Abstract
Our recent randomized, placebo-controlled study in Irritable Bowel Syndrome (IBS) patients with diarrhea or alternating bowel habits showed that the probiotic Bifidobacterium longum (BL) NCC3001 improves depression scores and decreases brain emotional reactivity. However, the involved metabolic pathways remain unclear. This analysis aimed to investigate the biochemical pathways underlying the beneficial effects of BL NCC3001 using metabolomic profiling. Patients received probiotic (1x 1010CFU, n=16) or placebo (n=19) daily for 6 weeks. Anxiety and depression were measured using the Hospital Anxiety and Depression Scale. Brain activity in response to negative emotional stimuli was assessed by functional Magnetic Resonance Imaging. Probiotic fecal abundance was quantified by qPCR. Quantitative measurement of specific panels of plasma host-microbial metabolites was performed by mass spectrometry-based metabolomics. Probiotic abundance in feces was associated with improvements in anxiety and depression scores, and a decrease in amygdala activation. The probiotic treatment increased the levels of butyric acid, tryptophan, N-acetyl tryptophan, glycine-conjugated bile acids, and free fatty acids. Butyric acid concentration correlated with lower anxiety and depression scores, and decreased amygdala activation. Furthermore, butyric acid concentration correlated with the probiotic abundance in feces. In patients with non-constipation IBS, improvements in psychological comorbidities and brain emotional reactivity were associated with an increased abundance of BL NCC3001 in feces and specific plasma metabolites, mainly butyric acid. These findings suggest the importance of a probiotic to thrive in the gut and highlight butyric acid as a potential biochemical marker linking microbial metabolism with beneficial effects on the gut-brain axis.
KEYWORDS: Irritable bowel syndrome (IBS); probiotic; depression; emotional reactivity; metabolomics, butyrate
Introduction
For millennia, traditional medicine has acknowledged the deep and complex relationships between the gut and the brain. More recently, growing scientific evidence has confirmed the existence of the gut-brain axis (GBA), the bidirectional communication between the gut and the brain that involves endocrine, neural, and immune signaling pathways.1 The gut microbiota is believed to interact with these pathways and to contribute with additional signaling, such that the concept is regularly extended to the microbiota-gut-brain axis. The microbiota has been associated with the modulation of gut homeostasis,2 and even with processes affecting brain development, physiology, behavior, and psychology.3 Gut bacteria have been shown to produce and degrade neuroactive compounds such as biogenic amines,4 γ-aminobutyric acid (GABA),5 serotonin, tryptophan,6 and short-chain fatty acids such as butyric acid7 that may reach the brain via circulation and the blood–brain barrier and/or activate neural pathways.8
Irritable bowel syndrome (IBS) is a common digestive disorder characterized by abdominal pain and altered bowel habits, and is frequently accompanied by psychiatric comorbidities.9 Disorders such as IBS, which were previously described as functional gastrointestinal disorders, have recently been defined as disorders of gut–brain interaction.10 Perturbation of bidirectional GBA is increasingly recognized as a conceptual model of IBS pathophysiology, involving dysfunction of the enteric, autonomic, and central nervous systems (CNS).11 Indeed, stress can trigger the onset or aggravate the severity of IBS symptoms (top-down model). On the other hand, intestinal symptoms can promote or aggravate psychological disorders (e.g., depression and anxiety) and decrease health-related quality of life (bottom-up model).12
Recent evidence obtained from germ-free mice colonized with human microbiota supports a role of the gut bacteria in IBS pathogenesis, including its psychological comorbidity.13 In multiple case-control studies, changes in microbiota composition and function have been linked to several disease states,14 ranging from gastrointestinal to neurological conditions. It has been postulated that modulation of the gut microbiome or consumption of specific probiotics might lead to beneficial changes in CNS functions.15
We have previously demonstrated that, in IBS patients with diarrhea or mixed bowel habits, a 6-week intervention with the probiotic Bifidobacterium longum subsp. longum (BL) NCC3001 reduced depression scores and responses to negative emotional stimuli in multiple brain areas, including the amygdala and fronto-limbic regions, compared to placebo.16 We found that gut metabolites were altered by BL NCC3001 consumption, despite having no major impact on the microbiota composition.16 Notably, the probiotic group had reduced urine levels of methylamines and metabolites derived from tyrosine compared with the placebo group. These observations suggest a shift in the bacterial metabolism of amines and amino acids, including a decrease in the production of the host-bacterial co-metabolite 4-cresol sulfate. This molecule inhibits dopamine β-hydroxylase, an enzyme involved in the conversion of dopamine to noradrenaline. However, the precise molecular mechanisms underlying the beneficial effects of BL NCC3001 in the brain remain poorly understood. Here, we applied a targeted quantitative metabolic profiling approach for plasma analysis combined with a measure of fecal BL NCC3001 abundance and integrated them with clinical and brain imaging outcomes to generate deeper insights into the potential mechanisms involved in the central effect of BL NCC3001.
Results
Study patients and biological samples
From the 38 patients who completed the study (BL NCC3001 = 18, placebo = 20), metabolomic analysis of blood samples was conducted in the 36 participants whose samples were available for both pre- and post-intervention (BL NCC3001 = 18, placebo = 18). The abundance of BL NCC3001 in feces was assessed in 35 participants (BL NCC3001 = 16, placebo = 19) and amygdala activation was measured in 25 participants (BL NCC3001 = 11, placebo = 14).
BL NCC3001 quantification and association with clinical outcomes
Using a quantitative PCR assay, we determined the relative abundance of BL NCC3001 in the feces of the participants (Figure 1a). A High BL NCC3001 abundance was detected only in the BL NCC3001 group, except for one subject in the placebo group at the third visit (end of treatment).
