Abstract
Background
While laparoscopic cholecystectomy is generally considered less painful than open surgery, pain is one of the important reasons for delayed discharge after day‐surgery and overnight stay following laparoscopic cholecystectomy. The safety and effectiveness of different pharmacological interventions such as non‐steroidal anti‐inflammatory drugs, opioids, and anticonvulsant analgesics in people undergoing laparoscopic cholecystectomy is unknown.
Objectives
To assess the benefits and harms of different analgesics in people undergoing laparoscopic cholecystectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded, and the World Health Organization International Clinical Trials Registry Platform portal (WHO ICTRP) to March 2013 to identify randomised clinical trials of relevance to this review.
Selection criteria
We considered only randomised clinical trials (irrespective of language, blinding, or publication status) comparing different pharmacological interventions with no intervention or inactive controls for outcomes related to benefit in this review. We considered comparative non‐randomised studies with regards to treatment‐related harms. We also considered trials that compared one class of drug with another class of drug for this review.
Data collection and analysis
Two review authors collected the data independently. We analysed the data with both fixed‐effect and random‐effects models using Review Manager 5 analysis. For each outcome, we calculated the risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
We included 25 trials with 2505 participants randomised to the different pharmacological agents and inactive controls. All the trials were at unclear risk of bias. Most trials included only low anaesthetic risk people undergoing elective laparoscopic cholecystectomy. Participants were allowed to take additional analgesics as required in 24 of the trials. The pharmacological interventions in all the included trials were aimed at preventing pain after laparoscopic cholecystectomy. There were considerable differences in the pharmacological agents used and the methods of administration. The estimated effects of the intervention on the proportion of participants who were discharged as day‐surgery, the length of hospital stay, or the time taken to return to work were imprecise in all the comparisons in which these outcomes were reported (very low quality evidence). There was no mortality in any of the groups in the two trials that reported mortality (183 participants, very low quality evidence). Differences in serious morbidity outcomes between the groups were imprecise across all the comparisons (very low quality evidence). None of the trials reported patient quality of life or time taken to return to normal activity. The pain at 4 to 8 hours was generally reduced by about 1 to 2 cm on the visual analogue scale of 1 to 10 cm in the comparisons involving the different pharmacological agents and inactive controls (low or very low quality evidence). The pain at 9 to 24 hours was generally reduced by about 0.5 cm (a modest reduction) on the visual analogue scale of 1 to 10 cm in the comparisons involving the different pharmacological agents and inactive controls (low or very low quality evidence).
Authors' conclusions
There is evidence of very low quality that different pharmacological agents including non‐steroidal anti‐inflammatory drugs, opioid analgesics, and anticonvulsant analgesics reduce pain scores in people at low anaesthetic risk undergoing elective laparoscopic cholecystectomy. However, the decision to use these drugs has to weigh the clinically small reduction in pain against uncertain evidence of serious adverse events associated with many of these agents. Further randomised clinical trials of low risk of systematic and random errors are necessary. Such trials should include important clinical outcomes such as quality of life and time to return to work in their assessment.
Keywords: Humans; Ambulatory Surgical Procedures; Analgesics; Analgesics/therapeutic use; Analgesics, Opioid; Analgesics, Opioid/therapeutic use; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Anticonvulsants; Anticonvulsants/therapeutic use; Cholecystectomy, Laparoscopic; Cholecystectomy, Laparoscopic/adverse effects; Elective Surgical Procedures; Length of Stay; Pain Measurement; Pain Measurement/methods; Pain, Postoperative; Pain, Postoperative/etiology; Pain, Postoperative/prevention & control; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Regular painkillers in people undergoing laparoscopic cholecystectomy
Background
About 10% to 15% of the adult western population have gallstones. Between 1% and 4% become symptomatic each year. Removal of the gallbladder (cholecystectomy) is the mainstay treatment for symptomatic gallstones. More than half a million cholecystectomies are performed per year in the US alone. Laparoscopic cholecystectomy (removal of gallbladder through a keyhole, also known as port) is now the preferred method of cholecystectomy.
Laparoscopic surgery is associated with less pain than open surgery for removal of the gallbladder but postoperative pain is one the major reasons for delayed hospital discharge after laparoscopic cholecystectomy. Administration of painkillers may be an effective way of decreasing the pain after laparoscopic cholecystectomy. The different types of painkillers include those that decrease the inflammation (non‐steroidal anti‐inflammatory drugs or NSAIDS), which include drugs that are available over‐the‐counter such as paracetamol and ibuprofen and other drugs that are not available over‐the‐counter such as diclofenac; opium‐like painkillers such as codeine and morphine, and some painkillers that are used to treat fits but also possess the ability to decrease the pain such as gabapentin and pregabalin. The last two classes of drugs are available only as prescription drugs except for low dose codeine in some countries. The benefits and harms of giving painkillers on a regular basis in people undergoing laparoscopic cholecystectomy is unknown. We sought to answer these questions by reviewing the medical literature and obtaining information from randomised clinical trials for benefits (where people are randomly allocated to one of two or more treatment groups) and comparative non‐randomised studies for treatment‐related harms. We compared the regular use of painkillers with no regular use of painkillers (ie, painkillers were administered as and when required) and the different type of painkillers.
Study characteristics
We identified 25 randomised clinical trials involving 2505 people undergoing laparoscopic cholecystectomy. Most participants in the trials were low anaesthetic risk people undergoing planned laparoscopic cholecystectomy. The choice of whether the participants received the different painkillers (or not) was determined by a method similar to the toss of coin so that the treatments compared were conducted in people who were as similar as possible. The treatments in all the included trials were aimed at decreasing the pain after laparoscopic cholecystectomy before the participants reported pain. Participants were allowed to take additional painkillers as required in most of the trials.
Key results
There were no deaths in either group in three trials (183 participants) that reported deaths. The differences in the serious complications between the groups was imprecise in all the comparisons. None of the trials reported quality of life or the time taken to return to normal activity. The differences in length of hospital stay and the time taken to return to work was imprecise in all the comparisons that reported these. Pain was lower in the participants who received painkillers compared with those who received controls at 4 to 8 hours and at 9 to 24 hours as measured by the visual analogue scale (a chart that rates the amount of pain on a scale of 1 to 10). This is a modest reduction and is comparable to other methods of pain reduction such as administering local anaesthetics (drugs that numb part of the body, similar to the ones used by the dentist to prevent the people from feeling pain) during the operation. In summary, different painkillers reduce pain scores in low anaesthetic risk people undergoing elective laparoscopic cholecystectomy. However, the decision to use these drugs has to weigh the clinically small reduction in pain against uncertain evidence of serious adverse events associated with many of these agents.
Quality of evidence
The overall quality of evidence was very low.
Future research
Further trials are necessary. Such trials should include outcomes such as quality of life, the time taken to return to normal activity, and the time taken to return to work, which are important for the person undergoing laparoscopic cholecystectomy and the people who provide funds for the treatment.
Summary of findings
Summary of findings for the main comparison. Various interventions compared with control for people undergoing laparoscopic cholecystectomy.
| Various interventions compared with control for people undergoing laparoscopic cholecystectomy | |||||
| Patient or population: people undergoing laparoscopic cholecystectomy Settings: secondary or tertiary Intervention: various interventions versus control | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Various interventions | ||||
| Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus no active intervention | |||||
| Morbidity | 59 per 1000 | 44 per 1000 (22 to 90) | RR 0.75 (0.37 to 1.53) | 543 (5 studies) | ⊕⊝⊝⊝ very low1,2 |
| Proportion discharged as day‐surgery | 603 per 1000 | 603 per 1000 (447 to 809) | RR 1 (0.74 to 1.34) | 116 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Length of hospital stay | The mean length of hospital stay in the control groups was 1.1 days | The mean length of hospital stay in the intervention group was 0.1 lower (0.72 lower to 0.52 higher) | ‐ | 119 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Pain (4 to 8 hours) | The mean pain (4 to 8 hours) in the control groups was 3.49 cm VAS | The mean pain (4 to 8 hours) in the intervention groups was 0.88 lower (1.07 to 0.7 lower) | ‐ | 999 (11 studies) | ⊕⊝⊝⊝ very low1,4 |
| Pain (9 to 24 hours) | The mean pain (9 to 24 hours) in the control groups was 2.2 cm VAS | The mean pain (9 to 24 hours) in the intervention groups was 0.5 lower (0.67 to 0.33 lower) | ‐ | 707 (9 studies) | ⊕⊝⊝⊝ very low1,4 |
| Mortality, patient quality of life, and return to normal activity were not reported in any trials. Return to work was not reported adequately in any of the trials. | |||||
| Opioids versus no active intervention | |||||
| Pain (4 to 8 hours) | The mean pain (4 to 8 hours) in the control groups was 4.00 cm VAS | The mean pain (4 to 8 hours) in the intervention groups was 2.51 lower (3.02 to 2.01 lower) | ‐ | 425 (3 studies) | ⊕⊕⊝⊝ low1 |
| Pain (9 to 24 hours) | The mean pain (9 to 24 hours) in the control groups was 2.76 cm VAS | The mean pain (9 to 24 hours) in the intervention groups was 0.32 lower (0.44 to 0.2 lower) | ‐ | 425 (3 studies) | ⊕⊕⊝⊝ low1 |
| Mortality, patient quality of life, hospital stay, and return to normal activity or work were not reported in any trials. Morbidity was reported adequately in any of the trials. | |||||
| Anticonvulsant analgesics versus no active intervention | |||||
| Mortality | There was no mortality in either group | Not estimable | 123 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Morbidity | 40 per 1000 | 120 per 1000 (13 to 1000) | RR 3 (0.33 to 26.92) | 50 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (4 to 8 hours) | The mean pain (4 to 8 hours) in the control groups was 4 cm VAS | The mean pain (4 to 8 hours) in the intervention groups was 2.52 lower (2.95 to 2.09 lower) | ‐ | 402 (3 studies) | ⊕⊝⊝⊝ very low1,4 |
| Pain (9 to 24 hours) | The mean pain (9 to 24 hours) in the control groups was 3 cm VAS | The mean pain (9 to 24 hours) in the intervention groups was 0.55 lower (0.68 to 0.42 lower) | ‐ | 402 (3 studies) | ⊕⊕⊝⊝ low1 |
| Patient quality of life, hospital stay, and return to normal activity were not reported in any trials. Return to work was not reported adequately in any of the trials. | |||||
| Opioids versus NSAIDs | |||||
| Only one trial was included in this comparison. None of the outcomes was reported adequately in this trial. | |||||
| Anticonvulsant analgesics versus NSAIDs | |||||
| Mortality | There was no mortality in either group | Not estimable | 60 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Morbidity | 37 per 1000 | 80 per 1000 (8 to 829) | RR 2.16 (0.21 to 22.38) | 52 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (4 to 8 hours) | The mean pain (4 to 8 hours) in the control groups was 4.3 cm VAS | The mean pain (4 to 8 hours) in the intervention groups was 2.5 lower (2.84 to 2.16 lower) | ‐ | 60 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Pain (9 to 24 hours) | The mean pain (9 to 24 hours) in the control groups was 2.1 cm VAS | The mean pain (9 to 24 hours) in the intervention groups was 0.5 lower (0.84 to 0.16 lower) | ‐ | 60 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Patient quality of life, hospital stay, and return to normal activity were not reported in any trials. Return to work was not reported adequately in any of the trials. | |||||
| Anticonvulsant analgesics versus opioids | |||||
| Pain (4 to 8 hours) | The mean pain (4 to 8 hours) in the control groups was 2.97 VAS | The mean pain (4 to 8 hours) in the intervention groups was 0.32 lower (0.92 lower to 0.28 higher) | ‐ | 306 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Pain (9 to 24 hours) | The mean pain (9 to 24 hours) in the control groups was 0.87 VAS | The mean pain (9 to 24 hours) in the intervention groups was 0.22 lower (0.34 to 0.1 lower) | ‐ | 306 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Mortality, patient quality of life, hospital stay, and return to normal activity or work were not reported in the only trial that was included in the comparison. Morbidity was not reported adequately in any of the trials. | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; NSAID: non‐steroidal anti‐inflammatory drug; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The trial(s) was (were) of high risk of bias (2 points). 2 The confidence intervals overlapped 1 and either 0.75 or 1.25 or both. The number of events in the intervention and control group was fewer than 300 (2 points). 3 There were fewer than 400 participants in total (1 point). 4 There was severe heterogeneity as noted by the I2statistic and the lack of overlap of confidence intervals (2 points).
Background
Description of the condition
About 5% to 25% of the adult western population have gallstones (GREPCO 1984; GREPCO 1988; Bates 1992; Halldestam 2004). The annual incidence of gallstones is about 1 in 200 people (NIH 1992). Only 2% to 4% of people with gallstones become symptomatic with biliary colic (pain), acute cholecystitis (inflammation), obstructive jaundice, or gallstone pancreatitis in a year (Attili 1995; Halldestam 2004). Cholecystectomy (removal of gallstones) is the preferred option in the treatment of symptomatic gallstones (Strasberg 1993) and every year, 1.5 million cholecystectomies are performed in the US and 60,000 in the UK (Dolan 2009; HES 2011). Approximately 80% of the cholecystectomies are performed laparoscopically (keyhole) (Ballal 2009).
While laparoscopic cholecystectomy is generally considered less painful than open surgery, pain is one of the important reasons for delayed discharge after laparoscopic cholecystectomy (Gurusamy 2008a; Gurusamy 2008b). The pain after laparoscopic cholecystectomy could be incisional pain, shoulder pain, or abdominal pain (Ng 2004). While the incisional pain is because of damage to the nerve endings because of the incision along with the associated inflammation, the aetiology of abdominal pain and shoulder pain after laparoscopic cholecystectomy is unclear. Peritoneal irritation, caused by carbonic acid and creation of space between diaphragm and liver, leading to loss of suction support of the heavy liver have been suggested as possible mechanisms of pain (Alexander 1987). However, use of an overnight drain to let out the gas has not been effective in the reduction of pain (Gurusamy 2013).
Description of the intervention
Analgesics provide pain relief (analgesia). There are different types of analgesics. The common analgesics used peri‐operatively can be broadly classified into non‐steroidal anti‐inflammatory drugs (NSAIDs), such as paracetamol, diclofenac, or ibuprofen; opioid analgesics (opium derivatives and synthetic substances that have similar action), such as tramadol or codeine; and anticonvulsant analgesics, such as gabapentin or pregabalin used to treat neuropathic pain (Argoff 2013). The analgesics can be administered by different routes including orally, sublingually, intravenously, subcutaneously, by transdermal patches, or rectally (Martindale 2011; Argoff 2013). The most common adverse events associated with short‐term use of NSAIDs include gastrointestinal disturbances, such as gastrointestinal discomfort, nausea, and diarrhoea; these are usually mild and reversible but in some people peptic ulceration and severe gastrointestinal bleeding may occur (Martindale 2011). The most common adverse events related to opioids used in usual doses include nausea, vomiting, constipation, drowsiness, confusion, difficulty in micturition, dry mouth, dizziness, sweating, facial flushing, headache, vertigo, bradycardia, tachycardia, palpitations, orthostatic hypotension, hypothermia, restlessness, changes of mood, decreased libido or potency, hallucinations, and raised intracranial pressure. Larger doses of opioids produce muscle rigidity, respiratory depression, hypotension with circulatory failure, and deepening coma (Martindale 2011). The most commonly reported adverse events associated with gabapentin are somnolence, dizziness, ataxia, and fatigue although psychiatric effects including confusion, depression, and nervousness can occur in some people (Martindale 2011). Common adverse events related to pregabalin include dizziness, somnolence, blurred vision, diplopia (double vision), dry mouth, constipation, vomiting, flatulence, euphoria, confusion, reduced libido, erectile dysfunction, irritability, vertigo, ataxia, tremor, dysarthria, paraesthesia, fatigue, oedema, and disturbances of attention, memory, co‐ordination, and gait (Martindale 2011).
How the intervention might work
NSAIDs inhibit cyclo‐oxygenase, an enzyme in the pathway of synthesis of prostaglandins, which play an important role in inflammation (Martindale 2011; Argoff 2013). NSAIDs may also have a central action in addition to their peripheral action (Martindale 2011). Opioid analgesics act on opioid receptors in the peripheral and central nervous system and inhibit the neuronal transmission (transmission by nerve) of pain sensation (Inturrisi 2002). Gabapentin and pregabalin are anticonvulsant drugs that inhibit the α2δ subunit of presynaptic, voltage‐gated calcium channels (Argoff 2013). This results in decreased excitability of nerves.
Why it is important to do this review
One systematic review by the Procedure Specific Postoperative Pain Management (PROSPECT) group recommended routine use of NSAIDs and recommended against routine use of opioid analgesics during or after laparoscopic cholecystectomy (Kehlet 2005). Another systematic review by Bisgaard et al. made similar recommendations as the PROSPECT group and, in addition, recommended against routine use of gabapentin during or after laparoscopic cholecystectomy (Bisgaard 2006). Reduction in pain may improve quality of life and allow earlier return to normal activity and work, which may have financial implications to the people undergoing the operations, their carers, and their employers. Reduction in pain may also improve the proportion of laparoscopic cholecystectomies performed as day‐surgery and decrease the length of hospital stay, which may be important for the people undergoing the procedure in a private‐funded healthcare system and may be important for state‐funded or insurance‐funded healthcare systems. We have been unable to identify any recent systematic reviews or Cochrane reviews assessing the role of different analgesics in people undergoing laparoscopic cholecystectomy.
Objectives
To assess the benefits and harms of different analgesics in people undergoing laparoscopic cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised clinical trials (irrespective of language, blinding, publication status, or sample size) for inclusion. We excluded quasi‐randomised trials (where the method of allocating participants to a treatment are not strictly random, for example, date of birth, hospital record number, alternation) and non‐randomised studies regarding assessment of benefit, but planned to include these studies regarding assessment of treatment‐related harms.
Types of participants
People undergoing laparoscopic cholecystectomy irrespective of age, elective or emergency surgery, and the reason why the laparoscopic cholecystectomy was performed.
