ABSTRACT
Metastatic (m) colorectal cancer (CRC) is an incurable disease with a poor prognosis and thus remains an unmet clinical need. Immune checkpoint blockade (ICB)-based immunotherapy is effective for mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) mCRC patients, but it does not benefit the majority of mCRC patients. NK cells are innate lymphoid cells with potent effector responses against a variety of tumor cells but are frequently dysfunctional in cancer patients. Memory-like (ML) NK cells differentiated after IL-12/IL-15/IL-18 activation overcome many challenges to effective NK cell anti-tumor responses, exhibiting enhanced recognition, function, and in vivo persistence. We hypothesized that ML differentiation enhances the NK cell responses to CRC. Compared to conventional (c) NK cells, ML NK cells displayed increased IFN-γ production against both CRC cell lines and primary patient-derived CRC spheroids. ML NK cells also exhibited improved killing of CRC target cells in vitro in short-term and sustained cytotoxicity assays, as well as in vivo in NSG mice. Mechanistically, enhanced ML NK cell responses were dependent on the activating receptor NKG2D as its blockade significantly decreased ML NK cell functions. Compared to cNK cells, ML NK cells exhibited greater antibody-dependent cytotoxicity when targeted against CRC by cetuximab. ML NK cells from healthy donors and mCRC patients exhibited increased anti-CRC responses. Collectively, our findings demonstrate that ML NK cells exhibit enhanced responses against CRC targets, warranting further investigation in clinical trials for mCRC patients, including those who have failed ICB.
KEYWORDS: Cetuximab, colorectal cancer, cytokines, immunotherapy, NK cells
Introduction
Colorectal cancer (CRC) is a major cause of cancer death in the US.1 While early-stage CRC is curable with surgery, long-term disease-free survival is limited in the 50% of the patients with advanced disease.2 The primary treatment for unresectable metastatic (m)CRC includes chemotherapy, targeted therapy, immunotherapy, or their combinations.2 Adjuvant therapy, guided by the tumor’s molecular profile, considers the evaluation of microsatellite instability (MSI), deficient/sufficient mismatch repair (MMR) as well as mutations in RAS family (KRAS, NRAS) and BRAF genes. Patients with MSI-H and dMMR CRC exhibit higher immune cell infiltration and are more sensitive to immune checkpoint blockade (ICB).3 However, only ~ 5–10% of mCRC patients show this profile, thus for >90% of mCRC patients there are no standard immunotherapy approaches. Patients with KRAS/NRAS/BRAF wild-type tumors can benefit from targeted therapy with anti-EGFR agents or VEGF inhibitors, however, many mCRC patients have mutations in KRAS or NRAS genes, which preclude them to receive targeted therapy,4 highlighting the urgency for developing new therapies.
Natural killer (NK) cells are cytotoxic innate lymphoid cells that potently kill tumor targets and orchestrate the immune response via cytokines and chemokines.5 NK cell recognition of targets is regulated by activating and inhibitory receptors stochastically expressed and modulated by cytokine receptors,6 allowing NK cells to circumvent immune evasion mechanisms (e.g., MHC-I downregulation) that occur in tumors resistant to ICB.5 NK cells are frequently deficient or dysfunctional in patients with cancer.7,8 A high frequency of circulating or tumor infiltrating NK cells have been associated with a favorable prognosis in CRC9,10 while a low NK cell function was predictive for an increased risk of subsequently developing cancer.11 NK cells from CRC patients exhibit reduced expression of activating receptors and increased expression of inhibitory receptors which correlated with reduced ability to produce cytokines and eliminate tumor cells.8,12 Thus, NK cells play a role in immune responses to CRC, and strategies that rescue or enhance their function may be effective treatments.
Memory-like (ML) differentiation of NK cells is induced after a brief stimulation with IL-12, IL-15, and IL-18, resulting in significantly improved NK cell persistence and anti-tumor functionality, including cytotoxicity and cytokine secretion, against several cancer types.13–15 Previous evidence from our group and others has shown that ML NK cell differentiation enhances their ability to infiltrate selected solid tumors.14–16 Strategies exploring NK cells as an approach for treating mCRC patients are under investigation,17 however, using a memory-like differentiation to broadly enhance NK cell anti-CRC responses remains unexplored. Here, we evaluate whether ML differentiation enhances human NK cell responses against CRC targets with and without tumor targeting monoclonal antibodies (mAb), using cell lines and primary cells, and in vitro and in vivo approaches.
Methods
PBMC and NK cell isolation
Healthy donor (HD) NK cells were isolated as previously described.14 CRC patients with metastatic disease (mCRC) were enrolled in an Institutional Review Board (IRB) – approved protocol for tissue collection. PBMC from mCRC patients were isolated using Ficoll (GE Health), frozen and then thawed for batch analysis. Cytokine-induced ML NK cells were generated from patient PBMC and HD NK cells as described.13–15
Cell lines
The colorectal cell lines HT29 (ATCC, HTB-38), HCT116 (ATCC, CCL-247), SW480 (ATCC, CCL-228), DLD-1 (ATCC, CCL-221), and K562 cells (ATCC, CCL-243) were obtained from ATCC. All lines were Mycoplasma free, and identity confirmed by STR profiling. In selected experiments, tumor cells were incubated with anti-EGFR antibody (cetuximab 1 µg/ml) or isotype control (IgG1k isotype control) for 30 min at 37°C and washed before the co-culture with NK cells.
Patient derived tumor spheroids generation
For generating patient-derived tumor spheroids, colon and rectum biopsy tissues from six CRC patients were collected through an IRB-approved protocol of Washington University School of Medicine. The samples were processed at The Precision Animal Models & Organoid Core from Washington University Department of Medicine. CRC patients’ spheroids were generated as previously described.18,19 For selected experiments, tumor spheroids were digested into single cell suspensions using TrypLE (Thermo Fisher Scientific) and used as targets to measure cytokine production, or partially digested for short-term killing assays.
Phenotypic characterization of CRC cells
Cell lines were collected by trypsinization (Trypsin-EDTA, Thermo Fischer Scientific) and patient spheroids were digested using TrypLE (Thermo Fischer Scientific) and rested before the staining. Tumor cells were stained with viability cell dye (Zombie NIR, Biolegend) before the staining with anti-CD155 (Clone SKII.4), anti-CD112 (Clone TX31), anti-B7-H6 (Clone # 875001), anti-CD58 (Clone TS2/9), anti-MICA/B (Clone GD4), anti-HLA-ABC (BD Biosciences), anti-PD-L1 (Clone MIH2), anti-HLA class I Bw4 (Clone REA274), anti-HLA-E (Clone 3D12), and anti-EGFR (Clone AY13). Staining of patient derived tumor spheroids was subjected to sample availability.
