Abstract
Background
In Australia, tixagevimab/cilgavimab 150 mg/150 mg was a government-funded pre-exposure prophylaxis for COVID-19 people with multiple sclerosis (pwMS) and other neuroimmunological conditions (pwNIc) treated with anti-CD20 antibodies or sphingosine-1-phosphate receptor modulators were eligible.
Objective
To analyse the roll-out, uptake and real-world efficacy of tixagevimab/cilgavimab in the prevention and severity of COVID-19. To assess compliance with uptake depending on the location of delivery.
Methods
We undertook a single-centre study. 440 pwMS and pwNIc were eligible. Logistic regression was used to assess predictors of COVID-19 during follow-up and to assess predictors of uptake among those who consented.
Results
Of the eligible pwMS and pwNIc in our service, 52.7% (233/440) requested a consultation and were included in this study. Consultation resulted in 71.7% of people (167/233) receiving the treatment. Of these, 94.0% (157/167) had received three or more COVID-19 vaccines. Among those who received a single dose of tixagevimab/cilgavimab, 19.16% (32/167) tested positive for COVID-19 during the observational window. The majority of these were on ocrelizumab (68.8% (22/32)). None of those with COVID-19 required hospitalisation or supplemental oxygen. There was no difference in odds of COVID-19 during the observation period between those who received and did not receive tixagevimab/cilgavimab (adjusted OR, aOR 2.16 (95% CI 0.82 to 6.85), p=0.43). Uptake of tixagevimab/cilgavimab was highest when offered at the hospital infusion centre (aOR 3.09 (95% CI 1.08 to 9.94) relative to referral to the local pharmacy, p=0.04).
Conclusion
Tixagevimab/cilgavimab administration did not protect against subsequent COVID-19 in our cohort. Compliance with uptake was influenced by administration location.
Keywords: MULTIPLE SCLEROSIS, COVID-19, HEALTH POLICY & PRACTICE
WHAT IS ALREADY KNOWN ON THIS TOPIC.
At the time of this study, clinical trials indicate that a single dose of tixagevimab/cilgavimab 150/150 mg reduces the risk of COVID-19 for up to 6 months.
WHAT THIS STUDY ADDS
This study confirms that tixagevimab/cilgavimab administration did not protect against subsequent COVID-19 in our cohort.
This study, however, demonstrates the importance of communication during a pandemic and highlights the importance of evidence-based service delivery/location to vulnerable people.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides further evidence that improvements in health information delivery and ease of service access to prophylactic treatments are required for optimal uptake of future public health initiatives.
Introduction
In Australia, available COVID-19 vaccines are highly effective and widely accessible. As of mid-August 2022, over 95% of the adult Australian population have received at least two doses of a COVID-19 vaccine, 72% of those eligible for a third dose have received at least three doses, and 38% of those eligible for a fourth dose have received four doses.1 However, people with multiple sclerosis (pwMS) and people with other neuroimmunological conditions (pwNIc) on anti-CD20 and anti-CD52 monoclonal antibody therapies or sphingosine-1-phosphate receptor modulators exhibit attenuated humoral responses to COVID-19 vaccines and may be at increased risk of severe COVID-19.2–7
These observations support the eligibility of these people for tixagevimab/cilgavimab.
Tixagevimab/cilgavimab is a combination of two human monoclonal antibodies which target the surface spike protein of SARS-CoV-2. It is the first non-vaccine medication in Australia for the prevention of COVID-19 in people who are at risk of infection but have not been exposed to the virus. Data from clinical trials indicate that a single dose of tixagevimab/cilgavimab 150/150 mg reduces the risk of COVID-19 for up to 6 months.8–10
The first delivery of tixagevimab/cilgavimab to the National Medical Stockpile in Australia was on 22 March 2022, with the state of Victoria receiving its allocated stockpile shortly after. The Victorian eligibility criteria to access tixagevimab/cilgavimab was expanded on 27 May 2022, to include pwMS and pwNIc who had received in the last 12 months one of the following medications: anti-CD20 antibodies (rituximab, obinutuzumab, ocrelizumab, ofatumumab), sphingosine-1-phosphate receptor modulators (fingolimod, siponimod, ozanimod) or anti-CD52 antibodies (alemtuzumab).11
Tixagevimab/cilgavimab for eligible pwMS and pwNIc was fully funded by the government, with no costs to eligible people. Treatment was available at different hospital and community locations from mid-2022. Here, we report the roll-out, uptake, real-world efficacy of tixagevimab/cilgavimab in pwMS and pwNIc during the COVID-19 pandemic in the second half of 2022.
