Abstract
Introduction
Breast cancer survivors have an increased risk for chronic fatigue and altered gut microbiota composition, both with negative health and quality of life affects. Exercise modestly improves fatigue and is linked to gut microbial diversity and production of beneficial metabolites. Studies suggest that gut microbiota composition is a potential mechanism underlying fatigue response to exercise. Randomised controlled trials testing the effects of exercise on the gut microbiome are limited and there is a scarcity of findings specific to breast cancer survivors. The objective of this study is to determine if fitness-related modifications to gut microbiota occur and, if so, mediate the effects of aerobic exercise on fatigue response.
Methods and analysis
The research is a randomised controlled trial among breast cancer survivors aged 18–74 with fatigue. The primary aim is to determine the effects of aerobic exercise training compared with an attention control on gut microbiota composition. The secondary study aims are to test if exercise training (1) affects the gut microbiota composition directly and/or indirectly through inflammation (serum cytokines), autonomic nervous system (heart rate variability) or hypothalamic-pituitary-adrenal axis mediators (hair cortisol assays), and (2) effects on fatigue are direct and/or indirect through changes in the gut microbiota composition. All participants receive a standardised controlled diet. Assessments occur at baseline, 5 weeks, 10 weeks and 15 weeks (5 weeks post intervention completion). Faecal samples collect the gut microbiome and 16S gene sequencing will identify the microbiome. Fatigue is measured by a 13-item multidimensional fatigue scale.
Ethics and dissemination
The University of Alabama at Birmingham Institutional Review Board (IRB) approved this study on 15 May 2019, UAB IRB#30000320. A Data and Safety Monitoring Board convenes annually or more often if indicated. Findings will be disseminated in peer-reviewed journals and conference presentations.
Trial registration number
ClinicalTrials.gov, NCT04088708.
Keywords: Breast tumours, ONCOLOGY, MICROBIOLOGY, Exercise Test
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is one of the very few randomised controlled trials testing the effects of exercise on the gut microbiome, especially in cancer survivors experiencing fatigue.
A standardised, energy-balanced diet reduces diet and body weight induced variance on gut microbiota yet no prior randomised exercise and gut microbiome study has provided the same diet for all participants, as being done in our study.
This study seeks to understand the mechanistic links (inflammation, autonomic nervous system or hypothalamic-pituitary-adrenal axis mediators) between exercise and the gut microbiome, and determine if the benefits of exercise on fatigue are directly and/or indirectly related to changes in the gut microbiota composition.
Although assessors are masked to study group allocation and a standard attention control condition is used, the intervention precludes participant masking to exercise type.
Introduction
Nearly 8 million individuals worldwide are living with a history of breast cancer.1 2 Breast cancer survivors are at increased risk of altered gut microbiota composition (ie, dysbiosis) that may worsen future cancer risk, comorbidities and quality of life.3 Factors that may contribute to the persistent gut microbiota composition changes include reduced physical activity and aerobic fitness, and detrimental changes in body composition after breast cancer diagnosis.4–7 Given its importance on health and well-being,8–12 strategies for reversing gut microbiota dysbiosis are needed, especially in breast cancer survivors.
While elucidating gut microbiota dysbiosis in breast cancer survivors remains imperative, it is relevant that the gut microbiome is associated with fatigue in breast cancer survivors13 and survivors rank fatigue as the number one priority related to quality of life.14 Additionally, breast cancer survivors are more likely to report fatigue than their age-matched controls15 and one in four suffer persistent fatigue years after their cancer diagnosis,16 which exacerbates post-cancer disability and reduces the quality of life.17 18 Furthermore, fatigue is associated with a greater risk of cancer recurrence and mortality.19 Interestingly, the benefits of supervised exercise for breast cancer survivors extend beyond the expected improvements in cardiometabolic parameters to include improvements in fatigue and other domains of quality of life.20 As we (and others) have reported, exercise is a well-established non-pharmacological therapy for fatigue, yet its effects are somewhat modest (weighted effect size of 0.30 in a recent meta-analysis).21–24 Hence, elucidating mechanisms underlying fatigue response is needed to optimise fatigue reductions for non-responders and increase effect sizes achievable with exercise.24–27 Moreover, our prior work and that of others suggest that gut microbiota composition is one such mechanism, but further research is needed.13 28
Exercise training also presents as a promising strategy for reversing dysbiosis as it is linked to gut microbial diversity, abundance of select microbes and production of beneficial metabolites (eg, acetate, butyrate, propionate), although, these phenomena are currently limited to animal models or cross-sectional29–36 and non-randomised prospective human studies.37 Randomised controlled trials testing the effects of exercise on the gut microbiome are limited38 and there is a scarcity of findings specific to breast cancer survivors.7 One randomised controlled trial in healthy overweight and obese individuals found vigorous-intensity exercise training was associated with increased microbe diversity.38 To support the importance of intensity in exercise training, we recently showed in breast cancer survivors, cardiorespiratory fitness was a better correlate of gut microbe diversity compared with free-living activity energy expenditure.7 It is unknown if the modulation of the microbiota by exercise occurs solely through direct means such as alterations to colonic transit time,39 40 or indirectly through inflammation,41–43 autonomic nervous system,44 45 or hypothalamic-pituitary-adrenal (HPA) axis.46–48 Additionally other lifestyle interventions such diet49 and body weight changes50 independently affect the gut microbiota, making controls for these variables critical in exercise trials. Rigorously testing the dysbiosis-exercise link while also exploring the bidirectional gut-brain axis pathways responsible for exercise effects51 52 can inform future exercise recommendations and multimodal interventions to counter the adverse effects of gut dysbiosis.
Given the potential benefits of exercise training on the gut microbiome and fatigue, a better understanding of their relationships in response to an exercise intervention among breast cancer survivors is warranted. Herein, we describe our ongoing randomised controlled trial testing aerobic exercise training as a potential strategy to attenuate dysbiosis in breast cancer survivors with fatigue while also standardising diet intake and maintaining energy balance. We further propose to determine if fitness-related modifications to gut microbiota mediate the effects of aerobic exercise on fatigue response. This is a critical next step for several reasons. First, to our knowledge, there are currently no completed randomised controlled trials using exercise training as a potential modifier for dysbiosis in breast cancer survivors.53 Additionally, no other trials exploring these variables have been performed with a standardised diet to: (1) mitigate the underlying variance on gut microbiota and (2) promote weight maintenance.54 55 Therefore, we describe our methods to facilitate future replicability.
Methods
Aims and hypotheses
The primary study aim is to determine the effects of a 10-week aerobic exercise training intervention compared with a flexibility/toning standard attention control on gut microbiota composition among breast cancer survivors with fatigue. All participants are following an energy-balanced controlled feeding diet. The gut microbiome is being collected by faecal sample and assessed by 16S rRNA at baseline, week 5 to explore interim changes, week 10 as our primary time point and week 15 to explore the durability of effects. The primary outcome measure will be the comparison of microbiome composition using standard diversity and taxa comparison metrics (table 1). We hypothesise that compared with the control, the exercise training group will demonstrate significant differences in gut microbial diversity with increased Firmicutes (p), Bacteroides (g),7 56 and Bifidobacterium (g), 57 and decreased Actinobacteria (p) and Proteobacteria (p).7
Table 1.
Outcome measures for the primary and secondary study aims
| Aim | Outcome of interest | Outcome measure |
| Primary aim: to determine the effects of a 10-week aerobic exercise training intervention compared with a flexibility/toning standard attention control on gut microbiota composition among breast cancer survivors with fatigue. | Gut microbiota composition assessed by 16S rRNA. | Diversity comparisons:
Taxa comparisons |
| Aim 2a: to test if exercise training affects the gut microbiota composition directly and/or indirectly through inflammation, autonomic nervous system or hypothalamic-pituitary-adrenal (HPA) axis mediators. | Inflammation. | Serum cytokines:
|
| Autonomic nervous system. | Heart rate variability:
|
|
| HPA axis. | Hair cortisol. | |
| Aim 2b: to test if the exercise training effect on fatigue is direct and/or indirect through changes in the gut microbiota composition. | Gut microbiota composition assessed by 16S rRNA. | Diversity comparisons:
Taxa comparisons |
| Fatigue. | 13-item multidimensional fatigue scale Fatigue Symptom Inventory. |
A secondary study aim is to test if exercise training affects the gut microbiota composition directly and/or indirectly through inflammation, autonomic nervous system or HPA axis mediators (table 1). We hypothesise that exercise training will have direct and indirect effects on gut microbiota composition through markers of the hypothesised mechanisms (interleukin (IL)-6, IL-10,41–43 heart rate variability,44–46 cortisol.46–48 Another secondary study aim is to test if the exercise training effect on fatigue is direct and/or indirect through changes in the gut microbiota composition. We hypothesise that exercise effects on fatigue will be mediated by changes in beta diversity,13 58 specifically frequency of Firmicutes (p),7 Actinobacteria (p)13 and Bacteroides (g).13 41 59
Overall mechanistic framework
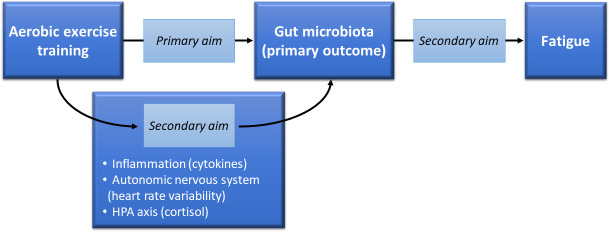
Given the relationships between cardiorespiratory fitness and gut microbiota composition,7 we have chosen an exercise intervention applying the principles of exercise prescription required to achieve an increase in cardiorespiratory fitness.60 The biological plausibility of a dysbiosis-exercise link also common to fatigue (eg, inflammation, autonomic nervous system and HPA axis)48 61–66 supports testing these potential mechanistic links in breast cancer survivors with fatigue. Thus, the overall mechanistic framework for our trial depicted in figure 1 can be applied to potentially optimising exercise interventions for the treatment of fatigue.
