Abstract
Background
Current classification for acute kidney injury (AKI) in critically ill patients with sepsis relies only on its severity-measured by maximum creatinine which overlooks inherent complexities and longitudinal evaluation of this heterogenous syndrome. The role of classification of AKI based on early creatinine trajectories is unclear.
Methods
This retrospective study identified patients with Sepsis-3 who developed AKI within 48-h of intensive care unit admission using Medical Information Mart for Intensive Care-IV database. We used latent class mixed modelling to identify early creatinine trajectory-based classes of AKI in critically ill patients with sepsis. Our primary outcome was development of acute kidney disease (AKD). Secondary outcomes were composite of AKD or all-cause in-hospital mortality by day 7, and AKD or all-cause in-hospital mortality by hospital discharge. We used multivariable regression to assess impact of creatinine trajectory-based classification on outcomes, and eICU database for external validation.
Results
Among 4197 patients with AKI in critically ill patients with sepsis, we identified eight creatinine trajectory-based classes with distinct characteristics. Compared to the class with transient AKI, the class that showed severe AKI with mild improvement but persistence had highest adjusted risks for developing AKD (OR 5.16; 95% CI 2.87–9.24) and composite 7-day outcome (HR 4.51; 95% CI 2.69–7.56). The class that demonstrated late mild AKI with persistence and worsening had highest risks for developing composite hospital discharge outcome (HR 2.04; 95% CI 1.41–2.94). These associations were similar on external validation.
Conclusions
These 8 classes of AKI in critically ill patients with sepsis, stratified by early creatinine trajectories, were good predictors for key outcomes in patients with AKI in critically ill patients with sepsis independent of their AKI staging.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-04935-x.
Keywords: Acute kidney injury, Creatinine trajectory, Critical care, Sepsis
Background
Acute kidney injury (AKI) is a common complication in up to 60% of patients with critical illness and is associated with high morbidity and mortality [1]. Sepsis is the most common cause of AKI among critically ill patients [2]. Sepsis patients with AKI, have mortality rates six to eight folds higher than those without AKI [3, 4]. AKI in critically ill patients with sepsis is also associated with a higher risk of worsening kidney function including acute kidney disease (AKD), chronic kidney disease (CKD) and end-stage kidney disease (ESKD) [5–7]. The risks of these complications increase with increasing severity of AKI as defined by Kidney Disease Improving Global Outcomes (KDIGO) AKI staging [2, 7, 8].
Recent evidence has shown that AKI in sepsis patients is a heterogenous syndrome [9] with multiple mechanisms. These include inflammation, mitochondrial dysfunction, metabolic reprogramming, and microcirculatory dysfunction [10]. Additionally, sepsis associated factors including use of nephrotoxic medications or associated complications such as volume overload can further contribute to AKI in patients with sepsis [11, 12]. Therefore, relying on the assessment of AKI severity and patient prognosis solely based on the maximum changes in serum creatinine, as current KDIGO AKI staging does, without considering the longitudinal characteristics of serum creatinine changes, is an oversimplification, as it overlooks the complexities of this heterogenous syndrome.
The trajectory of serum creatinine, identified by the trend of percent change in serum creatinine over time, has been shown to be an important dimension for risk stratification of patients with AKI after cardiac surgery [13]. As classification based on creatinine trajectories early in AKI course accounts for both absolute rise in creatinine and patient’s response to treatment, it could be a better tool for risk stratification of AKI. The role of classification of AKI based on early creatinine trajectories is unclear. Additionally, it is unknown whether such classification offers any advantages over current KDIGO AKI severity staging alone. We hypothesized that classification of AKI in critically ill patients with sepsis based on these trajectories will identify patients at risk for complications beyond KDIGO AKI staging.
Methods
Data sources
We used data from two independent databases—Medical Information Mart for Intensive Care IV (MIMIC-IV) and eICU Collaborative Research Database (eICU) [14, 15], for this study. MIMIC-IV is a single center database containing de-identified electronic health records of patients admitted to the Beth Israel Deaconess Medical Center from 2008 to 2019. We used the MIMIC-IV database, focusing on critically ill patients with sepsis who developed AKI within 48 h after their first ICU admission. Our aim was to develop AKI classification in critically ill patients with sepsis based on creatinine trajectories within the first 96 h (4 days) of ICU admission. We chose this time-period to adequately capture the early trajectory of the evolution of AKI. We further used this development cohort to develop prediction models to assess whether creatinine trajectories independently predict outcomes in sepsis patients with AKI. eICU is a multicenter database comprising de-identified health data from more than 200,000 ICU admissions across the United States during the period of 2014–2015. We used eICU as an independent external validation cohort.
