Abstract
Introduction
A prototype lateral flow device detecting cytokine biomarkers interleukin (IL)-1α and IL-1β has been developed as a point-of-care test—called the Genital InFlammation Test (GIFT)—for detecting genital inflammation associated with sexually transmitted infections (STIs) and/or bacterial vaginosis (BV) in women. In this paper, we describe the rationale and design for studies that will be conducted in South Africa, Zimbabwe and Madagascar to evaluate the performance of GIFT and how it could be integrated into routine care.
Methods and analysis
We will conduct a prospective, multidisciplinary, multicentre, cross-sectional and observational clinical study comprising two distinct components: a biomedical (‘diagnostic study’) and a qualitative, modelling and economic (‘an integration into care study’) part. The diagnostic study aims to evaluate GIFT’s performance in identifying asymptomatic women with discharge-causing STIs (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV) and Mycoplasma genitalium (MG)) and BV. Study participants will be recruited from women attending research sites and family planning services. Several vaginal swabs will be collected for the evaluation of cytokine concentrations (ELISA), STIs (nucleic acid amplification tests), BV (Nugent score) and vaginal microbiome characteristics (16S rRNA gene sequencing). The first collected vaginal swab will be used for the GIFT assay which will be performed in parallel by a healthcare worker in the clinic near the participant, and by a technician in the laboratory. The integration into care study aims to explore how GIFT could be integrated into routine care. Four activities will be conducted: user experiences and/or perceptions of the GIFT device involving qualitative focus group discussions and in-depth interviews with key stakeholders; discrete choice experiments; development of a decision tree classification algorithm; and economic evaluation of defined management algorithms.
Ethics and dissemination
Findings will be reported to participants, collaborators and local government for the three sites, presented at national and international conferences, and disseminated in peer-reviewed publications.
The protocol and all study documents such as informed consent forms were reviewed and approved by the University of Cape Town Human Research Ethics Committee (HREC reference 366/2022), Medical Research Council of Zimbabwe (MRCZ/A/2966), Comité d’Ethique pour la Recherche Biomédicale de Madagascar (N° 143 MNSAP/SG/AMM/CERBM) and the London School of Hygiene and Tropical Medicine ethics committee (LSHTM reference 28046).
Before the start, this study was submitted to the Clinicaltrials.gov public registry (NCT05723484).
Trial registration number
Keywords: sexually transmitted disease, bacteriology, public health, epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A multicountry evaluation of a new low-cost, rapid point-of-care (POC) test detecting vaginal inflammation to be conducted in three Sub-Saharan countries characterised by different sexually transmitted infection (STI)/bacterial vaginosis contexts.
Multidisciplinary approach, including social science, economic analysis and machine learning to inform how the GIFT device could be integrated into STI management algorithms for women.
The budget does not permit STI diagnosis using POC nucleic acid amplification testing in Madagascar, in contrast to South Africa and Zimbabwe, leading to delays in delivering the diagnostic results and providing treatment to the Malagasy study participants.
The user evaluation of the GIFT device does not include the evaluation nor quality assessment of the specimen extraction method.
Introduction
Sexually transmitted infections (STIs) continue to pose a significant health challenge globally, with over one million new cases reported daily. In 2020, approximately 377 million new cases of the four most common STIs (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), syphilis and Trichomonas vaginalis (TV)) were documented, with over 70% occurring in low-income and middle-income countries (LMICs), particularly in Sub-Saharan Africa and Latin America.1
STIs impact women more than men, as women endure long-lasting consequences. Additionally, women are more susceptible to STIs than men due to anatomical and immunological differences.2 3 The composition of the vaginal microbiome also plays a crucial role in sexual and reproductive health. Optimal vaginal microbiota, predominantly composed of Lactobacillus species, maintains an acidic pH, sustaining the vaginal epithelial barrier’s integrity and preventing pathogen colonisation and invasion. However, various genetic, behavioural and environmental factors can modulate this microbial composition and lead to bacterial vaginosis (BV) characterised by a decrease in protective Lactobacillus species along with an increase in bacterial diversity and vaginal pH.4–9
BV remains a persistent public health issue, with prevalences ranging from 20% to 30% globally, and higher rates observed in black populations compared with Caucasian or Asian populations.4 6 10 In Sub-Saharan African populations, prevalences are notably high (around 33%).10
Both STIs and BV induce elevated genital inflammation, marked by increased local production of proinflammatory cytokines and chemokines, irrespective of symptoms.7 11–15 Studies in Sub-Saharan African women cohorts have shown that common STIs such as CT, NG and TV significantly and consistently increased the concentrations of key genital tract cytokine biomarkers, including interleukin (IL)-1α and IL-1β.14 16 17 BV caused similar changes in these cytokine biomarkers, although was also shown to be associated with lower concentrations of the chemokine interferon-γ-induced protein of 10 kDa (IP-10).12 18 19 Genital inflammation caused by STIs and BV has in turn been associated with increased risk for the acquisition of other STIs, including HIV-1.20–25 This is particularly concerning in Sub-Saharan African countries, where a significant proportion of new HIV-1 infections occur. The HIV-1 prevalence among adults (15–49 years) was estimated at 17.8% and 11% in South Africa and Zimbabwe, respectively in 2022. Women are disproportionally affected by HIV than men, with young women (15–24 years) particularly vulnerable with prevalence rates estimated at 8.7% and 4.4% in South Africa and Zimbabwe, respectively.26
Both STIs and BV are also associated with other serious sexual and reproductive health complications, such as pelvic inflammatory disease which can lead to infertility and ectopic pregnancy and adversely affect a woman’s reproductive plans. For prevalent bacterial STIs and BV, intrauterine infection and/or vertical transmission to the unborn child or during birth can result in adverse pregnancy and birth outcomes, including preterm delivery, maternal and neonatal sepsis, low birth weight, pneumonia and conjunctivitis in the newborn.3 27
Despite the high prevalence of STIs and BV and their frequently asymptomatic nature, syndromic approaches remain the mainstay of management in LMICs.28 Immediate testing and results delivery are crucial to facilitate prompt diagnosis and accurate treatment, reducing both STI transmission and infection progression.29–31 Consequently, the inclusion of point-of-care (POC) tests has been highly recommended for the management of symptomatic STIs.28 Recently, nucleic acid amplification-based near-patient STI diagnostics have become available,32 33 but none of them fulfils the REASSURED (including user-friendly, rapid, robust, equipment-free and deliverable to end-users) criteria set out by WHO for POC tests.34
Genital inflammation has been associated with a significantly increased risk of HIV acquisition in women and the presence of STIs driving this inflammation has been well-described among South African women.11 16 22 25 To address the need for POC tests to improve STI/BV management for women in LMICs, Masson et al have focused on developing a POC lateral flow test to detect a combination of inflammation biomarkers (IL-1α, IL-1β and IP-10), measured in lateral vaginal wall swabs, to improve BV and STI case finding in women compared with clinical signs.20 This Genital InFlammation Test (GIFT) screening tool aims to improve case detection to help identify women at increased risk of HIV infection and to enhance the sexual and reproductive health of women in countries where only syndromic management is available.
