Abstract
Introduction: The rate of isolated locoregional recurrence after surgery for pancreatic adenocarcinoma (PDAC) approaches 25%. Ablative radiation therapy (A-RT) has improved outcomes for locally advanced disease in the primary setting. We sought to evaluate the outcomes of salvage A-RT for isolated locoregional recurrence and examine the relationship between subsequent patterns of failure, radiation dose, and treatment volume. Methods: We conducted a retrospective analysis of all consecutive participants who underwent A-RT for an isolated locoregional recurrence of PDAC after prior surgery at our institution between 2016 and 2021. Treatment consisted of ablative dose (BED10 98–100 Gy) to the gross disease with an additional prophylactic low dose (BED10 < 50 Gy), with the elective volume covering a 1.5 cm isotropic expansion around the gross disease and the circumference of the involved vessels. Local and locoregional failure (LF and LRF, respectively) estimated by the cumulative incidence function with competing risks, distant metastasis-free and overall survival (DMFS and OS, respectively) estimated by the Kaplan–Meier method, and toxicities scored by CTCAE v5.0 are reported. Location of recurrence was mapped to the dose region on the initial radiation plan. Results: Among 65 participants (of whom two had two A-RT courses), the median age was 67 (range 37–87) years, 36 (55%) were male, and 53 (82%) had undergone pancreaticoduodenectomy with a median disease-free interval to locoregional recurrence of 16 (range, 6–71) months. Twenty-seven participants (42%) received chemotherapy prior to A-RT. With a median follow-up of 35 months (95%CI, 26–56 months) from diagnosis of recurrence, 24-month OS and DMFS were 57% (95%CI, 46–72%) and 22% (95%CI, 14–37%), respectively, while 24-month cumulative incidence of in-field LF and total LRF were 28% (95%CI, 17–40%) and 36% (95%CI 24–48%), respectively. First failure after A-RT was distant in 35 patients (53.8%), locoregional in 12 patients (18.5%), and synchronous distant and locoregional in 10 patients (15.4%). Most locoregional failures occurred in elective low-dose volumes. Acute and chronic grade 3–4 toxicities were noted in 1 (1.5%) and 5 patients (7.5%), respectively. Conclusions: Salvage A-RT achieves favorable OS and local control outcomes in participants with an isolated locoregional recurrence of PDAC after surgical resection. Consideration should be given to extending high-dose fields to include adjacent segments of at-risk vessels beyond direct contact with the gross disease.
Keywords: pancreatic cancer, A-RT, pancreatic adenocarcinoma, salvage therapy, radiation therapy, ablative radiation, SABR, SBRT, hypofractionated RT, isolated local recurrence
1. Introduction
Although the management of pancreatic cancer is evolving, progress has been slow, with survival at 5 years estimated at 10% [1,2]. Complete surgical resection, when possible, is considered the best curative treatment [3]. For 15–20% of patients who present with resectable disease, 5-year overall survival after surgery ranges from 12–20% [4]. Isolated local recurrence has been reported in 28–34% [5,6,7,8,9,10,11] and is a significant cause of late disease-related mortality [12,13].
There is currently no established standard treatment for patients with isolated local or locoregional recurrence [14,15,16]. Local therapy options include resection and (chemo)radiotherapy (CRT) [17,18,19,20,21,22,23]. Surgery is rarely an option due to anatomic constraints. Conventionally fractionated standard CRT regimens to a total dose of 39–60 Gy in 1.8–2.0 Gy per fraction are associated with median disease-free and overall survival (DFS and OS, respectively) of 10–17 months and 10–19 months, respectively [20,21,22,24]. Small series using stereotactic body radiotherapy (SBRT) with standard or dose-escalated fractionation schemes including single fraction doses of 18–24 Gy or 5-fraction regimens to 20–50 Gy have demonstrated a median OS of 8.8–17.0 months [17,25,26,27,28,29].
In addition to SBRT, dose escalation to a biologically effective dose (BED) nearing or exceeding 100 Gy, assuming a standard tumor alpha/beta ratio of 10 (BED10), can be accomplished using a hypofractionated approach to a total dose of 67.5–75 Gy in 15–25 fractions [30]. Treatment to approximately BED10 of 100 Gy, also termed ablative radiotherapy (A-RT), using either SBRT or the hypofractionated approach has recently been shown to be associated with better overall survival than standard-dose RT in the setting of primary disease [31,32]. We sought to evaluate the outcomes of patients treated with A-RT for isolated locoregional recurrence and examine the relationship between the subsequent patterns of local progression, radiation dose, and treatment volume.
2. Methods
2.1. Patient Cohort
This is an institutional review board-approved (16–370) retrospective analysis of all consecutive patients who underwent A-RT for an isolated local or locoregional recurrence after prior pancreaticoduodenectomy or distal pancreatectomy at Memorial Sloan Kettering Cancer Center between June 2016 and 2021. A radiographic/clinical or pathologic diagnosis of recurrence was allowed.
2.2. Ablative Technique
The A-RT technique has been described previously [30,33,34]. Briefly, patients were simulated in a customized immobilization device using the Varian RPM system for motion management: deep-inspirational breath hold (preferred) or end-expiratory gating as tolerated by the patient. Target and organ-at-risk (OAR) volumes were delineated on a thin-slice pancreatic protocol contrast-enhanced CT. Prescription doses/fractionation schemes were based on the distance from the target to the luminal GI tract and included 75 Gy/25 fractions (BED10 = 97.5 Gy), 67.5 Gy/15 fractions (BED10 = 97.88 Gy), 60 Gy/10 fractions (BED10 = 96.0 Gy), and 50 Gy/5 fractions (BED10 = 100.0 Gy). Prophylactic low-dose (BED10 < 50 Gy) elective nodal and neural plexus coverage included peripancreatic, celiac axis, and superior mesenteric artery regions in all patients as well as porta hepatis/splenic hilum coverage in select patients depending on the tumor location; in general, 1 cm expansions around vessels were used. Luminal OAR Dmax, D2cc, and D5cc constraints were prioritized over PTV/GTV coverage. Treatments were delivered using daily cone-beam CT (CBCT) guidance. Adaptive re-planning was implemented selectively for patients with consistently unfavorable luminal OAR displacement toward the target on daily CBCTs.
