Abstract
Objectives
The aim of the study was to evaluate mortality and morbidity outcomes following open-heart isolated tricuspid valve surgery (TVSx) with medium to long-term follow-up.
Design
Retrospective cohort study.
Setting
New South Wales public and private hospital admissions between 1 January 2002 and 30 June 2018.
Participants
A total of 537 patients underwent open isolated TVSx during the study period.
Primary and secondary outcome measures
Primary outcome was all-cause mortality tracked from the death registry to 31 December 2018. Secondary morbidity outcomes, including admission for congestive cardiac failure (CCF), new atrial fibrillation (AF), infective endocarditis (IE), pulmonary embolism (PE) and insertion of a permanent pacemaker (PPM) or implantable cardioverter-defibrillator (ICD), were tracked from the Admitted Patient Data Collection database. Independent mortality associations were determined using the Cox regression method.
Results
A total of 537 patients underwent open isolated TVSx (46% male): median age (IQR) was 63.5 years (43.9–73.8 years) with median length of stay of 16 days (10–31 days). Main cardiovascular comorbidities were AF (54%) and CCF (42%); 67% had rheumatic tricuspid valve. In-hospital and total mortality were 7.4% and 39.3%, respectively (mean follow-up: 4.8 years). Cause-specific deaths were evenly split between cardiovascular and non-cardiovascular causes. Predictors of mortality included a history of CCF (HR=1.78, 95% CI 1.33 to 2.38, p<0.001) and chronic pulmonary disease (HR=2.66, 95% CI 1.63 to 4.33, p<0.001). In-hospital PPM rate was 10.0%. At 180 days, 53 (9.9%) patients were admitted for CCF, 25 (10.1%) had new AF, 7 (1.5%) had new IE and <1% had PE, post-discharge PPM or ICD insertion.
Conclusion
Open isolated TVSx carries significant mortality risk, with decompensated CCF and new AF the most common morbidities encountered after surgery. This report forms a benchmark to compare outcomes with newer percutaneous tricuspid interventions.
Keywords: Cardiothoracic surgery, CARDIOLOGY, Valvular heart disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A relatively large cohort of patients for an infrequently performed set of procedures.
Study cohort was derived from a state-wide unselected population from all public and private healthcare facilities that performed cardiothoracic surgery.
The use of a death registry with cause-specific data analysis adds detail to all-cause mortality figures.
Comprises a heterogeneous group of procedures: tricuspid valve surgery annuloplasty, repair and replacement.
Dataset lacks granular details such as echocardiographic data (eg, right ventricular size and function) or aetiology of tricuspid valve dysfunction.
Introduction
The burden of tricuspid valve (TV) disease is expected to increase with the increasing age of the Australian population. The prevalence of moderate or severe TV regurgitation of any cause in developed countries is 4.0% in those over the age of 75 years and 1.1% in those aged 65–74 years.1 The prevalence of TV stenosis, rare in developed countries, is not known.
The presence of tricuspid regurgitation (TR) is an independent predictor of increased mortality, both by itself (isolated functional TR) and for secondary aetiologies including left-sided valvular disease, heart failure, arrhythmogenic right ventricular (RV) cardiomyopathy and pulmonary arterial hypertension.2–10
TV surgery (TVSx) is largely performed in combination with other cardiac procedures, most frequently left-sided valve surgery.11 Society guidelines have consistently recommended isolated TVSx for patients with severe primary TR as their sole valvular lesion.12 13 More recently, the 2020 American Heart Association and 2021 European Society of Cardiology Valvular Heart Disease Guidelines have recommended TVSx for select patients with severe secondary TR regardless of the presence of an indication for concurrent left-sided valve surgery or a history of left-sided valve surgery.14 15
Open isolated TVSx has traditionally been associated with high mortality rates. Reported in-hospital mortality rates have varied over time from 8.8–19.0% in small (n<500) older studies16 17 to 8.8–9.7% in larger studies (n=1364 in Alqahtani et al and n=5005 in Zack et al) over the last 20 years,11 18 to as low as 3.2% in a recent single-centre study (n=95) involving carefully selected patients.19 However, longer term morbidity outcomes, including readmission for heart failure, permanent pacemaker (PPM) requirement, pulmonary embolism (PE) or new-onset atrial fibrillation (AF), are not well described. Moreover, while an association between TVSx and PE has not been described, worsening TR has been numerically (although not statistically) associated with PE, TR may result from chronic thromboembolic disease and PE is a plausible complication of TVSx given the association between left-sided valvular intervention and stroke.20–22
The primary aim of this study was to determine the incidence and temporal trends of open isolated TVSx in an Australian state-wide cohort and examine their mortality outcomes. The secondary aim was to characterise morbidity events after isolated TVSx.
