Abstract
Pariacoto virus (PaV) was recently isolated in Peru from the Southern armyworm (Spodoptera eridania). PaV particles are isometric, nonenveloped, and about 30 nm in diameter. The virus has a bipartite RNA genome and a single major capsid protein with a molecular mass of 39.0 kDa, features that support its classification as a Nodavirus. As such, PaV is the first Alphanodavirus to have been isolated from outside Australasia. Here we report that PaV replicates in wax moth larvae and that PaV genomic RNAs replicate when transfected into cultured baby hamster kidney cells. The complete nucleotide sequences of both segments of the bipartite RNA genome were determined. The larger genome segment, RNA1, is 3,011 nucleotides long and contains a 973-amino-acid open reading frame (ORF) encoding protein A, the viral contribution to the RNA replicase. During replication, a 414-nucleotide long subgenomic RNA (RNA3) is synthesized which is coterminal with the 3′ end of RNA1. RNA3 contains a small ORF which could encode a protein of 90 amino acids similar to the B2 protein of other alphanodaviruses. RNA2 contains 1,311 nucleotides and encodes the 401 amino acids of the capsid protein precursor α. The amino acid sequences of the PaV capsid protein and the replicase subunit share 41 and 26% identity with homologous proteins of Flock house virus, the best characterized of the alphanodaviruses. These and other sequence comparisons indicate that PaV is evolutionarily the most distant of the alphanodaviruses described to date, consistent with its novel geographic origin. Although the PaV capsid precursor is cleaved into the two mature capsid proteins β and γ, the amino acid sequence at the cleavage site, which is Asn/Ala in all other alphanodaviruses, is Asn/Ser in PaV. To facilitate the investigation of PaV replication in cultured cells, we constructed plasmids that transcribed full-length PaV RNAs with authentic 5′ and 3′ termini. Transcription of these plasmids in cells recreated the replication of PaV RNA1 and RNA2, synthesis of subgenomic RNA3, and translation of viral proteins A and α.
Nodaviruses are a family of small (30 nm in diameter), nonenveloped, spherical viruses with T=3 icosahedral symmetry (33). These viruses have a bipartite genome of messenger-sense RNAs which are capped but not polyadenylated (11, 13, 21, 28). The family Nodaviridae contains two genera: the alphanodaviruses, which primarily infect insects, and the betanodaviruses, which infect fish (45).
The best studied of the alphanodaviruses are Flock house virus (FHV), Black beetle virus (BBV), Nodamura virus (NoV), and Boolarra virus (BoV) (for a review, see reference 6). These four viruses were isolated from insects of the orders Coleoptera, Diptera, and Lepidoptera, but they all replicate well in larvae of the greater wax moth (Galleria mellonella), and all but NoV infect Drosophila melanogaster cells in culture. Furthermore, productive infections of some alphanodaviruses result from introducing viral RNA into plant (43), yeast (39), or mammalian (2, 5) cells.
The larger genomic segment, RNA1, contains about 3 kb and encodes protein A, the viral contribution to the RNA-dependent RNA polymerase (RdRp). During RNA replication, a subgenomic RNA3 is synthesized which is coterminal with RNA1 and encodes one or two small proteins (B1 and B2) with unknown functions. The smaller genomic segment, RNA2, contains about 1.4 kb and encodes the capsid protein precursor α. A single capsid is assembled from 180 copies of protein α which coencapsidate RNA1 and RNA2 (23, 30, 44). Following assembly, the capsid protein precursor of alphanodaviruses is autocatalytically cleaved near its C terminus to form the two mature capsid proteins β and γ, with approximate sizes of 40 and 4 kDa, respectively (20). The cleavage site, Asn/Ala, is conserved among all the alphanodaviruses that have been examined, as is an aspartate residue near the N terminus which catalyzes the cleavage (28, 47).
RNA2 sequences are available for four of the alphanodaviruses (NoV, BBV, FHV, and BoV) (11, 12) and for two of the betanodaviruses (Striped jack nervous necrosis virus and Dicenthrarchus labrax encephalitis virus) (15, 37), and partial RNA2 sequences from several other betanodaviruses have also been published (36, 37). Although the betanodaviruses have capsid proteins and virions of sizes similar to those of the alphanodaviruses, their amino acid sequences share less than 11% similarity (37). Furthermore, it has been suggested that the nodaviruses of fish have a capsid protein processing pathway different from that of their insect counterparts (15).
The nucleotide sequences of RNA1 are available for only two alphanodaviruses, BBV (13) and FHV (R. Dasgupta, GenBank accession no. X77156), and the RNA1 sequence of the betanodavirus Striped jack nervous necrosis virus was recently published (34). The BBV and FHV nucleotide sequences are 99% identical. The encoded amino acid sequences include a GDD box and other characteristic RdRp motifs in protein A, but they provide little insight into other conserved regions of the protein because they were so nearly identical. Protein A sequences from more divergent alphanodaviruses would allow further definition of important structural and functional domains.
The infectious cycle of FHV has been reconstructed from cDNA clones. FHV RNA1 transcripts made in vitro replicate autonomously when transfected into D. melanogaster cells, and they also support the replication of RNA2 transcripts and the production of infectious virus (14). In an alternative approach, cDNA copies of the FHV RNAs were transcribed in cultured baby hamster kidney (BHK-21) cells from specialized transcription plasmids that directed the synthesis of RNAs with authentic termini, which were found to be necessary for efficient RNA replication (2). This approach allowed more thorough investigation of the events involved in FHV replication (4, 7, 8, 26). The simplicity and robustness of the nodavirus replication cycle and the ability to manipulate it using DNA-based technology affords many advantages for the examination of RNA replication, virus structure, and virion assembly.
Pariacoto virus (PaV) was recently isolated from larvae of the southern armyworm Spodoptera eridania (46) which were found to be infected when collected from sweet potato plants near Pariacoto, Ancash province, Peru. In addition to its natural host, PaV multiplies in larvae of S. ochrea but not those of S. frugiperda (46); however, its ability to replicate in cell culture or in common laboratory-reared insects has not been tested. PaV particles are isometric, nonenveloped, and about 30 nm in diameter. The virus has a bipartite RNA genome and a single major capsid protein with an apparent molecular mass of 40.5 kDa, features that support its classification as a nodavirus (46). As such, PaV would be the first alphanodavirus isolated from outside Australasia.
In the work reported here, we characterized PaV at the molecular level and confirmed that PaV is a new and distinct member of the Alphanodavirus genus of the family Nodaviridae. We constructed transcription plasmids that contained full-length cDNA copies of each of the PaV genomic segments and used them to reconstruct viral RNA replication and protein synthesis in transfected BHK-21 cells. The sequence of these cDNA clones showed that PaV is the most distantly related of the characterized alphanodaviruses in both its capsid protein and its replicase protein. This work provides information and reagents to investigate the biology of PaV as well as potential applications of the nodaviruses.
MATERIALS AND METHODS
Virus.
The PaV isolate used in this study for inoculation of G. mellonella was an aliquot of sucrose gradient-purified virus from S. eridania larvae collected in the field in 1996 (46). All virus used for the experiments described below had been passaged only once or twice in G. mellonella larvae. Use of the vaccinia virus recombinant expressing T7 RNA polymerase (vTF7-3) (18) for the reconstruction of nodavirus RNA replication in vertebrate cells has been described previously (2).
Cells.
Baby hamster kidney (BHK-21) cells were grown at 37°C as monolayer cultures in Dulbecco's modified Eagle's medium (DMEM) containing 5% newborn calf serum and 5% fetal calf serum in an atmosphere containing 5% CO2. Drosophila line 2 (DL2) cells (41) were propagated as monolayer cultures at 28°C in Schneider's medium (Gibco/BRL) supplemented with 10% fetal calf serum.
