Abstract
One of the key problems of biology is how plants adapt to unfavorable conditions, such as low temperatures. A special focus is placed on finding ways to increase tolerance in important agricultural crops like wheat. Au-based nanoparticles (Au-NPs) have been employed extensively in this area in recent years. Au-NPs can be produced fast and easily using low-cost chemical reagents. When employed in microdoses, Au-NPs are often non-toxic to plants, animals, and people. In addition, Au-NPs mainly have favorable impacts on plants. In this study, we investigated the effect of Au-NP seed nanopriming (diameter 15.3 nm, Au concentration 5–50 µg mL−1) on cold tolerance, as well as some physiological, biochemical and molecular parameters, of cold-sustainable wheat (Triticum aestivum L.) genotype Zlata. The treatment with Au-NPs improved tolerance to low temperatures in control conditions and after cold hardening. Au-NPs treatment boosted the intensity of growth processes, the quantity of photosynthetic pigments, sucrose in leaves, and the expressions of encoded RuBisCo and Wcor15 genes. The potential mechanisms of Au-NPs’ influence on the cold tolerance of wheat varieties were considered.
Keywords: Au-based nanoparticles, low temperature tolerance, lipid peroxidation, photosynthetic pigments, sugars, Cor-genes, Triticum aestivum
1. Introduction
One of the main goals of biology is the study of the physiological, biochemical, and molecular mechanisms of plants’ adaptation to abiotic factors. The majority of arable land is currently located in an unstable agricultural region. This makes it relevant to study the adaptation strategies of such an important crop as wheat to low temperatures. Wheat is one of the cold- and freezing-tolerant crops that is able to survive even near-zero negative temperatures. The cultivation of wheat for centuries has resulted in the development of a wide range of cold- and freezing-tolerant varieties, and cold-sustainable genotypes respond differently to low temperatures [1]. No doubt, the diversity of wheat genotypes and the characteristics of their stress response make this crop an ideal subject for the research of physiological, biochemical, and molecular mechanisms that contribute to improving of their tolerance [2,3].
At low temperatures, cold-tolerant genotypes of wheat are able to maintain a stable content of photosynthetic pigments, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) activity, and photosynthetic intensity, and as a result, accumulate sugars required for the adaptation to low temperature [4,5,6]. It is known that more tolerant wheat genotypes at low temperatures enact a powerful response, including the expression of a number of Cor-genes (Cold-Regulated Genes) [7,8]. As for cold-sustainable genotypes, their set of mechanisms for maintaining cold and freezing tolerance may be significantly limited [4,5,9].
Many Cor-genes encode COR proteins, which are important in plant adaptation to low temperatures. These are hydrophilic low-molecular-weight proteins that function as chaperones in cells, limiting protein aggregation, cell water loss, and membrane degradation [10]. The WCS (Wheat Cold Specific) family is also referred to as COR proteins—dehydrins [7,11]. The molecular weights of these proteins encoded by the Wcs120 gene family range from 12 to 200 kD [8]. The accumulation of dehydrins and increased expression of genes encoding them are associated with the development of tolerance to low temperatures [7,12].
Plants also have cold-sensitive genes that encode chloroplast-targeted proteins [8]. These include the cold-sensitive wheat Wcor15 gene [13], which is related to Arabidopsis cor15 gene [14,15]. It encodes proteins that form an ordered structure when exposed to low temperature stress. These proteins can bind to chloroplast membranes in this condition, making them more tolerant [16].
The use of metal nanoparticles (NPs), which can have a positive effect on plant metabolism and stress tolerance in low concentrations, is a promising trend [17,18,19,20]. NPs cross cellular barriers and affect practically all processes in the plant organism due to their small size (less than 100 nm) and unique physical, electrical, optical, and chemical properties [17,18,21,22]. NPs are being employed more and more widely in biology, medicine, and agriculture as plant growth and development regulators, medicinal components, herbicidal/pesticidal chemicals, and nanofertilizers [19,21,23].
It is known that the effects of NPs on plants depend on a number of factors (for example, type of NPs, method of treatment, plant species, experimental conditions, etc.). First of all, the type of nanoparticles matters. In our research, we used Au-based NPs (Au-NPs). We should emphasize that Au-NPs occupy a special place among metallic NPs. On the one hand, Au-NPs can be produced fast and easily using low-cost chemical reagents [24,25,26]. On the other hand, when employed in microdoses up to 100 µg mL−1, Au-NPs are often non-toxic to plants, animals, and people [27,28,29,30]. According to the literature, Au-NPs mainly have favorable impacts on plants [26,29,30]. The increase in seed germination rate and growth intensity under the influence of Au-NPs was shown in maize [31], Arabidopsis [32], mungbean [33], mustard [34,35], gloriose [36], arugula [37], lavender [38], watermelon [39], onion [40] and barley [41]. Under the influence of Au-NPs, researchers observed increased photosynthetic intensity [33,42], and changes in fluorescence parameters [43], photosynthetic pigment content [31,33,34] and chloroplast ultrastructure [31]. The presence of unique optical properties associated with the excitation of localized plasmon resonances during light interaction is responsible for the worldwide scientific interest in the use of Au-NPs in biology and medicine as photoprotective substances that reduce the risks of oxidative stress [24,28,29,44,45,46]. In this regard, Au-NPs have recently attracted a lot of attention as substances with the potential to increase crop environmental tolerance. It is worth noting that there are a few studies on the impact of Au-NPs on plant metabolism and tolerance. According to studies in barley and Arabidopsis, Au-NPs that enter plants through the roots alter the expression of DGR1 and DGR2 genes that code cell wall proteins [29], as well as changing chemical composition and increasing the stiffness of the cell wall, which helps plants withstand stress [41]. The treatment of rice plant roots with nanocomposites containing Au-NPs reduced cadmium toxicity by limiting root cell uptake and lowering the risk of oxidative stress [47,48]. Under saline conditions, Au-NPs preserved ionic balance, reduced the formation of NO as a signaling molecule and regulator of many processes in the plant, and boosted the activity of antioxidant system enzymes [49]. The potential for further integrating Au-NPs into biological sciences and agriculture is quite intriguing in this regard. It is critical to research them as substances that improve plant tolerance to low temperatures. There are no such studies in the literature, in connection with which our research is groundbreaking.
In a previous study [50], we demonstrated for the first time that Au-NPs can increase the tolerance to low temperatures of the freezing-tolerant genotype of wheat Moskovskaya 39. However, it is an open question whether Au-NPs can increase the low temperature tolerance of more cold-sensitive genotypes. In this research, we explored the effects of Au-NPs on the low temperature tolerance of cold-sustainable wheat genotype Zlata. We chose nanopriming (soaking seeds in NPs solutions) as the mode of nanoparticle treatment. Nanopriming is largely regarded as the safest, simplest, and most cost-effective method of treating plants with NPs. This allows for scientific and agricultural applications. We attempted to discover potential mechanisms by which Au-NPs can influence plant stress tolerance. For this purpose, we assayed the following indices: survival of wheat seedlings after freezing, seed germination, length of the first leaf, the content of malondialdehyde (MDA), photosynthetic pigments and soluble sugars in leaves and the expression of some genes of photosynthetic apparatus (PSA) and Cor-genes, which are important in plant acclimation to low temperatures.
2. Results
It was necessary to assess the impacts of various Au-NPs concentrations (5, 10, 20 and 50 µg mL−1) on the low temperature tolerance of wheat genotype Zlata at the start of the study. The survival rate of wheat plants at 0 °C was 100% in all experiment variants. The survival rate of unhardened seedlings at −3 °C was increased by a factor of two to three after exposure to Au-NPs, but only at concentrations of 5 and 10 µg mL−1 (Table 1). When we dropped the temperature to −5 °C, all the plants died. The tolerance to cold was improved by low-temperature hardening and was shown to be 60%. The stimulating impact of Au-NPs on cold tolerance was clearly established at −5 °C. There, Au-NPs at a concentration of 10 µg mL−1 increased cold tolerance from 7% to 67% (Table 1).
