Abstract
Herpesviruses accomplish DNA replication either by expressing their own deoxyribonucleotide biosynthetic genes or by stimulating the expression of the corresponding cellular genes. Cytomegalovirus (CMV) has adopted the latter strategy to allow efficient replication in quiescent cells. In the present report, we show that murine CMV (MCMV) infection of quiescent fibroblasts induces both mRNA and protein corresponding to the cellular thymidylate synthase (TS) gene, which encodes the enzyme that catalyzes the de novo synthesis of thymidylic acid. The increase in TS gene expression was due to an increase in gene transcription, since the activity of a reporter gene driven by the mouse TS promoter was induced following MCMV infection. Mutagenesis of the potential E2F-responsive element immediately upstream from the TS essential promoter region abolished the virus-mediated stimulation of the TS promoter, suggesting that the transactivating activity of MCMV infection was E2F dependent. Cotransfection experiments revealed that expression of the viral immediate-early 1 protein was sufficient to mediate the increase in TS promoter activity. Finally, MCMV replication and viral DNA synthesis were found to be inhibited by ZD1694, a quinazoline-based folate analog that inhibits TS activity. These results demonstrate that upregulation of cellular TS expression is required for efficient MCMV replication in quiescent cells.
Cytomegalovirus (CMV) efficiently replicates in vivo in a restricted range of terminally differentiated cell types, such as epithelial and endothelial cells, fibroblasts, and some hematopoietic cell types in which the levels of deoxyribonucleoside triphosphates (dNTPs) are very low and the expression of cellular enzymes involved in their biosynthesis is stringently repressed (44). Analyses of viral DNA sequences have revealed that, unlike other herpesviruses, CMV does not encode dNTP biosynthetic enzymes, such as thymidine kinase, dihydrofolate reductase, thymidylate synthase (TS), and an active form of ribonucleotide reductase (16, 51), and must mainly rely upon host cell metabolism to ensure a sufficient supply of dNTPs for its DNA replication. It appears that CMV has developed strategies to stimulate the biochemical pathways involved in the biosynthesis of DNA precursors. Several reports have demonstrated that the early events occurring after CMV infection are similar to those observed in serum-deprived cells exposed to growth factors. These include nuclear translocation of Cdk2 (13), induction of cyclins E and B (14, 30, 52), pRb hyperphoshorylation (30), activation of E2F-dependent transcription (41, 50, 59), activation of c-myc, c-jun, and c-fos protooncogenes (9, 10), and a substantial increase in the activities of cellular enzymes involved in DNA metabolism, including thymidine kinase, ornithine decarboxylase, and topoisomerase II (6, 20, 27). Despite the induction of an S-phase-like state, CMV-infected cells fail to undergo cellular DNA replication and division as a result of blocks in cell cycle progression (14, 19, 30, 40, 52, 60) that prevent the host DNA replication machinery from competing with the virus for access to DNA precursors.
Among the cellular biochemical pathways involved in the production of DNA precursors, the de novo biosynthesis of thymidylic acid (dTMP) may be crucial for viral replication, since it only occurs in cells that are preparing for DNA synthesis. Infection of embryonic fibroblasts with human CMV (HCMV) in fact results in a 30-fold increase in the size of the TTP pool compared with mock-infected cells (7, 57).
TS is the enzyme that catalyzes the de novo synthesis of dTMP by reductive transfer of the methylene group from 5,10-methylene-tetrahydrofolate to the 5 position of the substrate, deoxyuridylic acid, to form dTMP and dihydrofolate. It is an essential enzyme in proliferating cells and an important target of a variety of anticancer drugs (31). TS activity and mRNA content are very low in quiescent cells but increase sharply at the G1-S border during a serum-induced transition from the resting (G0) to the growing state. However, nuclear run-on transcription assays have revealed that the rate of TS gene transcription does not change during the G1-S transition (3). Therefore, it appears that TS gene expression is primarily controlled at the posttranscriptional level in growth-stimulated cells (31). Proper S-phase regulation of transfected TS minigenes requires the presence of both the TS essential promoter region and a spliceable intron in the transcribed region and may involve some form of communication between the TS promoter and the RNA processing machinery (3, 34).
We have been studying the effects of CMV infection on expression of the enzymes involved in dTMP biosynthesis because knowledge of the molecular mechanisms of the virus-mediated regulation of this pathway could lead to the design of novel antiviral strategies. Here we report that murine CMV (MCMV) infection transcriptionally activates TS gene expression in quiescent fibroblasts, resulting in an increase in the TS enzyme needed for MCMV DNA synthesis during the productive replicative cycle. The increase appears to be mediated by the viral immediate-early (IE) 1 protein as well as a cellular E2F transcription factor.
MATERIALS AND METHODS
Cells and culture conditions.
NIH 3T3 murine fibroblasts were grown as monolayers in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL) supplemented with 10% calf serum (Gibco-BRL). Quiescent cells (arrested in G0/G1 phase) were obtained by culturing the subconfluent cultures for 48 h in DMEM supplemented with 0.5% calf serum. Flow cytometry demonstrated that more than 90% were growth arrested. C57BL/6 mouse embryo fibroblasts (C57BL/6-MEF) and the B6MEF cell line (an embryonic fibroblast cell line derived from C57BL/6 mice and immortalized through several culture passages) were maintained as monolayers in DMEM supplemented with 10% fetal calf serum.
Plasmids.
pMTS-687 contains the 687-nucleotide (nt) PstI fragment from the murine TS cDNA cloned into the pIBI30 vector (49). pTLG contains the mouse TS promoter region from −985 to −11 bp (relative to the AUG codon) linked to an intronless luciferase indicator gene derived from pGL2-basic (Promega). The simian virus 40 polyadenylation signal/small-T intron of pGL2-basic was replaced with the polyadenylation signal from the human β-globin gene (3). pTLG:I1d1.0 contains an internally deleted version of mouse TS intron 1 inserted into the polylinker region between the TS promoter and the luciferase coding region of pTLG (34). The intron retains approximately 440 nt from the 5′ end and 140 nt from the 3′ end of the intron. pTSWTGL3 was constructed by inserting the TS promoter region between the NheI and BglII sites of pGL3-basic (Promega). pTSWTGL3(−110) is a derivative of pTSWTGL3 in which the potential E2F element just upstream from the TS essential promoter region [(−115)GATTCTGGCGGCC(−103)] was mutated to (−115)GATTCGCTAGCCC(−103) (22). pCMVCAT contains a 1.2-kb PstI-NdeI segment from the HindIII fragment L of MCMV DNA, positioned upstream from the bacterial chloramphenicol acetyltransferase (CAT) reporter gene of pSVOCAT. The viral genomic segment contains the IE enhancer and the IE1/IE3 promoter of MCMV (24). pIE100/1 (pIE1) and pIE3 contain MCMV genomic fragments which encode the IE1 and IE3 proteins, respectively. Their expression is driven by the MCMV IE enhancer and the IE1/IE3 promoter (43). pE1CAT contains the CAT reporter gene under the control of the MCMV E1 early promoter (43).
Virus preparation and infections.
