Abstract
The antiapoptotic Bcl-2 and Bcl-xL proteins of mammals are converted into potent proapoptotic factors when they are cleaved by caspases, a family of apoptosis-inducing proteases (E. H.-Y. Cheng, D. G. Kirsch, R. J. Clem, R. Ravi, M. B. Kastan, A. Bedi, K. Ueno, and J. M. Hardwick, Science 278:1966–1968, 1997; R. J. Clem, E. H.-Y. Cheng, C. L. Karp, D. G. Kirsch, K. Ueno, A. Takahashi, M. B. Kastan, D. E. Griffin, W. C. Earnshaw, M. A. Veliuona, and J. M. Hardwick, Proc. Natl. Acad. Sci. USA 95:554–559, 1998). Gamma herpesviruses also encode homologs of the Bcl-2 family. All tested herpesvirus Bcl-2 homologs possess antiapoptotic activity, including the more distantly related homologs encoded by murine gammaherpesvirus 68 (γHV68) and bovine herpesvirus 4 (BHV4), as described here. To determine if viral Bcl-2 proteins can be converted into death factors, similar to their cellular counterparts, five herpesvirus Bcl-2 homologs from five different viruses were tested for their susceptibility to caspases. Only the viral Bcl-2 protein encoded by γHV68 was susceptible to caspase digestion. However, unlike the caspase cleavage products of cellular Bcl-2, Bcl-xL, and Bid, which are potent inducers of apoptosis, the cleavage product of γHV68 Bcl-2 lacked proapoptotic activity. KSBcl-2, encoded by the Kaposi's sarcoma-associated herpesvirus, was the only viral Bcl-2 homolog that was capable of killing cells when expressed as an N-terminal truncation. However, because KSBcl-2 was not cleavable by caspases, the latent proapoptotic activity of KSBcl-2 apparently cannot be released. The Bcl-2 homologs encoded by herpesvirus saimiri, Epstein-Barr virus, and BHV4 were not cleaved by apoptotic cell extracts and did not possess latent proapoptotic activities. Thus, herpesvirus Bcl-2 homologs escape negative regulation by retaining their antiapoptotic activities and/or failing to be converted into proapoptotic proteins by caspases during programmed cell death.
The bcl-2 gene was identified at chromosomal translocation breakpoints in follicular lymphomas and contributes to tumorigenesis by inhibiting programmed cell death rather than by stimulating cell growth (1, 59). Bcl-2 protein is normally expressed in a wide range of tissues and is required for normal development and maintenance of the immune system (61). More than 15 cellular Bcl-2-related proteins have been identified in a wide range of species. In addition, Bcl-2 homologs are also found in viral genomes, including oncogenic herpesviruses and the unrelated African swine fever virus (2, 23). Interestingly, all sequenced herpesviruses of the gamma subfamily, including Epstein-Barr virus (EBV), herpesvirus saimiri (HVS), mouse gammaherpesvirus 68 (γHV68), bovine herpesvirus 4 (BHV4) Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8, equine herpesvirus 2, and ateline herpesvirus 3 encode a Bcl-2-like protein, implying a conserved requirement for viral Bcl-2 proteins.
The function of cellular Bcl-2 family members is regulated in part by caspases. We and others have reported that caspase-3 cleaves Bcl-2 at Asp-34 and Bcl-xL at Asp-61 and Asp-76 to produce N-terminally truncated proteins that have lost their antiapoptotic activities (8, 13, 20, 22, 35). These cleavages are likely to be physiologically significant, as mutation of the cleavage sites in Bcl-2 and Bcl-xL enhances their antiapoptotic activities (8, 13). The caspase cleavage products of Bcl-2 and Bcl-xL are potently proapoptotic, based on transfection studies expressing protein fragments that are equivalent to caspase cleavage products (8, 13). Furthermore, apoptosis induced by these fragments is blocked by the baculovirus caspase inhibitor P35, suggesting that these fragments kill cells in a caspase-dependent manner. Thus, the generation of these fragments inside cells may accelerate cell death by amplifying the caspase cascade. In support of this hypothesis, N-terminally truncated Bcl-2 triggers the release of cytochrome c from mitochondria, similar to Bax (32, 35). Several groups have found that Bax and Bid are also cleaved during apoptosis, and their cleavage products are potently proapoptotic (35, 41, 43, 44, 66). Therefore, the cleavage products of Bcl-2-related proteins may be important facilitators of apoptosis in vivo.
The viral Bcl-2 homologs differ in interesting ways from their cellular counterparts with regard to their effects on cell cycle progression and their abilities to heterodimerize with other Bcl-2 family members (24). Here we report another important mechanistic difference between viral and cellular Bcl-2 proteins. Herpesvirus Bcl-2 homologs appear to have captured the antiapoptotic functions but eliminated the proapoptotic functions of their cellular counterparts. Thus, these viral proteins may represent constitutively active antiapoptotic versions that escape negative regulation by caspases because they fail to be converted into proapoptotic proteins.
MATERIALS AND METHODS
Plasmids and viruses.