Cytotoxicity toward cell line derived tumor spheroids
To generate tumor line spheroids, 2 × 103 GFP+ HT29 and HCT116 cells were seeded in Ultra-Low Adherence 96-well plates in replicates and incubated for 48 h to allow spheroid formation. cNK or ML NK cells stained with PKH26 (Millipore Sigma, St Louis, MO), were added at indicated effector to target (E:T) ratios. Untreated tumor spheroids were used as control. Fluorescent spheroids were imaged using the BioTek LionHeart FX automated microscope and the BioTek Gen5 Microplate reader (Agilent Technologies, Santa Clara, CA) at baseline and 24 h after addition of NK cells. Images were preprocessed using the Imager Software (Agilent Technologies) and analyzed using Fiji software (NIH). For the analysis, GFP images were subjected to a Gaussian blur filter with a radius of 2.00 pixels and the threshold adjusted to be the same for all images and all time points. Area of spheroids (GFP positive area) was analyzed using an ImageJ Macro, limiting the analysis to the selected threshold and selecting 20 as minimum particle size. Data was plotted using GraphPad Prism 9.0.
NK cell functional assays and blocking experiments
cNK and ML NK cells were incubated with tumor targets at 5:1 ET ratio as previously described.13,14 When indicated, cNK and ML NK cells were pre-incubated with anti-NKG2D, anti-NKp46, anti-NKp30, and anti-DNAM1 (5 µg/mL; all Biolegend) or a combination of anti-NKG2D, anti-NKp46 and anti-DNAM for 30 minutes before co-incubation with tumor targets. Cells incubated with Isotype control were used as control. At the end, the cells were stained with anti-CD56 (clone N901), anti-CD3 (clone UCHT1), anti-NKG2A (clone Z199), and anti-CD45 (clone J33) followed by intracellular staining with anti-IFN-γ (clone B27). Data was acquired in a Gallios flow cytometer (Beckman Coulter) and analyzed using FlowJo software (Tree Star v10.8).
NK cell cytotoxic assays
NK cells cytotoxicity was assessed in short term killing assays using a standard 4-hour 51Cr release assays and in long-term cytotoxic assays using IncuCyte Live-cell Analysis system as previously described.14,20 For Incucyte, GFP+ HT29 and HCT116 cells were used at indicated E:T ratios. Real time images were captured analyzed using the Incucyte software (Sartorious). Flow cytometry-based 4-hour killing assays were performed on cell line spheroids generated as above, with an additional labeling step with CellTrace Violet (Thermo Fisher) per manufacturer instructions prior to seeding. NK cells were added at different E:T ratios, and the plate spun for 1 min at 350 × g prior to incubation at 37°C for 4 hours. Then, samples were processed by transferring into 96-well V-bottom plate, spinning for 4 min at 700 × g, flicking the plate to remove coculture medium; washing with PBS and repeating spin and flick; adding trypsin to each well, incubating at 37°C for 5 minutes, and pipetting up and down with a P200 pipette to generate a single-cell suspension. After washing with annexin-V staining buffer and repeating spin and flick, the samples were then stained with 7-AAD and APC-conjugate annexin-V for 10 min at room temperature prior to data acquisition on Gallios flow cytometer. For flow-based assay using patient-derived organoids, organoid cultures were partially digested with TrypLE. A small aliquot of partially digested organoids was taken to calculate cell numbers by full digestion by alternating 37°C incubation with repeated pipetting up and down to generate a single-cell suspension. The remainder of partially digested organoids was stained with CellTrace Violet, then added to Ultra-Low Adherence 96-well plates along with NK cells at different E:T ratios. Subsequent incubation, staining, and data acquisition were identical to cell line spheroids as above.
NSG tumor control models
NSG (NOD/SCID/IL2rγnull) mice were sub-lethally irradiated with 250 cGy and 3 days later inoculated with 1 × 105 luciferase-expressing HCT116 intraperitoneally (i.p.). One day later, recipient mice were left untreated, or treated with 5 × 106 control or IL-12/15/18 activated NK cells. NK cells were supported in vivo with 50,000 U IL-2 i.p every 2–3 days. To monitor tumor burden, mice were assessed bioluminescent imaging (BLI) (Spectral instruments imaging ami).14,21
Statistical analysis
Between-group differences (mean ± SEM) were compared as described in figure legends. Analyses were performed using GraphPad Prism v10. p values were 2 sided, and p < 0.05 was considered significant.
Results
Memory-like NK cells exhibit enhanced functional responses against CRC targets encompassing different molecular profiles
ML NK cells have been shown to exhibit superior recall responses against leukemia,13,22,23 lymphoma,21 melanoma,14 head and neck15 and ovarian cancer.24 Since each malignancy is distinct in sensitivity to NK cell immunotherapy, we first evaluated ML NK cell functional responses against CRC cell lines that represent the molecular heterogeneity of CRC (Supplementary Table S1) (Figure 1a). ML NK cells exhibited significantly higher IFN-γ production in response to all CRC lines tested, compared to cNK cells (Figure 1(b,c)). As previously reported, degranulation in response to CRC targets was similar between ML NK and cNK cells (Supplementary Fig. S5A). Differences in IFN-γ production in response to the CRC cell lines were not explained by differences in activating ligands expressed by target cells (Supplementary Fig. S1A, B). Both HLA-I and PD-L1 were upregulated in HT29 but not HCT116 after 24 or 48 hours of coculture with NK cells, without clear difference between cNK and ML NK cells (Supplementary Fig. S1C). For subsequent experiments, we chose HCT116 and HT29 cells due to their distinct genetic profiles and differing magnitude of IFN-γ induction by NK cells. In 4-hour short-term killing assays, ML NK cells had enhanced HCT116 and HT29 target killing compared to cNK cells (Figure 1(d,e)). Interestingly, ML NK cell killing activity was comparable against both cell lines, despite the differences in IFN-γ production observed by ML NK cells (Figure 1c). The superior cytotoxic activity of ML NK cells was maintained over 72-hour cytotoxicity assays (Figure 1(f,g), Video 1). Together, these data demonstrated that ML NK cells exhibit enhanced cytokine response and cytotoxicity against CRC cell lines regardless of their molecular profile, and differences in IFN-γ production by NK cells are unlikely to be related to activating or inhibitory receptor ligand expression.
Figure 1.
