Abstract
Purpose
Human adenoviruses (HAdVs) have always been suggested as one of the main causes of gastroenteritis in children. However, no comprehensive report on the global epidemiology of these viruses in pediatric gastroenteritis is available.
Methods
A systematic search was conducted to obtain published papers from 2003 to 2023 in three main databases PubMed, Scopus, and Web of Science.
Results
The estimated global pooled prevalence of HAdV infection in children with gastroenteritis was 10% (95% CI: 9-11%), with a growing trend after 2010. The highest prevalence was observed in Africa (20%, 95% CI: 14–26%). The prevalence was higher in inpatients (11%; 95% CI: 8-13%) and patients aged 5 years old and younger (9%; 95% CI: 7-10%). However, no significant difference was observed between male and female patients (P = 0.63). The most prevalent species was found to be the species F (57%; 95% CI: 41-72%). The most common HAdVs observed in children with gastroenteritis were types 40/41, 38, and 2. Analysis of case-control studies showed an association between HAdV and gastroenteritis in children (OR: 2.28, 95% CI; 1.51–3.44).
Conclusion
This study provided valuable insights into the importance of HAdVs in children with gastroenteritis, especially in hospitalized and younger children. The results can be used in future preventive measurements and the development of effective vaccines.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09386-x.
Keywords: Gastroenteritis, Human adenoviruses, Pediatrics, Epidemiology, Children
Introduction
Acute gastroenteritis is a serious threat to health that affects individuals of any age. It is especially serious for the very young, such as newborns and young children [1, 2]. Because of their underdeveloped immunity, children are more susceptible to diarrheal illnesses. Different enteric pathogens, including bacteria, viruses, protozoa, helminths, and fungi, can cause diarrhea. These pathogens are typically transmitted by ingesting contaminated food, water, or things infected with feces [3]. Previous studies have shown that the virus is the most common cause of acute gastroenteritis in individuals younger than 18 years of age [4, 5]. The most common causes of acute gastroenteritis in children are rotavirus, norovirus (NoV), human adenovirus (HAdV), and human astrovirus (HAstV) [6, 7].
HAdV is a member of the Adenoviridae family and the Mastadenovirus genus. HAdV is a non-enveloped, medium-sized virus (70–100 nm) with an icosahedral nucleocapsid that contains a 34–45 kbp double-stranded linear DNA genome [8, 9]. HAdVs have been divided into seven species A to G based on pathogenicity and genetic features, with 115 distinct HAdVs genotypes being identified [10]. Based on the percentage of guanine plus cytosine in their DNA and other biochemical and biophysical criteria which are classified into 7 species (A-G). The word serotype is used to point to types up to 51 while newer types, which were differentiated by novel sequences or recombinant phylogeny in genes coding for major capsid proteins are known as genotype. Species G is composed of one type (type 52) and is extremely rare while other species are found in patients with various diseases including gastroenteritis, conjunctivitis, respiratory infections, and to a lesser extent in intussusception in infants, hemorrhagic cystitis, meningoencephalitis, myocarditis, and hepatitis [11]. HAdV infection, a highly infectious disease, can infect a range of organs, including upper and lower respiratory tracts, gastrointestinal tract, urinary tract, eye, and other systems [11]. . Tissue tropisms vary by species. It has been determined that the primary cause of acute gastroenteritis among the seven species is the HAdV F species, also known as enteric HAdV, which contains the HAdV-F40 and HAdV-F41 genotypes [12–15]. Enteric species F (genotypes F40/41) strains were predicted using a mathematical model to be the third most common agent responsible for mortality in diarrheal children under the age of five, after rotavirus and Shigella [16]. Furthermore, stool samples from patients with acute gastroenteritis have regularly revealed the presence of several other non-enteric HAdV species (HAdV A-E and G species), including HAdV A, B, C, and D [12, 13, 15, 17–20].
While there are many reports from different parts of the world, there is a gap of knowledge in understanding the epidemiology and association of HAdVs and pediatric gastroenteritis. This study aims to fulfill this gap by comprehensively analysis various factors including age group, gender, geographical teacher, clinical setting, diagnostics methods, species, and genotypes in pediatrics gastroenteritis for the first time to provide valuable insights into the current status of HAdVs in children with gastroenteritis.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline served as the foundation for this systematic review and meta-analysis approach [21].
Search strategy
To discover relevant papers, a systematic literature search was undertaken utilizing three electronic databases including PubMed, Scopus, and Web of Science. The literature search was restricted to the period between inception to June 24, 2023. Table S1 provides information about the search terms for each database. We manually searched the reference lists of pertinent articles to find further research that met the eligibility criteria. For data management, the systematic literature search was loaded into EndNote software version X8 (Thomson Reuters, California, USA).
Selection criteria
Studies were considered qualified if they reported: (1) case-control and cross-sectional studies providing data related to the prevalence of enteric and non-enteric HAdVs among children less than 18 years with gastroenteritis published in the English language in peer-reviewed journals; (2) the prevalence of HAdV genome in stool samples and rectal swabs; (3) studies detecting HAdV genome by polymerase chain reaction (PCR)-based methods; (4) studies detecting the prevalence of HAdV among inpatients and outpatients; (5) original articles and short communications with sufficient data. Studies that met any of the following criteria were excluded: (1) the prevalence of HAdV infection among adults patients with gastroenteritis; (2) the prevalence of HAdV infection among children presenting gastroenteritis with underlying conditions such as transplant recipients, HIV, immunocompromised status, and cancers; (3) the incidence of HAdV infection among children with gastroenteritis; (4) samples other than stool such as oral swabs, serum, cerebrospinal fluid, and conjunctival swabs; (5) detection of HAdV by assays othe than PCR-based methods such as antigen detection assays, immunochromatography, Loop-mediated isothermal amplification, next-generation sequencing-based viral metagenomics, microarray, latex agglutination, electronmicroscopy, DNA restriction enzyme analysis, enzyme immunoassay, culture techniques, immunoelectron microscopy, and nucleic acid hybridization; (6) seroprevalence of HAdV antibodies; (7) studies included patients with non-gastroenteritis symptoms such as respiratory symptoms, acute severe hepatitis, and asymptomatic; (8) letters, case series, notes, review articles, case reports, posters, and conference abstracts; (9) articles published in languages other than English.
Data extraction and quality assessment
Two reviewers separately examined the titles and abstracts of all identified papers, and studies that were unrelated to the study topic were eliminated. The reviewers got full texts of the selected papers and further analyzed them, and those that did not meet the inclusion criteria were excluded. Finally, any differences among reviewers were settled by consulting with a third reviewer. Utilizing a modified checklist based on strengthening the reporting of observational studies in epidemiology (STROBE), a quality assessment of the retrieved studies was carried out [22, 23]. The checklist consisted of 12 questions that addressed various methodological approaches. Studies that received a validity score of at least 8 out of a maximum of 12 were considered eligible for the main meta-analysis. One reviewer extracted the data listed below from each eligible article: first author’s last name, year of publication, year of sampling, study location, study design, sample size, age ranges of patients, age groups of patients, the gender of patients, number of HAdV-positive cases, HAdV detection methods, types of patient care, species, and genotype of HAdV. The retrieved data were entered into a pre-designed Excel spreadsheet (Microsoft Corporation, Redmond, Washington, USA).
Statistical analysis
We pooled the HAdV infection in children suffering from gastroenteritis using the metaprop package [24]. We applied the random-effects meta-analysis framework and subgroup analysis was conducted based on region, gender, age, detection method, sampling time, types of patient care, and genotype of HAdV. We also conducted meta-analyses of risk estimates for gastroenteritis and exposure to HAdV, and we reported pooled estimates of odds ratio (OR) and 95% CIs. DerSimonian and Laird method was used to compute the pooled estimate of OR with confidence interval (95% CI) using random models. Statistical heterogeneity between studies was evaluated with Cochran’s Q test and quantified by I2 statistic [25]. We investigated the presence and the effect of publication bias using a combination of the visual inspection of funnel plots that were constructed, plotting the logarithmically transformed ORs against the standard error of the associated log (OR) and Begg’s test and Egger’s test. All statistical tests were two-tailed and the significance level was considered less than 0.05 for all, except heterogeneity test that were set at less than 0.1, and statistical analyses were performed using Stata 14.1 (Stata Corp, College Station, TX, USA).
Results
Literature search
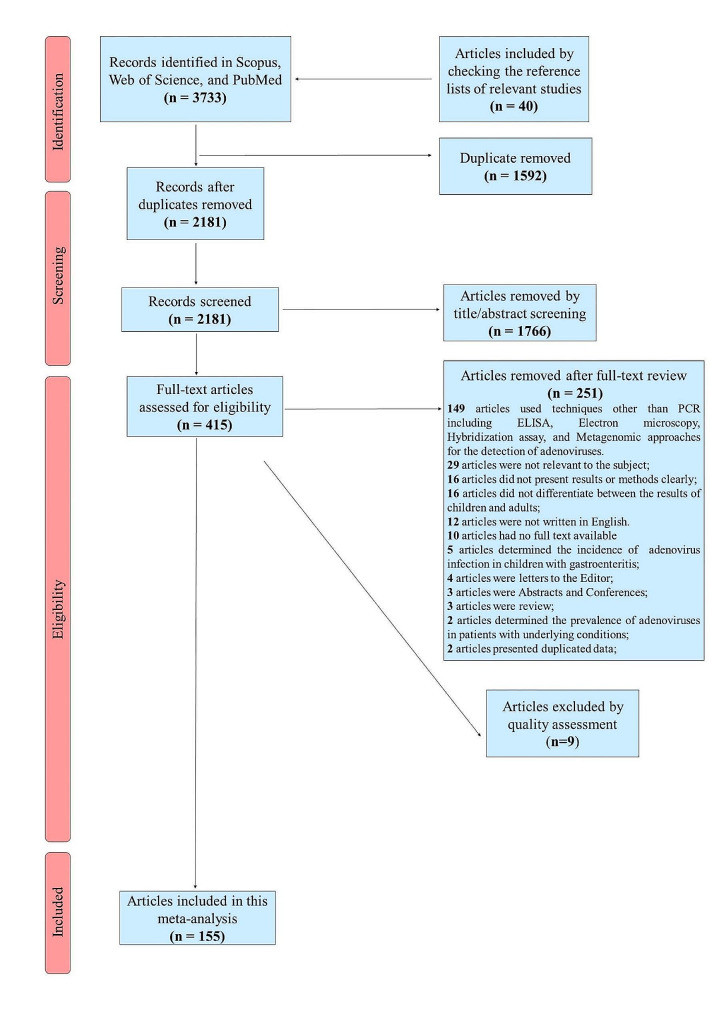
During the initial search, 3733 papers were identified, and 40 further papers were discovered by manually examining the reference lists of pertinent research. A total of 1592 duplicate papers were initially removed, and 1766 additional papers were removed after a manual check of titles and abstracts. After a thorough evaluation of the full text of the remaining 415 papers to determine their eligibility for the meta-analysis, 251 of them were removed. According to the modified STROBE checklist, 155 publications were deemed to be of good quality (scoring of 8 or higher), with 9 papers were failed to get a score of 8. Finally, this systematic review and meta-analysis contained 155 papers. An overview of the selection of relevant studies is depicted in Fig. 1.
Fig. 1.
Flowchart presenting the steps of literature search and selection
Study characteristics
Out of the 155 studies considered, 134 were cross-sectional and 21 were case-control in design. The articles’ publication dates varied from 2003 to 2023. The largest research involved 85,001 gastroenteritis cases [26], while the smallest contained 24 cases [27]. Out of the 155 papers included in this meta-analysis, 19 research examined the gender distribution of HAdV infection, and 80 studies looked into the genotype distribution of HAdVs. Specific primers for the detection of HAdVs group F (types 40 and 41) and universal primers identifying all types of HAdVs have been used in 37 and 118 studies, respectively. The majority of study populations (n = 103,815) were children under 5 years of age and 12,982 were children between the ages of 6 and 18 years old. The majority of studies (n = 29) were conducted in China, followed by Brazil (n = 12), India (n = 11), and Japan (n = 10). Regarding the continent, 84 were conducted in Asia, 26 in South America, 18 in Europe, 16 in North America, 9 in Africa, and 2 in Oceania. The characteristics of included studies in this systematic review and meta-analysis are summarized in Table 1.
Table 1.