MCMV (mouse salivary gland virus, strain Smith; ATCC VR.194) was purchased from the American Type Culture Collection. Viral stocks were first produced in the salivary glands of BALB/c mice and then propagated in vitro by infecting C57BL/6-MEF cells at a virus-to-cell ratio of 0.01. Cells were incubated in DMEM supplemented with 2% heat-inactivated calf serum, and virus was harvested at about 1 week postinfection (p.i.) by sonication and centrifugal clarification. Mock-infecting fluid was prepared from uninfected C57BL/6-MEF by the same procedure. A virus stock solution containing approximately 107 PFU/ml (as determined by plaque assay on B6MEF cells) was used in all infection experiments.
For RNA and protein determinations and transfection and enzyme assays, quiescent NIH 3T3 cells were infected with MCMV at a multiplicity of infection (MOI) of 5 unless otherwise stated. Mock-infected control cultures were exposed to an equal volume of mock-infecting fluid. Virus adsorptions were carried out for 2 h at 37°C. At the end of the adsorption period, which is defined as 0 h p.i., the low-serum medium removed before infection was restored to the cultures to avoid any cellular stimulation that might have resulted from the addition of fresh serum growth factors.
Inactivation of virus by UV light.
MCMV or mock-infecting fluid was placed in a 60-mm dish and irradiated (uncovered) in a UV-linker (Pbi International) with one pulse (0.6 J/cm2) of UV light. The preparations were irradiated just prior to use and then kept on ice. To prevent light-induced repair mechanisms, irradiated stocks were kept covered with aluminum foil, and infections were performed in the absence of fluorescent lights. Preliminary experiments demonstrated that no MCMV gene expression could be detected in NIH 3T3 cells infected with the UV-irradiated MCMV.
RNA analysis.
At the indicated times, cells were rinsed twice with ice-cold phosphate-buffered saline (PBS), and total cellular RNA was isolated by homogenization in 4 M guanidium isothiocyanate and centrifugation through a 5.7 M cesium chloride cushion (17). To determine the TS mRNA content, RNase protection assays were performed as described previously (4), using 30 μg of total cytoplasmic RNA and 105 cpm of probes corresponding to the 393-nt PstI-BamHI segment from mouse TS cDNA (49) and a 254-nt segment from mouse actin cDNA (2). Antisense RNA transcripts were generated by in vitro transcription with T3 RNA polymerase (Ambion). The RNase A- and RNase T1-resistant probe fragments were separated by electrophoresis on a 5% polyacrylamide–8 M urea gel. The dried gel was analyzed by autoradiography, and the amount of radioactivity was determined by scanning with a phosphoimager.
To study the transcriptional start site pattern, 30 μg of each sample were analyzed in an S1 nuclease protection assay as described previously (22). The probe was derived from an intronless TS minigene and was 5′ end labeled with 32P at the BamHI site in exon 3. The protected fragments were separated by electrophoresis on a 6% denaturing polyacrylamide gel and detected by autoradiography.
Transient transfections and reporter gene assays.
All plasmids were purified by cesium chloride centrifugation. For transient gene expression assays, cells were plated on the day before transfection in growth medium (10% calf serum) at a density of 2 × 105 cells/60-mm-diameter dish. The medium was changed 4 h before transfection. Cells were transfected for 18 h by the calcium phosphate procedure (4). The amount of DNA for each transfection was kept constant at 12 μg by adding an appropriate amount of carrier DNA (the inert pBluescript SK plasmid [Stratagene]). The transfected cells were washed twice with medium and incubated in DMEM supplemented with 0.5% calf serum (low-serum medium) for 48 h. To measure the luciferase activity, the cells were washed twice with PBS, scraped from the plates into PBS containing 1 mM EDTA, and collected by centrifugation. The pellets were resuspended in 100 μl of reporter lysis buffer (Promega), and soluble proteins were recovered after centrifugation. Supernatants were quantified for protein concentration, and aliquots were assayed with 100 μl of luciferin substrate (Promega) in a 1600CA Tri-Carb liquid scintillation analyzer (Packard). Reporter gene activity was normalized to the amount of plasmid DNA introduced into recipient cells by DNA dot blot analysis with a luciferase probe as previously described (1).
Preparation of protein extracts and immunoblotting.
Whole-cell extracts were prepared by resuspending pelleted cells in lysis buffer containing 125 mM Tris-Cl (pH 6.8), 3% sodium dodecyl sulfate (SDS), 20 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 4 μg of leupeptin, 4 μg of aprotinin, and 1 μg of pepstatin per ml. After a brief sonication, soluble proteins were collected by centrifugation at 15,000 × g. Supernatants were analyzed for protein concentration with a Bio-Rad Dc protein assay kit (Bio-Rad Laboratories) and stored at −70°C in 10% glycerol.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to Immobilon-P membranes (Millipore). Filters were blocked in 5% nonfat dry milk in 10 mM Tris-Cl (pH 7.5)–100 mM NaCl–0.1% Tween 20 and immunostained with the mouse anti-TS human monoclonal antibody (32) (clone TS106; Labvision Neomarkers) (diluted 300-fold), the rabbit anti-MCMV IE1 antibody (21) (diluted 5,000-fold), or the mouse antiactin monoclonal antibody (MAb) (Boehringer) (diluted 2,000-fold) at room temperature for 1 h. Immune complexes were detected with either sheep anti-mouse immunoglobulin (Ig) or goat anti-rabbit Ig antibodies conjugated to horseradish peroxidase (Amersham) and visualized by enhanced chemiluminescence (Super Signal; Pierce) according to the manufacturer's instructions.
Immunofluorescence microscopy.
Cells grown on coverslips and then incubated in low-serum medium for 48 h were infected with MCMV at an MOI of 0.5. At 48 h p.i. cells were washed with PBS, fixed with 1% paraformaldehyde for 20 min at room temperature, and then washed again with PBS. The cells were subsequently permeabilized with 0.2% Triton X-100 in PBS for 20 min at 4°C, washed with PBS–1% bovine serum albumin (BSA), and incubated with the anti-TS antibody (diluted 50-fold) and the anti-IE1 antibodies (diluted 250-fold) in PBS–1% BSA–0.2% Triton X-100 for 1 h at room temperature. After being washed with PBS–1% BSA–0.05% Tween 20, cells were incubated with fluorescein isothiocyanate-conjugated anti-mouse Ig and Texas Red-conjugated goat anti-rabbit Ig antibodies in PBS–1% BSA–0.2% Triton X-100 for 1 h. Finally, coverslips were washed with PBS–1% BSA–0.05% Tween 20 and mounted in 90% glycerol. Immunofluorescence microscopy was performed on an Olympus IX70 inverted confocal laser scanning microscope, equipped with a krypton-argon ion laser (488/568). Images derived from both channels (fluorescein and Texas Red) were recorded simultaneously at identical apertures. The fluorescein-derived image was assessed with a green color, and the Texas Red-derived image was assessed with a red color.
TS enzyme level.
TS levels were determined by measuring the formation of the ternary covalent complex between TS, 3H-labeled fluorodeoxy-UMP ([6-3H]FdUMP; Moravek Biochemicals), and N5,N10-methylene tetrahydrofolate, as previously described (45, 56).
Cytotoxicity assay.
