Abstract
A role for interferon (IFN) in modulating infection by dengue virus (DV) has been suggested by studies in DV-infected patients and IFN receptor-deficient mice. To address how IFN modulates DV type 2 infection, we have assayed IFN-α, -β, and -γ for the ability to enhance or diminish antibody-independent and antibody-dependent cell infection using a competitive, asymmetric reverse transcriptase-mediated PCR (RT-PCR) assay that quantitates positive and negative strands of viral RNA, a flow cytometric assay that measures viral antigen, and a plaque assay that analyzes virion production. Our data suggest that IFN-α and -β protect cells against DV infection in vitro. Treatment of hepatoma cells with IFN-α or -β decreases viral RNA levels greater than 1,000-fold, the percentage of cells infected 90 to 95%, and the amount of infectious virus secreted 150- to 100,000-fold. These results have been reproduced with several cell types and viral strains, including low-passage isolates. In contrast, IFN-γ has a more variable effect depending on the cell type and pathway of infection. Quantitative RT-PCR experiments indicate that IFN inhibits DV infection by preventing the accumulation of negative-strand viral RNA.
Dengue fever (DF), the most prevalent arthropod-borne viral illness in humans, is caused by dengue virus (DV). DV, a member of the Flaviviridae family, is related to the viruses that cause yellow fever, hepatitis C, and the Japanese, St. Louis, and West Nile encephalitides. Infection by the four serotypes of DV causes a spectrum of clinical disease ranging from an acute debilitating self-limited febrile illness (DF) to a life-threatening syndrome (dengue hemorrhagic fever/dengue shock syndrome [DHF/DSS]). One hundred million new cases of DF and 250,000 cases of DHF/DSS are estimated per year throughout the tropical and subtropical regions of the world (13, 40). At present, no effective antiviral treatment or vaccine exists, and therapy is largely supportive in nature.
In a primary DV infection, virus enters target cells after the envelope protein E adheres to an as yet uncharacterized receptor (13) that may display highly sulfated glycosaminoglycans (5). In a secondary infection with a different DV serotype, cell entry occurs both via a primary receptor and through antibody-dependent enhancement of infection (12, 13). In the latter case, Fcγ receptors I and II (39) also are believed to participate in viral entry. Immunopathologic studies of patients infected with DV suggest that many tissues may be involved, as viral antigens are expressed in liver, lymph node, spleen, and bone marrow (10, 19, 35). Although few details of the mechanism of either primary or secondary infection are known, the progression to DHF likely reflects a complex interplay between host and viral factors and the production of inflammatory cytokines (13, 35, 45).
For many viruses, an initial step in the establishment of infection is the evasion of the innate antiviral response provided by the cellular interferon (IFN) system. IFN-α and -β are secreted by virus-infected cells and exhibit multiple biologic properties including antiproliferative, antiviral, and immunomodulatory effects (42, 48). IFN-γ is secreted by activated T lymphocytes and NK cells and has antiviral activity directly, through the induction of effector molecules (e.g., nitric oxide), and indirectly, through enhanced antigen presentation and the induction of apoptosis (3). Induction and activation of specific host molecules by IFN block virus infection at several levels, including transcription, translation, and RNA degradation (8). Although several studies suggest that IFN-α, -β, and -γ modulate infection of members of the family Flaviviridae in vitro and in vivo (16, 20, 21, 41, 49), only one study has identified a specific inhibitory mechanism (37). Moreover, the role of IFN-γ in DV infection is still controversial. Although some studies suggest that it protects against DV infection (23, 47), others argue that it contributes to the pathogenesis of DHF (25, 32).
In this report, we assess the effect of different types of IFN on DV infection in vitro. Distinct cell types were treated with IFN-α, -β, and -γ, exposed to prototype and low-passage DV type 2 (DV2) strains, and evaluated for the production of positive- and negative-strand viral RNA, intracellular viral antigen, and infectious virus. We find that IFN-α and -β significantly inhibit antibody-dependent and antibody-independent infection when cells are treated prior to exposure to virus. The effect of IFN-γ is more variable, as it can inhibit, have little effect on, or even augment virus infection depending on the cell type and pathway of infection. Finally, the results of kinetic studies that assess the potency and durability of the IFN effect point to a critical step in viral pathogenesis that is impeded by IFN. These experiments suggest that IFN impairs either the production or stability of negative-strand viral RNA and that DV may be able to limit this antiviral response.
MATERIALS AND METHODS
Cell culture.
Human foreskin fibroblasts (HFF cells; obtained as a gift from M. Grigg and J. Boothroyd, Palo Alto, Calif., and used through passage 16), HepG2 hepatoma cells (American Type Culture Collection [ATCC], Manassas, Va.), K562 erythroleukemic cells (gift from L. Petruzzelli, Ann Arbor, Mich.), U937 myelomonocytes (ATCC), and THP-1 monocyte leukemic cells (gift from S. Goth, Berkeley, Calif.) were cultured in RPMI 1640 (Gibco BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS; Sigma Chemical Co., St. Louis, Mo.), penicillin G (100 U/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2. BHK-21 (clone 15; gift from S. Kliks, San Francisco, Calif.) hamster kidney cells were maintained in alpha minimal essential medium (α-MEM; Gibco BRL) containing 10% FBS, penicillin G (100 U/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2. C6/36 (ATCC), an Aedes albopictus cell line, was cultured in Leibovitz's L-15 medium (Gibco BRL) supplemented with 10% FBS, penicillin G (100 U/ml) and streptomycin (100 μg/ml) at 28°C in the absence of CO2. Monocytes were isolated from the whole blood of healthy volunteers by Ficoll-Hypaque gradient centrifugation (7), resuspended in endotoxin-free RPMI 1640 supplemented with 1% human AB serum (Sigma), and selected by adherence. Monocyte phenotype was confirmed by immunostaining with phycoerythrin-conjugated anti-CD14 antibodies (Becton Dickinson, Franklin Lakes, N.J.). The percentage of monocytes after adherence ranged from 88 to 95%.
Antibodies.
Murine hybridomas against human DV antigens (3H5-1, anti-DV2; 5D4-11, anti-DV3; and 2H2-9, anti-DV) or flavivirus antigens (4G2) were obtained from the ATCC and grown in Dulbecco's modified Eagle medium (Gibco BRL) supplemented with 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin G (100 U/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2. Supernatants were collected from cell cultures that had reached greater than 50% cell death, centrifuged, filtered, and stored at −20 or 4°C. For some investigations, monoclonal antibodies (MAbs) were purified after 45% NH4SO4 precipitation and protein A affinity chromatography and then directly conjugated to fluorescein isothiocyanate (FITC; Molecular Probes, Eugene, Oreg.) (17). Unless otherwise noted, in functional assays and immunostaining, purified immunoglobulin G was used at 10 to 20 μg/ml, and tissue culture supernatants were used at a 1/4 final dilution. FITC-labeled goat anti-mouse (Sigma) was used at a 1/250 dilution after centrifugation (14,000 rpm for 5 min) to remove insoluble debris.
Virus stocks.
