vascular fraction); tSVF (tissue stromal vascular fraction).
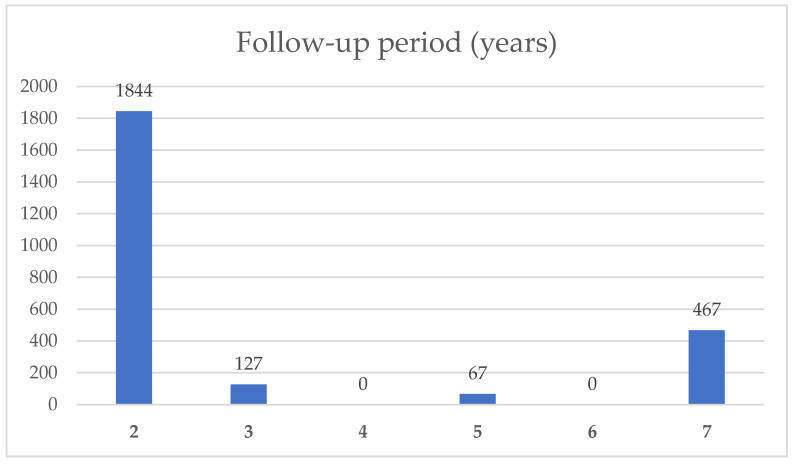
Of the 29 included publications, 28 investigated medial and/or lateral knee OA, while 1 study investigated patellofemoral OA [20]. In Figure 3, the cumulative number of patients with their respective follow-up period is indicated. Brief summaries of these studies were given in Table 2, while being sorted according to their mean follow-up periods. The included studies investigate patients with a wide range of OA severity, ranging from a Kellgren–Lawrence grade 0 to IV [21]. The abdomen (n = 19), followed by the gluteal region (n = 11), thigh region (n = 2), the flank (n = 2), and the infrapatellar fat pad (n = 1) were the chosen harvest sites of adipose tissue. Three studies did not contain the harvest site [19,22]. Three studies were presented using two rows in Table 2 because they compared two different FDO cohorts or methods: Kim et al. in two articles compared the application with and without the use of fibrin glue as a scaffold for arthroscopically guided application [23,24], and Yokota et al. compared the outcomes of tSVF and ASC [19]. In total, Table 2 contains 14 tSVF, 13 cSVF, and 5 ASC cohorts. Kim et al. used fibrin glue as a scaffold in five studies [23,24,25,26,27], while platelet-rich plasma (PRP) was injected as an additional substance in three studies [24,28,29]. Postoperative pain levels were available in 22 cohorts. Thus, pain reduction at the final follow-up examination was reported in all but one study: Screpsis et al. [30] reported a significant reduction after 6 and 12 months while pain levels were similar to baseline levels after 2 years. An improvement in functional scores could be detected in all studies. According to radiographic follow-up data, X-rays are available in 8 cohorts [19,31,32,33,34,35], while 14 studies report the effects of FDO on cartilage status via magnetic resonance imaging (MRI) [22,26,29,31,32,33,34,35,36,37,38,39]. Second-look arthroscopy was performed in four studies [23,24,28,40].
Figure 3.
Number of patients included in this review with their follow-up period.
Table 2.
Summary of the analyzed studies regarding the knee joint.
| Authors | Study Design/LoE | Control Group | QoE Score | Patients/Joints | Age (y) | Sex (F/M) | OA Stage | FuP (y) | Fat Origin | Mech./Enzym. | Addit. Inject. | Inject./Focal Implantation | Arthorsc. | VAS Pre. | VAS Post. | PROMs, Funct. Param. | Pre | Post | Rad. FuP (n) | Second. Arthro. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. [25] | retrosp./ IV |
- | 13/16 | 467/483 | 61 ± 6 | 333/150 | I, II | 7.2 ± 1.2 | gluteal region | e (1d) | fibrin glue | focal | guidance | n/a | n/a | IKDC, Tenger | IKDC | 39 ± 7 | 63 ± 9 | - | - |
| Zhang et al. [31] | RCT/ I |
HA | 7/8 | 56/56 | 54 ± 14 | 42/14 | II, III | 5 | abdomen | e | - | inject. | - | 4.0 ± 1.5 | 2.9 ± 1.8 | WOMAC | WOMAC | 33 ± 22 | 27 ± 22 | X-ray, MRI (51) | - |
| Kim et al. [32] | prosp./ III |
- | 16/16 | 11/11 | 61 ± 6 | 8/3 | II, III | 5 | abdomen | e + c (3w) | - | inject. | - | ≈6.6 | ≈2.6 | WOMAC | WOMAC | ≈60 | ≈35 | X-ray, MRI (11) | - |
| Tantuway et al. [41] | RCT/ I |
Saline | 4/8 | 58/115 | n/a | 36/22 | I, II, III | 3 | abdomen | m | - | inject. | - | 8.4 ± 0.6 | 3.2 ± 0.9 | KOOS | KOOS | 43 ± 12 | 79 ± 6 | - | - |
| Freitag et al. [36] | prosp./ II |
- | 12/16 | 27/27 | 54 ± 7 | 9/18 | IV | 3 | abdomen | e + c (within 1w) | 2. Inject. (6 m) | inject. | debrid., selective ME | 5.6 ± 2.7 | 1.5 ± 1.4 | KOOS, WOMAC, PGIC | KOOS-Pain | ≈53 | ≈84 | MRI (21) | - |
| Russo et al. [42] | retrosp./ IV |
- | 9/16 | 22/22 | 45 ± 11 | 8/14 | II, III, IV | 3 | abdomen | m | - | inject. | - | ≈5.8 | ≈2.8 | KOOS, IKDC, Tegner | KOOS | ≈52 | ≈80 | - | - |
| Çimen et al. [43] | prosp./III | - | 14/16 | 20/25 | 62 (50–76) | 18/2 | II, III | 3 | abdomen | m | - | inject. | - | 6.7 ± 2.2 | 5.7 ± 2.2 | WOMAC, Lysholm | WOMAC | 63 ± 19 | 50 ± 26 | - | - |
| Kim et al. [23] | retrosp./ III |
