Abstract
Background
Preterm birth, defined as birth between 20 and 36 completed weeks, is a major contributor to perinatal morbidity and mortality globally. Oxytocin receptor antagonists (ORA), such as atosiban, have been specially developed for the treatment of preterm labour. ORA have been proposed as effective tocolytic agents for women in preterm labour to prolong pregnancy with fewer side effects than other tocolytic agents.
Objectives
To assess the effects on maternal, fetal and neonatal outcomes of tocolysis with ORA for women with preterm labour compared with placebo or any other tocolytic agent.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 December 2013).
Selection criteria
We included all randomised controlled trials (published and unpublished) of ORA for tocolysis of labour between 20 and 36 completed weeks' gestation.
Data collection and analysis
Two review authors independently evaluated methodological quality and extracted trial data. When required, we sought additional data from trial authors. Results are presented as risk ratio (RR) for categorical and mean difference (MD) for continuous data with the 95% confidence intervals (CI). Where appropriate, the number needed to treat for benefit (NNTB) and the number needed to treat for harm (NNTH) were calculated.
Main results
This review update includes eight additional studies (790 women), giving a total of 14 studies involving 2485 women.
Four studies (854 women) compared ORA (three used atosiban and one barusiban) with placebo. Three studies were considered at low risk of bias in general (blinded allocation to treatment and intervention), the fourth study did not adequately blind the intervention. No difference was shown in birth less than 48 hours after trial entry (average RR 1.05, 95% CI 0.15 to 7.43; random‐effects, (two studies, 152 women), perinatal mortality (RR 2.25, 95% CI 0.79 to 6.38; two studies, 729 infants), or major neonatal morbidity. ORA (atosiban) resulted in a small reduction in birthweight (MD ‐138.86 g, 95% CI ‐250.53 to ‐27.18; two studies with 676 infants). In one study, atosiban resulted in an increase in extremely preterm birth (before 28 weeks' gestation) (RR 3.11, 95% CI 1.02 to 9.51; NNTH 31, 95% CI 8 to 3188) and infant deaths (up to 12 months) (RR 6.13, 95% CI 1.38 to 27.13; NNTH 28, 95% CI 6 to 377). However, this finding may be confounded due to randomisation of more women with pregnancy less than 26 weeks' gestation to atosiban. ORA also resulted in an increase in maternal adverse drug reactions requiring cessation of treatment in comparison with placebo (RR 4.02, 95% CI 2.05 to 7.85; NNTH 12, 95% CI 5 to 33). No differences were shown in preterm birth less than 37 weeks' gestation or any other adverse neonatal outcomes. No differences were evident by type of ORA, although data were limited.
Eight studies (1402 women) compared ORA (atosiban only) with betamimetics; four were considered of low risk of bias (blinded allocation to treatment and to intervention). No statistically significant difference was shown in birth less than 48 hours after trial entry (RR 0.89, 95% CI 0.66 to 1.22; eight studies with 1389 women), very preterm birth (RR 1.70, 95% CI 0.89 to 3.23; one study with 145 women), extremely preterm birth (RR 0.84, 95% CI 0.37 to 1.92; one study with 244 women) or perinatal mortality (RR 0.55, 95% CI 0.21 to 1.48; three studies with 816 infants). One study (80 women), of unclear methodological quality, showed an increase in the interval between trial entry and birth (MD 22.90 days, 95% CI 18.03 to 27.77). No difference was shown in any reported measures of major neonatal morbidity (although numbers were small). ORA (atosiban) resulted in less maternal adverse effects requiring cessation of treatment (RR 0.05, 95% CI 0.02 to 0.11; NNTB 6, 95% CI 6 to 6; five studies with 1161 women).
Two studies including (225 women) compared ORA (atosiban) with calcium channel blockers (CCB) (nifedipine only). The studies were considered as having high risk of bias as neither study blinded the intervention and in one study it was not known if allocation was blinded. No difference was shown in birth less than 48 hours after trial entry (average RR 1.09, 95% CI 0.44 to 2.73, random‐effects; two studies, 225 women) and extremely preterm birth (RR 2.14, 95% CI 0.20 to 23.11; one study, 145 women). No data were available for the outcome of perinatal mortality. One small trial (145 women), which did not employ blinding of the intervention, showed an increase in the number of preterm births (before 37 weeks' gestation) (RR 1.56, 95% CI 1.13 to 2.14; NNTH 5, 95% CI 3 to 19), a lower gestational age at birth (MD ‐1.20 weeks, 95% CI ‐2.15 to ‐0.25) and an increase in admission to neonatal intensive care unit (RR 1.70, 95% CI 1.17 to 2.47; NNTH 5, 95% CI 3 to 20). ORA (atosiban) resulted in less maternal adverse effects (RR 0.38, 95% CI 0.21 to 0.68; NNTB 6, 95% CI 5 to 12; two studies, 225 women) but not maternal adverse effects requiring cessation of treatment (RR 0.36, 95% CI 0.01 to 8.62; one study, 145 women). No longer‐term outcome data were included.
Authors' conclusions
This review did not demonstrate superiority of ORA (largely atosiban) as a tocolytic agent compared with placebo, betamimetics or CCB (largely nifedipine) in terms of pregnancy prolongation or neonatal outcomes, although ORA was associated with less maternal adverse effects than treatment with the CCB or betamimetics. The finding of an increase in infant deaths and more births before completion of 28 weeks of gestation in one placebo‐controlled study warrants caution. However, the number of women enrolled at very low gestations was small. Due to limitations of small numbers studied and methodological quality, further well‐designed randomised controlled trials are needed. Further comparisons of ORA versus CCB (which has a better side‐effect profile than betamimetics) are needed. Consideration of further placebo‐controlled studies seems warranted. Future studies of tocolytic agents should measure all important short‐ and long‐term outcomes for women and infants, and costs.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Albuterol; Albuterol/therapeutic use; Infant, Extremely Low Birth Weight; Obstetric Labor, Premature; Obstetric Labor, Premature/drug therapy; Oligopeptides; Oligopeptides/therapeutic use; Randomized Controlled Trials as Topic; Receptors, Oxytocin; Receptors, Oxytocin/antagonists & inhibitors; Ritodrine; Ritodrine/therapeutic use; Terbutaline; Terbutaline/therapeutic use; Tocolytic Agents; Tocolytic Agents/therapeutic use; Vasotocin; Vasotocin/analogs & derivatives; Vasotocin/therapeutic use
Plain language summary
Oxytocin receptor antagonists for inhibiting preterm labour
Tocolytic drugs suppress preterm labour and have the potential to postpone preterm birth long enough to, hopefully, improve infant outcome. This may be by allowing normal growth and maturation of the baby, or by allowing time for administration of magnesium sulphate to reduce risk of cerebral palsy and corticosteroids to help the baby's lungs and other organs to mature. They may also provide the opportunity, if necessary, for the mother to be transferred to a hospital that has facilities to provide neonatal intensive care. However, prolonging pregnancy may instead have adverse outcomes for the baby and so it is important to assess infant outcomes alongside duration of pregnancy. Oxytocin receptor antagonists (ORAs) are a group of tocolytic drugs, and we undertook this review to see if ORAs prolonged pregnancy and improved outcomes for infants compared with no treatment or with other tocolytic drugs.