Types of interventions
We included the following comparisons.
NSAIDs versus inactive controls (no intervention or placebo).
Opioid analgesics versus inactive controls (no intervention or placebo).
Anticonvulsant analgesics versus inactive controls (no intervention or placebo).
Comparison of one of the above three classes of drugs with another class.
We included only trials that compared the above analgesics administered orally, sublingually, intravenously, and rectally, which are the routes that are commonly used to administer the above agents. We excluded trials that compared administration of analgesics by intraperitoneal, intrathecal, or intrapleural routes; wound infiltration; or nerve blocks as we considered these as extensions of anaesthetic regimens. We excluded comparison of drugs within the same class of drugs, as inclusion of such trials would make the review very difficult to read. We planned to perform separate reviews for comparison of drugs within the same class if we found that one or more classes were safe and effective in people undergoing laparoscopic cholecystectomy. We excluded trials that involved a combination of two or more classes of drugs against inactive interventions. We excluded trials considering pharmacological agents not primarily meant for analgesia such as intravenous ketamine (used for its sedative property to perform short procedures) (Gottschling 2005), α2‐adrenoceptor antagonist, such as clonidine (aimed at improving the circulatory stability) (Yu 2003), and beta‐blockers such as esmolol (aimed at decreasing stress response) (Collard 2007). We excluded wound infiltration or intraperitoneal instillation of local anaesthetics because they have been considered in other reviews (Gurusamy 2014; Loizides 2014). We excluded epidural or intrathecal interventions because we consider these to be extensions of the anaesthetic regimen used.
We allowed co‐interventions if carried out equally in the trial groups.
Types of outcome measures
Primary outcomes
Mortality.
Serious adverse events defined as any event that would increase mortality, was life‐threatening, required hospitalisation, resulted in a persistent or significant disability, or any important medical event that might have jeopardised the person or required intervention to prevent it (ICH‐GCP 1997). We classified complications such as bile duct injury; re‐operations; intra‐abdominal collections requiring drainage (radiological or surgical); infected intra‐abdominal collections; bile leaks requiring drainage, stent, or surgery; gastrointestinal disturbances that required endoscopic investigations or treatment; respiratory depression that required monitoring and hence prolonged hospital stay as serious adverse events. We considered complications such as wound infections, bile leaks, abdominal collections, or minor gastrointestinal disturbances that did not require treatment and settled spontaneously to be non‐serious adverse events.
Patient quality of life (however defined by authors using a validated scale such as Euro‐QoL or 36‐item Short Form (SF‐36)).
Secondary outcomes
Hospital stay (length of hospital stay, proportion discharged as day‐surgery laparoscopic cholecystectomy).
Pain (overall pain) at different time points (4 to 8 hours and 9 to 24 hours) using visual analogue scale (VAS).
Return to activity.
Return to work.
We have reported all the outcomes with at least one trial in the Table 1.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded (Royle 2003), and the World Health Organization International Clinical Trials Registry Platform portal (WHO ICTRP) (apps.who.int/trialsearch/) to March 2013. The WHO ICTRP portal allows search of various trial registers including clinicaltrials.gov and ISRCTN among other registers. We have given the search strategies in Appendix 1 with the time span for the searches.
Searching other resources
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
We performed the systematic review according to the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2014).
Selection of studies
Two review authors (KSG and CT) identified the trials for inclusion independently of each other. We have also listed the excluded studies with the reasons for the exclusion (Characteristics of excluded studies).
Data extraction and management
Two review authors (JV and CT) extracted the following data independently of each other.
Year and language of publication.
Country in which the trial was conducted.
Year of trial.
Inclusion and exclusion criteria.
Sample size.
Elective surgery or acute cholecystitis.
Pharmacological agent used.
Dose of pharmacological agent.
Route of pharmacological agent.
Timing of administration.
Other co‐interventions.
Outcomes (Primary outcomes; Secondary outcomes).
Risk of bias (Risk of bias in included studies).
We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same participants ‐ completely or partially (by identifying common authors and centres) ‐ we planned to contact the authors of the trials to clarify whether the trial report had been duplicated.
We resolved any differences in opinion through discussion or arbitration of the third review author (BRD).
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2014). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012a; Savovic 2012b), the risk of bias of the trials was assessed based on the following bias risk domains.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (eg, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to introduce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes were likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes were likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were pre‐defined and reported, or all clinically relevant and reasonably expected outcomes were reported. For this purpose, the trial should have been registered either on the www.clinicaltrials.gov website or a similar register with sufficient evidence that the protocol had not been revised during the update, or there should be a protocol, for example, published in a paper journal. In the case when the trial was run and published in the years when trial registration was not required, we carefully scrutinized all publications reporting on the trial to identify the trial objectives and outcomes and determine whether usable data were provided in the publication results section on all outcomes specified in the trial objectives.
Uncertain risk of bias: it is unclear whether all pre‐defined and clinically relevant (mortality and morbidity) and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conductance, or results of the trial.
Uncertain risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by the industry or had received other type of for‐profit support.
We considered trials that were classified as low risk of bias in all the above domains as trials with low risk of bias and the remaining as trials with high risk of bias.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). We also calculated the risk difference with 95% CI. We planned to report the risk difference only if the conclusions were different from those of RR. Risk difference includes 'zero event trials' (trials in which both groups had no events) for calculating the summary treatment effect, while such trials will not be taken into account while calculating the summary treatment effect in the case of RR. For continuous variables, we calculated the mean difference (MD) with 95% CI for outcomes such as total hospital stay or standardised mean difference (SMD) with 95% CI for outcomes such as quality of life, where different authors used different scales of quality of life.
Unit of analysis issues
The units of analysis was the participant about to undergo laparoscopic cholecystectomy and randomised to the intraperitoneal local anaesthetic instillation or control.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). We imputed data for binary outcomes using various scenarios such as best‐best, best‐worst, worst‐best, and worst‐worst scenario (Gurusamy 2009; Gluud 2014).
For continuous outcomes, we used available‐case analysis. We imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and we used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CI, we planned to impute the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation would decrease the weight of the study for calculation of MDs and bias the effect estimate to no effect in the case of SMD (Higgins 2011).
Assessment of heterogeneity
We explored heterogeneity using the Chi2 test with significance set at a P value less than 0.10, and measured the quantity of heterogeneity using the I2 statistic (Higgins 2002). We also used overlapping of CIs on the forest plot to determine heterogeneity.
Assessment of reporting biases
We used visual asymmetry on a funnel plot to explore reporting bias since the search identified more than 10 trials (Egger 1997; Macaskill 2001). We used the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry. Selective reporting was also considered as evidence for reporting bias.
Data synthesis
We performed the meta‐analyses using the software package Review Manager 5 (RevMan 2012), and following the recommendations of The Cochrane Collaboration (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2014). We used both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) meta‐analysis. In the case of discrepancy between the two models, we have reported both results; otherwise, we have reported the results of the fixed‐effect model. We planned to use the generic inverse method to combine the hazard ratios for time‐to‐event outcomes.
Trial sequential analysis
Cumulative meta‐analyses run the risk of producing random errors of both type I and type II due to sparse data and repetitive analysis of accumulating data. The underlying assumption of trial sequential analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and if more than one trial was published in a year the trials were added alphabetically according to the last name of the first author. On the basis of the required information size, trial sequential monitoring boundaries were constructed. These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. In contrast, if the boundaries are not surpassed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010).
We applied trial sequential analysis (CTU 2011; Thorlund 2011) using a diversity‐adjusted required information size calculated from an alpha error of 0.05, a beta error of 0.20, a control event proportion obtained from the results, and a relative risk reduction of 20% for binary outcomes if there were two or more trials reporting the outcome to determine whether more trials are necessary on this topic (if the trial sequential alpha‐spending monitoring boundary or the futility zone is crossed, then more trials may be unnecessary) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). Since trial sequential analysis cannot be performed for SMD, we did not plan to perform the trial sequential analysis for quality of life. For pain, we calculated the diversity‐adjusted required information size from an alpha error of 0.05, a beta error of 0.20, the variance estimated from the meta‐analysis results of low risk of bias trials, and an MD of 1 cm on the VAS (Todd 1996). For length of hospital stay, return to work, and return to activity, we planned to calculate the required sample size using an MD of one day with the remaining parameters kept the same as that for pain.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials with low bias risk compared to trials with high bias risk.
Elective compared to emergency laparoscopic cholecystectomy.
Different times of administration (one to two hours before surgery, on induction, or at the end of surgery).
Different pharmacological agents.
With and without intraperitoneal local anaesthetic instillation.
With and without peri‐laparoscopic‐portal infiltration with local anaesthetic.
We used the 'test for subgroup differences' available through Review Manager 5 (RevMan 2012) to identify the differences between subgroups. We used the random‐effects model for this purpose.
Sensitivity analysis
We performed a sensitivity analysis by imputing data for binary outcomes using various scenarios such as best‐best, best‐worst, worst‐best, and worst‐worst scenario (Gurusamy 2009; Gluud 2014). We performed a sensitivity analysis by excluding the trials in which the mean and the standard deviation were imputed.
'Summary of findings' table
We have summarised the results of all the reported outcomes in the Table 1 prepared using GRADEPro 3.6 (ims.cochrane.org/revman/gradepro).
Results
Description of studies
Results of the search
We identified 1238 references through electronic searches of CENTRAL (n = 274), MEDLINE (n = 269), EMBASE (n = 302), and Science Citation Index Expanded (n = 393). We did not identify any new trials from the trial registers. We excluded 604 duplicates and 572 clearly irrelevant references through screening titles and reading abstracts. We retrieved 62 references for further assessment. We identified no references through scanning reference lists of the identified randomised trials. We excluded 25 references for the reasons listed in the Characteristics of included studies table. In total, 37 references of 36 completed randomised clinical trials met the inclusion criteria. This is summarised in the study flow diagram Figure 1. We did not identify any comparative non‐randomised studies that reported treatment‐related harms.
1.
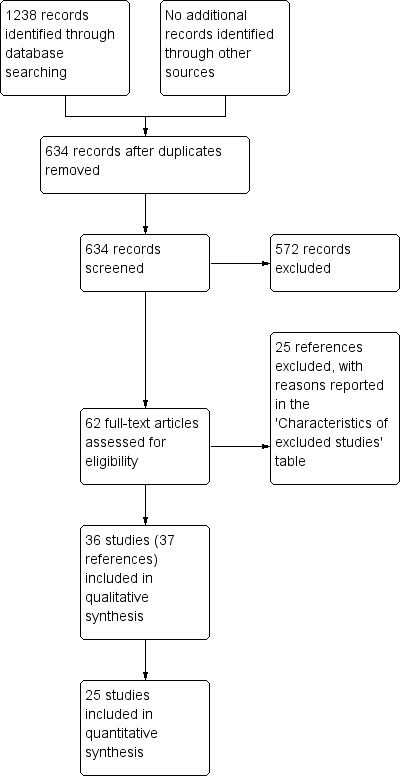
Study flow diagram.
Included studies
Of the 36 randomised clinical trials that reported the inclusion criteria, 10 trials did not provide any information for this systematic review (Liu 1993; Belzarena 1998; Muñoz 2002; Cheng 2004; Puura 2006; Akinci 2008; Fanelli 2008; Karakoc 2011; Balaban 2012; Gomez‐Vazquez 2012). These trials reported some specific aspects of pain, for example, shoulder pain or abdominal pain, used other scales of pain, or reported other outcomes such as stress response. One other trial did not report the number of participants randomised to the intervention and control groups (Schuster 2005). Thus, we included 25 randomised clinical trials including 2505 participants randomised to different interventions and controls in this review. In 15 trials, we included two arms in this review (Wilson 1994; Munro 1998; Chung 2004; Horattas 2004; Joshi 2004; Yeh 2004; Zajaczkowska 2004; Agarwal 2008; Akaraviputh 2009; Salihoglu 2009; Sen 2010; Sandhu 2011; Zhu 2011; Akarsu 2012; Sarakatsianou 2013), that is, although some of these trials randomised participants to more than two arms, only two arms were eligible for inclusion in this review. In the remaining 10 trials, we included more than two arms in this review (Forse 1996; Lane 1996; Dong 2003; Pandey 2004; Mebazaa 2008; Gilron 2009; Ji 2010; Peng 2010; Abdulla 2012; Nesek‐Adam 2012).
Participant characteristics
The pharmacological interventions in all the included trials were aimed at decreasing pain after laparoscopic cholecystectomy before the participants reported pain. Nineteen trials reported that they included only people undergoing elective laparoscopic cholecystectomy (Wilson 1994; Forse 1996; Chung 2004; Horattas 2004; Joshi 2004; Pandey 2004; Yeh 2004; Zajaczkowska 2004; Akaraviputh 2009; Gilron 2009; Salihoglu 2009; Peng 2010; Sen 2010; Sandhu 2011; Zhu 2011; Abdulla 2012; Akarsu 2012; Nesek‐Adam 2012; Sarakatsianou 2013). None of the remaining six trials stated whether people undergoing emergency laparoscopic cholecystectomy were included (Lane 1996; Munro 1998; Dong 2003; Agarwal 2008; Mebazaa 2008; Ji 2010). Fifteen trials stated that they included only people with American Society of Anesthesiologists (ASA) I or II status (Forse 1996; Lane 1996; Pandey 2004; Yeh 2004; Zajaczkowska 2004; Agarwal 2008; Mebazaa 2008; Gilron 2009; Salihoglu 2009; Ji 2010; Sen 2010; Sandhu 2011; Zhu 2011; Nesek‐Adam 2012; Sarakatsianou 2013). Three trials stated that they included only people with ASA I to III status (Peng 2010; Abdulla 2012; Akarsu 2012). The remaining seven trials did not state the ASA status of the people undergoing laparoscopic cholecystectomy (Wilson 1994; Munro 1998; Dong 2003; Chung 2004; Horattas 2004; Joshi 2004; Akaraviputh 2009).
Intervention and control
Eighteen trials compared NSAIDs with inactive control (Wilson 1994; Forse 1996; Lane 1996; Munro 1998; Dong 2003; Chung 2004; Horattas 2004; Joshi 2004; Yeh 2004; Mebazaa 2008; Akaraviputh 2009; Gilron 2009; Salihoglu 2009; Ji 2010; Sen 2010; Sandhu 2011; Abdulla 2012; Nesek‐Adam 2012). Four trials compared opioids versus inactive controls (Lane 1996; Pandey 2004; Zajaczkowska 2004; Zhu 2011). Five trials compared anticonvulsant analgesics versus inactive controls (Pandey 2004; Agarwal 2008; Gilron 2009; Peng 2010; Sarakatsianou 2013). Twenty‐one trials used placebo as control (Wilson 1994; Forse 1996; Lane 1996; Munro 1998; Chung 2004; Horattas 2004; Joshi 2004; Pandey 2004; Yeh 2004; Agarwal 2008; Akaraviputh 2009; Gilron 2009; Salihoglu 2009; Ji 2010; Peng 2010; Sen 2010; Sandhu 2011; Zhu 2011; Abdulla 2012; Nesek‐Adam 2012; Sarakatsianou 2013). Three trials used no intervention as control (Dong 2003; Zajaczkowska 2004; Mebazaa 2008). One trial compared opioid versus NSAID (Lane 1996). Two trials compared anticonvulsant analgesics versus NSAID (Gilron 2009; Akarsu 2012). One trial compared anticonvulsant analgesics versus opioid (Pandey 2004).
Co‐interventions
Intraperitoneal local anaesthetic instillation was used as a co‐intervention in one trial (Peng 2010). Intraperitoneal local anaesthetic instillation was not used as a co‐intervention in five trials (Lane 1996; Munro 1998; Joshi 2004; Mebazaa 2008; Sandhu 2011). The remaining trials did not provide this information.
Peri‐laparoscopic portal local anaesthetic infiltration was used as co‐intervention in three trials (Forse 1996; Gilron 2009; Peng 2010). Peri‐laparoscopic portal local anaesthetic infiltration was not used as co‐intervention in five trials (Lane 1996; Munro 1998; Joshi 2004; Zajaczkowska 2004; Sandhu 2011). The remaining trials did not provide this information.
Participants were allowed to take additional analgesics as required in 24 trials (Wilson 1994; Forse 1996; Lane 1996; Munro 1998; Chung 2004; Horattas 2004; Joshi 2004; Pandey 2004; Yeh 2004; Zajaczkowska 2004; Agarwal 2008; Mebazaa 2008; Akaraviputh 2009; Gilron 2009; Salihoglu 2009; Ji 2010; Peng 2010; Sen 2010; Sandhu 2011; Zhu 2011; Abdulla 2012; Akarsu 2012; Nesek‐Adam 2012; Sarakatsianou 2013). This information was not available from one trial (Dong 2003).
The other co‐interventions used in the trials is are shown in the Characteristics of included studies table.
Further details about sample size, participant characteristics, the inclusion and exclusion criteria used in the trials, post‐randomisation drop‐outs, intervention and control, comparisons, outcomes, and the risk of bias in the trials are shown in the Characteristics of included studies table.
Risk of bias in included studies
All the remaining trials were at high risk of bias. The risk of bias in the included trials is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
2.
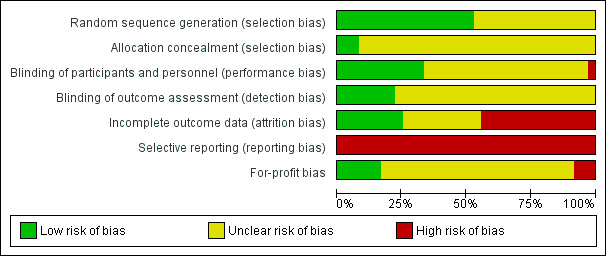
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only three trials (3/36 (8.3%)) described random sequence generation and allocation concealment adequately (Joshi 2004; Gilron 2009; Abdulla 2012). These three trials were considered to be at low risk of selection bias.
