Abstract
Activation of the TAL1 (or SCL) gene is the most frequent gain-of-function mutation in T-cell acute lymphoblastic leukemia (T-ALL). TAL1 belongs to the basic helix-loop-helix (HLH) family of transcription factors that bind as heterodimers with the E2A and HEB/HTF4 gene products to a nucleotide sequence motif termed the E-box. Reported to act both as an activator and as a repressor of transcription, the mechanisms underlying TAL1-regulated gene expression are poorly understood. We report here that the corepressor mSin3A is associated with TAL1 in murine erythroleukemia (MEL) and human T-ALL cells. Interaction mapping showed that the basic-HLH domain of TAL1 was both necessary and sufficient for TAL1-mSin3A interaction. TAL1 was found, in addition, to interact with the histone deacetylase HDAC1 in vitro and in vivo, and a specific histone deacetylase inhibitor, trichostatin A (TSA), relieved TAL1-mediated repression of an E-box-containing promoter and a GAL4 reporter linked to a thymidine kinase minimal promoter. Further, TAL1 association with mSin3A and HDAC1 declined during dimethyl sulfoxide-induced differentiation of MEL cells in parallel with a decrease in mSin3A abundance. Finally, TSA had a synergistic effect with enforced TAL1 expression in stimulating MEL cells to differentiate, while constitutive expression of mSin3A inhibited MEL cell differentiation. These results demonstrate that a corepressor complex containing mSin3A and HDAC1 interacts with TAL1 and restricts its function in erythroid differentiation. This also has implications for this transcription factor's actions in leukemogenesis.
Chromosomal rearrangements are frequent in hematological malignancies and typically involve genes encoding proteins, transcription factors in particular, that regulate cell growth, differentiation, or survival (8, 85). The TAL1 (or SCL) gene was initially identified as the transcriptional unit activated by a reciprocal (1;14) translocation in patients with T-cell acute lymphoblastic leukemia (T-ALL) (10, 17, 36). Activation of TAL1 transcription, generally without alteration of its coding potential, characterizes up to 60% of cases of T-ALL (9), making it the most frequent gain-of-function mutation observed in this disorder.
In addition to their expression in leukemic T cells, TAL1 transcripts and proteins have been detected in a number of hematopoietic and several nonhematopoietic cell types (10, 40, 41, 53, 71, 82, 101). A critical role for the gene in hematopoietic development was shown by the finding that Tal1−/− embryos died in midgestation with the complete absence of yolk sac blood cells (89, 93). Tal1−/− embryonic stem cells, in addition, failed to contribute to the hematopoietic lineages in Tal1−/−/Tal1+/+ chimeric mice, demonstrating that the gene is also required for blood cell formation postnatally (81, 88). Finally, studies involving enforced expression of TAL1 cDNAs or antisense sequences in murine erythroleukemia (MEL) cells (2) and normal human bone marrow cells (22, 33) indicate that TAL1 has a specific role in the differentiation of erythroid progenitors.
TAL1 belongs to the basic helix-loop-helix (bHLH) family of transcription factors, many of which regulate the differentiation of specific cellular lineages (reviewed in references 7, 24, 70, and 91). Like other tissue-restricted members of this group, TAL1 forms heterodimers with the protein products of more widely expressed HLH genes, including E2A and HEB/HTF4. These complexes were found to bind E-box sequence elements (49, 50, 103) and to stimulate transcription, particularly in association with other transcription factors or nuclear proteins (76, 77). However, transactivation of certain E-box-containing promoters was significantly lower with TAL1-E2A heterodimers than with complexes containing only E2A- or HEB/HTF4-encoded proteins (30, 51, 73), indicating that TAL1 could also act as a transcriptional repressor.
Considerable data indicate that the function of transcription factors can be modulated, if not determined, by their association with specific coregulators. A number of transcriptional coactivators, including GCN5 (13), TAFII250 (69), p300/CBP (75), PCAF (108), and SRC-1 (96), possess either intrinsic histone acetyltransferase activity or the ability to recruit proteins with such activity to chromatin. Histone acetylation is believed to destabilize nucleosomal structure, creating a permissive state for promoter activation (59). In contrast, transcriptional corepressors, including N-CoR (1, 47), SMRT (72), and mSin3 (1, 47, 72), act through recruitment of histone deacetylases (HDACs) to effect tighter nucleosomal packing and thereby restrict transcription factor accessibility. Direct physical interactions between components of coregulator complexes and the basal transcriptional machinery may also have a part in both gene repression (105) and activation (20).
By acting as integrators of signal transduction pathways, these corepressors and coactivators regulate critical steps in cellular proliferation and differentiation (reviewed in references 56, 57, and 98). p300 and CBP have been shown, in particular, to enhance the function of three erythroid transcription factors, GATA-1 (11, 12), erythroid Krüppel-like factor (112), and TAL1 (52). Further, [3H]acetate incorporation into histones increased with dimethyl sulfoxide (DMSO)-induced differentiation of wild-type but not mutant MEL cells incapable of differentiation (60), whereas the adenovirus protein E1A, an inhibitor of the histone acetyltransferases p300, CBP, and PCAF, blocked DMSO-induced MEL cell differentiation (11). In contrast, HDAC inhibitors, including trichostatin A (TSA) (109, 110) and a number of hybrid polar compounds (87), were found to induce MEL cells to differentiate.
Given the importance of both TAL1 and specific coregulators in erythroid differentiation, we investigated whether TAL1 interacts with two frequent components of nuclear corepressor complexes, mSin3A and HDAC1. We show that these proteins are associated with TAL1 in both MEL and T-ALL cell lines and that a corepressor complex containing mSin3A and HDAC1 restricts TAL1 action in erythroleukemia cell differentiation. These results have significant implications for how TAL1 function is regulated physiologically and for the mechanism by which this transcription factor acts as an oncoprotein.
MATERIALS AND METHODS
Cell culture and transient transfections.
MEL (F4-12B2 line) (23) and HeLa cells were cultured as described previously (52). HCD-57 cells (97) were cultured in Iscove's modified Dulbecco's medium with 20% fetal bovine serum (FBS) and 2 U of recombinant human erythropoietin per ml. Jurkat cells were cultured in RPMI 1640 medium containing 10% FBS. C3H10T1/2 cells were cultured in Dulbecco's modified Eagle medium with 10% FBS. Luciferase and chloramphenicol acetyltransferase (CAT) expression vectors were introduced into cells using Lipofectamine (Life Technologies, Gaithersburg, Md.) or SuperFect (Qiagen, Valencia, Calif.), and extracts were prepared 40 to 48 h after transfection. Luciferase and CAT assays were performed as described by De Wet et al. (27) and Nordeen et al. (74), respectively. The amount of plasmid DNA used in each transfection is detailed in the figure legends. The total mass of DNA transfected was equalized by addition of plasmid pCMV4, and each transfection was repeated three or more times.
Plasmids and constructs.
Plasmid pVZmSin3A was described by Ayer et al. (3) and provided by Robert Eisenman (University of Washington, Seattle). Eukaryotic expression vector pcDNA-mSin3A was prepared by subcloning the NotI and XbaI fragment of pVZmSin3A into vector pcDNA3.1 (Invitrogen, San Diego, Calif.). The cytomegalovirus promoter-driven plasmid pcDNA-Tal1 was described previously (52). Plasmid pSV2-CAT (39) was used as an internal control for variation in transfection efficiency. A full-length mouse Tal1 cDNA was cloned into the murine stem cell virus-based bicistronic retroviral vector MSCV-IRES-GFP (murine stem cell virus-internal ribosome entry site-green fluorescent protein) (80) provided by Arthur Nienhuis (St. Jude Children's Research Hospital, Memphis, Tenn.).
Plasmids expressing glutathione S-transferase (GST)–Tal1 fusion proteins were constructed by subcloning Tal1 cDNAs generated with PCR and corresponding to amino acids 1 to 330, 1 to 144, 142 to 330, 185 to 330, 242 to 330, and 185 to 240 into vectors pGEX-3X and pGEX-5X-1. GST-HD1 was described by Yang et al. (107) and provided by Edward Seto (University of South Florida, Tampa).
A full-length Tal1 cDNA was subcloned in frame with the DNA-binding domain of GAL4 (GAL41–147) in expression vector pSG424 (90). The E1bLUC-E6 reporter construct contains six copies of the preferred TAL1/E2A-binding motif 5′-AACAGATGGT-3′ (50) linked to an E1b-TATA-luciferase reporter. This plasmid was provided by Richard Baer (Texas Southwestern Medical Center, Dallas) and, except for the reporter gene, is identical to E1bCAT-E6 previously reported (51). The GAL4-thymidine kinase (GAL4-TK)-luciferase reporter construct was also described previously (35). All newly constructed plasmids were characterized by restriction enzyme mapping and DNA sequencing analysis.
Stable transduction.
