Abstract
The chemokine receptors CCR5 and CXCR4 were found to function in vivo as the principal coreceptors for M-tropic and T-tropic human immunodeficiency virus (HIV) strains, respectively. Since many primary cells express multiple chemokine receptors, it was important to determine if the efficiency of virus-cell fusion is influenced not only by the presence of the appropriate coreceptor (CXCR4 or CCR5) but also by the levels of other coreceptors expressed by the same target cells. We found that in cells with low to medium surface CD4 density, coexpression of CCR5 and CXCR4 resulted in a significant reduction in the fusion with CXCR4 domain (X4) envelope-expressing cells and in their susceptibility to infection with X4 viruses. The inhibition could be reversed either by increasing the density of surface CD4 or by antibodies against the N terminus and second extracellular domains of CCR5. In addition, treatment of macrophages with a combination of anti-CCR5 antibodies or β-chemokines increased their fusion with X4 envelope-expressing cells. Conversely, overexpression of CXCR4 compared with CCR5 inhibited CCR5-dependent HIV-dependent fusion in 3T3.CD4.401 cells. Thus, coreceptor competition for association with CD4 may occur in vivo and is likely to have important implications for the course of HIV type 1 infection, as well as for the outcome of coreceptor-targeted therapies.
Most of the cells that were found to be targets for human immunodeficiency virus (HIV) infection in vivo (i.e., T cells, macrophages, and dendritic cells) express both CD4 and multiple chemokine receptors. Among the chemokine receptors that were shown in recent years to function as coreceptors for HIV type 1 (HIV-1) viral entry in vitro, CCR5 and CXCR4 emerged as the predominant coreceptors for primary isolates in vivo. The potential of a given chemokine receptor to function as an HIV-1 coreceptor may depend on multiple parameters such as its surface density (29), posttranslational modifications (11), and interactions with other membrane components such as CD4 and other chemokine receptors. Previously, we demonstrated that exposure of human cell lines to soluble T-tropic HIV-1 envelope at 37°C can induce the formation of a trimolecular complex between CD4, gp120, and the chemokine receptor CXCR4 that was evidenced by their coimmunoprecipitation with CD4 (22). In the promonocytic cell line U937, a low-level coprecipitation of CD4 and CXCR4 was seen prior to treatment with gp120, suggesting that some constitutive association between CD4 and chemokine receptors may exist in certain cells. Recently, in a study on human monocytes and macrophages, we found preexisting CD4-CCR5 and CD4-CXCR4 complexes in the absence of prior exposure to HIV-1 or soluble gp120 (sgp120), which correlated with the fusion potential of the cells with X4 and R5 (CXCR4- and CCR5-dependent HIV) envelope-expressing cells (22). In a separate study, using either murine 3T3.CD4+ cells infected with a recombinant vaccinia-CCR5 virus (vCCR5) or primary human monocytes and macrophages, coprecipitation of CD4 with CCR5 was demonstrated in the absence of exposure to viral envelope (36). Together, these findings suggested that in certain cells with low CD4 densities, the relative levels of CCR5 and CXCR4 expression and their ability to associate with CD4 may influence the susceptibility of the cells to infection with X4 and R5 viruses, as was previously speculated (5). In the present study, we provide evidence that CCR5 and CXCR4, when expressed in the same cell, interfere with each other's function during HIV-1 envelope-mediated cell fusion and viral cell entry. This interference is likely manifested through competition for association with limiting CD4 molecules and can be reversed by various coreceptor-specific antibodies and β-chemokines.
MATERIALS AND METHODS
Recombinant vaccinia viruses and fusion assay.
Constructions of the recombinant vaccinia viruses vCB3 (human CD4 [huCD4]) (6), vCBFY1 (huCXCR4) (12), vHC-1 (huCCR5) (36), vCB28 (JR-FL envelope) (4), and vCB43 (Ba-L envelope) (4) were previously described. Syncytium formation was measured after 2.5 to 4 h (for T-tropic envelopes) and 5 to 18 h (for M-tropic envelopes) coculture (1:1 ratio, 105 cells each, in triplicates) of target cells with CD4 12E1 cells infected with recombinant vaccinia viruses expressing HIV-1 M-tropic envelopes (JR-FL [vCB28] and Ba-L [vCB43] at 10 PFU/cell) or with the human lymphoid cell line TF228.1.16, which stably expresses HIV-1 IIIB/BH10 (T-tropic) envelope (a gift from Z. L. Jonak, SmithKline Beechham Pharmaceuticals) (19). Where indicated, preimmune rabbit immunoglobulin G (IgG), rabbit anti-CXCR4, anti-CCR5, and anti-STRL33 (all produced in our laboratory) (22, 38) or monoclonal antibodies (MAbs) against CCR5 and CXCR4 (NIH AIDS Reagent Repository, R&D Systems, Minneapolis, Minn., or PharMingen, San Diego, Calif.) were added to the target cells for 1 h at 37°C at 10 μg/ml before the addition of envelope-expressing effector cells.
Flow cytometry.
The following antibodies were used: fluorescein isothiocyanate (FITC)-labeled mouse anti-huCD4 MAb (Leu3a; Becton Dickinson, San Jose, Calif.), MAb against CXCR4 (12G5) or CCR5 (2D7) (PharMingen), or murine isotype controls followed by FITC-conjugated goat anti-mouse IgG (Fc specific; Sigma). Gating on live cells was assisted by using propidium iodide at 5 μg/ml. Ten thousand events were collected per sample and analyzed by fluorescence-activated cell sorting (FACS) using the FL-1 (FITC channel) on a FACScan (Becton Dickinson) with CellQuest software. Delta mean fluorescence channels (ΔMFC) were calculated by subtracting the isotype control antibody MFC from the experimental values. In some experiments, cells infected with vCCR5 were sorted into CCR5neg, CCR5med, and CCR5hi subsets. The sorted cells were acquired on a Becton Dickinson FACStar Plus with a 5-W 488 Argon Laser Coherent Innova 90, using CellQuest (Becton Dickinson) on an Apple Macintosh Quadra 650. Coulter's Immuno-Check beads were used to maximize the PMTs, and Becton Dickinson's CaliBrite beads were used to show the efficiency of separation between positive and negative cells (linear scale). Live/dead separation of cells was based on side scatter on the x axis and forward scatter on the y axis. A live gate (R3) encompassed the live cells. The cell surface phenotypic marker was mouse 2D7 plus goat anti-mouse FITC. Therefore, the PMT of choice was FL-1. Appropriate filters floating positive gate (bright/dulls) (R1) and a negative gate (R2) were used to separate the positives from the negatives. The sort mode was set on normal R (high purity, high recovery). A 100-μm sorting nozzle was used, as well as a moderately slow event rate of approximately 2,200 events/s, and EDTA was added due to the size and clumping of the cells. The threshold forward scatter/side scatter was set at 20 to give a more accurate event count of nozzle passage.
Infection of A2.01.CD4.401 cells with X4 and R5 HIV-1.
