Abstract
Field isolates of foot-and-mouth disease virus (FMDV) have been shown to use the RGD-dependent integrin αvβ3 as a cellular receptor on cultured cells. However, several other RGD-dependent integrins may have the potential to act as receptors for FMDV in vivo. Of these, αvβ6 is a likely candidate for use as a receptor by FMDV as it is expressed on epithelial cells, which correlates with the tissue tropism of the virus. In this report, we show that human colon carcinoma cells (SW480) that are normally nonpermissive for FMDV become susceptible to infection as a result of transfection with the integrin β6 subunit and expression of αvβ6 at the cell surface. Integrin αvβ6 is the major site for virus attachment on the β6-transfected cells, and binding to αvβ6 serves to increase the rate of virus entry into these cells. In addition, we show that virus binding and infection of the β6-transfected cells is mediated through an RGD-dependent interaction that is specifically inhibited by a monoclonal antibody (10D5) that recognizes αvβ6. These studies establish a role for αvβ6 as a cellular receptor for FMDV.
The seven serotypes of Foot-and-mouth disease virus (FMDV) (types O, A, C, Asia-1, and the South African Territories [SAT] types 1, 2, and 3) are members of the Aphthovirus genus of the family Picornaviridae. Picornaviruses are small, nonenveloped, single-stranded, positive-sense RNA viruses which cause many important diseases of humans and animals (5). The virus capsid is made up of 60 copies each of four virus-encoded proteins, VP1 to VP4. Crystal structures of viruses representative of several FMDV serotypes have been determined, and a major feature of the outer capsid surface is a long, conformationally flexible loop (1, 14, 18, 27, 28, 29). This loop, the GH loop of VP1, includes at its apex a highly conserved arginine-glycine-aspartic acid (RGD) tripeptide motif.
FMDV is the causative agent of foot-and-mouth disease, an economically important and highly contagious disease of many domestic livestock, such as pigs, sheep, goats, and cattle. The primary route of infection by FMDV is through the upper respiratory tract. The predilection sites for initial virus replication are thought to be epithelial cells of the oropharynx and associated lymphoid tissues (11, 12, 13, 45). During the development of disease, virus is widely disseminated throughout the body, with secondary sites of replication in many epithelial tissues (13).
Two families of cellular receptors have been identified that mediate infection of FMDV, heparan sulfate proteoglycans (HSPG) and integrins. Several viruses that have been adapted for growth in cultured cell lines acquire a high affinity for heparan sulfate and, as a consequence, use HSPG as receptors for both attachment and subsequent internalization without the mediation of integrins (25, 37, 44). By contrast, field isolates of FMDV use RGD-dependent integrins as receptors through an interaction mediated by the RGD motif of the VP1 GH loop (24, 30, 31, 37, 44).
Integrins are a family of cell surface, α-β heterodimeric glycoproteins composed of at least 15 α and 8 β subunits which associate to form over 20 different α-β combinations (23, 47). Each subunit is composed of large extracellular domains, a transmembrane region, and, in most cases, a short cytoplasmic domain. Integrins contribute to a variety of processes, including adhesion between cells and between cells and the extracellular matrix and induction of signal transduction pathways that modulate various processes, including cell proliferation, morphology, migration, and apoptosis (16, 23, 34, 39, 47). Several integrins, including αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α5β1, and α8β1 (23, 38, 46, 47), bind their ligands by recognition of an RGD motif. To date, the vitronectin receptor αvβ3 is the only RGD-dependent integrin that has been shown to act as a receptor for FMDV (6); however, this integrin may not have a major role during the initial infection of an animal, since it is not normally expressed in epithelial cells and has limited expression in lymphoid cells (15, 33). However, several other RGD-dependent integrins, including α5β1, αvβ5, and αvβ6, are expressed on these cell types (15, 33, 47).
The integrin β6 subunit forms only a single heterodimer, αvβ6, which has been shown to be a receptor for the extracellular matrix proteins fibronectin (48), tenascin (43, 49), and vitronectin (22) and for latency-associated protein 1 (LAP-1), a protein involved in modulation of the activity of transforming growth factor β1 (36). Expression of αvβ6 is restricted to epithelial cells and has been observed at a variety of sites, including the epithelia of the uterus, bladder, respiratory tract, and salivary gland (10). Expression levels are moderate or low in normal healthy adult epithelia but are rapidly up regulated at sites of tissue injury and inflammation (9, 21). In addition, inactivation of the β6 subunit in mice has identified a role for αvβ6 in down regulation of inflammation in skin and the respiratory tract (21). αvβ6 also plays an important role in keratinocyte migration (22) and is expressed at the leading edges of healing cutaneous wounds (9, 19). αvβ6 has been shown to confer upon cells a growth advantage, an effect dependent on an 11-amino-acid COOH-terminal extension that is unique to the β6 subunit (2).
Recently, the pentapeptide DLXXL has been reported to be a ligand for αvβ6 in the absence of an RGD motif (26). This peptide shares sequence similarity with the region flanking the RGD motif (RGDLXXI) found in LAP-1, a recently identified high-affinity ligand for αvβ6 (36). Across the FMDV serotypes, a leucine is most commonly found as the residue immediately following the RGD motif (31), and virtually all virus isolates have a leucine residue in the RGD+4 position. Given the similarity between the residues flanking the RGD motifs of FMDV and LAP-1, we reasoned that αvβ6 could act as a receptor for FMDV. In this report, we show that cells that are normally nonpermissive for FMDV become susceptible to infection after transfection with the integrin β6 subunit and expression of αvβ6 at the cell surface. Evidence is also presented which shows that on the β6-transfected cells, αvβ6 functions as the major receptor for virus attachment and that the integrin serves to increase the rate of virus entry into the cell.