Study population
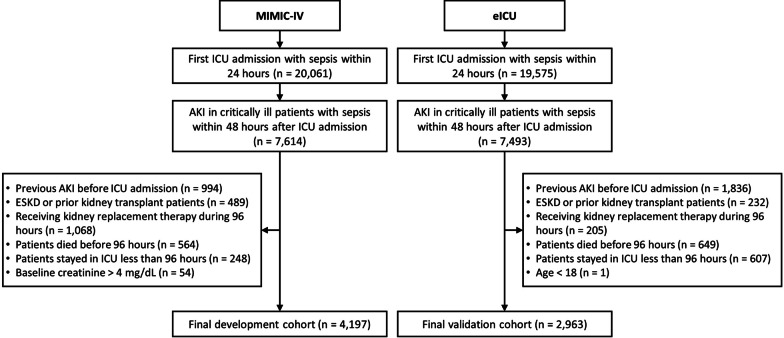
We included adult patients with sepsis who developed AKI within 48 h of intensive care unit (ICU) admission. We identified patients with sepsis based on a combination of suspicion of infection and increase in Sequential Organ Failure Assessment (SOFA) score by two or more within a 24-h period. To be consistent with prior literature [16, 17], we assumed a SOFA score of zero before ICU admission. We defined suspicion of infection as the co-occurrence of intravenous antibiotic treatment and collection of blood cultures such that if intravenous antibiotics were given first, then the cultures must have been obtained within 24 h [18]. In comparison, if cultures were obtained first, then intravenous antibiotics must have been ordered within 72 h. We identified onset of sepsis as the earlier of the suspicion of infection time and SOFA time if SOFA time occurred no more than 24 h before or 12 h after the suspected infection time [19]. As the eICU dataset provides limited data on body fluid samplings, we employed an alternative definition of suspected infection, which was determined by the occurrence of multiple antibiotic administrations. This alternative definition has been previously validated in a study comparing it to the original definition used in MIMIC-IV [20]. As per KDIGO guidelines, we defined AKI as an increase in serum creatinine by 0.3 mg/dL or more within 48 h or an increase by at least 1.5 times the reference serum creatinine within 7 days [8]. In congruence with previous literature [21] our specific inclusion criteria were—(1) Adult patients defined as those 18 years or older on admission, (2) who developed sepsis within 24 h of admission to ICU, and (3) developed AKI within 48 h after admission to ICU. For patients with multiple ICU admissions, we included data from only the first ICU admission. Figure S1 demonstrates the criteria to identify the definition and time of AKI in patients with sepsis onset [18–20]. We excluded patients under the age of 18 years, those with ESKD or prior kidney transplant, with a known baseline creatinine level > 4 mg/dL or with development of AKI prior to ICU admission. We also excluded patients receiving kidney replacement therapy or those who were discharged or died before 96 h after ICU admission. Details regarding the selection process employed in this study are given in Fig. 1.
Fig. 1.
Consort diagram of development and validation cohorts
Outcome
The primary outcome was development of AKD, defined as the continued meeting of AKI criteria in surviving patients beyond 7 days after development of AKI [22]. The secondary outcomes were—(1) a composite of AKD or all-cause in-hospital mortality by day 7 after AKI onset, and (2) a composite of AKD by hospital discharge or all-cause in-hospital mortality.
Feature extraction
We included data on patient demographics, comorbidities, baseline creatinine, laboratory values, vasopressor use and duration and exposure to nephrotoxic drugs [23] during the first 96 h of the ICU admission. Similar to prior literature, we determined baseline serum creatinine as the median serum creatinine within 12 months prior to hospital admission [24, 25]. For patients with missing previous serum creatinine values in this timeframe, as recommended by KDIGO, we estimated the baseline serum creatinine by assuming the Modification of Diet in Renal Disease estimated glomerular filtration rate of 75 mL/min per 1.73 m2 [8]. In accordance with previous studies, we then determined the reference serum creatinine as the lower of the baseline serum creatinine mentioned above and the first admission serum creatinine [24, 26]. Following published literature, we excluded variables with more than 40% missing values [27–29]. All other missing data were imputed using predictive mean matching techniques with five imputations based on Multivariate Imputation via Chained Equations (MICE) function in R [30].
Statistical analysis
We expressed descriptive results for the participant baseline characteristics as either mean with standard deviation or as median with interquartile range, depending on skewness. We compared categorical features using Chi-square test and continuous features using Student t test and Mann–Whitney U tests.
We utilized the latent class mixed models (LCMM) to identify classes based on serum creatinine trajectories defined by the percentage change in serum creatinine as ([serum creatinine–reference creatinine]/reference creatinine)*100. LCMM is a robust and validated statistical method designed to uncover clinically significant patient subgroups with similar longitudinal outcomes [31]. It integrates mixed models, which track individual-level growth in longitudinal outcomes through random effects, with latent class analysis that classifies patients into groups based on shared progression patterns. We calculated creatinine changes. We developed LCMM models with varying number of classes (2–10) in the development cohort. We selected the model with the lowest Bayesian Information Criterion (BIC). Subsequently, we computed the probability that a patient belongs to each class using this model and classified them into the class to which they had the highest probability of belonging. We assessed model discrimination using the mean posterior class membership probability (MPCMP). It is a class-specific metric that represents the mean probability of class membership for patients assigned to that class [32]. We then used mixed models to estimate marginal projection of the changes in creatinine levels across different LCMM classes. For external validation, we applied the trained LCMM model to the validation cohort, utilizing all estimated parameters derived from the model trained on the development cohort to classify creatinine trajectories with the validation cohort.
We used regression models to identify the impact of the classification of AKI in critically ill patients with sepsis based on early creatinine trajectories on various outcomes. We used logistic regression to assess the relationship between classification of AKI in critically ill patients with sepsis based on early creatinine trajectories and development of AKD. We used Kaplan–Meier and Cox regression analyses to evaluate the relationship between classification of AKI in critically ill patients with sepsis based on early creatinine trajectories and composite outcomes of AKD or all-cause in-hospital mortality by day 7, and AKD or all-cause in-hospital mortality by hospital discharge. To evaluate the independent effect of classification of AKI in critically ill patients with sepsis based on early creatinine trajectories on these outcomes, we adjusted the logistic and Cox regressions for age, gender, race, comorbidities, laboratory results, baseline creatinine level, SOFA score, initial and maximum AKI staging. We performed all analyses using R, version 4.2.2 [33].
Results
A total of 4197 patients with AKI in critically ill patients with sepsis from MIMIC-IV database satisfied the inclusion and exclusion criteria of this study and served as the development set. A total of 3963 patients with AKI in critically ill patients with sepsis from eICU served as external validation set. A comparison of baseline characteristics and outcomes between two cohorts is shown in Tables 1 and 2 respectively.
Table 1.