The tocolytic drugs, atosiban and barusiban, were the only ORAs we found that had been studied in trials; some trials compared with no treatment and others compared atosiban with betamimetics (another group of tocolytic drugs). We identified 14 studies, involving 2485 women. We found that, although atosiban resulted in fewer maternal side effects than other tocolytic drugs (especially betamimetics), atosiban was not effective in delaying or preventing preterm birth or improving neonatal outcome, and may possibly contribute to poorer infant outcomes. Further well‐designed studies would be helpful, especially in women with threatened preterm at low gestations where preterm birth puts babies at particularly high risk of death or disability.
Atosiban is no better than placebo or other drugs in delaying or preventing preterm birth but it has fewer maternal side effects compared to other tocolytics.
Background
Description of the condition
Preterm birth, defined as birth occurring between 20 and 36 completed weeks, is a major contributor to perinatal mortality and morbidity (Liu 2012; WHO 2012). Worldwide, it is estimated that more than one in 10 births is preterm, affecting 15 million babies annually (Blencowe 2012; WHO 2012). The incidence of preterm birth is 8.6% of births in high‐resource countries, and between 7.4% to 13.3% in low‐resource countries, and rose in both at least until the middle of the last decade (Chang 2013; WHO 2012).
In high‐income countries, very preterm birth (i.e. birth before 32 weeks' gestation) has an incidence of 1% to 2% (Tucker 2004) but despite the availability of perinatal and neonatal care, it is responsible for approximately one third to one half of all perinatal deaths (Dorling 2008; Zeitlin 2008). In high‐income countries, almost 95% of neonates born between 28 and 32 weeks' gestation will survive, with more than 90% surviving without impairment. In contrast, in many low‐income countries, only 30% of neonates born between 28 and 32 weeks will survive (WHO 2012).
Preterm birth is associated not only with high immediate costs attributable to neonatal intensive care, but also with substantial long‐term costs, including costs for special education (Petrou 2011), and other services for infants and children with intellectual and physical disability (Petrou 2011). In addition to the lengthy neonatal intensive care treatment required for many preterm infants, preterm birth often places stress on parents, which is greater with decreasing gestational age (Schappin 2013).
Approximately 65% to 70% are spontaneous preterm births either following spontaneous preterm labour (40% to 45%) and those following preterm rupture of membranes (25% to 30%) (Goldenberg 2008). While the cause of spontaneous preterm birth is often unclear, some risk factors have been identified including: maternal age (adolescence and advanced age); history of preterm birth; race; multiple pregnancy, short inter‐pregnancy interval; infections; medical conditions; poor nutrition; psychological factors and genetic predisposition (Goldenberg 2008; Plunkett 2008).
There has been little progress in reducing the incidence of preterm birth, even in high‐income countries despite intensive antenatal care programs aimed at high‐risk groups, the widespread use of pharmacological agents to inhibit preterm birth (tocolytics) and other preventive and therapeutic interventions. Nevertheless, short‐term prolongation of pregnancy has the potential to allow other interventions to improve outcomes, including maternal corticosteroid administration to accelerate maturation of fetal lungs (Roberts 2006) and other organs (Crowley 1996), magnesium sulphate administration to reduce risk of cerebral palsy (Doyle 2009) and maternal transfer before birth to a centre that can provide appropriate neonatal special or intensive care (Lasswell 2010). For these reasons, short‐term tocolytic therapy is commonly used to inhibit preterm labour and postpone preterm birth.
Description of the intervention
A range of tocolytic agents that have been used to inhibit preterm labour are the topics of Cochrane systematic reviews including: nitric oxide donors (glyceryl trinitrate) (Duckitt 2002), calcium channel blockers (CCB) (commonly nifedipine) (update of King 2003 in progress), betamimetics (Anotayanonth 2006), magnesium sulphate (Crowther 2002), cyclo‐oxygenase (COX) inhibitors (Khanprakob 2012) and progesterone (Su 2010). The betamimetics (ritodrine, salbutamol and terbutaline) have been shown to be effective in delaying delivery by seven days and longer, although no impact has yet been shown on perinatal mortality (Anotayanonth 2006; Gyetvai 1999; King 1988). Furthermore, betamimetics have a high frequency of unpleasant, sometimes severe maternal side effects including tachycardia, hypotension, tremor and a range of biochemical disturbances, and they have been associated with life‐threatening cardiovascular and respiratory events and deaths (FDA 2011). Compared with other tocolytic agents (mostly betamimetics), CCB prolonged pregnancy and improved short‐term neonatal outcomes, with fewer maternal adverse effects (update of King 2003 in progress). However, a fifth of women still delivered within 48 hours of CCB treatment, and nearly a third within seven days, so there is still a need for other safe, effective tocolytic agents, particularly at very early gestations.
A number of oxytocin receptor antagonists have been developed, and of these, three, atosiban, barusiban and retosiban have been investigated in humans as tocolytic agents. To date, only atosiban is in use outside of clinical trials. Atosiban is an oxytocin receptor antagonist which was specifically developed for the treatment of preterm labour (Melin 1994). Early reports of the use of atosiban for tocolysis showed promise both in vitro and in animal studies, and preliminary studies in pregnant and non‐pregnant humans suggested a very low incidence of maternal side effects (Goodwin 1996b; Goodwin 1998b). Potential maternal side effects include adverse injection site reaction, nausea, vomiting, headache, chest pain and hypotension (Moutquin 2000; Tsatsaris 2004).
How the intervention might work
Oxytocin is a peptide hormone produced in the pituitary, uterus, placenta and amnion. It has a variety of functions, which include stimulating myometrial activity (uterine contractions) as part of the pathway to normal and preterm labour. It binds receptors on myometrial cells, activating several intracellular pathways, which include protein kinase C phosphorylation of various proteins and a rise in intracellular calcium ions, both from intracellular stores via a GTP/phospholipase/inositol phosphate pathway and by activating voltage gated membrane channels allowing entry of extracellular calcium ions. Calcium ion binding to calmodulin then activates myosin light chain kinase, causing myometrial muscle contraction (Vrachnis 2011).
The oxytocin receptor antagonist, atosiban, is a peptide analogue of oxytocin that binds oxytocin receptors in the myometrial cell membrane, preventing the oxytocin‐induced rise in intracellular calcium and leading to relaxation of the myometrium (Melin 1994). Atosiban is an antagonist with high affinity to both the vasopressin receptor (V1a) and oxytocin receptor (Akerlund 1999).
Goodwin 1994 first described in 1994 the use of atosiban in humans for tocolysis. A previous review suggested that oxytocin antagonists could be effective and safe in preterm labour (Coomarasamy 2003). It has not been established whether the tocolytic effects of atosiban is due to its oxytocin or vasopressin receptor antagonist properties.
Barusiban is a selective oxytocin receptor antagonist with its effect on the vasopressin receptor (Nilsson 2003). Both atosiban and barusiban have a molecular structure very similar to the oxytocin molecule (a nonapeptide) (Vrachnis 2011). However, as peptide antagonists lack oral bioavailability, development of novel non‐peptide compounds with high oxytocin receptor selectivity is currently ongoing. Non‐peptides such as retosiban are small molecules, structurally not related to oxytocin (Borthwick 2013). It is currently not established how the molecular structure affects signalling pathways. It is plausible that the tocolytic effects of nonapeptides may differ from non‐peptide compounds based on their different binding affinity and selectivity to oxytocin receptors and also other receptors (Borthwick 2013; Vrachnis 2011).