This study protocol outlines a study to be conducted in three countries (South Africa, Zimbabwe and Madagascar) to evaluate the performance of the first-in-field GIFT prototype device and explore possible routes for integration into routine care.
Purpose and study objectives
This multidisciplinary, cross-sectional, prospective and multicentric study has two distinctive substudies, a diagnostic evaluation and an integration into care study. Each sub-study has several objectives (table 1).
Table 1.
GIFT study objectives
| Diagnostic study | Integration into care study | |
| Primary objective | To assess the sensitivity and specificity of the GIFT device at the point-of-care in South Africa, Zimbabwe and Madagascar | To evaluate how the GIFT device could be integrated into routine care |
| Secondary objectives |
|
|
| Exploratory objectives |
|
BV, bacterial vaginosis; GIFT, Genital InFlammation Test; NAAT, nucleic acid amplification test; STI, sexually transmitted infection.
The diagnostic study aims to evaluate the performance of GIFT in identifying women with inflammatory STIs (including CT, NG, TV and MG) and BV and who may, therefore, be at higher risk of STI and HIV infection and potential reproductive complications. Syndromic management in the absence of any diagnostic testing misses all asymptomatic reproductive tract infections by definition, and GIFT may detect the presence of asymptomatic subclinical inflammation. Syndromic management guidelines currently call for women with cervicovaginal discharge to be treated for all reproductive tract infections that might cause such discharge, but studies have shown that a substantial proportion of these women do not have any infection.29 In this scenario, GIFT may prevent overtreatment of symptomatic women without infection.
The primary objective is to assess the sensitivity and specificity of the GIFT device at the point-of-care in non-pregnant sexually active women aged 18–35 years accessing family planning services in South Africa, Zimbabwe and Madagascar against nucleic acid amplification tests (NAATs) for CT, NG, TV and MG plus BV by Nugent scoring (table 1). Secondary objectives include assessing the device’s predictive values; assessing performance in each country separately; assessing performance against syndromic management; assessing test reproducibility by comparing clinician-read and laboratory-read results (visual reading vs automated reader); and setting the device’s concentration cut-offs against gold standard cytokine concentrations (determined by ELISA).
The integrative part of the study aims to explore the feasibility, acceptability and cost-effectiveness of integrating GIFT into routine care in LMICs. The specific objectives include: assessing the user-experience, usability and acceptability of the GIFT device at the POC; examining patient preferences for various STI management aspects to develop STI management algorithms; generation of algorithms that integrate the GIFT device to optimise case finding and STI/vaginal infection management in women; and determine the cost-effectiveness and budget impact of the identified algorithms with the GIFT device.
Methods and design
Study design
The diagnostic study will target women attending family planning services in each of the three study sites in South Africa, Zimbabwe, and Madagascar. A series of vaginal swabs will be collected by a midwife or nurse (figure 1).
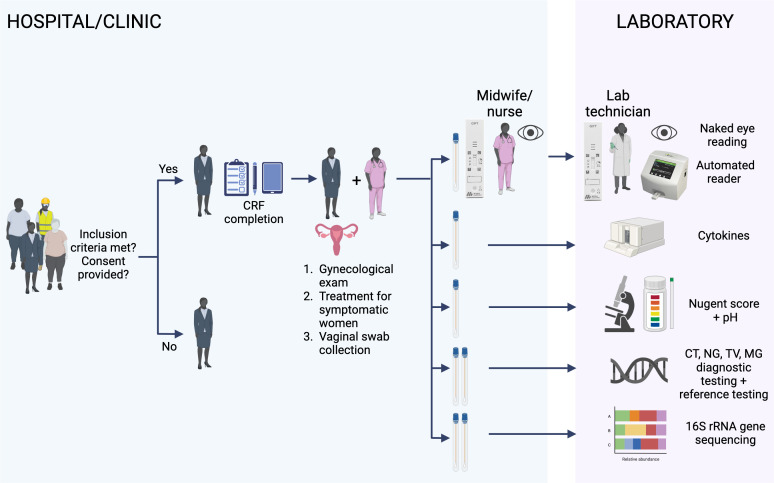
Figure 1.
Schematic of the GIFT diagnostic study. The recruited women who meet the inclusion criteria and consent to participate in the GIFT study will be administered a questionnaire. Data will be recorded on CRFs in a paper or electronic (tablet) format by the clinical nurse followed by a gynaecological examination. Vaginal swabs are collected: the first collected swab will be used for two GIFT assays; the first GIFT will be performed at the bedside by the midwife/nurse and the second GIFT by the lab technician. Each of them will read visually their own performed GIFT (naked eye reading). The two respective GIFT assays will then be read using a lateral flow automated reader. The other collected swabs will be used for different laboratory assays for the evaluation of the GIFT performance. HIV testing on fingerpick blood will be also included at the end of the medical examination. The figure was created with BioRender. CRFs, case report forms; CT, Chlamydia trachomatis; GIFT, Genital InFlammation Test; MG, Mycoplasma genitalium; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis.
The first swab will be used to test the performance of the GIFT device. For each woman, the GIFT will be performed twice and read four times: one GIFT will be performed at the clinical site near the participant and visually read by a midwife or study nurse and a second GIFT will be done and visually read by a technician in the laboratory. The GIFT assays carried out by the midwife/study nurse and the laboratory technician will secondly be read using an automated lateral flow reader (Axxin, Fairfield, Australia) (figure 1). The GIFT device performance in detecting (1) inflammation will be determined against cytokine ELISA; and (2) STIs/BV will be determined against the NAAT reference tests for CT, NG, TV and MG and Nugent scoring for BV (figure 1). All women will be offered treatment based on the on-site positive STI diagnostic results for CT, NG and TV. Additionally, treatment for BV will be offered based on the Nugent score analysis and at the clinician’s discretion.
