Abstract
This review aims to evaluate the efficacy of any vitamin administration(s) in preventing and managing COVID-19 and/or long-COVID. Databases were searched up to May 2023 to identify randomized clinical trials comparing data on the effects of vitamin supplementation(s) versus placebo or standard of care on the two conditions of interest. Inverse-variance random-effects meta-analyses were conducted to estimate pooled risk ratios (RRs) and 95% confidence intervals (CIs) for all-cause mortality between supplemented and non-supplemented individuals. Overall, 37 articles were included: two regarded COVID-19 and long-COVID prevention and 35 records the COVID-19 management. The effects of vitamin D in preventing COVID-19 and long-COVID were contrasting. Similarly, no conclusion could be drawn on the efficacy of multivitamins, vitamin A, and vitamin B in COVID-19 management. A few positive findings were reported in some vitamin C trials but results were inconsistent in most outcomes, excluding all-cause mortality (RR = 0.84; 95% CI: 0.72–0.97). Vitamin D results were mixed in most aspects, including mortality, in which benefits were observed in regular administrations only (RR = 0.67; 95% CI: 0.49–0.91). Despite some benefits, results were mostly contradictory. Variety in recruitment and treatment protocols might explain this heterogeneity. Better-designed studies are needed to clarify these vitamins’ potential effects against SARS-CoV-2.
Keywords: vitamin, COVID-19, systematic review, randomized clinical trials
1. Introduction
Viral infections are a significant global health concern [1]. They can lead to a wide range of illnesses, from the common cold to more severe and lethal diseases like influenza but also acquired immunodeficiency syndrome or COVID-19 [1,2]. Public health practices [3], including handwashing, social distancing, and other non-pharmacological treatments, are a cornerstone in reducing the spread of viral infections and minimizing their impact on individuals and communities [4]. Combined with vaccinations, they have mitigated the effects of the COVID-19 pandemic, during which searching for any effective supplemental treatment or preventive measure rekindled the interest in the role of micronutrients [5,6].
It is well-known that vitamins play a crucial role in infections [7] as they are essential for the proper functioning of the immune system. Clinical studies show that vitamin D deficiency is associated with a higher risk of respiratory infections, including COVID-19 [8]; some authors have reported the positive effect of vitamin A supplementation on the risk of severe diseases via an immunomodulatory and anti-inflammatory effect, replicable for COVID-19 [9,10], while a review has concluded that some reduction in duration and severity of common cold symptoms can be observed in regular supplementation trials [11].
As research progressed during the pandemic, data on the effects of vitamin administration in contributing to managing COVID-19 have accumulated [12,13,14,15] but evidence is still inconclusive [12,14] and their role in the prevention of the infection has not been systematically investigated to date. Additionally, some studies have explored the potential role of vitamins in managing the symptoms of long-COVID and post-acute sequelae of SARS-CoV-2 infection but with no clear conclusion [16]. Therefore, this systematic review and meta-analysis aimed to update the synthesis of evidence on the role of any vitamin supplementation, in any form or administration route, in the prevention and management of COVID-19 and/or long-COVID. The results could help clarify the clinical effects of these dietary supplements against SARS-CoV-2.
2. Materials and Methods
This systematic review was conducted according to the Cochrane Handbook for Systematic Reviews and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [17,18]. The review protocol was registered at PROSPERO, identifier CRD42022362055. Since this study did not involve primary data collection, the protocol was not submitted for institutional review board approval and did not require informed consent.
2.1. Inclusion and Exclusion Criteria
Eligible articles were randomized clinical trials (RCTs) conducted in any country, published in English or Italian, that compared data on the direct effects between (i) vitamin administration in any form, dosage, and route of administration and (ii) placebo or standard of care, in relation to the prevention and/or management of COVID-19 and/or long-COVID in people of any age. The following essential vitamins were considered eligible for inclusion [19], alone or in combination: vitamin A, vitamin C, vitamin D, vitamin E, vitamin K, vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folic acid), and vitamin B12 (cyanocobalamin). Long-COVID was defined as the continuation or development of new symptoms three months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least two months and no other explanation [20].
When a vitamin(s) was administered with other substances, the study was considered eligible only when the vitamin(s) effect could be isolated. Non-randomized trials, observational studies, studies using in vitro techniques, and studies conducted on animals, focusing only on the vitamin’s capacity to stimulate the participants’ immune response without a confirmed SARS-CoV-2 infection or investigating any indirect effect (i.e., any effect in non-supplemented individuals, such as the outcomes of vitamin supplementation in children born from women receiving the nutrient) were excluded.
2.2. Search Strategy
Two reviewers independently searched PubMed, Scopus, and Web of Science, from database inception to 26 May 2023. MedRxiv.org and bioRxiv.org were interrogated as pre-print databases using the “medrxivr” R package [21]. The following key terms were used: COVID-19; long-COVID; SARS-CoV-2; vitamin*; and provitamin*. The string was adapted to fit the search criteria of each database (Table S1). No filter was applied in the search strategy. Duplicate articles due to database overlap were removed and the titles and abstracts of the collected records were screened. Studies that clearly did not meet the inclusion criteria were excluded. Full texts of potentially relevant articles were retrieved and independently examined by two researchers. Disagreements were resolved through discussion and reasons for exclusion were recorded. The reference lists of retrieved articles were also manually searched to identify other potentially relevant studies.
2.3. Data Collection, Quality Assessment, and Data Synthesis
For each eligible study, two reviewers independently extracted the following information: general characteristics of the trial (i.e., first author, year of publication, and country); characteristics of the intervention (i.e., target population, sample size, vitamin administered, vitamin status at baseline, vitamin dose, vitamin route, vitamin frequency of administration, and follow-up time); area of evaluation (i.e., prevention or management of COVID-19 and/or long-COVID); main findings; and side effects or adverse events. Effects were classified into four categories: (i) immunological, hematological, and laboratory outcomes; (ii) clinical outcomes; (iii) length of hospitalization; and (iv) all-cause mortality. Articles providing data on different outcomes but from people enrolled in the same trial were grouped.
Two independent authors performed the quality assessment of the articles included in the systematic review using the revised Cochrane Risk-of-Bias tool version 2 for randomized studies [22]. Any discrepancy was resolved by consensus.
Then, given the high heterogeneity of the intervention protocols applied and the outcomes investigated, for each vitamin/multivitamin complex, a narrative synthesis of the main results was performed, distinguishing the two areas of evaluation (i.e., prevention or management of COVID-19 and/or long-COVID). In addition, for vitamins C and D, separate inverse-variance random-effects meta-analyses were performed to estimate pooled risk ratios (RRs) and 95% confidence intervals (CIs) for all-cause mortality, comparing the cumulative incidence of deceased patients between the intervention and control group. The Cochrane I2 metric was used to quantify heterogeneity. It was considered statistically significant at p-value < 0.05 and substantial heterogeneity was defined as I2 > 50% [23]. In the main analysis, we stratified by time-interval (i.e., mortality ≤ 14 days vs. mortality > 14 days from enrollment) considering the longest follow-up available, while in sensitivity analyses, whenever possible, we stratified by hospitalization setting (i.e., intensive care unit (ICU)-hospitalized patients vs. hospitalized patients vs. non-hospitalized patients), vitamin status at baseline (i.e., 100% vitamin deficient patients vs. other), route of administration (i.e., oral vs. intravenous administration), and frequency of administration (i.e., single vs. multiple administrations). Lastly, the small-study effect, potentially caused by publication bias, was investigated by visual inspection through funnel plot asymmetry.
Analyses were performed using Review Manager (RevMan, version 5.4, The Cochrane Collaboration, Copenhagen, Denmark), R Statistical Software (version 4.2.3; R Core Team 2023, R Foundation for Statistical Computing, Vienna, Austria), and the package “meta” [24].
3. Results
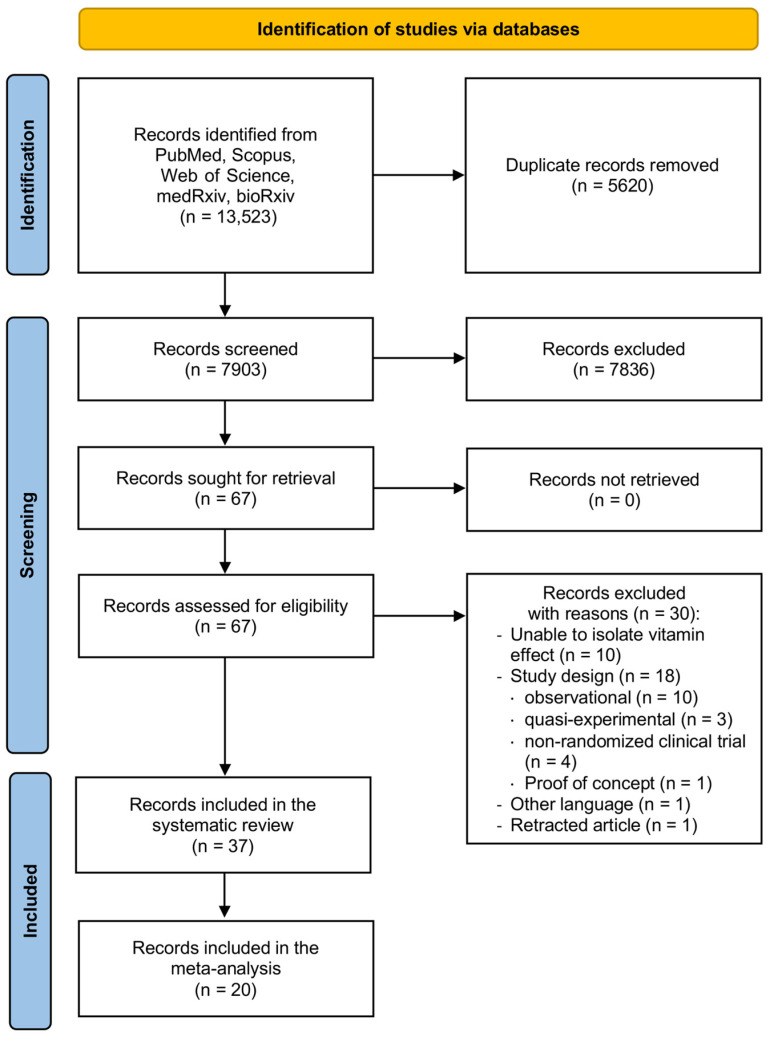
Overall, 13,523 records were identified by database search (Figure 1). After duplicate removal and screening by title and abstract, 67 articles were selected as eligible for full-text analysis, from which 30 were excluded with reasons, for a total of 37 articles ultimately included in the systematic review and 20 articles meta-analyzed.
Figure 1.
Flow diagram of the review process.