Why it is important to do this review
Preterm labour is often insidious in onset and difficult to anticipate, and the causes are likely to be multifactorial, so prevention by treating the underlying causes has proved elusive. Therefore, effective tocolysis in suspected or established preterm labour is likely to remain critical to reducing infant morbidity and mortality associated with preterm birth, and to mitigating the long‐term consequences of prematurity on developmental and health outcomes. Since oxytocin receptor antagonists have undergone clinical trials and are available in some countries for the management of women in preterm labour, this updated review is important to assist clinicians and women in informed decision making about which tocolytic to use.
Objectives
Primary objectives of the review
To assess the effects on maternal, fetal and neonatal outcomes of any oxytocin receptor antagonist administered as a tocolytic agent to women in preterm labour when compared with placebo.
To assess the effects on maternal, fetal and neonatal outcomes of any oxytocin receptor antagonist administered as a tocolytic agent to women in preterm labour when compared with other classes of tocolytic agents.
Secondary objective
A secondary objective of the review is to determine whether the effects of oxytocin receptor antagonists, when compared with placebo or any other tocolytic agent, are influenced by different population characteristics and duration of tocolytic therapy as follows: (i) women randomised before 28 weeks' gestation versus those randomised at 28 weeks or after; (ii) women with ruptured membranes at randomisation versus women with intact membranes; (iii) women with a singleton pregnancy versus women with a multiple pregnancy; (iv) women who received maintenance therapy* versus women who did not; and also by type of tocolytic agent as follows: (v) type of other tocolytic; betamimetics versus calcium channel blockers (CCB); (vi) type of oxytocin receptor antagonists (ORA); atosiban versus other ORA.
(*Use of continued tocolytic agents after successful suppression of threatened preterm labour.)
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised and cluster‐randomised studies in which oxytocin receptor antagonists were used for tocolysis in the management of preterm labour. Studies using quasi‐random methods of allocation and cross‐over studies were excluded.
Types of participants
Women assessed as being in preterm labour (between 20 and 36 completed weeks' gestation) and considered suitable for tocolysis.
Types of interventions
Oxytocin receptor antagonists administered as a tocolytic by any route compared with placebo.
Oxytocin receptor antagonists administered as a tocolytic by any route compared with other classes of tocolytic agents.
Types of outcome measures
This review aimed to assess the effects of oxytocin receptor antagonists on clinically relevant outcome measures relating to perinatal and infant short‐term and long‐term outcome as well as prolongation of pregnancy. Furthermore, maternal side effects and outcomes were also examined.
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Short‐term and long‐term serious infant outcome determined by the presence of any of the following.
Serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit).
Birth less than 48 hours after trial entry.
Serious infant outcome (defined as death or chronic lung disease [need for supplemental oxygen at 28 days of life or later], grade three or four intraventricular haemorrhage or periventricular leukomalacia, major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient (DQ) or intelligence quotient (IQ) less than 2 standard deviations below mean])).
Perinatal mortality (stillbirth defines as a birth not showing signs of life defined by any gestational age and birthweight in the trials and neonatal death up to 28 days).
Very preterm birth (before completion of 34 weeks of gestation).
Secondary outcomes
These include other measures of effectiveness, complications and health service use.
Maternal
Maternal adverse effects.
Maternal adverse effects requiring cessation of therapy.
Caesarean section.
Antepartum haemorrhage.
Postpartum haemorrhage.
Satisfaction with care.
Quality of life after the birth (measured by validated instruments).
Infant/child
Extremely preterm birth (before completion of 28 weeks of gestation).
Preterm birth (before completion of 37 weeks of gestation).
Preterm neonate delivered with full course of antenatal steroids.
Interval between trial entry and birth.
Gestational age at birth.
Birthweight.
Apgar score less than seven at five minutes.
Respiratory distress syndrome.
Use of mechanical ventilation.
Duration of mechanical ventilation.
Persistent pulmonary hypertension of the neonate.
Intraventricular haemorrhage.
Necrotising enterocolitis.
Retinopathy of prematurity.
Neonatal jaundice.
Neonatal sepsis.
Infant death.
Quality of life in childhood (measured by validated instruments).
Health service use
Admission to neonatal intensive care unit.
Neonatal length of hospital stay.
Maternal length of hospital stay.
In this update, primary and secondary outcomes measures were revised to enhance consistency across Cochrane tocolytic reviews and to better reflect important outcome measures.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (1 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Studies identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the studies identified in the previous version of this review, see Appendix 1.
For this update, we used the following methods when assessing all new and previously included studies.
Selection of studies
Two review authors independently assessed all potential studies identified from the search strategy for inclusion in this review. Any disagreement was resolved through discussion, or via consultation of a third author if required.
Data extraction and management
The authors used the standard methods of The Cochrane Collaboration and considered all potential studies for inclusion. At least two authors (D Papatsonis, V Flenady, H Reinebrant and E Tambimuttu) evaluated the methodological quality of studies and independently performed data extraction, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Any discrepancies were resolved through discussion, or via consultation of a third author. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the method as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed the method as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the method as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, results are presented as summary risk ratio with 95% confidence intervals (CI). Where appropriate, calculations for number needed to treat for benefit (NNTB) and number needed to treat for harm (NNTH) were performed.
Continuous data
For continuous data, the mean difference was used if outcome measures were comparable between studies. The standardised mean difference was intended for use to combine studies measuring comparable outcomes but using different methodology.
Unit of analysis issues
Cross‐over trials
Cross‐over trials were excluded from this review.
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but trials of this type may be identified and included in future updates.
If cluster‐randomised trials are included in future updates, they will be included in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Handbook (Higgins 2011) using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, this will be reported and sensitivity analyses will be conducted to investigate the effect of variation in the ICC. If both cluster‐randomised trials and individually‐randomised trials are identified, the relevant information will be synthesised. The authors consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. Heterogeneity in the randomisation unit will also be acknowledged and a sensitivity analysis will be performed to investigate the effects of the randomisation units.
Multi‐arm studies
For the subgroup comparisons undertaken, to avoid double counting, we planned to divide out data from the shared group approximately evenly among the comparisons as described in the Handbook 16.5.4 (Higgins 2011).
One study (Thornton 2009) compared four different dosage regimens of barusiban (0.3, 1, 3 or 10 mg) with placebo. For the analyses undertaken in this review, we have combined all doses for comparison with placebo.
Multiple pregnancy
Where multiple pregnancies are included, wherever possible, analyses should be adjusted for clustering to take into account the non‐independence of neonates from the same pregnancy (Gates 2004). Treating neonates from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than neonates from different pregnancies, will overestimate the sample size and give CIs that are too narrow. Each woman can be considered a cluster in a multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of relative risk and adjustment of CIs. Usually this will mean that the confidence intervals get wider. Although this may make little difference to the conclusion of a study, it avoids misleading results in those studies where the difference may be substantial.