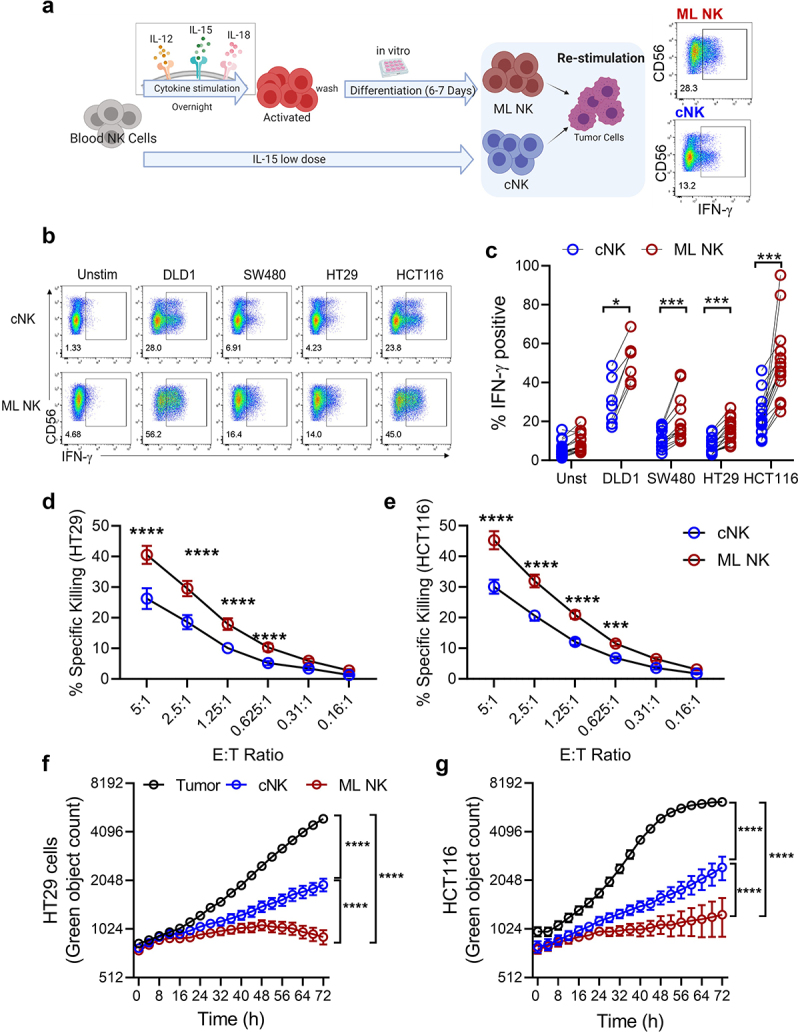
ML NK cells from healthy donors exhibit enhanced functionality against colorectal cell lines. a) schema shows the generation of ML NK cells and cNK cells. b, c) IFN-γ production by cNK (b, upper) and ML NK (b, lower) cells stimulated with all four CRC cell lines. Control and ML NK cells were incubated with HT29 and HCT116 at different E:T ratios and specific lysis was assessed in a 51Cr release assay (d, e) and incucyte (f, g). For incucyte, GFP expressing HT29 and HCT116 cells were incubated with NK cells at 2.5 E:T ratio for 72 h. F and G show one representative example from 3 different donors in 3 independent experiments. n = 7–12 in C, n = 3 in D and E. Two-way ANOVA with Sidak post test. *p < 0.05, ***p < 0.001, ****p < 0.0001.
ML NK cells efficiently eliminate tumor cells in 3D spheroid cultures
Tumor spheroids are used to simulate the complex solid tumor environment.25 To assess whether ML NK cells can eliminate tumor cells in 3D cultures, GFP+ HCT116 and HT29 spheroids were co-cultured with labeled cNK and ML NK cells. Over 24 hours of coculture, at high E:T ratios, ML NK cells reached almost complete elimination of HCT116 (Figure 2(a,b)) and HT29 (Figure 2(d,e)) spheroids. At lower E:T ratios, ML NK cells were significantly better at eliminating HCT116 (Figure 2(a,b)) but not HT29 (Figure 2(d,e)) tumor spheroids compared to cNK cells. In short-term killing assays, ML NK cells showed superior cytotoxicity than cNK cells at all E:T ratios in both HCT116 and HT29 (Figure 2(c,f), Supplementary Fig. S2A). These data demonstrated that ML NK cells can reduce tumor burden in 3D growing tumor spheroids.
Figure 2.
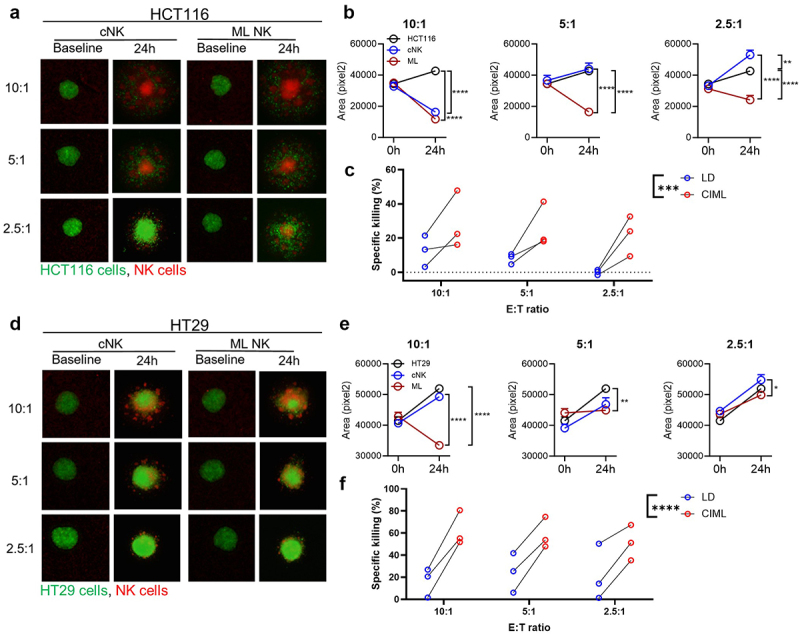
ML NK cells control CRC cells growing in spheroids more efficiently than cNK cells. spheroids generated from GFP+ HCT116 (a–c) and HT29 (d–f) cells were incubated with cNK or ML NK cells at different E:T ratios. (a, d) Representative images at baseline and at 24 h of spheroids (green) and labeled NK cells. (b, e) quantitation of GFP+ area of spheroid images. Untreated spheroids were used as control. (c, f) annexin V/7-AAD flow cytometry-based killing assay. Spheroids were co-cultured with cNK or ML NK cells at indicated E:T ratio for 4 hours and then dissociated for flow cytometric analysis. Percent of specific killing was calculated as the decrement in annexin V/7-AAD negative viable tumor cells compared to tumor cells alone. Two-way ANOVA with Tukey posttest for imaging, two-way repeated measures ANOVA for flow-based killing assay. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
NKG2D blockade inhibits ML NK cell responses against CRC cells
Loss of function with mAb blockade was used to provide mechanistic insight into CRC cells recognition by ML NK cells (Figure 3(a–d)). Anti-NKG2D single blockade significantly reduced IFN-γ production by ML NK cells upon stimulation with HCT116. DNAM-1 and NKp46 blockade decreased IFN-γ production by ML NK cells only when combined with anti-NKG2D (Figure 3(a,b)), suggesting that NKG2D is the main activating receptor driving ML NK cell responses against CRC cells. However, both NKG2D and NKp46 were required for optimal cytotoxicity as the ability to eliminate tumor cells was severely compromised after blocking either of these activating receptors (Figure 3(c,d)). Indeed, combined NKG2D, NKp46, and DNAM-1 blockade completely abrogated the ability of ML and cNK cells to eliminate HCT116 cells, suggesting an important synergistic contribution of these activating receptors to the overall killing ability of NK cells against CRC targets.
