Abstract
Venezuelan equine encephalitis virus (VEEV) is a highly infectious alphavirus endemic in parts of Central and South America. The disease is transmitted by mosquitoes, and the natural reservoir is the small rodent population, with epidemics occurring in horses and occasionally humans. Following infection, VEEV replicates in lymphoid tissues prior to invasion of the central nervous system. Treatment of VEEV-infected BALB/c mice with polyethylene glycol-conjugated alpha interferon (PEG IFN-α) results in a greatly enhanced survival from either a subcutaneous or an aerosol infection. Virus is undetectable within PEG IFN-α-treated individuals by day 30 postinfection (p.i.). Treatment results in a number of changes to the immune response characteristics normally associated with VEEV infection. Increased macrophage activation occurs in PEG IFN-α-treated BALB/c mice infected with VEEV. The rapid activation of splenic CD4, CD8, and B cells by day 2 p.i. normally associated with VEEV infection is absent in PEG IFN-α-treated mice. The high tumor necrosis factor alpha production by macrophages from untreated mice is greatly diminished in PEG IFN-α-treated mice. These results suggest key immunological mechanisms targeted by this lethal alphavirus that can be modulated by prolonged exposure to IFN-α.
Venezuelan equine encephalitis virus (VEEV), an Alphavirus in the family Togaviridae, is an arthropod-borne virus found throughout the Americas (25, 26). It is a single, positive-strand RNA virus with a genome size of approximately 11 kb. Infection with a virulent epizootic strain of VEEV from the 1A/B serogroup, like Trinidad Donkey (TrD), can cause an acute febrile illness and encephalomyelitis in horses as well as humans. The animal reservoir of VEEV is in the small rodent population, and outbreaks in humans often follow equine epidemics (18). Natural VEEV transmission usually occurs via the mosquito; however, the virus is also highly infectious when aerosolized (18, 22).
The most commonly used laboratory model of VEEV pathogenesis is the mouse, where infection via the subcutaneous or airborne route results in an encephalitis that is almost invariably fatal in as little as 5 to 7 days (17, 40). During the early stages of a systemic infection in mice, VEEV is strikingly lymphotrophic, with viral antigens detected by day 2 postinfection (p.i.) in the local draining lymph nodes and spleens of infected individuals (17, 40). Antigen-presenting cells have been identified as initial targets of infection, with dendritic cells (G. H. MacDonald, N. L. Davis, and R. E. Johnston, Abstr. 16th Annu. Meet. Am. Soc. Virol., p. 172, 1997) and macrophages (13) expressing virus shortly after infection. Little more is known about the early immune events following VEEV infection. A large increase in gamma interferon (IFN-γ) and interleukin-6 (IL-6) expression occurs in lymphoid tissue following VEEV infection, while IL-12, IL-10, and tumor necrosis factor alpha (TNF-α) expression is also upregulated (11). Extensive necrosis, which is characterized by lymphocyte destruction and an influx of polymorphonuclear leukocytes, occurs in the white pulp of the spleen (17).
Immune response studies of VEEV infection have focused mainly on the importance of vaccines or attenuated strains in the generation of neutralizing antibodies in virus clearance from the host (5, 10, 29). However, antibody-mediated immunity is not necessarily the only mechanism of protection during VEEV infection. Studies in which mice were treated with immune spleen cell culture supernatant have suggested that protection against a lethal challenge of VEEV can be mediated by IL-1 and IL-2 (15). Indeed, immune regulation by treatment with exogenous cytokines has proved to be effective in treating a variety of viral infections (2, 3). More importantly, antibody ablation studies have revealed a crucial role for IFN-α/β in immunity to VEEV, since disease is exacerbated in the absence of IFN-α/β (11). However, studies using IFN-α/β have proved unsuccessful in treating virulent VEEV (TrD) infection (34).
One solution to the ineffectiveness of conventional IFN-α treatment during VEEV infection would be to increase its bioavailability (and therefore its potency) within the host. We have obtained a novel formulation of recombinant IFN-α A/D that has had the polymer polyethylene glycol (PEG) chemically attached to the protein. This straight-chain amphiphilic polymer has been used to covalently modify several cytokines for a variety of clinical uses. PEG IL-2 has been used to treat human immunodeficiency virus-positive patients, as it exerts a prolonged immunostimulatory effect on patient CD4+ cells at low doses (30, 36). PEG TNF-α has also been used experimentally to treat Meth-A murine sarcoma, where greater antitumor potency of TNF-α than of unconjugated TNF-α was observed due to longer plasma half-life and higher tumor accumulation (38).
In this study we demonstrate that PEG IFN-α can prevent disease in animals exposed to both subcutaneous and inhalational challenge with VEEV (TrD). In addition, the use of flow cytometry to examine the host response to VEEV infection has allowed us to assess how PEG IFN-α treatment may mediate protection. We examined changes in leukocyte activation state, cytokine secretion, and viral antigen load in the splenic leukocytes of PEG IFN-α-treated and control mice following a subcutaneous infection with the TrD strain of VEEV. VEEV infection caused a massive overactivation of lymphocytes in untreated individuals which was absent in PEG IFN-α-treated mice. Expression of intracellular viral antigens seemed to be restricted to the macrophage population in both treated and untreated mice. Furthermore, we show that treatment with PEG IFN-α prevents the excessive inflammatory immune response to VEEV infection observed in untreated mice.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old female BALB/c mice were obtained from Charles River and used for all experiments. During the initial mortality studies, mice were culled as soon as they displayed terminal symptoms of VEEV infection, in other words, when the humane endpoint as defined by Wright and Phillpotts (41) had been reached. For this model of virulent VEEV infection, the humane endpoint for BALB/c mice was between days 6 and 7 p.i. As a result of these initial studies, the latter experiments that focused on viral load and the immune response to VEEV used time points in which mice would still be alive and yet display terminal symptoms; these were days 2, 4, and 6 p.i. Blood samples were taken by cardiac puncture under terminal anesthesia.
Virus and tissue preparation for assay.
Stocks of virulent VEEV (TrD) were kindly supplied by B. Shope, University of Texas Arbovirus Research Unit, Austin, and prepared as suckling mouse brain suspensions by standard methods. Virus was handled in vitro under Advisory Committee on Dangerous Pathogens category 3 containment.
