Abstract
Introduction
Even when total knee arthroplasty (TKA) is an extended treatment, most patients experience a suboptimal evolution after TKA. The objectives of this study are the following: (1) to determine the effectiveness of two different prosthesis stabilisation systems on the functionality in activities of daily life, and (2) to determine prognostic biomarkers of knee prosthesis function based on radiological information, quantification of cytokines, intra-articular markers and biomechanical functional evaluation to predict successful evolution.
Methods and analysis
The PROKnee trial was designed as a randomised controlled patient-blinded trial with two parallel groups that are currently ongoing. The initial recruitment will be 99 patients scheduled for their first TKA, without previous prosthesis interventions in lower limbs, who will be randomly divided into two groups that differed in the stabilisation methodology incorporated in the knee prosthesis: the MEDIAL-pivot group and the CENTRAL-pivot group. The maximum walking speed will be reported as the primary outcome, and the secondary results will be patient-reported questionnaires related to physical status, cognitive and mental state, radiological test, laboratory analysis and biomechanical instrumented functional performance, such as the 6-minute walking test, timed up-and-go test, gait, sit-to-stand, step-over, and ability to step up and down stairs. All the results will be measured 1 week before TKA and at 1.5, 3, 6 and 12 months after surgery.
Ethics and dissemination
All procedures were approved by the Ethical Committee for Research with Medicines of the University Clinical Hospital of Valencia on 8 October 2020 (order no. 2020/181). Participants are required to provide informed consent for the study and for the surgical procedure. All the data collected will be treated confidentially since they will be blinded and encrypted. The results from the trial will be published in international peer-reviewed scientific journals, regardless of whether these results are negative or inconclusive.
Trial registration number
ClinicalTrials.gov Registry (NCT04850300).
Keywords: Knee, REHABILITATION MEDICINE, Physical Examination
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study protocol describes a prospective triple-blinded, randomised controlled trial analysing the effect of central pivot and medial systems stabilisation of total knee prosthetics on functionality in patients under primary total knee arthroplasty (TKA).
This protocol has a battery of objective measures of functionality since it uses biomechanical tools to assess daily life motor gestures during 1 year post-TKA.
One limitation of the study is the large and complex instrumented assessment sessions that participants have to spend, which may influence the adherence to the study.
This is a one-centre study, which affects the capability of extrapolation of the future predictive model on functionality after TKA.
Introduction
Total knee arthroplasty (TKA) is currently the international standard treatment for end-stage degenerative and rheumatological diseases of the knee joint as well as certain types of fractures.1 Although TKA successfully relieves pain and corrects deformities, most patients never reach the functional level of age-matched healthy people even years after surgery2 or experience suboptimal outcomes, falls and readmission to the hospital.3 In the early post-surgery period (1 month), patients undergo loss of more than half of their pre-surgery strength,4–6 with quadriceps strength being the major determinant of general physical functions 1 year after TKA.7 Other authors have documented functional and balance limitations, which have been ascribed to deficits in the proprioceptive system8 produced by the loss of knee receptors located in structures such as the menisci, cruciate ligaments and cartilage, which are removed during the surgery for the prosthesis implant.9 This receptor loss results in impaired movement patterns, difficulties in walking and loss of postural control. The gait disturbances described after 1 year of post-surgery evolution are impairment of speed (40% less than that of healthy controls), cadence (17.48% less than that of healthy controls), and kinematic limitation during the loading response and swing phase.10 The aforementioned alterations influence functional gestures, such as getting up from a chair and walking, as measured by the timed up-and-go (TUG) test, where after 1 month of surgery, a 54.38% increase in execution time was observed in patients who received standard physiotherapy.4 A similar result occurs with stepping up and down stairs in patients without specialised physical therapy, where the measured time increased after 3 months post-TKA11 for stair ascent.
Owing to the limitations of functionality and poor recovery of motion, previous studies have analysed the possible predictors of referred functionality and satisfaction after TKA. Features such as pain,12 13 pre-surgery patient-reported outcome measure (PROM) scores related to functionality and quality of life,14 preoperative disease, and anxiety and depression13 were highly predictive of knee function after TKA. However, some variables, such as demographic data, surgical history or knee alignment, were less strongly correlated with TKA outcomes.15 Nonetheless, there are important limitations to the studies carried out to date that prevent the application of predicted models. On one hand, the clinical relevance of the improvements by the minimally clinically important difference is not universally valid across populations and varies by instrument. Moreover, the current ‘gold standard’ to measure functionality after TKA are PROM instruments related to motor execution, which have a well-defined ceiling effect16 produced by the ease of assessment items, such as standing up from a chair, for which evaluation depends on whether the patient performs the gesture, rather than motor execution quality; therefore, the extrapolation of predictive models to other populations is complex when inaccurate or subjective measures are used as predictors. Lastly, there is an important variability between the methodologies of the studies concerning measurements, follow-up period, patient population and analyses performed,15 which hamper the generation of predictive models applicable to daily clinical practice and support the decision-making among health professionals.
Although the alterations observed after TKA have been described, the progression achieved by patients in different day-to-day motor gestures in the short, medium and long term after surgery, as well as the objective indicators that best predict this functionality, remains uncertain. Therefore, the main objective of this study is to determine the effectiveness of two different prosthesis stabilisation procedures of TKA on the functionality achieved and perceived by the patient, as well as on the knee joint biomechanics during movement in activities of daily life. On the other hand, as a secondary objective, we propose determining prognostic biomarkers of knee prosthesis function based on radiological information, quantification of cytokines, intra-articular markers, and biomechanical functional evaluation that correlate and predict a correct evolution of patients with knee replacements. We hypothesised that both types of prosthesis stabilisation procedures will have similar functional outcomes and that the functional state before surgery impacts the results significantly more than the surgical characteristics, as the objective measures are better predictors than the usual clinical variables used in previous studies.
