Abstract
Background
Limited data existed on the burden of coronavirus disease 2019 (COVID-19) renal complications and the outcomes of the most critical patients who required kidney replacement therapy (KRT) during intensive care unit (ICU) stay. We aimed to describe mortality and renal function at 90 days in patients admitted for COVID-19 and KRT.
Methods
A retrospective cohort study of critically ill patients admitted for COVID-19 and requiring KRT from March 2020 to January 2022 was conducted in an Italian ICU from a tertiary care hospital. Primary outcome was mortality at 90 days and secondary outcome was kidney function at 90 days.
Results
A cohort of 45 patients was analyzed. Mortality was 60% during ICU stay and increased from 64% at the time of hospital discharge to 71% at 90 days. Among 90-day survivors, 31% required dialysis, 38% recovered incompletely, and 31% completely recovered renal function. The probability of being alive and dialysis-free at 3 months was 22%.
Conclusions
Critically ill patients with COVID-19 disease requiring KRT during ICU stay had elevated mortality rate at 90 days, with low probability of being alive and dialysis-free at 3 months. However, a non-negligible number of patients completely recovered renal function.
Keywords: COVID-19, Acute kidney injury, Kidney replacement therapy, Mortality, Kidney recovery
Introduction
The morbidity and mortality associated with severe illness from coronavirus disease 2019 (COVID-19) is attributed to multiorgan injury, including kidneys [1, 2]. Patients experiencing severe acute kidney injury (AKI) due to COVID-19 disease may require kidney replacement therapy (KRT).
AKI-KRT is one of the most severe complications and demands a significant allocation of resources. During COVID-19 pandemic where staff and material resources were scarce, clinicians faced the difficulty to decide to treat COVID-19-associated AKI with KRT, without knowing the long-term impact of this decision. The high morbidity and mortality showed in severely ill non-COVID19 patients may suggest a similar outcome, but data are limited.
Since the first COVID-19 victim was recorded in Europe in the North East of Italy, SARS-CoV-2 has become a relentless epidemic in Italy, and the surge of critically ill patients needing intensive care resulted in an unexpected crisis raised important questions about clinical course and prognosis for these patients. Efforts were made to prevent the breakdown of the healthcare system, by adopting local measures, increasing ICU and medical ward capacity, and sharing therapeutic strategies [3]. At that time, the impact of COVID-19 disease on the kidneys was undefined and unclear, and the taskforce did not consider the potential need for KRT machine and expert staff. It is now critical to understand the burden of COVID-19 renal complications and the outcomes of patients treated with KRT for resource planning and to support clinicians, patients, and their families in the process of shared decision.
We describe the clinical characteristics and outcomes of mortality and kidney recovery of critically ill patients with COVID-19 disease who developed severe AKI requiring KRT from a tertiary care hospital severely hit during the pandemic. We also aimed to estimate the probability of being alive and dialysis-free at 3 months since KRT initiation.
Material and methods
Population
All consecutive adult patients requiring KRT after being admitted to the general ICU of Padua from March 2020 to January 2022 with laboratory-confirmed COVID-19 disease were included in the retrospective study.
Exclusion criteria were as follows: (1) history of end-stage kidney disease; (2) reason for ICU admission not related with COVID-19 disease (example: acetaminophen intoxication in a patient that tested positive for COVID-19).
Indications for KRT, as well as the modality chosen, were determined by consensus between the attending intensivists and nephrologists and based on clinical status of the patient. Specifically, in the case of moderate/severe AKI, the indication was considered for patients who showed low tolerance to volume overload or metabolic acidosis; in the case of medically refractory hyperkalemia, metabolic acidosis, or severe hypoxia due to volume overload, the indication was mandatory.
Study outcomes
The primary outcome measure of the study was mortality at 90 days after initiation of KRT. The secondary outcome was kidney function at 90 days, measured as kidney recovery and dialysis dependence. Moreover, the probability to be alive and free of dialysis at 90 days was analyzed.
Definitions
The diagnosis of COVID-19 was defined as a positive RT-PCR (real-time polymerase chain reaction) result.
