Abstract
Signaling by the Wnt family of secreted proteins plays an important role in animal development and is often misregulated in carcinogenesis. Wnt signal transduction is controlled by the rate of degradation of β-catenin by a complex of proteins including glycogen synthase kinase 3 (GSK3), adenomatous polyposis coli, and Axin. Dishevelled is required for Wnt signal transduction, and its activation results in stabilization of β-catenin. However, the biochemical events underlying this process remain largely unclear. Here we show that Xenopus Dishevelled (Xdsh) interacts with a Xenopus Axin-related protein (XARP). This interaction depends on the presence of the Dishevelled-Axin (DIX) domains in both XARP and Xdsh. Moreover, the same domains are essential for signal transduction through Xdsh. Finally, our data point to a possible mechanism for signal transduction, in which Xdsh prevents β-catenin degradation by displacing GSK3 from its complex with XARP.
A central problem of molecular and cell biology is to understand how an extracellular signal is transmitted from the cell surface to the nucleus. One essential signaling pathway is the Wnt pathway, which controls cell fate and cell proliferation in embryonic development and is frequently activated during carcinogenesis (6, 41, 43). In vertebrate embryos, the Wnt signaling pathway is involved in specification of the dorsoventral axis (15, 35, 56). Ectopic Wnt signaling in Xenopus laevis leads to the activation of genes that are normally expressed in the dorsal signaling center, known as the Spemann organizer, resulting in the formation of a complete secondary body axis (32, 52, 53). Inhibition of this pathway at different levels abolishes the response to Wnt ligands and leads to deficient dorsal development (ventralization), providing a unique model system for signal transduction studies (2, 17, 34, 67, 68).
The canonical Wnt pathway is initiated by the interaction of Wnts with the Frizzled family of seven-transmembrane-domain receptors (6). The cytoplasmic protein Dishevelled (Dsh) is necessary to relay the signal further downstream, leading to an increase in the cytoplasmic level of β-catenin. In the absence of Wnt signaling, β-catenin is degraded by the complex of glycogen synthase kinase 3 (GSK3) (42, 66), the adenomatous polyposis coli (APC) gene product (43), PP2A (20, 50), and Axin (14, 61, 68). Wnt signaling leads to stabilization of β-catenin and its translocation to the nucleus (6, 8). In the nucleus, β-catenin forms a complex with transcription factors of the T-cell factor family and activates target gene expression (2, 5, 21, 34, 58).
Although Dsh is required for the cellular response to Wnt signals, the mechanism by which Dsh stabilizes β-catenin has not been elucidated. Dsh has three conserved protein domains. The DIX (Dishevelled-Axin) domain of Dsh is similar to the C-terminal domain of Axin (6, 68). Another conserved domain of Dsh belongs to the family of PDZ domains, which are found in many proteins interacting with transmembrane receptors and/or the cytoskeleton (45). The DEP domain of Dsh is also found in several proteins regulating G-protein signaling (44) and was recently proposed to be responsible for Jun kinase activation and for establishment of planar cell polarity (1, 4, 29). Thus, Dsh is a multidomain protein that is likely to function in different signal transduction pathways.
Dsh may operate by inhibiting the function of Axin, a negative regulator of the pathway. Mice with a mutation in the Fused locus that encodes Axin often develop duplicated embryonic axes, indicating that Axin is an inhibitor of dorsal development in vertebrates (11, 24, 68). Axin contains two conserved protein domains: the RGS domain, found in some regulators of G-protein signaling, and the C-terminal DIX domain with similarity to Dsh (68). When overexpressed in frog embryos, Axin inhibits dorsal development and blocks the ability of Xwnt8 to induce a secondary body axis. In contrast, a putative dominant-negative form of Axin, lacking the RGS domain, mimics Wnt signaling and dorsalizes the embryo, consistent with the proposed suppressive role for Axin in dorsal development (68).
Biochemical studies have shown that Axin and a related protein, Axil/Conductin, occupy a central position in Wnt signal transduction and form a complex with GSK3 and β-catenin in tissue culture cells (3, 16, 22, 38, 47, 62) and in Xenopus embryos (23). GSK3 is an essential negative regulator of the pathway (66). When the GSK3-binding domain of Axin is deleted, it is no longer able to inhibit axial development (23). Furthermore, the RGS domain of Axin was shown previously to interact with APC (3, 16, 25, 38). In the absence of the RGS domain, Axin behaves as a dominant-negative mutant (68) and fails to bind β-catenin in the embryo (23). These results suggest that Axin operates by allowing GSK3 to phosphorylate its putative substrate, β-catenin. Thus, a large protein complex of Axin, APC, and GSK3 functions to promote the phosphorylation and degradation of β-catenin.
In a Saccharomyces cerevisiae two-hybrid screen for proteins interacting with Xenopus Dishevelled (Xdsh), we identified a cDNA encoding a novel maternally encoded Xenopus Axin-related protein (XARP). Here we establish the biochemical association of XARP and Xdsh and define their interacting domains. We propose that the DIX domain of XARP is subject to regulation by Dsh and is essential for Wnt signal transduction. Our data suggest that, upon binding to XARP, Xdsh causes the release of GSK3 from the complex with XARP, thus providing a possible mechanism for Wnt signal transduction.
MATERIALS AND METHODS
Isolation of the XARP cDNA.
Proteins interacting with Xdsh were isolated in a yeast two-hybrid screen, according to the method of Gyuris et al. (13). In brief, a Xenopus gastrula cDNA expression library was constructed in the yeast pJG4-5 vector that is inducible by galactose and contains a transcriptional activation domain. The bait contained the fusion of the LexA DNA-binding domain and full-length Xdsh in the pEG202 vector. The bait and the library were transformed into the EGY48 yeast strain, which requires leucine for growth. Positive colonies were selected by growth in a leucine-free medium and on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing plates as described previously (13). Plasmid DNA was recovered from positive yeast colonies and sequenced. Two positive colonies contained inserts encoding 212 amino acids with similarity to the C terminus of the Axin protein. A full-length XARP cDNA was isolated by probing a Xenopus ovary cDNA λZAP library (27) with a short fragment of the XARP cDNA using standard procedures (49). pBluescript plasmids with the XARP cDNA inserts were rescued from the positive phage by an in vivo excision protocol (Stratagene). Both DNA strands of the XARP cDNA were sequenced using a 373A DNA sequencer (Applied Biosystems, Inc.).
DNA constructs.