Table 1.
Impact of Au-based nanoparticles (Au-NPs) on the survival rate (%) of unhardened and hardened (4 °C, 7 d) wheat seedlings of genotype Zlata (concentration test).
| Temperature of Freezing |
Concentration of Au-NPs, µg mL−1 | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 50 | |
| Unhardened seedlings | |||||
| −3 °C | 15 ± 3 c | 30 ± 5 b | 50 ± 5 a | 10 ± 3 c | 10 ± 3 c |
| −5 °C | 0 | 0 | 0 | 0 | 0 |
| Hardened seedlings | |||||
| −3 °C | 60 ± 3 b | 90 ± 3 a | 97 ± 3 a | 90 ± 3 a | 90 ± 3 a |
| −5 °C | 7 ± 3 c | 50 ± 3 b | 67 ± 3 a | 50 ± 3 b | 50 ± 3 b |
| −7 °C | 0 | 5 ± 3 a | 7 ± 3 a | 6 ± 3 a | 0 |
| −9 °C | 0 | 0 | 0 | 0 | 0 |
Survival rate was calculated as the percentage of undamaged seedlings from the total number of plants frozen at −3, −5, −7 and −9 °C for 24 h. In each variant of the treatment, 30 plants were used in 3 replications (90 plants in all). The experiment was repeated 2 times. In every line the values that significantly differ at p < 0.05 are denoted by different letters.
We assessed the impacts of various Au-NPs concentrations (5, 10, 20 and 50 µg mL−1) on the seed germination and growth of the first leaf of wheat seedlings (Figure 1). The stimulating impact of Au-NPs on seed germination was only noticed at a concentration of 20 µg mL−1 (Figure 1A). At the same time, Au-NPs treatment accelerated the growth of the first leaf of seedlings at all concentrations tested (Figure 1B). Under hardening, the growth of seedlings was completely stopped (Figure 1C).
Figure 1.
Impact of Au-based nanoparticles (Au-NPs) on seed germination (A) and leaf length before (B) and after (C) cold hardening (4 °C, 7 d) of wheat seedlings of genotype Zlata. In each variant of the treatment, 30 plants were used in 3 replications. The experiment was repeated 2 times. Different letters indicate mean values that are significantly different at p < 0.05.
Based on the results of concentration tests, a Au-NPs concentration of 10 µg mL−1 was chosen as causing the maximum effect on the low temperature tolerance of unhardened and hardened seedlings of wheat genotype Zlata (Figure 2 and Figure 3).
Figure 2.
Unhardened wheat seedlings of genotype Zlata after freezing at −3 °C for 24 h: (A) control seedlings; (B) seedlings grown from seeds treated with Au-based nanoparticles at 10 µg mL−1.
Figure 3.
Hardened (4 °C, 7 d) wheat seedlings of genotype Zlata after freezing at −5 °C for 24 h: (A) control seedlings; (B) seedlings grown from seeds treated with Au-based nanoparticles at 10 µg mL−1.
Table 2 displays data on the Au contents in roots, seeds and leaves of wheat. The study revealed that Au is present in Au-NPs-treated wheat roots and seeds. Furthermore, a trace of Au was discovered in the wheat leaves.
Table 2.
Au concentrations in roots, seed and leaves of wheat seedlings of genotype Zlata in control variant and in plants treated with Au-based nanoparticles (Au-NPs).
| Variant of Experiment | Au, µg g−1 Dry Weight | ||
|---|---|---|---|
| Roots | Leaves | Seeds | |
| Control | <0.05 | <0.05 | <0.05 |
| Au-NPs | 1.6 ± 0.1 | 0.28 ± 0.01 | 3.9 ± 0.2 |
In each variant of the treatment, 3 statistical repetitions were carried out. The experiment was repeated 2 times. Au-NPs concentration—10 µg mL−1 (as concentrations causing the maximum effect on tolerance to low temperature).
We studied MDA accumulation in wheat tissues as one of the end products of lipid peroxidation (LPO) of cell membranes. Treatment with Au-NPs both in control and hardening conditions did not affect MDA content in wheat leaves (Table 3).
Table 3.
Impact of Au-based nanoparticles (Au-NPs) on some physiological indicators of unhardened and hardened (4 °C, 7 d) wheat seedlings of genotype Zlata.
| Variant of Experiment |
MDA (µmol/g DW) | DW (%) | Monosaccharide (mg g−1 DW) | Sucrose (mg g−1 DW) | Sum of Sugars (mg g−1 DW) |
|---|---|---|---|---|---|
| Unhardened seedlings | |||||
| Control | 36.8 ± 1.8 a | 13.2 ± 0.2 c | 55.0 ± 1.5 a | 4.4 ± 0.2 d | 59.4 ± 3.0 b |
| Au-NPs | 36.4 ± 1.2 a | 13.3 ± 0.2 c | 42.8 ± 1.5 b | 7.6 ± 0.2 c | 50.4 ± 2.5 c |
| Hardened seedlings | |||||
| Control | 20.8 ± 1.0 b | 17.8 ± 0.3 b | 45.2 ± 1.2 b | 14.1 ± 0.2 b | 59.3 ± 3.0 b |
| Au-NPs | 19.8 ± 1.3 b | 19.1 ± 0.4 a | 44.2 ± 1.7 b | 24.5 ± 0.2 a | 68.7 ± 3.4 a |
In each variant of the treatment 3 statistical repetitions were carried out. The experiment was repeated 3 times. Au-NPs concentration—10 µg mL−1 (as concentrations causing maximum effect on tolerance to low temperature). DW—dry weight. In each column the values that significantly differ at p < 0.05 are denoted by different letters.
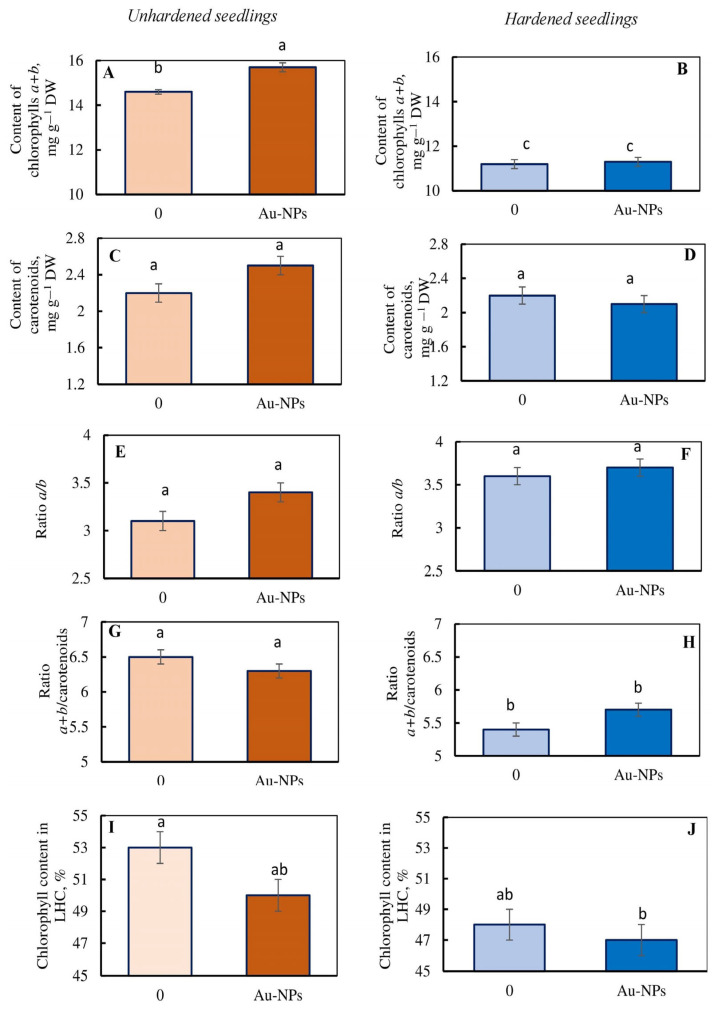
Under control conditions, Au-NPs treatment resulted in a significant increase in total chlorophyll content without affecting carotenoids concentration or pigment ratio in wheat leaves (Figure 4A,C,E,G,I). Au-NPs treatment had no influence on photosynthetic pigment quantity or ratio under low-temperature conditions (Figure 4B,D,F,H,J).