Figure 1.
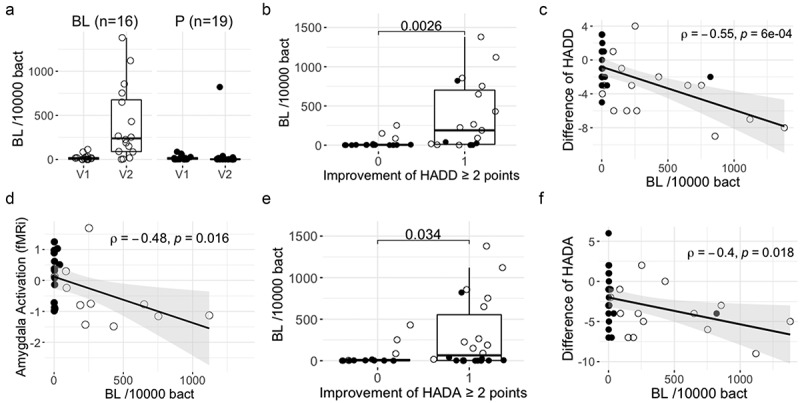
Fecal BL NCC3001 relative abundance and associations with clinical outcomes.
A) BL NCC3001 abundance in feces at baseline (V1) and at the end of intervention (V2) per group. B) Fecal BL NCC3001 abundance in responders [≥2 points improvement in HADD score (1)] and not responders [<2 points improvement in HADD score (0)]; p-value of Wilcoxon test. C) Correlation between the difference of HADD score (V2-V1) and BL NCC3001 abundance at V2; Spearman test. D) Correlation between amygdala activation (fMRi) and BL NCC3001 abundance at V2; Spearman test. E) Fecal BL NCC3001 abundance in responders [≥2 points improvement in HADA score (1)] and non-responders [<2 points improvement in HADA score (0)]; p-value of Wilcoxon test. F) Correlation between the difference in HADA score (V2-V1) and BL NCC3001 abundance at V2; Spearman test. BL NCC3001 (BL, open circle); Placebo (P, closed circle).
The reduction of two points or more in either HADD (Figure 1b) or HADA (Figure 1e) scores – used as primary outcome of the trial – was associated with an increased abundance of BL NCC3001 (Wilcoxon test; p = .003 and p = .034, respectively). The decrease in both scores (Figures 1c & f) correlated with the abundance of BL NCC3001 (rho = 0.55, p = 6e-04 and rho = −0.4, p = .018, respectively). Decreased amygdala activation in response to negative stimuli, as assessed by functional Magnetic Resonance Imaging (fMRI), was also correlated with probiotic abundance rho = −0.48, p = .016, Figure 1d).
Effect of the probiotic intervention on blood metabolites
OPLS discriminant analysis was applied using one predictive component and one orthogonal component to model the blood metabolic differences between the two groups (Figure 2). The model was statistically robust only for post-treatment analysis (R2X = 0.19, R2Y = 0.76, Q2Y = 0.26, where R2X: explained variance in the metabolomic data (plasma metabolites), R2Y: explained group variance (placebo and probiotic) and Q2Y: robustness of the model). Before treatment, there was no difference between the two groups (Q2Y <0) (Table 1), and when considered separately, none of the metabolites were significantly different, except for butyric acid, which was slightly higher in the placebo group (p = .0378, see below). Patients receiving BL NCC3001 for 6 weeks exhibited higher plasma concentrations of several fatty acid species (e.g., arachidonic, palmitic, and stearic acid; p < .05, Figures 3a–c), of the amino acid N-acetyl-tryptophan (p < .05, Figure 4d), and of butyric acid (p < .05, Figure 5a), compared to patients receiving placebo. These changes paralleled trends for higher concentrations of the branched-chain fatty acid ethylmethylacetic acid, several glycine conjugated bile acid species such as glycocholic acid (GCA) and glycochenodeoxycholic acid (GCDCA) and fatty acids (e.g., arachidic, docosatrienoic, and eicosapentaenoic acids), and in the amino acid tryptophan (p < .1, Figures 3c,d,e & 4a). Patients receiving BL NCC3001 also had lower plasma concentration of the bile acid TUDCA (p = .052).
Figure 2.
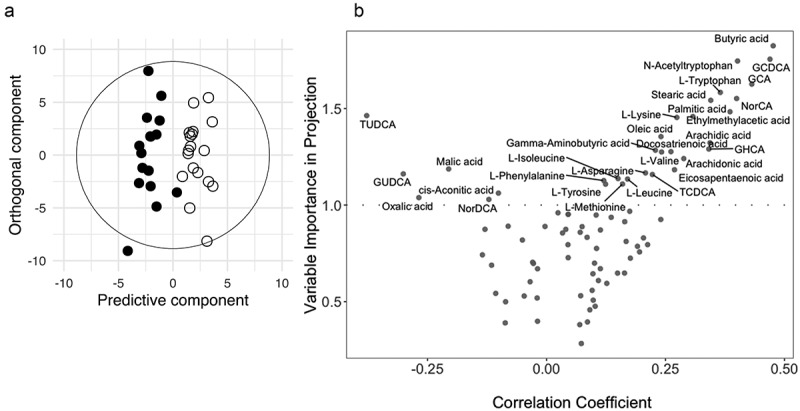
OPLS samples and variable plots.