Blinding
Five trials (5/36 (13.9%)) reported that the participants, healthcare personnel involved in patient care, and outcome assessors were blinded and were considered to be at low risk of performance and detection bias (Chung 2004; Joshi 2004; Agarwal 2008; Fanelli 2008; Abdulla 2012).
Incomplete outcome data
Nine trials (9/36 (25.0%)) had no post‐randomisation drop‐outs and were considered to be at low risk of attrition bias (Lane 1996; Cheng 2004; Fanelli 2008; Salihoglu 2009; Ji 2010; Abdulla 2012; Akarsu 2012; Balaban 2012; Gomez‐Vazquez 2012).
Selective reporting
None of the trials reported mortality and morbidity in the participants and so all the trials were considered to be at high risk of selective reporting bias.
Other potential sources of bias
Six trials (6/36 (16.7%)) were considered to be at low risk of 'for‐profit' bias (Puura 2006; Fanelli 2008; Akaraviputh 2009; Gilron 2009; Sandhu 2011; Akarsu 2012).
Effects of interventions
See: Table 1
The results are summarised in Table 1.
Non‐steroidal anti‐inflammatory drugs versus control
Mortality
None of the trials reported mortality.
Morbidity
Five trials reported serious adverse events (Chung 2004; Joshi 2004; Gilron 2009; Salihoglu 2009; Sandhu 2011). It is not clear whether any of the serious adverse events could be drug‐related. There was no significant difference in the proportion of people with serious adverse events between NSAID and control (RR 0.75; 95% CI 0.37 to 1.53; 543 participants; very low quality evidence) (Analysis 1.1). The results did not change by using the random‐effects model. Although the remaining trials did not report the overall morbidity, one other trial (52 participants) stated that there were no intraoperative complications (Forse 1996). Five other trials stated there were no drug‐related serious adverse events in any of the 226 participants who received NSAID (Wilson 1994; Lane 1996; Munro 1998; Abdulla 2012; Nesek‐Adam 2012). The trial sequential analysis revealed that the proportion of information accrued was only 4.5% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 4). The cumulative Z curve did not cross the conventional statistical boundaries. Sensitivity analysis by imputing missing outcomes according to different scenarios resulted in different results (Analysis 1.6).
1.1. Analysis.
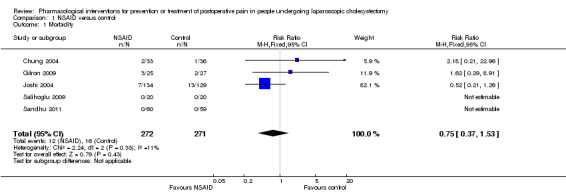
Comparison 1 NSAID versus control, Outcome 1 Morbidity.
4.

Trial sequential analysis of morbidity (non‐steroidal anti‐inflammatory drug (NSAID) versus control) The diversity‐adjusted required information size (DARIS) was calculated to 11,338 participants, based on the proportion of participants in the control group with the outcome of 5.90%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero‐event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z curve (blue line). After accruing 543 participants in five trials, only 4.79% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries have also not been crossed by the cumulative Z curve.
1.6. Analysis.

Comparison 1 NSAID versus control, Outcome 6 Morbidity (sensitivity analysis).
Patient quality of life
None of the trials reported patient quality of life.
Hospital stay
Proportion discharged as day‐surgery
One trial reported the proportion of participants discharged as day‐surgery (Horattas 2004). There were no significant differences in the proportion of participants discharged as day‐surgery between NSAID and control (RR 1.00; 95% CI 0.74 to 1.34; 116 participants; very low quality evidence) (Analysis 1.2). Trial sequential analysis was not performed because of the presence of only one trial. The results were robust to sensitivity analysis by imputing missing outcomes according to different scenarios (Analysis 1.7).
1.2. Analysis.

Comparison 1 NSAID versus control, Outcome 2 Proportion discharged as day‐surgery.
1.7. Analysis.
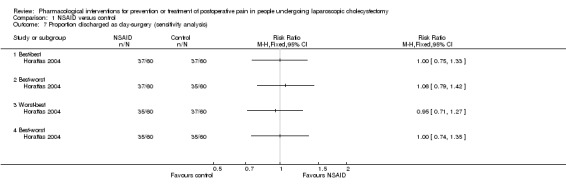
Comparison 1 NSAID versus control, Outcome 7 Proportion discharged as day‐surgery (sensitivity analysis).
Length of hospital stay
One trial reported length of hospital stay (Sandhu 2011). There were no significant differences in the length of hospital stay between the two groups (MD ‐0.10 days; 95% CI ‐0.72 to 0.52; 119 participants; very low quality evidence) (Analysis 1.3). Trial sequential analysis was not performed because of the presence of only one trial. The standard deviation was imputed from standard error. We did not perform the sensitivity analysis as this was the only trial included in this outcome.
1.3. Analysis.

Comparison 1 NSAID versus control, Outcome 3 Length of hospital stay.
Pain
Pain at 4 to 8 hours
Eleven trials reported pain at 4 to 8 hours (Wilson 1994; Munro 1998; Dong 2003; Chung 2004; Joshi 2004; Yeh 2004; Mebazaa 2008; Akaraviputh 2009; Ji 2010; Sen 2010; Abdulla 2012). The pain scores as measured using the VAS were significantly lower in the NSAID group than the control group (MD ‐0.88 cm VAS; 95% CI ‐1.07 to ‐0.70; 999 participants; very low quality evidence) (Analysis 1.4). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in seven trials (Wilson 1994; Munro 1998; Chung 2004; Joshi 2004; Yeh 2004; Mebazaa 2008; Akaraviputh 2009). Exclusion of these trials did not alter the results (MD ‐0.91 cm VAS; 95% CI ‐1.10 to ‐0.71) (Analysis 1.8). One trial contributed to more than 50% of the weight of the analysis (Sen 2010). It was not clear whether the values were standard deviation or standard error. Therefore, we performed another sensitivity analysis excluding this trial along with the other trials where mean or standard deviation was imputed. There was no change in the results by excluding this trial (MD ‐1.73 cm VAS; 95% CI ‐2.04 to ‐1.42). The trial sequential analysis revealed that the trial sequential monitoring boundaries were crossed by cumulative Z curve favouring NSAID. The findings were consistent with NSAID decreasing pain between 4 and 8 hours compared with inactive control with a low risk of random errors (Figure 5).
1.4. Analysis.
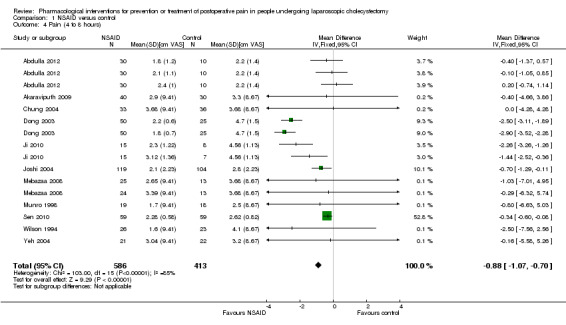
Comparison 1 NSAID versus control, Outcome 4 Pain (4 to 8 hours).
1.8. Analysis.
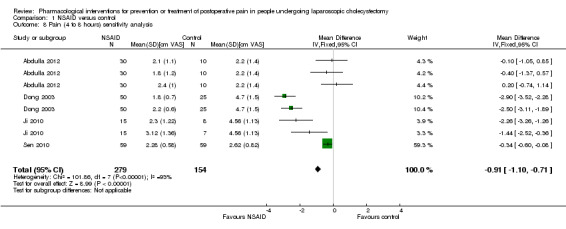
Comparison 1 NSAID versus control, Outcome 8 Pain (4 to 8 hours) sensitivity analysis.
5.
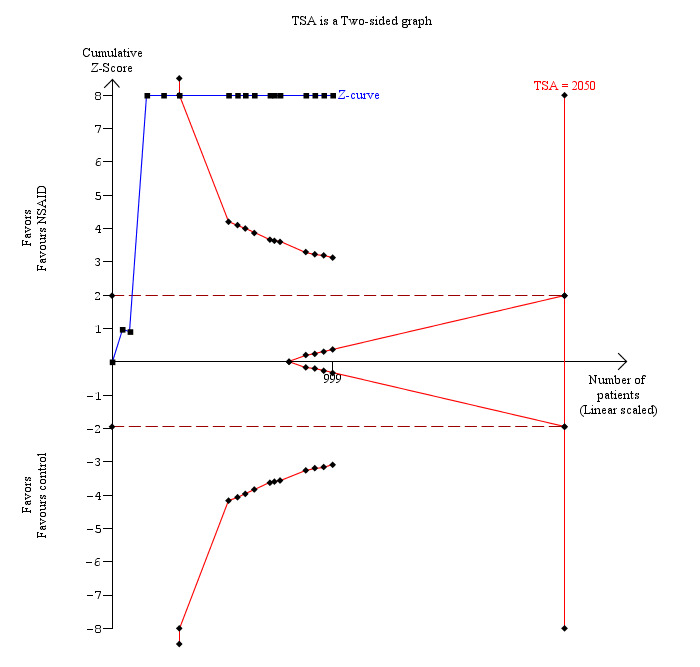
Trial sequential analysis of pain (4 to 8 hours) (non‐steroidal anti‐inflammatory drug (NSAID) versus control) The diversity‐adjusted required information size (DARIS) was 2050 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 4.51, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 93.07%. The conventional statistical boundaries (dotted red line) are crossed by the cumulative Z curve (blue line) after the third trial. The trial sequential monitoring boundaries (red line) are crossed by cumulative Z curve after the fifth trial. Although the DARIS has not been reached, the findings are consistent with NSAID decreasing pain between 4 and 8 hours compared with inactive control with low risk of random errors.
Pain at 9 to 24 hours
Nine trials reported pain at 9 to 24 hours (Wilson 1994; Munro 1998; Dong 2003; Yeh 2004; Mebazaa 2008; Akaraviputh 2009; Ji 2010; Sen 2010; Abdulla 2012). The pain scores as measured by VAS were significantly lower in the NSAID group than the control group (MD ‐0.50 cm VAS; 95% CI ‐0.67 to ‐0.33; 707 participants; very low quality evidence) (Analysis 1.5). On using the random‐effects model, there was no significant difference between the two groups (MD ‐0.65 cm VAS; 95% CI ‐1.37 to 0.08). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in five trials (Wilson 1994; Munro 1998; Yeh 2004; Mebazaa 2008; Akaraviputh 2009). Exclusion of these trials did not alter the results (MD ‐0.50 cm VAS; 95% CI ‐0.67 to ‐0.33) (Analysis 1.9). One trial contributed to more than 50% of the weight of the analysis (Sen 2010). It was not clear whether the values were standard deviation or standard error. Therefore, we performed another sensitivity analysis excluding this trial along with the other trials where mean or standard deviation was imputed. There was no change in the results by excluding this trial (MD ‐1.14 cm VAS; 95% CI ‐1.39 to ‐0.89). The trial sequential analysis revealed that the trial sequential monitoring boundaries were crossed by cumulative Z curve favouring NSAID. The findings were consistent with NSAID decreasing pain between 9 and 24 hours compared with inactive control with a low risk of random errors (Figure 6).
1.5. Analysis.
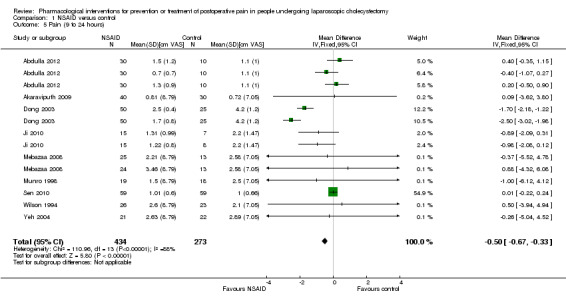
Comparison 1 NSAID versus control, Outcome 5 Pain (9 to 24 hours).
1.9. Analysis.
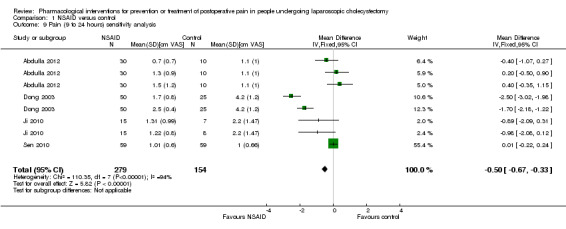
Comparison 1 NSAID versus control, Outcome 9 Pain (9 to 24 hours) sensitivity analysis.
6.
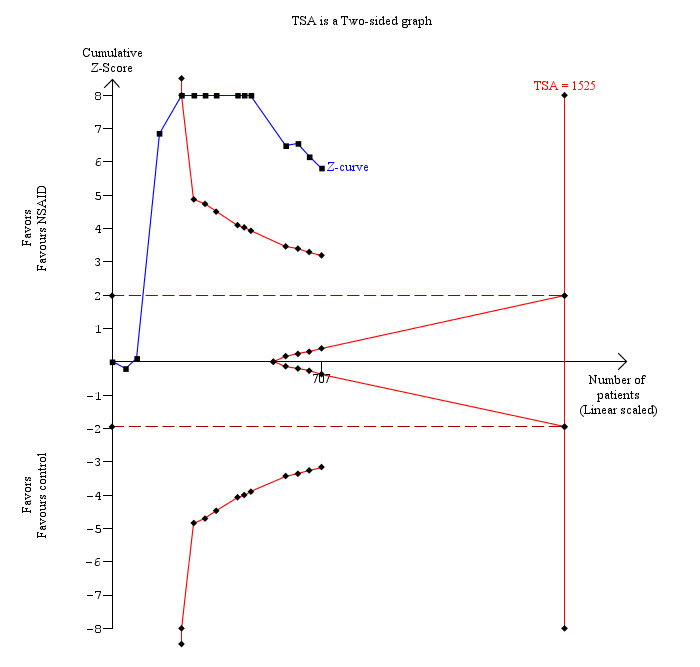
Trial sequential analysis of pain (9 to 24 hours) (non‐steroidal anti‐inflammatory drug (NSAID) versus control) The diversity‐adjusted required information size (DARIS) was 1525 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 2.62, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 94.56%. The conventional statistical boundaries (dotted red line) are crossed by the cumulative Z curve (blue line) after the third trial. The trial sequential monitoring boundaries (red line) are crossed by cumulative Z curve after the fifth trial. Although the DARIS has not been reached, the findings are consistent with NSAID decreasing pain between 9 and 24 hours compared with inactive control with low risk of random errors.
Return to normal activity
None of the trials reported return to normal activity.
Return to work
One trial (54 participants) reported return to work (Gilron 2009). The trial did not report the standard deviation. The trial reported that there were no significant differences in the time taken to return to work. Trial sequential analysis was not performed because of the presence of only one trial and because of the lack of standard deviation in the trial that reported this outcome (Gilron 2009).
Subgroup analysis
Only pain at 4 to 8 hours and pain at 9 to 24 hours were suitable for various subgroup analyses because of the paucity of data in the other outcomes. We did not perform the following subgroup analyses.
Trials with low bias risk compared to trials with high bias risk. None of the trials were at low risk of bias.
Elective compared with emergency laparoscopic cholecystectomy. None of the trials reported data for emergency laparoscopic cholecystectomy separately.
With and without intraperitoneal local anaesthetic instillation. None of the trials that provided information about intraperitoneal local anaesthetic instillation used local anaesthetic instillation.
With and without peri‐laparoscopic‐portal infiltration with local anaesthetic. None of the trials that provided information about local anaesthetic wound infiltration used local anaesthetic wound infiltration.
The results of the other two subgroup analyses are as follows.
Different times of administration (one to two hours before surgery, on induction, or at the end of surgery). The tests for subgroup differences were significant for both pain at 4 to 8 hours and for pain at 9 to 24 hours (P value < 0.00001). At both 4 to 8 hours and 9 to 24 hours, NSAID administration during the surgery appeared to be more effective than administration at other times.
Different pharmacological agents. The test for subgroup differences were significant for both pain at 4 to 8 hours and for pain at 9 to 24 hours (P value < 0.00001). At 4 to 8 hours, diclofenac, flurbiprofen, and lornoxicam appeared to be more effective than other agents (celecoxib, etofenomate, metamizol, paracetamol, parecoxib, and tenoxicam). At 9 to 24 hours, lornoxicam appeared to be more effective than other agents (celecoxib, diclofenac, etofenomate, fluribiprofen, metamizol, paracetamol, parecoxib, and tenoxicam).
Reporting bias
We explored reporting bias only for pain at 4 to 8 hours and for pain at 9 to 24 hours by funnel plots because of the presence of an adequate number of trials for these two outcomes only. The funnel plots did not reveal any evidence of reporting bias. The Egger's test did not reveal any evidence of reporting bias (pain at 4 to 8 hours: P value = 0.716; pain at 9 to 24 hours: P value = 0.871).
Opioids versus control
Mortality
None of the trials reported mortality.
Morbidity
None of the trials reported overall serious adverse events. Two trials reported drug‐related serious adverse event (Lane 1996; Pandey 2004). There were six serious adverse events (respiratory depression) in the opioid group compared with one serious adverse event (respiratory depression) in the control group in one trial (Pandey 2004). There were no drug‐related serious adverse events in the other trial (Lane 1996).
Patient quality of life
None of the trials reported patient quality of life.
Hospital stay
None of the trials reported the proportion of people discharged as day‐surgery or the length of hospital stay.
Pain
Pain at 4 to 8 hours
Three trials reported pain at 4 to 8 hours (Pandey 2004; Zajaczkowska 2004; Zhu 2011). The pain scores as measured by VAS were significantly lower in the opioid group than the control group (MD ‐2.51 cm VAS; 95% CI ‐3.02 to ‐2.01; 425 participants; low quality evidence) (Analysis 2.1). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in two trials (Zajaczkowska 2004; Zhu 2011). Exclusion of these trials did not alter the results (MD ‐2.56 cm VAS; 95% CI ‐3.07 to ‐2.05) (Analysis 2.3). Trial sequential analysis revealed that the trial sequential monitoring boundaries were crossed by cumulative Z curve favouring opioid. The findings were consistent with opioid decreasing pain between 4 and 8 hours compared with inactive control with a low risk of random errors (Figure 7).