Five mice from each treatment group were humanely culled on each time point (2, 4, and 6 p.i.). Spleen, lung, and brain tissues were passed through a metal gauze and resuspended in 2 ml of Leibowitz L-15 maintenance medium (MM) (Sigma) and stored at −80°C. Blood was collected into tubes containing 0.105 M sodium citrate (Becton Dickinson, Oxford, United Kingdom) and also stored at −80°C.
Plaque assays were performed with BHK-21 cells, grown in Glasgow minimal essential medium supplemented with 10% fetal calf serum (FCS) (all from Sigma). Samples were diluted down a log dilution series from 10−1 to 10−6 in L-15 MM and then plated onto BHK cells for plaque assay. The plaques were allowed to develop for 3 days under an overlay of L-15 MM with 3% FCS and 1.5% carboxymethyl cellulose (Sigma) before formalin fixation and staining of the residual cell sheet with crystal violet. Counts are expressed as PFU per milliliter of spleen cell suspension. For each assay, the limit of virus detection was 5 to 10 PFU/ml of tissue suspension.
Cytokine preparation.
Recombinant mouse IL-12 and PEG IFN-α A/D were gifts from Roche Discovery (Welwyn Garden City, United Kingdom). Recombinant unconjugated IFN-α B/D was a gift from Ciba-Geigy (Basel, Switzerland). IL-12 was diluted in 1% normal mouse serum (NMS) in Saline for Injection BP (N-Saline; Fresenius, Basingstoke, United Kingdom) and given as single daily doses of 100 ng of IL-12 i.p. on days −2 to +5 p.i. Unconjugated IFN-α B/D was diluted to a concentration of 106 U/ml in 10% NMS in N-saline (Antigen Ltd.) and given as single daily doses of 105 units intraperitoneally (i.p.). PEG IFN-α A/D was diluted in 1% NMS in N-saline (Fresenius) and given as single daily doses of 100 μl of PEG IFN-α containing 4 × 104 U given i.p. from days −2 to +5 p.i. In all experiments, negative control mice were given 1% NMS in N-saline (Fresenius). Unfortunately, a medium containing a dose of PEG equivalent to that in PEG IFN-α was not made available.
Subcutaneous challenge with VEEV (TrD).
Groups of mice were challenged subcutaneously with 25 median lethal doses (MLD) of TrD virus in 100 μl of L-15 MM. Titration of this strain of VEEV in BALB/c mice showed that 1 MLD was equivalent to 1 PFU of virus.
Inhalational challenge with VEEV (TrD).
Infection by the airborne route was achieved using a specially constructed small animal exposure box (27). Briefly, six groups of five mice were exposed loose in a box of 80-liter capacity, vented through a HEPA filter, to a virus-containing aerosol produced using a Collison nebulizer (8 liters/min). The virus suspending fluid was L-15 MM with 2% trehalose (Sigma), and the exposure time was 20 min. The air in the box was sampled with a glass impinger running at 1 liter/min, and the quantity of virus present in the Collison spray reservoir at the end of each run was determined by back-titration. The high-challenge group received approximately 103 MLD (or 103 PFU) of airborne VEEV; the low-challenge group received approximately 10 MLD (or 101 PFU) of airborne VEEV.
Stimulation of cells for intracellular cytokine production.
Single-cell suspensions of spleen cells from each mouse were maintained in L-15 MM containing 2% heat-inactivated FCS, 50 U of penicillin-streptomycin/ml, 2 mM l-glutamine, and 10 mM HEPES (all Sigma). Approximately 5 × 106 spleen cells were transferred into individual wells (24-well plate) and incubated with a 1/100 dilution of β-propriolactone-inactivated VEEV (TrD) antigen in the presence of 5 μl of Golgistop (Becton Dickinson, Mountain View, Calif.) for 4 h at 37°C. The VEEV antigen was prepared from a preinactivation stock with a VEEV (TrD) concentration of approximately 109 PFU/ml, obtained from the brains of suckling mice, and was a kind gift from R. Phillpotts (CBD). The spleen cells were then washed and stained by fluorescent antibodies as described below.
Flow cytometry.
Single-cell suspensions were harvested from the spleens of BALB/c mice, and erythrocytes were removed by incubation at room temperature with a lysing solution containing 0.85% NH4Cl in autoclaved water for 3 to 4 min. Following washing, cells were resuspended in a staining buffer containing 2% FCS and 0.1% sodium azide (both from Sigma) in phosphate-buffered saline. Cell surface markers were stained with a variety of monoclonal antibodies (MAbs) including Cy-chrome-conjugated anti-CD4, -CD8, -CD45R/B220, and -CD11b fluorescein isothiocyanate-conjugated anti-CD69 (all from PharMingen, San Diego, Calif.) at 4°C in the dark for 30 min. Following fixation in 4% paraformaldehyde, cells were incubated in a permeabilization buffer containing 2% FCS, 0.1% sodium azide (both from Sigma), and 1/10 dilution of Perm/Wash buffer (PharMingen). Intracellular VEEV antigen was detected using a biotinylated anti-VEEV E2 MAb (1A4A-1) (31) (a kind gift from J. T. Roehrig, Centers for Disease Control and Prevention, Fort Collins, Colo.) followed by incubation with phycoerythrin-streptavidin (Sigma). Intracellular cytokines were detected using fluorescein isothiocyanate-conjugated anti-TNF-α MAb (PharMingen). For flow cytometric analyses, 4 × 105 to 5 × 105 live cells (as determined by forward and side scatter characteristics) were acquired in a FACScan and analyzed using CellQuest software (both from Becton Dickinson).
Statistical analyses.
The significance of mortality data between treatment groups was assessed using Fisher's exact test. Student's t test was used to determine whether there was a significant difference between arithmetic means of treatment group data.
RESULTS
Cytokine treatment for VEEV infection.