Propagation of PaV in wax moth larvae.
Larvae of the greater wax moth (G. mellonella; Carolina Biologicals) were reared at 31°C on artificial medium containing 46% (wt/vol) baby rice cereal, 4% (wt/vol) dried yeast, 20% (wt/vol) honey, 20% (wt/vol) glycerol, 9.8% (wt/vol) water, and 0.2% (wt/vol) methyl-p-hydrobenzoate. PaV was injected into the hemocoel of late-instar larvae; after incubation at 31°C for 8 days, larvae were collected and stored at −20°C.
PaV purification.
PaV was purified by homogenizing frozen infected larvae in 0.05 M sodium phosphate (pH 7.2) containing 0.1% 2-mercaptoethanol (PB). The homogenate was clarified by centrifugation (9,000 × g, 15 min, 4°C), and virus was pelleted through a 30% sucrose cushion in PB (100,000 × g, 4 h, 4°C). The virus pellet was resuspended in PB and layered onto gradients of 15 to 45% sucrose in PB; following centrifugation (100,000 × g, 3 h, 10°C), the opalescent band of virus particles was harvested, diluted with PB, and pelleted (100,000 × g, 3 h, 4°C). Virus was resuspended in PB and stored at −80°C. Virus concentration was calculated using an extinction coefficient at a wavelength of 260 nm of 4.15/mg (32) and particle mass of 8 × 106 g/mol (24). Approximately 20 μg of virus was recovered per larva.
Infection and transfection of cells in culture.
For all infections and transfections, BHK-21 and DL2 cells were plated in 35-mm-diameter wells of six-well tissue culture plates and grown overnight to reach 80 to 100% confluence (corresponding to approximately 106 or 107 cells per well, respectively). For transfection of cells with virion RNA, cells were washed once with phosphate-buffered saline containing magnesium chloride and calcium chloride (PBSM). The cells were then overlaid with 1 ml of serum-free medium containing virion RNA (0.2 to 1 μg) and Lipofectamine (20 μg; BHK-21 cells) or Lipofectin (10 μg; DL2 cells) as instructed by the manufacturer (Gibco/BRL). Transfected cells were incubated at 28°C for 5 h before the medium was replaced with medium containing serum. Incubation of transfected cells was continued at 28°C for the times specified for the individual experiments.
For plasmid transfections, BHK-21 cells were washed twice with PBSM and infected with vTF7-3 at a multiplicity of infection (MOI) of 10 PFU/cell. vTF7-3 expresses T7 RNA polymerase which is necessary for primary RNA transcription from cDNA plasmids. The virus was allowed to adsorb for 60 min at room temperature before removal of the inoculum. Cells were washed twice with PBSM and then transfected with 2.5 μg of each plasmid, combined with 10 μg of Lipofectamine in serum-free DMEM. After incubation for 5 h at 28°C, the transfection medium was removed and replaced with DMEM containing serum. Incubation of transfected cells was continued at 28°C for the times specified for the individual experiments.
RNA labeling, extraction, and analysis.
The products of RNA replication were labeled by metabolic incorporation of [3H]uridine in the presence of actinomycin D as described previously (2). RNA was extracted, either from cells or from virion particles, by the acid phenol guanidinium thiocyanate method (10) as described previously (27).
cDNA synthesis, cloning, and sequence determination.
RNA extracted from purified PaV virions was used as the template for cDNA synthesis, using Superscript II reverse transcriptase (RT) at 42°C under reaction conditions recommended by the supplier (Gibco/BRL). To generate the initial cDNA clones, first-strand cDNA synthesis was primed with random hexamer oligonucleotides and second-strand synthesis was performed with RNase H and Escherichia coli DNA polymerase I (Gibco/BRL). Blunt-ended cDNAs were cloned, and their sequences were determined. Subsequent clones were constructed by RT-PCR primed with specific oligonucleotides designed according to the sequences of the initial clones. To obtain clones that included the 5′ ends of each of the viral genomic RNAs, rapid amplification of cDNA ends (RACE) (17) was performed using the 5′ RACE system with PaV-specific oligonucleotides under conditions recommended by the supplier (Gibco/BRL). The 5′ termini of PaV RNAs were also mapped by primer extension as previously described (7), using oligonucleotides that annealed to nucleotides (nt) 76 to 98 of RNA1, 98 to 119 of RNA2, or 95 to 115 of RNA3.
Clones were sequenced as double-stranded DNA by the dideoxy-chain termination method (40) using both vector-specific and PaV-specific oligonucleotide primers. Sequencing reactions were performed using dye terminators and analyzed on an automated sequencer. Overlapping cDNA clones that corresponded to the entire length of both RNA1 and RNA2 were sequenced completely in both directions.
Plasmid construction and analysis of full-length clones.
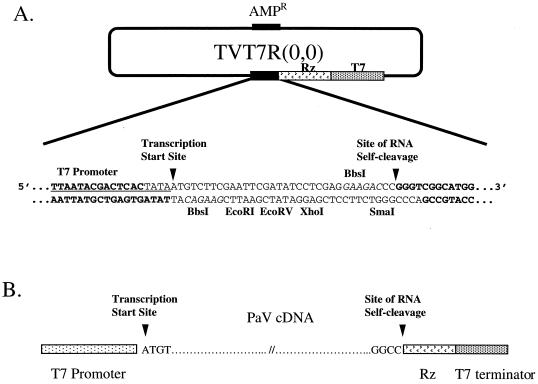
Full-length cDNA copies of genomic RNA segments 1 and 2 were synthesized by RT-PCR using oligonucleotides specific for the 5′ and 3′ termini of each RNA. These PCR products were ligated into transcription plasmids between a T7 promoter and cDNA sequences that encode the hepatitis delta virus (HDV) antigenomic ribozyme followed by a T7 terminator. The 5′ and 3′ nucleotides of the PaV cDNAs were positioned precisely at the sites of transcriptional initiation and ribozyme-mediated cleavage, respectively, in order to achieve transcription of RNAs which, following autocatalytic cleavage by the HDV ribozyme, had no additional nucleotides at either terminus. To construct a convenient version of the transcription vector which provided cohesive ends for the insertion of the cDNAs, Pfu DNA polymerase was used to amplify transcription vector (2,0) (38) as a template using primers VBbsI-T7 (5′-ATCGAATTCGAAGACATTATAGTGAGTCGTCGTATTATTTCGC-3′) and VBbsI-Rz (5′-ATCCTCGAGGAAGACCCGGGTCGGCATGGCATC-3′). The nucleotides in bold type anneal to the last 26 nt of the T7 promoter and first 16 nt of the HDV ribozyme sequence, respectively. In each case, these are preceded by an italicized sequence that corresponds to the recognition site for the remote cutting restriction enzyme BbsI, which cleaves double-stranded DNA to leave a 4-nt cohesive end (underlined). The primers also contained cleavage sites for either EcoRI or XhoI and half of an EcoRV site. Following circularization of the PCR product by blunt-end self-ligation and transformation into E. coli DH5α, plasmid TVT7R(0,0) (Fig. 1) was isolated. It was sequenced from the T7 promoter to the T7 terminator to confirm the insertion of the new sequences. Digestion of this plasmid with BbsI left cohesive termini that corresponded to the last 4 nt of the T7 promoter and the first 4 nt of the HDV ribozyme.
FIG. 1.