Cells were grown to subconfluence in 24-well plates and then incubated in low-serum medium for 48 h. Thereafter, the medium was replaced with the same low-serum medium or with fresh growth medium containing various concentrations of ZD1694 (Tomudex or Raltitrexed; Zeneca). After 4 days of incubation, the number of viable cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, as previously described (48).
Inhibition of viral replication and DNA synthesis.
To determine the extent of viral replication, cells were grown to subconfluence in 24-well plates, incubated in low-serum medium for 48 h, and infected with MCMV at an MOI of 1. One column per plate was mock infected and served as the cell control. The infected cultures were treated with different concentrations of ZD1694 in duplicate wells. One column per plate was not treated and served as the virus control. Cultures were incubated until the control cultures displayed 100% cytopathology. Thereafter, the cells and the supernatants from the anti-CMV assay were harvested and disrupted by sonication. The disrupted cells were centrifuged at 500 × g for 10 min, and the supernatant was assayed for infectivity by a standard plaque assay for MCMV using the cell line B6MEF. The number of plaques was plotted as a function of drug concentration, and the concentrations producing 50 and 90% reductions in plaque formation (EC50 and EC90, respectively) were determined.
To evaluate the inhibition of MCMV DNA synthesis, cells were grown to subconfluence in six-well plates, incubated in low-serum medium for 48 h, and infected with MCMV at an MOI of 1. One well per plate was mock infected and served as the cell control. The infected cultures were treated in low-serum medium with different concentrations of ZD1694. In some experiments, thymidine (20 μM) or hypoxanthine (100 μM) was added. One well per plate was not treated and served as the virus control. At 48 h p.i., cells were harvested, and total DNA was isolated by resuspending cell pellets in lysis buffer (10 mM Tris-Cl [pH 8.0], 25 mM EDTA, 100 mM NaCl, 0.5% SDS, 100 μg of proteinase K per ml) and incubating the mixtures at 50°C for 18 h. Digestion was followed by phenol-chloroform extraction, ethanol precipitation, and RNase treatment (1 μg of RNase A per ml for 1 h at 37°C). Twofold dilutions of the DNA samples were immobilized on a Zeta-Probe hybridization membrane (Bio-Rad). DNA samples were sequentially hybridized with 32P-labeled probes prepared from the XbaI-AvaI DNA fragment of the MCMV IE1 gene (exon 4) (35) and mouse glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA. The membranes were autoradiographed, and the hybridization signals were quantitated with a phosphoimager.
RESULTS
MCMV infection stimulates TS expression in quiescent NIH 3T3 cells.
To investigate the ability of MCMV to induce cellular TS gene expression in quiescent cells, serum-arrested NIH 3T3 cells were infected with MCMV (at an MOI of 5), and at different time points p.i. cell extracts were prepared and examined for TS expression. As shown in Fig. 1A, TS mRNA content increased by about threefold at 24 h and sevenfold at 48 h p.i. relative to mock-infected cells. Serum stimulation (10% calf serum for 24 h) resulted in an approximately fourfold increase in TS mRNA levels (data not shown).
FIG. 1.
Effects of MCMV infection on TS gene expression in quiescent NIH 3T3 cells. (A) MCMV infection increases TS mRNA content. NIH 3T3 cells were growth arrested in 0.5% calf serum for 48 h and then infected with MCMV (MOI of 5) or mock infected. Total RNA was isolated at the indicated times after infection and analyzed by RNase protection assays, as described in Materials and Methods. The probe segments protected by TS mRNA and actin mRNA were 393 and 254 nt in length, respectively. Radioactivity corresponding to the protected fragments was determined by scanning the gel with a phosphoimager. The increase in TS mRNA content was calculated by normalizing the amount of radioactivity corresponding to TS mRNA to that of actin mRNA (internal control) to correct for differences in RNA loading and recovery. The value at each time point was then normalized to the value observed with mock-infected cells, which was set at 1. (B) MCMV gene expression is required to increase TS enzyme levels: immunoblot analysis. NIH 3T3 cells were growth arrested in 0.5% calf serum and then infected with MCMV or UV-irradiated MCMV or mock infected. Total cell extracts were prepared at the indicated times after infection, fractionated by SDS-PAGE (50 μg of protein per lane), and analyzed by immunoblotting with the anti-TS MAb or with the anti-IE1 serum, as described in Materials and Methods. Actin immunodetection with an MAb was performed asan internal control. Cell extracts were isolated from mock-infected cells (lane 1), cells that were infected with MCMV for 6 h (lane 2), 12 h (lane 3), 18 h (lane 4), 24 h (lane 5), or 48 h (lane 6), or with UV-treated MCMV for 24 h (lane 7) or 48 h (lane 8). (C) MCMV gene expression is required to increase TS enzyme levels: [3H]FdUMP binding assay. NIH 3T3 cells were growth arrested in 0.5% calf serum and then infected with MCMV or UV-irradiated MCMV or mock infected. Total cytoplasmic extracts were isolated at the indicated times after infection and assayed for TS enzyme levels using the [3H]FdUMP binding assay, as described in Materials and Methods. The average values from two independent experiments are shown.
To determine if viral infection of quiescent cells would also lead to an increase in the TS protein, cell extracts were prepared at different times p.i. and analyzed by immunoblotting with an anti-TS MAb. As shown in Fig. 1B, TS protein was undetectable in mock-infected cells (lane 1), but the level began to increase at 12 h p.i. (lane 3) and peaked at 24 h p.i. (lane 5). To investigate whether MCMV gene expression is necessary to induce TS expression, quiescent cells were infected with UV-inactivated MCMV. Under the irradiation conditions used (a pulse of 0.6 J/cm2), IE1 gene expression was completely inactivated (Fig. 1B, lanes 7 and 8). When the same extracts were probed with the anti-TS MAb, no TS protein was detected at either 24 h p.i. (lane 7) or 48 h p.i. (lane 8), demonstrating that active viral gene expression is required to induce cellular TS expression. The TS enzyme level, assayed by the FdUMP binding assay, also increased by about 2.3-fold at 24 h p.i. and about 3.7-fold at 48 h (Fig. 1C). By contrast, cells infected with the UV-irradiated MCMV did not display any increase in TS level.
To obtain further evidence that viral gene expression is required for TS induction, immunofluorescence assays were performed on MCMV-infected cells that were costained with antibodies to the TS and MCMV IE1 proteins. Analysis by confocal laser microscopy showed that only cells that were expressing the IE1 antigen reacted with the anti-TS antibody (Fig. 2). The TS staining pattern appeared to be specific for virus-infected cells, because cells infected with UV-irradiated MCMV were not stained (data not shown). Attempts to establish the intracellular location of TS have produced variable results depending on the technique and cells used, and both nuclear and cytoplamsic locations have been reported (37, 53, 55). In the present study, the TS fluorescence in MCMV-infected cells had a predominantly granular cytoplasmic appearance, similar to that observed in human tumor tissues (32, 33).
FIG. 2.