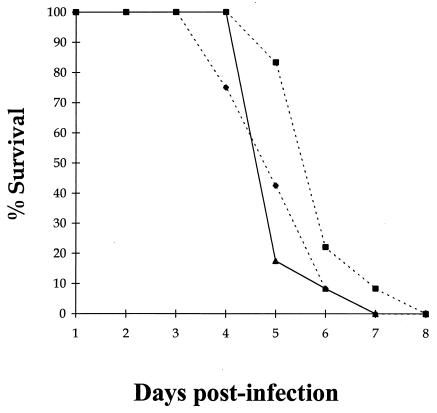
VEEV is a lymphotropic virus and so should be susceptible to modulation of the immune response by exogenous antiviral cytokines. However, treatment of BALB/c mice with either IFN-α or IL-12 was ineffective against a subcutaneous challenge of virulent VEEV, with no significant difference (P > 0.05) in the time to death between control animals and the different treatment groups (Fig. 1) according to Student's t test. Indeed, IL-12 treatment seemed to make individuals more susceptible to VEEV infection, with mortality beginning as early as day 3 p.i. Since cytokines that promote cell-mediated antiviral responses were ineffective, treatment with cytokines that promote either the humoral or innate parts of the immune system were used to treat VEEV infection in a separate experiment. Both IL-4 and granulocyte-macrophage colony-stimulating factor had no effect on the outcome of VEEV infection (data not shown). Taken together, these findings suggest that the primary immune response to VEEV is unable to protect against VEEV infection even in the presence of exogenous cytokines that should boost the host immune response.
FIG. 1.
Mortality in BALB/c mice given a subcutaneous infection with virus. Groups of 15 mice were given 100 μl i.p. of either 2 × 105 U of IFN-α in 10% NMS in N-saline (■), 100 ng of IL-12 in 1% NMS in N-saline (⧫), or just 1% NMS in N-saline (▴) on days −2 to +5 p.i. with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i.
PEG IFN-α is an effective treatment against subcutaneous and airborne challenge with VEEV.
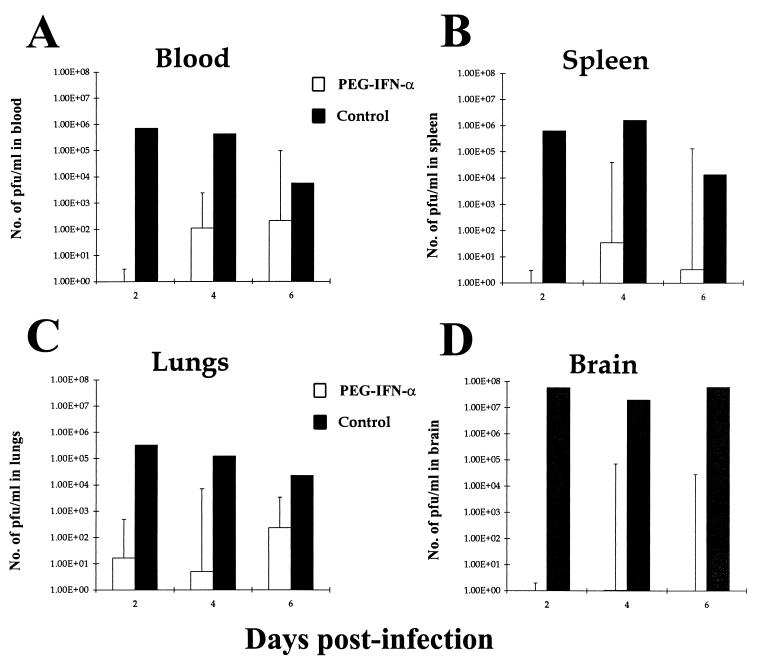
Conjugation of IFN-α to a carrier molecule like PEG considerably increases the half-life of IFN-α in the serum and therefore its effectiveness. BALB/c mice were treated with either PEG IFN-α, IL-12, or a combination of the two cytokines during a subcutaneous challenge with virulent VEEV. PEG IFN-α treatment alone provided highly significant (P < 0.001) protection against 25 MLD of VEEV, with over 75% of treated mice surviving to day 35 p.i. (Fig. 2). Virus was undetectable by plaque assay in surviving mice at day 30 p.i. (data not shown). Interestingly, combination treatment with PEG IFN-α and IL-12 resulted in 100% mortality by day 8 p.i., which indicates that IL-12 has a deleterious effect on the antiviral action of PEG IFN-α. Comparison of viremia in PEG IFN-α-treated and untreated BALB/c mice showed that PEG IFN-α treatment was effective at preventing viral replication in VEEV-infected individuals. No virus was detectable in the blood, spleen, lung, or brain tissues of PEG IFN-α-treated mice over the first 6 days p.i., while very high viremias were observed in untreated mice over the same period (Fig. 3).
FIG. 2.
Mortality in PEG IFN-α- and IL-12-treated BALB/c mice given a subcutaneous infection with VEEV. Groups of 15 mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (■), 100 ng of IL-12 in 1% NMS in N-saline (⧫), 4 × 104 U of PEG IFN-α in combination with 100 ng of IL-12 in 1% NMS in N-saline (●), or just 1% NMS in N-saline (▴) on days −2 to +5 after infection with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i.
FIG. 3.
VEEV titers in blood, spleen, lung, and brain tissues of PEG IFN-α-treated and untreated BALB/c given a subcutaneous challenge with virulent VEEV. Groups of 15 mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (□) or just 1% NMS in N-saline (■) on days −2 to +5 after infection with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i. Viral titers were calculated by standard plaque assay, incubating log dilutions of particular cell suspensions with BHK cells. Results shown are geometric mean viremias of five individual mice per time point + geometric confidence limits of 95%.
The success of PEG IFN-α against systemic challenge suggested that it may also protect against an aerosol infection with VEEV. PEG IFN-α-treated and untreated BALB/c mice were exposed to either a low (ca. 10 MLD) or a high (ca. 1,000 MLD) dose of virulent VEEV by aerosol. PEG IFN-α treatment provided significant protection against aerosolized infection with VEEV, with 36% survivors in the high-challenge group (P < 0.05) and 67% in the low-challenge group (P < 0.001) according to Fisher's exact test (Fig. 4). Although some animals died, their survival was prolonged by an interval equivalent to the length of the course of treatment. Comparison of viremias in PEG IFN-α-treated and untreated BALB/c mice given a high dose of VEEV demonstrated that PEG IFN-α treatment reduced viral load in the blood, spleen, lung, and brain over the first 6 days p.i. but that it did not eradicate the virus (Fig. 5). This suggests that PEG IFN-α was able to suppress virus replication, but in some animals it was not sufficient to eradicate VEEV. The high variance in viral load observed in the tissues of PEG IFN-α-treated BALB/c mice (Fig. 5) can be attributed to the variable survival of these mice when given a high dose of aerosolized VEEV.
FIG. 4.