Infections of A2.01.CD4.401 cells and PCR analyses were performed as previously described (39). Two million cells were infected with NL4-3 (multiplicity of infection [MOI] of 0.05) or LAI (MOI of 0.02). Viral stocks were treated with DNase for 45 min at 37°C before infection. After incubation with the virus for 1 h, cells were washed four to five times to remove unbound particles. After overnight incubation at 37°C, cells were recovered and counted. DNA lysates (the equivalent 104 cells/group) were amplified by PCR with gag-specific primers (SK38 and SK39), and the products were hybridized to a 32P-end-labeled SK19 probe as previously described (39). When indicated, target cells were preincubated with various murine anti-CCR5 MAbs, rabbit polyclonal anti-CCR5 IgGs, or control antibodies (at 10 μg/ml) for 1 h at 37°C before viral infection.
Coprecipitation of CD4 and CXCR4.
A2.01.CD4.401 cells were infected with a control recombinant vaccinia virus or with vCCR5 (15 PFU/cell, 7 h, 37°C) and were lysed at a concentration of 2 × 107 cells/ml in buffer containing 1% Brij 97 (22). CD4 was immunoprecipitated by mixing MAb OKT4-conjugated protein G-Sepharose beads with cell lysates at 4°C for 3 h. Preliminary data confirmed that under these conditions, CD4 was precipitated to completion. Beads were washed five times with lysis buffer and boiled with an equal volume of 2× Laemmli sample buffer containing 8 M urea. Samples (4 × 107 cells per lane) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels and electrophoretically transferred to nitrocellulose membranes. Membranes were blocked and incubated with a rabbit polyclonal antibody raised against a peptide corresponding to the N terminus of CXCR4. The membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody diluted in blocking buffer at 4°C (Amersham), followed by Supersignal Ultra chemiluminescent substrate (Pierce) for 5 min, and exposed to film. Membranes were stripped by wetting in 100% methanol and washing with blotting buffer. They were incubated in 0.5 M glycine (pH 2.5) with 0.05% Tween for 30 min at 60°C, washed in blotting buffer, and blocked. The membranes were reacted with rabbit polyclonal anti-CD4 (Intracel), HRP-conjugated anti-rabbit chemiluminescent reagent and were exposed to film. The relative amounts of CD4 and CXCR4 coimmunoprecipitated from cells infected with control vaccinia virus (vSC8) or vCCR5 were determined by densitometry.
Generation of MDM.
Elutriated monocytes and differentiated macrophages (MDM) were 100% CD3neg, >85% CD14+, and >95% HLA-DR+ as determined by flow cytometry. MDM were derived from elutriated monocytes in 5- to 7-day cultures in Dulbecco modified Eagle medium supplemented with recombinant human granulocyte-macrophage colony-stimulating factor (1,000 U/ml) and 10% pooled human serum (heat inactivated) (23, 38). Prior to use in the fusion assay, MDM were treated for 1 h at 37°C with various anti-CCR5 antibodies (10 μg/ml) or β-chemokines (1 to 100 ng/ml) and were then mixed (1:1 ratio) with target cells expressing X4 or R5 envelopes. Syncytium formation was scored after 18 h. In some experiments, MDM were first treated with pertussis toxin (PT; Sigma) at 1 μg/ml for 1 h at 37°C, washed twice, and then incubated with β-chemokines. This treatment with PT blocked protein G-dependent Ca2+ flux response to β-chemokines and SDF-1α (M. Zaitseva, unpublished data).
RESULTS
Introduction of CCR5 into CD4+ CXCR4+ cell lines results in a reduction of fusion with target cells expressing X4 envelopes.
To study the effects of CCR5 expression on CXCR4 function, a recombinant vaccinia virus expressing human CCR5 was used to infect CEM cells and two CEM-derived cell lines that express different levels of CD4. The A2.01.CD4.401 cells express tailless CD4 molecules (A2.01.401) (13). The 17D9 cell clone was derived from CEM cells in our laboratory (18). The rank order of surface CD4 expression levels was found to be CEM > A2.01.CD4.401 > 17D9. CXCR4 was expressed at similar levels on CEM and 17D9 cells and somewhat lower levels on A2.01.CD4.401 cells (Table 1). Infection of the three cell lines with vCCR5 did not affect CD4 expression and only modestly reduced CXCR4 surface expression (1 to 20% reduction in >20 experiments) (Table 1 and data not shown). The ability of these cells to fuse with X4 and R5 HIV-1 envelopes was evaluated in a syncytium assay using 12E cells (CD4neg) infected with recombinant vaccinia viruses expressing X4 or R5 envelope. In all cells, vCCR5 infection resulted in similar levels of surface CCR5 expression and the acquisition of fusion potential with various R5 envelopes such as JR-FL (Table 1), ADA, and Ba-L (not shown). The effect of CCR5 expression on fusion with X4 envelopes varied in accordance with the levels of CD4 expression. In the case of CEM cells (CD4high), CCR5 expression resulted in a moderate reduction (5 to 30% in 10 experiments) of fusion with IIIB envelope-expressing cells. In contrast, introduction of CCR5 into either 17D9 or A2.01.CD4.401 (CD4med) resulted in a reproducibly significant diminution of the X4 fusion (40 to 65% inhibition in >20 experiments [Table 1 and data not shown]). Similar results were obtained with other X4-dependent (RF and SF2) envelope-expressing effector cells (data not shown).
TABLE 1.
The relative density of surface CD4 on human T cells may determine the ability of CCR5 to reduce CXCR4-mediated cell fusion
| Cell | Vaccinia virus (PFU/cell) | Expression (ΔMFCa)
|
Mean no. of syncytiab ± SD (% reduction)
|
|||
|---|---|---|---|---|---|---|
| CD4 | CXCR4 | CCR5 | IIIB | JR-FL | ||
| CEM | vSC8 (15) | 403 | 258 | 7 | 433 ± 35 | 0 |
| vCCR5 (15) | 400 | 209 | 262 | 303 ± 6 | 328 ± 8 | |
| (30) | ||||||
| A2.01.401 | vSC8 (15) | 240 | 144 | 17 | 327 ± 48 | 0 |
| vCCR5 (15) | 246 | 131 | 392 | 152 ± 28 | 426 ± 17 | |
| (54) | ||||||
| 17D9 | vSC8 (15) | 186 | 283 | 2 | 329 ± 40 | 0 |
| vCCR5 (15) | 183 | 239 | 340 | 164 ± 18 | 301 ± 41 | |
| (50) | ||||||
Cells were stained with anti-CD4 (Leu3a), anti-CXCR4 (12G5), or anti-CCR5 (2D7) MAb, or with murine isotype control antibody and analyzed by FACS. ΔMFC was calculated by subtracting the MFC value of the control antibodies from the specific antibody staining.
Number of syncytia was determined 2 to 4 h after mixing of vSC8- or vCCR5-infected T cells (at 15 PFU/cell) with either TF228 cells (expressing IIIB/BH10 envelope) or 12E1 (CD4−) cells infected with vCB28 (JR-FL envelope), at a 1:1 ratio in triplicates. Data represent one of three experiments. Percent reduction was calculated as 100 − (no. of syncytia produced by vCCR5-infected cells/no. of syncytia produced by vSC8-infected cells) × 100.