Methods
This was a retrospective analysis of pwMS and pwNIc who were eligible for tixagevimab/cilgavimab attending a single academic tertiary multiple sclerosis (MS) and neuroimmunology (NI) service in Victoria, Australia. The study was conducted as an MSBase Registry substudy.12 Three administration sites became available to eligible people at our service. The hospital infusion centre became active on 14 June 2022. The infusion centre is a 22-chair centre within the hospital where people receive a wide range of infusible treatments. The hospital vaccination hub became active on 21 June 2022. This was an initiative by the hospital to provide a space in a non-clinical area of the hospital for people to receive tixagevimab/cilgavimab. The site, while staffed by the hospital, was not physically within the hospital building. Local supercare pharmacies were metropolitan or rural pharmacies that had a nurse available and private areas for administration 7 days a week from 18:00 to 22:00 hours became active on 15 July 2022.13
Participants and recruitment
Our service strategy was to provide timely access to tixagevimab/cilgavimab for eligible pwMS and pwNIc by rapid and concise communication as outlined below. All participants were enrolled in the MSBase registry with detailed treatment information recorded. We identified eligible pwMS and pwNIc through the site-specific MSBase registry and an electronic medical records (EMR) search. We developed a targeted approach for enrolment by first identifying and contacting eligible pwMS and pwNIc who had upcoming infusions at our hospital infusion centre. Text messaging (English only) was initially directed to these people and those who responded were emailed consumer medical information on tixagevimab/cilgavimab.14 This was followed by a telehealth or face-to-face consultation with a neurologist to discuss the risks and benefits and obtain informed consent formally.
Our second mass text message (English only), sent 10 days later, was focused on all other eligible pwMS/pwNIc who had attended the service in the last 12 months and who did not have an imminent infusion scheduled or were on oral therapies. Eligible people who responded to the message were sent further information and again scheduled for a neurologist consultation.
In this study, we included people who received formal telehealth or face-to-face consultation to discuss tixagevimab/cilgavimab from 14 June 2022 to 30 September 2022. Participants were followed up till 15 December 2022, to assess compliance and efficacy. Follow-up was undertaken by review of EMR, MSBase registry and phone/email to confirm administration and efficacy.
Dosage and administration
At the time of this study, SARS-CoV-2 Omicron variants such as BA.1.1 represented a minority of Omicron BA.1 sequences reported in Australia, supporting the use of the 150 mg/150 mg dosage of tixagevimab/cilgavimab as an effective pre-exposure prophylaxis strategy. In contrast, the US Food and Drug administration (FDA) had authorised increased tixagevimab/cilgavimab dosage to 300 mg/300 mg in February 2022 to combat the increased circulating variant of concern BA1.1 in the USA.15 16 Our site administration protocol was adjusted to include intramuscular injection of the vastus lateralis muscle for those who could not stand independently to receive injections in the gluteal muscle.
Data collection and assessments
We collected age, sex, disease-modifying therapy (DMT), the number of COVID-19 vaccines, Expanded Disability Status Scale Score (EDSS) and COVID-19 pre and post tixagevimab/cilgavimab. EDSS scores were categorised as mild (<3), moderate (≥3 and <6) and severe (≥6). In addition, we documented the location of the administration as per site allocation outlined above. We also enquired why people chose not to proceed with tixagevimab/cilgavimab at any of the above settings. We classified the lack of uptake/administration into the following categories: previous cardiovascular or thromboembolic events, previous severe vaccine reaction, family planning, recent COVID-19 and personal choice.
Administration of tixagevimab/cilgavimab was confirmed in several ways. First, we reviewed EMR to check administration at the hospital infusion centre and vaccination hub. Administration at a local supercare pharmacy was confirmed by telephone and/or email to the person. With the persons’ permission, we accessed the National Medical Record (My Health Record) and the Australian Immunisation Registry to confirm COVID-19 vaccine number. The MSBase registry and EMR were used to reconfirm vaccine and COVID-19 history.
Study endpoints
We assessed the uptake and efficacy of one dose of tixagevimab/cilgavimab and identified any associated compliance, administration location, demographic, vaccine history or disability up until 15 December 2022.
Statistical analysis
Baseline characteristics were described by summary statistics. Univariable and multivariable logistic regression was used to assess predictors of COVID-19 in the follow-up period and, second, predictors of follow-through with administration of tixagevimab/cilgavimab after consent. A p<0.05 was considered statistically significant. All statistical analyses were performed using R V.4.1.2.