Schematic representation of the transcription plasmid TVT7R(0,0). (A) DNA sequence of the region encompassing the T7 promoter (underlined), transcription start site, and site of RNA cleavage (both indicated with arrowheads). The BbsI recognition sequences are shown in italics, and the nucleotides that remain following excision of the short stuffer fragment by BbsI digestion are shown in bold. Positions of the HDV antigenomic ribozyme (Rz) and T7 terminator sequences are shown. (B) Positioning of PaV cDNAs in the transcription plasmid.
First-strand cDNA copies of RNA1 and RNA2 were synthesized at 50°C using Superscript II RT (Gibco/BRL), PaV virion RNA as the template, and oligonucleotide primers that annealed to the 3′ ends of the RNAs: RNA1 (5′-GCTCTAGACGTCTCTACCCGGCCGTGCGTTGGGATTTAC-3′) and RNA2 (5′-GC TCTAGACGTCTCTACCCGGCCATGGTTGTTTCTTTTATG-3′). These oligonucleotides were complementary to 3′-end sequences of the PaV RNA1 and RNA2 (shown in bold) and included recognition sequences for the remote cutting restriction enzyme BsmBI (italicized, with the cohesive end nucleotides underlined) and XbaI to facilitate cloning. The single-stranded cDNAs were used as templates for PCR using the above oligonucleotides and the following second primers: RNA1 (5′-GGGGTACCCGTCTCATATAATGTTGTAGTACGAAAGTACC-3′) and RNA2 (5′-GGGGTACCCGTCTCATATAATGTACAGGTATAACATCAAAGATG-3′). These sequences corresponded to the 5′ ends of RNA1 and RNA2 (shown in bold) next to the recognition sequences for BsmBI (italicized, with the cohesive end nucleotides underlined) and KpnI to facilitate cloning. PCR fragments that corresponded to full-length cDNAs of each RNA were gel purified, digested with BsmBI, and ligated into TVT7R vector that had been digested with BbsI. This strategy created the desired promoter-cDNA-ribozyme junction sequences shown in Fig. 1.
Sequence analysis.
Nucleotide sequences were assembled and analyzed using the University of Wisconsin Genetics Computer Group (GCG) programs (16). Other sequences were retrieved from the nonredundant nucleotide database of GCG and analyzed using BLAST (1). Amino acid sequences were aligned using PILEUP.
SDS-PAGE.
Proteins were labeled with [35S]methionine-cysteine, and cytoplasmic extracts were harvested as described previously (2). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by standard techniques (31).
MALDI-TOF (matrix-assisted laser desorption ionization–time-of-flight) mass spectrometry.
Samples were analyzed in the positive mode on a Voyager Elite mass spectrometer with delayed extraction technology (PerSeptive Biosystems, Framingham, Mass.). The acceleration voltage was set at 20 kV, and 10 to 50 laser shots were summed. Sinapinic acid (D13,460-0; Aldrich) dissolved in acetonitrile–0.1% trifluoroacetic acid (1:1) was used as the matrix. Virus samples (0.8 to 7.0 μg/μl) were diluted 1:10 with matrix, and 1 μl was pipetted onto a smooth plate.
Nucleotide sequence accession numbers.
The nucleotide sequences of PaV RNA1 and RNA2 were submitted to the GenBank database and assigned accession no. AF171942 and AF171943, respectively.
RESULTS
Replication of PaV in insects and in tissue culture cells.
PaV was initially purified from larvae of S. eridania (southern armyworm) collected near Pariacoto, Ancash province, Peru (46). To characterize the virus further, it was necessary to find a convenient system for the growth of PaV in the laboratory. Many of the alphanodaviruses grow well in larvae of G. mellonella (6), and so we tested the ability of PaV to infect these larvae. Following injection of virus into the hemocoel, the larvae became inactive, flaccid, and stunted in growth compared to uninoculated control insects, and some mortality was observed.
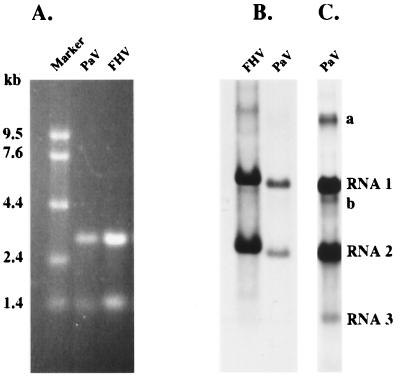
Virus was purified from groups of PaV-infected larvae, and the RNA and protein contents of the purified virus were analyzed. The virion RNAs were resolved by electrophoresis under denaturing conditions on an agarose gel alongside molecular size standards (0.24- to 9.5-kb RNA marker; Gibco/BRL) and visualized by staining with ethidium bromide (Fig. 2A). Two RNAs with estimated sizes of 3 and 1.4 kb were observed and designated RNA1 and RNA2, respectively, by analogy with other nodaviruses. PaV virions were analyzed by SDS-PAGE, and one major and one minor protein band, with estimated sizes of 39 and 42 kDa, respectively, were detected by Coomassie blue staining (data not shown). These results are similar to those observed previously for PaV purified from S. eridania by Zeddam et al. (46); however, in that publication the RNA markers were erroneously labeled in the figure (J. L. Zeddam, personal communication).
FIG. 2.
PaV RNAs and replication in BHK-21 cells. (A) RNAs extracted from purified PaV and FHV virions were resolved by electrophoresis in a 1% agarose-formaldehyde gel along with RNA markers (Gibco/BRL) and visualized by ethidium bromide staining. Sizes of the RNA markers are indicated on the left. (B) BHK-21 cells were transfected with 1 μg of PaV virion RNA or with 0.5 μg of FHV virion RNA and incubated at 28°C. After 22 h of incubation, actinomycin D was added at 5 μg/ml; 30 min later, replicating RNAs were metabolically labeled by incorporation of [3H]uridine for a period of 2 h before total cellular RNA was harvested. RNAs were resolved by electrophoresis on a 1% agarose-formaldehyde gel and visualized by fluorography. (C) The PaV lane of the autoradiogram in panel B was overexposed so that PaV RNA3 could be visualized. PaV RNA1, RNA2, and RNA3 are identified on the right, as are two additional minor RNAs, bands a and b.
We used PaV grown in G. mellanella larvae to attempt infection of insect cell lines from D. melanogaster (lines DL1 and DL2) and from S. frugiperda (Sf9 cells). In no case were viral RNAs metabolically labeled with [3H]uridine 24 h postinfection, even at MOIs of up to 104 particles per cell (data not shown). Furthermore, no RNA replication was detected following transfection of DL2 or Sf9 cells either with purified PaV virions (22) or with naked PaV RNA using four different lipid carriers: Cellfectin, Lipofectin, Lipofectamine, and DMRIE-C (Gibco/BRL) (data not shown). However, when four mammalian cell lines (BHK-21, Vero, BSC40, and HEp-2) were transfected with PaV RNAs using the same four lipid reagents, some replication of the viral RNAs was detected. The most abundant signal was seen in BHK-21 cells transfected with RNA using Lipofectamine. A very low level of PaV RNA replication was observed in Vero, BSC40, and HEp-2 cells (data not shown).
