Abstract
SCARECROW-LIKE6 (SCL6) plays a role in the formation and maintenance of the meristem. In Larix kaempferi (Lamb.) Carr., an important afforestation tree species in China, SCL6 (LaSCL6) has two alternative splicing variants—LaSCL6-var1 and LaSCL6-var2—which are regulated by microRNA171. However, their roles are still unclear. In this study, LaSCL6-var1 and LaSCL6-var2 were transformed into the Arabidopsis thaliana (L.) Heynh. genome, and the phenotypic characteristics of transgenic A. thaliana, including the germination percentage, root length, bolting time, flower and silique formation times, inflorescence axis length, and branch and silique numbers, were analyzed to reveal their functions. It was found that LaSCL6-var1 and LaSCL6-var2 overexpression shortened the root length by 41% and 31%, respectively, and increased the inflorescence axis length. Compared with the wild type, the bolting time in transgenic plants was delayed by approximately 2–3 days, the first flower and silique formation times were delayed by approximately 3–4 days, and the last flower and silique formation times were delayed by about 5 days. Overall, the life cycle in transgenic plants was prolonged by approximately 5 days. These results show that LaSCL6 overexpression inhibited the transitions from the vegetative meristem to inflorescence meristem and from the flower meristem to meristem arrest in A. thaliana, revealing the roles of LaSCL6-var1 and LaSCL6-var2 in the fate transition and maintenance of the meristem.
Keywords: HAM, Larix, life cycle, longevity, miR171, time
1. Introduction
Meristem cells can divide continuously to produce new cells. The cells of the meristem form other types of tissue through division, growth, and differentiation, which are directly related to the growth and development of plants [1,2,3]. Many studies have shown that GRAS (GAI-RGA-SCR) transcription factors are involved in the growth and development of plants by regulating meristem activity [1,4,5,6]; for example, they control the indeterminacy and proliferation of shoot apical meristems and the de novo formation of axillary meristems [1,6,7,8,9].
SCARECROW-LIKE6 (SCL6) belongs to the GRAS family [5,10]. The loss of function of SCL6 in Petunia hybrida leads to the early termination of shoot apical meristems, arrested axillary shoot development, and a reduced number of carpels and stamens [11]. In addition, the ham1 ham2 ham3 triple mutant leads to delayed inflorescence initiation, the early termination of shoot meristems, a disorganized meristem structure and morphology, and reduced axillary shoot branches [3,4,7,12]. These studies indicate that SCL6 regulates meristem activity.
SCL6 can be negatively regulated by microRNA171 (miR171) [12]. miR171 specifically recognizes and binds to the SCL6 mRNA to mediate its cleavage [13,14]. The miR171-SCL6 module participates in various developmental and physiological processes, including shoot branching [7,12,15,16], phase transition [1,17], root growth [12,18], inflorescence axis elongation [17,19,20,21], trichome initiation [22], silique production [20,21,23], and meristem development [9,18,24,25,26]. These findings indicate that the miR171-SCL6 module participates in the activity and fate transition of the meristem.
Larix kaempferi (Lamb.) Carr. is an important coniferous timber tree species in China. L. kaempferi SCL6 (LaSCL6) has two alternative splicing variants, LaSCL6-var1 and LaSCL6-var2, both of which can be regulated by miR171 [27,28,29]. However, their roles are still unclear. The objective of this study is to explore their roles in life cycle progression based on the meristem state. We constructed overexpression vectors of LaSCL6-var1 and LaSCL6-var2 and transformed them into the A. thaliana genome; by analyzing the phenotypes of transgenic A. thaliana with respect to life cycle progression, their functions were explored. This study aimed to provide additional functional information on SCL6.
2. Results
2.1. Successful Transformation of LaSCL6 into A. thaliana Genome
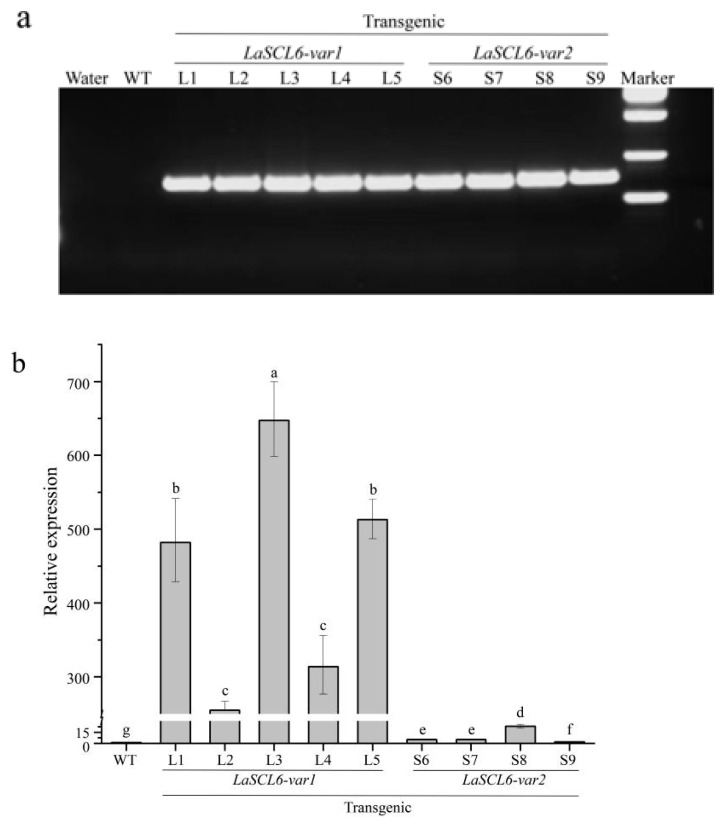
Five LaSCL6-var1 (L1, L2, L3, L4, and L5) and four LaSCL6-var2 (S6, S7, S8, and S9) overexpressing lines were randomly selected for the experiments. To verify the insertion of LaSCL6 into the A. thaliana genome, polymerase chain reaction (PCR) amplification was performed with LaSCL6-specific primers and with the A. thaliana DNA as a template. The results showed that the amplified fragments of LaSCL6 were detected in the transformed A. thaliana but not in the wild-type A. thaliana (Figure 1a). With the A. thaliana cDNA as a template, quantitative reverse transcription PCR (qRT-PCR) was performed to detect LaSCL6 expression. The results showed that it was expressed in all transformed plants at different levels, but not in the wild-type A. thaliana (Figure 1b). These results indicate that LaSCL6 was successfully integrated into the genome of A. thaliana and expressed.