AKI diagnosis and severity were based on Kidney Disease Improving Global Outcomes (KDIGO) criteria [4]. Baseline serum creatinine (SCr) was defined as the lowest value 365–7 days prior to hospitalization. If a prehospital baseline SCr was not available, we used SCr value obtained from Chronic Kidney Disease Epidemiology Collaboration estimation formula. Renal recovery was defined according to the Acute Disease Quality Initiative (ADQI) consensus as full recovery is the absence of AKI criteria; partial recovery can then be defined as a fall in AKI stage [5].
Data collection
We collected data by detailed chart review and used a standardized patient report form to enter data into a secure online database (REDCap). Patient-level data included demographics, such as age, sex, body mass index (BMI), and comorbidities, such as chronic kidney disease (CKD), hepatic failure, cardiac failure, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), antecedent solid neoplasm, active solid neoplasm, active hematological neoplasm, arterial hypertension, vasculopathy, and immunosuppression. We also collected the baseline SCr. Indication for KRT was recorded (fluid overload, uremia, hyperkalemia, acid–base disorders).
Characteristics at initiation of KRT were collected: heart rate, presence of arrhythmia, temperature, use of vasoactive, vasoactive inotropic score (VIS), mean arterial pressure (MAP), arterial partial pressure of oxygen over fractional inspiratory oxygen ratio (paO2/FiO2), arterial partial pressure of carbon dioxide (paCO2), pH, lactate, serum creatinine, urinary output (UO) during the day before start of KRT, urea, bilirubin, albumin, platelets count, fibrinogen, procalcitonin (PCT), C reactive protein (CRP), white blood cells count (WBC), need for mechanical ventilation, use of extracorporeal support, such as extracorporeal membrane oxygenation (ECMO) or extracorporeal dioxide removal (ECCO2R). Scores of illness severity (Sequential Organ Failure Assessment, SOFA, Simplified Acute Physiology Score, SAPS2) were collected at the time of KRT initiation.
We recorded the time from ICU admission and KRT initiation, as well as initial KRT modality and prescription (net ultrafiltration, prescribed dose, use of regional citrate anticoagulation or systemic heparin coagulation). We recorded complications related to KRT (such as hypomagnesemia, hypophosphatemia, hemorrhage, citrate accumulation, thromboembolism).
Kidney laboratory data were recorded also at ICU discharge, hospital discharge, and at 90 days. Data on long-term follow-up were gathered from patients’ electronic medical records.
Ethical approval
The study was approved with a waiver of informed consent by the institutional review board “Comitato Etico per la Sperimentazione Clinica della Provincia di Padova” with protocol number 70601 on 27/01/022.
Statistical analysis
The analysis was performed using R studio version 4.1.2.
Descriptive statistics were calculated for patients’ demographics, comorbidities, laboratory values, and hospital course and are presented as median (interquartile range) for continuous values or counts (percentage) for categorical values.
We evaluated differences between survivors and non-survivors using Mann–Whitney U test and chi-square test, as appropriate.
We used the package “survival” to estimate the probability of KRT independence at 90 days, with death as competing risk. The package “survminer” was used to plot the time-to-event function analysis. Patients were censored at 90 days.
In order to evaluate the influence of other variables on ICU mortality and 90-day mortality, we also performed backward stepwise multivariate analysis, using age, BMI, VIS, PaO2/FiO2, SAPS2, SOFA as continuous variables, and history of CKD, DM, arterial hypertension, or use of ECMO as categorical variables.
Data availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Results
Patients’ characteristics at baseline
During the study period, a total of 481 patients with COVID-19 positivity were admitted to ICU, among whom 54 patients (11.2%) were treated with KRT during ICU stay. We excluded eight patients that were on chronic dialysis before admission and one patient admitted to ICU for reasons unrelated to COVID-19 disease, as shown in Fig. 1. A final cohort of 45 patients was analyzed. The demographics of our cohort are outlined in Table 1. Basal creatinine was only present in 42% of our cohort and the median baseline sCr was 1 mg/dL (IQR 0.8–1.1); 27% had chronic kidney disease at any stage, with 22% with stages 3 and 4.
Fig. 1.