Figure 1.
Framework for testing exercise effects on gut microbiota and mechanistic links between exercise, gut microbiota and fatigue. HPA, hypothalamic-pituitary-adrenal.
Study overview and eligibility criteria
This two-arm, parallel group-controlled trial is randomising breast cancer survivors to 10 weeks of supervised aerobic exercise training or standard attention control (flexibility/toning) while on a controlled feeding diet. The trial is taking place at the University of Alabama at Birmingham (UAB) in Birmingham, Alabama, USA. Participant enrolment commenced 1 January 2020, was paused between March 2020 and August 2020 due to the COVID-19 pandemic, and is projected to end 1 January 2025. Institutional Review Board (IRB) approval has been obtained and all participants provide informed consent prior to participation (online supplemental materials 1and 2). Assessments occur at baseline and then at 5, 10 and 15 weeks. A study schema is provided in figure 2 and an overview of participants’ activities is provided in table 2. An electronic study manual of procedures is kept on a shared, Health Insurance Portability and Accountability Act (HIPAA)-compliant cloud server accessible to all study staff.
Figure 2.
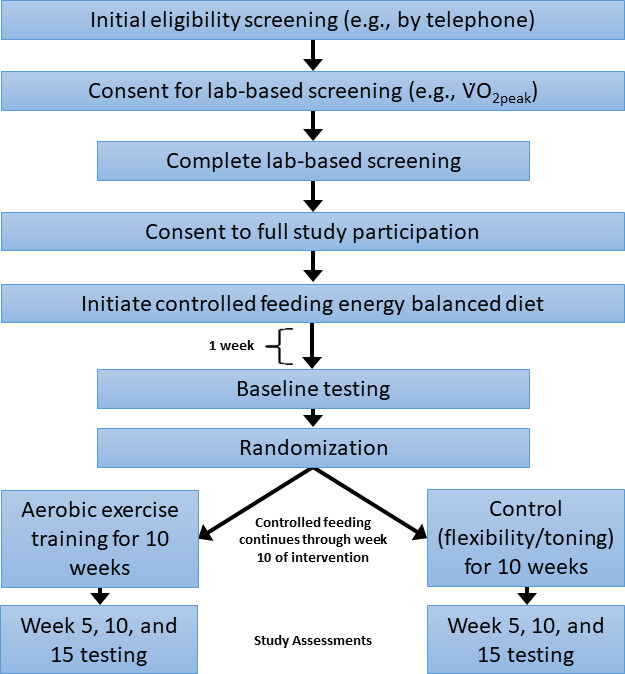
Study schema for testing aerobic exercise effects on gut microbiota composition and potential mechanistic links in breast cancer survivors.
Table 2.
Participant timeline (note: to facilitate temporal relationships, data collection is ordered within each assessment period as follows: #1—outcomes other than faecal sample and fatigue survey, #2—faecal sample 2–3 days after outcomes other than fatigue and #3—fatigue survey 2–3 days after faecal sample)
| Laboratory-based screening | Baseline assessment |
Exercise training or control | Follow-up assessments | ||
| Study week (preW=week leading up to randomisation (0); W=week after randomisation) | preW3 | preW2 – preW1 | preW1 –0 | W1 – W10 | W5, W10 and W15 |
| Laboratory-based screening consent, obtain medical clearance, complete laboratory-based screening (eg, V̇O2peak) | X | ||||
| Enrolment (consent for full participation) | X | ||||
| Controlled feeding diet (both study groups) | X | X | X | ||
| Self-administered questionnaire | X | X | |||
| Fatigue survey | X | X | |||
| Faecal sample collection for gut microbiota composition (with 3-day diet record) | X | X | |||
| Medication log (7 days prior to blood draw) | X | X | |||
| Fasted blood draw, heart rate variability, hair sample | X | X | |||
| Resting energy expenditure | X | X | |||
| Walking economy | X | X | |||
| V̇O2peak, weight, body mass index | X | X | |||
| Accelerometer with log sheet (7 days) | X | X | |||
| Dual-energy X-ray absorptiometry | X | X | |||
| Randomisation | X | ||||
| Exercise training or standard attention control | X | ||||
bmjopen-2023-081660supp001.pdf (271.7KB, pdf)
bmjopen-2023-081660supp002.pdf (919.7KB, pdf)
Inclusion criteria are as followed: (1) female breast cancer survivors ages 18–74 years with a history of ductal carcinoma in situ or stage 0, I, II, III breast cancer, (2) who are ≥1-year post-primary cancer treatment completion (chemotherapy and/or radiation), (3) average fatigue over the past week rated as ≥3 on a 1–10 Likert scales,67 (4) English speaking, (5) physician medical clearance for study participation, (6) able to ambulate without assistance, (7) no antibiotics for the past 90 days, (8) willing to avoid taking probiotics for the duration of the study and (9) after all other criteria are met, laboratory-based screening is used to confirm low fitness level (V̇O2peak <30 mL/kg/min). Exclusion criteria are as follows: (1) metastatic or recurrent cancer, (2) another diagnosis of cancer in the past 5 years (not including skin or cervical cancer in situ), (3) unstable angina, (4) New York Heart Association class II, III, IV congestive heart failure, (5) uncontrolled asthma, (6) interstitial lung disease, (7) current steroid use, (8) having been told by a physician to only do exercise prescribed by a physician, (9) dementia or organic brain syndrome, (10) schizophrenia or active psychosis, (11) connective tissue or rheumatological disease, (12) anticipate elective surgery during the study period, (13) anticipate changes in usual medications during the study period, (14) plan to move residence out of the local area during the study period, (15) plan to travel out of the local area >1 week during study participation, (16) contraindication to engaging in moderate-to-vigorous intensity aerobic exercise, (17) current or anticipated pregnancy during study participation, (18) live or work >50 miles from study site or do not have transportation to study site, (19) body mass index (BMI)>50 (confirmed during laboratory-based screening) or (20) anticipate needing antibiotics during the study period.
Recruitment and screening
Participants are being recruited through multiple recruitment strategies (eg, recruitment letters mailed to breast cancer survivors identified through the UAB O’Neal Comprehensive Cancer Center registry, UAB investigators’ waiting lists of cancer survivors inquiring about exercise and weight loss studies, newspaper advertising, cancer support groups, institutional websites and group emails, relevant non-institutional websites, flyers in waiting areas (hospitals, physicians’ offices)). Referrals from oncologists and other relevant healthcare providers are being requested using messaging (ie, electronic health records or institutional email) and face-to-face meetings; recruitment materials such as patient flyers are provided, as appropriate. Potential participants are given a description of the study and screened for eligibility based on a predetermined telephone script. In addition to questions related to the above eligibility criteria, participants are asked the following diet questions in the prescreening telephone screen to assess potential controlled feeding adherence and safety issues: (1) do you have any food allergies, restrictions, preferences or special diet (vegetarian, gluten-free, etc), (2) are you willing to eat the meals we provide, (3) do you drink alcohol? If yes, are you willing to refrain from alcohol during your participation in this study and (4) do you foresee any barriers to picking up the food, storing food or doing minimal meal preparation?
Enrolment and randomisation
Interested potential participants who pass the pre-screening telephone interview are invited to an orientation visit (in person or by videoconference) to complete administrative forms, sign laboratory-based screening consent (online supplemental material 1) and complete release forms for obtaining medical clearance with the study coordinator. Once medical clearance is received, the participant is scheduled for a laboratory-based screening visit which includes V̇O2peak to confirm cardiorespiratory fitness <30 mL/kg/min and BMI≤50 (see Section 3.5.3 for methods). If deemed eligible at the laboratory-based screening visit, informed consent for full study participation is obtained (online supplemental material 2), including optional permission to retain health information and biospecimens for future research. The participant is scheduled for initiation of controlled feeding and baseline assessment visits #1 and #2 (figure 3).
Figure 3.
Participant screening, enrolment and baseline assessment. A pre-screening telephone interview determines the potential eligibility of the participant. The orientation visit includes the completion of administrative forms, laboratory-based screening informed consent and release forms for obtaining medical clearance. Once medical clearance is received by the study team, the participant completes the laboratory-based screening visit, which includes collecting V̇O2peak and body mass index. If deemed eligible based on the screening visit, the individual will be invited to sign the consent for full study participation and be scheduled for controlled feeding initiation. Baseline assessment visit #1 is scheduled for at least 1 week after initiation of controlled feeding. Within 7 days of visit #1, (1) the participant is asked to collect the faecal sample at home 2–3 days after visit #1 and promptly overnight ships it to the laboratory, and then (2) complete the remaining assessment materials (eg, fatigue survey) 2–3 days after collecting the faecal sample and baseline visit #2 occurs to return these forms.
Participant randomisation is based on computer-generated random numbers and performed in blocks of four to facilitate an equal distribution between the two study groups. BMI is an important biological variable associated with gut microbiota composition,18 68 hence randomisation is stratified by BMI (<30 vs ≥30). The study statistician performed the computer generation of random numbers which were placed in sealed, opaque envelopes and delivered to the recruiting staff with written protocol for use. Assignments are made in the order in which participants complete baseline testing and are kept in a sealed envelope until the participant has completed all baseline testing. Once the study coordinator confirms the completion of baseline testing, the coordinator chooses the next envelope with group allocation. Participants remain partially blinded to study condition (eg, will not be told which study condition (exercise training or flexibility/toning intervention) is expected to yield more benefits and all receive a controlled diet which is potentially perceived as a ‘treatment’). Assessments, assays and data entry are conducted using objective and validated measures by staff who will remain blinded to study arm status.