BHK-21 cells were transfected with PaV virion RNA1 and -2 and labeled 24 h posttransfection by metabolic incorporation of [3H]uridine in the presence of actinomycin D. Two major and three minor RNA species were labeled under these conditions (Fig. 2B and C); the two major species comigrated with virion RNAs and ran slightly faster than RNA1 and -2 of FHV when analyzed on 1% agarose-formaldehyde gels (Fig. 2B). The smallest labeled RNA species (Fig. 2C) resembled the subgenomic RNA3 of FHV and was not detected in virions. It was therefore a good candidate for a subgenomic PaV RNA and was designated RNA3 accordingly. Two additional minor RNA species were visualized on longer exposures of the RNAs labeled in transfected cells (bands a and b in Fig. 2C). These RNAs have the mobilities expected for dimers of RNA1 (band a) and RNA2 (band b), and similar species have been observed during replication of FHV and NoV RNAs (2, 3). Despite the ability of PaV RNA to replicate in BHK-21 cells, no RNA replication was observed when BHK-21 cells were exposed to intact virus, even at MOIs of up to 7,000 virions per cell. In contrast to results previously reported for virions of FHV and NoV (22), no RNA replication was detected in BHK-21 cells transfected with intact PaV virions. Nevertheless, transfection of PaV RNAs into BHK-21 cells provided a system in which to examine RNA replication and a convenient source of replication intermediates.
Sequence determination of PaV RNAs and their termini.
The sequences of the two genomic RNAs were originally reconstructed from two families of overlapping cDNA clones. Initial sequence information was obtained from randomly primed cDNA clones, and gaps in the sequence were filled from clones generated by RT-PCR using PaV-specific primers. About 95% of the PaV genomic sequences were determined in this way, but it seemed unlikely that any of the clones contained the complete 5′ or 3′ termini of the genomic RNAs.
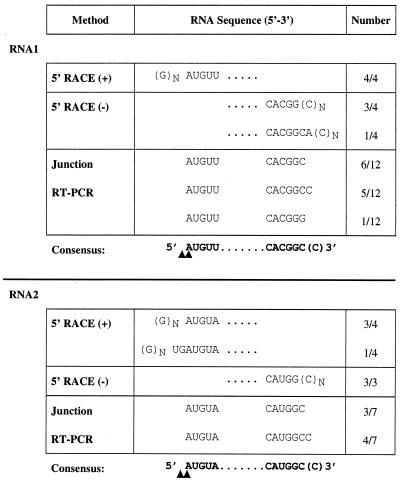
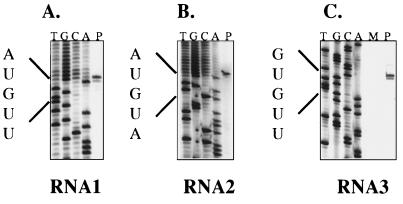
The 5′ termini of the two genomic RNAs were determined by examining clones generated by 5′ RACE. Virion RNA was used as the template for 5′ RACE using oligonucleotide primers specific for either RNA1 or RNA2. For each RNA, a single major PCR product was synthesized; these amplification products were cloned into plasmid vectors, and the sequences of four clones from each RNA were determined (Fig. 3). The sequences of all four RNA1 5′ RACE clones began with GnAUGUU. Three of the RNA2 5′ RACE clones began with the sequence GnAUGUA, and the fourth began with the sequence GnUGAUGUA. 5′ RACE, which involves adding a poly(C) tail to the product of first-strand cDNA synthesis, allows recovery of the exact 5′ end of an RNA molecule, but the method can leave some ambiguity at the junction between the 5′-terminal nucleotide of the RNA and the first residue of the homopolymeric tail. Therefore, the products of primer extension of PaV-specific oligonucleotides on virion RNAs were analyzed to map their 5′ termini (Fig. 4). For each RNA, we identified two primer extension products which differed in size by one nucleotide, the larger bands being more intense than the smaller. The primer extension products were sized by comparison with ladders generated using the same primer to sequence the corresponding 5′ RACE cDNA clone. For RNA1, the smaller band corresponded to an RNA terminus initiating 5′-AUGUU…-3′; for RNA2, the smaller band corresponded to the terminal sequence 5′-AUGUA…-3′. Assuming that PaV RNAs are capped, like those of other nodaviruses (11, 13, 28), the larger, more intense primer extension products most likely correspond to the capped versions of these sequences. The 5′ terminus of subgenomic RNA3 was also determined by primer extension (Fig. 4). Again, we detected two primer extension products differing in size by one nucleotide which indicated that the terminus of RNA3 lies at nt 2598 of RNA1 and begins with the capped sequence 5′-GUGUU…-3′.
FIG. 3.
Sequences of the 5′ and 3′ termini of PaV RNA1 and RNA2. The consensus sequences of the termini of PaV RNA1 and RNA2 were compiled using three independent methods, and the sequences generated by each method are shown. The homodimer junction sequences have been separated into their corresponding 5′ and 3′ termini for clarity of presentation. In each case, the number of clones (x) having the particular sequence out of the total number of clones examined (y) is expressed as x/y in the far right column. The consensus terminal sequences derived from the tabulated data are shown in bold for each RNA. The termini of the primer extension products corresponded to the nucleotides indicated by the arrowheads. We attribute the larger products to extension on capped RNAs.
FIG. 4.
Analysis of the 5′ termini of genomic RNA1 and RNA2 and subgenomic RNA3 by primer extension. (A and B) RNAs extracted from PaV virions were used as templates for extension of primers designed to anneal to nt 76 to 98 of RNA1 (A, lane P) and 98 to 119 of RNA2 (B, lane P). Dideoxynucleotide sequencing ladders of plasmids containing 5′ RACE products of RNA1 and RNA2 were generated using the same two primers and are shown for reference. (C) BHK-21 cells were transfected with 1 μg of PaV virion RNA (lane P) or mock transfected (lane M) and incubated at 28°C. After 24 h of incubation, total cellular RNA was harvested and used as a template for extension of a primer designed to anneal to nt 2692 to 2712 of RNA1. A dideoxynucleotide sequencing ladder generated using the same primer and plasmid pPaV1(0,0) is shown for reference. For simplicity, in all panels the individual lanes of the sequencing ladders are labeled with the complement of the terminating dideoxynucleotide.
As with other nodaviruses (11, 13, 21, 28), attempts to polyadenylate PaV genomic RNAs in vitro were unsuccessful (C. Albarino and L. A. Ball, unpublished data), and so the sequences of the 3′ ends of the virion RNAs were inferred from the 5′-terminal sequences of the respective negative-strand RNAs. Oligonucleotides complementary to the negative strand of either RNA1 or RNA2 were used to prime cDNA synthesis for 5′ RACE. In the case of RNA1, the oligonucleotide used for first-strand cDNA synthesis was designed so that it annealed outside the region encoding RNA3. In both cases, the template was total RNA extracted from BHK-21 cells 24 h after transfection with PaV virion RNAs. The sequences of four negative-strand RNA1 5′ RACE clones were examined, and the results are expressed in terms of the sequence of the 3′ end of the positive strand (Fig. 3). Three of the cDNA clones yielded the positive-strand RNA1 terminal sequence as 5′-…CACGGCn-3′, and the fourth yielded 5′…CACGGCACn-3′. The three 5′ RACE clones from negative-strand RNA2 that were examined all gave the positive-strand RNA2 terminal sequence as 5′-…CAUGGCn-3′ (Fig. 3).
Because the junctions between the templated cDNAs and the nontemplated poly(C) tails were indeterminate in these clones, we next examined the putative RNA dimers seen during PaV RNA replication (Fig. 2). In previous work with FHV and NoV, we detected homodimers of RNA1 and -2 during RNA replication and found that at least some of these molecules contained head-to-tail junctions that comprised perfectly juxtaposed termini (references 2 and 3; L. A. Ball, unpublished data). Using as template total cellular RNA extracted from BHK-21 cells that had been transfected 24 h earlier with PaV virion RNA, we primed cDNA synthesis with specific negative-sense oligonucleotides that annealed toward the 5′ terminus of the positive strand of PaV RNA1 or RNA2. The resulting first-strand cDNAs were then used as templates for PCR with positive-sense primers designed to amplify a DNA fragment that spanned the junction between the two adjacent RNA copies. We observed PCR products of the expected size to have been templated by positive-sense RNA1 dimers and by both positive- and negative-sense RNA2 dimers (data not shown).