Results
At our site, 46.7% (440/942) of people met the local eligibility criteria for tixagevimab/cilgavimab and received an alert of their eligibility. All eligible people were offered a neurologist consultation for further discussion of tixagevimab/cilgavimab. A total of 53.0% (233/440) pwMS and pwNIc responded and received tixagevimab/cilgavimab information during their scheduled consultation to discuss risks and benefits of administration. Demographics of the cohort are described in table 1.
Table 1.
Demographics pwMS and pwNIc who received a specialist consultation to discuss tixagevimab/cilgavimab access
| All pwMS and pwNIc who received tixagevimab/cilgavimab access consultation | pwMS and pwNIc who consented to tixagevimab/cilgavimab but failed to secure administration | pwMS and pwNIc who received tixagevimab/cilgavimab appointment but declined or were ineligible | |
| Total n=233 |
Total n=47 |
Total n=13 |
|
| Age (years) | |||
| Min | 18 | 29 | 24 |
| Max | 84 | 70 | 73 |
| Mean | 49.90 | 48.13 | 51.85 |
| Sex n (%) | |||
| Female | 169 (72.5%) | 32 (68%) | 7 (53.85%) |
| EDSS | |||
| Min | 0 | 0 | 1 |
| Max | 9 | 7.5 | 9 |
| Mean | 3.31 | 2.84 | 5.1 |
| DMT treatment n (%) | |||
| Ocrelizumab | 168 (72.10%) | 34 (72.34%) | 10 (76.92%) |
| Ofatumumab | 11 (4.72%) | 3 (6.38%) | 1 (7.69%) |
| Rituximab | 10 (4.29%) | 0 | 1 (7.69%) |
| Fingolimod | 29 (12.45%) | 7 (14.89%) | 0 |
| Siponimod | 7 (3.0%) | 1 (2.12%) | 1 (7.69%) |
| Other | 6 (2.58%) | 1 (2.12%) 1 missing data | 0 |
| COVID-19 Vaccine dose no n (%) | |||
| 2 | 19 (8.15%) | 9 (19.14%) 1 missing data | 2 (15.38%) |
| 3 | 63 (27.03%) | 11 (23.40%) | 1 (7.69%) |
| 4 | 111 (47.64%) | 25 (53.19%) | 6 (46.15%) |
| 5 | 38 (16.31) | 1 (2.13%) | 4 (30.77%) |
| Reason for not proceeding with tixagevimab/cilgavimab | |||
| Cardiac ineligible | 4 (30.77%) | ||
| Patient assessment of risk/benefit | 8 (61.54%) | ||
| Previous vaccine reaction | 1 (7.69%) | ||
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale Score; pwMS, people with multiple sclerosis; pwNIc, people with neuroimmunological conditions.
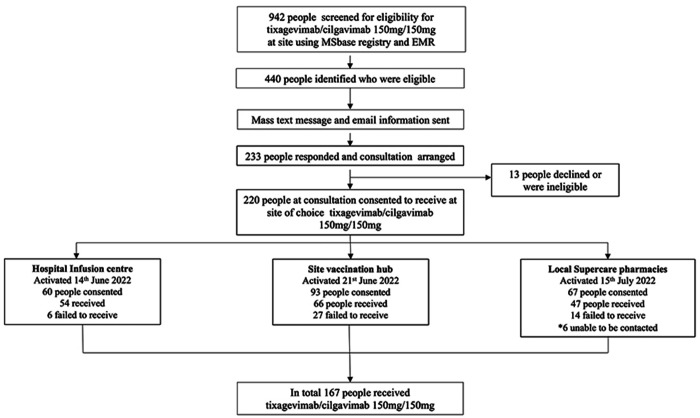
Following consultation, 13 people were excluded due to either a contraindication to the drug (cardiac risk factors (n=5, 38.5) or declined due to personal choice. (n=8, 615%). The remaining 220 people consented to receive tixagevimab/cilgavimab and were given a choice of location for administration. Of those who consented, 167 people received tixagevimab/cilgavimab, and 47 people confirmed did not, 6 people unable to be contacted. The roll-out of tixagevimab/cilgavimab administration at site from initial eligibility to administration at three available locations is described in figure 1.
Figure 1.
Roll-out of tixagevimab/cilgavimab 150 mg/150 mg at site. EMR, electronic medical record.
Hospital infusion centre
60 people who consented to receive tixagevimab/cilgavimab preferred administration at the familiar hospital infusion centre. Of those, 10% (6/60) did not end up receiving the treatment due to nursing error or personal preference not to wait any longer after the scheduled DMT infusion.