To construct Myc-XARP and XARP-cyanfluorescent protein (XARP-CFP), the 2.5-kb XARP cDNA insert, encoding amino acids 40 to 706 of XARP, was isolated from XARP-pBSSK digested with NcoI and XhoI and ligated in frame into pCS2-MT (57) or pECFP-C1 (Clontech). Other fragments of XARP cDNA were subcloned in frame into pXT7-Myc and pXT7-HA vectors (S. Sokol, unpublished data) to generate XARPΔDIX, encoding amino acids 40 to 584 of XARP, XARPΔRGS (amino acids 266 to 706), XARPΔRGSΔC (amino acids 266 to 394), and XARP-C (amino acids 506 to 706), using available restriction enzyme sites. Myc-Xdsh-pCS2 and Myc-Xdd1-pCS2 were described previously (55). Hemagglutinin (HA)-tagged Xdsh constructs were obtained by in-frame subcloning of full-length Xdsh cDNA into the pXT7-HA vectors. Truncated Xdsh variants were constructed using PstI (for Xdsh-A), SmaI (for Xdsh-Ab), BglII (for Xdsh-BC), and XhoI (for Xdsh-C and Xdsh-AB) sites. Xdsh-yellow fluorescent protein (Xdsh-YFP) was constructed by fusing the YFP insert (Clontech) to the 3′ terminus of the Xdsh cDNA by using the NcoI site next to the start codon of YFP and the third NcoI site in Xdsh (obtained by partial digestion). This construct lacks the C-terminal 12 amino acids of wild-type Xdsh. Further details of plasmid construction are available on request.
Embryo culture and analysis, RNA microinjections, and localization studies.
Capped synthetic RNAs were generated by in vitro transcription with Sp6, T7, and T3 RNA polymerases (28), using the mMessage mMachine kit (Ambion). Eggs and embryos were obtained from Xenopus females and cultured in 0.1× Marc's modified Ringer's medium (MMR) as described previously (39). Embryonic stages were determined according to the work of Nieuwkoop and Faber (40). For microinjection, embryos were transferred to 3% Ficoll in 0.5× MMR and injected at the four- to eight-cell stage with 10 nl of a solution containing 0.5 to 1 ng of RNA, unless specified otherwise. Injections were carried out in one ventral blastomere for secondary axis induction, two dorsal blastomeres for assaying the ventralizing activity of XARP, and all four blastomeres for protein analysis. Embryonic phenotypes were scored morphologically at the equivalent of stage 36. The results are combined for at least three independent experiments.
For subcellular localization studies, mRNAs encoding XARP-CFP and/or Xdsh-YFP (1 ng each) were injected into the animal pole of two-cell embryos. At stage 9 to 10.5, animal caps were dissected, fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min, washed three times in PBS, and mounted in 70% glycerol–PBS supplemented with 25 mg of DABCO [diazabicyclo(2,2,2)-octane; Sigma] per ml. Fluorescence was visualized using a Zeiss Axiophot microscope with an Omega XF102 filter for CFP, an XF22 filter for GFP, and an XF30 filter for YFP visualization.
Immunoprecipitations and Western blot analysis.
Injected embryos were lysed in 500 μl of lysis buffer (1% Triton X-100, 50 mM Tris-HCl at pH 7.5, 50 mM NaCl, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 1 mM Na3VO4) when sibling embryos had developed to late blastula to early gastrula stages (stage 9+ to stage 10+). Immunoprecipitation and Western blot analysis were carried out as described previously (23). For immunoprecipitations, 20 μl of 9E10 (anti-Myc) or 12CA5 (anti-HA) hybridoma supernatants was used per each sample. The equivalent of 0.14 embryo was loaded per lane for analysis of embryo lysates, and the equivalent of 4 to 24 embryos was used for analysis of immunoprecipitated proteins. GSK3 was detected by monoclonal antibodies from Transduction Labs, and APC was detected with antibodies from Oncogene Research Products. Peroxidase activity was visualized by enhanced chemiluminescence as described previously (10). When necessary, membranes were stripped for 15 min in 7 M guanidine-HCl–50 mM Tris-HCl (pH 8.0)–20 mM dithiothreitol–2 mM EDTA and reprobed with different antibodies. Each experiment was repeated at least three times.
Nucleotide sequence accession number.
The sequence of the XARP cDNA was deposited in GenBank (accession no. AF140243).
RESULTS
Dsh associates with XARP, a novel maternal Xenopus homologue of Axin.
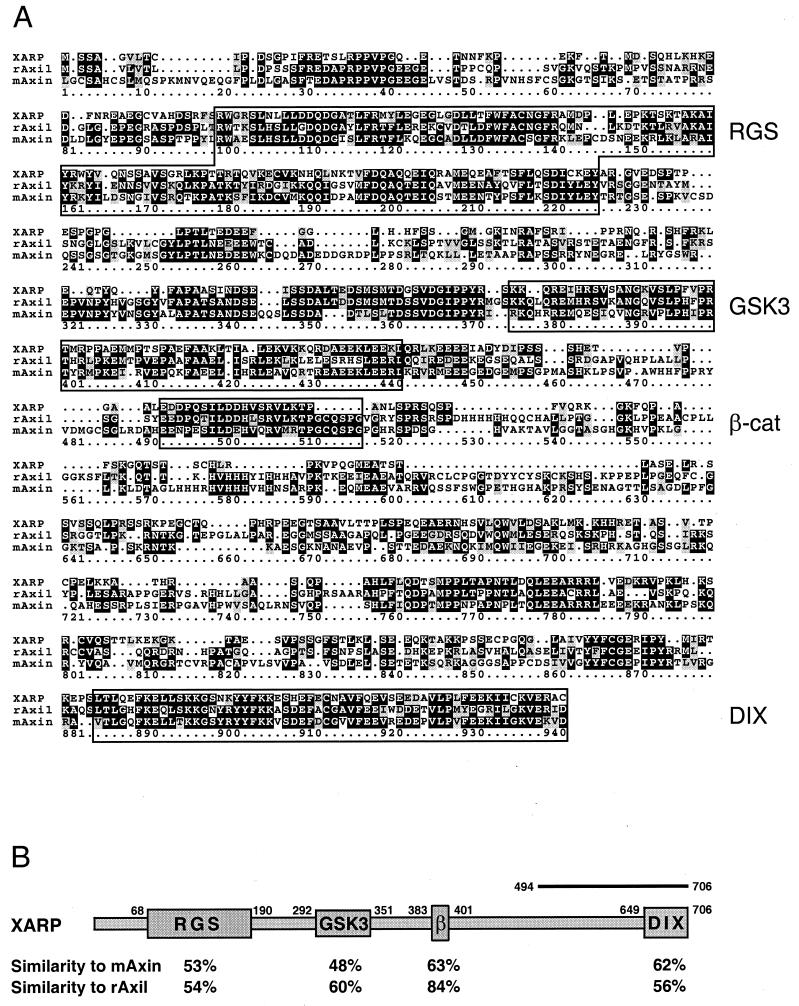
In a yeast two-hybrid screen for proteins interacting with Xdsh, we isolated a short cDNA fragment encoding a novel XARP. Repeated screening of a Xenopus ovary cDNA library with the XARP cDNA probe resulted in the isolation of a 3.0-kb XARP cDNA, which contained an open reading frame of 706 amino acids. At the amino acid level, XARP is 45% similar to mouse Axin (68) and 50% similar to rat Axil (62). Sequence alignment of XARP and other Axin homologues (Fig. 1) reveals the conserved RGS domain and the DIX domain, as well as the regions required for binding of GSK3 and β-catenin (3, 22, 38, 62). A Drosophila melanogaster Axin homologue is approximately 25% similar to both XARP and mAxin (14). XARP has only 43% similarity to the recently cloned Xenopus Axin (18). Thus, based on the analysis of their amino acid sequences, XARP, Axin, and Axil/Conductin are not orthologues but clearly belong to the same protein family.