Mortality in PEG IFN-α-treated BALB/c mice given either high or low doses of virus by aerosol. Groups of 15 mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline or just 1% NMS in N-saline on days −2 to +5 after infection. One group of PEG IFN-α-treated mice received a high dose of ca. 1,000 MLD (103 PFU) of VEEV (--■--), while another received a low dose of only 10 MLD (101 PFU) of VEEV by aerosol (--▴--) on day 0 p.i. At the same time, 1% NMS in N-saline-treated mice received similar high (—■—) and low (—▴—) doses of VEEV by aerosol on day 0 p.i.
FIG. 5.
VEEV titers in blood, spleen, lung, and brain tissues of PEG IFN-α-treated and untreated BALB/c given an aerosol challenge with 1,000 MLD of virulent VEEV. Groups of 15 mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (□) or just 1% NMS in N-saline (■) on days −2 to +5 after infection with ca. 1,000 MLD (103 PFU) of VEEV by aerosol on day 0 p.i. Viral titers were calculated by standard plaque assay, incubating log dilutions of particular cell suspensions with BHK cells. Results shown are geometric mean viremias of five mice per time point + geometric confidence limits of 95%.
The immune response to VEEV in PEG IFN-α-treated and untreated mice.
Since the host immune system can be manipulated by the addition of exogenous IFN-α to eradicate VEEV, we performed further experiments to assess which cell types were important in the response to VEEV infection. Population changes among the splenic leukocytes of both PEG IFN-α-treated and untreated BALB/c mice were studied using flow cytometry during a subcutaneous challenge with virulent VEEV.
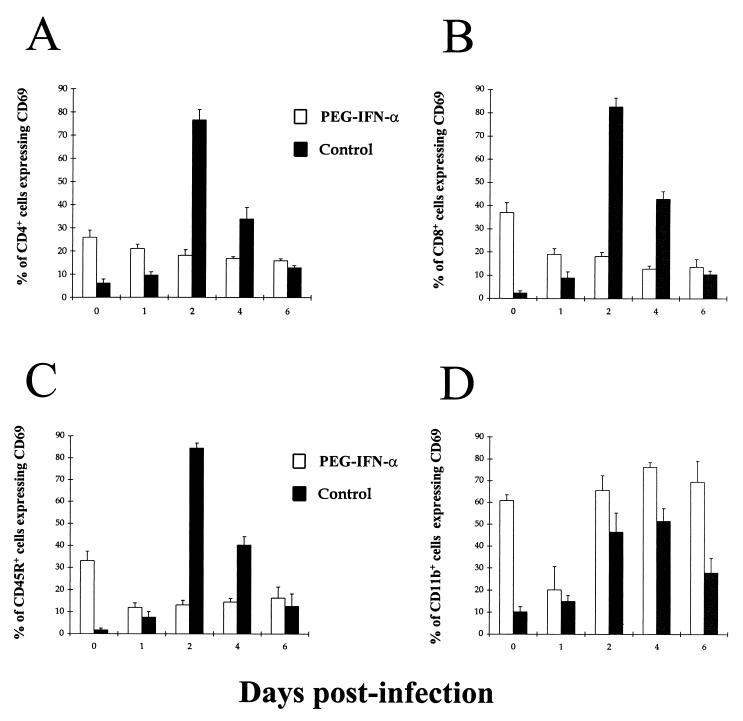
First, expression of the very early activation marker (CD69) over the first 6 days p.i. by both splenic lymphocytes and macrophages was assessed in PEG IFN-α-treated and untreated BALB/c mice (Fig. 6). In the CD4+, CD8+, and B-cell populations of untreated mice, CD69 expression increased rapidly by day 2 p.i. so that over 80% of each type of lymphocyte was activated in response to VEEV infection (Fig. 6A to C). However, by day 6 p.i., CD69 expression in these populations had decreased gradually to between 10 to 15% of lymphocytes. Similar results were observed in three separate experiments. By contrast, in treated mice, there was no such dramatic increase in CD69 expression among any of the lymphocyte populations at any time over the course of VEEV infection, with expression remaining relatively constant between 10 and 20% of lymphocytes (Fig. 6A to C). The splenic macrophage population behaved differently from the lymphocyte populations in both PEG IFN-α-treated and untreated mice during VEEV infection (Fig. 6D). In untreated mice, there was a gradual rise in CD69 expression that peaked at between 50 and 55% of macrophages by day 4 p.i. and then declined gradually to 25% by day 6 p.i. (Fig. 6D). In contrast, a high proportion (65 to 75%) of the splenic macrophage population of PEG IFN-α-treated mice expressed CD69 from days 2 through 6 p.i. (Fig. 6D). The effect of PEG IFN-α-treatment was also observed prior to infection (day 0), with much higher expression of CD69 in all lymphocyte as well as macrophage populations in treated than in untreated mice (Fig. 6).
FIG. 6.
Kinetics of CD69 expression by splenic leukocytes from PEG IFN-α-treated and untreated BALB/c mice given a subcutaneous challenge with virulent VEEV. Mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (□) or just 1% NMS in N-saline (■) on days −2 to +5 after infection with ca. 25 MLD of VEEV in 100 μl of L-15 MM on day 0 p.i. The data represents the fraction of splenic CD4+ (A), CD8+ (B), CD45R+ (C), or CD11b+ (D) cells expressing CD69. Results shown are the mean + 95% confidence limits of five mice per time point.
To assess whether this pattern of activation was a result of virus penetration of splenic leukocytes, the same populations were stained intracellularly with an anti-VEEV E2 antibody (Fig. 7). In both PEG IFN-α-treated and untreated individuals, there was very low level of expression of viral antigen among the CD4+, CD8+, and B-cell populations over the first 6 days p.i. (Fig. 7A to C). High levels of VEEV antigen expression were observed only in the splenic macrophage populations of both PEG IFN-α-treated and untreated mice, antigen expression being detectable from day 1 p.i. and peaking at day 4 p.i. (Fig. 7D).
FIG. 7.
Kinetics of intracellular VEEV E2 antigen expression by splenic leukocytes from PEG IFN-α-treated and untreated BALB/c mice given a subcutaneous challenge with virulent VEEV. Mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (□) or just 1% NMS in N-saline (■) on days −2 to +5 after infection with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i. The data represents the fraction of splenic CD4+ (A), CD8+ (B), CD45R+ (C), or CD11b+ (D) cells expressing VEEV E2 antigen. Results shown are the mean + 95% confidence limits of five mice per time point.