The demographics of the 54 people who received tixagevimab/cilgavimab are described in table 2. Of those who received tixagevimab/cilgavimab 14.8% (8/54) developed subsequent COVID-19 in the observation window at a mean time point of 125 days postadministration (SD 17.67).
Table 2.
Demographics: pwMS and pwNIc who received tixagevimab/cilgavimab by location
| pwMS and pwNIc received tixagevimab/cilgavimab by location | Total n=167 |
||
| Hospital infusion centre n=54 | Hospital vaccination hub n=66 | Local supercare pharmacy n=47 | |
| Age (years) | |||
| Mean | 51.35 | 49.80 | 51.36 |
| SD | 13.25 | 12.06 | 11.18 |
| Sex, n (%) | |||
| Female | (74.1%) | (71.2%) | (78.7%) |
| EDSS | |||
| Mean | 3.42 | 3.27 | 2.63 |
| SD | 2.48 | 2.48 | 2.16 |
| COVID-19 n (%) vaccine doses no 3=> | (90.7%) | (95.5%) | (94.0%) |
| DMT treatment n (%) | |||
| Ocrelizumab | 45 (83.33%) | 44 (66.67%) | 34 (72.34%) |
| Ofatumumab | 0 | 5 (7.58%) | 2 (4.26%) |
| Rituximab | 7 (12.96%) | 2 (3.03%) | 0 |
| Fingolimod | 0 | 7 (10.61%) | 10 (21.28%) |
| Siponimod | 0 | 5 (7.58%) | 1 (2.13%) |
| Other | 2 (3.70%) | 3 (4.55%) | 0 |
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale Score; pwMS, people with multiple sclerosis; pwNIc, people with neuroimmunological conditions.
Hospital vaccination hub
93 people consented to tixagevimab/cilgavimab administration at the hospital vaccination hub. People were given information on the booking process (telephone booking in English or by use of the hospital patient portal online booking). However, 29% (27/93) of people did not secure an appointment. Further demographics of this cohort are detailed in table 2.
Of those who received tixagevimab/cilgavimab at this site 16.2% (16/66) subsequently developed COVID-19 during the observation window at a mean time point of 87 days (SD 55.94).
Local supercare pharmacies in rural and metro community
67 people consented to receive tixagevimab/cilgavimab at their local supercare pharmacy. Of the people who consented and were referred to the local supercare pharmacy 22.9% (14/67) did not secure an appointment despite initially agreeing to do so; six people were not contactable in the observation period. The reasons provided for lack of attendance included: perceived change of risk/benefit of infection severity, logistical reasons, family planning and time constraints. The demographics of this cohort are outlined in table 2. Of those who received tixagevimab/cilgavimab at this site 17% (8/47) contracted COVID-19 at a mean time point of 91 days postadministration (SD 43.40).
COVID-19 during the observation period
Of the 167 people who received one dose of tixagevimab/cilgavimab 19.16% (32/167) tested positive, via rapid antigen test for COVID-19 during the observational window. None required hospitalisation or supplemental oxygen. The mean time from administration of tixagevimab/cilgavimab to COVID-19 in this cohort was 14 weeks 1 day (99 days) (table 3).
Table 3.
Demographics: pwMS and pwNIc who tested positive to COVID-19 post tixagevimab/cilgavimab administration
| pwMS and pwNIc who received tixagevimab/cilgavimab who tested positive to COVID-19 during observation follow-up | Total n=32 |
| Age (years) | |
| Min | 33 |
| Max | 67 |
| Mean | 49.47 |
| SD | 10.75 |
| Sex, n (%) | |
| Female | 25 (78.1%) |
| EDSS | |
| Min | 0 |
| Max | 6.5 |
| Mean | 2.45 |
| SD | 1.86 |
| DMT n (%) | |
| Ocrelizumab | 22 (68.75%) |
| Rituximab | 3 (9.38%) |
| Fingolimod | 5 (15.63%) |
| Siponimod | 1 (3.12%) |
| Other | 1 (3.12%) |
| COVID-19 n (%) vaccine dose no | |
| 2 | 2 (6.25%) |
| 3 | 9 (28.13%) |
| 4 | 17 (53.13%) |
| 5 | 4 (12.5%) |
| COVID-19 positive n (%) | |
| Tixagevimab/cilgavimab during observation window | 32/167 (19.16%) |
| Prior to tixagevimab/cilgavimab availability | 15/32 (46.88%) |
| Days from administration to COVID-19 positive n (days) | |
| Min | 3 |
| Max | 178 |
| Mean | 99 |
| SD | 52.34 |
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale Score; pwMS, people with multiple sclerosis; pwNIc, people with neuroimmunological conditions.