DV2 strains used in this study include a prototype DHF strain from Thailand (16681 [46]; kindly provided by the Centers for Disease Control and Prevention, Atlanta, Ga.), two recent DHF isolates from Thailand (C0477 and K0049 [43]; gift from R. Rico-Hesse, San Antonio, Tex.), and a recent DF isolate from Nicaragua (N9622, Jamaican subtype [1]); the recent DV2 isolates were recovered from serum samples and passaged once in C6/36 cells. All experiments used viral stocks from the same tissue culture passage: 16681, passage number unknown; C0477, passage 2; K0049, passage 2; and N9622, passage 2. Viral stocks were obtained by inoculating monolayers of C6/36 cells in 75-cm2 tissue culture flasks with virus diluted 1:5 to 1:10 in 1 ml of L-15–2% FBS. After 1 h, 14 ml of L-15 supplemented with 10% FBS was added, and the cells were cultured for 7 days. Cells and supernatant were then harvested by gentle pipetting. Cell debris was removed by centrifugation (2,000 × g for 5 min), and the viral supernatant was adjusted to 20% FBS, aliquoted, and stored at −80°C.
Virus titration by plaque assay.
Virus production was titrated by plaque assays using BHK-21 cells. BHK-21 cells were seeded in 6-well (6 × 105 cells/well) or 12-well (3 × 105 cells/well) plates in α-MEM with 10% FBS for 3 h at 37°C. Medium was removed, serial dilutions of virus supernatants were added (0.30 ml/well for 6-well plates; 0.15 ml/well for 12-well plates) in α-MEM with 2% FBS, and the cells were incubated for 2 h at 37°C. Subsequently, α-MEM containing 5% FBS and 1% low-melting-point agarose (3 ml/well for 6-well plates; 1.5 ml/well for 12-well plates) was added, and the plates were incubated at 37°C for 5 days. The plaques were visualized after fixation in 10% formaldehyde (>1 h at room temperature) and removal of the agarose plug by staining briefly (15 to 30 s) with a 1% crystal violet solution in 20% ethanol. Virus concentration was determined as PFU per milliliter.
Cell infection. (i) Antibody independent.
Cells were infected after adherence to tissue culture plastic (HFF and HepG2) or in suspension (U937, K562, and THP-1). Adherent cells (1 × 105 to 2 × 105 cells/well) were seeded in 12- or 24-well plates. At the time of infection, medium was removed, and virus was diluted in α-MEM with 2% FBS, added to monolayers or suspensions of cells at a given multiplicity of infection (MOI), and incubated at 37°C for 2 h. The viral supernatants were removed, and the cells were washed six times to remove residual virus and incubated at 37°C for 72 h prior to harvest. For cells infected in suspension, cells were washed in α-MEM with 2% FBS, exposed to viral supernatants (total volume, 200 μl), and incubated at 37°C for 2 h with agitation every 20 min to prevent cell pelleting. Cells were washed six times by centrifugation (900 × g for 3 min) and reseeded in 6- or 12-well plates for 72 h at 37°C. Supernatants were collected for plaque assay, and cells were harvested for flow cytometry and RNA determination. In some experiments, cells were pretreated with individual or combinations of recombinant IFN. Unless otherwise specified, IFN-α (gift from A. Wakil, California-Pacific Medical Center, San Francisco) was used at 100 IU/ml, IFN-β (gift from H. Daniel Perez, Berlex Biosciences, Richmond, Calif.) was used at 10 ng/ml (20 IU/ml), and IFN-γ (gift from Genentech, South San Francisco, Calif.) was used at 10 ng/ml (30 IU/ml). In some of the kinetic studies, IFN was added to cells after DV infection. For those time points in which IFN was used as a pretreatment, no additional IFN was added after infection.
(ii) Antibody dependent.
For antibody-dependent enhancement of DV infection studies, cells bearing Fcγ receptors (U937, THP-1, and K562 cells) were subjected to infection in the presence of subneutralizing concentrations of MAbs using a modification of previously published protocols (4, 15). Cells (2.5 × 105) were resuspended in 100 μl α-MEM with 2% FBS. MAb (200 ng of antiflavivirus antibody 4G2 or anti-DV antibody 3H5-1 in 50 μl) was mixed with 50 μl of diluted DV2 virus, added to cells, and incubated for 2 h at 37°C (subneutralizing concentration of MAb was 1 μg/ml). Cells were then washed extensively (eight times) to remove residual free virus, growth medium was replaced, and antibody (200 ng of 4G2 or 3H5) was added back as previously described (4). Cells were incubated for 96 h at 37°C prior to supernatant and cell harvest for plaque and flow cytometry assays, respectively.
Flow cytometry analysis.
For antibody-independent or antibody-dependent infection, DV2-infected and control cells were harvested at 72 or 96 h after infection. An aliquot (125 μl) of supernatant was removed for storage at −80°C for plaque assay, and the cells were divided into two pools, one for flow cytometric analysis and one for RNA quantitation. In addition, an aliquot of cells was removed for quantitation of the total number of cells by hemocytometer. For flow cytometric determination, harvested cells were aliquoted into individual wells of a 96-well U-bottom non-tissue culture plate. Cells were washed thrice in phosphate-buffered saline (PBS) by centrifugation, fixed in PBS with 4% paraformaldehyde for 10 min at room temperature, washed twice in PBS, and permeabilized in Hanks' balanced salt solution (Sigma) containing 10 mM HEPES (pH 7.3), 0.1% saponin (Aldrich Chemical, St. Louis, Mo.), and 0.02% NaN3 (HHSN). For indirect immunofluorescence experiments, cells were resuspended in HHSN (100 μl) and 25 μl of MAb, incubated for 1 to 2 h on ice, washed thrice in HHSN (4°C), resuspended in a 1/250 dilution of FITC-labeled goat anti-mouse immunoglobulin G (50 μl), and incubated for 1 h on ice in the dark. Cells were subsequently washed thrice in HHSN (4°C), fixed in 0.5% paraformaldehyde, and stored in the dark prior to flow cytometry. For direct immunofluorescence experiments (antibody-dependent studies), permeabilized cells were resuspended in HHSN (100 μl) and antibody (20 μg/ml FITC-labeled anti-DV (positive samples) or FITC-labeled anti-DV3 (negative samples) supplemented with 5% human serum (to saturate binding to Fcγ receptors) and incubated for 1 to 2 h on ice in the dark. Subsequently, cells were washed and fixed as described above for indirect immunofluorescence. Additional controls demonstrated that the background binding of the anti-DV2 MAb to uninfected cells was equivalent to the background binding of the anti-DV3 MAb used as a negative control (data not shown). Samples were analyzed on a FACScan flow cytometer using Cellquest software (Becton Dickinson, Franklin Lakes, N.J.).
RNA extraction and competitive RT-PCR.
Total RNA was harvested from infected cells using an RNEasy mini kit (Qiagen, Valencia, Calif.) and eluted in 100 μl of RNase-free double-distilled H2O. Positive- and negative-strand DV RNA was quantitated using a newly developed competitive, asymmetric reverse transcriptase (RT)-mediated PCR (RT-PCR) assay based on previous protocols (11, 22). Competitors for both the positive and the negative strand were designed by fusing sequences from the nonstructural protein NS3 of DV2 to nonfunctional fragments of the green fluorescent protein (GFP) gene. The positive-strand competitor was synthesized by PCR from a vector containing GFP (pEGFP; Clontech, Palo Alto, Calif.). The 5′ sense oligonucleotide (69-mer) contained (5′ to 3′) an EcoRI restriction site, the T7 promoter, 23 nucleotides (157 to 180) of the NS3 gene (NS3 sense; TTCCACACAATGTGGCACGTCAC), and 18 nucleotides (288 to 306) of GFP. The 3′ antisense oligonucleotide (46-mer) contained (5′ to 3′) a BamHI restriction site, 20 nucleotides (396 to 416) of the NS3 gene (NS3 antisense; GGAGATCCTGACGTTCCA/GGG), and 18 nucleotides (551 to 569) of GFP. The resultant PCR product was digested with EcoRI and BamHI, subcloned into pUC19, and confirmed by sequencing. The plasmid [pUC.NS3(+).GFP] was linearized with BamHI and purified by agarose gel electrophoresis, excision, and extraction using a QiaQuick gel extraction kit (Qiagen), and RNA was generated with T7 RNA polymerase using a T7 AmpliScribe transcription kit (Epicentre Technologies, Madison, Wis.). Subsequently, DNA template was degraded by the addition of RNase-free DNase I (106 U/μl; Calbiochem, San Diego, Calif.) for 15 min at 37°C, and residual DNA and nucleotides were removed using an RNA spin column (Qiagen). The positive-strand competitor RNA (ET7) generates a 324-nucleotide fragment after RT-PCR with NS3 oligonucleotides and was quantitated by spectrophotometry.