Figure 1.
Verification of transgenic Arabidopsis thaliana. (a) PCR amplification of LaSCL6 from wild-type (WT) and transgenic genomic DNA. (b) Relative expression levels of LaSCL6 measured via qRT-PCR with AtUBQ1 as internal control. Error bars represent standard deviations of three replicates. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters.
2.2. LaSCL6 Overexpression Inhibits Root Elongation in A. thaliana
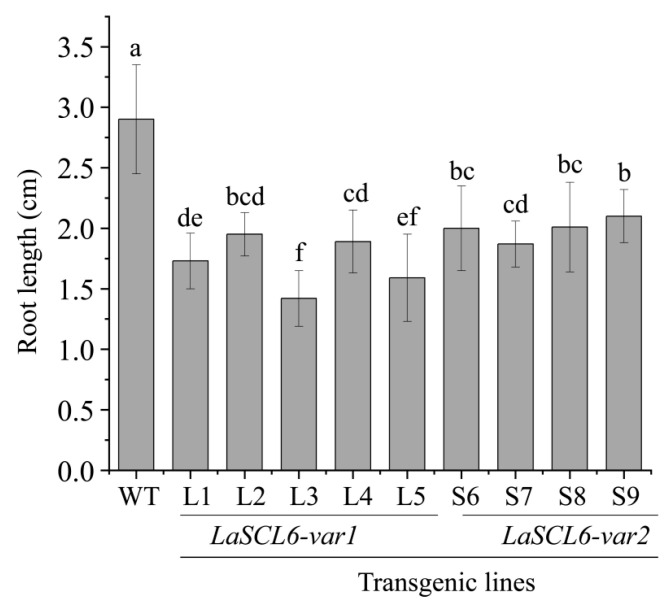
After measuring the root length of A. thaliana, we found that LaSCL6 overexpression inhibited root elongation because the root length in the transgenic A. thaliana was shorter than that in the wild-type A. thaliana (Figure 2). It was 2.90 cm (mean ± SD, 2.90 ± 0.45) in the wild-type A. thaliana, while it was 1.10–2.30 cm (mean ± SD, 1.71 ± 0.31; shortened by 41%) and 1.30–2.80 cm (mean ± SD, 1.99 ± 0.30; shortened by 31%) in the LaSCL6-var1- and LaSCL6-var2-overexpressing A. thaliana, respectively (Figure 2).
Figure 2.
Root lengths of wild-type (WT) and transgenic Arabidopsis thaliana. The root length was measured after the plants were transferred into the incubator for 8 days. For each line, twenty plants were counted. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters.
2.3. LaSCL6 Overexpression Has Almost No Influence on Reactivation of Dormant Meristem
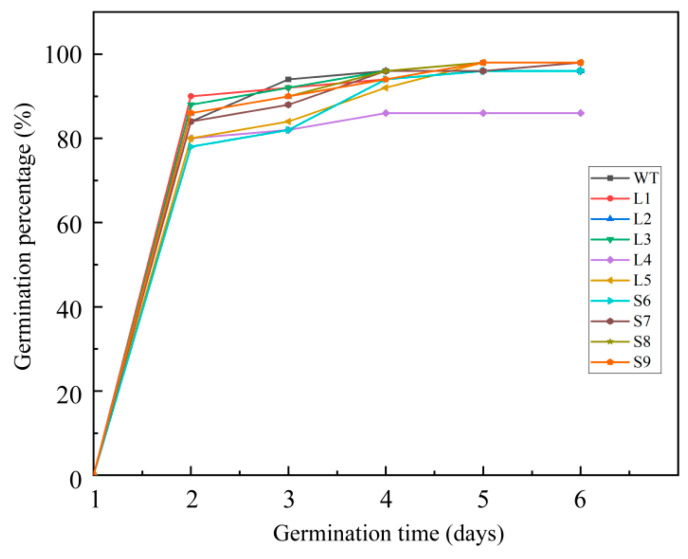
After 4 °C treatment, 78–90% of the transgenic seeds had germinated on the second day, while 84% of the wild-type seeds had germinated (Figure 3). On the fourth, fifth, and sixth days, the germination percentage of the seeds was more than 92% in both the wild-type and transgenic A. thaliana lines, with the exception of L4 (Figure 3). These data suggest that LaSCL6 overexpression does not affect the meristem’s reactivation from dormancy.
Figure 3.
Germination percentages of wild-type (WT) and transgenic Arabidopsis thaliana. LaSCL6-var1-overexpressing A. thaliana lines: L1, L2, L3, L4, L5. LaSCL6-var2-overexpressing A. thaliana lines: S6, S7, S8, S9. Fifty seeds were used in each line.