2.3. Study Definitions
Local and regional failures were defined according to modified RECIST 1.1 criteria. That is, local in-field failure required an increase of at least 20% in the sum of the largest diameters of target-irradiated lesions or the smaller diameters of pathologic irradiated nodes, along with an absolute increase of at least 5 mm. Regional nodal failure was defined as nodal failure that was not an initially targeted lesion. The following lymph node basins were considered regional (rather than metastatic): common bile duct, common hepatic artery, portal vein, right gastric/pyloric vein, posterior/anterior pancreaticoduodenal vasculature, superior mesenteric vein, and/or right lateral wall of the superior mesenteric artery, common hepatic artery, celiac axis, splenic artery, and/or splenic hilum. Para-aortic nodal basins were not considered regional failures (i.e., defined as metastatic failures).
The pattern of failure was characterized by the location of the recurrence with regard to the dose region on the initial radiation plan. Failures centered within the volume encompassed by the tumor prescription isodose line were defined as high-dose failures while those centered outside the tumor prescription isodose line but within the elective isodose line were defined as low-dose failures. Any lesion within the low-dose region was defined as a regional failure whether it appeared to be a lymph node or tumor extension along the vessel. Out-of-field failure was defined as any local or regional failure with its center outside of the elective low-dose prescription field.
Toxicities were graded based on CTCAE v.5 criteria. Toxicities were defined as acute and late if they occurred within or beyond 3 months of RT initiation, respectively. Attribution of adverse events to RT required confirmed or possible location within the RT field.
2.4. Statistical Analysis
In-field local failure (LF), locoregional failure (LRF), distant metastasis-free survival (DMFS), and overall survival (OS) were defined from the diagnosis of recurrence and from the start of RT. For LF, LRF, and DMFS, observations were censored at the date of last evaluation for the event of interest. For OS, patients were censored at the date of last contact. Kaplan–Meier methodology was used to estimate OS and DMFS, while cumulative incidence functions with competing risks of death were used to estimate LF and LRF.
The associations between baseline characteristics and outcomes (OS and LRF from RT start date) were analyzed using Cox proportional hazards regression and Fine–Gray competing risks regression.
All analyses were conducted using R version 4.2.2 (Vienna, Austria) with the tidyverse (v1.3.2), gtsummary (v1.6.2), tidycmprsk (v0.2.0), and ggsurvfit (v0.2.0) packages.
3. Results
3.1. Baseline Characteristics
Between June 2016 and January 2021, 65 participants received 67 A-RT courses for isolated local (n = 48) or locoregional recurrence (n = 17) of pancreatic cancer (Table 1). Median age at the time of RT was 67 (range 37–87) years and 36 participants (55%) were male. The primary surgery was pancreaticoduodenectomy (Whipple) for 53 (82%) participants and distal pancreatectomy for 12 (18%) participants. At the time of surgery, 57 (88%) participants had pT1/T2 and 42 (65%) participants had pN+ disease. Margins were negative (R0) in 34 (52%) participants, close (R0, tumor < 1 mm from surgical margin) in 14 (22%) participants, and positive (R1) in 17 (26%) participants. The median disease-free interval to diagnosis of recurrence was 16 months (range, 6–71 months). Median CA 19-9 at the time of recurrence diagnosis and following chemotherapy was 65 U/mL (range, 0–3919 U/mL) and 50 U/mL (IQR 0–561 U/mL), respectively.
Table 1.
(a) Baseline characteristics of the study cohort, (b) Treatment details for patients in the study cohort.
| (a) Baseline Characteristics of the Study Cohort | |
|---|---|
| Characteristic | N = 65 * |
| Age in years (range) | 67 (37–87) |
| Sex | |
| Female | 29 (45%) |
| Male | 36 (55%) |
| Histology | |
| Adenocarcinoma | 62 (95%) |
| Mucinous | 1 (2%) |
| Acinar cell | 1 (2%) |
| Colloid | 1 (2%) |
| T stage | |
| T1 | 16 (25%) |
| T2 | 41 (63%) |
| T3/T4 | 8 (12%) |
| N stage | |
| N0 | 23 (35%) |
| N1 | 26 (40%) |
| N2 | 16 (25%) |
| Initial pathologic stage | |
| I | 20 (31%) |
| II | 28 (43%) |
| III | 17 (26%) |
| Surgery type | |
| Distal | 12 (18%) |
| Whipple | 53 (82%) |
| Margin status at surgery | |
| Negative (R0) | 34 (52%) |
| Positive (R1) | 17 (26%) |
| Close (R0, tumor < 1 mm) | 14 (22%) |
| Disease-free interval, months (range) | 16 (6–71) |
| (b) Treatment Details for Patients in the Study Cohort | |
| Characteristic | N = 65 * |
| NACT | 27 (42%) |
| NACT regimen | |
| FOLFIRINOX | 13 (48%) |
| gem/Nab-paclitaxel | 12 (43%) |
| FOLFOX | 1 (4%) |
| G-FLIP | 1 (4%) |
| No NACT | 38 |
| Length of NACT, months (range) (N = 27) | 3.22 (0.46–13.96) |
| CA 19-9 at diagnosis, U/mL (range) | 65 (0–3919) |
| CA 19-9 post NACT, U/mL (range) (N = 27) | 50 (0–561) |
| Change in CA 19-9, (N = 27) | −3 (−3358, 135) |
| Salvage for local vs. nodal recurrence | |
| Local | 48 (74%) |
| Local and nodal | 10 (15%) |
| Nodal | 7 (11%) |
| Prior RT | 10 (15%) |
| Ablative RT dose (N = 67 A-RT courses) | |
| 75 Gy/25 fractions | 37 (55%) |
| 67.5 Gy/15 fractions | 23 (34%) |
| Other fractionation schemes | 7 (10%) |
* Unless otherwise stated. Abbreviations: NACT: neoadjuvant chemotherapy; FOLFIRINOX: folinic acid, fluorouracil, irinotecan hydrochloride, and oxaliplatin; FOLFOX: folinic acid, fluorouracil, and oxaliplatin; G-FLIP: gemcitabine, 5-fluorouracil, leucovorin, and cisplatin; RT: radiation therapy.