The negative-strand competitor was synthesized by PCR from pEGFP. The 5′ antisense oligonucleotide (66-mer) contained (5′ to 3′) a BamHI restriction site, a T7 promoter site, 20 nucleotides (396 to 416) of the NS3 gene, and 18 (641 to 659) nucleotides of GFP; the 3′ sense oligonucleotide (49-mer) contained (5′ to 3′) an EcoRI restriction site, 23 nucleotides (157 to 180) of the NS3 gene, and 18 nucleotides (370 to 388) of GFP. The resultant PCR product was digested with EcoRI and BamHI, subcloned into pUC19, and confirmed by sequencing. Plasmid [pUC.NS3(−).GFP] was linearized with EcoRI and purified by agarose gel electrophoresis, excision, and extraction, and RNA was generated with T7 RNA polymerase as described above. After DNase treatment and centrifugation through an RNA spin column, the negative-strand competitor RNA (BT7; 332 nucleotides in length) was quantitated by spectrophotometry.
For quantitative, asymmetric, competitive RT-PCR, serial dilutions of the competitor RNA were mixed with a fixed amount of DV2 RNA harvested from infected cells. cDNA was synthesized with the Rous-associated virus-2 RT (0.4 U per reaction; Amersham-Pharmacia, Piscataway, N.J.) using a previously published protocol (18), with slight modifications: only a single primer (NS3 antisense primer for the positive strand and NS3 sense primer for the negative strand) was included to generate cDNA from the strand of interest, and the reaction was performed at 55°C for 10 min to inhibit nonspecific oligonucleotide annealing and product formation. The RT was inactivated subsequently by a 2-min incubation at 80°C, a mixture of sense and antisense primers (1 μM) was added, and the newly synthesized cDNA was subjected to 28 (positive strand) or 31 (negative strand) rounds of PCR amplification consisting of 92°C for 30 s, 64°C for 45 s, and 72°C for 90 s on a PTC-200 thermocycler (M. J. Research, Waltham, Mass.). Reaction mixtures contained 0.2 mM each deoxynucleoside triphosphates (Gibco BRL), 5 mM dithiothreitol (Sigma), 30 mM tetramethyl ammonium chloride (Sigma), 500 mM betaine (Sigma), and Taq polymerase (0.25 U; Perkin-Elmer, Foster City, Calif.) in a total reaction volume of 25 μl. For both the positive- and negative-strand competitors, the absence of contaminating DNA was confirmed by the inability to amplify the competitor fragments when the reactions were performed without RT. PCR products were separated by 1.5% agarose gel electrophoresis. The amount of viral RNA was determined from the competitor concentration that produces competitor and DV bands of equal intensity. RNA per cell was calculated as follows: RNA per cell = {[competitor concentration in (copies/μl)][total volume of RNA/volume of RNA in RT-PCR]}/total number of cells.
RESULTS
Effect of IFN on antibody-independent DV2 infection. (i) Dose-response studies.
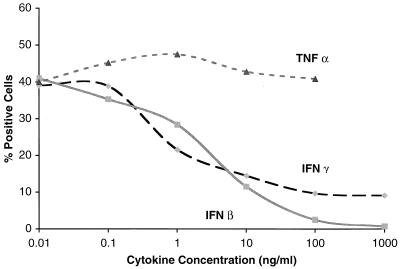
Because prior studies had reported conflicting data with respect to the effect of IFN-γ treatment on DV infection, we systematically assayed the ability of different IFNs to modulate DV infection. Two cell types that have been characterized in our laboratory as permissive for antibody-independent infection, a hepatoma cell line (HepG2) and primary HFF cells, were assayed. Initial studies were performed using a flow cytometry assay that detects cells that accumulate DV envelope (E) protein (M. S. Diamond et al., submitted for publication). When HepG2 cells were pretreated with physiologic concentrations of recombinant cytokine 24 h prior to infection (3 to 30 IU/ml; equivalent to 1 to 10 ng/ml), infection of cells with DV2 (strain 16681) was reduced 75% by IFN-β and 63% by IFN-γ, whereas tumor necrosis factor alpha (TNF-α) had little effect across a 4-log range of concentrations (Fig. 1). Similar results were obtained in dose-response studies with HFF cells, with a reduction of 71% by IFN-β and 56% by IFN-γ (data not shown). The inhibition by IFN was not due to a direct antiviral or toxic effect, as removal of IFN prior to infection did not alter the inhibition, and there was little difference in cell replication rates or cell viability after treatment (data not shown).
FIG. 1.
Dose response of cytokine pretreatment on DV2 infection of HepG2 cells. HepG2 cells were preincubated with increasing concentrations of IFN-β or -γ or TNF-α for 24 h and then exposed to DV2 (strain 16681) at an MOI of 2; 72 h later, cells (6 × 105) were detached and subjected to immunofluorescent flow cytometry after incubation with FITC-labeled anti-DV2 antibody. The results are expressed as the percentage of cells that express the E protein of DV2; 48% of cells that were infected without cytokine pretreatment (negative media control) were positive by flow cytometry. One representative experiment of two is shown.
(ii) Time course studies with HFF cells.