2.4. LaSCL6 Overexpression Prolongs Juvenile Period in A. thaliana
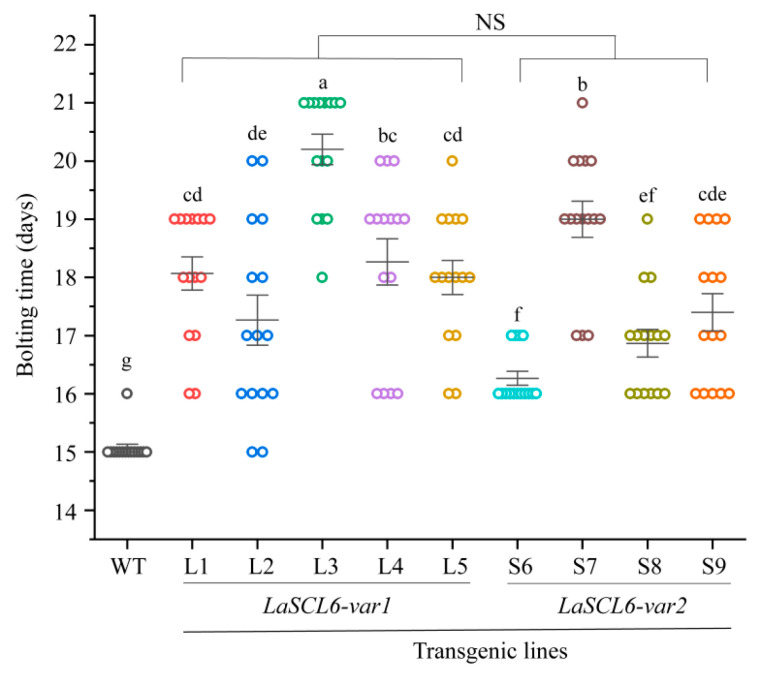
After determining the bolting time of A. thaliana, we found that it was delayed by LaSCL6 overexpression. For the wild type, ~15 days were needed to bolt after being transferred into the soil, while, for the transgenic A. thaliana, 15–21 days were needed (mean ± SD, 17.93 ± 1.60) (Figure 4). In addition, there was no difference in bolting time between the two types of transgenic A. thaliana. For the LaSCL6-var1-overexpressing A. thaliana, 15–21 days (mean ± SD, 18.36 ± 1.62) were needed to bolt; for the LaSCL6-var2-overexpressing A. thaliana, 16–21 days (mean ± SD, 17.38 ± 1.42) were needed. In conclusion, the transgenic A. thaliana bolted later than the wild-type A. thaliana, indicating that the transition from the vegetative meristem to inflorescence was delayed by LaSCL6 overexpression.
Figure 4.
Bolting times of wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters. Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups, p ≤ 0.05, and NS indicating no significant difference.
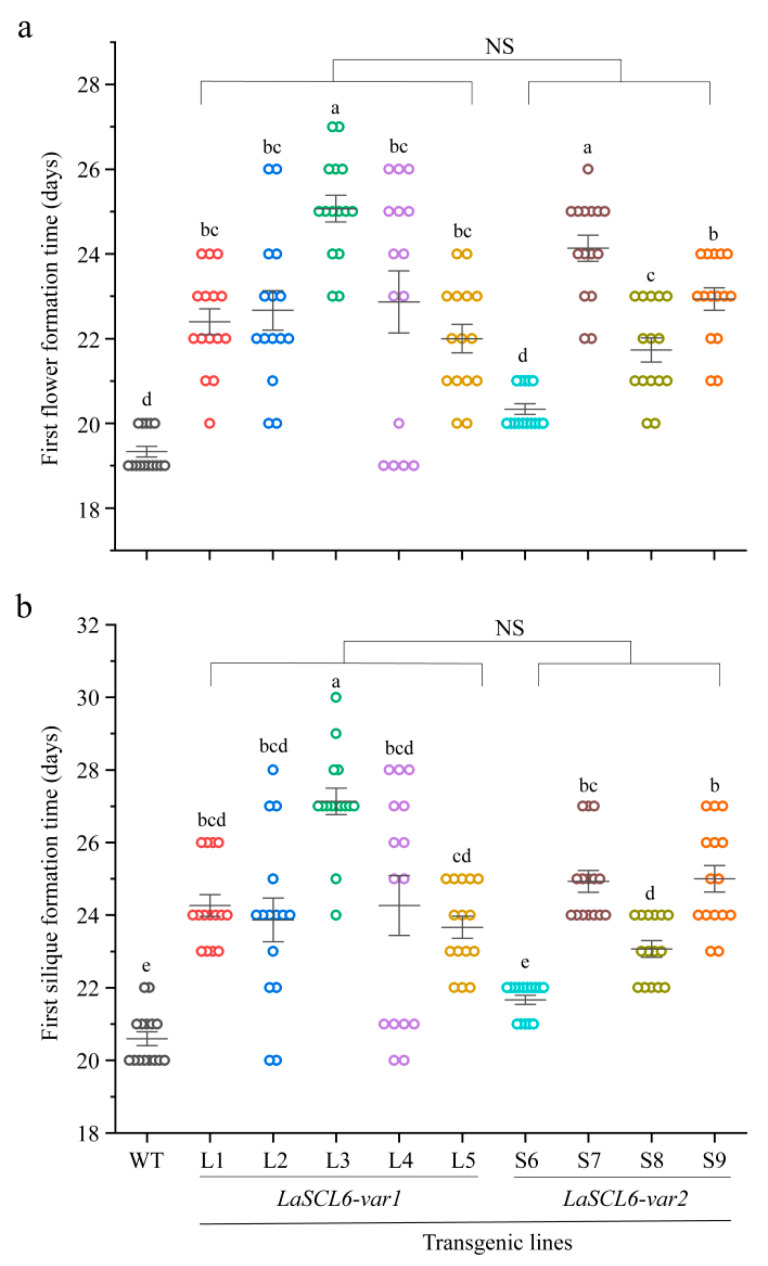
The transgenic A. thaliana started flowering later than the wild-type A. thaliana, because the transgenic A. thaliana required 19–27 days (mean ± SD, 22.68 ± 1.93) to produce the first flower, while the wild-type A. thaliana required ~19 days (Figure 5a). The transgenic A. thaliana started fruiting later than the wild-type A. thaliana because the transgenic A. thaliana required 20–30 days (mean ± SD, 24.21 ± 2.14) to produce the first silique, while the wild-type A. thaliana required ~21 days (Figure 5b). However, there was no difference between the two types of transgenic A. thaliana. In conclusion, the transgenic A. thaliana started flowering later than the wild-type A. thaliana, indicating that the transition from the inflorescence meristem to the flower meristem was delayed by LaSCL6 overexpression.