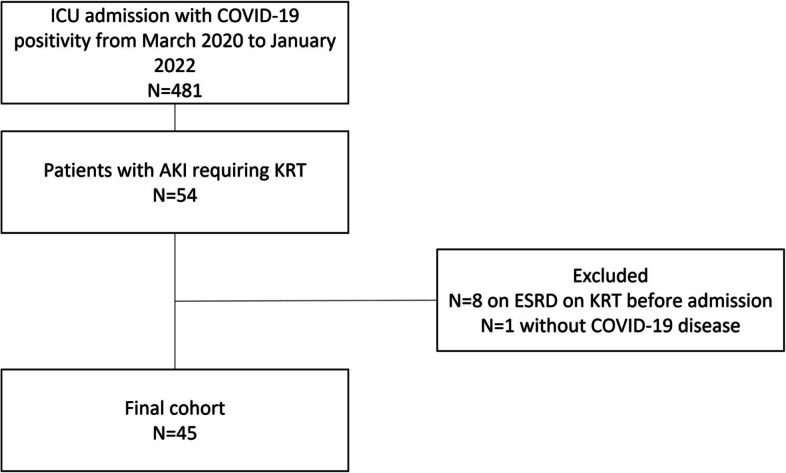
Study flowchart. ICU intensive care unit, COVID-19 coronavirus 2019, AKI acute kidney injury, KRT kidney replacement therapy, ESRD end-stage renal disease
Table 1.
Patients’ demographics and comorbidities. Differences between 90-day survivors and non-survivors are reported with p-value for significance
| Total cohort | 90-day survivors | 90-day non-survivors | p-value | |
|---|---|---|---|---|
| Number of patients | 45 | 13 | 32 | |
| Demographics | ||||
| Age, years | 69 (60–74) | 69 (58–78) | 68 (60–74) | 0.854 |
| Male sex, % | 82 | 85 | 81 | 0.789 |
| BMI, kg/m2 | 28 (26–33) | 28 (26–30) | 29 (26–33) | 0.997 |
| Comorbid conditions | ||||
| Baseline CKD, % | 27 | 31 | 25 | 0.692 |
| KDIGO stage 3 or more, % | 22 | 23 | 22 | 0.584 |
| Baseline SCr, mg/dl | 1 (0.8–1.1) | 1 (1–1.2) | 1 (0.8–1.1) | 0.854 |
| Hepatic failure, % | 2 | 0 | 3 | 0.519 |
| Cardiac failure, % | 2 | 0 | 3 | 0.519 |
| COPD, % | 2 | 0 | 3 | 0.519 |
| DM, % | 22 | 15 | 25 | 0.482 |
| Antecedent solid neoplasm, % | 9 | 15 | 6 | 0.329 |
| Active solid neoplasm, % | 4 | 8 | 3 | 0.5 |
| Active hematological neoplasm, % | 2 | 0 | 3 | 0.519 |
| Arterial hypertension, % | 67 | 77 | 62 | 0.352 |
| Vasculopathy, % | 18 | 23 | 16 | 0.553 |
| Immunosuppression, % | 9 | 8 | 9 | 0.857 |
| Characteristics at ICU admission | ||||
| Organ failure > 2, % | 22 | 25 | 15 | 0.482 |
BMI Body mass index, CKD Chronic kidney disease, KDIGO Kidney disease: improving global outcomes, SCr Serum creatinine, COPD Chronic obstructive pulmonary disease, DM Diabetes mellitus, ICU Intensive care unit, KRT Kidney replacement therapy
Primary outcome
ICU mortality was 60% with a median LOS of 29 [17–38] days. Mortality increased from 64% at the time of hospital discharge to 71% at 90 days. Ninety-day non-survivors had significantly lower paO2/FiO2 at the time of KRT initiation and experienced more complications related to KRT during ICU stay (Table 2). The major risk factor for ICU mortality was the.history of CKD, as shown in the multivariate analysis in Table 3, while no specific risk factor was found for 90-day mortality (Table 4).
Table 2.