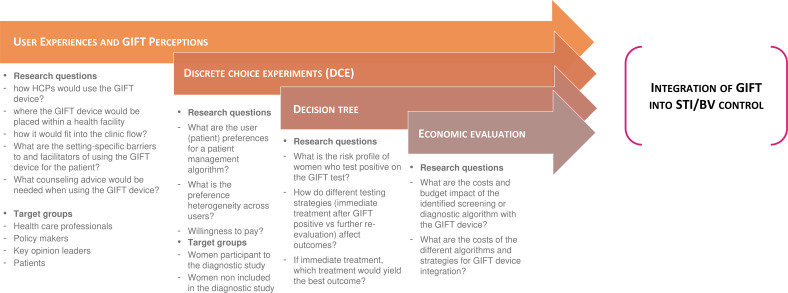
The integration into care study consists of four activities to determine how GIFT can be integrated in a feasible, acceptable and cost-effective way into routine care and national guidelines in South Africa, Zimbabwe and Madagascar (figure 2): (1) user experiences and/or perceptions of the GIFT device involving qualitative focus group discussions (FGDs) and in-depth interviews (IDIs) with key stakeholders; (2) discrete choice experiment (DCE); (3) development of a decision tree classification algorithm; and (4) economic evaluation of defined management algorithms.
Figure 2.
Integration study activities. Description of the four activities that will be performed for the GIFT integration into care study for improving the STI/BV diagnosis and control in women: (1) user experiences and/or perceptions of the GIFT device involving qualitative focus group discussions and in-depth interviews with key stakeholders; (2) discrete choice experiments; (3) development of a decision tree classification algorithm and (4) economic evaluation of defined management algorithms. In addition to the women already participating to the GIFT diagnostic study, HCPs, policymakers, key opinion leaders and patients coming for consultation will be included in the study. BV, bacterial vaginosis; GIFT, Genital InFlammation Test; HCPs, healthcare professionals; STI, sexually transmitted infection.
Study sites
The study will be performed in three countries: South Africa, Zimbabwe and Madagascar. In contrast to South Africa and Zimbabwe which are countries bearing a high burden of STIs, including HIV and BV,35–37 data on STI/BV prevalence are relatively scarce in Madagascar. BV, TV and cervical infections (CT and NG) prevalence measured in a large population sample of symptomatic Malagasy women (n=1066) were high at 53%, 24% and 17%, respectively, suggesting a significant burden of STIs/BV in the island.38 Finally, Madagascar is less affected by HIV with a prevalence<1%39 compared with South Africa and Zimbabwe, which were found to have an HIV-1 prevalence among adults (15–49 years) in 2022 of 17.8% and 11%, respectively.26
In South Africa, the study will be conducted at the Desmond Tutu Health Foundation (DTHF) Masiphumelele Youth Centre located in the South peninsula of Cape Town. The Masiphumelele Research Site has been recognised as a pioneer in HIV prevention and adolescent research. The STI and BV diagnostic testing of the South African participants will be performed in their on-site laboratory. In addition, vaginal swabs collected at all three study sites will be tested for selected cytokines including IL-1α, IL-1β and IP-10 by ELISAs and vaginal microbiome composition by 16S rRNA gene sequencing being conducted at the University of Cape Town (UCT), along with samples from the other two trial sites.
In Zimbabwe, the study will be conducted at the Spilhaus Family Planning Centre located in the capital Harare. The study site is run by the Zimbabwe National Family Planning Council (ZNFPC) in collaboration with the Zimbabwe Ministry of Health and Child Care. The study will be conducted by researchers from the Organisation for Public Health Interventions and Development (OPHID), equipped with a skilled medical and technical team. The study site’s laboratory will perform the STI and BV diagnostic testing for Zimbabwean study participants.
In Madagascar, the study will be conducted at the Centre Hospitalier Universitaire de Gynécologie et d’Obstétrique Gynéco-Obstétricaux de Befelatanana (CHU-GOB), a public university hospital that specialises in obstetrics located in the capital of Madagascar, Antananarivo. Outpatient services include prenatal consultations, family planning, obstetrics and gynaecology services. Due to the lack of recent data on STI/BV prevalence, a prestudy on CT, NG, TV and MG prevalence was conducted by the clinical and research team of Institut Pasteur Madagascar (IPM) from March to December 2022 to confirm the feasibility of conducting the GIFT study in the CHU-GOB in Antananarivo. The prestudy confirmed the appropriateness of the clinical study site in terms of the study population’s number and STI and BV prevalence rates (results will be presented elsewhere).
The unit of experimental bacteriology (UBEX) at the IPM will be the study site’s laboratory in Madagascar for diagnostic testing. UBEX is also designated as the reference laboratory in the GIFT project for BV and STI (CT, NG, TV and MG) comparator testing, as well as quantification of lactobacilli and BV-associated bacteria using quantitative PCR and molecular testing for the detection of Candida spp, and Y chromosome.
Study participants and recruitment
The diagnostic study will enrol its participants from sexually active women aged 18–35 years attending family planning services in each of the study’s clinical sites, irrespective of the symptoms. Before study initiation, advice will be sought from local Community Advisory Boards (CABs) or similar committees on the study material to be shared with potential study participants. The boards will be regularly consulted regarding language to use for behavioural and other sensitive questions included in the questionnaires. A CAB is currently not existing in Madagascar but will be installed as part of the study.
Due to the short recruitment period, sensitisation to the GIFT project will be extended in Antananarivo (Madagascar) to the family planning services of surrounding primary healthcare centres. In South Africa, the clinical study site offers family planning services to young women till the age of 25, and women outside of the study site will be recruited to enrol women between 25 and 35 years old.
Women interested in the diagnostic study will be invited to participate based on the inclusion and exclusion criteria detailed in table 2. Each woman will only be enrolled once.
Table 2.
Inclusion and exclusion criteria for both diagnostic and integration into care studies
| Diagnostic study | Integration into care study | |
| Inclusion criteria |
|
For all who are willing and able to provide informed consent to participate in the study.
|
| Exclusion criteria |
|
For all who are not willing or able to provide informed consent to participate in the study
|
GIFT, Genital InFlammation Test.
In addition to eligible women participating in the diagnostic study, participants for the integration into care study will include healthcare professionals (HCPs) including nurses, midwives and matrons working at the clinical site where the diagnostic study is being conducted, women attending gynaecological consultations at the study clinic, local and regional policymakers, programmers, other opinion leaders, and decision-makers according to the inclusion and exclusion criteria (table 2).