PCR-amplified full-length or truncated Bcl-2 open reading frames were cloned into pSG5 or a modified pSG5 vector containing a hemagglutinin (HA) epitope tag (pHYC79), and the correct sequence was confirmed by DNA sequencing. Restriction fragments containing the HA-tagged or untagged Bcl-2 family members were excised from the pSG5 derivatives and inserted at the BstEII site of the Sindbis virus vector dsTE12Q, and recombinant viruses were generated as previously reported (9, 39). Protein expression of the untagged constructs was confirmed by in vitro translation with [35S]methionine using T7 quick-coupled TNT (Promega) and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Cleavage assays.
In vitro cleavage reactions contained 1 μl of 35S-labeled in vitro translation mixture and 1 μl of purified caspase-1 (95 U), caspase-3 (1,600 U), or caspase-8 (260 U), where 1 U generates 1 pmol of 7-amino-4-methylcoumarin (AMC) per min by using saturating substrate Ac-YVAD-AMC or Ac-DEVD-AMC (Peptides International) at 25°C. Dithiothreitol was added to a final concentration of 10 mM, and caspase reaction buffer (100 mM HEPES [pH 7.5], 10% sucrose, 0.1% CHAPS [3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate]) was added to bring the total reaction volume to 10 μl. After digestion for 3 h at 37°C, the labeled proteins were analyzed by SDS-PAGE and autoradiography after enhancing with 1 M salicylic acid.
Apoptotic 293 cell extracts were prepared as previously described (18). Cleavage reactions contained 2 μl of 35S-labeled in vitro translation mix and 10 μl of 293 lysate or caspase reaction buffer. ATP (Boehringer Mannheim) was added to a final concentration of 1 mM, and the reaction mixtures were incubated at 37°C overnight and analyzed as described above.
Virus infection and cell transfection.
Low-passage-number (<15) BHK-21 cells (American Type Culture Collection) were infected with 5 PFU of recombinant Sindbis virus vectors per cell in a reduced volume of infection medium (Dulbecco's modified Eagle's medium supplemented with 1% fetal bovine serum) for 1 h and then returned to 10% serum for 48 h. Infections were performed in duplicate, blinded, and at least 500 cells were counted per sample.
BHK-21 or Cos-1 cells were transfected with 0.5 μg of lacZ reporter plasmid pCH110 and various amounts of Bcl-2 plasmid using Lipofectamine (Life Technologies). The total amount of plasmid transfected was held constant at 2.5 μg by using empty pSG5 vector. Alternatively, Cos-1 cells were transfected with 2 μg of plasmid containing procaspase-3 and 0.5 μg of bcl-2 plasmid. At 24 h posttransfection, the cells were fixed with 0.5% glutaraldehyde in phosphate-buffered saline and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (49). Cell viability of blinded samples was determined by counting the number of blue cells in 10 high-power fields and scoring for normal versus apoptotic morphology.
Immunoblot analysis.
Cos-1 cells were lysed at 24 h posttransfection in radioimmunoprecipitation assay buffer (150 mM NaCl, 1.0% NP-40, 0.5% deoxycholate, 1.0% SDS, 50 mM Tris [pH 8.0]) containing the protease inhibitors aprotinin, benzamidine, chymostatin, leupeptin, pepstatin A, and phenylmethylsulfonyl fluoride. Protein (50 μg, quantitated by the bicinchoninic acid assay; Pierce) was separated by SDS–15% PAGE, transferred to nitrocellulose (Schleicher and Schuell), probed with anti-hBcl-2 monoclonal antibody (MAb) (provided by David Mason), anti-HA MAb 12CA5 (Berkeley), or anti-caspase-3 antibody and detected using SuperSignal (Pierce).
RESULTS
Homology domains of viral and cellular Bcl-2 family members.
The mammalian Bcl-2 family is defined by the homology domains BH1 to BH4. The most conserved of these are the BH1 and BH2 domains, which are important for antiapoptotic activity and dimerization (9, 67). In addition, the BH1-BH2 region spans alpha helices 5 and 6, which are implicated in ion channel activity (48). A multiple alignment revealed that the BH1 homology domain is the most highly conserved domain among gamma herpesvirus Bcl-2 homologs (Fig. 1). The BH2 domain is also conserved with the exception of γHV68, which surprisingly lacks a recognizable BH2 domain (Fig. 1). The cell homologs and BHRF1 from Epstein-Barr virus were shown to be anchored to cytoplasmic membranes via their hydrophobic C termini (25, 52). The predicted amino acid sequence for the other viral genes also contains a stretch of hydrophobic residues followed by one or more positively charged residues at the C terminus. The original BHV4 genome sequence in GenBank apparently contains an error, causing a reading frameshift prior to the hydrophobic C terminus. The sequence of a genomic fragment of BHV4 provided by Vicky Van Santen (Auburn University) contains a 2-nucleotide insertion at position 615 within the open reading frame (Fig. 1). Both γHV68 and BHV4 have additional amino acids after the last charged residue, though the function of this C-terminal extension is not known. The BH-3 domain is implicated in the cell-killing activity of the proapoptotic Bcl-2 family members Bax and Bak (11, 64), as well as the Bcl-2 cleavage product (8) and the more distantly related proteins Bid and Bad (33, 65), but is poorly conserved in the viral homologs. The prodeath activity of the BH3 domain may be linked to its role in dimerization with other Bcl-2 family members. The N-terminal BH4 domain, which is required for the antiapoptotic activities of Bcl-2 and Bcl-xL (27, 30), is poorly conserved even among the cellular homologs. This domain is also poorly conserved in the viral proteins. Similar to Bcl-2 and Bcl-xL, the Bcl-2 homolog encoded by BHV4 contains a long “loop” domain stretching between BH4 and BH3. However, there is no significant amino acid similarity between any of the viral or cellular loop domains, suggesting that they possess unique functions (Fig. 1). The remaining viral Bcl-2 proteins have much shorter loop domains, many even shorter than that of Bax.