Figure 4.
Impact of Au-based nanoparticles (Au-NPs) on content of chlorophylls a + b (A,B), carotenoids (C,D), ratio a/b (E,F) and a + b/carotenoids (G,H), and content of chlorophyll in light harvesting complex (LCH) (I,J) in leaves of unhardened (A,C,E,G,I) and hardened (4 °C, 7 d) (B,D,F,H,J) wheat seedlings of genotype Zlata. In each variant of the treatment 3 statistical repetitions were carried out. The experiment was repeated 3 times. Different letters indicate mean values, which are significantly different at p < 0.05. Au-NPs concentration—10 µg mL−1 (as concentrations causing maximum effect on tolerance to low temperature). DW—dry weight.
Au-NPs treatment had no effect on the buildup of leaf dry weight of wheat leaves under control conditions. Dry leaf weight rose under hardening conditions, and Au-NPs treatment accelerated this process (Table 3).
Au-NPs treatment under control conditions considerably lowered the sugar content in wheat leaves by decreasing the content of monosaccharides (glucose and fructose). The content of sugars in leaves did not change under low-temperature conditions, whereas Au-NPs treatment increased them due to enhanced sucrose accumulation (Table 3).
We analyzed the effects of Au-NPs on the expression level of PSA genes RbcS and RbcL, encoding, respectively, the small and large subunits of RuBisCo. It was found that the expression level of RbcS and RbcL was increased in Au-NPs-treated plants under control conditions (Figure 5A,C). Under low-temperature hardening, Au-NPs had no significant effect on the expression levels of these genes (Figure 5B,D).
Figure 5.
Impact of Au-based nanoparticles (Au-NPs) on RbcL (A,B) and RbcS (C,D) gene transcription of unhardened (A,C) and hardened (4 °C, 7 d) (B,D) wheat seedlings of genotype Zlata. In each variant of the treatment 3 statistical repetitions were carried out. The experiment was repeated 3 times. Different letters indicate mean values, which are significantly different at p < 0.05. Au-NPs concentration—10 µg mL−1 (as concentrations causing maximum effect on tolerance to low temperature).
We also examined the impacts of Au-NPs on the expression of Wcor726 and Wcor15, encoding protective proteins. Expression of the Wcor15 gene increased 8-fold in wheat leaves, whereas the expression of the Wcor726 gene did not change (Figure 6A,C). Au-NPs treatment also slightly enhanced Wcor15 gene expression under low-temperature conditions (Figure 6B,D).
Figure 6.
Impact of Au-based nanoparticles (Au-NPs) on Wcor726 (A,B) and Wcor15 (C,D) gene transcription of unhardened (A,C) and hardened (4 °C, 7 d) (B,D) wheat seedlings of genotype Zlata. In each variant of the treatment 3 statistical repetitions were carried out. The experiment was repeated 3 times. Different letters indicate mean values, which are significantly different at p < 0.05. Au-NPs concentration—10 µg mL−1 (as concentrations causing maximum effect on tolerance to low temperature).
3. Discussion
In our experiments, wheat seeds of cold-susceptible genotype Zlata were soaked in Au-NPs solutions (5, 10, 20, and 50 µg mL−1) for 24 h. Based on data from the literature, concentrations of Au-NPs solutions below 50 μg mL−1 generally have a stimulating effect on plants [28,29]. The nanopriming procedure, which involves soaking seeds in NPs solution, is thought to be the mildest for such studies [31,39,51]. In this situation, NPs most likely enter the seeds passively with water, as well as through the damaged sections of the seed coat [52]. The mechanism of NPs continuing “work” in the plant organism is not entirely clear. Au-NPs are not only adsorbed and/or deposited in the treated seeds, but also penetrate further into the seedlings, where they spread through their tissues and cells. For instance, Au-NPs were found in the leaves of wheat, oat, and maize seedlings developed from seeds treated with Au-NPs, as demonstrated by fluorescence microscopy and optical emission spectroscopy [31,53]. Employing plasma-atomic emission and inductively coupled plasma mass spectrometry, we found Au in roots and seeds of treated seedlings. Minor concentrations of Au were found in the leaves of wheat grown from Au-NPs-treated seeds (Table 2). It is important to note that these plants differed from the control (untreated) in a number of indicators and, most critically, had a different adaptive status due to their ability to develop higher tolerance to low temperatures.
The Au-NPs concentration tests revealed that the nanopriming of wheat improved its tolerance to low temperatures in control conditions and after cold-hardening (4 °C, 7 d). The maximum effect was achieved in genotype Zlata after the treatment of seeds with Au-NPs at a concentration of 10 μg mL−1 (Table 1). Additionally, the treatment of seeds with Au-NPs induced the growth of plants (Figure 1B). We selected a concentration of 10 μg mL−1 for the further study of Au-NPs’ effects on physiological, biochemical and molecular parameters of wheat.
Similar results on the growth-promoting effects of low concentrations of Au-NPs were obtained in many other plant species, including maize, Arabidopsis, mungbean, mustard, gloriosa, arugula, lavender, and watermelon [31,32,34,35,38,40,41]. It is hypothesized that Au-NPs may participate in cell differentiation through molecular pathways [54]; for example, there is evidence of the ability of Au-NPs to activate genes that are involved in the regulation of aquaporins and responsible for the cell cycle [29,41]. Additionally, Au-NPs can affect cell differentiation through miRNAs—short, non-coding RNAs that regulate a variety of biological functions, including growth and stress responses. Thus, Au-NPs altered the expressions of miR398, miR408, miR164, miR167, and miR169 in Arabidopsis [32]. Furthermore, miR167 expression was linked to the activity of genes that regulate plant reproductive processes via affecting auxin signal transduction pathways. The altered expressions of miR169, miR398 and miR408 influenced the sizes of seedlings, as well as the development of their root systems, and was responsible for the early flowering of plants and hastened seed maturation [32].
We emphasize that the effects of Au-NP on PSA were expressed via a significant increase in the level of chlorophylls in the leaves of wheat under control conditions (Figure 4A). This could imply that the PSA is actively working, and that thylakoid membranes are stable. Another indication of the PSA’s stability is the consistent ratio of photosynthetic pigments of wheat under the impact of Au-NPs (Figure 4E–H). Other studies indicate that Au-NPs increased chlorophyll content in soybean, corn, and mustard plants [31,33,34]. Researchers observed an increase in the electronic transport rate and Hill reaction [33,43], as well as changes in fluorescence parameters and photosynthetic intensity [33,42], under the influence of Au-NPs. The peculiar features of Au-NPs in this area are attributed to the effect of plasmon resonance. The impact is that electrons on the surfaces of Au-NPs can dramatically enhance their activity due to their propensity to collectively oscillate in response to a certain wavelength of light (Hu and Xianyu 2021) [45]. Active electrons on the surfaces of Au-NPs can “trap” photons of light, facilitating the transfer of energy in the light-harvesting complex (LHC) [33,43]. Au-NPs’ effects on PSA parameters were shown not only in leaves sprayed with Au-NPs, but also in plants grown from treated seeds [55].