FIG. 1.
Sequence alignment of XARP, mouse Axin, and rat Axil. (A) Deduced amino acid sequences for XARP, mouse Axin (68), and rat Axil (62) were compared using the PILEUP program of the GCG software package. The RGS domain, the DIX domain, and the GSK3- and β-catenin-binding domains are indicated. (B) Conservation of different protein domains in XARP. A bar at the C terminus of XARP indicates the position of the XARP fragment that associated with Xdsh in the two-hybrid screen.
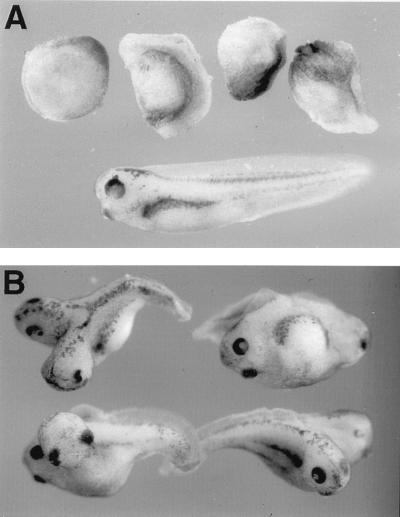
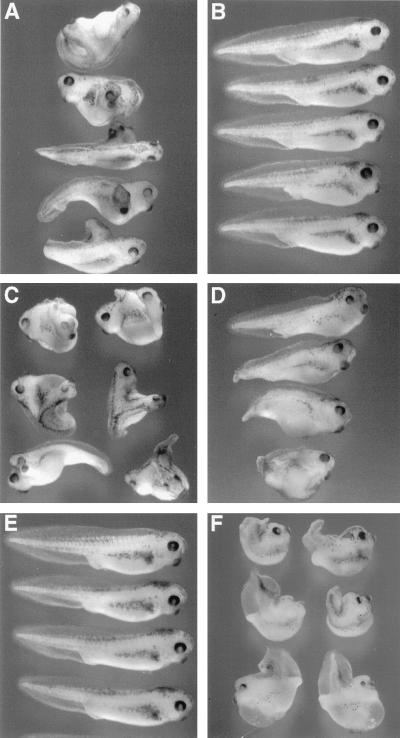
Since the XARP cDNA was isolated from an ovary cDNA library, XARP may be present maternally. Both maternal and zygotic XARP transcripts of approximately 3.5 kb in size were detected by Northern analysis (data not shown), consistent with the idea that XARP plays a role in early dorsoventral axis specification. Functional activity of XARP was evaluated by microinjecting XARP mRNA into Xenopus early embryos. Similar to mouse Axin (68), XARP inhibited axial development when overexpressed in dorsal blastomeres (Fig. 2A). Furthermore, a truncated form of XARP, lacking the RGS domain (XARPΔRGS), induced a complete secondary body axis in the embryo (Fig. 2B). XARPΔRGS thus appears to have a dominant-negative effect that is opposite to the ventralizing activity of the wild-type XARP. These findings suggest that XARP and Axin function similarly in dorsoventral axis determination.
FIG. 2.
Functional activities of XARP constructs. (A) Inhibition of dorsal axial development by XARP. Both dorsovegetal blastomeres of four- to eight-cell embryos were injected with 2 ng of XARP mRNA and allowed to develop until control siblings reached stage 36. An uninjected embryo is shown at the bottom. (B) Axis duplication caused by a truncated form of XARP lacking the RGS domain. Two nanograms of Myc-XARPΔRGS mRNA was injected into a ventrovegetal blastomere of four- to eight-cell embryos.
Biochemical interactions of XARP and Xdsh.
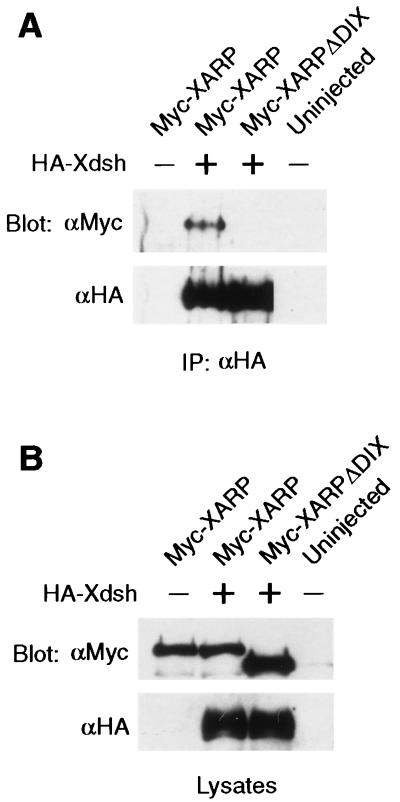
To confirm the biochemical association of Xdsh and XARP that was inferred from the yeast system, we tagged both proteins with different peptide epitopes (36) for immunoprecipitation analysis. Xenopus embryos were coinjected with mRNAs encoding HA-tagged Xdsh and Myc-tagged XARP (Fig. 3). At the early gastrula stage, protein complexes were precipitated from embryo lysates with anti-HA monoclonal antibodies. Western blot analysis with anti-Myc antibodies revealed the presence of Myc-XARP in the complex. In the absence of HA-Xdsh, Myc-XARP was not detected, illustrating the specificity of interactions between Xdsh and XARP (Fig. 3). Moreover, the association of XARP with Xdsh was dose dependent, and the complex was detected only weakly at lower doses of HA-Xdsh RNA (data not shown). These experiments reveal that Xdsh and XARP interact biochemically and form a complex in gastrula lysates.
FIG. 3.
Coprecipitation of Xdsh and XARP. Each blastomere of four-cell embryos was injected with 2 to 4 ng of mRNAs, encoding Myc-XARP, Myc-XARPΔDIX, and HA-Xdsh, as indicated. (A) Association of Xdsh and XARP was revealed by immunoprecipitation of Xdsh with anti-HA antibodies followed by Western analysis with anti-Myc antibodies. Myc-XARP, but not Myc-XARPΔDIX, coprecipitated with HA-Xdsh. The same membrane was stripped and probed with anti-HA antibodies. (B) Levels of protein expression are shown in corresponding embryo lysates.
The DIX domains mediate the association of XARP and Xdsh.
Initially, our two-hybrid screen resulted in the isolation of a small C-terminal fragment of XARP that can bind Xdsh in yeast. Since the DIX domain was the major conserved domain in the isolated fragment, we suspected that it might be essential for XARP-Xdsh interactions. The removal of the DIX domain in XARP impaired its ability to associate with Xdsh (Fig. 3 and 4A), demonstrating that this domain is required for the association of XARP and Xdsh. Moreover, the XARP-C construct encoding the DIX domain of XARP with the adjacent sequences was able to bind Xdsh on its own (Fig. 4B), consistent with the ability of a similar construct to interact with Xdsh in a two-hybrid assay (data not shown).