MATERIALS AND METHODS
Cells and viruses.
BHK cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum, 20 mM glutamine, penicillin (100 SI units/ml), and streptomycin (100 μg/ml). The human colon carcinoma cell line SW480 transfected to express the full-length human β6 integrin subunit and mock-transfected cells (48) were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 20 mM glutamine, penicillin (100 SI units/ml), streptomycin (100 μg/ml), and 1 mg of geneticin (Life Technologies)/ml. The viruses used in this study were the FMDV strains O1K-cad2, C-S8c1, and SAT-3 Zim 4/81 (SAT-3). These viruses were selected for this study because they do not appear to bind heparin (references 4 and 18 and unpublished observation). O1K-cad2 was used for binding studies, as this virus is readily purified to homogeneity and is recognized by a panel of available monoclonal antibodies (MAbs). However, the virus replicates relatively poorly in cultured cells, resulting in virus titers of ∼105 PFU/ml. Therefore, for infectivity studies we used the C-S8c1 and SAT-3 strains, as these viruses replicate well in BHK cells, resulting in virus titers of ∼107 to 108 PFU/ml. Virus stocks were prepared and virus titers were determined using BHK cells. In all assays, the multiplicity of infection (MOI) was based on the virus titer on BHK cells. Virus purification on sucrose gradients was done as described previously (14).
Antibodies and peptides.
The RGD peptide with its sequence derived from the GH loop of VP1 of type O FMDV (142-VPNLRGDLQVLA-153) and the control RGE version were synthesized at the peptide synthesis facility at the Oxford Centre for Molecular Science, New Chemistry Laboratory, Oxford, United Kingdom. The anti-integrin antibodies used in these studies were LM609 and 23C6 (anti-αvβ3), 25E11 (anti-β3), P1F6 (mouse immunoglobulin G1 [IgG1]; anti-αvβ5), and 10D5 (mouse IgG2a; anti-αvβ6), all from Chemicon, and SAM-1 (mouse IgG2b; anti-α5β1) from Serotec. The anti-FMDV MAbs, B2 (mouse IgG1) and D9 (mouse IgG2A), which recognize antigenic site 1 of type O FMDV (32), were purified using protein A (Pierce) according to the manufacturer's instructions.
Infectivity assays. (i) Infection conditions.
Cells (mock or β6 transfected) were seeded at 2.5 × 105 per well in 24-well plates or 1 × 106 per 35-mm-diameter dish 16 h prior to infection. The monolayers were washed with assay buffer (phosphate-buffered saline [PBS; pH 7.5] containing 2 mM CaCl2 and 1 mM MgCl2), and viruses, diluted in the same buffer, were added to the cells under the conditions indicated in the figure legends.
(ii) Growth curves.
Cells in 35-mm-diameter dishes were infected for 1 h at 37°C. Infectious virus that remained on the outsides of the cells was inactivated by incubating the cells with 0.1 M citric acid buffer (pH 5.2) in 140 mM NaCl for 1 to 2 min. The cells were then washed with assay buffer and cultivated in 2 ml of cell growth medium for 24 h. At various times, the cell culture medium was assayed for the presence of virus by standard plaque assay on BHK cells. Briefly, 100 μl of each virus dilution was layered onto BHK cells for 15 min at 37°C. The monolayers were then overlaid with 4 ml of molten Eagle's overlay (Eagle's medium supplemented with 0.6% indubiose, 5% tryptone phosphate broth, 1% fetal calf serum, 100 SI Units of penicillin/ml, and 100 μg of streptomycin/ml). The cells were then incubated at 37°C and 5% CO2 for 40 to 48 h. Plaques were visualized by staining the cell monolayer with methylene blue–4% formaldehyde in PBS.
(iii) Infectious-center assay.
Target cell monolayers in 35-mm-diameter dishes were infected at the temperature and MOI indicated on the figures. At the indicated time points, infectious virus that remained on the outsides of the cells was inactivated by acid treatment as described above. The cells were then rinsed three times with assay buffer, removed from the wells by using trypsin, collected by centrifugation, and resuspended in 300 μl of assay buffer supplemented with 0.5% fetal calf serum. The cells were counted, and dilutions of cells (100 μl), prepared in the same buffer, were mixed with 1 ml of molten Eagle's overlay and layered onto subconfluent monolayers of BHK cells prepared in 60-mm-diameter dishes. When the medium had solidified a further 3 ml of Eagle's overlay was layered onto each dish. Cells were incubated at 37°C and 5% CO2 for 40 to 48 h. Infectious centers were visualized as plaques by fixing and staining them as described above. Where peptides or anti-integrin antibodies were used to block infection, these reagents were added to the target cell monolayers at room temperature for 15 to 30 min prior to the addition of virus for a further 15 min at 37°C.
Flow cytometry analysis. (i) Standard assay.