Seven studies reported outcomes for twin pregnancies (European 2001; French/Australian 2001; Goodwin 1994; Moutquin 2000; Nonnenmacher 2009; Romero 2000; Salim 2012). Two of these studies (Goodwin 1994; Romero 2000) compared ORA (atosiban) versus placebo, one study (Salim 2012) compared ORA versus CCB, and four studies compared ORA (atosiban) versus betamimetics (European 2001; French/Australian 2001; Moutquin 2000; Nonnenmacher 2009).
For the studies with twin pregnancies, the sample sizes for reported newborn outcomes were adjusted using the methods described in the Handbook (Higgins 2011) using an estimate of the intra‐class correlation coefficient (ICC) of 0.2 as described by Yelland et al (Yelland 2011).
Dealing with missing data
For included studies, levels of attrition in the 'Risk of bias' table were noted. The authors planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. Analyses were, as far as possible, performed on an intention‐to‐treat basis for all outcomes. Attempts were made to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
Statistical heterogeneity was assessed in each meta‐analysis using the Tau², I² and Chi² statistics. Heterogeneity was regarded as substantial if the Tau² was greater than zero and either the I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If 10 or more studies had contributed data to meta‐analysis for any particular outcome, we planned to assess reporting biases (such as publication bias) using funnel plots. Possible asymmetry would have been assessed visually, and if asymmetry was suggested, we planned exploratory analyses for investigation. In this version of the review, insufficient data were available to allow us to carry out this planned analysis.
Data synthesis
Statistical analyses were performed using the Review Manager software (RevMan 2012). Fixed‐effect meta‐analysis was used for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect; i.e. where studies were examining the same intervention, and the studies’ populations and methods were judged sufficiently similar. If clinical heterogeneity was deemed sufficient to expect differences between studies in regards to the underlying treatment effects, or if substantial statistical heterogeneity was detected, random‐effects meta‐analysis was used to produce an overall summary. This was performed if an average treatment effect across studies was considered clinically meaningful.
In one study (Thornton 2009), which compared four dosing regimens of barusiban with placebo and one study (Goodwin 1996) comparing four different dosing regimens of atosiban with betamimetics, outcomes from all four dosing regimens were combined in the analyses.
The random‐effects summary was treated as the average range of possible treatment effects and the authors discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was deemed to be not clinically meaningful studies were not combined.
Where random‐effects analyses were used, the results are presented as the mean treatment effect with its 95% CI, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we performed investigations using subgroup analyses. Consideration was taken to whether an overall summary was meaningful, and if deemed relevant, we performed a random‐effects analysis. We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012).
A priori subgroup analyses
We planned the following subgroup analyses.
By population
Women randomised before 28 weeks' gestation versus those randomised at 28 weeks or after.
Women with ruptured membranes at randomisation versus women with intact membranes.
Women with a singleton pregnancy versus women with a multiple pregnancy.
Women who received maintenance therapy* versus women who did not.
(*Use of continued tocolytic agents after successful suppression of threatened preterm labour.)
By intervention
Oxytocin receptor antagonists compared with placebo, further subgrouped by type of oxytocin receptor antagonist.
Oxytocin receptor antagonists compared with other classes of tocolytic agents, further subgrouped by type of other tocolytic agent and type of oxytocin receptors antagonist.
We will assess the following outcomes in subgroup analyses.
Fetal/neonatal outcomes
Perinatal mortality (stillbirth and neonatal death up to 28 days).
Infant death (up to 12 months).
Major childhood sensorineural disability.
Extremely preterm birth (before completion of 28 weeks of gestation).
Very preterm birth (before completion of 34 weeks of gestation).
Preterm birth (before completion of 37 weeks of gestation).
Birth less than 48 hours after trial entry.
Respiratory distress syndrome.
Intraventricular haemorrhage.
Necrotising enterocolitis.
Retinopathy of prematurity.
Chronic lung disease (need for supplemental oxygen therapy at 36 weeks' postmenstrual age).
Admission to neonatal intensive care unit.
Neonatal length of hospital stay.
Quality of life in childhood (measured by validated instruments).
Maternal outcomes
Serious maternal outcome.
Maternal adverse effects requiring cessation of treatment.
Quality of life after the birth (measured by validated instruments).
Sensitivity analysis
We planned sensitivity analyses to explore the effect of study quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both. The intention was to exclude poor‐quality studies (including those assessed as low or unknown risk of bias) from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
In this review, 29 studies were identified as potentially eligible for inclusion. Nine studies were excluded (Al‐Omari 2006; de Heus 2008; Gagnon 1998; Husslein 2006; Husslein 2007; Poppiti 2009; Steinwall 2005; Valenzuela 1997; Valenzuela 2000). Another five studies are awaiting classification pending additional information from authors (de Heus 2009; Lenzen 2012; Neri 2009; Renzo 2003; Snidow 2013). For further information please seeCharacteristics of studies awaiting classification. One study is ongoing (APOSTEL III).
In this review update, an additional eight studies, involving 790 women testing the effects of oxytocin receptor antagonists for tocolysis in preterm labour, have been included, giving a total of 14 included studies, involving 2485 women.
Included studies
Fourteen studies, involving 2485 women, are included.
Four studies (854 women) compared an oxytocin receptor antagonists with placebo (Goodwin 1994; Richter 2005; Romero 2000; Thornton 2009). Eight studies compared an oxytocin receptor antagonist (atosiban) with betamimetics (Cabar 2008; European 2001; French/Australian 2001; Goodwin 1996; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Shim 2006). Two studies compared an oxytocin receptor antagonist (atosiban) with a calcium channel blocker (CCB) (nifedipine) (Kashanian 2005; Salim 2012).
Participants
The participants in these studies were reasonably homogenous. In the placebo‐controlled studies, the minimum gestational age at inclusion was 20 weeks, and the maximum gestational age at inclusion was 37 weeks. In the studies comparing atosiban with betamimetic agents, the minimum gestational age at study entry ranged from 20 to 23 weeks and the maximum from 33 to 35 weeks. The presence of ruptured membranes was an exclusion criterion in all studies, except one (Nonnenmacher 2009). Exclusion of women with ruptured membranes reflects the clinical uncertainty about the role of tocolytic agents in this situation because infection is more likely to be present and delay in delivery may harm the mother and baby. In all studies, standard maternal and fetal contraindications to tocolysis were reported, e.g. pre‐eclampsia and gestational hypertension. Exclusion criteria also included the use of non‐steroidal anti‐inflammatory agents 12 hours prior to randomisation in five studies (European 2001; French/Australian 2001; Lin 2009; Moutquin 2000; Shim 2006), and prior tocolytic therapy within 72 hours in one study (Goodwin 1996) and within seven days in one study (Thornton 2009). High‐order multiple gestations (triplets or more) were reported as excluded in six studies (European 2001; French/Australian 2001; Lin 2009; Moutquin 2000; Richter 2005; Salim 2012) and all multiple pregnancies were excluded in three studies (Goodwin 1996; Shim 2006; Thornton 2009).
Tocolysis
Three studies compared atosiban with placebo (Goodwin 1994; Richter 2005; Romero 2000), one study compared barusiban with placebo (Thornton 2009), two studies compared atosiban with a CCB (nifedipine) (Kashanian 2005; Salim 2012) and eight studies compared atosiban with betamimetics (ritodrine, fenoterol, salbutamol, terbutaline) (Cabar 2008; European 2001; French/Australian 2001; Goodwin 1996; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Shim 2006).