The junction PCR fragments were cloned, and the terminal RNA sequences derived from 12 clones of RNA1 are shown in Fig. 3. For clarity of presentation, we have separated the sequence of the dimer at the inferred junction and portrayed its 5′ and 3′ components as the termini of monomeric positive-strand RNA1. In 11 of the 12 clones, the sequences flanking the inferred 3′-5′ junction were identical to those previously found for the 3′- and 5′-terminal regions of RNA1. Similarly, the junction sequences of seven RNA2 dimer clones confirmed the terminal sequences determined for RNA2 (Fig. 3). The one discrepant RNA1 clone contained a single substituted residue (C to G) at the junction. Of the 18 junction clones (11 for RNA1 and 7 for RNA2) that confirmed the 5′ RACE results, the junction sequences were divided equally into two classes that differed by one nucleotide. One class predicted that the 3′ end of each RNA terminated with a single C residue (i.e., 5′-…GGC-3′), whereas the other class predicted two C residues at the termini (i.e., 5′-…GGCC-3′). Both junction sequences were consistent with the results of 5′ RACE from negative-strand RNA1 and RNA2 (i.e., 5′-…GGCn-3′). For construction of full-length cDNAs, the 3′-terminal sequence 5′-…GGCC-3′ was used for both RNAs.
Construction and functionality of full-length cDNA clones.
Full-length cDNAs were synthesized using primers that annealed to the termini of PaV RNA1 and -2 and ligated into the transcription vector TVT7R(0,0) as described in Materials and Methods. In each case, plasmids were constructed so that RNA transcripts made by T7 RNA polymerase would, when cleaved by the HDV ribozyme, have termini that corresponded to those determined for the PaV genomic RNAs. The resulting plasmids were named pPaV1(0,0) and pPaV2(0,0) for RNA1 and RNA2 cDNAs, respectively, the numbers in parentheses reflecting the absence of terminal extensions on the DNA-templated, ribozyme-cleaved transcripts.
To test whether the full-length cDNA clones were functional, plasmids pPaV1(0,0) and pPaV2(0,0) were transfected into BHK-21 cells that were infected with the vaccinia virus recombinant vTF7-3, and 22 h later the products of RNA replication were labeled by metabolic incorporation of [3H]uridine for 4 h in the presence of actinomycin D. Total cellular RNA was extracted, resolved by electrophoresis on an agarose-formaldehyde gel, and visualized by fluorography (Fig. 5A). In cells transfected with pPaV1(0,0) alone (Fig. 5A, lane 1), RNAs corresponding in size to authentic PaV RNA1 and -3 (Fig. 5A, lane 3) were labeled, indicating that pPaV1(0,0) encoded active PaV RNA replicase which catalyzed autonomous RNA1 replication and RNA3 synthesis. Cells cotransfected with pPaV1(0,0) and pPaV2(0,0) supported replication of both RNA1 and RNA2, indicating that pPaV2(0,0) encoded a replicable copy of RNA2 (Fig. 5A, lane 2). As expected, no replication of RNA2 was observed when cells were transfected with pPaV2(0,0) alone (data not shown). As observed with other nodaviruses, the presence of replicating RNA2 down-regulated the synthesis of RNA3 (6).
FIG. 5.
Replication of RNAs and synthesis of viral proteins in cells transfected with PaV cDNA clones. (A) BHK-21 cells were infected with vTF7-3 at an MOI of 10 PFU/cell. One hour postinfection, the cells were transfected with 5 μg of pPaV1(0,0) (lane 1), 2.5 μg each of pPaV1(0,0) and pPaV2(0,0) (lane 2) or 1 μg of PaV virion RNA (lane 3) and incubated at 28°C. After 22 h of incubation, actinomycin D was added at 5 μg/ml; 30 min later, replicating RNAs were metabolically labeled by incorporation of [3H]uridine (20 μCi/ml) for 4 h before total cellular RNA was harvested. RNAs were resolved by electrophoresis on a 1% agarose-formaldehyde gel and visualized by fluorography. PaV RNA1, RNA2, and RNA3 are identified on the left. (B) BHK-21 cells were infected with vTF7-3 at an MOI of 10 PFU/cell. One hour postinfection, the cells were transfected with water (lane 1), 1 μg of PaV virion RNA (lane 2), or 2.5 μg each of pPaV1(0,0) and pPaV2(0,0) (lane 3) and incubated at 28°C. After 44 h of incubation, actinomycin D was added at 5 μg/ml and incubation continued. Following a 0.5-h preincubation in methionine-cysteine-free medium, 48 h posttransfection proteins were labeled with [35S]methionine-cysteine for a period of 2 h. Cytoplasmic extracts were harvested and resolved by SDS-PAGE on a 12.5% gel, and the labeled proteins were visualized by autoradiography. PaV proteins A and α are identified on the right.
To determine whether the RNA2 encoded by pPaV2(0,0) could direct the synthesis of PaV capsid protein, BHK-21 cells infected with vTF7-3 were cotransfected with pPaV1(0,0) and pPaV2(0,0). Forty-eight hours later, proteins were labeled with [35S]methionine-cysteine, cytoplasmic extracts were resolved by SDS-PAGE, and the labeled proteins were visualized by autoradiography (Fig. 5B). Proteins of the sizes expected for both protein A and α were labeled in cells transfected with virion RNA (Fig. 5B, lane 2), and the band corresponding to protein α was also observed in extracts of cells transfected with pPaV1(0,0) and pPaV2(0,0) (Fig. 5B, lane 3). These results established that plasmids pPaV1(0,0) and pPaV2(0,0) were functional and could be used to reconstruct PaV RNA replication in BHK-21 cells.
Sequences of functional cDNA clones.
As described above, these plasmids successfully initiated PaV RNA replication in transfected cells; therefore, both strands of both viral cDNAs were fully sequenced to establish definitive, functional, nucleotide sequences of the two viral genome segments. Every nucleotide was sequenced at least four times from at least two independent cDNA clones, one of which was either pPaV1(0,0) or pPaV2(0,0). The sequence of pPaV2(0,0) was identical to that compiled from the overlapping cDNA clones, whereas the sequence of pPaV1(0,0) differed from the preliminary sequence by one nucleotide: position 1724 was T in pPaV1(0,0) and C in the preliminary sequence. The pPaV1(0,0) sequence encodes Phe at position 568 in protein A rather than Leu, and since this protein was found to be functional, this was the sequence submitted to GenBank and used here for further analysis.
Analysis of PaV genomic sequences.