2.1. Analysis.
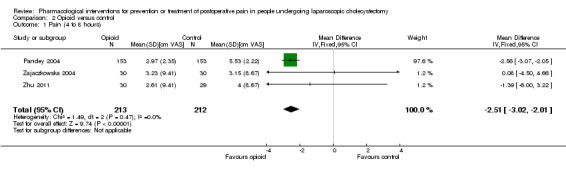
Comparison 2 Opioid versus control, Outcome 1 Pain (4 to 8 hours).
2.3. Analysis.
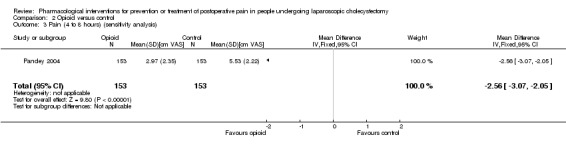
Comparison 2 Opioid versus control, Outcome 3 Pain (4 to 8 hours) (sensitivity analysis).
7.
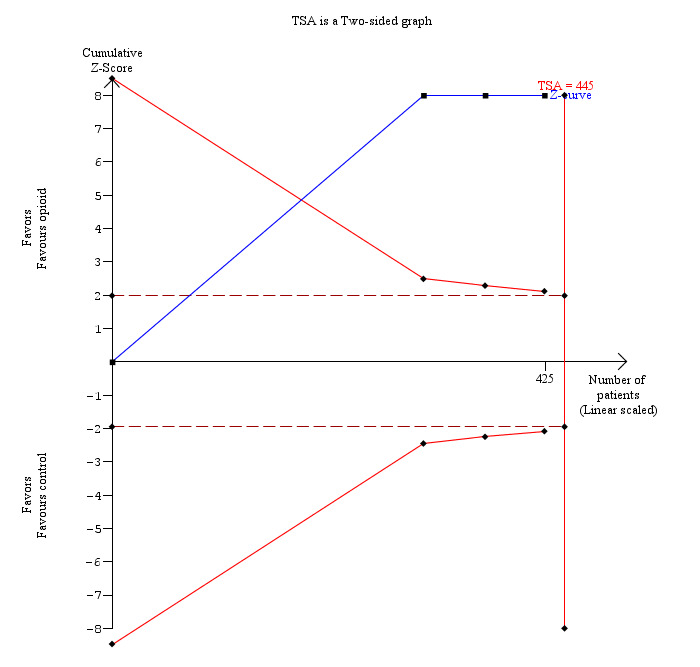
Trial sequential analysis of pain (4 to 8 hours) (opioid versus control) The diversity‐adjusted required information size (DARIS) was 445 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 14.16, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. The conventional statistical boundaries (dotted red line) and the trial sequential monitoring boundaries (red line) are crossed by the cumulative Z curve (blue line) after the first trial. Although the DARIS is not reached, the findings are consistent with opioid decreasing pain between 4 and 8 hours compared with inactive control with low risk of random errors.
Pain at 9 to 24 hours
Three trials reported pain at 9 to 24 hours (Pandey 2004; Zajaczkowska 2004; Zhu 2011). The pain scores as measured by VAS were significantly lower in the opioid group than the control group (MD ‐0.32 cm VAS; 95% CI ‐0.44 to ‐0.20; 425 participants; low quality evidence) (Analysis 2.2). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in two trials (Zajaczkowska 2004; Zhu 2011). Exclusion of these trials did not alter the results (MD ‐0.32 cm VAS; 95% CI ‐0.44 to ‐0.20) (Analysis 2.4). Trial sequential analysis revealed that the diversity‐adjusted required information size was 25 participants based on a minimal relevant difference (MIRD) of 1 cm on the VAS, a variance (VAR) of 0.78, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. As this was crossed by the first trial, the trial sequential boundaries were not drawn. A post hoc analysis with the MIRD revised to 0.25 cm was performed. The conventional statistical boundaries and the trial sequential monitoring boundaries were crossed by the cumulative Z curve after the second trial. The findings were consistent with opioid decreasing pain between 9 and 24 hours compared with inactive control with low risk of random errors (Figure 8).
2.2. Analysis.

Comparison 2 Opioid versus control, Outcome 2 Pain (9 to 24 hours).
2.4. Analysis.
Comparison 2 Opioid versus control, Outcome 4 Pain (9 to 24 hours) (sensitivity analysis).
8.

Trial sequential analysis of pain (9 to 24 hours) (opioid versus control) The diversity‐adjusted required information size (DARIS) was 25 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 0.78, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. As this was crossed by the first trial, the trial sequential boundaries were not drawn. A post‐hoc analysis with the MIRD revised to 0.25 cm was performed. The conventional statistical boundaries (dotted red line) and trial sequential monitoring boundaries (red line) are crossed by cumulative Z curve (blue line) after the first trial. Although the DARIS has not been reached, the findings are consistent with opioid decreasing pain between 9 and 24 hours compared with inactive control with low risk of random errors.
Return to normal activity
None of the trials reported return to normal activity.
Return to work
None of the trials reported return to work.
Subgroup analysis
We did not perform subgroup analysis because of the few trials included in this comparison.
Reporting bias
We did not assess the reporting bias by using funnel plots because of the few trials included in this comparison.
Anticonvulsant analgesics versus control
Mortality
One trial (123 participants) reported mortality (Peng 2010). There was no mortality in either group (0/82 (0%) in anticonvulsant analgesic group versus 0/41 (0%) in control group). Trial sequential analysis was not performed because of the presence of only one trial for this comparison.
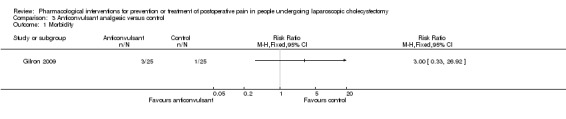
Morbidity
One trial reported morbidity (Gilron 2009). There was no significant difference in the morbidity between the two groups (RR 3.00; 95% CI 0.33 to 26.92; 50 participants; very low quality evidence) (Analysis 3.1). Two other trials reported drug‐related serious adverse events (Pandey 2004; Agarwal 2008).There was one respiratory depression in the anticonvulsant analgesic group (1/27 (3.7%)) compared with none in the control group (0/29 (0%)) in one trial (Agarwal 2008). There were no drug‐related serious adverse events (0/153 (0%)) compared with one respiratory depression in the control group (1/153 (0.7%)) in another trial (Pandey 2004). The severity of the respiratory depression was not reported. Trial sequential analysis was not performed because of the presence of only one trial that reported morbidity for this comparison. The results were robust to sensitivity analysis by imputing missing outcomes according to different scenarios (Analysis 3.4).
3.1. Analysis.
Comparison 3 Anticonvulsant analgesic versus control, Outcome 1 Morbidity.
3.4. Analysis.
Comparison 3 Anticonvulsant analgesic versus control, Outcome 4 Morbidity (sensitivity analysis).
Patient quality of life
None of the trials reported patient quality of life.
Hospital stay
Proportion discharged as day‐surgery
None of the trials reported the proportion of people discharged as day surgery or the length of hospital stay.
Pain
Pain at 4 to 8 hours
Three trials reported pain at 4 to 8 hours (Pandey 2004; Agarwal 2008; Sarakatsianou 2013). The pain scores as measured by VAS were significantly lower in the anticonvulsant analgesic group than the control group (MD ‐2.52 cm VAS; 95% CI ‐2.95 to ‐2.09; 402 participants; very low quality evidence) (Analysis 3.2). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in two trials (Agarwal 2008; Sarakatsianou 2013). Exclusion of these trials did not alter the results (MD ‐2.88 cm VAS; 95% CI ‐3.36 to ‐2.40) (Analysis 3.5). Trial sequential analysis revealed that there was a high risk of random errors even though there was a statistically significant reduction in pain in the anticonvulsant analgesic group compared with the control group (Figure 9), that is, more trials are needed before a firm conclusion about reduction in pain scores by anticonvulsants can be reached.
3.2. Analysis.
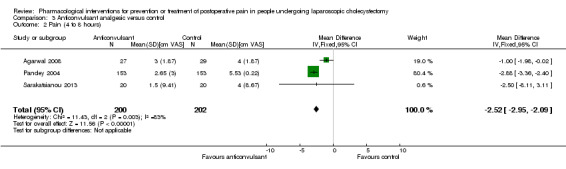
Comparison 3 Anticonvulsant analgesic versus control, Outcome 2 Pain (4 to 8 hours).
3.5. Analysis.
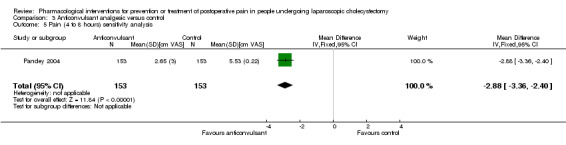
Comparison 3 Anticonvulsant analgesic versus control, Outcome 5 Pain (4 to 8 hours) sensitivity analysis.
9.
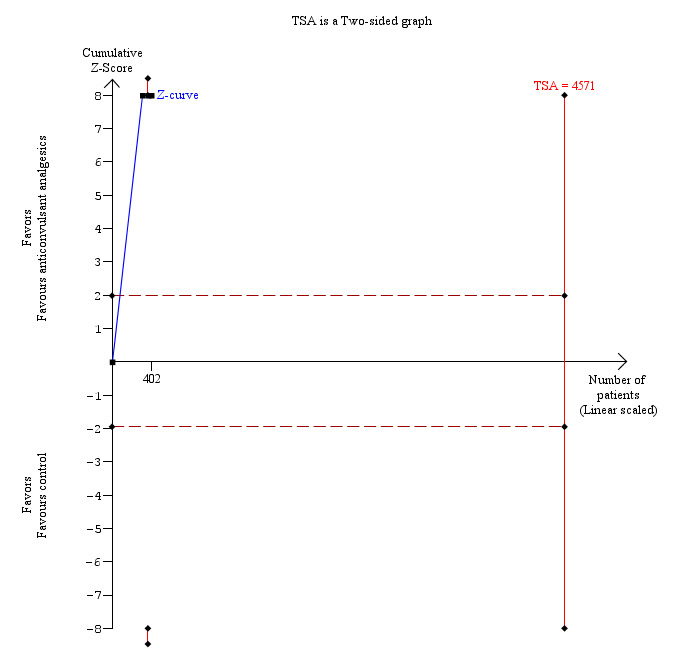
Trial sequential analysis of pain (4 to 8 hours) (anticonvulsant analgesics versus control) The diversity‐adjusted required information size (DARIS) was 4571 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 9.56, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 93.42%. The conventional statistical boundaries (dotted red line) are crossed by the cumulative Z curve (blue line) after the third trial. After accruing 402 participants in three trials, only 8.79% of DARIS has been reached. Accordingly, the futility area is not shown. The conventional monitoring boundaries (dotted red line) are crossed by the cumulative Z curve (blue line) after the first trial. The trial sequential monitoring boundaries (red line) are not crossed by cumulative Z curve. The findings are consistent with high risk of random errors even though there is a statistically significant reduction in pain in the anticonvulsant analgesic group compared with the control group.
Pain at 9 to 24 hours
Three trials reported pain at 9 to 24 hours (Pandey 2004; Agarwal 2008; Sarakatsianou 2013). The pain scores as measured by VAS were significantly lower in the anticonvulsant analgesic group than the control group (MD ‐0.55 cm VAS; 95% CI ‐0.68 to ‐0.42; 402 participants; very low quality evidence) (Analysis 3.3). There were no changes in the interpretation of results by using a random‐effects meta‐analysis. Either the mean or the standard deviation was imputed in two trials (Agarwal 2008; Sarakatsianou 2013). Exclusion of these trials did not alter the results (MD ‐0.54 cm VAS; 95% CI ‐0.67 to ‐0.41) (Analysis 3.6). Trial sequential analysis revealed that the diversity‐adjusted required information size (DARIS) was 25 participants based on a minimal relevant difference (MIRD) of 1 cm on the VAS, a variance (VAR) of 0.78, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. As this was crossed by the first trial, the trial sequential boundaries were not drawn. A post hoc analysis with the MIRD revised to 0.25 cm was performed. The conventional statistical boundaries and the trial sequential monitoring boundaries were crossed by the cumulative Z curve after the second trial. The findings were consistent with anticonvulsant analgesics decreasing pain between 9 and 24 hours compared with inactive control with low risk of random errors (Figure 10).
3.3. Analysis.
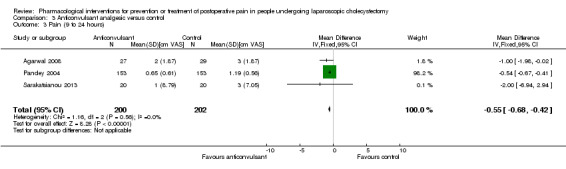
Comparison 3 Anticonvulsant analgesic versus control, Outcome 3 Pain (9 to 24 hours).
3.6. Analysis.

Comparison 3 Anticonvulsant analgesic versus control, Outcome 6 Pain (9 to 24 hours) sensitivity analysis.
10.
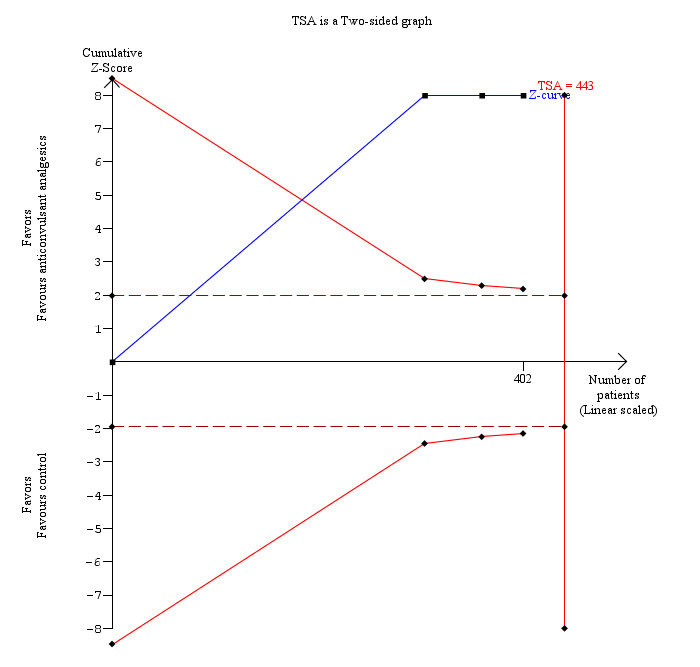
Trial sequential analysis of pain (9 to 24 hours) (anticonvulsant analgesics versus control) The diversity‐adjusted required information size (DARIS) was 28 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 0.88, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. As this was crossed by the first trial, the trial sequential boundaries were not drawn. A post‐hoc analysis with the MIRD revised to 0.25 cm was performed. The conventional statistical boundaries (dotted red line) and the trial sequential monitoring boundaries (red line) are crossed by the cumulative Z curve (blue line) after the first trial. Although the DARIS has not been reached, the findings are consistent with anticonvulsant analgesics decreasing pain between 9 and 24 hours compared with inactive control with low risk of random errors.
Return to normal activity
None of the trials reported return to normal activity.
Return to work
One trial (50 participants) reported return to work (Gilron 2009). The trial did not report the standard deviation. The trial reported that there were no significant differences in the time taken to return to work. Trial sequential analysis was not performed because of the presence of only one trial and because of the lack of standard deviation in the trial that reported this outcome (Gilron 2009).
Subgroup analysis
We did not perform subgroup analysis because of the few trials included in this comparison.
Reporting bias
We did not assess the reporting bias by using funnel plots because of the few trials included in this comparison.
Opioids versus non‐steroidal anti‐inflammatory drugs
Only one trial compared opioids versus NSAIDs. The only outcome reported in this trial was drug‐related serious adverse events. There were no drug‐related serious adverse events related to either group (0/51 (0%) in opioid group versus 0/51 (0%) in NSAID group). Trial sequential analysis, sensitivity analysis, subgroup analysis, and assessment of reporting bias by funnel plot were not performed because of the paucity of data.
Anticonvulsant analgesics versus non‐steroidal anti‐inflammatory drugs
Mortality
One trial reported mortality (Akarsu 2012). There was no mortality in either group in this trial (0/30 (0%) in anticonvulsant analgesic group versus 0/30 (0%) in NSAID group). Trial sequential analysis was not performed because of the presence of only one trial.
Morbidity
One trial reported morbidity (Gilron 2009). There was no significant difference in the morbidity between the two groups (RR 2.16; 95% CI 0.21 to 22.38; 52 participants; very low quality evidence) (Analysis 4.1). Another trial reported drug‐related serious adverse events (Akarsu 2012). There were no serious adverse events in the anticonvulsant analgesic group (0/30 (0%)) and one serious adverse event (respiratory depression) (1/30 (3.3%)) in the NSAID group. The severity of the respiratory depression was not reported (Akarsu 2012). Trial sequential analysis was not performed because of the presence of only one trial.
4.1. Analysis.
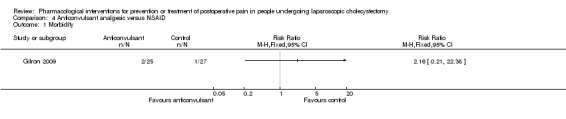
Comparison 4 Anticonvulsant analgesic versus NSAID, Outcome 1 Morbidity.
Patient quality of life
None of the trials reported patient quality of life.
Hospital stay
None of the trials reported the proportion of people discharged as day‐surgery or the length of hospital stay.
Pain
Pain at 4 to 8 hours
One trial reported pain at 4 to 8 hours (Akarsu 2012). The pain scores as measured by VAS were significantly lower in the anticonvulsant analgesic group than the NSAID group (MD ‐2.50 cm VAS; 95% CI ‐2.84 to ‐2.16; 60 participants; very low quality evidence) (Analysis 4.2). Neither the mean nor the standard deviation was imputed in this trial. Trial sequential analysis was not performed because of the presence of only one trial.