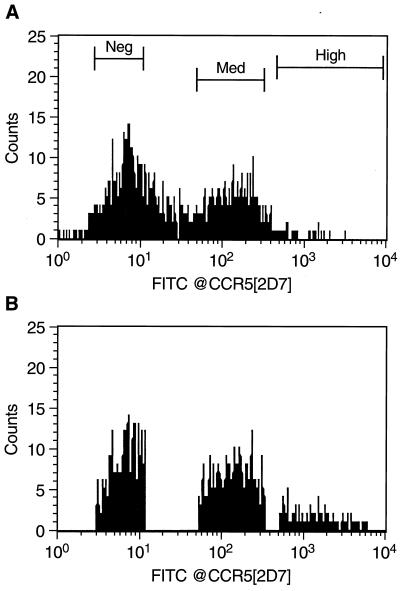
Since we often noted variations in the levels of surface CCR5 expression following vCCR5 infection, it was of interest to sort for cell subsets expressing different levels of surface CCR5 (Fig. 1). Importantly, no reduction in the expression of surface CXCR4 was seen even on the CCR5hi subset (data not shown). As can be seen in Table 2, the vCCR5-infected unsorted A2.01.CD4.401 cells exhibited 44% reduction in fusion with IIIB-expressing target cells compared with the control (vSC8)-infected cells. In contrast, the sorted CCR5med and CCR5hi populations showed 65 and 93% reduction in IIIB envelope-mediated fusion, respectively (Table 2). Thus, introduction of CCR5 into CXCR4+CD4med T cells interfered with their ability to fuse with an X4 envelope in a CCR5 surface concentration-dependent manner.
FIG. 1.
Sorting of vCCR5-infected A2.01.401 cells. Cells were infected with vCCR5-1107 at 10 PFU/cell for 6 h and then stained with the CCR5-specific MAb 2D7 (or isotype control antibody) followed by FITC-conjugated goat anti-mouse IgG. vSC8-infected cells were used as negative controls. The cells were analyzed and sorted on a Becton Dickinson FACSStar Plus. Profiles of the presorted cells (A) and of the three sorted populations (CCR5neg, CCR5med, and CCR5hi) (B) are shown. Data shown are from one of two experiments with similar results.
TABLE 2.
Sorting of surface CCR5hi A2.01.CD4.401 cells (vCCR5 infected) increases the observed reduction in fusion with T-tropic envelope-expressing cellsa
| Vaccinia virus | CCR5 (ΔMFC) | Mean no. of syncytia ± SD (% reduction)
|
|
|---|---|---|---|
| IIIB | JR-FL | ||
| vSC8 (presort) | 43 | 261 ± 38 | 0 |
| vCCR5 (presort) | 228 | 148 ± 2 (44) | 182 ± 10 |
| vCCR5 (CCR5neg) | 60 | 300 ± 32 (0) | 2 ± 1 |
| vCCR5 (CCR5med) | 341 | 107 ± 18 (65) | 191 ± 32 |
| vCCR5 (CCR5hi) | 438 | 21 ± 4 (93) | 305 ± 42 |
A2.01.CD4.401 cells were infected with vSC8 (control) or with vCCR5 at 10 PFU/cell. After 7 h of infection, the cells were left unsorted or were sorted into three subsets based on their surface CCR5 expression using MAb 2D7, as shown in Fig. 1. Syncytium formation and percent reduction were determined as described in the footnote to Table 1. Data represent one of two experiments with similar results.
Introduction of CCR5 into CD4+ CXCR4+ cells reduces their ability to be infected with a T-tropic HIV-1 strain.
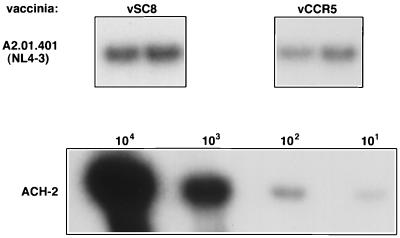
To further determine the biological relevance of the observed reduction in cell fusion, vSC8- and vCCR5-infected A2.01.CD4.401 cells were exposed to the T-tropic HIV-1 strain NL4-3 for 1 h. Viral entry was measured after 12 to 18 h by a viral DNA PCR analysis. As shown in Fig. 2, a significantly reduced signal was seen in vCCR5-infected cells compared with CCR5neg vSC8-infected cells. Based on the ACH-2-derived standard curve, the frequency of infected cells was reduced twofold in CCR5+ cells. Thus, introduction of CCR5 into CD4+ CXCR4+ cells resulted in twofold reduction in both X4 envelope fusion and X4 HIV-1 cell entry.
FIG. 2.
Introduction of CCR5 into CD4+ CXCR4+ A2.01.401 cells reduces their ability to be infected with a T-tropic HIV-1 strain. A2.01.401 cells (104) were infected (in duplicate) with vaccinia virus recombinant control (vSC8) or vCCR5-1107 at 20 PFU/cell and 7 h later were infected with the T-tropic HIV-1 strain NL4-3 (MOI of 0.02) for 24 h. DNA lysates were PCR amplified with gag-specific primers. In parallel, DNA extracts from serially diluted ACH-2 cells were amplified as an internal standard control. The data represent four experiments.
Competition for association with CD4 may be responsible for the reduction of fusion with X4 envelopes in cells expressing CCR5.
Several mechanisms may explain the observed reduction in the potential for fusion of CD4+ CXCR4+ cells, expressing surface CCR5 molecules, with X4 envelope-expressing cells. It is possible that CCR5 expression, especially after infection with a recombinant vaccinia virus, reduces CXCR4 (or CD4) transcription or interferes with their transport to the cell surface. Indeed, in murine 3T3.CD4.401 cells that were coinfected with recombinant vaccinia viruses expressing CXCR4 (vCBYF1; 5 PFU/cell) and CCR5 at increasing PFU per cell, a gradual reduction in surface CXCR4 expression was noted (data not shown). However, in all experiments with T-cell lines that express endogenous CXCR4, even high expression of surface CCR5 following infection with a recombinant vaccinia virus resulted in a minimal (Table 1) or no reduction in the surface expression of either CXCR4 or CD4 as mentioned before. Another possibility is that surface CCR5 and CXCR4 have a natural affinity for CD4 molecules and compete for association with limiting CD4 molecules either before or after encounter of the HIV-1 envelope.