Of those that consented to receive tixagevimab/cilgavimab but then failed to secure administration, 10.6% (5/47) tested positive for COVID-19 during the observational window. The five people were all receiving ocrelizumab (OCR) and had secured 3 or more covid vaccines. Hospitalisation or supplementary oxygen was not indicated for this cohorts’ COVID-19 treatment.
Notably, 53.2% (25/47) of this group had tested positive for COVID-19 prior to their tixagevimab/cilgavimab consultation.
We assessed factors predictive of COVID-19 during the follow-up period among those who consented to receive tixagevimab/cilgavimab. Severe disability as measured by the EDSS was associated with reduced odds of COVID-19 in both univariable and multivariable analyses (table 4). Administration of tixagevimab/cilgavimab was not significantly associated with the increased risk of COVID-19 infection.
Table 4.
Predictors of COVID-19 during follow-up observation in those who consented to receive tixagevimab/cilgavimab
| Unadjusted OR (95% CI; p value) | Adjusted OR (95% CI; p value) | |
| EDSS score | ||
| Mild | 1.00 | 1.00 |
| Moderate | 1.04 (0.91 to 1.19; 0.538) | 1.20 (0.48 to 2.88; 0.687) |
| Severe | 0.87 (0.77 to 0.99; 0.031) | 0.27 (0.06 to 0.88; 0.049) |
| Tixagevimab/cilgavimab administration | ||
| No | 1.00 | 1.00 |
| Yes | 1.09 (0.96 to1.23; 0.174) | 2.16 (0.82 to 6.85; 0.426) |
Additional covariates adjusted for in multivariable model were age, sex, prior COVID-19 vaccinations and prior COVID-19.
EDSS, Expanded Disability Status Scale.
Compliance
Of the 220 pwMS and pwNIc who consented to receive tixagevimab/cilgavimab, 21.4% (47/220) did not follow through with booking and administration. Compliance of administration was more favourable at the hospital infusion centre where people were already receiving infusible DMTs. Participants were the least likely to complete treatment at the hospital vaccination hub, with 29% of people allocated to this failing to book an appointment for administration. Local supercare pharmacy non-compliance was 22.9%. People assigned to the infusion centre had 3.1 times greater odds of being administered tixagevimab/cilgavimab relative to those assigned to the local supercare pharmacy (p=0.0438; table 5). The number of prior COVID-19 vaccinations was also predictive for tixagevimab/cilgavimab administration, with an 86% increase in odds of completing treatment for each additional vaccine dose.
Table 5.
Predictors of tixagevimab/cilgavimab administration among people who consented
| Unadjusted OR (95% CI; p value) | Adjusted OR (95% CI; p value) | |
| Assigned administration centre | ||
| Local supercare pharmacy | 1.00 | 1.00 |
| Hospital infusion centre | 1.13 (0.98 to 1.31; 0.097) | 3.09 (1.08 to 9.94; 0.044) |
| Vaccination hub | 0.94 (0.82 to 1.07; 0.338) | 0.82 (0.36 to 1.81; 0.622) |
| Number of prior COVID-19 vaccinations | 1.11 (1.04 to 1.19; 0.002) | 1.86 (1.20 to 2.94; 0.006) |
Additional covariates adjusted for in multivariable model were age, sex, EDSS score and prior COVID-19.
EDSS, Expanded Disability Status Scale.
Discussion
The COVID-19 pandemic has required MS and NI services to be highly adaptable and dedicate substantial resources to implement changes in local treatment and preventative guidelines. Vulnerable people such as those pwMS and pwNIc exposed to certain DMTs require specific and rapidly disseminated information to ensure their safety. Our results demonstrate the resources required to implement policy changes and the complexities of securing compliance in real-world health settings.
Just under half of the eligible peoples contacted did not respond to our offer to receive further consultation on the benefits of receiving pre-exposure prophylaxis. Our initial messaging was in English only and may have contributed to this. As Leask et al and Lewandowsky et al 17 18 have emphasised with vaccine communication, we prioritised the key groups of eligible people for targeted communication and utilised key senior neurologists as signatures of communication. Consultation was available in the persons’ language of choice using the hospital interpreter service. A response rate of just over 50% may also suggest health literacy or language barriers.19 20 Moreover, information was exclusively provided through specialist centres and hospitals with no specific involvement from primary healthcare providers such as general practitioners. A wider and more consistent information campaign is essential when communicating risk and needs to be considered in future health strategies during pandemics.