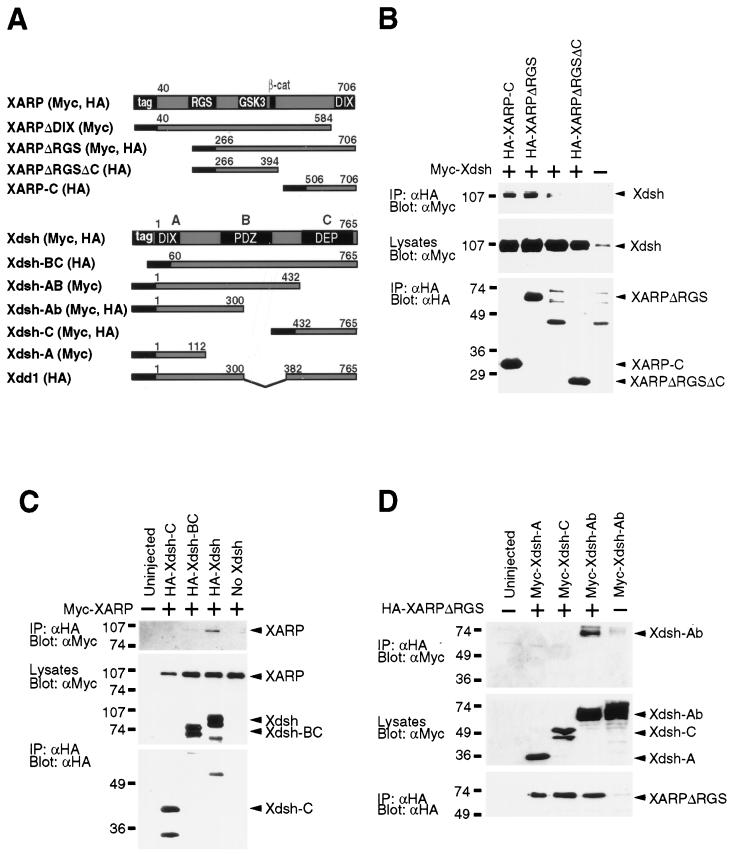
FIG. 4.
The association of Xdsh and XARP is mediated by the DIX domains. (A) Constructs of XARP and Xdsh used in this study. (B to D) Each blastomere of four-cell embryos was injected with mRNAs, encoding different tagged XARP and Xdsh constructs, as indicated. Immunoprecipitation (IP) with tag-specific antibodies was followed by Western analysis. Middle panels of each figure show relative protein expression levels in embryo lysates. Bottom panels show equal efficiency of immunoprecipitation with anti-HA antibodies. (B) The DIX domain of XARP binds Xdsh. Myc-Xdsh coprecipitated with HA-XARP-C but not with HA-XARPΔRGSΔC. A weak band of Xdsh was occasionally observed due to nonspecific binding of Xdsh to protein A-Sepharose. (C) The DIX domain of Xdsh is required for binding of XARP. Myc-XARP coprecipitated with HA-Xdsh, but not with HA-Xdsh-C, or HA-Xdsh-BC, lacking the DIX domain. (D) A truncated Xdsh (Xdsh-Ab) that lacks the DEP domain and the C-terminal half of the PDZ domain can bind XARP. HA-XARPΔRGS coprecipitates with Myc-Xdsh-Ab, whereas the binding of Xdsh-A and Xdsh-C is not detectable.
To determine which domain of Xdsh is involved in binding of XARP, a Myc-tagged XARP was overexpressed in frog embryos together with truncated HA-tagged Xdsh constructs (Fig. 4A). Protein complexes containing Xdsh were immunoprecipitated with anti-HA antibodies, and the presence of Myc-XARP was assessed with anti-Myc antibodies. We detected XARP in complex with the wild-type Xdsh, but not with Xdsh lacking the DIX domain, indicating that the DIX domain of Xdsh is required for its association with XARP (Fig. 4C). Moreover, in a similar experiment, the N-terminal part of Xdsh (Xdsh-Ab), containing the DIX domain and a part of the PDZ domain, was sufficient for this interaction to occur (Fig. 4D). No significant binding of XARP was detected for Xdsh-A, despite the presence of the DIX domain, suggesting that adjacent sequences are also required for efficient binding (Fig. 4D). Together, these findings show that the DIX domains play an essential role in the association of Xdsh and XARP.
Dsh and XARP colocalize to a punctate cytoplasmic compartment.
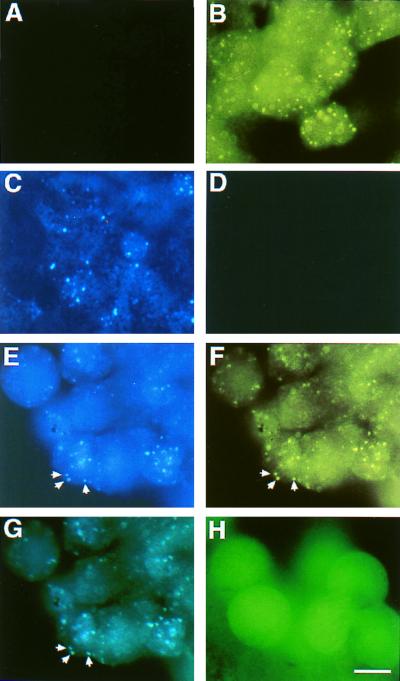
Although we demonstrated biochemical association of XARP and Xdsh in Xenopus embryos, as well as in the yeast system, the physiological relevance of this interaction for Wnt signal transduction was unclear. To assess whether Xdsh interacts with XARP in embryonic cells in vivo, we investigated the subcellular localization of these proteins (Fig. 5). XARP was tagged with the CFP, and Xdsh was tagged with the YFP. Embryos were injected with XARP-CFP mRNA, Xdsh-YFP mRNA, or both mRNAs together at the four-cell stage, and animal cap explants were analyzed at the late blastula stage. XARP-CFP was present in bright vesicular structures in the cytoplasm (Fig. 5C). This was reminiscent of the distribution pattern reported for Dsh (1, 65), suggesting that Wnt signal transduction may depend on the localization of some pathway component(s) in a specific cell compartment. In agreement with this, we found that Xdsh-YFP showed a similar punctate distribution (Fig. 5B). When expressed together, XARP-CFP and Xdsh-YFP colocalize to the same vesicular structures (Fig. 5E to G). In contrast, wild-type GFP was present in both the cytoplasm and the nucleus in a diffuse pattern (Fig. 5H). These data support our biochemical data showing that Xdsh and XARP associate in embryonic cells.
FIG. 5.
XARP colocalizes with Xdsh in Xenopus animal caps. Embryos were injected into the animal hemisphere with mRNAs encoding XARP and Xdsh that contain a fluorescent protein tag. The subcellular protein distribution was assessed by fluorescence in fixed animal pole cells. Xdsh-YFP is localized in a punctate pattern throughout the cell (B). This signal is not detectable through the CFP channel (A). XARP-CFP shows a similar distribution when monitored on the CFP channel (C) but not on the YFP channel (D). Coinjection of Xdsh-YFP and XARP-CFP RNAs results in colocalization of YFP (E and G) and CFP (F and G) fluorescence. Panel G shows a double exposure of panels E and F. Arrows point to three clear instances of colocalization. GFP alone is distributed evenly throughout the cytoplasm and nucleus (H). Bar, 40 μm.