Of 65 participants, 27 (42%) received chemotherapy prior to A-RT, including modified FOLFIRINOX (folinic acid, fluorouracil, irinotecan hydrochloride, and oxaliplatin; n = 14), gemcitabine/Nab-paclitaxel (n = 12), and other regimens (n = 2), for a median of 3.2 months (range, 0.5–14.0 months). Participants who did and did not receive chemotherapy began A-RT at a median of 4.2 months (range, 2–14.7 months) and 1.3 months (range, 0.2–6.1 months) after recurrence diagnosis, respectively. Most common fractionation schemes were 75 Gy/25 fx (N = 37) and 67.5 Gy/15 fx (N = 23). Full treatment details are summarized in Table 1.
3.2. Disease Outcomes
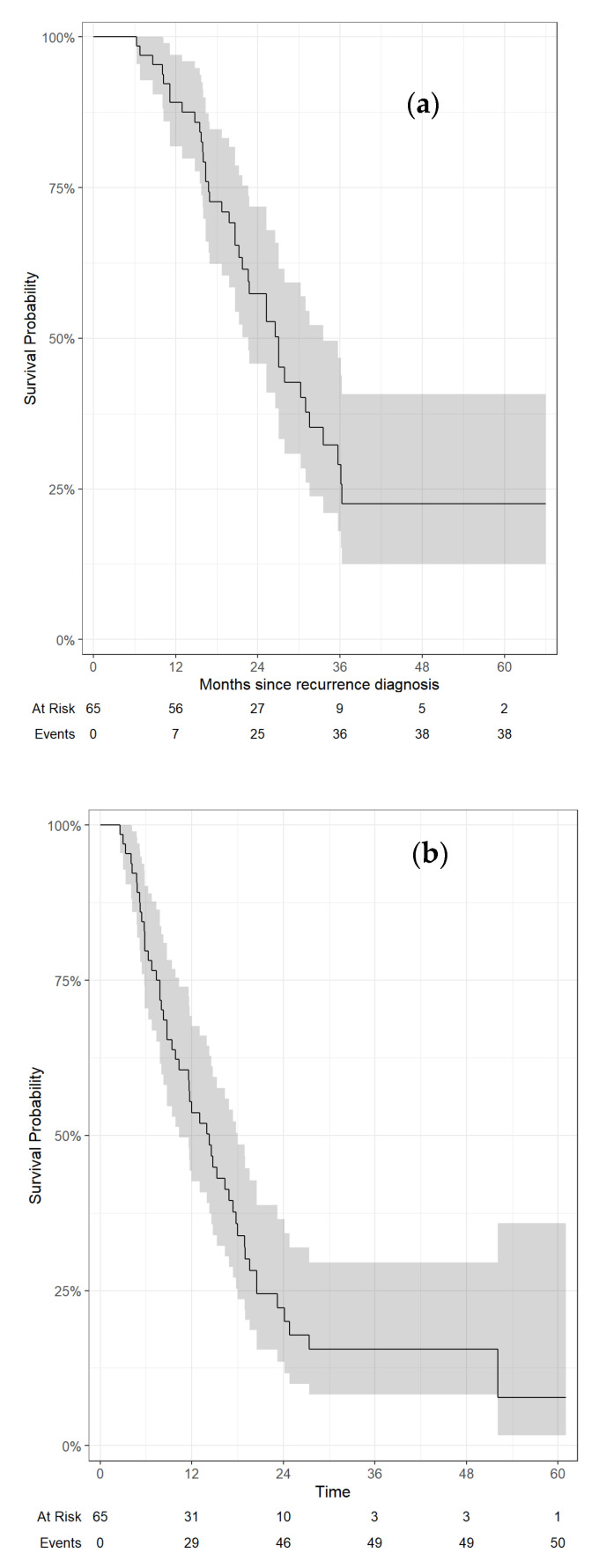
With a median follow-up of 21.0 months from diagnosis of recurrence (and 18.4 months from A-RT start), 38 of 65 (58.5%) patients had died. Median OS rates from diagnosis of recurrence and A-RT were 27 months (95%CI, 23–34 months) and 22 months (95%CI, 18–30 months), respectively. Twelve- and 24-month OS rates from diagnosis of recurrence were 89% (95%CI, 82–97%) and 57% (95%CI, 46–72%), respectively (Figure 1a). Twelve- and 24-month OS rates from A-RT were 81% (95%CI, 72–91%) and 45% (95%CI, 33–61%), respectively.
Figure 1.
Overall survival (a) and distant metastasis-free survival (b) from diagnosis of recurrence.
Median DMFS from diagnosis of recurrence was 14 months (95%CI, 10–18 months); median DMFS from A-RT was 10 months (95%CI, 7.5–14 months). Twelve- and 24-month DMFS rates from diagnosis of recurrence were 54% (95%CI, 43–68%) and 22% (95%CI, 14–37%) (Figure 1b), respectively. Twelve- and 24-month DMFS rates from A-RT were 42% (95%CI, 31–56%) and 18% (95%CI, 10–33%), respectively.