(A) OPLS Score plot derived from plasma metabolic profiles. The cross-validated scores plot showed statistically significant separations between the blood profiles obtained post-intervention from placebo (closed circle) and BL NCC3001 (open circle) treated patients. (B) OPLS variables plots describing influential variables contributing to the separation observed in the scores plots, according to variable importance in projection (threshold >1.0) and the correlation coefficient.
Table 1.
Overview of plasma metabolite differences according to treatment and time.
| Metabolites (unit) | OPLS parameters. |
Blood concentrations (Mean (SD)) |
P-value Pre | P-value Post | ||||
|---|---|---|---|---|---|---|---|---|
| Coeff | VIP | Placebo Pre | Placebo Post | BL NCC3001 Pre | BL NCC3001 Post | |||
| Arachidic acid (ng/mL) | 0.34 | 1.32 | 258.1 (233.6) | 143.4 (142.9) | 212 (143.7) | 241.5 (147.6) | 0.476 | 0.057 |
| Arachidonic acid (ng/mL) | 0.29 | 1.24 | 74831.5 (33085.7) | 73022.8 (23563.8) | 85945.1 (44065.4) | 95695.5 (43008.9) | 0.403 | 0.043 |
| Butyric acid (ng/mL) | 0.48 | 1.83 | 91.5 (45.9) | 72.0 (29.6) | 65 (25) | 103.5 (37.2) | 0.038 | 0.008 |
| Docosatrienoic acid (ng/mL) | 0.26 | 1.28 | 14736.4 (6929.5) | 14035.0 (6178.5) | 14103.9 (4891.4) | 18435.1 (7955) | 0.752 | 0.064 |
| Eicosapentaenoic acid (ng/mL) | 0.27 | 1.18 | 389870.2 (148890.7) | 387660.8 (140034.1) | 436787.9 (195879.5) | 475388.7 (171320.1) | 0.429 | 0.077 |
| Ethylmethylacetic acid (ng/mL) | 0.39 | 1.48 | 30.2 (63.9) | 34.5 (71.8) | 33.6 (67.3) | 89 (88.2) | 0.881 | 0.050 |
| GCA (nM) | 0.43 | 1.63 | 331.2 (632.3) | 258.7 (158) | 385.2 (291.2) | 602.2 (786.1) | 0.739 | 0.085 |
| GCDCA (nM) | 0.47 | 1.76 | 1069.2 (1042.4) | 980.8 (707.6) | 1371.9 (887.7) | 1807.2 (1632.6) | 0.347 | 0.061 |
| GUDCA (nM) | −0.30 | 1.16 | 35.6 (61.1) | 69.7 (128.5) | 54.9 (106) | 17.6 (23.4) | 0.508 | 0.108 |
| L-Asparagine (ng/mL) | 0.21 | 1.17 | 9083.2 (4325.8) | 9021.8 (4593.8) | 9555.4 (2702.9) | 10726 (1283.3) | 0.694 | 0.155 |
| L-Tryptophan (ng/mL) | 0.37 | 1.58 | 16181.8 (4381.5) | 15788.6 (4257.6) | 17504.5 (1917.2) | 17740.3 (1537.6) | 0.244 | 0.081 |
| Malic acid (ng/mL) | −0.21 | 1.19 | 255.1 (302.9) | 497.9 (679.3) | 340.7 (472.3) | 331.4 (404.5) | 0.528 | 0.372 |
| N-acetyl-tryptophan (ng/mL) | 0.40 | 1.75 | 36953.1 (12532.5) | 35349.5 (11388.5) | 40921.5 (8513.5) | 41968.6 (6827.7) | 0.271 | 0.044 |
| Oleic acid (ng/mL) | 0.24 | 1.35 | 407483.2 (119185) | 372474.5 (120852.2) | 377034.6 (140261.3) | 447659.5 (140379.6) | 0.491 | 0.131 |
| Palmitic acid (ng/mL) | 0.31 | 1.46 | 166557.1 (46476.1) | 156101.2 (44850.9) | 169164.7 (46423.7) | 189971.6 (40260.8) | 0.868 | 0.024 |
| Stearic acid (ng/mL) | 0.35 | 1.54 | 126420.4 (42195.5) | 117539.2 (29871.9) | 123284.8 (46273.5) | 141693.9 (34051.4) | 0.834 | 0.028 |
| TUDCA (nM) | −0.38 | 1.46 | 29.1 (27.4) | 51.6 (36) | 26.9 (25) | 30.3 (20.4) | 0.806 | 0.052 |
Legend: Coeff: OPLS Correlation coefficient, VIP: OPLS Variable Importance in Projection; p-value: Wilcoxon signed rank test between placebo and BL NCC3001 group post intervention.
Figure 3.
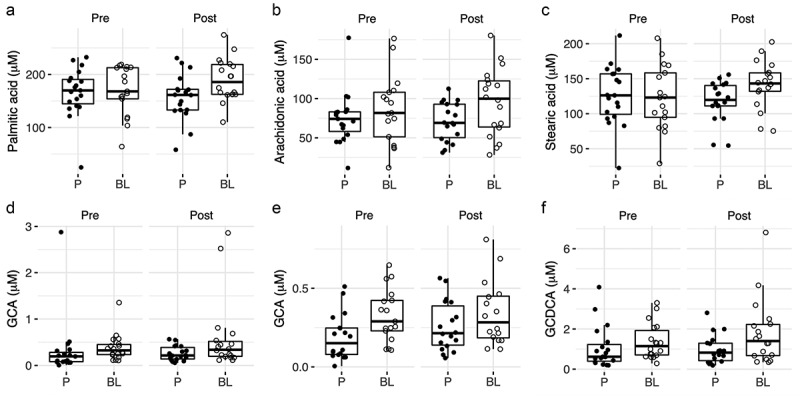
Overview of changes in plasma fatty acids and bile acids.