Figure 3.
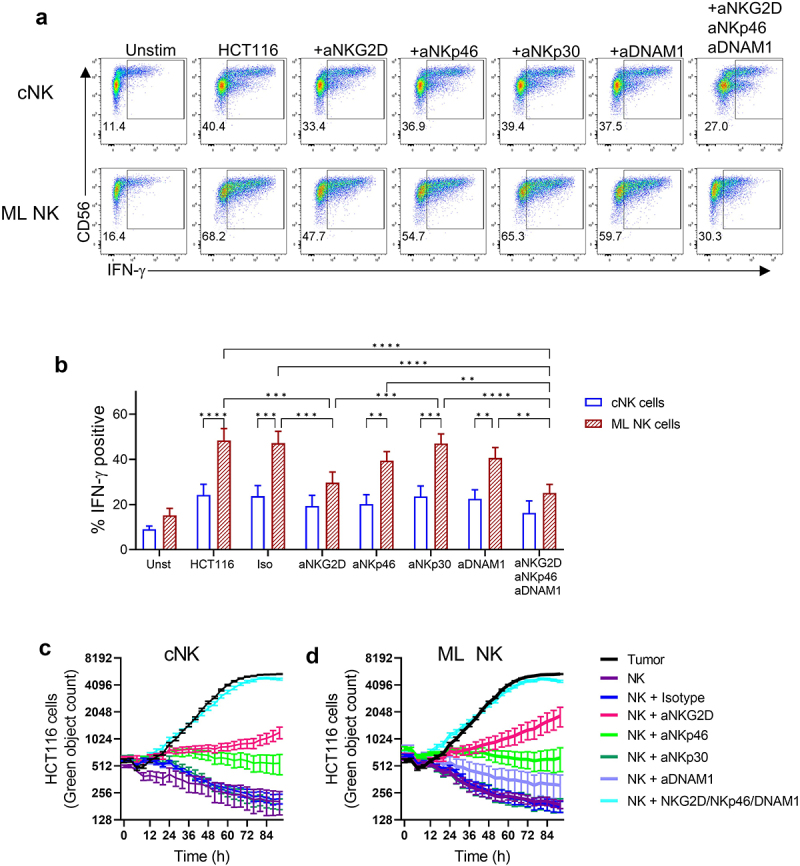
NKG2D is the main activating receptor driving ML NK cells against CRC cells. cNK and ML NK cells from healthy donors were incubated with blocking Abs for 30 min before the stimulation with HCT116 cells at 5:1 E:T ratio. a) Representative flow plots and b) summary data of IFN-γ production by cNK and ML NK cells stimulated with HCT116 cells without and with single and combined mAb blockade. Live imaging analysis using incucyte shows killing activity of c) cNK and d) ML NK cells against HCT116 cells in presence of antibody blockade. IgG1 isotype was used as control. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Memory-like NK cells exhibit enhanced response against CRC patient samples
We next sought to investigate ML NK cell responses against primary, patient-derived CRC cells. For this, tumor spheroids generated from four CRC patients were used as targets for functional assays (Figure 4a). Phenotypic characterization of the spheroids showed variable but positive expression of MHC-class I, CD155, MICA/B, and CD58, low expression of CD112, and were negative for B7-H6 (Supplementary Fig. S3). When co-cultured with primary CRC spheroids (as per Figure 1a), HD ML NK cells had significantly higher IFN-γ compared to the unstimulated condition (Figure 4(b,c)). IFN-γ production was similar between stimulated and unstimulated cNK cells. Furthermore, compared to cNK cells, ML NK cells exhibited greater cytotoxicity against two independent CRC patient-derived spheroid lines in a short-term killing assay (Figure 4d), Supplementary Fig. S2B). These results demonstrate that ML NK cells have superior cytokine production and cytotoxicity against primary CRC patient-derived cells in addition to CRC cell lines.
Figure 4.
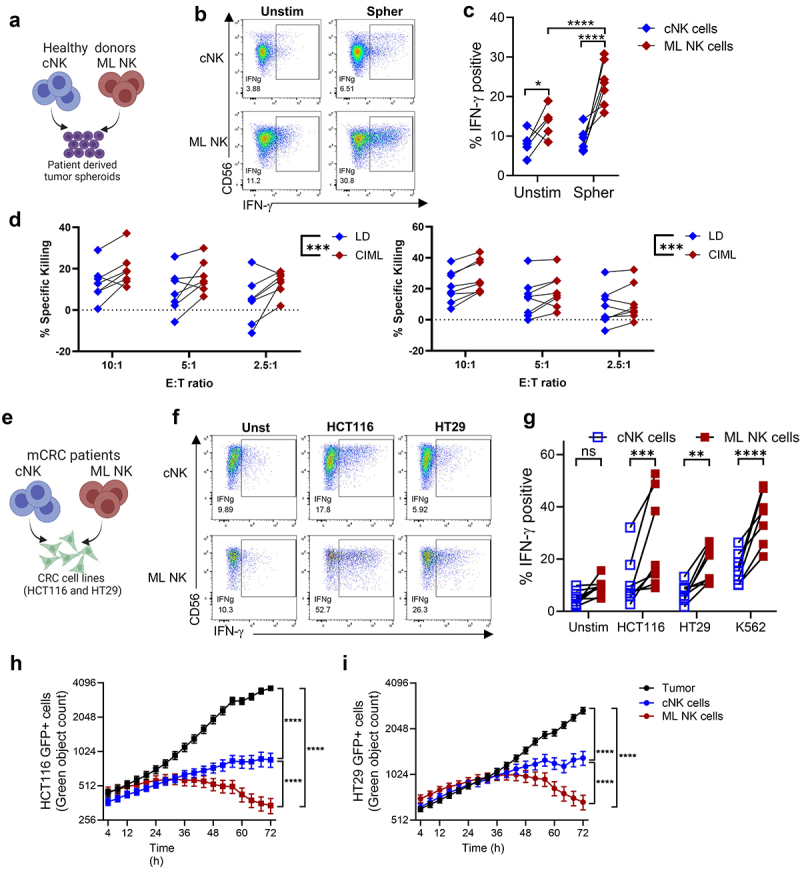
ML NK cell from HD and CRC patient samples have improved response against CRC cells, including patient derived spheroids. a) HD cNK and ML NK cells were stimulated with patient derived spheroids (5:1 E:T ratio). b) Representative dot plot and c) summary data show IFN-γ production by cNK and ML NK cells. n = 7. d) annexin V/7-AAD flow cytometry-based killing assay on two patient-derived spheroid lines. Spheroids were partially dissociated and co-cultured with healthy donor cNK or ML NK cells at indicated E:T ratio for 4 hours and then processed into a single cells for flow cytometric analysis. Percent of specific killing was calculated as the decrement in annexin V/7-AAD negative viable tumor cells compared to tumor cells alone, n = 7–8. E), patient derived ML NK cells and cNK cells were incubated with HCT116 and HT29 cells (5:1 E:T ratio). f, Representative flow plot and g) summary data show the frequency of IFN-γ producing cells by patient cNK and ML NK cells in response to CRC cell lines. n = 8. Cytotoxicity of cNK and ML NK cells against GFP expressing h) HCT116 and I) HT29 was assessed using incucyte. Two-way repeated measures ANOVA (d). Two-way ANOVA with Sidak (c, g) and holm-Sidak posttest (h, i). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