4.2. Analysis.
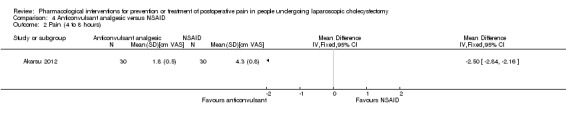
Comparison 4 Anticonvulsant analgesic versus NSAID, Outcome 2 Pain (4 to 8 hours).
Pain at 9 to 24 hours
One trial reported pain at 9 to 24 hours (Akarsu 2012). The pain scores as measured by VAS were significantly lower in the anticonvulsant analgesic group than the NSAID group (MD ‐0.50 cm VAS; 95% CI ‐0.84 to ‐0.16; 60 participants; very low quality evidence) (Analysis 4.3). Neither the mean nor the standard deviation was imputed in this trial. Trial sequential analysis was not performed because of the presence of only one trial.
4.3. Analysis.
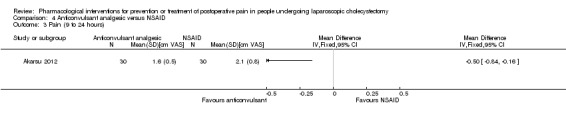
Comparison 4 Anticonvulsant analgesic versus NSAID, Outcome 3 Pain (9 to 24 hours).
Return to normal activity
None of the trials reported return to normal activity.
Return to work
One trial (52 participants) reported return to work (Gilron 2009). The trial did not report the standard deviation. The trial reported that there were no significant differences in the time taken to return to work. Trial sequential analysis was not performed because of the presence of only one trial and because of the lack of standard deviation in the trial that reported this outcome (Gilron 2009).
Subgroup analysis
We did not perform subgroup analysis because of the few trials included in this comparison.
Reporting bias
We did not assess reporting bias by using funnel plots because of the few trials included in this comparison.
Anticonvulsant analgesics versus opioids
Only one trial could be included under this comparison (Pandey 2004). The outcomes reported by this trial were drug‐related serious adverse events (respiratory depression) (0/153 (0%) in anticonvulsant analgesic group versus 6/153 (3.9%) in opioid group; severity of respiratory depression not known), pain at 4 to 8 hours, and pain at 9 to 24 hours. There were no significant differences in pain at 4 to 8 hours between the groups (MD ‐0.32 cm VAS; 95% CI ‐0.92 to 0.28; 306 participants; very low quality evidence) (Analysis 5.1). Pain at 9 to 24 hours was significantly lower in the anticonvulsant analgesic group versus opioid group (MD ‐0.22 cm VAS; 95% CI ‐0.34 to ‐0.10; 306 participants; very low quality evidence) (Analysis 5.2). Trial sequential analysis, sensitivity analysis, subgroup analysis, and assessment of reporting bias by funnel plot were not performed because of the paucity of data.
5.1. Analysis.
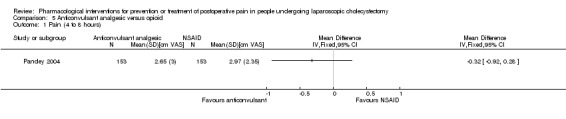
Comparison 5 Anticonvulsant analgesic versus opioid, Outcome 1 Pain (4 to 8 hours).
5.2. Analysis.
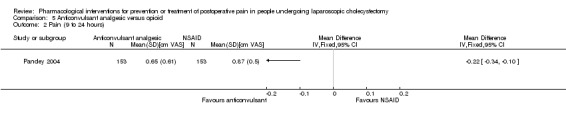
Comparison 5 Anticonvulsant analgesic versus opioid, Outcome 2 Pain (9 to 24 hours).
Discussion
Summary of main results
In this review, we have compared different pharmacological agents aimed at reducing pain during laparoscopic cholecystectomy. We included 25 randomised clinical trials including 2505 participants randomised to different groups and contributing to one or more of the outcomes. There were no significant differences in mortality or morbidity between the groups in different comparisons. The overall mortality after laparoscopic cholecystectomy is low (0.2%) (Giger 2011). In this review, the trials excluded high‐risk participants and we would anticipate that mortality would be even lower in these studies. To detect a 20% relative risk difference in mortality, more than 350,000 people are necessary. It is unlikely that trials will be powered to measure differences in mortality during laparoscopic cholecystectomy. Major complications during laparoscopic cholecystectomy are also rare. Although respiratory depression was reported as complications in some of the comparisons, the severity of the respiratory depression were not reported and whether these respiratory depressions were related to the drug per se or whether they were related to the anaesthetics that the participants received was not clear. Respiratory depression is one of the complications of opioids and anticonvulsant analgesics (Martindale 2011). Common adverse effects of opioids include nausea, vomiting, constipation, drowsiness, confusion, and urinary retention (Martindale 2011). Common adverse effects of anticonvulsant analgesics include drowsiness and sedation, although very serious adverse effects such as coma can occur rarely following overdose (Martindale 2011). Common adverse events related to NSAIDs include mild and reversible gastrointestinal discomfort, nausea, and diarrhoea, although in some people, peptic ulceration and severe gastrointestinal bleeding may occur (Martindale 2011). Various other rare adverse events include blood disorders such as anaemia; thrombocytopenia; neutropenia; eosinophilia; agranulocytosis; renal toxicity; central nervous system‐related adverse effects including depression, drowsiness, and insomnia; fluid retention; congestive heart failure; photosensitivity; and hypersensitivity reactions (Martindale 2011). The serious adverse events profile differs from one NSAID to another (Martindale 2011). Thus, all the drugs compared in this review have one of more potentially serious adverse events. To warrant routine use of these agents, the adverse events have to be balanced against the benefits that these agents may provide. Future trials should include drug‐related serious adverse events as an important outcome.
None of the trials reported quality of life or return to normal activity. There were no significant differences in the proportion of people discharged as day‐surgery, length of hospital stay, or the time taken to return to work in any of the comparisons that reported return to work. The main purpose of the pharmacological agents is to decrease pain enabling people to be discharged from hospital and to return to normal activity and work as early as possible. These outcomes are not only important for the person but are also important for the state‐funded health system. While quality of life is the outcome that is used for assessing the cost‐effectiveness of an intervention, return to normal activity and return to work may also have relevance to the state in terms of lack of productivity of the individual. Proportion of people discharged as day‐surgery and the length of the hospital stay are important for people in a private health setting and for the state in a state‐funded health system because of the costs associated with hospital stay. However, only a few trials reported one of more of these outcomes (Horattas 2004; Gilron 2009; Sandhu 2011). Future trials on this topic should include these outcomes.
Pain at 4 to 8 hours and at 9 to 24 hours were significantly reduced in the various comparisons. The findings were robust to different sensitivity analyses in most of the comparisons. Trial sequential analysis also confirmed the risk of random errors in concluding that the pharmacological intervention decreased pain was low in many of the comparisons. Although some subgroup analyses showed significant influence of some factors over the effect estimates, much importance should be not given to these subgroup analyses because of the presence of only one or two trials in the various subgroups. The mean reduction in pain was about 1 cm on the 0 to 10 cm VAS for 4 to 8 hours and about 0.5 cm on the 0 to 10 cm for 9 to 24 hours in most comparisons. Differences in pain scores of between 0.9 and 1.8 cm are generally considered clinically significant (Todd 1996). Thus, it appears that some pharmacological agents may have a role in increasing the proportion of laparoscopic cholecystectomies performed as day‐surgery since people undergoing day‐surgery laparoscopic cholecystectomy are discharged between 4 and 8 hours. There was no significant difference in the proportion of participants who were discharged as day‐surgery in this review. It does not appear from the description in the trials that day‐surgery was attempted in most trials. Future trials should investigate the role of different pharmacological agents in the day‐surgery laparoscopic cholecystectomy setting.
Surgical complications such as bile duct injury may increase the pain after laparoscopic cholecystectomy. However, the proportion of participants who develop serious complications after laparoscopic cholecystectomy is less than 0.5% (Giger 2011). It should be noted that the pharmacological interventions do not reduce the surgical complications and hence pharmacological interventions cannot be advocated routinely in all people undergoing laparoscopic cholecystectomy in order to decrease pain due to surgical complications.
Given that there are other alternatives that are safe and effective in reducing pain after laparoscopic cholecystectomy to a similar degree, for example, intraperitoneal local anaesthetic instillation (Gurusamy 2014) or local anaesthetic wound infiltration (Loizides 2014), the use of NSAIDs, opioids, and anticonvulsant analgesics can be questioned. Of course, local anaesthetic agents work only for a short time while NSAIDs, opioids, and anticonvulsant analgesics can be administered orally on a regular basis for a few days postoperatively. The question is whether such routine administration is more beneficial than administration as required or whether there is any benefit in administering prescription‐only agents compared with analgesics available over‐the‐counter (eg, NSAIDs such as paracetamol or ibuprofen), which are generally considered safe for short‐term use in most people. There is currently no evidence to suggest any clinical benefit in administering these agents routinely.
Overall completeness and applicability of evidence
Most of the trials included in this review included people undergoing elective laparoscopic cholecystectomy (Included studies; Characteristics of included studies). Most trials included only low anaesthetic risk participants (Included studies; Characteristics of included studies). The findings of this review are applicable only to such people.
Quality of the evidence
The overall quality of evidence was low to very low (Table 1). Although it is difficult to blind many interventions in surgery, this is one of the few interventions in which adequate blinding can be achieved and high‐quality evidence is possible. Nevertheless, this is the best evidence that is currently available.
Potential biases in the review process
We performed a thorough search of literature. However, we included 'pain' as one of the domains in this search strategy. Considering that reduction in pain is the main reason for the use of these treatments, we expected that all the trials related to the topic would be identified, and given the number of trials included in this review, it is likely that most of the trials on this topic have been identified, However, it is possible that trials did not mention pain or words related to pain, and such trials might have been missed by this search strategy. The impact of this is likely to be small since it is likely that most trials would have mentioned the purpose of the use of the intervention. At least two review authors independently identified trials for inclusion and extracted data, thus minimising errors. However, we imputed the mean and standard deviation when these were not available. We performed a sensitivity analysis excluding such trials but this did not change the results significantly thus demonstrating the minimal impact of missing mean or standard deviation.
Agreements and disagreements with other studies or reviews
A systematic review by Procedure Specific Postoperative Pain Management (PROSPECT) group recommended routine use of NSAIDs and recommended against routine use of opioid analgesics during laparoscopic cholecystectomy (Kehlet 2005). Another systematic review by Bisgaard et al. made similar recommendations as the PROSPECT group and in addition recommended against routine use of gabapentin during laparoscopic cholecystectomy (Bisgaard 2006). We do not recommend routine use of any of these pharmacological agents.
Authors' conclusions
Implications for practice.
There is evidence of very low quality that different pharmacological agents including non‐steroidal anti‐inflammatory drugs (NSAIDs), opioid analgesics, and anticonvulsant analgesics reduce pain scores in people at low anaesthetic risk undergoing elective laparoscopic cholecystectomy. However, the decision to use these drugs has to weigh the clinically small reduction in pain against uncertain evidence of serious adverse events associated with many of these agents.
Implications for research.
Further randomised clinical trials are necessary to evaluate the role of pharmacological agents in the emergency and in the elective set‐up particularly in the day‐surgery elective laparoscopic cholecystectomy.
Future trials should include drug‐related serious adverse events, quality of life, hospital stay, return to normal activity, and return to work as outcomes.
Future trials need to be designed according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines (www.spirit‐statement.org/) and conducted and reported according to the CONSORT (Consolidated Standards for Reporting of Trials) Statement (www.consort‐statement.org).
Notes
Change in review's author team. It is as follows: Kurinchi Selvan Gurusamy, Jessica Vaughan, Clare D Toon, Brian R Davidson.
Acknowledgements
To The Cochrane Hepato‐Biliary Group for the support that they have provided.
Peer reviewers: Anders Mark Christensen, Denmark; Achmet Ali, Turkey. Contact editor: Christian Gluud, Denmark.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: "The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health".
Appendices
Appendix 1. Search strategies for identification of studies
| Database | Period of search | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley) | Issue 3 of 12, 2013. | #1 laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop* #2 cholecystectom* #3 MeSH descriptor Cholecystectomy, Laparoscopic explode all trees #4 (( #1 AND #2) OR #3) #5 MeSH descriptor Pain explode all trees #6 MeSH descriptor Anesthetics, Local explode all trees #7 Intraperitoneal morphine OR intraperitoneal meperidine OR NSAID OR Ketorolac OR diclofenac OR Indomethacine OR Paracetamolo OR tenoxicam OR parecoxib OR valecoxib intraperitoneal bupivacaine OR thoracic epidural analgesia OR opioids analgesia OR preemptive gabapentin OR clonidine OR ketamine OR esmolol #8 (#4 OR #5 OR #6 ) #9 pain OR ache* OR suffering* #10 (#8 OR #9) #11 (#4 AND #5 AND #6 AND #10) |
| MEDLINE (PubMed) | 1987 to March 2013. | laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND (cholecystectom* OR "cholecystectomy, laparoscopic"[MeSH]) AND (Analgesia OR bupivacain* OR buvacaina OR sensorcaine OR marcain* OR svedocain* OR levobupivacaine OR ropivacaine OR Ketorolac OR diclofenac OR Indomethacine OR Paracetamolo OR tenoxicam OR parecoxib OR valecoxib OR ntraperitoneal morphine OR intraperitoneal meperidine OR intraperitoneal bupivacaine OR thoracic epidural analgesia OR opioids analgesia OR preemptive gabapentin OR clonidine OR ketamine OR esmolol AND ("Pain"[Mesh] OR pain OR ache* OR suffering*) AND (((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh])))) |
| EMBASE (OvidSP) | 1987 to March 2013. | 1. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 2. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 3. 1 or 2 4. (laparoscop* or coelioscop* or celioscop*OR peritoneoscop*).af. 5. exp laparoscopic surgery/ 6. 4 or 5 7. cholecystectom*.af. 8. exp cholecystectomy/ 9. 7 or 8 10. (analgesia or ketorolac or diclofenac or indomethacin or paracetamol or tenoxicam or parecoxib or valdecoxib or morphine or meperidine or opioid or opiate).af. 11. exp analgesia/ 12. 10 or 11 13. (pain or ache* or suffering*).af. 14. exp pain/ 15. 13 or 14 16. 3 and 6 and 9 and 12 and 15 |
| Science Citation Index Expanded (Web of Knowledge) | 1987 to March 2013. | #1 TS=(laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) #2 TS=(cholecystectom*) #3 TS=(pain OR ache* OR suffering* OR Preemptive analgesia OR pain control OR Multimodal analgesia) #4 TS=(NSAID OR Ketorolac OR diclofenac OR Indomethacine OR Paracetamolo OR tenoxicam OR parecoxib OR valecoxib OR intraperitoneal morphine OR intraperitoneal meperidine OR intraperitoneal bupivacaine OR thoracic epidural analgesia OR opioids analgesia OR preemptive gabapentin OR clonidine OR ketamine OR esmolol) #5 #3 OR #4 #6 #5 AND #4 AND #3 AND #2 AND #1 |
Data and analyses
Comparison 1. NSAID versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morbidity | 5 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.37, 1.53] |
| 2 Proportion discharged as day‐surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (4 to 8 hours) | 11 | 999 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.07, ‐0.70] |
| 5 Pain (9 to 24 hours) | 9 | 707 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.67, ‐0.33] |
| 6 Morbidity (sensitivity analysis) | 5 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.07] |
| 6.1 Best‐best | 5 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.53] |
| 6.2 Best‐worst | 5 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.23, 0.82] |
| 6.3 Worst‐best | 5 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.79, 2.67] |
| 6.4 Worst‐worst | 5 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.36] |
| 7 Proportion discharged as day‐surgery (sensitivity analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Best‐best | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Best‐worst | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Worst‐best | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Best‐worst | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pain (4 to 8 hours) sensitivity analysis | 4 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.10, ‐0.71] |
| 9 Pain (9 to 24 hours) sensitivity analysis | 4 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.67, ‐0.33] |
| 10 Pain (4 to 8 hours) stratified by drug | 11 | 999 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.07, ‐0.70] |
| 10.1 Celecoxib | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐7.01, 4.95] |
| 10.2 Diclofenac | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐7.56, 2.56] |
| 10.3 Etofenomate | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] |
| 10.4 Flurbiprofen | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐3.26, ‐1.26] |
| 10.5 Lornoxicam | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐3.13, ‐2.26] |
| 10.6 Metamizol | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.74, 1.14] |
| 10.7 Paracetamol | 3 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.02, 0.82] |
| 10.8 Parecoxib | 4 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.21, ‐0.31] |
| 10.9 Tenoxicam | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐4.42, 3.51] |
| 11 Pain (4 to 8 hours) stratified by time | 11 | 999 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.07, ‐0.70] |
| 11.1 Before | 4 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.60, ‐0.09] |
| 11.2 During | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐3.13, ‐2.26] |
| 11.3 After | 4 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.17, ‐0.29] |
| 11.4 Before and after | 2 | 293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.28, ‐0.11] |
| 12 Pain (9 to 24 hours) stratified by drug | 9 | 707 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.67, ‐0.33] |
| 12.1 Celecoxib | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐5.52, 4.78] |
| 12.2 Diclofenac | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐3.94, 4.94] |
| 12.3 Etofenomate | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.24] |
| 12.4 Flurbiprofen | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐2.08, 0.12] |
| 12.5 Lornoxicam | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.42, ‐1.72] |
| 12.6 Metamizol | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.35, 1.15] |
| 12.7 Paracetamol | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.48, 0.90] |
| 12.8 Parecoxib | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.08, 0.08] |
| 12.9 Tenoxicam | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.10, 2.89] |
| 13 Pain (9 to 24 hours) stratified by time | 9 | 707 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.67, ‐0.33] |
| 13.1 Before | 4 | 285 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.24] |
| 13.2 During | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.42, ‐1.72] |
| 13.3 After | 3 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.53, 0.20] |
| 13.4 Before and after | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐3.62, 3.80] |
1.10. Analysis.

Comparison 1 NSAID versus control, Outcome 10 Pain (4 to 8 hours) stratified by drug.