The amphotropic retroviral packaging cell line ΦNX-Ampho, provided by Garry Nolan (Stanford University, Stanford, Calif.), was cultured in Dulbecco's modified Eagle medium with 10% FBS. Infectious virus was produced by liposome-mediated transfection, and virus-containing supernatants were collected 48 h after transfection and frozen in aliquots at −70°C. For retroviral transduction, 105 exponentially growing MEL cells were mixed with virus particles in the presence of 6 μg of Polybrene per ml. The mixture was centrifuged for 1 h at 10,000 × g at room temperature to facilitate virus infection (4) and returned to culture. After 5 days, GFP-expressing MEL cells were then isolated by fluorescence-activated cell sorting and expanded in culture. MEL cells were transfected with pcDNA-mSin3A or pcDNA3.1 by electroporation as described previously (52), and transductants were selected for drug resistance in 500 μg of G418 per ml.
In vitro binding assays.
Translation-grade [35S]methionine was purchased from Amersham Pharmacia Biotech (Piscataway, N.J.). [35S]methionine-labeled mSin3A and TAL1 proteins were synthesized using a coupled in vitro transcription-translation system in reticulocyte lysates (TnT-coupled reticulocyte lysate system; Promega, Madison, Wis.). GST fusion proteins were expressed in the BL-21 strain of Escherichia coli and purified on glutathione-Sepharose 4B according to the manufacturer's instructions (Amersham Pharmacia Biotech). Equivalent amounts of GST fusion proteins were incubated with [35S]methionine-labeled proteins in a buffer containing 20 mM Tris HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, and 0.1 mg of bovine serum albumin per ml as previously described (52). Bound proteins were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and autoradiography.
Immunoprecipitation and immunoblot analysis.
Extracts were prepared by lysing cells in radioimmunoprecipitation assay (RIPA) buffer containing 10 mM Tris Cl (pH 8.0), 140 mM NaCl, 0.025% NaN3, 0.5% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, and 2 μg of aprotinin per ml. Lysates were incubated at 4°C with protein A-Sepharose 4B (Amersham Pharmacia Biotech), precleared with preimmune immunoglobulin for 30 min to prevent nonspecific binding, and incubated with the indicated antibody for 1.5 to 2 h at 4°C. Immune complexes were recovered with protein A-Sepharose 4B and washed three times with RIPA buffer.
Quantitative immunoprecipitation analysis was carried out with 107 cells. Total cellular extracts were prepared as described above in 1.0 ml of RIPA buffer. Following preclearing with preimmune immunoglobulin, extracts were incubated with 5 μl of Tal1 antibody, and the resulting immune complexes were collected with protein A-Sepharose 4B beads. The beads were collected by centrifugation and washed, and both the mSin3A protein that coimmunoprecipitated with Tal1 and that present in an aliquot of the remaining supernatant were detected by enhanced chemiluminescence after Western transfer as described below. The fraction of total cellular mSin3A protein associated with TAL1 was determined from image analysis (NIH Image software, version 1.5) of the X-ray film.
Proteins and immune complexes were prepared for immunoblot analysis by being boiled for 3 min in Laemmli loading buffer (58), fractionated by SDS-polyacrylamide gel electrophoresis, and electrotransferred to polyvinylidene difluoride membranes. Blots were blocked for 4 to 5 h in a buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 0.05% Tween 20, and 5% nonfat dry milk; incubated with the indicated primary antibodies overnight at 4°C; and incubated with horseradish peroxidase-conjugated secondary antibodies for 3 h at 4°C. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Rabbit antibody to mouse Tal1 was described previously (53). mSin3A (K-20) and β-actin antibodies were purchased from Santa Cruz Biotechnology (San Diego, Calif.) and Sigma Chemical Company (St. Louis, Mo.), respectively. HDAC1 and HDAC2 antibodies were generous gifts of Edward Seto.
HDAC assay.
[3H]acetyllysine-labeled histones were prepared from MEL cells metabolically labeled with sodium [3H]acetate (ICN Pharmaceuticals, Costa Mesa, Calif.) according to the method of Carmen et al. (14). Cellular lysates intended for analysis of HDAC activity were prepared in 100 mM Tris HCl (pH 7.5)–100 mM NaCl–1% NP-40. Immune complexes were formed as described above and collected with protein A-Sepharose 4B beads, and the beads were washed three times with 100 mM Tris HCl (pH 8.0)–150 mM NaCl–1 mM EDTA–10% glycerol. HDAC activity associated with these complexes was assayed in a 200-μl reaction volume containing 100 mM Tris HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 10% glycerol, and 40 μg of radiolabeled histone substrate (1.7 × 106 cpm/μg) at 37°C for 60 min. Reactions were stopped by the addition of 50 μl of 0.72 N hydrochloric acid–0.12 N acetic acid and extracted with 600 μl of ethyl acetate. The radioactivity from [3H]acetate in 500 μl of aqueous phase was determined by liquid scintillation counting. Control reactions lacking immune precipitates or containing immune precipitates coincubated with 50 nM TSA were carried out in parallel.
Northern blot analysis.
Northern blot analysis was performed as previously described (52). Following electrophoresis, mRNA samples were transferred to a Zeta-Probe GT blotting membrane (Bio-Rad Laboratories, Hercules, Calif.). A 1.2-kb murine β-globin genomic fragment provided by Maurice Bondurant (Vanderbilt University, Nashville, Tenn.) and 0.6-kb Tal1 and 1.4-kb glyceraldehyde phosphate dehydrogenase cDNAs were radiolabeled by random primer extension (Life Technologies). Hybridization was carried out at 65°C overnight in 0.25 M sodium phosphate (pH 7.2)–7% SDS. Membranes were washed twice at 65°C in 20 mM sodium phosphate (pH 7.2)–5% SDS and twice in 20 mM sodium phosphate (pH 7.2)–1% SDS before autoradiography or PhosphorImager analysis.
Cytochemical staining.
Cells were cytocentrifuged onto glass slides and stained for hemoglobin with a benzidine reagent as described previously (68). The fraction of hemoglobin-expressing cells was determined from counts of 500 consecutive cells.
RESULTS
TAL1 associates with the transcriptional corepressor mSin3A in vivo and in vitro.
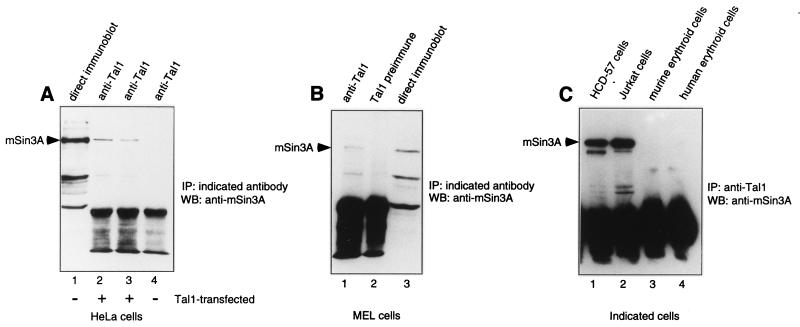
As an initial step in determining whether Tal1 and mSin3A interact, studies were carried out using transfected HeLa cells. Extracts from cells transfected with a Tal1 expression vector or parental vector were subjected to immunoprecipitation with a Tal1 antibody followed by immunoblot analysis with an mSin3A-specific antibody. mSin3A, which was detectable in HeLa cell extracts by direct immunoblot analysis (Fig. 1A), was specifically coimmunoprecipitated with Tal1 in extracts from Tal1-transfected cells, even with high-stringency washes, but not from cells transfected with the empty vector (Fig. 1A). To ascertain whether Tal1 also associates with mSin3A in cells that express both proteins physiologically, MEL cells were metabolically labeled with [35S]methionine and cellular extracts were immunoprecipitated with Tal1- or mSin3A-specific antibodies. A radiolabeled protein with the mobility predicted for mSin3A was immunoprecipitated with either antibody (data not shown), indicating that Tal1 and mSin3A interact in this MEL cell line. To further substantiate this interaction, coimmunoprecipitation analysis was carried out using MEL cellular extracts. Similar to the results obtained with Tal1-transfected cells, mSin3A was detected in immune precipitates obtained with the Tal1 antibody but not with preimmune immunoglobulin (Fig. 1B). These data thus demonstrate that Tal1 and mSin3A, expressed endogenously and following transfection, can interact in cells.
FIG. 1.
Tal1 interacts with mSin3A in Tal1-expressing cells. (A) Cellular lysates were prepared from mock-transfected (lane 4) or Tal1-transfected (lanes 2 and 3) HeLa cells and immunoprecipitated with Tal1 antibody (lanes 2 to 4). Immunoprecipitates were washed under low-stringency conditions with 1% Triton X-100 (lanes 2 and 4) or high-stringency conditions with 0.5% Triton X-100–1% deoxycholate–0.1% SDS (lane 3) and subjected to immunoblot analysis with mSin3A antibody. HeLa cell extracts were directly immunoblotted with mSin3A antibody as a control (lane 1). (B) Cellular lysates were prepared from MEL cells and immunoprecipitated with Tal1 antibody (lane 1) or Tal1 preimmune immunoglobulin (lane 2). Immunoprecipitates were then subjected to immunoblot analysis with mSin3A antibody (lanes 1 and 2). MEL cell extracts were directly immunoblotted with mSin3A antibody as a control (lane 3). (C) Cellular lysates were prepared from the HCD-57 cell line (lane 1), the Jurkat human T-ALL cell line (lane 2), Friend leukemia virus-induced murine erythroblasts induced to differentiate with erythropoietin (lane 3), and normal human blood-derived erythroid cells similarly induced to terminally differentiate in vitro (lane 4) and immunoprecipitated with antibody to mouse (lanes 1 and 3) or human (lanes 2 and 4) TAL1. Immunoprecipitates were then subjected to immunoblot analysis with mSin3A antibody (lanes 1 to 4). IP, immunoprecipitation; WB, Western blot.