Patients’ characteristics at initiation of KRT, KRT prescription, and KRT complications. Differences between 90-day survivors and non-survivors are reported with p-value for significance
| Total cohort | 90-day survivors | 90-day non-survivors | p-value | |
|---|---|---|---|---|
| Number of patients | 45 | 13 | 32 | |
| Characteristics at initiation of KRT | ||||
| SOFA score | 16 (14–18) | 16 (13–18) | 16 (14–18) | 0.924 |
| SAPS2 | 70 (62–75) | 63 (54–75) | 70 (64–75) | 0.709 |
| Mechanical ventilation, % | 100 | 100 | 100 | 1 |
| Vasoactive use, % | 84 | 85 | 84 | 0.793 |
| Extracorporeal support, % | 9 | 0 | 12 | 0.182 |
| ECMO, % | 7 | 0 | 9 | 0.253 |
| ECCO2R, % | 2 | 0 | 3 | 0.519 |
| Cardiac frequency, bpm | 95 (83–106) | 86 (64–101) | 96 (85–107) | 0.186 |
| Arrhythmya, % | 24 | 23 | 25 | 0.892 |
| MAP, mmHg | 79 (69–88) | 80 (71–85) | 79 (66–91) | 0.924 |
| Temperature, °C | 36.5 (35.9–37.0) | 36 (35.4–37.2) | 36.6 (36–37) | 0.573 |
| VIS | 23 (6–35) | 19 (7–29) | 25 (6–36) | 0.573 |
| paO2/FiO2, mmHg | 115 (85–165) | 151 (112–199) | 107 (75–151) | 0.024* |
| paCO2, mmHg | 42 (39–47) | 42 (39–44) | 42 (40–54) | 0.924 |
| Ph | 7.38 (7.32–7.42) | 7.38 (7.34–7.43) | 7.36 (7.31–7.42) | 0.806 |
| Renal characteristics | ||||
| Urine output, ml | 1990 (1210–2900) | 1800 (1390–2500) | 2300 (1055–3100) | |
| Fluid overload, % | 73 | 69 | 75 | 0.692 |
| Uremia, % | 56 | 46 | 59 | 0.419 |
| Hyperkalemia, % | 13 | 8 | 16 | 0.478 |
| Acid–base disorder, % | 9 | 8 | 9 | 0.857 |
| SCr at KRT initiation, mg/dl | 2.5 (1.9–4.1) | 3 (2.4–5.0) | 2.4 (1.8–3.9) | 0.451 |
| ICU to KRT length of stay, days | 7 (2–13) | 6 (1–13) | 8 (2–14) | 0.451 |
| Laboratory parameters at KRT initiation | ||||
| Lactate, umol/L | 2 (1.4–2.6) | 1.6 (1.3–2.5) | 2 (1.5–2.7) | 0.792 |
| Urea, mg/L | 145 (90–232) | 142 (76–231) | 148 (96–233) | 1 |
| Bilirubin, mg/L | 9 (5–19.5) | 11 (3–19) | 9 (5–20) | 0.845 |
| Platelet cells count, 103/µL | 207 (138–307) | 224 (139–290) | 202 (120–314) | 0.924 |
| Procalcitonine, ng/ml | 1.4 (0.5–3.6) | 1.9 (0.4–3.9) | 1.4 (0.6–3.5) | 0.925 |
| C reactive protein, mg/L | 110 (35–190) | 110 (35–154) | 110 (31–272) | 0.775 |
| White blood cells count, 109/µL | 15 (9.6–19.8) | 14.3 (9.7–17.9) | 16 (9.3–20.3) | 1 |
| KRT first prescription | ||||
| Continuous modality, % | 100 | 100 | 100 | 1 |
| Net ultrafiltration, ml | 100 (100–120) | 100 (100–100) | 100 (100–150) | 0.257 |
| Prescribed dose, ml/kg/h | 30 (28–35) | 29 (27–34) | 30 (27–35) | 0.944 |
| Anticoagulation, % | ||||
| Regional citrate anticoagulation, % | 69 | 69 | 69 | 0.912 |
| No anticoagulation, % | 11 | 15 | 9 | 0.525 |
| Systemic heparin, % | 9 | 0 | 12 | 0.345 |
| Complications related to KRT, % | 82 | 61 | 91 | 0.021* |
| Hypomagnesemia, % | 64 | 38 | 75 | 0.020* |
| Hypophosphatemia, % | 80 | 61 | 87 | 0.048* |
| Thromboembolism, % | 4 | 0 | 6 | 0.356 |
| Hemorrhage, % | 16 | 8 | 19 | 0.354 |
| Citrate accumulation, % | 2 | 0 | 3 | 0.519 |
KRT Kidney replacement therapy, SOFA Sequential organ failure assessment, SAPS2 Simplified acute physiology score, ECMO Extracorporeal membrane oxygenation, ECCO2R Extracorporeal carbon dioxide removal, MAP Mean arterial pressure, VIS Vasoactive inotropic score, paO2/FiO2 Partial Pressure of arterial oxygen content/fraction of inspired oxygen, paCO2 Partial pressure of carbon dioxide content
*p-value < 0.05
Table 3.