Materials and analysis
Study design
The PROKnee trial was designed as a randomised controlled and triple-blinded (patient, raters and data analysts) trial with two parallel groups. The Standard Protocol Items for Randomized Trials guideline17 was used during protocol development (online supplemental material 1). Additionally, we followed the extension for stepped, wedge, cluster, randomised trials of the Consolidated Standards of Reporting Trials statement.18 This trial was registered on 20 April 2021 at ClinicalTrials.gov (NCT04850300) with the approval of the Ethical Committee for Research with Drugs of the University Clinical Hospital of Valencia obtained (online supplemental material 2). The study begins with a baseline pre-surgical assessment, three interim follow-up measures at 6 weeks, at 3 months and at 6 months post-surgery, and the final follow-up assessment after 1 year of TKA intervention.
bmjopen-2023-077942supp001.pdf (85.9KB, pdf)
bmjopen-2023-077942supp002.pdf (823.8KB, pdf)
Participants, interventions and outcomes
Setting and eligibility criteria
This study recruited patients scheduled for total knee replacement surgery. Patient enrolment began on 13 September 2021 and is expected to be completed in August 2023. All patients will expect to have completed baseline testing by September 2022. All assessment procedures will be performed at the Evaluation Unit in Personal Autonomy, Dependence, and Mental Disorders of the Faculty of Medicine at the University of Valencia. The inclusion criteria are age 50–85 years and primary total knee prosthesis surgery indication with patellar fitting. The exclusion criteria are as follows: (1) previous lower limb joint prosthesis, (2) history of fracture or surgery in the lower limb or lumbar spine, (3) disabling contralateral knee pain, (4) lower limb length asymmetry >2 cm, (5) walking impairment due to other causes non-related to the knee pathology, (6) body mass index >39, and (7) severe surgical complications such as infection, aseptic loosening, deep vein thrombosis, periprosthetic fracture and arthrofibrosis.
Intervention
The intervention in this study consisted of TKA. Participants will be randomised and allocated to one of two groups of patients (figure 1), which differed in the stabilisation methodology incorporated in the knee prosthesis. The MEDIAL-pivot group used a medial condylar stabilisation prosthesis, which consists of an ultra-congruent tibial component medial surface and a free lateral compartment. The prosthetic model used will be the medial stabilisation prosthetic GMK sphere model from Medacta corporate (Medacta International, Switzerland). On the contrary, the CENTRAL-pivot group will use a prosthesis with a central pivot located between the medial and lateral prosthetic tibial surfaces to provide stability. For this group, the Persona prosthetic model with central pivot stabilisation and design from Zimmer Biomet (Zimmer Biomet, Warsaw, Indiana, USA) will be used.
Figure 1.
Flow of the study intervention and assessments. TKA, total knee arthroplasty.
Surgical technique
The surgical approach will be similar in both groups of patients. A standard midline incision with a medial parapatellar approach will be made in every case. Partial resection of the infrapatellar fat pad will be accomplished, and the patella will be everted. An intramedullary guide will be used on the femoral side and an extramedullary guide will be employed for the tibial replacement procedure. Gap balancing and soft tissue release will be performed if the knee is in flexion or extension. Block spacers will be used to check for soft-tissue tightness. After proper balance of gaps and verification of patellar tracking, cemented components will be inserted. Three degrees of external rotation will be applied in both designs to homogenise the procedures and to obtain friendly patellar tracking. Lateral release will be performed in cases of patellar subluxation or instability. Closure will be done with a non-absorbable suture for the parapatellar approach and stitches for the skin. Suction drainage will be performed in the joint for 24 hours. All surgeries will be performed by one of the two traumatologists specialising with more than 10 years of experience in joint surgery at the Orthopedic Surgery and Traumatology Service of the Valencia University Clinical Hospital, depending on the professionals’ work shifts.
Additionally, both groups of patients followed the outpatient physical rehabilitation protocol established in the hospital after TKA, which consists of 10–30 physical rehabilitation sessions (depending on the needs of each patient) that include, in addition to therapeutic exercise, conventional physiotherapy techniques for pain relief and control of the inflammatory process.
Outcomes and participant timeline
The participant timeline contained the enrolment, the basal (t0) assessment in the week before surgery and the surgery effect evaluations: the follow-up medium-term assessments at 6 weeks (t1), 3 months (t2), 6 months (t3) and long-term follow-up assessment at 1 year post-surgery (t4). The primary outcome of this study is the maximum walking speed (m/s),19 20 which is the ability to walk safely and as quickly as possible without running. Previous studies have determined that walking speed is associated with knee biomechanics, especially age-related decline,21 after bilateral TKA.20 The secondary outcomes of the study and measurement instruments are shown in table 1 and are organised into the following items: (1) patient-reported questionnaire related to physical status, (2) cognitive and mental status, (3) radiological and clinical analyses, and (4) biomechanical functional performance. All the outcomes proposed in this study will be measured at all measurement times mentioned above as long as the participant is confident to perform the test described hereunder. In cases where participants show insecurity about performing the requested test, they will be given the option of not performing the motor task in which the patient does not feel physically or mentally capable, in order to avoid falls or post-surgery discomfort.
Table 1.