Cells were harvested using EDTA (cell dissociation solution; Sigma), washed, and resuspended at 2 × 107 cells per ml in a solution of PBS (pH 7.5), 2 mM CaCl2, 1 mM MgCl2, 2% horse serum, 3% bovine serum albumin, and 0.1% sodium azide (buffer A). Cells (30 μl) were incubated with primary antibodies (10 μg/ml in buffer A) on ice for 20 min. The cells were then washed three times with buffer A and incubated on ice for 20 min with secondary antibodies conjugated with R-phycoerythrin (Southern Biotechnology Associates). The cells were then washed three times with buffer A and once with a solution of PBS (pH 7.5), 2 mM CaCl2, and 1 mM MgCl2 and resuspended in the same buffer containing 1% paraformaldehyde. Fluorescent staining was analyzed by flow cytometry using a FACSCalibur (Becton Dickinson) counting 10,000 cells per sample.
(ii) Virus binding assay.
Cells were prepared in buffer A as described above and incubated with O1K-cad2 (10 μg/ml) for 30 min on ice. The cells were then washed twice with buffer A and incubated sequentially with anti-type O MAb D9 (10 μg/ml), followed by a goat anti-mouse IgG2a-specific R-phycoerythrin conjugate.
(iii) Competition experiments.
In experiments where FMDV was used to block binding of integrin-specific antibodies, virus was incubated with the cells on ice for 30 min before the addition of the anti-integrin antibodies for a further 30 min. The cells were then washed three times with buffer A, followed by incubation with a goat anti-mouse IgG R-phycoerythrin conjugate. For experiments where integrin-specific antibodies or RGD peptides were used to block binding of FMDV, the antibodies and peptides were added to the cells for 30 min on ice before the addition of virus for a further 30 min. The cells were then washed three times with buffer A, and cell-bound virus was detected with an anti-type O FMDV MAb. When 10D5 (IgG2a) was used as a competitor, virus was detected with the MAb B2 (IgG1). When P1F6 (IgG1) was used as a competitor, virus was detected with the MAb D9 (IgG2a). Anti-FMDV antibodies were detected with goat anti-mouse IgG isotype-specific R-phycoerythrin conjugates.
RESULTS
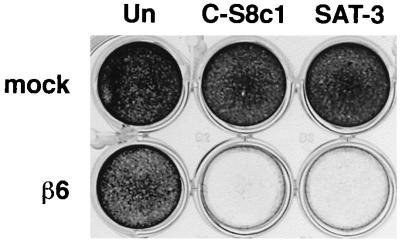
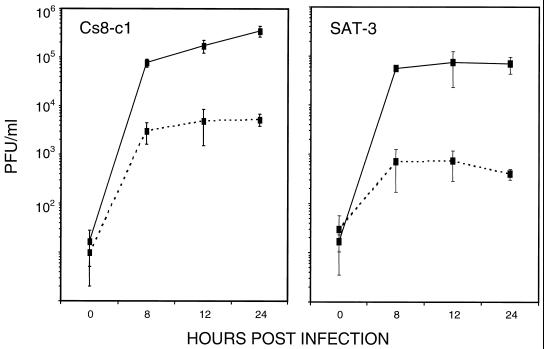
To determine whether expression of the integrin αvβ6 enhanced infection by FMDV, we compared SW480 cells that had been stably transfected to express the full-length human β6 subunit (β6-transfected) with cells transfected with the expression plasmid alone (mock transfected) (48). SW480 cells normally express α5β1 and αvβ5 as their only RGD-binding integrins but do not express the β6 subunit (48). However, when transfected with β6 cDNA, the αv subunit also has the opportunity of pairing with β6 so that they express αvβ6 at the cell surface as a functional heterodimer (36, 48). Initially, we confirmed by flow cytometry the integrin expression profiles for the RGD-binding integrins on the mock- and β6-transfected cells and that the β6-transfected cells express αvβ6 (data not shown). In addition, using three MAbs, LM609 and 23C6, which recognize αvβ3, and 25E11, which binds the β3 subunit, we confirmed that both the mock- and β6-transfected cells were negative for expression of αvβ3. The viruses used in these studies were FMDV strains, C-S8c1 and SAT-3. These viruses were selected because they are believed to depend completely on RGD-binding integrins for cellular internalization and to be unable to use HSPG as alternative receptors (reference 4 and unpublished observations). Figure 1 shows that extensive cytopathic effect was observed upon infection of the β6-transfected cells with FMDV, whereas the mock-transfected cells appeared resistant to infection even after a prolonged exposure to virus. Consistent with this observation, Fig. 2 shows that infection of the β6-transfected cells by FMDV results in the production of infectious virus whereas under the same assay conditions only a low level of virus is produced by the mock-transfected cells. The susceptibility of the mock-transfected cells to infection, although low, was greater for C-S8c1 than for SAT-3, as measured by both virus yield (Fig. 2) and the number of infectious centers (Table 1). The reason for this small difference was not investigated, but it is likely to be due to infection by an integrin-independent mechanism, most likely involving HSPG, as propagation of C-S8c1 in BHK cells has been shown to result in a virus population that binds heparin (4). To confirm that the reduction in virus yield produced by the mock-transfected cells was not due to an inability of these cells to support virus replication, we infected the mock- and β6-transfected cells with the O1BFS strain of FMDV. O1BFS binds to heparan sulfate and uses HSPG as alternative receptors for cell entry without the mediation of functional integrins (18, 25). The virus yields obtained using this virus were comparable for the mock- and β6-expressing cells (∼2 × 106 PFU/ml), indicating that the failure of the mock-transfected cells to support infection was not due to a general intracellular defect in virus replication (data not shown).
FIG. 1.