To assess the generality of the TAL1-mSin3A interaction, we also carried out coimmunoprecipitation assays on four other hematopoietic cell types representing different cellular lineages or stages of differentiation. Each expressed both TAL1 and mSin3A protein by immunoblot analysis (data not shown). mSin3A coimmunoprecipitated with TAL1 in extracts from the erythropoietin-dependent cell line HCD-57 and the Jurkat T-ALL cell line but not in extracts from Friend leukemia virus-induced splenic erythroblasts cultured with erythropoietin or normal human blood-derived erythroid progenitors similarly induced to terminally differentiate in culture (Fig. 1C). Quantitative immunoprecipitation analysis revealed that 6, 26, and 26% of total cellular mSin3A were complexed with TAL1 in MEL, HCD-57, and Jurkat cells, respectively. These results demonstrate that TAL1 interaction with mSin3A is not restricted to MEL cells and suggest that their association is influenced by the extent of cellular differentiation.
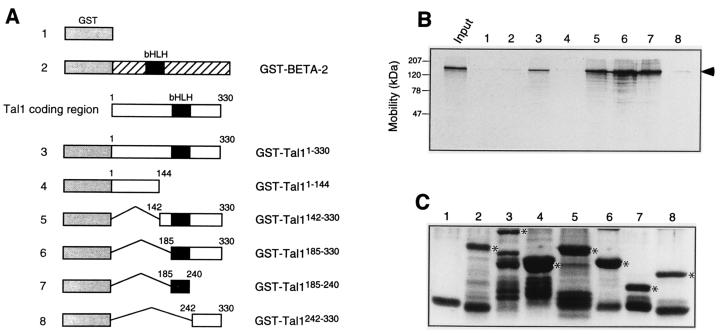
Both to test whether Tal1 and mSin3A could interact in vitro and to define the specific regions of the transcription factor involved, solution interaction assays were carried out using a series of GST-TAL1 fusion proteins (Fig. 2A) and in vitro-translated mSin3A. Radiolabeled mSin3A was found to interact with glutathione-Sepharose beads prebound with GST fusion proteins containing the Tal1 bHLH domain. In contrast, no binding was obtained to beads adsorbed with GST protein alone or with fusion proteins containing the N-terminal 144 amino acids of Tal1, the C-terminal 88 amino acids of Tal1, or the complete coding region of another bHLH transcription factor, BETA-2 (Fig. 2B). Comparable amounts of the GST fusion proteins were used (Fig. 2C), so that the differences in their ability to associate with mSin3A were related to the specific TAL1 protein sequence contained in the fusion rather than their abundance. In sum, the bHLH region of TAL1, comprising its DNA-binding and oligomerization domains, is both necessary and sufficient to mediate its interaction with mSin3A in vitro.
FIG. 2.
Tal1 interacts with mSin3A in vitro. (A and B) [35S]methionine-labeled mSin3A translated in vitro was incubated with the indicated bacterium-expressed GST-Tal1 or GST–BETA-2 fusion proteins (A) or GST that had been preadsorbed to glutathione-Sepharose beads. Specifically bound proteins were eluted from washed beads and visualized by fluorography following SDS-polyacrylamide gel electrophoresis (B). Input represents 10% of in vitro-translated mSin3A protein used for incubation with fusion proteins. Radiolabeled mSin3A is marked with an arrowhead. (C) Coomassie blue-stained SDS-polyacrylamide gel of GST (lane 1) and GST fusion proteins (lanes 2 to 8) used in pull-down experiments. Lanes are numbered as in panels A and B. Full-length fusion proteins are denoted with asterisks.
Tal1 associates with HDAC1 in vitro and in vivo.
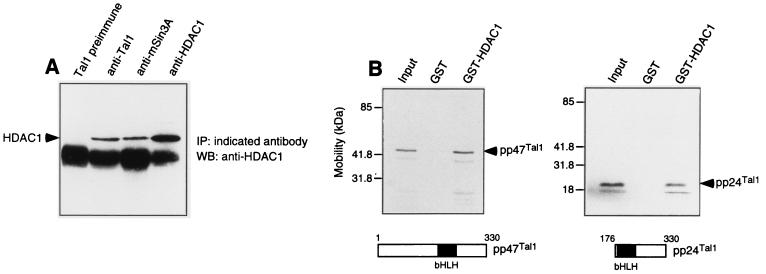
The primary mechanism by which the transcriptional corepressors mSin3A and mSin3B repress transcription is by recruitment of one or more HDACs (44, 45). To determine whether TAL1-associated complexes contain HDACs, MEL cellular extracts were subjected to immunoprecipitation with a Tal1 antibody followed by immunoblot analysis with HDAC-specific antibodies. As predicted, HDAC1 coimmunoprecipitated not only with mSin3A (44) but also with Tal1 (Fig. 3A). Moreover, the amount of HDAC1 protein precipitated with the Tal1 antibody was comparable to that precipitated with the mSin3A antibody (Fig. 3A). In contrast, HDAC2 did not coimmunoprecipitate with Tal1, even though HDAC activity and HDAC2 protein were detected in immune precipitates obtained from these cells with an HDAC2-specific antibody (data not shown). The reciprocal interactions demonstrated between Tal1, mSin3A, and HDAC1 in MEL cells, in addition to the similar kinetics with which these interactions changed with induction of these cells to differentiate (see below), suggest the existence of a single ternary complex containing all three proteins.
FIG. 3.
Tal1 interacts with HDAC1 in vivo and in vitro. (A) MEL cell extracts were immunoprecipitated with Tal1 preimmune immunoglobulin (Tal1 preimmune), Tal1 antibody (anti-Tal1), mSin3A antibody (anti-mSin3A), and HDAC1 antibody (anti-HDAC1) as indicated. Immunoprecipitates were subjected to immunoblot analysis with HDAC1 antibody. HDAC1 protein is marked with an arrowhead. IP, immunoprecipitation; WB, Western blot. (B) [35S]methionine-labeled pp47Tal1 (left) and pp24Tal1 (right) translated in reticulocyte lysates were incubated with a bacterium-expressed GST-HDAC1 fusion protein or GST preadsorbed to glutathione-Sephorose beads. Specifically bound proteins were eluted from washed beads and visualized by fluorography following SDS-polyacrylamide gel electrophoresis. Radiolabeled Tal1 proteins are marked with arrowheads.
To further characterize TAL1-HDAC1 interaction, GST pull-down assays were performed with [35S]methionine-labeled TAL1 proteins and a bacterium-expressed GST-HDAC1 fusion protein. Both TAL1 proteins detected in cells (19), pp47TAL1 and pp24TAL1, interacted with beads adsorbed with GST-HDAC1 but not with GST alone (Fig. 3B). Because of the possibility that another protein present in reticulocyte lysates, mSin3A in particular (32), could have mediated this interaction, it is not proven that TAL1 and HDAC1 contact each other directly. Indeed, the failure of HDAC1 to coimmunoprecipitate with Tal1 in extracts of DMSO-induced MEL cells containing both Tal1 and HDAC1 but not mSin3A (see below) suggests that mSin3A is an obligate intermediary in the Tal1-HDAC1 interaction. In any case, these results show that the two principal TAL1 isoforms can interact, directly or indirectly, with HDAC1 in vivo and in vitro.
Tal1-associated complexes exhibit HDAC activity.