15/24 | 37/39 | 58 ± 6 | 23/14 | I, II | 2.4 ± 0.3 | gluteal region | e (1d) | - | focal | guidance | n/a | n/a | IKDC, Lysholm | IKDC | 38 ± 8 | 62 ± 12 | - | x | |
| → | 17/17 | 58 ± 6 | 9/8 | I, II | 2.3 ± 0.3 | gluteal region | e (1d) | fibrin glue | focal | guidance | n/a | n/a | IKDC, Lysholm | IKDC | 36 ± 6 | 64 ± 12 | - | ||||
| Kim et al. [24] | retrosp./ III |
18/24 | 20/20 | 59 ± 3 | 13/7 | I, II | 2.4 ± 0.4 | gluteal region | e (1d) | PRP | focal | guidance | n/a | n/a | IKDC, Tegner | IKDC | 39 ± 9 | 56 ± 15 | - | x | |
| → | 37/68 | 59 ± 3 | 13/7 | I, II | 2.4 ± 0.3 | gluteal region | e (1d) | fibrine glue + PRP | focal | guidance | n/a | n/a | IKDC, Tegner | IKDC | 37 ± 5 | 65 ± 13 | - | ||||
| Bakowski et al. [44] | retrosp./ IV |
- | 9/16 | 20/24 | 58 ± 8 | 21/16 | I, II, III, IV | 2.3 ± 0.5 | abdomen | m | - | inject. | - | 5.0 ± 2.2 | 4.1 ± 2.0 | IKDC, WOMAC, KOOS, EQ-5D, 5× STS, TUG, 10 m WT | KOOS | 59 ± 17 | 66 ± 16 | - | - |
| Kim et al. [26] | prosp./ II |
- | 13/16 | 20/24 | 58 ± 6 | 9/11 | I, II | 2.3 (2–2.8) | gluteal region | e (1d) | fibrin glue | focal | guidance | n/a | n/a | IKDC, Tegner | IKDC | 38 ± 7 | 67 ± 11 | MRI (24) | - |
| Koh et al. [40] | retrosp./ IV |
- | 11/16 | 56/60 | 57 ± 5 | 34/22 | I, II | 2.2 ± 0.2 | gluteal region | e (1d) | - | focal | debrid., guidance | n/a | n/a | IKDC, Lysholm | IKDC | 38 ± 7 | 61 ± 11 | - | x |
| Kim et al. [27] | retrosp./ IV |
- | 12/16 | 49/55 | 58 (48–69) | 29/26 | I, II | 2.2 (2–3) | gluteal region | e (1d) | fibrin glue | focal | guidance | n/a | n/a | IKDC, Lysholm | IKDC | 38 ± 6 | 67 ± 10 | - | - |
| Ulivi et al. [33] | RCT/ I |
arthrosc. debrid. | 6/8 | 28/28 | 61 ± 8 | n/a | III, IV | 2.2 ± 0.8 | abdomen or thigh | m | - | inject. | debrid. | −4 | KOOS, WOMAC, SF-12 | KOOS-Pain | ≈+20 | X-ray, mri (28) | - | ||
| Borg et al. [45] | retrosp./ IV |
- | 13/16 | 386/386 | n/a | 192/194 | I, II, III, IV | 2 | abdomen or flank | m | - | inject. | - | n/a | n/a | OKS | † | † | - | - | |
| Freitag et al. [46] | prosp./ II |
- | 14/16 | 297/297 | 59 ± 12 | 130/199 | I, II, III, IV | 2 | abdomen or thigh | e + c (n/a) | - | inject. | - | 5.2 ± 2.3 | 2.4 ± 2.2 | WOMAC, KOOS, ROM | KOOS-Pain | ≈58 | ≈78 | - | - |
| Heidari et al. [47] | prosp./ II |
- | 13/16 | 220/344 | n/a | 95/125 | III, IV | 2 | abdomen | m | - | inject. | - | n/a | n/a | OKS, EQ-5D | † | † | - | - | |
| Screpis et al. [30] | prosp./ II |
- | 13/16 | 202/202 | 54 ± 9 | 105/97 | I, II, III, IV | 2.0 ± 0.8 | abdomen or flank | m | - | inject. | - | ≈5 | ≈6 | KOOS | KOOS | ≈55 | ≈75 | - | - |
| Bistolfi et al. [48] | retrosp./ IV |
- | 12/16 | 78/78 | 60 ± 10 | 43/35 | I, II, III | 2 | abdomen | m | - | inject. | - | 7.1 ± 2.0 | 2.4 ± 2.9 | IKS, Lysholm, FJS, KOOS | KOOS-Pain | 41 ± 14 | 76 ± 15 | - | - |
| Gobbi et al. [49] | retrosp./ IV |
- | 13/16 | 75/120 | 70 | 79/26 | II, III, IV | 2 | abdomen or supragluteal region | m | - | focal and inject. | - | n/a | n/a | KOOS | KOOS-Pain | ≈53 | ≈76 | - | - |
| Fujita et al. [39] | retrosp./ IV |
- | 15/24 | 54/54 | 69 ± 10 | 40/14 | II, III, IV | 2 | abdomen or gluteal region | e | - | inject. | - | 6.5 ± 2.6 | 4.3 ± 2.4 | WOMAC, ROM, Force | WOMAC | 26 ± 12 | 17 ± 12 | MRI (n/a) | - |
| Zaffagnini et al. [34] | RCT/ I |
PRP | 6/8 | 50/50 | 55 ± 12 | 25/28 | I, II, III, IV | 2 | abdomen | m | - | inject. | - | 6.6 ± 2.0 | –1.5 ± 2.4 | IKDC, KOOS, EQ-5D, EQ-VAS | KOOS-Pain | 58 ± 16 | 10 ± 18 | X-ray, MRI (50) | - |
| Gobbi et al. [50] | RCT/ I |
PRP + HA (3×) | 7/8 | 40/40 | 63 ± 13 | 23/17 | 0, I, II | 2 | abdomen | m | - | inject. | - | 5.0 ± 2.0 | 4.0 ± 2.6 | IKDC, Tegner, MKM, KOOS | KOOS-Pain | 67 ± 18 | 73 ± 22 | - | - |
| Koh et al. [28] | n/a | - | 9/16 | 30/30 | 70 (65–80) | 25/5 | II, III, IV | 2 | gluteal region | e (1d) | PRP | focal | guidance | 4.7 ± 1.6 | 1.7 ± 1.4 | KOOS, Lysholm | KOOS-Pain | ≈30 | ≈58 | - | x |
| Yokota et al. [19] | prosp./ II |
20/24 | 25/25 | 73 ± 9 | 20/5 | II, III, IV | 2 | n/a | m | - | inject. | - | ≈7.5 | ≈5.0 | KOOS, OARSI | KOOS | ≈38 | ≈54 | X-ray (25) | - | |
| → | 35/35 | 70 ± 9 | 28/7 | II, III, IV | 2 | n/a | e + c (n/a) | - | inject. | - | ≈7.2 | ≈3.8 | KOOS, OARSI | KOOS | ≈42 | ≈60 | X-ray (35) | - | |||