In the placebo‐controlled studies, two studies (Richter 2005; Romero 2000) administered atosiban as a initial bolus dose of 6.75 mg intravenously (i.v.) followed by an infusion of 300 µg/min for three hours, then 100 µg/minutes for 45 hours. In Romero 2000, maintenance therapy was thereafter administered via subcutaneous injections up to 36 weeks. One study (Goodwin 1994) administered atosiban as a initial bolus dose of 6.75 mg i.v. followed by an infusion of 300 µg/minutes for two hours. One study administered barusiban as a single bolus dose (1 mL of either 0.3 mg, 1 mg, 3 mg or 10 mg barusiban, i.v.). Two of the placebo‐controlled studies included rescue tocolysis as a part of the study protocol. In Goodwin 1994, the primary aim was to determine the effect of atosiban on uterine activity during an infusion limited to two hours. In the atosiban group, 19.6% of the participants required an additional rescue tocolytic agent versus 32% in the placebo group. In this study (Goodwin 1994), maintenance therapy after the two hour infusion was not instituted. In Goodwin 1994, of the 120 women enrolled, 29 (11 atosiban and 18 placebo) required additional tocolysis with magnesium sulphate (n = 23) or subcutaneous terbutaline (n = 6). There is, however, no description of the doses or duration of this additional tocolysis. In Romero 2000, rescue therapy was given in 42% of the atosiban group and in 51% of the placebo group. Participants received rescue tocolytic therapy with an alternate tocolytic of the investigator's choice after discontinuation of the study drug. Rescue tocolysis was considered in this study when preterm labour has progressed after at least one hour of observation and either of the following occurred: (1) cervical effacement of ≥ 75% (≤ 0.5 cm) with no decrease in the frequency or intensity of contractions and continued cervical change (at least a 1 cm change in dilatation or effacement); or (2) cervical dilatation of ≥ 4 cm with a 1 cm increase since the last cervical examination. Maintenance therapy was started with either atosiban or placebo in women who achieved uterine quiescence with a subcutaneous infusion of 0.004 mL (30 µg/minute for atosiban) and was ceased at the end of the 36th week of gestation, at delivery, or if progression of labour necessitated an alternate tocolytic agent. In the third placebo‐controlled study (Richter 2005), rescue tocolysis was not performed. In cases of successful tocolysis but with persisted cervical dilatation, the woman was informed of the option of a cerclage and/or total occlusion of the cervix. One study (Thornton 2009) did not allow any tocolytics as rescue therapy.
In the studies comparing atosiban with nifedipine, one study (Salim 2012), administered atosiban as a initial bolus dose of 6.75 mg i.v. followed by an infusion of 300 µg/minute for three hours, then 100 µg/minute for 45 hours. The other study (Kashanian 2005) administered atosiban as an i.v. infusion of 300 µg/minute up to 12 hours, or six hours after contractions ceased. In one of the studies comparing atosiban with nifedipine (Kashanian 2005), rescue tocolysis was not performed. Nifedipine was given at an initial dose of 10 mg (one capsule) sublingually every 20 minutes for four doses. Maintenance dose with nifedipine was continued orally (20 mg) every six hours for the first 24 hours, and then every eight hours for the following 24 hours, and finally 10 mg every eight hours for the last 24 hours. In the other nifedipine‐controlled study (Salim 2012), rescue tocolysis was performed if progression of labour was determined after one hour but before 48 hours, or if adverse effects of the drug were noted, a cross‐over of the study drugs was performed and the alternative tocolytic drug (i.e. rescue treatment) was initiated. Nifedipine was given as a loading dose of 20 mg orally followed by another two doses of 20 mg, 20 to 30 minutes apart as needed. Maintenance was started after six hours with 20 to 40 mg four times daily for a total of 48 hours. If tocolysis failed from both drugs before 48 hours or admission at a gestational age below 28 weeks, indomethacin as a second rescue agent was started. Initial dose of indomethacin was two 100 mg per rectum tablets, one hour apart, followed by oral tablets of 25 mg four times daily up to a total of 48 hours.
In most of the studies comparing atosiban with betamimetics (Cabar 2008; European 2001; French/Australian 2001; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Shim 2006), an initial bolus dose of 6.75 mg (i.v.) atosiban was given, followed by a continuous infusion of 300 µg/minute for three hours, then 100 µg/minute for a maximum of 15 to 48 hours. One study (Goodwin 1996) tested four atosiban regimens: 6.5 mg bolus dose followed by infusion 300 µg/mL, two mg bolus dose followed by infusion of 100 µg/mL, 0.6 mg bolus dose followed by infusion of 30 µg/minute. All treatments were continued up to 12 hours. In the studies comparing atosiban with betamimetics, betamimetic therapy was administered i.v. for a maximum of 48 hours. Rescue tocolytic therapy was reported as a part of the study protocol for all studies in this comparison. In the European study (European 2001), administration of an alternative tocolytic agent was dependent on both efficacy and tolerability of study medication and could be administered when there was recurrence or progression of preterm labour. In the French/Australian study (French/Australian 2001), if labour was progressing or women experienced intolerable adverse effects of study drug administration, an alternative tocolytic agent could be given. There were 58% (n = 69) in the atosiban group versus 63.1% (n = 77) in the salbutamol group who needed an alternate tocolytic agent. Goodwin 1996 included an alternate tocolytic agent to be used when: (1) the cervix dilated 1 cm or more during therapy; (2) uterine contraction persisted at a same or higher rate; or (3) labour was not controlled, according to the judgement of the investigator. In Shim 2006, rescue tocolysis could be given if the study drug failed either by progression of labour or intolerable adverse events judged by the investigator. Alternative drugs could be ritodrine or magnesium sulphate, but not atosiban as rescue tocolytic in case of failure for women in the ritodrine group. In Lin 2009, re‐treatment with the study drug was allowed when there was recurrence of preterm labour. In Moutquin 2000, an alternative tocolytic agent could be given after the study treatment was stopped if labour was progressing, or if any woman had an intolerable adverse event. Maintenance therapy was used in at least one study in this comparison (Goodwin 1996); however, the details of the regimen were not provided. One study reported that maintenance therapy was not a part of the study protocol (French/Australian 2001). Maintenance therapy was not used in the remaining six studies where atosiban therapy was administered i.v. for a maximum of 48 hours.
Please seeCharacteristics of included studies for further details.
Outcome measures
Most of the studies included reported on the important clinical outcome of respiratory distress syndrome (except Cabar 2008; Kashanian 2005; Nonnenmacher 2009; Richter 2005). Many studies also reported maternal adverse drug reaction (European 2001; Goodwin 1994; Kashanian 2005; Romero 2000; Salim 2012; Shim 2006; Thornton 2009). The outcome of birth within 48 hours of initiation of treatment was reported in 12 (Cabar 2008; European 2001; French/Australian 2001; Goodwin 1994; Goodwin 1996; Kashanian 2005; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Richter 2005; Salim 2012; Shim 2006) of the 14 included studies and perinatal mortality in four studies (European 2001; French/Australian 2001; Moutquin 2000; Romero 2000). Other important outcomes were inconsistently reported including preterm birth, which was reported in four studies (European 2001; Romero 2000; Salim 2012; Thornton 2009), and major neonatal morbidity, which was largely not well reported across the studies.