Assessments
Schedule and masking
Assessments occur at baseline (pre-intervention), 5 weeks (mid-point intervention), 10 weeks (immediately post intervention) and 15 weeks (5 weeks post intervention) and are performed by staff who are masked to participant study group allocation. Table 2 presents the timeline of data and measures collected at each assessment visit. If eligible based on laboratory-based screening and the participant consents to full study participation (online supplemental material 2), then controlled feeding preparations are made and the baseline visit #1 is scheduled for 1 week after controlled feeding begins (figure 3). For each assessment, the participant completes two visits to the exercise testing laboratory. In preparation for assessment visit #1, participants are provided instructions for the laboratory-based measurements (location, parking, 12-hour fast, appropriate clothing, etc). During assessment visit #1, the participant provides a hair sample, completes the fasted blood draw, resting energy expenditure by indirect calorimeter, resting heart rate variability (Actiheart), dual-energy X-ray absorptiometry (DXA) and walking economy (ie, net V̇O2). Because the V̇O2peak and BMI measurements are taken at the screening visit, these are not repeated at baseline but are repeated at the follow-up assessments. During assessment visit #1, study staff provide the participant with the additional assessment materials (survey, accelerometer with log, 3-day diet record, medication log, faecal sample kit, etc) and related instructions. The participant ships the faecal sample back to the UAB microbiome laboratory within 7 days of visit #1 and returns the remaining assessment materials at assessment visit #2. To better align the temporal relationship between the gut microbiome and fatigue, the fatigue scale is collected at assessment visit #2 (ie, several days after faecal sample collection).
Gut microbiota composition
Participants are provided with a stool collection kit at each baseline and follow-up assessment visit #1 to self-collect the stool sample at home according to provided instructions. Briefly, the instructions are to collect the sample in a clean dry study-provided collection hat and scoop a small amount into the provided Para-Pak vials (Meridian Biosciences; Cincinnati, Ohio, USA) pre-labelled with participant identification and assessment time point, and then ship the sample back to our site via pre-paid overnight shipping materials. Once received by the microbiome laboratory, each sample is aliquoted into labelled cryovials and stored at −80°C until time for DNA extraction and 16S rRNA processing. One cryovial of precisely 100 µL is retained and labelled for future metabolomics assays (if indicated and funds can be obtained).
With each sample collection, the participant completes a faecal sample questionnaire69 and returns it to the research staff. The questionnaire asks the participant to report changes in normal diet and vitamin supplements; recent gastrointestinal symptoms (eg, nausea, vomiting, diarrhoea and constipation); and usual frequency or changes in probiotic supplements, yoghurt intake and high-fibre foods or fibre supplements. Participants also report recent medical treatments such as antibiotics, chemotherapy or radiation therapy and if they have ever had a major bowel resection, gastric bypass surgery, an inflammatory bowel disease (such as Crohn’s disease, ulcerative colitis, indeterminate colitis) or irritable bowel syndrome. The participant is also asked to complete a 3-day diet record capturing dietary intake 2 days prior to and the day of faecal sample.
Cardiorespiratory fitness (V̇O2peak)
Participants perform a graded treadmill (Trackmaster TMX428CP; Full Vision; Newton, Kansas, USA) test in accordance with the modified-Balke protocol to elicit V̇O2peak (ie, the highest measured rate of oxygen uptake expressed in mL/kg/min). Initially, V̇O2 is stabilised over a 3 min period of standing rest, after which, participants begin walking at 2.0 mph at 0% grade for 2 min. Grade is then increased by 3.5% every 2 min until the 12 min, at which point, grade is decreased to 12% and speed increased to 3.0 mph. Grade is increased by 2.5% each minute (as needed) until volitional exhaustion. V̇O2 and related gas exchange measures are aggregated in 30 s bins and determined by open-circuit spirometry (TrueOne 2400 system; ParvoMedics, Salt Lake City, Utah, USA). Gas analysers and flowmeter are calibrated prior to each test using standard gases and 3 L syringe, respectively. Heart rate and rating-of-perceived exertion (RPE; Borg 6–20, 6=no exertion at all, relaxed and 20=maximal exertion)70 are recorded in the final 30 s of each stage. Blood pressure is measured via auscultation at minutes 6, 10, 14, 16 and/or the final stage of the graded treadmill test.
Serum cytokines
Inflammatory cytokines, IL-6 and IL-10, are collected by blood samples. Participants are instructed to abstain from vigorous exercise, smoking and alcohol for 24 hours prior and fast for 12 hours prior to the blood draw. Blood samples are collected, processed and stored (−80°C) using standard operating procedure consistent with expert consensus recommendations71 and batch analysed according to manufacturer’s instructions by staff who are blinded to the participant’s group allocation.64 Serum cytokine assays will be analysed by the UAB Metabolism Core using an MSD imager (Meso Scale Discovery, Gaithersburg, Maryland, USA; chemiluminescence technology; multiplex platform). Blood and serum samples are being processed and stored so that future metabolomic/functional metabolic studies can be done if indicated and funds can be obtained. A 7-day medication log is collected with each blood sample for medication changes between assessments that may influence study outcomes (eg, anti-inflammatory agents).
Heart rate variability
Heart rate variability is evaluated with the Actiheart 5 (CamNtech, Cambridgeshire, UK) device. First, a urine sample is collected from participants to measure urine specific gravity—an indicator of hydration status. In accordance with manufacturer guidelines, skin is prepped with a 70% isopropyl alcohol wipe before positioning a two-lead electrode arrangement in the upper left quadrant across the participant’s chest. Measurements are collected during 5 min of quiet rest in the seated position. High-frequency sampling is used to measure inter-beat intervals wherein Actiheart software is used to perform offline analyses. The primary variables of interest include heart rate and root mean square of successive R-wave interval differences as well as the low-frequency, high-frequency components derived from the fast-Fourier transform. Procedures are performed in the morning hours in a dimly-lit, temperature-controlled room.
Hair cortisol
Hair specimens are collected by trained study staff. For participants whose hair is longer than 1.5–3 cm, a thin layer of hair (one to two hairs thick) parallel to the floor is cut from a point close to the scalp across a 4–5 cm length (laterally), to obtain a minimum of 50 strands of hair. For participants with shorter hair, the lateral cut is 6–8 cm (2 cm vertical × 5 cm lateral for long hair, >2 cm vertical × 7 cm lateral for shorter hair). String is used to indicate the end of the hair closest to the scalp; hair specimens are folded tightly into aluminium foil and placed in a small labelled bag at room temperature until being sent for assay at the Department of Biopsychology at Technische Universität Dresden in Dresden, Germany.
Fatigue
Fatigue is measured by a 13-item multidimensional fatigue scale (ie, Fatigue Symptom Inventory).72 On a 1–10 scale (1=not at all fatigued, 10=as fatigued as I could be), participants are asked to rate their level of fatigue on the day they felt most and least fatigued in the last week, the average level of fatigue in the last week and the level of fatigue at the time of survey. Participants report how much fatigue interferes (1=no interference, 10=extreme interference) with their general level of activity, ability to bathe and dress, their normal work activity, ability to concentrate, relations with other people, enjoyment of life and mood. Participants report how many days in the past week they felt fatigued for any part of the day and how much of the day on average the participant experienced fatigue (1=none of the day, 10=the entire day). Since our prior studies have demonstrated that exercise effects on fatigue may vary by dimension (ie, intensity vs interference; intensity=mean of four items; interference=mean of seven items, 0–10 scale) our final analyses will focus on fatigue interference.
Potential covariates
Self-administered survey measures age, race/ethnicity, education level, annual household income, marital status, smoking history, alcohol intake, employment status and a number of recent sick days, cancer-related factors (date of diagnosis, stage, subtypes (eg, receptor status), current and past cancer treatment type (including, but not limited to, radiation, chemotherapy and anti-oestrogen therapy)), caffeine intake, dietary supplements (including prebiotic, probiotic and vitamins), current medications (including over the counter medications), any antibiotic medications over the last 6 months, any steroid medications or injections over the last 6 months, current/past diagnosis of and treatment for anxiety or depression, treatment duration, time since treatment completion), medical comorbidities73 (including but not limited to endocrine or hormone disorders), history of surgeries, menopausal status6 and history of COVID-19 diagnosis. If a participant is not able to recall medical-related information, a medical release form is completed allowing study staff to request this information from the participant’s physician.
Because stress, depression, anxiety, sleep quality, pain and fatigue may cluster and be associated with inflammation,74–76 stress is measured by Perceived Stress Scale-10,77 depression and anxiety is measured by 14-item Hospital Anxiety and Depression Scale,78 sleep dysfunction is measured subjectively using the Pittsburgh Sleep Quality Index79 and pain is measured by the Patient-Reported Outcomes Measurement Information System (PROMIS; http://www.nihpromis.org/default.aspx).80 Because post-traumatic stress symptoms are associated with psychosocial outcomes and gut microbiota composition,81 82 post-traumatic stress is measured using the Post-traumatic Stress Disorder Checklist.83–86
To assess free-living physical activity, participants are given the same ActiGraph accelerometer (ActiGraph; Pensacola, Florida, USA) device for each assessment to be worn at the waist for seven consecutive days during waking hours (non-dominant hip; same side each time). Participants are instructed to remove the accelerometer while bathing, showering or swimming and are asked to complete an accelerometer log (times device removed, exercise not detectable by device, sleep times, etc). The accelerometer is set for 30 s epochs and monitoring is repeated if less than four valid days are recorded. Non-wear time is defined when no motion is detected for 60 min. A valid day is defined as at least 10 hours of valid wear time. The following cut points are planned: Sedentary: 0–99 counts/min; inactive: 100–499 counts/min; light: 500–1951 counts/min; moderate: 1952–5724 counts/min; and vigorous: 5725+counts/min.87 88 Leisure-time physical activity is measured using the Godin Leisure Time Exercise Questionnaire which asks for the average weekly frequency of leisure-time exercise for periods exceeding 10 min over the past month per three activity intensity levels (light, moderate or vigorous).89 90
BMI is calculated from weight and height (weight (kg)/height (m2)) obtained from a scale (in light clothing) and wall stadiometer (without shoes). DXA scans assess lean mass and fat mass using the Lunar Dual Energy X-ray Absorptiometry Scanner (iDXA; Lunar Radiation Madison, Wisconsin, USA). Pre-menopausal women at risk for pregnancy undergo a urine pregnancy test prior to each DXA scan.