We have also observed that the DIX domain of XARP is essential for its localization to the vesicular structures, since removal of the DIX domain from GFP-XARP eliminated the punctate staining (data not shown). Moreover, overexpression of Xdsh mRNA at higher doses (2 to 4 ng) disrupted the punctate distribution of GFP-XARP, and this effect depended on the presence of the DIX domain in Xdsh (data not shown). These studies provide independent evidence for functional interactions of Xdsh and XARP in vivo.
The DIX domain of Xdsh is required for its functional activity.
Since our experiments indicated that the association of XARP and Xdsh requires intact DIX domains, we wanted to test whether these domains are functionally involved in transduction of Wnt signals. If the DIX domain of Dsh is involved in Wnt signal transduction, Xdsh lacking this domain should not be able to induce a secondary axis when injected into a ventral blastomere, as was reported for the wild-type Xdsh (54). Consistent with this prediction, all embryos injected with Xdsh-BC, in which the DIX domain was deleted, developed normally (n = 84), whereas the wild-type Xdsh induced a complete secondary axis in 76% of injected embryos (n = 86) (Fig. 4A and 6A and B). Western analysis of embryo lysates with anti-HA antibodies confirmed equal protein expression levels for Xdsh and Xdsh-BC (data not shown). Thus, the DIX domain of Xdsh is essential for the functional activity of the protein. Although both Xdd1 and Xdsh-Ab can bind XARP (Fig. 4A and D; see also Fig. 7B and C), they are unable to induce secondary axes (reference 55 and data not shown), suggesting that the association of Xdsh and XARP is not sufficient for signal transduction and that sequences outside of the DIX domain are necessary for Xdsh to function.
FIG. 6.
The requirement for the DIX domains of Xdsh and XARP in signal transduction. Four- to eight-cell embryos were injected into a single ventrovegetal blastomere with 1 ng of HA-Xdsh mRNA (A) or HA-Xdsh-BC mRNA (B), 1 pg of Xwnt8 mRNA (C), or 1 pg of Xwnt8 mRNA with 2 ng of XARP-C mRNA (D). Secondary axes and other morphological abnormalities were scored when uninjected sibling embryos (E) reached stages 36 to 39. The results show that the DIX domain of Xdsh is required for its functional activity (A and B), whereas the DIX domain of XARP blocks Wnt signaling (C to E). Dorsal injections of XARP-C mRNA (2 ng) do not eliminate the primary axis but suppress morphogenetic movements in the trunk-tail region (F).
FIG. 7.
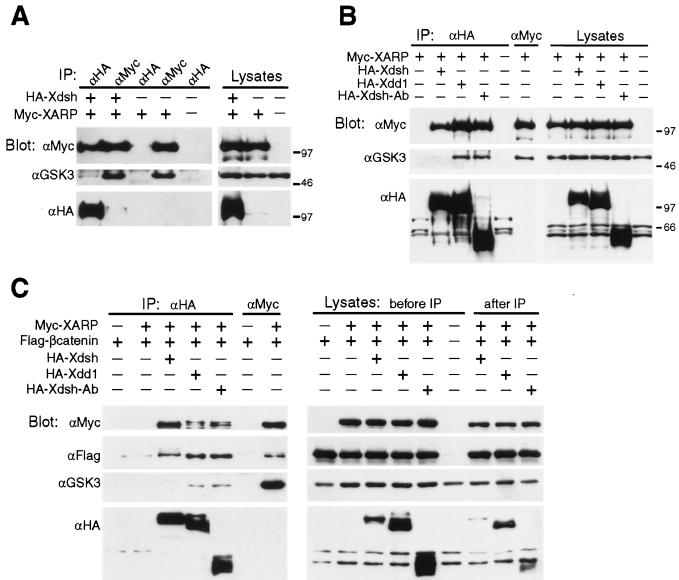
GSK3 is not retained in the Xdsh-XARP complex. Embryos overexpressing HA-Xdsh, HA-Xdd1, HA-Xdsh-Ab, Myc-XARP, and Flag–β-catenin, as indicated, were cultured until early gastrula stages for protein analysis. Each lysate was subjected to two sequential precipitations, first with anti-HA antibodies and then with anti-Myc antibodies. Protein complexes were analyzed by immunoblotting with anti-HA, anti-Myc, anti-GSK3, and anti-Flag antibodies, as indicated. (A) Protein complexes containing XARP in association with Xdsh did not significantly bind GSK3, whereas the pool of XARP that is not bound to Xdsh retained GSK3. (B) The complex of XARP with Xdd1 and Xdsh-Ab, but not with Xdsh, retained GSK3. Protein levels in corresponding embryo lysates are shown (A and B). (C) The Xdsh-XARP complex retains β-catenin. The panel on the right shows that the Xdsh and XARP proteins are not fully depleted from embryo lysates. IP, immunoprecipitation.
The DIX domain of XARP blocks Wnt signaling.
Whereas the DIX domain of Dsh is required for its ability to transduce a signal, the removal of the DIX domain of Axin or XARP does not impair its ability to inhibit dorsal development (reference 23 and data not shown). We therefore hypothesized that this domain of Axin-XARP functions to receive a signal from Xdsh, a more upstream component of the Wnt pathway. If the DIX domain of XARP is involved in this process, it would compete with endogenous XARP for binding to Xdsh, resulting in inhibition of Wnt signal transduction.
To test this prediction, we evaluated whether a short form of XARP, containing the DIX domain with adjacent sequences (XARP-C [Fig. 4A]), can suppress the ability of ventrally injected Xwnt8 mRNA to induce a complete secondary axis (53). Consistent with our expectations, we found that XARP-C blocked the axis-inducing activity of Xwnt8 (Fig. 6C and D). Whereas Xwnt8 mRNA induced complete secondary axes in 90% of injected embryos (n = 60), the majority of embryos coinjected with XARP-C mRNA developed a single axis, and only 14.5% of injected embryos were similar to embryos overexpressing Xwnt8 (n = 62). In contrast, Xdsh-A, containing the DIX domain of Xdsh, did not inhibit Wnt signaling (data not shown), in agreement with our observation that this domain does not bind XARP on its own (Fig. 4D) and arguing that the effect of XARP-C is specific. These findings suggest that the DIX domain of XARP is required for Wnt signal transduction.
To test the possibility that Wnt signaling upstream of XARP is involved in dorsoventral axis specification, XARP-C mRNA was injected into both dorsal blastomeres of four-cell embryos. We found that XARP-C did not inhibit axis development even at the highest dose of RNA tested (2 to 4 ng) but resulted in embryos with shortened trunks (Fig. 6F). This activity is similar to the effect of a dominant-negative form of Xdsh that failed to block dorsal development and yet interfered with morphogenetic movements during gastrulation and neurulation (55). These data suggest that XARP is unlikely to be regulated by Xdsh during initial specification of the dorsoventral axis in Xenopus.