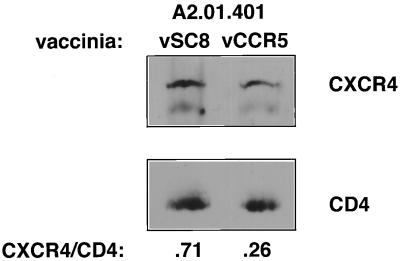
To test this hypothesis, A2.01.CD4.401 cells were infected with either vCCR5 (15 PFU/cell) or vCCR5 plus vCD4 (vCB3; 3 PFU/cell). Fusion with IIIB Env-expressing cells was inhibited by 60% after infection with vCCR5 compared with control (vSC8)-infected cells. However, this inhibition was completely reversed in cells coinfected with vCCR5 and vCD4, even though only a modest increase in the density of surface CD4 was measured (ΔMFC, 206 and 251, respectively) (Table 3). These findings support the hypothesis put forward by Pratt et al. (29) and by our previous study (23) that the fusion potential of a cell with X4 or R5 envelopes may depend on threshold concentrations of CD4 and coreceptors (and possibly of CD4-CCR5 and CD4-CXCR4 complexes). Thus, even a modest increase in CD4 surface concentration may be sufficient to increase the interactions between CD4 and CXCR4 above the threshold required for initiation of fusion with T-tropic envelope-expressing cells (despite the presence of CCR5 at high density). In a second approach, we used a biochemical assay that directly evaluates CD4-CXCR4 interactions. We have previously demonstrated that coimmunoprecipitation of surface CD4 and CXCR4 from CD4+ CXCR4+ human cell lines was achieved after exposure of cells to sgp120 at 37°C (22). In the present study, vSC8- or vCCR5-infected A2.01.401 cells were incubated with sgp120 (LAI) for 1 h at 37°C followed by cell lysis and immunoprecipitation with OKT4-conjugated Sepharose beads. The relative amounts of CXCR4 and CD4 in the immunoprecipitates were determined by Western blotting using anti-CXCR4 and anti-CD4 polyclonal antibodies (Fig. 3). In four experiments, the ratios of CXCR4/CD4 precipitation in CCR5-expressing A2.01.CD4.401 cells were two- to fourfold lower than in cells infected with control vaccinia virus (Fig. 3). Thus, direct evidence was provided in support of the hypothesis that in cells expressing both CXCR4 and CCR5, the ability of CXCR4 to associate with CD4 molecules (after exposure to HIV-1 envelope) is reduced.
TABLE 3.
Increased density of surface CD4 on human T cells can reverse the ability of CCR5 to reduce CXCR4-mediated cell fusiona
| Vaccinia virus (PFU/cell) | Surface expression (ΔMFC)
|
Mean no. of syncytia ± SD (% reduction)
|
|||
|---|---|---|---|---|---|
| CD4 | CXCR4 | CCR5 | IIIB | JR-FL | |
| vSC8 (15) | 200 | 227 | 17 | 192 ± 10 | 0 |
| vCCR5 (15) | 206 | 229 | 266 | 77 ± 8 (60) | 230 ± 4 |
| vCCR5 (15+) | 251 | 241 | 269 | 205 ± 1 | 243 ± 13 |
| vCD4 (3) | (0) | ||||
A2.01.CD4.401 cells were infected with the indicated recombinant vaccinia viruses for 7 h. Surface staining and syncytium formation were determined as described in the footnote to Table 1. Data represent two experiments.
FIG. 3.
Reduced coprecipitation of CD4 and CXCR4 from A2.01.401 cells infected with vCCR5. vSC8- or vCCR5-infected A2.01.CD4.401 cells (7-h infection, 15 PFU/cell) were incubated with gp120 (LAI; Intracel, Seattle, Wash.) at 10 μg/ml for 1 h at 37°C. Cell lysates were immunoprecipitated with antibody OKT4 covalently linked to protein G-Sepharose beads. Eluted samples were analyzed by Western blotting with rabbit polyclonal IgG raised against the N terminus of CXCR4. The same membranes were reacted with polyclonal rabbit antiserum against huCD4, followed by HRP-conjugated antibody against rabbit IgG. The bands were detected using chemiluminescence, and the CXCR4/CD4 ratios in the immunoprecipitates were determined by densitometric analysis. The data are representative of four experiments.
Anti-CCR5 antibodies can reverse the reduction in fusion with X4 envelopes seen in A2.01.401 cells infected with vCCR5.
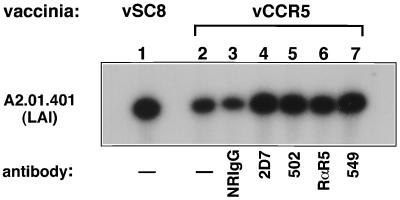
The data described above support the possibility that CCR5 competes with CXCR4 for association with CD4 molecules. This competition may take place either after binding of the HIV-1 envelope or due to constitutive interactions between CD4 and coreceptors as previously demonstrated (9, 23, 36). To further dissect these interactions, a panel of murine MAbs and rabbit polyclonal antibodies specific for either the N terminus (identified by “N”) or the three extracellular loops (EC1, EC2, and EC3) of CCR5 (17, 24) were used. These antibodies were tested in parallel for their ability to block the fusion of vCCR5-infected A2.01.CD4.401 cells with R5 (JR-FL) Env-expressing cells and to reverse the reduction in fusion of the same cells with X4 Env-expressing cells (Table 4). Of the panel of antibodies used, MAb 2D7 (EC2) was the most effective in blocking R5-envelope fusion (85%). However, MAbs 502 (CCR5/N) and rabbit anti-CCR5 (N peptide) also blocked R5 envelope fusion by 50 to 60% (Table 4 and reference 37). In the same experiment, infection of A2.01.CD4.401 cells with vCCR5 resulted in 45% inhibition of their fusion with X4 Env-expressing cells. This reduction was reversed by the same murine anti-CCR5 MAbs (2D7; R&D product no. 502) and rabbit anti-CCR5 (N) IgG that blocked the R5-envelope fusion. Interestingly, one MAb (R&D product no. 549) did not block R5 Env-mediated cell fusion but did reverse the inhibition of fusion with X4 Env-expressing cells. This MAb was recently categorized as recognizing a multidomain (MD) CCR5 epitope (24). In a separate set of experiments, the same panel of antibodies were tested for the ability to reverse the reduction in LAI (or NL4-3) entry into A2.01.CD4.401 cells infected with vCCR5. In the infectivity experiments, vCCR5 infection of A2.01.CD4.401 cells resulted in 53% reduction of X4 viral entry. In three experiments, the CCR5-specific MAbs 2D7 (EC2), 502 (N), and 549 (MD) and rabbit polyclonal IgG (CCR5/N) restored viral entry to 80 to 95% of level for control (vSC8)-infected A2.01.401 cells, as determined by densitometry (Fig. 4 and data not shown). Together, these data suggest that the reduction in X4-envelope fusion potential resulting from the introduction of CCR5 into cells expressing endogenous CD4 and CXCR4 was mediated by the surface CCR5 molecules in a specific manner.
TABLE 4.