Methods
Study population
The Centre for Health Record Linkage (CHeReL), established in 2006, holds one of the largest data linkage systems in Australia containing high-quality linked health data of residents in the state of New South Wales (NSW).23 From its Admitted Patient Data Collection (APDC) database, which includes ≥97% of all healthcare facilities in the state, we identified consecutive admissions that involved open-heart surgery (excluding percutaneous approach) for TV pathology (see online supplemental table 1 for relevant Australian Classification of Health Intervention (ACHI) procedure codes) either as primary or secondary procedures coded under the ACHI coding system between 1 July 2001 and 31 December 2018. Our research group has published detailed outcome studies using data obtained from the APDC database.24–29
bmjopen-2023-080804supp002.pdf (1.8MB, pdf)
Data sources
Variables obtained from the APDC database for each hospital admission that involved TVSx include admission date, age, gender, country of birth, admission referral source, length of admission and in-hospital mortality.
The primary and all secondary diagnoses (potentially up to 50 secondary diagnoses) associated with each admission were retrieved from the APDC database. Each diagnosis was coded in the APDC database according to the International Classification of Diseases, Tenth Revision, Australian Modification (ICD-10-AM). For this study, we prespecified the indication for cardiac valve surgery during admission as either for endocarditis (as primary or secondary diagnosis) or as non-endocarditis valve surgery, and if concomitant coronary artery bypass graft (CABG) surgery was performed in the same admission (see online supplemental table 1 for relevant ICD-10-AM and ACHI codes). In addition, whether rheumatic TV was documented during admission was recorded. Additional comorbidities extracted for this study include ischaemic heart disease, prior percutaneous coronary interventions and/or CABG surgery, congestive cardiac failure (CCF), stroke, peripheral vascular disease, prosthetic heart valve, AF, primary or secondary pulmonary hypertension, cardiac risk factors (including hypertension, hyperlipidaemia, diabetes and current/ex-smoker), malignancy, chronic pulmonary disease, neurodegenerative disease, chronic kidney disease and history of intravenous drug use (IVDU). In addition, the overall comorbid status of each patient was quantified using the Charlson Comorbidity Index (CCI).30 A value of 0 indicates no comorbidity, while higher values represent an increasing burden of comorbid illnesses. Conditions included in the CCI include age (1 point for every decade after 40), myocardial infarction, CCF, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease (mild vs moderate to severe), diabetes (with or without organ damage), hemiplegia, moderate to severe renal disease, any tumour (within the last 5 years), lymphoma, leukaemia, metastatic solid tumour and AIDS.
Study outcomes
The primary outcome of the study was all-cause and cause-specific death rates, tracked from the state-wide death registry also held by CHeReL. For mortality analysis, cases were limited to only NSW state residents to minimise incomplete tracking. The end-of-study date was set at 31 December 2018. All death certificates were reviewed to ascertain cause-specific death rates. Each death was coded independently by two reviewers (ACCN and VC) according to general principles set by the WHO.31 Reviewers were blinded to the patient’s background comorbid illnesses during coding. Disparities were resolved by consensus. Cause-specific mortality was based on prior published classifications.26 In brief, cardiovascular cause was defined as death due to acute myocardial infarction, CCF, stroke, cardiac-related causes (when more than one cardiac cause of death was recorded) or PE. Non-cardiovascular causes included death due to sepsis, malignancy, other non-cardiovascular causes or undefined. Patients with multiple potential causes of death on their death certificates were classified as ‘undefined’ and labelled as non-cardiovascular death for the purposes of the present study.
Secondary outcomes of the study were tracked from the APDC database using linkage method to determine morbidity events during follow-up after surgery. These include first readmission for CCF, development of new AF or infective endocarditis, PE and the need for a PPM or implantable cardioverter-defibrillator (ICD) implantation.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Statistical analysis
To determine the incidence and temporal trend on case volumes of open isolated TVSx state-wide during the study period, all admissions between 1 January 2002 and 31 December 2018 were included. For the rest of the analyses, the study cohort was limited to NSW state residents and confined to the index admission between 1 January 2002 and 30 June 2018, enabling a minimum of 6 months of follow-up. Thus, for those who had repeat TVSx during the study period (recurring patients), only their initial admission was included. End-of-study follow-up was prespecified at 31 December 2018.
All continuous variables are expressed as mean±SD, unless otherwise stated, and categorical data given in absolute numbers and percentages. Linear regression was used to determine trends in TVSx case load per annum over the study period, excluding 2018 to minimise ascertainment bias as the APDC database receives six monthly updates. To identify predictors of mortality after TVSx, Cox proportional hazards regression method was used. Univariables considered include age (dichotomised by mean age), gender, admission referral source, year groups of surgery (stratified into 2002–2005, 2006–2009, 2010–2013, 2014–2018), indication for surgery (infective endocarditis), rheumatic TV status, types of TVSx, concomitant CABG, other cardiovascular and non-cardiovascular comorbidities. Univariables with p<0.05 were included in the multivariable Cox regression analysis, except for age and gender which were included irrespective of significance. The proportional hazards assumption was checked with log-minus-log plots.
All analyses were performed using SPSS V.25.0 (IBM) and Stata V.16.1. A two-tailed probability value <0.05 was considered statistically significant. No sponsors had a role in study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication.
Patient and public involvement
Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research.