Our results demonstrate the highest compliance with tixagevimab/cilgavimab administration occurred when the treatment was offered while the person was already on-site, for instance, receiving their usual DMT in the hospital infusion centre. In contrast, the lowest compliance was seen in people who opted to receive treatment through the hospital vaccination hub. Scheduling this additional appointment required several person-driven steps and, for some, significant travel time. People cited a change of risk perception to COVID-19 and logistical reasons, as reasons for not obtaining a booking. These observations are important as they suggest that preprophylaxis treatment for any future pandemics may be more successful at opportunistic moments such as in hospital infusion centre or outpatient consultations.
Our results demonstrate that at least one in five people who received tixagevimab/cilgavimab developed symptomatic COVID-19 during the 6-month observation period. This breakthrough infection rate is substantially higher than the published efficacy rate in the PROVENT study on unvaccinated participants. In this pivotal trial, symptomatic COVID-19 occurred in 8 of 3441 participants (0.2%) in the AZD7442 group and 17 of 1731 participants (1.0%) in the placebo group. Severe infections occurred (0.1%) in the placebo group in contrast to our study where none were observed. Our study, in contrast, demonstrates high levels of COVID-19 vaccination among eligible people. Our breakthrough COVID-19 positive incidence of 19.16% at the end of 2022 supports other recent studies that tixagevimab/cilgavimab had weakening neutralising effectiveness against Omicron BA.5, BR.2, BA2.75, BQ.1 and XBB.21–24 The severity of COVID-19 in both treated and untreated groups was mild, with no hospitalisation required.
Completion of tixagevimab/cilgavimab administration did not decrease the risk of COVID-19 in the observational period when compared with those who did not receive the treatment. However, we observed that people with higher disability levels were less likely to develop COVID-19, which suggests that these people may have been more compliant with social distancing and other precautions than those with less disability. This observation gives important significance to the social behaviours of vulnerable groups during health emergencies.
Vaccination is one of the most effective strategies in reducing severity of COVID-19. Tixagevimab/cilgavimab was approved in Australia to provide additional protection against COVID-19 for vulnerable/immunocompromised people who may not mount adequate vaccine responses. However, at the time when access to tixagevimab/cilgavimab was granted, the Australian population, including pwMS and pwNIc, were already highly vaccinated. Australia’s variant of concern had changed from Delta to Omicron with the first known Omicron sub lineages BA.1, BA.2 and BA.5 being reported in Australia in November 2021. In Victoria mid-April 2022, the BA.2 sublineage was the dominant strain in Victoria comprised at 97% of all COVID-19 variants, BA.4 was detected at 3% and BA.2.12.1 less than 1%.25 The dominant variant BA.2 at the time of roll-out supported the use of tixagevimab/cilgavimab as an effective neutralising treatment. Victoria did not see a change in variants till July 2022, when BA.5 and sublineages became dominant, however were, still neutralised by tixagevimab/cilgavimab. By December 2022, variants in Victoria were polyclonal mix including XBF, BA.2.75 sublineages and BQ.1 and BQ.1.1. The USA FDA warned on 1 June 2022 that tixagevimab/cilgavimab 150 mg/150 mg might not be effective at neutralising new emerging variants of concern and by December 2022, the effectiveness of tixagevimab/cilgavimab administration against these variants was shown to be reduced to less than 25%.26 In addition, infections from the new variants were reported to result in less severe infections.27–29 This information was widely available through online resources and may be one of the reasons 53% of eligible pwMS and pwNIc did not respond to our message to discuss securing tixagevimab/cilgavimab administration. It also is plausible that by late 2022 pandemic fatigue had caused our poor response rate. Eligible people now had not only complex individual health-related messaging, but competing community economic, social and educational aspects to consider and prioritise.30
This study has several limitations to generalisability, as it took place in only one tertiary centre, and the numbers although high for a single centre, may be not reflective of the wider population and health sector. It was not possible to follow up on eligible people who did not seek tixagevimab/cilgavimab consultation and their reasons for not contacting the service. Further research to explore optimal pre-exposure prophylaxis uptake and social behaviours during a pandemic is warranted to ensure healthcare services communicate important health information effectively and plan administration locations based on minimal steps required from patients.