Anti-CCR5 MAb and rabbit IgG that block fusion with cells expressing M-tropic envelope can reverse the reduction in fusion with T-tropic envelope due to CCR5 expression in A2.01.CD4.401 cellsa
| Vaccinia virus (PFU/cell) | MAb or rabbit IgG during fusion | Mean no. of syncytia ± SD (% reduction)
|
|
|---|---|---|---|
| IIIB | JR-FL | ||
| vSC8 (15) | 275 ± 45 | 0 | |
| vCCR5 (15) | 152 ± 8 (45) | 200 ± 26 | |
| Mouse IgG2a | 138 ± 38 (50) | 191 ± 10 (5) | |
| 5C7 (CCR5/N) | 162 ± 3 (42) | 193 ± 41 (4) | |
| 502 (CCR5/N) | 282 ± 49 (0) | 83 ± 24 (59) | |
| 2D7 (CCR5/EC2) | 293 ± 45 (0) | 31 ± 8 (85) | |
| 523 (CCR5/EC2) | 130 ± 9 (53) | 187 ± 11 (7) | |
| 531 (CCR5/EC2) | 132 ± 6 (52) | 197 ± 13 (2) | |
| 549 (CCR5/MD) | 263 ± 16 (4) | 186 ± 15 (7) | |
| Normal rabbit IgG | 144 ± 7 (48) | 185 ± 21 (7) | |
| R (CCR5/N) | 283 ± 6 (0) | 79 ± 2 (60) | |
| R (CCR5/EC1) | 155 ± 14 (44) | 198 ± 21 (1) | |
| R (CCR5/EC2) | 135 ± 17 (51) | 194 ± 4 (3) | |
| R (CCR5/EC3) | 141 ± 12 (49) | 202 ± 15 (0) | |
| R (STRL33/N) | 138 ± 1 (50) | 199 ± 2 (1) | |
A2.01.CD4.401 cells infected with vCCR5 were preincubated with a control antibody (mouse IgG2a or normal rabbit IgG), with an anti-CCR5 MAb (from the NIH AIDS Reagent Repository or R&D), or with rabbit (R) polyclonal IgG antibodies specific for different extracellular domains of CCR5 (or the N terminus of CCR5 or STRL33). After 1 h of incubation with antibodies (10 μg/ml) at 37°C, cells were mixed (at 1:1 ratio) with effector cells expressing IIIB/BH10 or JR-FL envelopes. Syncytia were scored after 2.5 h (IIIB) or 5 h (JR-FL). Percent reduction in syncytium formation compared to vSC8-infected cells (IIIB/BH10) or to vCCR5-infected cells (JR-FL; untreated) was calculated as for Table 1. Data represent four experiments.
FIG. 4.
Treatment of vCCR5-infected A2.01.401 cells with anti-CCR5 antibodies can increase their susceptibility to infection with a T-tropic HIV-1 strain. A2.01.401 cells were infected with recombinant vaccinia virus vSC8 or vCCR5 (20 PFU/cell) for 7 h. When indicated, cells were treated for 1 h with anti-CCR5 or control antibodies at 37°C. Treated and untreated cells were exposed to HIV-1 strain LAI (MOI of 0.02) for 1 h at 37°C, after which the virus and antibodies were washed away. DNA extraction and PCR analysis (gag primers) were conducted after 24 h as described in Material and Methods and in the legend to Fig. 2. The data represent one out of four experiments with similar results. NRIgG, normal rabbit IgG.
Overexpression of CXCR4 can reduce CCR5-dependent cell fusion with R5 envelope-expressing cells.
Thus far, it was found that a competition for association with CD4 molecules favors CCR5-CD4 association and results in an inhibition of CXCR4-dependent fusion. It was of interest to determine if this competition could be reversed by increasing the expression of CXCR4 in the same cells. To address this question, 3T3.CD4.401 (14) cells were coinfected with vCXCR4 or vCCR5 at different ratios. Since this CD4.401-transfected cell line expresses a higher level of CD4 than do A2.01.401 cells, no significant inhibition of fusion with either a T-tropic or M-tropic envelope was seen in cells infected with vCCR5 and vCXCR4 at a 1:1 ratio (each at 5 PFU/cell) (data not shown). However, coinfection with vCCR5 and vCXCR4 at a 1:4 ratio resulted in a reduced fusion capacity with target cells expressing the JR-FL, Ba-L, or ADA R5 envelope (Table 5 and data not shown). Under these conditions, the cells expressed 20 to 30% lower levels of surface CCR5 compared with cells infected only with vCCR5. However, the reduction in fusion ranged between 50 and 65% in three separate experiments. The role of surface CXCR4 in this inhibition was demonstrated by treatment with phorbol ester myristate acetate (PMA), which was shown to induce downmodulation of CXCR4 but not of CCR5 (15). While CD4 molecules are normally highly susceptible to PMA-induced downmodulation, the tailless CD4.401 molecules do not internalize following PMA treatment (1, 14). As shown in Table 5, PMA-treated cells lost 80% of their surface CXCR4 molecules, but no increase in surface CCR5 was observed. However, the PMA-treated cells fully recovered their ability to fuse with R5 envelopes (Table 5 and data not shown). These data suggest that under conditions of CXCR4 overexpression compared with CCR5, cells may be more predisposed to infection with T-tropic (X4-dependent) viral strains. In subsequent experiments, we tested a panel of commercially available CXCR4-specific MAbs, as well as our rabbit polyclonal anti-CXCR4 IgG. We found that the only antibodies that could inhibit X4-envelope fusion were 12G5 (30 to 40% inhibition) and rabbit anti-CXCR4 (N) IgG (45 to 55% inhibition). These antibodies could also reverse the reduction of fusion with R5 Env-expressing cells observed when 3T3.CD4.401 cells were coinfected with vCCR5 and vCXCR4 at a 1:4 ratio (data not shown).
TABLE 5.
Overexpression of CXCR4 can reduce CCR5-dependent cell fusion with R5 envelope-expressing cellsa
| Vaccinia virus (PFU/cell) | PMA | FACS (ΔMFC)
|
Mean no. of syncytia ± SD (% reduction)
|
|||
|---|---|---|---|---|---|---|
| CD4 | CXCR4 | CCR5 | IIIB | JR-FL | ||
| vSC8 (20) | − | 368 | 7 | 2 | 6 ± 2 | 8 ± 1 |
| vCCR5 (5) | − | 396 | 9 | 305 | 7 ± 1 | 476 ± 18 |
| vCCR5 (5)+ | − | 369 | 459 | 230 | 1,042 ± 21 | 207 ± 1 (56) |
| vCXCR4 (20) | ||||||
| vCCR5 (5)+ | + | 347 | 101 | 225 | 191 ± 4 | 531 ± 36 (0) |
| vCXCR4 (20) | ||||||
NIH.3T3.CD4.401 cells (11) were infected with vCCR5 and vCXCR4 (or with control virus [vSC8]) for 7 h. When indicated, cells were treated with the phorbol ester PMA at 100 ng/ml for the last 3 h before they were stained and used in a fusion assay with IIIB/BH10 or JR-FL envelope-expressing cells as described in the footnote to Table 1. Data represent three experiments.
Treatment of macrophages with anti-CCR5 antibodies or β-chemokines can enhance their fusion with X4 envelope-expressing cells.
