Abstract
Objectives
Patients receiving chiropractic spinal manipulation (CSM) for low back pain (LBP) are less likely to receive any opioid prescription for subsequent pain management. However, the likelihood of specifically being prescribed tramadol, a less potent opioid, has not been explored. We hypothesised that adults receiving CSM for newly diagnosed radicular LBP would be less likely to receive a tramadol prescription over 1-year follow-up, compared with those receiving usual medical care.
Design
Retrospective cohort study.
Setting
US medical records-based dataset including >115 million patients attending academic health centres (TriNetX, Inc), queried 9 November 2023.
Participants
Opioid-naive adults aged 18–50 with a new diagnosis of radicular LBP were included. Patients with serious pathology and tramadol use contraindications were excluded. Variables associated with tramadol prescription were controlled via propensity matching.
Interventions
Patients were divided into two cohorts dependent on treatment received on the index date of radicular LBP diagnosis (CSM or usual medical care).
Primary and secondary outcome measures
Risk ratio (RR) for tramadol prescription (primary); markers of usual medical care utilisation (secondary).
Results
After propensity matching, there were 1171 patients per cohort (mean age 35 years). Tramadol prescription was significantly lower in the CSM cohort compared with the usual medical care cohort, with an RR (95% CI) of 0.32 (0.18 to 0.57; p<0.0001). A cumulative incidence graph demonstrated that the reduced incidence of tramadol prescription in the CSM cohort relative to the usual medical care cohort was maintained throughout 1-year follow-up. Utilisation of NSAIDs, physical therapy evaluation and lumbar imaging was similar between cohorts.
Conclusions
This study found that US adults initially receiving CSM for radicular LBP had a reduced likelihood of receiving a tramadol prescription over 1-year follow-up. These findings should be corroborated by a prospective study to minimise residual confounding.
Keywords: complementary medicine, rehabilitation medicine, pain management
Strengths and limitations of this study.
We adhered to an a priori protocol which was developed and registered by a multidisciplinary team, making minimal modifications to minimise bias.
This study used extensive selection criteria to make cohorts more comparable, including only adults with new, non-serious radicular low back pain, who had not received an opioid prescription in the previous year.
While we made cohorts similar via propensity score matching, markers of socioeconomic status, pain severity or low back-related disability were poorly represented or unavailable in the dataset.
Although this study was drawn from a large national US dataset, it may only be generalisable to patients attending academic health centres rather than private practice settings.
The retrospective cohort design may be subject to residual confounding and should be corroborated by a prospective study.
Background
Tramadol is an atypical, synthetic opioid with comparatively lower potency that has been increasingly prescribed in the USA for low back pain (LBP).1 2 Chiropractors are healthcare clinicians who often treat LBP using spinal manipulation (ie, chiropractic spinal manipulation (CSM)), a manual therapy directed to the joints of the spine.3 4 While previous studies have found that individuals with LBP receiving CSM are less likely to be prescribed any opioid,5–9 no study has specifically focused on the likelihood of tramadol prescription.
Tramadol functions via dual mechanisms, whereby it both stimulates opioid receptors and inhibits norepinephrine and serotonin reuptake.10 11 The strength of tramadol as measured by morphine milligram equivalents (MMEs) is comparatively low at 0.2 MME, as compared with other prescription opioids, such as morphine and hydrocodone, which are both 1.0 MME.11 In addition, tramadol is short-acting, with a half-life of approximately 6 hours.10 According to the US Food and Drug Administration, tramadol is a schedule IV drug, whereas other stronger potency prescription opioids (eg, morphine, hydrocodone) are schedule II drugs and thus more stringently regulated.12 In the setting of increased overuse, misuse and abuse of opioids in the USA, tramadol may be prescribed in place of stronger potency opioids as it is often perceived as a safer alternative,13–15 with fewer and less severe associated adverse events such as constipation, pruritus and neuropsychological symptoms.16 17 However, several major clinical practice guidelines for LBP do not provide recommendations for or against tramadol prescription.18–21
Globally, tramadol is the most consumed opioid in terms of MME per 1000 inhabitants per day.22 According to one study based on a large, nationally representative US sample (n=50 201), overall opioid prescriptions slightly declined from 2005 to 2018; however, prescription of tramadol increased during this time period.13 In another survey of US military members (n=809), 77% of whom had LBP, 35% reported being prescribed tramadol over the preceding 3 months.1 In addition, tramadol is often prescribed for individuals with radicular LBP,23 24 which is characterised by radiating pain into the lower extremity, with or without corresponding neurological deficits (ie, weakness, sensory loss).
While respiratory depression, constipation and safety events requiring hospitalisation are less prevalent with tramadol compared with other prescription opioids,10 16 tramadol still carries the risk of chronic, persistent opioid use, and may trigger deleterious sequelae such as neurobehavioural disorders.25 One retrospective cohort study of cancer-free, opioid-naive individuals in the USA undergoing elective surgery (n=444 764) found that those receiving tramadol had a 6% increased risk of additional opioid prescription over the following 180 days compared with those receiving other short-acting opioids.12 However, it remains unclear whether tramadol prescribed for LBP increases the risk of long-term opioid use.24
As portal-of-entry clinicians, chiropractors frequently care for patients with LBP, often using CSM.3 4 8 26 CSM has been found to be effective for radicular LBP27 and is recommended by numerous clinical practice guidelines for treatment of this condition.20 28 29 While chiropractors do not prescribe opioids, several studies have found that individuals receiving CSM for LBP are less likely receive any opioid prescription.5 6 8 9 However, to our understanding, these studies did not differentiate between individual types of opioid prescriptions. Therefore, it is unclear if previous studies’ findings reflected a decrease in tramadol versus non-tramadol prescription opioids.
Prior studies have proposed several potential mechanisms for the association between initial CSM and reduced opioid prescribing. First, spinal manipulation may reduce pain and increase back-related function, thereby reducing the need for opioids.5 Second, as chiropractors do not prescribe medications, their patients would not receive an opioid prescription at an initial visit and could only receive a prescription if subsequently visiting a medical clinician.5 6 These mechanisms may apply broadly to other types of non-pharmacological care, such as physical therapy,5 8 9 examination of which is beyond the scope of the present study.