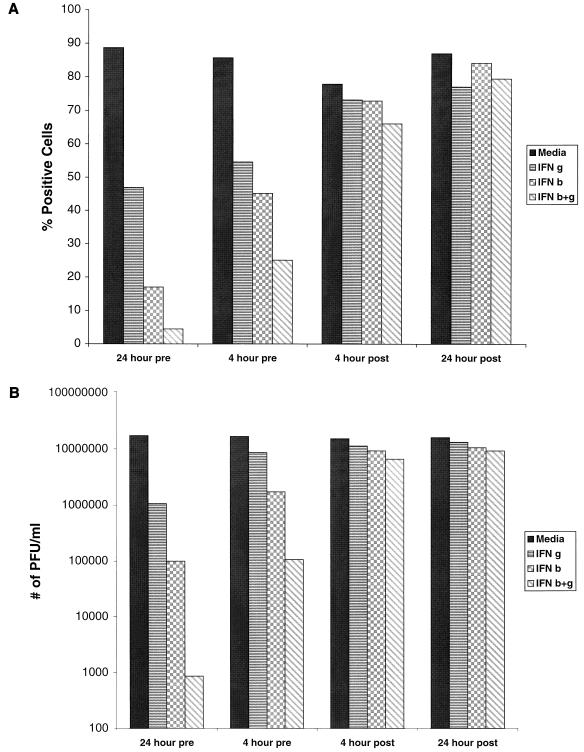
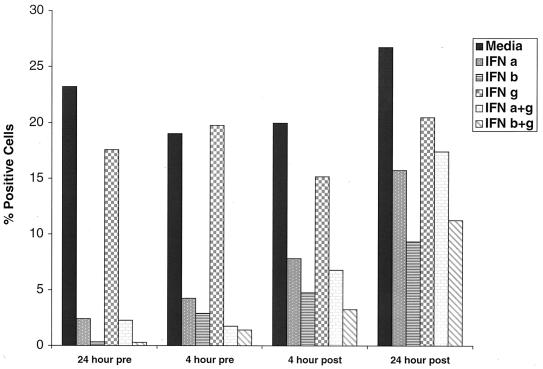
To better assess the kinetics of the effect of IFN on DV2 infection, time course studies were initiated, and the effects were monitored using both flow cytometric analysis and viral plaque assays. HFF cells were exposed to IFN-β and -γ, individually and in combination (IFN-β+γ) either before (24 or 4 h) or after (4 or 24 h) infection with the 16681 prototype DV2 strain and were evaluated for the percentage of cells expressing E protein and for the quantity of infectious virus produced in cell supernatants. Pretreatment of HFF cells with IFN-β and -γ individually or in combination 24 h prior to infection (Fig. 2) dramatically reduced the percentage of cells that express the viral E protein (β, 80% inhibition; γ, 47%; β+γ, 95%) and the ability to generate infectious virions (β, >2-log inhibition; γ, >1 log; β+γ, >4 log). A significant proportion of this effect was retained when cells were incubated with IFN 4 h prior to infection, as E protein accumulation (β, 47%; γ, 36%; β+γ, 71%) and virion production (β, 1-log inhibition; γ, 0.5 log; β+γ, >2 log) still were reduced. However, treatment of cells with IFN as little as 4 h after exposure to DV2 revealed a marked loss in the inhibitory effect of either individual or combinations of IFN on E protein accumulation (β, 6% inhibition; γ, 6%; β+γ, 15%) and virus secretion (β, 0.5 log inhibition; γ, <0.5 log; β+γ, <1 log).
FIG. 2.
Time course of the effect of IFN pretreatment on DV2 infection of HFF cells. HFF cells were exposed to DV2 (strain 16681) and incubated for 72 h. Cells (2 × 105) were processed for flow cytometry (A), and supernatants were harvested for plaque assays (B) using BHK-21 cells. In each case, cells were exposed to medium IFN-γ (10 ng/ml), IFN-β (10 ng/ml), or IFN-β+γ (10 ng/ml) either 24 or 4 h before (pre) or after (post) incubation with virus. The flow cytometric data are presented as the percentage of cells that express the E protein of DV2, and the plaque assay data are expressed as the number of PFU per milliliter. One representative experiment of three is shown.
(iii) Time course studies with HepG2 cells.
A similar trend was observed in time course studies with HepG2 hepatoma cells (data not shown). As with HFF cells, pretreatment of HepG2 cells with IFN 24 h prior to exposure to the prototype DV2 strain markedly reduced E protein accumulation (α, 86% inhibition; β, 94%; γ, 53%; α+γ, 99%; β+γ, 100%) and virus production (α, >2-log inhibition; β, >5 log; γ, >1 log; α+γ, >5 log; β+γ, >5 log). Pretreatment with IFN-α demonstrated an inhibitory effect on DV2 infection similar to that of IFN-β. Most of the inhibition of HepG2 cells effected by IFN was retained even when treatment occurred only 2 h prior to virus exposure. In contrast, incubation of HepG2 with IFN as little as 4 h after exposure to DV revealed a marked loss in the inhibitory effect of either individual or combinations of IFN in flow cytometric assays (α, 28% inhibition; β, 49%; γ, 16%; α+γ, 46%; β+γ 51%) and plaque assays (α, <0.5 log inhibition in titer; β, <1 log; γ <1 log; α+γ, <1 log; β+γ, <1 log, data not shown).
(iv) Studies with low-passage isolates.
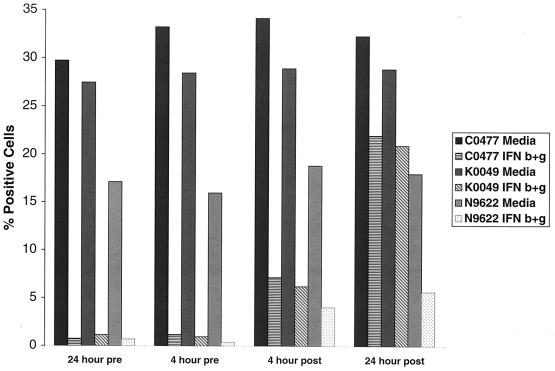
To confirm that the inhibitory effect of IFN was not limited to the prototype DV2 strain (16681), two low-passage, recent isolates from Thailand (C0477 and K0049), and one recent isolate from Nicaragua (N9622) were studied (Fig. 3). Pretreatment of HepG2 cells with individual or combinations of IFN dramatically reduced the percentage of cells that expressed viral antigen after exposure to recent DV2 isolates (for C0047, β, 93% inhibition; γ, 55%; β+γ, 97%; for K0049, β, 91%; γ, 46%; β+γ, 96%; for N9622, β+γ, 96%) as well as the ability to produce infectious virus (for C0047, β+γ, >4-log reduction; for K0049, β+γ, >4 log; for N9622, β+γ, 3 log [data not shown]). As seen with the prototype DV2 strain, the inhibitory effect of IFN was decreased significantly when the cells were treated with cytokine a few hours after exposure to virus.
FIG. 3.
Time course of the effect of IFN pretreatment on DV2 infection by low-passage viral isolates. HepG2 cells were exposed to DV2 (Thai strains C0477 and K0049; Nicaraguan strain N9622) at an MOI of 2 and incubated for 72 h. In each case, cells were exposed to medium or combinations of IFN-β and -γ (10 ng/ml each) as described for Fig. 2. One representative experiment of two is shown.
Effect of IFN on antibody-dependent DV2 infection.
DV enters cells through an as yet uncharacterized receptor(s) that may display glycosaminoglycans (5). In the presence of enhancing antibodies, DV presumably enters both via its primary receptor and through antibody-dependent enhancement of infection (12, 13), in which Fcγ receptors are believed to participate (14, 39). To assess whether the inhibitory effect of IFN-α, -β, and -γ was limited to antibody-independent DV entry, cells that bear Fcγ receptors were pretreated with IFN and exposed to DV2 in the presence of enhancing MAbs.
Initial studies were performed on monocytes isolated from peripheral blood. In the absence of enhancing antibodies, monocytes that were exposed to high levels of input virus (MOI > 10) produced no DV2 antigen or infectious virus (data not shown). When enhancing MAbs were added, virus was sometimes detected at low levels (103 PFU/ml from 106 cells) in supernatants from a subset of monocyte donors that were infected with the prototype DV2 strain at high MOI. When recent DV2 isolates K0049 and N9622 were used, even at the highest possible MOI, no appreciable infectious virus was detected in monocyte supernatants regardless of the presence of enhancing antibody.
(i) THP-1 cells.