NPs also change the intensity of photosynthetic pigment synthesis and the activity of PSA by regulating the expression of genes involved. For example, zinc oxide NPs increased the expression of genes encoding photosystem structural units (PSAD2, PSAE2, PSAK) and photosynthetic pigment synthesis genes (CAO, CHLG, GGPS6, PSY, PDS, ZDS) [56]. Silicon oxide NPs enhanced the expression of PsbH, PsbB, and PsbD genes encoding photosystem II (PS II) proteins [57]. Titanium oxide particles enhanced the expression of genes involved in the synthesis of RuBisCo [58]. Au-NPs’ effects on RbcL and RbcS genes were shown in our study under control conditions (Figure 5A,C). The expression levels of RbcL and RbcS genes encoding the large and small subunits of RuBisCo were increased upon Au-NPs treatment, which may indicate an increase in RuBisCo as a key PSA enzyme.
Under low-temperature hardening, Au-NPs caused an additional increase in the cold tolerance of wheat seedlings (Table 1). However, the effects of Au-NPs on growth processes and PSA activity were almost completely cancelled out by the effect of low temperature. At the same time, under the Au-NPs treatment, the process of increasing cold tolerance was accompanied by a significant increase in sucrose content (Table 3). The buildup of soluble sugars is known to be the most important nonspecific plant response to abiotic stress, which is required for survival under low temperatures [59,60]. Frost tolerance (resistance to sub-zero temperatures) in cereals is related to the outflow of water from cells into intercellular spaces, and the formation of intercellular ice. At the same time, the high content of sugars in the cells reduces the risks of dehydration and decreases ice nucleation temperature. Sugars act as osmolytes and cryoprotectants; they stabilize membranes and act as antioxidants [61,62]. Furthermore, sugars are reserve substances, and their accumulation is required for the energy-intensive cold adaptation process [63,64,65].
It is understood that Au-NPs, as alien exogenous particles to the plant organism, can operate as triggers for oxidative stress [66]. The development of LPO products, on the other hand, is a critical link in the chain of plant cell-adaptive responses to abiotic stress [59]. The products of LPO (including reactive oxygen species, MDA and ET) created in plant cells during oxidative stress development can induce the ICE-CBF/DREB1 ((ICE)-C-Repeat Binding Factor/Dehydration Responsive Element Binding Factor 1) signaling pathway of cold adaptation in plants, which triggers the expression of Cor-genes [60,67]. Additionally, they stimulate the antioxidant system (AOS), which in plants is the most significant non-specific protective enzymatic mechanism. It was shown in watermelon and mustard that exposure to Au-NPs was accompanied not only by an increase in MDA in leaves, but also by an increase in the activity of AOS enzymes [35,39]. We found that under either control or hardening conditions, the Au-NPs treatment had no effect on the MDA contents of wheat leaves (Table 3). It can be concluded that in our case, NPs do not cause oxidative stress.
We studied the effects of Au-NPs on the expression of the Cor-genes Wcor15 and Wcor726. Note that the Wcor726 gene belongs to the Wcs120 family, which is specific to wheat [68,69]. These genes are regulated by low temperatures and encode WCS protein accumulation, which directly correlates with the ability of plants to increase tolerance to low temperatures [68]. According to some research [7], Wcor726 is a member of the family D11 of the large class LEA (Late Embryogenesis Abundant) proteins, which are dehydrins involved in plant defense against a variety of stresses.
WCOR15 appears to belong to group III LEA proteins that target chloroplasts [70,71]. The Wcor15 gene encoding chloroplast proteins contains CRT/DRE cis-elements similar to Wcs120 [13]. There is proof that the Wcor15 gene produces the WCOR15 protein, which builds up in chloroplasts and reduces the degree of photoinhibition of PS II [72,73].
According to our studies, under the influence of Au-NPs, in control conditions an 8-fold increase in the expression of the Wcor15 gene was observed (Figure 6C). Under low-temperature hardening, the expressions of Wcor726 and Wcor15 in untreated wheat plants were significantly increased. Au-NPs treatment caused a small additional increase in the expressions of these genes. As shown in other studies, plants of cold-sensitive wheat genotypes tended to accumulate less Wcor15 transcripts [13]. However, our data suggest that in wheat genotype Zlata, the increase in freezing tolerance under the influence of Au-NPs seems to be related to the expression of this gene. Most likely, this ensured an increase in their freezing tolerance.
This research demonstrates that the nanopriming method can successfully deliver Au-NPs to seeds, and the NPs then permeate the plant organism. Plants grown from treated seeds differed from control (untreated) plants not only in physiological, biochemical and molecular characteristics, but also in adaptive status. The treatment with Au-NPs improved tolerance to low temperatures in control conditions and after cold hardening. Au-NPs treatment boosted the intensity of growth processes, the quantity of photosynthetic pigments, sucrose in leaves, and the expression of genes, such as encoded RuBisCo and the Wcor15 gene (Figure 7). The mechanisms of cold tolerance enhancement under the influence of Au-NPs are not completely clear. It can be assumed based on our data that improvements in wheat’s low temperature tolerance are associated with a high content of soluble sugars and an increased level of Wcor15 gene expression.
Figure 7.
Some physiological, biochemical and molecular mechanisms of freezing tolerance improvement due to Au-NPs’ effects in seedlings of wheat of the cold-susceptible genotype Zlata.
Comparing biochemical and molecular processes related to this phenomenon in the freezing-tolerant genotype Moskovskaya 39 (presented in previous study [50]) and the cold-susceptible genotype Zlata revealed that, under control conditions, Au-NPs treatment increased the content of photosynthetic pigments and the expression of the Wcor15 gene in both wheat genotypes. There were also differences between genotypes. In the freezing-tolerant genotype Moskovskaya 39, the rise in cold tolerance was followed by an increase in peroxide processes, but in cold-susceptible genotype Zlata, the increase in peroxide processes was not detected, while the concentrations of suitable osmolytes in tissues rose. The considerable improvement in cold tolerance under Au-NPs treatment in freezing-tolerant genotype Moskovskaya 39 under low-temperature hardening conditions was related to a complex of adaptation changes: increased chlorophyll and carotenoids content, sucrose, and the expression of Wcor15 and Wcor726 genes. The increase in cold tolerance of cold-susceptible genotype Zlata under these conditions was connected with the expression of the Wcor15 gene and a rise in multiple sugars.
4. Materials and Methods
4.1. Experimental Design
The experiments were carried out according to the following scheme (Figure 8). The first stage is the chemical synthesis of Au-NPs solutions. Based on data from the literature, we selected four concentrations of Au-NPs solutions—5, 10, 20 and 50 μg mL−1—since it is known that concentrations of Au-NPs solutions below 50 μg mL−1 generally have a stimulating effect on plants [28,29].
Figure 8.
Design of experiment. Au-NPs—Au-based nanoparticles.
The second step was the nanopriming (soaking for 24 h) of seeds in Au-NPs solutions at the selected concentrations. The next step involved growing plants from treated seeds under controlled conditions for 10 days.
Then, we determined the tolerance of plants to low temperatures (concentration tests) using growth indicators and survival rates after freezing. As a result, the concentrations of Au-NPs solutions with the maximum effect on low temperature tolerance were selected.
All further experiments were conducted under control conditions (at 22 °C) and after cold hardening (4 °C, 7 d) using Au-NPs concentrations giving the maximum effect on the low temperature tolerance of plants.