Sample size
A total number of 675 women (225 women per site) will be enrolled for the diagnostic evaluation of the prototype GIFT device. The sample size calculation is based on 7% precision, and an assumed sensitivity of 77% and a specificity of 71% of GIFT compared with NAATs for STIs and Nugent scoring for BV.40
No sample size calculations were performed for the qualitative research. A purposive sampling strategy will be adopted to reflect maximum variation, as feasible, across the sample. It is anticipated that the proposed number of participants per activity (FGDs and IDIs), per country, will enable adequate data to be collected and saturation to be reached in the thematic analysis.41 42 Data collection may be finalised before the planned sample size is reached, if saturation is reached first.
For the DCE component of the study, using econometric criteria (including choice probability, confidence level, accuracy level, attrition rate and number of choice tasks), subgroups of more than 30 individuals are required to conduct meaningful statistical analysis.43 44 Because the DCE study will undertake subgroup analysis on four to five groups (including respondent age group, socioeconomic status, location of residence and experience with the GIFT device), up to 200 participants per site will be recruited for the DCE study, with a quarter of the participants recruited on the diagnostic study of the GIFT device, and reminder from women coming for gynaecological consultations at the study clinic.
No sample size calculation is needed for the decision tree algorithm study, which will use data from the diagnostic study. Equally, there are no sample size requirements for the economic evaluation.
Study procedures and data collection
Diagnostic study
The diagnostic study participant and sample flow from screening to laboratory results is described in figure 1 and detailed in online supplemental figure 1—Diagnostic study flow steps. After confirmation that participants were eligible for the study (table 2) and written informed consent obtained, enrolled women will be interviewed using a structured questionnaire with questions including demographics, sexual behaviour, hygiene habits, current medical and reproductive history, medication and symptoms. The data will be captured on paper or electronic case report forms (CRFs) (figure 1). The clinician, midwife or study nurse will perform a physical examination including a pelvic examination, and will collect fingerprick blood for HIV testing and vaginal swabs using regular flocked swabs (Copan, Italy) (figure 1 and online supplemental figure 1—Diagnostic study flow steps).
bmjopen-2024-084918supp001.pdf (13.1KB, pdf)
The first inserted swab will be eluted in buffer and the nozzle-sample (provided with the GIFT kit) will be applied to two prototype GIFT devices, one near the patient and the other in the laboratory (figure 1). A second swab will serve for cytokine ELISA testing, and a third swab for pH testing using a pH indicator strip and to prepare a vaginal smear slide for BV (figure 1). The next swabs will be collected and used for CT, NG and TV diagnosis in the laboratory, for reference STI testing including MG, quantitative NAAT of vaginal microbiome bacteria, the detection of Candida spp and Y chromosome for the presence of semen. The last two collected swabs will be used to characterise the vaginal microbiome by using 16S rRNA PCR and sequencing (figure 1).
Integration into care study
The integration study is composed of four activities aiming to understand how GIFT can be integrated into STI control (figure 2).
For the qualitative study assessing the user experience, usability and acceptability of the GIFT device at the point of care, data will be collected through IDIs and FGDs. Target groups will be: HCPs at the health facilities; local or regional policymakers, programmers, other opinion leaders and decision-makers; and women who are eligible for the diagnostic study (table 2), but who are not part of the diagnostic study. HCP participants will be identified through the diagnostic study sites and will be contacted directly to ask if they would be interested in participating. Policymakers recommended for inclusion through snowball sampling will be contacted and asked if they would be interested in participating. All women presenting at the family planning service facilities for inclusion in the diagnostic study will be informed about the integration study. For the DCE, the study team will seek to recruit women participating and not-participating in the GIFT diagnostic study to explore heterogeneity in patient preferences for various STI attributes. For all population groups, those who agree to participate will provide written informed consent, separate from the diagnostic study.
For the decision tree classification algorithm, data will be obtained from the diagnostic study participants, and no additional participants will be recruited. For the economic evaluation, HCPs at health facilities involved in GIFT device implementation and able to complete timesheets will be recruited.
For the user experience activity (figure 2, activity 1), up to 10–12 IDIs and/or 1–3 FGDs will be conducted with policymakers, up to 8–10 IDIs and/or 3 FGDs will be conducted with HCPs, and up to 12–15 IDIs and/or 3 FGDs will be conducted with women. FGDs will include participatory tools, such as card mapping. The FGDs with HCPs may additionally include role plays of how the providers would discuss the GIFT device with women, and how providers would discuss GIFT device results and subsequent care and management with women. IDIs will explore more detailed personal perceptions of the potential structures of integration.
User experience data (qualitative and FGDs) on the patient experience will help inform the development of attributes for the DCE (figure 2, activity 2). The DCE aims to identify the relative importance of factors influencing patients’ preferences for the use of GIFT devices. For the DCE study, face-to-face interviews using a questionnaire will be conducted, with the questionnaire asking respondents to state preferences for hypothetical alternatives, each described by several attributes. A D-efficient design will be used to identify the choice tasks to present to respondents. Each choice task presents two STI screening test options that are defined using different levels of the attributes and then ask respondents to choose their preferred option, with an opt-out option available (to choose neither of the two options). The results of the DCE will inform the development of STI management algorithms that integrate the GIFT device, to increase the acceptability of the STI management programme.
A decision tree will be developed to classify symptomatic and asymptomatic women using the following attributes: sociodemographic information, signs and symptoms, medical history, sexual behaviour, the GIFT output and vaginal pH. Different classifications will be tested by varying the combinations of the attributes and the number of levels of the tree. The algorithm will be developed using a training dataset (data from previous studies). After the development of the algorithm based on the training dataset, the algorithm will be tested on the data to be collected in the diagnostic study and refined if necessary. The algorithm will be designed to be as simple as possible (minimum number of nodes and minimum number of scenarios) and usable in routine practice. Finally, the management outcomes will be compared with those obtained with the WHO recommendations.
The economic evaluation (figure 2, activity 4) will involve the collection of cost and service utilisation data from a provider’s perspective at each of the clinical sites, using an ingredients-based approach. After obtaining approval from the facility and/or district, relevant facility management staff will be approached to share financial and other facility-level data. The cost and utilisation data will be collected from individual facility records and financial reports through cost data collection tools at each study site. The time that it takes to perform and analyse the GIFT assay will be obtained from clinical study staff. The time required for the delivery of routine family planning and STI services will be assessed through timesheets completed by selected healthcare workers at the study sites. These data will be captured in the Provider Cost Data Collection Tool by data collectors. This activity will determine the cost and budget impact of the identified screening or diagnostic algorithm with the GIFT device and will model the cost-effectiveness of the different strategies of integration of GIFT into care.