It would therefore seem that there is no difference in the behavior of splenic macrophages between PEG IFN-α-treated and untreated BALB/c mice since there is high activation and intracellular VEEV antigen in both treatment groups. However, further examination of CD69 and intracellular VEEV antigen coexpression in splenic macrophage populations showed significant differences between the treatment groups (Fig. 8). In PEG IFN-α-treated mice, there was a consistently higher proportion of splenic macrophages that express both CD69 and VEEV antigen than in untreated mice over the course of infection. Comparison of splenic viral load (Fig. 3) with intracellular E2 antigen expression by splenic macrophages reveals that expression of E2 antigen in untreated BALB/c mice is related to the presence of whole intact virulent VEEV. However, since no VEEV was detected by plaque assay in the spleens of PEG IFN-α-treated mice (Fig. 3), then expression of E2 antigen in the corresponding macrophage population must be related to either inactivated nonviable VEEV or viral antigen that is in the process of being presented to the immune system. This finding suggests that macrophages play a key role in immunity to VEEV infection since those from PEG IFN-α-treated mice display functional characteristics different from those from untreated mice.
FIG. 8.
Expression of CD69 and intracellular VEEV E2 antigen by splenic macrophages from PEG IFN-α-treated and untreated BALB/c mice during a subcutaneous challenge with virulent VEEV. Mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline or just 1% NMS in N-saline daily on days −2 to +5 after infection with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i. The data are representative of five mice per time point and show CD69 and VEEV E2 antigen expression in gated populations of macrophages.
The inflammatory response of VEEV in PEG IFN-α-treated and untreated mice.
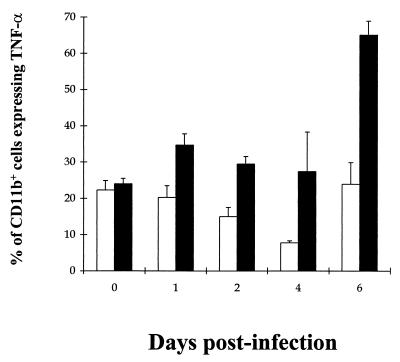
The extent of the inflammatory response to VEEV infection was assessed by measuring intracellular TNF-α levels by splenic leukocytes over the first 6 days p.i. (Fig. 9). TNF-α could not be detected in the T- and B-lymphocyte populations of both PEG IFN-α-treated and untreated mice (data not shown). However, TNF-α was detected in the splenic macrophage populations in both treatment groups. TNF-α expression was consistently and significantly higher in untreated mice than in PEG IFN-α-treated mice on each day postinfection (P < 0.05) according to Student's t test. During the latter stages of VEEV infection, TNF-α expression reached a peak of over 65% of macrophages in untreated mice which coincided with the clinical symptoms of advanced VEEV infection such as piloerection and cachexia.
FIG. 9.
Kinetics of intracellular TNF-α expression by splenic macrophages from PEG IFN-α-treated and untreated BALB/c mice given a subcutaneous challenge with virulent VEEV. Mice were given 100 μl i.p. of either 4 × 104 U of PEG IFN-α in 1% NMS in N-saline (□) or just 1% NMS in N-saline (■) on days −2 to +5 after infection with ca. 25 MLD (25 PFU) of VEEV in 100 μl of L-15 MM on day 0 p.i. The data represent the fraction of splenic CD11b+ cells expressing intracellular TNF-α following 4 h of culture at 37°C with Golgistop and VEEV E2 antigen. Results shown are the mean + 95% confidence limits of five mice per time point.
DISCUSSION
The data presented show that PEG IFN-α given prophylactically protects mice from VEEV. This differs from the work of previous authors, who have shown that VEEV is relatively resistant to IFN treatment and that the more virulent strains are the most resistant (34). Our work confirms that while the TrD strain of VEEV is resistant to conventional IFNs such as IFN-α B/D, IFN-α does play a key role in the host response to VEEV infection, as antibody ablation studies have already demonstrated (11). More recent evidence for this role of IFN comes from work on IFN receptor knockout mice, which are extremely susceptible to the virus with an accelerated course of disease (14).
The dose of PEG IFN-α A/D in units of IFN used in this experiment was one-fifth of that used to control St. Louis encephalitis in mice (3) and one-fifth of the dose of unconjugated IFN-α B/D used unsuccessfully to control VEEV (TrD) in this experiment. The mode of action of PEG is not fully understood, but PEG-conjugated cytokines show a longer half-life and delayed renal clearance compared to native protein (19, 36). Although IFN-α induces the synthesis of a series of intracellular antiviral proteins that continue to exert an antiviral effect after the disappearance of the cytokine from the serum (33), there is invariably a seesaw pattern of effector protein levels following single daily dosing of unconjugated cytokine. VEEV replicates in a wide range of cells and would be able to replicate significantly between doses of conventional IFN-α. It is likely that PEG conjugation of IFN maintains serum levels of IFN for a longer period, with continuing stimulation of IFN receptors and expression of effector proteins. This effect appears to be very important in PEG IFN-α treatment of herpes simplex virus infection in mice and treatment of hepatitis C in humans (M. Mulqueen [Roche Discovery], personal communication) and is probably involved to some extent here.
IL-12 has been used successfully to treat a number of viral diseases in animal models, including those caused by herpes virus (4), lymphocytic choriomeningitis virus (2), vesicular stomatitis virus (20), and St. Louis encephalitis virus (T. Brooks, unpublished data). However, IL-12 treatment may also be detrimental during viral infections (24) as was the case during VEEV infection, with mice treated with either IL-12 alone or in combination with PEG IFN-α becoming more susceptible to infection than untreated mice. This would suggest that the IL-12-induced cell-mediated immune response is inappropriate in combating VEEV infection and that IL-12 has a role in the pathogenesis of disease.
Aerosol infection of mice and humans with VEEV is well documented (17, 28, 32), and in mice the incubation period and clinical progress of the disease are very similar to those of subcutaneous infection. The virus enters the brain by both the olfactory nerve and vascular spread (32), and similar pathogenic mechanisms appear to be involved. Although VEEV may bypass the immune system by direct entry to the brain along the olfactory tract, most of the virus delivered is deposited in other parts of the airways and causes a systemic infection analogous to that introduced by the subcutaneous route. The effect of PEG IFN-α is less for inhalational than for subcutaneous challenges, possibly because those that succumb to infection may have been primarily affected by direct central nervous system invasion of the virus before the immune system developed a protective response. Virus in the central nervous system would also be less susceptible to the antiviral actions induced by PEG IFN-α.