Results
Temporal trend of TV cases
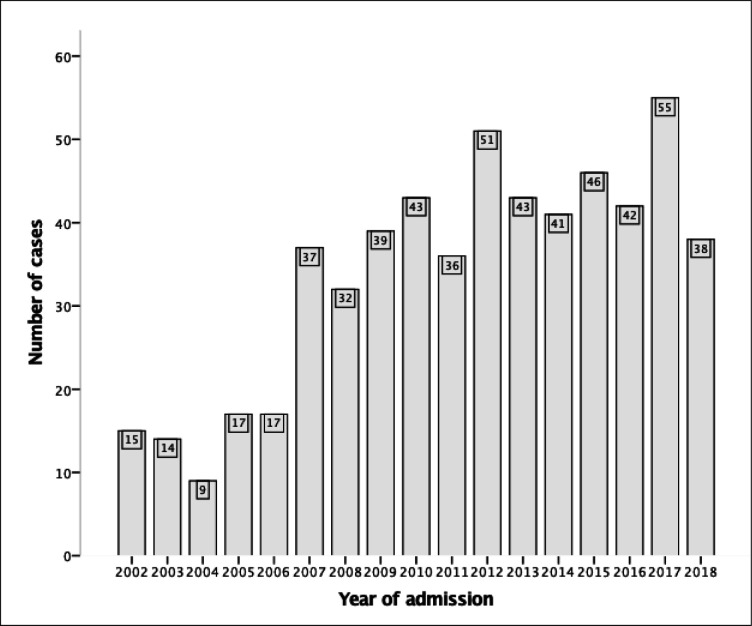
There were 575 cases of open isolated TVSx in the calendar years of 2002–2018, averaging 34±14 cases per annum (online supplemental figure 1). There was a significant increase in case numbers by an average of 2.73 cases per annum over the study period (95% CI 1.95 to 3.50, p<0.001) (figure 1). The bulk of TVSx cases was TV annuloplasty (n=272) and replacement (n=245), with case volume for both surgeries increasing during the study period (online supplemental figures 2 and 3). A smaller number of non-annuloplasty TV repairs (n=85) and valvotomies (n=5) were performed. While there were significant increases in TV repair case loads during the study period, TV valvotomy case loads were so small as to preclude trend analysis (online supplemental figures 4 and 5).
Figure 1.
Temporal trend of annual volume of isolated tricuspid valve surgery during study period (n=575), with a mean (±SD) of 34±14 cases per annum. Annual case volumes significantly increased over the study period with an average rise of 2.73 cases per year (95% CI 1.95 to 3.50, p<0.001).
Baseline demographic and surgical characteristics of study cohort
The study cohort’s median age was 63.5 years (43.9–73.8 years) and was 46.4% male (table 1). A total of 14.3% of patients had concomitant CABG, and endocarditis was the indication for TVSx in 10.4% of patients. A rheumatic TV was documented in 66.5% of patients.
Table 1.
Study cohort demographic and surgical characteristics
| Parameters | Open isolated TVSx (n=537) |
| Demographics | |
| Age, years | 58.2±20.1 |
| Median (IQR) | 63.5 (43.9–73.8) |
| Males | 249 (46.4) |
| Country of birth | |
| Australia plus territories/New Zealand | 379 (70.6) |
| Europe | 77 (14.3) |
| Asia | 33 (6.1) |
| Other | 48 (8.9) |
| Comorbidities | |
| Cardiovascular disease* | 454 (84.5) |
| Ischaemic heart disease | 104 (19.4) |
| Prior PCI/CABG | 31 (5.8) |
| Congestive cardiac failure | 200 (37.2) |
| Stroke | 11 (2.0) |
| Peripheral vascular disease | 25 (4.7) |
| Prosthetic heart valve | 59 (11.0) |
| Atrial fibrillation/flutter | 289 (53.8) |
| Cardiac risk factors* | 323 (60.1) |
| Hypertension | 138 (25.7) |
| Hyperlipidaemia | 16 (3.0) |
| Diabetes | 82 (15.3) |
| Current/ex-smoker | 198 (36.9) |
| Primary PHT | 11 (2.0) |
| Secondary PHT | 74 (13.8) |
| Malignancy | 10 (1.9) |
| Chronic pulmonary disease | 31 (5.8) |
| Neurodegenerative disease* | 3 (0.6) |
| Chronic kidney disease | 73 (13.6) |
| IVDU history | 54 (10.1) |
| Charlson Comorbidity Index score† | 1.4±1.9 |
| Median (IQR) | 1 (0–2) |
| Surgical characteristics | |
| Indication for valve surgery | |
| Endocarditis | 56 (10.4) |
| Non-endocarditis | 481 (89.6) |
| Rheumatic tricuspid valve | 357 (66.5) |
| Concomitant CABG‡ | 77 (14.3) |
| Types of TVSx§ | |
| Annuloplasty | 262 (48.8) |
| Replacement | 217 (40.4) |
| Repair | 83 (15.5) |
| Open valvotomy | 5 (0.9) |
| Others | 15 (2.8) |
| Length of hospital stay, days | 24.4±23.5 |
| Median (IQR) | 16 (10–31) |
Plus-minus values represent mean±SD; all others represent numbers of patients with values in parentheses representing percentages, or otherwise stated.