1.11. Analysis.
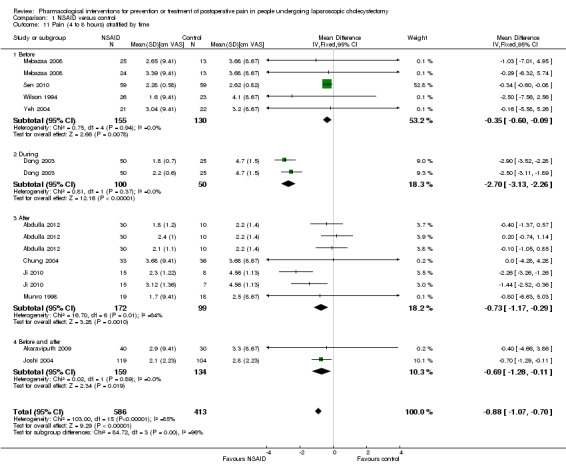
Comparison 1 NSAID versus control, Outcome 11 Pain (4 to 8 hours) stratified by time.
1.12. Analysis.

Comparison 1 NSAID versus control, Outcome 12 Pain (9 to 24 hours) stratified by drug.
1.13. Analysis.
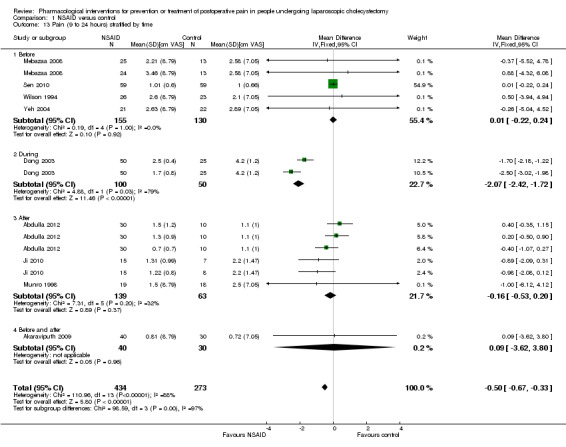
Comparison 1 NSAID versus control, Outcome 13 Pain (9 to 24 hours) stratified by time.
Comparison 2. Opioid versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (4 to 8 hours) | 3 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐2.51 [‐3.02, ‐2.01] |
| 2 Pain (9 to 24 hours) | 3 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.44, ‐0.20] |
| 3 Pain (4 to 8 hours) (sensitivity analysis) | 1 | 306 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐3.07, ‐2.05] |
| 4 Pain (9 to 24 hours) (sensitivity analysis) | 1 | 306 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.44, ‐0.20] |
Comparison 3. Anticonvulsant analgesic versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pain (4 to 8 hours) | 3 | 402 | Mean Difference (IV, Fixed, 95% CI) | ‐2.52 [‐2.95, ‐2.09] |
| 3 Pain (9 to 24 hours) | 3 | 402 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.68, ‐0.42] |
| 4 Morbidity (sensitivity analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Best‐best | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Best‐worst | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Worst‐best | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Worst‐worst | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pain (4 to 8 hours) sensitivity analysis | 1 | 306 | Mean Difference (IV, Fixed, 95% CI) | ‐2.88 [‐3.36, ‐2.40] |
| 6 Pain (9 to 24 hours) sensitivity analysis | 1 | 306 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.67, ‐0.41] |
Comparison 4. Anticonvulsant analgesic versus NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pain (4 to 8 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain (9 to 24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Morbidity (sensitivity analysis) | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.50, 2.01] |
| 4.1 Best‐best | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 4.2 Best‐worst | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.52] |
| 4.3 Worst‐best | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.62, 40.28] |
| 4.4 Worst‐worst | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.44] |
4.4. Analysis.
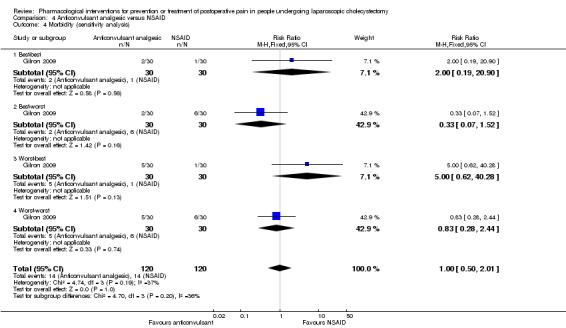
Comparison 4 Anticonvulsant analgesic versus NSAID, Outcome 4 Morbidity (sensitivity analysis).
Comparison 5. Anticonvulsant analgesic versus opioid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (4 to 8 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain (9 to 24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulla 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 120. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 120. Mean age: 52 years. Females: 90 (75%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 4 groups. Group 1: postoperative saline IV (n = 30). Group 2: postoperative parecoxib 40 mg IV twice daily (n = 30). Group 3: postoperative metamizol 1 mg IV 3 times daily (n = 30). Group 4: postoperative paracetamol (acetaminophen) 1 mg IV 3 times daily (n = 30). | |
| Outcomes | Drug‐related serious adverse events and pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomisation table used". |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignment code retained until the conclusion of the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Group assignment code retained until the conclusion of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Group assignment code retained until the conclusion of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Agarwal 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: India. Number randomised: 60. Post‐randomisation drop‐outs: 4 (6.7%). Revised sample size: 56. Mean age: 46 years. Females: 19 (33.9%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: pregabalin 150 mg orally 1 h before surgery (n = 27). Group 2: placebo 1 h before orally surgery (n = 29). | |
| Outcomes | Drug‐related serious adverse events and pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: conversion to open cholecystectomy (n = 3), re‐exploration on account of postoperative bleeding (n = 1). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated table of random numbers used". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Selected using sealed envelopes to be opened by anesthesia resident". Comment: Further details not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All the medications…were identical, and were administered…by a staff nurse who was not involved in the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcomes were assessed by an independent anaesthesia registrar blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Akaraviputh 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Thailand. Number randomised: 70. Post‐randomisation drop‐outs: not stated. Revised sample size: 70. Mean age: 57 years. Females: 41 (58.6%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: parecoxib 20 mg infusion 30 min before induction of anaesthesia and at 12 h after the first dose (n = 40). Group 2: saline infusion 30 min before induction of anaesthesia and at 12 h after the first dose (n = 30). | |
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelope technique used". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The degree of postoperative pain was assessed…by nursing staff who were unaware of the perioperative intervention". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Comment: Supported by Faculty of Medicine Siriraj Hospital Research Project Grant. |
Akarsu 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 60. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 60. Mean age: 59 years. Females: 24 (40%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: pregabalin 300 mg orally 1 h before surgery (n = 30). Group 2: diclofenac 75 mg IM 15 to 20 min before completion of surgery (n = 30). | |
| Outcomes | Mortality, drug‐related serious adverse events, and pain. | |
| Notes | Attempts were made to contact authors in August 2013. Authors provided replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single‐blind study, subjects do not know the methodology applied. The investigator knows" (author replies). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessors were blinded" (author replies). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Overall morbidity was not reported. |
| For‐profit bias | Low risk | Quote: "The study was funded by us" (author replies). |
Akinci 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 41. Post‐randomisation drop‐outs: not stated. Revised sample size: 41. Mean age: 45 years. Females: 27 (65.9%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: tramadol 100 mg IV (n = 21). Group 2: placebo (n = 20). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computerised allocation schedule used". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Coded syringes used". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Balaban 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 90. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 90. Mean age: 53 years. Females: 69 (76.7%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: pregabalin 150 mg orally 1 h before surgery (n = 30). Group 2: pregabalin 300 mg orally 1 h before surgery (n = 30). Group 3: placebo orally (n = 30). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation achieved sealed envelope assignment". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Belzarena 1998.
| Methods | Randomised clinical trial. | |
| Participants | Country: Portugal. Number randomised: 90. Post‐randomisation drop‐outs: not stated. Revised sample size: 90. Mean age: 42 years. Females: 65 (72.2%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: tenoxicam 20 mg in 4‐mL saline IV (n = 30). Group 2: tenoxicam 40 mg in 4‐mL saline IV (n = 30). Group 3: saline IV (n = 30). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Venipuncture was performed by a nurse who was unaware of the nature of the study". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Cheng 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 60. Post‐randomisation drop‐outs: 1 (1.7%). Revised sample size: 59. Mean age: 50 years. Females: 37 (62.7%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: celecoxib 200 mg orally before surgery (n = 30). Group 2: placebo orally before surgery (n = 29). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: non‐standardisation of the anaesthetic drugs (n = 1). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized into two groups by sealed envelopes". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A post‐anaesthetic care unit nurse, who was unaware of the study drug recorded the time of the first dose of PCA morphine and evaluated the post operative pain and intensity". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was a post‐randomisation drop‐out but this was unlikely to be related to the intervention. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Chung 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 84. Post‐randomisation drop‐outs: 15 (17.9%). Revised sample size: 69. Mean age: 48 years. Females: not stated. Inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups.
Group 1: paracetamol (acetaminophen) 300 mg orally every 6 h for 48 h (n = 33).
Group 2: placebo (n = 36). Co‐intervention: codeine 48 h postoperatively |
|
| Outcomes | Serious adverse events and pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: lack of pain (n = 4), loss to follow‐up (n = 3), adverse events (n = 3), inadequate pain control (n = 6) (note that there was discrepancy in the number of post‐randomisation drop‐outs and the reasons for drop‐outs in the report). Attempts were made to contact the authors. Replies were received from authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated sequence (author replies)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐dummy technique, with matching placebo used". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome blinded" (author replies). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported. |
| For‐profit bias | High risk | Quote: "This study was partially supported by a grant from Purdue Pharma (Canada) Inc". |
Dong 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: not stated.
Number randomised: 150.
Post‐randomisation drop‐outs: not stated.
Revised sample size: 150.
Mean age: 48 years.
Females: not stated. Inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: lornoxicam 16 mg (8 mg IM and 8 mg IV) (n = 50). Group 2: lornoxicam 24 mg (8 mg IM and 16 mg IV) (n = 50). Group 3: control (n = 50). | |
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Fanelli 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 50. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 50. Mean age: 57 years. Females: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: oxycodone, 10 mg for people with age ≥ 60 years and 20 mg for those aged < 60 years, orally 1 h before surgery and 12 h after the first administration (n = 25). Group 2: placebo 1 h before surgery and 12 h after the first administration (n = 25). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random number table used". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo tablets were apparently identical to the active drug tablets and all treatments were given to patients double blindly". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient was first asked by a blind observer to express his/her pain". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Quote: "Sources of financial support for the work: University of Parma". |
Forse 1996.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 60. Post‐randomisation drop‐outs: 8 (13.3%). Revised sample size: 52. Mean age: 47 years. Females: 32 (61.5%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups.
Group 1: ketorolac IM before surgery (n = 17). Group 2: indomethacin rectally before surgery (n= 17). Group 3: placebo (n = 18). |
|
| Outcomes | Operative complications. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed numbered envelopes". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Gilron 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 89. Post‐randomisation drop‐outs: 12 (13.5%). Revised sample size: 77. Mean age: 46 years. Females: 64 (83.1%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups:
Group 1: meloxicam 15 mg daily orally, 1 h before until 2 days after surgery (n = 25).
Group 2: gabapentin 1200 to 1600 mg daily orally 1 h before until 2 days after surgery (n = 27). Group 3: meloxicam 15 mg and gabapentin 1200 to 1600 mg daily orally 1 h before until 2 days after surgery (n = 25). |
|
| Outcomes | Serious adverse events and return to work. | |
| Notes | Reasons for post‐randomisation drop‐outs: surgery cancellation (n = 2), dizziness (n = 3), liver laceration (n = 1), hypoxaemia (n = 1), intra‐operative electrocardiogram changes (n =1), personal reasons (n = 1), protocol withdrawal (n =1), reflux (n = 1), pruritus (n = 1). Attempts were made to contact the authors in August 2013. Replies from authors were received in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A concealed, computer‐generated random treatment allocation schedule, which randomized... three treatments". |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigational pharmacist and the biostatistician determine the treatment randomization sequence and block size without sharing this information with trial investigators and research personnel (author replies)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medications were encapsulated in identically appearing red (rofecoxib or "rofecoxib" placebo) and gray (gabapentin or "gabapentin" placebo) gelatin capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported. |
| For‐profit bias | Low risk | Quote: "Supported by Physicians’ Services Incorporated Foundation Grant no. 03‐30 and by Canadian Institutes of Health Research Grant no. MSH‐55041". |
Gomez‐Vazquez 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Mexico. Number randomised: 40. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 40. Mean age: 30 years. Females: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups: Group 1: morphine 0.15 mg/kg IV postoperatively for 40 min (n = 20). Group 2: ketorolac 0.7 mg/kg IV postoperatively for 40 min (n = 20). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Horattas 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA.
Number randomised: 120.
Post‐randomisation drop‐outs: 4 (3.3%).
Revised sample size: 116.
Mean age: 49 years.
Females: 85 (73.3%).
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: rofecoxib 50 mg orally preoperatively (n = 58). Group 2: placebo (n = 58). | |
| Outcomes | Proportion discharged as day‐surgery. | |
| Notes | Reasons for post‐randomisation drop‐outs: incomplete data extraction (n = 2), conversion to open cholecystectomy (n = 2). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The hospital pharmacist prepared individual unmarked 30 cc bottles of rofecoxib elixir (50 mg), or an identical amount of indistinguishable strawberry‐flavored placebo elixir. Bottles were randomly identified by number and then sequentially administered to each participating patient in the presurgery unit 1 to 2 hours before their surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Ji 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 30. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 30. Mean age: not stated. Females: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: flurbiprofen axetil 1.0 mg/kg parenteral (IV or IM not stated) postoperative (n = 15). Group 2: parecoxib 0.8 mg/kg parenteral (IV or IM not stated) postoperative (n = 15). Group 3: saline 10 mL postoperative (n = 15). | |
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Joshi 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 276. Post‐randomisation drop‐outs: 13 (4.7%). Revised sample size: 263. Mean age: 44 years. Females: 215 (81.7%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: parecoxib 40 mg IV 30 to 45 min before surgery plus oral valdecoxib 40 mg 6 to 12 h after IV parecoxib (n = 134). Group 2: placebo IV 30 to 45 min before surgery and oral placebo 6 to 12 h after IV placebo (n = 129). | |
| Outcomes | Serious adverse events and pain. | |
| Notes | Attempts were made to contact the authors in August 2013. Reasons for post‐randomisation drop‐outs: did not receive medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Based on the computer generated randomization scheme". |
| Allocation concealment (selection bias) | Low risk | Quote: "The hospital pharmacist who was not involved with patient care or data collection prepared the IV study drugs (2 mL solution identical in appearance) and provided them to the investigator". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The hospital pharmacist who was not involved with patient care or data collection prepared the IV study drugs (2 mL solution identical in appearance) and provided them to the investigator". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The hospital pharmacist who was not involved with patient care or data collection prepared the IV study drugs (2 mL solution identical in appearance) and provided them to the investigator". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported. |
| For‐profit bias | High risk | Quote: "Supported, in part, by Pharmacia Corporation and Pfizer Inc". |
Karakoc 2011.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 80. Post‐randomisation drop‐outs: not stated. Revised sample size: 80. Mean age: not stated. Females: not stated. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: dexketoprofen trometamol 50 mg IV 30 min before the completion of surgery (n = not stated). Group 2: 0.9% normal saline IV 30 min before the completion of surgery (n = not stated). Co‐intervention: morphine at end of surgery in both groups, then delivered by patient‐controlled analgesia. | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Lane 1996.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA.
Number randomised: 125.
Post‐randomisation drop‐outs: 0 (0%).
Revised sample size: 125.
Mean age: 44.
Females: 107.
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 5 groups: Group 1: placebo (n = 23) Group 2: meripedine 100 mg IM intra‐operatively, pre‐procedure (n = 31). Group 3: meripedine 100 mg IM intra‐operatively, post‐procedure (n = 20). Group 4: ketorolac tromethamine 60 mg IM intra‐operatively, pre‐procedure (n = 25). Group 5: ketorolac tromethamine 60 mg IM intra‐operatively, post‐procedure (n = 26). |
|
| Outcomes | Drug‐related serious adverse events. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Liu 1993.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 60. Post‐randomisation drop‐outs: not stated. Revised sample size: 60. Mean age: 46 years. Females: 15 (25%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups.
Group 1: ketorolac 60 mg in 2 mL IM 20 to 40 min before surgery (n = 31).
Group 2: saline 2 mL IM 20 to 40 min before surgery (n = 29). Co‐intervention: midazolam 2 mg. |
|
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Medication was prepared in a 2‐mL numbered (unlabeled) syringe by the pharmacy". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recorded by a blinded nurse observer". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Mebazaa 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 75. Post‐randomisation drop‐outs: not stated. Revised sample size: 75. Mean age: 46 years. Females: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: paracetamol (acetaminophen) 1000 mg orally 1 h before induction (n = 24). Group 2: celecoxib 200 mg orally 1 h before induction (n= 25). Group 3: control (n = 26). | |
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Munro 1998.
| Methods | Randomised clinical trial. | |
| Participants | Country: not stated.
Number randomised: 40.
Post‐randomisation drop‐outs: 3 (7.5%).
Revised sample size: 37.
Mean age: 51 years.
Females: 30 (81.1%).