3.1. Characteristics of the Included Studies in the Prevention of COVID-19 and/or Long-COVID
Two studies [25,26] published in 2022 and conducted in the United Kingdom [25] and Mexico [26], respectively, investigated vitamin D supplementation in the prevention of COVID-19, one of which extended the analysis to include long-COVID data [25] (Table 1). While one study [25] recruited a large number of non-hospitalized subjects (over 5000 people), the other RCT [26] enrolled a smaller sample of healthcare workers (less than 350 participants). In both cases [25,26], approximately three-quarters of the sample were females, while the proportion of individuals with vitamin D deficiency ranged from 67% in one trial [26] to 100% in the other RCT [25]. Intervention protocols included the oral supplementation of cholecalciferol/vitamin D3 with different dosages, from 800 international units (IU) [25] to 4000 IU [26], administered once daily for six months [25] or 30 days [26], with a follow-up of 6 months [25] and 45 days [26], respectively. The risk of bias was judged as high in both studies [25,26] (Table S2).
Table 1.
Characteristics of the studies retrieved from the literature search and included in the systematic review that analyzed vitamin supplementation and prevention of COVID-19 and/or long-COVID.
| Author, Year | Country | Population | Vitamin Status at Baseline | Vitamin Dose, Form, Route of Administration | Frequency of Administration | Follow-Up Time | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| COVID-19 | |||||||
| Jolliffe, 2022 # [25] | United Kingdom | 5979 non-hospitalized subjects, adults, 77% female | 25-OH-vitamin D levels [mean ± SD)]: Group I: 40.9 ± 16.4 nmol/L Group II: 41.5 ± 18.0 nmol/L Group III: >75 nmol/L 100% vitamin D deficient subjects in Group I and Group II (≤75 nmol/L) 100% vitamin D non-deficient subjects in Group III (>75 nmol/L) |
Vitamin D3 3200 or 800 IU, oral | Once daily for 6 months | 6 months | High |
| Villasis-Keever, 2022 [26] | Mexico | 321 healthcare workers, SARS-CoV-2 negative, adults, 70% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 18.3 (14.6–22.9) ng/mL Group II: 17.1 (13.6–21.3) ng/mL 67% vitamin D deficient subjects (<20 ng/mL) |
Cholecalciferol 4000 IU, oral | Once daily for 30 days | 45 days | High |
| Long-COVID | |||||||
| Jolliffe, 2022 # [25] | United Kingdom | 5979 non-hospitalized subjects, adults, 77% female | 25-OH-vitamin D levels [mean ± SD)]: Group I: 40.9 ± 16.4 nmol/L Group II: 41.5 ± 18.0 nmol/L Group III: >75 nmol/L 100% vitamin D deficient subjects in Group I and Group II (≤75 nmol/L) 100% vitamin D non-deficient subjects in Group III (>75 nmol/L) |
Vitamin D3 3200 or 800 IU, oral | Once daily for 6 months | 6 months | High |
COVID-19: Coronavirus disease 2019. IQR: interquartile range. SD: standard deviation. # This trial evaluated two different outcomes.
3.2. Characteristics of the Included Studies on the Management of COVID-19 by Vitamin Type
3.2.1. Vitamin Co-Administration
Three studies investigated multivitamin supplementation in managing adult patients with COVID-19, two of which were conducted in Iran [9,27] and one in Mexico [28] (Table 2). The number of individuals enrolled was small in all trials (less than 100 patients), with the proportion of females ranging from 35% [28] to 49% [9]. The baseline levels of vitamins were measured in one case only [9]. Protocol interventions were heterogeneous: in one study [9], vitamin A, vitamin D, vitamin E, vitamin C, and vitamin B-complex were administrated intravenously with a varying dosage and frequency (from four times a day to only once in 7 days); in the second trial [27], vitamins C and E were administered orally once a day until discharge; in the last study [28], vitamins C and D were administered orally two times a day for 21 days. Follow-up ranged from 7 [9] to 40 days [28] or until hospital discharge [27]. The overall risk of bias was considered with some concerns [9], high [27] and low [28] (Table S2).
Table 2.
Characteristics of the studies included in the systematic review that analyzed the effects of vitamin supplementation in the management of COVID-19 by vitamin type.
| Author, Year | Country | Population | Vitamin Status at Baseline | Vitamin Dose, Form, and Route of Administration | Frequency of Administration | Follow-Up Time | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Vitamin co-administration | |||||||
| Beigmohammadi, 2021 [9] | Iran | 60 ICU-hospitalized patients with severe COVID-19, adults (20–60 years), 49% female | Group I: Vitamin A (median ± IQR): 0.20 ± 0.20 ng/mL Vitamin D (median ± IQR): 22.00 ± 9.07 ng/mL Vitamin E (mean ± SD): 11.30 ± 3.60 µg/mL Vitamin C (median ± IQR): 0.20 ± 0.20 mg/dL, Vitamin B9 (mean ± SD): 7.90 ± 3.80 ng/mL Vitamin B12 (mean ± SD): 480.34 ± 292.7 pg/mL Group II: Vitamin A (median ± IQR): 0.20 ± 0.22 ng/mL Vitamin D (median ± IQR): 22.00 ± 12.35 ng/mL Vitamin E (mean ± SD): 11.01 ± 2.53 µg/mL Vitamin C (median ± IQR): 0.10 ± 0.10 mg/dL Vitamin B9 (mean ± SD): 6.54 ± 3.10 ng/mL Vitamin B12 (mean ± SD): 521.25 ± 324.67 pg/mL |
Vitamin A: 25,000 IU, intravenous Vitamin D: 600,000 IU, intravenous Vitamin E: 300 IU, intravenous Vitamin C: 0.5 g, intravenous Vitamin B-complex: thiamine nitrate 3.1 mg, sodium riboflavin phosphate 4.9 mg, nicotinamide 40 mg, pyridoxine hydrochloride 4.9 mg, sodium pantothenate 16.5 mg, biotin 60 μg, folic acid 400 μg, and cyanocobalamin 5 μg, intravenous |
Vitamin A: once daily for 7 days Vitamin D: once Vitamin E: 2 times Vitamin C: 4 times/day for 7 days Vitamin B-complex: once daily for 7 days |
7 days | SC |
| Hakamifard, 2022 [27] | Iran | 73 hospitalized patients with non-severe COVID-19, adults (≥18 years), 37% female | NA | Vitamin C: 1 g, oral Vitamin E: 400 IU, oral |
Once daily until discharge | Until hospital discharge | High |
| Leal-Martínez, 2022 [28] | Mexico | 80 hospitalized patients with severe COVID-19, adults (30–75 years), 35% female | NA | Vitamin C: 2 g, oral Vitamin D: 4000 IU, oral Vitamin B-complex: thiamin 100 mg, cyanocobalamin 10 mg, pyridoxine 100 mg, folic acid 5 mg, intramuscular |
Vitamin C and D: 2 times/day for 21 days Vitamin B-complex: once daily for 5 days |
40 days | Low |
| Vitamin A | |||||||
| Rohani, 2022 [29] | Iran | 182 outpatients with COVID-19, adults (18–75 years), 41.8% female | NA | Vitamin A 25,000 IU, oral | Once daily for 10 days | Ten days | SC |
| Somi, 2022 [30] | Iran | 30 hospitalized patients with COVID-19, adults (≥18 years), 36.7% female | NA | Vitamin A 50,000 IU, intramuscular | Once daily for two weeks | Until hospital discharge | Low |
| Vitamin B | |||||||
| Majidi, 2022 [31] | Iran | 85 ICU-hospitalized patients with severe COVID-19, adults (35–85 years), 49.0% female | NA | Vitamin B complex, including thiamine (10 mg), riboflavin (4 mg), nicotinamide (40 mg), and dexpanthenol (6 mg), intramuscular | Daily for two weeks | Two weeks | SC |
| Hu, 2022 [32] | China | 24 hospitalized patients with COVID-19 and lymphopenia, adults (18–85 years), 54.2% female | NA | Nicotinamide 100 mg, not specified the route of administration | Five times daily for 2 days | Two days | High |
| Vitamin C | |||||||
| Coppock, 2022 [33] | United States | 66 hospitalized patients COVID-19, adults (≥18 years), 50% female | NA | Ascorbic acid 0.3 g/kg on day 0, 0.6 g/kg on day 1, 0.9 g/kg on days 2–5, intravenous | Once daily for 5 days | Until hospital discharge | Low |
| Fogleman, 2022 [34] | United States | 104 non-hospitalized patients with mild or moderate COVID-19, adults (≥40 years), 80% female | NA | 1 g, oral | Once daily for 14 days | 30 days | High |
| Jamali Moghadam Siahkali, 2021 [35] | Iran | 60 hospitalized patients with severe COVID-19, adults (>18 years), 50% female | NA | 1.5 g, intravenous | 4 times/day for 5 days | Until hospital discharge | High |
| Kumar, 2022 [36] | India | 60 ICU-hospitalized patients with moderate or severe COVID-19, adults (>18 years), 22% female | NA | 1 g, intravenous | 3 times/day for 4 days | Until hospital discharge | Low |
| Kumari, 2020 [37] | Pakistan | 150 hospitalized patients with severe COVID-19, adults (mean age: 52.5 years), 43% female | NA | 50 mg/kg, intravenous | Once daily, not specified the intervention duration | Until hospital discharge | High |
| Labbani-Mothlag, 2022 [38] | Iran | 90 hospitalized patients with moderate or severe COVID-19, adults (>18 years), 43% female | NA | 12 g, intravenous | 2 times/day for 4 days | Until hospital discharge | Low |
| Majidi, 2021 [39] | Iran | 120 ICU-hospitalized patients with severe COVID-19 and enteral nutrition, adults (35–75 years), 50% female | NA | 0.5 g, oral (enteral feeding) | Once daily for 14 days | Until hospital discharge | High |
| Ried, 2021 [40] | Australia, Turkey | 237 hospitalized patients with COVID-19, adults (22–99 years), 50% female | NA | 50 mg/kg or 100 mg/kg, intravenous | 4 times/day for 8 days | 45 days | High |
| Tehrani, 2021 [41] | Iran | 54 hospitalized patients with COVID-19, adults (>18 years), 61% female | NA | 2 g, intravenous | 4 times/day for 5 days | Until hospital discharge | High |
| Thomas, 2021 [42] | United States | 214 non-hospitalized patients with COVID-19, adults (≥18 years), 62% female | NA | 8 g, oral | 2 or 3 times/day for 10 days | 28 days | SC |
| Zhang, 2021 [43] | China | 56 ICU-hospitalized patients with severe COVID-19, adults (18–80 years), 44% female | NA | 12 g, intravenous | 2 times/day for 7 days | 28 days | SC |
| Vitamin D | |||||||
| Abroug, 2023 [44] | Tunisia | 117 individuals in isolation centers with COVID-19, adults (≥18 years), 44% female | NA | Cholecalciferol 200,000 IU/1 mL, oral | Single administration | 1 year after admission | SC |
| Bishop, 2022 [45] | United States | 171 non-hospitalized patients with mild or moderate COVID-19, adults (18–71 years), 46% female | 25-OH-vitamin D levels (mean ± SD): Group I: 37.7 ± 12.1 ng/mL Group II: 37.1 ± 15.6 ng/mL |
Calcifediol 30 μg, oral | 300 μg on days 1 to 3 and 60 μg on days 4 to 27 | 28 days | High |
| Bugarin, 2023 [46] | Croatia | 152 ICU-hospitalized patients with COVID-19, adults (>18 years), 72% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 25.3 (17.9–36.9) nmol/L Group II: 27.3 (16.0–37.3) nmol/L |
Cholecalciferol 10,000 IU, oral | Once daily for ICU stay or at least 14 days | Until hospital discharge | Some concerns |