Concentrations of metabolites in plasma at baseline (V1) and end of intervention (V2) per group: A) palmitic acid, B) arachidonic acid, C), stearic acid, D) glycine conjugated cholic acid (GCA), E) glycine conjugated cholic acid (GCA) without outliers, F) glycine-conjugated chenodeoxy cholic acid (GCDCA). BL NCC3001 (BL, open circle); Placebo (P, closed circle).
Figure 4.
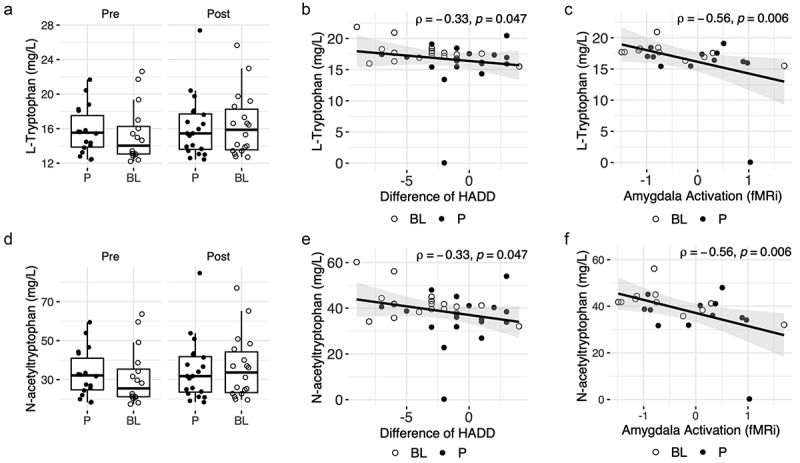
Overview of changes in plasma tryptophan, N-Acetyl tryptophan, and associations with clinical endpoints.
A) Concentrations of tryptophan in plasma at baseline (V1) and end of intervention (V2) per group, B) Correlation between the difference of HADD score (V2-V1) and tryptophan concentration in plasma at V2; Spearman test. C) Correlation between amygdala activation (fMRi) and tryptophan concentration in plasma at V2; Spearman test. D) Concentrations of N-acetyltryptophan in plasma at baseline (V1) and end of intervention (V2) per group, E) Correlation between the difference in HADD score (V2-V1) and N-acetyltryptophan concentration in plasma at V2; Spearman test. F) Correlation between amygdala activation (fMRi) and plasma N-acetyl-tryptophan concentration at V2; Spearman test. BL NCC3001 (BL, open circle); Placebo (P, closed circle).
Figure 5.
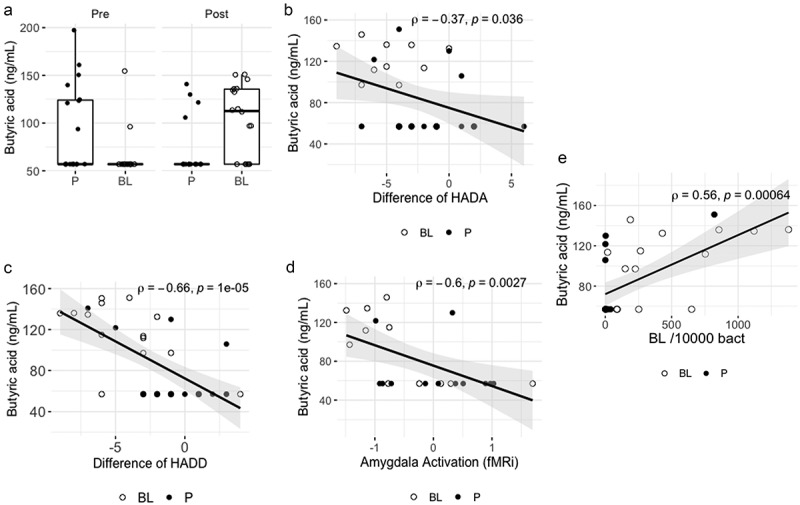
Overview of changes in plasma butyric acid and associations with clinical endpoints and fecal BL NCC3001 counts.
A) Concentrations of butyric acid in plasma at baseline (V1) and end of intervention (V2) per group, B) Correlation between the difference in HADA score (V2-V1) and butyric acid concentration in plasma at V2; Spearman test, C) Correlation between the difference in HADD score (V2-V1) and butyric acid concentration in plasma at V2; Spearman test. D) Correlation between amygdala activation (fMRi) and butyric acid concentration in the plasma at V2; Spearman test. E) Correlation between fecal BL NCC3001 abundance and butyric acid concentration in plasma at V2; Spearman test. BL NCC3001 (BL, open circle); Placebo (P, closed circle).
Associations of metabolites with clinical outcomes and fecal BL NCC3001 abundance
Several metabolites were associated with changes in clinical outcomes and abundance of BL NCC3001 (Supplementary Table S1). There was a strong correlation between the levels of butyric acid and the reduction in the HADD score in the BL NCC3001 group and in the entire study population rho = −0.67, p = .002 and rho= −0.66, p = .00001, respectively; Figure 5c). Butyric acid levels in the whole study population also correlated with a decrease in amygdala activation (rho= −0.50, p = .016, Figure 5d) and HADA scores (rho= −0.35, p = .034, Figure 5b).