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: tenoxicam 40 mg IV on skin closure (n = 19). Group 2: placebo on skin closure (n = 18). | |
| Outcomes | Drug‐related serious adverse events and pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: conversion to open cholecystectomy (n = 3). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Muñoz 2002.
| Methods | Randomised clinical trial. | |
| Participants | Country: Chile. Number randomised: 120. Post‐randomisation drop‐outs: not stated. Revised sample size: 120. Mean age: 42 years. Females: 80 (66.7%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 4 groups. Group 1: morphine 0.15 mg/kg IV < 20 min before surgery (n = 33). Group 2: morphine 0.15 mg/kg IV 20 to 40 min before surgery (n = 30). Group 3: morphine 0.15 mg/kg IV > 40 min before surgery (n= 27). Group 4: placebo (n = 30). | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Nesek‐Adam 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Croatia. Number randomised: 80. Post‐randomisation drop‐outs: not stated. Revised sample size: 80. Mean age: 51 years. Females: 58 (72.5%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 4 groups. Group 1: diclofenac 1 mg/kg IV 20 min before induction (n = 20). Group 2: saline 100 mL IV 20 min before induction (n = 20). Group 3: same as group 1 with ketamine IV as co‐intervention (n = 20). Group 4: same as group 2 with ketamine IV as co‐intervention (n = 20). | |
| Outcomes | Drug‐related serious adverse events. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An envelope containing group assignment was prepared, sealed, and numbered for each patient". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "On the morning of surgery, anesthesia technician opened the envelope and prepared completely identical syringes and infusion that were labeled "infusion" and "skin bolus" in equal volume to make the study double blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Pandey 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: India. Number randomised: 459. Post‐randomisation drop‐outs: not stated. Revised sample size: 459. Mean age: 42 years. Females: 308 (67.1%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: gabapentin 300 mg orally 2 h before surgery (n = 153). Group 2: tramadol 100 mg orally 2 h before surgery (n = 153). Group 3: placebo 2 h before surgery (n = 153). | |
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated table of random numbers used". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A senior resident, who was not part of the anesthesia team recorded the pain score". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Peng 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 165. Post‐randomisation drop‐outs: 42 (25.5%). Revised sample size: 123. Mean age: 45 years. Females: 95 (77.2%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: pregabalin 50 mg orally 1 h before surgery and then every 12 h after operation for 3 doses (n = 42). Group 2: pregabalin 75 mg orally 1 h before surgery and then every 12 h after operation for 3 doses. (n = 40). Group 3: placebo 1 h before surgery and then every 12 h after operation for 3 doses (n = 41). | |
| Outcomes | Mortality. | |
| Notes | Reasons for post‐randomisation drop‐outs: protocol violation (n = 23), questionnaire not completed or returned (n = 19). Attempts were made to contact authors in August 2013. Authors provided replies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to a computer‐generated randomization list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study medications were prepared in capsules of identical colour and appearance and were packaged by the hospital pharmacy". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Morbidity was not reported. |
| For‐profit bias | High risk | Quote: "This research was funded by the Pfizer Global Investigator Initiated Grant. The medications in this study were provided by Pfizer Inc". |
Puura 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: Finland. Number randomised: 75. Post‐randomisation drop‐outs: 3 (4%). Revised sample size: 72. Mean age: 46 years. Females: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 groups. Group 1: etoricoxib 120 mg plus paracetamol (acetaminophen) 1 g orally 1.5 h before surgery (n = 25). Group 2: etoricoxib 120 mg orally 1.5 h before surgery (n = 24). Group 3: placebo 1.5 h before surgery (n = 23). | |
| Outcomes | No outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: cirrhosis (n = 1), needed open cholecystectomy (n = 2). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Drug‐containing bags used". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by a grant from the Medical Research Fund of Tampere University Hospital". |
Salihoglu 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 40. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 40. Mean age: 42 years. Females: 31 (77.5%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: paracetamol (acetaminophen) 1 g IV after intubation (n = 20). Group 2: saline IV after intubation (n = 20). | |
| Outcomes | Serious adverse events. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random number generator". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetist who intubated and followed the patient during surgery, and the surgical team were also unaware about which patient received paracetamol or not". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The researchers were unaware about the patients or the anaesthetists, who were taken into the study while the study was going on". Comment: Further details not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Sandhu 2011.
| Methods | Randomised clinical trial. | |
| Participants | Country: Thailand. Number randomised: 120. Post‐randomisation drop‐outs: 1 (0.8%). Revised sample size: 119. Mean age: 53 years. Females: 78 (65.5%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: etoricoxib 120 mg (route not stated) 1 h before surgery (n = 60). Group 2: placebo 1 h (route not stated) before surgery (n = 59). | |
| Outcomes | Length of hospital stay. | |
| Notes | Reasons for post‐randomisation drop‐outs: conversion to open cholecystectomy (n = 1). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There was one post‐randomisation drop‐out. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by the Prasert Prasarttong‐Osoth Research Fund, Medical Association of Thailand". |
Sarakatsianou 2013.
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece. Number randomised: 50. Post‐randomisation drop‐outs: 10 (20%). Revised sample size: 40. Mean age: 54 years. Females: 24 (60%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: pregabalin 600 mg orally divided in 2 doses, the night before surgery and 1 h preoperatively (n = 20). Group 2: placebo divided in 2 doses, the night before surgery and 1 h preoperatively (n = 20). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: converted to open cholecystectomy (n = 4), use of drain (n = 6). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated table of random numbers with sex stratification". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised by a computer‐generated, blinded randomisation list". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All medications were provided by the hospital pharmacy, and they were identical in shape and colour". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Schuster 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 80. Post‐randomisation drop‐outs: 8 (10%). Revised sample size: 72. Mean age: not stated. Females: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: rofecoxib 25 mg orally presurgically (n = not stated). Group 2: placebo (n = not stated). | |
| Outcomes | Serious adverse events, hospital stay, and pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: converted to open cholecystectomy (n = 2), refused (n = 2), acute cholecystitis (n = 3), postoperative NSAID use (n = 1). Attempts were made to contact authors in August 2013. There were no serious adverse events in either group. There was no significant difference in the length of hospital stay and pain in either group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random card pull design method". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patient, surgeon and researcher were all blinded to the patient's treatment group". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The patient, surgeon and researcher were all blinded to the patient's treatment group". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Sen 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomised: 120. Post‐randomisation drop‐outs: 2 (1.7%). Revised sample size: 118. Mean age: 54 years. Females: 81 (68.6%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: etofenomate 1 g (2 mL) IM 1 h before surgery (n = 59). Group 2: saline IM 1 h before surgery (n = 59). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: protocol violation (n = 2). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated, blinded randomisation list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Wilson 1994.
| Methods | Randomised clinical trial. | |
| Participants | Country: England.
Number randomised: 55.
Post‐randomisation drop‐outs: 6 (10.9%).
Revised sample size: 49.
Mean age: 48 years.
Females: 40 (81.6%).
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: diclofenac 75 mg IM (n = 26). Group 2: placebo (n = 23). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: incomplete data extraction (n = 3), conversion to open (n = 3). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer random number generation". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Pharmacy provided identical pre‐packed syringes". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Yeh 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Taiwan. Number randomised: 44. Post‐randomisation drop‐outs: 1 (2.3%). Revised sample size: 43. Mean age: 49 years. Females: 27 (62.8%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: tenoxicam 40 mg IV 30 min before incision (n = 21). Group 2: saline 30 min before incision (n = 22). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: ineligibility for laparoscopic cholecystectomy (n = 1). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study drugs were prepared by the hospital pharmacy in identical, consecutively numbered containers marked with the name of the project, the investigator’s name and route of administration". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There was one post‐randomisation drop‐out. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Zajaczkowska 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Poland. Number randomised: 60. Post‐randomisation drop‐outs: not stated. Revised sample size: 60. Mean age: 51 years. Females: 44 (73.3%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: morphine SC (n = 30). Group 2: no intervention (n = 30). |
|
| Outcomes | Pain. | |
| Notes | Attempts were made to contact authors in August 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random‐number table". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelope method used". Comment: Further details not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Zhu 2011.
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 60. Post‐randomisation drop‐outs: 1 (1.7%). Revised sample size: 59. Mean age: 44 years. Females: 25 (42.4%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: dezocine 0.1 mg/kg IM 10 min before induction (n = 30). Group 2: saline IM 10 min before induction (n = 29). | |
| Outcomes | Pain. | |
| Notes | Reasons for post‐randomisation drop‐outs: conversion to open cholecystectomy (n = 1). Attempts were made to contact authors in August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated table used". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There was one post‐randomisation drop‐out. |
| Selective reporting (reporting bias) | High risk | Comment: Some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
ASA: American Society of Anesthesiologists; h: hour; IM: intramuscular; IV: intravenous; min: minute; NSAID: non‐steroidal anti‐inflammatory drug; SC: subcutaneous.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aftab 2008 | Quasi‐randomisation used. |
| Akca 2004 | Separate data for laparoscopic cholecystectomy not available. |
| Bajaj 2004 | Separate data for laparoscopic cholecystectomy not available. |
| Boccara 2005 | Compares different NSAIDs. |
| Collard 2007 | Compares different opioids. |
| Elhakim 2000 | Compares different routes of administration of NSAIDs. |
| Gan 2004 | Combination of ≥ 2 classes of drug used. |
| Hernandez‐Palazon 2003 | Trial features intraperitoneal installation of local anaesthetics. |
| Kocaayan 2007 | Compares different NSAIDs. |
| Koch 2008 | Compares different NSAIDs. |
| Kokki 2012 | Combination of ≥ 2 classes of drug used. |
| Matkap 2011 | Compares different routes of administration of opioids. |
| Naguib 1998 | Compares different opioids. |
| O'Hanlon 2002 | Compares different routes of administration of opioids. |
| Ozkocak 2002 | Trial features intraperitoneal installation of local anaesthetics. |
| Sanchez‐Rodriguez 2010 | Comparison of steroids not included in this review. |
| Sozbilen 2007 | Trial features local anaesthetics. |
| Stempin 2007 | Trial features intraperitoneal installation of local anaesthetics. |
| Tiippana 2008 | Not a randomised clinical trial. |
| Wininger 2010 | Separate data for laparoscopic cholecystectomy not available. |
| Wu 1999 | Intervention not intended primarily for analgesia. |
| Wu 2005 | Intervention not intended primarily for analgesia. |
| Yamazaki 2003 | Not a randomised clinical trial. |
| Zambouri 2002 | Comparison of 2 opioids. |
| Zghidi 2011 | Comparison of steroids not included in this review. |
NSAID: non‐steroidal anti‐inflammatory drug.
Characteristics of studies awaiting assessment [ordered by study ID]
Gan 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text unavailable. |
Differences between protocol and review
The outcomes have been revised based on importance to the participants and not according to the expected treatment effect. As a result, pain was moved to a secondary outcome as mortality was considered more important than pain. With regards to quality of life, we accepted only validated scales of quality of life such as Euro‐QoL, SF‐36 since using unvalidated other scales may be misleading. With regards to pain, we limited pain to 4 to 8 hours and 9 to 24 hours since this is the time period that most people are discharged. We also included only trials that reported pain on the visual analogue scale. Even this is difficult to interpret (see Discussion) but using other scales would make it even more difficult to interpret. We added other outcomes such as return to normal activity and return to work, which are very important to the patient. We have excluded analgesic requirement, which was considered a surrogate to pain. Any clinically significant differences in pain would be captured by quality of life, return to normal activity, and return to work. By altering the outcomes, we have harmonised the outcomes in this review with other reviews aimed at decreasing pain in people undergoing laparoscopic cholecystectomy.
We have added a section on trial sequential analysis to control for random errors.
We have restricted the comparisons to six main comparisons to ensure that the review is readable.
Contributions of authors
KS Gurusamy performed the analysis, interpreted the information, and wrote the review.
J Vaughan and C Toon independently assessed the trials for inclusion and extracted data on included trials.
BR Davidson critically commented on the review.
All authors agreed on the final version of the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known.
New
References
References to studies included in this review
Abdulla 2012 {published data only}
- Abdulla S, Eckhardt R, Netter U, Abdulla W. A randomized, double‐blind, controlled trial on non‐opioid analgesics and opioid consumption for postoperative pain relief after laparoscopic cholecystectomy. Acta Anaesthesiologica Belgica 2012;63(1):43‐50. [PubMed] [Google Scholar]
Agarwal 2008 {published data only}
- Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. British Journal of Anaesthesia 2008;101(5):700‐4. [DOI] [PubMed] [Google Scholar]
Akaraviputh 2009 {published data only}
- Akaraviputh T, Leelouhapong C, Lohsiriwat V, Aroonpruksakul S. Efficacy of perioperative parecoxib injection on postoperative pain relief after laparoscopic cholecystectomy: a prospective, randomized study. World Journal of Gastroenterology 2009;15(16):2005‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akarsu 2012 {published data only}
- Akarsu T, Tur H, Bolat C, Ozkaynak I. Comparison of pre‐emptive pregabalin with placebo and diclofenac combination for postoperative analgesia and cognitive functions after laparoscopic cholecystectomy. Turkiye Klinikleri Journal of Medical Sciences 2012;32(4):963‐70. [Google Scholar]
Akinci 2008 {published data only}
- Akinci SB, Ayhan B, Aycan IO, Tirnaksiz B, Basgul E, Abbasoglu O, et al. The postoperative analgesic efficacy of intraperitoneal tramadol compared to normal saline or intravenous tramadol in laparoscopic cholecystectomy. European Journal of Anaesthesiology 2008;25(5):375‐81. [DOI] [PubMed] [Google Scholar]
Balaban 2012 {published data only}
- Balaban F, Yagar S, Ozgok A, Koc M, Gullapoglu H. A randomized, placebo‐controlled study of pregabalin for postoperative pain intensity after laparoscopic cholecystectomy. Journal of Clinical Anesthesia 2012;24(3):175‐8. [DOI] [PubMed] [Google Scholar]
Belzarena 1998 {published data only}
- Belzarena SD. Intravenous tenoxicam for postoperative pain relief after laparoscopic cholecystectomy. A comparison among placebo, 20 and 40 mg of tenoxicam [Tenoxicam venoso para analgesia pos operatoria em colecistectomia videolaparascopica. Comparacao entre placebo, 20 e 40 mg de tenoxicam]. Revista Brasileira De Anestesiologia 1998;48(1):7‐13. [Google Scholar]
Cheng 2004 {published data only}
- Cheng PGB, Lim MJ, Onsiong MK, Chiu KYW, Chan MK, Li KWM, et al. Celecoxib premedication in post‐operative analgesia for laparoscopic cholecystectomy. Acute Pain 2004;6(1):23‐8. [Google Scholar]
Chung 2004 {published data only}
- Chung F, Tong D, Miceli PC, Reiz J, Harsanyi Z, Darke AC, et al. Controlled‐release codeine is equivalent to acetaminophen plus codeine for post‐cholecystectomy analgesia. Canadian Journal of Anaesthesia 2004;51(3):216‐21. [DOI] [PubMed] [Google Scholar]
Dong 2003 {published data only}
- Dong FT, Yang YL, Guo J. Postoperative analgesia with lornoxicam in patients undergoing laparoscopic cholecystectomy. Academic Journal of Kunming Medical College 2003;24(2):71‐3. [Google Scholar]
Fanelli 2008 {published data only}
- Fanelli G, Ghisi D, Berti M, Troglio R, Ortu A, Consigli C, et al. Preoperative administration of controlled‐release oxycodone as a transition opioid for total intravenous anaesthesia in pain control after laparoscopic cholecystectomy. Surgical Endoscopy 2008;22(10):2220‐8. [DOI] [PubMed] [Google Scholar]
Forse 1996 {published data only}
- Forse A, Beheiry H, Butler PO, Pace RF. Indomethacin and ketorolac given preoperatively are equally effective in reducing early postoperative pain after laparoscopic cholecystectomy. Canadian Journal of Surgery 1996;39(1):26‐30. [PMC free article] [PubMed] [Google Scholar]