*Cardiovascular disease includes history of ischaemic heart disease (includes PCI and/or CABG), stroke, congestive cardiac failure, peripheral vascular disease, prosthetic heart valve and/or atrial fibrillation/flutter. Cardiac risk factors include history of hypertension, hyperlipidaemia, diabetes and/or smoking (current/previous). Neurodegenerative disease includes dementia, central nervous systemic atrophies, Parkinson’s disease, basal ganglia degeneration and/or nervous systemic degenerative diseases.
†Conditions included in the Charlson Comorbidity Index include myocardial infarction, congestive cardiac failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease (mild vs moderate to severe), diabetes (with or without organ damage), hemiplegia, moderate to severe renal disease, any tumour (within the last 5 years), lymphoma, leukaemia, metastatic solid tumour and AIDS.
‡Concomitant CABG performed during same admission for cardiac valve surgery.
§More than one type of TVSx might be performed on a patient during the same admission.
CABG, coronary artery bypass graft; IVDU, intravenous drug use; PCI, percutaneous coronary intervention; PHT, pulmonary hypertension; TVSx, tricuspid valve surgery.
AF was the most common cardiovascular comorbidity (58.3%), followed by CCF (37.2%) and ischaemic heart disease (19.4%). A history of smoking (36.9%), hypertension (25.7%) and diabetes (15.3%) was common. Of the non-cardiovascular comorbidities, secondary pulmonary hypertension (13.8%) and chronic kidney disease (13.6%) were the most common. Concomitant malignancy was rare, comprising 1.9% of the cohort. 10.1% had a documented history of IVDU. The median CCI was 1 (IQR 0–2). The median length of stay was 16 days (IQR 10–31 days). Rheumatic TV disease was common in our cohort, representing 66.5% of our cohort.
All-cause and cause-specific mortality
A total of 211 (39.3%) patients died during a mean follow-up of 4.82±3.94 years (table 2). In-hospital mortality rate was 7.4%, with 62 (11.5%) patients dying within 180 days after open isolated TVSx. A cardiovascular cause of death occurred in 45% of in-hospital deaths and in 52% of post-discharge deaths (table 3). Of the cardiovascular causes of death, heart failure was the most frequent cause, representing 10.0% (n=4) of in-hospital deaths and 25.2% (n=43) of post-discharge deaths. Sepsis was the most identified non-cardiovascular cause of death documented in 7 (17.5%) in-hospital deaths and 37 (21.6%) post-discharge deaths.
Table 2.
Morbidity and mortality outcomes following open isolated TVSx
| Cumulative incidence, n (%) | 30 days | 180 days | 2 years | End of study* |
| Congestive cardiac failure | 11 (2.0) | 53 (9.9) | 109 (20.2) | 157 (29.2) |
| Atrial fibrillation† | 10 (4.0) | 25 (10.1) | 40 (16.1) | 68 (27.4) |
| Infective endocarditis† | 4 (0.9) | 7 (1.5) | 10 (2.1) | 26 (5.6) |
| Pulmonary embolism | 1 (0.2) | 2 (0.4) | 5 (0.9) | 7 (1.3) |
| Permanent pacemaker | 2 (0.4) | 3 (0.6) | 20 (3.7) | 40 (7.5) |
| Implantable cardioverter-defibrillator | 1 (0.2) | 3 (0.6) | 4 (0.8) | 13 (2.4) |
| All-cause death | 18 (3.4) | 62 (11.5) | 108 (20.1) | 211 (39.3) |
*End of study was 31 December 2018.
†Atrial fibrillation (AF) and infective endocarditis (IE) incidences were based on patients without baseline AF (n=248) or IE (n=466) during open isolated tricuspid valve surgery (TVSx) admission.
Table 3.
Cause-specific death outcomes
| Categories | In-hospital (n=40) n (%)* |
Post-discharge (n=171) n (%)* |
| Cardiovascular causes | 18 (45.0) | 89 (52.0) |
| Acute myocardial infarction | 0 (0) | 6 (3.5) |
| Heart failure | 7 (17.5) | 43 (25.2) |
| Stroke | 4 (10.0) | 15 (8.8) |
| Pulmonary embolism | 0 (0) | 2 (1.17) |
| Cardiac related† | 7 (17.5) | 23 (13.5) |
| Non-cardiovascular causes | 22 (55.0) | 82 (48.0) |
| Sepsis | 7 (17.5) | 37 (21.6) |
| Malignancy | 1 (2.5) | 15 (8.8) |
| Other | 7 (17.5) | 17 (10.0) |
| Undefined | 7 (17.5) | 13 (7.6) |
*n (%) represents total number of deaths from each specific cause and value in parenthesis represents the percentage out of total deaths.
†Cardiac-related cause of death is coded when more than one cardiac cause of death is recorded on the death certificate.
Morbidity outcomes
Table 2 shows the cumulative incidence of the study’s prespecified morbidity events after open isolated TVSx. The development of new AF (in those without a history of AF at index TVSx) and admissions for CCF were the most frequent morbidities documented during follow-up: the cumulative incidence of AF at 180 days and by end of study was 10.1% and 27.4% of patients, respectively, while 53 (9.9%) patients had an admission for CCF within the first 180 days following TVSx, reaching 29.2% by end-of-study follow-up. Across the study period the rate of PE admission was low at 1.3%. 10.0% of patients had PPM implanted during their index TVSx admission. A further 40 (7.5%) and 13 (2.4%) patients required PPM and ICD implantations by end-of-study follow-up, respectively.