FIG. 1.
Amino acid alignment of viral and cellular Bcl-2 homologs. Human Bcl-2, human Bcl-xL, and human Bax are compared with the gammaherpesvirus Bcl-2 homologs ORF16 from KSHV (KSBcl-2), M11 of γHV68 (γ68), ORF16 of HVS, BHRF1 of EBV, and BORFB2 of BHV4. Identical (dark shade) and similar (light shade) amino acids occurring in four of the eight entries are marked. Homology domains BH1 to BH4 and the transmembrane domain (TM) are marked with horizontal lines. The caspase recognition sites in Bcl-2 and Bcl-xL are in bold; arrowheads mark the N termini of truncated mutants. Stars indicate the hydrophobic residues in the BH3 domain of Bak that are important for binding to Bcl-xL. The GenBank accession number for the corrected sequence data for BORFB2 of BHV4 is AF129421.
γHV68 and BHV4 Bcl-2 homologs possess antiapoptotic activity.
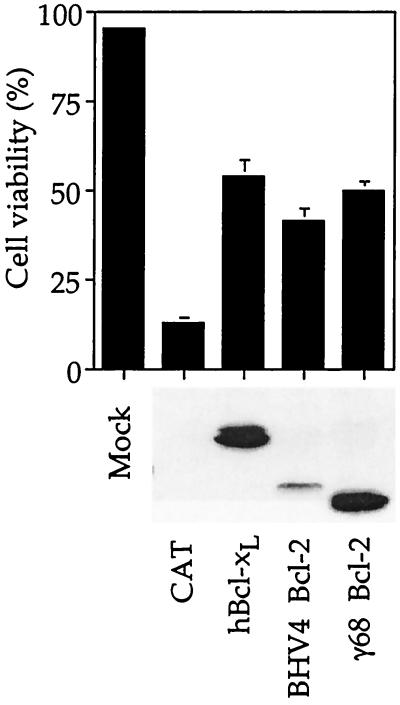
The viral Bcl-2 homologs from EBV, KSHV, and HVS were shown previously to possess antiapoptotic activity (10, 15, 26, 46, 50, 53). Therefore, to determine if the more distantly related Bcl-2 homologs encoded by γHV68 and BHV4 also function as apoptosis inhibitors, they were cloned into the Sindbis virus vector and tested for their ability to inhibit Sindbis virus-induced apoptosis in BHK cells. Sindbis virus induces all the classic morphological and biochemical characteristics of apoptosis in many cell types, including BHK cells, and has proven to be a useful model for studying a variety of cell death regulators, including viral Bcl-2 proteins (9, 10, 16, 40, 42, 51, 60). Both γHV68 and BHV4 Bcl-2 homologs with N-terminal HA tags were capable of inhibiting apoptosis induced by Sindbis virus almost as efficiently as HA-tagged Bcl-xL despite lower expression levels of the BHV4 Bcl-2, as measured by immunoblot analysis with anti-HA antibody (Fig. 2). In contrast, a control protein (chloramphenicol acetyltransferase [CAT]) lacked protective activity when expressed from the Sindbis virus vector. Similar to the cellular proteins, deletion of the BH4 domain of γHV68 abolished its ability to block Sindbis virus-induced apoptosis (data not shown).
FIG. 2.
BHV4 and γHV68 (γ68) Bcl-2 proteins inhibit apoptosis. Apoptotic cell morphology and viability were determined by light microscopy and trypan blue dye exclusion, respectively, at 48 h postinfection of BHK cells with Sindbis virus vectors encoding the indicated Bcl-2 homolog or control CAT. The means and standard errors of the mean (SEM) are shown for three independent experiments. All Bcl-2 homologs have N-terminal HA tags. A corresponding immunoblot analysis with anti-HA antibody is shown.
Viral homologs escape cellular regulatory mechanisms.
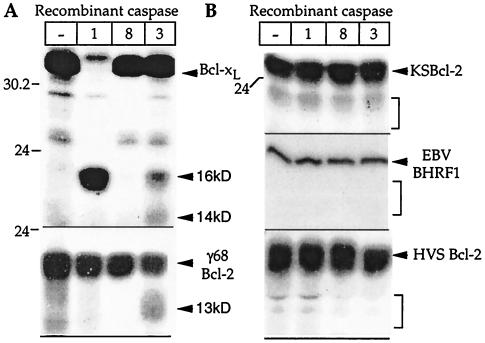
To determine whether viral Bcl-2 homologs are susceptible to caspase digestion, the viral proteins were translated in vitro and treated with active recombinant purified caspase-1 (which cleaves Bcl-xL), caspase-3 (which cleaves Bcl-xL and Bcl-2), and caspase-8 (which cleaves Bid). Of the viral proteins, only γHV68 Bcl-2 was susceptible to partial cleavage by caspase-3 (Fig. 3A), and none of the viral homologs was cleaved by caspase-1 or caspase-8 (Fig. 3B) (data not shown). In contrast, Bcl-xL was cleaved by caspase-1 to produce a 16-kDa fragment and by caspase-3 to produce both the 16- and 14-kDa fragments observed previously in vitro and in apoptotic cells (13, 20).