Characteristics of studies included in the systematic review and meta-analysis
| Author (Ref) | Publication Year | Location | Study design | Age Range | Number of cases | No. Positive in cases | Number of controls | No. Positive in controls |
|---|---|---|---|---|---|---|---|---|
| Oh [28] | 2003 | Germany | Cross-Sectional | 29 days to 15.5 years | 217 | 31 | ||
| Phan [29] | 2004 | Japan | Cross-Sectional | 2 months to 14 years | 236 | 9 | ||
| Yan [30] | 2004 | China | Cross-Sectional | Under 7 years | 207 | 12 | ||
| Akihara [31] | 2005 | Japan | Case-Control | 1 month to 2 years | 88 | 11 | 833 | 96 |
| Logan [32] | 2006 | Ireland | Cross-Sectional | Under 18 years | 220 | 11 | ||
| Phan [33] | 2006 | Japan | Cross-Sectional | 5 months to 8 years | 125 | 1 | ||
| Reither [34] | 2007 | Ghana | Case-Control | Under 12 years | 243 | 67 | 124 | 39 |
| Chen [35] | 2007 | Taiwan | Cross-Sectional | 3 months to 18 years | 257 | 51 | ||
| Fabiana [36] | 2007 | Italy | Cross-Sectional | 2 months to 12 years | 313 | 29 | ||
| Nguyen [37] | 2007 | Vietnam | Cross-Sectional | 37 days to 9 years | 1010 | 32 | ||
| Shimizu [38] | 2007 | Japan | Cross-Sectional | 3 months to 14 years | 337 | 27 | ||
| Gomara [39] | 2008 | UK | Cross-Sectional | Under 6 years | 685 | 66 | ||
| Jin [40] | 2008 | China | Cross-Sectional | Under 5 years | 1110 | 85 | ||
| Silva [41] | 2008 | Ghana | Cross-Sectional | Under 11 years | 367 | 73 | ||
| Verma [42] | 2008 | India | Cross-Sectional | Under 5 years | 439 | 34 | ||
| Dey [43] | 2009 | Japan | Cross-Sectional | Under 10 years | 628 | 28 | ||
| Dey [44] | 2009 | Bangladesh | Cross-Sectional | 2 months to 3.2 years | 917 | 17 | ||
| Jin [45] | 2009 | China | Cross-Sectional | Under 5 years | 544 | 18 | ||
| Kittigul [46] | 2009 | Thailand | Cross-Sectional | Under 15 years | 131 | 4 | ||
| Li [47] | 2009 | Hong Kong | Cross-Sectional | Under 18 years | 209 | 7 | ||
| Nakanishi [48] | 2009 | Japan | Cross-Sectional | Under 14 years | 877 | 33 | ||
| Podkolzin [49] | 2009 | Russia | Cross-Sectional | Under 14 years | 3208 | 119 | ||
| Sdiri-Loulizi [50] | 2009 | Tunisia | Cross-Sectional | Under 12 years | 788 | 18 | ||
| Cunliffe [51] | 2010 | UK | Cross-Sectional | Under 16 years | 576 | 83 | ||
| Rasanen [52] | 2010 | Finland | Cross-Sectional | Under 15 years | 50 | 5 | ||
| Zhang [53] | 2011 | China | Case-Control | Under 5 years | 201 | 10 | 53 | 5 |
| Khamrin [54] | 2011 | Japan | Cross-Sectional | Under 5 years | 235 | 8 | ||
| Rimoldi [55] | 2011 | Italy | Cross-Sectional | Under 18 years | 273 | 4 | ||
| Braun [27] | 2012 | USA | Case-Control | Under 2 years | 24 | 11 | 78 | 43 |
| Chaimongkol [56] | 2012 | Thailand | Cross-Sectional | Under 5 years | 160 | 3 | ||
| Grant [57] | 2012 | USA | Cross-Sectional | Under 9 months | 247 | 3 | ||
| Lee [58] | 2012 | South Korea | Cross-Sectional | Under 18 years | 2064 | 113 | ||
| Ouyang [59] | 2012 | China | Cross-Sectional | Under 5 years | 766 | 135 | ||
| Rezaei [60] | 2012 | Iran | Cross-Sectional | Under 5 years | 100 | 8 | ||
| Seo [61] | 2012 | South Korea | Cross-Sectional | Under 10 years | 310 | 48 | ||
| Chhabra [62] | 2013 | USA | Case-Control | Under 5 years | 782 | 93 | 499 | 9 |
| Al-Thani [63] | 2013 | Qatar | Cross-Sectional | Under 10 years | 121 | 11 | ||
| Chen [64] | 2013 | China | Cross-Sectional | Under 5 years | 811 | 22 | ||
| Chen [65] | 2013 | Taiwan | Cross-Sectional | Under 18 years | 755 | 69 | ||
| Dey [66] | 2013 | Japan | Cross-Sectional | Under 15 years | 7185 | 565 | ||
| Ren [67] | 2013 | China | Cross-Sectional | Under 5 years | 477 | 30 | ||
| So [68] | 2013 | South Korea | Cross-Sectional | 1 month to 11 years | 186 | 0 | ||
| Zhu [69] | 2013 | China | Cross-Sectional | Under 3 years | 749 | 6 | ||
| Kabayiza [70] | 2014 | Rwanda | Case-Control | Under 5 years | 544 | 216 | 162 | 68 |
| Chhabra [71] | 2014 | Soviet Union | Cross-Sectional | Under 5 years | 495 | 20 | ||
| Kabayiza [72] | 2014 | Rwanda | Cross-Sectional | Under 5 years | 880 | 216 | ||
| Liu [73] | 2014 | China | Cross-Sectional | Under 6 years | 2233 | 219 | ||
| Mitui [74] | 2014 | Turkey and Bangladesh | Cross-Sectional | Under 5 years | 288 | 168 | ||
| Raboni [75] | 2014 | Brazil | Cross-Sectional | Under 5 years | 225 | 45 | ||
| Soli [76] | 2014 | New Guinea | Cross-Sectional | Under 5 years | 199 | 23 | ||
| Amaral [77] | 2015 | Brazil | Cross-Sectional | Under 5 years | 591 | 12 | ||
| Chen [78] | 2015 | Taiwan | Cross-Sectional | Under 5 years | 2810 | 105 | ||
| Khoshdel [79] | 2015 | Iran | Cross-Sectional | Under 5 years | 100 | 22 | ||
| La Rosa [19] | 2015 | Albania | Cross-Sectional | 2 months to 7 years | 142 | 33 | ||
| Lekana-Douki [80] | 2015 | Gabon | Cross-Sectional | Under 5 years | 317 | 62 | ||
| Liu [81] | 2015 | China | Cross-Sectional | Under 5 years | 2171 | 150 | ||
| Lu [82] | 2015 | China | Cross-Sectional | Under 5 years | 436 | 31 | ||
| Mladenova [83] | 2015 | Bulgaria | Cross-Sectional | Under 3 years | 115 | 11 | ||
| Osborne [84] | 2015 | USA | Cross-Sectional | Under 18 years | 941 | 95 | ||
| Patil [85] | 2015 | India | Cross-Sectional | Under 9 years | 950 | 12 | ||
| Thongprachum [86] | 2015 | Japan | Cross-Sectional | Under 15 years | 2381 | 134 | ||
| Yu [87] | 2015 | China | Cross-Sectional | Under 5 years | 18,266 | 879 | ||
| Zhang [88] | 2015 | China | Cross-Sectional | Under 14 years | 1128 | 76 | ||
| Li [89] | 2016 | China | Case-Control | Under 5 years | 461 | 50 | 461 | 12 |
| Ouédraogo [90] | 2016 | Burkina Faso | Case-Control | Under 5 years | 263 | 82 | 50 | 25 |
| Steyer [91] | 2016 | Slovenia | Case-Control | Under 6 years | 297 | 22 | 88 | 0 |
| Brown [92] | 2016 | UK | Cross-Sectional | Under 18 years | 1393 | 146 | ||
| Dashti [93] | 2016 | Iran | Cross-Sectional | Under 5 years | 2682 | 132 | ||
| Jin [94] | 2016 | South Korea | Cross-Sectional | 1 month to 16 years | 345 | 26 | ||
| Liu [95] | 2016 | China | Cross-Sectional | Under 5 years | 3147 | 324 | ||
| Nakamura [96] | 2016 | Japan | Cross-Sectional | Under 15 years | 1796 | 88 | ||
| Reis [97] | 2016 | Brazil | Cross-Sectional | Under 12 years | 377 | 47 | ||
| Shen [98] | 2016 | China | Cross-Sectional | Under 18 years | 137 | 3 | ||
| Colak [99] | 2017 | Turkey | Cross-Sectional | Under 5 years | 180 | 25 | ||
| Cornejo-Tapia [100] | 2017 | Peru | Cross-Sectional | Under 5 years | 117 | 17 | ||
| Costa [101] | 2017 | Brazil | Cross-Sectional | Under 2 years | 172 | 74 | ||
| Hawash [102] | 2017 | Saudi Arabia | Cross-Sectional | Under 18 years | 76 | 5 | ||
| Kim [103] | 2017 | South Korea | Cross-Sectional | Under 16 years | 415 | 56 | ||
| Lu [104] | 2017 | China | Cross-Sectional | Under 5 years | 674 | 32 | ||
| Stockmann [105] | 2017 | USA | Cross-Sectional | Under 18 years | 1089 | 71 | ||
| Zaki [106] | 2017 | Egypt | Cross-Sectional | Under 5 years | 100 | 20 | ||
| Qiu [107] | 2018 | China | Case-Control | Under 18 years | 273 | 79 | 361 | 26 |
| Adam [108] | 2018 | Sudan | Cross-Sectional | Under 5 years | 437 | 7 | ||
| Alcala [109] | 2018 | Venezuela | Cross-Sectional | Under 5 years | 227 | 26 | ||
| Biscaro [110] | 2018 | Italy | Cross-Sectional | 2 months to 15 years | 510 | 35 | ||
| Primo [111] | 2018 | Brazil | Cross-Sectional | Under 10 years | 2009 | 107 | ||
| Yu [112] | 2018 | Taiwan | Cross-Sectional | Under 5 years | 837 | 13 | ||
| Hassan [113] | 2019 | USA | Case-Control | Under 2 years | 330 | 75 | 272 | 44 |
| Iturriza-Gomara [114] | 2019 | Malawi | Case-Control | Under 5 years | 684 | 199 | 527 | 14 |
| Lima [115] | 2019 | Brazil | Case-Control | 2 months to 3 years | 588 | 19 | 573 | 5 |
| Shen [116] | 2019 | China | Case-Control | Under 18 years | 273 | 24 | 361 | 16 |
| Tilmanne [117] | 2019 | Belgium | Case-Control | Under 16 years | 178 | 12 | 165 | 5 |
| Arashkia [118] | 2019 | Iran | Cross-Sectional | Under 5 years | 376 | 16 | ||
| Arowolo [119] | 2019 | Nigeria | Cross-Sectional | Under 5 years | 175 | 9 | ||
| Elmahdy [120] | 2019 | Egypt | Cross-Sectional | Under 5 years | 60 | 17 | ||
| Gaensbauer [121] | 2019 | Guatemala | Cross-Sectional | 6 to 35 months | 316 | 41 | ||
| Gelaw [13] | 2019 | Ethiopia | Cross-Sectional | Under 5 years | 450 | 144 | ||
| Goldar [122] | 2019 | India | Cross-Sectional | 6 months to 5 years | 80 | 27 | ||
| Harb [123] | 2019 | Iraq | Cross-Sectional | Under 5 years | 155 | 53 | ||
| Kumthip [12] | 2019 | Thailand | Cross-Sectional | Under 5 years | 2312 | 165 | ||
| Portal [124] | 2019 | Brazil | Cross-Sectional | Under 9 years | 219 | 110 | ||
| Pratte-Santos [125] | 2019 | Brazil | Cross-Sectional | Under 12 years | 134 | 81 | ||
| Tatte [126] | 2019 | India | Cross-Sectional | Under 5 years | 185 | 5 | ||
| Theamboonlers [127] | 2019 | Thailand | Cross-Sectional | Under 15 years | 442 | 87 | ||
| Farfan-Garcia [128] | 2020 | Colombia | Case-Control | Under 5 years | 431 | 14 | 430 | 1 |
| Pabbaraju [129] | 2020 | Canada | Case-Control | Under 18 years | 3347 | 629 | 1355 | 97 |
| Dey [130] | 2020 | Bangladesh | Cross-Sectional | Under 15 years | 574 | 24 | ||
| Kim [131] | 2020 | South Korea | Cross-Sectional | Under 5 years | 740 | 7 | ||
| Lambisia [132] | 2020 | Kenya | Cross-Sectional | Under 5 years | 984 | 120 | ||
| Mohammadi [133] | 2020 | Iran | Cross-Sectional | Under 5 years | 103 | 3 | ||
| Mousavi Nasab [134] | 2020 | Iran | Cross-Sectional | Under 5 years | 120 | 6 | ||
| Romo-Saenz [135] | 2020 | Mexico | Cross-Sectional | Under 5 years | 57 | 8 | ||
| Sharif [136] | 2020 | Bangladesh | Cross-Sectional | Under 15 years | 387 | 22 | ||
| Zhu [137] | 2020 | China | Cross-Sectional | Under 5 years | 1220 | 37 | ||
| Alsuwaidi [138] | 2021 | UAE | Case-Control | Under 5 years | 203 | 35 | 73 | 2 |
| Harrison [139] | 2021 | USA | Case-Control | Under 11 years | 660 | 51 | 624 | 9 |
| Huang [140] | 2021 | China | Case-Control | Under 5 years | 383 | 21 | 327 | 13 |
| Mero [141] | 2021 | Guinea-Bissau | Case-Control | Under 5 years | 228 | 40 | 201 | 32 |
| Abdel-Rahman [142] | 2021 | Qatar | Cross-Sectional | 3 months and 14 years | 901 | 59 | ||
| Barsoum [143] | 2021 | Ireland | Cross-Sectional | Under 3 years | 150 | 19 | ||
| Chandra [144] | 2021 | India | Cross-Sectional | Under 5 years | 3882 | 351 | ||
| Chang [145] | 2021 | China | Cross-Sectional | Under 18 years | 2692 | 193 | ||
| De Francesco [146] | 2021 | Italy | Cross-Sectional | Under 18 years | 476 | 34 | ||
| Gopalkrishna [147] | 2021 | India | Cross-Sectional | Under 5 years | 308 | 25 | ||
| Huang [14] | 2021 | China | Cross-Sectional | Under 5 years | 656 | 49 | ||
| Lu [148] | 2021 | China | Cross-Sectional | Under 5 years | 804 | 28 | ||
| Ndjangangoye [149] | 2021 | Gabon | Cross Sectional | Under 15 years | 66 | 54 | ||
| Olivares [150] | 2021 | Brazil | Cross-Sectional | Under 5 years | 458 | 139 | ||
| Rossouw [151] | 2021 | South Africa | Cross-Sectional | Under 5 years | 221 | 15 | ||
| Souza [152] | 2021 | Brazil | Cross-Sectional | Under 18 years | 1992 | 166 | ||
| Souza [153] | 2021 | Brazil | Cross-Sectional | Under 14 years | 3419 | 171 | ||
| Wang [26] | 2021 | China | Cross-Sectional | Under 18 years | 85,001 | 2284 | ||
| Abbasi [154] | 2022 | Iran | Cross-Sectional | Under 7 years | 173 | 4 | ||
| Allayeh [155] | 2022 | Egypt | Cross-Sectional | Under 5 years | 447 | 35 | ||
| Al-Nasrawy [156] | 2022 | Iraq | Cross-Sectional | Under 3 years | 450 | 150 | ||
| Colito [157] | 2022 | Cape Verde | Cross-Sectional | Under 12 years | 105 | 7 | ||
| do Nascimento [158] | 2022 | Brazil | Cross-Sectional | Under 18 years | 1012 | 227 | ||
| Dong [159] | 2022 | China | Cross-Sectional | Under 5 years | 897 | 106 | ||
| Gelaw [160] | 2022 | Ethiopia | Cross-Sectional | Under 5 years | 38 | 7 | ||
| Jo [161] | 2022 | South Korea | Cross-Sectional | Under 9 years | 184 | 1 | ||
| Li [162] | 2022 | China | Cross-Sectional | Under 14 years | 160 | 15 | ||
| Mihala [163] | 2022 | Australia | Cross-Sectional | Under 2 years | 11,111 | 2171 | ||
| Mitra [164] | 2022 | India | Cross-Sectional | Under 5 years | 3157 | 276 | ||
| Othma [165] | 2022 | Egypt | Cross-Sectional | Under 5 years | 50 | 3 | ||
| Shams [166] | 2022 | Iran | Cross-Sectional | Under 15 years | 130 | 23 | ||
| Tang [20] | 2022 | China | Cross-Sectional | Under 14 years | 1352 | 60 | ||
| Yılmaz [167] | 2022 | Turkey | Cross-Sectional | Under 18 years | 94 | 13 | ||
| Bhat [168] | 2023 | India | Cross-Sectional | 1 month to 18 years | 109 | 0 | ||
| Borkakoty [169] | 2023 | India | Cross-Sectional | Under 5 years | 407 | 187 | ||
| Eifan [170] | 2023 | Saudi Arabia | Cross-Sectional | Under 18 years | 97 | 6 | ||
| Hugho [3] | 2023 | Tanzania | Cross-Sectional | Under 5 years | 146 | 29 | ||
| Joshi [16] | 2023 | India | Cross-Sectional | Under 5 years | 1167 | 61 | ||
| Lu [171] | 2023 | China | Cross-Sectional | Under 15 years | 1048 | 97 | ||
| Ndjangangoye [172] | 2023 | Gabon | Cross Sectional | Under 15 years | 284 | 75 | ||
| Potgieter [173] | 2023 | South Africa | Cross-Sectional | Under 5 years | 275 | 52 |
Prevalence of HAdV infection among children with gastroenteritis
The estimated global pooled prevalence of HAdV infection among 222,267 gastroenteritis-affected children from 51 countries was 10% (95% CI: 9-11%; I²=98.6%; P < 0.001). By age, children aged 13 to 24 months had a slightly greater prevalence of HAdV (14%; 95% CI: 9-20%) than children of other ages (P = 0.56). The frequency of HAdV infection was similar between males and females (8%; 95% CI: 6-11% vs. 8%; 95% CI: 6-10%, respectively; P = 0.63) (Table 2).