Objectives
Considering the recent increase in tramadol prescribing for LBP, the current study aimed to examine the association between receipt of CSM and tramadol prescription among adults with a new diagnosis of radicular LBP. We hypothesised that adults receiving CSM on the index date of radicular LBP diagnosis would have a reduced likelihood of tramadol prescription relative to those receiving non-chiropractic usual medical care over 1-year follow-up.
Materials and methods
Study design
We used a retrospective cohort design with new-user, active-comparator features to reduce bias,30 31 and registered our protocol a priori in the Open Science Framework.32 Study reporting follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.33 This study was deemed Not Human Subjects Research by the University Hospitals Institutional Review Board (Cleveland, Ohio, USA, STUDY20230159).
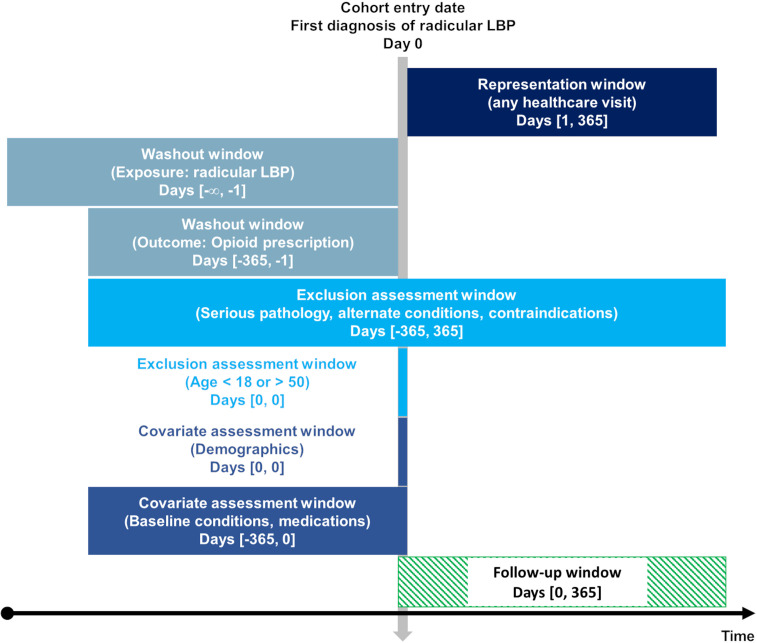
Considering that the prescribing of tramadol has increased over the past decade,13 34 we only used data from 1 January 2017 to the query date (9 November 2023). Patients were included up to 1 year prior to the query date (9 November 2022), to allow for sufficient follow-up time to capture the outcome. To limit loss to follow-up, patients were required to have an additional healthcare visit any time from 1 day to 1 year after radicular LBP diagnosis (figure 1). We made two alterations to our a priori protocol wherein we (1) controlled for receipt of any prescription medication over the previous year via propensity matching, as a strategy to reduce any potential selection bias related to patients’ preference towards receiving pharmacological versus non-pharmacological care,5 35–37 and serve as an improvement on an E-value sensitivity analysis,38 and (2) added a cumulative incidence graph as a sensitivity analysis to provide greater insights into the timing of tramadol prescription between cohorts.
Figure 1.
Study design. The vertical arrow represents the index date of diagnosis of radicular LBP. Assessment windows to the left of this arrow represent time windows occurring before the index date over a period of days (#,#). ‘∞’ indicates that the time window extends as far retrospectively as data permit per patient. The follow-up window occurring after the index date is shown by a green striped rectangle. Image by Robert Trager using a Creative Commons template from Schneeweiss et al. 77 LBP, low back pain.
Setting and data source
The present study derived data from a US research database (TriNetX, Inc) which includes aggregated deidentified medical records from over 115 million patients seeking care across 80 academic healthcare institutions.39 The TriNetX platform uses several strategies to safeguard patient information, such as maintaining anonymity of the specific participating healthcare institutions and geographic locations. This database may be searched using standardised nomenclature, such as the International Classification of Diseases, 10th Edition (ICD-10) diagnosis codes. The TriNetX data are routinely examined for completeness,39 40 and one validation study reported that its data for medications were at least 87% complete.41 Although specific healthcare sites cannot be revealed, chiropractors employed in the types of academic, integrated settings included in this network typically have at least 21 years of clinical experience.42 Only 5% of chiropractors are employed within large integrative health settings such as those included in the TriNetX dataset.4
Participants
Eligibility criteria
Inclusions
We included adults aged 18–50 on the index date of a diagnosis of radicular LBP, which was defined as the first occurrence of this diagnosis in the dataset. Rather than all types of LBP, radicular LBP was chosen for analysis to create homogeneity between the CSM and usual medical care cohorts, considering that tramadol could be more often prescribed in this subset of LBP.23 24 In addition, radicular LBP is diagnosed in the presence of specific signs and symptoms (ie, radiating, dermatomal pain, paresthesia and/or segmental neurological deficits).43 Thus, specifically analysing patients with radicular LBP helped ensure that both cohorts had a similar degree of LBP complexity.
Young to middle-aged adults were included, given that radicular LBP is typically the result of a lumbar disc herniation in patients in this age range.44 45 In contrast, older adults with radicular LBP may have lumbar stenosis, an often chronic disorder with different pathophysiology and potentially different management.46 Narrowing the study population by age and diagnosis aimed to create a more predictable case definition for radicular LBP and thus allow for greater comparability between cohorts.
Patients with radicular LBP were included by requiring the presence of at least one of several diagnosis codes describing lumbar or sacral radiculopathy or sciatica (online supplemental table 1).47 We did not include diagnoses describing disc degeneration or displacement, which may cause localised LBP without radicular symptoms.48
bmjopen-2023-078105supp001.pdf (120.3KB, pdf)
We divided patients into two cohorts depending on whether they did or did not receive CSM on the index date of radicular LBP diagnosis. Those receiving any of the Current Procedural Terminology (CPT) codes specifying CSM (online supplemental table 2), which are used almost entirely by chiropractors in the USA,49 were included in the CSM cohort. Those not receiving CSM on the index date of diagnosis formed the usual medical care cohort. For the purposes of this study, usual medical care was considered any of a range of medical services, such as medications, interventional procedures, surgery, healthcare encounters, physical therapy, or exercise, barring CSM.