| Khoury et al. [22] | retrosp./ IV |
PRP | 18/24 | 23/23 | 56 ± 9 | 7/16 | I, II, III | 2 | n/a | e | - | inject. | - | 6.0 ± 0.7 | 2.9 ± 0.7 | KOOS | KOOS-Pain | 50 ± 5 | 73 ± 4 | MRI (23) | - |
| Koh et al. [29] | retrosp./IV | - | 12/16 | 18/18 | 55 (41–69) | 12/6 | III, IV | 2 (2–2.2) | infrapatellar fat pad | e | PRP | inject. | debr., selective ME, guidcance | 4.8 ± 1.6 | 2.0 ± 1.1 | WOMAC, Lysholm | WOMAC | 50 ± 12 | 30 ± 9 | MRI (18) | - |
| Jo et al. [35] | prosp./ III |
- | 14/16 | 17/17 | 62 ± 7 | 15/3 | III, IV | 2 | abdomen | e + c (3w) | - | inject. | - | 7.9 ± 0.2 | 4.6 ± 0.8 | WOMAC, KOOS, IKS | KOOS-Pain | 43 ± 4 | 76 ± 5 | X-ray, MRI (17) | - |
| Boric et al. [37] | prosp./ III |
- | 12/16 | 10/10 | 69 ± 12 | 3/3 | III, IV | 2 | abdomen | m | - | inject. | - | 7.7 ± 1.4 | 3.4 ± 1.7 | n/a | n/a | n/a | n/a | MRI (10) | - |
Legends: Arthrosc. (arthroscopic technique); Addit. Inject (additional Injection); debrid. (debridement); EQ-5D (EuroQol 5 dimensions); F/M (female/male); FJS (Forgotten Joint Score); PROMs, Funct. Param. (Patient-Reported Outcome Measures, functional parameter/s); HA (hyaluronic acid); inject./focal implantation (intra-articular injection/focal implantation at arthritically degenerated cartilage); IKDC (Subjective International Knee Documentation Committee); IKS (International Knee Society score); Inject. (injection); K-L (Kellgren–Lawrence); KOOS (Knee Injury and Osteoarthritis Outcome Score); LoE (level of evidence); m/e/e + c (mechanical procession/enzymatical procession/enzymatical procession including cluture); Lysholm (Lysholm knee score); ME (meniscectomy); MKM (Marx knee measure); MQ (methodological quality); MRI (magnetic resonance imaging); n/a (not available); OA (osteoarthritis); OARSI (outcome measures in rheumatology–osteoarthritis research society international); OKS (Oxford knee score); PGIC (patients’ global impression of change scale); post. (postoperatively); pre. (preoperatively); prosp. (prospective study); PRP (platelet-rich plasma); RCT (randomized controlled trial); retrosp. (retrospective study); SF-12 (short form health survey-12); rad. FuP(n) (radiological follow-up (including number of patients)) ROM (range of motion); Second. arthro. (Secondary arthroscopy); Tegner (Tegner activity scale); TUG (timed up and go test); VAS (visual analogue scale for pain); WOMAC (western Ontario and McMaster universities); Y (years); 5× STS (5 times sit to stand test); 10 m WT (10 m walk test); → (indicating a second FDO method within one study); † (due to a gender-based assessment, no values for the total cohort are provided; however, improvements could be detected in both cohorts).
4. Discussion
Cell-based tissue engineering has emerged as a promising approach in the treatment of OA in the last decade. Adipose tissue has emerged as the most attractive source of MSC because of its abundance, ease of accessibility, as well as regenerative capabilities [10]. ASCs have the potential to differentiate into chondrocytes, especially when exposed to hydrostatic pressure [51], the functional characteristics of articular cartilage tissue are defined by far more than its cellular components.
When discussing the therapeutic effects of FDO, paracrine effects of ASC, i.e., the secretion of a wide range of bioactive molecules, are recently considered even more important than the differentiation into parenchymal cells [52,53]. These beneficial effects of ASC’s numerous factors and cytokines on recipient cells leading to anti-apoptosis, angiogenesis, immunomodulation, support of the differentiation and growth of local stem and progenitor cells, chemo attraction, and anti-scarring [10] are collectively known as the “secretome” theory [54,55]. In vitro studies suggest that the synovial fluid has the capacity to initialize macrophage differentiation [56]. Therefore, the paracrine effects of FDO may also have an impact on the synovial fluid niche by modulating macrophage polarization and the subsequent inflammatory response.