Baseline characteristics
| MIMIC-IV (4197) | eICU (3963) | p-value | |
|---|---|---|---|
| Age (years) | 70 (59, 80) | 67 (59, 78) | 0.001 |
| Male (%) | 2577 (61%) | 2228 (56%) | < 0.001 |
| Race (%) | < 0.001 | ||
| White | 2883 (69%) | 3171 (80%) | |
| Black | 375 (9%) | 434 (11%) | |
| Hispanic | 139 (3%) | 64 (2%) | |
| Others | 800 (19%) | 294 (7%) | |
| Height (cm) | 170 (163, 178) | 170 (163, 178) | 0.07 |
| Weight (kg) | 80 (68, 96) | 84.7 (70, 104) | < 0.001 |
| Underlying diseases (%) | |||
| Diabetes mellitus | 951 (23%) | 467 (12%) | < 0.001 |
| Congestive heart failure | 968 (23%) | 560 (14%) | < 0.001 |
| Arrhythmia | 774 (18%) | 587 (15%) | < 0.001 |
| Chronic lung disease | 620 (15%) | 379 (10%) | < 0.001 |
| Liver disease | 328 (8%) | 180 (5%) | < 0.001 |
| Laboratorya | |||
| Hemoglobin (g/dL) | 8.4 (7.5, 9.6) | 8.8 (7.6, 10.2) | < 0.001 |
| Hematocrit (%) | 25 (23, 29) | 27 (23, 31) | < 0.001 |
| White blood cell count (× 109/L) | 15.4 (11.5, 20.5) | 15.3 (11.3, 20.8) | 0.54 |
| Platelet (× 1000/mm3) | 123 (82, 180) | 130 (85, 185) | 0.02 |
| PT | 16 (14, 20) | 17 (14, 22) | < 0.001 |
| PTT | 37 (31, 55) | 38 (32, 55) | 0.22 |
| INR | 1.5 (1.3, 1.9) | 1.4 (1.2, 2) | 0.001 |
| BUN (mg/dL) | 38 (26, 57) | 44 (31, 61) | < 0.001 |
| Sodium (mmo/L) | 142 (139, 145) | 142 (139, 146) | < 0.001 |
| Potassium (mmo/L) | 4.8 (4.4, 5.2) | 4.7 (4.3, 5.1) | < 0.001 |
| Chloride (mmo/L) | 109 (106, 113) | 109 (105, 114) | 0.97 |
| Bicarbonate (mmo/L) | 20 (17, 23) | 20 (16, 23) | 0.01 |
| Calcium (mg/dL) | 8.6 (8.2, 9) | 8.6 (8.1, 9) | < 0.001 |
| SGOT (U/L) | 54 (28, 137) | 46 (25, 118) | < 0.001 |
| SGPT (U/L) | 34 (18, 100) | 31 (18, 79) | 0.01 |
| ALP (U/L) | 86 (62, 129) | 84 (61, 125) | 0.21 |
| Albumin (d/dL) | 2.9 (2.4, 3.2) | 2.5 (2.1, 2.9) | < 0.001 |
| Glucose (mg/dL) | 171 (140, 229) | 183 (147, 246) | < 0.001 |
| pH | 7.30 (7.24, 7.35) | 7.31 (7.24, 7.37) | < 0.001 |
| pO2 | 64 (40, 87) | 75 (62, 95) | < 0.001 |
| pCO2 | 47 (42, 55) | 43 (37, 52) | < 0.001 |
| pO2/FiO2 | 319 (234, 414) | 310 (217, 376) | < 0.001 |
| Lactate (mg/dL) | 2.4 (1.7, 3.7) | 2 (1.3, 3.6) | < 0.001 |
| Baseline creatinine (mg/dL)b | 1 (0.8, 1.2) | 0.98 (0.8, 1) | < 0.001 |
| First AKI staging (%) | < 0.001 | ||
| 1 | 2755 (66%) | 2047 (52%) | |
| 2 | 667 (16%) | 1114 (28%) | |
| 3 | 775 (18%) | 802 (20%) | |
| MAX AKI Staging (%) | < 0.001 | ||
| 1 | 2421 (58%) | 1508 (38%) | |
| 2 | 788 (19%) | 1204 (30%) | |
| 3 | 988 (23%) | 1251 (32%) | |
| MAX SOFA score | 7 (5, 10) | 8 (5, 10) | < 0.001 |
| Vasopressor use (%)c | 2603 (62%) | 1019 (26%) | < 0.001 |
| Vasopressor duration (minutes) | 1285 (419, 3101) | 2731 (960, 4906) | < 0.001 |
| Nephrotoxins (%) | 1441 (34%) | 2883 (73%) | < 0.001 |
Data are presented as count (percent) or median (interquartile range [IQR])
AKI acute kidney injury, BUN blood urea nitrogen, SGPT serum glutamic pyruvic transaminase, SGOT serum glutamic-oxaloacetic transaminase, SOFA sequential organ failure assessment
aLaboratory values were selected by the most abnormal results
bBaseline serum creatinine was determined as the lower of the median serum creatinine level within 12 months prior to hospital admission and the first admission creatinine measure. For patients with missing previous serum creatinine level, we calculated a baseline serum creatinine level by using the Modification of Diet in Renal Disease equation as recommended in the KDIGO AKI guideline, assuming a glomerular filtration rate of 75 mL/min per 1.73 m2
cVasopressors included norepinephrine, dopamine, epinephrine, phenylephrine, or vasopressin
Table 2.