FIG. 3.
Except for γHV68 (γ68) Bcl-2, viral Bcl-2 homologs are not cleaved by caspases. The indicated 35S-labeled, in vitro-translated proteins were digested with the indicated recombinant caspases or incubated with caspase buffer only (lane —). Proteins were analyzed by SDS-PAGE and autoradiography. Caspase activity was determined by using peptide substrates as described in Materials and Methods. Minor bands that are present in both digested and undigested lanes are presumably premature terminations, internal initiations, or nonspecific degradation products. For example, the ∼26-kDa fragment of Bcl-xL is due to initiation at an internal Met at position 45 (13). Molecular size markers are indicated (in kilodaltons). Brackets indicate the approximate positions of viral proteins lacking the N-terminal BH4 domain.
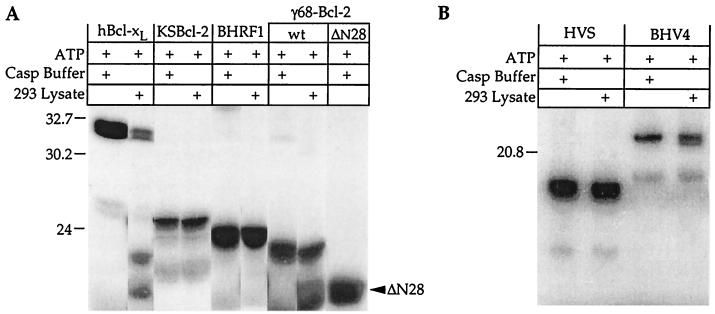
To further explore the possibility that viral Bcl-2 homologs could be cleaved by caspases or other proteases during apoptosis, in vitro-translated proteins were treated with apoptotic extracts prepared from 293 cells, which contain a number of activated caspases (18). Again only Bcl-xL and γHV68 Bcl-2 were cleaved (Fig. 4A and B). By analogy with Bcl-2 and Bcl-xL, the γHV68 homolog is expected to be cleaved in the loop region between BH4 and BH3. Immunoblot analysis verified that the γHV68 protease site is located in the N terminus, because the cleavage product is not detected with an antibody to the N-terminal HA tag (data not shown). Caspases cleave exclusively after Asp residues, and there are three Asp residues present in the loop region of γHV68 Bcl-2 (residues 28, 31, and 37). The consensus cleavage site for caspase-3 is DXXD (58), consistent with the γHV68 Bcl-2 sequence DCVD31 (bold in Fig. 1). Furthermore, the cleavage product of γHV68 Bcl-2 migrates only slightly faster than that of a deletion mutant lacking amino acids 2 to 28 (ΔN28), consistent with cleavage at Asp-31, Asp-37, or both (Fig. 4, compare last two lanes). Except for KSBcl-2, encoded by KSHV, the viral Bcl-2 proteins contain at least one Asp residue in this region, though none is a consensus caspase-3 site. Other sequences as well as structural features are required to constitute a caspase cleavage site because caspases are known to cleave only at specific Asp residues. Therefore, it appears that caspases cleave and inactivate only one of the herpesvirus Bcl-2 homologs tested. However, it is not known if caspase cleavage of γHV68 Bcl-2 occurs during virus infection of mice.
FIG. 4.
Except for γHV68 (γ68) Bcl-2, viral Bcl-2 proteins are resistant to cleavage by apoptotic cell extracts. In vitro-translated, 35S-labeled proteins were digested with apoptotic 293 cell extracts (18). Proteins were analyzed by SDS-PAGE and autoradiography. Treated and untreated samples were run on the same gel with the same exposure, though the lanes were rearranged for display. Longer gels confirmed the absence of detectable small cleavage products (data not shown). For an explanation of minor bands in both digested and undigested samples, see the legend to Fig. 3. Molecular size markers are indicated (in kilodaltons). wt, wild type.