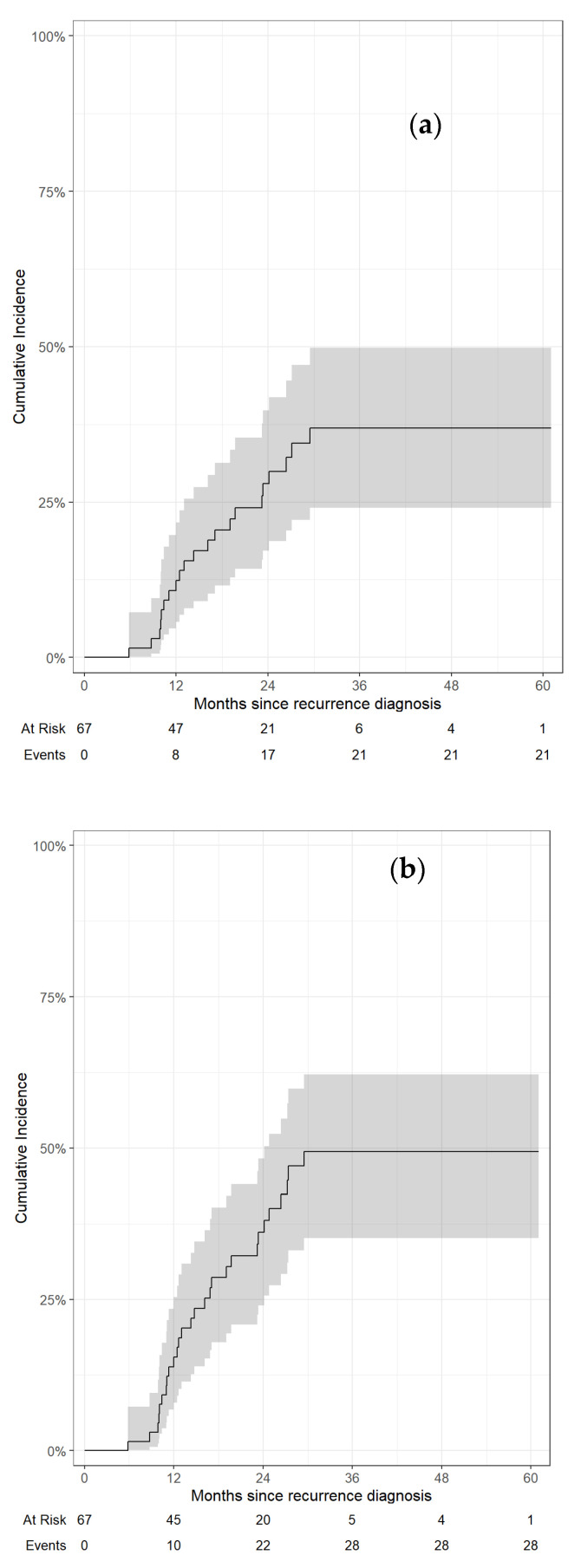
Twenty-eight total locoregional failures were recorded, including 13 in-field failures of the irradiated target alone. Twelve- and 24-month rates of in-field LF from diagnosis of recurrence were 12% (95%CI, 5.7–22%) and 28% (95%CI, 17–40%), respectively, while 12- and 24-month rates of total LRF from diagnosis of recurrence were 15% (95%CI, 7.9–25%) and 36% (95%CI, 24–48%), respectively. (Figure 2). Twelve- and 24-month rates of in-field LF from A-RT were 16% (95%CI, 8.0–26%) and 32% (95%CI, 21–35%), respectively. Twelve- and 24-month rates of total LRF from A-RT were 20% (95%CI, 11–31%) and 44% (95%CI 31–56%), respectively.
Figure 2.
Local in-field failure (a) and cumulative locoregional failure (b) from diagnosis of recurrence.
3.3. Patterns of Failure
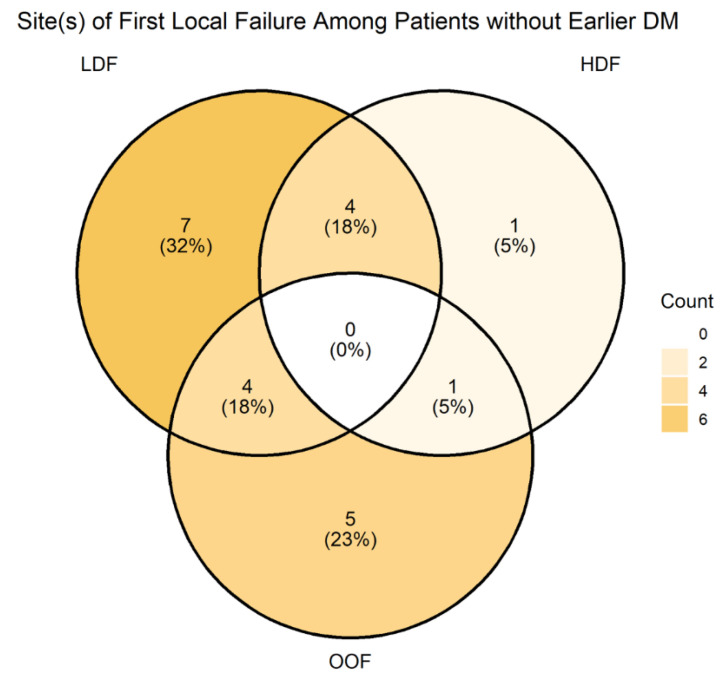
The site of first progression after A-RT was distant in 35 patients (53.8%), locoregional in 12 patients (18.5%), and synchronous distant and locoregional in 10 patients (15.4%). Of the 22 first locoregional failures, 17 occurred at least partly in field (i.e., covered by either ablative or elective dose volumes) and 5 were entirely out of field (Figure 3). Of the five isolated out-of-field failures, all were located within 1 cm of the RT field edge, two were in the left para-aortic basin, one was in the gastrohepatic ligament basin, and two were along SMA/SMV just inferior to the treated recurrence.
Figure 3.
First locoregional failures by RT dose (LDF, low-dose field; HDF, high-dose field; OOF, out-of-field).
3.4. Predictors of OS and LRF
In the univariable analysis of baseline factors, only reduction in CA 19-9 after chemotherapy was significantly associated with LRF (HR = 0.93; 95%CI, 0.89–0.97, p = 0.002) (Table 2). No clinical factors tested, including stage of the primary tumor, time to recurrence, margin status, or receipt of chemotherapy for recurrence were significantly associated with OS after A-RT (Table 2).
Table 2.