Figure 5.
Formation times for the first flower (a) and silique (b) in wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters. Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups, p ≤ 0.05, and NS indicating no significant difference.
2.5. LaSCL6 Overexpression Delays Global Proliferative Arrest (GPA) in A. thaliana
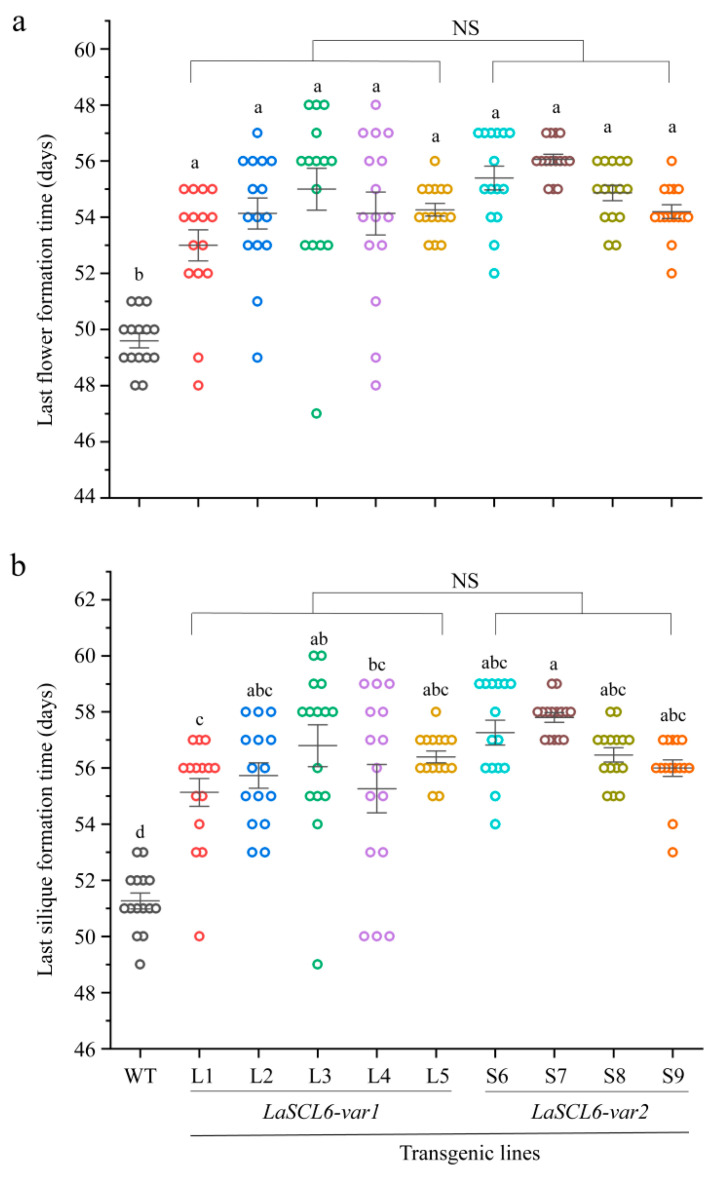
After calculating the formation time for the last flower of A. thaliana, we found that the last flower formed later in the transgenic A. thaliana than the wild-type A. thaliana, because the transgenic A. thaliana required 47–58 days (mean ± SD, 54.44 ± 1.88) to produce the last flower, while the wild-type A. thaliana required ~50 days (Figure 6a). We counted the number of siliques each day until it became stable. We found that it stopped increasing in the transgenic A. thaliana at 49–60 days (mean ± SD, 56.13 ± 2.01) and in the wild-type A. thaliana at ~51 days (Figure 6b). However, there was no difference between the two types of transgenic A. thaliana. These data indicate that GPA occurred later in the transgenic A. thaliana, and it was delayed by LaSCL6 overexpression. In addition, there was almost no difference in the timing of the formation of the last flower or silique between the two types of transgenic A. thaliana.
Figure 6.
Formation times for the last flower (a) and silique (b) in wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters. Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups, p ≤ 0.05, and NS indicating no significant difference.
2.6. LaSCL6 Overexpression Increases Inflorescence Axis Length of A. thaliana
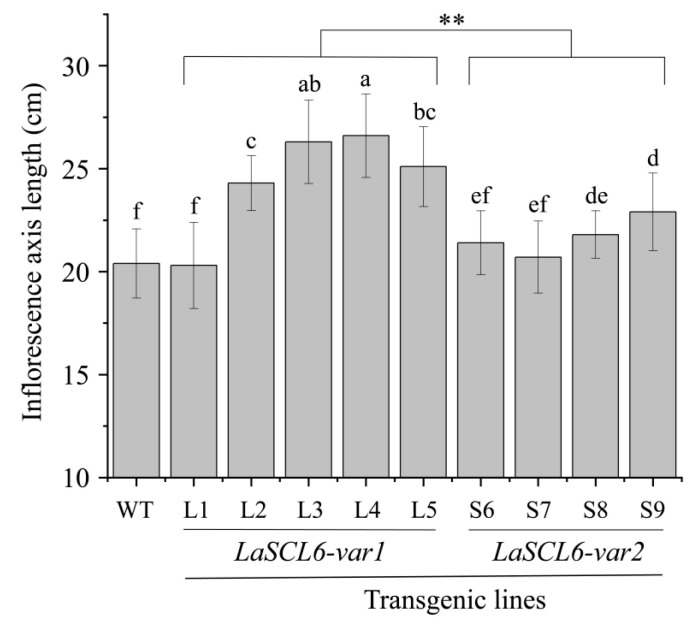
After measuring the length of the inflorescence axis of A. thaliana, we found that LaSCL6 overexpression promoted the elongation of the inflorescence axis because it was longer in the transgenic A. thaliana than in the wild-type A. thaliana (Figure 7). It was 17–29 cm (mean ± SD, 24.50 ± 2.92) and 19–26 cm (mean ± SD, 21.70 ± 1.75) in the LaSCL6-var1- and LaSCL6-var2-overexpressing A. thaliana, respectively, while it was 18–22 cm (mean ± SD, 20.40 ± 1.68) in the wild-type A. thaliana (Figure 7). In addition, it was longer in the LaSCL6-var1-overexpressing A. thaliana than in the LaSCL6-var2-overexpressing A. thaliana (Figure 7). In conclusion, the transgenic A. thaliana had a longer inflorescence axis.