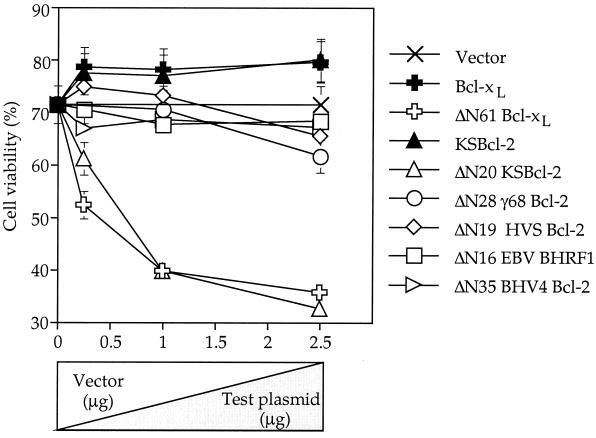
To determine whether viral Bcl-2 proteins harbor latent proapoptotic activity, C-terminal fragments of the viral proteins were expressed in transfected cells. Constructs were generated to mimic potential caspase cleavage fragments, such that all truncated proteins lacked the BH4 homology domain and retained the BH3 domain. The arrowheads in Fig. 1 mark the new N termini (plus an initiation Met). The exact positions of the newly generated N termini may not be critical, as the position of the caspase cleavage site is not conserved between Bcl-2 and Bcl-xL. That is, Bcl-2 is cleaved on the N-terminal side of the ∼50-amino-acid loop domain, while Bcl-xL is cleaved on the C-terminal side of the loop (bold in Fig. 1). Furthermore, the 16-kDa Bcl-xL, 14-kDa Bcl-xL, and 23-kDa Bcl-2 fragments all possess equivalent proapoptotic activities in cultured cells (8, 20) (data not shown). Transfection of the N-terminally truncated viral Bcl-2 constructs had no effect on cell viability except for ΔN20 KSBcl-2, which killed cells in a dose-dependent manner, similar to ΔN61 Bcl-xL (16-kDa fragment) (Fig. 5). Similar results were obtained in Cos-1 cells (data not shown). However, because the KSBcl-2 protein has no potential caspase cleavage sites between BH4 and BH3 and was not cleaved by recombinant caspases or apoptotic cell extracts, its proapoptotic function appears to remain latent. The caspase cleavage product of γHV68 Bcl-2, the only cleavable viral homolog, was not capable of killing BHK cells (Fig. 5). Similar results were obtained in Cos-1 cells (data not shown). Because of the lack of appropriate antibodies and because N- and C-terminal tags impair the prodeath activities of truncated Bcl-xL and KSBcl-2, all fragments were expressed without tags. Therefore, all plasmid inserts were completely sequenced, and protein expression was confirmed by in vitro translation of the same plasmids used for transfection (Fig. 4 and data not shown).
FIG. 5.
Except for ΔN20 KSBcl-2, N-terminally truncated viral Bcl-2 proteins lack proapoptotic activity. Plasmids encoding wild-type Bcl-2 family members or N-terminal truncations lacking the indicated number of amino acids were transfected into BHK cells at the indicated DNA concentrations. Cell viability was determined at 24 h posttransfection by scoring the percentage of live/nonapoptotic versus total transfected cells (counting >250 lacZ-positive cells per sample). The data presented are the means and SEM for three to six independent experiments.
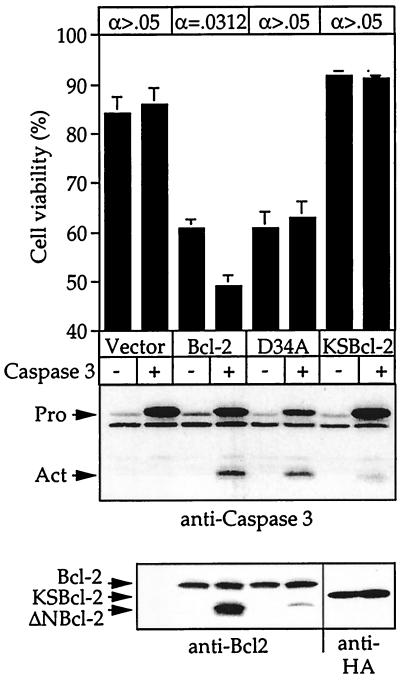
These findings suggest that viral Bcl-2 homologs escape cellular regulatory mechanisms by retaining their antiapoptotic activities and/or by failing to be converted into proapoptotic proteins when caspases are activated during apoptosis. To compare KSBcl-2 with a cellular homolog in the presence of activated caspases, cell viability was monitored in Cos-1 cells that had been transfected with procaspase-3 and a Bcl-2 homolog (Fig. 6). Caspase-3 was selected for this experiment because it is an abundant downstream caspase and the only caspase that cleaves viral and cellular Bcl-2 proteins (35). Overexpression of procaspase-3 alone had no effect on cell viability. However, overexpression of Bcl-2 alone exhibited some intrinsic proapoptotic activity, a phenomenon previously observed in many laboratories, including ours (8). When procaspase-3 was cotransfected with human Bcl-2, cell viability was further reduced concomitant with cleavage of Bcl-2 to its 23-kDa signature fragment. The Bcl-2 cleavage product was shown previously to activate caspases by inducing release of cytochrome c from mitochondria in a feed-forward pathway to accelerate cell death (35). Consistent with this finding, cotransfection of Bcl-2 and procaspase-3 resulted in processing of procaspase-3 to its active form (Fig. 6). The caspase-3-mediated enhancement of cell death was abolished by mutation of the caspase-3 cleavage site in Bcl-2 (D34A). The faint Bcl-2 cleavage product observed with the D34A mutant in the presence of caspase-3 is probably due to inefficient cleavage at Asp-31 (the P4 position in the DAGD34 site). Taken together, these data indicate that the cell killing function of Bcl-2 is enhanced when the proapoptotic fragment of Bcl-2 is released by caspase cleavage. The observation that Bcl-2 induced cell death (without cotransfected caspase-3) suggests that the proapoptotic function of full-length Bcl-2 may be unleashed by mechanisms other than caspase cleavage. In contrast to human Bcl-2, KSBcl-2 lacked intrinsic proapoptotic activity and failed to enhance cell death relative to the control vector when cotransfected with procaspase-3 (Fig. 6). In addition, KSBcl-2 had almost no ability to induce the processing of procaspase-3 to its active form. Thus, KSBcl-2 was not converted to a proapoptotic form by caspase-3 or other cell factors.