Univariable regression for OS and LRF.
| Characteristic | OS 1 | LRF 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Event N | HR 3 | 95% CI 3 | p-Value 4 | N | Event N | HR 3 | 95% CI 3 | p-Value 4 | ||
| Central tumor high dose | 65 | 38 | 0.92 | 67 | 28 | 0.4 | |||||
| No | — | — | — | — | |||||||
| Yes | 1.05 | 0.41, 2.69 | 1.55 | 0.59, 4.10 | |||||||
| Change in CA 19-9 (divided by 100) | 26 | 16 | 1.00 | 0.93, 1.06 | 0.91 | 26 | 11 | 0.93 | 0.89, 0.97 | 0.002 | |
| Salvage for primary vs. nodal recurrence | 65 | 38 | 0.31 | 67 | 28 | 0.056 | |||||
| Primary | — | — | — | — | |||||||
| Both | 1.94 | 0.82, 4.57 | 2.04 | 0.82, 5.03 | |||||||
| Nodal | 0.81 | 0.25, 2.67 | 2.89 | 1.10, 7.61 | |||||||
| NACT | 65 | 38 | 0.78 | 67 | 28 | 0.6 | |||||
| No | — | — | — | — | |||||||
| Yes | 0.91 | 0.48, 1.74 | 1.19 | 0.56, 2.51 | |||||||
| Length of NACT | 27 | 17 | 1.02 | 0.86, 1.21 | 0.82 | 27 | 12 | 1.06 | 0.93, 1.22 | 0.4 | |
| Prior RT | 65 | 38 | 0.69 | 67 | 28 | 0.7 | |||||
| No | — | — | — | — | |||||||
| Yes | 1.20 | 0.50, 2.90 | 0.78 | 0.24, 2.53 | |||||||
| Surgery type | 65 | 38 | 0.33 | 67 | 28 | 0.14 | |||||
| Whipple | — | — | — | — | |||||||
| Distal | 0.66 | 0.28, 1.58 | 1.86 | 0.81, 4.28 | |||||||
| Disease free interval | 65 | 38 | 0.98 | 0.95, 1.01 | 0.18 | 67 | 28 | 1.00 | 0.98, 1.02 | 0.91 | |
| T stage | 65 | 38 | 0.63 | 67 | 28 | 0.65 | |||||
| T1 | — | — | — | — | |||||||
| T2 | 1.21 | 0.56, 2.60 | 1.69 | 0.60, 4.74 | |||||||
| T3/T4 | 0.78 | 0.26, 2.34 | 1.56 | 0.47, 5.16 | |||||||
| N stage | 65 | 38 | 0.085 | 67 | 28 | 0.2 | |||||
| N0 | — | — | — | — | |||||||
| N1 | 1.53 | 0.70, 3.34 | 0.45 | 0.19, 1.06 | |||||||
| N2 | 2.57 | 1.13, 5.87 | 0.62 | 0.24, 1.63 | |||||||
| Initial pathologic stage | 65 | 38 | 0.24 | 67 | 28 | 0.5 | |||||
| I | — | — | — | — | |||||||
| II | 1.46 | 0.65, 3.28 | 0.63 | 0.29, 1.41 | |||||||
| III | 2.11 | 0.89, 5.04 | 0.61 | 0.22, 1.69 | |||||||
| Margin status at surgery | 65 | 38 | 0.97 | 67 | 28 | >0.9 | |||||
| Negative | — | — | — | — | |||||||
| Positive | 1.05 | 0.48, 2.33 | 0.92 | 0.34, 2.49 | |||||||
| Close | 1.11 | 0.50, 2.45 | 1.10 | 0.48, 2.50 |
1 Cox proportional hazards regression, 2 Fine–Gray competing risks regression, 3 HR = Hazard Ratio, CI = Confidence Interval, 4 Global p-value.
3.5. Adverse Events
Of 67 treatment courses among 65 participants, only one case of acute grade 3 toxicity (fatigue) was noted (Table 3). The most common acute toxicity (all grade ≤ 2) was nausea (58.2%), followed by fatigue (55.2%), and diarrhea (34.3%).
Table 3.
Adverse events by CTCAE v.5.
| Adverse Event | Grade | ||||
|---|---|---|---|---|---|
| Acute Toxicity | 1 | 2 | 3 | 4 | 5 |
| Nausea | 38 (56.7) | 1 (1.5) | 0 | 0 | 0 |
| Fatigue | 29 (43.4) | 7 (10.4) | 1 (1.5) | 0 | 0 |
| Diarrhea | 19 (28.4) | 4 (6.0) | 0 | 0 | 0 |
| Anorexia | 17 (25.4) | 1 (1.5) | 0 | 0 | 0 |
| Subacute toxicity | 1 | 2 | 3 | 4 | 5 |
| Gastrointestinal toxicity | 4 (6.0) | 1 (1.5) | 1 (1.5) | ||
Percentages reported out of 67 treatment courses among 65 patients.
In the sub-acute setting, four (6.0%) cases of grade 3 and one (1.5%) case of grade 4 gastrointestinal toxicity were noted. Grade 4 gastrointestinal bleeding occurred in a participant who received two A-RT treatments one year apart. In addition, one patient died of gastrointestinal bleeding that occurred outside of the irradiated field and was adjudicated to be unrelated to radiation.
4. Discussion
Isolated local and locoregional recurrences are common in patients with resected PDAC and appear to benefit from local therapy [19,23]. Herein, we demonstrate that similar to the primary setting, a novel A-RT approach delivering BED10 of 98–100 Gy achieved favorable local control and OS with a low risk of treatment-related morbidity in the salvage setting.
In this very selected cohort, the median OS of 27 months and 2-year 57% OS rate from diagnosis of recurrence compared favorably to those reported in cohorts that underwent re-resection [17]. For instance, Strobel et al. reported on 41 patients who underwent re-resection, with a median survival from reoperation of 26 months [23]. Miyazaki et al. reported on 11 patients who had a median OS after re-resection of 25 (3–61) months [19]. It is important to note that only a small minority of patients with local recurrence—estimated at 2%—are candidates for surgical salvage [17]. While prior surgery may complicate eligibility for salvage surgery, it may actually facilitate delivery of ablative radiation doses in some cases by removing or moving the luminal GI tract farther away from the origin of SMA and celiac axis where these recurrences are often seen. This may contribute to a slightly lower risk of bleeding in this cohort compared to patients treated in the primary setting [33]. The feasibility of A-RT coupled with low rates of grade 3 or higher toxicity make it a viable option in a larger proportion of patients with isolated local recurrence compared to surgery.