| Bychinin, 2022 [47] | Russia | 110 ICU-hospitalized patients with severe COVID-19, adults (≥18 years), 50% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 9.6 (5.6–21.0) ng/mL Group II: 11.0 (8.6–15.0) ng/mL 51% severe vitamin D deficient patients (<10 ng/mL) |
Cholecalciferol 60,000 IU or 5000 IU, oral | High dose once/week followed by low dose once/day until discharge | Until hospital discharge | Low |
| Cannata-Andía, 2022 [48] | Spain, Argentina, Guatemala, Chile | 548 hospitalized patients with moderate to severe COVID-19, adults (>18 years), 37% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 17.0 (11.8–22.0) ng/mL Group II: 16.1 (11.5–22.0) ng/mL |
Cholecalciferol 100,000 IU, oral | Single administration | Until hospital discharge | SC |
| De Niet, 2022 [49] | Belgium | 50 hospitalized patients with COVID-19 and vitamin D deficiency, adults (≥18 years), 40% female | 25-OH-vitamin D levels (mean ± SD): Group I: 17.9 ± 10.2 ng/mL, Group II: 6.9 ± 9.5 ng/mL, 100% Vitamin D deficient patients (<20 ng/mL) |
Vitamin D3 25,000 IU, oral | Once daily for 4 consecutive days, then once weekly until hospital discharge or for 36 days | 9 weeks | SC |
| Elamir, 2022 [50] | United States | 50 hospitalized patients with COVID-19, adults (≥18 years), 50% female | NA | Calcitriol 0.5 μg, oral | Once daily for 14 days or hospital discharge | Until hospital discharge | SC |
| Entrenas Castillo, 2020 [51] | Spain | 76 hospitalized patients with severe COVID-19, adults (≥18 years), 61% female | NA | Calcifediol 0.532 mg or 0.266 mg, oral | 3 times/week in the first week, followed by once weekly until discharge or ICU admission | Until hospital discharge | Low |
| Karonova, 2022 [52] | Russia | 129 hospitalized patients with COVID-19, adults (18–75 years), 49% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 17.8 (11.7–25.4) ng/mL Group II: 15.4 (11.0–22.9) ng/mL 81% vitamin D deficient patients (<30 ng/mL) |
Cholecalciferol 50,000 IU, oral | 2 times, on day 1 and day 8 | 9 days | SC |
| Maghbooli, 2021 [53] | Iran | 106 hospitalized patients with COVID-19 and vitamin D deficiency, adults (>18 years), 40% female | 25-OH-vitamin D levels (mean ± SD): Group I: 19 ± 8 ng/mL Group II: 18 ± 8 ng/mL 100% Vitamin D deficient patients (<30 ng/mL) |
Calcifediol 25 μg (3000–6000 IU), oral | Once daily for 60 days | Two months after hospital discharge | High |
| Mariani, 2022 [54] | Argentina | 218 hospitalized patients with mild or moderate COVID-19 and at least one risk factor for disease progression, adults (≥18 years), 47% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 32.5 (27.2–44.2) ng/mL Group II: 30.5 (22.5–36.2) ng/mL |
Cholecalciferol 500,000 IU, oral | Single administration | Until hospital discharge | High |
| Murai [§], 2021 A [55] | Brazil | 240 hospitalized patients with moderate or severe COVID-19, adults (≥18 years), 43% female | 25-OH-vitamin D levels (mean ± SD): Group I: 21.2 ± 10.1 ng/mL Group II: 20.6 ± 8 ng/mL 48% vitamin D deficient patients (<20 ng/mL) |
Vitamin D3 200,000 IU, oral | Single administration | Until hospital discharge | Low |
| Murai [§], 2021 B [56] | Brazil | 32 hospitalized patients with moderate or severe COVID-19 and severe vitamin D deficiency, adults (≥18 years), 44% female | 25-OH-vitamin D levels (mean ± SD): Group I: 7.7 ± 1.6 ng/mL Group II: 7.7 ± 1.9 ng/mL 100% severe vitamin D deficient patients (<10 ng/mL) |
High | |||
| Fernandes [§], 2022 [57] | Brazil | 240 hospitalized patients with moderate or severe COVID-19, adults (≥18 years), 43% female | 25-OH-vitamin D levels (mean ± SD): Group I: 21.1 ± 10.1 ng/mL Group II: 20.2 ± 8.1 ng/mL |
4 months | High | ||
| Rastogi, 2021 [58] | India | 40 hospitalized patients with mild or moderate COVID-19, adults, 50% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 8.6 (7.1–13.1) ng/mL Group II: 9.54 (8.1–12.5) ng/mL 100% severe vitamin D deficient patients (<20 ng/mL) |
Cholecalciferol 60,000 IU, oral | Once daily for 7 days, followed by once weekly for the following 7 days (if 25-OH-vitamin D levels > 50 ng/mL) or once daily for the following 7 days (if 25-OH-vitamin D levels <50 ng/mL) | 21 days | High |
| Sánchez-Zuno, 2021 [59] | Mexico | 42 non-hospitalized patients with mild or moderate COVID-19, adults (>18 years), 52% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 20.2 (12.2–45.9) ng/mL Group II: 23.4 (12.1- 45.6) ng/mL 80% vitamin D deficient or insufficient patients (<30 ng/mL) |
Vitamin D3 10,000 IU, oral | Once daily for 14 days | 14 days | SC |
| Zurita-Cruz, 2022 [60] | Mexico | 45 hospitalized patients with moderate COVID-19, pediatric patients (1–17 years), 60% female | 25-OH-vitamin D levels [median (IQR)]: Group I: 13.8 (10.8–18.4) ng/mL Group II: 11.4 (8.7–13.1) ng/mL 100% severe Vitamin D deficient patients (<20 ng/mL) |
Vitamin D3 1000 IU/day among children < 1 year or 2000 IU/day among children 1–17 years, oral | Once daily for 7–14 days | 14 days | High |
§ studies with the same symbol included participants from the same trial. COVID-19: coronavirus disease 2019. ICU: intensive care unit. IQR: interquartile range. IU: international unit. SC: some concerns. SD: standard deviation.
3.2.2. Vitamin A
Two studies conducted in 2022 in Iran examined vitamin A supplementation in the management of COVID-19 in hospitalized [30] and non-hospitalized patients [29], respectively (Table 2). In both cases, the sample was small (less than 200 participants) with a female proportion of around 40%. Vitamin A levels were not tested at baseline but it was supplemented intramuscularly at a dosage of 50,000 IU in hospitalized patients [30] and orally at half of the dosage in outpatients [29]. In both studies, patients took vitamin A daily, with a similar duration of treatment (two weeks in hospitalized patients and 10 days in non-hospitalized patients). Participants were followed until hospital discharge [30] or for 10 days [29]. The overall risk of bias was low [30] and with some concerns [29] (Table S2).
3.2.3. Vitamin B
Vitamin B supplementation in COVID-19 management was investigated in two studies conducted in 2022 in Iran [31] and in China [32] (Table 2). Participants were enrolled among hospitalized patients with COVID-19 [32] and in one case they were recruited from the ICU setting [31]. The population was small, ranging from 24 [32] to 85 [31] individuals, of whom about 50% were females. The levels of vitamin B at baseline were not assessed in any study and the route of administration was specified only in one case [31], in which vitamin B was supplemented intramuscularly. Vitamin form and dosage differed between the two studies as well as the duration of treatment and follow-up. Specifically, in one case, vitamin B complex was supplemented daily for two weeks with an additional two weeks of follow-up [31], while only nicotinamide was administered five times daily for a shorter time and follow-up (two days for both) [32]. The overall risk of bias was considered with some concerns [31] and high [32] (Table S2).
3.2.4. Vitamin C
A total of 11 RCTs analyzed vitamin C supplementation in the COVID-19 management (Table 2). They were all conducted between 2020 and 2022 in Iran (n = 4) [35,38,39,41], the United States (n = 3) [33,34,42], India (n = 1) [36], Pakistan (n = 1) [37], Australia and Turkey (n = 1) [40], and China (n = 1) [43]. The sample size was relatively small, ranging from 54 [41] to 237 adults [40]. Participants were mainly recruited among hospitalized patients with moderate or severe COVID-19 (n = 6) [33,35,37,38,40,41], in three cases among ICU patients [36,39,43], and in the other two studies among non-hospitalized adults with mild or moderate COVID-19 [34,42]. The female proportion ranged from 22% [36] to 80% [34]. Vitamin C levels were not tested at baseline in any study but it was administered orally in three cases [34,39,42] and intravenously in the other eight studies [33,35,36,37,38,40,41,43]. The dosage was heterogeneous, from 0.5 g [36] to 12 gr [38], similar to the frequency of administration, from once daily for 5 days [33,34,39,42] to 4 times/day for at least 5 days [35,40,41]. Patients were followed until hospital discharge in seven studies [33,35,36,37,38,39,41] and for approximately one month in the other cases [34,40,42,43]. The risk of bias was judged as high in six studies [34,35,37,39,40,41], with some concerns in two trials [42,43], and low in the remaining three cases [33,36,38] (Table S2).
3.2.5. Vitamin D
In total, 17 articles referring to 15 RTCs reported data on vitamin D supplementation in COVID-19 management (Table 2). They were conducted in Tunisia (n = 1 RCT) [44], the United States (n = 2 RCTs) [45,50], Croatia (n = 1 RCT) [46], Russia (n = 2 RCTs) [47,52], Belgium (n = 1 RCT) [49], Spain (n = 1 RCT) [51], Iran (n = 1 RCT) [53], Argentina (n = 1 RCT) [54], India (n = 1 RCT) [58], Mexico (n = 2 RCTs) [59,60], Brazil (n = 1 RCT, n = 3 papers) [55,56,57], and one RCT in four countries [48] (Table 2). The population enrolled was relatively small, ranging from 40 [58] to 240 individuals [55,56,57], with a varying proportion of females, from 37% [48] to 72% [46]. All but three trials [44,45,59] recruited hospitalized patients with mild, moderate, or severe COVID-19 and, among these, only one study considered the pediatric population [60]. Vitamin D at baseline was assessed in nine RCTs, with the proportion of vitamin D-deficient individuals ranging from 48% [55] to 100% [49,53,58,60]. Vitamin D was always administered orally in the form of cholecalciferol/vitamin D3 in 11 RCTs [44,46,47,48,49,52,54,55,58,59,60], calcifediol in 3 trials [45,51,53], and calcitriol in the remaining 1 RCT [50]. Dosage and frequency of administration were strongly heterogeneous: a single administration in four RCTs [44,48,54,55], two administrations in one trial [52], once daily for 7–14 days [46,50,59,60] or 60 days [53] in three cases, respectively, and in RCTs it consisted of de-escalation schedules [45,47,49,51,58]. Follow-up time ranged from 9 days [52] to one year [44,57] but about half of the studies followed participants until hospital discharge [46,47,48,50,51,54,55]. In seven papers [45,53,54,56,57,58,60], the risk of bias was judged as high, in the other seven studies [44,46,48,49,50,52,59] with some concerns, and in the remaining three cases [47,51,55] low (Table S2).