Expression of αvβ6 correlates with cell death on infection by FMDV. Mock-transfected (mock) and β6-transfected (β6) SW480 cells in 24-well plates were either uninfected (Un) or infected with FMDV strain C-S8c1 (5 × 105 PFU) or SAT-3 (4 × 106 PFU) at 37°C. At 48 h postinfection, the cells were fixed and stained as described in Materials and Methods.
FIG. 2.
Comparison of FMDV production by mock-transfected and β6-transfected SW480 cells. Mock-transfected (dashed lines) and β6-transfected (solid lines) cells in 35-mm-diameter dishes were infected with FMDV strain C-S8c1 or SAT-3 at MOIs of 2 and 10 PFU/cell, respectively, for 1 h at 37°C. Infectious virus that remained on the outsides of the cells was inactivated with acid, and the cells were cultivated in cell growth medium. The appearance of infectious virus in the cell culture medium (PFU/ml) was analyzed with time (hours postinfection) by plaque assay on BHK cells. The error bars indicate standard deviations.
TABLE 1.
Expression of αvβ6 correlates with increased infection by FMDVa
| Virus | Infection | Expression of αvβ6b | Incubation with virus (°C/min) | No. of infectious centersc |
|---|---|---|---|---|
| SAT-3 | Mock | − | 4/15 | <10 |
| Mock | − | 4/30 | <10 | |
| β6 | + | 4/15 | <10 | |
| β6 | + | 4/30 | <10 | |
| C-S8c1 | Mock | − | 4/15 | <10 |
| Mock | − | 4/30 | 12 | |
| β6 | + | 4/15 | <10 | |
| β6 | + | 4/30 | <10 | |
| SAT-3 | Mock | − | 37/15 | <10 |
| Mock | − | 37/30 | <10 | |
| β6 | + | 37/15 | 28,000 | |
| β6 | + | 37/30 | 35,500 | |
| C-S8c1 | Mock | − | 37/15 | 25 |
| Mock | − | 37/30 | 90 | |
| β6 | + | 37/15 | 31,500 | |
| β6 | + | 37/30 | 70,500 |
Triplicate monolayers of target cells (mock-transfected [Mock] or β6-transfected [β6]) were infected with virus at an MOI of ∼1 PFU/cell under the conditions of time and temperature indicated. Infected cells were then used in an infectious-center assay and layered onto monolayers of indicator cells (BHK).
+, present; −, absent.
Number of infectious centers per 5 × 105 target cells infected.
In order to quantitate the difference in the susceptibilities of the mock- and β6-transfected cells to FMDV, we compared the number of productive infectious events occurring on infection using an infectious-center assay (Table 1). When the target cells (mock or β6 transfected) were infected at 4°C to limit virus entry, virtually no infectious centers were observed for either cell line, indicating that inactivation of the virus that remained on the outsides of the cells following infection had been effective (see Materials and Methods). However, when the infection was allowed to proceed under conditions that permit virus entry (37°C), the β6-transfected cells were found to be more susceptible to infection than the mock-transfected cells by 2 to 3 orders of magnitude (Table 1). Taken together, the above data show that expression of αvβ6 at the cell surface correlates with a lytic infection of SW480 cells by FMDV and that the integrin is acting to increase the rate at which the virus enters the cell.
To investigate further the role of αvβ6 in infection, we analyzed the ability of FMDV to bind to the mock- and β6-transfected cells by flow cytometry. Figure 3 shows that binding of FMDV to the mock-transfected cells could not be detected, whereas virus binding to the β6-transfected cells was clearly evident. These data indicate that the failure of the mock-transfected cells to support infection by FMDV is due, at least in part, to the inability of these cells to support virus binding.
FIG. 3.
Flow cytometric analysis of FMDV binding to mock-transfected and β6-transfected SW480 cells. FMDV strain O1K-cad2 (10 μg/ml) was bound to mock-transfected (A) and β6-transfected (B) cells, and the cells were analyzed by flow cytometry using the anti-FMDV antibody D9 (10 μg/ml) and a goat anti-mouse IgG2a-specific R-phycoerythrin conjugate as the secondary antibody (solid histogram). The open histogram (negative control) shows the cells incubated with the secondary antibody in the absence of both the virus and D9. Additional negative controls where only the virus or MAb D9 was omitted from the assay gave results nearly identical to those with the control shown and, for clarity, are not shown.
As the β6-transfected cells express several different RGD-dependent integrins, we next used FMDV as a competitor in experiments designed to inhibit the binding of MAbs that recognize specific integrin heterodimers. All of the MAbs used for this study are functional blocking antibodies, i.e., they block binding of the natural ligands to their integrin receptors. We reasoned that if a large multivalent ligand, such as FMDV, bound to the RGD-binding site on the integrin, then the virus would be expected to block binding of the MAbs. Figure 4 shows that preincubation of the β6-transfected cells with O1K-cad2, a non-heparin binding strain of FMDV, inhibited binding of MAb 10D5 (anti-αvβ6), whereas under identical conditions, O1K-cad2 failed to inhibit binding of the MAbs P1F6 and SAM-1, which recognize αvβ5 and α5β1, respectively. The same result was obtained when the SAT-3 virus was used as the competitor (data not shown). The above data imply that FMDV is binding to αvβ6 on the β6-transfected cells and not to the other RGD-dependent integrin expressed on these cells. That virus was binding to αvβ6 was confirmed by reciprocal experiments where anti-integrin antibodies were used to block binding of virus to the β6-transfected cells. Consistent with the data shown in Fig. 4, MAb 10D5 was found to inhibit virus binding to the β6-transfected cells by >90%, implying that αvβ6 serves as the major receptor for virus attachment on these cells (Fig. 5). Under identical conditions, P1F6 (anti-αvβ5) had no effect on virus binding.