Multivariate analysis for ICU mortality
| Variables | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Age | 0.94 | 0.86–1.02 | 0.14 |
| BMI | 1.03 | 0.88–1.21 | 0.69 |
| CKD | 6.95 | 1.04–46.5 | 0.045* |
| DM | 0.75 | 0.11–5.03 | 0.76 |
| Arterial hypertension | 0.93 | 0.16–5.38 | 0.94 |
| VIS | 1.04 | 0.97–1.11 | 0.24 |
| PaO2/FiO2 | 0.99 | 0.98–1.01 | 0.77 |
| SAPS2 | 1.02 | 0.95–1.1 | 0.49 |
| SOFA | 0.77 | 0.56–1.07 | 0.12 |
| ECMO | 1.55 | 0.95–4.72 | 0.14 |
BMI Body mass index, CKD Chronic kidney disease, DM Diabetes mellitus, SOFA Sequential organ failure assessment, SAPS2 Simplified acute physiology score, ECMO Extracorporeal membrane oxygenation, VIS Vasoactive inotropic score, paO2/FiO2 Partial pressure of arterial oxygen content/fraction of inspired oxygen
*p-value < 0.05
Table 4.
Multivariate analysis for 90-day mortality
| Variables | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Age | 0.96 | 0.88–1.05 | 0.47 |
| BMI | 1.01 | 0.85–1.22 | 0.85 |
| CKD | 1.26 | 0.18–8.66 | 0.81 |
| DM | 0.52 | 0.07–4.1 | 0.53 |
| Arterial hypertension | 1.62 | 0.25–10.6 | 0.61 |
| VIS | 1.03 | 0.96–1.1 | 0.41 |
| PaO2/FiO2 | 0.99 | 0.98–1.01 | 0.32 |
| SAPS2 | 1.08 | 0.98–1.19 | 0.102 |
| SOFA | 0.81 | 0.58–1.13 | 0.21 |
| ECMO | 1.17 | 0.91–2.98 | 0.28 |
BMI Body mass index, CKD Chronic kidney disease, DM Diabetes mellitus, SOFA Sequential organ failure assessment, SAPS2 Simplified acute physiology score, ECMO Extracorporeal membrane oxygenation, VIS Vasoactive inotropic score, paO2/FiO2 Partial pressure of arterial oxygen content/fraction of inspired oxygen
Secondary outcomes
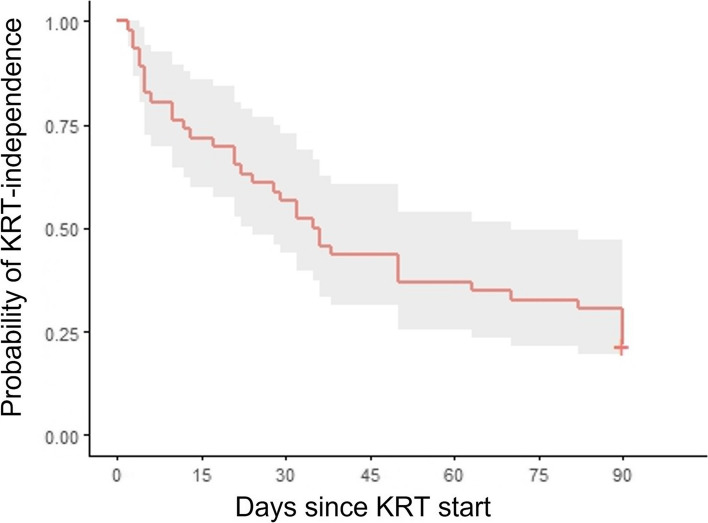
Among ICU survivors, the median ICU LOS was 32 [21–45] days, with a median time from KRT initiation to liberation of 20 [6–43] days. Among 90-day survivors, 4 patients (31%) still required dialysis, 5 patients (38%) recovered incompletely with a median eGFR of 42 [32–49] ml/min per 1.73 m2, and 4 patients (31%) completely recovered renal function with a median eGFR of 75 [68–77] ml/min per 1.73 m2. As shown in Fig. 2, the probability to be alive and free of dialysis at 90 days for a patient who developed AKI-KRT during the ICU stay for COVID-19 disease was 22%.