Exclusions
We excluded individuals prescribed opioids at baseline, thus only including opioid-naive patients as part of our new-user design. We excluded patients with any opioid prescription within the preceding year, a duration at the high end of previous studies50–52 (online supplemental table 3). We also excluded several conditions over a 1-year window preceding and following the index date of radicular LBP diagnosis to make cohorts more comparable: serious pathology (ie, cancer, infection, fracture and cauda equina syndrome), fibromyalgia, lumbar surgery, lumbosacral plexopathy, multiple sclerosis, myelopathy, scoliosis and spondylolisthesis; and those with contraindications to tramadol prescription (ie, monoamine oxidase inhibitor prescription,53 opioid sensitivity10 and pregnant women).10
Variables
Tramadol
We examined tramadol prescription by identifying any occurrence of its RxNorm code (10689) over a 1-year follow-up after the index date of radicular LBP diagnosis. As radicular LBP typically improves over the course of 3 months to a year, we considered a 1-year follow-up window to be clinically relevant.54 55
We chose to focus our study on tramadol prescription rather than other opioids for several reasons. First, tramadol is commonly prescribed for radicular LBP.1 23 24 Second, tramadol has a unique pharmacological profile, including the inhibition of norepinephrine and serotonin reuptake. In contrast to other opioids, it is contraindicated in individuals who are also prescribed a monoamine oxidase inhibitor,53 thus requiring a customised study design. Third, our exclusion of patients with serious pathology would likely lead to underestimates of the prescription rates for more potent opioids (eg, morphine and fentanyl), which are often prescribed for cancer-related pain.56
Potential confounders
To reduce selection bias, we used propensity-score matching to balance confounding variables between cohorts associated with tramadol prescription.30 Variables present within 1 year preceding and including the index date of radicular LBP diagnosis were eligible for matching. Confounders with a negative or positive association with tramadol prescription were selected. To help account for any potential patient preference to avoid prescription medications in the CSM cohort,35 we controlled for receipt of any prescriptions in the year preceding the index date (online supplemental table 4):
Antidepressants (negative).57
Asthma (positive)58
Demographics: age, sex, race, ethnicity (positive or negative)58 59
Medication prescription in the preceding year (positive)35
Gastrointestinal disorders (negative)58
Radiographs (positive).60
Social determinants of health: problems related to education59 and economic circumstances (positive)60
Study size
We calculated a total required sample size of 396 using G*Power (V.3.1.9.7, University of Kiel, DE). We used a z-test for determining the difference between two proportions, using data from a recent study regarding opioid prescription and CSM versus medical care (ie, 0.35 vs 0.19),61 two tails, alpha error of 0.05 and power of 0.95. Comparison with cohort sizes from our previous work examining radicular LBP and CSM using the same database suggested that we would have a sufficient sample.62
Statistical methods
We compared key features of both cohorts using a Pearson χ2 or independent-samples t test. Propensity-score matching was conducted in real-time within the TriNetX dataset viewing platform using a greedy nearest-neighbour algorithm with one-to-one matching and a calliper width of 0.1 pooled SD, using a standardised mean difference (SMD) of <0.1 as a marker of successful covariate balance.63 The risk ratio (RR) for tramadol prescription was derived by dividing the risk (incidence proportion) in the CSM cohort by the risk in the usual medical care cohort. There were no imputations for missing data. To provide better insight regarding the timing of prescription over follow-up, we conducted a post-hoc sensitivity analysis to graph the cumulative incidence of tramadol prescription per cohort with 95% CIs.
To examine the proportion and RR of various treatments during follow-up we added secondary outcomes of CSM in the usual medical care cohort (CPT: 98940, 98941, 98942), and markers of usual medical care in both cohorts including physical therapy evaluations (CPT: 1029677), non-steroidal anti-inflammatory drugs (NSAIDs (Veterans Health Administration National Drug File: CN104 and MS102)), and a composite outcome of lumbar spine imaging including lumbosacral radiography (CPT: 1010381), lumbar computed tomography (CPT: 1010395) and lumbar magnetic resonance imaging (CPT: 1010405 and 1010408).64 We deemed RR point estimates less than 0.73 and greater than 1.38 to represent meaningful between-cohort difference.65
Patient and public involvement
No patient or public involvement.
Results
Participants
Our query identified eligible patients from several healthcare organisations (CSM: 9; usual medical care: 73). The total CSM population within TriNetX at the time of the query was 141 532. Only 2467 patients met the base CSM population definition, receiving CSM on the first date of a diagnosis of radicular LBP. This cohort size was further reduced by 20% when applying age criteria, by 25% after exclusions for diagnoses and contraindications, by 19% after excluding those with prior opioid prescriptions and reduced by 3% when restricting to the desired time window.
Before propensity-score matching there were 1171 patients in the CSM cohort and 258 041 patients in the usual medical care cohort (table 1; available data for baseline characteristics).66 After matching, there were 1171 patients in both cohorts (mean age: 35 years, SD=8) as this process discarded usual care patients who did not match a CSM patient. Before matching, patients in the CSM cohort were younger, less often identified as Asian, Hispanic or Latino, or black or African American, and less often were prescribed any medication, yet more often prescribed a selective serotonin reuptake inhibitor or serotonin and norepinephrine reuptake inhibitor antidepressant over the preceding year (SMD >0.1 for each). After propensity matching there were no meaningful between-cohort differences for any matched variable (SMD <0.1 for each).
Table 1.