Xdsh displaces GSK3 from the XARP–GSK3–β-catenin complex.
Whereas the complex of Axin, APC, and GSK3 serves to degrade cytoplasmic β-catenin (43, 56), activation of Dsh by Wnt signaling leads to accumulation of β-catenin (6). Our results suggest that Xdsh may affect interactions of XARP with other proteins that are present in the same complex and mediate β-catenin degradation.
Since the association of GSK3 with Axin appears to be critical for the ability of Axin to inhibit axial development (23), we evaluated whether Xdsh and GSK3 are present in the same complex with XARP or form alternative complexes. Embryos were injected with Myc-XARP and HA-Xdsh mRNAs, and protein complexes containing Xdsh were precipitated with anti-HA antibodies (Fig. 7). Subsequently, anti-Myc antibodies were applied to the same lysates to immunoprecipitate Myc-XARP protein complexes not containing Xdsh. Western analysis of immunoprecipitates with anti-GSK3 antibodies revealed endogenous GSK3 in complex with XARP (Fig. 7A). Thus, XARP, similar to Axin, associates with GSK3. At the same time, GSK3 was barely detectable in the Xdsh-XARP complex, indicating that Xdsh may function by displacing GSK3 from the complex with XARP (Fig. 7A). This finding is consistent with the hypothesis that Wnt signaling prevents β-catenin degradation through the formation of alternative protein complexes.
We next wanted to determine whether this effect of Xdsh on the association of XARP and GSK3 is causally connected to the ability of Xdsh to transduce a signal and stimulate secondary axis formation in the embryo. We tested whether Xdd1 and Xdsh-Ab (Fig. 4A), which do not induce an axis, form a complex with XARP and GSK3. In contrast to the wild-type Xdsh, Xdd1 and Xdsh-Ab failed to release GSK3 from its complex with XARP, although they retained the ability to bind XARP (Fig. 7B). Together, these findings strongly suggest that the regulation of GSK3 binding to XARP by Xdsh is essential for signal transduction.
The Xdsh-Axin and Xdsh-XARP complexes contain β-catenin but not APC.
We further asked whether β-catenin and APC, which can also bind Axin, were present in the Xdsh-XARP protein complex. In a similar experimental setup, Flag–β-catenin (31) was detected at approximately equal levels both in the Xdsh-XARP complex and in the complex of XARP with Xdd1 or Xdsh-Ab (Fig. 7C). This finding suggests that Xdsh does not eliminate the binding of β-catenin to XARP. Similarly, the complex of Xdsh with mouse Axin also contained decreased amounts of GSK3, but not β-catenin (Fig. 8A and data not shown).
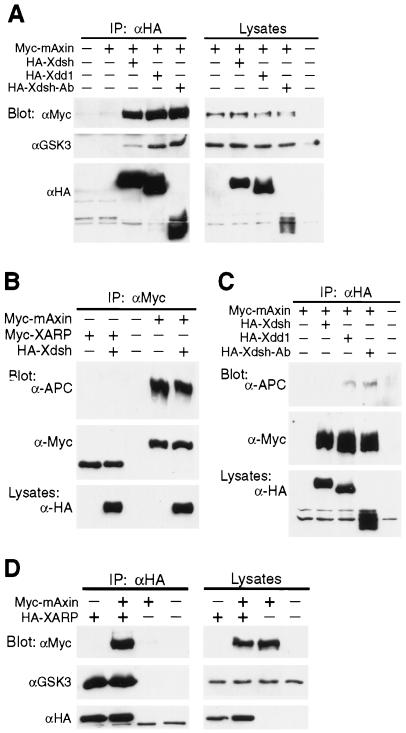
FIG. 8.
The comparative analysis of mouse Axin and XARP properties. (A and C) GSK3 (A) and APC (C) are not well retained in the Xdsh-mAxin complex in comparison with the Xdd1-mAxin and Xdsh-Ab-mAxin complexes. The experimental design is similar to the one described in the Fig. 7 legend. (B) Overexpressed Myc-XARP or Myc-mAxin was immunoprecipitated with anti-Myc antibodies followed by Western analysis with anti-APC and anti-Myc antibodies. Xdsh does not significantly alter the amount of Axin or APC in the complex. In contrast to mAxin, XARP does not appear to bind endogenous APC. (D) Axin and XARP can form heterodimers. Myc-mAxin and HA-XARP were coexpressed in early embryos, and HA-XARP was precipitated with anti-HA antibodies followed by Western analysis with anti-Myc antibodies. Binding of GSK3 to XARP is not affected by overexpressed Axin. IP, immunoprecipitation.
We also wanted to examine if the association of APC with XARP or Axin is affected by Xdsh. Unexpectedly, endogenous APC did not appear to bind XARP (Fig. 8B), indicating that despite the structural similarity, the biochemical properties of XARP and Axin are different. APC was not detectable in the Xdsh-mAxin complex but was retained in the complex of mAxin with Xdd1 or Xdsh-Ab (Fig. 8C). These data show that the negative regulators of Wnt signaling, GSK3 and APC, are selectively eliminated from the Xdsh-XARP and Xdsh-Axin complexes, whereas β-catenin remains associated with both XARP and Axin, suggesting a possible mechanism for Wnt signal transduction.
Association of XARP and Axin.
Axin has been reported to dimerize via its C-terminal region, which includes the DIX domain (19, 20, 48). Since the C terminus of XARP bears a significant similarity to Axin, we tested if XARP and Axin can form heterodimers. Following microinjection of embryos with Myc-mAxin and HA-XARP mRNAs, HA-XARP-containing protein complexes were precipitated with anti-HA antibodies for analysis with anti-Myc antibodies. Clear association of HA-XARP with Myc-mAxin was observed (Fig. 8D).
Since Axin is known to bind GSK3, it is possible that GSK3 associates with XARP only indirectly, through Axin. Overexpression of mAxin did not significantly alter the amount of GSK3 bound to XARP (Fig. 8D), indicating that GSK3 binding to XARP is not likely to be mediated by Axin. Moreover, the presence of a conserved GSK3-binding domain in XARP suggests that XARP can bind GSK3 directly. In support of this view, a short construct of XARP, XARPΔRGSΔC, encompassing the putative GSK3-binding domain, was still capable of binding GSK3 (data not shown).
DISCUSSION
In this study, we have found that Dsh associates with XARP, a Xenopus Axin-related protein, which is not an orthologue of Axin and yet functions similarly. This interaction, initially discovered in a yeast two-hybrid system, was confirmed biochemically in embryonic lysates. In addition, we have demonstrated colocalization of Xdsh and XARP in embryonic cells in vivo. The interaction of Xdsh and XARP is mediated predominantly by their DIX domains, because their removal eliminates binding. Whereas the DIX domain of Xdsh is required for the ability of Xdsh to transduce a signal, the DIX domain of XARP is not essential for the activity of XARP but appears to be important for the regulation of XARP by Xdsh. Finally, our data point to a possible mechanism of signal transduction, in which Xdsh operates by displacing GSK3 from its complex with XARP.