Localization of TS protein in MCMV-infected quiescent NIH 3T3 cells by indirect immunofluorescence. NIH 3T3 cells grown on coverslips were growth arrested in 0.5% calf serum for 48 h and then infected with MCMV (MOI of 0.5 PFU/cell) or UV-irradiated MCMV or mock infected. At 48 h p.i., cells were fixed with paraformaldehyde, permeabilized, and costained with MCMV IE1 (A) and TS (B) antibodies, as described in Materials and Methods. The merged image is shown in panel C. The immunolocalization experiments were repeated three times, and representative results are presented.
Altogether, these results demonstrate that MCMV infection of quiescent NIH 3T3 cells leads to an increase in TS mRNA and protein levels that depends on active viral gene expression.
MCMV infection transactivates the TS gene promoter in quiescent NIH 3T3 cells.
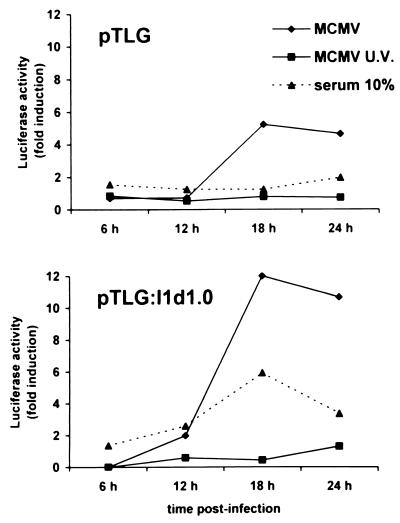
To determine if the increase in TS mRNA levels correlated with an increase in the activity of the TS promoter, we analyzed the effects of MCMV infection on the expression of a transiently transfected luciferase reporter gene driven by the TS promoter. Previous studies have demonstrated that S-phase-specific expression of stably transfected TS minigenes requires the presence of both the TS promoter region and a spliceable intron in the transcribed region (3, 34). To evaluate the contribution of the TS promoter and intron sequences to virus-mediated regulation, we compared the effects of MCMV infection on the activity of two indicator plasmids, pTLG, which contains an intronless luciferase gene driven by 1 kb of the 5′-flanking region of the mouse TS gene, and pTLG:I1d1.0, in which an internally deleted version of TS intron 1 was inserted into the polylinker region of pTLG between the TS promoter region and the luciferase gene. After transfection, cells were serum starved and then infected with MCMV or UV-inactivated virus or stimulated with serum. At different time points p.i., cell extracts were prepared and assayed for luciferase activity. As shown in Fig. 3, in agreement with previous observations, serum stimulation led to an increase in luciferase activity only in cells transfected with the intron-containing TS construct pTLG:I1d1.0. By contrast, MCMV infection (at 18 h p.i.) increased the luciferase activity by about 5-fold in cells transfected with the intronless pTLG and by about 12-fold in cells transfected with pTLG:I1d1.0. Although the magnitude of the increase was somewhat greater for the intron-containing reporter gene, MCMV infection transactivated both the intronless and the intron-containing TS-luciferase constructs. For both constructs, luciferase activity peaked at 18 h p.i. UV-inactivated virus did not increase luciferase activity, demonstrating that MCMV-mediated transactivation requires virus gene expression.
FIG. 3.
MCMV infection transactivates the TS promoter in quiescent NIH 3T3 cells. DNA (2 μg) from construct pTLG or pTLG:I1d1.0 was transiently transfected along with carrier DNA (10 μg of pBluescript SK) into NIH 3T3 cells as described in Materials and Methods. After 18 h, cells were washed and growth arrested in 0.5% calf serum for 48 h. Thereafter, transfectants were infected with MCMV or UV-irradiated MCMV, mock infected, or serum stimulated to reenter the cell cycle. Total cytoplasmic extracts were isolated at the indicated times after virus infection or serum stimulation and assayed for luciferase activity. Reporter gene activity was normalized to the amount of plasmid DNA introduced into recipient cells by DNA dot blot analysis. The resulting luciferase activity is expressed as fold induction relative to basal levels measured in cells transfected with pTLG or pTLG:I1d1.0 and then mock infected, which was set at 1. The experiment was repeated twice, and representative results are reported.
Altogether, these results indicate that MCMV regulates TS gene expression primarily at the transcriptional level, and the presence of a spliceable intron contributes to the overall response of the TS gene to virus infection.
E2F binding site of TS promoter crucial for MCMV transactivation.
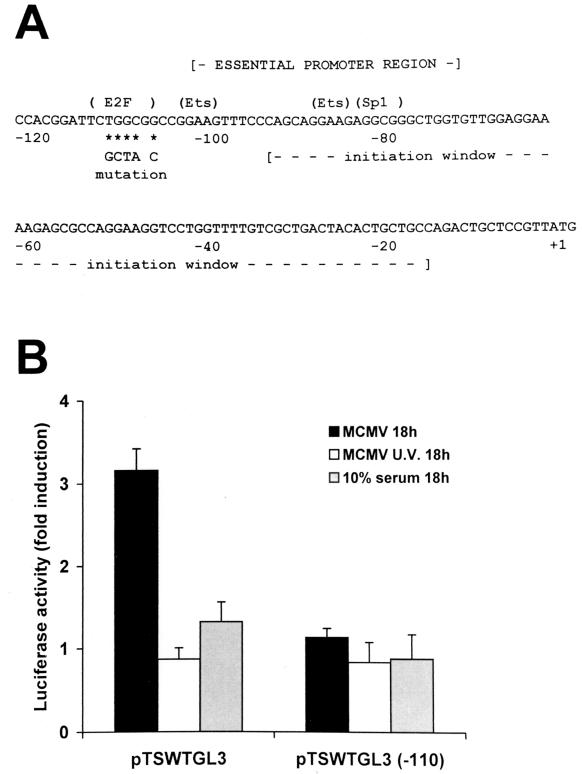
The mouse TS minimal promoter region is located between nt 75 and 105 upstream from the AUG start codon. The presence of a potential E2F binding site just upstream from this region (Fig. 4A) and the observation that HCMV stimulates cellular E2F-dependent transcription (41, 50, 59) prompted us to investigate whether the E2F site may contribute to the regulation of TS promoter activity following MCMV infection. We therefore compared the effects of MCMV infection on the expression of two indicator plasmids: pTSWTGL3, which contains the luciferase gene of pGL3 driven by 1 kb of the 5′-flanking region of the mouse TS gene, and the pTSWTGL3(−110) construct, in which the E2F binding site at −110 (TCTGGCGG) has been mutated to TCGCTAGC. NIH 3T3 cells were transfected with the plasmids, serum starved, and then infected with active or UV-inactivated MCMV or stimulated with serum. At 18 h p.i., cell extracts were prepared and assayed for luciferase activity. The results (Fig. 4B) show that inactivation of the E2F site at −110 abolished the activation of TS promoter activity following MCMV infection. Once again, UV-inactivated MCMV as well as serum failed to stimulate the transcriptional activity of the TS promoter. These results suggest that activation of the TS promoter by MCMV is E2F dependent and confirm that TS gene induction is brought about by mechanisms that are different from those activated by serum factors.
FIG. 4.