Baseline characteristics before and after propensity-score matching
| Variable (n (%) or mean (SD)) | Before matching | After matching | ||||
| CSM | Usual medical care | SMD | CSM | Usual medical care | SMD | |
| N | 1171 | 258 253 | 1171 | 1171 | ||
| Age at Index | 34.9 (8.0) | 35.7 (7.6) | 0.105 | 34.9 (8.0) | 34.9 (7.9) | 0.009 |
| Sex | ||||||
| Female | 649 (55%) | 140 224 (54%) | 0.023 | 649 (55%) | 647 (55%) | 0.003 |
| Male | 521 (44%) | 108 660 (42%) | 0.049 | 521 (44%) | 523 (45%) | 0.003 |
| Race | ||||||
| American Indian or Alaska native | 10 (1%) | 1113 (%) | 0.053 | 10 (1%) | 10 (1%) | 0.000 |
| Asian | 16 (1%) | 8235 (3%) | 0.122 | 16 (1%) | 17 (1%) | 0.007 |
| Black or African American | 81 (7%) | 38 684 (15%) | 0.260 | 81 (7%) | 83 (7%) | 0.007 |
| Native Hawaiian or Other Pacific Islander | 10 (1%) | 1291 (1%) | 0.043 | 10 (1%) | 10 (1%) | 0.000 |
| White | 826 (71%) | 151 784 (59%) | 0.248 | 826 (71%) | 827 (71%) | 0.002 |
| Other Race | 16 (1%) | 16 338 (6%) | 0.260 | 16 (1%) | 14 (1%) | 0.015 |
| Ethnicity | ||||||
| Hispanic or Latino | 37 (3%) | 27 706 (11%) | 0.301 | 37 (3%) | 34 (3%) | 0.015 |
| Not Hispanic or Latino | 960 (82%) | 159 603 (62%) | 0.461 | 960 (82%) | 961 (82%) | 0.002 |
| Diagnoses | ||||||
| Asthma | 74 (6%) | 17 355 (7%) | 0.016 | 74 (6%) | 73 (6%) | 0.004 |
| Diseases of the digestive system | 183 (16%) | 45 521 (18%) | 0.054 | 183 (16%) | 185 (16%) | 0.005 |
| Mood (affective) disorders | 143 (12%) | 31 278 (12%) | 0.003 | 143 (12%) | 136 (12%) | 0.018 |
| Problems related to education and literacy | 0 (0%) | 94 (%) | 0.027 | 0 (0%) | 0 (0%) | 0.000 |
| Problems related to housing and economic circumstances | 0 (0%) | 784 (%) | 0.078 | 0 (0%) | 0 (0%) | 0.000 |
| Medications and procedures | ||||||
| Medications (VANDF: any) | 831 (71%) | 206 281 (80%) | 0.208 | 831 (71%) | 827 (71%) | 0.008 |
| Antidepressants (SSRIs/SNRIs) | 238 (20%) | 37 293 (14%) | 0.156 | 238 (20%) | 231 (20%) | 0.015 |
| Monoamine oxidase inhibitor antidepressants | 0 (0%) | 0 (0%) | 0.000 | 0 (0%) | 0 (0%) | 0.000 |
| Radiological examination, spine, lumbosacral | 226 (19%) | 58 083 (22%) | 0.079 | 226 (19%) | 212 (18%) | 0.031 |
CSM, chiropractic spinal manipulation; SD, standard deviation; SMD, standardised mean difference; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; VANDF, Veterans Health Administration National Drug File.
Descriptive data
The mean number of data points per patient per cohort was sufficient (CSM: 1287; usual medical care: 850). After propensity matching, the frequency of unknown demographic variables was similar for both cohorts: unknown ethnicity (15%, SMD=0.005), unknown sex (<1%, SMD=0) and unknown age (0%, SMD=0). A graph of the propensity score density showed that the cohorts were well-balanced following matching (online supplemental figure 1; available data for figure67). These findings suggested that there were negligible between-cohort differences with regards to missing data, data density, and covariate balance.
Key results
The proportion of patients who received a tramadol prescription over 1 year following the index event of radicular LBP diagnosis was lower in the CSM cohort compared with the usual care cohort (table 2). After propensity matching, 1.3% of the CSM cohort had received a tramadol prescription, compared with 4.0% of the usual medical care cohort, yielding an RR (95% CI) of 0.32 (0.18 to 0.57; p<0.0001) for our primary outcome.
Table 2.
Key results before and after propensity-score matching
| Before matching | After matching* | |||
| CSM n=1171 |
Usual medical care n=2 59 581 |
CSM n=1171 |
Usual medical care n=1171 |
|
| Tramadol n (%) | 15 (1.3%) | 10 805 (4.2%) | 15 (1.3%) | 47 (4.0%) |
| RR (95% CI) | 0.31 (0.19 to 0.51; p<0.0001) | (reference) | 0.32 (0.18 to 0.57; p<0.0001) | (reference) |
95% CI, number (n) and percentage (%) of patients receiving a tramadol prescription.
*Indicates our primary outcome.
CSM, chiropractic spinal manipulation; RR, risk ratio.
Sensitivity analysis
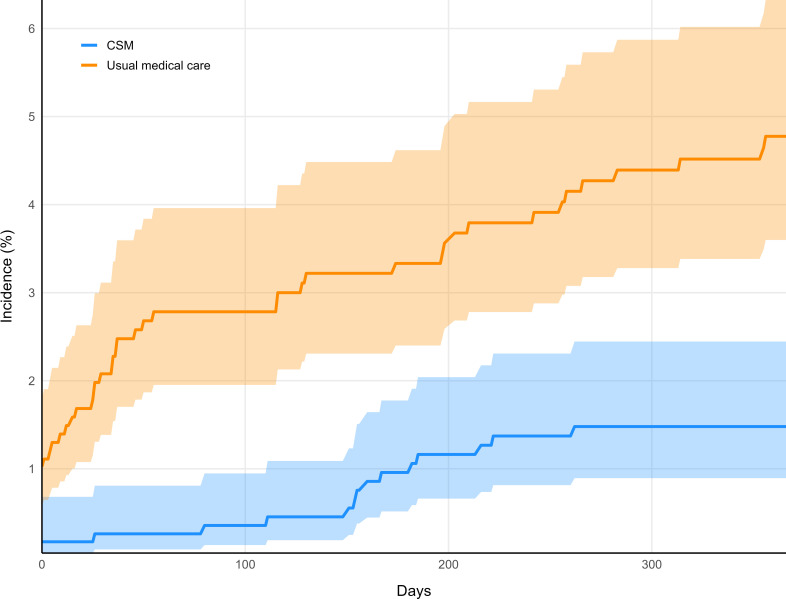
A cumulative incidence graph suggested that the incidence of tramadol prescription in the usual medical care cohort increased relative to the CSM cohort early during follow-up (figure 2; available data for cumulative incidence).68 The cumulative incidence curves did not converge during or at the end of the 1-year follow-up window, suggesting that a significant difference in prescription incidence was maintained.
Figure 2.
Cumulative incidence graph. Incidence curves of tramadol prescription in the chiropractic spinal manipulation cohort (CSM; blue) and usual medical care cohort (orange) are shown over the 1-year follow-up window (365 days). Shaded regions indicate 95% CIs.