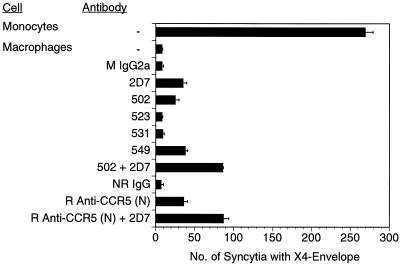
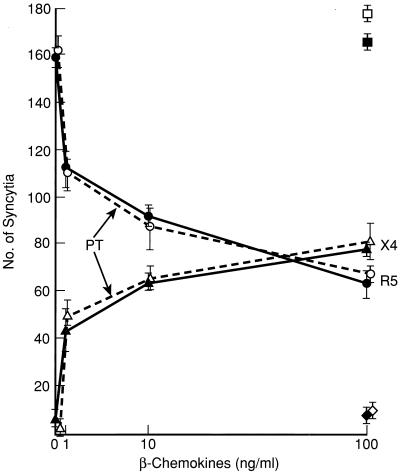
Based on the above findings in cell lines, It was important to determine if the coreceptor competition for association with CD4 is operative in vivo. To address this question, we chose to examine primary cells known to express multiple coreceptors and a relatively low number of surface CD4 molecules. It was previously shown by several laboratories that MDM preferentially fuse with M-tropic (R5) HIV envelopes and support infection with M-tropic better than T-tropic lab-adapted viral strains (4, 7, 8, 30). This restricted susceptibility could not be attributed simply to lack of CXCR4 expression on MDM (9, 23, 30, 38) and may, at least partially, be due to a decrease in “fusion-active” CXCR4 molecules on macrophages (23). In addition, this restricted tropism profile could also be explained by the competition phenomenon described above (9, 23). Thus, we treated MDM with the panel of anti-CCR5 MAbs and rabbit IgGs, alone or in combination, and tested their ability to fuse with cells expressing the IIIB envelope. As depicted in Fig. 5, elutriated monocytes generated high numbers of syncytia with TF228 (IIIB/BH10 Env) cells (269 ± 5 syncytia), while the MDM derived from them fused very poorly with the same cells (8 ± 1 syncytia). Treatment with MAb 2D7, 502, or 549 or with the rabbit anti-CCR5 (N) antibody increased the number of syncytia only modestly (25 to 35 syncytia per well). However, treatment of cells with a combination of antibodies against the EC2 (2D7) and N terminus (MAb 502 or rabbit polyclonal IgG) resulted in a more significant increase in fusion with IIIB Env-expressing cells (87 ± 7 syncytia). Since β-chemokine analogs are under development as therapeutic agents for HIV-1-infected individuals (33) it was important to determine whether treatment of MDM with CCR5-binding β-chemokines could result in augmented fusion with X4 envelopes. As depicted in Fig. 6, treatment of MDM with increasing concentrations of MIP-1α-, MIP-1β-, and RANTES (1 to 100 ng/ml) resulted in a dose-dependent reduction of R5-fusion (JR-FL) and in a gradual increase in the X4 (IIIB) syncytium formation. No such increase was seen in MCP-1 (or I309 [not shown])-treated MDM. The observed increase in X4 fusion could not be attributed to protein G signaling via the CCR5 receptor (20), since treatment of cells with PT did not prevent it (Fig. 6). Thus, one likely explanation for both findings is that binding of the β-chemokines (or anti-CCR5 antibodies) either reversed or prevented the association of CCR5 to surface CD4 molecules, which in turn allowed better CD4-CXCR4 association and improved the ability of cells to fuse with T-tropic envelopes. We still observed considerably fewer IIIB syncytia than with the elutriated monocytes of the same donor, suggesting that several mechanisms may be responsible for the reduced function of CXCR4 molecules in differentiated macrophages. We have previously shown that the predominant form of CXCR4 on the surface MDM (but not in monocytes) appears as high-molecular-weight species rather than as a monomer. These high-molecular-weight species do not associate with CD4 molecules, as determined by coimmunoprecipitation experiments (23). In contrast, CCR5 molecules were coprecipitated equally well with CD4 in monocytes and MDM. Thus, the functionality of CXCR4 molecules in primary cells such as monocytes/macrophages may be determined by multiple factors including posttranslational modifications, relative densities of other coreceptors, and a competition for association with surface CD4 molecules.
FIG. 5.
Anti-CCR5 antibody treatment can modestly increase the fusion efficiency of macrophages with X4 envelopes. Elutriated monocytes (Mon) and the macrophages derived from them after 5-day cultures (MDM) were untreated or treated with various CCR5-specific murine (mouse [M] or normal rabbit [NR]) MAbs (or isotype control antibody) or with rabbit (R) polyclonal anti-CCR5 IgG (or preimmune IgG) at 10 μg/ml for 1 h at 37°C; the cells were then mixed at 1:1 ratio with TF228 cells expressing IIIB/BH10 envelope in triplicates. Syncytia were scored after 18 h. Data represent one of four experiments.
FIG. 6.
Treatment of MDM with β-chemokines result in a PT-resistant reduction of R5 fusion and a parallel increase in X4 fusion. MDM were generated as described in the legend to Fig. 5. MDM were treated (open symbols) or untreated (closed symbols) with PT (1 μg/ml) at 37°C for 1 h and washed twice. Treated and untreated cells were incubated with a combination of MIP-1α, MIP-1β, and RANTES at increasing concentrations (1, 10, and 100 ng/ml) for 1 h at 37°C. All groups were then mixed at 1:1 ratio with either 12E1/vCB28 (JR-FL envelope) (○, ●) or TF228 cells expressing IIIB/BH10 envelope (▴, ▵). Control cells were treated with MCP-1 at 100 ng/ml and were mixed with 12E1/vCB28 (■, □) or TF228 cells (⧫, ◊). Syncytia were scored after 18 h. The experiment was repeated three times.
DISCUSSION
This study was designed to test the hypothesis that the efficiency of HIV-1–cell fusion is influenced not only by the presence of CD4 and the appropriate coreceptor (CXCR4 or CCR5) but also by the levels of other coreceptors expressed by the same target cells. Therefore, in the setting of limited surface CD4, the presence of both CCR5 and CXCR4 at given concentrations will yield fewer successful viral interactions than in the presence of a single chemokine receptor at the same concentration, using viruses (or target cells expressing viral envelopes) of the appropriate tropism. This is an important issue to address since most of the target cells for HIV-1 infections in vivo express multiple coreceptors and may be subjected to in vivo factors that differentially affect the levels of individual coreceptors. Among the cellular targets for HIV-1 at the mucosal surfaces are dendritic cells and macrophages, both of which cell types express relatively low levels of surface CD4 (25). In previous studies, we and others provided evidence that CD4, the primary receptor of HIV-1, may have a natural affinity for the chemokine receptors CCR5 and CXCR4 (9, 23, 32, 35, 36). Thus, the susceptibility of cells with limited CD4 to infection with viral strains of different tropism may be influenced not only by the density of the appropriate coreceptors but also by a competition for association with CD4 either before or after exposure to viral envelopes. In this study, we tested this hypothesis by introducing CCR5 into CD4+ CXCR4+ cells and measuring their ability to fuse with X4 and R5 envelopes and their susceptibility to infection with T-tropic HIV-1 strains. We also coinfected NIH 3T3.CD4.401 cells with recombinant vaccinia viruses expressing CCR5 or CXCR4 at different ratios.