Other relevant measurements
Resting energy expenditure measurement is required to more accurately assess participant’s calorie needs for the controlled feeding which facilitates energy balance and resultant weight maintenance during the study. Hence, resting energy expenditure is measured by ventilated hood indirect calorimetry (TrueOne 2400 system; ParvoMedics, Salt Lake City, Utah, USA) while lying quietly on an examination table. Participants must fast for at least 6 hours prior (4 hours if they are diabetic), avoid physical activity for 12 hours and avoid any caffeine or nicotine for at least 2 hours prior to this test.
Although not originally proposed, walking economy (ie, net V̇O2) was added because it reflects oxygen uptake during ambulation, an important alternative measure of (mobility) independence in older women.91 Participants wear a hip-worn accelerometer and complete a fixed-workload task by walking on a treadmill at 2.0 mph (0% grade) for 6 min during which steady-state V̇O2 is reached. RPE (Borg 6–20, 6=no exertion at all, relaxed and 20=maximal exertion)70 is collected at minutes 3 and 6. At minute 5, the participant reports perceived difficulty of the test using a Visual Analogue Scale (100 mm line). Blood pressure is measured at rest and while standing. Blood pressure is also measured at the 1, 2 and 5 min time points during walking. Participants remain quietly seated for at least 10 min between the walking economy and V̇O2peak tests during the follow-up assessments.
Quality of life is measured with The Functional Assessment of Cancer Therapy-Breast (FACT-B)92 because of its relation to fatigue, relevance for breast cancer populations and repeated use in prior studies which allow for comparison of study results. The FACT-B is a 37-item instrument using 5-point Likert scales and includes the subscales of physical well-being, social well-being, emotional well-being, functional well-being and additional concerns.92
Since cognitive function is associated with the gut microbiome93 and physical activity in breast cancer survivors,94 cognitive function is measured with the 10-item Frequency of Forgetting scale.95 The summed score will assess subjective memory impairment (total score) along with four memory subscales (general memory, frequency of forgetting, frequency of forgetting when reading and remembering past events).
To improve adherence to future, similar exercise training protocols, the self-administered survey assesses social cognitive theory constructs: exercise self-efficacy (barriers and walking), enjoyment, social support, barriers and outcome expectations. Barriers self-efficacy (ie, confidence in ability to overcome barriers) is measured using a 9-item scale specifically designed for breast cancer patients.96 The scale uses frequently reported barriers among patients with breast cancer (eg, ‘How confident are you that you can exercise when you are tired?’). Walking task self-efficacy scale is assessed with a 6-item scale asking participants to rate confidence in their ability to walk at a moderately fast pace for 5, 10, 15, 20, 25 and 30 min.97 Analyses for barriers and walking task self-efficacy are using the mean score for the Likert scale (0%=not at all confident to 100%=extremely confident). Perceived exercise barriers (or barriers interference) are measured by asking participants to rate on a 5-point Likert scale (1=never to 5=very often) how often 21 different barriers (eg, lack of time, weather) interfere with exercise. The items are summed for a perceived barriers score.98–100 Physical activity enjoyment is measured with a single question (5-point Likert scale).100 Social support is measured by asking for the frequency with which friends (two items) or family (two items) encourage or offer to exercise with the participant. Items are summed for a friends, family and total social support score.101 102 For outcome expectations, participants are asked to rate their agreement on a 5-point Likert scale (1=strongly disagree to 5=strongly agree) with the statement that exercise would result in 17 potential benefits or risks. 14 positive benefits (eg, feel less depressed) and 3 negative outcomes (eg, increased joint pain) are included. Responses are summed for positive outcome expectations and negative outcome expectations.100 The participants answer the outcome expectation questions twice: once considering stretching and light resistance exercises and again considering aerobic exercise.
Participant satisfaction
At the 15-week assessment, participants are asked to provide a written evaluation of the study staff and procedures. All participants are asked to report their agreement (Likert scale; 1=strongly disagree to 5=strongly agree) with 10 statements relating to the clarity of study information, helpfulness of staff interactions, palatability of the provided food and ease of following the menu, the likelihood of recommending this study to others and overall satisfaction with the study staff and activities. One open-ended question seeks any additional information they would like to share with the study team.
Data quality control
Multiple strategies are being used to minimise missing data (eg, baseline testing and controlled feeding before randomisation provides a ‘run-in’ period, monetary and non-monetary incentives, up to date contact information, ongoing review of source documents by study coordinator for immediate rectification of missing data).103 Study staff are trained by the investigator with the relevant expertise using an electronic manual of procedures with regular review of source documents for quality. Multiple trained staff are present during in-person assessment activities increasing accountability and immediate identification of potential drift in protocol adherence. All most recent IRB-approved study forms are stored on a shared, HIPAA-compliant cloud server.
Interventions
Supervised exercise sessions
Participants are randomised to 10 weeks of either an aerobic exercise intervention or a flexibility/toning attention control condition. Sessions occur on non-consecutive days of the week at the study site and are supervised by experienced exercise specialists who are not involved in the collection of outcome assessments.
Aerobic exercise sessions
Aerobic exercise sessions, supervised by trained exercise specialists, are primarily performed using the treadmill. However, the cycle ergometer may be used if preferred by the participant. The training target heart rate zone for each session corresponds with the heart rate at a given percentage of V̇O2peak measured at the most recent assessment. Training sessions commence with a 5 min warm-up consisting of light treadmill walking and stretching. During the first week of training, after warm-up, participants perform 20 min of exercise at ≈60% maximum heart rate (equivalent to ≈45–50% V̇O2peak). Over the next 3 weeks, exercise duration is increased by 5 min intervals, as tolerated, so that by the beginning of the fifth week participants are exercising for 40 min (up to a total of 60 min with warm-up and stretching time). This coincides with an elevation in exercise intensity equating to ≈75% of maximum heart rate (≈55–60% of V̇O2peak) by the fifth week. Following each exercise bout, participants cool down for 3–5 min. To mitigate stagnation, and facilitate continued improvement of V̇O2peak,104 high-intensity interval exercise is added during weeks 5–10 as described in table 3. 8–10 work-intervals are performed at a workload to elicit ≈85–90% maximum heart rate for 60 s with rest intervals of 3 min with the total exercise duration ranging from 20 to 40 min.
Table 3.
Aerobic exercise progression (based on maximum heart rate; high intensity added in later weeks to facilitate continued cardiorespiratory fitness improvement)
| Week | Intensity | Max heart rate (%) | Duration (min) | Frequency per week |
| 1–4 | Moderate-intensity, continuous | 60–75 | 20–35 | 3 |
| 5–7 | Moderate-intensity, continuous | 75 | 40 | 2 |
| High-intensity interval | 85–90 | 20–22 | 1 | |
| 8–10 | Moderate-intensity, continuous | 75 | 40 | 1 |
| High-intensity interval | 85–90 | 22–28 | 2 |
Standard attention controls
The non-aerobic exercise attention control condition controls for the effects of attention and social interaction through the administration of flexibility/range-of-motion activities using light resistance bands delivered at the same frequency as the aerobic condition (ie, three times per week). The sessions last about 40 min and target the head/neck, shoulder, elbow/forearm, hand/wrist, trunk/hip and ankle/foot. The progression of activities over the 10-week period involves performing additional exercises and sets (ie, Thera-bands) that provide minimal resistance (ie, sham). The first 5 weeks of the control condition involve performing body stretches without resistance (20–30 s for one to two sets). In weeks 6–7, the light resistance Thera-band is used to perform the stretches for the upper extremities once per week for 8–10 repetitions for two sets, and the other two sessions are body stretches without resistance. In weeks 8–10, the light resistance Thera-band is used twice per week for 8–10 repetitions for two sets, and one session will be body weight stretches without resistance. Such a progression is not expected to induce aerobic fitness adaptations and is designed to maintain participant interest and expectation of treatment benefit. Control condition participants are asked to not undertake additional exercise (eg, not join a gym and begin exercising) during the 10-week intervention period.
Missed exercise and control sessions
Session attendance is tracked weekly and missed sessions are made up as soon as possible during the intervention period. No more than four supervised aerobic sessions will occur in 1 week. Exercise specialists encourage exercise adherence by discussing social cognitive theory-based educational newsletters with participants at six time points during the 10 weeks of aerobic exercise and standard attention control.105
Controlled feeding
Controlled feeding provided by the UAB Center for Clinical and Translational Science Metabolic Kitchen standardises dietary intake across all participants. The menus are designed to provide 55% of energy as carbohydrate primarily through complex sources (fibre: 21–38 g/day), 23% as fat, and a minimum of 22% as protein (≈0.8 g/kg). Dietary sodium intake and the polyunsaturated:saturated (P:S) fat ratio are held constant (sodium <3500 mg/d, P:S fat ratio of 1 and saturated fat less than 30% of total fat intake).