Based on the results of this review, FDO appears to result in subjective improvement of symptoms of knee OA. Apart from 1 study out of 29 [30], beneficial outcomes were reported throughout via pain reduction and/or improvement of functional scores. In addition to subjective scores, the effects of FDO on the articular cartilage were investigated macroscopically through arthroscopy and radiographically:
In four studies, second-look arthroscopy was used to macroscopically monitor cartilage lesions [23,24,28,40]. In three studies, the second procedure was performed 1 year postoperatively, and the chondral lesions were evaluated using the International Cartilage Repair Society Macroscopic Evaluation of Cartilage Repair (ICRS) [23,24,40]. The researchers found a significant negative correlation between ICRS repair grade and functional parameters, i.e., International Knee Documentation Committee (IKDC) subjective knee form and Tegner Activity Scale. Additionally, patients with a lower body mass index and a defect size smaller than 5.4 cm2 were associated with better macroscopic defect repair and better functional parameters [23,40]. Conversely, functional parameters appear to reliably indicate the macroscopic chondral status. Moreover, cellular stromal vascular fraction (cSVF) implantation using fibrin glue as a scaffold, in comparison to cSVF injection alone, showed 58–65% vs. 23–35% ICRS grade I–II chondral regeneration [23,24]. Slightly better ICRS grades could also be achieved if cSVF was injected in combination with PRP: ICRS grade I–II 35% vs. 24% [23,24,40]. In one study with second-look arthroscopy after 2 years, 62% of patients resulted in “positive” or “very positive” results, indicating at least newly forming cartilage partially covering the lesion [28].
Radiological follow-up examinations using X-ray and MRI were performed 0.5 to 5 years postoperatively. Up to 3 years of follow-up, eight studies [22,26,29,33,35,36,37,39] showed favorable signs of cartilage regeneration, while two studies [31,34] reported no changes after FDO injection. In terms of comparable MRI scores, four studies reported the MRI observation of cartilage repair tissue (MOCART) score which assesses the cartilage repair including the surrounding tissue in nine parameters with a maximal total score of 100 indicating perfect hyaline-like repair [57]: the final results were 63 after 1.5 years [58], 89 after 2 years [38], and 70 as well as 76 after 3 years [26,36]. One study showed even a progression compared to the assessment after 1 year [36]. Three studies used the Whole-Organ Magnetic Resonance Imaging Score (WORMS) ranging from 0 (completely normal joint) to 332 [59], while Zaffagnini et al. [34] showed no change after 2 years and Koh et al. proved a significant reduction after 2 years (from baseline 60 to 48 points) [25]. Kim et al. reported a reduction up to 3 years postoperatively (67 points) and recurrence to baseline values after 4 and 5 years (73 points) [32]. However, the cartilage defect area was still reduced after 5 years compared to baseline values. After 5 years, Zhang et al. [31] reported an 8% decrease in full-thickness cartilage volume, which was significantly lower compared to the hyaluronic acid (HA) group. Of all patients, 92% and 84% showed a better or constant full-thickness defect and Kellgren–Lawrence grade, respectively. Studies assessing changes in different knee compartments found the lowest regenerative potential of FDO in medial tibial cartilage defects, especially in varus knee deformities [33,35,37]. In summary, the improvements observed in pain levels and functional scores may be mirrored by chondral regeneration for at least up to a minimum of 3 years of follow-up, as assessed by MRI analyses.
The basic idea of MSC inducing cartilage regeneration was inspired by the approaches suggested by Pridie and Steadman [2,3], whose regenerative effects due to the differentiation of bone marrow MSC are well known [60,61,62]. Thus, autologous bone marrow MSC transplantation was proposed as a further evolution of this treatment approach. However, the current state of knowledge suggests that SVF has been found to have a higher content of MSC [63] with greater proliferative capacity [64] and more predictable cellular differentiation, while also being less invasive to harvest and carrying a lower risk of donor site morbidity including infection and pain [63,65]. Moreover, a recent meta-analysis also reported SVF to be more effective than bone marrow aspirate in pain reduction [66]. Recently, some research groups also investigated the effects of FDO combined with arthroscopic microfracture. Accordingly, three studies reported even better clinical outcomes when FDO was added to arthroscopic microfracture [60,61,62]. Leukocyte-poor PRP represents another substance pursuing a regenerative approach in OA treatment. Compared to FDO treatment, the methodological advantage of PRP is a lower material requirement and lower spatial demand for substrate harvesting and procession. Regarding the clinical outcomes, the current review involves three publications comparing FDO to PRP [22,34,50]. While two authors report similar functional improvements after 2 years [34,50], Khoury et al. report that cSVF outperformed PRP after 1 and 2 years in clinical and radiological parameters [22].
The strength of this systematic review is its focus on clinical mid-term results of FDO treatment in knee OA, while other reviews regarding FDO treatment discuss a wide range of indications [12,65,67], methodological details [9] cell compositions [68], or short-term results in knee OA [14]. This clear topic was chosen because the sustainability of symptom relief is deemed a major element in OA treatments. The main limitation of this review is that it contains plain descriptive outcomes of studies fulfilling the inclusion criteria regardless of their study design. Due to a lack of studies comparing FDO to a uniform control group, it was not possible to phrase a reasonable research question for a meta-analysis investigating the superiority of FDO. Therefore, there is a need for further RCT comparing FDO to commonly applied treatment options, i.e., PRP, to gain further insights into the efficacy of FDO. Regarding the included studies investigating FDO, the heterogeneity of study quality and treatment methods should be kept in mind when assessing the descriptive outcomes. Autologous fat tissue may be the common substrate of FDO, but it is ultimately applied after minimal or, to some extent, maximal manipulation [9]. The categories tSVF, cSVF and ASC aim to categorize the techniques, but even within these subcategories, a wide range of different methods are used.