ML differentiation restores dysfunctional NK cells from CRC patients
Since NK cells from CRC patients have been reported to be functionally defective,8,12 we evaluated whether ML differentiation rescues dysfunctional patient NK cells. ML NK cells from eight mCRC patients (Supplementary Table S2) were generated as previously described14 and assessed against CRC cell lines in functional and killing assays (Figure 4d). Patient-derived ML NK cells produced significantly more IFN-γ compared to cNK cells (Figure 4(e,f)). IFN-γ production by mCRC ML NK cells was comparable to that in HD ML NK cells when stimulated with HT29 but significantly lower in response to HCT116 stimulation (Supplementary Fig. S4). ML NK cells from mCRC patients also exhibited a superior cytotoxic activity against HCT116, and HT29 cells (Figure 4(g,h)). Collectively, these data indicate that primary CRC patient NK cells have the potential for enhancement via ML NK cell differentiation, representing a promising approach to restore dysfunctional mCRC patient NK cells.
ML NK cells better control CRC cells in vivo
To investigate whether ML NK cells can efficiently control CRC cells in vivo, we used a xenograft model of CRC using immunodeficient NSG mice. Briefly, 1 × 105 luciferase expressing HCT116 cells were injected i.p. into NSG mice and one day later, mice were treated with a single injection of 5 × 106 cNK or ML NK cells (Figure 5). Non-treated mice were used as control. Tumor burden was not different between untreated and cNK cell-treated mice; however, ML NK cell-treated mice had significantly lower tumor burden compared to both untreated mice and cNK cell-treated mice (Figure 5(b,c)). Thus, ML NK cells exhibited superior control of CRC cells compared to cNK cells in vivo in NSG mice.
Figure 5.
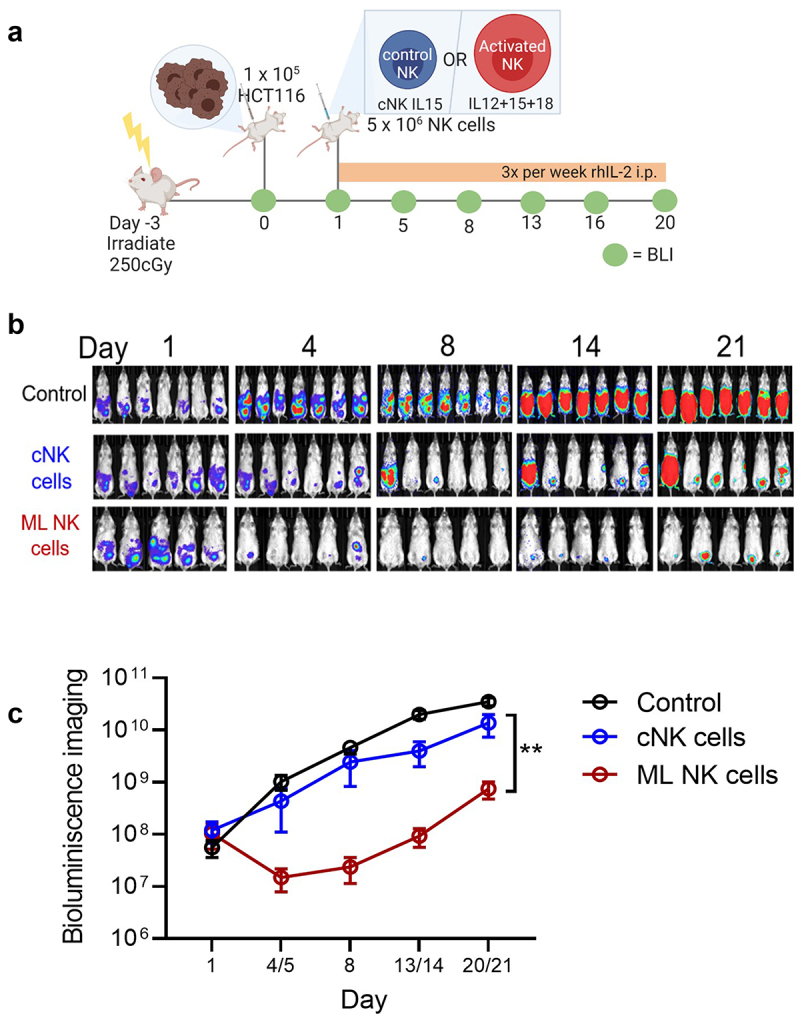
ML NK cells control HCT116 cells better than cNK cells in a xenograft model in NSG mice. a) irradiated NSG mice (day −3) were injected with 1 × 105 (ip.) Luc+ HCT116 cells at day 0. At day 1, 5 × 106 cNK or ML NK cells were injected (ip) and the tumor burden assessed biweekly by BLI. b) BLI images in representative from one of three independent experiments. c) summary data indicate that allogenic ML NK cells control CRC cells in vivo better than cNK cells. Data from all mice from the three experiments (total untreated = 17 mice, cNK cells = 11 mice, and ML NK cells = 11 mice). Two-way ANOVA – mixed-effect model with Tukey posttest. **p < 0.001.