Figure 7.
Statistical diagram of inflorescence axis lengths of wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters. Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups, p ≤ 0.01, and ** was used.
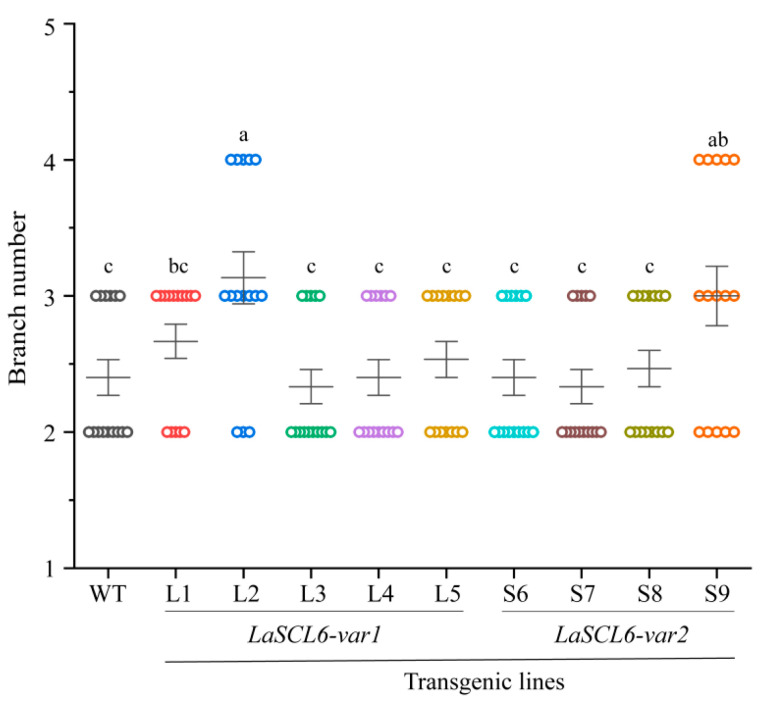
Regarding the branch number in A. thaliana, we found that it was two to three in both the wild-type and transgenic A. thaliana, with the exception of some L2 plants (Figure 8), indicating that LaSCL6 overexpression had almost no influence on the occurrence and activity of the axillary meristem.
Figure 8.
Branch numbers of wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters.
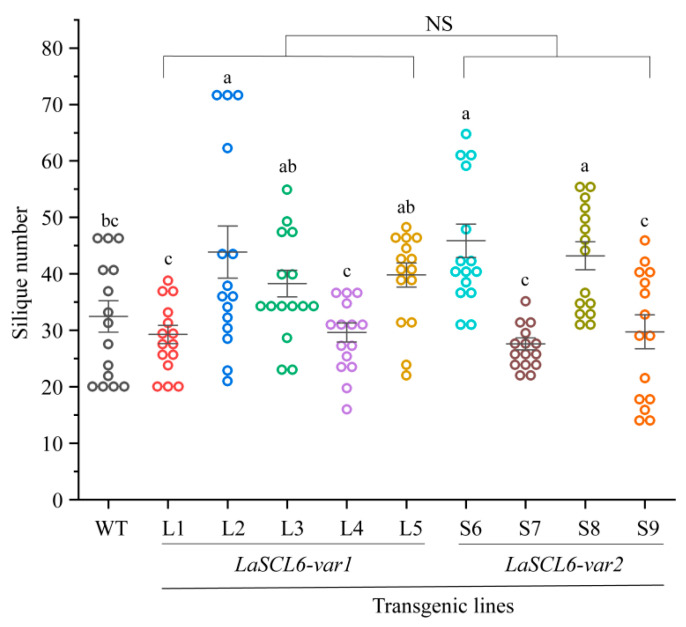
2.7. LaSCL6 Overexpression Influences Silique Yield of A. thaliana
After determining the fruit number of A. thaliana, we found that LaSCL6 overexpression had varying influences on fruit production in each transgenic line. It was ~32 in the wild-type A. thaliana, while it was 16–73 (mean ± SD, 36.16 ± 11.78) and 14–66 (mean ± SD, 36.60 ± 12.44) in the LaSCL6-var1- and LaSCL6-var2-overexpressing A. thaliana, respectively (Figure 9). However, there was no difference between the two types of transgenic A. thaliana. These results indicate that LaSCL6 overexpression affected the silique yield of A. thaliana.
Figure 9.
The number of siliques in wild-type (WT) and transgenic Arabidopsis thaliana. The differences between each line were analyzed using LSD, p ≤ 0.05, indicated by lowercase letters. Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups, p ≤ 0.05, and NS indicating no significant difference.