Regarding tSVF, more than 17 different isolation systems are currently available and described in the medical literature [9,69]. This review shows large geographical differences in FDO methods: in Europe (and also in the United States), mainly tSVF is used because of very strict regulations if fat tissue is not obtained and applied during the same surgical procedure. However, emerging evidence suggests that mechanically disrupting adipose tissue (tSVF) has better regenerative effects compared to enzymatically processed lipoaspirate. Although the cell number and density are higher in the cSVF [70], the surface markers in tSVF are twice as high [71]. Moreover, in tSVF, adipocytes are selectively removed without damaging key components of the extracellular matrix. As mentioned before, an intact extracellular structure represents a niche and scaffold for cell modulation, migration, signaling, interaction, and differentiation [62,72,73]. While subcutaneous fat tissue is the basic material, further processing substantially defines its cell composition, biological properties, and terminology used for the respective method. In this regard, the chosen liposuction technique proves to have an impact on the cell composition and MSC quality [74,75,76]. Furthermore, the injection volume, the number and composition of cells or MSC content during application are not standardized. Moreover, individual patient prerequisites, e.g., age or stage of OA, also vary largely in the current literature. The patient’s mobilization, ranging between immediate full-weight bearing [32] and 2 months of non-weight bearing [35] in this review, might also represent an underestimated factor in providing the optimal environment for cartilage regeneration. Concomitant treatments or injections may also change or amplify the effect of FDO: PRP is often used to reduce inflammation and increase FDO’s paracrine effects (Supercharged Liparthroplasty), while fibrin glue should work as a degradable scaffold for ASC to differentiate into mature chondrocytes.
5. Conclusions
The present review demonstrates that the intra-articular administration of fat-derived orthobiologics (FDO) is a promising treatment option for patients with knee OA. It provides a wide range of beneficial mid-term results, including symptom reduction and preservation of the affected joint, which may postpone the need for arthroplasty. The review also revealed that 28 out of 29 studies showed pain reduction and functional improvement in knee OA treated with FDO. Moreover, a limited number of studies were able to demonstrate cartilage regeneration via MRI examinations and second-look arthroscopy. However, further research is necessary to determine the optimal processing, dosage and administration (including additional substances) of FDO as well as postoperative mobilization to define its ideal role in the treatment regimen of knee OA.
Acknowledgments
Johannes Kepler University Open Access Publishing Fund.
Abbreviations
| ASC | Adipose-tissue-derived stromal/stem cells |
| cSVF | Cellular stromal vascular fraction |
| EQ-5D | EuroQol 5 dimensions |
| EQ-VAS | EuroQol 5 visual analogue scale |
| FDO | Fat-derived orthobiologics |
| FJS | Forgotten Joint Score |
| HA | Hyaluronic acid |
| ICRS | International Cartilage Repair Society Macroscopic Evaluation of Cartilage Repair |
| IKDC | Subjective International Knee Documentation Committee |
| IKS | International Knee Society score |
| K-L | Kellgren–Lawrence |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| KKS | Knee Society score |
| LoE | Level of evidence |
| Lysholm | Lysholm knee score |
| ME | Meniscectomy |
| MRI | Magnetic resonance imaging |
| MKM | Marx knee measure |
| MOCART | MRI observation of cartilage repair tissue |
| MQ | Methodological quality |
| MSC | Mesenchymal stem cell |
| OA | Osteoarthritis |
| OARSI | Outcome measures in rheumatology–osteoarthritis research society international |
| OKS | Oxford knee score |
| PGIC | Oatients’ global impression of change scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRP | Platelet-rich plasma |
| PROMs | Patient-Reported Outcome Measures |
| RCT | Randomized controlled trial |
| ROM | Range of motion |
| SF-12 | Short form health survey-12 |
| SVF | Stromal vascular fraction |
| Tegner | Tegner activity scale |
| tSVF | Tissue stromal vascular fraction |
| TUG | Timed up and go test |
| WOMAC | Western Ontario and McMaster universities |
| 10 m WT | 10 m walk test |
| 5 × STS | 5 times sit to stand test |
Author Contributions
Conceptualization, D.D. and M.H.; methodology, M.H.; software, M.H.; validation, D.D. and S.-F.T.K.; formal analysis, D.D.; investigation, M.H. and C.S.; resources, M.H.; data curation, M.H.; writing—original draft preparation, M.H.; writing—review and editing, E.P., P.W.W. and D.D.; visualization, M.H.; supervision, D.D., L.P. and T.G.; project administration, D.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews. The review protocol was registered at the Inplasy register (DOI: 10.37766/inplasy2024.1.0107).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. Liparthroplasty and Supercharged Liparthroplasty are Trademarks owned by Dominik Duscher.