FIG. 4.
FMDV inhibits binding of MAb 10D5 (anti-αvβ6) to β6-transfected SW480 cells. β6-transfected cells in duplicate wells were incubated with FMDV O1K-cad2 prior to the addition of anti-integrin antibodies (final concentration, 5 μg/ml). The antibodies used were the functional blocking MAbs P1F6 (anti-αvβ5), 10D5 (anti-αvβ6), and SAM-1 (anti-α5β1). The cells were analyzed for anti-integrin antibodies by flow cytometry using a goat anti-mouse IgG R-phycoerythrin conjugate. For each of the antibodies, 100% binding was the fluorescence determined in the absence of virus. Background fluorescence (mean fluorescence intensity, 2.5) was determined by incubating the cells in the presence of the secondary antibody alone. One experiment representative of two is shown.
FIG. 5.
Anti-αvβ6 MAb 10D5 inhibits binding of FMDV to β6-transfected SW480 cells. β6-transfected cells in triplicate wells were incubated with the antibody P1F6 (anti-αvβ5) or 10D5 (anti-αvβ6) prior to the addition of virus (O1K-cad2; 10 μg/ml), and the cells were analyzed for bound virus by flow cytometry. When 10D5 (mouse IgG2a) was used as a competitor, virus was detected with the anti-FMDV MAb B2 (mouse IgG1; 10 μg/ml). When P1F6 (mouse IgG1) was used as a competitor, virus was detected with the anti-FMDV MAb D9 (mouse IgG2a; 10 μg/ml). Anti-FMDV antibodies were detected using goat anti-mouse IgG isotype-specific R-phycoerythrin-conjugated secondary antibodies. Background fluorescence was determined by incubating the cells in the presence of the secondary antibodies alone and was subtracted from the data. One experiment representative of two is shown. The error bars indicate standard deviations.
To verify that the virus was binding to αvβ6 through an RGD-dependent interaction, we next sought to inhibit virus binding to αvβ6 on the β6-transfected cells using an RGD-containing peptide with its sequence derived from the GH loop of type O FMDV. Figure 6 shows that FMDV binding to αvβ6 is inhibited by >95% by the RGD peptide, demonstrating that the virus does indeed bind to αvβ6 through an RGD-mediated interaction. Inhibition was specific, as the control RGE version of the peptide had a minimal effect on virus binding at the highest concentration used.
FIG. 6.
Binding of FMDV to β6-transfected SW480 cells is specifically inhibited by an RGD peptide. β6-transfected cells in triplicate wells were incubated with the peptide VPNLRGDLQVLA or VPNLRGELQVLA (RGE) prior to the addition of O1K-cad2 (10 μg/ml). Cell-bound virus was detected by flow cytometry using the anti-FMDV antibody D9 (10 μg/ml) and a goat anti-mouse IgG2a-specific R-phycoerythrin-conjugated secondary antibody. Background fluorescence was determined by incubating the cells in the presence of the secondary antibody alone and was subtracted from the data. One experiment representative of two is shown. The error bars indicate standard deviations.
The above data show that FMDV binds to αvβ6 on the surfaces of β6-transfected SW480 cells and that this interaction can be specifically inhibited by an RGD-containing peptide and the anti-αvβ6 MAb, 10D5. Figures 7 and 8 show that the inhibitory effects of these reagents on virus binding correlate with the ability to inhibit infection. Thus, the RGD peptide was found to specifically inhibit infection of the β6-transfected cells by FMDV (Fig. 7). Similarly, Fig. 8A shows that infection of the β6-transfected cells by FMDV C-S8c1 is inhibited by MAb 10D5. Inhibition by 10D5 was found to be concentration dependent, with 50% inhibition seen at an antibody concentration of ∼5 μg/ml. The same result was obtained when SAT-3 was used as the infecting virus. Figure 8B shows that the inhibitory effect of MAb 10D5 was specific, as under the conditions where 10D5 inhibited infection by >99%, antibodies to α5β1 and αvβ5 had no effect on infection.
FIG. 7.
Infection of β6-transfected SW480 cells is specifically inhibited by an RGD peptide. β6-transfected cells in triplicate 35-mm-diameter dishes were incubated with 200 μl of 0.2 mM peptide (VPNLRGDLQVLA or VPNLRGELQVLA [RGE]) in PBS containing 2 mM CaCl2 and 1 mM MgCl2, (assay buffer) or with assay buffer alone (control) prior to infection by FMDV C-S8c1 or SAT-3 at an MOI of ∼0.2 PFU/cell. The infected cells were used in an infectious-center assay. The number of infectious centers (mean ± standard deviation) is shown as a percentage of the control. One experiment representative of two is shown.
FIG. 8.