Conclusion
MS and NI services need to have strategies and resources to rapidly disseminate crucial health information during a pandemic to vulnerable/immune-compromised people. Information needs to be multilingual, targeted and at a health literacy level that the majority would understand to make meaningful decisions. While a shared decision-making framework was followed during consultation, pwMS and pwNIc who consented to tixagevimab/cilgavimab still had poor compliance when responsible for booking and receiving the treatment. Compliance tended to be highest in the hospital infusion centre compared with hospital vaccination hub and pharmacy, suggesting preprophylaxis treatments may have greater uptake if people can access the therapy at DMT infusions or outpatient clinics. We demonstrate that in second half of 2022 one dose of tixagevimab/cilgavimab had limited efficacy in the prevention of COVID-19 in a highly vaccinated cohort of pwMS and pwNIc.
Acknowledgments
Thank you to the people who attended Alfred Health MSNI service during the pandemic.
Footnotes
Contributors: LR, WZY, AvdW and MM were responsible for planning and developing the research concept and are responsible for the overall content. LR wrote the manuscript with support from WZY, MM and AvdW. WZY derived the models and analysed the data. AR collected data. YF, RW, MZ, TT, NS, FB, OS, CN and HB contributed to the final manuscript. All authors discussed the results and contributed to the final manuscript. AvdW is responsible as the guarantor for this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AvdW served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck and Roche She has received speaker’s honoraria and travel support from Novartis, Roche and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. MM has served on advisory board for Merck, has received speaker honoraria from Merck and Biogen. Her institution receives funding from Merck, Australian National Health Medical Research Council, Brain Foundation, Charles and Sylvia Viertel Foundation, Bethlehem Griffith Foundation and MS Research Australia. WZY has received speaker honoraria from Merck & Novartis. LR served on advisory boards from Biogen, Merck and Roche She has received speaker’s honoraria and travel support from Biogen, Novartis, Roche and Merck. HB has received compensation for consulting, talks, advisory/steering board activities from Biogen, Merck, Novartis, Genzyme, Alfred Health; research support from Novartis, Biogen, Roche, Merck, NHMRC, Pennycook Foundation, MSRA; received compensation for same activities from Oxford Health Policy Forum, Merck, Biogen, Novartis. OS received travel support, speaker honoraria for Biogen, Merck, Genzyme and Novartis and served on scientific advisory board of Merck and Biogen. RW has received travel support from Merck, Roche and Honoraria from Biogen. MZ has received conference support from Roche. CN has no conflicts of interest. AR has no conflicts of interest. FB has received travel support from Biogen. NS has received conference support from Roche. TT has no conflicts of interest. YF has received travel support from Biogen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Alfred Hospital Melbourne Australia Ethics CommitteeHREC, approval no: 528/12. Participants gave informed consent to participate in the study before taking part.
References
- 1. Australian Government Department of Health . Vaccination numbers and Statistics data as of 15 February 2023. Available: https://www.health.gov.au/our-work/covid-19-vaccines/vaccination-numbers-and-statistics [Accessed 20 Feb 2023].
- 2. Shields AM, Venkatachalam S, Shafeek S, et al. SARS-Cov-2 vaccine responses following Cd20-depletion treatment in patients with haematological and Rheumatological disease: a West Midlands research consortium study. Clin Exp Immunol 2022;207:3–10. 10.1093/cei/uxab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwarz T, Otto C, Jones TC, et al. Preserved T cell responses to SARS-Cov-2 in anti-Cd20 treated multiple sclerosis. Mult Scler 2022;28:1041–50. 10.1177/13524585221094478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedotti R, Muros-Le Rouzic E, Raposo C, et al. Understanding the impacts of COVID-19 pandemic in people with multiple sclerosis treated with Ocrelizumab. Mult Scler Relat Disord 2021;55. 10.1016/j.msard.2021.103203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hughes R, Whitley L, Fitovski K, et al. COVID-19 in Ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord 2021;49. 10.1016/j.msard.2020.102725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-Cd20-Depleting therapy in autoimmune diseases. Clin Exp Immunol 2020;202:149–61. 10.1111/cei.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021;97:e1870–85. 10.1212/WNL.0000000000012753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular Azd7442 (Tixagevimab–Cilgavimab) for prevention of COVID-19. N Engl J Med 2022;386:2188–200. 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Australian Government Department of Health . Tixagevimab and Cilgavimab 150/150mg fact sheet for health professionals. Available: https://www.health.gov.au/resources/publications/evusheld-fact-sheet-for-health-professionals [Accessed 1 Apr 2023].