FIG. 6.
Antiapoptotic activity of KSBcl-2 is resistant to inactivation by caspase-3. Cell viability of Cos-1 cells transfected with the indicated plasmids was determined as described in the legend to Fig. 5. The data are the means and SEM. The effect of cotransfected procaspase-3 was statistically significant only for wild-type Bcl-2 using a Wilcoxon signed-rank test for paired analysis of seven independent experiments (indicated at the top). Representative immunoblots of transfected cell lysates with the indicated antibodies are shown below. Pro, unprocessed form of procaspase-3; Act, active cleavage product of caspase-3.
DISCUSSION
Herpesvirus genomes contain large blocks of conserved genes required for housekeeping functions. These blocks are separated by genes that are unique to herpesvirus subfamilies or unique to a particular virus. Unlike other herpesviruses, the gammaherpesvirus subfamily encodes a number of proteins with obvious homology to cellular factors, such as cyclin D, OX2, interleukin-8 receptor, interleukin-6, chemokines, chemokine receptors, interferon regulatory factors, FLIP proteins, Bcl-2, and others (21). These factors were presumably acquired as adaptations to a particular host environment and are candidate perpetrators of the distinct diseases and cancers associated with these viruses. Some of these viral homologs have expanded functions or escape regulatory mechanisms to which their cellular counterparts are subject. KSHV encodes a G-protein-coupled receptor (ORF74) that stimulates cell proliferation and angiogenesis by a constitutive, agonist-independent mechanism (3, 5). The viral chemokine encoded by KSHV, vMIP-II, binds to a broader range of receptors with higher affinity, functions as an antagonist of chemotaxis, and is a potent angiogenic factor, unlike cellular MIP-1α and RANTES (7, 36). Our data suggest that like these factors, the gammaherpesvirus Bcl-2 homologs may be constitutively active. In this way, viral Bcl-2 proteins are unlike several cellular Bcl-2 family members that become potent killer proteins following proteolytic cleavage. Consistent with this model, HVS Bcl-2 was shown to protect Jurkat cells from Fas-induced apoptosis, in contrast to human Bcl-2, which is cleaved by caspases in Jurkat cells following Fas ligation (8, 15). While our paper was in review, Wang et al. reported that γHV68 Bcl-2 inhibits Fas- and tumor necrosis factor-induced apoptosis in HeLa cells (63). Therefore, the Sindbis virus-induced apoptosis utilized in our studies also reflects the results obtained with other cell death stimuli.
Viral Bcl-2 proteins differ in other ways from their cellular homologs. In contrast to Bcl-2, which suppresses cell cycle progression (28), BHRF1 was reported to stimulate cell cycle progression in some situations (14, 29). However, another group reported that BHRF1 interferes with Ras-induced proliferation, which can be relieved by amino acid substitutions in the BH3 domain of BHRF1 (56). These disparate results could potentially be explained by cell type-specific factors that modulate BHRF1 function (19).
Like human Bcl-2, herpesvirus Bcl-2 homologs can cooperate with adenovirus E1A and c-Myc to facilitate cell transformation (17, 56), raising the possibility that viral Bcl-2 proteins may contribute directly to the tumorigenic potential of several of these viruses. This is consistent with the finding that the γHV68 Bcl-2 homolog appears to be expressed during latency in infected mice (62). In addition, viral Bcl-2 homologs may serve to prevent premature cell death during virus replication, fitting with the observation that several of the viral Bcl-2 homologs are synthesized during the lytic phase of the virus life cycle (4, 10). Although an EBV mutant lacking its Bcl-2 homolog (BHRF1) has no detectable phenotype in cell culture (38, 45), natural isolates of EBV retain a functional BHRF1, further suggesting its importance to the biology of the virus (34). However, by analogy with other large DNA viruses, antiapoptotic functions may be redundantly encoded (23). In fact, a second Bcl-2 homolog encoded by EBV was recently reported (46). Furthermore, the KSHV, equine herpesvirus 2, BHV4, HVS, and ateline herpesvirus 3 viruses all encode viral FLIP proteins that are implicated in blocking caspase recruitment to cell death receptors (6, 57).