Cetuximab enhances ML NK cell responses against CRC cell lines
We next investigated whether cetuximab, an EGFR inhibitor, could further improve ML NK cell responses against CRC cells by providing an additional activating signal via CD16/FcγRIIIa26 (Supplementary Fig. S1). Cetuximab increased IFN-γ in all NK cells but mainly ML NK cells when stimulated with HT29 cells (Figure 6a). No changes were found on CD107a expression (Supplementary Fig. S5A). Cetuximab did not increase IFN-γ production in response to HCT116, but it did increase CD107a in both cNK cells and ML NK cells to a similar extent (Supplementary Fig. S5B, S5C). We assessed the contribution of cetuximab to the ADCC mediated cytotoxicity of CRC targets, we found that ML NK cells killed significantly more isotype-treated HT29 and HCT116 cells compared to cNK cells. Moreover, in the presence of cetuximab, ML NK cells killing was significantly increased (Figure 6(b,c)). No biological effect on tumor cell growth was observed when tumor cells were treated with cetuximab alone or isotype control (Supplementary Fig. S6).
Figure 6.
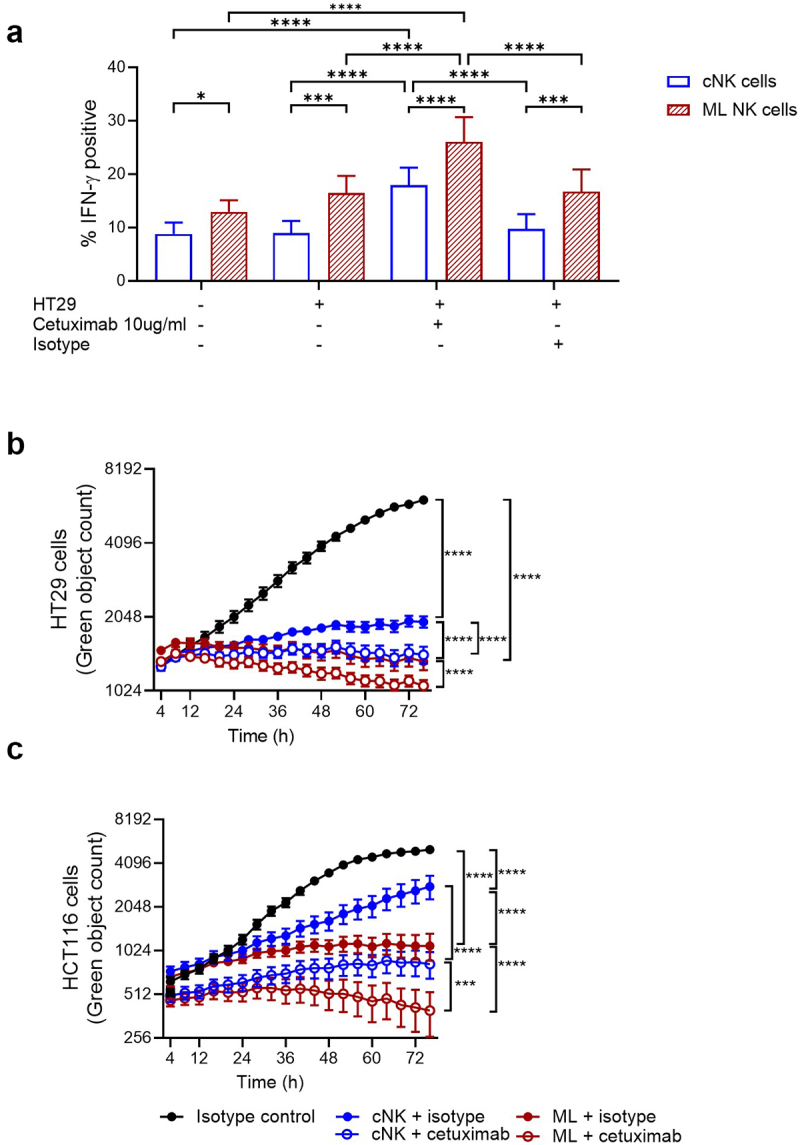
Cetuximab enhances functional response of cNK and ML NK cells against CRC cell lines regardless their molecular profile. a) IFN-γ production by HD cNK and ML NK cells incubated with untreated, isotype treated (human IgG1) and cetuximab-treated HT29 and HCT116 cells was assessed by flow cytometry. n = 5. Cytotoxicity of b) HT29 and c) HCT116 cells by isotype or cetuximab-treated cNK and ML NK cells was evaluated using incucyte. One representative example from three replicates is shown in b and c. Two-way ANOVA with a) Sidak and b, c) Tukey posttest. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
NK cell-based cellular therapies have emerged as potential immunotherapy for treating mCRC patients.17 Here, we demonstrated that ML NK cell differentiation results in a potent NK cell response against CRC cells regardless of their molecular profile. ML NK cells generated from HD or CRC patients exhibited potent anti-CRC tumor properties including cytokine secretion and cytotoxicity when tested in vitro against CRC cell lines or CRC patient tumor samples. This was also observed when testing ML NK cells against CRC targets in vivo in NSG xenograft models. Mechanistically, ML NK cell anti-tumor responses against CRC targets were primarily dependent on NKG2D as its mAb blockade compromised multiple ML NK cell functions. ML NK cell function was further potentiated when directed by cetuximab, regardless of the KRAS/BRAF status of the tumor cell lines. Collectively, these pre-clinical data provide the first proof-of-principle that ML NK cells alone or in combination with mAb may be harnessed as a cellular therapy for patients with advanced CRC, who have failed previous therapies or who are not candidates for current targeted immunotherapy.
Multiple studies have demonstrated quantitative, phenotypic, and functional defects on cancer patients’ NK cells. Reduced expression of activating receptors on patient NK cells contributes to their inability to recognize and eliminate CRC cells.8,12 We had previously demonstrated that ML differentiation upregulates the expression of many activating receptors on NK cells13 and rescues the dysfunctional phenotype of patient NK cells.13,14 Here, we found that the enhanced anti-CRC activity of ML NK cells was mediated mainly by the activating receptor NKG2D, highly expressed in ML NK cells.13 Interestingly, NKG2D and NKp46 were similarly required to mediate killing activity of both ML NK cells and cNK cells against CRC targets, suggesting additional dependency on these activating receptors to eliminate CRC regardless of the NK cell activation state.