The principal findings in this current study were as follows. (i) Infection of CD4+ CXCR4+ T cells with recombinant vCCR5 resulted in a significant reduction in their fusion with effector cells expressing X4 envelopes. Consistent with these findings, a similar reduction was seen in their susceptibility to infection with the T-tropic strains LAI and NL4-3 (Table 1; Fig. 2). (ii) The reduction in X4-dependent fusion and infection was inversely correlated with the density of surface CD4 and positively correlated with the levels of CCR5 surface expression (Tables 1 to 3; Fig. 1 and 2; data not shown). (iii) CCR5 surface expression played a direct role in the reduction of CXCR4-dependent fusion, since several antibodies against the N terminus and second extracellular loop of CCR5 (EC2) reversed this inhibition. (iv) The inhibitory effects of CCR5 correlated with a competition for association with CD4, since coimmunoprecipitation of CD4 and CXCR4 molecules was reduced two- to threefold in vCCR5-infected cells. Furthermore, the inhibition was reversed by increasing the levels of surface CD4 (Table 3). (v) Overexpression of CXCR4 compared with CCR5 resulted in reduced fusion with R5 Env-expressing cells. (vi) Treatment of macrophages with a combination of anti-CCR5 antibodies (N plus EC2) or with a mixture of CCR5-binding β-chemokines resulted in augmented fusion with X4 Env-expressing cells.
Platt et al. (29) have shown that the concentrations of CD4 and CCR5 required for efficient infection of HeLa cells by M-tropic HIV-1 are interdependent, and the requirement for each is increased when the other component is present in a limiting amount. Our data suggest that this interdependence is further complicated by the presence of other coreceptors in the same cells. Several mechanisms may explain the observed competition. (i) Overexpression of one chemokine receptor may result in a reduction in the expression of other coreceptors (i.e., transport interference). However, in most of the experiments described in this study, expression of CCR5 in CD4+ CXCR4+ T cells reduced their X4 fusion without significant alterations in their CXCR4 surface expression. (ii) According to the second model, the fusion process is a multistep event that requires recruitment first of CD4 and then of the appropriate coreceptor into a stable trimolecular complex. Unlike the high-affinity binding between envelope and CD4, the binding of envelope to the coreceptors is of much lower affinity. The additional interactions between the coreceptors and CD4 within the same complexes may further stabilize them. As shown in this study, the recruitment of the appropriate coreceptor into the fusion complex may be negatively affected by the presence (and density) of other coreceptors on the cell membrane. According to this model, agents that can bind specifically to relevant regions of the wrong coreceptor could indirectly promote recruitment of the appropriate coreceptor into the trimolecular complex. Such a mechanism is at least partially supported by our finding that several antibodies against CCR5 (or CXCR4) N terminus and EC2 could reverse the fusion inhibition observed in our experimental system. It is also supported by a recent study (10) in which the half-life of CD4-Env binding was calculated to be <1 min, while the half-life for coreceptor recruitment was 5.8 min (for JR-FL envelope). (iii) According to the third model, CCR5 and CXCR4 may have a tendency to constitutively associate with CD4 molecules in some cells. In cells with limited CD4 molecules, the ratio of preexisting CD4-CCR5 to CD4-CXCR4 complexes may contribute to the susceptibility profile of the cells. In this scenario, changes in the relative densities of the coreceptors could have a major impact due to competition for association with a limited number of CD4 molecules. Two independent studies from our laboratories provided evidence that CD4-coreceptor complexes can be coimmunoprecipitated from monocytes and macrophages by using either an anti-CD4 (OKT4 MAb) or anticoreceptor antibody as the precipitating agent (9, 23, 36). This spontaneous association may be stronger for CCR5-CD4 than for CXCR4-CD4, although no direct measurements of their association rates were attempted in this or previous studies. Furthermore, in this study, we demonstrated that treatment of macrophages with combinations of anti-CCR5 antibodies or with β-chemokines resulted in a dose-dependent reduction in R5 fusion and an increase in R4 fusion. Importantly, the increase in X4 fusion was not sensitive to treatment of cells with the G-protein inhibitor PT. Thus, the observed effect was not due to signaling and/or cell activation as previously suggested by Kinter et al., based on experiments with peripheral blood mononuclear cells (20). In a separate set of experiments, it was found that treatment of macrophages with a combination of anti-CCR5 antibodies (2D7 and 3C3) plus staphylococcal protein G, and with RANTES, resulted in a significant increase (78%) in their fusion with LAV Env-expressing cells (T. Stanchev and C. Broder, unpublished data).
Both mechanisms may be operational in vivo to various degrees in different cell types. We and others have demonstrated constitutive and gp120-induced association between CD4 and coreceptors (9, 23, 32, 36). Such mechanisms may also shed some light on the recent findings by Yi et al. that the dualtropic viruses 89.6 and DH12 utilize primarily CCR5 during infection of normal macrophages but use only CXCR4 during infection of CCR5neg macrophages (37). It is conceivable that the preferential use of CCR5 in normal macrophages reflects the higher density of preexisting CD4-CCR5 complexes in these cells (23). Competition for association with CD4 may be a contributing factor to the efficiency of viral cell entry and cell-to-cell transmission in vivo, since most HIV-1-susceptible cells express multiple chemokine receptors. Furthermore, several important target cells of HIV-1, such as macrophages and dendritic cells, express relatively low concentrations of surface CD4 molecules (26).
Coreceptor competition may also explain earlier studies with human thymocytes in which preferential infection of double-positive thymocytes with T-tropic viruses was observed (3, 21, 28, 33), despite expression of low-level CCR5 in both double-positive and single-positive thymocyte subpopulations as recently demonstrated (39). Significantly higher expression of CXCR4 compared with CCR5 was found on immature double-positive thymocytes (but not on the mature single-positive thymocytes) (21, 39). Based on the findings in the present study with 3T3.CD4.401 cells (Table 5), high surface CXCR4/CCR5 ratios may result in the inhibition of CCR5-mediated cell fusion with M-tropic envelope-expressing cells.
In addition to the absolute numbers of coreceptor molecules, posttranslational modifications may affect their ability to form complexes with CD4 and/or HIV-1 envelope, resulting in a reduced or enhanced fusion potential. In addition to previously published studies (11, 23), it was recently found that CXCR4 with removed N-linked glycosylation sites can function as a coreceptor for R5 HIV-1 envelope glycoproteins (D. J. Chabot, X. Xiao, D. S. Dimitrov, and C. C. Broder, submitted for publication).
Coreceptor competition in vivo may also be affected by the cytokine milieu in the tissues and mucosal surfaces. Several cytokines were shown to upregulate or downmodulate CD4 and coreceptor surface expression in various cell types (16, 26, 40). Thus, changes in the cytokine milieu due to infections or vaccinations, and high local production of β-chemokines, may result in either increase or decrease in the susceptibility of target cells to infection with X4 and R5 viruses. Recent findings in our laboratory showed that macrophages treated with several proinflammatory cytokines are more susceptible to infection with X4 viruses while demonstrating increased resistance to R5 infection. Both phenomena could not be attributed to changes in surface expression of CCR5 or CXCR4 but correlated better with β-chemokine production (M. Zaitseva and S. Lee, submitted for publication).