The BH3 domain is required and sufficient for the proapoptotic activity of Bax and Bak in some assays (8, 11). Given that the viral Bcl-2 proteins have lost their latent proapoptotic activities (except for KSBcl-2), it is not surprising that the BH3 domain is less well conserved in the viral proteins. Based on the nuclear magnetic resonance structure of a peptide of Bak bound to Bcl-xL, the BH3 domain of Bak forms an alpha-helix that inserts into a hydrophobic cleft on Bcl-xL, probably inactivating its antiapoptotic activity (54). A comparison of the structures of cleaved and uncleaved Bid suggests that cleavage of Bid by caspase-8 exposes the Bid BH3 domain and may contribute to reorientation of the Bid BH3 domain, making it more available for binding partners (12, 47). Of the four hydrophobic amino acids in the Bak/Bid BH3 domain that insert into the hydrophobic groove on Bcl-xL, only three of these are conserved in the viral homologs (the positions of these hydrophobic amino acids are marked with stars in Fig. 1). However, in comparing their BH3 domains, it is not apparent why N-terminally truncated KSBcl-2 possesses proapoptotic activity while the other viral proteins lack this activity. Perhaps a cleavage-dependent conformational change that exposes the binding face of the BH3 domain of Bcl-2 and Bcl-xL does not occur in the herpesvirus homologs.
The role of heterodimerization between proapoptotic and antiapoptotic Bcl-2 family members in blocking cell death is not fully understood. Although Bcl-2 and Bcl-xL can prevent cell death by mechanisms other than sequestering Bax and Bak, heterodimerization may serve to titrate the intracellular concentrations of these partners (9, 37). However, no consistent picture has emerged with regard to heterodimerization of viral Bcl-2 proteins. HVS Bcl-2 appears to be capable of binding and perhaps suppressing the activity of Bax (10, 50, 56), while other viral homologs fail to heterodimerize with Bax (e.g., KSBcl-2 and BHRF1) and potentially escape inactivation by Bax. KSBcl-2 was recently demonstrated to bind a new member of the Bcl-2 family, Diva/Boo, though there is controversy about whether Diva/Boo is an antiapoptotic or proapoptotic protein (31, 55). Like Bcl-2, Bcl-xL, and perhaps Diva/Boo, Bax can also flip its function and become an antiapoptotic factor (41). Thus, while both pro- and antiapoptotic cellular Bcl-2 family proteins can reverse their functions, viral Bcl-2 homologs appear to be locked into the antiapoptotic mode.
The inability of herpesvirus Bcl-2 proteins to be cleaved by caspases and their lack of proapoptotic activity strongly indicate that these viral factors have eliminated key features of the cellular homologs from which they were likely derived. If low levels of caspases become activated in healthy cells, the generation of proapoptotic fragments from target substrates such as Bcl-2 family proteins may be necessary to amplify the apoptotic pathway and facilitate cell death. Indeed, the cleavage fragment of Bcl-2 and Bid can induce the release of cytochrome c from mitochondria (35, 43). Cytochrome c serves as an essential cofactor for Apaf-1 to activate procaspase-9, which in turn amplifies the caspase cascade (68). Overexpression of viral Bcl-2 proteins that fail to facilitate cell death could potentially serve to tip the balance in favor of cell survival.
ACKNOWLEDGMENTS
We thank Vicky van Santen for BHV4 DNA, John Nicholas for HVS DNA, and Nancy Thornberry for purified caspases.
This work was supported by research grant RO1 CA73581 from the National Institutes of Health (J.M.H.).
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Afonso C L, Neilan J G, Kutish G F, Rock D L. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. 1996;70:4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature (London) 1998;385:347–349. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 4.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature (London) 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 5.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature (London) 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 6.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G-H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 8.Cheng E H-Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 9.Cheng E H-Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng E H-Y, Nicholas J, Bellows D S, Hayward G S, Guo H-G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi's sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou J J, Li H, Salvesen G, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 13.Clem R J, Cheng E H-Y, Karp C L, Kirsch D G, Ueno K, Takahashi A, Kastan M B, Griffin D E, Earnshaw W C, Veliuona M A, Hardwick J M. Modulation of cell death by Bcl-xL through caspase interaction. Proc Natl Acad Sci USA. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson C W, Dawson J, Jones R, Ward K, Young L S. Functional differences between BHRF1, the Epstein-Barr virus-encoded Bcl-2 homolog, and Bcl-2 in human epithelial cells. J Virol. 1998;72:9016–9024. doi: 10.1128/jvi.72.11.9016-9024.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derfuss T, Fickenscher H, Kraft M S, Henning G, Lengenfelder D, Fleckenstein B, Meinl E. Antiapoptotic activity of the herpesvirus saimiri-encoded Bcl-2 homolog: stabilization of mitochondria and inhibition of caspase-3-like activity. J Virol. 1998;72:5897–5904. doi: 10.1128/jvi.72.7.5897-5904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duckett C S, Nava V E, Gedrich R W, Clem R J, Van Dongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. A conserved family of apoptosis inhibitors related to the baculovirus iap gene. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 17.Fanidi A, Hancock D C, Littlewood T D. Suppression of c-Myc-induced apoptosis by the Epstein-Barr virus gene product BHRF1. J Virol. 1998;72:8392–8395. doi: 10.1128/jvi.72.10.8392-8395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearnhead H O, McCurrach M E, O'Neill J, Zhang K, Lowe S W, Lazebnik Y A. Oncogene-dependent apoptosis in extracts from drug-resistant cells. Genes Dev. 1997;11:1266–1276. doi: 10.1101/gad.11.10.1266. [DOI] [PubMed] [Google Scholar]