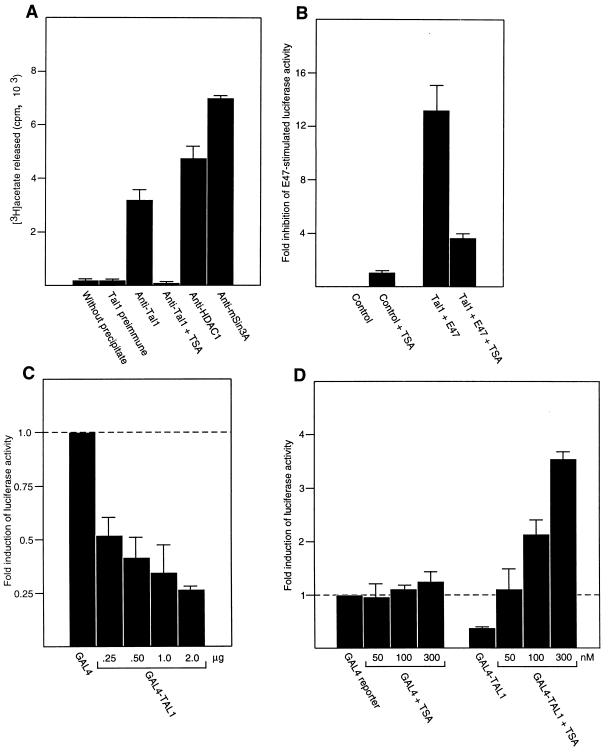
Given that HDAC1 protein coimmunoprecipitated with Tal1 in extracts from erythroleukemia cells, the Tal1-associated corepressor complex would be expected to exhibit HDAC enzymatic activity. To investigate this issue, immune complexes from MEL cellular extracts selected with antibodies to Tal1, mSin3A, and specific HDACs were assayed for deacetylase activity using soluble [3H]acetyllysine-labeled histone as substrate. As expected (44, 45), complexes immunoprecipitated with antibodies to mSin3A or HDAC1 showed activity toward radiolabeled histones (Fig. 4A). Complexes immunoprecipitated with Tal1 antibody, but not those selected with preimmune immunoglobulin, also showed significant HDAC activity, the specificity of which was confirmed by its complete inhibition with TSA (Fig. 4A). These results indicate that TAL1 associates with both immunoreactive and enzymatically active HDAC1 protein in uninduced MEL cells.
FIG. 4.
TSA inhibits Tal1-associated HDAC activity and relieves Tal1-directed transcriptional repression. (A) MEL cell extracts were immunoprecipitated with Tal1 preimmune immunoglobulin (Tal1 preimmune), Tal1 antibody (anti-Tal1), HDAC1 antibody (anti-HDAC1), or mSin3A antibody (anti-mSin3A) as indicated. Immunoprecipitates were incubated with radiolabeled hyperacetylated histone protein as described in Materials and Methods, and the amount of [3H]acetate released was quantitated by liquid scintillation counting. The specificity of the assay was ensured by incubating [3H]acetate-labeled histone without immunoprecipitate (without precipitate) and by adding 50 nM TSA to Tal1-containing immunoprecipitate (anti-Tal1 + TSA). (B) A luciferase reporter linked to a promoter containing six copies of the preferred TAL1/E47 binding sequence (1.0 μg) was cotransfected into C3H10T1/2 cells with E47 (0.5 μg) or Tal1 (2.0 μg) plus E47 (0.5 μg) expression vectors (Tal1 + E47). Transfected cells and control cells transfected with the reporter construct alone (control) were then treated with (+ TSA) or without 300 nM TSA for 24 h. Reporter activity was corrected for variation in transfection efficiency through cotransfection of pSV2-CAT and measurement of CAT activity. The corrected luciferase activity obtained with reporter alone was assigned a value of 1, and the fold inhibition of E47-stimulated reporter activity by Tal1 was calculated. Plotted is mean repression ± standard error. (C) MEL cells were transfected with a luciferase reporter linked to four copies of the GAL4 DNA-binding site and a minimal TK promoter (1.0 μg), an expression vector for the GAL4–full-length TAL1 fusion protein (micrograms indicated) or the GAL4 DNA-binding domain GAL41–147 (micrograms indicated), and plasmid pSV2-CAT (1.0 μg). Transfected cells and control cells transfected with the reporter construct alone were cultured for an additional 48 h and lysed for measurement of luciferase activity. The corrected luciferase activity obtained with GAL41–147 (GAL4) was assigned a value of 1, and the fold induction of reporter activity by the GAL4-TAL1 fusion (GAL4-TAL1) was calculated. Plotted is mean induction ± standard error. (D) MEL cells were transfected with a luciferase reporter linked to four copies of the GAL4 DNA-binding site and a minimal TK promoter (1.0 μg), an expression vector (1.0 μg) for a GAL4–full-length TAL1 fusion protein or the GAL4 DNA-binding domain GAL41–147, and pSV2-CAT. Transfected and control cells were cultured for an additional 24 h with or without TSA at the indicated concentrations and lysed for measurement of luciferase activity. The corrected luciferase activity obtained with the reporter alone was assigned a value of 1, and the fold induction of reporter activity by the GAL4-TAL1 fusion (GAL4-TAL1) was calculated. Plotted is mean induction ± standard error.
Tal1-mediated transcriptional repression is relieved by TSA.
We reasoned that if HDAC1 were required for TAL1-mediated repression, TAL1-mediated inhibition of transcription could be reversed with the specific HDAC inhibitor TSA. As no target genes for this transcription factor have been unequivocally established, a model promoter containing multiple copies of the preferred TAL1-E2A DNA-binding site linked to a luciferase reporter gene was employed in transient-transfection assays. Tal1 strongly inhibited luciferase activity induced by the E2A gene product E47 in transfected C3H10T1/2 cells, consistent with previous results using this promoter (51), while addition of 300 nM TSA to culture relieved this repression by more than 70% (Fig. 4B).
Both to exclude the possibility of TAL1 affecting expression by interfering with E47 binding and to determine whether TAL1 sequences could function in cis to repress transcription, a Tal1 cDNA was fused to the DNA-binding domain of the yeast transcription factor GAL4, and the resultant GAL4-TAL1 fusion was expressed in MEL cells with a luciferase reporter plasmid containing four GAL4 binding sites upstream of a minimal TK promoter. In contrast to the transcriptional activation observed with GAL41–147 fused to the coding sequences of the bHLH protein BETA-2 (data not shown), the GAL4-TAL1 fusion protein repressed reporter activity in a concentration-dependent manner (Fig. 4C), and a GAL4 fusion containing only the bHLH domain of TAL1 inhibited the activity of the TK promoter to a similar extent (data not shown). As TSA's activity in relieving repression mediated by similar GAL4-transcription factor fusions correlated with the ability of these proteins or regions of proteins to interact with HDACs (15, 26, 105), it was of interest to test the effect that this HDAC inhibitor had on the repressive function of the GAL4-TAL1 fusion protein. Similar to the E-box-containing promoter, TSA relieved GAL4-TAL1 repression of a minimal TK promoter, even stimulating reporter activity at higher concentrations, while having little or no effect on transactivation by GAL41–147 (Fig. 4D). The obvious limitations imposed by the lack of knowledge of its target genes notwithstanding, these findings suggest the involvement of an HDAC in TAL1-mediated transcriptional repression and, considered with the results of the GST pull-down and coimmunoprecipitation assays, point to a role for HDAC1 specifically.
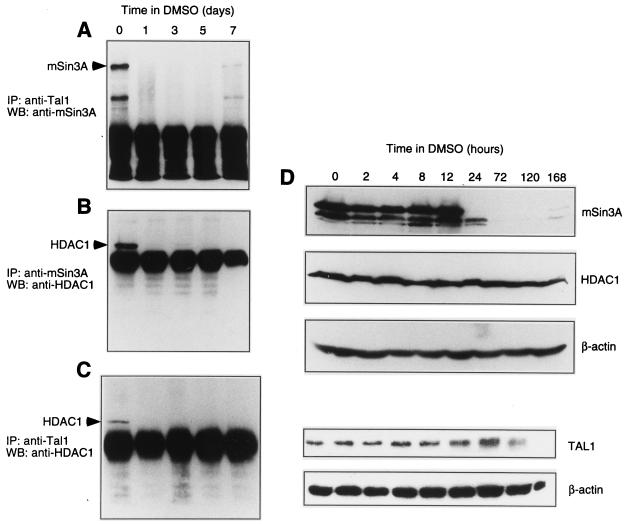
The abundance of the Tal1-associated corepressor complex decreases with DMSO-induced MEL cell differentiation.
Coimmunoprecipitation studies with different erythroid populations (Fig. 1C) suggested that interaction of TAL1 and mSin3A might be affected by the extent of cellular differentiation. To characterize the kinetics of Tal1 interaction with mSin3A and HDAC1 in differentiating MEL cells, cell lysates were prepared at timed intervals following the addition of DMSO to culture, the lysates were subjected to immunoprecipitation with Tal1 or mSin3A antibodies, and the presence of specific proteins in the resulting immune complexes was determined by immunoblot analysis. Tal1- and mSin3A-containing complexes became essentially undetectable 24 h following addition of DMSO (Fig. 5A), coincident with the time period during which these cells become committed to differentiate (reviewed in reference 99). The abundance of mSin3A- and HDAC1-containing (Fig. 5B) and Tal1- and HDAC1-containing (Fig. 5C) complexes also decreased rapidly upon differentiation, paralleling that of the Tal1- and mSin3A-containing complex. Taken together, these data suggest that the concentration of a single Tal1-associated corepressor complex containing both mSin3A and HDAC1 decreases upon DMSO-induced MEL cell differentiation.
FIG. 5.