Infection of β6-transfected SW480 cells is specifically inhibited by the MAb 10D5 (anti-αvβ6). (A) Duplicate monolayers of β6-transfected cells in 35-mm-diameter dishes were incubated with 100 (a), 10 (b), or 1 (c) μg of 10D5/ml diluted in PBS (pH 7.5)–2 mM CaCl2–1 mM MgCl2 (assay buffer) or with assay buffer alone (d) before the addition of virus (C-S8c1; MOI, ∼0.2 PFU/cell), and the infected cells were used in an infectious-center assay. The infectious centers for one cell dilution are shown. Other cell dilutions used to quantitate the percent inhibition for each concentration of 10D5 are not shown. (B) Triplicate monolayers of β6-transfected cells in 35-mm-diameter dishes were incubated with 200 μl (50 μg/ml) of the functional blocking MAbs P1F6 (anti-αvβ5), 10D5 (anti-αvβ6), and SAM-1 (anti-α5β1) in assay buffer or with assay buffer alone (control) prior to infection by FMDV C-S8c1 (MOI, ∼0.2 PFU/cell), and the infected cells were used in an infectious-center assay. The number of infectious centers is shown as a percentage of the control. The data show the mean (± standard deviation) of three separate experiments.
DISCUSSION
Field isolates of FMDV are believed to use RGD-dependent integrins as cellular receptors for virus internalization in vivo (37). In this study, we show that the RGD-dependent integrin αvβ6 functions as a cellular receptor for FMDV. The main evidence in support of this finding is (i) SW480 cells, which are normally nonpermissive for FMDV, become susceptible to infection upon transfection with the integrin β6 subunit and expression of αvβ6 at the cell surface as a result of an increased rate of virus entry; (ii) αvβ6 serves as the major receptor for attachment of FMDV on β6-transfected cells, as virus binding is inhibited by >90% by a MAb (10D5) that specifically recognizes αvβ6 and inhibits binding of its natural ligands; and (iii) consistent with the above observations, infection of the β6-transfected cells by FMDV is also inhibited by >99% by the same antibody (10D5). In addition, an RGD-containing peptide with its sequence derived from the GH loop of type O FMDV inhibits virus attachment and infection of the β6-transfected cells.
SW480 cells express two other RGD-dependent integrins, namely, α5β1 and αvβ5. Ligation and/or cross-linking of an integrin at the cell surface by natural protein ligands, small ligand-mimetic peptides, or anti-integrin antibodies has been shown to modulate the functions of other species of integrins (the target integrin) expressed on the same cell. This process is dependent on intracellular signaling and has been termed integrin cross talk (7). In some cases, the effects of cross talk are to stimulate the functions of the target integrin (40, 42). In interpreting our data, it is therefore important to consider the possibility that ligation of αvβ6 by a multivalent RGD ligand, such as FMDV, could result in the transient activation of other RGD-dependent integrins expressed on SW480 cells. However, the failure of functional blocking antibodies to either α5β1 or αvβ5 to inhibit infection by FMDV makes this scenario unlikely. Similarly, it may also be possible that a low level of virus binding to either α5β1 or αvβ5 could be sufficient to cross-link these integrins and trigger integrin cross talk pathways that result in activation of αvβ6 in order for αvβ6 to function as a receptor for FMDV. As we found that FMDV did not appear to inhibit binding of functional blocking antibodies to either α5β1 or αvβ5 (Fig. 4), implying that virus was not able to bind to these integrins, this scenario would also appear to be unlikely. Therefore, our data show that, under the assay conditions used in this study, neither α5β1 nor αvβ5 appears to have a role in infection of β6-transfected SW480 cells by FMDV. However, the functions of integrins, including their ligand binding or “activation state” (17, 23, 35, 41), and the rates at which they are internalized (8) are regulated by several complex mechanisms, which may differ depending on the cell type, the cellular environment, and the state of differentiation of the cell. How these mechanisms are controlled in vivo is at present unclear. Therefore, our data cannot rule out the possibility that, on different cell types or under different experimental conditions that affect the activation state of integrins, α5β1 and αvβ5 could serve as receptors for FMDV. Similarly, we cannot rule out the possibility that these integrin species could serve as receptors for FMDV on cells derived from the natural hosts.
Taken together, our data demonstrate that expression of αvβ6 on SW480 cells is required for infection by non-heparin binding strains of FMDV and that this species of integrin appears to be the sole representative of the RGD-binding integrins expressed on these cells that functions as a receptor for FMDV. However, our data cannot rule out the possibility that an as-yet-unidentified coreceptor(s) may be required for a post-receptor binding event that is necessary for virus internalization.
Expression of αvβ6 has been shown to enhance a lytic infection of SW480 cells mediated by another picornavirus, coxsackievirus B1 (3). Since, this virus lacks an RGD motif on its surface and binds to alternative receptors on SW480 cells, the role of integrin αvβ6 in the infection process is at present unclear (3). The same uncertainty does not apply to FMDV, since we have shown that αvβ6 is essential for binding and infection of SW480 cells. Across the FMDV serotypes, the majority of viruses have either a leucine (e.g., C-S8c1) or methionine (e.g., SAT-3) residue immediately following the RGD motif (RGD+1), and a leucine residue is highly conserved at the RGD+4 position (31). Leucine residues at these positions have been shown to be important for receptor recognition by FMDV, as mutations at these sites dramatically reduced the abilities of peptides derived from the RGD-containing loop of FMDV C-S8c1 to inhibit infection of BHK cells by that strain of virus (31). The similarity between the residues following the RGD motif of FMDV and those of LAP-1 and the observation that the pentapeptide DLXXL can inhibit the interaction between αvβ6 and fibronectin suggest that conservation of the leucine residues located at the RGD+1 and RGD+4 positions in FMDV may be driven by the requirement for virus binding to its integrin receptors, αvβ6 and αvβ3. αvβ3 is a multifunctional receptor that binds a broad range of RGD-containing ligands (23). Consistent with this role, ligand binding to αvβ3 has been shown to tolerate several different amino acids flanking the RGD, including those at the RGD+1 and RGD+4 positions (20). By contrast, αvβ6 binds to relatively few ligands and serves as a high-affinity receptor for LAP-1 (36). Thus, if conservation of the GH loop leucine residues in FMDV is driven by integrin binding, then it follows that αvβ6 rather than αvβ3 would be more likely to influence this process. Interestingly, the extracellular matrix protein tenascin, which is also a ligand for αvβ6, has a methionine residue immediately following its RGD, which is the second most common amino acid seen at this site in FMDV.