CRC spheroid models have been used to evaluate potential of NK cells-based immunotherapy for solid tumors. Cytokine primed NK cells have been shown to infiltrate and eliminate CRC spheroids more efficiently than resting NK cells.27,28 In those studies, the efficacy of the anti-CRC NK cell activity was dose dependent and not related to their molecular profile. In contrast, we found that ML NK cells destroy CRC spheroids in a cell line dependent manner with HCT116 being more sensitive than HT29. This was intriguing since we did not find differences in NK cell activity against those CRC cells in 2D cultures. These differences are unlikely explained by changes in the repertoire of ligands for NK cell activating receptors in the 3D culture versus 2D cultures as previously demonstrated.27,29 Furthermore, different primary patient derived spheroids showed different susceptibility to NK cell-mediated killing. It is possible that other receptors or secretion of inhibitory molecules (e.g., TGF-β) may play a role in regulating tumor cell sensitivity to ML NK cell mediated killing.30 These findings are of high interest as NK cell infiltration within tumor sites have been associated with better prognosis in CRC.31
Cetuximab was the first FDA-approved agent for treating KRAS wild type CRC that operates partially via ADCC.26 In prior studies, cetuximab as monotherapy was shown to be ineffective against RAS/BRAF mutated tumors but its combination with IL-2 and IL-15 enhanced NK cells killing activity against CRC cell lines irrespective of their RAS/BRAF status.8,32 In line with those reports, we showed that cetuximab alone did not impact tumor growth when added as monotherapy in absence of NK cells. While we did see an increase in IFN-γ secretion and cytotoxicity on cNK cells after cetuximab treatment, these anti-tumor functions were significantly higher in ML NK cells upon stimulation with CRC cell lines, regardless of their RAS/BRAF status. Altogether, this data suggests that cetuximab potentiates ML NK cells functions and tumor elimination through ADCC and their combination represents an attractive therapy for patients who are not candidates to receive cetuximab therapy alone.
Cytokine activated and expanded NK cells from autologous or allogeneic sources used as single therapy or in combination with targeted therapy have been used in clinical trials for mCRC patients.33,34 The therapy has been shown to be safe, do not induce GvHD, CRS, or ICANS but the induction of clinical response was variable suggesting that additional approaches are needed to enhance the overall anti-tumor response. ML NK cells have been shown to overcome several challenges of the NK cell based therapy, including enhanced functionality, sustained persistence in vivo,13–15,23 and increased trafficking to tumor sites.16 First evaluated for the treatment of hematological malignancies, allogeneic ML NK cells were safe and induced composite complete remissions in patients with relapsed/refractory AML.13,35 In a separate trial, immune compatible ML NK cells persisted in the patient for over 3 months and exhibited enhanced ex vivo functionality when compared to cNK cells.23 The late effects of ML NK cell-based therapies are an active area of investigation and are expected to be impacted by the NK cellular source. In the allogeneic setting, ML NK cells are rejected by the recipient immune system after 3–4 week and stop exerting active effects, contrasted to an immune-compatible setting where ML NK cells may persist for several months. In both situations, NK cell responses may impact the tumor microenvironment, and potentially tumor-specific T cell response. These later effects of ML NK cells are not assessed in the current study and require evaluation in ML NK cell clinical trials or immunocompetent in vivo models. The immunobiology of ML NK cells and the pre-clinical data presented here supports the translation of point-of-care ML NK cells that may be used in autologous or allogeneic setting. Indeed, allogeneic donor ML NK cells for patients with CRC are under evaluation in a phase 1 clinical trial (NCT05674526).
Supplementary Material
Acknowledgment
We would like to acknowledge the Siteman Flow Cytometry Core at Washington University School of Medicine. Schemas were created using Biorender.com.
Funding Statement
NIH/NIGMS: T32GM139799 (JAF).NIH/NIGMS: F31GM146361-01 (JT).NIH/NHLBI: T32 HL007088 (PW, DAR-G, WMS).NIH/NIAID: F30AI161318 (CCC).NIH/NIDDK: P30 DK052574 (MAC)NIH/NIDDK: R01 DK109384 (MAC)NIH/NIDDK: T32 DK007130 (QAA, MAC)NIH: P50 CA171963 (TAF, MMB-E)NIH/NCI: Leukemia SPORE P50CA171063 (TAF/AFC/MMB-E).NIH/NCI: R01CA205239 (TAF).NIH/NCI: Cancer Center Support Grant P30CA91842 (TAF).P30CA91842 (TAF).K12CA167540 (MTJ)Washington University: MGI Pilot Grant (TAF)Siteman Cancer Center Investment Program (TAF, MAC)Leukemia and Lymphoma Society (TAF)
Disclosure statement
M.M.B.-E. and T.A.F. are inventors on patent/patent applications (15/983,275, 62/963,971, and PCT/US2019/060005) licensed to Wugen Inc. and held/submitted by Washington University that involve ML NK cells. This results in potential royalties to M.M.B.-E., T.A.F., and Washington University from Wugen Inc. M.M.B.-E. has equity, consulting, and royalty interest in Wugen Inc. T.A.F. reports research funding from HCW Biologics Inc., Wugen, Affimed and the NIH during the conduct of the study. T.A.F. has equity, research funding, consulting, and royalty interest in Wugen Inc. Unrelated to this work, T.A.F. also reports consulting for Affimed, AI Proteins, Smart Immune, and advises (equity interest) Indapta and OrcaBio. Unrelated to this work, J.A.F. is an inventor on patent/patent application (WO 2019/152387, US 63/018,108) licensed to Kiadis Inc. and held/submitted by Nationwide Children’s Hospital on TGF-β resistant, expanded NK cells. Unrelated to this work, J.A.F. has a monoclonal antibody unrelated to the present work licensed to EMD Millipore. Unrelated to this work, C.C.C. reports equity in Pionyr Immunotherapeutics. Unrelated to this work, D.A.R.-G. receives consulting fees from Cartography Inc. All other authors declare that they have no competing interests.
Author contributions
Conceptualization & Methodology: NDM, TAF.
Formal Analysis: NDM, TAF.
Investigation: NDM, MB-H, QAA, LM, NS, MMB-E, MF, JAF, JT, PW, CCC, PP, KW, AYZ, MTJ, TS, WMS, DAR-G.
Resources: RCF, MAC, TAF
Writing – original drafts: NDM, TAF.
Writing – review and editing: All authors.
Supervision: TAF.
Funding acquisition: TAF
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2348254
References
- 1.Rawla P, Sunkara T, Barsouk A.. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Przegla̜d Gastroenterol. 2019;14(2):89. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society Inc . Cancer-Facts-and-Figures-2023. 2023.