Summary of outcomes, measurement instruments and assessment times
| Domain/outcomes | Measurement instrument | Enrolment | Study period | ||||
| Basal | Post 6 weeks | Post 3 months | Post 6 months | Post 1 year | |||
| t0 | t1 | t2 | t3 | t4 | |||
| Enrolment | |||||||
| Eligibility screen | x | ||||||
| Informed consent | x | ||||||
| Patient-reported outcomes and measures related to physical status | |||||||
| Function | KOOS | – | x | x | x | x | x |
| Pain, stiffness and function | WOMAC | – | x | x | x | x | x |
| Physical activity | UCLA | – | x | x | x | x | x |
| Knee, hip and lumbar pain | EVA | – | x | x | x | x | x |
| Health-related quality of life | EQ-5D-5L; SF-12 | – | x | x | x | x | x |
| Cognitive and mental status | |||||||
| Cognitive state | SCIP | – | x | x | x | x | x |
| Joint awareness | FJS-12 | – | x | x | x | x | x |
| Anxiety and depression | HADS | – | x | x | x | x | x |
| Radiological and clinical laboratory analyses | |||||||
| Anatomical femoral–tibial angle (°) | X-ray | – | x | x | x | x | x |
| Mechanical femoral–tibial angle (°) | – | x | x | x | x | x | |
| Posterior tibial slope (°) | – | x | x | x | x | x | |
| Prosthetic rotation (°) | – | – | x | x | x | x | |
| Interleukin 6 | Blood test | – | x | x | x | x | x |
| C reactive protein | – | x | x | x | x | x | |
| Erythrocyte sedimentation rate | – | x | x | x | x | x | |
| Biomechanical functional performance | |||||||
| Active knee range of motion (°) | Manual goniometer | – | x | x | x | x | x |
| Passive knee range of motion (°) | x | x | x | x | x | ||
| Isometric knee strength flexion (N) | Electronic dynamometer | – | x | x | x | x | x |
| Isometric knee strength extension (N) | – | x | x | x | x | x | |
| Lower limb proprioception (cm) | PPA | – | x | x | x | x | x |
| Six-minute walking test | |||||||
| Distance (m) | IMU integrated into an Android system | – | x | x | x | x | x |
| CoM vertical displacement (mm) | – | x | x | x | x | x | |
| CoM ML displacement (mm) | – | x | x | x | x | x | |
| Jerk ML (m/s3) | – | x | x | x | x | x | |
| Jerk AP (m/s3) | – | x | x | x | x | x | |
| Power spectral density in turning (W/Hz) | – | x | x | x | x | x | |
| Timed up-and-go test | |||||||
| Total execution time (s) | IMU integrated into an Android system | – | x | x | x | x | x |
| CoM vertical range displacement (mm) | – | x | x | x | x | x | |
| CoM ML displacement (mm) | – | x | x | x | x | x | |
| Power spectral density in turning (W/Hz) | – | x | x | x | x | x | |
| Jerk sit (m/s3) | – | x | x | x | x | x | |
| Jerk stand (m/s3) | – | x | x | x | x | x | |
| Modified timed up-and-go test | |||||||
| ML and AP displacement (mm) during Romberg part | IMU integrated into an Android system (Fallskip software) | – | x | x | x | x | x |
| Vertical and ML displacement CoM (mm) during gait part | – | x | x | x | x | x | |
| Turn-to-sit-time (s) | – | x | x | x | x | x | |
| Sit-to-stand power (W) | – | x | x | x | x | x | |
| Jerk sit (m/s3) | – | x | x | x | x | x | |
| Jerk stand (m/s3) | – | x | x | x | x | x | |
| Total execution time (s) | – | x | x | x | x | x | |
| Reaction time (s) | – | x | x | x | x | x | |
| Gait | |||||||
| Velocity of gait (m/s) | 3D photogrammetry Dynamometric platform |
– | x | x | x | x | x |
| Stride length (m) | – | x | x | x | x | x | |
| Cadence (steps/min) | – | x | x | x | x | x | |
| Stance time (%) | – | x | x | x | x | x | |
| Maximum knee extension during stance (°) | – | x | x | x | x | x | |
| Maximum knee flexion during swing (°) | – | x | x | x | x | x | |
| GRV force during weight acceptance (N) | – | x | x | x | x | x | |
| GRV force during toe off (N) | – | x | x | x | x | x | |
| Sit-to-stand | 3D photogrammetry Dynamometric platform |
||||||
| Maximum vertical force (N) | – | x | x | x | x | x | |
| Knee momentum (Nm) | – | x | x | x | x | x | |
| Knee range of motion (°) | – | x | x | x | x | x | |
| Knee angular velocity (°/seg) | – | x | x | x | x | x | |
| Step-over | |||||||
| Maximum GRV force during the single-stance step (N) | 3D photogrammetry Dynamometric platform |
– | x | x | x | x | x |
| Maximum GRV force in landing (N) | |||||||
| Knee momentum during the single-stance step (Nm) | – | x | x | x | x | x | |
| Knee range of motion (°) | – | x | x | x | x | x | |
| Knee angular velocity during swing (°/s) | – | x | x | x | x | x | |
| Stepping up and down stairs | |||||||
| Maximum knee flexion (°) | 3D photogrammetry Dynamometric platform |
– | x | x | x | x | x |
| Knee angular velocity (°/s) | – | x | x | x | x | x | |
| Maximum GRV force in single support (N) | – | x | x | x | x | x | |
| Flexion/extension knee momentum (Nm) | – | x | x | x | x | x | |
| Varus/valgus knee momentum (Nm) | – | x | x | x | x | x | |
The outcomes from sit-to-stand test will be estimated for sitting and standing separately. The outcomes from the stepping up and down stairs test will be estimated for up and down separately.
AP, anterior–posterior; CoM, centre of mass; 3D, three-dimensional; EQ-5D-5L, EuroQol Group 5-dimensions-Level 5; EVA, Visual Analogue Scale; FJS-12, Forgotten Joint Score-12; GRV, ground reaction vertical; HADS, Hospital Anxiety and Depression Scale; IMU, Inertial Measurement Unit; KOOS, Knee Osteoarthritis Outcome Score; ML, medial–lateral; PPA, Physiological Profile Assessment; SCIP, Screen for Cognitive Impairment in Psychiatry; SF-12, Short Form-12 Physical Functioning; UCLA, University of California, Los Angeles Physical Activity Questionnaire; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Following the list of variables in table 1, an electronic handheld dynamometer and the NedDiscapacidad/IBV (V.4.2, Instituto de Biomecánica de Valencia) measurement software will be used to measure muscle strength. The 6-minute walking test, TUG test and its modified version will be assessed with an inertial sensor composed of a high-performance six-axis MEMS MotionTracking running on an Android system. To analyse the modified TUG test, the Fallskip/IBV software (V.2.2.0, Instituto de Biomecánica de Valencia) was installed in the Android system.22 23 In addition, a three-dimensional photogrammetry system with 12 smart-cams (Kinescan/IBV V.7.1.0, Instituto de Biomecánica de Valencia) and 2 force platforms (Dinascan/IBV V.5.6.0, Instituto de Biomecánica de Valencia) will be used to measure sit-to-stand, step-over, and stepping up and down stairs daily living activities. Python software (V.3.8) will used to estimate the parameter values from the ground reaction forces and three-dimension landmark position coordinates from the biomechanical models. Finally, gait will be registered with NedAMH+/IBV software (V.1.1.1, Institute of Biomechanics of Valencia, Spain), which uses two red-light photocells to measure gait speed, in addition to the photogrammetry system and dynamometric platform described above.