Secondary outcomes
After matching, compared with the CSM cohort, patients in the usual medical care cohort seldom received CSM during follow-up (≤10 patients, <1%), yielding an RR of 105.60 (95% CI: 56.95 to 195.80). Markers of usual medical care were similar between cohorts during follow-up, including NSAIDs (CSM: 24%; usual medical care: 27%; RR=0.90 (95% CI: 0.78 to 1.03); p=0.1277), lumbar spine imaging (CSM: 14%; usual medical care: 18%; RR=0.75 (95% CI: 0.62 to 0.90); p=0.0020) and physical therapy evaluation (CSM: 9%; usual medical care: 8%; RR=1.03 (95% CI: 0.79 to 1.35); p=0.8233).
Discussion
To our knowledge, this retrospective cohort study was the first to examine the association between CSM and tramadol prescription, and used a propensity-matched sample of opioid-naive US adults. These real-world findings support our hypothesis that adults initially receiving CSM for a new diagnosis of radicular LBP have a reduced likelihood of receiving a tramadol prescription over 1-year follow-up.
Analysis of care utilisation markers suggested that patients in the usual medical care cohort seldom received CSM following the index date. In addition, both cohorts demonstrated similar utilisation of usual medical care (ie, NSAIDs, physical therapy and lumbar imaging).64 Therefore, the cohorts were distinct with respect to CSM use, but similar with respect to usual medical care services. These findings further strengthen our results by highlighting that tramadol prescribing differed while other markers of usual medical care remained similar between cohorts.
We suggest that receiving initial CSM for radicular LBP could reduce the likelihood of tramadol prescription either via direct pain relief mechanisms or due to entry into a non-pharmacological care pathway. First, the known efficacy of CSM in alleviating radicular LBP27 could provide patients with substantial pain relief, therefore obviating the need for pharmacological treatment. Second, radicular LBP has a favourable natural history;54 55 thus, if patients first visited a non-pharmacological clinician for CSM, they may not have presented to a clinician with a scope of practice capable of prescribing tramadol when most symptomatic.
In the present study, we found a relatively low incidence of tramadol prescription in both cohorts compared with previous studies (eg, <5% for both cohorts compared with >15% in prior studies).1 23 One study reported that 35% of US military members, mostly with LBP, received a tramadol prescription over a span of 3 months.1 In another study conducted in Switzerland, 16% of patients with LBP received a tramadol prescription over a 6-month follow-up.23 The comparatively lower incidence of tramadol prescription in our study is likely explained by our methods of (1) excluding patients who received an opioid prescription over the preceding year, (2) excluding patients with serious pathology who would be more likely to receive an opioid prescription and (3) including patients with a new radicular LBP diagnosis, which helped exclude patients more likely to receive pharmacologic pain management for chronic LBP.
Although our CSM cohort had a small absolute reduction in tramadol prescription (~3%), we cannot rule out a clinically important effect of this care pathway. Considering that tramadol users may develop addiction, physical dependence or long-term use,10 12 a longer-duration follow-up study that expressly investigates these outcomes is warranted. In addition, some evidence suggests that adults with LBP receiving CSM have a significantly reduced odds of adverse drug events.69 As tramadol is also associated with adverse sequelae such as seizures and serotonin syndrome,25 it would be beneficial to investigate these potential adverse events in a similar follow-up study. Such a study would require a larger sample size and consider tramadol dosage and other medications concurrently prescribed, features which were not feasible in the present study.
Our findings are similar to previous studies which found that patients receiving CSM for LBP were less likely to receive an opioid, benzodiazepine or gabapentin prescription.6 9 38 62 These medications are similarly not endorsed by clinical practice guidelines for treating LBP.18–21 Accordingly, our study adds to existing evidence that patients initiating care for LBP with CSM are more likely to enter a guideline-concordant pathway with respect to medication prescribing.6 9 62 Furthermore, our findings corroborate previous authors’ suggestions that chiropractors, along with other non-pharmacological care clinicians (eg, physical therapists) may serve as an initial point of contact for LBP.5 8 70 71
As a retrospective, real-world study, our findings should be corroborated or extended with future research. A prospective observational study or pragmatic trial would minimise sources of confounding and enable measurement of several key medications used for the management of LBP in tandem, such as types of opioids, gabapentinoids, benzodiazepines and non-steroidal anti-inflammatory medications.72 Further, other measures of pain severity, disability, adverse events and health-related quality-of-life could be examined alongside these pharmacological outcomes. Future studies could also focus on a different population (eg, older adults, paediatric patients, chronic LBP or private practice healthcare environments).
Further research is also needed to determine whether there is a broad impact of non-pharmacological care on tramadol prescribing for radicular LBP. Ideally this would include several cohorts representing pharmacological clinicians, such as primary care physicians, orthopaedists and physical medicine and rehabilitation specialists, as well as non-pharmacological clinicians, such as chiropractors, physical therapists and acupuncturists. Given the differences in direct access among non-pharmacological providers,26 73 the study would be modified from our current design to implement a flexible inclusion window to account for a potential lag between the date of diagnosis and date of initiating care. Such an analysis is beyond the scope and capacity of our present study and methods.
Limitations
The main limitation of this study is the potential for unmeasured confounders to influence results. Certain variables that may influence the likelihood of tramadol prescription are unavailable or poorly represented in the TriNetX dataset, including pain severity,58 60 income,60 insurance type,60 geographic location,60 type of medical clinician seen,74 75 education level,59 marital status,59 patients’ requests for medication (ie, pressure to prescribe), or conversely, reluctance to consider taking a prescription opioid.36 37 76 Although the TriNetX database contains medical record data, we did not have access to detailed patient charts to directly validate our results due to the deidentified, aggregated nature of the dataset sourced from multiple healthcare organisations. As such, we may have misclassified patients according to having a new diagnosis of radicular LBP. Variables included in propensity matching could also be incorrect or missing for each patient. While tramadol prescriptions were temporally associated with the index radicular LBP diagnosis, it is possible these may have been prescribed for another condition or procedure. This possibility was minimised by accounting for common indications for tramadol via exclusion (eg, serious pathology)18 and propensity matching (eg, fibromyalgia)14. We were unable to access data regarding MMEs, which limited our insight into possible tramadol dosing variability between cohorts during follow-up. The results of this study may only be generalisable to patients receiving care and chiropractors delivering CSM at large academic healthcare organisations in the USA. Other regions may have different drug scheduling status for tramadol, prescription guidelines, or management strategies for LBP.