Prior to initiating controlled feeding, the participant meets with a study registered dietitian to review the study menu and collect information about food allergies and intolerances. Changes to the menu based on dietary preferences are attempted if substitutions are accessible to the Metabolic Kitchen and maintain the standardised diet protocol. The participant and study dietitian meet a second time to review the final menus and discuss approved beverages and seasonings. Each participant starts weekly meal pick up from the Metabolic Kitchen at least 1 week before baseline assessment visit #1.
To allow the Metabolic Kitchen time to prepare the controlled feeding, the daily calorie need (total energy expenditure) is estimated pre-baseline using the Harris Benedict equation and an activity factor to promote weight maintenance. This estimate is then updated once resting energy expenditure data is available at the baseline assessment. The estimate of total energy expenditure is further updated for participants randomised to the aerobic exercise condition using the individual’s V̇O2peak and resting energy expenditure data based on prior work by the investigative team (equation provided in online supplemental material 3).106 107 The total energy expenditure estimates for all participants are updated, if appropriate, based on the week 5 assessment of V̇O2peak and resting energy expenditure. A study registered dietitian monitors body weight weekly and uses these changes and participant dietary preferences to further refine the calorie content and menus.
bmjopen-2023-081660supp003.pdf (128.4KB, pdf)
Controlled feeding adherence
Menu checklists are included with each weekly food pick up and participants are asked to log how much of the provided foods they consume and report additional foods and beverages along with the amounts consumed. The menu checklists are returned at exercise and control sessions on a weekly basis and reviewed by the dietitian for adherence. Participants with potential adherence issues or missing or incomplete checklists are called by a study dietitian for reminders and instruction.
Staff training
Staff are trained using a variety of electronic manuals, protocols and up-to-date IRB-approved study forms and scripts. An electronic manual of procedures is maintained in a shared, HIPAA-compliant cloud server for reference by staff. Given the range of staff responsibilities (ie, exercise intervention, diet), additional supplemental role-specific protocols are also maintained (eg, exercise progression prescription for exercise specialist and controlled feeding menu review scripts for dietitian).
Intervention fidelity plan
The exercise and controlled feeding intervention fidelity plans include the five domains recommended by National Institutes of Health (NIH) Behaviour Change Consortium108 (ie, study design, provider training, treatment delivery, treatment receipt and enactment of treatment skills). Fidelity is facilitated with the electronic manual of procedures, standardised scripts and participant education materials. Data sources for tracking exercise intervention include a review of all exercise session record sheets (ie, attendance, if exercise goals are met and if exercise progression is administered according to protocol) and direct observation by each interventionist at least once a month. The main data source for tracking controlled feeding fidelity are menu checklists on which the participant reports the provided foods consumed and any additional foods/beverages consumed. The food included in each controlled feeding pick up is reviewed for accuracy and completeness by a trained research staff before the food is given to the participant. Further, study registered dietitians offer the same food substitutions for all participants requesting a change. Monthly reports are presented to the study team to monitor the fidelity of both the exercise and controlled feeding so that fidelity concerns can be rectified in a timely manner.
Statistical analysis
Sample size and power considerations
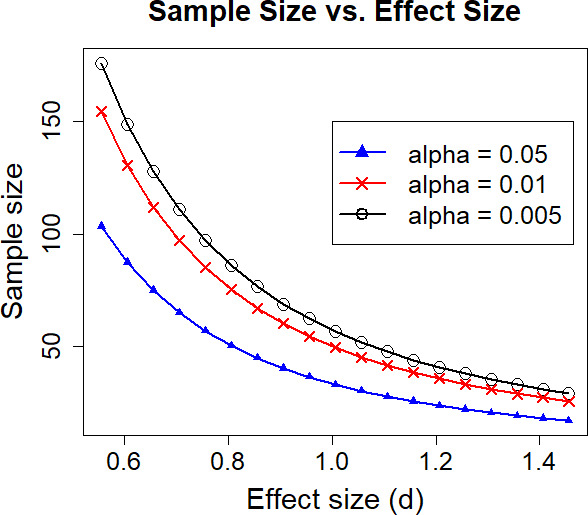
Sample size is based on detecting alpha diversity and beta diversity taxa comparisons. The power calculation is based on two-tailed test at power of 0.8 using software G*Power V.3.1.9.2.109 110 Our pre-COVID-19 pandemic sample size was estimated at 126 (63 in each group) with 100 (50 per study group) remaining after dropouts. This sample size would have allowed us to detect a medium effect size (d=0.57; power of 0.8, p<0.05) in alpha diversity which is sufficient for detecting effects related to associations with fatigue and intervention effects falling midway between that found in our two pilot studies. Relevant to taxa comparisons, we have >0.8 power to detect the effect of any of the taxa after multiple testing correction (q value <0.05).111–113 Due to the detrimental impact of the COVID-19 pandemic on recruitment into on-site, supervised exercise trials, we provide revised contingency power calculations in figure 4, where we can see that with sample size decreasing, the effect size we can detect changes from moderate to large. For example, for enrolling at 100%, 75% (74 samples with 37 per group) and 50%, the effect size that can be detected changes from 0.57, to 0.67, and to 0.81 (with power of 0.8 and alpha of 0.05). Of note, larger effect sizes are possible in this study (compared with our pilot studies) because the study will provide controlled feeding (reducing variability), select low fit individuals (greater chance of improvement) and manipulate the exercise exposure (standardise the exercise exposure). Also relevant, the sample sizes in our pilot studies (N=12 and 37) were smaller than our proposed study even with dropped enrolment yet yielded statistically significant results (eg, a significant association between alpha diversity and cardiorespiratory fitness in 37 breast cancer survivors).7 13
Figure 4.
Revised contingency power curve.
Data management and analysis considerations
Microbiome 16S gene sequence data is analysed using the QIIME114 analysis package, our in-house developed automated analysis pipeline QWRAP69 and DADA2115 to provide a robust error model for sample filtering and clustering. Data quality is assessed using FastQC, with low-quality data filtered out using the FASTX toolset. Filtering, denoising and clustering of reads into Amplicon Sequence Variants is done using DADA2. Taxon assignment is performed using Mothur116 and the SILVA 16S rDNA database.117 Alignment and phylogenetic inference is then performed using PyNAST118 and FastTree.119 Comparative analytical tools such as UniFrac120 are used to assess differences between samples and sample groups using principal coordinates analysis. To expedite sample processing and reporting, QWRAP automates the running of these tools using a single command line argument on UAB’s high-performance computing cluster, Cheaha.
Survey and other data entry and checking is conducted by trained research staff masked to study group allocation using password protected Research Electronic Data Capture. Data analyses will be carried out on an intent-to-treat basis. A multiple imputation approach will be employed to handle any missing data that cannot be rectified and we will conduct sensitivity analysis to assess the robustness of our findings.103 121 SAS software, V.9.3 (SAS Institute, Cary, North Carolina, USA) and R software, V.4.3.1122 will be used for data analysis. Transformations and non-parametric procedures will be performed when needed. The false discovery rate (FDR) will be used for multiple testing correction and the statistical significance threshold will be FDR q≤0.05 (q value is a p value after FDR correction). Each element (ie, alpha diversity, beta diversity and taxa level comparisons) describes a different perspective on gut microbiota changes and is integrated for interpretation (eg, does exercise change the relative abundance of organisms and, if so, which organisms). We will assess the microbiota composition change over time using mixed-effects models.123 All mediation analyses will conduct indirect effects analysis with the bootstrap method developed by Hayes.124 Week 10 is our primary time point yet we will also analyse week 5 to assess interim changes that occur and week 15 to assess durability.
Participant safety and withdrawal
Risk management and safety
Participant safety is facilitated by obtaining medical clearance, limiting to a BMI<50, collecting a medical history and the PAR-Q (Physical Activity Readiness Questionnaire) before the laboratory-based screening, and consulting clinical investigators, if indicated. Exercise sessions are supervised by exercise specialists who have experience training cancer survivors or chronic disease populations. Additionally, physician supervision is provided during fitness testing when deemed appropriate based on American College of Sports Medicine (ACSM) guidelines.125 Information about food allergies and intolerances are screened for and collected before initiating controlled feeding and throughout participation and these are communicated to the Metabolic Kitchen to minimise allergen contamination.
Adverse event reporting
Adverse events are identified spontaneously (eg, reported to research staff during contact time) or non-spontaneously (structured interview done at each assessment time point). Reported adverse events are reviewed promptly by the Principal Investigator (Rogers) and reported to the IRB according to local requirements. A Data and Safety Monitoring Board (DSMB) is convened annually or more often if indicated.
Handling of withdrawals
Participants are informed of their right to withdraw at any time without consequences in the informed consent forms and during the signing of consent forms. Participants will be withdrawn from the study if any social, psychological or physical conditions arise that may unduly increase risk of participating in the study. Data will be analysed on an intention-to-treat basis.
Unexpected required antibiotics
Given the effect of antibiotics on the gut microbiota composition, participants unexpectedly requiring intensive antibiotic therapy while enrolled in the study will be withdrawn from the study. Intensive antibiotic therapy is defined as intravenous, extended use (ie, ≥2 weeks), or combined therapy (multiple broad-spectrum agents). Less intensive antibiotic use will be tracked by self-administered survey and considered during the analyses.
Patient and public involvement
Patients and members of the public were not involved in the design of the trial.
Ethics and dissemination
The UAB IRB approved this study, 15 May 2019, UAB IRB#30 000 320. The trial is registered with ClinicalTrials.gov. A DSMB convenes annually or more often if indicated. Any amendments will be submitted to the IRB and DSMB for approval. Research findings will be disseminated in peer-reviewed journals and conference presentations.