The abundance of the Tal1-associated corepressor complex declines with DMSO-induced MEL cell differentiation. (A to C) Cellular lysates from MEL cells incubated with 1.8% DMSO for the indicated number of days were subjected to immunoprecipitation (IP) with Tal1 antibody (A and C) or mSin3A antibody (B) as indicated. Immunoprecipitates were then subjected to Western blot (WB) analysis with mSin3A antibody (A) or HDAC1 antibody (B and C). mSin3A and HDAC1 protein were visualized by enhanced chemiluminescence and are marked by arrowheads. (D) Cellular lysates from MEL cells incubated with 1.8% DMSO for the indicated number of hours were fractionated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene membrane, which was incubated sequentially with specific antibodies to mSin3A, HDAC1, and β-actin. Immunoblot analysis was similarly carried out with Tal1 antibody. Antibody binding was visualized by enhanced chemiluminescence.
To determine whether the abundance of this complex could be related to the expression of any of its component proteins, steady-state levels of Tal1, mSin3A, and HDAC1 were examined by immunoblot analysis at timed intervals following addition of DMSO to culture. Tal1 increased by 72 h and then fell at late time points as previously described (40), and HDAC1 was unchanged, while mSin3A levels declined dramatically within 24 h of DMSO induction (Fig. 5D). These results suggest that assembly of the TAL1-associated corepressor complex could be regulated by mSin3A concentration.
Enforced expression of Tal1 and chemical inhibition of HDAC activity synergistically induce MEL cell differentiation.
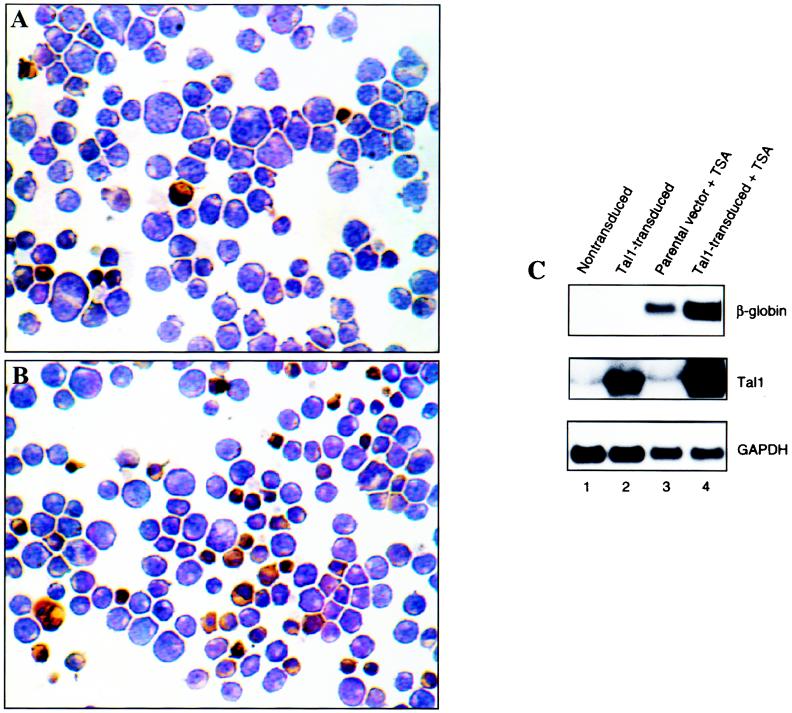
Immunoblot analysis demonstrated that DMSO effected both an increase in Tal1 and a decrease in mSin3A protein expression (Fig. 5D). As TAL1 overexpression had previously been shown to enhance DMSO-induced differentiation of MEL cells (2) and a number of chemical inhibitors of HDAC activity are inducers themselves (86, 110), we predicted that combining Tal1 overexpression with TSA treatment could have a synergistic effect on the differentiation of these cells. To test this possibility, MEL cells were infected with a retroviral vector expressing a full-length Tal1 cDNA and a GFP gene from a bicistronic transcript or the parental GFP-expressing vector alone, and polyclonal populations of transduced cells were selected by fluorescence-activated cell sorting. The cells were then treated with 50 nM TSA for 8 days and assessed for differentiation by histochemical staining for hemoglobin in cytospin preparations and Northern blot analysis of β-globin mRNA expression. From two independently sorted and TSA-treated polyclonal populations, a mean of 12% of cells transduced with the parental virus stained with the benzidine reagent (Fig. 6A). Addition of TSA to the Tal1 transductants, in contrast, resulted in morphological differentiation and histochemical evidence of hemoglobin expression in a mean of 44% of cells (Fig. 6B), with few to no cells showing evidence of differentiation in the absence of TSA (data not shown) (2). Northern blot analysis of β-globin mRNA confirmed the synergy between HDAC inhibition and enforced TAL1 expression (Fig. 6C).
FIG. 6.
TSA cooperates with Tal1 overexpression in inducing MEL cell differentiation. (A and B) Polyclonal populations of MEL cells transduced with a retroviral vector expressing the GFP gene (A) or both a full-length Tal1 cDNA and the GFP gene (B) were selected in a fluorescence-activated cell sorter and incubated for 8 days in the presence of 50 nM TSA. Cells were then cytocentrifuged onto glass slides and stained with a benzidine reagent to detect hemoglobin. Dark brown cells are hemoglobin expressing. Original magnification, ×200. (C) Polyclonal populations of nontransduced MEL cells and cells transduced with a retroviral vector expressing a full-length Tal1 cDNA and GFP gene (Tal1-transduced) or the parental GFP-expressing vector (parental vector) were selected in a fluorescence-activated cell sorter and incubated for 8 days in the presence (+ TSA) or absence of 50 nM TSA. Total cellular RNA was fractionated in a formaldehyde-agarose gel and transferred to a Nytran membrane, which was sequentially hybridized with radiolabeled β-globin, Tal1, and glyceraldehyde phosphate dehydrogenase (GAPDH) probes. mRNAs were detected by autoradiography.
Because of the possibility that TSA could have enhanced expression from the retroviral vector employed (18), Northern blot analysis was also carried out for Tal1 mRNA. TSA treatment did increase Tal1 expression in Tal1 retroviral transductants, but it had no effect on Tal1 expression in nontransduced cells or in cells transduced with the parental GFP virus (Fig. 6C). As Tal1 transductants showed greatly increased Tal1 expression without any evidence of differentiation in the absence of added DMSO or TSA, it is unlikely that the increase in retroviral transcription of Tal1 noted with TSA treatment was responsible in itself for the biological effects observed. In sum, these results suggest that an mSin3A- and HDAC1-containing corepressor complex acts to restrict the ability of TAL1 to induce terminal erythroid differentiation.
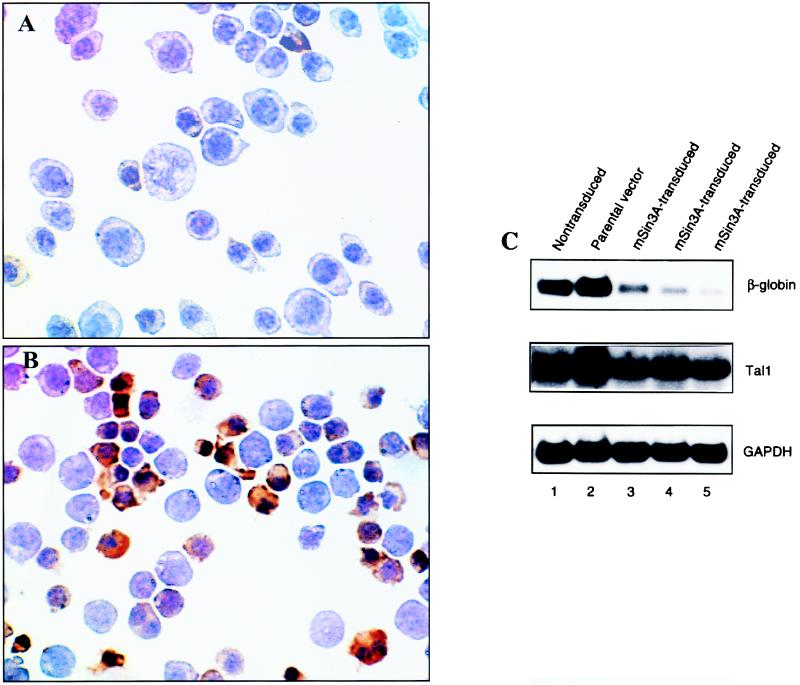
Enforced expression of mSin3A inhibits DMSO-induced MEL cell differentiation.
The dramatic decline in mSin3A protein expression observed after addition of DMSO to MEL cells suggested that this could in fact be required for them to differentiate. To investigate this issue, MEL cells were stably transfected with a plasmid expression vector containing a full-length mSin3A cDNA or with the empty vector, polyclonal populations of transduced cells were selected for G418 resistance, and the extent of DMSO-induced differentiation was determined. Immunoblot analysis showed that mSin3A-transfected cells expressed two- to threefold more mSin3A protein than did either nontransfected cells or cells transduced with the parental vector (data not shown). DMSO-induced differentiation was significantly reduced in mSin3A transductants compared to that in control cells. Specifically, a mean of 23% of mSin3A-transfected cells stained with benzidine after 5 days in culture with DMSO (Fig. 7A) compared to 67% of parental vector controls (Fig. 7B), and the mSin3A-overexpressing cells also appeared much less differentiated morphologically. Finally, in accord with cytochemical staining, β-globin mRNA levels were significantly reduced in mSin3A-transfected cells following DMSO induction compared to those in nontransfected populations and parental vector controls (Fig. 7C). In contrast to its effect on differentiation, enforced mSin3A expression did not alter Tal1 expression significantly (Fig. 7C) or affect cell viability, and mSin3A transductants actually exhibited higher growth rates than those of control cells (data not shown). In aggregate, these results indicate that the decline in mSin3A protein expression is not only associated with but necessary for DMSO-induced MEL cell differentiation.