- 10. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of Intramuscular administration of Tixagevimab-Cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet Respiratory Medicine 2022;10:985–96. 10.1016/S2213-2600(22)00180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tixagevimab-and-Cilgavimab-PSD-September-2022. Available: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2022-09/Tixagevimab-cilgavimab-Evusheld-PSD-September-2022 [Accessed 1 May 2023].
- 12. Msbase: an international, online Registry and platform for collaborative outcomes research in multiple sclerosis. n.d. Available: https://www.msbase.org [DOI] [PubMed]
- 13. Victorian Supercare Pharmacy available from www.health.Vic.Gov.au/primary-care/Victorian-Supercare-pharmacies. Available: https://www.betterhealth.vic.gov.au/sites/default/files/2022-09/victorian-supercare-pharmacies-english-sept-2022.pdf [Accessed 20 Mar 2023].
- 14. Consumer medical information . Summary: Evusheld. Available: https://www.tga.gov.au/sites/default/files/evusheld-cmi.pdf [Accessed 1 May 2023].
- 15. US Food and Drug Administration . EVUSHELD: emergency use authorization (EAU)—Full fact sheet for Healthcare providers. 2022. Available: https://www.fda.gov/media/154701/download [Accessed 3 Mar 2023].
- 16. VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-Cov-2 B. 1.1. 529 Omicron virus escapes neutralization by therapeutic Monoclonal antibodies. Nat Med 2022;28:490–5. 10.1038/s41591-021-01678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leask J, Carlson SJ, Attwell K, et al. Communicating with patients and the public about COVID-19 vaccine safety: recommendations from the collaboration on social science and Idmmunisation. Med J Aust 2021;215:9–12. 10.5694/mja2.51136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewandowsky S, Cook J, Schmid P, et al. The COVID-19 vaccine communication Handbook. In: A practical guide for improving vaccine communication and fighting misinformation. Bristol, UK: University of Bristol, 2021. Available: https://hackmd.io/@scibehC19vax/home [Google Scholar]
- 19. Lupton D, Lewis S. Sociomaterialities of health, risk and care during COVID-19: experiences of Australians living with a medical condition. Soc Sci Med 2022;293. 10.1016/j.socscimed.2021.114669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCaffery KJ, Dodd RH, Cvejic E, et al. Health literacy and disparities in COVID-19–related knowledge, attitudes, beliefs, and Behaviours in Australia. Public Health Res Pract 2020;30. 10.17061/phrp30342012 [DOI] [PubMed] [Google Scholar]
- 21. Department of health, Victoria chief health office update ; 23RdDecember 2022. 2022. Available: https://www.health.vic.gov.au/media-releases/chief-health-officer-update-23-december-2022 [Accessed 22 May 2024].
- 22. Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-Cov-2Neutralizing antibodies. Nature 2022;602:657–63. 10.1038/s41586-021-04385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-Cov-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic Monoclonal antibodies. Nat Med 2022;28:490–5. 10.1038/s41591-021-01678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dejnirattisai W, Huo J, Zhou D, et al. SARS-Cov-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022;185:467–84. 10.1016/j.cell.2021.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. COVID-19 Australia: epidemiology report 73 reporting period ending 9 April 2023. COVID-19 Epidemiology and Surveillance Team. Available: http://health.gov.au/cdi [Accessed 2 May 2023]. [Google Scholar]
- 26. Chang SL, Nguyen QD, Martiniuk A, et al. Persistence of the Omicron variant of SARS-Cov-2 in Australia: the impact of fluctuating social distancing. PLOS Glob Public Health 2023;3:e0001427. 10.1371/journal.pgph.0001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Case JB, Mackin S, Errico J, et al. Resilience of s309 and azd7442 monoclonal antibody treatments against infection by sars-cov-2 omicron lineage strains. Microbiology [Preprint]. 10.1101/2022.03.17.484787 [DOI] [PMC free article] [PubMed]
- 28. Tuekprakhon A, Huo J, Nutalai R. Further antibody escape by Omicron Ba.4 and Ba.5 from vaccine and Ba.1 serum. Microbiology [Preprint]. 10.1101/2022.05.21.492554 [DOI]
- 29. NSW health - NSW respiratory surveillance reports - COVID-19 and influenza – weekly reports from. 2023. Available: https://www.health.nsw.gov.au/Infectious/covid-19/Pages/weekly-reports.aspx [Accessed 1 Jan 2023].
- 30. Lazarus JV, Romero D, Kopka CJ, et al. A multinational Delphi consensus to end the COVID-19 public health threat. Nature 2022;611:332–45. 10.1038/s41586-022-05398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.