Conclusion
This propensity-matched retrospective cohort study found that US adults initially receiving CSM for a new index diagnosis of radicular LBP had a reduced likelihood of receiving a tramadol prescription over 1-year follow-up compared with those receiving usual medical care. Our findings may not be broadly generalisable given they derive from academic healthcare settings in the US and should be corroborated by a prospective study to minimise residual confounding.
Supplementary Material
Acknowledgments
This publication was made possible through the support of the Clinical Research Center of University Hospitals Cleveland Medical Center (UHCMC) and the Case Western Reserve University Clinical and Translational Science Collaborative (CTSC) 4UL1TR000439. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of UHCMC or National Institutes of Health.
Footnotes
Contributors: RJT, ZAC, RS, RMC, JAP and JAD conceived of and designed the study protocol and methods. RJT, RMC and JAP directly accessed the study software. RJT, ZAC and JAD formally analysed and interpreted data. JAD provided mentorship and supervision. RJT drafted the initial manuscript, while all authors contributed to, critically revised and approved of the final manuscript. RJT was the study guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The views expressed are those of the authors and do not necessarily reflect the official policy or position of the US Department of Veterans Affairs or the US Government.
Competing interests: Dr Trager reports he has received book royalties as the author of two texts on the topic of sciatica. The other authors report no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Minimal, de-identified, aggregated datasets that support the findings of this study are openly available in Figshare at: https://doi.org/10.6084/m9.figshare.24539908 (baseline characteristics), https://doi.org/10.6084/m9.figshare.24539917 (cumulative incidence data) and https://doi.org/10.6084/m9.figshare.24539920 (propensity score density data).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was declared Not Human Subjects Research by the University Hospitals Institutional Review Board (Cleveland, OH, USA, STUDY20221445).
References
- 1. Coyne KS, Barsdorf AI, Currie BM, et al. Insight into chronic pain in the United States: descriptive results from the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ) validation study. Curr Med Res Opin 2021;37:483–92. 10.1080/03007995.2020.1865889 [DOI] [PubMed] [Google Scholar]
- 2. Ly DP. Evaluation and treatment patterns of new low back pain episodes for elderly adults in the United States, 2011–2014. Med Care 2020;58:108–13. 10.1097/MLR.0000000000001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beliveau PJH, Wong JJ, Sutton DA, et al. The chiropractic profession: a scoping review of utilization rates, reasons for seeking care, patient profiles, and care provided. Chiropr Man Therap 2017;25:35. 10.1186/s12998-017-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Himelfarb I, Hyland J, Ouzts N, et al. National Board of Chiropractic Examiners: Practice Analysis of Chiropractic 2020. Greeley, CO: NBCE, 2020. Available: https://www.nbce.org/practice-analysis-of-chiropractic-2020/ [Google Scholar]
- 5. Kazis LE, Ameli O, Rothendler J, et al. Observational retrospective study of the association of initial healthcare provider for new-onset low back pain with early and long-term opioid use. BMJ Open 2019;9:e028633. 10.1136/bmjopen-2018-028633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corcoran KL, Bastian LA, Gunderson CG, et al. Association between Chiropractic use and opioid receipt among patients with spinal pain: a systematic review and meta-analysis. Pain Med 2020;21:e139–45. 10.1093/pm/pnz219 [DOI] [PubMed] [Google Scholar]
- 7. Whedon JM, Toler AWJ, Kazal LA, et al. Impact of chiropractic care on use of prescription opioids in patients with spinal pain. Pain Med 2020;21:3567–73. 10.1093/pm/pnaa014 [DOI] [PubMed] [Google Scholar]
- 8. Bise CG, Schneider M, Freburger J, et al. First provider seen for an acute episode of low back pain influences subsequent health care utilization. Phys Ther 2023;103:pzad067. 10.1093/ptj/pzad067 [DOI] [PubMed] [Google Scholar]
- 9. Harwood KJ, Pines JM, Andrilla CHA, et al. Where to start? A two stage residual inclusion approach to estimating influence of the initial provider on health care utilization and costs for low back pain in the US. BMC Health Serv Res 2022;22:694. 10.1186/s12913-022-08092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subedi M, Bajaj S, Kumar MS, et al. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother 2019;111:443–51. 10.1016/j.biopha.2018.12.085 [DOI] [PubMed] [Google Scholar]
- 11. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep 2022;71:1–95. 10.15585/mmwr.rr7103a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thiels CA, Habermann EB, Hooten WM, et al. Chronic use of tramadol after acute pain episode: cohort study. BMJ 2019;365:l1849. 10.1136/bmj.l1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Wu D, Chan A, et al. Temporal trend of opioid and nonopioid pain medications: results from a national in-home survey, 2001 to 2018. PAIN Rep 2022;7:e1010. 10.1097/PR9.0000000000001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep 2019;23:76. 10.1007/s11916-019-0777-x [DOI] [PubMed] [Google Scholar]
- 15. Mullins PM, Mazer-Amirshahi M, Pourmand A, et al. Tramadol use in United States emergency departments 2007–2018. J Emerg Med 2022;62:668–74. 10.1016/j.jemermed.2022.01.004 [DOI] [PubMed] [Google Scholar]
- 16. Musich S, Wang SS, Schaeffer JA, et al. Safety events associated with tramadol use among older adults with osteoarthritis. Popul Health Manag 2021;24:122–32. 10.1089/pop.2019.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grond S, Radbruch L, Meuser T, et al. High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage 1999;18:174–9. 10.1016/s0885-3924(99)00060-3 [DOI] [PubMed] [Google Scholar]
- 18. Price MR, Cupler ZA, Hawk C, et al. Systematic review of guideline-recommended medications prescribed for treatment of low back pain. Chiropr Man Therap 2022;30:26. 10.1186/s12998-022-00435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med 2017;166:514–30. 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 20. Kreiner DS, Matz P, Bono CM, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J 2020;20:998–1024. 10.1016/j.spinee.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 21. Chou R, Côté P, Randhawa K, et al. The global spine care initiative: applying evidence-based guidelines on the non-invasive management of back and neck pain to low-and middle-income communities. Eur Spine J 2018;27:851–60. 10.1007/s00586-017-5433-8 [DOI] [PubMed] [Google Scholar]