Fig. 2.
Probability of KRT independence at 90 days since KRT initiation. Time-to-event analysis with death as a competing risk
Discussion
In this monocenter cohort study of 481 critically ill patients with COVID-19, we found that almost 10% of all patients required KRT for AKI over the disease course. During ICU stay, 60% of AKI-KRT patients died and we found history of CKD as only risk factor. The mortality rate of AKI-KRT patients rose to 71% at 90 days, while more than 20% of survivors still needed dialysis at 3 months. According to our estimation, the probability of being alive and dialysis-free at 3 months since KRT initiation was 22%. Cumulatively, these findings indicate that AKI-KRT is associated with high rates of mortality and morbidity. The present study is one of the few investigating mortality and renal recovery in the subpopulation of critically ill patients requiring KRT during the course of COVID-19 disease.
Several studies investigated long-term outcomes in critically ill patients with COVID-related AKI [2, 6–9], but few focused on the subpopulation of critically ill patients requiring KRT during COVID-19 disease.
During the first wave of pandemic, there were reports with a limited number of patients worldwide that showed different rates of mortality. In a small cohort of 13 Dutch patients, the ICU mortality rate was 39% [10], while a larger cohort of 114 patients in New York showed a 60-day mortality of 70% [11]. The reports did not specify many characteristics of the population analyzed particularly the severity and the comorbidities prior to ICU admission. Another small cohort of 34 Brazilian patients found a 60-day mortality of 35.4% in critically ill patients all mechanically ventilated and with high rate of ECMO [12]. Stevens et al. analyzed again in New York a cohort of 115 patients, of which 99% mechanically ventilated and 84% needing vasopressors, with a SOFA score of 15 [13]. The authors found a 51% mortality at follow-up. They identified COPD and coronary artery disease as main risk factors for mortality even though a small proportion of patients had history of these comorbidities. Another study by Eriksson et al. on 82 patients (SAPS2 of 61, all mechanically ventilated, with 8.5% use of ECMO) in Sweden demonstrated a rate of ICU and 30-day mortality of 39% and 45%, respectively [14]. The larger cohort represented by 876 AKI-KRT patients with COVID-19 from 67 ICUs in the USA was investigated in the STOP-COVID study by Gupta et al., which showed a 28-day mortality rate of 67% [15]. The analyzed cohort required mechanical ventilation in 98.1% of patients and 41% of the population had pre-existing kidney chronic dysfunction. Other more recent investigations reported a hospital mortality rate of 67.5%, 72.5%, 68.1%, and 43.4% in cohorts in Boston [16], Brazil [17], India, and Pakistan [18], respectively.
As discussed by Gupta et al., the different rates of mortality might be due to many patient-level (history of pre-existing CKD or high-risk population like Black and Hispanic races or severity of COVID-19 disease) and hospital-level factors (resource limit or high burden hospital) [15].
The population that we described was very sick, as expressed by severity scores, such as SAPS 2 and SOFA scores of 70 and 16, respectively. The entire cohort of 45 patients needed invasive mechanical ventilation and 84% required vasopressor use during ICU stay. Moreover, the pre-existing CKD was an independent risk factor for ICU mortality, while we could not find any independent factor associated with 90-day mortality. Pre-existing CKD has been among the most robust predictors of severe and critical illness in patients with COVID-19. A meta-analysis with data from 9 studies found that CKD was associated with a threefold higher risk of mortality in COVID-19 hospitalized patients [19]. Consistent with our finding, Eriksson et al. found that the baseline serum creatinine was a predictor for survival in critically ill patients with AKI-KRT [14]. Other predictors of mortality in COVID-19 critical patients needing KRT were age [14] and a higher number of organ dysfunction [17].
Renal recovery after AKI-KRT is important for patients, families, and all clinicians involved. Our study shows 9% (31% among survivors) of dialysis dependence at 90 days, while 9% (31% among survivors) completely recovered renal function according to sCr at follow-up.