The role of the DIX domains in Wnt signaling.
Our findings reveal the importance of the DIX domains in Wnt signal transduction. We have found that the DIX domain of XARP is essential for its interactions with Xdsh. Furthermore, the DIX domain of Xdsh is also required for the association of the two proteins, indicating that the two DIX domains bind each other. Interestingly, recent studies reported that the C terminus of Axin is capable of homophilic interactions (19, 20, 48). Furthermore, we observed that XARP can heterodimerize with Axin (Fig. 8D), although the significance of these findings is not fully clear. The physiological relevance of interactions of Xdsh and XARP is supported by our observation that Xdsh disrupts cytoplasmic localization of GFP-XARP depending on the expression levels, and this property of Xdsh correlated with the presence of the Xdsh-DIX domain (data not shown).
The removal of the DIX domain of Xdsh resulted in a complete loss of the axis-inducing activity of Xdsh (Fig. 6A and B), indicating that this domain is required for the ability of Xdsh to transduce a signal. This finding corroborates earlier studies in Drosophila, in which Dsh lacking the DIX domain does not elevate the cytoplasmic levels of Armadillo in tissue culture cells and fails to rescue dsh embryos (1, 64). On the other hand, the DIX domains of Xdsh and Xdsh-Ab (Fig. 4A) do not have significant axis-inducing activity (data not shown), indicating that other regions of Xdsh must contribute to the effector function of the protein.
Since the deletion of the DIX domain of XARP or Axin does not affect its ability to ventralize frog embryos (reference 23 and data not shown), we hypothesized that this domain is subject to regulation by the upstream components of the pathway such as Dsh. This hypothesis is strongly supported by our observation that XARP-C, which contains the DIX domain with adjacent sequences, blocked the ability of Xwnt8 to induce a secondary axis (Fig. 6C and D). Thus, the DIX domain is not required for functional activity of XARP but is likely to be involved in the reception of a signal.
In contrast to Xwnt8-induced secondary axis, XARP-C failed to suppress the primary axis (Fig. 6F), suggesting that endogenous axis does not depend on signaling from Xdsh to XARP. Since overexpression of a dominant-negative Xdsh resulted in similar embryonic phenotypes (55), these observations are consistent with the view that the endogenous pathway leading to dorsal development does not seem to involve Xdsh. Although maternal Xdsh was reported to be enriched at the dorsal side of the embryo (33), our data fail to provide additional support for a role of Xdsh in primary axis specification.
Signal transduction by Dsh and XARP-Axin.
β-Catenin appears to be a central target of Wnt signal transduction (12, 43). In the absence of Wnt signals, β-catenin is degraded by the complex of Axin, GSK3, and APC. The binding of GSK3 to Axin is essential for the ability of Axin to ventralize Xenopus embryos (23). Wnt signaling prevents degradation of β-catenin, which then enters the nucleus and activates target gene expression.
Although Wnt signaling has been shown to require the function of Dsh, the mechanism by which Dsh regulates signaling was not clear. Our findings suggest that Xdsh may function by displacing GSK3 from the XARP-GSK3 complex, thereby allowing β-catenin to escape degradation. The alternative explanation of these data is that Xdsh preferentially associates with the pool of XARP that is not bound to GSK3. This possibility seems unlikely, because other Xdsh constructs, such as Xdd1 and Xdsh-Ab, are able to associate with the XARP-GSK3 complex, indicating that the Xdsh-interacting domain of XARP is accessible even in the presence of GSK3 (Fig. 7B and C).
Since Xdd1 lacks the C-terminal half of the PDZ domain (55), the PDZ domain may be involved in the regulation of XARP-GSK3 interactions. Consistent with this idea, the removal of the PDZ domain abolishes the ability of Dsh to stabilize Armadillo in Drosophila cells (64). However, Dsh lacking the PDZ domain can suppress the segment polarity phenotype of dsh flies (1), indicating that the PDZ domain may not be absolutely required for the downstream function of Dsh and that other regions of the protein may suffice in some experimental situations.
XARP and Axin are indistinguishable in our functional assays, consistent with the significant conservation of their structural domains. Despite this similarity, there is a clear difference in the biochemical properties, since only Axin, not XARP, binds endogenous APC (Fig. 8B). It is thus possible that APC is not an essential component of Wnt signal transduction. Alternatively, since several APC gene products are known to exist in Drosophila and mammals (37, 59), they may be engaged in specific interactions with different Axin homologues. At present, it remains unclear whether there is a direct homologue of XARP in mammals. Although there are multiple Axin homologues in the human and mouse genome, the database search did not reveal a gene that is more closely related to XARP than to Axin or Axil/Conductin (data not shown).
Since Wnt signaling was shown to inhibit the enzymatic activity of GSK3 (7, 23, 46), it is possible that the association of Dsh and Axin inactivates GSK3 and, as a result, lowers the affinity of GSK3 for Axin. Consistent with the possible displacement of GSK3 from the Axin-XARP-GSK3 complex, Ikeda et al. (22) observed that inactive forms of GSK3 do not bind Axin. Thus, downregulation of GSK3 activity and the removal of GSK3 from the complex with Axin-XARP and β-catenin may be coupled. Moreover, the study of Willert et al. suggested that phosphorylation of Axin by GSK3 is decreased in response to Wnt3a (60). Although Dsh was reported to stimulate degradation of Axin in tissue culture (63), our experiments did not reveal significant changes in the amount of XARP or Axin in embryos overexpressing Xdsh.
Our model predicts that the formation of the complex between Xdsh and XARP-Axin is essential for Wnt signal transduction. This notion is supported by recent independent reports of functional interactions and colocalization of Dsh and Axin (9, 26, 30, 51). At present, we have not detected a significant change in the amount of Xdsh-XARP or GSK3-XARP complexes in embryos injected with Xwnt8 RNA (data not shown). It is possible that such change cannot be detected in our assays, because only a small proportion of the total pool of Xdsh is associated with XARP. Moreover, since Axin-XARP blocks Wnt signaling (reference 68 and data not shown), it is likely that overexpressed XARP easily overcomes the effects of limiting endogenous upstream regulators. The analysis of endogenous proteins will be necessary to investigate how XARP is controlled by Wnt signaling and to clarify a role for XARP in dorsoventral axis determination.
ACKNOWLEDGMENTS
We thank K.-M. Yao and G. Wong for help in the yeast two-hybrid screen, R. Brent for yeast strains and plasmid vectors, F. Costantini and X. He for plasmids, and T. Komiya for the Xenopus ovary cDNA λZAP library. We also thank A. Lugovskoy for help with Fig. 1 and V. Krupnik, J. Weber, M. Fan, and T. Schultheiss for comments on the manuscript.
This work was supported by the grants from the March of Dimes Birth Defects Foundation and NIH to S.S.