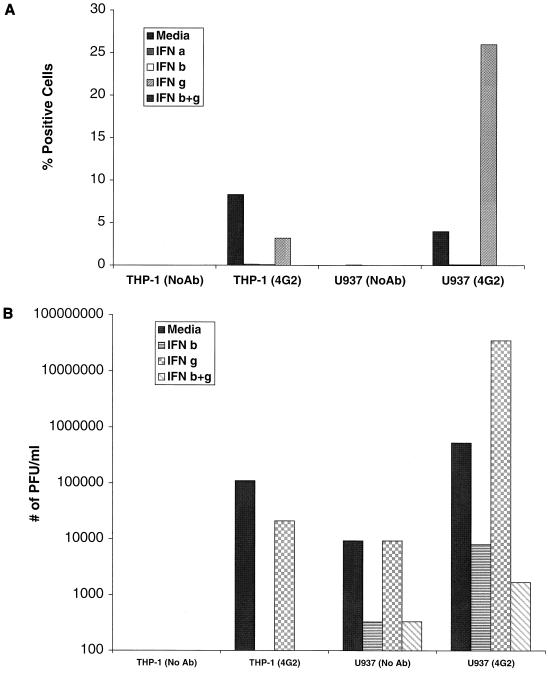
Because of the variability in infection assays observed in monocytes, we chose to perform antibody-dependent DV2 infection experiments with the human cell lines THP-1, U937, and K562, which express Fcγ receptors. THP-1 monocyte leukemic cells were used because infection of these cells by DV2 requires enhancing antibodies (34, 38). Without enhancing antibodies, even at high MOI (>10), we did not detect intracellular E protein or virus production in cell supernatants (Fig. 4 and data not shown). In the presence of an enhancing MAb (4G2 [4]), greater than 8% of the cells expressed viral antigen and produced infectious virions. A 24-h pretreatment of cells with IFN-α or -β abrogated viral antigen accumulation (decreased to <0.1% of cells) and virion production (2- to 5-log reduction). Of note, in THP-1 cells, IFN-γ pretreatment had a modest inhibitory effect on DV2 infection (61% decrease in cells that express viral antigen and <1-log reduction in viral titer). Even when a 6-log range of IFN-γ concentrations (0.01 ng/ml to 1 μg/ml) were tested, no enhancement of infection was observed (data not shown).
FIG. 4.
Effect of IFN pretreatment on antibody-dependent DV2 infection of THP-1 and U937 cells. THP-1 and U937 cells were exposed to DV2 (strain 16681) at an MOI of 10 in the absence (No Ab) or presence (4G2) of an enhancing MAb. After incubation for 96 h, cells were processed for flow cytometry (A), and supernatants were harvested for plaque assays (B). In each case, cells were pretreated 24 h prior to exposure to virus with medium, IFN-α (100 IU/ml), IFN-β (10 ng/ml), IFN-γ (10 ng/ml), or IFN-β+γ (each at 10 ng/ml). The data are expressed as in Fig. 2. One representative experiment of three is shown.
(ii) U937 cells.
Because a prior study reported that treatment with IFN-γ augmented DV infection in U937 cells (25), we investigated its effect on DV2 infection in these cells. In the absence of antibodies, the flow cytometry assay did not detect individual cells that expressed significant viral antigen (Fig. 4), although collectively, these U937 cells (1.4 × 106) produced a measurable viral titer (9.3 × 103 PFU/ml). Exposure of U937 cells to DV2 in the presence of enhancing MAbs resulted in a marked increase in the percentage of cells that expressed viral antigen (from 0 to 4%) and in the amount of infectious virus produced (from 1.3 × 104 to 6.0 × 105 PFU/ml), results that concur with previous studies (4). Pretreatment of U937 cells with either IFN-α or -β virtually abolished antibody-dependent DV2 infection, as judged by flow cytometry or plaque assay. However, in contrast to THP-1 cells, IFN-γ augmented viral replication in U937 cells, confirming previous results (25). This effect required the presence of enhancing MAbs and resulted an increase in the number of cells generating viral antigen (26%) and in the amount of virus produced (3.5 × 107 PFU/ml). Finally, the enhancement was overcome by IFN-β, as pretreatment with IFN-β+γ blocked antigen and virus production.
(iii) K562 cells.
We also assessed the effect of IFN-α, -β, and -γ on DV2 infection of K562 cells. This erythroleukemic cell line expresses high levels of Fcγ receptor II (CD32) and has been used extensively because it is susceptible to DV infection by an antibody-dependent pathway at low MOI and by an antibody-independent pathway at high MOI (39). Exposure to DV2 at a low MOI resulted in a low percentage (4%) of positive cells by flow cytometry and a moderate plaque titer (2 × 105 PFU/ml [data not shown]). Addition of the enhancing MAb 3H5 augmented the percentage of cells expressing viral antigen and the amount of infectious virus produced in the supernatant (Fig. 5). Pretreatment with IFN-α or β 24 h prior to infection significantly blocked antigen expression (α, 90% inhibition; β, 99%) and accumulation of infectious virus in the supernatant (α, >1.5-log decrease; β, >2.5 log [data not shown]). As noted for antibody-independent infection, the inhibitory effect was greatest when K562 cells were treated prior to infection but diminished rapidly if IFN-α or -β was added after exposure to virus. Treatment as few as 4 h after virus resulted in a noticeable loss of inhibition, both in the percentage of cells that expressed viral antigen (α, 60% inhibition; β, 76%) and the production of infectious virus (α, <1-log reduction; β, 1 log [data not shown]).
FIG. 5.
Time course of the effect of IFN pretreatment on antibody-dependent DV2 infection of K562 cells. K562 cells were exposed to DV2 (strain 16681) at an MOI of 0.005 in the presence of an enhancing MAb (3H5). After incubation for 96 h, cells (106) were processed for flow cytometry. In each case, cells were exposed to medium, IFN-α (100 IU/ml), IFN-β (10 ng/ml), IFN-γ (10 ng/ml), or a combination of IFNs as described for Fig. 2. One representative experiment of two is shown.
Mechanism of IFN inhibition of DV infection.
The studies described above demonstrate that IFN-α and -β markedly inhibit antigen accumulation and virus production regardless of the effect of enhancing antibodies. We began to investigate whether IFN inhibited DV at any of the steps prior to protein accumulation. We used an immunofluorescent virus binding assay (2) to assess the effect of IFN on DV binding to cells. Pretreatment with individual or combinations of IFN-α, -β, and -γ did not alter virus attachment to HepG2 cells (data not shown).
Since IFN did not appear to modulate DV binding to its cell surface receptor(s), we investigated its effect on steady-state levels of positive- and negative-strand DV2 RNA. To assess this, a quantitative, competitive RT-PCR assay was developed. Briefly, competitor RNA that contains sequences of NS3 fused to a nonfunctional fragment of GFP was synthesized and quantitated (see Materials and Methods). Serial dilutions of competitor were added to fixed quantities of RNA harvested from DV-infected cells and subjected to RT-PCR. The RT step was performed asymmetrically using either a sense or antisense oligonucleotide primer to distinguish the polarity of the viral RNA.
HepG2 cells were pretreated with IFN-β and/or -γ for 24 h prior to exposure to DV and harvested 24 h after infection. IFN-β treatment caused a 3-log reduction in the levels of the positive and negative strands of viral RNA (Fig. 6), whereas IFN-γ had a modest inhibitory effect (0.5- to 1-log reduction). The combination IFN-β+γ diminished the positive strand levels by 3 logs and virtually abolished accumulation of the negative strand. To determine if the kinetics of viral RNA accumulation paralleled antigen and virus production, we performed a time course experiment that quantitated the positive and negative strands of viral RNA from HepG2 cells that were treated with IFN at different times relative to virus exposure. In agreement with the flow cytometry and plaque assay results, treatment of HepG2 cells with IFN-β+γ prior to exposure to the low-passage DV2 isolate K0049 markedly attenuated the production of the positive and negative strands by several orders of magnitude. Pretreatment (24 or 4 h) decreased the amount of positive- and negative-strand viral RNA per cell (Fig. 7A) so that less competitor RNA was required to reach an equivalence point (Fig. 7B). Moreover, when IFN-β+γ was added 4 h after exposure to DV, much of the inhibition was lost, with only a 1- to 1.5-log reduction in the steady-state level of positive or negative strand. When added 24 h after the virus, there was no appreciable difference in positive strand and only a 1-log reduction in negative strand.