With regard to other radiotherapy options, A-RT demonstrates superior outcomes compared to standard-dose (BED10 53–72 Gy) conventionally fractionated CRT or standard-dose SBRT [21,24,25,27]. An older series of patients who underwent chemoradiotherapy demonstrated a median OS from time of treatment ranging from 10 months to 19 months [21,24]. The largest retrospective analysis of 51 patients using 5-fraction SBRT to a total dose of 25–33 Gy showed a median OS and local progression-free survival of 16 and 10 months, respectively [35]. Perhaps the most robust evidence comes from a prospective phase 2 trial in a locally recurrent population (with KRAS mutation and PD-L1 positivity), which randomized 170 participants to receive 5-fraction SBRT to 35–40 Gy (BED10 59.5–72 Gy) with either immunotherapy or gemcitabine, and showed a median OS of 12.8–14.9 months [28]. Subsequent dose–response analysis that dichotomized patients to BED10 of 60–65 Gy vs. > 65 Gy showed a possible association with PFS in the immunotherapy group but not the chemotherapy group, and no association with OS [36]. The dose–response relationship was also examined in a small cohort of 19 patients from Johns Hopkins treated with standard-dose 5-fraction SBRT to a total dose of 25–33 Gy (median BED10 of 54.8 Gy and range of 37.5–54.8 Gy) with a median OS of 17.1 months [29]. Interestingly, in this analysis, BED10 < 54.8 Gy was associated with lower local progression-free survival (1 year, 25.0% vs. 80.2%, p < 0.009) [29]. Collectively, these findings suggest that dose escalation may be beneficial, but they failed to test ablative dosing.
Ablative-dose SBRT was given to some of the patients in a 24-participant retrospective cohort reported by Zeng et al. with a median BED10 of 85.50 Gy (range, 71.4–100 Gy) [27]. However, the wide range of dosing and heterogeneity of the patient population where 21% of participants had distant metastasis complicated interpretation of the 1-year local control of 83% and mOS of 12.2 months.
Ablative-dose RT to BED10 of 98–100 Gy using either the hypofractionated technique or 5-fraction SBRT has shown improved local control and OS outcomes in the primary treatment of locally advanced pancreatic cancer [31,33,37,38,39]. The current series constitutes the first experience using dose-escalated RT for salvage therapy and builds on our prior work showing that, despite a high metastatic potential, localized pancreatic cancer patients benefit from more aggressive local therapy.
There are no consensus volumes for irradiation of local recurrence. Our institutional practice has been to extend the elective prophylactic coverage isometrically around the gross disease as well as along the involved mesenteric vessels, making sure to include the origin of the involved mesenteric arteries thought to contain the neural plexi mediating pancreatic cancer spread. Interestingly, most locoregional failures occurred in the field encompassed by low-dose elective coverage volume or immediately beyond it, with some, like the para-aortic basin, within regions that may have been included by the Radiation Therapy Oncology Group 0848 contouring atlas created for the adjuvant setting [40]. This suggests that locoregional failures are predictable and could be more effectively addressed with larger volumes receiving a higher dose of RT. We have now changed our institutional practice to asymmetrically extend the high-dose clinical target volume beyond the gross disease to cover at least 0.5–1.0 cm of the length of any involved mesenteric vessels and the origin of arteries whenever normal tissue constraints allow. Furthermore, a higher microscopic dose exceeding BED10 of 50 Gy may be needed to improve local control. Finally, coverage of para-aortic basins or selective coverage of adjacent non-regional basins (i.e., lymph nodes around the lesser curvature of the stomach for a celiac recurrence) with elective volume may also be considered.
5. Limitations
Our findings are limited by the retrospective nature, highly selected single-institution cohort, and relatively small sample size of our study. However, the potential benefit compared to historical results is compelling and consistent with findings in the primary setting. Thus, these findings support further exploration of the role of A-RT for locally recurrent pancreatic adenocarcinoma.
6. Future Directions
Although A-RT achieves favorable local control for patients with ILR, rates of in-field local failure nonetheless underscore the need for further improvement. From a dosimetric and treatment planning standpoint, our institutional practice has evolved to include the origin of the involved mesenteric vessels to target neural plexi that may harbor microscopic disease. However, ablative doses are constrained by the dose tolerances of nearby OARs. Newer technologies, such as MR-guided RT systems, facilitate daily adaptive planning to manage movement of luminal gastrointestinal organs [38].
Another consideration is our evolving understanding of the biology of PDAC. The tumor microenvironment (TME) consists of cellular elements (such as cancer-associated fibroblasts and tumor-associated neutrophils) and molecular structures (such as the extracellular matrix) that surround PDAC cells [41]. The TME may promote radiation resistance through factors including an immune-inhibitory environment, increased numbers of mitochondria associated with cancer-associated fibroblasts, and increased tumor hypoxia [41]. Therefore, efforts to target the TME have been explored in PDAC, although success in modulating stromal desmoplasia and immunosuppressive pathways has been limited [42]. Efforts to synergize interventions targeting the stroma and immune elements are under exploration [42]. Furthermore, how radiation can be leveraged to disrupt the TME [43] and potentiate systemic treatments—beyond the integration of RT with immunotherapy—should be explored further.
7. Conclusions
In this single-institution series of 65 participants with isolated locoregional recurrence of PDAC after prior resection, we report that A-RT to BED10 of 98–100 Gy achieves favorable local control and overall survival outcomes compared to historical controls treated with surgical resection or standard-dose conventionally fractionated RT or SBRT. Consideration should be given to expanding high-dose clinical target volumes to include adjacent segments of at-risk vessels beyond contact with the gross disease whenever normal tissue constraints allow. In addition, increasing the dose to elective clinical target volume where feasible and selective inclusion of adjacent non-regional nodal basins in the low-dose clinical target volume should be routinely considered. Prospective studies examining A-RT for locally recurrent PDAC are warranted.