Discussion
The ROME study is the first randomised controlled exercise training study in fatigued breast cancer survivors testing exercise effects on gut microbiota composition while standardising dietary intake with rigorous attention to energy balance. Our careful attention to diet and energy balance is critical to more fully understanding the role that exercise can play in altering dysbiosis in breast cancer survivors, a group at increased risk for detrimental changes in gut microbiota composition. Also, understanding the potential mechanistic links between aerobic exercise training, gut microbiota composition and fatigue in cancer survivors has great potential to improve the lives of the breast cancer survivors suffering fatigue.
Thus, we describe a highly rigorous trial that is especially appropriate for studying exercise, gut microbiome and fatigue in breast cancer survivors because it integrates a standard attention control condition and energy-balanced controlled feeding. The standard attention control condition is critical to detecting exercise effects on this patient-reported outcome beyond staff attention alone.126 Further, few randomised trials testing exercise effects on the gut microbiome have attempted to standardise diet intake with energy-balanced controlled feeding, a critical element given the strong association between diet, body weight and the gut microbiome characteristics.49 53 127
Given the careful attention to the temporal relationships and randomised study design, this study will explore mechanistic pathways heretofore most frequently studied in animal models rather than humans. With regard to the potential mechanisms through which exercise influences the gut microbiome, we will explore exercise induced changes to inflammation, the autonomic nervous system and the HPA axis. Exercise training in breast cancer survivors positively impacts inflammatory markers.128 In particular we have previously observed beneficial changes in IL-10 and tumour necrosis factor-α.25 A better understanding of the bidirectional communication between the microbiome and inflammation, HPA and autonomic nervous system is needed. Microbes influence cytokine production and T-cell activation33 129 and they and their metabolic by-products can also directly stimulate immune cells with a resultant influence on cytokine release.33 130 Similarly, pro-inflammatory cytokines influence serotonin availability, serotonin and norepinephrine synaptic reuptake pumps, HPA axis and regional brain activity.42 Gut microbes also influence the autonomic nervous system through the vagus nerve,48 as exemplified by reduced anxiety and depression-related behaviour in mice given Lactobacillus rhamnosus, with this effect absent in vagotomised mice.131 In a separate animal study, mice pretreated with a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175), then exposed to a water avoidance stressor, exhibited attenuated HPA axis and autonomic nervous system activity.132 Given that exercise alters the microbiome, inflammation, HPA and autonomic nervous system, a better understanding of the direct and/or indirect relationships are needed.
Recent interest related to our primary aim to test exercise effects on gut microbiome has grown. Allen et al 58 observed significant changes in gut microbiome beta diversity after 6 weeks of supervised exercise training in healthy adults (20–45 years old) and showed the changes reversed post-intervention. Additionally, positive changes to the gut microbiome have been observed in older adults participating in exercise interventions.57 59 Yet, the literature in cancer populations connecting exercise to changes in the microbiome warrants additional scrutiny. Sampsell et al 133 recently conducted a 12-week exercise intervention in 10 breast cancer survivors with reassessment after a 12-week washout period. No statistically significant pre-post differences in alpha or beta diversity were detected yet a follow-up mouse study yielded a trend toward lower tumour development in mice colonised with post-exercise microbiota versus those colonised with pre-exercise microbiota.
Others report on the relationship between fatigue and gut microbiota composition in cancer survivors,134 135 but we were the first to focus on breast cancer survivors and observe fatigue was associated with alpha diversity and differences in beta diversity representing shifts in taxa relative abundance.13 Additionally, understanding the role of exercise on the gut microbiota composition in fatigue response can be leveraged to identify new therapeutic strategies warranting testing in larger trials. Further, exercise is a well-known therapy for alleviating fatigue136 yet not all cancer survivors report fatigue improvements with exercise.26 Thus, a better understanding of the potential mediating effects of the microbiome can lead to exercise recommendations that optimise fatigue reductions.
As no research study is perfect, several limitations warrant discussion. Notably, the high scientific rigour made possible by the supervised exercise and controlled feeding may limit the translatability of the results to less controlled interventions. However, this is offset by the opportunities for exploring potential mechanistic links related to exercise, gut microbiome and fatigue. Moreover, the study inclusion and exclusion criteria may limit generalisability of the results to other cancer types or individuals with higher baseline cardiorespiratory fitness or BMI over 50. Finally, the COVID-19 pandemic’s detrimental impact on our anticipated sample size may preclude detecting smaller effect sizes and mediating factors. This is offset by several a priori design features that enhance study power: (1) controlled feeding (reduces variability), (2) selecting low-fit and fatigued individuals (greater chance of improvement), (3) manipulating the exercise exposure (standardises the exercise exposure) and (4) stratifying randomisation by BMI (reduces type 1 error and improves study power in trials with <200 participants per study condition).137
We will report findings in peer-reviewed journals and present them at conferences.
Supplementary Material
Acknowledgments
The authors would like to thank Josh Graham, Kaitlyn Waugaman, Sydney McGlawn, Victoria Roughsedge and Ildiko Nyikos for their administrative assistance in the setup and ongoing management of the study, and David Bryan for administrative support of assessments and conducting fitness tests.
Footnotes
Contributors: LQR conceived the study design, led and integrated the multidisciplinary input and achieved funding through the National Cancer Institute as the lead investigator. SJC, RWM, GH, NL, HK, EJL and BT helped design the final study protocol and choice of outcome measures and provided intellectual contributions in their expert areas. RBL led the development of sample process tracking and operationalising controlled feeding implementation and fidelity monitoring; she also completed the initial draft of the manuscript. AC and ES assisted with exercise intervention fidelity and adherence tracking protocols. AC completed a literature review to guide data management related to current medication use. All authors assisted with drafting the manuscript and have read, edited and approved the final manuscript.
Funding: This study is supported by the following National Institute of Health grants: R01CA235598, P30DK056336, T32 A047888, UL1TR003096, and R25CA76023.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Intl Journal of Cancer 2021;149:778–89. 10.1002/ijc.33588 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Bodai BI, Nakata TE. Breast cancer: lifestyle, the human gut Microbiota/Microbiome, and survivorship. Perm J 2020;24. 10.7812/TPP/19.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabiston CM, Brunet J, Vallance JK, et al. Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the Teachable moment. Cancer Epidemiol Biomarkers Prev 2014;23:1324–30. 10.1158/1055-9965.EPI-13-1179 [DOI] [PubMed] [Google Scholar]
- 5. Pradhan KR, Stump TE, Monahan P, et al. Relationships among attention function, exercise, and body mass index: a comparison between young breast cancer survivors and acquaintance controls. Psychooncology 2015;24:325–32. 10.1002/pon.3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vance V, Mourtzakis M, McCargar L, et al. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev 2011;12:282–94. 10.1111/j.1467-789X.2010.00805.x [DOI] [PubMed] [Google Scholar]
- 7. Carter SJ, Hunter GR, Blackston JW, et al. Gut Microbiota diversity is associated with cardiorespiratory fitness in post-primary treatment breast cancer survivors. Exp Physiol 2019;104:529–39. 10.1113/EP087404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thursby E, Juge N. Introduction to the human gut Microbiota. Biochem J 2017;474:1823–36. 10.1042/BCJ20160510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly DL, Lyon DE, Yoon SL, et al. The Microbiome and cancer: implications for oncology nursing science. Cancer Nurs 2016;39:E56–62. 10.1097/NCC.0000000000000286 [DOI] [PubMed] [Google Scholar]
- 10. Jiang H, Ling Z, Zhang Y, et al. Altered fecal Microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–94. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 11. Fu J, Bonder MJ, Cenit MC, et al. The gut Microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res 2015;117:817–24. 10.1161/CIRCRESAHA.115.306807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatt AP, Redinbo MR, Bultman SJ. The role of the Microbiome in cancer development and therapy. CA Cancer J Clin 2017;67:326–44. 10.3322/caac.21398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paulsen JA, Ptacek TS, Carter SJ, et al. Gut Microbiota composition associated with alterations in cardiorespiratory fitness and Psychosocial outcomes among breast cancer survivors. Support Care Cancer 2017;25:1563–70. 10.1007/s00520-016-3568-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollen PJ, Msaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: results of a survey of 1072 patients. Breast Cancer Res Treat 2015;151:679–86. 10.1007/s10549-015-3420-5 [DOI] [PubMed] [Google Scholar]
- 15. Carreira H, Williams R, Dempsey H, et al. Quality of life and mental health in breast cancer survivors compared with non-cancer controls: a study of patient-reported outcomes in the United Kingdom. J Cancer Surviv 2021;15:564–75. 10.1007/s11764-020-00950-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maass SWMC, Brandenbarg D, Boerman LM, et al. n.d. Fatigue among long-term breast cancer survivors: A controlled cross-sectional study. Cancers 13:1301. 10.3390/cancers13061301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv 2016;10:51–61. 10.1007/s11764-015-0450-2 [DOI] [PubMed] [Google Scholar]
- 18. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groenvold M, Petersen MA, Idler E, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 2007;105:209–19. 10.1007/s10549-006-9447-x [DOI] [PubMed] [Google Scholar]
- 20. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. breast cancer Res. Breast Cancer Res 2018;20:124. 10.1186/s13058-018-1051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers LQ, Courneya KS, Anton PM, et al. Effects of a Multicomponent physical activity behavior change intervention on fatigue, anxiety, and depressive Symptomatology in breast cancer survivors: randomized trial. Psychooncology 2017;26:1901–6. 10.1002/pon.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dennett AM, Peiris CL, Shields N, et al. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother 2016;62:68–82. 10.1016/j.jphys.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 23. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol 2017;3:961–8. 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2008;11. 10.1002/14651858.CD006145.pub2 [DOI] [PubMed] [Google Scholar]
- 25. Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther 2013;12:323–35. 10.1177/1534735412449687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogers LQ, Vicari S, Trammell R, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc 2014;46:1077–88. 10.1249/MSS.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2010;4:87–100. 10.1007/s11764-009-0110-5 [DOI] [PubMed] [Google Scholar]
- 28. Inglis JE, Lin P-J, Kerns SL, et al. Nutritional interventions for treating cancer-related fatigue: A qualitative review. Nutr Cancer 2019;71:21–40. 10.1080/01635581.2018.1513046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsumoto M, Inoue R, Tsukahara T, et al. Voluntary running exercise alters Microbiota composition and increases N-butyrate concentration in the rat Cecum. Biosci Biotechnol Biochem 2008;72:572–6. 10.1271/bbb.70474 [DOI] [PubMed] [Google Scholar]