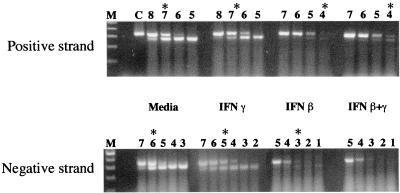
FIG. 6.
IFN effect on the levels of positive and negative strands of DV2 RNA in HepG2 cells. HepG2 cells were exposed to DV2 (strain 16681) at an MOI of 2 and incubated for 24 h. In each case, cells were exposed to medium, IFN-γ (10 ng/ml), IFN-β (10 ng/ml), or IFN-β+γ (each at 10 ng/ml) 24 h prior to incubation with virus. Cells (6 × 105) were harvested, total RNA was isolated, and quantitative asymmetric RT-PCR was performed with fixed amounts of cellular RNA in the presence of 10-fold decreasing concentrations of positive- or negative-strand competitor. The RT-PCR product was subjected to agarose gel electrophoresis. M refers to the molecular weight marker, C denotes an RT-PCR with only competitor RNA, and the number above each lane represents the log number of copies of competitor used. The amount of viral RNA was determined from the competitor concentration that produces competitor and DV bands of equal intensity (denoted by asterisk). RNA per cell is calculated as defined in Materials and Methods. For the positive strand, the equivalence point and RNA copies per cell were as follows: media, 107 and 833; IFN-γ, 5 × 106 and 417; IFN-β, 104 and 0.8; IFN-β+γ, 104 and 0.8. For the negative strand, the corresponding values were as follows: media, 106 and 110; IFN-γ, 105 and 11; IFN-β, 103 and 0.1; IFN-β+γ, undetectable.
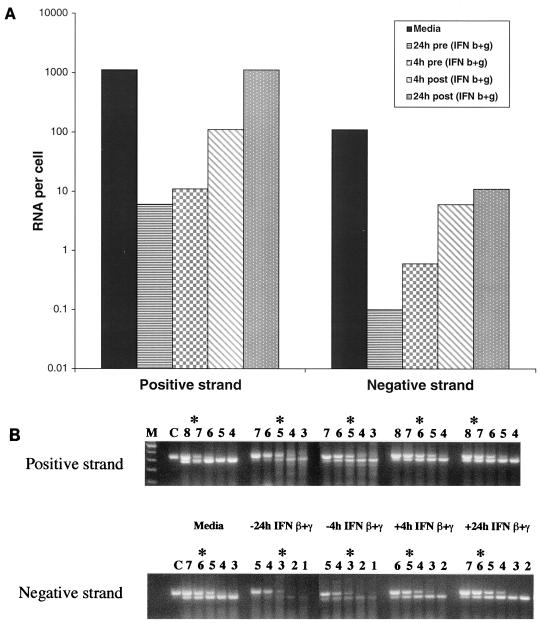
FIG. 7.
Time course of IFN effect on the levels of positive and negative strands of DV2 RNA in HepG2 cells. HepG2 cells were exposed to DV2 (Thai strain K0049) at an MOI of 2 and incubated for 72 h. In each case, cells were exposed to medium or IFN-β+γ (each at 10 ng/ml) either 24 or 4 h before (pre) or after (post) incubation with virus. Cells (6 × 105) were harvested, total RNA was isolated, and asymmetric RT-PCR was performed with fixed amounts of cellular RNA in the presence of 10-fold decreasing concentrations of positive- or negative-strand competitor. The amount of viral RNA per cell (A) was determined after agarose gel electrophoresis (B) as described for Fig. 6.
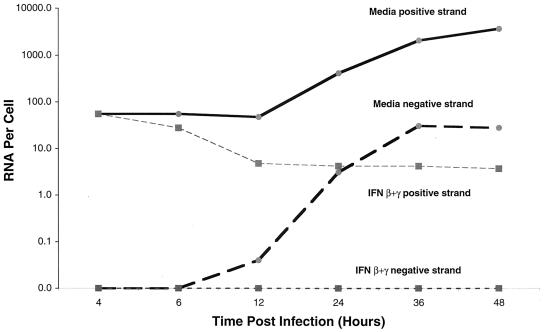
To begin to address the mechanism of how IFN affects viral RNA accumulation, HepG2 cells were pretreated for 24 h with medium or the combination IFN-β+γ, exposed to the 16681 DV2 strain, harvested at various time points after infection, and analyzed for positive- and negative-strand viral RNA. At early time points (4 and 6 h postinfection), IFN-β+γ did not appear to affect the steady-state level of the positive or negative strand (Fig. 8). The positive strand present at early time points likely represents the input virus prior to de novo RNA synthesis. Consistent with this, there was virtually no detectable negative strand in either the medium or IFN-treated samples. A constant level of positive strand and absence of negative strand at early time points is predicted by a lag in viral replication due to the time required for viral entry, nucleocapsid penetration, initial translation of the infectious RNA, and generation of the nonstructural proteins that comprise the viral replicase. At later time points (24, 36, and 48 h postinfection), however, IFN-β+γ dramatically reduced the steady-state level of positive strand. By 48 h after infection, there was a greater than 1,000-fold difference in the amount of positive-strand viral RNA per cell. Moreover, pretreatment virtually abolished the accumulation of negative strand, whereas the medium control exhibited a time-dependent increase in its steady-state levels.
FIG. 8.
Time course of the levels of positive and negative strands of DV2 RNA after IFN treatment of HepG2 cells. HepG2 cells were exposed to DV2 (strain 16681) at an MOI of 2 and incubated as indicated prior to harvest. In each case, cells were pretreated with medium or IFN-β+γ (each at 10 ng/ml) for 24 h prior to exposure to virus. At each time point after infection, cells were harvested and counted by hemocytometry, and total RNA was isolated. Subsequently, asymmetric RT-PCR was performed with fixed amounts of cellular RNA in the presence of 10-fold decreasing concentrations of positive- or negative-strand competitor. The product was subjected to agarose gel electrophoresis, and the amount of viral RNA per cell was determined as described for Fig. 6.
DISCUSSION
In this paper, we demonstrate that infection of human cells in vitro by DV is prevented by pretreatment of cells with IFN-α or -β. This conclusion is based on experiments showing that antibody-independent or antibody-dependent infection by DV2 in hepatoma cells, primary fibroblasts, and leukemic and myeloid cells is dramatically reduced when cells are exposed to IFN-α or -β several hours prior to virus infection. Using newly developed competitive RT-PCR and flow cytometry assays as well as plaque assays, we demonstrate that IFN inhibits DV infection by significantly reducing the levels of viral RNA, intracellular DV antigen, and infectious virus that are secreted into cell supernatants. The effect of IFN-γ is variable, as it inhibits antibody-independent infection but enhances antibody-dependent infection in a subset of myeloid cells. The antiviral effects of IFN are dose dependent, time dependent, and observed with primary cell lines and recent DV isolates that have not been laboratory adapted by repeated passage. The inhibitory effect of IFN-α, -β, and -γ is not due to cellular toxicity, as there is little change in the rate of cell division or viability after treatment. Dose-response studies demonstrate that the concentrations of IFN-α, -β, and -γ that prevent DV2 infection (3 to 100 IU/ml) are physiologically relevant. Time course studies suggest that the antiviral effect is not direct, as removal of the cytokine before, during, or after infection does not affect the level of inhibition, as long as cells have been pretreated with IFN for several hours.