FIG. 7.
Enforced expression of mSin3A inhibits DMSO-induced MEL cell differentiation. (A and B) Polyclonal populations of MEL cells stably transduced with an expression vector (pcDNA3) containing a full-length mSin3A cDNA (A) or the parental vector (B) were incubated with 1.5% DMSO for 3 days and standard culture medium for an additional 2 days. Cells were then cytocentrifuged onto glass slides and stained with a benzidine reagent to detect hemoglobin. Dark brown cells are hemoglobin expressing. Original magnification, ×400. (C) Polyclonal populations of nontransduced MEL cells and cells stably transduced with a plasmid vector expressing a full-length mSin3A cDNA (mSin3A-transduced) or parental vector were incubated for 5 days in 1.5% DMSO. Total RNA was extracted and subjected to Northern blot analysis with radiolabeled β-globin, Tal1, and glyceraldehyde phosphate dehydrogenase (GAPDH) probes. mRNAs were detected by autoradiography. The last three lanes represent independent determinations from polyclonal populations of mSin3A-transduced cells.
DISCUSSION
We have determined that a corepressor complex interacts with the hematopoietic transcription factor TAL1 and restricts its ability to induce erythroid differentiation. We demonstrate that the corepressor mSin3A and HDAC1 are associated with TAL1 in erythroleukemia and T-ALL cell lines but not in terminally differentiated murine or human erythroid cells. The specific HDAC inhibitor TSA both relieved TAL1-mediated transcriptional repression and acted synergistically with enforced TAL1 expression in inducing MEL cells to differentiate. In contrast, mSin3A and, by inference, HDAC1 inhibited DMSO-induced MEL cell differentiation. These results provide new insights into the regulation of TAL1 transcriptional activity and define a function for mSin3A in a specific biological process.
The reduced transcriptional potency of TAL1-E2A heterodimers has been attributed both to a functional incompatibility of the two proteins' activation domains (79) and to the ability of specific regions of TAL1 to act in cis to repress transcription (48, 79). TAL1 has also been shown to inhibit MyoD-directed transcription by competing for common E protein partners and thereby preventing MyoD binding to DNA, similar to the actions of the Id proteins (38). Our finding that TAL1-directed repression could be relieved by a specific chemical inhibitor of HDACs (Fig. 4) indicates yet another mechanism by which TAL1 can inhibit transcription. The development of thymic lymphomas in E2A-deficient (6) and Id1-overexpressing (54) mice and the induction of apoptosis by overexpression or restoration, respectively, of E2A activity in TAL1-expressing Jurkat cells (78) and E2A-deficient lymphoblasts (34) provide considerable support for inhibition of E2A function being important for TAL1's actions as an oncoprotein.
Although it appears to function under a number of circumstances as a transcriptional repressor, TAL1 can also stimulate transcription (21, 76, 77). In this regard, we recently observed that the coactivator p300 augments TAL1-directed transcription and that both p300 (52) and another histone acetyltransferase, the p300/CBP-associated factor PCAF (unpublished data), coimmunoprecipitate with TAL1 in extracts from DMSO-induced MEL cells. TAL1-E2A heterodimers, in addition, interact with the products of two genes, LMO1 and LMO2, that encode transcriptional coactivators (76, 77) and are themselves activated by chromosomal translocation in T-ALL (100, 102). As the binding preferences of TAL1-E2A heterodimers (50) differ from those of complexes containing TAL1, GATA-1, Ldb-1, and LMO1 or LMO2 (103), specific sequence elements could by dictating the composition of the DNA-binding complexes influence whether TAL1 stimulates or represses transcription. For MEL cells at least, the decline in mSin3A (data herein) and Id (62, 63, 94) expression that accompanies their differentiation would also facilitate TAL1 functioning as a transcriptional activator.
In light of the differentiation-associated changes noted in mSin3A expression, it is of considerable interest that mSin3A (Fig. 2), p300 (52), PCAF (unpublished data), and LMO1 and LMO2 (102) each apparently interact with TAL1 through the bHLH domain. While competition for a common binding site has not yet been established, this observation suggests that TAL1 could function as a molecular switch, repressing transcription when associated with an mSin3A- and HDAC1-containing complex and transactivating these same or other genes upon interaction with the coactivators p300/CBP, PCAF, and/or LMO1-LMO2. In fact, the transforming growth factor β-activated transcription factor Smad2 was shown recently to interact with a p300/CBP-containing coactivator complex and a corepressor complex containing the homeodomain protein TGIF and HDAC1 (106). The nuclear steroid receptors are also regulated by a process in which corepressors and HDACs associate with the receptor in its unliganded state, with ligand binding promoting the dissociation of this corepressor complex and its replacement by a coactivator complex (reviewed in reference 16). Even the prototypic bHLH transcription factor MyoD was shown recently to interact with the nuclear corepressor N-CoR (5) in addition to the coactivators p300/CBP (31, 83, 92, 111) and PCAF (84). This ability of transcription factors to interact with both coactivators and corepressors likely facilitates on-off decision making, which may be especially useful in developmental contexts (reviewed in reference 66).
One of our more interesting findings was that the inducibility of MEL cells for DMSO-stimulated differentiation was affected by the concentration and/or activity of the mSin3A- and HDAC1-containing corepressor complex. The formation of a corepressor complex for the thyroid hormone and retinoic acid receptors was shown, similarly, to be regulated by changes in corepressor, in that case N-CoR, expression (95). Further, a decline in the expression of the Smad corepressor TGIF augmented Smad2-directed transcriptional activity and cellular responsiveness to transforming growth factor β (106), while increased expression of the coactivator PCAF stimulated the activity of interferon regulatory factors 1 and 2 and enhanced the responsiveness of a monocytic cell line to interferons (67). Analogous to mSin3A in MEL cells, the expression of N-CoR, which was found to interact with MyoD through its bHLH domain, decreased with differentiation of C2C12 myoblasts into myotubes, and enforced expression of this corepressor in C3H10T1/2 cells inhibited their MyoD-induced differentiation (5). This work thus highlights what is likely to be a general mechanism by which the sensitivity of cells to extracellular stimuli is regulated—differential association of transcription factors with coregulator proteins.
While our intent in using the widely employed MEL cell model of erythroid differentiation was to elucidate the physiologic importance of TAL1-corepressor interaction, the finding that TAL1 interacts with mSin3A in an erythroleukemia and, especially, a T-ALL cell line has significant implications for its actions in leukemogenesis. A number of other leukemia-associated oncoproteins, including AML1-ETO (37, 65, 104), TEL-AML1 (15, 35), inv(16) fusion protein (64), BCL6/LAZ3 (25, 28, 29), PML-RARα (42, 43, 46, 61), and PLZF-RARα (25, 42, 43, 46, 61), also interact with corepressors and HDACs. In what may be particularly relevant to TAL1, the ability of the normal AML1 protein to activate transcription and to induce differentiation of a myeloid cell line was associated with binding of p300/CBP (55), while interaction of the chimeric AML1-ETO oncoprotein with mSin3A and N-CoR was implicated in transcriptional repression (37, 65, 104) and inhibition of differentiation (37). Further, TSA was shown to relieve transcriptional repression mediated by a number of these proteins (42, 43, 46, 65) and to potentiate or restore retinoid-induced differentiation in cell lines expressing the leukemia-associated retinoic acid receptor fusions (46, 61). Finally, the ability of mSin3A to inhibit differentiation and stimulate proliferation (data herein), two phenotypic hallmarks of the neoplastic state, shows directly that these transcriptional corepressors and associated HDACs can function as oncogenic cofactors. The fact that a diverse group of transcription factors interact with what are likely functionally equivalent corepressor complexes suggests that common pathogenetic mechanisms underlie both myeloid and lymphoid leukemias despite their association with different chromosomal translocations.
ACKNOWLEDGMENTS
We thank Scott Hiebert for many helpful discussions and for reviewing the manuscript; Bart Lutterbach and Rebecca Shattuck-Brandt for their suggestions on the manuscript; Yubin Shi for assistance with expression of GST fusion proteins; Robert Eisenman, Edward Seto, Garry Nolan, Arthur Nienhuis, Richard Baer, Scott Hiebert, Mark Koury, Maurice Bondurant, Roland Stein, and Sanford Krantz for providing reagents; and Victoria Richon for advice on purification of radiolabeled histones and HDAC assays.
This work was supported by National Institutes of Health grant R01 HL49118 (to S.J.B.), a Merit Review Award from the Department of Veterans Affairs (to S.J.B.), and an American Society of Hematology Fellow Scholar Award (to S.H.). Photomicroscopy was carried out in the Vanderbilt University Medical Center Imaging Resource supported by National Institutes of Health grants CA68485 and DK20593.