The decrease in amygdala activation was also strongly associated with higher plasma levels of tryptophan (rho= −0.66, p = .031 and rho= −0.56, p = .006, respectively; Figure 4c), N-acetyl tryptophan (rho= −0.66, p = .031 and rho= −0.56, p = .006, respectively; Figure 4f), and the fatty acid pentadecanoic acid (rho= −0.66, p = .031 and rho= −0.55, p = .007, respectively) in the BL NCC3001 group and the entire study population (Table 1). The increases in plasma levels of tryptophan (rho= −0.33, p = .047, Figure 4b), N-acetyl tryptophan (rho= −0.33, p = .047, Figure 4e), and arachidic acid (rho= −0.36, p = .029) were also correlated with decreased HADD scores in the whole study population (Supplementary Table S1).
The plasma level of butyric acid was positively associated with the abundance of BL NCC3001 measured in the feces (rho = 0.59, p = .016 and rho = 0.56, p = .00064, Figure 5e) in the BL NCC3001 group and the entire study population, respectively.
Discussion
We previously showed in this randomized, placebo-controlled study that a 6-week administration of BL NCC3001 lowered depression scores and decreased responses to fearful stimuli in multiple brain areas involved in the processing of emotions, including the amygdala and fronto – limbic regions. Here, we demonstrated that these changes were associated with the abundance of BL NCC3001 as measured in feces and plasma levels of several metabolites, including butyric acid, tryptophan, N-acetyl tryptophan, glycine-conjugated bile acids, and free fatty acids. Of these, butyric acid was strongly correlated with lower anxiety and depression scores and decreased amygdala activation, suggesting that it could play a key role in the beneficial effect of the probiotic.
Measurements of BL NCC3001 abundance in feces revealed that there was overall good compliance with probiotic intake. Interestingly, a high level of BL NCC3001 was detected in one patient in the placebo group at the end of treatment. Although Bifidobacterium longum subsp. longum is common in the adult gut microbiota,17 this marked increase suggests that the patient likely consumed a dietary product or probiotic supplement containing a very closely related strain, since it was detected by our assay targeting a strain-specific chromosomal region of the bacterium. The genomic signature of the strain detected in this sample was further investigated by three PCR assays targeting other strain-specific regions of the NCC3001 chromosome (the sites of insertion of mobile genetic elements), which were all positive, further demonstrating the relatedness of this strain with BL NCC3001 (data not shown). It is noteworthy that the same study participant showed an improvement in the HADD and HADA scores, consistent with the strain-specificity of the probiotic effect.
The supplementation with BL NCC3001 modulated the blood plasma biochemical composition, from which only a few metabolites changed and correlated with improvements in amygdala reactivity, anxiety, or depression scores – namely bile acids, short chain fatty acids, and amino acids.
Amongst major metabolic changes not associated with clinical outcomes were increased plasma fatty acids, which may relate to other external factors, such as lifestyle.18 Here, BL NCC3001 administration increased saturated and polyunsaturated fatty acids (PUFAs), namely palmitate (C16), stearate (C18), arachidonate (C20:4), and oleate (C18:1), possibly by modulating fatty acid absorption and bioavailability.19 IBS patients have been reported to have a distinct composition of polyunsaturated fatty acids (PUFAs) in gut biopsies and blood samples.20,21 PUFAs have been shown to regulate brain function through multiple mechanisms, including the hypothalamic-pituitary-adrenal axis, neuroendocrine, and immune regulations. 19–21 Arachidonic acid may impact the pathophysiology of depression by affecting serotonin transport22,23 whilst blood oleic acid is associated with a lower incidence of depression,24 although its underlying mechanisms are not well understood.16,25–27 BL NCC3001 increased the levels of two primary bile acids, GCA and GCDCA, which correlated with improvement in anxiety scores. The probiotics may exert some of its benefits, directly or indirectly, through metabolic functions related to bile acid absorption and enterohepatic recirculation, factors shown to be impaired in 40% of IBS patients.28 The shift in the bile acid pattern toward glycine-conjugates can be explained by a reduction in bile salt hydrolase activity by bacteria in the small intestine. As the conjugation of bile acids increases their solubility and lipid emulsification properties,29 higher levels of GCA and GCDCA, and fatty acids in the BL NCC3001 group may indicate increased lipid emulsification and absorption. Recent studies have suggested that bile acids have neuroprotective effects in models of Huntington’s disease30 and Alzheimer’s disease.31,32 Upon absorption and blood circulation, bile acids may exert their central effects via the bile acid receptors FXR and TGR5 signaling pathways.33,34 The G-protein coupled receptor TGR5 is expressed in various brain regions and acts as a neurosteroid receptor,33 suggesting a potential cross talk between neurosteroids and bile acids. Neurosteroids classically act to modulate GABAergic tone. In fact, CDCA has been recently shown to antagonize GABAA and NMDA receptors.35
Our data showed that tryptophan metabolism may also play an important role in mediating the central benefits of BL NC3001, as the probiotic increased the circulating plasma levels of both L-tryptophan (+8%) and N-acetylated-tryptophan (+18%). Although N-acetylated-tryptophan concentrations are more than double those of tryptophan, both metabolites are correlated with decreased amygdala reactivity and lower anxiety in patients with IBS. Tryptophan, a precursor of serotonin, mediates serotonergic activity in the brain and exerts beneficial effects on cognition, mood, and anxiety.36 N-acetyl-tryptophan exerts a neuroprotective effect by blocking substance P-mediated neuroinflammation, reducing oxidative stress, exhibiting anti-apoptotic properties, and contributing to improved motor and cognitive functions in models of Parkinson’s diseases.37,38 Since plasma concentrations of these amino acids are strictly dependent on dietary sources, BL NCC3001-induced changes are indicative of a shift in protein and aromatic amino acid metabolism by the gut microbiota, a feature already revealed by the perturbation of 4-cresol sulfate metabolism described previously.16
The changes in plasma butyric acid and 2-methylbutyric acid induced by probiotics intervention are an additional indicator of a shift in protein and carbohydrate (including fibers and complex carbohydrates) metabolism by the gut microbiota. We found that the plasma concentration of butyric acid was correlated with both the fecal abundance of BL NCC3001 and improvements in depression scores and amygdala reactivity. We hypothesize that BL NCC3001 increases butyric acid production by cross-feeding, as bifidobacteria are acetate producers that cross-feed butyric acid-producing colonic bacteria.39 Butyric acid is known to reverse depressive behavior, increase serotonin concentration and BDNF expression, and restore blood–brain barrier impairments.8,40–42 Furthermore, butyric acid contributes to dopamine and norepinephrine synthesis as well as dopaminergic function, by modulating tyrosine hydroxylase and dopamine-β-hydroxylase genes.40 In addition, butyric acid-related changes in microbiota have been associated with changes in neuroinflammation through modulation of microglia activation, which may also contribute to the observed benefits.43 However, none of the other Bifidobacterium spp previously tested in a mouse model improved anxiety-like behavior (WO2009127566). Therefore, in addition to the contribution of BL NCC3001 to a cross-feeding chain producing butyrate, other factors that are not conserved in all bifidobacteria may explain the observed specificity of the effect, such as the capacity of this strain to survive in the gastro-intestinal tract and/or the production of distinct metabolites (herein reported or undescribed).