3.3. Main Findings of Vitamin Administration in the Prevention of COVID-19 and/or Long-COVID
One of the two articles [25] that analyzed the effects of administering vitamin D in COVID-19 and long-COVID prevention compared a high dose of supplementation, a low dose of supplementation, and no supplementation at all, while the other RCT [26] compared a high dose of supplementation to a placebo (Table 3). While vitamin D administration seemed to not influence the prevention of long-COVID symptoms at month six in the only study that analyzed it [25], data about the prevention of COVID-19 risk were contrasting, with one study [25] reporting a non-significant difference between treated and untreated in the prevention of SARS-CoV-2 infection, risk of hospitalization, and mortality at six months, whereas the Mexican RCT [26] found a significantly lower proportion of infections among vitamin D-supplemented individuals. Side effects did not differ between the intervention and control groups in both studies [25,26].
Table 3.
Main effects of vitamin administration in the prevention of COVID-19 and/or long-COVID.
| Author, Year | Intervention | Clinical Outcomes | Mortality | Side Effects or Adverse Events |
|---|---|---|---|---|
| COVID-19 | ||||
| Jolliffe, 2022 [25] # | Group I: High dose vitamin D Group II: Low dose vitamin D Group III: No vitamin |
|
NA |
|
| Villasis-Keever, 2022 [26] | Group I: vitamin D3 Group II: Placebo |
|
NA |
|
| Long-COVID | ||||
| Jolliffe, 2022 [25] # | Group I: High dose vitamin D Group II: Low dose vitamin D Group III: No vitamin |
|
|
|
COVID-19: Coronavirus disease 2019. FACIT: functional assessment of chronic illness therapy. MRC: United Kingdom Medical Research Council. NA: not assessed. # this trial evaluated two different outcomes.
3.4. Main Findings of Vitamin Administration in the Management of COVID-19 by Vitamin Type
3.4.1. Vitamin Co-Administration
In the three trials investigating multivitamin supplementation, the combination of vitamins was compared with no supplementation in two cases [9,28] or with the standard of care [27] (Table 4). Results were contrasting: a reduction in some inflammatory parameters in supplemented patients was registered in one study [9] but not in the other [27], whereas Leal-Martínez et al. [28] reported a significantly higher hydric balance and lower gastrointestinal distension among treated individuals but a non-significant difference in post-COVID syndrome, weight decrease, and gastrointestinal symptoms at day 40 from enrollment. Similarly, in the intervention group, a lower gravity score and length of hospitalization were found in the trial by Beigmohammadi et al. [9]; a lower mortality and higher saturation were registered by Leal-Martínez et al. [28], while Hakamifard et al. [27] did not report any difference between the two arms in clinical outcomes such as ICU-admission rate, temperature, and pulse rate, apart from a lower respiratory rate that was reported in patients who had received vitamins C and E. Side effects were not reported in the only study in which they were assessed [9].
Table 4.
Main effects of supplementation with multivitamins and vitamin A, vitamin B, and vitamin C in the management of COVID-19.
| Author, Year | Intervention | Immunological, Hematological, and Laboratory Outcomes |
Clinical Outcomes and Mortality | Length of Hospitalization | Side Effects or Adverse Events |
|---|---|---|---|---|---|
| Vitamin co-administration | |||||
| Beigmohammadi, 2021 [9] | Group I: Vitamin A + Vitamin D + Vitamin E + Vitamin C + Vitamin B (in combination) Group II: No vitamin |
|
|
|
|
| Hakamifard, 2022 [27] | Group I: Vitamin C + Vitamin E + SoC Group II: SoC |
|
|
|
NA |
| Leal-Martínez, 2022 [28] | Group I: Vitamin C + Vitamin D + Vitamin B (in combination) Group II: No vitamin |
|
|
NA | NA |
| Vitamin A | |||||
| Rohani, 2022 [29] | Group I: Vitamin A + SoC Group II: SoC + placebo |
|
|
NA | NA |
| Somi, 2022 [30] | Group I: Vitamin A + SoC Group II: SoC |
NA |
|
|
|
| Vitamin B | |||||
| Majidi, 2022 [31] | Group I: Vitamin B complex Group II: nutritional support without Vitamin B complex |
|
|
NA | NA |
| Hu, 2022 [32] | Group I: Vitamin B + SoC Group II: SoC |
|
|
NA | NA |
| Vitamin C | |||||
| Coppock, 2022 [33] | Group I: Vitamin C + SoC Group II: SoC |
NA |
|
|
|
| Fogleman, 2022 [34] | Group I: Vitamin C Group II: Melatonin Group III: Placebo |
NA |
|
NA | NA |
| Jamali Moghadam Siahkali, 2021 [35] | Group I: Vitamin C + SoC Group II: SoC |
NA |
|
|
|
| Kumar, 2022 [36] | Group I: Vitamin C + SoC Group II: Placebo + SoC |
NA |
|
NA | NA |
| Kumari, 2020 [37] | Group I: Vitamin C + SoC Group II: SoC |
NA |
|
|
NA |
| Labbani-Mothlag, 2022 [38] | Group I: Vitamin C + SoC Group II: Placebo + SoC |
|
|
|
NA |
| Majidi, 2021 [39] | Group I: Vitamin C Group II: No vitamin |
|
|
NA |
|
| Ried, 2021 [40] | Group I: Vitamin C + Vitamin D + SoC Group II: Vitamin D + SoC |
NA |
|
NA |
|
| Tehrani, 2021 [41] | Group I: Vitamin C + SoC Group II: SoC |
|
|
|
NA |
| Thomas, 2021 [42] | Group I: Vitamin C Group II: Zinc gluconate Group III: Zinc + Vitamin C Group IV: SoC |
NA |
|
NA |
|
| Zhang, 2021 [43] | Group I: Vitamin C + SoC Group II: Placebo + SoC |
|
|
|
|
BG: blood glucose. BUN: blood urea nitrogen. CBC: cell blood count. CRP: C-reactive protein. CS: Glasgow Coma Scale. ESR: erythrocyte sedimentation rate. ICU: intensive care unit. IFN: interferon. IL: interleukin. IMV: invasive mechanical ventilation. INR: international normalized ratio of prothrombin time of blood coagulation. LDH: lactate dehydrogenase. MAP: mean arterial pressure. NA: not assessed. NEWS: national early warning score. NLR: neutrophil-to-lymphocyte ratio. RBC: red blood cell. SoC: standard of care. SOFA: sequential organ failure assessment. SF ratio: SpO2/FiO2 ratio. TNF: tumor necrosis factor. WBC: white blood cell. WURSS: Wisconsin Upper Respiratory Symptom Survey.
3.4.2. Vitamin A
The effects of vitamin A were evaluated by comparing its administration with the standard of care with [29] or without a placebo [30] (Table 4). No significant difference was found in the trial of Somi et al. between the two groups, neither in clinical outcomes such as ICU-admission rate, need for invasive or non-invasive mechanical ventilation, time for clinical response and treatment strategies, nor in mortality and in length of hospitalization. By contrast, a significant improvement in some clinical symptoms such as fever, body aches, weakness, and fatigue and also significant differences in immunological response were detected in the other trial [29]. Only one study [30] investigated side effects and found no events.
3.4.3. Vitamin B
The effects of vitamin B were investigated by comparing the administration of vitamin B complex with nutritional complex without vitamin B in one trial [31] and vitamin B with the standard of care in the other one [32] (Table 4). No immunological difference was detected in both trials between the two arms, as well as no difference in hematological parameters or in organ function indexes [31,32]. Similarly, clinical outcomes such as oxygenation parameters, clinical aggravation, and mortality showed no difference between groups in the two studies [31,32]. The effects of vitamin B on the duration of hospitalization were not evaluated in the studies, in which the side effects were also not investigated [31,32].
3.4.4. Vitamin C
The effects of vitamin C were mainly evaluated by comparing its administration with the standard of care, with [36,38,43] or without a placebo [33,35,37,41,42] (Table 4). No difference was observed in the four trials [38,39,41,43] investigating immunological response and other hematological and laboratory parameters, except for lower levels of Interleukin-6 detected after one week among patients treated with vitamin C in the study of Zhang et al. [43]. All studies assessed the clinical outcomes: of these, five trials [34,36,38,39,43] did not find any difference in severity scores, including SOFA (sequential organ failure assessment) or GCS (Glasgow Coma Scale), while five trials [33,35,36,37,43] did not report any difference in clinical improvement, oxygenation parameters, need for invasive or non-invasive mechanical ventilation, intubation, or admission to the ICU. By contrast, three authors [35,41,43] registered better respiratory parameters during the first day of hospitalization among supplemented individuals, even though the overall clinical improvement was not found to differ in two trials [42,43], was faster in one case [37], or was greater in another study [40].
As for length of hospitalization, four trials found no difference between the intervention and control group [33,38,41,43], one study [37] found it was shorter among treated individuals, whereas another study [35] found that it was longer, but with no difference after restriction to the ICU-admitted patient subgroup. Out of the six studies that analyzed side effects [33,35,39,40,42,43], only one found a higher proportion of events in the intervention group [42].