Prior to this study, αvβ3 was the only member of the integrin family that had been shown to act as a receptor for FMDV (6), but that integrin has limited expression on epithelial cells and cells of lymphoid origin (15, 33, 47), and it is these cell types in which FMDV is likely to reside during the initial phase of infection. By contrast, αvβ6 is expressed exclusively on epithelial cells, including sites where initial virus replication is believed to occur, making this integrin a more likely candidate as the receptor used by FMDV during the initial phase of infection in an animal.
ACKNOWLEDGMENTS
We thank M. Pitkeathly and S. Shah for the peptides and Stephen Archibald for help with the figures.
This work was supported by MAFF.
REFERENCES
- 1.Acharya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9Å resolution. Nature. 1989;337:709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- 2.Agrez M, Chen A, Cone R I, Pytela R, Sheppard D. The αvβ6 integrin promotes proliferation of colon carcinoma cells through a unique region of the β6 cytoplasmic domain. J Cell Biol. 1994;127:545–556. doi: 10.1083/jcb.127.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrez M, Shafren D R, Gu X, Cox K, Sheppard D, Barry R D. Integrin αvβ6 enhances coxsackievirus B1 lytic infection of human colon carcinoma cells. Virology. 1997;239:71–77. doi: 10.1006/viro.1997.8831. [DOI] [PubMed] [Google Scholar]
- 4.Baranowski E, Sevilla N, Verdaguer N, Ruiz-Jarabo C M, Beck E, Domingo E. Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J Virol. 1998;72:6362–6372. doi: 10.1128/jvi.72.8.6362-6372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belsham G J. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family: aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol. 1993;69:241–260. doi: 10.1016/0079-6107(93)90016-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berinstein A, Roivainen M, Hovi T, Mason P W, Baxt B. Antibodies to the vitronectin receptor (integrin αvβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69:2664–2666. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blystone S D, Slater S E, Williams M P, Crow M T, Brown E. A molecular mechanism of integrin crosstalk: αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretscher M S. Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 1992;11:405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuss J M, Gallo J, DeLisser H M, Kilmanskaya I V, Folkesson H G, Pittet J F, Nishimura S L, Aldape K, Landers D V, Carpenter W, Gillett N, Sheppard D, Matthay M A, Albelda S M, Krammer R H, Pytela R. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodelling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 10.Breuss J M, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin β6 messenger RNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 11.Brown C C, Meyer R F, Olander H J, House C, Mebus C A. A pathogenesis study of foot-and-mouth disease virus in cattle, using in situ hybridisation. Can J Vet Res. 1992;56:189–193. [PMC free article] [PubMed] [Google Scholar]
- 12.Brown C C, Olander H J, Meyer R F. A preliminary study of the pathogenesis of foot-and-mouth disease virus, using in situ hybridisation. Vet Pathol. 1991;28:216–222. doi: 10.1177/030098589102800305. [DOI] [PubMed] [Google Scholar]
- 13.Burrows R, Mann J A, Garland A J M, Greig A, Goodridge D. The pathogenesis of natural and stimulated natural foot-and-mouth disease virus infection in cattle. J Comp Pathol. 1981;91:599–609. doi: 10.1016/0021-9975(81)90089-x. [DOI] [PubMed] [Google Scholar]
- 14.Curry S, Fry E, Blakemore W E, Abu-Ghazaleh R, Jackson T, King A, Lea S, Newman J, Rowlands D, Stuart D. Perturbations in the surface structure of A22 Iraq foot-and-mouth disease virus accompanying coupled changes in host cell specificity and antigenicity. Structure. 1996;4:135–145. doi: 10.1016/s0969-2126(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 15.Damjanovich L, Albelda S M, Mette S A, Buck C A. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am J Respir Cell Mol Biol. 1992;6:197–206. doi: 10.1165/ajrcmb/6.2.197. [DOI] [PubMed] [Google Scholar]
- 16.Dedhar S, Hannigan G E. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 17.Finnemann S, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic and photoreceptor compete for αvβ3 and αvβ5 integrins, protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry E, Lea S M, Jackson T, Newman J W I, Ellard F M, Blakemore W E, Abu-Ghazaleh R, Samuel A, King A M Q, Stuart D I. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Krammer R, Clark R, Uitto V, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Investig Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 20.Healy J M, Murayama O, Maeda T, Yoshino K, Sekiguchi K, Kikuchi M. Peptide ligands for integrin αvβ3 selected from random phage display libraries. Biochemistry. 1995;34:3948–3955. doi: 10.1021/bi00012a012. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Wu J F, Cass D, Erle D J, Corey D, Young S G, Farese R V, Jr, Sheppard D. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lungs and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Wu J F, Spong S, Sheppard D. The integrin αvβ6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111:2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 23.Hynes R O. Integrins: versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 24.Jackson T, Sharma A, Abu-Ghazaleh R, Blakemore W E, Ellard F M, Simmons D L, Newman J W I, Stuart D I, King A M Q. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth disease virus to the purified integrin αvβ3 in vitro. J Virol. 1997;71:8357–8361. doi: 10.1128/jvi.71.11.8357-8361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson T, Ellard F M, Abu-Ghazaleh R, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W I, King A M Q. Efficient infection of cells in culture by type O foot-and mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraft S, Diefenbach B, Mehta R, Jonczyk A, Luckenbach A, Goodman S L. Definition of an unexpected ligand recognition motif for αvβ6 integrin. J Biol Chem. 1999;274:1979–1985. doi: 10.1074/jbc.274.4.1979. [DOI] [PubMed] [Google Scholar]