Independent predictors for all-cause mortality
Independent predictors for all-cause mortality following open isolated TVSx were age ≥59 years, a background history of CCF, chronic pulmonary disease and malignancy (table 4). Malignancy was the strongest predictor of mortality (adjusted HR (aHR)=3.49, 95% CI 1.73 to 7.07, p<0001), followed by a history of chronic pulmonary disease (aHR=2.21, 95% CI 1.36 to 3.59, p<0.001). Neither gender, indication for surgery, rheumatic TV status, concomitant CABG, history of ischaemic heart disease, stroke, diabetes, pulmonary hypertension, chronic kidney disease, smoking status nor history of IVDU were associated with the primary outcome (online online supplemental tables 2 and 3). While univariate analysis showed TV replacement was associated with increased mortality (HR=1.35, 95% CI 1.03 to 1.77, p=0.03) and TV repair was associated with reduced mortality (HR=0.50, 95% CI 0.32 to 0.79, p=0.003), type of TVSx was not associated with the primary outcome in multivariate analysis (online supplemental tables 2 and 3).
Table 4.
Independent predictors for all-cause mortality
| Multivariable analysis* | Parameters | aHR (95% CI) | P value |
| All-cause death during follow-up (4.82±3.94 years) |
Age ≥59 years (mean age) | 1.76 (1.26 to 2.47) | 0.001 |
| Congestive cardiac failure | 1.78 (1.33 to 2.38) | <0.001 | |
| Chronic pulmonary disease | 2.21 (1.36 to 3.59) | <0.001 | |
| Malignancy | 3.49 (1.73 to 7.07) | <0.001 |
Plus-minus value represents mean±SD.
*Multivariable Cox regression method was used to identify independent predictors for all-cause mortality. Only significant independent predictors are shown in the above table (see online supplemental table 3 for complete multivariable analysis results).
aHR, adjusted HR.
Discussion
The present study examined the case load and outcomes of open isolated TVSx over a 17-year period in an unselected Australian state-wide population. The main findings were: (1) open isolated TVSx case volumes have increased significantly over the study period; (2) high postoperative mortality rates in the short term and intermediate term comparable to those in international studies; (3) heart failure and sepsis were the most common specific causes of death in both in-hospital and post-discharge follow-up; (4) new AF and admissions for CCF were the two most common morbidities encountered after surgery; and (5) age ≥59 years and history of CCF, chronic pulmonary disease and malignancy were associated with increased mortality risk.
TVSx case loads
Alqahtani et al demonstrated a significant increase in the case load of both open isolated TV repairs and replacements in the USA between 2003 and 2014 using the Nationwide Inpatient Sample (NIS).11 While the NIS captured about 20% of US admissions during this period, our study showed similarly increasing case load findings in a state-wide population where ≥97% of hospital admissions are captured, with the state of NSW approximating 32% of Australia’s overall population. While the increase in case load was significant, the procedure is still relatively rare as shown in our study, with open isolated TVSx cases representing only 1.8% of total open-heart cardiac valve surgery. We postulate the increased case load reflects the growth and ageing of the NSW population over this timeframe.
Prior studies mostly limited to in-hospital outcomes
Existing literature has been mostly limited to in-hospital outcomes after open isolated TVSx. There are two larger US-based studies examining in-hospital mortality and morbidity in addition to several smaller studies.11 32 Our study showed an in-hospital mortality (7.4%) that is lower than the 8.8–9.7% reported in recent studies using similar administrative datasets,11 18 but higher than the 3.4% rate reported in a recent single-centre study based on carefully selected patients.19 In-hospital PPM implantation rates (10.0%) in our study were also at the lower end of reported figures, which range from 9.5% to 24.4%.11 19 32
Cause-specific deaths following open isolated TVSx
This study is the first of its scale to examine cause-specific mortality after open isolated TVSx. The two leading causes of death both in-hospital and post-discharge were sepsis and CCF. Fatal decompensated CCF may reflect unsuccessful attempted medical and/or surgical management of severe TV regurgitation with associated heart failure—indeed a history of CCF predicted a near 70% increased mortality risk in our multivariable analysis. On the other hand, the large proportion of deaths by sepsis is likely driven by the baseline comorbidities in our population. This is supported by our study’s demonstration of strong independent associations between increased mortality and the presence of malignancy, older age and chronic pulmonary disease. While more conservative case selection may reduce mortality rates, the goal of surgery in these unwell patients may have been to improve quality of life (a parameter not directly measured in this administrative dataset) rather than longevity.