E2F binding site of the TS promoter required for MCMV-mediated transactivation. (A) Sequence of the mouse TS promoter region. The locations of the essential promoter region, the transcriptional initiation window, the potential binding sites for E2F, Ets, and SP1, and the AUG translational start codon are indicated. The nucleotide changes made in the E2F element in the −110 TS promoter mutation are shown under the wild-type sequence. (B) Effect of inactivation of the E2F element. NIH 3T3 cells were transiently transfected with 2 μg of the indicator construct pTSWTGL3, which contains the wild-type TS promoter region, or pTSWTGL3(−110), which contains the E2F mutation, and 10 μg of carrier DNA (pBluescript SK). After 18 h, cells were washed and growth arrested in 0.5% calf serum for 48 h. Thereafter, transfectants were infected with MCMV or UV-irradiated MCMV, mock infected, or serum stimulated to reenter the cell cycle. Total cytoplasmic extracts were isolated at 18 h after virus infection or serum stimulation and assayed for luciferase activity. Reporter gene activity was normalized to the amount of plasmid DNA introduced into recipient cells by DNA dot blot analysis. The resulting luciferase activity is expressed as fold induction relative to basal levels measured in cells transfected with pTSWTGL3 or pTSWTGL3(−110) and then mock infected, which was set at 1. The data shown are the averages of three experiments ± the standard errors of the means (error bars).
Transcriptional start site pattern of the TS gene does not change in response to MCMV infection.
The mouse TS promoter lacks both a TATA box and an initiator element and has a complex pattern of transcriptional initiation sites over an 80-nt region (Fig. 4A). The elements within the TS essential promoter region establish the strength of the promoter as well as the boundaries of the transcriptional initiation window (22). Previous studies (18, 22) have shown that inactivation or deletion of the E2F element has no effect on promoter activity or the pattern of transcriptional start sites in exponentially growing cells. However, since the E2F element plays an important role in the induction of TS promoter activity following viral infection, it was important to determine whether the E2F-mediated activation of the TS promoter would lead to a significant change in the pattern of transcriptional start sites. RNA was isolated at different time points following MCMV infection or serum stimulation of quiescent NIH 3T3 cells, and the 5′-terminal structure of TS mRNA was analyzed in an S1 nuclease protection assay. As shown in Fig. 5, activation of the TS promoter following MCMV infection did not lead to a significant change in the boundaries of the transcriptional initiation window or the pattern of transcriptional start sites.
FIG. 5.
Complex transcriptional start site pattern of the TS gene does not change in response to MCMV infection. NIH 3T3 cells maintained in medium supplemented with 0.5% calf serum for 48 h were either mock infected (mock), infected with MCMV, or stimulated with 10% serum (serum) for the indicated times. The transcriptional start site pattern was analyzed by an S1 nuclease protection assay as described in Materials and Methods. The protected fragments were separated by gel electrophoresis and detected by autoradiography. The approximate locations of the start sites relative to the AUG codon are indicated.
MCMV IE1 protein plays a role in regulation of the TS promoter.
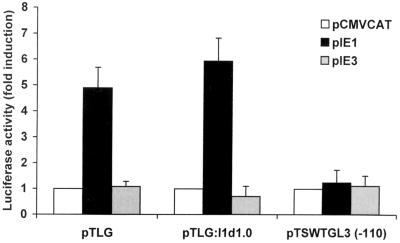
Previous studies have shown that transfection of cells with a construct expressing HCMV IE1 protein resulted in the activation of the dihydrofolate reductase (DHFR) promoter and that this transactivation was dependent on a functional E2F element (41). To examine the potential role of the MCMV IE gene products in the regulation of the TS promoter, we cotransfected an expression plasmid for the IE1 or IE3 protein with the pTLG, pTLG:I1d1.0, or pTSWTGL3(−110) construct into NIH 3T3 cells. To rule out the possibility that the MCMV promoter titrates out negative regulators of the TS promoter, thereby appearing to activate it, the amount of MCMV promoter included in the transfection mixtures was kept constant by including appropriate amounts of pCMVCAT, which contains the regulatory sequences of the MCMV IE region (the IE enhancer and IE1/IE3 promoter) linked to the CAT protein coding region. Figure 6 demonstrates that only the IE1 product transactivated the wild-type TS promoter. The extent of activation was fivefold for pTLG and more than sixfold for pTLG:I1d1.0 relative to the control. Mutagenesis of the E2F site at −110 abolished the activation of the TS promoter by IE1, suggesting that MCMV IE1 transactivated the TS promoter through an E2F-dependent mechanism. The ability of the IE1 or the IE3 construct to express functional proteins was verified by cotransfection assays with the pCMVCAT and pE1CAT indicator plasmids, respectively. As previously observed (43), IE1 expression increased the activity of the MCMV IE enhancer and IE1/IE3 promoter of pCMVCAT, whereas IE3 expression resulted in transactivation of the MCMV E1 early promoter of pE1CAT (data not shown). Furthermore, titration of the IE1 expression plasmid demonstrated a dose-dependent response (data not shown).
FIG. 6.
Transactivation of the TS promoter by MCMV IE1 protein. NIH 3T3 cells were transiently cotransfected with 2 μg of the indicator plasmid pTLG, pTLG:I1d1.0, or pTSWTGL3(−110) and 1 μg of the expression vector for MCMV IE1 protein, MCMV IE3 protein, or CAT, respectively. At 18 h after transfection, cells were washed and then maintained in medium containing 0.5% calf serum for 48 h. Thereafter, total cytoplasmic extracts were isolated and assayed for luciferase activity, as described in Materials and Methods. The resulting luciferase activity is expressed as fold induction relative to basal levels measured in cells transfected with pTLG, pTLG:I1d1.0, or pTSWTGL3(−110) and the CAT-expressing vector, which was set at 1. The data shown are the averages of three experiments ± the standard errors of the means (error bars).
MCMV replication and DNA synthesis are blocked by a TS inhibitor.
These findings suggest that induction of TS activity by MCMV is necessary to ensure a sufficient supply of dTMP for viral DNA replication in quiescent cells. To further test this hypothesis, we examined the effect of the quinazoline-based TS inhibitor ZD1694 on MCMV replication and DNA synthesis. Quiescent cells were infected at an MOI of 1, and after virus adsorption, medium containing 10−3 to 10 μM ZD1694 was added. Culture supernatants collected 4 days after infection were assayed for virus yield on B6MEF cells. ZD1694 produced a significant dose-related reduction in MCMV yield at concentrations much lower than those that produced cytotoxic effects. The calculated EC50 and EC90 were 0.006 and 0.01 μM, respectively. Cell toxicity assays demonstrated that ZD1694 did not affect the viability of mock-infected cells at concentrations up to 1 μM and that the 50% cytotoxic concentration was >10 μM for quiescent NIH 3T3 cells.
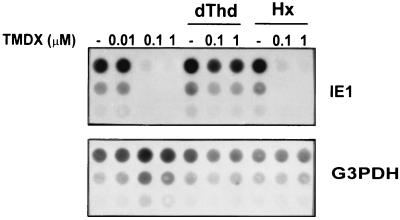
To evaluate the effects of ZD1694 on MCMV DNA synthesis, intracellular viral DNA levels were quantified 48 h p.i. by dot blot analysis and hybridization with a radiolabeled viral probe. As shown in Fig. 7, a strong reduction in the MCMV DNA level was observed in cultures treated with ZD1694. The EC50 of ZD1694 was about 0.05 μM, which was more than 200-fold lower than the 50% cytotoxic concentration for quiescent cells. Therefore, the observed effects of ZD1694 on MCMV DNA synthesis were not due to a generalized cellular toxicity of the drug.