During subcutaneous or airborne challenge, PEG IFN-α treatment results in either undetectable or significantly lower viremias, respectively, than in untreated controls. In particular, the undetectable viremia observed in PEG IFN-α-treated BALB/c mice during subcutaneous challenge with VEEV suggests that PEG IFN-α-dependent effector mechanisms clear the virus by day 2 p.i. Very little variance in viral load was observed in untreated mice challenged either subcutaneously or by aerosol and remained relatively high in the periphery throughout the course of infection (e.g., VEEV blood titer of ca. 103 on day 6 p.i.). These observations differ from those of previous studies which suggest that VEEV is cleared from the periphery by days 3 to 5 p.i. (7, 12). This discrepancy may be explained by differences in the strain of mouse used as well as virus passage level and/or route of inoculation (18).
While many studies of the immune response to VEEV infection have concentrated on the secondary response induced by vaccine treatment (28), few have described the primary immune response to VEEV in great detail (11). In this study, we were able to describe the primary immune response to VEEV in both a susceptible population and a population rendered resistant by prophylaxis with PEG IFN-α during a subcutaneous infection with virulent VEEV.
In untreated susceptible mice, VEEV infection induces a rapid and high expression of the very early activation marker CD69 in the splenic lymphocyte populations by day 2 p.i., while in PEG IFN-α-treated mice this overactivation is absent. Such a rapid high expression of CD69 has been described in other models of virus infection in which viral antigens from either mouse mammary tumor virus, rhinovirus, or African swine fever virus induced high CD69 expression among various lymphocyte populations (8, 39, 42).
These observations imply a number of conclusions about the interactions of VEEV with the host. First, the high expression of CD69 is not attributable to the presence of VEEV itself inside the lymphocytes, since low levels of viral E2 antigen were detected in both untreated and PEG IFN-α-treated mice. Second, the interaction between host and pathogen is unlikely to be that of classical antigen-specific cell activation seen during a normal adaptive immune response since the normal frequency of antigen-specific T cells is generally less than 1 in 1,000, while during VEEV infection a very high proportion of lymphocytes were activated. Instead it is likely that VEEV infection induces a very large nonspecific immune response in untreated mice which is prevented in PEG IFN-α-treated mice.
Other studies of virus-induced nonspecific cell activation have suggested that macrophages or monocytes induce this nonspecific expression of CD69 via the secretion of soluble factors in situ (8, 39). VEEV E2 expression seemed to be restricted to the splenic macrophage population in both untreated and PEG IFN-α-treated mice. In the same macrophage populations, CD69 expression also increased gradually over the first 6 days p.i. Both IFN-α and IL-12 are able to upregulate CD69 expression on T cells under certain conditions (1, 9). It has been suggested that cytokines like IFN-α and IL-12 secreted by monocytes and macrophages during viral infections could be responsible for such nonspecific cell activation (8). Our findings confirm those of other studies which indicate that IFN-α is able to upregulate CD69 expression on lymphocytes (35) since exogenous PEG IFN-α treatment prior to infection (day 0) causes a significant proportion of lymphocytes and macrophages to express CD69. However, by contrast, CD69 expression in the same treatment group is low throughout VEEV infection. It is unlikely that PEG IFN-α-treatment directly downregulates lymphocyte and macrophage CD69 expression during VEEV infection. Instead, we suggest that PEG IFN-α-treatment suppresses viral load by inducing antiviral mechanisms in effector cells (like macrophages) prior to infection so that most or all of the VEEV particles in the inoculum are destroyed before the virus has a chance to spread. It is the resulting absence of VEEV that prevents cellular exposure to the endogenously produced soluble mediators, like IFN-α, that are secreted upon infection in untreated individuals and hence low levels of CD69 expression.
Closer examination of splenic macrophage VEEV E2 antigen and CD69 expression revealed that the macrophages of PEG IFN-α-treated mice behaved differently from those of untreated mice. PEG IFN-α treatment results in a greater coexpression of viral antigen and CD69 than in untreated mice with no viable VEEV present in the spleen. This would suggest that macrophages may play an important role in generating an effective primary immune response to VEEV infection. In vitro studies of mouse macrophage activity in cultures of VEEV-infected cells have demonstrated that activation of macrophages either by lipopolysaccharide or IFN-α/β dramatically enhances their ability to kill infected cells (21). PEG IFN-α prophylaxis may therefore upregulate macrophage antiviral activity, either directly against VEEV or against VEEV-infected cells, to such an extent that the infection is controlled before the virus can replicate in sufficient numbers to induce the terminal events of VEEV infection.
Under the same experimental conditions of use as PEG IFN-α, IL-12 alone does not upregulate CD69 expression on any of the leukocyte populations described above in uninfected mice (our unpublished data). The deleterious effect of IL-12 treatment alone or in combination with PEG IFN-α points to a possible role in the pathogenesis of VEEV infection. IL-12-dependent antiviral effector mechanisms seem to be inappropriate in combating VEEV infection, and it may be that treatment with PEG IFN-α downregulates this arm of the host response and promotes beneficial IFN-α-dependent effector mechanisms (6). Addition of exogenous IL-12 at the same time as PEG IFN-α may overcome the inhibitory effects of IFN-α and promote a proinflammatory IL-12-dependent response to VEEV and ultimately death of the host. Clearly, further studies of the effect of IL-12 on VEEV infection are needed to gain a better understanding of this possible mechanism of pathogenesis of virulent VEEV infection.