Morbidity following open isolated TVSx
Morbidity after TVSx may provide a surrogate for quality of life, and providing data surrounding long-term morbidity forms an important aspect of informed consent prior to surgery. These data also form a benchmark against which to compare newer percutaneous interventions. In the present study, the main morbidities encountered after discharge were readmission for decompensated CCF (9.9%) and new AF (10.1%), although low rates of admissions for IE, PE, PPM and ICD insertions (all <1% except for IE at 1.5%) were also observed within the first 180 days. Two smaller studies have examined medium to long-term morbidity outcomes following open isolated TVSx. Dreyfus et al described a 38% incidence of heart failure hospitalisation at 5 years after discharge in a French cohort of 466 patients who underwent open isolated TVSx.33 Wong et al described a much lower rate of 13.8% heart failure hospitalisation after discharge during a mean follow-up of 4.9 years in a younger Taiwanese cohort (n=333) compared with 29.2% of patients in our study with a similar mean follow-up duration.34 While Dreyfus et al did not report on rates of post-discharge PPM insertion, Wong et al observed a 5.2% incidence of post-discharge PPM insertion by end of study, compared with 7.6% in our study. Notably, these and other studies have not reported on rates of PE, ICD insertion or de novo infective endocarditis after discharge. Reassuringly, these events appear to be low.
TVSx for rheumatic TV
Our cohort had a high proportion (66.5%) of patients with rheumatic TV disease. While increasingly uncommon in developed countries, rheumatic heart disease (RHD) remains prevalent in Indigenous Australians and immigrants from countries with endemic RHD.35 Organic TV involvement occurs in 4.9–9% of RHD, although autopsy studies have suggested much higher rates of involvement.36 Both repair and replacement are established surgical techniques for treating organic RHD TV disease, although several studies have reported higher mortality rates with TV replacement compared with repair.36–38 While highly prevalent in our population, a rheumatic TV was not associated with increased mortality (online supplemental table 2).
Pathomechanistic reasons for high mortality and morbidity associated with open isolated TVSx
There are two main hypotheses which attempt to explain why open isolated TVSx has consistently been associated with high in-hospital mortality and morbidity rates, despite not being considered technically more difficult than left-sided valvular surgery. The first hypothesis is that patients are referred late for surgery, by which time the consequences of severe TR are, at best, partly remediable by surgery (eg, RV dilation and/or dysfunction, cardiac cirrhosis).32 33 Furthermore, patients with impaired RV size and/or function may not tolerate the increased afterload created by surgical correction of TR, and consequently further decompensate. Supporting this hypothesis, Hamandi et al 19 reported a dramatically lower in-hospital mortality of 3.2%, highlighting early referral as a defining feature of their single-centre 95-patient cohort study. However, in-hospital mortality in their cohort was still higher than that reported for left-sided valve surgery in the literature.11 16–18 The second hypothesis is that patients with severe TR form a more comorbid cohort of patients, whose comorbidities often exacerbate the severity of their TR (eg, pulmonary disease). Indeed, our study showed chronic pulmonary disease to be associated with a 2.7-fold increased risk of death after surgery. Interestingly, while type of TVSx was associated with mortality in the univariate analysis, with replacement being higher risk than repair, this association did not persist in multivariate analysis. One explanation may be that those with more advanced disease are more likely to require replacement and not be appropriate candidates for repair.
Comparison with percutaneous TV interventions
There are presently little published data on outcomes following isolated TV intervention, and no long-term data. Published international registry data (n=312) have reported a 30-day all-cause mortality rate of 3.6% following percutaneous TV intervention, varying depending on the technique used from 2.8% with MitraClip to 7.6% with Cardioband (mean age 76.6 years).39 More recently, the Trial to Evaluate Cardiovascular Outcomes in Patients Treated with the Tricuspid Valve Repair System (TRILUMINATE) trial (n=85), an international, prospective, single-arm study examining safety and efficacy of the TriClip edge-to-edge repair system, reported a 1-year all-cause mortality rate of 7.1%.40 Mean ages for patients in both above trials were greater than 75 years. While comparison between open isolated TVSx and percutaneous interventions is currently limited by their different cohorts with respect to age, comorbidities and indication, our data form an important benchmark against which to compare emerging data on medium to long-term outcomes following percutaneous TV intervention.
Strengths and limitations
This study’s strengths lie in the large cohort of patients who underwent open isolated TVSx, a relatively rare procedure compared with other cardiac valve surgery. In addition, our study cohort was derived from a state-wide unselected population and included patients from all public and private healthcare facilities that performed cardiothoracic surgery, thus reflecting real-world clinical practice. Our long study period also allows for longitudinal trend analysis of medium to long-term outcomes including identifying important clinical predictors of mortality. The use of a death registry with cause-specific data analysis adds important detail to all-cause mortality figures.
However, this study is limited by its retrospective study design, which limits the imputation of causal links in our multivariable analysis. There was also no propensity-matched control group that did not undergo surgery against which to compare outcomes after TVSx. Additionally, this is an observational study reflecting current practice on open isolated TVSx which includes a heterogeneous group of procedures (eg, annuloplasty, repair, replacement) with less clear evidence on the best approach compared with aortic or mitral valve procedures. Furthermore, while redo valvular surgery has been reported to be associated with poorer outcomes, we were unable to assess the impact of redo surgery on outcomes as our dataset only extends back to 2001.41 42 Also, our anonymised dataset does not allow for analysis of the association of operator experience with patient outcomes, a parameter that has been shown to be significant in other open valvular surgery.43 44 While our study used the CCI as a measure of comorbidity and operative risk, our dataset does not have the necessary data to calculate values for more conventional cardiothoracic risk preoperative scores such as the Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) or the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II). Finally, our administrative data lack important granular details such as echocardiographic data (eg, RV size and function), functional class, medication usage, biochemical data or organ function such as creatinine or liver function tests, exact aetiology of TV disease or its severity, or indication for surgery (longevity vs quality of life). This speaks to the need for a national registry of TVSx with such granular detail, especially with the development of newer TV interventions.