FIG. 7.
Inhibition of MCMV DNA synthesis by ZD1694. Cultures of NIH 3T3 cells were growth arrested in 0.5% calf serum for 48 h and then mock infected or infected with MCMV at an MOI of 1 PFU/cell. After virus adsorption, cells were incubated with no drug (lanes —) or with the indicated concentrations of ZD1694 (TMDX) alone or in combination with 20 μM thymidine (dThd) or 100 μM hypoxanthine (Hx). At 48 h p.i., DNA was purified, and threefold dilutions (6, 2, and 0.66 μg) were immobilized on a hybridization membrane by a dot blot apparatus. The same filter was sequentially hybridized with 32P-labeled MCMV IE1 and mouse G3PDH probes. Hybridization signals were quantitated with a phosphoimager.
To be certain that the antiviral activity of ZD1694 was due to inhibition of TS, we determined the effects of thymidine on MCMV replication in ZD1694-treated cells. The observation (Fig. 7) that 20 μM thymidine (but not 100 μM hypoxanthine) abrogated the antiviral activity of ZD1694 confirms that TS is the intracellular target of ZD1694. The observation that a TS inhibitor can suppress MCMV DNA synthesis strongly supports the conclusion that virus-induced TS enzyme activity is absolutely required for efficient viral replication in quiescent cells.
DISCUSSION
MCMV does not encode enzymes involved in the de novo biosynthesis of thymidylic acid, such as DHFR and TS (51). Previous studies have shown that an expansion of the cellular dTTP pool occurs during HCMV infection (7, 57). However, the mechanism by which this is brought about was not known. In the present study, we show for the first time that the expression of the cellular TS gene is upregulated following MCMV infection of quiescent mouse fibroblasts and that TS activity is required for efficient viral replication. The mechanism responsible for the increase in TS gene expression in MCMV-infected cells appears to be different from that which occurs in growth-stimulated cells.
Several earlier reports have shown that CMV infection stimulates the expression of a number of cellular genes important for cell cycle regulation and DNA synthesis, presumably to facilitate the cell's ability to support viral replication. This regulation has been reported to depend on either viral binding to the cell surface (11, 12, 61) or viral IE protein expression (15, 23, 25, 36, 41, 50, 59, 63). We have observed in this study that inactivation of MCMV by UV exposure abolished the induction of TS protein and mRNA as well as transactivation of the TS promoter. These results suggest that viral gene expression, rather than interaction of viral particles with the cell surface, is required to stimulate TS gene expression. As we have previously observed for the DHFR promoter (38), both MCMV infection and the product of the IE1 gene transactivated the TS promoter, and the virus-dependent transactivation was observed during the time frame in which IE1 protein was expressed. The regulatory properties of the MCMV IE1 and IE3 proteins have been demonstrated previously. The IE1 protein regulates MCMV gene expression by stimulating the activity of the IE enhancer. The IE3 protein strongly transactivates the early gene E1 and shows an autoregulatory function by repression of the IE enhancer (43). Moreover, the IE1 protein acts as a general transcriptional activator, since it transactivates heterologous viral promoters (e.g., simian virus 40 early) (36) and cellular promoters (e.g., c-fos, NF-κB1, and DHFR) (23, 38, 54). It is likely that IE1-dependent activation of these cellular promoters is important for the viral replicative cycle. For example, the increase in NF-κB1 gene expression could give rise to a large increase in NF-κB required for the transcriptional induction of viral IE gene expression. Furthermore, stimulation of c-fos, DHFR, and TS gene expression is required for viral DNA replication in the lytic pathway.
In serum-stimulated cells, the increase in TS gene expression during the G1-to-S transition is regulated at the posttranscriptional level. S-phase regulation of transfected TS minigenes in such cells requires the presence of both the TS essential promoter region and a spliceable intron in the transcribed region (3, 34, 39). Inactivation of the E2F motif upstream from the essential promoter region has no effect on TS promoter activity (22) and does not prevent the increase in TS gene expression in serum-stimulated fibroblasts (3). Thus, it appears that S-phase regulation of TS gene expression in growth-stimulated cells may involve some form of communication between the promoter and the RNA processing machinery (3, 34). In line with these earlier observations, we found that only the intron-containing TS-luciferase construct was stimulated in response to serum growth factors (Fig. 3).
In contrast, we found that MCMV infection stimulated the expression of both an intronless and an intron-containing TS indicator gene. This finding suggests that MCMV controls TS gene expression primarily at the transcriptional level rather than at the posttranscriptional level. Although the mechanism leading to the induction of TS gene transcription remains to be determined, specific regulatory sequences located in the TS 5′-flanking region appear to be important. In particular, MCMV infection had no effect on a TS promoter in which the putative E2F site was mutated, suggesting that one or more members of the E2F family of transcription factors play an important role in the viral induction of TS gene expression. In line with this, it has been reported that MCMV and HCMV infection results in an E2F-dependent activation of the DHFR promoter and the formation of an E2F-p107-cyclin A-cdk2 complex during the time frame in which the DHFR gene is activated (38, 41, 50, 59). Furthermore, HCMV IE1 protein was shown to be a kinase with a selective substrate specificity for different E2F members, and this activity was found to be required for the E2F-dependent activation of DHFR gene transcription (47).
The dependence of MCMV replication on TS enzyme activity is further supported by the results obtained with the folate analog ZD1694, a powerful TS inhibitor that has already been approved for trials in clinical oncology. This drug, which interacts with the TS folate binding site, inhibits DNA synthesis and repair by blocking the de novo synthesis of thymidylic acid (8, 29). We have observed that ZD1694 strongly inhibits the replication of MCMV in quiescent NIH 3T3 cells. A 90% reduction in virus yield was achieved with ZD1694 concentrations (10−2 μM) that are well below those required for its cytotoxic activity (>1 μM). This selectivity may depend on the very low TS activity levels of quiescent uninfected cells. Inhibition of viral DNA synthesis is likely the mechanism by which ZD1694 inhibits MCMV replication, since late gene expression but not IE gene expression was inhibited by 10−2 μM ZD1694 (data not shown). Therefore, the inhibitory effect of ZD1694 on MCMV DNA replication and its abrogation by 20 μM thymidine demonstrated that TS activity is required for efficient CMV replication in quiescent cells.
We have recently observed that MCMV infection of quiescent NIH 3T3 cells also leads to an increase in other cellular enzymes involved in the synthesis of DNA precursors, such as DHFR and folyl polyglutamate synthetase (FPGS) (38; D. Lembo, G. Gribaudo, and S. Landolfo, unpublished data). FPGS converts the reduced folates transported into the cell via the folate carrier system to polyglutamated forms retained by the cell and in this way catalyzes an important step in the biochemical pathway that supplies reduced cofactors to TS (5, 26, 42, 46). Therefore, the selective induction of TS and FPGS in CMV-infected cells could be exploited to develop anti-CMV therapeutic strategies with anti-TS drugs such as ZD1694, whose biological activity depends on its active uptake via the reduced folate cell membrane carrier and subsequent metabolism by FPGS to polyglutamated forms that are approximately 100-fold more active as TS inhibitors than the unmodified analog and are not effluxed from the cell (28, 58). Inhibition of TS by ZD1694 is likely to have little effect on uninfected resting cells even though the drug is highly deleterious to viral replication.