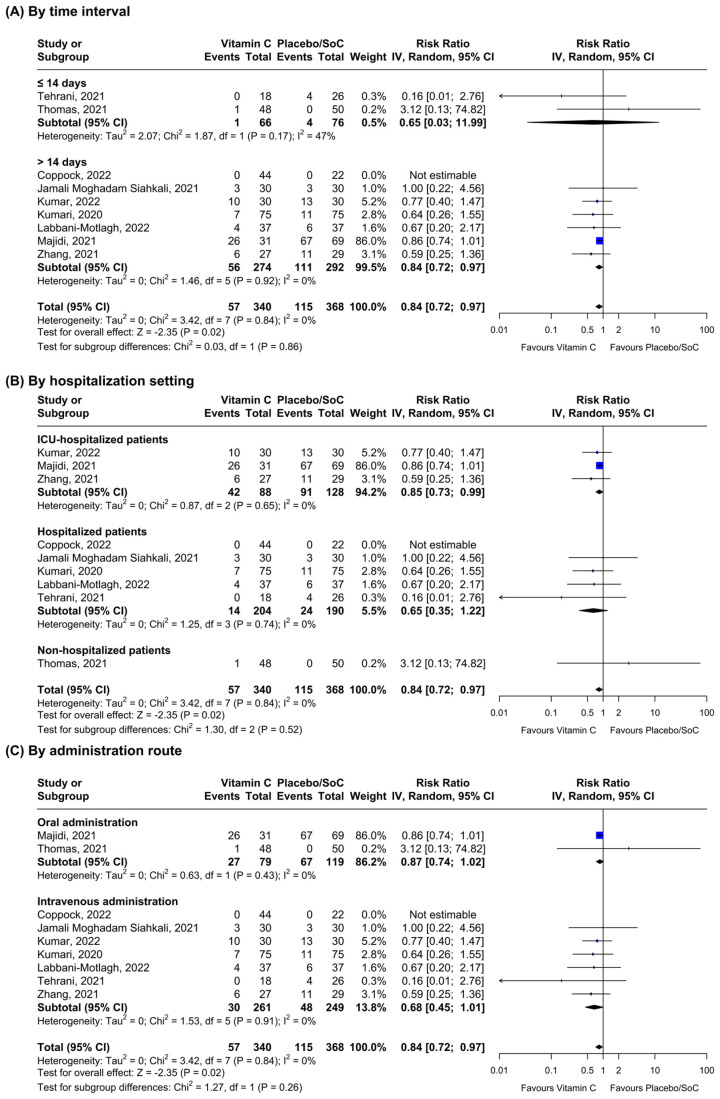
Regarding all-cause mortality, nine RCTs provided data on the outcome and were included in the meta-analysis (Figure 2). Vitamin C supplementation seemed to reduce mortality in the overall analysis (n = 9, RR = 0.84; 95% CI: 0.72–0.97, I2 = 0.0%) and in the subgroup in which mortality was quantified after 14 days from enrollment (n = 7, RR = 0.84; 95% CI: 0.72–0.97, I2 = 0.0%). Sensitivity analyses by hospitalization setting showed significant results among ICU-hospitalized patients only (n = 3, RR = 0.85; 95% CI: 0.73–0.99, I2 = 0.0%), whereas vitamin C administration did not lead to reduced mortality in any group following stratification by administration route (oral supplementation: n = 2, RR = 0.87; 95% CI: 0.74–1.02, I2 = 0.0%, and intravenous supplementation: n = 6, RR = 0.68; 95% CI: 0.45–1.01, I2 = 0.0%, respectively). Heterogeneity between the subgroups was always non-significant (p > 0.05). Funnel plot analysis showed some evidence of asymmetry (Figure S1).
Figure 2.
Stratified inverse-variance random-effects meta-analyses for all-cause mortality comparing patients receiving vitamin C vs. placebo or standard of care (SoC) [33,35,36,37,38,39,41,42,43].
3.4.5. Vitamin D
Vitamin D administration was compared to placebo in five trials [44,45,47,54,55], to standard of care in two studies [46,59], and no supplementation in two RCTs [48,52], whereas it was added to standard of care (with or without placebo) in the remaining six trials [49,50,51,53,58,60] (Table 5). Eight authors [45,46,48,51,55,57,58,59] did not report a difference between the intervention and control group in any of the immunological, hematological, and laboratory parameters investigated, whereas three trials [47,52,53] registered better outcomes during hospital stay but only in a few factors, including neutrophil cell count, neutrophil-to-lymphocyte ratio, and natural killer cell count. A significantly longer duration of viral RNA conversion was also reported in the supplemented group by Abroug et al. [44]. As for the clinical effects, severity scores such as SOFA were not found to differ [54], whereas some respiratory parameters were better among treated individuals in two cases [49,50] but not in another trial [52]. A need for intubation, respiratory support, and ICU admission rate were non significantly different between the two groups in most cases [46,47,48,49,50,52,54,55,56], even though two trials reported positive results favoring supplemented individuals [48,60]. Signs or symptoms of greater clinical improvement during hospital stay or at discharge were mentioned in two [49,59] out of the six RCTs that investigated such outcomes [44,45,46,48,49,59]. Furthermore, no difference was found in the duration of infection or the proportion of patients with re-infection at one year between the two groups [44,59]. Length of hospitalization was found to be higher among supplemented individuals in one trial [47], it was shorter in another trial [49], and did not differ in the other RCTs, considering both the overall patients [46,49,50,53,55,56], only those admitted to the ICU [46,49,53,54], or only those with vitamin D deficiency at baseline [52,55,56]. Side effects were not detected in five trials [46,47,49,50,53] or were not found to differ in the other four RCTs that were investigated [51,54,55,60].
Table 5.
Main effects of vitamin D administration in the management of COVID-19.
| Author, Year | Intervention | Immunological, Hematological, and Laboratory Outcomes |
Clinical Outcomes | Length of Hospitalization | Side Effects or Adverse Events |
|---|---|---|---|---|---|
| Abroug, 2023 [44] | Group I: Vitamin D Group II: Placebo |
|
|
NA | NA |
| Bishop, 2022 [45] | Group I: Vitamin D Group II: Placebo |
|
|
NA | NA |
| Bugarin, 2023 [46] | Group I: Vitamin D Group II: SoC |
|
|
|
|
| Bychinin, 2022 [47] | Group I: Vitamin D Group II: Placebo |
|
|
|
|
| Cannata-Andía, 2022 [48] | Group I: Vitamin D Group II: No vitamin |
|
|
|
NA |
| De Niet, 2022 [49] | Group I: Vitamin D + SoC Group II: Placebo + SoC |
NA |
|
|
|
| Elamir, 2022 [50] | Group I: Vitamin D + SoC Group II: SoC |
NA |
|
|
|
| Entrenas Castillo, 2020 [51] | Group I: Vitamin D + SoC Group II: SoC |
|
|
NA |
|
| Karonova, 2022 [52] | Group I: Vitamin D Group II: No vitamin |
|
|
|
NA |
| Maghbooli, 2021 [53] | Group I: Vitamin D + SoC Group II: Placebo + SoC |
|
|
|
|
| Mariani, 2022 [54] | Group I: Vitamin D Group II: Placebo |
NA |
|
|
|
| Murai [§], 2021 A [55] | Group I: Vitamin D Group II: Placebo |
|
|
|
|
| Murai [§], 2021 B [56] | Group I: Vitamin D Group II: Placebo |
NA |
|
|
NA |
| Fernandes [§], 2022 [57] | Group I: Vitamin D Group II: Placebo |
|
NA | NA | NA |
| Rastogi, 2021 [58] | Group I: Vitamin D + SoC Group II: Placebo + SoC |
|
|
NA | NA |
| Sánchez-Zuno, 2021 [59] | Group I: Vitamin D Group II: SoC |
|
|
NA | NA |
| Zurita-Cruz, 2022 [60] | Group I: Vitamin D + SoC Group II: SoC |
NA |
|
NA |
|
§ Studies with the same symbol included participants from the same trial. CRP: C-reactive protein. eGFR: estimated glomerular filtration rate. IFN: interferon. GM-CSF: granulocyte-macrophage colony-stimulating factor. IL: interleukin. ICU: intensive care unit. LDH: lactate dehydrogenase. MCP-1: monocyte chemoattractant protein-1. MIP-1β: macrophage inflammatory protein-1β. NA: not assessed. NLR: neutrophil-to-lymphocyte ratio. NK: natural killer. NKT: natural killer T. RBC: red blood cell. SoC: standard of care. SOFA: sequential organ failure assessment. qSOFA: quick SOFA. rSOFA: respiratory SOFA. ESR: erythrocyte sedimentation rate. TLC: total leucocyte count. TNF: tumor necrosis factor. VEGF: vascular endothelial growth factor. WBC: white blood cell.
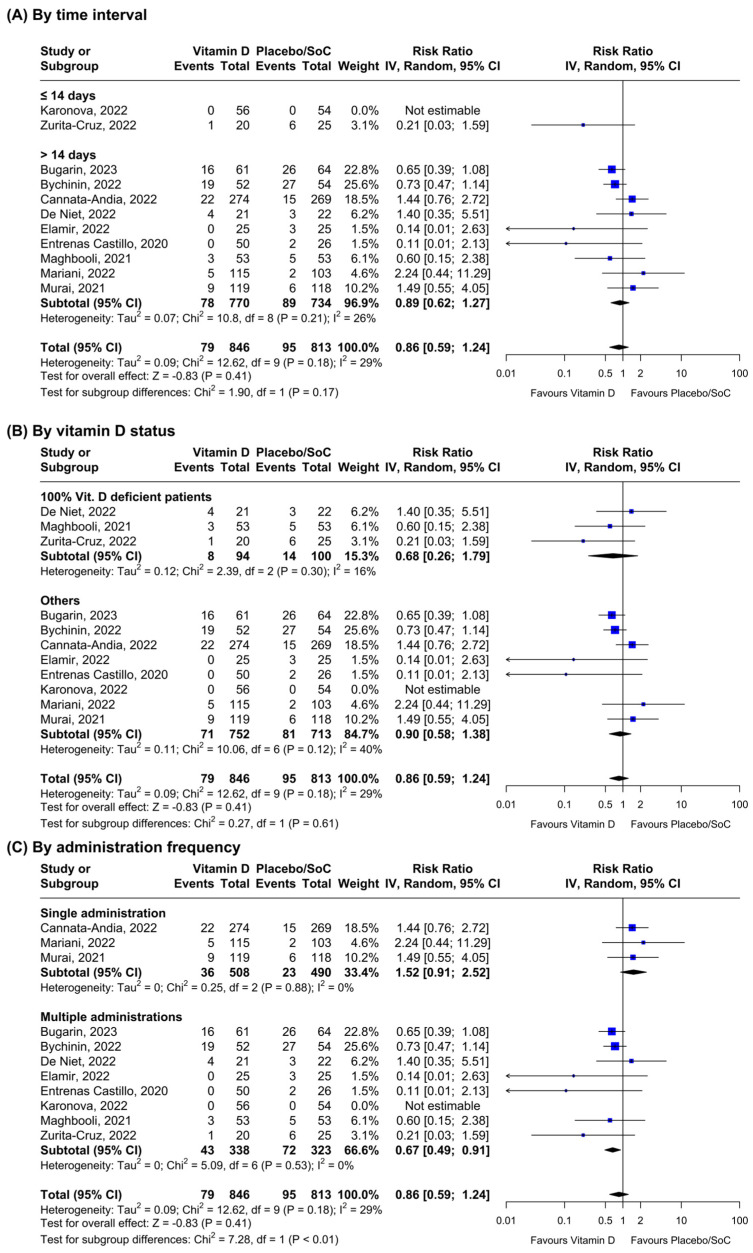
As for all-cause mortality, 11 RCTs provided data on vitamin D and all-cause mortality (Figure 3). The results did not show any significant reduction in the outcome incidence in the overall (n = 10, RR = 0.86; 95% CI: 0.59–1.24, I2 = 29%) and time-stratified analyses (mortality ≤ 14 days: n = 1, RR = 0.21; 95% CI: 0.03–1.59; and mortality > 14 days: n = 9, RR = 0.89; 95% CI: 0.62–1.27, I2 = 26%, respectively). At sensitivity analyses, vitamin D supplementation did not seem to reduce mortality according to the vitamin level at baseline (100% vitamin D deficient patients: n = 3, RR = 0.68; 95% CI: 0.26–1.69, I2 = 16%; and others: n = 7, RR = 0.90; 95% CI: 0.58–1.38, I2 = 40%, respectively). However, multiple administrations of vitamin D seemed to have a positive effect on the outcome (n = 7, RR = 0.67; 95% CI: 0.49–0.91, I2 = 0%) compared to the single administration subgroup (n = 3, RR = 1.52; 95% CI: 0.91–2.52, I2 = 0%). Heterogeneity between the subgroups was always non-significant apart from the frequency of administration stratification (p < 0.01). Funnel plot analysis revealed moderate asymmetry (Figure S2).