Together, these data contribute to understanding the possible effects of in vivo alterations in CD4 and coreceptor densities (or coreceptor availability) on HIV-1 cell entry and disease progression. Furthermore, the development of β-chemokine analogs as antiviral agents (28, 32, 35) should take into account their potential to alter coreceptor-CD4 interactions and to alter the susceptibility of target cells to infection with viruses of different coreceptor usage (2, 27, 31).
ACKNOWLEDGMENTS
Shirley Lee and Cheryl K. Lapham made equal contributions to this study.
We thank Zdenka Jonak for providing the TF228 cell line; Keith Peden for providing viral stocks; and Keith Peden, Marina Zaitseva, and Joshua Farber for critical reviews of the manuscript.
This work was supported in part by a grant from the NIH Intramural AIDS Targeted Antiviral Program (H.G.), by a grant from the Office of Women's Health, FDA (H.G.), and by NIH grant RO1AI43885 (C.C.B.).
REFERENCES
- 1.Bedinger P, Moriarty A, von Borstel II R C, Donovan N J, Steimer K S, Littman D R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 4.Broder C C, Berger E A. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broder C C, Dimitrov D S. HIV and the 7-transmembrane domain receptors. Pathobiology. 1996;64:171–179. doi: 10.1159/000164032. [DOI] [PubMed] [Google Scholar]
- 6.Broder C C, Dimitrov D S, Blumenthal R, Berger E A. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s) Virology. 1993;193:483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- 7.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Quiroga M, Tung J W, Dina D, Levy J A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990;64:4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitrov D S, Norwood D, Stantchev T S, Feng Y, Xiao X, Broder C C. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology. 1999;259:1–6. doi: 10.1006/viro.1999.9747. [DOI] [PubMed] [Google Scholar]
- 10.Doranz B J, Baik S W, Doms R W. Use of a gp120 binding assay to dissect the requirements and kinetics of human immunodeficiency virus fusion events. J Virol. 1999;73:10346–10358. doi: 10.1128/jvi.73.12.10346-10358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farzan M, Mirzabekov T, Kolchinksy P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13.Golding H, Dimitrov D S, Manischewitz J, Broder C C, Robinson J, Fabian S, Littman D R, Lapham C K. Phorbol ester-induced down modulation of tailless CD4 receptors requires prior binding of gp120 and suggests a role for accessory molecules. J Virol. 1995;69:6140–6148. doi: 10.1128/jvi.69.10.6140-6148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golding H, Manischewitz J, Vujcic L, Blumenthal R, Dimitrov D S. The phorbol ester phorbol myristate acetate inhibits human immunodeficiency virus type 1 envelope-mediated fusion by modulating an accessory component(s) in CD4-expressing cells. J Virol. 1994;68:1962–1969. doi: 10.1128/jvi.68.3.1962-1969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golding H, Ouyang J, Zaitseva M, Broder C C, Dimitrov D S, Lapham C. Increased association of glycoprotein 120-CD4 with HIV type 1 coreceptors in the presence of complex-enhanced anti-CD4 monoclonal antibodies. AIDS Res Hum Retroviruses. 1999;15:149–159. doi: 10.1089/088922299311574. [DOI] [PubMed] [Google Scholar]
- 16.Hariharan D, Douglas S D, Lee B, Lai J P, Campbell D E, Ho W Z. Interferon-gamma upregulates CCR5 expression in cord and adult blood mononuclear phagocytes. Blood. 1999;93:1137–1144. [PubMed] [Google Scholar]
- 17.Hill C M, Kwon D, Jones M, Davis C B, Marmon S, Daugherty B L, DeMartino J A, Springer M S, Unutmaz D, Littman D R. The amino terminus of human CCR5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology. 1998;248:357–371. doi: 10.1006/viro.1998.9283. [DOI] [PubMed] [Google Scholar]
- 18.Hillman K, Shapira-Nahor O, Gruber M F, Hooley J, Manischewitz J, Seeman R, Vujcic L, Geyer S J, Golding H. Chemically induced CD4 mutants of a human T cell line. Evidence for dissociation between binding of HIV I envelope and susceptibility to HIV I infection and syncytia formation. J Immunol. 1990;144:2131–2139. [PubMed] [Google Scholar]
- 19.Jonak Z L, Clark R K, Matour D, Trulli S, Craig R, Henri E, Lee E V, Greig R, Debouck C. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res Hum Retroviruses. 1993;9:23–32. doi: 10.1089/aid.1993.9.23. [DOI] [PubMed] [Google Scholar]
- 20.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, Jackson R, Ostrowski M, Hoxie J, Offord R, Fauci A S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4(+) T cells: role of signal transduction. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 23.Lapham C K, Zaitseva M B, Lee S, Romanstseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Sharron M, Montaner L J, Weissman D, Doms R W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriuchi M, Moriuchi H, Turner W, Fauci A S. Exposure to bacterial products renders macrophages highly susceptible to T-tropic HIV-1. J Clin Investig. 1998;102:1540–1550. doi: 10.1172/JCI4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosier D E, Picchio G R, Gulizia R J, Sabbe R, Poignard P, Picard L, Offord R E, Thompson D A, Wilken J. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 32.Ugolini S, Moulard M, Mondor I, Barois N, Demandolx D, Hoxie J, Brelot A, Alizon M, Davoust J, Sattentau Q J. HIV-1 gp120 induces an association between CD4 and the chemokine receptor CXCR4. J Immunol. 1997;159:3000–3008. [PubMed] [Google Scholar]
- 33.Uittenbogaart C H, Anisman D J, Jamieson B D, Kitchen S, Schmid I, Zack J A, Hays E F. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS. 1996;10:F9–F16. doi: 10.1097/00002030-199606001-00001. [DOI] [PubMed] [Google Scholar]
- 34.Vila-Coro A J, Mellado M, Martin de Ana A, Martinez A C, Rodriguez-Frade J M. Characterization of RANTES- and aminooxypentane-RANTES-triggered desensitization signals reveals differences in recruitment of the G protein-coupled receptor complex. J Immunol. 1999;163:3037–3044. [PubMed] [Google Scholar]
- 35.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 36.Xiao X, Wu L, Stantchev T S, Feng Y R, Ugolini S, Chen H, Shen Z, Riley J L, Broder C C, Sattentau Q J, Dimitrov D S. Constitutive cell surface association between CD4 and CCR5. Proc Natl Acad Sci USA. 1999;96:7496–7501. doi: 10.1073/pnas.96.13.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi Y, Isaacs S N, Williams D A, Frank I, Schols D, De Clercq E, Kolson D L, Collman R G. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J Virol. 1999;73:7117–7125. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 39.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 40.Zoeteweij J P, Golding H, Mostowski H, Blauvelt A. Cytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J Immunol. 1998;161:3219–3223. [PubMed] [Google Scholar]