REFERENCES
- 1.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Aplan P D, Nakahara K, Orkin S H, Kirsch I R. The SCL gene product: a positive regulator of erythroid differentiation. EMBO J. 1992;11:4073–4081. doi: 10.1002/j.1460-2075.1992.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayer D E, Lawrence Q A, Eisenman R N. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 4.Bahnson A B, Dunigan J T, Baysal B E, Mohney T, Atchison R W, Nimgaonkar M T, Ball E D, Barranger J A. Centrifugal enhancement of retroviral mediated gene transfer. J Virol Methods. 1995;54:131–143. doi: 10.1016/0166-0934(95)00035-s. [DOI] [PubMed] [Google Scholar]
- 5.Bailey P, Downes M, Lau P, Harris J, Chen S L, Hamamori Y, Sartorelli A, Muscat G E O. The nuclear receptor corepressor N-Cor regulates differentiation: N-Cor directly interacts with MyoD. Mol Endocrinol. 1999;13:1155–1168. doi: 10.1210/mend.13.7.0305. [DOI] [PubMed] [Google Scholar]
- 6.Bain G, Engel I, Robanus Maandag E C, te Riele H P, Voland J R, Sharp L L, Chun J, Huey B, Pinkel D, Murre C. E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain G, Murre C. The role of E-proteins in B- and T-lymphocyte development. Semin Immunol. 1998;10:143–153. doi: 10.1006/smim.1998.0116. [DOI] [PubMed] [Google Scholar]
- 8.Barr F G. Translocations, cancer and the puzzle of specificity. Nat Genet. 1998;19:121–124. doi: 10.1038/475. [DOI] [PubMed] [Google Scholar]
- 9.Bash R O, Hall S, Timmons C F, Crist W M, Amylon M, Smith R G, Baer R. Does activation of the TAL1 gene occur in a majority of patients with T-cell acute lymphoblastic leukemia? A Pediatric Oncology Group study. Blood. 1995;86:666–676. [PubMed] [Google Scholar]
- 10.Begley C G, Aplan P D, Denning S M, Haynes B F, Waldmann T A, Kirsch I R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci USA. 1989;86:10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blobel G A, Nakajima T, Eckner R, Montminy M, Orkin S H. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc Natl Acad Sci USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 13.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 14.Carmen A A, Rundlett S E, Grunstein M. HDA1 and HDA3 are components of a yeast histone deacetylase (HDA) complex. J Biol Chem. 1996;271:15837–15844. doi: 10.1074/jbc.271.26.15837. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti S R, Nucifora G. The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSin3A. Biochem Biophys Res Commun. 1999;264:871–877. doi: 10.1006/bbrc.1999.1605. [DOI] [PubMed] [Google Scholar]
- 16.Chen J D, Li H. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit Rev Eukaryot Gene Expr. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Cheng J-T, Tsai L-H, Schneider N, Buchanan G, Carroll A, Crist W, Ozanne B, Siciliano M J, Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990;9:415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W Y, Bailey E C, McCune S L, Dong J Y, Townes T M. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci USA. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J T, Hsu H L, Hwang L Y, Baer R. Products of the TAL1 oncogene: basic helix-loop-helix proteins phosphorylated at serine residues. Oncogene. 1993;8:677–683. [PubMed] [Google Scholar]
- 20.Cho H, Orphanides G, Sun X, Yang X J, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen-Kaminsky S, Maouche-Chretien L, Vitelli L, Vinit M A, Blanchard I, Yamamoto M, Peschle C, Romeo P H. Chromatin immunoselection defines a TAL-1 target gene. EMBO J. 1998;17:5151–5160. doi: 10.1093/emboj/17.17.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condorelli G, Vitelli L, Valtieri M, Marta I, Montesoro E, Lulli V, Baer R, Peschle C. Coordinate expression and developmental role of Id2 protein and TAL1/E2A heterodimer in erythroid progenitor differentiation. Blood. 1995;86:164–175. [PubMed] [Google Scholar]
- 23.Coppola J A, Cole M D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986;320:760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- 24.Dambly-Chaudiere C, Vervoort M. The bHLH genes in neural development. Int J Dev Biol. 1998;42:269–273. [PubMed] [Google Scholar]
- 25.David G, Alland L, Hong S H, Wong C W, DePinho R A, Dejean A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene. 1998;16:2549–2556. doi: 10.1038/sj.onc.1202043. [DOI] [PubMed] [Google Scholar]
- 26.De Luca P, Majello B, Lania L. Retinoblastoma protein tethered to promoter DNA represses TBP-mediated transcription. J Cell Biochem. 1998;70:281–287. doi: 10.1002/(sici)1097-4644(19980801)70:2<281::aid-jcb13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.De Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhordain P, Albagli O, Lin R J, Ansieau S, Quief S, Leutz A, Kerckaert J P, Evans R M, Leprince D. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc Natl Acad Sci USA. 1997;94:10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhordain P, Lin R J, Quief S, Lantoine D, Kerckaert J P, Evans R M, Albagli O. The LAZ3(BCL-6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression. Nucleic Acids Res. 1998;26:4645–4651. doi: 10.1093/nar/26.20.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle K, Zhang Y, Baer R, Bina M. Distinguishable patterns of protein-DNA interactions involving complexes of basic helix-loop-helix proteins. J Biol Chem. 1994;269:12099–12105. [PubMed] [Google Scholar]
- 31.Eckner R, Yao T-P, Oldread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 32.Eilers A L, Billin A N, Liu J, Ayer D E. A 13-amino acid amphipathic α-helix is required for the functional interaction between the transcriptional repressor Mad1 and mSin3A. J Biol Chem. 1999;274:32750–32756. doi: 10.1074/jbc.274.46.32750. [DOI] [PubMed] [Google Scholar]
- 33.Elwood N J, Zogos H, Pereira D S, Dick J E, Begley C G. Enhanced megakaryocyte and erythroid development from normal human CD34(+) cells: consequence of enforced expression of SCL. Blood. 1998;91:3756–3765. [PubMed] [Google Scholar]
- 34.Engel I, Murre C. Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc Natl Acad Sci USA. 1999;96:996–1001. doi: 10.1073/pnas.96.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenrick R, Amann J M, Lutterbach B, Wang L, Westendorf J J, Downing J R, Hiebert S W. Both TEL and AML-1 contribute repression domains to the t(12;21) fusion protein. Mol Cell Biol. 1999;19:6566–6574. doi: 10.1128/mcb.19.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finger L R, Kagan J, Christopher G, Kurtzberg J, Hershfield M S, Nowell P C, Croce C M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci USA. 1989;86:5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelmetti V, Zhang J, Fanelli M, Minucci S. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol. 1998;18:7185–7191. doi: 10.1128/mcb.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldfarb A N, Lewandowska K. Inhibition of cellular differentiation by the SCL/tal oncoprotein: transcriptional repression by an Id-like mechanism. Blood. 1995;85:465–471. [PubMed] [Google Scholar]
- 39.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green A R, Lints T, Visvader J, Harvey R, Begley C G. SCL is coexpressed with GATA-1 in hemopoietic cells but is also expressed in developing brain. Oncogene. 1992;7:653–660. [PubMed] [Google Scholar]
- 41.Green A R, Salvaris E, Begley C G. Erythroid expression of the ‘helix-loop-helix’ gene, SCL. Oncogene. 1991;6:475–479. [PubMed] [Google Scholar]
- 42.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara F F, Zamir I, Seiser C, Grignani F, Lazar M A, Minucci S, Pelicci P G. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 43.Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARα underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]
- 44.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 45.Hassig C A, Tong J K, Fleischer T C, Owa T, Grable P G, Ayer D E, Schreiber S L. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc Natl Acad Sci USA. 1998;95:3519–3524. doi: 10.1073/pnas.95.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He L Z, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 47.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann T J, Cole M D. The Tal1/Scl basic helix-loop-helix protein blocks myogenic differentiation and E-box dependent transactivation. Oncogene. 1996;13:617–624. [PubMed] [Google Scholar]
- 49.Hsu H-L, Cheng J-T, Chen Q, Baer R. Enhancer-binding activity of the tal-1 oncoprotein in association with the E47/E12 helix-loop-helix proteins. Mol Cell Biol. 1991;11:3037–3042. doi: 10.1128/mcb.11.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu H-L, Huang L, Tsan J T, Funk W, Wright W E, Hu J-S, Kingston R E, Baer R. Preferred sequences for DNA recognition by the TAL1 helix-loop-helix proteins. Mol Cell Biol. 1994;14:1256–1265. doi: 10.1128/mcb.14.2.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu H-L, Wadman I, Tsan J T, Baer R. Positive and negative transcriptional control by the Tal1 helix-loop-helix protein. Proc Natl Acad Sci USA. 1994;91:5947–5951. doi: 10.1073/pnas.91.13.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang S, Qiu Y, Stein R W, Brandt S J. p300 functions as a transcriptional coactivator for the TAL1/SCL oncoprotein. Oncogene. 1999;18:4958–4967. doi: 10.1038/sj.onc.1202889. [DOI] [PubMed] [Google Scholar]
- 53.Kallianpur A R, Jordan J E, Brandt S J. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83:1200–1208. [PubMed] [Google Scholar]