Table 2.
Subgroup analysis of the prevalence of HAdV infection among pediatric patients with gastroenteritis
| Group | Number of studies | Pooled prevalence (%) (95%CI) | Heterogeneity test I2%, p-value |
Differences between subgroups; χ2 test (p-value) |
|
|---|---|---|---|---|---|
| Overall prevalence | - | 155 | 0.10 (0.09–0.11) | 98.63, < 0.001 | |
| Study design | Cross-sectional | 134 | 0.10 (0.08–0.11) | 98.58, < 0.001 | P = 0.01 |
| Case-control | 21 | 0.15 (0.11–0.20) | 97.32, < 0.001 | ||
| Method | Nested PCR | 8 | 0.23 (0.12–0.37) | 98.17, < 0.001 | P < 0.001 |
| Multiplex PCR | 44 | 0.05 (0.04–0.06) | 94.27, < 0.001 | ||
| Real-time PCR | 29 | 0.15 (0.12–0.19) | 98.27, < 0.001 | ||
| Conventional PCR | 61 | 0.09 (0.08–0.11) | 98.09, < 0.001 | ||
| Multiplex Real-time PCR | 13 | 0.17 (0.10–0.26) | 97.74, < 0.001 | ||
| Primer | Universal | 118 | 0.10 (0.09–0.12) | 98.84, < 0.001 | P = 0.60 |
| Group F | 37 | 0.10 (0.08–0.12) | 95.87, < 0.001 | ||
| Sampling time | 1996–2000 | 2 | 0.32 (0.26–0.37) | NA | P < 0.001 |
| 2001–2005 | 16 | 0.08 (0.04–0.12) | 97.74, < 0.001 | ||
| 2006–2010 | 36 | 0.08 (0.06–0.09) | 95.15, < 0.001 | ||
| 2011–2015 | 42 | 0.12 (0.10–0.15) | 98.88, < 0.001 | ||
| 2016–2020 | 51 | 0.11 (0.08–0.13) | 97.21, < 0.001 | ||
| 2021–2022 | 8 | 0.13 (0.06–0.21) | 99.14, < 0.001 | ||
| Continent | South America | 26 | 0.16 (0.10–0.22) | 98.61, < 0.001 | P < 0.001 |
| Asia | 84 | 0.07 (0.06–0.08) | 97.70, < 0.001 | ||
| Europe | 18 | 0.09 (0.07–0.12) | 92.45, < 0.001 | ||
| Africa | 9 | 0.20 (0.14–0.26) | 97.93, < 0.001 | ||
| North America | 16 | 0.12 (0.08–0.18) | 96.96, < 0.001 | ||
| Oceania | 2 | 0.19 (0.19–0.20) | NA | ||
| Gender | Male | 19 | 0.08 (0.06–0.11) | 90.64, < 0.001 | P = 0.63 |
| Female | 19 | 0.08 (0.06–0.10) | 87.95, < 0.001 | ||
| Age (month) | 0–6 | 18 | 0.08 (0.05–0.12) | 93.26, < 0.001 | P = 0.56 |
| 7–12 | 17 | 0.09 (0.06–0.13) | 95.36, < 0.001 | ||
| 13–24 | 19 | 0.14 (0.09–0.20) | 95.31, < 0.001 | ||
| 25–36 | 12 | 0.11 (0.04–0.19) | 90.97, < 0.001 | ||
| 37–48 | 8 | 0.10 (0.02–0.22) | 86.23, < 0.001 | ||
| 49–60 | 8 | 0.06 (0.00-0.16) | 80.80, < 0.001 | ||
| Age (year) | 0–5 | 38 | 0.11 (0.08–0.13) | 98.49, < 0.001 | P < 0.001 |
| 6–18 | 23 | 0.04 (0.02–0.05) | 83.87, < 0.001 | ||
| Patient type | Outpatients | 27 | 0.07 (0.05–0.08) | 95.88, < 0.001 | P = 0.09 |
| Inpatients | 62 | 0.09 (0.07–0.10) | 96.95, < 0.001 | ||
| Species | A | 7 | 0.05 (0.01–0.11) | 71.62, < 0.001 | P < 0.001 |
| B | 6 | 0.05 (0.01–0.11) | 80.03, < 0.001 | ||
| C | 8 | 0.29 (0.16–0.44) | 88.67, < 0.001 | ||
| D | 5 | 0.09 (0.02–0.19) | 88.08, < 0.001 | ||
| E | 2 | 0.05 (0.01–0.12) | NA | ||
| F | 7 | 0.57 (0.41–0.72) | 89.01, < 0.001 |
According to our subgroup analysis, the highest prevalence of HAdV infection was seen in pediatric gastroenteritis patients from Gabon (42%, 95% CI: 16-70%), followed by Iraq (34%, 95% CI: 30-37%), Ethiopia (31%, 95% CI: 27-35%), and Rwanda (30%, 95%CI: 28-33%). Figure 2 depicts the global distribution of HAdV infection among children with gastroenteritis.
Fig. 2.
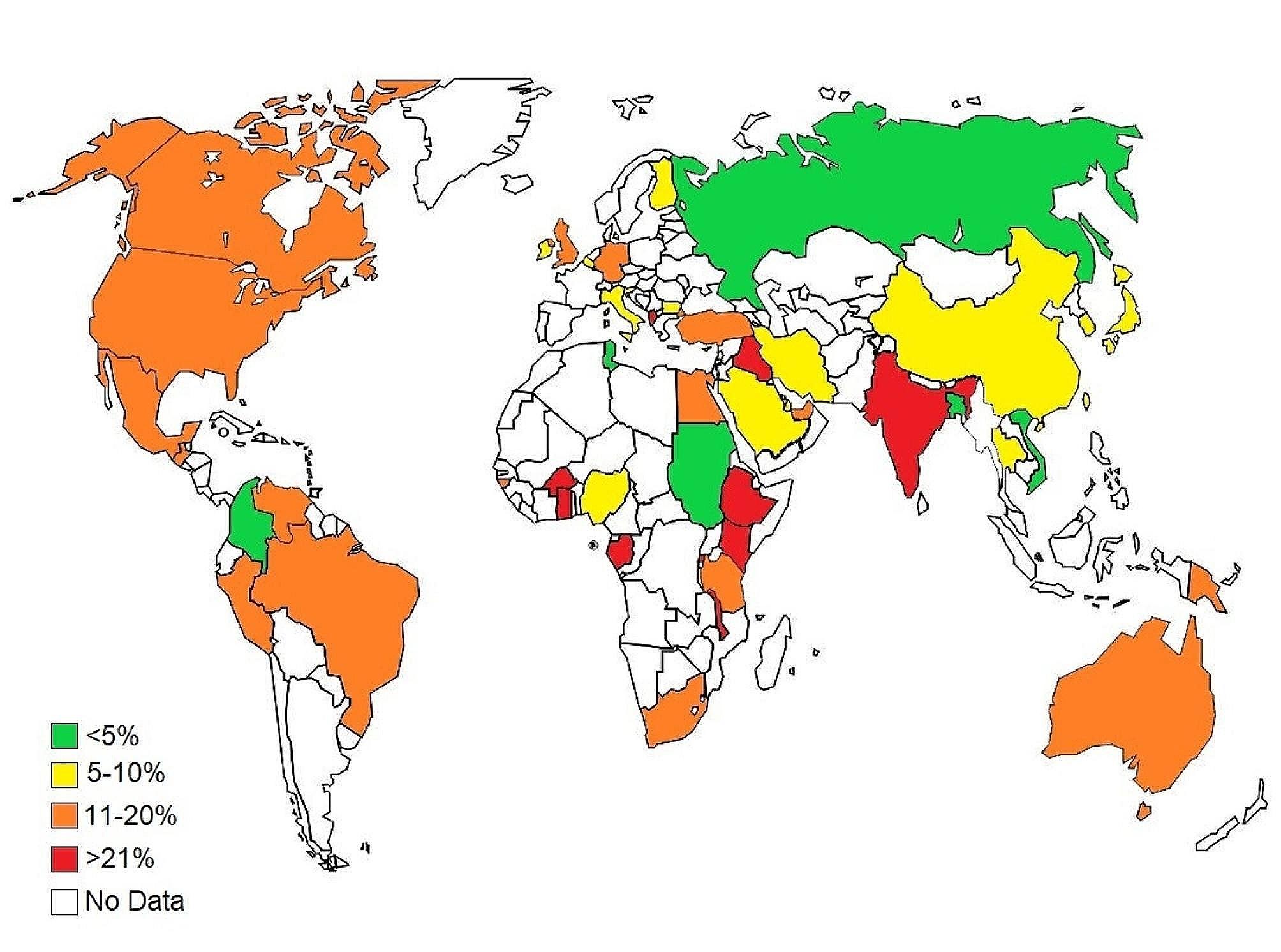
The global map presents the geographical variations in the prevalence of HAdV infection among pediatric patients with gastroenteritis in a period of 11 years (2003–2023)
With respect to HAdV detection methods, Nested PCR, Multiplex PCR, Real-time PCR, Conventional PCR, and Multiplex Real-time PCR methods were used. The prevalence of HAdV was 23% (95% CI: 12–37%), 5% (95% CI: 4–6%), 15% (95% CI: 12–19%), 9% (95% CI: 8–11%), and 17% (95% CI: 10–26%), when Nested PCR, Multiplex PCR, Real-time PCR, Conventional PCR, and Multiplex Real-time PCR methods were used, respectively (P < 0.001). Regarding patient setting, the higher prevalence of HAdV was found in inpatients than in outpatients (9%; 95% CI: 7-10% vs. 7%; 95% CI: 5-8%, respectively); however, the difference was not statistically significant (P = 0.09) (Table 2).
A time trend analysis was conducted to assess variations in the prevalence of HAdV infection over time throughout the world. According to this analysis, the prevalence of HAdV was the highest (32%; 95% CI: 26-37%) between the years of 1996 and 2000. Since 2001 until 2010, the number of HAdV-positive cases among pediatric patients with gastroenteritis was dramatically decreased, so that the prevalence was 8% (95% CI: 4-12%) between the years of 2001 and 2005, and 8% (95% CI: 6-9%) between the years of 2006 and 2010. However, the prevalence of HAdV infection was remarkably increased after the year 2010, reaching a peak of 13% (95% CI: 6-21%) during the years of 2021 and 2022 (P < 0.001) (Table 2).
Regarding the continent, Africa showed a higher prevalence of HAdV in pediatric patients with gastroenteritis (20%, 95% CI: 14–26%) compared to Oceania (19%, 95% CI: 19–20%), the South America (16%, 95% CI: 10–22%), the North America (12%, 95% CI: 8–18%), Europe (9%, 95% CI: 7–12%), and the Asia (7%, 95% CI: 6–8%) (P < 0.001) (Table 2).
Distribution of species and types of HAdVs
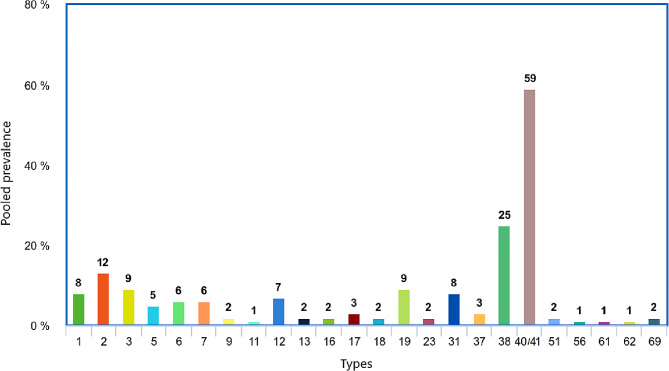
Our results showed that the majority of HAdVs circulating in pediatric patients with gastroenteritis belonged to species F (57%; 95% CI: 41-72%) and species C (29%; 95% CI: 16-44%) (P < 0.001). Overall, twenty-eight types of HAdVs were detected among pediatric patients with gastroenteritis across studies. The most prevalent HAdVs observed in children with gastroenteritis were types 40/41 (59%, 95% CI: 49–68%), 38 (25%, 95% CI: 0–79%), and 2 (12%, 95% CI: 7–17%). Figure 3 shows more details on the frequency of HAdV types in children with gastroenteritis. Types 6 (20%; 95% CI: 12-28%) and 37 (5%; 95% CI: 1-14%) in Africa, types 1 (15%; 95% CI: 0-42%), 2 (29%; 95% CI: 17-43%) and 5 (12%; 95% CI: 3-28%) in Europe, types 3 (13%; 95% CI: 3-18%), 4 (1%; 95% CI: 0-2%), and 18 (4%; 95% CI: 0-10%) in Asia, and types 7 (8%; 95% CI: 2-20%), 12 (17%; 95% CI: 8-30%), 40/41 (66%; 95% CI: 17-100%) in South America were the most prevalent types in each one of the mentioned geographical areas. Africa and South America had equally the highest percentage of type 31 (Africa:12%; 95% CI: 0-33%; South America: 12%; 95% CI: 6-19% ) Analysis of other types in different continents was not possible due to lack or low number of reports.