Laboratory procedures
The BV diagnosis will be obtained from a vaginal smear carried out with the third swab collected during the gynaecological examination (figure 1). The slide will be transferred from the clinic to the respective on-site laboratory for Gram staining and Nugent scoring.40
CT, NG and TV diagnosis from collected vaginal swabs (figure 1) will be performed using the diagnostic tests available in each of the three sites. In South Africa and Zimbabwe, GeneXpert tests (Cepheid, Sunnyvale, USA) will be used for CT and NG detection, and TV detection will be performed with the OSOM trichomonas rapid test or TV GeneXpert test, depending on the availability. In Madagascar, the vaginal swabs will be tested by UBEX (IPM) using validated quantitative PCR (qPCR) protocols for CT, NG, and TV detection.45 46
The swabs collected for STI reference testing in South Africa and Zimbabwe will be shipped to the IPM (Madagascar) for reference testing using validated qPCR,45–47 vaginal bacteria-specific quantitative qPCR testing, molecular detection of Candida spp and presence of semen (Y chromosome testing).
Three swabs per participant will be sent to the UCT, for cytokine ELISA measurement and 16S rRNA gene PCR and sequencing. The results obtained with the prototype GIFT device will be compared with the cytokine concentrations resulting from the ELISA measurements. 16S rRNA gene sequencing and Candida spp NAAT will be used to evaluate the proportion of cases of genital inflammation that could be explained by vaginal bacterial or fungal dysbiosis, respectively.
All testing will be performed blinded to the clinical information and results obtained at each of the study sites, including the GIFT results.
The swabs will be stored in each study site at −80°C until shipment using dry ice to the respective laboratories as stipulated above.
Monitoring, quality assurance, and control
This study will be monitored per regulations applicable to diagnostic study, including International Committee on Harmonisation of Good Clinical Practice (ICH-GCP) and GCLP (Good Clinical Laboratory Practices) requirements, and sponsor-specific standard operating procedures (SOPs). The diagnostic study will be monitored by the Epidemiology and Clinical Research Unit at the IPM. Routine monitoring will be conducted throughout the study and at the study closure. All laboratory activities including specimen transport, processing, testing, result reporting and storage will be conducted following clinical trial quality requirements. The GCLP guidelines will be followed and the designated laboratories will perform testing according to the SOPs which will be documented in the analytical plan.
A batch of known negative and positive specimens for CT/NG, TV, pregnancy test and slides for Nugent scoring will be provided by the UBEX laboratory at IPM for external quality control. The external quality control will consist of three panels to be tested at the start, middle and end of the study or every 3 months. Before the use of the GIFT devices, a lot of validation will be performed using the quality control panel provided by the manufacturer according to the SOP.
Data management
The REDCap platform will be used as a clinical data management system (CDMS) for the clinical study. Depending on the organisation in each study site data will be collected partially or fully using paper CRF (pCRF) before being entered into REDCap daily. In addition, data may be collected and entered in real-time into REDCap using tablets.
The GIFT database will be managed by the Epidemiology and Clinical Research Unit at the IPM. The quality and accuracy of CRF data transcription into the database will be verified weekly. Missing or overdue forms will be identified and tracked using the REDCap field comment log. A control script developed by the central data manager will check the double data entry, and any transcription differences found will be noted. Status reporting including the list of inconsistencies will be done weekly by the central data management team. The weekly reports will also resume the progress of data collection and entry in each study site. All reports will be sent to the operational team for verification and correction of the data, if required.
External data sources not described on the CRF like cytokine ELISA data, the STI reference testing data, and 16S rRNA sequencing data will be reconciled against the CDMS at the end of the study.
For the user experience study, FGD and IDI data will be audiorecorded and electronically transcribed into the national and/or local language of the study sites. The DCE questionnaire data will be captured electronically using REDcap and securely stored on a password-protected computer. Data for the economic evaluation will be collected in a Provider Cost: Data Collection Tool, and timesheets will be completed in Excel by HCPs.
Data analysis
Diagnostic study
Primary analysis: sensitivity and specificity estimated of the GIFT device with 95% CIs using any positive etiological NAATs for STIs (CT, NG, TV and MG) or Nugent score result for BV as reference standards will be calculated. Visual readings of the GIFT device performed by the clinicians and technicians will be included separately in this analysis.
Secondary analysis: positive and negative predictive values with 95% CIs of the GIFT device will be compared with the likelihood ratios using NAAT and Nugent scoring as reference standards overall and in each country (which may have different STI prevalence that will impact these predictive values).
The performance of the GIFT device will be compared with the performance of syndromic management for detecting STIs. Both methods (GIFT and syndromic management) will be compared with any positive results of NAAT (for CT, NG, TV and MG) and/or BV detected by Nugent scoring. Agreement between the GIFT device results read visually by both the clinician and the laboratory technician and on an automated reader will be determined.
The concordance of band intensity results from the GIFT device and cytokine ELISA measurements will be analysed. This analysis will be used to optimise the cytokine concentration cut-offs for interpretation of the GIFT device performance, in terms of sensitivity, specificity and predictive values.
The impact of additional characteristics and/or determinants (such as intermediate microbiota, Candida, age, parity and sexual activity) on the prediction of STI/BV status in women and the impact on the accuracy of the device will be determined.
Integration into care study
For the user experience study, iterative thematic analysis will be applied to the IDI and FGD data, conducted by the qualitative research team. Sekhon’s framework of acceptability of healthcare interventions will be used.48
For the DCE, data will be analysed in STATA using the utility function modelled from two alternatives: a systematic (explainable) component and a random (unexplainable) component. To estimate the trade-offs that respondents are willing to make between attributes, willingness to pay (WTP) for marginal improvements in attributes will be estimated for all attributes. WTP estimates will be calculated as the ratio of the coefficient of interest to the negative of the coefficient on the attribute with continuous variables. Subgroup analyses will be undertaken to investigate how preferences are influenced by demographic characteristics, residential area, socioeconomic status and experience of POC testing.