Conclusion
Open isolated TVSx carries a significant risk of postoperative mortality, with admission for decompensated CCF and new AF the most common morbidities encountered after surgery. Independent predictors of mortality include age ≥59 years and comorbidities including history of cardiac failure, chronic pulmonary disease and malignancy. This study forms a benchmark against which to compare outcomes with newer percutaneous TV interventions.
bmjopen-2023-080804supp001.pdf (77.3KB, pdf)
Supplementary Material
Footnotes
Contributors: GH, VC, IR, DBB, LK and ACCN were responsible for study concept and design; data collection, analysis and interpretation; and manuscript preparation. GH and ACCN drafted the initial manuscript, and all authors contributed to data interpretation and critical revision of the report. All authors had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. ACCN is the guarantor of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the New South Wales Population and Health Services Research Ethics Committee, (reference number: 2013/09/479). The ethics committees granted a waiver of the usual requirement for the consent of the individual to the use of their health information. All patient data were deidentified and analysed anonymously.
References
- 1. Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019;12:433–42. 10.1016/j.jcmg.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 2. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. 10.1016/j.jacc.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 3. Ruel M, Rubens FD, Masters RG, et al. Late incidence and predictors of persistent or recurrent heart failure in patients with mitral prosthetic valves. J Thorac Cardiovasc Surg 2004;128:278–83. 10.1016/j.jtcvs.2003.11.048 [DOI] [PubMed] [Google Scholar]
- 4. Hahn RT, Asch F, Weissman NJ, et al. Impact of tricuspid regurgitation on clinical outcomes: the COAPT trial. J Am Coll Cardiol 2020;76:1305–14. 10.1016/j.jacc.2020.07.035 [DOI] [PubMed] [Google Scholar]
- 5. Mehr M, Karam N, Taramasso M, et al. Combined tricuspid and mitral versus isolated mitral valve repair for severe MR and TR: an analysis from the trivalve and TRAMI registries. JACC Cardiovasc Interv 2020;13:543–50. 10.1016/j.jcin.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 6. McCarthy FH, Vemulapalli S, Li Z, et al. Association of tricuspid regurgitation with transcatheter aortic valve replacement outcomes: a report from the society of thoracic surgeons/American college of cardiology transcatheter valve therapy registry. Ann Thorac Surg 2018;105:1121–8. 10.1016/j.athoracsur.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 7. Benfari G, Antoine C, Miller WL, et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation 2019;140:196–206. 10.1161/CIRCULATIONAHA.118.038946 [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Larsen CM, Le RJ, et al. The prognostic significance of tricuspid valve regurgitation in pulmonary arterial hypertension. Clin Respir J 2018;12:1572–80. 10.1111/crj.12713 [DOI] [PubMed] [Google Scholar]
- 9. Pinamonti B, Dragos AM, Pyxaras SA, et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J 2011;32:1105–13. 10.1093/eurheartj/ehr040 [DOI] [PubMed] [Google Scholar]
- 10. Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185–94. 10.1016/j.jcmg.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 11. Alqahtani F, Berzingi CO, Aljohani S, et al. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc 2017;6:e007597. 10.1161/JAHA.117.007597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 2014;63:2438–88. 10.1016/j.jacc.2014.02.537 [DOI] [PubMed] [Google Scholar]
- 13. Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616–64. 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 14. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart Association joint committee on clinical practice guidelines. Circulation 2021;143:450–500. 10.1161/CIR.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 15. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic surgery (EACTS). Eur Heart J 2022;43:2022. 10.1093/eurheartj/ehac051 [DOI] [PubMed] [Google Scholar]
- 16. Staab ME, Nishimura RA, Dearani JA. Isolated tricuspid valve surgery for severe tricuspid regurgitation following prior left heart valve surgery: analysis of outcome in 34 patients. J Heart Valve Dis 1999;8:567–74. [PubMed] [Google Scholar]
- 17. Do QB, Pellerin M, Carrier M, et al. Clinical outcome after isolated tricuspid valve replacement: 20-year experience. Can J Cardiol 2000;16:489–93. [PubMed] [Google Scholar]
- 18. Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–60. 10.1016/j.jacc.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 19. Hamandi M, Smith RL, Ryan WH, et al. Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg 2019;108:11–5. 10.1016/j.athoracsur.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 20. Chorin E, Rozenbaum Z, Topilsky Y, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging 2020;21:157–65. 10.1093/ehjci/jez216 [DOI] [PubMed] [Google Scholar]