4.2. The synthesis of Au-NPs
Gold spherical nanoparticles were created using the citrate method [25] by reducing chloroauric acid (Sigma-Aldrich, St. Louis, MO, USA) with sodium citrate (Fluka, St. Gallen, Switzerland). The reduction was accomplished by heating 250 mL of a 0.01% aqueous solution of chloroauric acid to 100 °C in an Erlenmeyer flask on a magnetic stirrer with a reflux water condenser. In the next step, 7.75 mL of 1% aqueous sodium citrate solution was added, and the mixture was boiled for an additional 30 min until a crimson sol formed. Freshly produced Au-NP solutions were transferred to sterile glass vials with tight-fitting lids and kept at 4 °C.
The resulting Au-NPs were studied by transmission electron microscopy, spectroscopy, and dynamic light scattering [74]. According to the measurement results, the average diameter of Au-NPs was 15.3 nm. Detailed characterizations and TEM images of Au-NPs have been presented in our previous study [50].
4.3. Growth Conditions
Seeds of cold-susceptible wheat (Triticum aestivum L., Poaceae) genotype Zlata (Federal Research Center “Nemchinovka”, Moscow, Russia) were used in the investigations. The seeds were immersed in Au-NPs solutions for 24 h, and then were washed in distilled water. The seeds were germinated in distilled water at 22 °C, 60–70% relative humidity, and a photoperiod of 16 h (illumination 100 μmol photons m−2 s−1) with OSRAM L 80W/640 lamps (Osram, Smolensk, Russia). The seedlings were grown on distilled water under the same conditions until 10 days of age. Then, a part of plants was hardened at 4 °C for 7 days in a KBW-240 climatic chamber (Binder, Tuttlingen, Germany).
4.4. Parameters of Growth
Wheat seed germination was measured on day 7 and reported as a percentage. The initial leaf’s length was measured both at 10 days of growth and at 17 days following hardening. In each variant of the treatment, 30 plants were used in 3 replications (90 plants in all). The experiment was repeated 2 times.
4.5. Survival of Plants after Freezing
In order to identify the concentrations of Au-NPs that have the maximum protective effect on wheat plants of genotype Zlata, the degree of tolerance to low temperatures was determined by direct freezing. The rate of survival of seedlings was measured after freezing in a climatic chamber MIR-153 (Sanyo, Osaka, Japan) at 0 °C, −3 °C, −5 °C, −7 °C and −9 °C, with a 24 h interval. After freezing, the plants were preserved for one day at 4 °C in the dark before being transplanted to normal temperatures (22 °C, daylight) for 72 h. The number of undamaged seedlings as a percentage of the total number of frozen plants was used to calculate the survival rate. The choice of temperatures for freezing was made on the basis of our own research and literature data. In each variant of the treatment, 30 plants were used in 3 replications. The experiment was repeated 2 times.
4.6. Quantification of Au
We analyzed tissues of 10-day-old seedlings grown at 22 °C from seeds treated with Au-NPs at a concentration of 10 μg mL−1. Roots, seeds, and leaves of wheat seedlings were oven-dried at 70 °C for 72 h, weighed, and digested (the seeds were first cleaned and dried). The digestion was performed using microwave-assisted digestion (UltraClave III, Milestone, Santa Clara, CA, USA) at 105 °C for 15 min with 3 mL of plasma pure HNO3. Then, the volume of the sample was adjusted to 10 mL using deionized water. The concentration of Au was measured using an inductively coupled plasma-atomic emission spectrometer (Agilent Technologies, Santa Clara, CA, USA) and an inductively coupled plasma mass spectrometer 820 (Bruker, Bremen, Germany). In each variant of the treatment, 3 statistical repetitions were carried out. The experiment was repeated 2 times.
4.7. Dry Matter Content
Given that plants lose water at low temperatures, the data for all indicators were converted to dry weight. The dry matter content was determined by drying them in a thermostat (at 100–105 °C) to a constant weight and expressed as a percentage of the sample’s initial wet weight.
4.8. Lipid Peroxidation Level (LPO)
LPO level was evaluated as the content of MDA [75] with slight modifications. Leaf samples (300 mg) were homogenized in 5 mL of extraction medium (0.35 M NaCl in 0.1 M Tris-HCl buffer, pH 7.6). The homogenate (3 mL) mixed with 2 mL 0.5% thiobarbituric acid in 20% trichloroacetic acid was incubated (95 °C for 30 min), then cooled and filtered. The extraction medium with the reagent was employed as a control. Results were adjusted for nonspecific absorbance by subtracting the values observed at 532 and 600 nm. A Genesys 10UV spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA) was used in the study. MDA concentration was determined using a molar extinction coefficient (ε = 1.56·105 M−1 cm−1) in μM g−1 dry weight of leaves. In each variant of the treatment, 3 statistical repetitions were carried out. The experiment was repeated 3 times.
4.9. Content of Chlorophylls and Carotenoids
Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Car) concentration in the leaves were measured spectrophotometrically at wavelengths 663, 646, and 470 nm, respectively, in an 80% acetone solution. The contents of pigments were calculated using the formula [76]:
| Ccar = (1000D470 − 3.27Ca − 104Cb)/198, Ca = 12.21D663 − 2.81D646, Cb = 20.13D646 − 5.03D663. |
Total chlorophyll was determined as the sum of chlorophylls a and b. The pigment content was expressed as mg g−1 dry weight of leaves.
We calculated chlorophyll portion in light harvesting complex (LHC), assuming that practically all Chl b was located in LHC and the chlorophyll ratio Chl a/Chl b in this complex was equal to 1.2 [77]: LHC (%) = (Chl b + 1.2 × Chl b)/(Chl a + Chl b) × 100.
In each variant of the treatment, 3 statistical repetitions were carried out. The experiment was repeated 3 times.
4.10. Soluble Sugars (Glucose, Fructose, and Sucrose) Content
Solution of sugars were obtained from 500 mg of plant tissue after ethanol extraction three times. The fructose content was evaluated via the interaction of ketoses with resorcinol, and then the sucrose concentration was recalculated [78]. The content of glucose was measured by the glucose oxidase method using the Olvex diagnosticum Kit (Vital Diagnostics, Saint Petersburg, Russia). In each variant of the treatment, 3 statistical repetitions were carried out. The experiment was repeated 3 times.
4.11. Total RNA Extraction and cDNA Synthesis
Total RNA was isolated from 50 mg of leaf tissue samples using the Spectrum Plant Total RNA Kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions with modification of the homogenization step according to [79]. The quality and quantity of purified RNA was determined by the NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed by 2% agarose gel electrophoresis. To remove residual genomic DNA impurities, total RNA preparations were treated with DNase I (Thermo Fisher Scientific, USA).
cDNA was synthesized using a RevertAid reverse transcription kit (Thermo Fisher Scientific, USA).
4.12. Gene Expression by RT-qPCR
Real-time quantitative PCR (RT-qPCR) was carried out on the CFX96 Touch™ (Bio-Rad, Hercules, CA, USA), using the SYBR Green I intercalating dye (Evrogen, Moscow, Russia). The reaction mixture for quantitative PCR in a volume of 25 μL contained 5 μL of qPCRmix HS SYBR (Evrogen), 0.2 μM of each primer, and 15 ng of the cDNA template. The following amplification conditions were used: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s.
Gene-specific primers (Table 4) for the amplification of large and small subunits of RuBisCo (RbcS and RbcS) target genes were borrowed from [80]; gene-specific primers for amplification of the target genes (Wcor726, Wcor15) and reference genes (TaAct7, TaRP15) were selected using the Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 13 March 2024) and OligoAnalyzer™ Tool (https://eu.idtdna.com/pages/tools/oligoanalyzer, accessed on 13 March 2024) online resources. Primers were designed to bind to all three wheat sub-genomes [80].