The assessment of the decision tree classification algorithm will be based on the accuracy of the classification compared with the results of the gold standard methods (NAATs and Nugent score). Following this classification, different scenarios of management will be tested: immediate treatment, referral, re-evaluation and no treatment. These scenarios will be optimised in order to avoid undertreatment and overtreatment by also considering immediate specific rapid diagnosis tests (if available) or risk scores for immediate presumptive treatment.
For the economic evaluation, models will be developed in Microsoft Excel. For the cost analysis, an ingredient-based model will be constructed, and for the budget impact analysis, an expenditure-based model will be applied. A decision-analysis model to estimate the cost and health outcomes associated with different GIFT device implementation strategies will be developed. The main outcome will measure the effectiveness of each approach in correctly diagnosing an STI and/or BV in women, proxied by the sensitivity measure of the diagnostic test or approach, in comparison to the gold standard NAATs and Nugent scoring. Secondary outcomes such as the performance of the device vers syndromic management and the variation in device results when determined by a clinician, laboratory technician or an automated reader, will be assessed by means of scenario analyses. Univariate sensitivity analyses will be carried out to test the robustness of the findings.
Ethics and dissemination
The study will be carried out according to the principles stated in the Declaration of Helsinki (as amended in Seoul in 2008) and any further updates, all applicable national and international regulations, and according to the most recent applicable principles of the GCP-ICH E6. All the participants will be informed about the diagnostic results. The choice of treatment for STIs or BV will be based on the current national guidelines in the three study sites. The protocol and all study documents such as informed consent forms were reviewed and approved by the UCT Human Research Ethics Committee (HREC reference 366/2022), Medical Research Council of Zimbabwe (MRCZ/A/2966), Comité d’Ethique pour la Recherche Biomédicale de Madagascar (N° 143 MNSAP/SG/AMM/CERBM) and the London School of Hygiene and Tropical Medicine ethics committee (LSHTM reference 28046).
Findings will be reported to participants, collaborators and local government for the three sites, presented at national and international conferences and disseminated in peer-review publications.
Before the start, this study was submitted to the Clinicaltrials.gov public registry (NCT05723484).
Patient and public involvement
Before study initiation, we will engage with established local CABs or similar boards at each site. We will present the details of the study and ask for their input. According to the specific procedures in place in each site, we may allow the CAB to comment on the written protocol, the flyers and the posters. We will also ask the CAB for their feedback on the language used in the flyers, posters and informed consent forms. During the study, the study investigators will regularly attend CAB meetings to update on the progress of the study. We will also ensure that the CAB and the community are the first to receive the results of the study.
Discussion
Here, we have described the diagnostic and integration studies that will be used to validate the GIFT device as a proof-of-principle prototype for implementing a novel POC cytokine test to detect vaginal inflammation associated with STIs and/or BV among women in differing clinical settings.
The development of low-cost rapid POC tests has become a major focus in the management of STIs/BV to replace or improve syndromic management widely adopted in Africa. The availability of these tests would offer all sexually active women the opportunity to be screened and treated. This may have particular benefit to women at high risk of STIs, including young women, pregnant women, female sex workers and those with HIV.
The results from the prospective diagnostic study will be closely combined with the results of the qualitative research, modelling and economic evaluation. The feasibility and acceptability studies will inform how the GIFT device could be integrated into national guidelines. The GIFT feasibility study consists of a ‘user experience and GIFT perceptions’ prototype involving a large spectrum of people selected from the general population (HCPs, sexually active women, policymakers) that will help extend STI/BV screening into family planning services in primary healthcare services at affordable costs for LMICs, with the aim that women should know about their health without delaying their treatment. The qualitative part of the study will improve our understanding of the different key factors contributing to the successful implementation of a novel screening device for STIs and BV in LMICs.
Besides evaluating a POC test for screening of STIs and BV, this study will be among the first of its kind, focusing on STI/BV prevalence and risk factors including up to 675 women mainly from urban areas in three sub-Saharan countries in different STI/BV contexts. The GIFT study will also improve our knowledge in both biological and molecular characterisation of the three most common STIs (CT, NG and TV) and MG in association with the different states of the vaginal microbiota and will provide new insights into the interplay between STI and BV.
Our study protocol has some limitations. First of all, the user evaluation of the GIFT device, evaluating the performance of the device when applied by different types of users, does not include the evaluation of the extraction step. The extraction step of the vaginal swab will be performed by the medical staff only and not in parallel with a duplicate vaginal swab by a laboratory professional. Second, due to budget constraints, the STI diagnostic testing in Madagascar will be conducted by batch testing in the laboratory and not by POC NAAT testing as will be done in South Africa and Zimbabwe. Consequently, Malagasy participants will receive their results and treatment, if needed, after a delay of up to 10 days. This delay in treatment may facilitate further STI transmission. However, the study clinician may decide on presumptive treatment in alignment with the routine care practice in place.
In conclusion, the multidisciplinary study will be instrumental in developing strategies to improve STI and BV management in LMICs.
Supplementary Material
Acknowledgments
We thank in advance all women who will participate in this study and the healthcare workers who will be involved in the recruitment and enrolment of the study participants. We would also like to thank EDCTP and the members of GIFT study group for making this study possible. We thank the members of the community advisory boards for their valuable advice. We are extremely grateful to the members of the scientific advisory board for the precious scientific discussions and suggestions for improving our study.
Footnotes
@chidodc
SR and TC contributed equally.
LM and J-AP contributed equally.