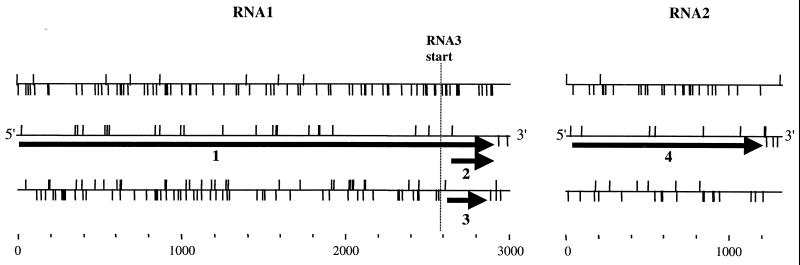
The organization of the PaV genome revealed by these sequences resembles that of other alphanodaviruses (Fig. 6). The larger genome segment RNA1 is 3,011 nt in length and encodes two overlapping open reading frames (ORFs) flanked by 5′ and 3′ untranslated regions of 22 and 69 nt, respectively. The larger ORF starts at the first AUG codon of RNA1 (nt 23 to 25) and terminates at nt 2941. A smaller ORF is found in the 3′ region of the RNA and extends from nt 2622 to 2891. The larger ORF encodes a 973-amino-acid protein with a calculated size of 108 kDa which contains a GDD sequence and other motifs characteristic of viral RdRps (29). The deduced translation of this ORF also shares 26% sequence identity with protein A of FHV. The smaller ORF encodes a 90-amino-acid protein with a calculated size of 10.3 kDa. This small protein shares 26% sequence identity with FHV B2 and is encoded in an analogous position in the PaV genome, within the sequence of subgenomic RNA3. By analogy with the other alphanodaviruses, the two proteins encoded by PaV RNA1 have been designated proteins A and B2. The RNA1 sequences of FHV and BBV also encode a second small ORF on RNA3 that directs the synthesis of a protein called B1 which corresponds to the C-terminal region of protein A (13). A similar ORF was identified in PaV RNA3, but in contrast to FHV and BBV, its AUG lies downstream of that for the B2 ORF, 60 nt from the 5′ end of the RNA3. The smaller genomic RNA segment, RNA2, contains 1,311 nt and encodes a single large ORF that encompasses nt 23 to 1225, flanked by 5′ and 3′ untranslated regions of 22 and 86 nt, respectively. Comparisons with other alphanodaviruses indicate that the RNA2 ORF encodes protein α, the precursor to the viral capsid proteins; it contains 401 amino acids and has a calculated size of 43.3 kDa.
FIG. 6.
Schematic representation of the arrangement of the ORFs encoded by the PaV genomic RNA1 and RNA2. The horizontal lines represent the RNAs, with vertical lines above and below the RNA indicating positions of methionine codons and termination codons, respectively. For each RNA, the three frames on the positive-sense RNA are shown. The 5′ end of the subgenomic RNA3 is indicated by the dotted vertical line. Bold arrows indicate the four major ORFs which are predicted to encode protein A (RdRp catalytic subunit), protein B1, protein B2, and capsid precursor protein α (1 to 4, respectively). The scale indicates length of RNAs in nucleotides.
Comparison of PaV proteins with those of other viruses.
The amino acid sequence of PaV protein A contains the core motifs defined for RdRps including the characteristic GDD box. PaV protein A is 25 amino acids shorter than the homologous FHV protein, with which it shares 26% amino acid identity (35% similarity). Pairwise alignment of the PaV and FHV B2 proteins show that they also share 26% sequence identity. Further analysis of the sequences of RNA1, protein A, and protein B2 will be reported elsewhere.
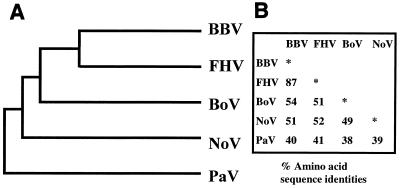
The amino acid sequence of the PaV capsid protein α was compared with the other available alphanodavirus sequences—those of NoV (AF174534), FHV (X15959), BBV (X00956), and BoV (X15960) (references 11 and 12; K. L. Johnson and L. A. Ball, unpublished data)—in a pairwise manner using the program GAP (16). This analysis showed that PaV is the most distantly related of the alphanodaviruses and shares the following levels of protein α sequence identity with the other viruses in this genus: BBV, 40%; FHV, 41%; NoV, 39%; and BoV, 38%. These protein α sequences were aligned with that of PaV (Fig. 7) by using the program PILEUP. We included in the analysis the capsid protein sequences of two betanodaviruses (Dicentrarchus labrax encephalitis virus [U39876] and Striped jack nervous necrosis virus [D30814] [15, 37]), but they shared less than 20% identical amino acids with each of the alphanodaviruses and are not shown in Fig. 7. The data in Fig. 8 summarize the extent of relatedness of the α proteins of all the alphanodaviruses and show that PaV is the most distant.
FIG. 7.
Alignment of amino acid sequences of capsid protein precursors α from all available alphanodaviruses, generated using the GCG program PILEUP. The number of amino acids from the N terminus of protein α is shown at the left. Amino acids that are conserved in three or more of the viruses are shaded. The site of cleavage of the capsid protein precursor α into the two mature capsid proteins β and γ is indicated with an arrow below the alignment. The conserved catalytic Asp residue (75 for FHV) is indicated with an asterisk. Above the alignment, the secondary structural elements as determined from the crystal structure of BBV and labeled according to Johnson and Reddy (25) are indicated as follows: arrows, regions of β sheet; solid lines, regions of α helix; dotted lines; other regions of peptide that are visible in the BBV crystal structure.
FIG. 8.
Genotypic relationships among the capsid protein precursors of five alphanodaviruses. (A) Phenogram produced based on the distance matrix generated from the alignment shown in Fig. 7; (B) percent amino acid identity among protein α of the five viruses.
The amount of conservation varies along the length of the alignment of the capsid protein sequences, and much but not all of this variation correlates with the placement of the region of the polypeptide chain within the capsid structure. The positions of the secondary structural elements identified from the well-defined BBV crystal structure (23–25, 28) are shown in Fig. 7. The 50 N-terminal residues show only a low level of conservation in aligned residues. However, all five sequences contain a very high percentage of basic residues (28% for PaV); since much of this region of the protein lies within the interior of the capsid, it has previously been suggested that these residues interact with the phosphate backbone of the encapsidated RNA (28). The next 40 residues (56 to 90 for BBV) are highly conserved among the viruses and are located on the interior of the capsid shell. Several regions which are found toward the outside of the capsid are less well conserved, including the regions encompassing the antiparallel β sheets C′/C" and E′/E", and also a loop region from residues 247 to 292 (BBV).
Another interesting feature of the capsid protein alignment involves the site at which the capsid protein precursor α is cleaved during virion maturation to form the two mature capsid proteins, β and γ (20) (Fig. 7). The cleavage site Asn/Ala, which is conserved in all other alphanodaviruses, aligns unambiguously to an Asn-Ser dipeptide (residues 361 and 362) in PaV. In fact, the dipeptide Asn-Ala does not occur anywhere in the PaV capsid protein, which suggests that if the PaV protein is cleaved, cleavage must occur at a novel sequence. Asp 68 of PaV α aligns in a region of high sequence conservation with Asp 75 of FHV α (Fig. 7), which catalyzes the autoproteolytic cleavage reaction in FHV virions (47).
Detection of protein γ.
Candidates for both capsid precursor α and the mature protein β were resolved by SDS-PAGE analysis of PaV virion proteins (data not shown), and conservation of the catalytic Asp residue suggested that PaV protein α may be cleaved into the mature capsid proteins β and γ. However, PaV protein γ was not detected in purified virions on SDS-polyacrylamide gels even under conditions of gel analysis where the γ proteins of FHV and NoV could be readily visualized (data not shown).