Table 4.
Primers for qRT-PCR analysis.
| Gene | Primer | Primer Sequences |
|---|---|---|
| TaAct7 | Forward | TGCTATCCTTCGTTTGGACCTT |
| Reverse | AGCGGTTGTTGTGAGGGAGT | |
| TaRP15 | Forward | TCATTGTGGAGGACTCGTGG |
| Reverse | GCAGACATAGCCCACACAT | |
| RbcS | Forward | GGATTCGACAACATGCGCCAGG |
| Reverse | ATATGGCCTGTCGTGAGTGAGC | |
| RbcL | Forward | ACCATTTATGCGCTGGAGAGACC |
| Reverse | CAAGTAATGCCCCTTGATTTCACC | |
| Wcor726 | Forward | ACTGGAATGACCGGCTCG |
| Reverse | TGTCCCGACTTCCCGTAGTT | |
| Wcor15 | Forward | CCACCCATCCATCAGCAGTT |
| Reverse | CTTGGAGCGTTCTGCAGGC |
Wcor726 encodes WCOR 726 dehydrin, which refers to Wheat Cold Specific family proteins that are important in plant cold and freezing adaptation. Wcor15 encodes the WCOR15 chloroplast-targeted COR protein. The transcript levels were normalized to the expression of the reference genes TaAct7 (actin) [81] and TaRP15 (RNA polymerase I, II, and III, 15 kDa subunit) [82]. The relative expression levels were calculated using the Pfaffl method [83]. Each qRT-PCR reaction was performed in three biological and two technical replicates.
4.13. Statistical Analysis
Subject to the method used, the experiments were repeated three times to obtain similar reproducible results. Statistical significance was calculated via one-way ANOVA with a Tukey’s test (p < 0.05) using Origin 7.0 software. The tables and figures show the mean values and their standard errors.
5. Conclusions
Our results demonstrate that Au-NPs can “reprogram” the plant organism by altering the metabolism and the expression of genes involved in stress responses. It is important that Au-NPs act not only on freezing-tolerant, plants as we have previously established [50], but also on varieties whose genetic tolerance to low temperatures is reduced. As a result, plants are able to withstand low temperatures much better. Au-NPs regulated growth processes, increased the content of photosynthetic pigments and soluble sugars in leaves, and also increased the expression of COR genes and PSA genes. It should be mentioned that more research in this area is needed to better understand the effects of Au-NPs on plants, and the mechanisms of action in the plant organism. Perhaps in the future, Au-NPs can be used as growth and development stimulants as well as potential adaptogens. The development of strategies for the use of NPs should aim to reduce agricultural risks, i.e., focus on the development of sustainable agriculture.
Acknowledgments
We thank L.A. Dykman of Institute of Biochemistry and Physiology of Plants and Microorganisms (Saratov Scientific Centre of the Russian Academy of Sciences, Saratov, Russia) for his help in this investigation.
Author Contributions
Conceptualization, Y.V. and A.D.; investigation, Y.V., A.D. and K.Z.; writing—original draft preparation, Y.V.; writing—review and editing, Y.V., A.D. and K.Z.; supervision, Y.V.; project administration, A.D. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was supported by the Russian Science Foundation, grant no. 23-26-00054 (https://rscf.ru/project/23-26-00054/, accessed on 13 March 2024).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hosseini M., Maali-Amiri R., Mahfoozi S., Fowler D.B., Mohammadi R. Developmental regulation of metabolites and low temperature tolerance in lines of crosses between spring and winter wheat. Acta Physiol. Plant. 2016;38:87. doi: 10.1007/s11738-016-2103-0. [DOI] [Google Scholar]
- 2.Hassan M.A., Xiang C., Farooq M., Muhammad N., Yan Z., Hui X., Yuanyuan K., Bruno A.K., Lele Z., Jincai L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021;12:676884. doi: 10.3389/fpls.2021.676884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darkó É., Pál M., Janda T. Abiotic stress responses and tolerance in wheat under climate change. In: Liu F., Li X., Hogy P., Jiang D., Brestic M., Liu B., editors. Sustainable Crop Productivity and Quality under Climate Change Responses of Crop Plants to Climate Change. Elsevier; Amsterdam, The Netherlands: Academic press; Cambridge, MA, USA: 2022. pp. 137–155. [DOI] [Google Scholar]
- 4.Hurry V.M., Strand A., Tobiaeson M., Gardestrom P., Oquist G. Cold Hardening of Spring and Winter Wheat and Rape Results in Differential Effects on Growth, Carbon Metabolism, and Carbohydrate Content. Plant Physiol. 1995;109:697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venzhik Y., Talanova V., Titov A. Features of the Photosynthetic Apparatus in Winter and Spring Wheat Plants with Different Cold Tolerance. Russ. Agric. Sci. 2014;40:233–236. doi: 10.3103/S1068367414040181. [DOI] [Google Scholar]
- 6.Janmohammadi M., Enayati V., Sabaghnia N. Impact of cold acclimation, de-acclimation and re-acclimation on carbohydrate content and antioxidant enzyme activities in spring and winter wheat. Icel. Agric. Sci. 2012;25:3–11. [Google Scholar]
- 7.Winfield M.O., Lu C., Wilson I.D., Coghill J.A., Edwards K.J. Plant responses to cold: Transcriptome analysis of wheat. Plant Biotechnol. J. 2010;8:749–771. doi: 10.1111/j.1467-7652.2010.00536.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsvetanov S., Atanassov A., Nakamura C. Gold Responsive Gene/Protein Families and Cold/Freezing Tolerance in Cereals. Biotechnol. Biotechnol. Equip. 2014;14:3–11. doi: 10.1080/13102818.2000.10819054. [DOI] [Google Scholar]
- 9.Savitch L.V., Harney T., Huner N.P.A. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol. Plant. 2000;108:270–278. doi: 10.1034/j.1399-3054.2000.108003270.x. [DOI] [Google Scholar]
- 10.Goyal K., Walton L.J., Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005;388:151–157. doi: 10.1042/bj20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler S., Thomashow M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talanova V., Titov A.F., Topchieva L., Malysheva I., Venzhik Y., Frolova S.A. Expression of WRKY Transcription Factor and Stress Protein Genes in Wheat Plants during Cold Hardening and ABA Treatment. Russ. J. Plant Physiol. 2009;56:702–708. doi: 10.1134/S1021443709050173. [DOI] [Google Scholar]
- 13.Takumi S., Koike A., Nakata M., Kume S., Ohno R., Nakamura C. Cold-specific and light-stimulated expression of a wheat (Triticum aestivum L.) Cor gene Wcor15 encoding a chloroplast-targeted protein. J. Exp. Bot. 2003;54:2265–2274. doi: 10.1093/jxb/erg247. [DOI] [PubMed] [Google Scholar]
- 14.Lin C., Thomashow M.F. A cold-regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem. Biophys. Res. Commun. 1992;183:1103–1108. doi: 10.1016/s0006-291x(05)80304-3. [DOI] [PubMed] [Google Scholar]
- 15.Artus N.N., Uemura M., Steponkus P.L., Gilmour S.J., Lin C., Thomashow M.F. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proc. Natl. Acad. Sci. USA. 1996;93:13404–13409. doi: 10.1073/pnas.93.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thalhammer A., Bryant G., Sulpice R., Hincha D.K. Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014;166:190–201. doi: 10.1104/pp.114.245399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarraf M., Vishwakarma K., Kumar V., Arif N., Das S., Johnson R., Janeeshma E., Puthur J.T., Aliniaeifard S., Chauhan D.K., et al. Metal/Metalloid-Based Nanomaterials for Plant Abiotic Stress Tolerance: An Overview of the Mechanisms. Plants. 2022;11:316. doi: 10.3390/plants11030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azameti M.K., Imoro A.-W.M. Nanotechnology: A promising field in enhancing abiotic stress tolerance in plants. Crop Des. 2023;2:100037. doi: 10.1016/j.cropd.2023.100037. [DOI] [Google Scholar]