Fig. 3.
Distribution of HAdV types in children with gastroenteritis
Prevalence of HAdV infection before and after coronavirus disease 2019 (COVID-19)
Our analysis indicated that the prevalence of HAdV among children with gastroenteritis in studies with the sampling time in 2019 and earlier was (10%, 95% CI: 9–11%) while the prevalence in studies with sampling time from 2020 and later was (13%, 95% CI: 6–21%), showing a statistically significant difference (P < 0.001).
Association of HAdV infection with gastroenteritis among children
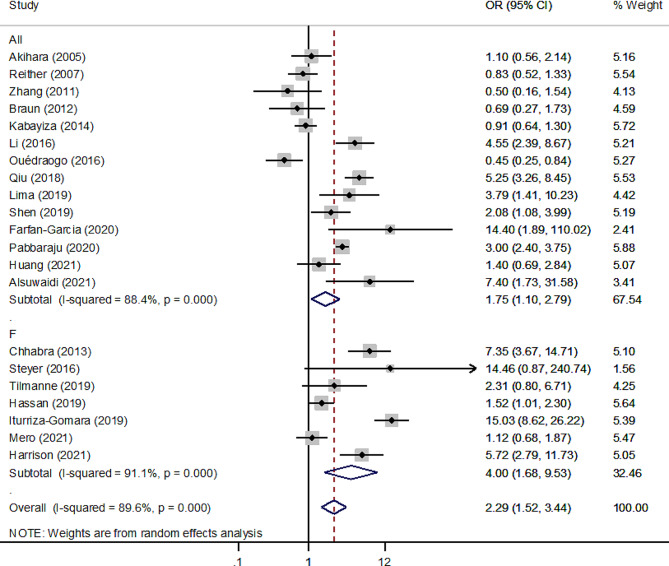
The second analysis used data from case-control studies to look into the relationship between HAdV infection and the risk of gastroenteritis in children. There were 10,482 gastroenteritis patients and 7618 controls in 21 case-control studies. The results showed that the overall pooled odds ratio (OR) of the association of HAdV infection (detected by universal + species F primers) and gastroenteritis was 2.29 (95% CI: 1.52–3.44; I2 = 89.6%) (Fig. 4). The association was much stronger between HAdV species F (detected by species F primers) and gastroenteritis (4.0; 95% CI: 1.68–9.53; I2 = 91.1%) than between all types of HAdV (detected by universal primers) and gastroenteritis (1.75; 95% CI: 1.10–2.79; I2 = 88.4%).
Fig. 4.
Forest plot of the association between HAdV infection and gastroenteritis risk in pediatric patients according to the random effect model in case case-control studies using universal and F primers for the detection of HAdV
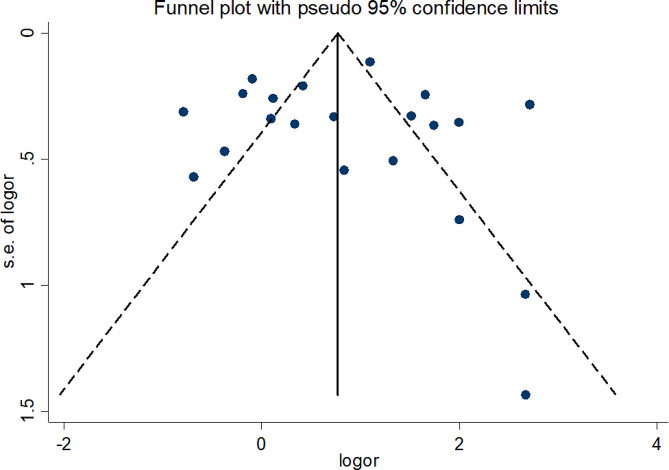
Based on the funnel plot (Fig. 5) there was no evidence of publication bias in the meta-analysis, which was statistically supported by Begg’s test (p = 0.55) and Egger’s test (p = 0.82).
Fig. 5.
Funnel plot for assessment of publication bias
Sensitivity analysis
In a sensitivity analysis by successively removing a particular study at a time to assess the influence of every single study on pooled results, a significant positive association [range of summary ORs 2.14–2.43] between HAdV infection and gastroenteritis among children was observed consistently and did not alter the pooled results, which indicated that the meta-analysis model is robust.
Discussion
Acute gastroenteritis is still a prominent global health threat for children, especially in developing countries. In recent years, the improvements in sanitation have led to a decrease in the prevalence of bacterial and parasitical agents in the development of acute gastroenteritis, making viruses the main causative agent of the disease. While individuals of all ages can be infected by HAdVs, children are the main targets of these viruses. To the best of our knowledge, no systematic review and meta-analysis has been performed on the prevalence of HAdVs and pediatric patients with gastroenteritis. Our results showed a high (10%) prevalence of HAdVs in children with gastroenteritis, which highlights the role of HAdVs as a main cause of gastroenteritis among children worldwide. While rotavirus was known as the main cause of pediatric gastroenteritis, the introduction of rotavirus vaccine is changing the pattern [174, 175]. We exhibited a higher prevalence of HAdVs infection in studies published after 2010, which show the increasing trend of HAdVs in the pathogenesis of pediatric gastroenteritis. Furthermore, the analysis of case-control studies indicated an association (OR: 2.28, 95% CI; 1.51–3.44) between HAdV infection and gastroenteritis in children. Therefore, in addition to respiratory infections [176], HAdVs are important pathogens in gastroenteritis among children.
Among the different regions, the highest prevalence was observed in Africa. Low micronutrition (such as vitamins and trace elements) intake as a result of malnutrition is a key factor that abates the ability of both innate and adaptive immune systems to fight pathogens [177]. Also, poor sanitation and hygiene can be a key contributor that facilitates the infection of the gastrointestinal tract by enteric viruses [178]. Other continents with high prevalence were South America and Oceania. Therefore, our results delineate an epidemiologic pattern with higher prevalence in the southern hemisphere. This can be due to malnutrition and lack of proper hygiene, which make children prone to infectious gastroenteritis by negatively affecting the immune system and exposing children to viral agents [174]. Interestingly, there was a significantly higher prevalence of HAdV in pediatric gastroenteritis cases after the initiation of the COVID-19 pandemic. It might be due to the fact that despite the effects of social distancing and mask mandate on respiratory infections, their effects on preventing viral gastroenteritis were limited and in some regions, schools were less focused on preventing gastroenteritis and therefore, school authorities could not efficiently report gastroenteritis cases, which enables the viruses to be transmitted to other children [179]. Moreover, the rise of malnutrition due to financial restrictions and lack of access to school meals for children [180] is another factor, which makes children more vulnerable to viral infection by weakening the immune system [181].
Our results did not indicate a significant difference (P value = 0.06) between male and female patients. This suggests that the occurrence of HAdVs-related gastroenteritis does not exhibit a gender-based pattern among children. After puberty, different compartments of the immune system are affected by sex hormones. For example, androgens including dihydrotestosterone (DHT) and testosterone can suppress immune cell activities in post-pubertal individuals [182]. However, the impacts of sex hormones are not significant in this study due to the age of the included patients. Studies on various viral infections in children resulted in different results and therefore, a specific sex is not prone to viral infections [183].
Exploring the age-specific prevalence of HAdVs in pediatric gastroenteritis showed intriguing patterns in the distribution of infections across different age groups. Notably, the prevalence of HAdVs was significantly higher (P < 0.001) in children younger than 5 years old, which shows the higher susceptibility of this age group. This difference aligns with the well-established notion that young children are more susceptible to viral infections. This vulnerability is primarily due to the immaturity of their immune systems, which does not provide robust protection against viral pathogens [184]. Additionally, younger children often have limited pre-existing immunity and may lack previous exposure to HAdVs, which makes them more prone to infection [185]. The increased possibility of close contact in daycare settings, preschools, and households may further contribute to a higher risk of transmission in this age group. As children advance to later childhood, their immune systems become more mature [184] and exposure in early life fosters a natural immunity to pathogens [174, 184]. Behavioral changes such as improved hygiene practices may also help the reduction of the risk of diarrhea [186]. These findings highlight the importance of monitoring HAdV transmission and infection in childcare and healthcare settings to avoid future outbreaks.
More than half of typed HAdVs in our study belonged to species F, which consisted of types 40/41 that are known for their role in gastroenteritis [11]. In addition, no significant difference (P = 0.06) was observed between universal primers and those that were designed to only detect species F, which shows the remarkable prevalence of this species in HAdVs-related gastroenteritis cases. Interestingly, we observed that species C, which is known to cause respiratory symptoms [11], is the second most prevalent species in pediatric gastroenteritis cases. This underscores the clinical importance of species C as a causative agent of respiratory and gastrointestinal infections among children. These data are good indicators of the most prevalent species and can be used to design effective vaccines.
In the context of clinical settings, the pooled prevalence for inpatients was found to be slightly higher than the prevalence among outpatients. While this difference did not reach statistical significance (P = 0.09), the trend suggests a potential association between HAdV infections and increased disease severity necessitating hospitalization. Noteworthy, the lack of statistical significance may stem from various factors, including heterogeneity in study populations, variations in healthcare practices, and potential underreporting in outpatient settings. Further research into the specific factors contributing to the observed prevalence differences between inpatients and outpatients can provide valuable insights into the clinical implications of HAdVs-associated gastroenteritis.
This systematic review and meta-analysis faced some limitations. There were no studies from some countries in various geographic regions such as Africa and Europe. We recommend researchers to conduct epidemiologic studies in those countries with no previous reports to gain a comprehensive insight into the HAdV epidemiology in pediatric gastroenteritis. Some reports did not mention the characteristics of isolated viruses including species and genotypes. Finally, in the context of systematic review and meta-analysis studies, publication bias and study heterogeneity are inevitable limitations.
Conclusion
This systematic review and meta-analysis highlights HAdVs as significant and increasing causes of pediatric gastroenteritis globally, particularly affecting children under 5 years old. The prevalence is considerably high in Africa, with also remarkable rates in South America and Oceania, which shows a southern hemisphere predominance possibly linked to factors such as malnutrition and poor sanitation. Furthermore, the absence of a gender-based pattern suggests equal susceptibility among male and female pediatric patients. Variations in diagnostic approaches indicate the importance of choosing sensitive tests such as Nested PCR. The dominance of species F adenoviruses, genotypes 40/41, shows potential targets for vaccine development. A higher prevalence among inpatients can be indicative of the potential of HAdVs to cause severe gastrointestinal symptoms. These results suggest future epidemiologic investigations, particularly in underrepresented regions to address existing gaps in HAdVs epidemiology in pediatric gastroenteritis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
A.T designed and administrated the study. H.S performed all statistical analyses. P.K, M.H.R, and S.G wrote the initial draft. M.H.R and H.S constructed all maps and graphs. A.M, J.S, V.P, and S.S performed intellectual interpretation. P.K, A.T, Z.S, M.H.H, and M.H.R performed search strategy and data extraction. All authors read and approved the final draft.