Clinical outcomes
| MIMIC-IV (4197) | eICU (3963) | p-value | |
|---|---|---|---|
| Outcomes | |||
| AKD day 7 (%) | 674 (16%) | 1000 (25%) | < 0.001 |
| Death within 7 days (%) | 198 (5%) | 184 (5%) | 0.87 |
| In hospital mortality (%) | 591 (14%) | 512 (13%) | 0.12 |
| Composite of AKD and death by day 7 (%) | 872 (21%) | 1184 (30%) | < 0.001 |
| Composite of AKD or in-hospital mortality by hospital discharge (%) | 772 (18%) | 1008 (25%) | < 0.001 |
| ICU length of stay (days) | 3.9 (2, 7.2) | 4.1 (2.2, 7.4) | 0.004 |
| Hospital length of stay after ICU admission (days) | 9.2 (6.1, 15.4) | 9.1 (6.3, 14.4) | 0.002 |
Classification of AKI in critically ill patients with sepsis based on early creatinine trajectories
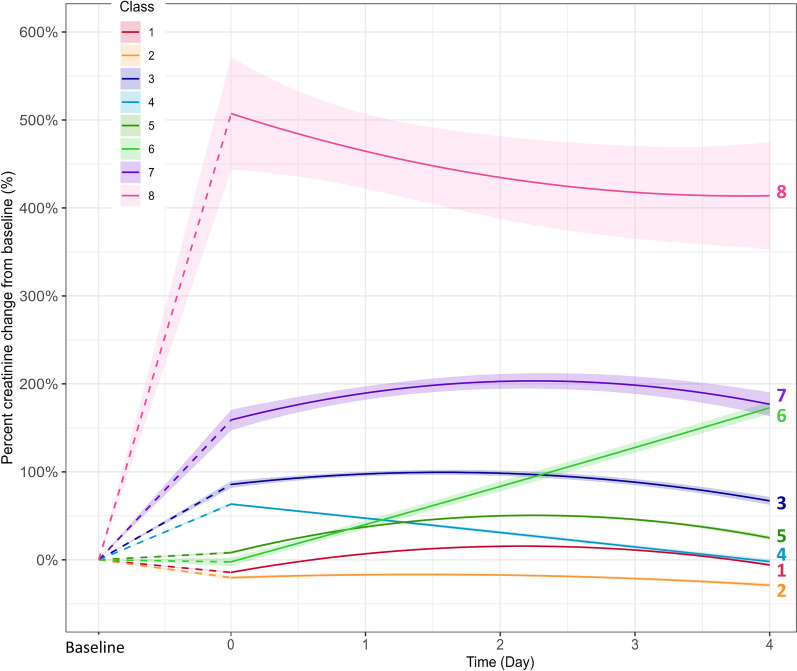
With the lowest BIC for an eight-class model (Additional file 2: Table S1), we identified 8 distinct Classes of creatinine trajectories in the development cohort using LCMM. MPCMP values for class assignment ranged from 62 to 89% (Additional file 2: Table S2), indicating good discrimination between classes. These classes showed differences in AKI staging, rate, timing, and recovery (Fig. 2).
Class 1. Transient AKI—Creatinine trajectory in this class showed a minor nadir from baseline on ICU admission, followed by a mild rise that peaked at AKI stage 1 on day 2–3, with subsequent recovery.
Class 2. Minor Transient AKI—Creatinine trajectory in this class started with a minor decrease from baseline, followed by a mild rise in creatinine that peaked on day 1, with subsequent recovery of AKI.
Class 3. Early Mild AKI with Persistence—Creatinine trajectory in this class started with stage I AKI at ICU admission with continued rise in creatinine till day 1 to 2, followed by a sustained decline but persistence of AKI.
Class 4. Early Mild AKI followed by Recovery—Creatinine trajectory in this class started with an initial stage 1 AKI on admission to ICU which then showed a sustained decline to AKI resolution.
Class 5. Late Mild AKI with persistence—This class had a mild rise in creatinine on ICU admission that continued worsening with peak at AKI stage 1 by day 2, followed by a sustained decline but persistence of AKI.
Class 6. Late Mild AKI with persistence and worsening—This class had a mildly elevated creatinine on ICU admission that then showed a sustained increase.
Class 7. Moderate AKI with persistence—This class had stage II AKI on ICU admission with continued increased in creatinine for a peak of AKI stage 2–3 by day 2–3. This was followed by a mild decline but with persistence of AKI.
Class 8. Severe AKI with mild improvement but persistence—This class had AKI stage 3 on ICU admission followed by a small, sustained decline thereafter but with continued persistence of AKI.
Fig. 2.
AKI in critically ill patients with sepsis classification based on serum creatinine trajectories in development cohort
The class observed most was Transient AKI (Class 1) (23.5%), followed by Late Mild AKI with Persistence (Class 5) (22%) and Early Mild AKI with Persistence (Class 3) (17%). There were significant differences among Classes in age (p < 0.001), hemoglobin (p = 0.009), white blood cell counts (p = 0.02), lactate levels (p = 0.009), maximum SOFA score (p < 0.001), vasopressor duration (p < 0.001) (Additional file 2: Table S3).
Classification of AKI in critically ill patients with sepsis based on early creatinine trajectories and outcomes in the development cohort (MIMIC-IV)
Development of AKD
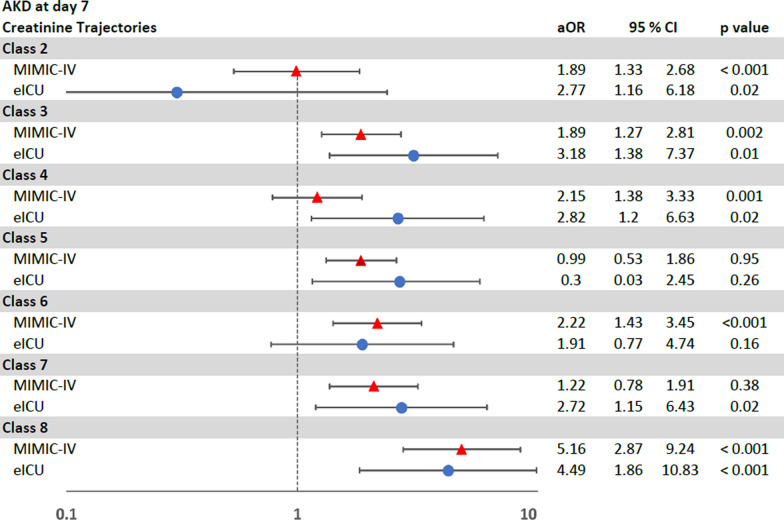
In development cohort, 674 (16%) patients developed AKD (Table 2). There were significant differences among Classes in development of AKD (p < 0.001) (Additional file 2: Table S4). On regression analysis, the creatinine trajectories were associated with differing risks of development of AKD. In comparison to patients with transient AKI (Class 1), the highest risk for development of AKD was seen in patients in severe AKI with mild improvement but persistence (Class 8) (OR 9.02; 95% CI 5.94–13.7; p < 0.001), followed by Class 6, 7, 3, and 5 respectively (Table 3). This difference in the risks of development of AKD by classes based on creatinine trajectories persisted on multivariable logistic regression analysis where Class 8 still showed the highest risk for development of AKD with Class 1 as the reference group (OR 5.16; 95% CI 2.87–9.24; p < 0.001) (Fig. 3).