Taken as whole, the available data demonstrate that the levels of expression of at least three cellular enzymes involved in de novo thymidylic acid biosynthesis, namely, FPGS, DHFR, and TS, are increased in CMV-infected quiescent cells and that this induction is a consequence of an increase in gene transcription. However, the mechanisms exploited by the virus to transcriptionally activate FPGS appear to be different from those involved in the regulation of DHFR and TS transcription. Both the DHFR and TS promoters appear to be transactivated by CMV through the involvement of an E2F-dependent pathway. In contrast, the occurrence of several SP1 sites in the FPGS promoter and the finding that MCMV infection brings about a simultaneous increase in SP1 binding activity and FPGS mRNA content suggest that MCMV infection may stimulate the FPGS promoter at least in part through an SP1-mediated event (Lembo et al., unpublished data). In line with this, induction of an SP1 binding activity was previously demonstrated to regulate the promoter activity of the NF-κB p65 subunit gene during HCMV infection of embryonic lung fibroblasts (62, 63).
In conclusion, the results of the present as well as previous studies demonstrate that MCMV infection of quiescent cells leads to the coordinated stimulation of cellular enzymes that are involved in dTMP synthesis. It should be possible to exploit these observations to develop novel antiviral strategies.
ACKNOWLEDGMENTS
We thank Martin Messerle for providing plasmid pE1CAT.
This work was supported by grants from MURST-CNR Biotechnology program L. 95/95 to G.G., from the AIDS Research Project (grant number 50B.25) to S.L., and by grants from the National Institute for General Medical Sciences (GM29356) and the National Cancer Institute (CA16058) to L.F.J. T.L.R. was supported by a training grant (T32 CA09498) from the National Cancer Institute.
REFERENCES
- 1.Abken H, Reifenrath B. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 1992;20:3527. doi: 10.1093/nar/20.13.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse: evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 3.Ash J, Liao W-C, Ke Y, Johnson L F. Regulation of mouse thymidylate synthase gene expression in growth-stimulated cells: upstream S phase control elements are indistinguishable from the essential promoter elements. Nucleic Acids Res. 1995;23:4649–4656. doi: 10.1093/nar/23.22.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 5.Barredo J, Moran R G. Determinants of antifolate cytotoxicity: folylpolyglutamate synthetase activity during cellular proliferation and development. Mol Pharmacol. 1992;42:687–694. [PubMed] [Google Scholar]
- 6.Benson J D, Huang E-S. Human cytomegalovirus induces expression of cellular topoisomerase II. J Virol. 1990;64:9–15. doi: 10.1128/jvi.64.1.9-15.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron K K, Fyfe J A, Stanat S C, Leslie L K, Sorrell J A, Lambe C U, Coen D M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-[2-hydroxy-1-(hydroxymethyl)ethoxy]methylguanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci USA. 1986;83:8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackledge G. New developments in cancer treatment with the novel thymidylate synthase inhibitor raltitrexed (‘Tomudex’) Br J Cancer. 1998;77:29–37. doi: 10.1038/bjc.1998.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boldogh I, AbuBakar S, Albrecht T. Activation of protooncogenes: an immediate early event in human cytomegalovirus infection. Science. 1990;247:961–964. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- 10.Boldogh I, AbuBakar S, Deng C Z, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65:1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldogh I, AbuBakar S, Millinoff D, Deng C Z, Albrecht T. Cellular oncogenes activation by human cytomegalovirus. Lack of correlation with virus infectivity and immediate early gene expression. Arch Virol. 1991;118:163–177. doi: 10.1007/BF01314027. [DOI] [PubMed] [Google Scholar]
- 12.Boyle K A, Pietropaolo R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresnahan W A, Thompson E A, Albrecht T. Human cytomegalovirus infection results in altered Cdk2 subcellular localization. J Gen Virol. 1997;78:1993–1997. doi: 10.1099/0022-1317-78-8-1993. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 15.Bresnahan W A, Albrecht T, Thompson E A. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J Biol Chem. 1998;273:22075–22082. doi: 10.1074/jbc.273.34.22075. [DOI] [PubMed] [Google Scholar]
- 16.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison III C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 17.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonucleases. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 18.Deng T, Li Y, Yolliff K, Johnson L F. The mouse thymidylate synthase promoter: essential elements are in close proximity to the transcriptional initiation sites. Mol Cell Biol. 1989;9:4079–4082. doi: 10.1128/mcb.9.9.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estes J E, Huang E-S. Stimulation of cellular thymidine kinase by human cytomegalovirus. J Virol. 1977;24:13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariglio M, Foresta P, Sacchi C, Lembo M, Hertel L, Landolfo S. Suppression of high mobility group protein T160 expression impairs mouse cytomegalovirus replication. J Gen Virol. 1997;78:665–670. doi: 10.1099/0022-1317-78-3-665. [DOI] [PubMed] [Google Scholar]
- 22.Geng Y, Johnson L F. Lack of an initiator element is responsible for multiple transcriptional initiation sites of the TATA-less mouse thymidylate synthase promoter. Mol Cell Biol. 1993;13:4894–4903. doi: 10.1128/mcb.13.8.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gribaudo G, Ravaglia S, Guandalini L, Cavallo R, Gariglio M, Landolfo S. The murine cytomegalovirus immediate early 1 protein stimulates NF-κB activity by transactivating the NF-κB p105/p50 promoter. Virus Res. 1996;45:15–27. doi: 10.1016/0168-1702(96)01356-1. [DOI] [PubMed] [Google Scholar]
- 24.Gribaudo G, Ravaglia S, Gaboli M, Gariglio M, Cavallo R, Landolfo S. Interferon-α inhibits the murine cytomegalovirus immediate-early gene expression by down-regulating NF-κB activity. Virology. 1995;211:251–260. doi: 10.1006/viro.1995.1398. [DOI] [PubMed] [Google Scholar]
- 25.Hayhurst G P, Bryant L A, Caswell R C, Walker S M, Sinclair J H. CCAAT box-dependent activation of the TATA-less human DNA polymerase α promoter by the human cytomegalovirus 72-kilodalton major immediate-early protein. J Virol. 1995;69:182–188. doi: 10.1128/jvi.69.1.182-188.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilton J G, Cooper B A, Rosenblatt D S. Folate polyglutamate synthesis and turnover in cultured human fibroblasts. J Biol Chem. 1979;254:8393–8403. [PubMed] [Google Scholar]
- 27.Isom H C. Stimulation of ornithine decarboxylase by human cytomegalovirus. J Gen Virol. 1979;42:265–278. doi: 10.1099/0022-1317-42-2-265. [DOI] [PubMed] [Google Scholar]
- 28.Jackman A L, Gibson W. Polyglutamation of the thymidylate synthase inhibitor, ZD1694 (Tomudex) in mormal mouse tissues. Proc Am Assoc Cancer Res. 1995;36:376. [Google Scholar]