Figure 3.
Stratified inverse-variance random-effects meta-analyses for all-cause mortality comparing patients receiving Vitamin D vs. placebo or standard of care (SoC) [46,47,48,49,50,51,52,53,54,55,60].
4. Discussion
Despite growing evidence [61,62] that proves the role of supplement compounds in supporting the immune system, the debate about the use of natural agents in the prevention and management of viral infections is still far from solved [63,64,65]. Indeed, although it is well-known that vitamins are critical to making the immune system work properly [66,67], the results coming from clinical trials on the role of these substances in COVID-19 disease are inconclusive [13,68]. It is not surprising that many reviews have already been published on the topic [13,15,69,70] but they mostly focused on one vitamin only [14,15,68,71] or a specific outcome in COVID-19 patients [13,69,70]. Alternatively, or in addition, they had different inclusion criteria [12,14,68], such as the investigation of vitamin administration in combination with other substances or they were not updated [14,69,71,72,73]. Therefore, we systematically reviewed all evidence on the role of any vitamin in the prevention and management of COVID-19 but also long-COVID, with the aim to provide healthcare professionals with a wider overview of the topic, including the latest available evidence, and considering simultaneously several aspects, such as different populations, settings, vitamin dosages, and route of administrations. We found that the prevention of COVID-19 was little investigated and in relation to vitamin D only. Moreover, given the contrasting findings, no clear conclusion could be drawn on the potential role of such vitamins in reducing the SARS-CoV-2 infection risk [25,26]. Similarly, even though vitamin D seems to provide some benefits in the long-term complications of COVID-19 [74], its effects in the prevention of long-COVID symptoms were poorly studied, together with the effects of vitamin co-administration in the management of COVID-19 patients, both of which did not show any robust result. However, considering the low quality of most trials included and the relatively safe profile of these substances, additional data could be produced in the near future, allowing a more conclusive judgment on the potential benefits of vitamin D as a prevention therapy and vitamin co-administration as a supplemental treatment for COVID-19 patients [75,76].
As for the efficacy of individual vitamin supplementation in managing SARS-CoV-2 infections, we found limited evidence on vitamins A and B, with most outcomes showing no additional benefit [29,30,31,32]. As for vitamin C, even though its role as an antioxidant agent [77,78] and necessary micronutrient for leukocyte function is well recognized [79], a few positive findings were reported in some trials but results were largely inconsistent in relation to most outcomes. Among these, all-cause mortality was the only one in which COVID-19 vitamin-supplemented individuals seemed to have significant benefits, in line with a recent meta-analysis that found reduced mortality rates after vitamin C administration in many diseases [80]. These results also align with other similar reviews conducted on COVID-19 subjects [13,15,71] and might be explained by the fact that patients with pneumonia, sepsis, and/or multiple organ failure, as happens in critically ill COVID-19 patients, have usually high oxidative stress [13,81]. Furthermore, vitamin C may have a role in various immunity and inflammation pathways against COVID-19 by regulating the growth and function of innate and adaptive immune cells, the production of antibodies, and the suppression of pro-inflammatory cytokines, potentially helping to balance the immune response and mitigate excessive inflammation [82,83]. Nevertheless, most randomized trials that investigated all-cause mortality incidence had some concerns or were at high risk of bias [37,39,41,42]. In addition, the treatment protocols were largely heterogeneous as for dose, route, and frequency of administration. For this reason, further studies are necessary to confirm our results, especially well-conducted RCTs that would contribute to populating the funnel plot and probably mitigate any potential presence of publication bias [84].
As for vitamin D, it is widely recognized for its role in the regulation of the immune system and how its deficiency is linked to inflammation. On the one hand, SARS-CoV-2 infection can cause an inflammation status leading to vitamin D deficiency [85], while, on the other hand, lower serum vitamin D levels may contribute to a dysregulation of the renin–angiotensin system and thus may increase the risk of developing a cytokine storm in COVID-19 [86,87]. For these reasons, it has been the object of many investigations in the last years [14,73], but its efficacy in COVID-19 management is still contradictory [68], as also found in this systematic review, in which the effects of vitamin D supplementation were mostly inconsistent. In this regard, it is worth mentioning the small sample sizes and low quality of the retrieved RCTs, together with the considerable heterogeneity among the included studies in terms of drug dosing, population characteristics, COVID-19 severity, and treatment strategies that may have affected these findings [68]. Interestingly, despite the hypothesis that individuals with low baseline 25-OH vitamin D levels may benefit to a different extent from the supplementation [68,88,89], we did not detect any increased effect, as already reported in the literature [68]. By contrast, this review found a reduced mortality rate in individuals supplemented regularly with vitamin D, a finding that aligns with a large population study in which an observed inverse association between habitual use of vitamin D supplements and COVID-19 infection was observed [90] and that suggests the presence of a positive effect of this vitamin on several inflammatory mechanisms induced by SARS-CoV-2 [90,91,92,93].
Additionally, it is known that treating SARS-CoV-2 infections is more effective in the early stages of the disease and therefore, by the time COVID-19 patients are hospitalized due to their critical conditions, the treatment is likely to be less effective [94]. This may be the case for vitamin administration as well, as both vitamin C and D help counterbalance the cytokine excess in the early phases of SARS-CoV-2 infection [83,87]. Moreover, the choice between different vitamin D forms may be relevant in this context due to their different pharmacokinetic profiles [95]. However, since most studies included patients already hospitalized, further research is needed to explore how the timing of vitamin supplementation affects the outcomes in COVID-19 patients.
However, as several trials are still ongoing or have not been published yet [96], new data could soon become available that may allow us to alleviate some uncertainty about the findings and may contribute to making the funnel plot more balanced. Alternatively, or in addition, given the high tolerability consistently mentioned across the studies, large RCTs with standardized recruitment and treatment protocols should be designed to further explore the clinical benefits of vitamin D supplementation in COVID-19 patients and better clarify the mechanisms by which it acts [68].
This study has some strengths and limitations. The main strength is the updated and comprehensive collection of data on the topic. Indeed, to the best of our knowledge, this is the first review that synthesizes available evidence on the role of any vitamin in both the prevention and management of COVID-19 and long-COVID, providing results for multivitamin complex and vitamins A, B, C, and D. Moreover, we studied all effects related to vitamin supplementation in patients with confirmed SARS-CoV-2 infection, including any immunological, hematological, laboratory, and clinical outcomes. By contrast, the limitations of the current review are mostly related to the primary studies included. Since most of them were conducted in low-middle income countries, enrolled a small sample size, and/or great proportions of individuals with vitamin deficiency, the generalizability of our findings may be limited. In addition, none of the studies provided information on the patients’ SARS-CoV-2 vaccination status or reported outcome data based on age groups and/or type or number of comorbidities, factors that could have been considered in the stratified analyses. Moreover, the included studies were conducted across different countries and at different times during the COVID-19 pandemic and they did not specify the SARS-CoV-2 variant responsible for the infections [97], nor did they assess the effect of supplements on the management or prevention of infections caused by different SARS-CoV-2 variants, limiting the opportunity to consider these aspects in the analyses. Furthermore, large heterogeneity in the recruitment and treatment protocols, as well as in the outcome definitions, was found, limiting the opportunity to provide a quantitative synthesis for most aspects. Lastly, the concerning quality of the trials, coupled with some uncertainties in the findings due to the potential presence of publication bias, made the interpretation of the results particularly challenging. This was not unexpected, given that, as highlighted by Honarmand et al. [98], several biases affected the largest part of the RTCs on COVID-19 management and prevention, making it imperative to prioritize rigorous trial design to address the demand for effective and safe treatment and prevention options during healthcare crises. For all these reasons, despite some positive findings, further and better-designed studies are needed to clarify the potential benefits of vitamin administration in relation to both the prevention and management of COVID-19 and long-COVID, using common pre-established daily dosages and standardized recruitment and intervention protocols.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16091345/s1. Table S1: Search string used in the systematic review by database; Table S2: Quality assessment of the articles included in the systematic review by alphabetic order. Revised Cochrane risk-of-bias tool for randomized trials (RoB2) was used; Figure S1: Funnel plot of randomized controlled trials comparing all-cause mortality between patients receiving Vitamin C vs. placebo or standard of care; Figure S2: Funnel plot of randomized controlled trials comparing all-cause mortality between patients receiving Vitamin D vs. placebo or standard of care.
Author Contributions
The authors’ responsibilities are as follows—A.S. (Alessandra Sinopoli): conceived this review; A.S. (Antonio Sciurti) and V.B.: contributed to perform data extraction and analysis; C.I.: performed data extraction; A.S. (Alessandra Sinopoli) and V.B.: contributed to draft the manuscript; all authors (A.S. (Alessandra Sinopoli), A.S. (Antonio Sciurti), C.I., M.M.S. and V.B.): critically reviewed the draft and provided detailed commentary and changes that were incorporated in its revision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This research does not contain primary clinical or patient data and an approval by an ethics committee was not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lucas S. Pandemics and Pathology: A Reflection on Influenza, HIV/AIDS and SARS (COVID-19) Pandemic Infections. Diagn. Histopathol. 2021;27:128–133. doi: 10.1016/j.mpdhp.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrington C., Coates P., Duprex W. Viruses and Disease: Emerging Concepts for Prevention, Diagnosis and Treatment. J. Pathol. 2015;235:149–152. doi: 10.1002/path.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Si R., Yao Y., Zhang X., Lu Q., Aziz N. Investigating the Links Between Vaccination Against COVID-19 and Public Attitudes Toward Protective Countermeasures: Implications for Public Health. Front. Public Health. 2021;9:702699. doi: 10.3389/fpubh.2021.702699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferson T., Dooley L., Ferroni E., Al-Ansary L., van Driel M., Bawazeer G., Jones M., Hoffmann T., Clark J., Beller E., et al. Physical Interventions to Interrupt or Reduce the Spread of Respiratory Viruses. Cochrane Database Syst. Rev. 2023:CD006207. doi: 10.1002/14651858.CD006207.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenkin A. Micronutrients in Health and Disease. Postgrad. Med. J. 2006;82:559–567. doi: 10.1136/pgmj.2006.047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speakman L.L., Michienzi S.M., Badowski M.E. Vitamins, Supplements and COVID-19: A Review of Currently Available Evidence. Drugs Context. 2021;10:2021-6-2. doi: 10.7573/dic.2021-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alpert P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017;29:199–202. doi: 10.1177/1084822317713300. [DOI] [Google Scholar]
- 8.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does Vitamin D Deficiency Increase the Severity of COVID-19? Clin. Med. 2020;20:e107–e108. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigmohammadi M.T., Bitarafan S., Hoseindokht A., Abdollahi A., Amoozadeh L., Soltani D. The Effect of Supplementation with Vitamins A, B, C, D, and E on Disease Severity and Inflammatory Responses in Patients with COVID-19: A Randomized Clinical Trial. Trials. 2021;22:802. doi: 10.1186/s13063-021-05795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R., Wu K., Li Y., Liang X., Tse W.K.F., Yang L., Lai K.P. Revealing the Targets and Mechanisms of Vitamin A in the Treatment of COVID-19. Aging. 2020;12:15784–15796. doi: 10.18632/aging.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemilä H., Chalker E. Vitamin C for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2013:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ao G., Li J., Yuan Y., Wang Y., Nasr B., Bao M., Gao M., Qi X. Intravenous Vitamin C Use and Risk of Severity and Mortality in COVID-19: A Systematic Review and Meta-Analysis. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2022;37:274–281. doi: 10.1002/ncp.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kow C.S., Hasan S.S., Ramachandram D.S. The Effect of Vitamin C on the Risk of Mortality in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Inflammopharmacology. 2023;31:3357–3362. doi: 10.1007/s10787-023-01200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawat D., Roy A., Maitra S., Shankar V., Khanna P., Baidya D.K. Vitamin D Supplementation and COVID-19 Treatment: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102189. doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olczak-Pruc M., Swieczkowski D., Ladny J.R., Pruc M., Juarez-Vela R., Rafique Z., Peacock F.W., Szarpak L. Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:4217. doi: 10.3390/nu14194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrea L., Verde L., Grant W.B., Frias-Toral E., Sarno G., Vetrani C., Ceriani F., Garcia-Velasquez E., Contreras-Briceño J., Savastano S., et al. Vitamin D: A Role Also in Long COVID-19? Nutrients. 2022;14:1625. doi: 10.3390/nu14081625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 19.Manetti S., Dugdale D.C., Conaway B. Vitamins. [(accessed on 26 May 2023)]; Available online: https://medlineplus.gov/ency/article/002399.htm.