Funding
This study was not financially supported by any individual, agency, or institution.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Troeger CE, Khalil IA, Blacker BF, Biehl MH, Albertson SB, Zimsen SR, Rao PC, Abate D, Ahmadi A, brahim Ahmed MLC. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the global burden of Disease Study 2017. Lancet Infect Dis. 2020;20(1):37–59. doi: 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugho EA, Kumburu HH, Amani NB, Mseche B, Maro A, Ngowi LE, Kyara Y, Kinabo G, Thomas KM, Houpt ER et al. Enteric Pathogens Detected in Children under Five Years Old Admitted with Diarrhea in Moshi, Kilimanjaro, Tanzania. Pathogens 2023, 12(4). [DOI] [PMC free article] [PubMed]
- 4.Wang L-P, Zhou S-X, Wang X, Lu Q-B, Shi L-S, Ren X, Zhang H-Y, Wang Y-F, Lin S-H, Zhang C-H. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1):2464. doi: 10.1038/s41467-021-22551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kõivumägi K, Geller J, Toompere K, Soeorg H, Kallas E, Jõgeda EL, Huik K, Lutsar I. Norovirus strains among children aged 0–18 years hospitalized with acute gastroenteritis in Estonia 2015–2016. J Med Virol. 2022;94(6):2632–9. doi: 10.1002/jmv.27495. [DOI] [PubMed] [Google Scholar]
- 6.Farahmand M, Khales P, Salavatiha Z, Sabaei M, Hamidzade M, Aminpanah D, Tavakoli A. Worldwide prevalence and genotype distribution of human astrovirus in gastroenteritis patients: a systematic review and meta-analysis. Microb Pathog. 2023;181:106209. doi: 10.1016/j.micpath.2023.106209. [DOI] [PubMed] [Google Scholar]
- 7.Farahmand M, Moghoofei M, Dorost A, Shoja Z, Ghorbani S, Kiani SJ, Khales P, Esteghamati A, Sayyahfar S, Jafarzadeh M, et al. Global prevalence and genotype distribution of norovirus infection in children with gastroenteritis: a meta-analysis on 6 years of research from 2015 to 2020. Rev Med Virol. 2022;32(1):e2237. doi: 10.1002/rmv.2237. [DOI] [PubMed] [Google Scholar]
- 8.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. CLIN MICROBIOL REV. 2014;27(3):441–62. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadhim Jwaziri A, Karbalaie Niya MH, Khales P, Kachooei A, Sabaei M, Rahmani Fard S, Tavakoli A. Molecular prevalence and genotype distribution of human adenovirus in Iranian children with gastroenteritis. Fetal Pediatr Pathol. 2023;42(6):901–13. doi: 10.1080/15513815.2023.2262576. [DOI] [PubMed] [Google Scholar]
- 10.Kurskaya OG, Prokopyeva EA, Dubovitskiy NA, Solomatina MV, Sobolev IA, Derko AA, Nokhova AR, Anoshina AV, Leonova NV, Simkina OA et al. Genetic Diversity of the Human Adenovirus C Isolated from Hospitalized Children in Russia (2019–2022). Viruses 2024, 16(3):386. [DOI] [PMC free article] [PubMed]
- 11.Shieh W-J. Human adenovirus infections in pediatric population-an update on clinico–pathologic correlation. Biomed J. 2022;45(1):38–49. doi: 10.1016/j.bj.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumthip K, Khamrin P, Ushijima H, Maneekarn N. Enteric and non-enteric adenoviruses associated with acute gastroenteritis in pediatric patients in Thailand, 2011 to 2017. PLoS ONE 2019, 14(8). [DOI] [PMC free article] [PubMed]
- 13.Gelaw A, Pietsch C, Liebert UG. Genetic diversity of human adenovirus and human astrovirus in children with acute gastroenteritis in Northwest Ethiopia. Arch Virol. 2019;164(12):2985–93. doi: 10.1007/s00705-019-04421-8. [DOI] [PubMed] [Google Scholar]
- 14.Huang D, Wang Z, Zhang G, Sai L. Molecular and epidemiological characterization of human adenoviruses infection among children with acute diarrhea in Shandong Province, China. Virol J 2021, 18(1). [DOI] [PMC free article] [PubMed]
- 15.Öner SZ, Kaleli̇ İ, Demi̇r M, Mete E, Çalişkan A. Rotavirus and adenovirus prevalence in patients with acute viral gastroenteritis in Denizli, Turkey, 2017–2021. J Med Virol. 2022;94(8):3857–62. doi: 10.1002/jmv.27834. [DOI] [PubMed] [Google Scholar]
- 16.Joshi MS, Sukirti V, Chavan NA, Walimbe AM, Potdar VA, Vipat VC, Lavania M, Gopalkrishna V. Enteric and non-enteric adenoviruses in children with acute gastroenteritis in Western India. Infec Genet Evol 2023, 112. [DOI] [PubMed]
- 17.Wu X, Zhang J, Lan W, Quan L, Ou J, Zhao W, Wu J, Woo P, Seto D, Zhang Q. Molecular typing and rapid identification of human adenoviruses associated with respiratory diseases using universal PCR and sequencing primers for the three major capsid genes: Penton base, hexon, and fiber. Front Microbiol. 2022;13:911694. doi: 10.3389/fmicb.2022.911694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afrad MH, Avzun T, Haque J, Haque W, Hossain ME, Rahman A, Ahmed S, Faruque AG, Rahman MZ, Rahman M. Detection of enteric- and non-enteric adenoviruses in gastroenteritis patients, Bangladesh, 2012–2015. J Med Virol. 2018;90(4):677–84. doi: 10.1002/jmv.25008. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa G, Della Libera S, Petricca S, Iaconelli M, Donia D, Saccucci P, Cenko F, Xhelilaj G, Divizia M. Genetic diversity of human adenovirus in children with acute gastroenteritis, Albania, 2013–2015. BioMed Res Int 2015, 2015. [DOI] [PMC free article] [PubMed]
- 20.Tang X, Hu Y, Zhong X, Xu H. Molecular Epidemiology of Human Adenovirus, Astrovirus, and Sapovirus among Outpatient Children with Acute Diarrhea in Chongqing, China, 2017–2019. Front Pediatr 2022, 10. [DOI] [PMC free article] [PubMed]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eslamipour F, Afshari Z, Najimi A. Prevalence of orthodontic treatment need in permanent dentition of Iranian population: a systematic review and meta-analysis of observational studies. Dent Res J. 2018;15(1):1. doi: 10.4103/1735-3327.223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moosazadeh M, Nekoei-moghadam M, Emrani Z, Amiresmaili M. Prevalence of unwanted pregnancy in Iran: a systematic review and meta‐analysis. Int J Health Plann Manag. 2014;29(3):e277–90. doi: 10.1002/hpm.2184. [DOI] [PubMed] [Google Scholar]
- 24.Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA. Metan: fixed-and random-effects meta-analysis. Stata J. 2008;8(1):3–28. doi: 10.1177/1536867X0800800102. [DOI] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Wang LP, Zhou SX, Wang X, Lu QB, Shi LS, Ren X, Zhang HY, Wang YF, Lin SH, Zhang CH et al. Etiologicpidemiological, and clinical features of acute diarrhea in China. Nat Commun 2021, 12(1). [DOI] [PMC free article] [PubMed]
- 27.Braun LE, Renaud C, Fairchok MP, Kuypers J, Englund JA, Martin ET. Human parechovirus and other enteric viruses in childcare attendees in the era of rotavirus vaccines. J Pediatr Infect Dis Soc. 2012;1(2):136–43. doi: 10.1093/jpids/pis005. [DOI] [PubMed] [Google Scholar]
- 28.Oh DY, Gaedicke G, Schreier E. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J Med Virol. 2003;71(1):82–93. doi: 10.1002/jmv.10449. [DOI] [PubMed] [Google Scholar]
- 29.Phan TG, Nishimura S, Okame M, Nguyen TA, Khamrin P, Okitsu S, Maneekarn N, Ushijima H. Virus diversity and an outbreak of group C rotavirus among infants and children with diarrhea in Maizuru City, Japan during 2002–2003. J Med Virol. 2004;74(1):173–9. doi: 10.1002/jmv.20162. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, Nguyen TA, Phan TG, Okitsu S, Li Y, Ushijima H. Development of RT-multiplex PCR assay for detection of adenovirus and group A and C rotaviruses in diarrheal fecal specimens from children in China. Kansenshogaku Zasshi. 2004;78(8):699–709. doi: 10.11150/kansenshogakuzasshi1970.78.699. [DOI] [PubMed] [Google Scholar]
- 31.Akihara S, Phan TG, Nguyen TA, Hansman G, Okitsu S, Ushijima H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch Virol. 2005;150(10):2061–75. doi: 10.1007/s00705-005-0540-y. [DOI] [PubMed] [Google Scholar]
- 32.Logan C, O’Leary JJ, O’Sullivan N. Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J CLIN MICROBIOL. 2006;44(9):3189–95. doi: 10.1128/JCM.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan TG, Trinh QD, Yagyu F, Sugita K, Okitsu S, Müller WEG, Ushijima H. Outbreak of sapovirus infection among infants and children with acute gastroenteritis in Osaka City, Japan during 2004–2005. J Med Virol. 2006;78(6):839–46. doi: 10.1002/jmv.20632. [DOI] [PubMed] [Google Scholar]
- 34.Reither K, Ignatius R, Weitzel T, Seidu-Korkor A, Anyidoho L, Saad E, Djie-Maletz A, Ziniel P, Amoo-Sakyi F, Danikuu F, et al. Acute childhood diarrhoea in northern Ghana: epidemiological, clinical and microbiological characteristics. BMC Infect Dis. 2007;7:104. doi: 10.1186/1471-2334-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SY, Chang YC, Lee YS, Chao HC, Tsao KC, Lin TY, Ko TY, Tsai CN, Chiu CH. Molecular epidemiology and clinical manifestations of viral gastroenteritis in hospitalized pediatric patients in Northern Taiwan. J CLIN MICROBIOL. 2007;45(6):2054–7. doi: 10.1128/JCM.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabiana A, Donia D, Gabrieli R, Petrinca AR, Cenko F, Bebeci D, Altan AMD, Buonomo E, Divizia M. Influence of enteric viruses on gastroenteritis in Albania: epidemiological and molecular analysis. J Med Virol. 2007;79(12):1844–9. doi: 10.1002/jmv.21001. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TA, Yagyu F, Okame M, Phan TG, Trinh QD, Yan H, Hoang KT, Cao ATH, Le Hoang P, Okitsu S, et al. Diversity of viruses associated with acute gastroenteritis in children hospitalized with diarrhea in Ho Chi Minh City, Vietnam. J Med Virol. 2007;79(5):582–90. doi: 10.1002/jmv.20857. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Phan TG, Nishimura S, Okitsu S, Maneekarn N, Ushijima H. An outbreak of adenovirus serotype 41 infection in infants and children with acute gastroenteritis in Maizuru City, Japan. Infec Genet Evol. 2007;7(2):279–84. doi: 10.1016/j.meegid.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Iturriza Gómara M, Simpson R, Perault AM, Redpath C, Lorgelly P, Joshi D, Mugford M, Hughes CA, Dalrymple J, Desselberger U, et al. Structured surveillance of infantile gastroenteritis in East Anglia, UK: incidence of infection with common viral gastroenteric pathogens. Epidemiol Infect. 2008;136(1):23–33. doi: 10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin M, Xie HP, Duan ZJ, Liu N, Zhang Q, Wu BS, Li HY, Cheng WX, Yang SH, Yu JM, et al. Emergence of the GII4/2006b variant and recombinant noroviruses in China. J Med Virol. 2008;80(11):1997–2004. doi: 10.1002/jmv.21308. [DOI] [PubMed] [Google Scholar]
- 41.Silva PA, Stark K, Mockenhaupt FP, Reither K, Weitzel T, Ignatius R, Saad E, Seidu-Korkor A, Bienzle U, Schreier E. Molecular characterization of enteric viral agents from children in Northern Region of Ghana. J Med Virol. 2008;80(10):1790–8. doi: 10.1002/jmv.21231. [DOI] [PubMed] [Google Scholar]
- 42.Verma H, Chitambar SD, Varanasi G. Identification and characterization of enteric adenoviruses in infants and children hospitalized for acute gastroenteritis. J Med Virol. 2009;81(1):60–4. doi: 10.1002/jmv.21331. [DOI] [PubMed] [Google Scholar]
- 43.Dey SK, Thongprachum A, Ota Y, Phan TG, Nishimura S, Mizuguchi M, Okitsu S, Ushijima H. Molecular and epidemiological trend of rotavirus infection among infants and children in Japan. Infec Genet Evol. 2009;9(5):955–61. doi: 10.1016/j.meegid.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Dey SK, Shimnizu H, Phan TG, Hayakawa Y, Islam A, Salim AFM, Khan AR, Mizuguchi M, Okitsu S, Ushijima H. Molecular epidemiology of adenovirus infection among infants and children with acute gastroenteritis in Dhaka City, Bangladesh. Infect Genet Evol. 2009;9(4):518–22. doi: 10.1016/j.meegid.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Jin Y, Cheng W, Xm Y, Jin M, Zhang Q, Xu Zq Y, Jm, Zhu L, Yang S, Liu N, et al. Viral agents associated with acute gastroenteritis in children hospitalized with diarrhea in Lanzhou, China. J Clin Virol. 2009;44(3):238–41. doi: 10.1016/j.jcv.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Kittigul L, Pombubpa K, Taweekate Y, Yeephoo T, Khamrin P, Ushijima H. Molecular characterization of rotaviruses, noroviruses, sapovirus, and adenoviruses in patients with acute gastroenteritis in Thailand. J Med Virol. 2009;81(2):345–53. doi: 10.1002/jmv.21380. [DOI] [PubMed] [Google Scholar]
- 47.Li CSY, Chan PKS, Tang JW. Prevalence of diarrhea viruses in hospitalized children in Hong Kong in 2008. J Med Virol. 2009;81(11):1903–11. doi: 10.1002/jmv.21611. [DOI] [PubMed] [Google Scholar]
- 48.Nakanishi K, Tsugawa T, Honma S, Nakata S, Tatsumi M, Yoto Y, Tsutsumi H. Detection of enteric viruses in rectal swabs from children with acute gastroenteritis attending the pediatric outpatient clinics in Sapporo, Japan. J Clin Virol. 2009;46(1):94–7. doi: 10.1016/j.jcv.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Podkolzin AT, Fenske EB, Yu Abramycheva N, Shipulin GA, Sagalova OI, Mazepa VN, Ivanova GN, Semena AV, Tagirova ZG, Alekseeva MN, et al. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005–2007. J Infect Dis. 2009;200(SUPPL 1):S228–33. doi: 10.1086/605054. [DOI] [PubMed] [Google Scholar]