3. Discussion
The meristem is an indispensable part of a plant that determines its morphology and function. The root meristem can continuously divide to produce new root cells, thereby promoting the growth of the roots. Many studies have shown that SCL6 regulates the growth of roots [7,12,18]. Transgenic A. thaliana plants with miR171c overexpression and the scl6-II scl6-III scl6-IV triple mutant exhibited reduced root lengths [12]. The root lengths of SCL6-overexpressing and STTM171-silenced plants become longer, and the number of roots increased, while the root length of miR171-overexpressing Lilium pumilum DC. Fisch became shorter and the number of roots decreased [18]. However, the root length of SlGRAS24-overexpressing Solanum lycopersicum L. cv. Micro-Tom was obviously shortened [20]. In this study, the root lengths of two types of LaSCL6-overexpressing A. thaliana were shorter, showing that LaSCL6 has a similar role to SlGRAS24. These data indicate that SCL6 plays a role in the growth of plant roots via different regulatory mechanisms, and further research is needed to explore these differences.
Previous studies have shown that the miR171-SCL6 module affects the plant height [17,20,21]. For example, miR171b-overexpressing rice was taller [21], which was consistent with the results obtained for S. lycopersicum [20]. Meanwhile, the height of barley became shorter after miRNA171 overexpression [17]. In this study, LaSCL6 overexpression promoted inflorescence axis elongation, which was consistent with the result obtained for barley. In addition, the inflorescence axis was longer in LaSCL6-var1-overexpressing A. thaliana than in LaSCL6-var2-overexpressing A. thaliana.
The miR171-SCL6 module affects shoot branching [7,12,15,30]. For example, miR171a- or miR171c-overexpressing plants had a reduced shoot branch number [12,15]. In scl6-II scl6-III scl6-IV triple mutant A. thaliana, there was a significant decrease in the branch number. In addition, Petunia HAM mutant plants had no lateral organs, and a ring mark structure was formed in the missing organs [7]. These studies indicate that SCL6 plays a positive role in the regulation of shoot branching by controlling the meristem’s activity in the lateral bud. However, in this study, there was almost no change in the branch number of A. thaliana after LaSCL6 overexpression (Figure 8). Notably, after LaSCL6-var2 (LkHAM) was overexpressed in the A. thaliana ham123 mutant, normal branches were able to initiate from the cauline leaves of transgenic A. thaliana [31]. These data add complexity to the study of the functional mechanism of LaSCL6 in shoot branching.
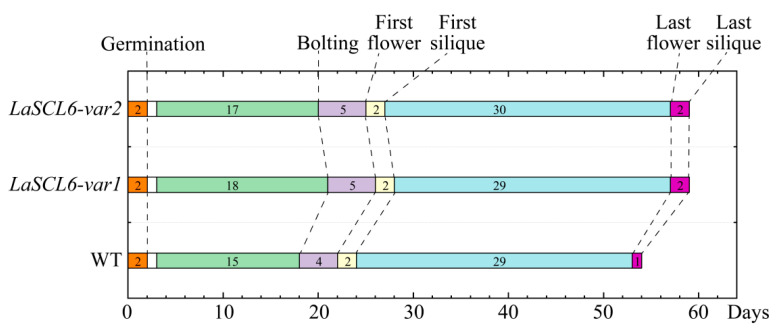
The miR171-SCL6 module also has an effect on the timing of the plant phase transition [17,20]. The first flower of Sly-miR171-overexpressing plants formed late [20]. In barley, miR171 overexpression altered the vegetative to reproductive phase transition by activating the miR156 pathway and repressing the expression of the THIRD OUTER GLUME and Hordeum vulgare L. cv. Golden promise Plastochron1 genes [17]. In this study, the bolting time and the formation time of the flower (the first and the last) and silique (the first and the last) of LaSCL6-overexpressing A. thaliana were later than those of the wild-type A. thaliana (Figure 4, Figure 5 and Figure 6). After determining the timing of these life cycle events in A. thaliana, we found that the juvenile period in LaSCL6-overexpressing plants was longer, and the bolting time in LaSCL6-var1 and LaSCL6-var2 was 3 and 2 days later than that in the wild-type A. thaliana, respectively (Figure 10). The time from bolting to the first flower formation was 1 day later in the LaSCL6-overexpressing A. thaliana than in the wild-type A. thaliana, but there was no difference between the two types of transgenic plants (Figure 10). Overall, the life cycle in transgenic plants was prolonged by approximately 5 days. These data show that LaSCL6 overexpression inhibited the transitions from the vegetative meristem to inflorescence meristem and from the flower meristem to meristem arrest in A. thaliana.
Figure 10.
Occurrence of life cycle events in wild-type and transgenic Arabidopsis thaliana. When A. thaliana seeds were transported to a growth chamber for two days, the germination percentage was more than 78%; when the seedlings had 2–3 true leaves, the plants were transferred into the soil, and the timings of life cycle events including bolting and flower and silique formation were recorded. The white boxes indicate the number of days before the seedlings were transferred into the soil. The numbers in the boxes indicate the duration of each stage.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The seeds of A. thaliana ecotype Columbia (Col-0), stored in our laboratory, were disinfected in a 0.8% NaClO solution and then inoculated on 1/2 Murashige and Skoog medium at 4 °C for 3 days. Then, the seeds were transported to a growth chamber with a 16 h photoperiod, a temperature of 22 °C, and relative humidity of 75–85%. When the seedlings had 2–3 true leaves, twenty plants of each line were transferred into 1:1 mixed roseate and nutrient soil and some were sampled for genomic DNA and total RNA extraction. After sampling, the materials were immediately frozen in liquid nitrogen and then stored at −80 °C.