Collaborators: Eneyi Kpokiri (London School of Hygiene & Tropical Medicine, United Kingdom), Bahiah Meyer (Division of Medical Virology, Department of Pathology, University of Cape Town, South Africa), Phumla Radebe (Division of Medical Virology, Department of Pathology, University of Cape Town, South Africa), Conita Lombard (Division of Medical Virology, Department of Pathology, University of Cape Town, South Africa), Celia Mehou-Loko (Division of Medical Virology, Department of Pathology, University of Cape Town, South Africa), Yacoeb Ganief (Division of Medical Virology, Department of Pathology, University of Cape Town, South Africa), Rezeen Daniels (Desmond Tutu HIV Centre, University of Cape Town, South Africa), Anda Madikida (Desmond Tutu HIV Centre, University of Cape Town, South Africa), Karabo Mahlangu (Desmond Tutu HIV Centre, University of Cape Town, South Africa), Tinashe Mwaturura (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Mutsawashe Chisenga (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Joseph F Chipanga (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Tsitsi Bandason (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Patricia Makunyire (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Felicia Mhangami (Organization for Public Health Interventions and Development, Zimbabwe; The Biomedical Research and Training Institute, Zimbabwe), Jayjay Karumazondo (The Biomedical Research and Training Institute, Zimbabwe), Jason Naidoo (The Biomedical Research and Training Institute, Zimbabwe), Karen Webb (Organization for Public Health Interventions and Development, Zimbabwe), Lyndon Mungur (Medical Diagnostech, Cape Town, South Africa), Ashley Uys (Medical Diagnostech, Cape Town, South Africa), Darryl Uys (Medical Diagnostech, Cape Town, South Africa), Elise Smith (Health Economics Unit, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa), Saberi Marias (Research Contracts and Innovation, University of Cape Town, South Africa), Solange Rasoanandrianina (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Dimitri Ravoavison (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Patrick Andry Rakotonirina (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Andrilaina Razakarivony (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Hantamalala Randria (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Alphonsine Rahantarimalala (Centre Hospitalier Universitaire de Gynécologie et Obstétrique de Befelatanana, Antananarivo, Madagascar), Lala Rafetrarivony (Institut Pasteur de Madagascar, Madagascar), Barivola Bernardson (Institut Pasteur de Madagascar, Madagascar), Tsiry Rasolofomanana (Institut Pasteur de Madagascar, Madagascar), Norohasina Randriamange (Institut Pasteur de Madagascar, Madagascar), Antsanirina Nomenjanahary (Institut Pasteur de Madagascar, Madagascar), Laurah Rabarisoa (Institut Pasteur de Madagascar, Madagascar), Dimitri Rasoloson (Institut Pasteur de Madagascar, Madagascar), Sahara Raveloson (Institut Pasteur de Madagascar, Madagascar), Laura Randrianantenaina (Institut Pasteur de Madagascar, Madagascar), Hanitratiavina Rakotonindriana (Institut Pasteur de Madagascar, Madagascar), Fenosoa Voanarivolalao (Institut Pasteur de Madagascar, Madagascar), Vaomalala Ranarinandra (Institut Pasteur de Madagascar, Madagascar), Farai Machinga (National Family Planning Council, Zimbabwe).
Contributors: SR and TC wrote the first draft of the manuscript. LM designed figure 1, EMH-E and SR designed figure 2. All authors contributed to the writing, and reviewed and approved the manuscript. SR reformatted the manuscript. LM and J-AP validated the final version. All authors were involved in the conception of the study and contributed to the design of the study protocol. All authors contributed to the elaboration of the study protocol. TC led the design of the diagnostic study with the participation of KG, LDB, JHHMvdW, AHarimanana, TMG, RVR, RM, CDC, KK, NT, DA, FRT, MM, ML, FK, LM, J-AP. EMH-E led the design of the integration into care study with the participation of B-TH, CF, CRSM-Y, SB, ES, AHonda, SCF, LM, J-AP. The protocol development was facilitated by TP; and validated by LM, J-AP principal investigators of the study.
Funding: The study is funded by the EDCTP through the EDCTP2 programme supported by the European Union (grant number EDCTP-RIA2020I-3297-GIFT).
Disclaimer: The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP.
Competing interests: The last authors, J-AP and LM, declare sharing a patent for the biomarkers for GIFT: patent number PCT/IB 2014/065740, October 2014.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: the GIFT study group, Eneyi Kpokiri, Bahiah Meyer, Phumla Radebe, Conita Lombard, Celia Mehou-Loko, Yacoeb Ganief, Rezeen Daniels, Anda Madikida, Karabo Mahlangu, Tinashe Mwaturura, Mutsawashe Chisenga, Joseph F Chipanga, Tsitsi Bandason, Patricia Makunyire, Felicia Mhangami, Jayjay Karumazondo, Jason Naidoo, Karen Webb, Lyndon Mungur, Ashley Uys, Darryl Uys, Elise Smith, Saberi Marias, Solange Rasoanandrianina, Dimitri Ravoavison, Patrick Andry Rakotonirina, Andrilaina Razakarivony, Hantamalala Randria, Alphonsine Rahantarimalala, Lala Rafetrarivony, Barivola Bernardson, Tsiry Rasolofomanana, Norohasina Randriamange, Antsanirina Nomenjanahary, Laurah Rabarisoa, Dimitri Rasoloson, Sahara Raveloson, Laura Randrianantenaina, Hanitratiavina Rakotonindriana, Fenosoa Voanarivolalao, Vaomalala Ranarinandra, and Farai Machinga
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019;97:548–562P. 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tuddenham S, Ravel J, Marrazzo JM. Protection and risk: male and female genital microbiota and sexually transmitted infections. J Infect Dis 2021;223:S222–35. 10.1093/infdis/jiaa762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Gerwen OT, Muzny CA, Marrazzo JM. Sexually transmitted infections and female reproductive health. Nat Microbiol 2022;7:1116–26. 10.1038/s41564-022-01177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 2003;95:201–12. [PMC free article] [PubMed] [Google Scholar]
- 5. Yotebieng M, Turner AN, Hoke TH, et al. Effect of consistent condom use on 6-month prevalence of bacterial vaginosis varies by baseline BV status. Tropical Med Int Health 2009;14:480–6. 10.1111/j.1365-3156.2009.02235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 2013;209:505–23. 10.1016/j.ajog.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 7. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015;42:965–76. 10.1016/j.immuni.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jespers V, van de Wijgert J, Cools P, et al. The significance of lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 2015;15:115. 10.1186/s12879-015-0825-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hickey RJ, Zhou X, Pierson JD, et al. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 2012;160:267–82. 10.1016/j.trsl.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peebles K, Velloza J, Balkus JE, et al. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019;46:304–11. 10.1097/OLQ.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 11. Adapen C, Réot L, Menu E. Role of the human vaginal Microbiota in the regulation of inflammation and sexually transmitted infection acquisition: contribution of the non-human primate model to a better understanding Front Reprod Health 2022;4:992176. 10.3389/frph.2022.992176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyongo JK, Crucitti T, Menten J, et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol 2015;22:526–38. 10.1128/CVI.00762-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 2014;209:1989–99. 10.1093/infdis/jiu004 [DOI] [PubMed] [Google Scholar]