- 50.Sdiri-Loulizi K, Gharbi-Khelifi H, De Rougemont A, Hassine M, Chouchane S, Sakly N, Pothier P, Guédiche MN, Aouni M, Ambert-Balay K. Molecular epidemiology of human astrovirus and adenovirus serotypes 40/41 strains related to acute diarrhea in Tunisian children. J Med Virol. 2009;81(11):1895–902. doi: 10.1002/jmv.21586. [DOI] [PubMed] [Google Scholar]
- 51.Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, Kitchin N, Nakagomi O, Nakagomi T, Hart CA, Regan M. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010;16(1):55–62. doi: 10.3201/eid1601.090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Räsänen S, Lappalainen S, Kaikkonen S, Hämäläinen M, Salminen M, Vesikari T. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. EPIDEMIOL INFECT. 2010;138(9):1227–34. doi: 10.1017/S0950268809991671. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Chen TH, Wang J, Dong C, Pan J, Moe C, Chen W, Yang L, Wang X, Tang H, et al. Symptomatic and asymptomatic infections of rotavirus, norovirus, and adenovirus among hospitalized children in Xi’an, China. J Med Virol. 2011;83(8):1476–84. doi: 10.1002/jmv.22108. [DOI] [PubMed] [Google Scholar]
- 54.Khamrin P, Okame M, Thongprachum A, Nantachit N, Nishimura S, Okitsu S, Maneekarn N, Ushijima H. A single-tube multiplex PCR for rapid detection in feces of 10 viruses causing diarrhea. J Virol Methods. 2011;173(2):390–3. doi: 10.1016/j.jviromet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Rimoldi SG, Stefani F, Pagani C, Chenal LL, Zanchetta N, Di Bartolo I, Lombardi A, Ruggeri FM, Di Lillo D, Zuccotti GV, et al. Epidemiological and clinical characteristics of pediatric gastroenteritis associated with new viral agents. Arch Virol. 2011;156(9):1583–9. doi: 10.1007/s00705-011-1037-5. [DOI] [PubMed] [Google Scholar]
- 56.Chaimongkol N, Khamrin P, Suantai B, Saikhreang W, Thongprachum A, Malasao R, Ukarapol N, Kongsricharoern T, Ushijima H, Maneekarn N. A wide variety of diarrhea viruses circulating in pediatric patients in Thailand. Clin Lab. 2012;58(1–2):117–23. [PubMed] [Google Scholar]
- 57.Grant L, Vinjé J, Parashar U, Watt J, Reid R, Weatherholtz R, Santosham M, Gentsch J, O’Brien K. Epidemiologic and clinical features of other enteric viruses associated with acute gastroenteritis in American Indian infants. J Pediatr. 2012;161(1):110–e115111. doi: 10.1016/j.jpeds.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 58.Lee JI, Lee GC, Chung JY, Han TH, Lee YK, Kim MS, Lee CH. Detection and molecular characterization of adenoviruses in Korean children hospitalized with acute gastroenteritis. MICROBIOL IMMUNOL. 2012;56(8):523–8. doi: 10.1111/j.1348-0421.2012.00469.x. [DOI] [PubMed] [Google Scholar]
- 59.Ouyang Y, Ma H, Jin M, Wang X, Wang J, Xu L, Lin S, Shen Z, Chen Z, Qiu Z, et al. Etiology and epidemiology of viral diarrhea in children under the age of five hospitalized in Tianjin, China. Arch Virol. 2012;157(5):881–7. doi: 10.1007/s00705-012-1235-9. [DOI] [PubMed] [Google Scholar]
- 60.Rezaei M, Sohrabi A, Edalat R, Siadat SD, Gomari H, Rezaei M, Gilani SM. Molecular epidemiology of acute gastroenteritis czaused by subgenus F (40, 41) enteric adenoviruses in inpatient children. Lab Med. 2012;43(1):10–5. doi: 10.1309/LMJG3UEBIBWBJPH4. [DOI] [Google Scholar]
- 61.Seo SY, Jung IA, Kim JH, Cho KS, Bin JH, Kim HH, Lee HJ, Lee W. Prevalence of viruses with diarrhea among hospitalized children west Gyeonggi Province. Korean J Pediatr Infect Dis. 2012;19(1):28–36. doi: 10.14776/kjpid.2012.19.1.28. [DOI] [Google Scholar]
- 62.Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, Wikswo M, Nix WA, Lu XY, Parashar UD, et al. Etiology of viral gastroenteritis in children < 5 years of age in the United States, 2008–2009. J Infect Dis. 2013;208(5):790–800. doi: 10.1093/infdis/jit254. [DOI] [PubMed] [Google Scholar]
- 63.Al-Thani A, Baris M, Al-Lawati N, Al-Dhahry S. Characterising the aetiology of severe acute gastroenteritis among patients visiting a hospital in Qatar using real-time polymerase chain reaction. BMC Infect Dis 2013, 13(1). [DOI] [PMC free article] [PubMed]
- 64.Chen Y, Li Z, Han D, Cui D, Chen X, Zheng S, Yu F, Liu J, Lai S, Yan Y, et al. Viral agents associated with acute diarrhea among outpatient children in southeastern China. PEDIATR RES. 2013;32(7):e285–90. doi: 10.1097/INF.0b013e31828c3de4. [DOI] [PubMed] [Google Scholar]
- 65.Chen SY, Tsai CN, Chen CL, Chao HC, Lee YS, Lai MW, Chen CC, Huang WL, Chiu CH. Severe viral gastroenteritis in children after suboptimal rotavirus immunization in Taiwan. Pediatr Infect Dis J. 2013;32(12):1335–9. doi: 10.1097/INF.0b013e3182a5f5b6. [DOI] [PubMed] [Google Scholar]
- 66.Dey SK, Hoq I, Okitsu S, Hayakawa S, Ushijima H. Prevalence, seasonality, and peak age of infection of enteric adenoviruses in Japan, 1995–2009. EPIDEMIOL INFECT. 2013;141(5):958–60. doi: 10.1017/S0950268812001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren ZZ, Kong YM, Wang J, Wang QQ, Huang AL, Xu HM. Etiological study of enteric viruses and the genetic diversity of norovirus, sapovirus, adenovirus, and astrovirus in children with diarrhea in Chongqing, China. BMC Infect Dis 2013, 13. [DOI] [PMC free article] [PubMed]
- 68.So CW, Sup Kim D, Taek Yu S, Cho JH, Duck Kim J. Acute viral gastroenteritis in children hospitalized in Iksan, Korea during December 2010-june 2011. Korean J Pediatr. 2013;56(9):383–8. doi: 10.3345/kjp.2013.56.9.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu M, Cui S, Lin L, Xu B, Zhao J, Xia S, Deng W, Xie Z, Zhang J, Wang Z, et al. Analysis of the aetiology of diarrhoea in outpatients in 2007, Henan province, China. EPIDEMIOL INFECT. 2013;141(3):540–8. doi: 10.1017/S0950268812000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabayiza JC, Andersson ME, Nilsson S, Bergström T, Muhirwa G, Lindh M. Real-time PCR identification of agents causing diarrhea in Rwandan children less than 5 years of age. Pediatr Infect Dis J. 2014;33(10):1037–42. doi: 10.1097/INF.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 71.Chhabra P, Samoilovich E, Yermalovich M, Chernyshova L, Gheorghita S, Cojocaru R, Shugayev N, Sahakyan G, Lashkarashvili M, Chubinidze M, et al. Viral gastroenteritis in Rotavirus negative hospitalized children < 5 years of age from the independent states of the former Soviet Union. Infec Genet Evol. 2014;28:283–8. doi: 10.1016/j.meegid.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Kabayiza JC, Andersson ME, Nilsson S, Baribwira C, Muhirwa G, Bergström T, Lindh M. Diarrhoeagenic microbes by real-time PCR in Rwandan children under 5 years of age with acute gastroenteritis. Clin Microbiol Infect. 2014;20(12):O1128–35. doi: 10.1111/1469-0691.12698. [DOI] [PubMed] [Google Scholar]
- 73.Liu L, Qian Y, Zhang Y, Deng J, Jia L, Dong H. Adenoviruses associated with acute diarrhea in children in Beijing, China. PLoS ONE 2014, 9(2). [DOI] [PMC free article] [PubMed]
- 74.Mitui MT, Bozdayi G, Ahmed S, Matsumoto T, Nishizono A, Ahmed K. Detection and molecular characterization of diarrhea causing viruses in single and mixed infections in children: a comparative study between Bangladesh and Turkey. J Med Virol. 2014;86(7):1159–68. doi: 10.1002/jmv.23744. [DOI] [PubMed] [Google Scholar]
- 75.Raboni SM, Damasio GAC, Ferreira CEO, Pereira LA, Nogueira MB, Vidal LR, Cruz CR, Almeida SM. Acute gastroenteritis and enteric viruses in hospitalised children in southern Brazil: Aetiology, seasonality and clinical outcomes. Mem Inst Oswaldo Cruz. 2014;109(4):428–35. doi: 10.1590/0074-0276140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soli KW, Maure T, Kas MP, Bande G, Bebes S, Luang-Suarkia D, Siba PM, Morita A, Umezaki M, Greenhill AR, et al. Detection of enteric viral and bacterial pathogens associated with paediatric diarrhoea in Goroka, Papua New Guinea. Int J Infect Dis. 2014;27:54–8. doi: 10.1016/j.ijid.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 77.Amaral MSC, Estevam GK, Penatti M, Lafontaine R, Lima ICG, Spada PKP, Gabbay YB, Matos NB. The prevalence of norovirus, astrovirus and adenovirus infections among hospitalised children with acute gastroenteritis in Porto Velho, state of Rondonia, western Brazilian Amazon. Mem Inst Oswaldo Cruz. 2015;110(2):215–21. doi: 10.1590/0074-02760140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CJ, Wu FT, Huang YC, Chang WC, Wu HS, Wu CY, Lin JS, Huang FC, Hsiung CA. Clinical and epidemiologic features of severe viral gastroenteritis in children: a 3-year surveillance, multicentered study in Taiwan with partial rotavirus immunization. Medicine. 2015;94(33):e1372. doi: 10.1097/MD.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khoshdel A, Parvin N, Doosti A, Famouri F. Prevalence of nosocomial diarrhea due to adenoviruses 40 and 41 in a paediatric ward in Iran. J Clin Diagn Res. 2015;9(12):SC15–7. doi: 10.7860/JCDR/2015/15353.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lekana-Douki SE, Kombila-Koumavor C, Nkoghe D, Drosten C, Drexler JF, Leroy EM. Molecular epidemiology of enteric viruses and genotyping of rotavirus A, adenovirus and astrovirus among children under 5 years old in Gabon. Int J Infect Dis. 2015;34:90–5. doi: 10.1016/j.ijid.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Liu XN, Meng L, Li JS, Liu XF, Bai YN, Yu DS, Ren XW, Liu HX, Shen XP, Wang P, et al. Etiological epidemiology of viral diarrhea on the basis of sentinel surveillance in children younger than 5 years in Gansu, northwest China, 2009–2013. J Med Virol. 2015;87(12):2048–53. doi: 10.1002/jmv.24283. [DOI] [PubMed] [Google Scholar]
- 82.Lu L, Jia R, Zhong H, Xu M, Su L, Cao L, Dong Z, Dong N, Xu J. Molecular characterization and multiple infections of rotavirus, norovirus, sapovirus, astrovirus and adenovirus in outpatients with sporadic gastroenteritis in Shanghai, China, 2010–2011. Arch Virol. 2015;160(5):1229–38. doi: 10.1007/s00705-015-2387-1. [DOI] [PubMed] [Google Scholar]
- 83.Mladenova Z, Steyer A, Steyer AF, Ganesh B, Petrov P, Tchervenjakova T, Iturriza-Gomara M. Aetiology of acute paediatric gastroenteritis in Bulgaria during summer months: prevalence of viral infections. J Med Microbiol. 2015;64(3):272–82. doi: 10.1099/jmm.0.000018. [DOI] [PubMed] [Google Scholar]
- 84.Osborne CM, Montano AC, Robinson CC, Schultz-Cherry S, Dominguez SR. Viral gastroenteritis in children in Colorado 2006–2009. J Med Virol. 2015;87(6):931–9. doi: 10.1002/jmv.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patil PR, Chitambar SD, Gopalkrishna V. Molecular surveillance of non-polio enterovirus infections in patients with acute gastroenteritis in Western India: 2004–2009. J Med Virol. 2015;87(1):154–61. doi: 10.1002/jmv.23992. [DOI] [PubMed] [Google Scholar]
- 86.Thongprachum A, Takanashi S, Kalesaran AFC, Okitsu S, Mizuguchi M, Hayakawa S, Ushijima H. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol. 2015;87(7):1141–8. doi: 10.1002/jmv.24155. [DOI] [PubMed] [Google Scholar]
- 87.Yu J, Jing H, Lai S, Xu W, Li M, Wu J, Liu W, Yuan Z, Chen Y, Zhao S, et al. Etiology of diarrhea among children under the age five in China: results from a five-year surveillance. J Infect. 2015;71(1):19–27. doi: 10.1016/j.jinf.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang DM, Ma MM, Wen WT, Zhu X, Xu L, He ZJ, He X, Wu JH, Hu YW, Zheng Y, et al. Clinical epidemiology and molecular profiling of human bocavirus in faecal samples from children with diarrhoea in Guangzhou, China. EPIDEMIOL INFECT. 2015;143(11):2315–29. doi: 10.1017/S0950268814003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li LL, Liu N, Humphries EM, Yu JM, Li S, Lindsay BR, Stine OC, Duan ZJ. Aetiology of diarrhoeal disease and evaluation of viral-bacterial coinfection in children under 5 years old in China: A matched case-control study. Clin Microbiol Infect 2016, 22(4):381.e389-381.e316. [DOI] [PMC free article] [PubMed]
- 90.Ouédraogo N, Kaplon J, Bonkoungou IJO, Traoré AS, Pothier P, Barro N, Ambert-Balay K. Prevalence and genetic diversity of enteric viruses in children with diarrhea in Ouagadougou, Burkina Faso. PLoS ONE 2016, 11(4). [DOI] [PMC free article] [PubMed]
- 91.Steyer A, Jevšnik M, Petrovec M, Pokorn M, Grosek Š, Fratnik Steyer A, Šoba B, Uršič T, Kišek TC, Kolenc M, et al. Narrowing of the diagnostic gap of acute gastroenteritis in children 0–6 years of age using a combination of classical and molecular techniques, delivers challenges in syndromic approach diagnostics. Pediatr Infect Dis J. 2016;35(9):e262–70. doi: 10.1097/INF.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown JR, Shah D, Breuer J. Viral gastrointestinal infections and norovirus genotypes in a paediatric UK hospital, 2014–2015. J Clin Virology: Official Publication Pan Am Soc Clin Virol. 2016;84:1–6. doi: 10.1016/j.jcv.2016.08.298. [DOI] [PubMed] [Google Scholar]