- 19.Foghsgaard L, Jaattela M. The ability of BHRF1 to inhibit apoptosis is dependent on stimulus and cell type. J Virol. 1997;71:7509–7517. doi: 10.1128/jvi.71.10.7509-7517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-xL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- 21.Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning? Cell. 1998;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 22.Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel M R. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998;17:1268–1278. doi: 10.1093/emboj/17.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardwick J M. Viral interference with apoptosis. Semin Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 24.Hardwick J M. Comparing and contrasting BHRF1 with human Bcl-2. Epstein-Barr Virus Rep. 1998;5:31–35. [Google Scholar]
- 25.Hardwick J M, Lieberman P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson S, Hue D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang D C S, Adams J M, Cory S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 1998;17:1029–1039. doi: 10.1093/emboj/17.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang D C S, O'Reilly L A, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, Pan X H, Zhou S M, Li Z J, Yu L, Kong X T, Zheng Q P. Flow cytometric analysis of BHRF1 expression prohibiting apoptosis induced by radiation. Ann Otol Rhinol Laryngol. 1999;108:481–484. doi: 10.1177/000348949910800511. [DOI] [PubMed] [Google Scholar]
- 30.Hunter J J, Bond B L, Parslow T G. Functional dissection of the human Bcl-2 protein: sequence requirements for inhibition of apoptosis. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inohara N, Gourley T S, Carrio R, Muniz M, Merino J, Garcia E, Koseki T, Hu Y, Chen S, Nunez G. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J Biol Chem. 1998;273:32479–32486. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- 32.Jurgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelekar A, Chang B S, Harlan J E, Fesik S W, Thompson C B. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-xL. Mol Cell Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanim F, Dawson C, Meseda C A, Dawson J, Mackett M, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, is conserved at both the sequence and functional level in different Epstein-Barr virus isolates. J Gen Virol. 1997;78:2987–2999. doi: 10.1099/0022-1317-78-11-2987. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch D G, Doseff A, Chau B N, Lin D-S, de Souza-Pinto N C, Hansford R, Kastan M B, Lazebnik Y A, Hardwick J M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 36.Kledal T N, Rosenkilde M M, Coulin F, Simmons G, Johnsen A H, Alouani S, Power C A, Luttichau H R, Gerstoft J, Clapham P R, Clark-Lewis I, Wells T N C, Schwartz T W. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 37.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 38.Lee M-A, Yates J L. BHRF1 of Epstein-Barr virus, which is homologous to human proto-oncogene bcl-2, is not essential for transformation of B cells or for virus replication in vitro. J Virol. 1992;66:1899–1906. doi: 10.1128/jvi.66.4.1899-1906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature (London) 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 41.Lewis J, Oyler G A, Ueno K, Fannjiang Y-R, Chau B N, Vornov J, Korsmeyer S J, Zou S, Hardwick J M. Inhibition of virus-induced neuronal apoptosis by Bax. Nat Med. 1999;5:832–835. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- 42.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Sindbis virus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 44.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 45.Marchini A, Tomkinson B, Cohen J I, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bcl-2, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall W L, Yim C, Gustafson E, Graf T, Sage D R, Hanify K, Williams L, Fingeroth J, Finberg R W. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonnell J M, Fushman D, Milliman C L, Korsmeyer S J, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 48.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 49.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Induction of apoptosis in fibroblasts by IL-1-beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 50.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearson G R, Luka J, Petti L, Sample J, Birkenbach M, Braun D, Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987;160:151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 53.Sarid R, Sato T, Bohenzky R A, Russo J J, Chang Y. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 54.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, Thompson C B, Fesik S W. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 55.Song Q, Kuang Y, Dixit V M, Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J. 1999;18:167–178. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theodorakis P, D'Sa-Eipper C, Subramanian T, Chinnadurai G. Unmasking of a proliferation-restraining activity of the anti-apoptosis protein EBV BHRF1. Oncogene. 1996;12:1707–1713. [PubMed] [Google Scholar]
- 57.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature (London) 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 58.Thornberry N A, Ranon T A, Pieterson E P, Rasper D M, Timkey T, Garciacalvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B—functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 59.Tsujimoto Y, Bashir M M, Givol I, Cossman J, Jaffe E, Croce C M. DNA rearrangements in human follicular lymphoma can involve the 5′ or the 3′ region of the bcl-2 gene. Proc Natl Acad Sci USA. 1987;84:1329–1331. doi: 10.1073/pnas.84.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veis D J, Sorenson C M, Shutter S R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 62.Virgin H W, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang G H, Garvey T L, Cohen J I. The murine gammaherpesvirus-68 M11 protein inhibits Fas- and TNF-induced apoptosis. J Gen Virol. 1999;80:2737–2740. doi: 10.1099/0022-1317-80-10-2737. [DOI] [PubMed] [Google Scholar]
- 64.Wang K, Gross A, Waksman G, Korsmeyer S J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang K, Yin X-M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 66.Wood D E, Thomas A, Devi L A, Berman Y, Beavis R C, Reed J C, Newcomb E W. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 67.Yin X-M, Oltvai Z N, Korsmeyer S J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 68.Zou H, Li Y, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]