Whilst this was the first randomized trial to show that BL NCC3001 fecal abundance and several plasma metabolites associated with the improvement of psychological comorbidities in IBS patients, there are some limitations that are important to emphasize. First, this was a pilot study with a limited number of participants, which included only IBS patients with diarrhea or mixed-stool phenotypes. Of note, we are currently conducting a confirmatory trial in a larger cohort of patients (NCT05054309), including those with IBS with constipation. This multicentric trial conducted in Canada will also offer the opportunity to validate our results based on a larger sample size. In terms of technical limitations, lipidome analysis would be required to enable a comprehensive study of all saturated and unsaturated fatty acids levels and their implications for depression disorders. Furthermore, the strain-specificity of the BL NCC3001 fecal abundance quantification method by qPCR is valid as long as there is no very closely related strain in the microbial environment, as discussed above. Noteworthy, we did not use a quantification assay that would distinguish live cells from dead cells.44 A rapid calculation allows to show that the differences in the BL NCC3001 abundances measured in feces reflect the capacity of the probiotic to thrive in the gut (see supplementary discussion), and therefore the viability of the detected cells is not important in the current context.
In conclusion, improvement in psychological comorbidities and decreased brain emotional reactivity in non-constipated IBS patients was associated with an increased abundance of BL NCC3001 detected in feces and with several plasma metabolites, mainly butyric acid. These new findings suggest that the capacity of the probiotic BL NCC3001 to thrive in the gastrointestinal tract is an important requisite for its beneficial effects and that butyric acid may be a biochemical biomarker linking probiotic metabolism with its bioactivity.
Materials and methods
Clinical study design
We conducted a randomized, double-blind, placebo-controlled, single-center pilot study16 in adult IBS patients with diarrhea or a mixed-stool pattern diagnosed based on the Rome III criteria.45 The study was approved by the Hamilton Health Sciences and St Joseph’s Health Care Research Ethics Boards, and all participants provided informed consent. This study was registered at ClinicalTrials.gov on January 13, 2011 (NCT01276626). These patients also exhibited mild-to-moderate anxiety and/or depression based on the Hospital Anxiety and Depression (HAD) scale46 (HADA or HADD score 8–14).
This study included four hospital visits. At the screening visit, clinical history and symptoms were assessed, and physical examination and complete bloodwork were performed. At the second visit, the inclusion and exclusion criteria and symptoms were reassessed, and baseline stool, urine, and blood samples were collected. Psychological and intestinal symptoms were assessed using the HADS and the Birmingham IBS questionnaires, respectively, and a functional magnetic resonance imaging (fMRI) study was conducted. Patients were randomized to either spray-dried BL NCC3001 (1x 1010CFU/1 gram powder with maltodextrin) or placebo containing 1 g of maltodextrin, to be taken daily at breakfast for a period of 6 weeks. The patients were asked not to change their eating habits or fiber intake. The study products were indistinguishable in terms of packaging, color, taste, and consistency. Compliance was measured by recording the participants’ treatment intake, that is, the empty sachets were collected at the end of the intervention (the third visit). Symptom assessment, fMRI study, and samples collection were repeated at the third visit. Blood was collected in EDTA, and plasma was obtained by centrifugation. Finally, patients’ symptoms were reassessed at a follow-up visit (4 weeks after treatment completion).
Clinical study endpoints
The primary endpoint was a reduction in anxiety (HADA) and/or depression (HADD) scores of ≥ 2 points on the HAD scale45 at the end of the treatment. These cutoffs were based on the previously established main clinically important differences for the anxiety and depression scores on the HAD scale of 1.3 and 1.4, respectively.47 Secondary endpoints included, among others, improvement in anxiety and depression scores (HADA and HADD, continuous data), changes in brain activation patterns measured by fMRI, plasma metabolomics, and fecal BL NCC3001 counts.