4.2. Plasmid Construction and Genetic Transformation
The primers were designed based on our published LaSCL6-var1 (GenBank: MK501379) and LaSCL6-var2 (GenBank: JX280920) mRNA sequences. After the Noc I restriction site was added to the forward primers and the PmL I restriction site was added to the reverse primers, the primers 5′-ACGGGGGACTCTTGACCATGGGGATGAACGGGATGCTAAGCAGG-3′ and 5′-CTGGTCACCAATTCACACGTGTTAAGGCGGGGGCCCGCACCT-3′ were used to clone LaSCL6-var1 and the primers 5′-ACGGGGGACTCTTGACCATGGGGATGGAAGATTTGGAGAGTATG-3′ and 5′-CTGGTCACCAATTCACACGTGTTAAGGCGGGGGCCCGCACCT-3′ were used to clone LaSCL6-var2 into the binary vector pCAMBIA1305.1. Then, these vectors were used to transform A. thaliana ecotype Col-0 using the floral dip method, mediated by the Agrobacterium tumefaciens strain GV3101. The homozygous T3 transgenic plants, which were cultured in the same way as the wild-type A. thaliana, were used for the phenotype investigation.
4.3. Extraction of Nucleic Acids, PCR, and qRT-PCR
Genomic DNA was extracted from A. thaliana using the Plant Genomic DNA Extraction Kit (BioTeke, Beijing, China), following the manufacturer’s protocol. The quality of the DNA was determined using a spectrophotometer and agarose gel electrophoresis. Then, the DNA was used for PCR with the specific primers of LaSCL6: 5′-TCCCACATTGTCTAACCAGCC-3′ and 5′-GCGGGATTCGAACCGTAGAC-3′.
The total RNA was extracted from A. thaliana using the Easy Pure RNA Kit (TRANS; Beijing, China), following the manufacturer’s protocol. Then, 2 µg of total RNA was reverse-transcribed into cDNA with the TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix (TRANS; Beijing, China). The qRT-PCR was performed with the Bio-Rad CFX96 PCR system using TB Green® Premix Ex Taq™ (Tli RNase H Plus) (Takara; Shiga, Japan). AtUBQ1 (AT3G52590) was used as the internal control with the specific primers 5′-GCCAAGATCCAAGACAAAGAAG-3′ and 5′-CTGATTGTACTTACGAGCAAGC-3′ [32]. The relative gene expression levels were calculated using the 2−∆∆Ct method. The qRT-PCR was performed with three replicates, and the data are presented as the mean ± SD.
4.4. Phenotypic Observation and Statistical Analysis
Fifty seeds were used for germination percentage measurement after they were transferred to the incubator. Twenty seedlings were randomly selected for root length measurement after they were transferred to the incubator for 8 days.
Fifteen seedlings in each line were randomly selected from twenty seedlings planted in the soil to measure the bolting time, the flower and silique formation times, the length of the inflorescence axis, and the number of branches and siliques.
Excel was used for data statistics and analysis, and Origin was used for drawing. The significance of the differences between wild-type and transgenic A. thaliana was analyzed with Statistical Product and Service Solutions (SPSS Statistics 26, IBM Corp., New York, NY, USA) software using analysis of variance (ANOVA). Student’s t-tests were used to compare LaSCL6-var1 and LaSCL6-var2 groups.
5. Conclusions
Taken together, our results show that LaSCL6 plays a role in the transition and maintenance of the meristem, as well as the growth of roots and the plant height, providing more functional information about LaSCL6 with respect to the whole life cycle.
Author Contributions
J.-X.X. carried out the study, analyzed the data, and wrote the manuscript. Q.-L.Z. performed the genetic transformation. Z.-L.Y. helped to investigate the phenotypes of A. thaliana. W.-F.L. designed the study, analyzed the data, and revised the manuscript. L.Y. and L.-W.Q. provided suggestions on the experimental design and analyses. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32271904).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fan T., Li X., Yang W., Xia K., Ouyang J., Zhang M. Rice osa-miR171c mediates phase change from vegetative to reproductive development and shoot apical meristem maintenance by repressing four OsHAM transcription factors. PLoS ONE. 2015;10:e0125833. doi: 10.1371/journal.pone.0125833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S., Chen Q., Zhang Q., Zhang Y., Hao N., Ou C., Wang F., Li T. Pyr-miR171f-targeted PyrSCL6 and PyrSCL22 genes regulate shoot growth by responding to IAA signaling in pear. Tree Genet. Genomes. 2018;14:20. doi: 10.1007/s11295-018-1233-5. [DOI] [Google Scholar]
- 3.Han H., Geng Y., Guo L., Yan A., Meyerowitz E.M., Liu X., Zhou Y. The overlapping and distinct roles of HAM family genes in Arabidopsis shoot meristems. Front. Plant Sci. 2020;11:541968. doi: 10.3389/fpls.2020.541968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulze S., Schafer B.N., Parizotto E.A., Voinnet O., Theres K. LOST MERISTEMS genes regulate cell differentiation of central zone descendants in Arabidopsis shoot meristems. Plant J. 2010;64:668–678. doi: 10.1111/j.1365-313X.2010.04359.x. [DOI] [PubMed] [Google Scholar]
- 5.Grimplet J., Agudelo-Romero P., Teixeira R.T., Martinez-Zapater J.M., Fortes A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016;7:353. doi: 10.3389/fpls.2016.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendelman A., Kravchik M., Stav R., Frank W., Arazi T. Tomato HAIRY MERISTEM genes are involved in meristem maintenance and compound leaf morphogenesis. J. Exp. Bot. 2016;67:6187–6200. doi: 10.1093/jxb/erw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom E.M., Andersen C.M., Gumulak-Smith J., Hu J., Orlova E., Sozzani R., Bowman J.L. Arabidopsis homologs of the Petunia HAIRY MERISTEM gene are required for maintenance of shoot and root indeterminacy. Plant Physiol. 2011;155:735–750. doi: 10.1104/pp.110.168757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David-Schwartz R., Borovsky Y., Zemach H., Paran I. CaHAM is autoregulated and regulates CaSTM expression and is required for shoot apical meristem organization in pepper. Plant Sci. 2013;203–204:8–16. doi: 10.1016/j.plantsci.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y., Yan A., Han H., Li T., Geng Y., Liu X., Meyerowitz E.M. HAIRY MERISTEM with WUSCHEL confines CLAVATA3 expression to the outer apical meristem layers. Science. 2018;361:502–506. doi: 10.1126/science.aar8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Galea P., Huang L.F., Chua N.H., Bolle C. The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome A responses. Mol. Genet. Genom. 2006;276:13–30. doi: 10.1007/s00438-006-0123-y. [DOI] [PubMed] [Google Scholar]