- 93.Sanaei Dashti A, Ghahremani P, Hashempoor T, Karimi A. Molecular Epidemiology of Enteric Adenovirus Gastroenteritis in under-Five-Year-Old Children in Iran. Gastroenterol Res Pract 2016, 2016:2045697. [DOI] [PMC free article] [PubMed]
- 94.Jin HI, Lee YM, Choi YJ, Jeong SJ. Recent viral pathogen in acute gastroenteritis: a retrospective study at a tertiary hospital for 1 year. Korean J Pediatr. 2016;59(3):120–5. doi: 10.3345/kjp.2016.59.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu LY, Qian Y, Zhang Y, Zhao LQ, Jia LP, Dong HJ. Epidemiological aspects of rotavirus and adenovirus in hospitalized children with diarrhea: a 5-year survey in Beijing. BMC Infect Dis 2016, 16. [DOI] [PMC free article] [PubMed]
- 96.Nakamura N, Kobayashi S, Minagawa H, Matsushita T, Sugiura W, Iwatani Y. Molecular epidemiology of enteric viruses in patients with acute gastroenteritis in Aichi prefecture, Japan, 2008/09-2013/14. J Med Virol. 2016;88(7):1180–6. doi: 10.1002/jmv.24445. [DOI] [PubMed] [Google Scholar]
- 97.Reis TAV, Assis ASF, do Valle DA, Barletta VH, de Carvalho IP, Rose TL, Portes SAR, Leite JPG. Da Rosa E Silva ML: the role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after Rotavirus vaccination. Braz J Microbiol. 2016;47(1):243–50. doi: 10.1016/j.bjm.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen H, Zhang J, Li Y, Xie S, Jiang Y, Wu Y, Ye Y, Yang H, Mo H, Situ C et al. The 12 gastrointestinal pathogens spectrum of acute infectious diarrhea in a sentinel hospital, Shenzhen, China. Front Microbiol 2016, 7(NOV). [DOI] [PMC free article] [PubMed]
- 99.Çolak M, Bozdayı G, Altay A, Yalaki Z, Ahmed K, Özkan S. Detection and molecular characterisation of adenovirus in children under 5 years old with diarrhoea. Turk J Med Sci. 2017;47(5):1463–71. doi: 10.3906/sag-1510-94. [DOI] [PubMed] [Google Scholar]
- 100.Cornejo-Tapia A, Orellana-Peralta F, Weilg P, Bazán-Mayra J, Cornejo-Pacherres H, Ulloa-Urizar G, Aguilar-Luis MA, Pons MJ, del Valle-Mendoza J. Etiology, epidemiology and clinical characteristics of acute diarrhea in hospitalized children in rural Peru. J Infect Developing Ctries. 2017;11(11):826–32. doi: 10.3855/jidc.7881. [DOI] [PubMed] [Google Scholar]
- 101.Costa LCPN, Siqueira JAM, Portal TM, Sousa Júnior EC, Linhares AC, Gabbay YB, Resque HR. Detection and genotyping of human adenovirus and sapovirus in children with acute gastroenteritis in Belém, Pará, between 1990 and 1992: first detection of GI.7 and GV.2 sapoviruses in Brazil. REV SOC BRAS MED TROP. 2017;50(5):621–8. doi: 10.1590/0037-8682-0198-2017. [DOI] [PubMed] [Google Scholar]
- 102.Hawash YA, Ismail KA, Almehmadi M. High frequency of enteric protozoan, viral, and bacterial potential pathogens in community-acquired acute diarrheal episodes: evidence based on results of Luminex gastrointestinal pathogen panel assay. Korean J Parasitol. 2017;55(5):513–21. doi: 10.3347/kjp.2017.55.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim A, Chang JY, Shin S, Yi H, Moon JS, Ko JS, Oh S. Epidemiology and factors related to clinical severity of Acute Gastroenteritis in Hospitalized Children after the introduction of Rotavirus Vaccination. J Korean Med Sci. 2017;32(3):465–74. doi: 10.3346/jkms.2017.32.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu LJ, Zhong HQ, Su LY, Cao LF, Xu MH, Dong NN, Xu J. Detection and Molecular Characterization of Human Adenovirus Infections among Hospitalized Children with Acute Diarrhea in Shanghai, China, 2006–2011. Canadian Journal of Infectious Diseases & Medical Microbiology 2017, 2017. [DOI] [PMC free article] [PubMed]
- 105.Stockmann C, Pavia AT, Graham B, Vaughn M, Crisp R, Poritz MA, Thatcher S, Korgenski EK, Barney T, Daly J, et al. Detection of 23 gastrointestinal pathogens among children who present with Diarrhea. J Pediatr Infect Dis Soc. 2017;6(3):231–8. doi: 10.1093/jpids/piw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaki ME, El Kheir NA. Molecular study of astrovirus, adenovirus and norovirus in community acquired diarrhea in children: one Egyptian center study. Asian Pac J Trop Biomed. 2017;7(11):987–90. doi: 10.1016/j.apjtb.2017.10.003. [DOI] [Google Scholar]
- 107.Qiu FZ, Shen XX, Li GX, Zhao L, Chen C, Duan SX, Guo JY, Zhao MC, Yan TF, Qi JJ et al. Adenovirus associated with acute diarrhea: a case-control study. BMC Infect Dis 2018, 18(1). [DOI] [PMC free article] [PubMed]
- 108.Adam MA, Wang J, Enan KA, Shen HW, Wang H, El Hussein AR, Musa AB, Khidir IM, Ma XJ. Molecular Survey of viral and bacterial causes of Childhood Diarrhea in Khartoum State, Sudan. Front Microbiol 2018, 9. [DOI] [PMC free article] [PubMed]
- 109.Alcalá AC, Pérez K, Blanco R, González R, Ludert JE, Liprandi F, Vizzi E. Molecular detection of human enteric viruses circulating among children with acute gastroenteritis in Valencia, Venezuela, before rotavirus vaccine implementation. Gut Pathogens 2018, 10(1). [DOI] [PMC free article] [PubMed]
- 110.Biscaro V, Piccinelli G, Gargiulo F, Ianiro G, Caruso A, Caccuri F, De Francesco MA. Detection and molecular characterization of enteric viruses in children with acute gastroenteritis in Northern Italy. Infec Genet Evol. 2018;60:35–41. doi: 10.1016/j.meegid.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 111.Primo D, Pacheco GT, Timenetsky M, Luchs A. Surveillance and molecular characterization of human adenovirus in patients with acute gastroenteritis in the era of rotavirus vaccine, Brazil, 2012–2017. J Clin Virology: Official Publication Pan Am Soc Clin Virol. 2018;109:35–40. doi: 10.1016/j.jcv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Yu WJ, Chen SY, Tsai CN, Chao HC, Kong MS, Chang YJ, Chiu CH. Long-term impact of suboptimal rotavirus vaccines on acute gastroenteritis in hospitalized children in Northern Taiwan. J Formos Med Assoc. 2018;117(8):720–6. doi: 10.1016/j.jfma.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Hassan F, Kanwar N, Harrison CJ, Halasa NB, Chappell JD, Englund JA, Klein EJ, Weinberg GA, Szilagyi PG, Moffatt ME, et al. Viral etiology of Acute Gastroenteritis in < 2-Year-old US children in the Post-rotavirus Vaccine Era. J Pediatr Infect Dis Soc. 2019;8(5):414–21. doi: 10.1093/jpids/piy077. [DOI] [PubMed] [Google Scholar]
- 114.Iturriza-Gomara M, Jere KC, Hungerford D, Bar-Zeev N, Shioda K, Kanjerwa O, Houpt ER, Operario DJ, Wachepa R, Pollock L, et al. Etiology of Diarrhea among Hospitalized Children in Blantyre, Malawi, following Rotavirus Vaccine introduction: a case-control study. J Infect Dis. 2019;220(2):213–8. doi: 10.1093/infdis/jiz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lima AAM, Oliveira DB, Quetz JS, Havt A, Prata MMG, Lima IFN, Soares AM, Filho JQ, Lima NL, Medeiros PHQS et al. Etiology and severity of diarrheal diseases in infants at the semiarid region of Brazil: a case-control study. PLoS Negl Trop Dis 2019, 13(2). [DOI] [PMC free article] [PubMed]
- 116.Shen XX, Qiu FZ, Li GX, Zhao MC, Wang J, Chen C, Zhao L, Qi JJ, Liu H, Zhang Y, et al. A case control study on the prevalence of enterovirus in children samples and its association with diarrhea. Arch Virol. 2019;164(1):63–8. doi: 10.1007/s00705-018-4021-5. [DOI] [PubMed] [Google Scholar]
- 117.Tilmanne A, Martiny D, Quach C, Wautier M, Vandenberg O, Lepage P, Hallin M. Enteropathogens in paediatric gastroenteritis: comparison of routine diagnostic and molecular methods. Clin Microbiol Infect. 2019;25(12):1519–24. doi: 10.1016/j.cmi.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 118.Arashkia A, Bahrami F, Farsi M, Nejati B, Jalilvand S, Nateghian A, Rahbarimanesh A, Shoja Z. Molecular analysis of human adenoviruses in hospitalized children < 5 years old with acute gastroenteritis in Tehran, Iran. J Med Virol. 2019;91(11):1930–6. doi: 10.1002/jmv.25539. [DOI] [PubMed] [Google Scholar]
- 119.Arowolo KO, Ayolabi CI, Lapinski B, Santos JS, Raboni SM. Epidemiology of enteric viruses in children with gastroenteritis in Ogun State, Nigeria. J Med Virol. 2019;91(6):1022–9. doi: 10.1002/jmv.25399. [DOI] [PubMed] [Google Scholar]
- 120.Elmahdy EM, Ahmed NI, Shaheen MNF, Mohamed EB, Loutfy SA. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J Water Health. 2019;17(2):287–94. doi: 10.2166/wh.2019.303. [DOI] [PubMed] [Google Scholar]
- 121.Gaensbauer JT, Lamb M, Calvimontes DM, Asturias EJ, Kamidani S, Contreras-Roldan IL, Dominguez SR, Robinson CC, Zacarias A, Berman S, et al. Identification of enteropathogens by multiplex PCR among rural and urban Guatemalan children with acute diarrhea. AM J TROP MED HYG. 2019;101(3):534–40. doi: 10.4269/ajtmh.18-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goldar S, Rajbongshi G, Chamuah K, Alam ST, Sharma A. Occurrence of viral gastroenteritis in children below 5 years: a hospital-based study from Assam, India. Indian J Med Microbiol. 2019;37(3):415–7. doi: 10.4103/ijmm.IJMM_19_79. [DOI] [PubMed] [Google Scholar]
- 123.Harb A, Abraham S, Rusdi B, Laird T, O’Dea M, Habib I. Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with Acute Diarrhea in Thi-Qar Governorate, Iraq. Int J Environ Res Public Health 2019, 16(9). [DOI] [PMC free article] [PubMed]
- 124.Portal TM, Reymao TKA, Neto GAQ, Fiuza MKD, Teixeira DM, Lima ICG, Sousa EC, Bandeira RD, De Deus DR, Justino MCA, et al. Detection and genotyping of enteric viruses in hospitalized children with acute gastroenteritis in Belem, Brazil: occurrence of adenovirus viremia by species F, types 40/41. J Med Virol. 2019;91(3):378–84. doi: 10.1002/jmv.25321. [DOI] [PubMed] [Google Scholar]
- 125.Pratte-Santos R, Miagostovich MP, Fumian TM, Maciel EL, Martins SA, Cassini ST, Keller R. High prevalence of enteric viruses associated with acute gastroenteritis in pediatric patients in a low-income area in Vitória, Southeastern Brazil. J Med Virol. 2019;91(5):744–50. doi: 10.1002/jmv.25392. [DOI] [PubMed] [Google Scholar]
- 126.Tatte VS, Gopalkrishna V. Detection of different enteric viruses in children with diarrheal disease: evidence of the high frequency of mixed infections. Access Microbiol. 2019;1(2):e000010. doi: 10.1099/acmi.0.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theamboonlers A, Duang-in A, Vichaiwattana P, Thongmee T, Poovorawan Y, HUMAN ADENOVIRUS IN PATIENTS WITH INFLUENZA-LIKE ILLNESS AND / OR ACUTE GASTROENTERITIS IN THAILAND., 2016. SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH 2019, 50(2):229–239.
- 128.Farfán-García AE, Imdad A, Zhang C, Arias-Guerrero MY, Sánchez-álvarez NT, Iqbal J, Hernández-Gamboa AE, Slaughter JC, Gómez-Duarte OG. Etiology of acute gastroenteritis among children less than 5 years of age in Bucaramanga, Colombia: a case-control study. PLoS Negl Trop Dis. 2020;14(6):1–20. doi: 10.1371/journal.pntd.0008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pabbaraju K, Tellier R, Pang XL, Xie J, Lee BE, Chui L, Zhuo R, Vanderkooi OG, Ali S, Tarr PI et al. A clinical epidemiology and molecular attribution evaluation of adenoviruses in Pediatric Acute Gastroenteritis: a case-control study. J Clin Microbiol 2020, 59(1). [DOI] [PMC free article] [PubMed]
- 130.Dey SK, Sharif N, Sarkar OS, Sarkar MK, Talukder AA, Phan T, Ushijima H. Molecular epidemiology and surveillance of circulating rotavirus among children with gastroenteritis in Bangladesh during 2014–2019. PLoS ONE. 2020;15(11):e0242813. doi: 10.1371/journal.pone.0242813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim GR, Kim SH, Jeon GW, Shin JH. Prevalence of eleven infectious viruses causing diarrhea in Korea. Jpn J Infect Dis. 2020;73(6):427–30. doi: 10.7883/yoken.JJID.2020.069. [DOI] [PubMed] [Google Scholar]
- 132.Lambisia AW, Onchaga S, Murunga N, Lewa CS, Nyanjom SG, Agoti CN. Epidemiological trends of five common diarrhea-associated enteric viruses pre-and post-rotavirus vaccine introduction in coastal Kenya. Pathogens. 2020;9(8):1–14. doi: 10.3390/pathogens9080660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohammadi J, Amini R, Akbari A, Amraei M, Mahmoudvand S, Jalilian FA. Prevalence and Seasonal frequency of acute viral gastroenteritis in children less than 5 years in Ilam, Iran. Entomol Appl Sci Lett. 2020;7(3):66–74. [Google Scholar]
- 134.Nasab SDM, Zali F, Kaghazian H, Aghasadeghi MR, Mardani R, Gachkar L, Vasmehjani AA, Ahmadi N, Ghasemzadeh A. Prevalence of astrovirus, adenovirus, and sapovirus infections among Iranian children with acute gastroenteritis. Gastroenterol Hepatol Bed Bench. 2020;13:S122–7. [PMC free article] [PubMed] [Google Scholar]
- 135.Romo-Saenz CI, Medina-Soltero MR, Delgado-Gardea MCE, de la Serna F, Tamez-Guerra P, Contreras-Palma M, Gonzalez-Horta MD, Erosa-de la Vega G, Gomez-Flores R, Contreras-Cordero JE et al. Human enteric circulating viruses and co-infections among hospitalized children with severe Acute Gastroenteritis in Chihuahua, Mexico, during 2010–2011. Jundishapur J Microbiol 2020, 13(2).