Melero et al. investigated a subpopulation of critically ill COVID-19 patients who required KRT and reported a zero rate of dialysis dependence, but a high rate of renal non-recovery at 1 year [20]; in contrast with our analysis, only patients with normal baseline serum creatinine were analyzed, while in our cohort, 27% had history of CKD at any stage. In fact, Ng et al. published the outcomes among patients hospitalized with COVID-19 and AKI [21]. Among those with AKI-KRT who survived, 30.6% remained on dialysis at discharge, and pre-hospitalization CKD was the only independent risk factor associated with needing dialysis at discharge. Hsu et al. noticed that the more severe the pre-existing kidney dysfunction, the greater the risk for dialysis dependence in critically ill patients with AKI-KRT [22]. In another report from New York, a population of ICU patients with AKI-KRT, only 26% of survivors were able to be weaned from KRT before hospital discharge [11]. Chand et al. investigated specifically critically ill patients who developed AKI-KRT and survived; among 35 survivors, 77% were KRT liberated and 57% had complete renal recovery [23].
Comparing with other studies, we report a low rate of renal recovery, and there might be many possible explanations: the baseline comorbid state of our population (27% was known to have CKD at any stage), the severity of COVID-19 disease treated in our unit (where almost exclusively mechanically ventilated patients were admitted), the management of fluid therapy and drug stewardship, that were not taken into account in this analysis.
There are several potential limitations concerning the results of the present study. First, this was a single-center observational study which might impact the generalization of findings. Second, due to the retrospective nature of the study, some laboratory results were not available for all patients. We had incomplete information on treatment before ICU admission. Third, baseline sCr was available in less than half the patients: we evaluated renal recovery according to sCr value at follow-up and this might overestimate or underestimate the rate of recovery. Hence, further research might help shed light on the effect of COVID-19 on mortality and kidney disease.
Conclusion
Critically ill patients with COVID-19 disease requiring KRT during ICU stay had elevated mortality rate at 90 days, with low probability of being alive and dialysis-free at 3 months. However, a non-negligible number of patients completely recovered renal function.
Acknowledgements
Not applicable.
Abbreviations
- ADQI
Acute disease quality initiative
- AKI
Acute kidney injury
- BMI
Body mass index
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CRP
C reactive protein
- DM
Diabetes mellitus
- ECCO2R
Extracorporeal dioxide removal
- ECMO
Extracorporeal membrane oxygenation
- eGFR
Estimated glomerular filtration rate
- ICU
Intensive care unit
- KDIGO
Kidney disease improving global outcomes
- KRT
Kidney replacement therapy
- MAP
Mean arterial pressure
- paCO2
Arterial partial pressure of carbon dioxide
- paO2/FiO2
Arterial partial pressure of oxygen over fractional inspiratory oxygen ratio
- PCT
Procalcitonin
- RT-PCR
Real-time polymerase chain reaction
- SAPS2
Simplified acute physiology score 2
- sCr
Serum creatinine
- SOFA
Sequential organ failure assessment
- UO
Urinary output
- VIS
Vasoactive inotropic score
- WBC
White blood count
Authors’ contributions
Ilaria Godi: conceptualization, methodology, formal analysis, writing—original draft; Laura Pasin: methodology, writing—review and editing; Andrea Ballin: data curation, writing—review and editing; Gabriele Martelli: data curation, writing—review and editing; Claudio Bonanno: data curation, writing—review and editing; Francesco Terranova: data curation, writing—review and editing; Enrico Tamburini: investigation, writing—review and editing; Caterina Simoni: investigation, writing—review and editing; Ginevra Randon: investigation, writing—review and editing; Nicola Franchetti: investigation, writing—review and editing; Leda Cattarin: data curation, writing—review and editing; Federico Nalesso: data curation, writing—review and editing; Lorenzo Calò: writing—review and editing, supervision; Ivo TIberio: conceptualization, writing—review and editing, supervision. All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
Funding
The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasin L, Sella N, Correale C, Boscolo A, Rosi P, Saia M, et al. Regional COVID-19 network for coordination of SARS-CoV-2 outbreak in Veneto, Italy. J Cardiothorac Vasc Anesth. 2020;34(9):2341–2345. doi: 10.1053/j.jvca.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Practice Guidelines for Acute Kidney Injury (2012) Kidney International Supplements. 2(1):1. http://www.kdigo.org/clinicalpracticeguidelines/AKI.php