- 3.Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol. 2020. Aug 12;11. doi: 10.3389/fimmu.2020.02039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021. Feb 16;325(7):669–11. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008. May 18;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16(1):359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 7.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases – elimination, equilibrium and escape. Curr Opin Immunol. 2014. Apr;27(1):16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocca YS, Roberti MP, Juliá EP, Pampena MB, Bruno L, Rivero S, Huertas E, Sánchez Loria F, Pairola A, Caignard A. et al. Phenotypic and functional dysregulated blood NK cells in colorectal cancer patients can be activated by cetuximab plus IL-2 or IL-15. Front Immunol. 2016. Oct 10;7(OCT):413. doi: 10.3389/fimmu.2016.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca S, Perez-Piqueras J, Martinez D, Colmenajero A, Saez MA, Vallejo C, Martos JA. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma BACKGROUND. Natural killer (NK) cells have a spontaneous cytotoxic capacity. Cancer. 1997;79(12):2320–2328. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Tang YP, Xie MZ, Li KZ, Li JL, Cai ZM, Hu BL. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020. Feb 7;20(1). doi: 10.1186/s12876-020-1177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai K, Matsuyama S, Miyake S, Suga K, Yu H, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000. Nov 25;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 12.Rocca YS, Roberti MP, Arriaga JM, Amat M, Bruno L, Pampena MB, Huertas E, Loria FS, Pairola A, Bianchini M. et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013. Feb;19(1):76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 13.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016. Sep 21;8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin ND, Krasnick BA, Becker-Hapak M, Conant L, Goedegebuure SP, Berrien-Elliott MM, Robbins KJ, Foltz JA, Foster M, Wong P. et al. Memory-like differentiation enhances NK cell responses to melanoma. Clin Cancer Res. 2021. Sep 1;27(17):4859–4869. doi: 10.1158/1078-0432.CCR-21-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs MT, Wong P, Zhou AY, Becker-Hapak M, Marin ND, Marsala L, Foster M, Foltz JA, Cubitt CC, Tran J, et a. Memory-like differentiation, tumor-targeting mAbs, and chimeric antigen receptors enhance natural killer cell responses to head and neck cancer. Clin Cancer Res. Aug 9;2023;29(20): 4196–4208. doi: 10.1158/1078-0432.CCR-23-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18–preactivated NK cells against established tumors. J Exp Med. 2012/12/05 ed. 2012. Dec 17;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Chiesa M, Setti C, Giordano C, Obino V, Greppi M, Pesce S, Marcenaro E, Rutigliani M, Provinciali N, Paleari L. et al. NK cell-based immunotherapy in colorectal cancer. Nato Adv Sci Inst Se. 2022;10(7):1033. doi: 10.3390/vaccines10071033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015. Jun 1;64(6):911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandussen KL, Sonnek NM, Stappenbeck TS. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res. 2019;37(101430):101430. doi: 10.1016/j.scr.2019.101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker-Hapak MK, Shrestha N, McClain E, Dee MJ, Chaturvedi P, Leclerc GM, Marsala LI, Foster M, Schappe T, Tran J. et al. A fusion protein complex that combines IL-12, IL-15, and IL-18 signaling to induce memory-like NK cells for cancer immunotherapy. Cancer Immunol Res. 2021. Sep 1;9(9):1071–1087. doi: 10.1158/2326-6066.CIR-20-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gang M, Marin NDD, Wong P, Neal CCC, Marsala L, Foster M, Schappe T, Meng W, Tran J, Schaettler M. et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020. Nov 12;136(20):2308–2318. doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bednarski JJ, Zimmerman C, Berrien-Elliott MM, Foltz JA, Becker-Hapak M, Neal CC, Foster M, Schappe T, McClain E, Pence PP. et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood. 2021/12/07 ed. 2022. Mar 17;139(11):1670–1683. doi: 10.1182/blood.2021013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrien-Elliott MM, Foltz JA, Russler-Germain DA, Neal CC, Tran J, Gang M, Wong P, Fisk B, Cubitt CC, Marin ND. et al. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci Transl Med. 2022. Feb 23;14(633):eabm1375. doi: 10.1126/scitranslmed.abm1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uppendahl LD, Felices M, Bendzick L, Ryan C, Kodal B, Hinderlie P, Boylan KLM, Skubitz APN, Miller JS, Geller MA. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol Oncol. 2019/01/20 ed. 2019. Apr 1;153(1):149–157. doi: 10.1016/j.ygyno.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poggi A, Villa F, Fernadez JLC, Costa D, Zocchi MR, Benelli R. Three-dimensional culture models to study innate anti-tumor immune response: advantages and disadvantages. Cancers. 2021. Jul 2;13(14):3417. doi: 10.3390/cancers13143417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean L, Kane M. 2020. Cetuximab therapy and RAS and BRAF genotype. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US) 2012. Nov 24. [Google Scholar]
- 27.Sargenti A, Musmeci F, Bacchi F, Delprete C, Cristaldi DA, Cannas F, Bonetti S, Pasqua S, Gazzola D, Costa D. et al. Physical characterization of colorectal cancer spheroids and evaluation of NK cell infiltration through a flow-based analysis. Front Immunol. 2020;11(December):1–13. doi: 10.3389/fimmu.2020.564887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanuza PM, Vigueras A, Olivan S, Prats AC, Costas S, Llamazares G, Sanchez-Martinez D, Ayuso JM, Fernandez L, Ochoa I. et al. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. OncoImmunology. 2018. Apr 3;7(4). doi: 10.1080/2162402X.2017.1395123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanuza PM, Alonso MH, Hidalgo S, Uranga-Murillo I, García-Mulero S, Arnau R, Santos C, Sanjuan X, Santiago L, Comas L. et al. Adoptive NK cell transfer as a treatment in colorectal cancer patients: analyses of tumour cell determinants correlating with efficacy in vitro and in vivo. Front Immunol. 2022. Jun 7;13. DOI: 10.3389/fimmu.2022.890836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, De Lima M, Wald DN. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PloS One. 2018. Jan 1;13(1):e0191358. doi: 10.1371/journal.pone.0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, Ferrelli F, Coppola A, Arriga R, Lauro D. et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. OncoImmunology. 2014;3(8):1–6. doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veluchamy JP, Spanholtz J, Tordoir M, Thijssen VL, Heideman DAM, Verheul HMW, de Gruijl TD, van der Vliet HJ. Combination of NK cells and cetuximab to enhance anti-tumor responses in RAS mutant metastatic colorectal cancer. PloS One. 2016. Jun 1;11(6):e0157830. doi: 10.1371/journal.pone.0157830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, Mineno J, Yoshida N, Katada K, Kamada K. et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142(12):2599–2609. doi: 10.1002/ijc.31285. [DOI] [PubMed] [Google Scholar]
- 34.Otegbeye F, Cooper B, Caimi P, Zamborsky K, Reese-Koc J, Hillian A, Hernandez-Collazo Y, Lee G, Boughan K, Tomlinson B. et al. A phase I study to determine the maximum tolerated dose of ex vivo expanded natural killer cells derived from unrelated, HLA-Disparate adult donors. Transplant Cell Ther. 2022. May 1;28(5):.e250.1–.e250.8. doi: 10.1016/j.jtct.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA, Foster M, Schappe T, Desai S, McClain E. et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020. Dec 21;10(12):1854–1871. doi: 10.1158/2159-8290.CD-20-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