Long‐term outcomes up to two years of age were reported as an abstract (Goodwin 1998a) for infants enrolled in one placebo‐controlled study (Romero 2000). Unfortunately, data were not reported in a format suitable for inclusion In this review. The authors have been contacted for further details before publication of the previous version of this review, but no further information has been forthcoming. The following outcomes were assessed: (1) illness, accidents, and physical abnormalities; (2) measurements of infant weight, length, and head circumference; (3) neurological examinations; (4) Bayley II assessment of mental and motor development; and (5) infant deaths. Although the report stated all infants were followed up and infant death up to 12 months was reported, only 55% of the infants who were originally included in the study were assessed for Bayley II Mental and Motor Development Index (Mean ± SD) and neurological examination at two years. One study comparing barusiban and placebo reported long‐term outcomes (Thornton 2009) for Bayley Scale evaluations of psychomotor developmental index (PDI) and mental developmental index (MDI) and physical growth. The long‐term outcomes were assessed at one year of age (Thornton 2009) and, as this time point was not prespecified, these data have not been included in this review.
In Romero 2000, data for the outcomes of birth less than 48 hours after trial entry and birth less than seven days after trial entry were reported only for women who did not receive alternative tocolytics and therefore these data were not included in the review.
Please seeCharacteristics of included studies for further details.
Excluded studies
We excluded nine studies.
One study was excluded on the basis of quasi‐random allocation (Al‐Omari 2006). Eight studies were excluded as they did not fulfil the intervention inclusion criteria as follows: A study in term labour (de Heus 2008); studies of maintenance tocolysis (Gagnon 1998; Valenzuela 2000); no comparison between different dosing regimens or tocolytic treatment (Husslein 2006); undefined treatment in control group (Husslein 2007); repeat course treatment with tocolysis (Poppiti 2009); did not use an oxytocin receptor antagonist (Steinwall 2005); study aimed to measure oestradiol levels before and after treatment (Valenzuela 1997a).
Please seeCharacteristics of excluded studies for further details.
Risk of bias in included studies
Overall the quality of the included studies was considered fair to good. Refer to Characteristics of included studies for further details and to Figure 1 and Figure 2 for a summary of 'Risk of bias' assessment.
1.
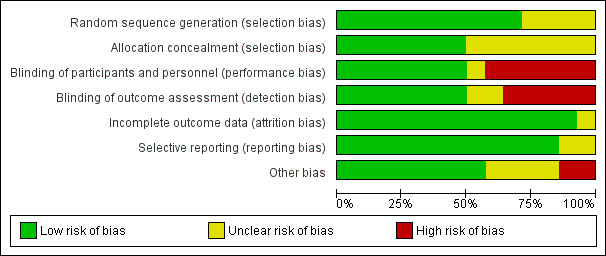
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
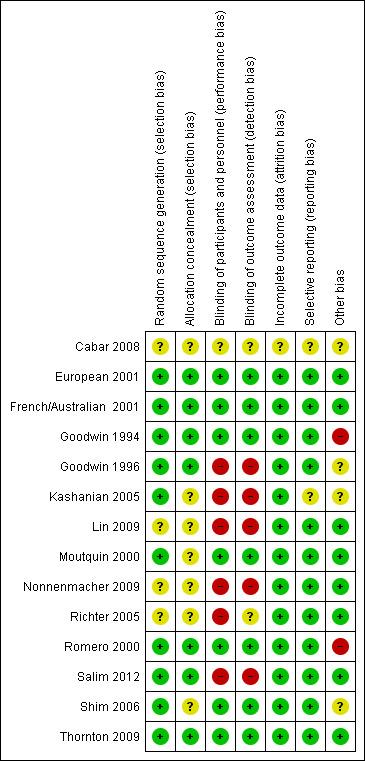
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The randomisation sequence generation was judged as adequate in 10 of the 14 included studies (European 2001; French/Australian 2001; Goodwin 1994; Goodwin 1996; Kashanian 2005; Moutquin 2000; Romero 2000; Salim 2012; Shim 2006; Thornton 2009) and therefore assessed as having a low risk of selection bias. In the remaining four studies, the randomisation sequence generation process was unclear.
Allocation to treatment was adequately concealed in seven of the 14 included studies (European 2001; French/Australian 2001; Goodwin 1994; Goodwin 1996; Romero 2000; Salim 2012; Thornton 2009) and therefore assessed as having low risk of selection bias. In the remaining studies the allocation process was unclear.
Blinding
Blinding of the intervention and outcome assessment was performed in seven of the 14 included studies (European 2001; French/Australian 2001; Goodwin 1994; Moutquin 2000; Romero 2000; Shim 2006; Thornton 2009). These studies were placebo controlled. For one study, the blinding of the intervention process was unclear (Cabar 2008). In one study, while a saline infusion control group was used, as different dosing regimens were used, the study was assessed as having a high risk of bias(Richter 2005). For the remaining studies, the lack of blinding of the intervention may be, in part, as a result of the difficulties with adequately blinding such interventions, i.e. presentation of the intervention as either oral versus intravenous and the well‐known side effects of certain interventions.
Incomplete outcome data
The majority (13 of the 14 included studies) had minimal or no attrition and were therefore assessed as having a low risk of attrition bias. For the remaining study, it was unclear whether outcome data were complete (Cabar 2008).
Selective reporting
In 12 of the 14 included studies, no reporting bias was evident (European 2001; French/Australian 2001; Goodwin 1994; Goodwin 1996; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Richter 2005; Romero 2000; Salim 2012; Shim 2006; Thornton 2009) and these studies were judged to be at low risk of bias. In the remaining two studies it was unclear whether reporting bias was present (Cabar 2008; Kashanian 2005).
Other potential sources of bias
In eight of the 14 included studies, no other potential sources of bias were apparent European 2001; French/Australian 2001; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Richter 2005; Salim 2012; Thornton 2009) based on baseline characteristics being similar and no other biases were evident. In four studies, the risk of other bias was unclear (Cabar 2008; Goodwin 1996; Kashanian 2005; Shim 2006). In two studies, the risk of other bias was judged to be high (Goodwin 1994; Romero 2000). These two studies (Goodwin 1994; Romero 2000) included women of lower gestational age in the atosiban group compared with the placebo group. One of these studies (Goodwin 1994) also recruited women from five different centres, and the inclusion criteria differed between the centres, which may have introduced bias. In the Romero 2000 trial, there were nearly twice as many atosiban‐treated patients enrolled at < 26 weeks’ gestation. Among the women enrolled at less than 26 weeks' gestation, the number who had advanced cervical dilation was higher in the atosiban group compared with the placebo group.
Assessment of studies that included multiple pregnancies
Seven studies included data from women with a multiple pregnancy (European 2001; French/Australian 2001; Goodwin 1994; Moutquin 2000; Nonnenmacher 2009; Romero 2000; Salim 2012). Two of these studies (Goodwin 1994; Romero 2000) compared ORA (atosiban) versus placebo, one study (Salim 2012) compared ORA versus CCB, and four studies compared ORA (atosiban) versus betamimetics (Nonnenmacher 2009; European 2001; French/Australian 2001; Moutquin 2000). As described previously (Methods), we have adjusted for clustering in the analyses of infant outcomes.