Author Contributions
Conceptualization, V.C.N., C.H.C. and M.R.; methodology, E.C.D., V.C.N., S.M.L., Z.Z., C.H.C. and M.R.; validation, M.R.; formal analysis, E.C.D., V.C.N., S.M.L., Z.Z. and M.R.; investigation, E.C.D., V.C.N. and M.R.; resources, M.R.; data curation, V.C.N. and M.R.; writing—original draft, E.C.D., V.C.N. and M.R.; writing—review & editing, E.C.D., V.C.N., E.M.O., A.C.W., S.M.L., A.M.V., M.Z., P.B.R., A.J.W., C.H., J.J.C., D.N.K., W.P., K.H.Y., Z.Z., J.A.D., W.R.J., C.H.C. and M.R.; visualization, M.R.; supervision, E.M.O., A.C.W., A.M.V., M.Z., P.B.R., A.J.W., C.H., J.J.C., D.N.K., W.P., K.H.Y., Z.Z., J.A.D., W.R.J., C.H.C. and M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
All authors are funded in part through the Cancer Center Support Grant from the National Cancer Institute (P30 CA008748).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Survival Rates for Pancreatic Cancer. 2023. [(accessed on 28 November 2023)]. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
- 2.Bajaj S.S., Jain B., Dee E.C., Wo J.Y., Qadan M. ASO Research Letter: Trends in Location of Death for Individuals with Pancreatic Cancer in the United States. Ann. Surg. Oncol. 2022;29:2766–2768. doi: 10.1245/s10434-021-11058-y. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y., Sohal D.P.S. Pancreatic Adenocarcinoma Management. JCO Oncol. Pract. 2023;19:19–32. doi: 10.1200/OP.22.00328. [DOI] [PubMed] [Google Scholar]
- 4.Wong J.C., Raman S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom. Imaging. 2010;35:471–480. doi: 10.1007/s00261-009-9539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperti C., Pasquali C., Piccoli A., Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J. Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 6.Hishinuma S., Ogata Y., Tomikawa M., Ozawa I., Hirabayashi K., Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J. Gastrointest. Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Kayahara M., Nagakawa T., Ueno K., Ohta T., Takeda T., Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::aid-cncr2820720710>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Van den Broeck A., Sergeant G., Ectors N., Van Steenbergen W., Aerts R., Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos J.P., Stocken D.D., Friess H., Bassi C., Dunn J.A., Hickey H., Beger H., Fernandez-Cruz L., Dervenis C., Lacaine F., et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N. Engl. J. Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M., Faluyi O., O’Reilly D.A., Cunningham D., Wadsley J., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 11.Regine W.F., Winter K.A., Abrams R., Safran H., Hoffman J.P., Konski A., Benson A.B., Macdonald J.S., Rich T.A., Willett C.G. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the, U.S. Intergroup/RTOG 9704 phase III trial. Ann. Surg. Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobuzio-Donahue C.A., Fu B., Yachida S., Luo M., Abe H., Henderson C.M., Vilardell F., Wang Z., Keller J.W., Banerjee P., et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J. Clin. Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane C.H., Varadhachary G.R., Yordy J.S., Staerkel G.A., Javle M.M., Safran H., Haque W., Hobbs B.D., Krishnan S., Fleming J.B., et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: Correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J. Clin. Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., Chiorean E.G., Chung V., Czito B., Del Chiaro M., et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 15.Sunamura M., Egawa S., Shibuya K., Shimamura H., Takeda K., Kobari M., Matsuno S. Therapeutic strategy for the recurrence of pancreatic cancer following pancreatectomy. Nihon Geka Gakkai Zasshi. 1999;100:200–205. [PubMed] [Google Scholar]
- 16.Gangl O., Fröschl U., Dutta-Függer B., Függer R. Elective pancreatic reresection—Report of a series and review of the literature. Eur. Surg. 2010;42:91–95. doi: 10.1007/s10353-010-0527-0. [DOI] [Google Scholar]
- 17.Groot V.P., van Santvoort H.C., Rombouts S.J.E., Hagendoorn J., Borel Rinkes I.H.M., van Vulpen M., Herman J.M., Wolfgang C.L., Besselink M.G., Molenaar I.Q. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB. 2017;19:83–92. doi: 10.1016/j.hpb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Lavu H., Nowcid L.J., Klinge M.J., Mahendraraj K., Grenda D.R., Sauter P.K., Rosato E.L., Kennedy E.P., Yeo C.J. Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J. Surg. Res. 2011;170:89–95. doi: 10.1016/j.jss.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M., Yoshitomi H., Shimizu H., Ohtsuka M., Yoshidome H., Furukawa K., Takayasiki T., Kuboki S., Okamura D., Suzuki D., et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: Is it worthwhile? Surgery. 2014;155:58–66. doi: 10.1016/j.surg.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Wilkowski R., Thoma M., Bruns C., Dühmke E., Heinemann V. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP. 2006;7:34–40. [PubMed] [Google Scholar]
- 21.Zhang Y., Frampton A.E., Kyriakides C., Bong J.J., Habib N., Ahmad R., Jiao L.R. Loco-recurrence after resection for ductal adenocarcinoma of the pancreas: Predictors and implications for adjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2012;138:1063–1071. doi: 10.1007/s00432-012-1165-7. [DOI] [PubMed] [Google Scholar]
- 22.Habermehl D., Brecht I.C., Bergmann F., Welzel T., Rieken S., Werner J., Schirmacher P., Büchler M.W., Debus J., Combs S.E. Chemoradiation in patients with isolated recurrent pancreatic cancer—Therapeutical efficacy and probability of re-resection. Radiat. Oncol. 2013;8:27. doi: 10.1186/1748-717X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel O., Hartwig W., Hackert T., Hinz U., Berens V., Grenacher L., Bergmann F., Debus J., Jäger D., Büchler M., et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann. Surg. Oncol. 2013;20:964–972. doi: 10.1245/s10434-012-2762-z. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto D., Chikamoto A., Ohmuraya M., Sakata K., Miyake K., Kuroki H., Watanabe M., Beppu T., Hirota M., Baba H. Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg. Today. 2014;44:1313–1320. doi: 10.1007/s00595-013-0708-0. [DOI] [PubMed] [Google Scholar]