Funding Statement
ACP funding was supported by Johannes Kepler University Open Access Publishing Fund.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vogelmann T., Roessler P.P., Buhs M., Ostermeier S., Gille J., Hoburg A., Zöllner Y., Schwarz S., Schubert T., Grebe M., et al. Long-term cost-effectiveness of matrix-associated chondrocyte implantation in the German health care system: A discrete event simulation. Arch. Orthop. Trauma Surg. 2023;143:1417–1427. doi: 10.1007/s00402-021-04318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin R., Jakob R.P. Review of K.H. Pridie (1959) on “A method of resurfacing osteoarthritic knee joints”. J. ISAKOS. 2022;7:39–46. doi: 10.1016/j.jisako.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Chen X., Tong Y., Luo J., Bi Q. Development and Prospect of Intra-Articular Injection in the Treatment of Osteoarthritis: A Review. J. Pain Res. 2020;13:1941–1955. doi: 10.2147/JPR.S260878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 7.Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: A case series. J. Med. Case Rep. 2011;5:296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivisonno A., Alexander R.W., Baldari S., Cohen S.R., Di Rocco G., Gentile P., Magalon G., Magalon J., Miller R.B., Womack H., et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019;8:1265–1271. doi: 10.1002/sctm.19-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vargel İ., Tuncel A., Baysal N., Hartuç-Çevik İ., Korkusuz F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. Int. J. Mol. Sci. 2022;23:13517. doi: 10.3390/ijms232113517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ude C.C., Shah S., Ogueri K.S., Nair L.S., Laurencin C.T. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen. Eng. Transl. Med. 2022;8:210–224. doi: 10.1007/s40883-021-00226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Lan Z., Yan J., Tang Z., Zhou L., Jin D., Jin Q. Effect of intra-knee injection of autologous adipose stem cells or mesenchymal vascular components on short-term outcomes in patients with knee osteoarthritis: An updated meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2023;25:147. doi: 10.1186/s13075-023-03134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal N., Mak C., Bojanic C., To K., Khan W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells. 2021;10:1365. doi: 10.3390/cells10061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razak H.R.B.A., Corona K., Totlis T., Chan L.Y.T., Salreta J.F., Sleiman O., Vasso M., Baums M.H. Mesenchymal stem cell implantation provides short-term clinical improvement and satisfactory cartilage restoration in patients with knee osteoarthritis but the evidence is limited: A systematic review performed by the early-osteoarthritis group of ESSKA-European knee associates section. Knee Surg. Sports Traumatol. Arthrosc. 2023;31:5306–5318. doi: 10.1007/s00167-023-07575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremy H., Library J.L., Paul G., Trish G., Carl H., Alessandro L., Ivan M., Bob P., Hazel T., Olive G., et al. The Oxford Levels of Evidence 2. [(accessed on 22 March 2024)]. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 17.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 19.Yokota N., Lyman S., Hanai H., Shimomura K., Ando W., Nakamura N. Clinical Safety and Effectiveness of Adipose-Derived Stromal Cell vs Stromal Vascular Fraction Injection for Treatment of Knee Osteoarthritis: 2-Year Results of Parallel Single-Arm Trials. Am. J. Sports Med. 2022;50:2659–2668. doi: 10.1177/03635465221107364. [DOI] [PubMed] [Google Scholar]
- 20.Vasso M., Corona K., Capasso L., Toro G., Schiavone Panni A. Intraarticular injection of microfragmented adipose tissue plus arthroscopy in isolated primary patellofemoral osteoarthritis is clinically effective and not affected by age, BMI, or stage of osteoarthritis. J. Orthop. Traumatol. 2022;23:7. doi: 10.1186/s10195-022-00628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KELLGREN J.H., LAWRENCE J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury M.A., Chamari K., Tabben M., Alkhelaifi K., Papacostas E., Marín Fermín T., Laupheimer M., D Hooghe P. Knee Osteoarthritis: Clinical and MRI Outcomes After Multiple Intra-Articular Injections with Expanded Autologous Adipose-Derived Stromal Cells or Platelet-Rich Plasma. Cartilage. 2023;14:433–444. doi: 10.1177/19476035231166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.S., Choi Y.J., Suh D.S., Heo D.B., Kim Y.I., Ryu J.-S., Koh Y.G. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold? Am. J. Sports Med. 2015;43:176–185. doi: 10.1177/0363546514554190. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.S., Kwon O.R., Choi Y.J., Suh D.S., Heo D.B., Koh Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015;43:2738–2746. doi: 10.1177/0363546515599632. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.S., Suh D.S., Tak D.H., Chung P.K., Koh Y.G. Mesenchymal Stem Cell Implantation in Knee Osteoarthritis: Midterm Outcomes and Survival Analysis in 467 Patients. Orthop. J. Sports Med. 2020;8:2325967120969189. doi: 10.1177/2325967120969189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y.S., Choi Y.J., Lee S.W., Kwon O.R., Suh D.S., Heo D.B., Koh Y.G. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: A prospective study. Osteoarthr. Cartil. 2016;24:237–245. doi: 10.1016/j.joca.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.S., Choi Y.J., Koh Y.G. Mesenchymal stem cell implantation in knee osteoarthritis: An assessment of the factors influencing clinical outcomes. Am. J. Sports Med. 2015;43:2293–2301. doi: 10.1177/0363546515588317. [DOI] [PubMed] [Google Scholar]
- 28.Koh Y.-G., Choi Y.-J., Kwon S.-K., Kim Y.-S., Yeo J.-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:1308–1316. doi: 10.1007/s00167-013-2807-2. [DOI] [PubMed] [Google Scholar]