Effects of interventions
Two main comparisons were undertaken as follows.
Comparison 1: Oxytocin receptor antagonists compared with placebo further subgrouped by type of oxytocin receptor antagonist. Comparison 2: Oxytocin receptor antagonists compared with other classes of tocolytic agents by type of other tocolytic agent.
We did not undertake other planned subgroup analyses by population characteristics and by intervention due to insufficient data.
Comparison 1: Oxytocin receptor antagonists (ORA) compared with placebo, further subgrouped by type of ORA
Four studies are included in this analysis. Three studies including 691 women comparing atosiban and placebo (Goodwin 1994; Richter 2005; Romero 2000) and one study (163 women) (Thornton 2009) comparing barusiban with placebo are included in this comparison. For the comparison between atosiban and placebo, the Romero and Goodwin studies (Goodwin 1994; Romero 2000) contributed the majority of data (651 women) and the Richter study (Richter 2005; 40 women) contributed to two outcomes only; stillbirth and birth less than 48 hours after trial entry.
Primary outcomes
Birth less than 48 hours after trial entry (Analysis 1.1)
1.1. Analysis.
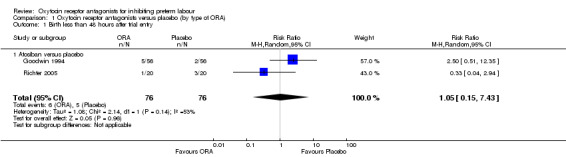
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 1 Birth less than 48 hours after trial entry.
Comparing ORA (atosiban) with placebo, no difference was shown in birth less within 48 hours after trial entry (average risk ratio (RR) 1.05, 95% confidence interval (CI) 0.15 to 7.43; random‐effects, Tau² = 1.08, Chi² = 2.14, df = 1 (P = 0.14), I² = 53%) (two studies with 152 women) Analysis 1.1. A moderate degree of statistically heterogeneity was evident for this outcome measure. However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
No data were available for the subgroup of barusiban versus placebo.
Perinatal mortality, Stillbirth, Neonatal death and Infant death (Analysis 1.2) (Analysis 1.3) (Analysis 1.4) (Analysis 1.5)
1.2. Analysis.
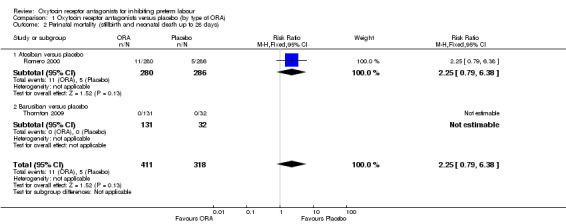
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 2 Perinatal mortality (stillbirth and neonatal death up to 28 days).
1.3. Analysis.
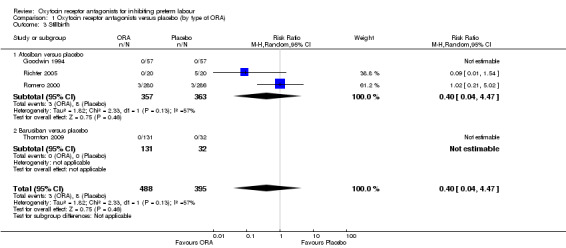
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 3 Stillbirth.
1.4. Analysis.
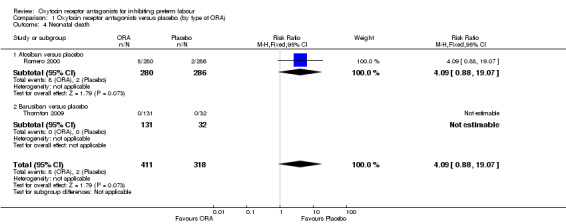
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 4 Neonatal death.
1.5. Analysis.
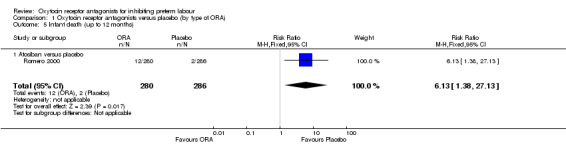
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 5 Infant death (up to 12 months).
Comparing ORA versus placebo no difference was shown in perinatal mortality (RR 2.25, 95% CI 0.79 to 6.38; two studies with 729 infants) (Analysis 1.2), stillbirth (average RR 0.40, 95% CI 0.04 to 4.47 random‐effects; four studies with 883 infants) (Analysis 1.3), or neonatal death (RR 4.09, 95% CI 0.88 to 19.07; two studies with 729 infants) (Analysis 1.4). These findings are driven by the subgroup of trials comparing atosiban versus placebo as no events were reported for the barusiban subgroup including a single trial (Thornton 2009).
A moderate degree of statistical heterogeneity was evident for the outcome measure of stillbirth (Heterogeneity: Tau² = 1.82; Chi² = 2.33, df = 1 (P = 0.13); I² = 57%). However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
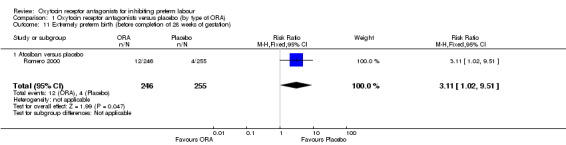
Infant death (up to 12 months) was increased with the use of the ORA atosiban in one trial (Romero 2000; 566 infants) (RR 6.13, 95% CI 1.38 to 27.13; number needed to treat for harm (NNTH) 28, 95% CI 6 to 377) (Analysis 1.5).
As mentioned in Other potential sources of bias, it is likely that the adverse infant outcomes associated with atosiban in the Romero 2000 trial are due to an imbalance in randomisation which resulted in more women under 26 weeks' gestation and fewer over 32 weeks assigned to the atosiban group. It is of note that in the publication of the trial results, the authors used the denominator of women who were enrolled less than 28 weeks' gestation and reported a non‐statistically significant increase in birth less than 28 weeks' gestation.
Serious maternal outcome (Analysis 1.6)
1.6. Analysis.
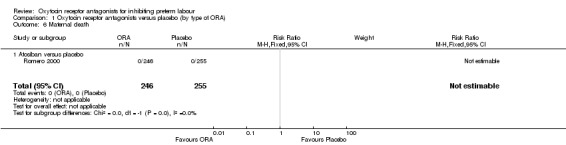
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 6 Maternal death.
One study Romero 2000 comparing ORA (atosiban only) with placebo (501 women) reported that no maternal deaths occurred during the trial period.
No data were available on other serious maternal outcomes or the other primary outcomes measure of very preterm birth before 34 weeks' gestation or long‐term outcomes for the child.
Secondary outcomes
For the infant/child
Preterm birth (before 37 weeks gestation) (Analysis 1.10) and Extremely preterm birth (before completion of 28 weeks of gestation) (Analysis 1.11)
1.10. Analysis.
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 10 Preterm birth (before completion of 37 weeks of gestation).
1.11. Analysis.
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 11 Extremely preterm birth (before completion of 28 weeks of gestation).