The procedures for the biomechanical measurements of the daily living activities are:
Knee range of motion
The participant is assessed in the prone position, with the knee on the edge of the examination table. A manual goniometer (accurate to 1°) is used to measure the active movement of maximum flexion and extension, and then the passive non-painful movement is measured. The landmarks used in the measurements are the greater trochanter of the femur, proximal head of the fibula and the lateral malleolus.24
Maximum strength of knee flexors and extensors
Muscle strength is evaluated through the maximum voluntary contraction of the knee flexors and extensor muscles. The patients are seated on an examination table with knees and hips positioned at 90° flexion, after which they are instructed to remain seated in an upright position and place both hands on the thighs to avoid compensation. An electronic handheld dynamometer (figure 2A) is located in the anterior and posterior distal thirds of the leg to resist the gesture of extension and flexion, respectively. Each participant receives three trials. For the analysis, the mean maximal strength of the measures is calculated and corrected for body weight.25
Figure 2.
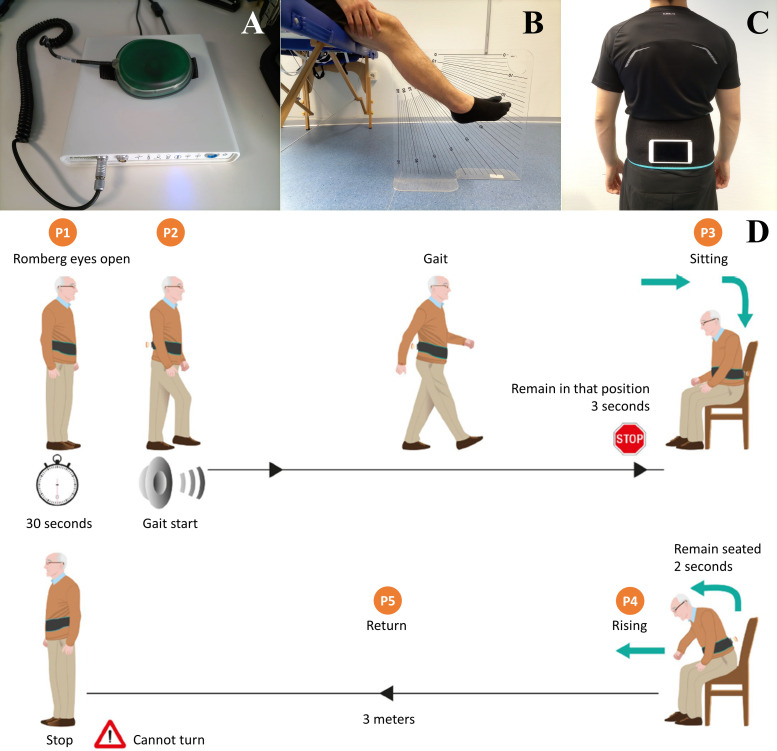
Accessories and tools for the measurement of muscle strength, proprioception and clinical test. (A) Electronic handheld dynamometer. (B) Vertical acrylic board (60×60×1 cm) inscribed with a protractor and placed between the legs of the participant to measure proprioception. (C) Inertial sensor built in an Android system, fixed to a sacrum area through a Velcro strap. (D) Measurement protocol of the modified timed up-and-go test (extracted with authorisation from Pérez-Ros et al 23).
Lower limb proprioception
The proprioception part of the Physiological Profile Assessment tool is used.26 27 In this test, participants are seated with their eyes closed and are asked to align their lower limbs simultaneously on either side of a vertical clear acrylic sheet (60×60×1 cm) inscribed with a protractor and placed between their legs27 (figure 2B). The outcome registered consists of the degrees of difference observed by the misalignment of the limbs, represented by the mismatch of the big toes of both feet on the graduated acrylic sheet. Five repetitions are recorded after the two practice attempts.
Six-minute walking test
This test assesses the distance covered within 6 min.28 A pair of cones is placed on the ground at 20 m, and the participant has to walk in a straight line around the cones for 6 min. Additionally, the patient is instrumented with an inertial sensor in the sacral area (figure 2C) using a Velcro strap.
TUG test
This test assesses the time required for patients to stand from a 46 cm (seat height) chair, walk around a cone located 3 m away, turn around, and return to take a seat as quickly and safely as possible. Patients are instructed to walk as quickly as possible while feeling safe and comfortable.29 The fastest number of trials is used for further analysis. Up to 1 min of rest is allowed between the trials. A stopwatch is used to measure the time required to complete the test. Additionally, an inertial sensor is used, as shown in figure 2C.