- 30. Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014;63:1913–20. 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- 31. Lambert JE, Myslicki JP, Bomhof MR, et al. Exercise training modifies gut Microbiota in normal and diabetic mice. Appl Physiol Nutr Metab 2015;40:749–52. 10.1139/apnm-2014-0452 [DOI] [PubMed] [Google Scholar]
- 32. Choi JJ, Eum SY, Rampersaud E, et al. Exercise attenuates PCB-induced changes in the Mouse gut Microbiome. Environ Health Perspect 2013;121:725–30. 10.1289/ehp.1306534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bermon S, Petriz B, Kajėnienė A, et al. The Microbiota: an exercise Immunology perspective. Exerc Immunol Rev 2015;21:70–9. [PubMed] [Google Scholar]
- 34. Campbell SC, Wisniewski PJ, Noji M, et al. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS One 2016;11:e0150502. 10.1371/journal.pone.0150502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a Predictor of intestinal microbial diversity and distinct Metagenomic functions. Microbiome 2016;4:42. 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Houghton D, Stewart CJ, Stamp C, et al. Impact of age-related mitochondrial dysfunction and exercise on intestinal Microbiota composition. J Gerontol A Biol Sci Med Sci 2018;73:571–8. 10.1093/gerona/glx197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verheggen R, Konstanti P, Smidt H, et al. Eight-week exercise training in humans with obesity: marked improvements in insulin sensitivity and modest changes in gut Microbiome. Obesity (Silver Spring) 2021;29:1615–24. 10.1002/oby.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kern T, Blond MB, Hansen TH, et al. Structured exercise alters the gut Microbiota in humans with overweight and obesity-A randomized controlled trial. Int J Obes (Lond) 2020;44:125–35. 10.1038/s41366-019-0440-y [DOI] [PubMed] [Google Scholar]
- 39. Roager HM, Hansen LBS, Bahl MI, et al. Colonic transit time is related to bacterial metabolism and Mucosal turnover in the gut. Nat Microbiol 2016;1:16093. 10.1038/nmicrobiol.2016.93 [DOI] [PubMed] [Google Scholar]
- 40. Dainese R, Serra J, Azpiroz F, et al. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med 2004;116:536–9. 10.1016/j.amjmed.2003.12.018 [DOI] [PubMed] [Google Scholar]
- 41. Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social Stressor alters the structure of the intestinal Microbiota: implications for Stressor-induced Immunomodulation. Brain Behav Immun 2011;25:397–407. 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral Comorbidities in patients with cancer. J Clin Oncol 2008;26:971–82. 10.1200/JCO.2007.10.7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen AMW, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol 2006;57 Suppl 10:43–51. [PubMed] [Google Scholar]
- 44. Lavín-Pérez AM, Collado-Mateo D, Hinojo González C, et al. High-intensity exercise prescription guided by heart rate variability in breast cancer patients: a study protocol for a randomized controlled trial. BMC Sports Sci Med Rehabil 2023;15:28. 10.1186/s13102-023-00634-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niederer D, Vogt L, Thiel C, et al. Exercise effects on HRV in cancer patients. Int J Sports Med 2013;34:68–73. 10.1055/s-0032-1314816 [DOI] [PubMed] [Google Scholar]
- 46. Toohey K, Pumpa K, McKune A, et al. The impact of high-intensity interval training exercise on breast cancer survivors: a pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 2020;20:787. 10.1186/s12885-020-07295-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russell E, Koren G, Rieder M, et al. Hair Cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012;37:589–601. 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 48. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut Microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. 10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asnicar F, Berry SE, Valdes AM, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply Phenotyped individuals. Nat Med 2021;27:321–32. 10.1038/s41591-020-01183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Remely M, Tesar I, Hippe B, et al. Gut Microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes 2015;6:431–9. 10.3920/BM2014.0104 [DOI] [PubMed] [Google Scholar]
- 51. Ribeiro FM, Silva MA, Lyssa V, et al. The molecular signaling of exercise and obesity in the Microbiota-gut-brain axis. Front Endocrinol (Lausanne) 2022;13. 10.3389/fendo.2022.927170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Javed I, Cui X, Wang X, et al. Implications of the human gut-brain and gut-cancer axes for future Nanomedicine. ACS Nano 2020;14:14391–416. 10.1021/acsnano.0c07258 [DOI] [PubMed] [Google Scholar]
- 53. Sampsell K, Hao D, Reimer RA. The gut Microbiota: A potential gateway to improved health outcomes in breast cancer treatment and survivorship. Int J Mol Sci 2020;21. 10.3390/ijms21239239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ryu J, Lee E-Y, Min J, et al. Effect of a 1-year tailored exercise program according to cancer Trajectories in patients with breast cancer: study protocol for a randomized controlled trial. BMC Cancer 2023;23:200. 10.1186/s12885-023-10664-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Newton RU, Christophersen CT, Fairman CM, et al. Does exercise impact gut Microbiota composition in men receiving androgen deprivation therapy for prostate cancer? A single-blinded, two-armed, randomised controlled trial. BMJ Open 2019;9:e024872. 10.1136/bmjopen-2018-024872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang Y, Shi Y, Wiklund P, et al. n.d. The association between cardiorespiratory fitness and gut Microbiota composition in premenopausal women. Nutrients 9:792. 10.3390/nu9080792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Erlandson KM, Liu J, Johnson R, et al. An exercise intervention alters stool Microbiota and metabolites among older, sedentary adults. ther Adv infect dis. Ther Adv Infect Dis 2021;8. 10.1177/20499361211027067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut Microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018;50:747–57. 10.1249/MSS.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 59. Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal Bacteroides in healthy elderly women. Nutrients 2019;11:868. 10.3390/nu11040868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Nutrition, Energy, and Human Performance. Wolters Kluwer Health/Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 61. Fagundes C, LeRoy A, Karuga M. Behavioral symptoms after breast cancer treatment: A Biobehavioral approach. J Pers Med 2015;5:280–95. 10.3390/jpm5030280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013;30 Suppl:S48–57. 10.1016/j.bbi.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peterson CT, Sharma V, Elmén L, et al. Immune homeostasis, Dysbiosis and therapeutic modulation of the gut Microbiota. Clin Exp Immunol 2015;179:363–77. 10.1111/cei.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010;411:785–93. 10.1016/j.cca.2010.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al-Majid S, Gray DP. A Biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs 2009;10:381–91. 10.1177/1099800408324431 [DOI] [PubMed] [Google Scholar]
- 66. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 2011;11:625–32. 10.1038/nri3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Donovan KA, Jacobsen PB, Small BJ, et al. Identifying clinically meaningful fatigue with the fatigue symptom inventory. J Pain Symptom Manage 2008;36:480–7. 10.1016/j.jpainsymman.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dieli-Conwright CM, Mortimer JE, Schroeder ET, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer 2014;14. 10.1186/1471-2407-14-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar R, Eipers P, Little RB, et al. Getting started with Microbiome analysis: sample acquisition to Bioinformatics. Curr Protoc Hum Genet 2014;82:18. 10.1002/0471142905.hg1808s82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Borg GAV. Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise 1982;14:377. 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 71. Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 2009;8:113–7. 10.1021/pr800545q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the fatigue symptom inventory. Qual Life Res 1998;7:301–10. 10.1023/a:1024929829627 [DOI] [PubMed] [Google Scholar]
- 73. Groll DL, To T, Bombardier C, et al. The development of a Comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595–602. 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 74. Saligan LN, Kim HS. A systematic review of the association between Immunogenomic markers and cancer-related fatigue. Brain Behav Immun 2012;26:830–48. 10.1016/j.bbi.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal Transduction theory of depression. Psychol Bull 2014;140:774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thomas BC, Waller A, Malhi RL, et al. A longitudinal analysis of symptom clusters in cancer patients and their Sociodemographic predictors. J Pain Symptom Manage 2014;47:566–78. 10.1016/j.jpainsymman.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 77. Taylor JM. Psychometric analysis of the ten-item perceived stress scale. Psychol Assess 2015;27:90–101. 10.1037/a0038100 [DOI] [PubMed] [Google Scholar]
- 78. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 79. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 80. Cook KF, Dunn W, Griffith JW, et al. Pain assessment using the NIH Toolbox. Neurology 2013;80:S49–53. 10.1212/WNL.0b013e3182872e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morrill EF, Brewer NT, O’Neill SC, et al. The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology 2008;17:948–53. 10.1002/pon.1313 [DOI] [PubMed] [Google Scholar]
- 82. Leclercq S, Forsythe P, Bienenstock J. Posttraumatic stress disorder: does the gut Microbiome hold the key Can J Psychiatry 2016;61:204–13. 10.1177/0706743716635535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther 1996;34:669–73. 10.1016/0005-7967(96)00033-2 [DOI] [PubMed] [Google Scholar]
- 84. Cordova MJ, Andrykowski MA, Kenady DE, et al. Frequency and correlates of Posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol 1995;63:981–6. 10.1037//0022-006x.63.6.981 [DOI] [PubMed] [Google Scholar]
- 85. Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther 2001;39:977–86. 10.1016/s0005-7967(00)00084-x [DOI] [PubMed] [Google Scholar]
- 86. Parikh D, De Ieso P, Garvey G, et al. Post-traumatic stress disorder and post-traumatic growth in breast cancer patients--a systematic review. Asian Pac J Cancer Prev 2015;16:641–6. 10.7314/apjcp.2015.16.2.641 [DOI] [PubMed] [Google Scholar]
- 87. Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications. Med Sci Sports Exerc 1998;30:777–81. 10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 88. Sirard JR, Melanson EL, Li L. Field evaluation of the computer science and applications. Inc Physical Activity Monitor Med Sci Sports Exerc Mar 2000;32:695–700. 10.1097/00005768-200003000-00022 [DOI] [PubMed] [Google Scholar]
- 89. Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health 1986;77:359–62. [PubMed] [Google Scholar]
- 90. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985;10:141–6. [PubMed] [Google Scholar]
- 91. Carter SJ, Baranauskas MN, Singh H, et al. ARTE index Revisited: linking biomarkers of Cardiometabolic health with free-living physical activity in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 2022;322:R292–8. 10.1152/ajpregu.00075.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 1997;15:974–86. 10.1200/JCO.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 93. Gareau MG. Cognitive function and the Microbiome. Int Rev Neurobiol 2016;131:227–46. 10.1016/bs.irn.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 94. Ehlers DK, Rogers LQ, Courneya KS, et al. Effects of BEAT cancer randomized physical activity trial on subjective memory impairments in breast cancer survivors. Psychooncology 2018;27:687–90. 10.1002/pon.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zelinski EM, Gilewski MJ. A 10-item Rasch modeled memory self-efficacy scale. Aging Ment Health 2004;8:293–306. 10.1080/13607860410001709665 [DOI] [PubMed] [Google Scholar]
- 96. Rogers LQ, Courneya KS, Verhulst S, et al. Exercise barrier and task self-efficacy in breast cancer patients during treatment. Support Care Cancer 2006;14:84–90. 10.1007/s00520-005-0851-2 [DOI] [PubMed] [Google Scholar]
- 97. McAuley E, Mihalko S. Advances in sport and exercise psychology measurement. measuring exercise-related self-efficacy fitness information technology. 1998;371–89.