Gilron 2009 {published data only}
- Gilron I, Orr E, Tu D, Mercer CD, Bond D. A randomized, double‐blind, controlled trial of perioperative administration of gabapentin, meloxicam and their combination for spontaneous and movement‐evoked pain after ambulatory laparoscopic cholecystectomy. Anesthesia and Analgesia 2009;108(2):623‐30. [DOI] [PubMed] [Google Scholar]
Gomez‐Vazquez 2012 {published data only}
- Gomez‐Vazquez ME, Hernandez‐Salazar E, Novelo‐Otanez JD, Cabrera‐Pivaral CE, Davalos‐Rodriguez IP, Salazar‐Paramo M. Effect of endovenous morphine vs. ketorolac on proinflammatory cytokines during postoperative analgesia in laparoscopic cholecystectomy. Cirugia y Cirujanos 2012;80(1):56‐62. [PubMed] [Google Scholar]
Horattas 2004 {published data only}
- Horattas MC, Evans S, Sloan‐Stakleff KD, Lee C, Snoke JW. Does preoperative rofecoxib (Vioxx) decrease postoperative pain with laparoscopic cholecystectomy?. American Journal of Surgery 2004;188(3):271‐6. [DOI] [PubMed] [Google Scholar]
Ji 2010 {published data only}
- Ji FH, Jin X, Yang JP, Zan LL. Analgesic effect of parecoxib and flurbiprofen axetil for patients undergoing laparoscopic cholecystectomy and their influences on platelet aggregation. Chinese Medical Journal 2010;123(12):1607‐9. [PubMed] [Google Scholar]
Joshi 2004 {published data only}
- Gan TJ, Joshi GP, Viscusi E, Cheung RY, Dodge W, Fort JG, et al. Preoperative parenteral parecoxib and follow‐up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery. Anesthesia and Analgesia 2004;98(6):1665‐73. [DOI] [PubMed] [Google Scholar]
- Joshi GP, Viscusi ER, Gan TJ, Minkowitz H, Cippolle M, Schuller R, et al. Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX‐2 specific inhibitor. Anesthesia and Analgesia 2004;98(2):336‐42. [DOI] [PubMed] [Google Scholar]
Karakoc 2011 {published data only}
- Karakoc F, Akcaboy EY, Akcaboy ZN, Gogus N. The effects of intravenous dexketoprofen trometamol on postoperative analgesia and morphine consumption undergoing laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2011;2:E165‐E166. [Google Scholar]
Lane 1996 {published data only}
- Lane GE, Lathrop JC, Boysen DA, Lane RC. Effect of intramuscular intraoperative pain medication on narcotic usage after laparoscopic cholecystectomy. American Surgeon 1996;62(11):907‐10. [PubMed] [Google Scholar]
Liu 1993 {published data only}
- Liu J, Ding Y, White PF, Feinstein R, Shear JM. Effects of ketorolac on postoperative analgesia and ventilatory function after laparoscopic cholecystectomy. Anesthesia and Analgesia 1993;76(5):1061‐6. [DOI] [PubMed] [Google Scholar]
Mebazaa 2008 {published data only}
- Mebazaa MS, Frikha N, Hammouda NB, Mestiri T, Mestiri H, Khalfallah T, et al. Postoperative analgesia after laparoscopic cholecystectomy: comparison of the preoperative administration of celecoxib with paracetamol?. Tunisie Medicale 2008;86(10):869‐73. [PubMed] [Google Scholar]
Muñoz 2002 {published data only}
- Muñoz HR, Guerrero ME, Brandes V, Cortínez LI. Effect of timing of morphine administration during remifentanil‐based anaesthesia on early recovery from anaesthesia and postoperative pain. British Journal of Anaesthesia 2002;88(6):814‐8. [DOI] [PubMed] [Google Scholar]
Munro 1998 {published data only}
- Munro FJ, Young SJ, Broome IJ, Robb HM, Wardall GJ. Intravenous tenoxicam for analgesia following laparoscopic cholecystectomy. Anaesthesia and Intensive Care 1998;26(1):56‐60. [DOI] [PubMed] [Google Scholar]
Nesek‐Adam 2012 {published data only}
- Nesek‐Adam V, Grizelj‐Stojcic E, Mrsic V, Rasic Z, Schwarz D. Preemptive use of diclofenac in combination with ketamine in patients undergoing laparoscopic cholecystectomy: a randomized, double‐blind, placebo‐controlled study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2012;22(3):232‐8. [DOI] [PubMed] [Google Scholar]
Pandey 2004 {published data only}
- Pandey CK, Priye S, Singh S, Singh U, Singh RB, Singh PK. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Canadian Journal of Anaesthesia 2004;51(4):358‐63. [DOI] [PubMed] [Google Scholar]
Peng 2010 {published data only}
- Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, et al. Use of low‐dose pregabalin in patients undergoing laparoscopic cholecystectomy. British Journal of Anaesthesia 2010;105(2):155‐61. [DOI] [PubMed] [Google Scholar]
Puura 2006 {published data only}
- Puura A, Puolakka P, Rorarius M, Salmelin R, Lindgren L. Etoricoxib pre‐medication for post‐operative pain after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2006;50(6):688‐93. [DOI] [PubMed] [Google Scholar]
Salihoglu 2009 {published data only}
- Salihoglu Z, Yildirim M, Demiroluk S, Kaya G, Karatas A, Ertem M, et al. Evaluation of intravenous paracetamol administration on postoperative pain and recovery characteristics in patients undergoing laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2009;19(4):321‐3. [DOI] [PubMed] [Google Scholar]
Sandhu 2011 {published data only}
- Sandhu T, Paiboonworachat S, Ko‐iam W. Effects of preemptive analgesia in laparoscopic cholecystectomy: a double‐blind randomized controlled trial. Surgical Endoscopy 2011;25(1):23‐7. [DOI] [PubMed] [Google Scholar]
Sarakatsianou 2013 {published data only}
- Sarakatsianou C, Theodorou E, Georgopoulou S, Stamatiou G, Tzovaras G. Effect of pre‐emptive pregabalin on pain intensity and postoperative morphine consumption after laparoscopic cholecystectomy. Surgical Endoscopy 2013;27(7):2504‐11. [DOI] [PubMed] [Google Scholar]
Schuster 2005 {published data only}
- Schuster R, Stewart D, Schuster L, Greaney G, Waxman K. Preoperative oral rofecoxib and postoperative pain in patients after laparoscopic cholecystectomy: a prospective, randomized, double‐blinded, placebo‐controlled trial. American Surgeon 2005;71(10):827‐9. [PubMed] [Google Scholar]
Sen 2010 {published data only}
- Sen M, Inan A, Sert H, Akpinar A, Dener C. Preemptive use of etofenamate in laparoscopic cholecystectomy: a randomized, placebo‐controlled, double‐blind study. European Journal of General Medicine 2010;7(1):50‐5. [Google Scholar]
Wilson 1994 {published data only}
- Wilson YG, Rhodes M, Ahmed R, Daugherty M, Cawthorn SJ, Armstrong CP. Intramuscular diclofenac sodium for postoperative analgesia after laparoscopic cholecystectomy: a randomised, controlled trial. Surgical Laparoscopy & Endoscopy 1994;4(5):340‐4. [PubMed] [Google Scholar]
Yeh 2004 {published data only}
- Yeh CC, Wu CT, Lee MS, Yu JC, Yang CP, Lu CH, et al. Analgesic effects of preincisional administration of dextromethorphan and tenoxicam following laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2004;48(8):1049‐53. [DOI] [PubMed] [Google Scholar]
Zajaczkowska 2004 {published data only}
- Zajaczkowska R, Wnek W, Wordliczek J, Dobrogowski J. Peripheral opioid analgesia in laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2004;29(5):424‐9. [DOI] [PubMed] [Google Scholar]
Zhu 2011 {published data only}
- Zhu Y, Jing G, Yuan W. Preoperative administration of intramuscular dezocine reduces postoperative pain for laparoscopic cholecystectomy. Journal of Biomedical Research 2011;25(5):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aftab 2008 {published data only}
- Aftab S, Rashdi S. Comparison of intravenous ketorolac with diclofenac for postoperative analgesia. Journal of Surgery Pakistan 2008;13(2):62‐6. [Google Scholar]
Akca 2004 {published data only}
- Akca T, Colak T, Kanik A, Yaylak F, Caglikulekci M, Aydin S. The effect of preoperative intravenous use of tenoxicam: a prospective, double‐blind, placebo‐controlled study. Journal of Investigative Surgery 2004;17(6):333‐8. [DOI] [PubMed] [Google Scholar]
Bajaj 2004 {published data only}
- Bajaj P, Ballary CC, Dongre NA, Baliga VP, Desai AA. Role of parecoxib in pre‐emptive analgesia: comparison of the efficacy and safety of pre‐ and postoperative parecoxib in patients undergoing general surgery. Journal of the Indian Medical Association 2004;102(5):272‐8. [PubMed] [Google Scholar]
Boccara 2005 {published data only}
- Boccara G, Chaumeron A, Pouzeratte Y, Mann C. The preoperative administration of ketoprofen improves analgesia after laparoscopic cholecystectomy in comparison with propacetamol or postoperative ketoprofen. British Journal of Anaesthesia 2005;94(3):347‐51. [DOI] [PubMed] [Google Scholar]
Collard 2007 {published data only}
- Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesthesia and Analgesia 2007;105(5):1255‐62. [DOI] [PubMed] [Google Scholar]
Elhakim 2000 {published data only}
- Elhakim M, Amine H, Kamel S, Saad F. Effects of intraperitoneal lidocaine combined with intravenous or intraperitoneal tenoxicam on pain relief and bowel recovery after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2000;44(8):929‐33. [DOI] [PubMed] [Google Scholar]
Gan 2004 {published data only}
- Gan TJ, Joshi GP, Zhao SZ, Hanna DB, Cheung RY, Chen C. Presurgical intravenous parecoxib sodium and follow‐up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid‐related adverse effects. Acta Anaesthesiologica Scandinavica 2004;48(9):1194‐207. [DOI] [PubMed] [Google Scholar]
Hernandez‐Palazon 2003 {published data only}
- Hernandez‐Palazon J, Tortosa JA, Rosa VN, Gimenez‐Viudes J, Ramirez G, Robles R. Intraperitoneal application of bupivacaine plus morphine for pain relief after laparoscopic cholecystectomy. European Journal of Anaesthesiology 2003;20(11):891‐6. [DOI] [PubMed] [Google Scholar]
Kocaayan 2007 {published data only}
- Kocaayan E, Ozkardeşler S, Ozzeybek D, Bayindir S, Akan M. Comparison of effects of preoperatively administered lornoxicam and tenoxicam on morphine consumption after laparoscopic cholecystectomy. European Journal of Anaesthesiology 2007;24(8):714‐9. [DOI] [PubMed] [Google Scholar]
Koch 2008 {published data only}
- Koch S, Ahlburg P, Spangsberg N, Brock B, Tonnesen E, Nikolajsen L. Oxycodone vs. fentanyl in the treatment of early post‐operative pain after laparoscopic cholecystectomy: a randomised double‐blind study. Acta Anaesthesiologica Scandinavica 2008;52(6):845‐50. [DOI] [PubMed] [Google Scholar]
Kokki 2012 {published data only}
- Kokki M, Broms S, Eskelinen M, Neuvonen PJ, Halonen T, Kokki H. The analgesic concentration of oxycodone with co‐administration of paracetamol ‐ a dose‐finding study in adult patients undergoing laparoscopic cholecystectomy. Basic & Clinical Pharmacology & Toxicology 2012;111(6):391‐5. [DOI] [PubMed] [Google Scholar]
Matkap 2011 {published data only}
- Matkap E, Bedirli N, Akkaya T, Gümü H. Preincisional local infiltration of tramadol at the trocar site versus intravenous tramadol for pain control after laparoscopic cholecystectomy. Journal of Clinical Anesthesia 2011;23(3):197‐201. [DOI] [PubMed] [Google Scholar]
Naguib 1998 {published data only}
- Naguib M, Seraj M, Attia M, Samarkandi AH, Seet M, Jaroudi R. Perioperative antinociceptive effects of tramadol. A prospective, randomized, double‐blind comparison with morphine. Canadian Journal of Anaesthesia 1998;45(12):1168‐75. [DOI] [PubMed] [Google Scholar]
O'Hanlon 2002 {published data only}
- O'Hanlon DM, Colbert S, Ragheb J, McEntee GP, Chambers F, Moriarty DC. Intraperitoneal pethidine versus intramuscular pethidine for the relief of pain after laparoscopic cholecystectomy: randomized trial. World Journal of Surgery 2002;26(12):1432‐6. [DOI] [PubMed] [Google Scholar]
Ozkocak 2002 {published data only}
- Ozkocak I, Kirdemir P, Rasa K, Aksu C, Gogus N. The comparison of preemptive intraperitoneal analgesic effects of tramadol, pethidine and bupivacaine. Anestezi Dergisi 2002;10(1):49‐52. [Google Scholar]
Sanchez‐Rodriguez 2010 {published data only}
- Sanchez‐Rodriguez PE, Fuentes‐Orozco C, Gonzalez‐Ojeda A. Effect of dexamethasone on postoperative symptoms in patients undergoing elective laparoscopic cholecystectomy: randomized clinical trial. World Journal of Surgery 2010;34(5):895‐900. [DOI] [PubMed] [Google Scholar]
Sozbilen 2007 {published data only}
- Sozbilen M, Yeniay L, Unalp O, Makay O, Pirim A, Ulukaya S, et al. Effects of ropivacaine on pain after laparoscopic cholecystectomy: a prospective, randomized study. Advances in Therapy 2007;24(2):247‐57. [DOI] [PubMed] [Google Scholar]
Stempin 2007 {published data only}
- Stempin S, Gajdosz R. Intraperitoneal morphine for prevention of postoperative shoulder pain after laparoscopic cholecystectomy. Anestezjologia Intensywna Terapia 2007;39(1):18‐20. [Google Scholar]
Tiippana 2008 {published data only}
- Tiippana E, Bachmann M, Kalso E, Pere P. Effect of paracetamol and coxib with or without dexamethasone after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2008;52(5):673‐80. [DOI] [PubMed] [Google Scholar]
Wininger 2010 {published data only}
- Wininger SJ, Miller H, Minkowitz HS, Royal MA, Ang RY, Breitmeyer JB, et al. A randomized, double‐blind, placebo‐controlled, multicenter, repeat‐dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clinical Therapeutics 2010;32(14):2348‐69. [DOI] [PubMed] [Google Scholar]
Wu 1999 {published data only}
- Wu CT, Yu JC, Yeh CC, Liu ST, Li CY, Ho ST, et al. Preincisional dextromethorphan treatment decreases postoperative pain and opioid requirement after laparoscopic cholecystectomy. Anesthesia and Analgesia 1999;88(6):1331‐4. [DOI] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu CT, Borel CO, Lee MS, Yu JC, Liou HS, Yi HD, et al. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesthesia and Analgesia 2005;100(2):448‐53. [DOI] [PubMed] [Google Scholar]
Yamazaki 2003 {published data only}
- Yamazaki E, Murao K, Asai T, Matsumoto S, Shingu K. Comparison of analgesic effects of intravenous flurbprofen and suppository indomethacin after laparoscopic cholecystectomy. Masui. The Japanese Journal of Anesthesiology 2003;52(11):1186‐90. [PubMed] [Google Scholar]
Zambouri 2002 {published data only}
- Zambouri A, Petropoulou P, Petra K, Ralli M, Douvantzi A, Papachristou D. Do early postoperative pain, nausea and vomiting really differ when remifentanil or fentanyl are used in laparoscopic cholecystectomy?. 10th World Society of Pain Clinicians of the International Pain Clinic; 2002 May 04‐08, Sardinia, Italy. World Society of Pain Clinicians, 2002:257‐63.
Zghidi 2011 {published data only}
- Zghidi SM, Jaoua H, Ghariani S, Saada S, Laabidi S, Khemiri K, et al. Effectiveness of dexamethasone in postoperative analgesia after laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2011;2:E278‐9. [Google Scholar]
References to studies awaiting assessment
Gan 2003 {published data only}
- Gan TJ, Joshi G, Viscusi E, Chen C, Cheung R. Postdischarge recovery experience after single presurgery does of IV parecoxib sodium, a novel COX‐2 inhibitor, followed by oral valdecoxib for pain after laparoscopic cholecystectomy. International Journal of Obstetrics & Gynecology 2003;83(3):23. [Google Scholar]
Additional references
Alexander 1987
- Alexander JI, Hull MG. Abdominal pain after laparoscopy: the value of a gas drain. British Journal of Obstetrics and Gynaecology 1987;94(3):267‐9. [DOI] [PubMed] [Google Scholar]
Argoff 2013
Attili 1995
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO group. Hepatology 1995;21(3):655‐60. [DOI] [PubMed] [Google Scholar]
Ballal 2009
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. [DOI] [PubMed] [Google Scholar]
Bates 1992
- Bates T, Harrison M, Lowe D, Lawson C, Padley N. Longitudinal study of gall stone prevalence at necropsy. Gut 1992;33(1):103‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisgaard 2006
- Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology 2006;104(4):835‐46. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ trial sequential analysis, 2011. ctu.dk/tsa/ (accessed 25 March 2014).
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dolan 2009
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giger 2011
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. British Journal of Surgery 2011;98(3):391‐6. [DOI] [PubMed] [Google Scholar]
Gluud 2014
- Nikolova D, Klingenberg SL, Gluud C, Als‐Nielsen B, Bjelakovic G, Casazza G, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 2. Art. No.: LIVER.
Gottschling 2005
- Gottschling S, Meyer S, Krenn T, Reinhard H, Lothschuetz D, Nunold H, et al. Propofol versus midazolam/ketamine for procedural sedation in pediatric oncology. Journal of Pediatric Hematology/Oncology 2005;27(9):471‐6. [DOI] [PubMed] [Google Scholar]
GREPCO 1984
- GREPCO. Prevalence of gallstone disease in an Italian adult female population. Rome group for the epidemiology and prevention of cholelithiasis (GREPCO). American Journal of Epidemiology 1984;119(5):796‐805. [PubMed] [Google Scholar]
GREPCO 1988
- GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part i. Prevalence data in men. The Rome group for epidemiology and prevention of cholelithiasis (GREPCO). Hepatology 1988;8(4):904‐6. [PubMed] [Google Scholar]
Gurusamy 2008a
- Gurusamy K, Junnarkar S, Farouk M, Davidson B. Day‐case versus overnight stay for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006798.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2008b
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Koti R, Davidson BR. Routine abdominal drainage versus no abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD006004.pub4] [DOI] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Nagendran M, Guerrini GP, Toon CD, Zinnuroglu M, Davidson BR. Intraperitoneal local anaesthetic instillation versus no intraperitoneal local anaesthetic instillation for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007337.pub3] [DOI] [PubMed] [Google Scholar]
Halldestam 2004
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. [DOI] [PubMed] [Google Scholar]
HES 2011
- HESonline. Hospital episode statistics. Main procedures and interventions: 3 character, 2011. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=205 (accessed on 25 March 2014).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Inturrisi 2002
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clinical Journal of Pain 2002;18(4 Suppl):S3‐13. [DOI] [PubMed] [Google Scholar]
Kehlet 2005
- Kehlet H, Gray AW, Bonnet F, Camu F, Fischer HB, McCloy RF, et al. A procedure‐specific systematic review and consensus recommendations for postoperative analgesia following laparoscopic cholecystectomy. Surgical Endoscopy 2005;19(10):1396‐415. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Loizides 2014
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007049.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Martindale 2011
- Sweetman S (editor). Martindale: the complete drug reference (online version), 37th edition, 2011. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference (accessed 25 March 2014).
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Ng 2004
NIH 1992
- NIH. NIH consensus statement on gallstones and laparoscopic cholecystectomy, 1992. consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm (accessed 25 March 2014). [PubMed]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savovic 2012b
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Strasberg 1993
- Strasberg SM, Clavien PA. Overview of therapeutic modalities for the treatment of gallstone diseases. American Journal of Surgery 1993;165(4):420‐6. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 25 March 2014).
Todd 1996
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2003
- Yu HP, Hseu SS, Yien HW, Teng YH, Chan KH. Oral clonidine premedication preserves heart rate variability for patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2003;47(2):185‐90. [DOI] [PubMed] [Google Scholar]