One of the main clinical signs of VEEV infection in mice is the strong inflammatory response characterized by piloerection and pyrexia that become apparent from day 2 p.i. (41). This coincides with the peak CD69 expression in the splenic lymphocyte population which would suggest that the VEEV-induced illness and CD69 expression are somehow linked. Although the functional significance of CD69 expression on T cells has not been clearly defined, there is evidence to suggest that CD69 may potentiate inflammatory responses via its role as a lectin receptor capable of signal transduction. While a ligand for CD69 has not yet been identified, anti-CD69 MAbs have been shown to provide a costimulatory signal sufficient to cause cytokine secretion and proliferation in T cells (37). In addition, CD69 has been implicated as a key receptor involved in stimulatory signals sent from activated T cells to monocytes, as anti-CD69 MAbs block the ability of activated T cells to induce IL-1β production from monocytes (16, 23). It is therefore possible that activated T cells, which already express high amounts of CD69, may stimulate the release of inflammatory cytokines like IL-1β and TNF-α from macrophages during VEEV infection. Indirect evidence for this is shown by intracellular expression of TNF-α by macrophages in untreated mice which coincides with increased CD69 expression by these macrophages following VEEV infection.
TNF-α is typically secreted during a nonspecific immune response and at low levels causes nonspecific cell activation which can be protective in the early stages of some infections. However, in large amounts it can lead to overstimulation, pyrexia, and an inappropriate inflammatory response. High levels are indicative of the systemic inflammatory response syndrome commonly associated with the latter stages of VEEV infection. Macrophages from PEG IFN-α-treated mice expressed consistently low levels of TNF-α, while those from untreated mice were high throughout infection. These observations correlate well with the clinical state of the animals: untreated mice were emaciated, ruffled, and sluggish from days 4 to 5 p.i. onwards, while PEG IFN-α-treated mice showed no outward signs of illness. This would suggest that PEG IFN-α treatment prevents or suppresses the very strong inflammatory response associated with virulent VEEV infection, again probably by inducing immune effector cells to destroy the virus early during infection before the inflammatory cascade has been triggered.
From these observations, we propose that the immune response to VEEV infection involves a complex interaction between macrophages, lymphocytes, and the virus itself. It is likely that the initial interaction of VEEV with macrophages shapes the outcome of infection. In untreated individuals, the binding of VEEV to macrophages may activate a small number of antigen-specific T cells via normal antigen presentation to antigen-specific T cells. However, the interaction between VEEV and macrophages also causes a nonspecific activation, as indicated by increased CD69 expression, of the majority of lymphocytes. In addition, the macrophages themselves become activated and a large proportion express CD69 as well. Following this overactivation of leukocytes, a strong inflammatory response occurs, with increased TNF-α production by macrophages and physical signs such as piloerection and pyrexia manifesting themselves from day 2 p.i. onward.
PEG IFN-α-treated animals are able to survive VEEV infection, and we suggest that this may be due to the prevention or suppression of the inappropriate immune response observed in untreated mice. Lymphocyte CD69 expression remains consistently low over the first 6 days of infection, as do macrophage TNF-α levels. Perhaps more importantly, macrophages from PEG IFN-α-treated mice seem to be more activated and express more viral antigen (possibly from destroyed virus) than their counterparts in untreated mice. It would seem, therefore, that PEG IFN-α treatment may affect macrophages strongly to induce an early clearance of virus before it has a chance to trigger the nonspecific inflammatory response observed in untreated mice.
The use of these two models of susceptibility and IFN-α-induced resistance to VEEV infection will help to increase our understanding of the early immune events that precede the characteristic encephalomyelitis associated with infection. More detailed definition of the immune response to VEEV infection will not only reveal other antiviral host defense mechanisms but also clarify the pathogenic processes associated with VEEV as well as other alphavirus infections.
ACKNOWLEDGMENTS
We gratefully acknowledge the gift of recombinant cytokines from Mike Mulqueen, Roche Discovery, United Kingdom. We also thank R. Phillpotts for helpful comments and discussion during manuscript preparation.
REFERENCES
- 1.Alzona M, Jack H M, Fisher R I, Ellis T M. IL-12 activates IFN-g production through the preferential activation of CD30+ T cells. J Immunol. 1995;154:9–16. [PubMed] [Google Scholar]
- 2.Biron C A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Brooks T J G, Phillpotts R J. Interferon-alpha protects mice against lethal infection with St. Louis encephalitis virus delivered by the aerosol and subcutaneous routes. Antiviral Res. 1999;41:57–64. doi: 10.1016/s0166-3542(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 4.Carr J A, Rogerson J, Mulqueen M J, Roberts N A, Booth R F. Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J Virol. 1997;71:7799–7803. doi: 10.1128/jvi.71.10.7799-7803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles P C, Brown K W, Davis N L, Hart M K, Johnston R E. Mucosal immunity induced by parenteral immunisation with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology. 1997;228:153–160. doi: 10.1006/viro.1996.8381. [DOI] [PubMed] [Google Scholar]
- 6.Coussens L P, Peterson R, Hsu S, Dorner A, Altman J D, Biron C A. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis N L, Grieder F B, Smith J F, Greenwald G F, Valenski M L, Sellon D C, Charles P C, Johnston R E. A molecular genetic approach to the study of Venezuelan equine encephalitis virus pathogenesis. Arch Virol Suppl. 1994;9:99–109. doi: 10.1007/978-3-7091-9326-6_11. [DOI] [PubMed] [Google Scholar]
- 8.Gern J E, Vritis R, Kelly E A B, Dick E C, Busse W W. Rhinovirus produces non-specific activation of lymphocytes through a monocyte-dependent mechanism. J Immunol. 1996;157:1605–1612. [PubMed] [Google Scholar]
- 9.Gerosa F, Tomassi M, Garra G, Gandini G, Tridente G, Benati C. Different sensitivity to interleukin 4 of interleukin 2 and interferon alpha-induced CD69 expression in human resting NK cells and CD3+, CD4−, CD8− lymphocytes. Cell Immunol. 1992;141:342–351. doi: 10.1016/0008-8749(92)90153-g. [DOI] [PubMed] [Google Scholar]