The present study extends and clarifies how IFN regulates DV infection in cells. Previous investigations have suggested a role for IFN in modulating DV infection both in vivo and in vitro. Studies from patients with DF or DHF demonstrated increased serum levels of IFN-α (29) and IFN-γ (9, 28, 30) within days of disease presentation, and in vitro experiments showed that peripheral blood mononuclear cells generated elevated levels of IFN-α (26, 27) and IFN-γ (6, 31, 32) after DV infection. Other investigations showed that IFN-β protected dermal fibroblasts from DV2 infection (33), IFN-α protected monocytes from infection (31), and IFN-γ reduced virus production 10- to 100-fold in peripheral blood monocytes (47). Consistent with these results, AG129 mice that are deficient in the IFN-α, -β, and -γ receptor genes are killed by intraperitoneal administration of a mouse-adapted DV, whereas control mice survive (23). However, prior reports also postulated that IFN-γ contributed to the pathogenesis of DV infection by enhancing antibody-dependent viral entry in myeloid cells, presumably by increasing the expression of Fcγ receptors (25, 31, 32).
Overall, our data suggests that at least in vitro, IFN-α, -β, and -γ protect cells against antibody-independent DV infection. IFN-α and -β appear to have a more potent and durable inhibitory effect than IFN-γ. The similarity in effect of IFN-α and -β is anticipated, as both cytokines bind to receptors that share a common subunit (48). Pretreatment with IFN-α or IFN-β reduces antigen accumulation and virus production by several orders of magnitude for both antibody-dependent and antibody-independent infection. Consistent with prior studies, we observed a variable effect of IFN-γ on antibody-dependent DV2 infection. IFN-γ pretreatment partially inhibited viral antigen expression and virus production in THP-1 monocyte leukemic cells but augmented infection in U937 monomyelocytes. Although we attempted to assess the effect of IFN-γ on DV infection in peripheral blood monocytes, variable infection results among donors and the lack of productive infection with recent DV isolates made interpretation difficult. Because one prior study reported that IFN-γ did not augment DV2 infection of peripheral blood monocytes (47), the physiologic relevance of IFN-γ-mediated enhancement of antibody-dependent DV infection remains unclear.
Our results define an anti-DV effect by IFN-α, -β, and -γ that is consistent with the IFN-mediated antiviral activity that has been described for other RNA and DNA viruses (48). We have begun to investigate the critical molecular step(s) at which IFN exerts its anti-DV capacity. Virus binding and competitive RT-PCR experiments indicate that IFN does not prevent DV from binding to or entering cells but instead inhibits productive infection by preventing the intracellular accumulation of DV RNA. Kinetic studies in HepG2 cells demonstrate that the reduction in RNA temporally corresponds to decreases in antigen and virus production. By using asymmetric RT-PCR, we show that despite the presence of large quantities of positive-strand viral RNA in IFN-pretreated cells, productive infection is aborted. Moreover, we demonstrate that IFN pretreatment prevents the accumulation of the negative strand of DV2. This may be due either to a block in the translation of the input virus positive strand that is needed for production of the viral replicase, a block in transcription of the negative strand, or an acceleration of negative-strand degradation.
The kinetics of the IFN response demonstrate that pretreatment is required for complete inhibition of viral RNA, antigen, and virion production. Although there was some cell type and viral strain variation, in general, a significant percentage of the inhibitory effect was lost if cells were treated as little as a few hours after infection. Two non-mutually exclusive interpretations are possible: (i) IFN protects against de novo viral infection but cannot impede an established infection because it inhibits at an early step, and (ii) DV actively interrupts the antiviral effect of IFN. Several RNA and DNA viruses have evolved specific mechanisms to subvert IFN antiviral effects through the synthesis of proteins that mimic and interfere with host proteins (48). Studies are currently under way to define the mechanism by which the IFN-mediated block of DV infection is attenuated.
A substantial amount of the data in this study was obtained from experiments that used primary cell lines and recent DF and DHF viral isolates. The use of recent low-passage DV isolates is essential, as high-passage laboratory strains can accumulate dominant mutations that confer phenotypes that may not be physiologically relevant (24, 43). Additional studies are under way with a more extensive collection of recent DF and DHF isolates with accompanying epidemiologic data to determine whether a response in vitro to IFN demonstrates any correlation with disease severity in vivo. The extension of the IFN studies to other DV strains is critical, as determinants that are genotype specific and/or serotype specific could confer virulence through IFN resistance; clinical and laboratory studies suggest that some DV2 strains cause a more severe syndrome than others, but the molecular basis for this remains unclear (13, 35, 36, 44, 50).
The data we present in this paper suggest that IFN-α, -β, and -γ limit DV infection by making uninfected cells resistant to viral replication. This observation is sustained with several different cell lines and recent viral isolates. By using an asymmetric, quantitative RT-PCR to measure the levels of the positive and negative viral RNA strands, we conclude that IFN blocks DV infection by preventing the accumulation of the negative viral RNA strand. Future research will explore how IFN exerts its antiviral effect on DV: whether it inhibits negative-strand viral RNA accumulation directly by blocking transcription or accelerating degradation, or whether it blocks a more proximal step, possibly the translation of the input positive strand. These studies should define the specific IFN-induced mechanism that hampers viral infection and address why this inhibitory effect is diminished when IFN is added after an established infection.
ACKNOWLEDGMENTS
We thank R. Beatty for critical comments on the manuscript, L. Petruzzelli, L. Klickstein, S. Goth, M. Grigg, J. Boothroyd, and S. Kliks for providing cell lines and vectors, R. Rico-Hesse for providing recent DV isolates, S. Halstead and S. Kliks for helpful discussions and advice, P. Dazin for assistance with flow cytometry, and E. Lipner for technical assistance.
The work was supported by National Institutes of Health grants to E. Harris (AI-42052) and J. Ernst (HL-51992 and 56001) and by fellowships from the Giannini Foundation of the Bank of America and the Infectious Diseases Society of America to M. Diamond.