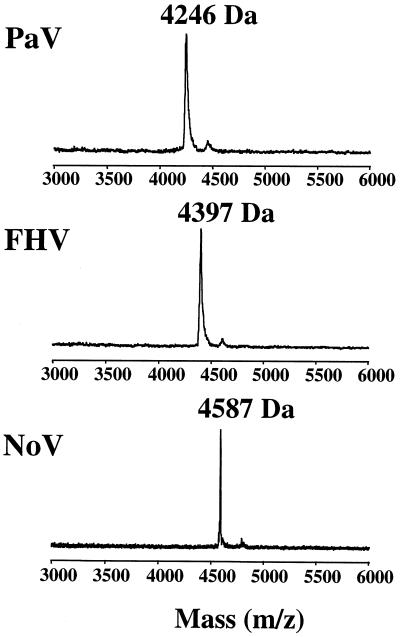
We therefore sought an alternative method to investigate the existence of protein γ in PaV virions. Since the γ protein in FHV virions can be detected using mass spectrometry (9), we applied MALDI-TOF mass spectrometry to intact sucrose gradient-purified PaV virions to see if we could detect protein γ. The spectrum of PaV in Fig. 9 shows a clear peak of Mr 4,246. The 40-amino-acid peptide which would be liberated following cleavage of protein α between Asn 361 and Ser 362 has a calculated Mr of 4,244. Similar analyses of purified FHV and NoV showed γ peptides with Mrs of 4,397 for FHV (calculated, 4,396) and 4,587 for NoV (calculated, 4,584) (Fig. 9), confirming the reliability of this method for the analysis of nodavirus γ peptides in general. These results showed not only that PaV protein α was cleaved, but that it was cleaved between Asn 361 and Ser 362, a novel cleavage site sequence for nodavirus capsid maturation.
FIG. 9.
MALDI-TOF spectrum of protein γ. Whole virus particles were analyzed by MALDI-TOF mass spectrometry. The part of the spectrum which contains protein γ is shown for three nodaviruses: PaV, FHV, and NoV. In each case, the molecular mass of the γ peak is indicated.
DISCUSSION
The results presented above confirmed the placement of PaV in the Alphanodavirus genus of the Nodaviridae family and revealed several interesting features of the viral genome. Comparison of the capsid protein sequence with those of other nodaviruses indicated that PaV is the most distantly related member of the Alphanodavirus genus, which contains the nodaviruses of insects. This is consistent with PaV being the first alphanodavirus to be isolated outside the Australasian region. The sequence divergence extends even as far as the cleavage site of the capsid protein precursor, which is Asn/Ala for all other alphanodaviruses but Asn/Ser for PaV. Cleavage at this site was confirmed using mass spectrometry, which proved to be an easy and reliable method for detecting the small γ protein from intact nodavirus particles. Despite the novel cleavage sequence, the catalytic Asp residue was conserved at position 68 of PaV protein α, suggesting that the cleavage mechanism may also be conserved (47). Analysis of the crystal structure of PaV capsids will provide further insight into this issue.
Full-length cDNA clones of both genomic segments were constructed and used to recreate PaV RNA replication in BHK-21 cells. For this purpose, we developed the new generic T7 transcription plasmid TVT7R(0,0), which readily accommodated cDNAs such that the corresponding T7 transcripts contained no terminal extensions (Fig. 1). This was advantageous because extraneous terminal nucleotides interfere with the replication of other nodaviral RNAs (3, 4, 7). Transcription of the full-length PaV clones in BHK-21 cells led to the replication of RNA1 and RNA2, synthesis of the subgenomic RNA3, and translation of proteins A and α (Fig. 5). As with other nodaviruses (4, 19), RNA1 encodes protein A, the likely catalytic subunit of the viral RdRp. PaV RNA1 transcribed from the cDNA clone replicated autonomously and supported the replication of RNA2 transcripts in trans (Fig. 5), establishing that both cDNA clones were functional for RNA replication. A detailed comparative analysis of the sequences and structures of RNA1 and protein A from six nodavirus species will be presented elsewhere (Johnson et al., unpublished data).
Although RNA-dependent replication of PaV RNAs was observed following transfection of BHK-21 cells with clones of RNA1 and RNA2, the level of replication was significantly lower than that observed with FHV cDNA clones. It remains to be determined whether this is because BHK-21 cells provide a suboptimal environment or whether vaccinia virus coinfection interferes with PaV RNA replication. Another possibility is that the second C residue at the 3′ termini of the synthetic transcripts may interfere with optimal RNA replication. We are currently screening several cell lines in an attempt to identify one which is susceptible to infection with PaV and able to support more robust RNA replication.
Since the 3′ ends of nodavirus RNAs are not polyadenylated (35, 42) or reactive with poly(A) polymerase or RNA ligase (11, 13, 21, 28), determination of their extreme 3′-terminal sequences is not straightforward. However, previous work in this laboratory has shown that head-to-tail homodimers of RNA1 and -2 accumulate to low levels during replication of FHV and NoV RNAs in cell culture (2, 8), and these molecules provide an accessible source of information about the sequences at both termini of the monomeric RNAs. Since PaV also produced homodimers of both RNAs during replication, we determined the sequences across the head-to-tail junctions and combined the results with those generated by 5′ RACE on strands of both polarities. This approach yielded unambiguous sequences for the termini of each RNA molecule and suggested that the replicating RNA population might show microheterogeneity in having one or two 3′-terminal C residues. The origin of this microheterogeneity is unclear at present, as is the function of the RNA dimers themselves, but their presence during replication of three diverse alphanodaviruses suggests that dimeric RNAs may be a general feature of nodaviral RNA replication.
We have described the molecular characterization of PaV, the first Alphanodavirus to be isolated from outside Australasia. In accordance with its geographic origin, PaV's genome sequence reveals it to be the most distantly related of the known alphanodaviruses; as such, it has several features worthy of further investigation. The functional cDNA clones of the two viral genomic segments have served both to confirm the sequences of functional PaV RNAs and to provide us with an experimental system that will be the basis of future research on the biology of this novel nodavirus.
ACKNOWLEDGMENTS
We thank Cesar Albarino for testing whether PaV virion RNAs could be polyadenylated, Sean Whelan for advice on the strategy for constructing vector TVT7R(0,0), UAB Microbiology Department core DNA sequencing facility, and Lori Coward for conducting the MALDI-TOF analyses.