- 22. Jayawardana S, Forman R, Johnston-Webber C, et al. Global consumption of prescription opioid analgesics between 2009-2019: a country-level observational study. EClin Med 2021;42:101198. 10.1016/j.eclinm.2021.101198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Gangi S, Pichierri G, Zechmann S, et al. Prescribing patterns of pain medications in unspecific low back pain in primary care: A retrospective analysis. J Clin Med 2021;10:1366. 10.3390/jcm10071366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manniche C, Stokholm L, Ravn S, et al. The prevalence of long-term opioid therapy in spine center outpatients following initiation of tramadol: the Spinal Pain Opioid Cohort (SPOC). Chron Pain Manag 2022;6:145. [Google Scholar]
- 25. Raj K, Chawla P, Singh S. Neurobehavioral consequences associated with long term tramadol utilization and pathological mechanisms. CNS Neurol Disord Drug Targets 2019;18:758–68. 10.2174/1871527318666191112124435 [DOI] [PubMed] [Google Scholar]
- 26. Chang M. The chiropractic scope of practice in the United States: a cross-sectional survey. J Manipulative Physiol Ther 2014;37:363–76. 10.1016/j.jmpt.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 27. Lewis RA, Williams NH, Sutton AJ, et al. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. Spine J 2015;15:1461–77. 10.1016/j.spinee.2013.08.049 [DOI] [PubMed] [Google Scholar]
- 28. Van Wambeke P, Desomer A, Ailiet L, et al. Low back pain and Radicular pain: assessment and management. good clinical practice (GCP) Brussels: Belgian health care knowledge centre (KCE). 2017. Available: https://kce.fgov.be/sites/default/files/2021-11/KCE_287_Low_back_pain_Report_2.pdf
- 29. Bernstein IA, Malik Q, Carville S, et al. Low back pain and sciatica: summary of NICE guidance. BMJ 2017;356:i6748. 10.1136/bmj.i6748 [DOI] [PubMed] [Google Scholar]
- 30. Gokhale M, Stürmer T, Buse JB. Real-world evidence: the devil is in the detail. Diabetologia 2020;63:1694–705. 10.1007/s00125-020-05217-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franklin JM, Schneeweiss S. When and how can real world data analyses substitute for randomized controlled trials Clin Pharmacol Ther 2017;102:924–33. 10.1002/cpt.857 [DOI] [PubMed] [Google Scholar]
- 32. Trager RJ, Cupler ZA, Srinivasan R, et al. Association between chiropractic spinal manipulation and tramadol prescription in adults with radicular low back pain: retrospective cohort study using United States data. Med and Health Sci 2023. 10.17605/OSF.IO/C2K47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 34. Seago S, Hayek A, Pruszynski J, et al. Change in prescription habits after Federal rescheduling of Hydrocodone combination products. Proc (Bayl Univ Med Cent) 2016;29:268–70. 10.1080/08998280.2016.11929431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma R, Haas M, Stano M. Patient attitudes, insurance, and other determinants of self-referral to medical and chiropractic physicians. Am J Public Health 2003;93:2111–7. 10.2105/ajph.93.12.2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emary PC, Brown AL, Oremus M, et al. The association between chiropractic integration in an Ontario community health centre and continued prescription opioid use for chronic non-cancer spinal pain: a sequential explanatory mixed methods study. BMC Health Serv Res 2022;22:1313. 10.1186/s12913-022-08632-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Emary PC, Brown AL, Oremus M, et al. Association of chiropractic care with receiving an opioid prescription for noncancer spinal pain within a Canadian community health center: a mixed methods analysis. J Manipulative Physiol Ther 2022;45:235–47. 10.1016/j.jmpt.2022.06.009 [DOI] [PubMed] [Google Scholar]
- 38. Trager RJ, Cupler ZA, Srinivasan R, et al. Association between chiropractic spinal manipulation and gabapentin prescription in adults with radicular low back pain: retrospective cohort study using US data. BMJ Open 2023;13:e073258. 10.1136/bmjopen-2023-073258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topaloglu U, Palchuk MB. Using a federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform 2018;2:1–10. 10.1200/CCI.17.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfaff ER, Girvin AT, Gabriel DL, et al. Synergies between centralized and Federated approaches to data quality: a report from the national COVID cohort collaborative. J Am Med Inform Assoc 2022;29:609–18. 10.1093/jamia/ocab217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evans L, London JW, Palchuk MB. Assessing real-world medication data completeness. J Biomed Inform 2021;119:103847. 10.1016/j.jbi.2021.103847 [DOI] [PubMed] [Google Scholar]
- 42. Salsbury SA, Goertz CM, Twist EJ, et al. Integration of doctors of chiropractic into private sector health care facilities in the United States: a descriptive survey. J Manipulative Physiol Ther 2018;41:149–55. 10.1016/j.jmpt.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 43. Tarulli AW, Raynor EM. Lumbosacral radiculopathy. Neurol Clin 2007;25:387–405. 10.1016/j.ncl.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 44. Konstantinou K, Dunn KM, Ogollah R, et al. Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet Disord 2015;16:332. 10.1186/s12891-015-0787-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jönsson B, Strömqvist B. Influence of age on symptoms and signs in lumbar disc herniation. Eur Spine J 1995;4:202–5. 10.1007/BF00303410 [DOI] [PubMed] [Google Scholar]
- 46. Miyakoshi N, Hongo M, Kasukawa Y, et al. Prevalence, spinal alignment, and mobility of lumbar spinal stenosis with or without chronic low back pain: a community-dwelling study. Pain Res Treat 2011;2011:340629. 10.1155/2011/340629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stynes S, Konstantinou K, Dunn KM. Classification of patients with low back-related leg pain: a systematic review. BMC Musculoskelet Disord 2016;17:226. 10.1186/s12891-016-1074-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suri P, Boyko EJ, Goldberg J, et al. Longitudinal associations between incident lumbar spine MRI findings and chronic low back pain or radicular symptoms: retrospective analysis of data from the Longitudinal Assessment of Imaging and Disability of the Back (LAIDBACK). BMC Musculoskelet Disord 2014;15:1:152. 10.1186/1471-2474-15-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whedon JM, Haldeman S, Petersen CL, et al. Temporal trends and geographic variations in the supply of clinicians who provide spinal manipulation to Medicare beneficiaries: a serial cross-sectional study. J Manipulative Physiol Ther 2021;44:177–85. 10.1016/j.jmpt.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: characteristics of prescriptions and association with long-term use. Ann Emerg Med 2018;71:326–36. 10.1016/j.annemergmed.2017.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnson DG, Ho VT, Hah JM, et al. Prescription quantity and duration predict progression from acute to chronic opioid use in opioid-naïve medicaid patients. PLOS Digit Health 2022;1:e0000075. 10.1371/journal.pdig.0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;176:1286–93. 10.1001/jamainternmed.2016.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park SH, Wackernah RC, Stimmel GL. Serotonin syndrome: is it a reason to avoid the use of tramadol with antidepressants J Pharm Pract 2014;27:71–8. 10.1177/0897190013504957 [DOI] [PubMed] [Google Scholar]