- 29.Koh Y.-G., Jo S.-B., Kwon O.-R., Suh D.-S., Lee S.-W., Park S.-H., Choi Y.-J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Screpis D., Natali S., Farinelli L., Piovan G., Iacono V., de Girolamo L., Viganò M., Zorzi C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022;11:1268. doi: 10.3390/jcm11051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S., Xu H., He B., Fan M., Xiao M., Zhang J., Chen D., Tong P., Mao Q. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Res. Ther. 2022;13:105. doi: 10.1186/s13287-022-02788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.-I., Lee W.-S., Kim J.-H., Bae J.-K., Jin W. Safety and Efficacy of the Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritic Knee: A 5-Year Follow-up Study. Stem Cells Transl. Med. 2022;11:586–596. doi: 10.1093/stcltm/szac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulivi M., Meroni V., Viganò M., Colombini A., Lombardo M.D.M., Rossi N., Orlandini L., Messina C., Sconfienza L.M., Peretti G.M., et al. Micro-fragmented adipose tissue (mFAT) associated with arthroscopic debridement provides functional improvement in knee osteoarthritis: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2022;31:3079–3090. doi: 10.1007/s00167-022-07101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaffagnini S., Andriolo L., Boffa A., Poggi A., Cenacchi A., Busacca M., Kon E., Filardo G., Di Martino A. Microfragmented Adipose Tissue Versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up. Am. J. Sports Med. 2022;50:2881–2892. doi: 10.1177/03635465221115821. [DOI] [PubMed] [Google Scholar]
- 35.Jo C.H., Chai J.W., Jeong E.C., Oh S., Shin J.S., Shim H., Yoon K.S. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017;45:2774–2783. doi: 10.1177/0363546517716641. [DOI] [PubMed] [Google Scholar]
- 36.Freitag J., Wickham J., Shah K., Li D., Norsworthy C., Tenen A. Mesenchymal stem cell therapy combined with arthroscopic abrasion arthroplasty regenerates cartilage in patients with severe knee osteoarthritis: A case series. Regen. Med. 2020;15:1957–1977. doi: 10.2217/rme-2020-0128. [DOI] [PubMed] [Google Scholar]
- 37.Borić I., Hudetz D., Rod E., Jeleč Ž., Vrdoljak T., Skelin A., Polašek O., Plečko M., Trbojević-Akmačić I., Lauc G., et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes. 2019;10:1051. doi: 10.3390/genes10121051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitag J., Shah K., Wickham J., Li D., Norsworthy C., Tenen A. Evaluation of autologous adipose-derived mesenchymal stem cell therapy in focal chondral defects of the knee: A pilot case series. Regen. Med. 2020;15:1703–1717. doi: 10.2217/rme-2020-0027. [DOI] [PubMed] [Google Scholar]
- 39.Fujita M., Matsumoto T., Sobajima S., Tsubosaka M., Matsushita T., Iwaguro H., Kuroda R. Clinical and Radiological Comparison of Single and Double Intra-articular Injection of Adipose-Derived Stromal Vascular Fraction for Knee Osteoarthritis. Cell Transplant. 2023;32:9636897231190175. doi: 10.1177/09636897231190175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koh Y.G., Choi Y.J., Kwon O.R., Kim Y.S. Second-Look Arthroscopic Evaluation of Cartilage Lesions After Mesenchymal Stem Cell Implantation in Osteoarthritic Knees. Am. J. Sports Med. 2014;42:1628–1637. doi: 10.1177/0363546514529641. [DOI] [PubMed] [Google Scholar]
- 41.Tantuway V., Thomas W., Parikh M.B., Sharma R., Jeyaraman N., Jeyaraman M. Clinical Outcome of Minimally Manipulated, Mechanically Isolated Autologous Adipose Tissue-Derived Stromal Vascular Fraction (Sahaj Therapy®) in Knee Osteoarthritis-Randomized Controlled Trial. Indian J. Orthop. 2023;57:1646–1658. doi: 10.1007/s43465-023-00981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo A., Screpis D., Di Donato S.L., Bonetti S., Piovan G., Zorzi C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: An update at 3 year follow-up. J. Exp. Orthop. 2018;5:52. doi: 10.1186/s40634-018-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Çimen O., Irgıt K.S., Bekmezci T., Büyüktopçu Ö., Şahbat Y., Korucu A. Midterm results of intra-articular stromal vascular fraction injection for the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2023;31:5012–5017. doi: 10.1007/s00167-023-07555-0. [DOI] [PubMed] [Google Scholar]
- 44.Bąkowski P., Kaszyński J., Baka C., Kaczmarek T., Ciemniewska-Gorzela K., Bąkowska-Żywicka K., Piontek T. Patients with stage II of the knee osteoarthritis most likely benefit from the intra-articular injections of autologous adipose tissue-from 2 years of follow-up studies. Arch. Orthop. Trauma Surg. 2021;143:55–62. doi: 10.1007/s00402-021-03979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borg T.-M., Heidari N., Noorani A., Slevin M., Cullen A., Olgiati S., Zerbi A., Danovi A., Wilson A. Gender-Specific Response in Pain and Function to Biologic Treatment of Knee Osteoarthritis: A Gender-Bias-Mitigated, Observational, Intention-to-Treat Study at Two Years. Stem Cells Int. 2021;2021:6648437. doi: 10.1155/2021/6648437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freitag J., Wickham J., Shah K., Tenen A. Real-world evidence of mesenchymal stem cell therapy in knee osteoarthritis: A large prospective two-year case series. Regen. Med. 2022;17:355–373. doi: 10.2217/rme-2022-0002. [DOI] [PubMed] [Google Scholar]
- 47.Heidari N., Borg T.-M., Olgiati S., Slevin M., Danovi A., Fish B., Wilson A., Noorani A. Microfragmented Adipose Tissue Injection (MFAT) May Be a Solution to the Rationing of Total Knee Replacement: A Prospective, Gender-Bias Mitigated, Reproducible Analysis at Two Years. Stem Cells Int. 2021;2021:9921015. doi: 10.1155/2021/9921015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bistolfi A., Roato I., Fornelli G., Sabatini L., Massè A., Ferracini R. Treatment of knee osteoarthritis by intra-articular injection of concentrated autologous adipose tissue: A twenty four month follow-up study. Int. Orthop. 2021;45:627–633. doi: 10.1007/s00264-020-04923-0. [DOI] [PubMed] [Google Scholar]
- 49.Gobbi A., Dallo I., Rogers C., Striano R.D., Mautner K., Bowers R., Rozak M., Bilbool N., Murrell W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: A multi-centric, international study. Int. Orthop. 2021;45:1179–1188. doi: 10.1007/s00264-021-04947-0. [DOI] [PubMed] [Google Scholar]