Modified TUG test
This assessment is a modification of the TUG test that integrates a balance test measured with an inertial sensor located, as shown in figure 2C. The test consists of the following phases (figure 2D): (1) the patient completes the initial Romberg test with open eyes for 30 s; (2) the device emits a sound, and at that moment, the patient must start a walk through a 3 m corridor in a straight line, in the direction of a chair; (3) seating and rising: upon reaching the end of the walking corridor, the patient has to sit down and get up from a chair; and (4) the patient walks in the opposite direction until they reach the starting position of the test.23
Gait
A 10 m-long, straight, flat walkway is used for gait assessment. The biomechanical model consists of a simplified gait model implemented in the sagittal plane on the right and left sides. The anatomical placement of the motion capture markers is shown in figure 3A. All participants walk at a self-selected speed. 10 kinematic records are analysed, 5 of which correspond to the force data of the right footprint and 5 of which correspond to that of the left footprint. Before recording gait, the participants are allowed to walk along the corridor a few times.
Figure 3.
Biomechanical model used in gait, sit-to-stand and step-over functional activities. (A) Gait model composed by tuberosity of fifth metatarsal (5MTT), posterior surface of calcaneus (CAL), greater trochanter of the femur (GT), lateral condyle of the knee (LC) and lateral malleolus of the ankle (LM). (B) Sit-to-stand assessment set-up. (C) Modification of the model in 5.A for the sit-to-stand test: spinous process of the seventh cervical vertebra (C7) and sacro (S). (D) 20 cm step configuration for a step-over assessment. (E) Step-over assessment configuration started with the right foot.
Sit-to-stand
The participant starts from the standing position and sits on a stool without a backrest that allows 90° knee flexion in the sitting position to be maintained.30 31 To avoid interruptions during the gesture, each participant is able to perform a few repetitions of practice while looking carefully at the stool where they are going to sit. The sitting position is standardised by marking the location of the stool and feet on the floor, leaving 10 cm of distance between the base of the stool and heel of the participants (figure 3B). After practising the gesture a few times, five cycles of movement are recorded, that is, sitting down and getting up again, leaving 2 s between each part. The biomechanical model that is used consists of 10 markers, as shown in figure 3A, with the exception that the landmarks from both calcaneus will be relocated to the spinous process of the seventh cervical vertebra and sacrum (figure 3C).
Step-over
A step of 20 cm is used, with a configuration as shown in figure 3D, to evaluate the gesture of moving up and down a single step. The test is standardised using the following sequence (figure 3E): (1) the participant starts from the standing position to put one foot in a step; (2) the contralateral foot goes over the step and is placed just in front of it; (3) the foot on the step is used to take a step forward, passing the contralateral foot; and (4) the rear foot advances to finish in the standing position. Six independent repetitions are recorded; in three of them, the participants start with the right leg, and in the other three repetitions, the participants start with the left leg. The biomechanical model is shown in figure 3A.
Stepping up and down stairs
The configuration in figure 4 is used to evaluate the gesture of going up and down stairs, which is standardised with two rungs 20 and 40 cm high. The biomechanical model that is used is illustrated in figure 4A. After recording the standing calibration scene, markers representing the knee and ankle joint axes are removed. Next, 12 repetitions are recorded: 6 to go up the stairs and another 6 to go down, starting with the right and left legs three times for each part of the activity. To go up the stairs, the participant starts by taking a step directly on the first activated platform, which determines the start of the test with the right or left foot (figure 4B). Subsequently, the participant has to raise the leg opposite the first rung of the stairs, represented by the smaller step located on the second active platform. The upstairs gesture ends with the foot that started the test on the highest rung and the contralateral foot is placed at the same height. To go down the stairs (figure 4C), the participant initiates the gesture by lowering the foot to a smaller step and then places the contralateral foot on the free active platform. The gesture ends by taking the last step outside the force measurement zone.
Figure 4.
Set-up for biomechanical measurement of up and down stairs. (A) Biomechanical model: anterior vertex triangle forming the thigh segment (THIGH), posterior vertex triangle of the leg segment (LEG), right and left lateral condyle of the knee (LC), and right and left lateral malleolus (LM). (B) Sequence to go upstairs started with the right leg. (C) Sequence to go downstairs started with the right leg.
Sample size and recruitment
The sample size was determined using G*Power V.3.1. Due to the lack of previous studies with a similar methodology to that proposed in this protocol, the sample size estimation was based on the expected differences on gait between two participant groups who received two different prosthetic at 6-month follow-up post-TKA,32 in whose work the medial stabilisation model proposed in this protocol is used. A small-medium effect (f=0.15), a statistical significance of 5% at the two-tailed level and a power of 90% are set, which gives a total of 82 people to be recruited. If 20% of the possible dropouts are considered, the initial recruitment will be of 99 people. From the beginning of the study, the coordinating researcher and head of the Orthopedic Surgery and Traumatology Service has reviewed the scheduled surgeries and asks each patient with scheduled TKA if they are interested in participating. All patients who show interest are informed, and the criteria for participation in their usual pre-surgical trauma appointment are evaluated. The study recruitment period will remain open for 1 year.
Assignment of interventions
Because patient recruitment will occur over a prolonged period, the assignment to the groups will be performed as block randomisation33 34 with a 1:1 allocation. This restrictive permuted block sample randomisation is performed by an external investigator and will allow us to ensure a periodical balance in the number of participants assigned to each intervention group. For this, 13 different blocks were created among themselves, with a homogeneous distribution (8) of letters A and B representing each prosthetic option.34 The order in which the letters A and B were arranged varied in all the blocks. Then, the order of the blocks was randomised from 1 to 13 and the distribution of the letters A and B was followed depending on the order after randomisation. This order of prosthesis assignment will be used to assign each prosthesis model to the participants who will be recruited. The coordinating researcher of the project is the only member who knows about the randomisation sequence and is the one who indicates to the two surgeons in the study what type of surgery to perform according to the randomised sequence. Stratification factors are not considered in the randomisation. Regarding the blinding of the study, the study participants, researchers who perform the functional assessments and data analyst are unaware of the type of prosthetic stabilisation used and they will not have access to the hospital medical history where the surgical procedure performed will be specified. The two surgeons in the study know the type of prosthesis used in each patient, and if the patient requests it, the technical specifications of the stabilisation used are disclosed.