- 10.Greenway T E, Eldridge J H, Ludwig G, Staas J K, Smith J F, Gilley R M, Michalek S M. Induction of protective immune responses against Venezuelan equine encephalitis (VEE) virus aerosol challenge with microencapsulated VEE virus vaccine. Vaccine. 1998;16:1314–1323. doi: 10.1016/s0264-410x(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 11.Grieder F B, Davis B K, Zhou X D, Chen S J, Finkelman F D, Gause W C. Kinetics of cytokine expression and regulation of host protection following infection with molecularly cloned Venezuelan equine encephalitis virus. Virology. 1997;233:302–312. doi: 10.1006/viro.1997.8617. [DOI] [PubMed] [Google Scholar]
- 12.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 13.Grieder F B, Nguyen H T. Virulent and attenuated mutant Venezuelan equine encephalitis virus show marked differences in replication in infection in murine macrophages. Microb Pathog. 1996;21:85–95. doi: 10.1006/mpat.1996.0045. [DOI] [PubMed] [Google Scholar]
- 14.Grieder F B, Vogel S N. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257:106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- 15.Huprikar J, Dal Canto M C, Rabinowitz S G. Protection against lethal Venezuelan equine encephalitis (VEE) virus infection by cell-free supernatant obtained from immune spleen cells. J Neurol Sci. 1990;97:143–153. doi: 10.1016/0022-510x(90)90213-7. [DOI] [PubMed] [Google Scholar]
- 16.Isler P, Vey E, Zhang J H, Dayer J M. Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Netw. 1993;4:15–23. [PubMed] [Google Scholar]
- 17.Jackson A C, SenGupta S K, Smith J F. Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet Pathol. 1991;28:410–418. doi: 10.1177/030098589102800509. [DOI] [PubMed] [Google Scholar]
- 18.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Strauss S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1995. pp. 843–898. [Google Scholar]
- 19.Katre N V, Knauf M J, Laird W J. Chemical modification of recombinant interleukin 2 by polyethylene glycol increases its potency in the murine Meth A sarcoma model. Proc Natl Acad Sci USA. 1987;84:1487–1491. doi: 10.1073/pnas.84.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu T, Barna M, Reiss C S. Interleukin-12 promotes recovery from viral encephalitis. Viral Immunol. 1997;10:35–47. doi: 10.1089/vim.1997.10.35. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc P A. Macrophage activation for cytolysis of virally infected target cells. J Leukoc Biol. 1989;45:345–352. doi: 10.1002/jlb.45.4.345. [DOI] [PubMed] [Google Scholar]
- 22.Lennette E H, Koprowski H. Human infection with Venezuelan equine encephalomyelitis virus. JAMA. 1943;123:1088–1095. [Google Scholar]
- 23.Manie S, Kubar J, Limouse M, Ferrua B, Ticchioni M, Breittmayer J P, Peyron J F, Schaffar L, Rossi B. CD3-stimulated Jurkat T cells mediate IL-1 beta production in monocytic THP-1 cells: role of LFA-1 molecule and participation of CD69 T cell antigen. Eur Cytokine Netw. 1993;4:7–13. [PubMed] [Google Scholar]
- 24.Orange J S, Wolf S F, Biron C A. Effects of IL-12 on the response and susceptibility of experimental viral infections. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 25.Pan American Health Organization. Proceedings of the Workshop. Symposium on Venezuelan encephalitis virus. Washington, D.C.: Pan American Health Organization; 1972. Venezuelan encephalitis; pp. 7–16. [Google Scholar]
- 26.Peters C J, Dalrymple J M. Alphaviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 713–761. [Google Scholar]
- 27.Phillpotts R J, Brooks T J G, Cox C S. A simple device for the exposure of animals to infectious microorganisms by the airborne route. Epidemiol Infect. 1997;118:71–75. doi: 10.1017/s0950268896007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillpotts R J, Wright A J. TC-83 vaccine protects against airborne or subcutaneous challenge with heterologous mouse-virulent strains of Venezuelan equine encephalitis virus. Vaccine. 1999;17:982–988. doi: 10.1016/s0264-410x(98)00315-6. [DOI] [PubMed] [Google Scholar]
- 29.Pittman P R, Makuch R S, Mangiafico J A, Cannon T L, Gibbs P H, Peters C J. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14:337–343. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran R, Katzenstein D A, Winters M A, Kundu S K, Merigan T C. Polyethylene glycol-modified interleukin-2 and thymosin alpha 1 in human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:1005–1008. doi: 10.1093/infdis/173.4.1005. [DOI] [PubMed] [Google Scholar]
- 31.Roehrig J T, Day J W, Kinney R M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982;118:269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- 32.Ryzhikov A B, Tkacheva N V, Sergeev A N, Ryabchikova E I. Venezuelan equine encephalitis virus propagation in the olfactory tract of normal and immunized mice. Biomed Sci. 1991;2:607–614. [PubMed] [Google Scholar]
- 33.Samuel C E. Antiviral actions of interferon, interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 34.Spotts D R, Reich R M, Kalkhan M A, Kinney R M, Roehrig J T. Resistance to alpha/beta interferons correlates with the epizootic and virulence potential of Venezuelan equine encephalitis viruses and is determined by the 5′ noncoding region and glycoproteins. J Virol. 1998;72:10286–10291. doi: 10.1128/jvi.72.12.10286-10291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun S, Zhang X, Tough D F, Sprent J. Type 1 interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teppler H, Kaplan G, Smith K A, Montana A L, Meyn P, Cohn Z A. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin 2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993;177:483–492. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Testi R, Phillips J H, Lanier L L. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–1128. [PubMed] [Google Scholar]
- 38.Tsutsumi Y, Kihira T, Tsunoda S, Kamada H, Nakagawa S, Kaneda Y, Kanamori T, Mayumi T. Molecular design of hybrid tumour necrosis factor-alpha III: polyethylene glycol-modified tumour necrosis factor-alpha has markedly enhanced antitumour potency due to longer plasma half-life and higher tumour accumulation. J Pharmacol Exp Ther. 1996;278:1006–1011. [PubMed] [Google Scholar]
- 39.Vilanova M, Tavares D, Ferreira P, Oliveira L, Nobrega A, Appelberg R, Arala-Chaves M. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand J Immunol. 1996;43:155–163. doi: 10.1046/j.1365-3083.1996.d01-25.x. [DOI] [PubMed] [Google Scholar]
- 40.Vogel P, Abplanalp D, Kell W, Ibrahim M S, Downs M B, Pratt W D, Davis K J. Venezuelan equine encephalitis in BALB/c mice: kinetic analysis of central nervous system infection following aerosol or subcutaneous inoculation. Arch Pathol Lab Med. 1996;120:164–172. [PubMed] [Google Scholar]
- 41.Wright A W, Phillpotts R J. Humane endpoints are an objective measure of morbidity in Venezuelan encephalomyelitis virus infection in mice. Arch Virol. 1998;141:71–75. doi: 10.1007/s007050050363. [DOI] [PubMed] [Google Scholar]
- 42.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J D, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]