- 50.Gobbi A., Dallo I., D’Ambrosi R. Autologous microfragmented adipose tissue and leukocyte-poor platelet-rich plasma combined with hyaluronic acid show comparable clinical outcomes for symptomatic early knee osteoarthritis over a two-year follow-up period: A prospective randomized clinical trial. Eur. J. Orthop. Surg. Traumatol. 2022;33:1895–1904. doi: 10.1007/s00590-022-03356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa R., Mizuno S., Murphy G.F., Orgill D.P. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng. Part A. 2009;15:2937–2945. doi: 10.1089/ten.TEA.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang X., Ding Y., Zhang Y., Tse H.-F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 53.Baglio S.R., Pegtel D.M., Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceccarelli S., Pontecorvi P., Anastasiadou E., Napoli C., Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020;8:236. doi: 10.3389/fcell.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salgado A.J.B.O.G., Reis R.L.G., Sousa N.J.C., Gimble J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni P., Srivastava V., Tootsi K., Electricwala A., Kharat A., Bhonde R., Koks S., Martson A., Harsulkar A. Synovial Fluid in Knee Osteoarthritis Extends Proinflammatory Niche for Macrophage Polarization. Cells. 2022;11:4115. doi: 10.3390/cells11244115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marlovits S., Singer P., Zeller P., Mandl I., Haller J., Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Spasovski D., Spasovski V., Baščarević Z., Stojiljković M., Vreća M., Anđelković M., Pavlović S. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J. Gene Med. 2018;20:e3002. doi: 10.1002/jgm.3002. [DOI] [PubMed] [Google Scholar]
- 59.Peterfy C.G., Guermazi A., Zaim S., Tirman P.F.J., Miaux Y., White D., Kothari M., Lu Y., Fye K., Zhao S., et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Koh Y.-G., Kwon O.-R., Kim Y.-S., Choi Y.-J., Tak D.-H. Adipose-Derived Mesenchymal Stem Cells with Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthroscopy. 2016;32:97–109. doi: 10.1016/j.arthro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen P.D., Tran T.D.-X., Nguyen H.T.-N., Vu H.T., Le P.T.-B., Phan N.L.-C., Vu N.B., Phan N.K., van Pham P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017;6:187–195. doi: 10.5966/sctm.2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bisicchia S., Bernardi G., Pagnotta S.M., Tudisco C. Micro-fragmented stromal-vascular fraction plus microfractures provides better clinical results than microfractures alone in symptomatic focal chondral lesions of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2020;28:1876–1884. doi: 10.1007/s00167-019-05621-0. [DOI] [PubMed] [Google Scholar]
- 63.Oedayrajsingh-Varma M.J., van Ham S.M., Knippenberg M., Helder M.N., Klein-Nulend J., Schouten T.E., Ritt M.J.P.F., van Milligen F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 64.Hass R., Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun. Signal. 2012;10:26. doi: 10.1186/1478-811X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunze K.N., Burnett R.A., Wright-Chisem J., Frank R.M., Chahla J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020;13:264–280. doi: 10.1007/s12178-020-09624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolia I.K., Bougioukli S., Hill W.J., Trasolini N.A., Petrigliano F.A., Lieberman J.R., Weber A.E. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients with Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022;50:1451–1461. doi: 10.1177/03635465211014500. [DOI] [PubMed] [Google Scholar]
- 67.Tang Q., Zhao X.-S., Guo A., Cui R.-T., Song H.-L., Qi Z.-Y., Pan Y., Yang Y., Zhang F.-F., Jin L. Therapeutic applications of adipose-derived stromal vascular fractions in osteoarthritis. World J. Stem Cells. 2022;14:744–755. doi: 10.4252/wjsc.v14.i10.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z., Zhang S., Cao M., Lin Z., Kong L., Wu X., Guo Q., Ouyang Y., Song Y. What is the optimal dose of adipose-derived mesenchymal stem cells treatment for knee osteoarthritis? A conventional and network meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2023;14:245. doi: 10.1186/s13287-023-03475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberbauer E., Steffenhagen C., Wurzer C., Gabriel C., Redl H., Wolbank S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015;4:7. doi: 10.1186/s13619-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winnier G.E., Valenzuela N., Peters-Hall J., Kellner J., Alt C., Alt E.U. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS ONE. 2019;14:e0221457. doi: 10.1371/journal.pone.0221457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiryaki T., Condé-Green A., Cohen S.R., Canikyan S., Kocak P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open. 2020;8:e2652. doi: 10.1097/GOX.0000000000002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimozono Y., Fortier L.A., Brown D., Kennedy J.G. Adipose-Based Therapies for Knee Pain-Fat or Fiction. J. Knee Surg. 2019;32:55–64. doi: 10.1055/s-0038-1672155. [DOI] [PubMed] [Google Scholar]
- 73.Nürnberger S., Lindner C., Maier J., Strohmeier K., Wurzer C., Slezak P., Suessner S., Holnthoner W., Redl H., Wolbank S., et al. Adipose-tissue-derived therapeutic cells in their natural environment as an autologous cell therapy strategy: The microtissue-stromal vascular fraction. Eur. Cell. Mater. 2019;37:113–133. doi: 10.22203/eCM.v037a08. [DOI] [PubMed] [Google Scholar]
- 74.Chung M.T., Zimmermann A.S., Paik K.J., Morrison S.D., Hyun J.S., Lo D.D., McArdle A., Montoro D.T., Walmsley G.G., Senarath-Yapa K., et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl. Med. 2013;2:808–817. doi: 10.5966/sctm.2012-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duscher D., Atashroo D., Maan Z.N., Luan A., Brett E.A., Barrera J., Khong S.M., Zielins E.R., Whittam A.J., Hu M.S., et al. Ultrasound-Assisted Liposuction Does Not Compromise the Regenerative Potential of Adipose-Derived Stem Cells. Stem Cells Transl. Med. 2016;5:248–257. doi: 10.5966/sctm.2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duscher D., Luan A., Rennert R.C., Atashroo D., Maan Z.N., Brett E.A., Whittam A.J., Ho N., Lin M., Hu M.S., et al. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J. Transl. Med. 2016;14:126. doi: 10.1186/s12967-016-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.