Data collection, management and analysis
An electronic template was prepared with ‘required fields’ to ensure that there will not be missing items. Each evaluator enters the data collected in the different areas described in table 1, under the code each participant will be assigned for this study (coding). In this way, the data dump to a database is carried out under the supervision of one of the evaluators who, in turn, has the task of guaranteeing anonymisation of the participants in the outcomes registry. All patient-reported outcomes are evaluated by one of the study evaluators, so that the participants do not answer any of the questions from home or alone. The patients do not receive any incentives or compensation for participation in this study. Personal data about the patient are located separately from the main dataset on a local computer to protect confidentiality during all trial phases. The raw dataset will be maintained for 10 years after the completion of the trial with indefinite restricted access due to sensitive data. No individual data from participants will be openly available. Instead, all additional information to the results presented in future publications will be provided through the corresponding supplemental material to each publication.
If adherence issues occur, all analyses will be evaluated using intention-to-treat principles, with a level of significance of 0.05. The statistical software SPSS Statistics V.24.0 (IBM Corporation) and R V.4.1.0 (R Core Team, R Foundation for Statistical Computing) are used for all analyses. Categorical variables are presented as frequency and percentage, and continuous variables are presented as mean and SD or median, depending on normality tests. To answer the main aim of the study, a two-factor mixed multivariate analysis of variance (MANOVA) is conducted to analyse the effects of within-subject factors (surgical intervention) and between-subject factors (group) on the outcomes registered (table 1). Previously, the principal component analysis method is applied to reduce the number of outcomes from each test evaluated running in the MANOVA analysis. Bonferroni adjustment is used for post-hoc comparisons, and differences are declared statistically significant if the p value is less than 0.05. Differences in demographic outcomes are verified with a one-way MANOVA. Furthermore, to test for sex differences between the groups, Χ2 analysis is performed.35
To address the second objective of this study, the data collected will be randomly divided into two sets. Part one of the dataset (70% of patients) will be used to build the prediction models, and the remaining 30% of the dataset will be used to validate the models.36 A prediction model will be explored for all study participants and for women and men separately, taking into account the sex differences in the results after TKA.37 A linear multiple regression analysis will be performed for the primary outcome, and regression models will be explored for the biomechanical dependent variables. For the prediction model assessment, the Akaike Information Criterion analysis is used in order to identify the model with the largest likelihood under the constraint of the smallest number of predictors.38 Lastly, in both statistical analyses, the percentage of variance attributed to the surgeon performing the TKA will be reported. In the MANOVA analysis, it will be included as a covariance, while in the regression analysis, it will be included as a predictor factor.
Monitoring
This study did not integrate external professionals or staff from sponsors for data-monitoring procedures. Instead, data monitoring is coordinated by an internal Data Monitoring Committee integrated with one member of each clinical service participating in the research group. Participant monitoring is carried out weekly by telephone, which allows us to promote participant retention and encourage participants to complete the follow-up. The following information is recorded: (1) symptoms related to the surgical area, adverse events such as falls or other interventions, and medication (Traumatology Service); (2) number of rehabilitation sessions received, as well as the physiotherapy techniques applied (Rehabilitation Service); and (3) anxiety or depressive symptoms and individual perception of the evolution process (Functional Assessment Unit). The Data Monitoring Committee decides whether trial participation should be discontinued based on monitoring reports. If participants decide to withdraw, they are given the option to continue the follow-up of the clinical scales, which can be carried out by telephone.
Patient and public involvement
Patients and/or the public were not involved in the design of this protocol.
Ethics and dissemination
All procedures were approved by the Ethical Committee for Research with Medicines of the University Clinical Hospital of Valencia on 8 October 2020 (order no. 2020/181). We did not consider future changes in the study protocol reported by the committee.
Eligible participants are informed about the study and the privacy protection concept before participating. They sign an informed consent form for the study (online supplemental material 3), which includes a detailed explanation of the research milestones, in accordance with the Declaration of Helsinki. Patients sign a second consent form related exclusively to the surgical procedure. Both consent forms are signed during regular medical appointments with trauma specialists. All the data and information collected regarding this trial are treated confidentially (blinded and encrypted) by the researchers connected to the trial.39 Finally, all results from the trial will be published in international peer-reviewed scientific journals, regardless of whether these results are negative or inconclusive.
bmjopen-2023-077942supp003.pdf (5.7MB, pdf)
Discussion
The intervention in this study aimed to improve decision quality for total knee replacement and publicise the functionality achieved objectively in different activities of daily life during the first year after surgery with two different knee prosthesis stabilisation systems. The stabilisation of knee prostheses has been thoroughly debated for many years. To imitate knee kinematics, new designs with a more congruent medial compartment and a more permissive lateral compartment have been introduced. Indeed, the medial pivot model addressed the paradoxical anterior femoral translation encountered by the posterior-stabilised model and seemed to have better patient satisfaction, minor TUG score, improved self-paced walking test speed40 and better performed tasks in closed chain movement41 in comparison with other models with central pivot. On the other hand, there is also evidence that finds no differences in perception or objective functionality between the medial pivot model and other stabilisation systems,42 43 even in the long term after surgery.44 A careful evaluation of the objective functionality outcomes will be conducted in this study to help clarify the best functional scope of the studied models.
Additionally, although previous studies have analysed possible predictors of functionality measured through PROMs,45 46 to our knowledge, no quantitative analysis has been made to determine which biomechanical components of daily life activities have a slow or altered evolution after TKA. Owing to this approach of objective assessment of daily living, one of the difficulties of this study is the time required to evaluate and record the data in each evaluation session, which could limit the acceptance of participation, adherence and retention of the participants during the follow-up. Even so, independent of the intrinsic mechanics of the different prosthesis designs, biomechanical characterisation of functional gestures in prosthetic knee patients is necessary to determine if the remaining capacities after surgery are sufficient to perform normal functionality in activities where the knee plays a key role, although patients present less muscle strength.4 11 39 In this sense, multiple variable analysis is essential for assessing the independent contribution of each feature to the prediction of postoperative outcomes.