Metabolomic analysis
Metabolomic analysis was conducted in plasma-EDTA samples collected before and at the end of the treatment to measure specific panels of bile acids and other host-gut microbial metabolites. Samples were extracted and prepared according to previously published methods.48,49 All standards for bile acid analysis were obtained from Steraloids, Inc. (Newport, RI, USA) and TRC Chemicals (Toronto, ON, Canada). The calibration curve samples were prepared in a blank matrix and processed in the same manner as the real biological samples. An ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA) was used to quantify bile acids in the human plasma samples based on previously published protocols.48,50 Data acquisition was performed using MassLynx version 4.1 and bile acid quantification was performed using the TargetLynx applications manager version 4.1 (Waters, Milford, MA, USA). For other host-gut microbial metabolite analyses, samples were analyzed using a previously published targeted host-microbial metabolic profiling method using gas-chromatography mass spectrometry (LECO, Saint Joseph, MI).49 This second method enabled the quantification of 63 metabolites, including amino acids and derivatives, carboxylic acids, fatty acids, hydroxy acids, keto-acids, and aromatics.
Fecal BL NCC3001 abundance analysis
Fecal DNA was extracted using the QIAamp FAST DNA Stool Mini Kit (51604, Qiagen, Germany). DNA concentrations were measured using the PicoGreen fluorescence method (Thermo Fisher).
A chromosomal region spanning the insertion site of a mobile element was previously used to specifically detect the strain BL NCC3001.51,52 A TaqMan MGB assay targeting this strain-specific region was designed (Primer Express 3.0, Applied Biosystems) to measure the abundance of the probiotic in fecal samples (BL NCC3001_Fw 5’-GTGATAACCTCAACAACCGACAAC-3’, BL NCC3001_Pr (FAM) 5’- ATCTGCCCTTAACGGC-3’ (MGB), BL NCC3001_Rev 5’- GCATCACCTCGTTCTCGACAA-3’). The MasterMix LightCycler® 1536 DNA Green Master (05573092001, Roche) was used with a final concentration of 0.9 µM for each primer and 0.25 µM of the probe. Each data point was run in technical triplicate, and a standard curve was constructed in serial 10-fold dilutions of BL NCC3001 genomic DNA. The assay was performed on an LC480 II cycler (Roche) under the following PCR conditions: 7 min at 95°C for Taq activation, 10 s at 95°C for denaturation, and 30 s at 60°C for annealing and extension for 40 cycles, followed by cooling for 30 s at 40°C.
Another TaqMan MGB assay was used to normalize the abundance of BL NCC3001 relative to bacterial load.53
Statistical analysis
Chemometric analysis was performed on metabolomic data using the software package SIMCA-P+ (version 16.0, Sartorius Stedim Biotech, Sweden). Principal component analysis (PCA) and a modification of Partial Least Squares Regression (PLSR), which removes all information orthogonal to the response variable during the fitting process were employed. This variant, Orthogonal Projection to Latent Structures (O-PLS)54 provides sparser models (improving their interpretability) with the same degree of fit as PLSR. Variable Importance in Projection (VIP) was used to highlight the weight of individual variables in the model, with a value above 1 used as a threshold by convention. Univariate analysis was conducted using unpaired and paired t-tests for group comparisons, and Spearman correlations between metabolites and HAD, amygdala endpoints, and bacterial counts were computed. Statistical analysis was performed using R 4.0.5 (2021-03-31). Data were visualized using the R packages ggplot2 (2_3.3.5) and ggpubr (0.4.0), particularly by applying their respective functions, ggboxplot, and ggscatter. Spearman correlation and Wilcoxon signed-rank exact tests were used to determine the significance levels between metabolites and fecal BL NCC3001 abundance after interventions and clinical endpoints.
Access to data
The authors will consider sharing anonymized participant data upon request directed to the corresponding author. The sponsor, investigators, and collaborators will review and approve the requests based on scientific rigor and ethical compliance of the proposal.
Supplementary Material
Acknowledgments
The authors thank Nicolas Sauvageot for support with data analysis, Tiago Nunes, and Jane Natividad for valuable scientific discussions, and Marcus Böhme for support during the finalization of the manuscript.
Funding Statement
The work was funded by Société des Produits Nestlé S. A.
Disclosure statement
GB, BB, SC, JM, FPM, and OC are employees of Société des Produits Nestlé S.A., Switzerland. WJ, GX, and PB received research support from Société des Produits Nestlé, SA. PB holds the Richard Hunt-AstraZeneca Chair in Gastroenterology.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2347715
References
- 1.Collins SM, Surette M, Bercik P.. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–12. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV. et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 4.Lyte M, Brown DR, Yamashita A. Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of lactobacillus: implications for interkingdom communication within the microbiota-gut-brain axis. PloS One. 2018;13(1):e0191037. doi: 10.1371/journal.pone.0191037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A. et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 8.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;150:1393–1407. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Hasler WL. Rome IV—functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Karantanos T, Markoutsaki T, Gazouli M, Anagnou NP, Karamanolis DG. Current insights in to the pathophysiology of irritable bowel syndrome. Gut Pathog. 2010;2(1):3. doi: 10.1186/1757-4749-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine–immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. 2012;47(11):1177–1185. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 13.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S. et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9(379):eaaf6397. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 14.Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216(1):20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salami M. Interplay of good bacteria and central nervous system: cognitive aspects and mechanistic considerations. Front Neurosci. 2021;15:613120. doi: 10.3389/fnins.2021.613120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin F-P, Cominetti O, Welsh C, Rieder A. et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–59 e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Arboleya S, Watkins C, Stanton C, Ross RP. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautzova T, Hockley JRF, Perez-Berezo T, Pujo J, Tranter MM, Desormeaux C, Barbaro MR, Basso L, Le Faouder P, Rolland C. et al. 5-oxoETE triggers nociception in constipation-predominant irritable bowel syndrome through MAS-related G protein–coupled receptor D. Sci Signal. 2018;11(561):eaal2171. doi: 10.1126/scisignal.aal2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song C. Essential fatty acids as potential anti-inflammatory agents in the treatment of affective disorders. Mod Trends Pharmacopsychiatry. 2013;28:75–89. [DOI] [PubMed] [Google Scholar]