- 19.Ijaz M., Khan F., Ahmed T., Noman M., Zulfiqar F., Rizwan M., Chen J., Siddique K.H.M., Li B. Nanobiotechnology to advance stress resilience in plants: Current opportunities and challenges. Mater. Today Bio. 2023;22:100759. doi: 10.1016/j.mtbio.2023.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zafar H., Javed R., Zia M. Nanotoxicity assessment in plants: An updated overview. Environ. Sci. Poll. Res. 2023;30:93323–93344. doi: 10.1007/s11356-023-29150-z. [DOI] [PubMed] [Google Scholar]
- 21.Sanzari I., Leone A., Ambrosone A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019;7:120. doi: 10.3389/fbioe.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solano R., Patino-Ruiz D., Tejeda-Benitez L., Herrera A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: A review on the applications, nanotoxicological effects, and risk control strategies. Env. Sci. Pollut. Res. Int. 2021;28:16962–16981. doi: 10.1007/s11356-021-12996-6. [DOI] [PubMed] [Google Scholar]
- 23.Goswami P., Yadav S., Mathur J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci. Today. 2019;6:232. doi: 10.14719/pst.2019.6.2.502. [DOI] [Google Scholar]
- 24.Alaqad K., Saleh T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016;6:384. doi: 10.4172/2161-0525.1000384. [DOI] [Google Scholar]
- 25.Dykman L., Khlebtsov N. Methods for chemical synthesis of colloidal gold. Russ. Chem. Rev. 2019;88:229. doi: 10.1070/RCR4843. [DOI] [Google Scholar]
- 26.Bandi R., Dadigala R., Alle M. Emerging role of gold nanoparticles for healthier crop plants growth and enhanced yield. In: Husen A., editor. Engineered Nanomaterials for Sustainable Agricultural Production, Soil Improvement and Stress Management. Elsevier; Amsterdam, The Netherlands: 2022. pp. 125–143. [Google Scholar]
- 27.Siegel J., Zaruba K., Svorcik V., Kroumanova K., Burketova L., Martinec J. Round-shape gold nanoparticles: Effect of particle size and concentration on Arabidopsis thaliana root growth. Nanoscale Res. Lett. 2018;13:95. doi: 10.1186/s11671-018-2510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramalingam V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid. Interface Sci. 2019;271:101989. doi: 10.1016/j.cis.2019.101989. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari E., Barbero F., Busquets-Fite M., Franz-Wachtel M., Kohler H.R., Puntes V., Kemmerling B. Growth-Promoting Gold Nanoparticles Decrease Stress Responses in Arabidopsis Seedlings. Nanomaterials. 2021;11:3161. doi: 10.3390/nano11123161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venzhik Y., Moshkov I., Dykman L. Gold Nanoparticles in Plant Physiology: Principal Effects and Prospects of Application. Russ. J. Plant Physiol. 2021;68:401–412. doi: 10.1134/S1021443721020205. [DOI] [Google Scholar]
- 31.Mahakham W., Theerakulpisut P., Maensiri S., Phumying S., Sarmah A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 32.Kumar V., Guleria P., Kumar V., Yadav S.K. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013;461–462:462–468. doi: 10.1016/j.scitotenv.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Das S., Debnath N., Pradhan S., Goswami A. Enhancement of photon absorption in the light-harvesting complex of isolated chloroplast in the presence of plasmonic gold nanosol—A nanobionic approach towards photosynthesis and plant primary growth augmentation. Gold Bull. 2017;50:247–257. doi: 10.1007/s13404-017-0214-z. [DOI] [Google Scholar]
- 34.Arora S., Sharma P., Kumar S., Nayan R., Khanna P., Zaidi M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012;66:303–310. doi: 10.1007/s10725-011-9649-z. [DOI] [Google Scholar]
- 35.Gunjan B., Zaidi M.G.H., Sandeep A. Impact of gold nanoparticles on physiological and biochemical characteristics of Brassica juncea. J. Plant Biochem. Physiol. 2014;2:3. doi: 10.4172/2329-9029.1000133. [DOI] [Google Scholar]
- 36.Gopinath K., Gowri S., Karthika V., Arumugam A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superb. J. Nanostructure Chem. 2014;4:115–125. doi: 10.1007/s40097-014-0115-0. [DOI] [Google Scholar]
- 37.Zaka M., Abbasi B.H., Rahman L.U., Shah A., Zia M. Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa. IET Nanobiotechnol. 2016;10:134–140. doi: 10.1049/iet-nbt.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadczak P., Kulpa D., Bihun M., Przewodowski W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ Cult. (PCTOC) 2019;139:191–197. doi: 10.1007/s11240-019-01656-w. [DOI] [Google Scholar]
- 39.Wan Y., Li J., Ren H., Huang J., Yuan H. Physiological investigation of gold nanorods toward watermelon. J. Nanosci. Nanotechnol. 2014;14:6089–6094. doi: 10.1166/jnn.2014.8853. [DOI] [PubMed] [Google Scholar]
- 40.Debnath P., Mondal A., Hajra A., Das C., Mondal N.K. Cytogenetic effects of silver and gold nanoparticles on Allium cepa roots. JGEB. 2018;16:519–526. doi: 10.1016/j.jgeb.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milewska-Hendel A., Gepfert V., Zubko M., Kurczyńska E. Morphological, histological and ultrastructural changes in Hordeum vulgare (L.) roots that have been exposed to negatively charged gold nanoparticles. Appl. Sci. 2022;12:3265. doi: 10.3390/app12073265. [DOI] [Google Scholar]
- 42.Avellan A., Yun J., Zhang Y., Spielman-Sun E., Unrine J.M., Thieme J., Li J., Lombi E., Bland G., Lowry G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano. 2019;13:5291–5305. doi: 10.1021/acsnano.8b09781. [DOI] [PubMed] [Google Scholar]
- 43.Torres R., Diz V.E., Lagorio M.G. Effects of gold nanoparticles on the photophysical and photosynthetic parameters of leaves and chloroplasts. Photochem. Photobiol. Sci. 2018;17:505–516. doi: 10.1039/c8pp00067k. [DOI] [PubMed] [Google Scholar]
- 44.Alkilany A.M., Murphy C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J., Xianyu Y. When nano meets plants: A review on the interplay between nanoparticles and plants. Nano Today. 2021;38:101143. doi: 10.1016/j.nantod.2021.101143. [DOI] [Google Scholar]
- 46.Lou-Franco J., Das B., Cao c., Elliott C. Gold Nanozymes: From Concept to Biomedical Applications. Nano-Micro Lett. 2020;13:1–36. doi: 10.1007/s40820-020-00532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai S., Wang B., Song Y., Xie Z., Li C., Li S., Huang Y., Jiang M. Astaxanthin and its gold nanoparticles mitigate cadmium toxicity in rice by inhibiting cadmium translocation and uptake. Sci. Total Environ. 2021;786:147496. doi: 10.1016/j.scitotenv.2021.147496. [DOI] [PubMed] [Google Scholar]
- 48.Jiang M., Dai S., Wang B., Xie Z., Li J., Wang L., Li S., Tan Y., Tian B., Shu Q., et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano. 2021;8:1042–1056. doi: 10.1039/D0EN01172J. [DOI] [Google Scholar]