Table 3.
Univariate logistic regression models for AKD and univariate cox regression models for composite outcomes in the development cohort
| AKD | AKD or mortality by day 7 | AKD at discharge or in-hospital mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Class 1 (reference group) | 1 | 1 | 1 | ||||||
| Class 2 | 0.99 | 0.60, 1.63 | 0.97 | 0.99 | 0.68, 1.47 | 0.99 | 1.14 | 0.82, 1.58 | 0.43 |
| Class 3 | 3.99 | 2.87, 5.55 | < 0.001 | 2.31 | 1.75, 3.04 | < 0.001 | 1.51 | 1.17, 1.94 | 0.001 |
| Class 4 | 1.30 | 0.85, 2.01 | 0.23 | 0.85 | 0.58, 1.23 | 0.38 | 0.57 | 0.39, 0.83 | 0.003 |
| Class 5 | 2.52 | 1.80, 3.55 | < 0.001 | 1.92 | 1.45, 2.54 | < 0.001 | 1.32 | 1.02, 1.70 | 0.03 |
| Class 6 | 7.11 | 4.81, 10.53 | < 0.001 | 4.98 | 3.60, 6.89 | < 0.001 | 3.48 | 2.56, 4.74 | < 0.001 |
| Class 7 | 5.59 | 3.99, 7.83 | < 0.001 | 3.02 | 2.28, 3.99 | < 0.001 | 1.79 | 1.37, 2.33 | < 0.001 |
| Class 8 | 9.02 | 5.94, 13.69 | < 0.001 | 3.38 | 2.36, 4.85 | < 0.001 | 1.93 | 1.31, 2.82 | < 0.001 |
Fig. 3.
Multivariable logistic regression analysis of the risk of AKD
Composite of AKD or mortality in seven days
In the development cohort, 872 (21%) patients developed AKD or died within seven days after AKI onset.
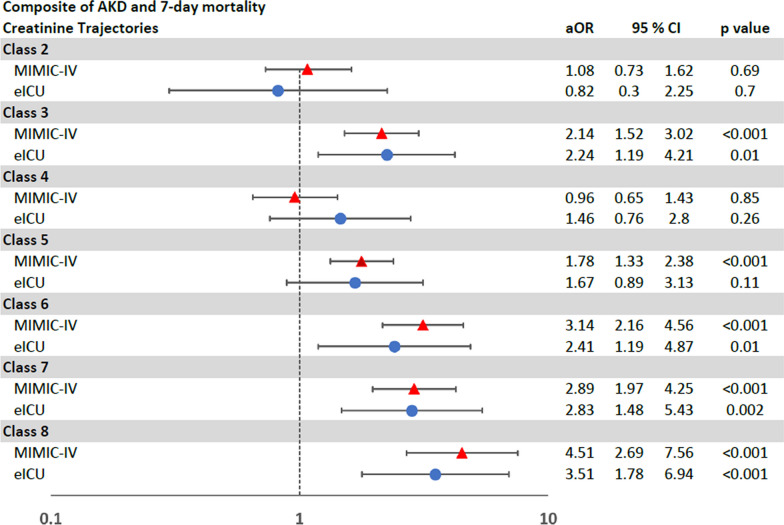
On univariable cox regression analysis, creatinine trajectories were associated with differing risks of development of a composite of AKD or all-cause in-hospital mortality by day 7 after AKI onset. In comparison to Transient AKI (Class 1), Late Mild AKI with Persistence and Worsening (Class 6) showed the highest risk for development of a composite of AKD or mortality in 7 days (HR 4.98; 95% CI 3.60–6.89; p < 0.001), followed by Severe AKI with Mild Improvement but Persistence (Class 8) (HR 3.38; 95% CI 2.36–4.85; p < 0.001) and Moderate AKI with Persistence (Class 7) (HR 3.02; 95% CI 2.28–3.99; p = < 0.001), respectively (Table 3 and Additional file 1: Figure S2).
This difference in the risks of development of a composite of AKD or all-cause in-hospital mortality by day 7 after AKI onset by classes based on creatinine trajectories persisted on adjusted survival analysis (Fig. 4).
Fig. 4.
Multivariable Cox regression analysis of a composite of AKD and 7-day mortality
Composite of AKD or total all-cause in-hospital mortality by discharge
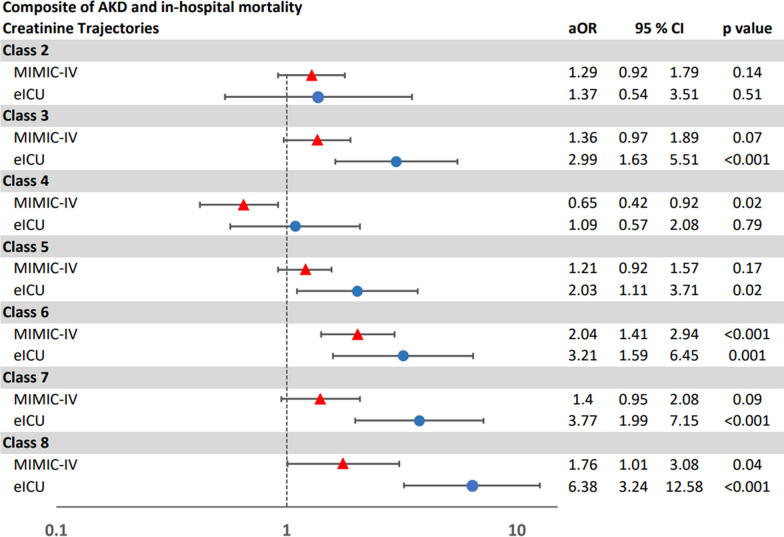
A total 772 (18%) patients had AKD by discharge or died during the hospital admission in the development cohort (Table 2). The classes of creatinine trajectories were associated with differing risks of development of a composite of AKD or all-cause in-hospital mortality by discharge in both unadjusted (Table 3 and Additional file 1: Figure S3) and adjusted analyses (Fig. 5).