The large battery of biomechanical tests designed for this study will allow us to study the most relevant variables to predict functionality after surgery and to provide clear guidelines on the evaluation of patients before and after TKA. To extrapolate the predictive model, the sample size plays a key role in hindering the validity of the model if a sufficiently large sample is not obtained.36 The fact that this study is not multicentric and does not use participants from different countries may represent another difficulty related to the extrapolation of the predictive model. However, differences in surgical methodology and the characteristics of the patients themselves between countries may have important unequal percentages of explained variance in functionality; thus, a local population model may be the most appropriate. The evaluation model of this study can serve as a starting point for other authors because it includes variables that can be modified in the short term prior to surgery, such as muscle strength, gait speed, proprioception and balance.
Supplementary Material
Acknowledgments
We would like to thank all patients, caregivers, and all persons and institutions who have collaborated in this project.
Footnotes
@Constanza_SnMV, @chromosome8
Contributors: All authors read and approved the final manuscript. Conceptualisation—CSMV, RT-S, JFP-S and ASM. Funding acquisition—RT-S and ASM. Methodology—CSMV, RT-S, APR, PC-G, JFP-S and ASM. Project administration—RT-S and ASM. Resources—RT-S and ASM. Supervision—RT-S and ASM. Visualisation—CSMV and JFP-S. Writing (original draft)—CSMV. Writing (review and editing)—CSMV, RT-S, APR, PC-G, JFP-S and ASM.
Funding: This work is part of the project 'Efficiency evaluation of the methodology for the follow-up of patients with knee prostheses' of the INCLIVA Biomedical Research Institute, University Clinical Hospital of Valencia, and was financed by the Mutua-Madrileña Foundation in its annual call for aid for research projects in 2020 (grant number: AP174612020).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Gao J, Xing D, Dong S, et al. The primary total knee arthroplasty: a global analysis. J Orthop Surg Res 2020;15:190. 10.1186/s13018-020-01707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schache MB, McClelland JA, Webster KE. Lower limb strength following total knee arthroplasty: a systematic review. Knee 2014;21:12–20. 10.1016/j.knee.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Causey-Upton R, Howell DM, Kitzman PH, et al. Factors influencing discharge readiness after total knee replacement. Orthopaedic Nursing 2019;38:6–14. 10.1097/NOR.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 4. Paravlic AH, Maffulli N, Kovač S, et al. Home-based motor imagery intervention improves functional performance following total knee arthroplasty in the short term: a randomized controlled trial. J Orthop Surg Res 2020;15:451. 10.1186/s13018-020-01964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther 2011;41:932–41. 10.2519/jospt.2011.3734 [DOI] [PubMed] [Google Scholar]
- 6. Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012;92:210–26. 10.2522/ptj.20110124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizner RL, Petterson SC, Stevens JE, et al. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol 2005;32:1533–9. [PubMed] [Google Scholar]
- 8. Labanca L, Iovine R, Bragonzoni L, et al. Instrumented platforms for balance and proprioceptive assessment in patients with total knee replacement: a systematic review and meta-analysis. Gait Posture 2020;81:230–40. 10.1016/j.gaitpost.2020.07.080 [DOI] [PubMed] [Google Scholar]
- 9. Bragonzoni L, Rovini E, Barone G, et al. How proprioception changes before and after total knee arthroplasty: a systematic review. Gait Posture 2019;72:1–11. 10.1016/j.gaitpost.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 10. Pellegrino P, Conti A, Pautasso A, et al. Gait analysis: comparative evaluation of conventional total knee replacement and modular distal femoral megaprosthesis. Knee 2020;27:1567–76. 10.1016/j.knee.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 11. Hepperger C, Gföller P, Hoser C, et al. The effects of a 3-month controlled hiking programme on the functional abilities of patients following total knee arthroplasty: a prospective, randomized trial. Knee Surg Sports Traumatol Arthrosc 2017;25:3387–95. 10.1007/s00167-016-4299-3 [DOI] [PubMed] [Google Scholar]
- 12. Baker PN, van der Meulen JH, Lewsey J, et al. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br 2007;89:893–900. 10.1302/0301-620X.89B7.19091 [DOI] [PubMed] [Google Scholar]
- 13. Judge A, Arden NK, Cooper C, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51:1804–13. 10.1093/rheumatology/kes075 [DOI] [PubMed] [Google Scholar]
- 14. Baker PN, Deehan DJ, Lees D, et al. The effect of surgical factors on early patient-reported outcome measures (PROMS) following total knee replacement. J Bone Joint Surg Br 2012;94:1058–66. 10.1302/0301-620X.94B8.28786 [DOI] [PubMed] [Google Scholar]
- 15. Batailler C, Lording T, De Massari D, et al. Predictive models for clinical outcomes in total knee arthroplasty: a systematic analysis. Arthroplast Today 2021;9:1–15. 10.1016/j.artd.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckhard L, Munir S, Wood D, et al. The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop Traumatol Surg Res 2021;107:102758. 10.1016/j.otsr.2020.102758 [DOI] [PubMed] [Google Scholar]
- 17. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito H, Ichihara K, Tamari K, et al. Factors characterizing gait performance of patients before and soon after knee arthroplasty. J Phys Ther Sci 2021;33:274–82. 10.1589/jpts.33.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ro DH, Han H-S, Lee DY, et al. Slow gait speed after bilateral total knee arthroplasty is associated with suboptimal improvement of knee biomechanics. Knee Surg Sports Traumatol Arthrosc 2018;26:1671–80. 10.1007/s00167-017-4682-8 [DOI] [PubMed] [Google Scholar]