- 20.Post COVID-19 Condition (Long COVID) [(accessed on 14 December 2023)]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition.
- 21.McGuinness L.A., Schmidt L. Medrxivr: Accessing and Searching medRxiv and bioRxiv Preprint Data in R. J. Open Source Softw. 2020;5:2651. doi: 10.21105/joss.02651. [DOI] [Google Scholar]
- 22.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring Inconsistency in Meta-Analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balduzzi S., Rücker G., Schwarzer G. How to Perform a Meta-Analysis with R: A Practical Tutorial. BMJ Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolliffe D.A., Holt H., Greenig M., Talaei M., Perdek N., Pfeffer P., Vivaldi G., Maltby S., Symons J., Barlow N.L., et al. Effect of a Test-and-Treat Approach to Vitamin D Supplementation on Risk of All Cause Acute Respiratory Tract Infection and COVID-19: Phase 3 Randomised Controlled Trial (CORONAVIT) BMJ. 2022;378:e071230. doi: 10.1136/bmj-2022-071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villasis-Keever M.A., López-Alarcón M.G., Miranda-Novales G., Zurita-Cruz J.N., Barrada-Vázquez A.S., González-Ibarra J., Martínez-Reyes M., Grajales-Muñiz C., Santacruz-Tinoco C.E., Martínez-Miguel B., et al. Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial. Arch. Med. Res. 2022;53:423–430. doi: 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakamifard A., Soltani R., Maghsoudi A., Rismanbaf A., Aalinezhad M., Tarrahi M.J., Mashayekhbakhsh S., Dolatshahi K. The Effect of Vitamin E and Vitamin C in Patients with COVID-19 Pneumonia; a Randomized Controlled Clinical Trial. Immunopathol. Persa. 2022;8:e08. doi: 10.34172/ipp.2022.08. [DOI] [Google Scholar]
- 28.Leal-Martínez F., Abarca-Bernal L., García-Pérez A., González-Tolosa D., Cruz-Cázares G., Montell-García M., Ibarra A. Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health. 2022;19:1172. doi: 10.3390/ijerph19031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohani M., Mozaffar H., Mesri M., Shokri M., Delaney D., Karimy M. Evaluation and Comparison of Vitamin A Supplementation with Standard Therapies in the Treatment of Patients with COVID-19. East. Mediterr. Health J. 2022;28:673–681. doi: 10.26719/emhj.22.064. [DOI] [PubMed] [Google Scholar]
- 30.Somi M.H., Faghih Dinevari M., Taghizadieh A., Varshochi M., Sadeghi Majd E., Abbasian S., Nikniaz Z. Effect of Vitamin A Supplementation on the Outcome Severity of COVID-19 in Hospitalized Patients: A Pilot Randomized Clinical Trial. Nutr. Health. 2022:1–6. doi: 10.1177/02601060221129144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majidi N., Bahadori E., Shekari S., Gholamalizadeh M., Tajadod S., Ajami M., Gholami S., Shadnoush M., Ahmadzadeh M., Dehnadi Moghadam A., et al. Effects of Supplementation with Low-Dose Group B Vitamins on Clinical and Biochemical Parameters in Critically Ill Patients with COVID-19: A Randomized Clinical Trial. Expert Rev. Anti-Infect. Ther. 2022:1–7. doi: 10.1080/14787210.2022.2125867. [DOI] [PubMed] [Google Scholar]
- 32.Hu Q., Zhang Q.-Y., Peng C.-F., Ma Z., Han Y.-L. Efficiency of Nicotinamide-Based Supportive Therapy in Lymphopenia for Patients with Ordinary or Severe COVID-19: A Randomized Controlled Trial. Medicine. 2022;101:43. doi: 10.1097/MD.0000000000031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppock D., Violet P.-C., Vasquez G., Belden K., Foster M., Mullin B., Magee D., Mikell I., Shah L., Powers V., et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life. 2022;12:453. doi: 10.3390/life12030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogleman C., Cohen D., Mercier A., Farrell D., Rutz J., Bresz K., Vernon T. A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19. J. Am. Board Fam. Med. 2022;35:695–707. doi: 10.3122/jabfm.2022.04.210529. [DOI] [PubMed] [Google Scholar]
- 35.Jamali Moghadam Siahkali S., Zarezade B., Koolaji S., SeyedAlinaghi S., Zendehdel A., Tabarestani M., Sekhavati Moghadam E., Abbasian L., Dehghan Manshadi S.A., Salehi M., et al. Safety and Effectiveness of High-Dose Vitamin C in Patients with COVID-19: A Randomized Open-Label Clinical Trial. Eur. J. Med. Res. 2021;26:20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V., Bhushan D., Supriya S., Ganapule A.A., Lohani P., Shyama, Pandey S., Majhi P.K., Anand U., Kumar R., et al. Efficacy of Intravenous Vitamin C in Management of Moderate and Severe COVID-19: A Double Blind Randomized Placebo Controlled Trial. J. Fam. Med. Prim. Care. 2022;11:4758–4765. doi: 10.4103/jfmpc.jfmpc_2437_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumari P., Dembra S., Dembra P., Bhawna F., Gul A., Ali B., Sohail H., Kumar B., Memon M.K., Rizwan A. The Role of Vitamin C as Adjuvant Therapy in COVID-19. Cureus. 2020;12:e11779. doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labbani-Motlagh Z., Amini S., Aliannejad R., Sadeghi A., Shafiee G., Heshmat R., Jafary M., Talaschian M., Akhtari M., Jamshidi A., et al. High-Dose Intravenous Vitamin C in Early Stages of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Double-Blind, Randomized, Controlled Clinical Trial. J. Res. Pharm. Pract. 2022;11:64–72. doi: 10.4103/jrpp.jrpp_30_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majidi N., Rabbani F., Gholami S., Gholamalizadeh M., BourBour F., Rastgoo S., Hajipour A., Shadnoosh M., Akbari M.E., Bahar B., et al. The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial. Front. Immunol. 2021;12:717816. doi: 10.3389/fimmu.2021.717816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ried K., BinJemain T., Sali A. Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, and Vitamin D3 With or Without Intravenous Vitamin C: An International, Multicenter, Randomized Trial. Cureus. 2021;13:e19902. doi: 10.7759/cureus.19902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tehrani S., Yadegarynia D., Abrishami A., Moradi H., Gharaei B., Rauofi M., Maghsoudi Nejad F., Sali S., Khabiri N., Abolghasemi S. An Investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial. Urol. J. 2022;19:460–465. doi: 10.22037/uj.v18i.6863. [DOI] [PubMed] [Google Scholar]
- 42.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., Luo G., Meng Z., De Backer D., Xiang H., et al. Pilot Trial of High-Dose Vitamin C in Critically Ill COVID-19 Patients. Ann. Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abroug H., Maatouk A., Bennasrallah C., Dhouib W., Ben Fredj M., Zemni I., Kacem M., Mhalla S., Nouira S., Ben Belgacem M., et al. Effect of Vitamin D Supplementation versus Placebo on Recovery Delay among COVID-19 Tunisian Patients: A Randomized-Controlled Clinical Trial. Trials. 2023;24:123. doi: 10.1186/s13063-023-07114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop C.W., Ashfaq A., Melnick J.Z., Vazquez-Escarpanter E., Fialkow J.A., Strugnell S.A., Choe J., Kalantar-Zadeh K., Federman N.C., Ng D., et al. REsCue Trial: Randomized Controlled Clinical Trial with Extended-Release Calcifediol in Symptomatic COVID-19 Outpatients. Nutrition. 2023;107:111899. doi: 10.1016/j.nut.2022.111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domazet Bugarin J., Dosenovic S., Ilic D., Delic N., Saric I., Ugrina I., Stojanovic Stipic S., Duplancic B., Saric L. Vitamin D Supplementation and Clinical Outcomes in Severe COVID-19 Patients-Randomized Controlled Trial. Nutrients. 2023;15:1234. doi: 10.3390/nu15051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bychinin M.V., Klypa T.V., Mandel I.A., Yusubalieva G.M., Baklaushev V.P., Kolyshkina N.A., Troitsky A.V. Effect of Vitamin D3 Supplementation on Cellular Immunity and Inflammatory Markers in COVID-19 Patients Admitted to the ICU. Sci. Rep. 2022;12:18604. doi: 10.1038/s41598-022-22045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannata-Andía J.B., Díaz-Sottolano A., Fernández P., Palomo-Antequera C., Herrero-Puente P., Mouzo R., Carrillo-López N., Panizo S., Ibañez G.H., Cusumano C.A., et al. A Single-Oral Bolus of 100,000 IU of Cholecalciferol at Hospital Admission Did Not Improve Outcomes in the COVID-19 Disease: The COVID-VIT-D-a Randomised Multicentre International Clinical Trial. BMC Med. 2022;20:18604. doi: 10.1186/s12916-022-02290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Niet S., Trémège M., Coffiner M., Rousseau A.-F., Calmes D., Frix A.-N., Gester F., Delvaux M., Dive A.-F., Guglielmi E., et al. Positive Effects of Vitamin D Supplementation in Patients Hospitalized for COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2022;14:3048. doi: 10.3390/nu14153048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elamir Y.M., Amir H., Lim S., Rana Y.P., Lopez C.G., Feliciano N.V., Omar A., Grist W.P., Via M.A. A Randomized Pilot Study Using Calcitriol in Hospitalized COVID-19 Patients. Bone. 2022;154:116175. doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., Quesada Gomez J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karonova T.L., Golovatyuk K.A., Kudryavtsev I.V., Chernikova A.T., Mikhaylova A.A., Aquino A.D., Lagutina D.I., Zaikova E.K., Kalinina O.V., Golovkin A.S., et al. Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients. 2022;14:2602. doi: 10.3390/nu14132602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maghbooli Z., Sahraian M.A., Jamalimoghadamsiahkali S., Asadi A., Zarei A., Zendehdel A., Varzandi T., Mohammadnabi S., Alijani N., Karimi M., et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021;27:1242–1251. doi: 10.1016/j.eprac.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariani J., Antonietti L., Tajer C., Ferder L., Inserra F., Cunto M.S., Brosio D., Ross F., Zylberman M., López D.E., et al. High-Dose Vitamin D versus Placebo to Prevent Complications in COVID-19 Patients: Multicentre Randomized Controlled Clinical Trial. PLoS ONE. 2022;17:e0267918. doi: 10.1371/journal.pone.0267918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., Macedo M.B., Dalmolin H.H.H., et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murai I.H., Fernandes A.L., Antonangelo L., Gualano B., Pereira R.M.R. Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19. Clinics. 