- 14. Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial Vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 2014;90:580–7. 10.1136/sextrans-2014-051601 [DOI] [PubMed] [Google Scholar]
- 15. Mtshali A, Ngcapu S, Mindel A, et al. HIV susceptibility in women: the roles of genital inflammation, sexually transmitted infections and the genital microbiome. J Reprod Immunol 2021;145:103291. 10.1016/j.jri.2021.103291 [DOI] [PubMed] [Google Scholar]
- 16. Masson L, Arnold KB, Little F, et al. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect 2016;92:186–93. 10.1136/sextrans-2015-052072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filardo S, Di Pietro M, Tranquilli G, et al. Selected immunological mediators and cervical microbial signatures in women with chlamydia trachomatis infection. mSystems 2019;4:mSystems. 10.1128/mSystems.00094-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jespers V, Kyongo J, Joseph S, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 2017;7:11974. 10.1038/s41598-017-12198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masson L, Barnabas S, Deese J, et al. Inflammatory cytokine biomarkers of asymptomatic sexually transmitted infections and vaginal dysbiosis: a multicentre validation study. Sex Transm Infect 2019;95:5–12. 10.1136/sextrans-2017-053506 [DOI] [PubMed] [Google Scholar]
- 20. Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42. 10.1038/nrmicro794 [DOI] [PubMed] [Google Scholar]
- 21. Borgdorff H, Tsivtsivadze E, Verhelst R, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 2014;8:1781–93. 10.1038/ismej.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masson L, Passmore J-AS, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015;61:260–9. 10.1093/cid/civ298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. The Lancet Infectious Diseases 2018;18:554–64. 10.1016/S1473-3099(18)30058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarrett OD, Srinivasan S, Richardson BA, et al. Specific vaginal bacteria are associated with an increased risk of trichomonas vaginalis acquisition in women. J Infect Dis 2019;220:1503–10. 10.1093/infdis/jiz354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 2019;35:219–28. 10.1089/AID.2018.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. GBD 2015 HIV Collaborators . Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the global burden of disease study 2015. Lancet HIV 2016;3:e361–87. 10.1016/S2352-3018(16)30087-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fettweis JM, Serrano MG, Brooks JP, et al. The vaginal Microbiome and Preterm birth. Nat Med 2019;25:1012–21. 10.1038/s41591-019-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Guidelines for the management of symptomatic sexually transmitted infections: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization, 2021. [PubMed] [Google Scholar]
- 29. Verwijs MC, Agaba SK, Sumanyi J-C, et al. Targeted point-of-care testing compared with syndromic management of Urogenital infections in women (WISH): a cross-sectional screening and diagnostic accuracy study. Lancet Infect Dis 2019;19:658–69. 10.1016/S1473-3099(18)30724-2 [DOI] [PubMed] [Google Scholar]
- 30. Garrett N, Mtshali A, Osman F, et al. Impact of point-of-care testing and treatment of sexually transmitted infections and bacterial vaginosis on genital tract inflammatory cytokines in a cohort of young South African women. Sex Transm Infect 2021;97:555–65. 10.1136/sextrans-2020-054740 [DOI] [PubMed] [Google Scholar]
- 31. Asare K, Andine T, Naicker N, et al. Impact of point-of-care testing on the management of sexually transmitted infections in South Africa: evidence from the Hvtn702 human immunodeficiency virus vaccine trial. Clin Infect Dis 2023;76:881–9. 10.1093/cid/ciac824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adamson PC, Loeffelholz MJ, Klausner JD. Point-of-care testing for sexually transmitted infections: a review of recent developments. Arch Pathol Lab Med 2020;144:1344–51. 10.5858/arpa.2020-0118-RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization . The diagnostics landscape for sexually transmitted infections. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization, 2023. [Google Scholar]
- 34. Land KJ, Boeras DI, Chen X-S, et al. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 2019;4:46–54. 10.1038/s41564-018-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torrone EA, Morrison CS, Chen P-L, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018;15:e1002511. 10.1371/journal.pmed.1002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wand H, Reddy T, Dassaye R, et al. Estimating prevalence and incidence of sexually transmitted infections among South African women: implications of combined impacts of risk factors. Int J STD AIDS 2020;31:1093–101. 10.1177/0956462420915388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delany-Moretlwe S, Mgodi N, Bekker L-G, et al. High prevalence and incidence of gonorrhoea and chlamydia in young women eligible for HIV pre-exposure prophylaxis in South Africa and Zimbabwe: results from the HPTN 082 trial. Sex Transm Infect 2023;99:433–9. 10.1136/sextrans-2022-055696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behets F, Andriamiadana J, Rasamilalao D, et al. Sexually transmitted infections and associated socio-demographic and behavioural factors in women seeking primary care suggest Madagascar’s vulnerability to rapid HIV spread. Trop Med Int Health 2001;6:202–11. 10.1046/j.1365-3156.2001.00690.x [DOI] [PubMed] [Google Scholar]
- 39. UNAIDS . Country factsheets. 2023. Available: https://www.unaids.org/en/regionscountries/countries/madagascar
- 40. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosisis improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. 10.1128/jcm.29.2.297-301.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dworkin SL. Specimen size policy for qualitative studies using in-depth interviews. Arch Sex Behav 2012;41:1319–20. 10.1007/s10508-012-0016-6 [DOI] [PubMed] [Google Scholar]
- 42. Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability specimen sizes. Field Methods 2017;29:3–22. 10.1177/1525822X16639015 [DOI] [Google Scholar]
- 43. Louviere JJ, Hensher DA, Swait JD, et al. Stated choice methods: analysis and application. Cambridge, United Kingdom: Cambridge University Press, 2000. Available: https://www.cambridge.org/core/product/identifier/9780511753831/type/book [Google Scholar]
- 44. Ryan M, Gerard K, Amaya-Amaya M, eds. Using discrete choice experiments to value health and health care. Dordrecht, the Netherlands: Springer, 2008. [Google Scholar]
- 45. Goffin Molecular Technologies . PRESTO CT/NG combined qualitative real time CT/NG Assay (Kit # GM CG 160500). the Netherlands, [Google Scholar]
- 46. Crucitti T, Van Dyck E, Tehe A, et al. Comparison of culture and different PCR assays for detection of trichomonas vaginalis in self collected vaginal SWAB specimens. Sex Transm Infect 2003;79:393–8. 10.1136/sti.79.5.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Müller EE, Venter JME, Magooa MP, et al. Development of a rotor-gene real-time PCR assay for the detection and quantification of mycoplasma genitalium. J Microbiol Methods 2012;88:311–5. 10.1016/j.mimet.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 48. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:88. 10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-084918supp001.pdf (13.1KB, pdf)