Fig. 5.
Multivariable Cox regression analysis of a composite of AKD at discharge and in-hospital mortality
Validation of creatinine trajectories in independent external cohort (eICU)
We validated the LCMM model and identified 8 creatinine trajectories in the eICU which served as our external validation cohort. Discrimination among classes was good, with MPCMP ranging from 60 to 91% (Additional file 2: Table S5). There were significant differences among Classes in age (p < 0.001), hemoglobin (p < 0.001), white blood cell counts (p < 0.001), lactate levels (p < 0.001), maximum SOFA score (p < 0.001), vasopressor duration (p < 0.001), and development of AKD (p < 0.001) (Additional file 2: Table S6, S7).
Among 3963 AKI with sepsis patients, 1000 (25%) patients developed AKD at day 7 (Table 2). Like the development cohort, these classes of creatinine trajectories in the external validation cohort were associated with differing risks of development of AKD, and composite of both AKD or all-cause in-hospital morality by day 7 and AKD or all-cause in-hospital by discharge, in both unadjusted and adjusted analyses (Table 4 and Figs. 3, 4, 5).
Table 4.
Univariate logistic regression models for AKD and univariate cox regression models for composite outcomes in the validation cohort
| AKD | AKD or mortality by day 7 | AKD at discharge or in-hospital mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Class 1 (reference group) | 1 | 1 | 1 | ||||||
| Class 2 | 0.39 | 0.05, 3.21 | 0.38 | 0.90 | 0.33, 2.44 | 0.84 | 1.24 | 0.49, 3.16 | 0.65 |
| Class 3 | 7.58 | 3.38, 17.01 | < 0.001 | 3.57 | 1.96, 6.49 | < 0.001 | 3.46 | 1.95, 6.16 | < 0.001 |
| Class 4 | 2.53 | 1.07, 5.95 | 0.03 | 1.47 | 0.77, 2.82 | 0.24 | 1.16 | 0.61, 2.21 | 0.65 |
| Class 5 | 3.69 | 1.61, 8.45 | 0.002 | 1.92 | 1.03, 3.58 | 0.04 | 2.25 | 1.24, 4.09 | 0.007 |
| Class 6 | 9.26 | 3.85, 22.32 | < 0.001 | 4.47 | 2.28, 8.76 | < 0.001 | 4.59 | 2.37, 8.88 | < 0.001 |
| Class 7 | 13.32 | 5.95, 29.83 | < 0.001 | 4.23 | 2.33, 7.69 | < 0.001 | 3.61 | 2.03, 6.42 | < 0.001 |
| Class 8 | 19.97 | 8.79, 45.4 | < 0.001 | 4.66 | 2.52, 8.62 | < 0.001 | 4.82 | 2.66, 8.74 | < 0.001 |
Discussion
In this study using data from two large, independent critical care databases we derived and validated 8 different classes of early creatinine trajectories early in AKI in critically ill patients with sepsis. These classes were heterogenous in both baseline characteristics and outcomes of patients with AKI in critically ill patients with sepsis. Additionally, we show that membership in these classes is an independent predictor for AKD and composite of AKD or mortality by day 7 and composite of AKD or mortality by hospital discharge.
AKI is common in critically ill patients and is associated with high morbidity and mortality. However, not all instances of AKI are the same as is demonstrated by the recognition of various stages of AKI [8]. The current staging for AKI relies on the severity of AKI as identified by maximum change in serum creatinine or minimum urine output over a period. The recent discovery of different phenotypes of AKI [9] suggests that classification of AKI just by severity is inadequate, particularly when considering different etiologies of AKI and effects of early management strategies of AKI in patients with sepsis [9, 25]. Use of an unbiased methodology to identify novel and clinically relevant sub-phenotypes of AKI based on early trajectory of serum creatinine provides a novel approach to classify AKI in critically ill patients with sepsis. An added advantage of this technique is that it enhances and further personalizes the current AKI classification, which relies solely on its severity.
The trajectories of serum creatinine have been shown to be associated with different outcomes in patients with AKI. Bhatraju et al. [34] studied the creatinine trajectory in first 72 h of ICU stay among critically ill patients with AKI and showed that patients who have a non-resolving AKI subphenotype in that timeframe have a higher risk of mortality in comparison to patients with a resolving subphenotype of AKI (RR 1.68; 95% CI 1.15–2.33). Similarly, Kellum et al. [35] delineated five distinct recovery sub-phenotypes among critical care patients with AKI during the first week following the onset of AKI. These sub-phenotypes were classified based on patterns of reversal, relapse, or recovery. Sub-phenotypes with patients who never recovered or had reversal with relapse had the longest lengths of stay and the worst prognosis, while patients recovering late did better than those with no recovery, but not as well as those recovering early.
Most recently, Andrew et al. [13] employed LCMM technique to identify twelve different trajectories of serum creatinine within the first 4 days after cardiac surgery. Among them, they found that there were four creatinine trajectories that were associated with higher risk of death. These results are similar to our study where we identified eight creatinine trajectories, with distinct clinical characteristics and outcomes, in patients with AKI in critically ill patients with sepsis. With increasing recognition of the importance of AKD, we focused our attention in this study on studying the impact of these trajectories on development of AKD.