- 21. Clark DJ, Manini TM, Fielding RA, et al. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol 2013;48:358–63. 10.1016/j.exger.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Pascual J, Hurtado Abellán J, Inglés M, et al. P 151 – reliability of variables measured with an android device during a modified timed up and go test in patients with Alzheimer’s disease. Gait & Posture 2018;65:484–5. 10.1016/j.gaitpost.2018.07.072 [DOI] [Google Scholar]
- 23. Pérez-Ros P, Sanchis-Aguado MA, Durá-Gil JV, et al. Fallskip device is a useful tool for fall risk assessment in sarcopenic older community people. Int J Older People Nurs 2022;17:e12431. 10.1111/opn.12431 [DOI] [PubMed] [Google Scholar]
- 24. Mutsuzaki H, Takeuchi R, Mataki Y, et al. Target range of motion for rehabilitation after total knee arthroplasty. J Rural Med 2017;12:33–7. 10.2185/jrm.2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koblbauer IFH, Lambrecht Y, van der Hulst MLM, et al. Reliability of maximal Isometric knee strength testing with modified hand-held dynamometry in patients awaiting total knee arthroplasty: useful in research and individual patient settings? A reliability study. BMC Musculoskelet Disord 2011;12:249. 10.1186/1471-2474-12-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levinger P, Menz HB, Wee E, et al. Physiological risk factors for falls in people with knee osteoarthritis before and early after knee replacement surgery. Knee Surg Sports Traumatol Arthrosc 2011;19:1082–9. 10.1007/s00167-010-1325-8 [DOI] [PubMed] [Google Scholar]
- 27. Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther 2003;83:237–52. 10.1093/ptj/83.3.237 [DOI] [PubMed] [Google Scholar]
- 28. Moffet H, Collet J-P, Shapiro SH, et al. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil 2004;85:546–56. 10.1016/j.apmr.2003.08.080 [DOI] [PubMed] [Google Scholar]
- 29. Podsiadlo D, Richardson S. “The timed “UP & go”: a test of basic functional mobility for frail elderly persons”. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 30. Boonstra MC, De Waal Malefijt MC, Verdonschot N. How to quantify knee function after total knee arthroplasty? Knee 2008;15:390–5. 10.1016/j.knee.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 31. Boonstra MC, Schwering PJA, De Waal Malefijt MC, et al. Sit-to-stand movement as a performance-based measure for patients with total knee arthroplasty. Phys Ther 2010;90:149–56. 10.2522/ptj.20090119 [DOI] [PubMed] [Google Scholar]
- 32. Gray HA, Guan S, Young TJ, et al. Comparison of posterior-stabilized, cruciate-retaining, and medial-stabilized knee implant motion during gait. J Orthop Res 2020;38:1753–68. 10.1002/jor.24613 [DOI] [PubMed] [Google Scholar]
- 33. Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health 2011;8:15–20. 10.3390/ijerph8010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train 2008;43:215–21. 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. San Martín Valenzuela C, Moscardó LD, López-Pascual J, et al. Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: results of randomized controlled DUALGAIT trial. Arch Phys Med Rehabil 2020;101:1849–56. 10.1016/j.apmr.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 36. Tolk JJ, Waarsing JEH, Janssen RPA, et al. Development of preoperative prediction models for pain and functional outcome after total knee arthroplasty using the Dutch arthroplasty register data. J Arthroplasty 2020;35:690–8. 10.1016/j.arth.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 37. Volkmann ER, FitzGerald JD. Reducing gender disparities in post-total knee arthroplasty expectations through a decision aid. BMC Musculoskelet Disord 2015;16:16. 10.1186/s12891-015-0473-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19:716–23. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 39. Jørgensen SL, Bohn MB, Aagaard P, et al. Efficacy of low-load blood flow restricted resistance exercise in patients with knee osteoarthritis scheduled for total knee replacement (Exknee): protocol for a multicentre randomised controlled trial. BMJ Open 2020;10:e034376. 10.1136/bmjopen-2019-034376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kulshrestha V, Sood M, Kanade S, et al. Early outcomes of medial pivot total knee arthroplasty compared to posterior-stabilized design: a randomized controlled trial. Clin Orthop Surg 2020;12:178–86. 10.4055/cios19141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beach A, Regazzola G, Neri T, et al. The effect of knee prosthesis design on tibiofemoral biomechanics during extension tasks following total knee arthroplasty. Knee 2019;26:1010–9. 10.1016/j.knee.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 42. Lee QJ, Wai Yee EC, Wong YC. No difference in patient preference for medial pivot versus posterior-stabilized design in staged bilateral total knee arthroplasty: a prospective study. Knee Surg Sports Traumatol Arthrosc 2020;28:3805–9. 10.1007/s00167-020-05867-z [DOI] [PubMed] [Google Scholar]
- 43. Lin Y, Chen X, Li L, et al. Comparison of patient satisfaction between medial pivot prostheses and posterior-stabilized prostheses in total knee arthroplasty. Orthop Surg 2020;12:836–42. 10.1111/os.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenny J-Y, Bercovy M, Cazenave A, et al. No difference in 13-year survival after medial pivot or central pivot mobile bearing total knee arthroplasty. A propensity matched comparative analysis. Knee Surg Sports Traumatol Arthrosc 2021;29:3648–53. 10.1007/s00167-020-06355-0 [DOI] [PubMed] [Google Scholar]
- 45. Disantis AY, Piva SR, Irrgang JJ. Standardized patient reported outcomes do not capture functional deficits of patients following contemporary total knee replacement: descriptive study. J Exerc Sports Orthop 2018;5. 10.15226/2374-6904/5/1/00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moutzouri M, Gleeson N, Coutts F, et al. Early self-managed focal sensorimotor rehabilitative training enhances functional mobility and sensorimotor function in patients following total knee replacement: a controlled clinical trial. Clin Rehabil 2018;32:888–98. 10.1177/0269215518757291 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077942supp001.pdf (85.9KB, pdf)
bmjopen-2023-077942supp002.pdf (823.8KB, pdf)
bmjopen-2023-077942supp003.pdf (5.7MB, pdf)