REFERENCES
- 1.Balmaseda A, Sandoval E, Perez L, Gutierrez C M, Harris E. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am J Trop Med Hyg. 1999;61:893–897. doi: 10.4269/ajtmh.1999.61.893. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt-Ohmann H. Analysis of antibody-independent binding of dengue viruses and dengue virus envelope protein to human myelomonocytic cells and B lymphocytes. Virus Res. 1998;57:63–79. doi: 10.1016/s0168-1702(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Brandt W E, McCown J M, Gentry M K, Russell P K. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect Immun. 1982;36:1036–1041. doi: 10.1128/iai.36.3.1036-1041.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 6.Elbishbishi E A, Agarwal R, Raghupathy R, Nagar R, Tandon R, Pacsa A S, Younis O I, Azizieh F. Sequential production of cytokines by dengue virus-infected human peripheral blood leukocyte cultures. J Med Virol. 1999;59:335–340. doi: 10.1002/(sici)1096-9071(199911)59:3<335::aid-jmv13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.English D, Anderson B R. Single-step separation of red blood cells, granulocytes, and mononuclear cells on discontinuous density gradients of ficoll-hypaque. J Immunol Methods. 1974;5:249–253. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 8.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 9.Green S, Vaughn D W, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis B L, Kurane I, Rothman A L, Ennis F A. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 10.Guzman M G, Alvarez M, Rodriguez R, Rosario D, Vazquez S, Valdés L, Cabrera M V, Kouri G. Fatal dengue hemorrhagic fever in Cuba, 1997. Int J Infect Dis. 1999;3:130–135. doi: 10.1016/s1201-9712(99)90033-4. [DOI] [PubMed] [Google Scholar]
- 11.Haberhausen G, Pinsl J, Kuhn C C, Markert-Hahn C. Comparative study of different standardization concepts in quantitative competitive reverse transcription-PCR assays. J Clin Microbiol. 1998;36:628–633. doi: 10.1128/jcm.36.3.628-633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11(Suppl. 4):S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 13.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 14.Halstead S B, O'Rourke E J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 15.Halstead S B, Porterfield J S, O'Rourke E J. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg. 1980;29:638–642. doi: 10.4269/ajtmh.1980.29.638. [DOI] [PubMed] [Google Scholar]
- 16.Harinasuta C, Wasi C, Vithanomsat S. The effect of interferon on Japanese encephalitis virus in vitro. Southeast Asian J Trop Med Public Health. 1984;15:564–568. [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 18.Harris E, Roberts T G, Smith L, Selle J, Kramer L D, Valle S, Sandoval E, Balmaseda A. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol. 1998;36:2634–2639. doi: 10.1128/jcm.36.9.2634-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hase T, Summers P L, Eckels K H. Flavivirus entry into cultured mosquito cells and human peripheral blood monocytes. Arch Virol. 1989;104:129–143. doi: 10.1007/BF01313814. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa H, Satake Y, Kobayashi Y. Effect of cytokines on Japanese encephalitis virus production by human monocytes. Microbiol Immunol. 1990;34:459–466. doi: 10.1111/j.1348-0421.1990.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle J H. Therapy of acute and chronic viral hepatitis. Adv Intern Med. 1994;39:241–275. [PubMed] [Google Scholar]
- 22.Jiang Y H, Davidson L A, Lupton J R, Chapkin R S. Rapid competitive PCR determination of relative gene expression in limiting tissue samples. Clin Chem. 1996;42:227–231. [PubMed] [Google Scholar]
- 23.Johnson A J, Roehrig J T. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontny U, Kurane I, Ennis F A. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988;62:3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurane I, Ennis F A. Induction of interferon alpha from human lymphocytes by autologous, dengue virus-infected monocytes. J Exp Med. 1987;166:999–1010. doi: 10.1084/jem.166.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurane I, Ennis F A. Production of interferon alpha by dengue virus-infected human monocytes. J Gen Virol. 1988;69:445–449. doi: 10.1099/0022-1317-69-2-445. [DOI] [PubMed] [Google Scholar]
- 28.Kurane I, Innis B L, Hoke C H, Jr, Eckels K H, Meager A, Janus J, Ennis F A. T cell activation in vivo by dengue virus infection. J Clin Lab Immunol. 1995;46:35–40. [PubMed] [Google Scholar]
- 29.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Ennis F A. High levels of interferon alpha in the sera of children with dengue virus infection. Am J Trop Med Hyg. 1993;48:222–229. doi: 10.4269/ajtmh.1993.48.222. [DOI] [PubMed] [Google Scholar]
- 30.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis F A. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Investig. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Rothman A L, Livingston P G, Janus J, Ennis F A. Human immune responses to dengue viruses. Southeast Asian J Trop Med Public Health. 1990;21:658–662. [PubMed] [Google Scholar]
- 32.Kurane I, Innis B L, Nisalak A, Hoke C, Nimmannitya S, Meager A, Ennis F A. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Investig. 1989;83:506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurane I, Janus J, Ennis F A. Dengue virus infection of human skin fibroblasts in vitro production of IFN-beta, IL-6 and GM-CSF. Arch Virol. 1992;124:21–30. doi: 10.1007/BF01314622. [DOI] [PubMed] [Google Scholar]
- 34.Kurane I, Kontny U, Janus J, Ennis F A. Dengue-2 virus infection of human mononuclear cell lines and establishment of persistent infections. Arch Virol. 1990;110:91–101. doi: 10.1007/BF01310705. [DOI] [PubMed] [Google Scholar]
- 35.Kurane I, Rothman A L, Livingston P G, Green S, Gagnon S J, Janus J, Innis B L, Nimmannitya S, Nisalak A, Ennis F A. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch Virol Suppl. 1994;9:59–64. doi: 10.1007/978-3-7091-9326-6_7. [DOI] [PubMed] [Google Scholar]
- 36.Leitmeyer K C, Vaughn D W, Watts D M, Salas R, Villalobos de Chacon I, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y L, Huang Y L, Ma S H, Yeh C T, Chiou S Y, Chen L K, Liao C L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y L, Liao C L, Chen L K, Yeh C T, Liu C I, Ma S H, Huang Y Y, Huang Y L, Kao C L, King C C. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Littaua R, Kurane I, Ennis F A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 40.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto A J, Morahan P S, Brinton M, Stewart D, Gavin E. Comparative therapeutic efficacy of recombinant interferons-alpha, -beta, and -gamma against alphatogavirus, bunyavirus, flavivirus, and herpesvirus infections. J Interferon Res. 1990;10:293–298. doi: 10.1089/jir.1990.10.293. [DOI] [PubMed] [Google Scholar]
- 42.Platanias L C, Uddin S, Domanski P, Colamonici O R. Differences in interferon α and β signalling. J Biol Chem. 1996;271:23630–23633. doi: 10.1074/jbc.271.39.23630. [DOI] [PubMed] [Google Scholar]
- 43.Rico-Hesse R, Harrison L M, Nisalak A, Vaughn D W, Kalayanarooj S, Green S, Rothman A L, Ennis F A. Molecular evolution of dengue type 2 virus in Thailand. Am J Trop Med Hyg. 1998;58:96–101. doi: 10.4269/ajtmh.1998.58.96. [DOI] [PubMed] [Google Scholar]
- 44.Rico-Hesse R, Harrison L M, Salas R A, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa M T, Nogueira R M, da Rosa A T. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 45.Rothman A L, Ennis F A. Immunopathogenesis of Dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 46.Russell P K, Udomsakdi S, Halstead S B. Antibody response in dengue and dengue hemorrhagic fever. Jpn J Med Sci Biol. 1967;20:103–108. [PubMed] [Google Scholar]
- 47.Sittisombut N, Maneekarn N, Kanjanahaluethai A, Kasinrerk W, Viputtikul K, Supawadee J. Lack of augmenting effect of interferon-gamma on dengue virus multiplication in human peripheral blood monocytes. J Med Virol. 1995;45:43–49. doi: 10.1002/jmv.1890450109. [DOI] [PubMed] [Google Scholar]
- 48.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 49.Vithanomsat S, Wasi C, Harinasuta C, Thongcharoen P. The effect of interferon on flaviviruses in vitro: a preliminary study. Southeast Asian J Trop Med Public Health. 1984;15:27–31. [PubMed] [Google Scholar]
- 50.Watts D M, Porter K R, Putvatana P, Vazquez B, Calampa C, Hayes C G, Halstead S B. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]