The mass spectrometer was purchased with funds from an NIH Shared Instrumentation Grant (S10 RR11329) and from a Howard Hughes Medical Institute infrastructure support grant to UAB. Its operation was supported in part by an NCI Core Research Support Grant to the Comprehensive Cancer Center (P30 CA13148-27). This work was supported by NIH grant AI18270.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ball L A. Cellular expression of a functional nodavirus RNA replicon from vaccinia virus vectors. J Virol. 1992;66:2335–2345. doi: 10.1128/jvi.66.4.2335-2345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball L A. Replication of the genomic RNA of a positive-strand RNA animal virus from negative-sense transcripts. Proc Natl Acad Sci USA. 1994;91:12443–12447. doi: 10.1073/pnas.91.26.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball L A. Requirements for the self-directed replication of flock house virus RNA 1. J Virol. 1995;69:720–727. doi: 10.1128/jvi.69.2.720-727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball L A, Amann J M, Garrett B K. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. J Virol. 1992;66:2326–2334. doi: 10.1128/jvi.66.4.2326-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball L A, Johnson K L. Nodaviruses of insects. In: Miller L K, Ball L A, editors. The insect viruses. New York, N.Y: Plenum Publishing Corporation; 1998. pp. 225–267. [Google Scholar]
- 7.Ball L A, Li Y. cis-acting requirements for the replication of flock house virus RNA2. J Virol. 1993;67:3544–3551. doi: 10.1128/jvi.67.6.3544-3551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball L A, Wohlrab B, Li Y. Nodavirus RNA replication: mechanism and harnessing to vaccinia virus recombinants. Arch Virol Suppl. 1994;9:407–416. doi: 10.1007/978-3-7091-9326-6_40. [DOI] [PubMed] [Google Scholar]
- 9.Bothner B, Dong X F, Bibbs L, Johnson J E, Siuzdak G. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J Biol Chem. 1998;273:673–676. doi: 10.1074/jbc.273.2.673. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta R, Ghosh A, Dasmahapatra B, Guarino L A, Kaesberg P. Primary and secondary structure of black beetle virus RNA2, the genomic messenger for BBV coat protein precursor. Nucleic Acids Res. 1984;12:7215–7223. doi: 10.1093/nar/12.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta R, Sgro J-Y. Nucleotide sequences of three nodavirus RNA's: the messengers for their coat protein precursors. Nucleic Acids Res. 1989;17:7525–7526. doi: 10.1093/nar/17.18.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasmahapatra B, Dasgupta R, Ghosh A, Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985;182:183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasmahapatra B, Dasgupta R, Saunders K, Selling B, Gallagher T, Kaesberg P. Infectious RNA derived from transcription from cloned cDNA copies of the genomic RNA of an insect virus. Proc Natl Acad Sci USA. 1986;83:63–66. doi: 10.1073/pnas.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delsert C, Morin N, Comps M. Fish nodavirus lytic cycle and semipermissive expression in mammalian and fish cell cultures. J Virol. 1997;71:5673–5677. doi: 10.1128/jvi.71.7.5673-5677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohman M A. RACE: rapid amplification of cDNA ends. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press; 1990. pp. 28–38. [Google Scholar]
- 18.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher T M, Friesen P D, Rueckert R R. Autonomous replication and expression of RNA1 from black beetle virus. J Virol. 1983;46:481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher T M, Rueckert R R. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J Virol. 1988;62:3399–3406. doi: 10.1128/jvi.62.9.3399-3406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarino L A, Ghosh A, Dasmahapatra B, Dasgupta R, Kaesberg P. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology. 1984;139:199–203. doi: 10.1016/0042-6822(84)90342-8. [DOI] [PubMed] [Google Scholar]
- 22.Hiscox J A, Ball L A. Cotranslational disassembly of flock house virus in a cell-free system. J Virol. 1997;71:7974–7977. doi: 10.1128/jvi.71.10.7974-7977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosur M V, Schmidt T, Tucker R C, Johnson J E, Gallagher T M, Selling B H, Rueckert R R. Structure of an insect virus at 3.0 angstrom resolution. Protein Struct Funct Genet. 1987;2:167–176. doi: 10.1002/prot.340020302. [DOI] [PubMed] [Google Scholar]
- 24.Hosur M V, Schmidt T, Tucker R C, Johnson J E, Selling B H, Rueckert R R. Black beetle virus-crystallization and particle symmetry. Virology. 1984;133:119–127. doi: 10.1016/0042-6822(84)90430-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J E, Reddy V. Structural studies of nodaviruses and tetraviruses. In: Miller L K, Ball L A, editors. The insect viruses. New York, N.Y: Plenum Publishing Corporation; 1998. pp. 171–223. [Google Scholar]
- 26.Johnson K L, Ball L A. Replication of flock house virus RNAs from primary transcripts made in cells by RNA polymerase II. J Virol. 1997;71:3323–3327. doi: 10.1128/jvi.71.4.3323-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K N, Christian P D. A molecular taxonomy for cricket paralysis virus including two new isolates from Australian populations of Drosophila (Diptera: Drosophilidae) Arch Virol. 1996;141:1509–1522. doi: 10.1007/BF01718251. [DOI] [PubMed] [Google Scholar]
- 28.Kaesberg P, Dasgupta R, Sgro J-Y, Wery J-P, Selling B H, Hosur M V, Johnson J E. Structural homology among four nodaviruses as deduced by sequencing and X-ray crystallography. J Mol Biol. 1990;214:423–435. doi: 10.1016/0022-2836(90)90191-N. [DOI] [PubMed] [Google Scholar]
- 29.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 30.Krishna N K, Schneemann A. Formation of an RNA heterodimer upon heating of nodavirus particles. J Virol. 1999;73:1699–1703. doi: 10.1128/jvi.73.2.1699-1703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Longworth J F, Carey G P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoptera: Scarabaeidae] J Gen Virol. 1976;33:31–40. doi: 10.1099/0022-1317-33-1-31. [DOI] [PubMed] [Google Scholar]
- 33.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy: sixth report of the International Committee on Taxonomy of Viruses. New York, N.Y: Springer-Verlag; 1995. [Google Scholar]
- 34.Nagai T, Nishizawa T. Sequence of the non-structural protein gene encoded by RNA 1 of striped jack nervous necrosis virus. J Gen Virol. 1999;80:3019–3022. doi: 10.1099/0022-1317-80-11-3019. [DOI] [PubMed] [Google Scholar]
- 35.Newman J F E, Brown F. Absence of poly(A) from the infective RNA of Nodamura virus. J Gen Virol. 1976;30:137–140. doi: 10.1099/0022-1317-30-1-137. [DOI] [PubMed] [Google Scholar]
- 36.Nishizawa T, Furuhashi M, Nagai T, Nakai T, Muroga K. Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl Environ Microbiol. 1997;63:1633–1636. doi: 10.1128/aem.63.4.1633-1636.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishizawa T, Mori K-i, Furuhashi M, Nakai T, Furusawa I, Muroga K. Comparison of the coat protein genes of five fish nodaviruses, the causative agents of viral nervous necrosis in marine fish. J Gen Virol. 1995;76:1563–1569. doi: 10.1099/0022-1317-76-7-1563. [DOI] [PubMed] [Google Scholar]
- 38.Pattnaik A K, Ball L A, LeGrone A W, Wertz G W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992;69:1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- 39.Price B D, Rueckert R R, Ahlquist P. Complete replication of an animal virus and maintenance of expression vectors derived from it in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:9465–9470. doi: 10.1073/pnas.93.18.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27:353–365. [PubMed] [Google Scholar]
- 42.Scotti P D, Dearing S, Mossop D W. Flock house virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae) Arch Virol. 1983;75:181–189. doi: 10.1007/BF01315272. [DOI] [PubMed] [Google Scholar]
- 43.Selling B H, Allison R F, Kaesberg P. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc Natl Acad Sci USA. 1990;87:434–438. doi: 10.1073/pnas.87.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selling B H, Rueckert R R. Plaque assay for black beetle virus. J Virol. 1984;51:251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Regenmortel M H V, Fauquet C M, Bishop D H L, Carstens E B, Estes M K, Lemon S M, McGeoch D J, Maniloff J, Mayo M A, Pringle C R, Wickner R B, editors. Virus taxonomy, classification and nomenclature of viruses. 7th ed. San Diego, Calif: Academic Press; 2000. [Google Scholar]
- 46.Zeddam J L, Rodriguez J L, Ravallec M, Lagnaoui A. A noda-like virus isolated from the sweetpotato pest Spodoptera eridania (Cramer) (Lep.; Noctuidae) J Invert Pathol. 1999;74:267–274. doi: 10.1006/jipa.1999.4881. [DOI] [PubMed] [Google Scholar]
- 47.Zlotnick A, Reddy V S, Dasgupta R, Schneemann A, Ray W J, Jr, Rueckert R R, Johnson J E. Capsid assembly in a family of animal viruses primes an autoproteolytic maturation that depends on a single aspartic acid residue. J Biol Chem. 1994;269:13680–13684. [PubMed] [Google Scholar]