- 5.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Acute Disease Quality Initiative Workgroup 16 et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–57. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 6.Neyra JA, Ortiz-Soriano V, Liu LJ, Smith TD, Li X, Xie D, et al. Prediction of mortality and major adverse kidney events in critically ill patients with acute kidney injury. Am J Kidney Dis. 2023;81(1):36–47. doi: 10.1053/j.ajkd.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezerra R, Teles F, Mendonca PB, Damte T, Likaka A, Ferrer-Miranda E, et al. Outcomes of critically ill patients with acute kidney injury in COVID-19 infection: an observational study. Ren Fail. 2021;43(1):911–918. doi: 10.1080/0886022X.2021.1933530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11(1):123. doi: 10.1186/s13613-021-00914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez ADLV, Pérez AN, Pérez-Carrasco M, Sonet MT, Buendia YD, Ballujera PO, et al. Acute kidney injury in critically ill patients with COVID–19: the AKICOV multicenter study in Catalonia. PLoS One. 2023;18(4):e0284248. doi: 10.1371/journal.pone.0284248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilbers TJ, Koning MV. Renal replacement therapy in critically ill patients with COVID-19: a retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care. 2020;60:103–105. doi: 10.1016/j.jcrc.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkar J, Chand S, Aboodi MS, Gone AR, Alahiri E, Schecter DE, et al. Characteristics, outcomes and 60-day hospital mortality of ICU patients with COVID-19 and acute kidney injury. Kidney360. 2020;1(12):1339–44. doi: 10.34067/KID.0004282020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doher MP, De Carvalho Torres FR, Scherer PF, Matsui TN, Ammirati AL, Da Silva Caldin B, et al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif. 2021;50(4–5):520–30. doi: 10.1159/000513425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens JS, King KL, Robbins-Juarez SY, Khairallah P, Toma K, Alvarado Verduzco H, et al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One. 2020;15(12):e0244131. doi: 10.1371/journal.pone.0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson KE, Campoccia-Jalde F, Rysz S, Rimes-Stigare C. Continuous renal replacement therapy in intensive care patients with COVID-19; survival and renal recovery. J Crit Care. 2021;64:125–130. doi: 10.1016/j.jcrc.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI Treated with renal replacement therapy in critically ill patients with COVID-19. JASN. 2021;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Mouhayyar C, Dewald J, Cabrales J, Tighiouart H, Moraco AH, Jaber BL, et al. Factors associated with severity of acute kidney injury and adverse outcomes in critically ill patients with COVID-19. Nephron. 2022;146(6):584–592. doi: 10.1159/000524657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaan F, De Paula Carneiro E, De Lima Souza FBG, Mendes LFC, Rossi PRG, Freitas RAP, et al. COVID-19-associated acute kidney injury patients treated with renal replacement therapy in the intensive care unit: a multicenter study in São Paulo, Brazil. PLoS One. 2022;17(1):e0261958. doi: 10.1371/journal.pone.0261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anandh U, Noorin A, Kazmi SKS, Bannur S, Shah SSA, Farooq M, et al. Acute kidney injury in critically ill COVID-19 infected patients requiring dialysis: experience from India and Pakistan. BMC Nephrol. 2022;23(1):308. doi: 10.1186/s12882-022-02931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melero R, Mijaylova A, Rodriguez-Benitez P, Macıas N, Aragoncillo I, Rodriguez-Ferrero ML et al (2011) Renal long-term outcome of critically ill COVID-19 patients with acute kidney failure and continuous renal replacement therapy. Clin Kidney J 14(11):2449–2450 [DOI] [PMC free article] [PubMed]
- 21.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215.e1. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CM, Gupta S, Tighiouart H, Goyal N, Faugno AJ, Tariq A, et al. Kidney Recovery and death in critically ill patients with COVID-19–associated acute kidney injury treated with dialysis: the STOP-COVID cohort study. Am J Kidney Dis. 2022;79(3):404–416.e1. doi: 10.1053/j.ajkd.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chand S, Kapoor S, Naqvi A, Thakkar J, Fazzari MJ, Orsi D, et al. Long-term follow up of renal and other acute organ failure in survivors of critical illness due to Covid-19. J Intensive Care Med. 2022;37(6):736–742. doi: 10.1177/08850666211062582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.