- 21. Russo A, Grigioni F, Avierinos J-F, et al. Thromboembolic complications after surgical correction of mitral regurgitation: incidence, predictors, and clinical implications. J Am Coll Cardiol 2008;51:1203–11. 10.1016/j.jacc.2007.10.058 [DOI] [PubMed] [Google Scholar]
- 22. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–25. 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 23. Centre for health record linkage (CHeReL). n.d. Available: http://www.cherel.org.au
- 24. Lee A-H, Ng ACC, Yong ASC, et al. Outcomes of 1,098 patients following transcatheter aortic valve implantation: a statewide population-linkage cohort study. Heart Lung Circ 2021;30:1213–20. 10.1016/j.hlc.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 25. Cheng Y-Y, Brieger D, Bannon P, et al. Outcomes following triple cardiac valve surgery over 17-years: a multicentre population-linkage study. Heart Lung Circ 2023;32:269–77. 10.1016/j.hlc.2022.09.018 [DOI] [PubMed] [Google Scholar]
- 26. Ng ACC, Chung T, Yong ASC, et al. Long-term cardiovascular and Noncardiovascular mortality of 1023 patients with confirmed acute pulmonary embolism. Circ Cardiovasc Qual Outcomes 2011;4:122–8. 10.1161/CIRCOUTCOMES.110.958397 [DOI] [PubMed] [Google Scholar]
- 27. Ng ACC, Lau JK, Chow V, et al. Outcomes of 4838 patients requiring temporary transvenous cardiac pacing: a statewide cohort study. Int J Cardiol 2018;271:98–104. 10.1016/j.ijcard.2018.05.112 [DOI] [PubMed] [Google Scholar]
- 28. Brieger DB, Ng ACC, Chow V, et al. Falling hospital and postdischarge mortality following CABG in New South Wales from 2000 to 2013. Open Heart 2019;6:e000959. 10.1136/openhrt-2018-000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng Y-Y, Chow V, Brieger D, et al. Outcomes of 16,436 patients requiring isolated aortic valve surgery: a statewide cohort study. Int J Cardiol 2021;326:55–61. 10.1016/j.ijcard.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 30. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying Prognostic Comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 31. National Center for Health Statistics . Instructions for classifying the underlying cause-of-death, ICD-10. 2008. 1–259. Available: http://www.cdc.gov/nchs/about/major/dvs/im.htm [accessed 10 Jun 2009]
- 32. Kawsara A, Alqahtani F, Nkomo VT, et al. Determinants of morbidity and mortality associated with isolated tricuspid valve surgery. J Am Heart Assoc 2021;10:e018417. 10.1161/JAHA.120.018417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304–17. 10.1093/eurheartj/ehaa643 [DOI] [PubMed] [Google Scholar]
- 34. Wong W-K, Chen S-W, Chou A-H, et al. Late outcomes of valve repair versus replacement in isolated and concomitant tricuspid valve surgery: a nationwide cohort study. J Am Heart Assoc 2020;9:e015637. 10.1161/JAHA.119.015637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katzenellenbogen JM, Bond-Smith D, Seth RJ, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc 2020;9:e016851. 10.1161/JAHA.120.016851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sultan FAT, Moustafa SE, Tajik J, et al. Rheumatic tricuspid valve disease: an evidence-based systematic overview. J Heart Valve Dis 2010;19:374–82. [PubMed] [Google Scholar]
- 37. Bernal JM, Pontón A, Diaz B, et al. Surgery for rheumatic tricuspid valve disease: a 30-year experience. J Thorac Cardiovasc Surg 2008;136:476–81. 10.1016/j.jtcvs.2008.02.065 [DOI] [PubMed] [Google Scholar]
- 38. Moutakiallah Y, Aithoussa M, Atmani N, et al. Reoperation for isolated rheumatic tricuspid regurgitation. J Cardiothorac Surg 2018;13:104. 10.1186/s13019-018-0793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taramasso M, Alessandrini H, Latib A, et al. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international trivalve registry. JACC Cardiovasc Interv 2019;12:155–65. 10.1016/j.jcin.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 40. Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 2021;77:229–39. 10.1016/j.jacc.2020.11.038 [DOI] [PubMed] [Google Scholar]
- 41. Fukunaga N, Okada Y, Konishi Y, et al. Clinical outcomes of redo valvular operations: a 20-year experience. Ann Thorac Surg 2012;94:2011–6. 10.1016/j.athoracsur.2012.06.045 [DOI] [PubMed] [Google Scholar]
- 42. Fukunaga N, Sakata R, Koyama T. Short‐and long‐term outcomes following redo valvular surgery. J Card Surg 2018;33:56–63. 10.1111/jocs.13534 [DOI] [PubMed] [Google Scholar]
- 43. Bolling SF, Li S, O’Brien SM, et al. Predictors of mitral valve repair: clinical and surgeon factors. Ann Thorac Surg 2010;90:1904–11. 10.1016/j.athoracsur.2010.07.062 [DOI] [PubMed] [Google Scholar]
- 44. Moon MR, Henn MC, Maniar HS, et al. Impact of surgical experience on operative mortality after reoperative cardiac surgery. Ann Thorac Surg 2020;110:1909–16. 10.1016/j.athoracsur.2020.04.077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-080804supp002.pdf (1.8MB, pdf)
bmjopen-2023-080804supp001.pdf (77.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data are available upon reasonable request.