2021;76:e3549. doi: 10.6061/clinics/2021/e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandes A.L., Murai I.H., Reis B.Z., Sales L.P., Santos M.D., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., et al. Effect of a Single High Dose of Vitamin D3 on Cytokines, Chemokines, and Growth Factor in Patients with Moderate to Severe COVID-19. Am. J. Clin. Nutr. 2022;115:790–798. doi: 10.1093/ajcn/nqab426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., Puri G.D., Malhotra P. Short Term, High-Dose Vitamin D Supplementation for COVID-19 Disease: A Randomised, Placebo-Controlled, Study (SHADE Study) Postgrad. Med. J. 2022;98:87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 59.Sánchez-Zuno G.A., González-Estevez G., Matuz-Flores M.G., Macedo-Ojeda G., Hernández-Bello J., Mora-Mora J.C., Pérez-Guerrero E.E., García-Chagollán M., Vega-Magaña N., Turrubiates-Hernández F.J., et al. Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. J. Clin. Med. 2021;10:2378. doi: 10.3390/jcm10112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurita-Cruz J., Fonseca-Tenorio J., Villasís-Keever M., López-Alarcón M., Parra-Ortega I., López-Martínez B., Miranda-Novales G. Efficacy and Safety of Vitamin D Supplementation in Hospitalized COVID-19 Pediatric Patients: A Randomized Controlled Trial. Front. Pediatr. 2022;10:943529. doi: 10.3389/fped.2022.943529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients. 2020;12:2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ali S., Alam M., Khatoon F., Fatima U., Elasbali A.M., Adnan M., Islam A., Hassan M.I., Snoussi M., De Feo V. Natural Products Can Be Used in Therapeutic Management of COVID-19: Probable Mechanistic Insights. Biomed. Pharmacother. 2022;147:112658. doi: 10.1016/j.biopha.2022.112658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atluri K., Aimlin I., Arora S. Current Effective Therapeutics in Management of COVID-19. J. Clin. Med. 2022;11:3838. doi: 10.3390/jcm11133838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinopoli A., Isonne C., Santoro M.M., Baccolini V. The Effects of Orally Administered Lactoferrin in the Prevention and Management of Viral Infections: A Systematic Review. Rev. Med. Virol. 2022;32:e2261. doi: 10.1002/rmv.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandra R.K. Nutrition and the Immune System: An Introduction. Am. J. Clin. Nutr. 1997;66:460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 67.Sinopoli A., Caminada S., Isonne C., Santoro M.M., Baccolini V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients. 2022;14:4081. doi: 10.3390/nu14194081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Li J., Yang M., Wang Q. Effect of Vitamin D Supplementation on COVID-19 Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2023;10:11311033. doi: 10.3389/fnut.2023.1131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tentolouris N., Samakidou G., Eleftheriadou I., Tentolouris A., Jude E.B. The Effect of Vitamin D Supplementation on Mortality and Intensive Care Unit Admission of COVID-19 Patients. A Systematic Review, Meta-Analysis and Meta-Regression. Diabetes/Metab. Res. Rev. 2022;38:e3517. doi: 10.1002/dmrr.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaazouee M.S., Eleisawy M., Abdalalaziz A.M., Elhady M.M., Ali O.A., Abdelbari T.M., Hasan S.M., Almadhoon H.W., Ahmed A.Y., Fassad A.S., et al. Hospital and Laboratory Outcomes of Patients with COVID-19 Who Received Vitamin D Supplementation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023;396:607–620. doi: 10.1007/s00210-022-02360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawat D., Roy A., Maitra S., Gulati A., Khanna P., Baidya D.K. Vitamin C and COVID-19 Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. 2021;15:102324. doi: 10.1016/j.dsx.2021.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pal R., Banerjee M., Bhadada S.K., Shetty A.J., Singh B., Vyas A. Vitamin D Supplementation and Clinical Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2022;45:53–68. doi: 10.1007/s40618-021-01614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah K., Saxena D., Mavalankar D. Vitamin D Supplementation, COVID-19 and Disease Severity: A Meta-Analysis. QJM Mon. J. Assoc. Physicians. 2021;114:175–181. doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiwari A.V., Dangore-Khasbage S. Vitamin D and COVID-19: An Update on Evidence and Potential Therapeutic Implications. Cureus. 2023;15:e46121. doi: 10.7759/cureus.46121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergman P. Can Vitamin D Protect against Covid-19? BMJ. 2022;378:o1822. doi: 10.1136/bmj.o1822. [DOI] [Google Scholar]
- 76.Vollbracht C., Kraft K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022;13:899198. doi: 10.3389/fphar.2022.899198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S., Tan H.-Y., Wang N., Zhang Z.-J., Lao L., Wong C.-W., Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borrelli E., Roux-Lombard P., Grau G.E., Girardin E., Ricou B., Dayer J., Suter P.M. Plasma Concentrations of Cytokines, Their Soluble Receptors, and Antioxidant Vitamins Can Predict the Development of Multiple Organ Failure in Patients at Risk. Crit. Care Med. 1996;24:392–397. doi: 10.1097/00003246-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Carr A.C., Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu C., Yi T., Tan S., Xu H., Hu Y., Ma J., Xu J. Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis. Nutrients. 2023;15:1848. doi: 10.3390/nu15081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schorah C.J., Downing C., Piripitsi A., Gallivan L., Al-Hazaa A.H., Sanderson M.J., Bodenham A. Total Vitamin C, Ascorbic Acid, and Dehydroascorbic Acid Concentrations in Plasma of Critically Ill Patients. Am. J. Clin. Nutr. 1996;63:760–765. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 82.Moore A., Khanna D. The Role of Vitamin C in Human Immunity and Its Treatment Potential Against COVID-19: A Review Article. Cureus. 2023;15:e33740. doi: 10.7759/cureus.33740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miranda-Massari J.R., Toro A.P., Loh D., Rodriguez J.R., Borges R.M., Marcial-Vega V., Olalde J., Berdiel M.J., Riordan N.H., Martinez J.M., et al. The Effects of Vitamin C on the Multiple Pathophysiological Stages of COVID-19. Life. 2021;11:1341. doi: 10.3390/life11121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin L., Chu H. Quantifying Publication Bias in Meta-Analysis. Biometrics. 2018;74:785–794. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smolders J., van den Ouweland J., Geven C., Pickkers P., Kox M. Letter to the Editor: Vitamin D Deficiency in COVID-19: Mixing up Cause and Consequence. Metabolism. 2021;115:154434. doi: 10.1016/j.metabol.2020.154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benskin L.L. A Basic Review of the Preliminary Evidence That COVID-19 Risk and Severity Is Increased in Vitamin D Deficiency. Front. Public Health. 2020;8:513. doi: 10.3389/fpubh.2020.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for Possible Association of Vitamin D Status with Cytokine Storm and Unregulated Inflammation in COVID-19 Patients. Aging Clin. Exp. Res. 2020;32:2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bignardi P.R., de Andrade Castello P., de Matos Aquino B., Delfino V.D.A. Is the Vitamin D Status of Patients with COVID-19 Associated with Reduced Mortality? A Systematic Review and Meta-Analysis. Arch. Endocrinol. Metab. 2023;67:276–288. doi: 10.20945/2359-3997000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmad A.S., Juber N.F., Al-Naseri H., Heumann C., Ali R., Oliver T. Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study. Nutrients. 2023;15:4818. doi: 10.3390/nu15224818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma H., Zhou T., Heianza Y., Qi L. Habitual Use of Vitamin D Supplements and Risk of Coronavirus Disease 2019 (COVID-19) Infection: A Prospective Study in UK Biobank. Am. J. Clin. Nutr. 2021;113:1275–1281. doi: 10.1093/ajcn/nqaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kast J.I., McFarlane A.J., Głobińska A., Sokolowska M., Wawrzyniak P., Sanak M., Schwarze J., Akdis C.A., Wanke K. Respiratory Syncytial Virus Infection Influences Tight Junction Integrity. Clin. Exp. Immunol. 2017;190:351–359. doi: 10.1111/cei.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F., Borregaard N., Modlin R.L., Hewison M. Vitamin D-Directed Rheostatic Regulation of Monocyte Antibacterial Responses1. J. Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanff T.C., Harhay M.O., Brown T.S., Cohen J.B., Mohareb A.M. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin. Infect. Dis. 2020;71:870–874. doi: 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haddad F., Dokmak G., Karaman R. A Comprehensive Review on the Efficacy of Several Pharmacologic Agents for the Treatment of COVID-19. Life. 2022;12:1758. doi: 10.3390/life12111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouillon R., Quesada Gomez J.M. Comparison of Calcifediol with Vitamin D for Prevention or Cure of Vitamin D Deficiency. J. Steroid Biochem. Mol. Biol. 2023;228:106248. doi: 10.1016/j.jsbmb.2023.106248. [DOI] [PubMed] [Google Scholar]
- 96.Cara K.C., Beauchesne A.R., Li R., Chung M. Cochrane Review Summary on “Vitamin D Supplementation for the Treatment of COVID-19: A Living Systematic Review”. J. Diet. Suppl. 2022;19:143–145. doi: 10.1080/19390211.2022.2008601. [DOI] [PubMed] [Google Scholar]
- 97.Modes M.E., Directo M.P., Melgar M., Johnson L.R., Yang H., Chaudhary P., Bartolini S., Kho N., Noble P.W., Isonaka S., et al. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance—One Hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:217–223. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honarmand K., Penn J., Agarwal A., Siemieniuk R., Brignardello-Petersen R., Bartoszko J.J., Zeraatkar D., Agoritsas T., Burns K., Fernando S.M., et al. Clinical Trials in COVID-19 Management & Prevention: A Meta-Epidemiological Study Examining Methodological Quality. J. Clin. Epidemiol. 2021;139:68–79. doi: 10.1016/j.jclinepi.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.