- 136.Sharif N, Nobel NU, Sakib N, Liza SM, Khan ST, Billah B, Parvez AK, Haque A, Talukder AA, Dey SK. Molecular and epidemiologic analysis of diarrheal pathogens in children with acute gastroenteritis in Bangladesh during 2014–2019. Pediatr Infect Dis J 2020:580–5. [DOI] [PubMed]
- 137.Zhu Y-N, Ye Y-H, Zhang Z, Wu Y-J, Chen L, Wang J, Tang Y-J, Meng J, Zhang H-L, Hu G-F. Prevalence and molecular characterization of parechovirus A in children with acute gastroenteritis in Shenzhen, 2016–2018. Arch Virol. 2020;165:1377–84. doi: 10.1007/s00705-020-04587-6. [DOI] [PubMed] [Google Scholar]
- 138.Alsuwaidi AR, Al Dhaheri K, Al Hamad S, George J, Ibrahim J, Ghatasheh G, Issa M, Al-Hammadi S, Narchi H. Etiology of diarrhea by multiplex polymerase chain reaction among young children in the United Arab Emirates: a case-control study. BMC Infect Dis 2021, 21(1). [DOI] [PMC free article] [PubMed]
- 139.Harrison CJ, Hassan F, Lee B, Boom J, Sahni LC, Johnson C, Dunn J, Payne DC, Wikswo ME, Parashar U et al. Multiplex PCR Pathogen Detection in Acute Gastroenteritis among hospitalized US children compared with healthy controls during 2011–2016 in the Post-rotavirus Vaccine Era. Open Forum Infect Dis 2021, 8(12). [DOI] [PMC free article] [PubMed]
- 140.Huang Z, He Z, Wei Z, Wang W, Li Z, Xia X, Qin D, Zhang L, Guo J, Li J et al. Correlation between prevalence of selected enteropathogens and diarrhea in children: a case-control study in China. Open Forum Infect Dis 2021, 8(10). [DOI] [PMC free article] [PubMed]
- 141.Mero S, Timonen S, Lääveri T, Løfberg S, Kirveskari J, Ursing J, Rombo L, Kofoed P-E, Kantele A. Prevalence of diarrhoeal pathogens among children under five years of age with and without diarrhoea in Guinea-Bissau. PLoS Negl Trop Dis. 2021;15(9):e0009709. doi: 10.1371/journal.pntd.0009709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abdel-Rahman ME, Mathew S, Al Thani AA, Al Ansari K, Yassine HM. Clinical manifestations associated with acute viral gastroenteritis pathogens among pediatric patients in Qatar. J Med Virol. 2021;93(8):4794–804. doi: 10.1002/jmv.26859. [DOI] [PubMed] [Google Scholar]
- 143.Barsoum Z. Paediatric viral gastroenteritis and regional predominant viral pathogens in the post-rotavirus vaccination year: prospective Irish regional study. Sudan J Paediatrics. 2021;21(1):36–41. doi: 10.24911/SJP.106-1598279768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chandra P, Lo M, Mitra S, Banerjee A, Saha P, Okamoto K, Deb AK, Ghosh SK, Manna A, Dutta S, et al. Genetic characterization and phylogenetic variations of human adenovirus-F strains circulating in eastern India during 2017–2020. J Med Virol. 2021;93(11):6180–90. doi: 10.1002/jmv.27136. [DOI] [PubMed] [Google Scholar]
- 145.Chang H, Guo J, Wei Z, Huang Z, Wang C, Qiu Y, Xu X, Zeng M. Aetiology of acute diarrhoea in children in Shanghai, 2015–2018. PLoS ONE 2021, 16(4 April). [DOI] [PMC free article] [PubMed]
- 146.De Francesco MA, Lorenzin G, Meini A, Schumacher RF, Caruso A. Nonenteric adenoviruses Associated with gastroenteritis in Hospitalized Children. Microbiol Spectr. 2021;9(1):1–8. doi: 10.1128/Spectrum.00300-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gopalkrishna V, Joshi MS, Chavan NA, Shinde MS, Walimbe AM, Sawant PM, Kalrao VR, Dhongade RK, Bavdekar AR. Prevalence and genetic diversity of gastroenteritis viruses in hospitalized children < 5 years of age in Maharashtra state, Western India, 2017–2019. J Med Virol. 2021;93(8):4805–16. doi: 10.1002/jmv.27085. [DOI] [PubMed] [Google Scholar]
- 148.Lu L, Zhong H, Xu M, Su L, Cao L, Jia R, Xu J. Molecular and epidemiological characterization of human adenovirus and classic human astrovirus in children with acute diarrhea in Shanghai, 2017–2018. BMC Infect Dis 2021, 21(1). [DOI] [PMC free article] [PubMed]
- 149.Ndjangangoye NK, Lekana-Douki SE, Lekolo GM, Mve-Ella OB, Oyegue-Liabagui SL, Lekana-Douki JB. High prevalence and prolonged shedding with enteric viruses among children with acute diarrhea in Franceville, Southeast of Gabon. J Clin Virol Plus 2021, 1(4).
- 150.Olivares AIO, Leitão GAA, Pimenta YC, Cantelli CP, Fumian TM, Fialho AM, da Silva e Mouta S, Delgado IF, Nordgren J, Svensson L, et al. Epidemiology of enteric virus infections in children living in the Amazon region. Int J Infect Dis. 2021;108:494–502. doi: 10.1016/j.ijid.2021.05.060. [DOI] [PubMed] [Google Scholar]
- 151.Rossouw E, Brauer M, Meyer P, du Plessis NM, Avenant T, Mans J. Virus etiology, diversity and clinical characteristics in South African children hospitalised with gastroenteritis. Viruses 2021, 13(2). [DOI] [PMC free article] [PubMed]
- 152.Souza YFVPD, Souza EVD, Azevedo LSD, Medeiros RS, Timenetsky MDCST, Luchs A. Enteric adenovirus epidemiology from historical fecal samples in Brazil (1998–2005): pre-rotavirus vaccine era. Infec Genet Evol 2021, 94. [DOI] [PubMed]
- 153.Souza EV, de Souza YFVP, Medeiros RS, de Azevedo LS, de Queiroz TGA, Sanz-Duro RL, Marinho RSS, Komninakis SV, Timenetsky MCST, Luchs A. Diversity of enteric and non-enteric human adenovirus strains in Brazil, 2006–2011. Arch Virol. 2021;166(3):897–903. doi: 10.1007/s00705-020-04946-3. [DOI] [PubMed] [Google Scholar]
- 154.Abbasi E, Mondanizadeh M, van Belkum A, Ghaznavi-Rad E. Low frequency of Adenovirus, Rotavirus, and Norovirus in Pediatric Diarrheal samples from Central Iran. Arch Pediatr Infect Dis 2022, 10(2).
- 155.Allayeh AK, Al-Daim SA, Ahmed N, El-Gayar M, Mostafa A. Isolation and genotyping of adenoviruses from Wastewater and Diarrheal Samples in Egypt from 2016 to 2020. Viruses 2022, 14(10). [DOI] [PMC free article] [PubMed]
- 156.Al-Nasrawy LM, Jawad SM, Al-Nasrawy WD. Human adenoviruses 40/41 and Cytokines Response in Children with Diarrhoea. Egypt J Hosp Med. 2022;89(2):7025–30. doi: 10.21608/ejhm.2022.272502. [DOI] [Google Scholar]
- 157.Colito DA, Dorta-Guerra R, Da Costa Lima HS, Pina C, Gonçalves D, Valladares B, Foronda P. Epidemiological investigations of diarrhea in children in Praia city, Cape Verde. Front Microbiol 2022, 13. [DOI] [PMC free article] [PubMed]
- 158.do Nascimento LG, Fialho AM, de Andrade J, de Assis RMS, Fumian TM. Human enteric adenovirus F40/41 as a major cause of acute gastroenteritis in children in Brazil, 2018 to 2020. Sci Rep. 2022;12(1):11220. doi: 10.1038/s41598-022-15413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong XX, Qi Y, Chai RY, Xu H, Wang J, Wang YS, Chen Y, Zhang LL, Lu Y, Chen HJ, et al. Viral infection among children under the age of 5 with diarrhea in Shenyang from 2018 to 2020: a hospital-based study. J Med Virol. 2022;94(6):2662–8. doi: 10.1002/jmv.27511. [DOI] [PubMed] [Google Scholar]
- 160.Gelaw A, Liebert UG. Molecular detection of enteric viruses in under-five children with Diarrhea in Debre Tabor, Northwest Ethiopia. Infect Drug Resist. 2022;15:1981–94. doi: 10.2147/IDR.S364142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jo SJ, Kang HM, Kim JO, Cho H, Heo W, Yoo IY, Park YJ. Evaluation of the biofire gastrointestinal panel to detect diarrheal pathogens in pediatric patients. Diagn 2022, 12(1). [DOI] [PMC free article] [PubMed]
- 162.Li W, Li W, Li L, Guo Y, Chen J, Shang S, Mao J. Multiplex detection of eight different viral enteropathogens in clinical samples, combining RT-PCR technology with melting curve analysis. Virol J 2022, 19(1). [DOI] [PMC free article] [PubMed]
- 163.Mihala G, Ware RS, Lambert SB, Bialasiewicz S, Whiley DM, Sarna M, Sloots TP, Nissen MD, Grimwood K. Potentially pathogenic organisms in Stools and their Association with Acute Diarrheal illness in children aged < 2 years. J Pediatr Infect Dis Soc. 2022;11(5):199–206. doi: 10.1093/jpids/piab130. [DOI] [PubMed] [Google Scholar]
- 164.Mitra S, Lo M, Saha R, Deb AK, Debnath F, Miyoshi SI, Dutta S, Chawla-Sarkar M. Epidemiology of major entero-pathogenic viruses and genetic characterization of Group A rotaviruses among children (≤ 5 years) with acute gastroenteritis in eastern India, 2018–2020. J Appl Microbiol. 2022;133(2):758–83. doi: 10.1111/jam.15594. [DOI] [PubMed] [Google Scholar]
- 165.Othma AAS, Gomaa HEE, El Anany MG, Rahman EMA, Hassan EM, Abd Elbaky A, Soliman MMS, Awadallah E. Use of multiplex PCR in diagnosis of childhood acute viral diarrhoea caused by rotavirus, norovirus, astrovirus and adenovirus in Upper Egypt. Egypt J Med Hum Genet 2022, 23(1).
- 166.Shams S, Tafaroji J, Aghaali M, Ahmadi N, Heydari H, Nasab SDM, Maurya VK. Prevalence of enteric adenovirus and co-infection with rotavirus in children under 15 years of age with gastroenteritis in Qom, Iran. Gastroenterol Hepatol Bed Bench. 2022;15(3):256–62. doi: 10.22037/ghfbb.v15i3.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yllmaz F, Kaya H, Özdemir M. Investigation of children with Acute Gastroenteritis by Multiplex PCR method in Central Part of Turkey. J Pediatr Infect Dis. 2022;17(1):48–52. doi: 10.1055/s-0041-1740372. [DOI] [Google Scholar]
- 168.Bhat A, Rao SS, Bhat S, Vidyalakshmi K, Dhanashree B. Molecular diagnosis of bacterial and viral diarrhoea using multiplex-PCR assays: an observational prospective study among paediatric patients from India. Indian J Med Microbiol. 2023;41:64–70. doi: 10.1016/j.ijmmb.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 169.Borkakoty B, Bali NK, Jakaria A, Hazarika R, Temsu T, Gohain M, Kaur H. Norovirus gastroenteritis in children under-five years hospitalized for diarrhea in two cities of northeast India: a retrospective study. Indian J Med Microbiol 2023, 45. [DOI] [PubMed]
- 170.Eifan S, Nour I, Hanif A, Alhetheel A, Al-Ashkar I. Molecular Epidemiology and Surveillance of Human Adenovirus and Rotavirus A Associated Gastroenteritis in Riyadh, Saudi Arabia. Trop Med Infect Dis 2023, 8(5). [DOI] [PMC free article] [PubMed]
- 171.Lu L, Jia R, Zhong H, Duan S, Xu M, Su L, Cao L, Xu J. Surveillance and epidemiological characterization of human adenovirus infections among outpatient children with acute gastroenteritis during the COVID-19 epidemic in Shanghai, China. Virol J. 2023;20(1):133. doi: 10.1186/s12985-023-02105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ndjangangoye NK, Lekana-Douki SE, Oyegue-Liabagui SL, Kouna LC, Ndong KAN, Onanga R, Lekana-Douki JB. Molecular Prevalence of Diarrheal Pathogens in children with Acute Diarrhea in Southeastern Gabon. AM J TROP MED HYG. 2023;108(4):829–36. doi: 10.4269/ajtmh.22-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Potgieter N, Heine L, Ngandu JPK, Ledwaba SE, Zitha T, Mudau LS, Becker P, Traore AN, Barnard TG. High Burden of Co-infection with multiple enteric pathogens in children suffering with Diarrhoea from Rural and Peri-urban communities in South Africa. Pathogens 2023, 12(2). [DOI] [PMC free article] [PubMed]
- 174.Razizadeh MH, Pourrostami K, Kachooei A, Zarei M, Asghari M, Hamldar S, Khatami A. An annoying enteric virus: a systematic review and meta-analysis of human astroviruses and gastrointestinal complications in children. Rev Med Virol. 2022;32(6):e2389. doi: 10.1002/rmv.2389. [DOI] [PubMed] [Google Scholar]
- 175.Lee B, Damon CF, Platts-Mills JA. Pediatric acute gastroenteritis due to adenovirus 40/41 in low-and middle-income countries. CURR OPIN INFECT DIS. 2020;33(5):398. doi: 10.1097/QCO.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang J, Zhu Y, Zhou Y, Gao F, Qiu X, Li J, Yuan H, Jin W, Lin W. Pediatric adenovirus pneumonia: clinical practice and current treatment. Front Med 2023, 10. [DOI] [PMC free article] [PubMed]
- 177.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nguyen GT, Phan K, Teng I, Pu J, Watanabe T. A systematic review and meta-analysis of the prevalence of norovirus in cases of gastroenteritis in developing countries. Medicine (USA) 2017, 96(40). [DOI] [PMC free article] [PubMed]
- 179.Lu Y, Zhang Z, Xie H, Su W, Wang H, Wang D, Lu J. The rise in Norovirus-related Acute Gastroenteritis during the fight against the COVID-19 pandemic in Southern China. Front Public Health 2022, 9. [DOI] [PMC free article] [PubMed]
- 180.Ashikkali L, Carroll W, Johnson C. The indirect impact of COVID-19 on child health. Paediatrics Child Health. 2020;30(12):430–7. doi: 10.1016/j.paed.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Foolchand A, Ghazi T, Chuturgoon AA. Malnutrition and Dietary habits alter the Immune System which may consequently influence SARS-CoV-2 virulence: a review. Int J Mol Sci. 2022;23(5):2654. doi: 10.3390/ijms23052654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 183.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;209(suppl3):S120–6. doi: 10.1093/infdis/jiu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B: Biological Sciences 2015, 282(1821):20143085. [DOI] [PMC free article] [PubMed]
- 185.Lynch JP, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med: 2011. © Thieme Medical; 2011. pp. 494–511. [DOI] [PubMed]
- 186.Agustina R, Sari TP, Satroamidjojo S, Bovee-Oudenhoven IM, Feskens EJ, Kok FJ. Association of food-hygiene practices and diarrhea prevalence among Indonesian young children from low socioeconomic urban areas. BMC Public Health. 2013;13(1):1–12. doi: 10.1186/1471-2458-13-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.