- 49.Wahid I., Rani P., Kumari S., Ahmad R., Hussain S.J., Alamri S., Tripathy N., Khan M.I.R. Biosynthesized gold nanoparticles maintained nitrogen metabolism, nitric oxide synthesis, ions balance, and stabilizes the defense systems to improve salt stress tolerance in wheat. Chemosphere. 2022;287:132142. doi: 10.1016/j.chemosphere.2021.132142. [DOI] [PubMed] [Google Scholar]
- 50.Venzhik Y., Deryabin A., Popov V., Dykman L., Moshkov I. Gold nanoparticles as adaptogens increasing the freezing tolerance of wheat seedlings. Environ. Sci. Poll. Res. 2022;9:55235–55249. doi: 10.1007/s11356-022-19759-x. [DOI] [PubMed] [Google Scholar]
- 51.Rhaman M.S., Tania S.S., Imran S., Rauf F., Kibria M.G., Ye W., Hasanuzzaman M., Murata Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil Sci. Plant Nutr. 2022;22:4047–4062. doi: 10.1007/s42729-022-01007-3. [DOI] [Google Scholar]
- 52.Parveen A., Mazhari B.B.Z., Rao S. Impact of bio-nanogold on seed germination and seedling growth in Pennisetum glaucum. Enzym. Microb. Technol. 2016;95:107–111. doi: 10.1016/j.enzmictec.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Joshi A., Nayyar H., Dharamvir K., Verma G. Detection of gold nanoparticles signal inside wheat (Triticum aestivum L) and oats (Avena sativa) seedlings. AIP Conf. Proc. 2018;1953:030058. doi: 10.1063/1.5032393. [DOI] [Google Scholar]
- 54.Pissuwan D., Poomrattanangoon S., Chungchaiyart P. Trends in using gold nanoparticles for inducing cell differentiation: A review. ACS Appl. Nano Mater. 2022;5:3110–3120. doi: 10.1021/acsanm.1c04173. [DOI] [Google Scholar]
- 55.Falco W.F., Botero E.R., Falcão E.A., Santiago E.F., Bagnato V.S., Caires A.R.L. In vivo observation of chlorophyll fluorescence quenching induced by gold nanoparticles. J. Photochem. Photobiol. A Chem. 2011;225:65–71. doi: 10.1016/j.jphotochem.2011.09.027. [DOI] [Google Scholar]
- 56.Wang X., Yang X., Chen S., Li Q., Wang W., Hou C., Gao X., Wang L., Wang S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2015;6:1243. doi: 10.3389/fpls.2015.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan H., Alatawi A., Abdulmajeed A., Emam M., Khattab H. Roles of Si and SiNPs in Improving Thermotolerance of Wheat Photosynthetic Machinery via Upregulation of PsbH, PsbB and PsbD Genes Encoding PSII Core Proteins. Horticulturae. 2021;7:16. doi: 10.3390/horticulturae7020016. [DOI] [Google Scholar]
- 58.Hasanpour H., Maali Amiri R., Zeinali H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 2015;62:779–787. doi: 10.1134/S1021443715060096. [DOI] [Google Scholar]
- 59.Kreslavskii V., Los D., Allakhverdiev S., Kuznetsov V. Signaling Role of Reactive Oxygen Species in Plants under Stress. Russ. J. Plant Physiol. 2012;59:141–154. doi: 10.1134/S1021443712020057. [DOI] [Google Scholar]
- 60.John R., Anjum N.A., Sopory S.K., Akram N.A., Ashraf M. Some key physiological and molecular processes of cold acclimation. Biol. Plant. 2016;60:603–618. doi: 10.1007/s10535-016-0648-9. [DOI] [Google Scholar]
- 61.Keunen E., Peshev D., Vangronsveld J., Van Den Ende W., Cuypers A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013;36:1242–1255. doi: 10.1111/pce.12061. [DOI] [PubMed] [Google Scholar]
- 62.Kolupaev Y., Karpets Y., Kabashnikova L. Antioxidative System of Plants: Cellular Compartmentalization, Protective and Signaling Functions, Mechanisms of Regulation (Review) Appl. Biochem. Microbiol. 2019;55:441–459. doi: 10.1134/S0003683819050089. [DOI] [Google Scholar]
- 63.Rihan H.Z., Al-Issawi M., Fuller M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017;12:143–157. doi: 10.1080/17429145.2017.1308568. [DOI] [Google Scholar]
- 64.Chang C.Y., Brautigam K., Huner N.P.A., Ensminger I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021;229:675–691. doi: 10.1111/nph.16904. [DOI] [PubMed] [Google Scholar]
- 65.Deryabin A.N., Trunova T.I. Colligative effects of solutions of low-molecular sugars and their role in plants under hypothermia. Biol. Bull. 2021;48:29–37. doi: 10.1134/S1062359021060042. [DOI] [Google Scholar]
- 66.Khan M.R., Adam V., Rizvi T.F., Zhang B., Ahamad F., Josko I., Zhu Y., Yang M., Mao C. Nanoparticle-Plant Interactions: Two-Way Traffic. Small. 2019;15:e1901794. doi: 10.1002/smll.201901794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding Y., Shi Y., Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–1704. doi: 10.1111/nph.15696. [DOI] [PubMed] [Google Scholar]
- 68.Ouellet F., Vazquez-Tello A., Sarhan F. The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Lett. 1998;423:324–328. doi: 10.1016/s0014-5793(98)00116-1. [DOI] [PubMed] [Google Scholar]
- 69.Rehman S., Khushi M., Sher H., Que Y., Ali R., Ali S., Hassan I., Murad A., Rahat M. Molecular Analysis of Cold Responsive (COR) Genes in Selected Sugarcane and Saccharum spontaneum L. Adv. Life Sci. 2022;9:547–551. [Google Scholar]
- 70.NDong C., Danyluk J., Wilson K.E., Pocock T., Huner N.P., Sarhan F. Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses. Plant Physiol. 2002;129:1368–1381. doi: 10.1104/pp.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu F., Si H., Wang C., Sun G., Zhou E., Chen C., Ma C. Molecular evolution of Wcor15 gene enhanced our understanding of the origin of A, B and D genomes in Triticum aestivum. Sci. Rep. 2016;6:31706. doi: 10.1038/srep31706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi Y., Yamamoto S., Minami H., Kagaya Y., Hattori T. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C.W., Huang Y.C., Chang H.Y. CIA2 coordinately up-regulates protein import and synthesis in leaf chloroplasts. Plant Physiol. 2009;150:879–888. doi: 10.1104/pp.109.137240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dykman L., Khlebtsov N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 75.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 76.Wellburn A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 77.Lichtenthaler H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148C:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- 78.Nakamura M. Determination of Fructose in the Presence. Agric. Biol. Chem. 1968;32:701–706. doi: 10.1271/bbb1961.32.701. [DOI] [Google Scholar]
- 79.Kalinowska E., Chodorska M., Paduch-Cichal E., Mroczkowska K. An improved method for RNA isolation from plants using commercial extraction kits. Acta Biochim. Pol. 2012;59:391–393. doi: 10.18388/abp.2012_2127. [DOI] [PubMed] [Google Scholar]
- 80.Perdomo J.A., Buchner P., Carmo-Silva E. The relative abundance of wheat Rubisco activase isoforms is post-transcriptionally regulated. Photosynth. Res. 2021;148:47–56. doi: 10.1007/s11120-021-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudziak K., Sozoniuk M., Szczerba H., Kuzdraliński A., Kowalczyk K., Börner A., Nowak M. Identification of stable reference genes for qPCR studies in common wheat (Triticum aestivum L.) seedlings under short-term drought stress. Plant Methods. 2020;16:58. doi: 10.1186/s13007-020-00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue G.-P., Sadat S., Drenth J., McIntyre C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014;65:539–557. doi: 10.1093/jxb/ert399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to ethical reasons.