- 98. Lewis CE, Raczynski JM, Heath GW. n.d. Promoting physical activity in low-income African-American communities: the PARR project. Ethnicity & Disease 1993:106–18. [PubMed] [Google Scholar]
- 99. Rogers LQ, McAuley E, Courneya KS, et al. Correlates of physical activity self-efficacy among breast cancer survivors. Am J Health Behav 2008;32:594–603. 10.5555/ajhb.2008.32.6.594 [DOI] [PubMed] [Google Scholar]
- 100. Rogers LQ, Shah P, Dunnington G, et al. Social cognitive theory and physical activity during breast cancer treatment. Oncology Nursing Forum 2005;32:807–15. 10.1188/05.ONF.807-815 [DOI] [PubMed] [Google Scholar]
- 101. Sallis JF, Grossman RM, Pinski RB, et al. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine 1987;16:825–36. 10.1016/0091-7435(87)90022-3 [DOI] [PubMed] [Google Scholar]
- 102. Sallis JF, Hovell ]Melbourne F., Richard Hofstetter C, et al. A multivariate study of determinants of vigorous exercise in a community sample. Preventive Medicine 1989;18:20–34. 10.1016/0091-7435(89)90051-0 [DOI] [PubMed] [Google Scholar]
- 103. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012;367:1355–60. 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martin EA, Battaglini CL, Hands B, et al. Higher-intensity exercise results in more sustainable improvements for Vo2Peak for breast and prostate cancer survivors. Oncol Nurs Forum 2015;42:241–9. 10.1188/15.ONF.42-03AP [DOI] [PubMed] [Google Scholar]
- 105. Motl RW, Backus D, Neal WN, et al. Rationale and design of the STEP for MS trial: comparative effectiveness of supervised versus Telerehabilitation exercise programs for multiple sclerosis. Contemp Clin Trials 2019;81:110–22. 10.1016/j.cct.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 106. Hunter GR, Bickel CS, Fisher G, et al. Combined aerobic and strength training and energy expenditure in older women. Medicine & Science in Sports & Exercise 2013;45:1386–93. 10.1249/MSS.0b013e3182860099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hunter GR, Fisher G, Neumeier WH, et al. Exercise training and energy expenditure following weight loss. Medicine & Science in Sports & Exercise 2015;47:1950–7. 10.1249/MSS.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment Fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychology 2004;23:443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 109. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Research Methods 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 110. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and BIOMEDICAL sciences. Behavior Research Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 111. Bi R, Liu P. n.d. Sample size calculation while controlling false discovery rate for differential expression analysis with RNA-sequencing experiments. BMC Bioinformatics 17:146. 10.1186/s12859-016-0994-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pounds S, Cheng C. Sample size determination for the false discovery rate. Bioinformatics 2005;21:4263–71. 10.1093/bioinformatics/bti699 [DOI] [PubMed] [Google Scholar]
- 113. Liu P, Hwang JTG. Quick calculation for sample size while controlling false discovery rate with application to Microarray analysis. Bioinformatics 2007;23:739–46. 10.1093/bioinformatics/btl664 [DOI] [PubMed] [Google Scholar]
- 114. Navas-Molina JA, Peralta-Sánchez JM, González A, et al. Advancing our understanding of the human Microbiome using QIIME. Methods Enzymol 2013;531:371–444. 10.1016/B978-0-12-407863-5.00019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Callahan BJ, McMurdie PJ, Rosen MJ, et al. Dada2: high-resolution sample inference from Illumina Amplicon data. Nat Methods 2016;13:581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schloss PD, Westcott SL, Ryabin T, et al. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75:7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yilmaz P, Parfrey LW, Yarza P, et al. “The SILVA and "all-species living tree project (LTP)" Taxonomic frameworks”. Nucleic Acids Res 2014;42:D643–8. 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Caporaso JG, Bittinger K, Bushman FD, et al. Pynast: a flexible tool for Aligning sequences to a template alignment. Bioinformatics 2010;26:266–7. 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Price MN, Dehal PS, Arkin AP. Fasttree 2--Approximately maximum-likelihood trees for large alignments. PLoS One 2010;5:e9490. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lozupone C, Hamady M, Knight R. Unifrac--an online tool for comparing microbial community diversity in a Phylogenetic context. BMC Bioinformatics 2006;7:371. 10.1186/1471-2105-7-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li P, Stuart EA, Allison DB. Multiple imputation: A flexible tool for handling missing data. JAMA 2015;314:1966–7. 10.1001/jama.2015.15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. R: A language and environment for statistical computing. 2022. Available: https://www.R-project.org
- 123. Chen EZ, Li H. A two-part mixed-effects model for analyzing longitudinal Microbiome compositional data. Bioinformatics 2016;32:2611–7. 10.1093/bioinformatics/btw308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. In: Guilford publications. 2017. [Google Scholar]
- 125. Liguori G. American college of sports medicine. In: ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, 2020. [Google Scholar]
- 126. Tock WL, Maheu C, Johnson NA. Considerations of control conditions designs in randomized controlled trials of exercise interventions for cancer survivors. Can J Nurs Res 2022;54:377–91. 10.1177/08445621211062467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hossain F, Majumder S, David J, et al. Obesity modulates the gut Microbiome in triple-negative breast cancer. Nutrients 2021;13:3656. 10.3390/nu13103656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. de Jesus Leite MA, Gonçalves Á, Portari G, et al. Application of physical exercise therapies in breast cancer survivors and their effects on the inflammatory profile: A narrative review. Journal of Bodywork and Movement Therapies 2020;24:536–45. 10.1016/j.jbmt.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 129. Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 130. Rescigno M. Intestinal Microbiota and its effects on the immune system. Cell Microbiol 2014;16:1004–13. 10.1111/cmi.12301 [DOI] [PubMed] [Google Scholar]
- 131. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the Vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–5. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ait‐Belgnaoui A, Colom A, Braniste V, et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterology Motil 2014;26:510–20. 10.1111/nmo.12295 [DOI] [PubMed] [Google Scholar]
- 133. Sampsell K, Wang W, Ohland C, et al. Exercise and Prebiotic fiber provide gut Microbiota-driven benefit in a survivor to germ-free Mouse Translational model of breast cancer. Cancers (Basel) 2022;14. 10.3390/cancers14112722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hajjar J, Mendoza T, Zhang L, et al. n.d. Associations between the gut Microbiome and fatigue in cancer patients. Sci Rep 11:5847. 10.1038/s41598-021-84783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xiao C, Fedirko V, Beitler J, et al. The role of the gut Microbiome in cancer-related fatigue: pilot study on epigenetic mechanisms. Support Care Cancer 2021;29:3173–82. 10.1007/s00520-020-05820-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mishra SI, Scherer RW, Snyder C, et al. Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncology Nursing Forum 2014;41:E326–42. 10.1188/14.ONF.E326-E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kernan WN, Viscoli CM, Makuch RW, et al. Stratified randomization for clinical trials. Journal of Clinical Epidemiology 1999;52:19–26. 10.1016/S0895-4356(98)00138-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081660supp001.pdf (271.7KB, pdf)
bmjopen-2023-081660supp002.pdf (919.7KB, pdf)
bmjopen-2023-081660supp003.pdf (128.4KB, pdf)