- 54. Haugen AJ, Grøvle L, Brox JI, et al. Estimates of success in patients with sciatica due to lumbar disc herniation depend upon outcome measure. Eur Spine J 2011;20:1669–75. 10.1007/s00586-011-1809-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vroomen PCAJ, de Krom MCTFM, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract 2002;52:119–23. [PMC free article] [PubMed] [Google Scholar]
- 56. Edinoff AN, Kaplan LA, Khan S, et al. Full opioid agonists and tramadol: pharmacological and clinical considerations. Anesth Pain Med 2021;11:e119156. 10.5812/aapm.119156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shatin D, Gardner JS, Stergachis A, et al. Impact of mailed warning to prescribers on the co-prescription of tramadol and antidepressants. Pharmacoepidemiol Drug Saf 2005;14:149–54. 10.1002/pds.961 [DOI] [PubMed] [Google Scholar]
- 58. Cammarota S, Conti V, Corbi G, et al. Predictors of opioid prescribing for non-malignant low back pain in an Italian primary care setting. J Clin Med 2021;10:3699. 10.3390/jcm10163699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. King C, Liu X. Racial and ethnic disparities in opioid use among US adults with back pain. Spine (Phila Pa 1976) 2020;45:1062–6. 10.1097/BRS.0000000000003466 [DOI] [PubMed] [Google Scholar]
- 60. Gwam CU, Emara AK, Chughtai N, et al. Trends and risk factors for opioid administration for non-emergent lower back pain. World J Orthop 2021;12:700–9. 10.5312/wjo.v12.i9.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Whedon JM, Toler AWJ, Goehl JM, et al. Association between utilization of chiropractic services for treatment of low-back pain and use of prescription opioids. J Altern Complement Med 2018;24:552–6. 10.1089/acm.2017.0131 [DOI] [PubMed] [Google Scholar]
- 62. Trager RJ, Cupler ZA, DeLano KJ, et al. Association between chiropractic spinal manipulative therapy and benzodiazepine prescription in patients with radicular low back pain: a retrospective cohort study using real-world data from the USA. BMJ Open 2022;12:e058769. 10.1136/bmjopen-2021-058769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Z, Kim HJ, Lonjon G, et al. Balance diagnostics after propensity score matching. Ann Transl Med 2019;7:16. 10.21037/atm.2018.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamper SJ, Logan G, Copsey B, et al. What is usual care for low back pain? A systematic review of health care provided to patients with low back pain in family practice and emergency departments. Pain 2020;161:694–702. 10.1097/j.pain.0000000000001751 [DOI] [PubMed] [Google Scholar]
- 65. Nordahl-Hansen A, Øien RA, Volkmar F, et al. Enhancing the understanding of clinically meaningful results: a clinical research perspective. Psychiatry Res 2018;270:801–6. 10.1016/j.psychres.2018.10.069 [DOI] [PubMed] [Google Scholar]
- 66. Trager RJ. Baseline characteristics. figshare, 2023. Available: 10.6084/m9.figshare.24539908.v1 [DOI]
- 67. Trager RJ. Propensity score density graph data. figshare, 2023. Available: 10.6084/m9.figshare.24539920.v1 [DOI]
- 68. Trager RJ. Cumulative incidence data. figshare, 2023. Available: 10.6084/m9.figshare.24539917.v1 [DOI]
- 69. Whedon JM, Toler AWJ, Goehl JM, et al. Association between utilization of chiropractic services for treatment of low back pain and risk of adverse drug events. J Manipulative Physiol Ther 2018;41:383–8. 10.1016/j.jmpt.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 70. Buchbinder R, Underwood M, Hartvigsen J, et al. The lancet series call to action to reduce low value care for low back pain: an update. Pain 2020;161 Suppl 1:S57–64. 10.1097/j.pain.0000000000001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. George SZ, Goertz C, Hastings SN, et al. Transforming low back pain care delivery in the United States. PAIN 2020;161:2667–73. 10.1097/j.pain.0000000000001989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goertz CM, Long CR, English C, et al. Patient-reported physician treatment recommendations and compliance among US adults with low back pain. J Altern Complement Med 2021;27:S99–105. 10.1089/acm.2020.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hon S, Ritter R, Allen DD. Cost-effectiveness and outcomes of direct access to physical therapy for musculoskeletal disorders compared to physician-first access in the United States: systematic review and meta-analysis. Phys Ther 2021;101:pzaa201. 10.1093/ptj/pzaa201 [DOI] [PubMed] [Google Scholar]
- 74. Tucker J, Salas J, Zhang Z, et al. Provider specialty and odds of a new codeine, hydrocodone, oxycodone and tramadol prescription before and after the CDC opioid prescribing guideline publication. Prev Med 2021;146:106466. 10.1016/j.ypmed.2021.106466 [DOI] [PubMed] [Google Scholar]
- 75. Tannoury C, Bhale R, Koh J, et al. Opioid use among orthopaedic patients and comparison of opioid prescribing patterns among spine surgeons and other orthopaedic subspecialists in the United States. MRAJ 2022;10. 10.18103/mra.v10i8.2984 [DOI] [Google Scholar]
- 76. Turk D, Boeri M, Abraham L, et al. Patient preferences for osteoarthritis pain and chronic low back pain treatments in the United States: a discrete-choice experiment. Osteoarthritis and Cartilage 2020;28:1202–13. 10.1016/j.joca.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 77. Schneeweiss S, Rassen JA, Brown JS, et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 2019;170:398. 10.7326/M18-3079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078105supp001.pdf (120.3KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Minimal, de-identified, aggregated datasets that support the findings of this study are openly available in Figshare at: https://doi.org/10.6084/m9.figshare.24539908 (baseline characteristics), https://doi.org/10.6084/m9.figshare.24539917 (cumulative incidence data) and https://doi.org/10.6084/m9.figshare.24539920 (propensity score density data).