- 29.Jackman A L, Taylor G A, Gibson W, Kimbell R, Brown M, Calvert A H, Judson I, Hughes L R. ZD1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- 30.Jault F M, Jault J-M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson L F. Posttranscriptional regulation of thymidylate synthase gene expression. J Cell Biochem. 1994;54:387–392. doi: 10.1002/jcb.240540405. [DOI] [PubMed] [Google Scholar]
- 32.Johnston P G, Liang C-M, Henry S, Chabner B A, Allegra C J. Production and characterization of monoclonal antibodies that localize human thymidylate synthase in the cytoplasm of human cells and tissue. Cancer Res. 1991;51:6668–6676. [PubMed] [Google Scholar]
- 33.Johnston P G, Drake J C, Trepel J, Allegra C J. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992;52:4306–4312. [PubMed] [Google Scholar]
- 34.Ke Y, Ash J, Johnson L F. Splicing signals are required for S-phase regulation of the mouse thymidylate synthase gene. Mol Cell Biol. 1996;16:376–383. doi: 10.1128/mcb.16.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keil G M, Ebeling-Keil A, Koszinowski U H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987;61:1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koszinowski U H, Keil G M, Volkmer H, Fibi M R, Ebeling-Keil A, Munch K. The 89,000-Mr murine cytomegalovirus immediate-early protein activates gene transcription. J Virol. 1986;58:59–66. doi: 10.1128/jvi.58.1.59-66.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucera R, Paulus H. Localization of the deoxyribonucleotide biosynthetic enzymes ribonucleotide reductase and thymidylate synthase in mouse L cells. Exp Cell Res. 1986;167:417–428. doi: 10.1016/0014-4827(86)90182-5. [DOI] [PubMed] [Google Scholar]
- 38.Lembo D, Angeretti A, Gariglio M, Landolfo S. Murine cytomegalovirus induces expression and enzyme activity of dihydrofolate reductase in quiescent cells. J Gen Virol. 1998;78:2803–2808. doi: 10.1099/0022-1317-79-11-2803. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Li D, Osborn K, Johnson L F. The 5′-flanking region of the mouse thymidylate synthase gene is necessary but not sufficient for normal regulation in growth-stimulated cells. Mol Cell Biol. 1991;11:1023–1029. doi: 10.1128/mcb.11.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margolis M J, Pajovic S, Wong E L, Wade M, Jupp R, Nelson J A, Azizkhan J C. Interaction of the 72-kilodalton human cytomegalovirus IE1 gene product with E2F1 coincides with E2F-dependent activation of dihydrofolate reductase transcription. J Virol. 1995;69:7759–7767. doi: 10.1128/jvi.69.12.7759-7767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGuire J J, Bertino J R. Enzymatic synthesis and function of folylpolyglutamates. Mol Cell Biochem. 1981;38:19–48. doi: 10.1007/BF00235686. [DOI] [PubMed] [Google Scholar]
- 43.Messerle M, Buhler B, Keil G M, Koszinowski U H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992;66:27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocarski E S. Cytomegalovirus and their replication. In: Fields B N, Knipe D M, Howley P H, Chanock R M, Menlick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 45.Moran R G, Spears C P, Heidelberger C. Biochemical determinants of tumor sensitivity to 5-fluorouracil: ultrasensitive methods for the determinattion of 5-fluoro-2′-deoxyuridylate, 2′-deoxyuridylate, and thymidylate synthetase. Proc Natl Acad Sci USA. 1979;76:1456–1460. doi: 10.1073/pnas.76.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moran R G, Werkheiser W C, Zakrewski S F. Folate metabolism in mammalian cells in culture. J Biol Chem. 1976;251:3569–3575. [PubMed] [Google Scholar]
- 47.Panjovic S, Wong E L, Black A R, Azizkhan J C. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol Cell Biol. 1997;17:6459–6464. doi: 10.1128/mcb.17.11.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Hederwijin P, Desmyter J, De Clerq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 49.Perryman S M, Rossana C, Deng T, Vanin E F, Johnson L F. Sequence of a cDNA for mouse thymidylate synthase reveals striking similarity with the prokaryotic enzyme. Mol Biol Evol. 1986;3:313–321. doi: 10.1093/oxfordjournals.molbev.a040400. [DOI] [PubMed] [Google Scholar]
- 50.Poma E E, Kowalik T F, Zhu L, Sinclair J H, Huang E-S. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J Virol. 1996;70:7867–7877. doi: 10.1128/jvi.70.11.7867-7877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvant B S, Fortunato E A, Spector D H. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J Virol. 1998;72:3729–3741. doi: 10.1128/jvi.72.5.3729-3741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samsonoff W A, Reston J, McKee M, O'Connor B, Galivan J, Maley G, Maley F. Intracellular location of thymidylate synthase and its state of phosphorylation. J Biol Chem. 1997;272:13281–13285. doi: 10.1074/jbc.272.20.13281. [DOI] [PubMed] [Google Scholar]
- 54.Schickedanz J, Philipson L, Ansorge W, Pepperkork R, Klein R, Koszinowski U H. The 89,000-Mr murine cytomegalovirus immediate-early protein stimulates c-fos expression and cellular DNA synthesis. J Virol. 1988;62:3341–3347. doi: 10.1128/jvi.62.9.3341-3347.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibui S, Hoshino T, Iwasaki K, Nomura K, Jastreboff M M. Cell cycle phase dependent emergence of thymidylate synthase studied by monoclonal antibody (M-TS-49) Cell Tissue Kinet. 1989;22:259–268. doi: 10.1111/j.1365-2184.1989.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 56.Spears C P, Shahinian A H, Moran R G, Heidelberger C, Corbett T H. In vivo kinetics of thymidylate synthetase inhibition in 5-fluorouracil-sensitive and -resistant murine colon adenocarcinomas. Cancer Res. 1982;42:450–456. [PubMed] [Google Scholar]
- 57.Suzuki S, Saneyoshi M, Nakayama C, Nishiyama Y, Yoshida S. Mechanism of selective inhibition of human cytomegalovirus replication by 1-β-d-arabinofuranosyl-5-fluorouracil. Antimicrob Agents Chemother. 1985;28:326–330. doi: 10.1128/aac.28.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Synold T W, Willits E M, Barredo J C. Role of folylpolyglutamate synthetase (FPGS) in antifolate chemotherapy: a biomedical and clinical update. Leuk Lymphoma. 1996;21:9–15. doi: 10.3109/10428199609067573. [DOI] [PubMed] [Google Scholar]
- 59.Wade M, Kowalik T F, Mudryj M, Huang E-S, Azizkhan J C. E2F mediates dihydrofolate reductase promoter activation and multiprotein complex formation in human cytomegalovirus infection. Mol Cell Biol. 1992;12:4364–4374. doi: 10.1128/mcb.12.10.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiebusch L, Hagemeier C. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G1. J Virol. 1999;73:9274–9283. doi: 10.1128/jvi.73.11.9274-9283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yurochko A D, Mayo M W, Poma E E, Baldwin A S, Jr, Huang E-S. Induction of the transcription factor SP1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yurochko A D, Kowalik T F, Huong S M, Huang E-S. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoter. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]