- 25.Wild A.T., Hiniker S.M., Chang D.T., Tran P.T., Khashab M.A., Limaye M.R., Laheru D.A., Le D.T., Kumar R., Pai J.S., et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J. Gastrointest. Oncol. 2013;4:343–351. doi: 10.3978/j.issn.2078-6891.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagoglu N., Callery M., Moser J., Tseng J., Kent T., Bullock A., Miksad R., Mancias J.D., Mahadevan A. Stereotactic Body Radiotherapy (SBRT) Reirradiation for Recurrent Pancreas Cancer. J. Cancer. 2016;7:283–288. doi: 10.7150/jca.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng M.-B., Wang H.-H., Wu Z.-Q., Song Y.-C., Zhuang H.-Q., Dong Q., Li F.-T., Zhao L.-J., Yuan Z.-Y., Zeng X.-L., et al. Stereotactic body radiation therapy for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph nodes or postoperative stump including pancreatic stump and other stump. Onco Targets Ther. 2016;9:3985–3992. doi: 10.2147/OTT.S102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X., Cao Y., Liu W., Ju X., Zhao X., Jiang L., Ye Y., Jin G., Zhang H. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: An open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23:e105–e115. doi: 10.1016/S1470-2045(22)00066-3. [DOI] [PubMed] [Google Scholar]
- 29.Reddy A.V., Hill C.S., Sehgal S., He J., Zheng L., Herman J.M., Meyer J., Narang A.K. Stereotactic body radiation therapy for the treatment of locally recurrent pancreatic cancer after surgical resection. J. Gastrointest. Oncol. 2022;13:1402–1412. doi: 10.21037/jgo-22-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyngold M., Parikh P., Crane C.H. Ablative radiation therapy for locally advanced pancreatic cancer: Techniques and results. Radiat. Oncol. 2019;14:95. doi: 10.1186/s13014-019-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudra S., Jiang N., Rosenberg S.A., Olsen J.R., Roach M.C., Wan L., Portelance L., Mellon E.A., Bruynzeel A., Lagerwaard F., et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan S., Chadha A.S., Suh Y., Chen H.-C., Rao A., Das P., Minsky B.D., Mahmood U., Delclos M.E., Sawakuchi G.O., et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyngold M., O’reilly E.M., Varghese A.M., Fiasconaro M., Zinovoy M., Romesser P.B., Wu A., Hajj C., Cuaron J.J., Tuli R., et al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 2021;7:735–738. doi: 10.1001/jamaoncol.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane C.H., O’Reilly E.M. Ablative Radiotherapy Doses for Locally Advanced: Pancreatic Cancer (LAPC) Cancer J. 2017;23:350–354. doi: 10.1097/PPO.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 35.Ryan J.F., Groot V.P., Rosati L.M., Hacker-Prietz A., Narang A.K., McNutt T.R., Jackson J.F., Le D.T., Jaffee E.M., Zheng L., et al. Stereotactic Body Radiation Therapy for Isolated Local Recurrence After Surgical Resection of Pancreatic Ductal Adenocarcinoma Appears to be Safe and Effective. Ann. Surg. Oncol. 2018;25:280–289. doi: 10.1245/s10434-017-6134-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X., Liu W., Cao Y., Ju X., Zhao X., Jiang L., Ye Y., Zhang H. Effect of stereotactic body radiotherapy dose escalation plus pembrolizumab and trametinib versus stereotactic body radiotherapy dose escalation plus gemcitabine for locally recurrent pancreatic cancer after surgical resection on survival outcomes: A secondary analysis of an open-label, randomised, controlled, phase 2 trial. EClinicalMedicine. 2023;55:101764. doi: 10.1016/j.eclinm.2022.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolissaint J.S., Reyngold M., Bassmann J., Seier K.P., Gönen M., Varghese A.M., Yu K.H., Park W., O’reilly E.M., Balachandran V.P., et al. Local Control and Survival After Induction Chemotherapy and Ablative Radiation Versus Resection for Pancreatic Ductal Adenocarcinoma with Vascular Involvement. Ann. Surg. 2021;274:894–901. doi: 10.1097/SLA.0000000000005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tringale K.R., Tyagi N., Reyngold M., Romesser P.B., Wu A., O’Reilly E.M., Varghese A.M., Scripes P.G., Khalil D.N., Park W., et al. Stereotactic ablative radiation for pancreatic cancer on a 1.5 Telsa magnetic resonance-linac system. Phys. Imaging Radiat. Oncol. 2022;24:88–94. doi: 10.1016/j.phro.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh P.J., Lee P., Low D.A., Kim J., Mittauer K.E., Bassetti M.F., Glide-Hurst C.K., Raldow A.C., Yang Y., Portelance L., et al. A Multi-Institutional Phase 2 Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023;117:799–808. doi: 10.1016/j.ijrobp.2023.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Goodman K.A., Regine W.F., Dawson L.A., Ben-Josef E., Haustermans K., Bosch W.R., Turian J., Abrams R.A. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:901–908. doi: 10.1016/j.ijrobp.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taghizadeh-Hesary F. “Reinforcement” by Tumor Microenvironment: The Seventh “R” of Radiobiology. Int. J. Radiat. Oncol. Biol. Phys. 2023 doi: 10.1016/j.ijrobp.2023.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Ho W.J., Jaffee E.M., Zheng L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farren M.R., Sayegh L., Ware M.B., Chen H.R., Gong J., Liang Y., Krasinskas A., Maithel S.K., Zaidi M., Sarmiento J.M., et al. Immunologic alterations in the pancreatic cancer microenvironment of patients treated with neoadjuvant chemotherapy and radiotherapy. J. Clin. Investig. Insight. 2020;5:e130362. doi: 10.1172/jci.insight.130362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.