- 11.Stuurman J., Jaggi F., Kuhlemeier C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes Dev. 2002;16:2213–2218. doi: 10.1101/gad.230702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Mai Y.X., Zhang Y.C., Luo Q., Yang H.Q. MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol. Plant. 2010;3:794–806. doi: 10.1093/mp/ssq042. [DOI] [PubMed] [Google Scholar]
- 13.Rhoades M.W., Reinhart B.J., Lim L.P., Burge C.B., Bartel B., Bartel D.P. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 14.Llave C., Xie Z., Kasschau K.D., Carrington J.C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- 15.Song L., Axtell M.J., Fedoroff N.V. RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr. Biol. 2010;20:37–41. doi: 10.1016/j.cub.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 16.Li H., Zhang Q., Li L., Yuan J., Wang Y., Wu M., Han Z., Liu M., Chen C., Song W., et al. Ectopic overexpression of bol-miR171b increases chlorophyll content and results in sterility in broccoli (Brassica oleracea L var. italica) J. Agric. Food Chem. 2018;66:9588–9597. doi: 10.1021/acs.jafc.8b01531. [DOI] [PubMed] [Google Scholar]
- 17.Curaba J., Talbot M., Li Z.Y., Helliwell C. Over-expression of microRNA171 affects phase transitions and floral meristem determinancy in barley. BMC Plant Biol. 2013;13:6. doi: 10.1186/1471-2229-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan R., Song S., Li H., Sun H. Functional analysis of the eTM-miR171-SCL6 module regulating somatic embryogenesis in Lilium pumilum DC. Fisch. Hortic. Res. 2022;9:uhac045. doi: 10.1093/hr/uhac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensel L.L., Nelson M.A., Richmond T.A., Bleecker A.B. The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 1994;106:863–876. doi: 10.1104/pp.106.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W., Peng S., Xian Z., Lin D., Hu G., Yang L., Ren M., Li Z. Overexpression of a Tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 2017;15:472–488. doi: 10.1111/pbi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Tong Y., He X., Zhu Y., Li T., Lin X., Mao W., Ghulam Nabi Gishkori Z., Zhao Z., Zhang J., et al. The rice miR171b-SCL6-IIs module controls blast resistance, grain yield, and flowering. Crop J. 2022;10:117–127. doi: 10.1016/j.cj.2021.05.004. [DOI] [Google Scholar]
- 22.Xue X.Y., Zhao B., Chao L.M., Chen D.Y., Cui W.R., Mao Y.B., Wang L.J., Chen X.Y. Interaction between two timing microRNAs controls trichome distribution in Arabidopsis. PLoS Genet. 2014;10:e1004266. doi: 10.1371/journal.pgen.1004266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Z., Zhang Y., Gao Y., Shen Y., Huang Y. GRAS family transcription factor FaSCL8 regulates FaVPT1 expression mediating phosphate accumulation and strawberry fruit ripening. Fruit Res. 2023;3:15. doi: 10.48130/FruRes-2023-0015. [DOI] [Google Scholar]
- 24.Greb T., Clarenz O., Schafer E., Muller D., Herrero R., Schmitz G., Theres K. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003;17:1175–1187. doi: 10.1101/gad.260703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kravchik M., Stav R., Belausov E., Arazi T. Functional characterization of microRNA171 family in Tomato. Plants. 2019;8:10. doi: 10.3390/plants8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Y., Zhou Y. HAM gene family and shoot meristem development. Front. Plant Sci. 2021;12:800332. doi: 10.3389/fpls.2021.800332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang Q.L., Li W.F., Qi L.W. Regulation of LaSCL6 expression by genomic structure, alternative splicing, and microRNA in Larix kaempferi. Tree Genet. Genomes. 2019;15:1–7. doi: 10.1007/s11295-019-1362-5. [DOI] [Google Scholar]
- 28.Zang Q.L., Zhang Y., Han S.Y., Li W.F., Qi L.W. Transcriptional and post-transcriptional regulation of the miR171-LaSCL6 module during somatic embryogenesis in Larix kaempferi. Trees. 2021;35:145–154. doi: 10.1007/s00468-020-02026-2. [DOI] [Google Scholar]
- 29.Li W.F., Zhang S.G., Han S.Y., Wu T., Zhang J.H., Qi L.W. The post-transcriptional regulation of LaSCL6 by miR171 during maintenance of embryogenic potential in Larix kaempferi (Lamb.) Carr. Tree Genet. Genomes. 2014;10:223–229. doi: 10.1007/s11295-013-0668-y. [DOI] [Google Scholar]
- 30.Yang L., Chen D.L., Peng Z.H., Zhao H.S., Gao Z.M. Cloning of transcription factor DlSCL6 from Dendrocalamus latiflorus and its ectopic expression in Arabidopsis thaliana. Sci. Silvae Sin. 2014;50:52–57. [Google Scholar]
- 31.Geng Y., Guo L., Han H., Liu X., Banks J.A., Wisecaver J.H., Zhou Y. Conservation and diversification of HAIRY MERISTEM gene family in land plants. Plant J. 2021;106:366–378. doi: 10.1111/tpj.15169. [DOI] [PubMed] [Google Scholar]
- 32.Ye Z.L., Zang Q.L., Cheng D.X., Li X.Y., Qi L.W., Li W.F. Over-expression of larch DAL1 accelerates life-cycle progression in Arabidopsis. Forests. 2022;13:953. doi: 10.3390/f13060953. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.