Development of AKD is associated with development of CKD, ESKD, longer length of stay, and greater risk of mortality [36, 37]. Additionally, the AKD period itself represents a critical time window during which interventions could be initiated to potentially alter the natural history of kidney disease. Though mortality is a critical endpoint, use of AKD for risk stratification of AKI in critically ill patients allows for a more holistic assessment by incorporating both risk for increased mortality with AKD and increased risk for further decline in kidney function. Additionally, it allows for identification of high-risk patients where targeted novel therapies could decrease the onset of AKD, and subsequent long term adverse consequences. In this study, we show that classification based on early creatinine trajectories in sepsis patients with AKI identifies patients at risk for AKD. Moreover, these classes further identify patients at risk for a composite of AKD or mortality by day 7 and AKD or mortality by discharge. Risk stratification of patients with AKI in critically ill patients with sepsis by creatinine trajectories is independent of AKI staging, thus providing a method to further personalize care. It has been shown that certain patients with AKI respond differently to vasopressin therapy [38]. As classification by creatinine trajectories incorporates information about both severity of AKI and its response to early management strategies, this may allow for further identification of differential responses to therapeutic interventions across these classes. This classification would also be important for timely allocation of appropriate resources for patients, such as need for follow up in nephrology clinic on discharge.
It is important to acknowledge the limitations of this study. Most importantly, this is a retrospective study and the impact of these classes on clinical care needs to be evaluated in prospective studies. Furthermore, our study aims to classify early AKI in critically ill patients with sepsis based on initial 96-h serum creatinine trajectory after ICU admission. We, therefore, utilized creatinine-based criteria to define AKI, while focusing on patients who developed AKI within first 48 h of ICU admission and survived the initial 96 h. As kidney replacement therapy directly affects serum creatinine measurements, we excluded patients receiving kidney replacement therapy during the first 96 h after ICU admission. Despite these limitations, our study is a significant step towards personalization of risk stratification of AKI in critically ill patients with sepsis.
Conclusions
We identified eight distinct classes of AKI in critically ill patients with sepsis based-on serum creatinine trajectories within the first 96 h after AKI. These classes identified patients with distinct clinical characteristics and risk for development of AKD, AKD or mortality by day 7 and AKD or mortality by hospital discharge. This risk stratification by creatinine trajectories was independent of AKI staging and validated independently. Further studies are needed to identify therapeutic and management implications of creatinine trajectories.
Supplementary Information
Supplementary Material 1: Figure S1. Criteria to identify time of AKI in critical ill patients with sepsis onset. Figure S2. Kaplan Meier survival curve for AKD or 7-day mortality for creatinine trajectories in development cohort (A) and validation cohort (B). Figure S3. Kaplan Meier survival curve for AKD at discharge or in-hospital mortality in development cohort (A) and validation cohort (B).
Supplementary Material 2: Table S1. Fit statistics for latent class mixed models in development cohort. Table S2. Mean Posterior Probability of Membership in Class in MIMIC-IV. Table S3. Baseline characteristics by creatinine trajectory in MIMIC-IV. Table S4. Clinical outcomes by creatinine trajectory in MIMIC-IV. Table S5. Mean Posterior Probability of Membership in Class in eICU database. Table S6. Baseline characteristics by creatinine trajectory in eICU database. Table S7. Clinical outcomes by creatinine trajectory in eICU database.
Acknowledgements
None.
Abbreviations
- AKD
Acute kidney disease
- AKI
Acute kidney injury
- BIC
Bayesian information criterion
- CKD
Chronic kidney disease
- eICU
Electronic Intensive Care Unit
- ESKD
End-stage kidney disease
- ICU
Intensive care unit
- KDIGO
Kidney Disease Improving Global Outcomes
- LCMM
Latent class mixed models
- MICE
Multivariate imputation via chained equations
- MIMIC-IV
Medical Information mart for intensive care IV
- MPCMP
Mean posterior class membership probability
Author contributions
Conceptualization: Kullaya T, Wonsuk O, Ankit S. Funding acquisition: Girish NN, Ankit S. Methodology: Kullaya T, Wonsuk O, Ankit S. Data curation: Kullaya T, Wonsuk O, Ankit S. Data analysis: Kullaya T, Wonsuk O, Ankit S. Interpretation of data and analysis: Kullaya T, Wonsuk O, Lili C, Ira H, Khaled S, Monica K, Neomi S, Roopa K, Girish NN, Ankit S. Drafting of Manuscript: Kullaya T, Wonsuk O, Ankit S. Critical revision of the manuscript for important intellectual content: Kullaya T, Wonsuk O, Lili C, Ira H, Khaled S, Monica K, Neomi S, Roopa K, Girish NN, Ankit S. Supervision: Lili C, Ira H, Girish NN, Ankit S. Final approval of version to be published: Kullaya T, Wonsuk O, Lili C, Ira H, Khaled S, Monica K, Neomi S, Roopa K, Girish NN, Ankit S.
Funding
This study was supported by NIDDK grant 5K08DK131286 (AS) and R01DK127139 (GNN). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Publicly available datasets were analyzed in this study. The dataset used in this study, MIMIC-IV, is available at https://mimic.physionet.org/, and the eICU dataset is available at https://github.com/mit-lcp/eicu-code.
Declarations
Ethics approval and consent to participate
Consent obtained for use of MIMIC and eICU databases.
Consent for publication
Not applicable.
Competing interests
GNN is a founder of Renalytix, Pensieve, Verici and provides consultancy services to AstraZeneca, Reata, Renalytix, Siemens Healthineer and Variant Bio, serves a scientific advisory board member for Renalytix and Pensieve. He also has equity in Renalytix, Pensieve and Verici. LC is a consultant for Vifor Pharma INC and has received honorarium from Fresenius Medical Care. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kullaya Takkavatakarn and Wonsuk Oh contributed equally as first author.
Girish N. Nadkarni and Ankit Sakhuja contributed equally as senior author.