- 21.Provensi G, Schmidt SD, Boehme M, Bastiaanssen TFS, Rani B, Costa A, Busca K, Fouhy F, Strain C, Stanton C. et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci U S A. 2019;116(19):9644–9651. doi: 10.1073/pnas.1820832116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopaldas M, Zanderigo F, Zhan S, Ogden RT, Miller JM, Rubin-Falcone H, Cooper TB, Oquendo MA, Sullivan G, Mann JJ. et al. Brain serotonin transporter binding, plasma arachidonic acid and depression severity: a positron emission tomography study of major depression. J Affect Disord. 2019;257:495–503. doi: 10.1016/j.jad.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuboi H, Watanabe M, Kobayashi F, Kimura K, Kinae N. Associations of depressive symptoms with serum proportions of palmitic and arachidonic acids, and α-tocopherol effects among male population – a preliminary study. Clin Nutr. 2013;32(2):289–293. doi: 10.1016/j.clnu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe AR, Ogbonna EM, Lim S, Li Y, Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(6):972–977. doi: 10.1016/j.pnpbp.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes MF, Mutch DM, Leri F. The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients. 2017;9(3):298. doi: 10.3390/nu9030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain G, Schmitt F, Loeffler J-P, Gonzalez De Aguilar J. Fatting the brain: a brief of recent research. Front Cell Neurosci. 2013;7. doi: 10.3389/fncel.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA. et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139(6):2102–12 e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery IB, Das A, O’Herlihy E, Coughlan S, Cisek K, Moore M, Bradley F, Carty T, Pradhan M, Dwibedi C. et al. Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Gastroenterology. 2020;158(4):1016–28 e8. doi: 10.1053/j.gastro.2019.11.301. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong MJ, Carey MC. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res. 1982;23(1):70–80. doi: 10.1016/S0022-2275(20)38175-X. [DOI] [PubMed] [Google Scholar]
- 30.Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A. 2002;99(16):10671–10676. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM. Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med. 2008;14(2):54–62. doi: 10.1016/j.molmed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Sola S, Amaral JD, Borralho PM, Ramalho RM, Castro RE, Aranha MM, Steer CJ, Rodrigues CMP. Functional modulation of nuclear steroid receptors by tauroursodeoxycholic acid reduces amyloid β-peptide-induced apoptosis. Mol Endocrinol. 2006;20(10):2292–2303. doi: 10.1210/me.2006-0063. [DOI] [PubMed] [Google Scholar]
- 33.Keitel V, Gorg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58(15):1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 34.Mertens KL, Kalsbeek A, Soeters MR, Eggink HM. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci. 2017;11:617. doi: 10.3389/fnins.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes J, Mudgal J, Rao CM, Arora D, Basu Mallik S, Pai KSR, Nampoothiri M. N-acetyl-L-tryptophan, a substance-P receptor antagonist attenuates aluminum-induced spatial memory deficit in rats. Toxicol Mech Methods. 2018;28(5):328–334. doi: 10.1080/15376516.2017.1411412. [DOI] [PubMed] [Google Scholar]
- 38.Sirianni AC, Jiang J, Zeng J, Mao LL, Zhou S, Sugarbaker P, Zhang X, Li W, Friedlander RM, Wang X. et al. N-acetyl-l-tryptophan, but not N-acetyl-d-tryptophan, rescues neuronal cell death in models of amyotrophic lateral sclerosis. J Neurochem. 2015;134(5):956–968. doi: 10.1111/jnc.13190. [DOI] [PubMed] [Google Scholar]
- 39.De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149(1):73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. 2019;6(10):454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, McArthur S. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome. 2018;6(1):55. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehme M, Guzzetta KE, Wasén C, Cox LM. The gut microbiota is an emerging target for improving brain health during ageing. Gut Microbiome. 2023;4:e2. doi: 10.1017/gmb.2022.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papanicolas LE, Wang Y, Choo JM, Gordon DL, Wesselingh SL, Rogers GB. Optimisation of a propidium monoazide based method to determine the viability of microbes in faecal slurries for transplantation. J Microbiol Methods. 2019;156:40–45. doi: 10.1016/j.mimet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 46.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 47.Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6(1):46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z. et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. Faseb J. 2013;27(9):3583–3593. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L, Ni Y, Su M, Li H, Dong F, Chen W, Wei R, Zhang L, Guiraud SP, Martin F-P. et al. High throughput and quantitative measurement of microbial metabolome by gas chromatography/mass spectrometry using automated alkyl chloroformate derivatization. Anal Chem. 2017;89(10):5565–5577. doi: 10.1021/acs.analchem.7b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KLR, Sidney Barritt A. Altered bile acid metabolome in patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60(11):3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouge C, Goldenberg O, Ferraris L, Berger B, Rochat F, Legrand A, Göbel UB, Vodovar M, Voyer M, Rozé J-C. et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe. 2010;16(4):362–370. doi: 10.1016/j.anaerobe.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu CM, Aziz M, Kachur S, Hsueh PR, Huang YT, Keim P, Price LB. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol. 2012;12(1):56. doi: 10.1186/1471-2180-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trygg J, Wold S. O2-PLS, a two-block (X–Y) latent variable regression (LVR) method with an integral OSC filter. JChemom. 2003;17(1):53–64. doi: 10.1002/cem.775. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