- 27.Lea S, Abu-Ghazaleh R, Blakemore W E, Curry S, Fry E, Jackson T, King A, Logan D, Newman J, Stuart D. Structural comparison of two strains of foot-and-mouth disease virus subtype O1 and a laboratory antigenic variant, G67. Structure. 1995;3:571–580. doi: 10.1016/s0969-2126(01)00191-5. [DOI] [PubMed] [Google Scholar]
- 28.Lea S, Hernández J, Blakemore W E, Brocchi E, Curry S, Domingo E, Fry E, Abu-Ghazaleh R, King A, Newman J, Stuart D, Mateu M G. The structure and antigenicity of a type C foot-and-mouth disease virus. Structure. 1994;2:123–139. doi: 10.1016/s0969-2126(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 29.Logan D, Abu-Ghazaleh R, Blakemore W E, Curry S, Jackson T, King A, Lea S, Lewis R, Newman J W I, Parry N, Rowlands D, Stuart D, Fry E. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature. 1993;362:566–568. doi: 10.1038/362566a0. [DOI] [PubMed] [Google Scholar]
- 30.Mason P W, Reider E, Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateu M G, Luz Valero M, Andreu D, Domingo E. Systematic replacement of amino acid residues within an Arg-Gly-Asp-containing loop of foot-and-mouth disease virus and effects on cell recognition. J Biol Chem. 1996;271:12814–12819. doi: 10.1074/jbc.271.22.12814. [DOI] [PubMed] [Google Scholar]
- 32.McCahon D, Crowther J R, Belsham G J, Kitson J D A, Duchesne M, Have P, Meloen R H, Morga D O, de Simone F. Evidence for at least 4 antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple monoclonal antibody-resistant mutants. J Gen Virol. 1989;70:639–645. doi: 10.1099/0022-1317-70-3-639. [DOI] [PubMed] [Google Scholar]
- 33.Mette S A, Pilewski J, Buck C A, Albelda S M. Distribution of integrin cell adhesion receptors in normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am J Respir Cell Mol Biol. 1993;8:562–572. doi: 10.1165/ajrcmb/8.5.562. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery A M P, Reisfeld R A, Cheresh D A. Integrin αvβ3 rescues melanoma cells from apoptosis in three dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mould A P, Akiyama S K, Humphries M J. Regulation of integrin α5β1-fibronectin interactions by divalent cations. J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- 36.Munger J S, Huang X, Kawakatsu H, Griffiths M D J, Dalton S L, Wu J, Pittet J F, Kaminski N, Garat C, Matthay M A, Rifkin D B, Sheppard D. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 37.Neff S, Sa-Carvalho D, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura S L, Sheppard D, Pytela R. Integrin αvβ8. Interaction with vitronectin and functional divergence of the β8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- 39.O'Toole T E, Katagiri Y, Faull R J, Peter R K, Tamura R, Quaranta R, Loftus J C, Shattil S J, Ginsberg M H. Integrin cytoplasmic domains mediate inside-out signal-transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pacifici R, Roman J, Kimble R, Civitelli R, Brownfield C M, Bizzarri C. Ligand binding to monocyte α5β1 integrin activates the α2β1 receptor via the α5 subunit cytoplasmic domain and protein kinase C. J Immunol. 1994;153:2222–2233. [PubMed] [Google Scholar]
- 41.Pampori N, Hato T, Stupack D G, Aidoudi S, Cheresh D A, Nemerow G R, Shattil S J. Mechanisms and consequences of affinity modulation of integrin αvβ3 detected with a novel patch-engineered monovalent ligand. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- 42.Pijuan-Thompson V, Gladson C L. Ligation of integrin α5β1 is required for internalization of vitronectin by integrin αvβ3. J Biol Chem. 1997;272:2736–2743. doi: 10.1074/jbc.272.5.2736. [DOI] [PubMed] [Google Scholar]
- 43.Prieto A L, Edelman G M, Crossin K L. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci USA. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sa-Carvalho D, Rieder E, Baxt B, Rodarte R, Tanuri A, Mason P W. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol. 1997;71:5115–5123. doi: 10.1128/jvi.71.7.5115-5123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salt J S. Persistent infection with foot-and-mouth disease virus. Top Trop Virol. 1998;1:77–129. [Google Scholar]
- 46.Schnapp L M, Hatch N, Ramos D M, Kilmanskaya I V, Sheppard D, Pytela R. The human integrin α8β1 functions as a receptor for tenascin, fibronectin and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- 47.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 48.Weinacker A, Chen A, Agrez M, Cone R I, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin αvβ6 in cell attachment to fibronectin. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 49.Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins α9β1, αvβ3 and αvβ6 on cell proliferative responses to tenascin. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]