References
- 1.Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a veterans administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 5.Murugan R, Kellum JA. Acute kidney injury: What's the prognosis? Nat Rev Nephrol. 2011;7(4):209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou SM, Lee KH, Tsai MT, Tseng WC, Chu YC, Tarng DC. Sepsis and the risks of long-term renal adverse outcomes in patients with chronic kidney disease. Front Med. 2022;9:809292. doi: 10.3389/fmed.2022.809292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neyra JA, Mescia F, Li X, et al. Impact of acute kidney injury and CKD on adverse outcomes in critically Ill septic patients. Kidney Int Rep. 2018;3(6):1344–1353. doi: 10.1016/j.ekir.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary K, Vaid A, Duffy A, et al. Utilization of deep learning for subphenotype identification in sepsis-associated acute kidney injury. Clin J Am Soc Nephrol. 2020;15(11):1557–1565. doi: 10.2215/CJN.09330819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–553. doi: 10.1097/MCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil VP, Salunke BG. Fluid overload and acute kidney injury. Indian J Crit Care Med. 2020;24(Suppl 3):S94–S97. doi: 10.5005/jp-journals-10071-23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasrebi-de Kom IAR, Dongelmans DA, Abu-Hanna A, et al. Acute kidney injury associated with nephrotoxic drugs in critically ill patients: a multicenter cohort study using electronic health record data. Clin Kidney J. 2023 doi: 10.1093/ckj/sfad160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrew BY, Pieper CF, Cherry AD, et al. Identification of trajectory-based acute kidney injury phenotypes among cardiac surgery patients. Ann Thorac Surg. 2022;114(6):2235–2243. doi: 10.1016/j.athoracsur.2021.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV clinical database demo (version 2.2). PhysioNet. 2023. 10.13026/dp1f-ex47.
- 15.Johnson A PT, Badawi O, Raffa J. eICU collaborative research database demo (version 2.0.1). PhysioNet. 2021. 10.13026/4mxk-na84.
- 16.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raith EP, Udy AA, Bailey M, et al. Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 18.Kamaleswaran R, Lian J, Lin DL, et al. Predicting volume responsiveness among sepsis patients using clinical data and continuous physiological waveforms. AMIA Annu Symp Proc. 2020;2020:619–628. [PMC free article] [PubMed] [Google Scholar]
- 19.Reyna MA, Josef CS, Jeter R, et al. Early prediction of sepsis from clinical data: The PhysioNet/computing in cardiology challenge 2019. Crit Care Med. 2020;48(2):210–217. doi: 10.1097/CCM.0000000000004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moor M, Bennett N, Plecko D, et al. Predicting sepsis using deep learning across international sites: a retrospective development and validation study. EClinicalMedicine. 2023;62:102124. doi: 10.1016/j.eclinm.2023.102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wu R, Zhao W, et al. Machine learning algorithm to predict mortality in critically ill patients with sepsis-associated acute kidney injury. Sci Rep. 2023;13(1):5223. doi: 10.1038/s41598-023-32160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 23.Goswami E, Ogden RK, Bennett WE, et al. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm. 2019;76(22):1869–1874. doi: 10.1093/ajhp/zxz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peerapornratana S, Priyanka P, Wang S, et al. Sepsis-associated acute kidney disease. Kidney Int Rep. 2020;5(6):839–850. doi: 10.1016/j.ekir.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–417. doi: 10.1038/s41581-023-00683-3. [DOI] [PubMed] [Google Scholar]
- 26.Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25(12):3911–3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhang G, Goyal H, Mo L, Hong Y. Identification of subclasses of sepsis that showed different clinical outcomes and responses to amount of fluid resuscitation: a latent profile analysis. Crit Care. 2018;22(1):347. doi: 10.1186/s13054-018-2279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong K, Lee HK, Yu K, Xie X, Li J. A prediction and interpretation framework of acute kidney injury in critical care. J Biomed Inform. 2021;113:103653. doi: 10.1016/j.jbi.2020.103653. [DOI] [PubMed] [Google Scholar]
- 29.Park N, Kang E, Park M, et al. Predicting acute kidney injury in cancer patients using heterogeneous and irregular data. PLoS ONE. 2018;13(7):e0199839. doi: 10.1371/journal.pone.0199839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2015 doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- 32.Weller B, Bowen N, Faubert S. Latent class analysis: a guide to best practice. J Black Psychol. 2020;46.
- 33.R Core Team: A language and environment for statistical computing. Vienna, Austria. URL [R version 4.2.2 for Windows https://www.R-project.org/: R Foundation for Statistical Computing October 2022.
- 34.Bhatraju PK, Mukherjee P, Robinson-Cohen C, et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit Care. 2016;20(1):372. doi: 10.1186/s13054-016-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gameiro J, Marques F, Lopes JA. Long-term consequences of acute kidney injury: a narrative review. Clin Kidney J. 2021;14(3):789–804. doi: 10.1093/ckj/sfaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su CC, Chen JY, Chen SY, et al. Outcomes associated with acute kidney disease: a systematic review and meta-analysis. EClinicalMedicine. 2023;55:101760. doi: 10.1016/j.eclinm.2022.101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatraju PK, Zelnick LR, Herting J, et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med. 2019;199(7):863–872. doi: 10.1164/rccm.201807-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1. Criteria to identify time of AKI in critical ill patients with sepsis onset. Figure S2. Kaplan Meier survival curve for AKD or 7-day mortality for creatinine trajectories in development cohort (A) and validation cohort (B). Figure S3. Kaplan Meier survival curve for AKD at discharge or in-hospital mortality in development cohort (A) and validation cohort (B).
Supplementary Material 2: Table S1. Fit statistics for latent class mixed models in development cohort. Table S2. Mean Posterior Probability of Membership in Class in MIMIC-IV. Table S3. Baseline characteristics by creatinine trajectory in MIMIC-IV. Table S4. Clinical outcomes by creatinine trajectory in MIMIC-IV. Table S5. Mean Posterior Probability of Membership in Class in eICU database. Table S6. Baseline characteristics by creatinine trajectory in eICU database. Table S7. Clinical outcomes by creatinine trajectory in eICU database.
Data Availability Statement
Publicly available datasets were analyzed in this study. The dataset used in this study, MIMIC-IV, is available at https://mimic.physionet.org/, and the eICU dataset is available at https://github.com/mit-lcp/eicu-code.