- 54.Kim D, Peng X-C, Sun X-H. Massive apoptosis in T-cell-deficient Id1 transgenic mice. Mol Cell Biol. 1999;19:8240–8253. doi: 10.1128/mcb.19.12.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitabayashi I, Yokoyama A, Shimizu K, Ohki M. Interaction and functional cooperation of the leukemia-associated factors AML1 and p300 in myeloid cell differentiation. EMBO J. 1998;17:2994–3004. doi: 10.1093/emboj/17.11.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev. 1999;9:40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 57.Kuo M H, Allis C D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 58.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 59.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 60.Leiter J M E, Helliger W, Puschendorf B. Increase in histone acetylation and transitions in histone variants during Friend cell differentiation. Exp Cell Res. 1984;155:222–231. doi: 10.1016/0014-4827(84)90783-3. [DOI] [PubMed] [Google Scholar]
- 61.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 62.Lister J, Forrester W C, Baron M H. Inhibition of an erythroid differentiation switch by the helix-loop-helix protein Id1. J Biol Chem. 1995;270:17939–17946. doi: 10.1074/jbc.270.30.17939. [DOI] [PubMed] [Google Scholar]
- 63.Lister J A, Baron M H. Induction of basic helix-loop-helix protein-containing complexes during erythroid differentiation. Gene Expr. 1998;7:25–38. [PMC free article] [PubMed] [Google Scholar]
- 64.Lutterbach B, Hou Y, Durst K L, Hiebert S W. The inv(16) encodes an acute myeloid leukemia 1 transcription corepressor. Proc Natl Acad Sci USA. 1999;96:12822–12827. doi: 10.1073/pnas.96.22.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutterbach B, Westendorf J J, Linggi B, Patten A, Moniwa M, Davie J R, Huynh K D, Bardwell V J, Lavinsky R M, Rosenfeld M G, Glass C, Seto E, Hiebert S W. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol. 1998;18:7176–7184. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mannervik M, Nibu Y, Zhang H, Levine M. Transcriptional coregulators in development. Science. 1999;284:606–609. doi: 10.1126/science.284.5414.606. [DOI] [PubMed] [Google Scholar]
- 67.Masumi A, Wang I M, Lefebvre B, Yang X J, Nakatani Y, Ozato K. The histone acetylase PCAF is a phorbol-ester-inducible coactivator of the IRF family that confers enhanced interferon responsiveness. Mol Cell Biol. 1999;19:1810–1820. doi: 10.1128/mcb.19.3.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLeod D L, Shreeve M M, Axelrad A A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974;44:517–534. [PubMed] [Google Scholar]
- 69.Mizzen C A, Yang X J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 70.Molkentin J D, Olson E N. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mouthon M-A, Bernard O, Mitjavila M-T, Romeo P-H, Vainchenker W, Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993;81:647–655. [PubMed] [Google Scholar]
- 72.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 73.Nielsen A L, Nørby P L, Pedersen F S, Jørgensen P. E-box sequence and context-dependent TAL1/SCL modulation of basic helix-loop-helix protein-mediated transcriptional activation. J Biol Chem. 1996;271:31463–31469. doi: 10.1074/jbc.271.49.31463. [DOI] [PubMed] [Google Scholar]
- 74.Nordeen S K, Green III P P, Fowlkes D M. Laboratory methods. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987;6:173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- 75.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 76.Ono Y, Fukuhara N, Yoshie O. TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3. Mol Cell Biol. 1998;18:6939–6950. doi: 10.1128/mcb.18.12.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ono Y, Fukuhara N, Yoshie O. Transcriptional activity of TAL1 in T cell acute lymphoblastic leukemia (T-ALL) requires RBTN1 or -2 and induces TALLA1, a highly specific tumor marker of T-ALL. J Biol Chem. 1997;272:4576–4581. doi: 10.1074/jbc.272.7.4576. [DOI] [PubMed] [Google Scholar]
- 78.Park S T, Nolan G P, Sun X-H. Growth inhibition and apoptosis due to restoration of E2A activity in T cell acute lymphoblastic leukemia cells. J Exp Med. 1999;189:501–508. doi: 10.1084/jem.189.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S T, Sun X-H. The Tal1 oncoprotein inhibits E47-mediated transcription. J Biol Chem. 1998;273:7030–7037. doi: 10.1074/jbc.273.12.7030. [DOI] [PubMed] [Google Scholar]
- 80.Persons D A, Allay J A, Allay E R, Smeyne R J, Ashmun R A, Sorrentino B P, Nienhuis A W. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 81.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt F W, Orkin S H. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 82.Pulford K, Lecointe N, Leroy-Viard K, Jones M, Mathieu-Mahul D, Mason D Y. Expression of TAL-1 proteins in human tissues. Blood. 1995;85:675–684. [PubMed] [Google Scholar]
- 83.Puri P L, Avantaggiati M L, Balsano C, Sang N L, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 1997;16:369–383. doi: 10.1093/emboj/16.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puri P L, Sartorelli V, Yang X-J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y J, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 85.Rabbitts T H. Perspective: chromosomal translocations can affect genes controlling gene expression and differentiation—why are these functions targeted? J Pathol. 1999;187:39–42. doi: 10.1002/(SICI)1096-9896(199901)187:1<39::AID-PATH235>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 86.Richon V M, Emiliani S, Verdin E, Webb Y, Breslow R. A class of hybrid polar inducers of transformed cell differentiation inhibit histone deacetylases. Proc Natl Acad Sci USA. 1998;95:3003–3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richon V M, Webb Y, Merger R, Sheppard T, Jursic B, Ngo L, Civoli F, Breslow R, Rifkind R A, Marks P A. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci USA. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robb L, Elwood N J, Elefanty A G, Kontgen F, Li R, Barnett L D, Begley C G. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15:4123–4129. [PMC free article] [PubMed] [Google Scholar]
- 89.Robb L, Lyons I, Li R, Hartley L, Köntgen F, Harvey R P, Metcalf D, Begley C G. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci USA. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sadowski I, Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989;17:7339. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sander M, German M S. The beta cell transcription factors and development of the pancreas. J Mol Med. 1997;75:327–340. doi: 10.1007/s001090050118. [DOI] [PubMed] [Google Scholar]
- 92.Sartorelli V, Huang J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shivdasani R A, Mayer E L, Orkin S H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 94.Shoji W, Yamamoto T, Obinata M. The helix-loop-helix protein Id inhibits differentiation of murine erythroleukemia cells. J Biol Chem. 1994;269:5078–5084. [PubMed] [Google Scholar]
- 95.Soderstrom M, Vo A, Heinzel T, Lavinsky R M, Yang W M, Seto E, Peterson D A, Rosenfeld M G, Glass C K. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- 96.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 97.Spivak J L, Pham T, Isaacs M, Hankins W D. Erythropoietin is both a mitogen and a survival factor. Blood. 1991;77:1228–1233. [PubMed] [Google Scholar]
- 98.Torchia J, Glass C, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 99.Tsiftsoglou A S, Wong W. Molecular and cellular mechanisms of leukemic hemopoietic cell differentiation: an analysis of the Friend system. Anticancer Res. 1985;5:81–99. [PubMed] [Google Scholar]
- 100.Valge-Archer V E, Osada H, Warren A J, Forster A, Li J, Baer R, Rabbitts T H. The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells. Proc Natl Acad Sci USA. 1994;94:8617–8621. doi: 10.1073/pnas.91.18.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Visvader J, Begley C G, Adams J M. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene. 1991;6:187–194. [PubMed] [Google Scholar]
- 102.Wadman I, Li J, Bash R O, Forster A, Osada H, Rabbitts T H, Baer R. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 1994;13:4831–4839. doi: 10.1002/j.1460-2075.1994.tb06809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wadman I A, Hirotaka O, Grutz G G, Agulnick A D, Westphal H, Forster A, Rabbitts T H. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Lbd1/NL1 protein. EMBO J. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Hoshino T, Redner R L, Kajigaya S, Liu J M. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-Cor/mSin3/HDAC1 complex. Proc Natl Acad Sci USA. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong C W, Privalsky M L. Transcriptional repression by the SMRT-mSin3 corepressor: multiple interactions, multiple mechanisms, and a potential role for TFIIB. Mol Cell Biol. 1998;18:5500–5510. doi: 10.1128/mcb.18.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wotton D, Lo R S, Lee S, Massagué J. A Smad transcriptional corepressor. Cell. 1999;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 107.Yang W-M, Inouye C, Zeng Y, Bearss D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 109.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 110.Yoshida M, Nomura S, Beppu T. Effects of trichostatins on differentiation of murine erythroleukemia cells. Cancer Res. 1987;47:3688–3691. [PubMed] [Google Scholar]
- 111.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W, Bieker J J. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci USA. 1998;95:9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]