REFERENCES
- 1.Axelrod J D, Miller J R, Shulman J M, Moon R T, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 3.Behrens J, Jerchow B-A, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 4.Boutros M, Paricio N, Strutt D I, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 5.Brunner E, Peter O, Schweizer L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 6.Cadigan K M, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 7.Cook D, Fry M J, Hughes K, Sumathipala R, Woodgett J R, Dale T C. Wingless inactivates glycogen synthase kinase-3 via an intracellular signaling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 8.Fagotto F, Glück U, Gumbiner B M. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 9.Fagotto F, Jho E-H, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan M J, Sokol S Y. A role for Siamois in Spemann organizer formation. Development. 1997;124:2581–2589. doi: 10.1242/dev.124.13.2581. [DOI] [PubMed] [Google Scholar]
- 11.Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner B M. A balance between β-catenin and APC. Curr Biol. 1997;7:R443–R446. doi: 10.1016/s0960-9822(06)00214-4. [DOI] [PubMed] [Google Scholar]
- 13.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 14.Hamada F, Tomoyasu Y, Takatsu Y, Nakamura M, Nagai S, Suzuki A, Fujita F, Shibuya H, Toyoshima K, Ueno N, Akiyama T. Negative regulation of wingless signaling by D-axin, a Drosophila homolog of axin. Science. 1999;283:1739–1742. doi: 10.1126/science.283.5408.1739. [DOI] [PubMed] [Google Scholar]
- 15.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 16.Hart M J, de los Santos R, Albert I N, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 17.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Yoshida Noro C, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 18.Hedgepeth C M, Deardorff M A, Klein P S. Xenopus Axin interacts with glycogen synthase kinase-3 beta and is expressed in the anterior midbrain. Mech Dev. 1999;80:147–151. doi: 10.1016/s0925-4773(98)00203-2. [DOI] [PubMed] [Google Scholar]
- 19.Hedgepeth C M, Deardorff M A, Rankin K, Klein P S. Regulation of glycogen synthase kinase 3β and downstream Wnt signaling by Axin. Mol Cell Biol. 1999;19:7147–7157. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 21.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B G, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh K, Krupnik V E, Sokol S Y. Axis determination of Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and β-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs-Cohen R J, Spiegelman M S, Cookingham J C, Bennett D. Knobbly, a new dominant mutation in the mouse that affects embryonic ectoderm organization. Genet Res. 1984;43:43–50. doi: 10.1017/s0016672300025702. [DOI] [PubMed] [Google Scholar]
- 25.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 26.Kishida S, Yamamoto H, Hino S-I, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komiya T, Itoh K, Ikenishi K, Furusawa M. Isolation and characterization of a novel gene of the DEAD box protein family which is specifically expressed in germ cells of Xenopus laevis. Dev Biol. 1994;162:354–363. doi: 10.1006/dbio.1994.1093. [DOI] [PubMed] [Google Scholar]
- 28.Krieg P A, Melton D A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Yuan H, Xie W, Mao J, Caruso A M, McMahon A, Sussman D J, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Yuan H, Weaver C D, Mao J, Far III G H, Sussman D J, Jonker J, Kimelman D, Wu D. Axin and Frat1 interact with Dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Kato Y, Zhang Z, Do V M, Yankner B A, He X. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMahon A P, Moon R T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 33.Miller J R, Rowning B A, Larabell C A, Yang-Snyder J A, Bates R L, Moon R T. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of Dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 35.Moon R T, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays. 1998;20:536–545. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Munro S, Pelham H R. Use of peptide tagging to detect proteins expressed from cloned genes: deletion mapping functional domains of Drosophila hsp 70. EMBO J. 1984;3:3087–3093. doi: 10.1002/j.1460-2075.1984.tb02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa H, Murata Y, Koyama K, Fujiyama A, Miyoshi Y, Monden M, Akiyama T, Nakamura Y. Identification of a brain-specific APC homologue, APCL, and its interaction with β-catenin. Cancer Res. 1998;58:5176–5181. [PubMed] [Google Scholar]
- 38.Nakamura T, Hamada F, Ishidate T, Anai K-I, Kawahara K, Toyoshima K, Akiyama T. Axin, an inhibitor of the Wnt signaling pathway, interacts with β-catenin, GSK-3β and APC and reduces the β-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 39.Newport J, Kirschner M. A major developmental transition in early Xenopus embryos. I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwkoop P D, Faber J. Normal table of Xenopus laevis (Daudin). Amsterdam, The Netherlands: North-Holland Publishing Co.; 1967. [Google Scholar]
- 41.Parr B A, McMahon A P. Wnt genes and vertebrate development. Curr Opin Genet Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- 42.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 43.Polakis P. The oncogenic activation of β-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 44.Ponting C P, Bork P. Pleckstrin's repeat performance: a novel domain in G-protein signaling? Trends Biochem Sci. 1996;21:245–246. [PubMed] [Google Scholar]
- 45.Ponting C P, Phillips C, Davies K E, Blake D J. PDZ domains: targeting signaling molecules to sub-membranous sites. Bioessays. 1997;19:469–479. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- 46.Ruel L, Stambolic V, Ali A, Manoukian A S, Woodget J R. Regulation of the protein kinase activity of ShaggyZeste-white3 by components of the wingless pathway in Drosophila cells and embryos. J Biol Chem. 1999;274:21790–21796. doi: 10.1074/jbc.274.31.21790. [DOI] [PubMed] [Google Scholar]
- 47.Sakanaka C, Weiss J B, Williams L T. Bridging of β-catenin and glycogen synthase kinase-3β by Axin and inhibition of β-catenin-mediated transcription. Proc Natl Acad Sci USA. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakanaka C, Williams L T. Functional domains of Axin. J Biol Chem. 1999;274:14090–14093. doi: 10.1074/jbc.274.20.14090. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Seeling J M, Miller J R, Gil R, Moon R T, White R, Virshup D M. Regulation of β-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 51.Smalley M J, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer L G, Hutchinson L, Fry M J, Dale T C. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 1999;18:2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith W C, Harland R M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- 53.Sokol S, Christian J L, Moon R T, Melton D A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 54.Sokol S Y, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homologue of dishevelled. Development. 1995;121:1637–1647. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- 55.Sokol S Y. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 56.Sokol S Y. Wnt signaling and dorsoventral axis specification in vertebrates. Curr Opin Genet Dev. 1999;6:405–410. doi: 10.1016/S0959-437X(99)80061-6. [DOI] [PubMed] [Google Scholar]
- 57.Turner D L, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 58.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo co-activates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 59.van Es J H, Kirkpatrick C, van de Wetering M, Molenaar M, Miles A, Kuipers J, Destrée O, Clevers H. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr Biol. 1999;9:105–108. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- 60.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of Axin release β-catenin from the Axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willert K, Logan C Y, Arora A, Fish M, Nusse R. A Drosophila Axin homologue, Daxin, inhibits Wnt signaling. Development. 1999;126:4165–4173. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 64.Yanagawa S-I, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The Dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- 65.Yang-Snyder J, Miller J R, Brown J D, Lai C-J, Moon R T. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 66.Yost C, Torres M, Miller J R, Huang E, Kimelman D, Moon R T. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 67.Yost C, Farr III G H, Pierce S B, Ferkey D M, Chen M M, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- 68.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry W L, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]