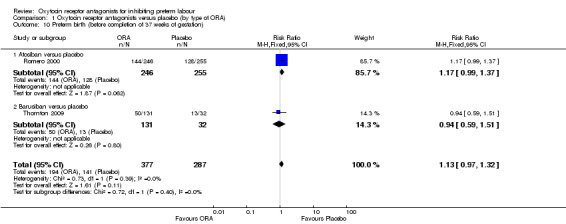
Comparing ORA versus placebo, no difference was found in preterm birth (before 37 weeks' gestation) (RR 1.13, 95% CI 0.97 to 1.32; two studies with 664 women). (Analysis 1.10).
Comparing ORA (atosiban only) with placebo, one trial (Romero 2000) (501 women) showed an increase in extremely preterm birth (before completion of 28 weeks of gestation) (RR 3.11, 95% CI 1.02 to 9.51; NNTH 31, 95% CI 8 to 3188).
No data were available for the subgroup of barusiban versus placebo.
No differences were evident according to type of ORA.
Gestational age (Analysis 1.12) and Birthweight (Analysis 1.13)
1.12. Analysis.
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 12 Gestational age (weeks).
1.13. Analysis.
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 13 Birthweight (grams).
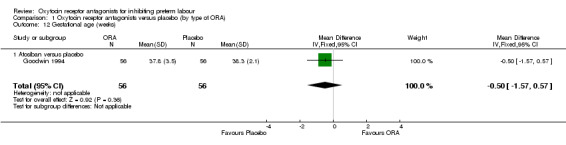
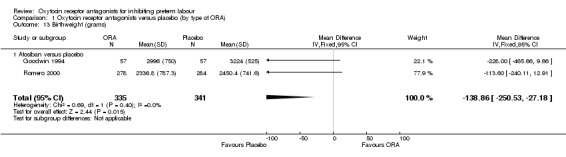
No difference was shown in gestational age at birth comparing ORA (atosiban only) with placebo (mean difference (MD) ‐0.50 weeks, 95% CI ‐1.57 to 0.57; one study with 112 women) (Analysis 1.12). ORA (atosiban) was associated with lower birthweight (MD ‐138.86 g, 95% CI ‐250.53 to ‐27.18; two studies with 676 infants) (Analysis 1.13), however the clinical importance of this difference (139 g) is questionable.
No data were available for the subgroup of barusiban versus placebo.
Neonatal morbidity
No difference was shown when comparing ORA versus placebo overall (or by type of ORA where data were available for these comparisons) for the following outcomes measures.
Respiratory distress syndrome (Analysis 1.14)
1.14. Analysis.
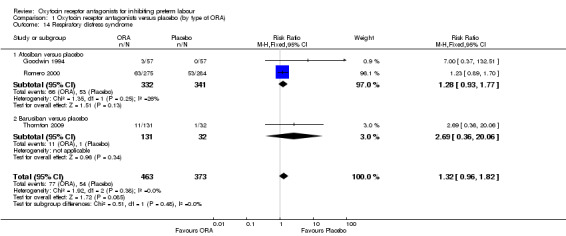
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 14 Respiratory distress syndrome.
For the comparison ORA versus placebo, no difference was shown in respiratory distress syndrome (RR 1.32; 95% CI 0.96 to 1.82; three studies with 836 infants).
Intraventricular haemorrhage (Analysis 1.15)
1.15. Analysis.
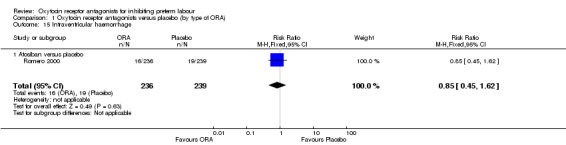
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 15 Intraventricular haemorrhage.
ORA (atosiban only) versus placebo: RR 0.85, 95% CI 0.45 to 1.62 (one study with 475 infants).
Necrotising enterocolitis (Analysis 1.16)
1.16. Analysis.
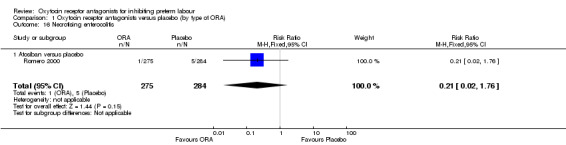
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 16 Necrotising enterocolitis.
ORA (atosiban only) versus placebo: RR 0.21, 95% CI 0.02 to 1.76 (one study with 559 infants).
Neonatal jaundice (Analysis 1.17)
1.17. Analysis.
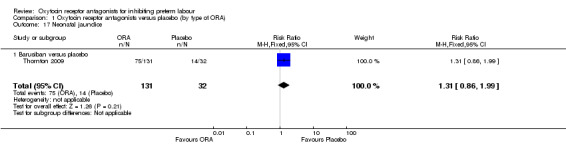
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 17 Neonatal jaundice.
ORA (barusiban only) versus placebo: RR 1.31, 95% CI 0.86 to 1.99 (one study with 163 infants).
Admission to neonatal intensive care unit (Analysis 1.18)
1.18. Analysis.
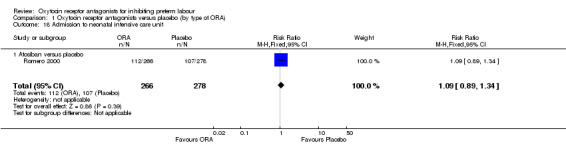
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 18 Admission to neonatal intensive care unit.
ORA (atosiban only) versus placebo: RR 1.09, 95% CI 0.89 to 1.34 (one study with 544 infants).
For the woman
Maternal adverse effects (Analysis 1.7)
1.7. Analysis.
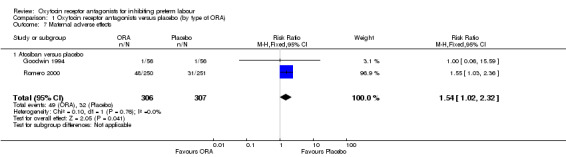
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 7 Maternal adverse effects.
ORA (atosiban only) resulted in a significant increase in maternal adverse effects (RR 1.54, 95% CI 1.02 to 2.32; two studies with 613 women; NNTH 18, 95% CI 8 to 480); representing 19% of women having an adverse effect in the ORA group versus 14% in the placebo.
Maternal adverse effects requiring cessation of treatment (Analysis 1.8)
1.8. Analysis.
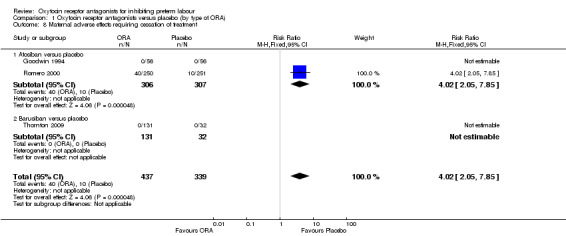
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 8 Maternal adverse effects requiring cessation of treatment.
Maternal adverse effects requiring cessation of treatment was increased for the ORA group when compared to placebo (RR 4.02, 95% CI 2.05 to 7.85; NNTH 12; 95% CI 5 to 33; three studies with 776 women); representing 16% of women having an adverse effect in the ORA group versus 4% in the placebo.
These findings are driven by the subgroup of trials comparing atosiban versus placebo as no events were reported for the barusiban subgroup including a single trial (Thornton 2009).
Caesarean section (Analysis 1.9)
1.9. Analysis.
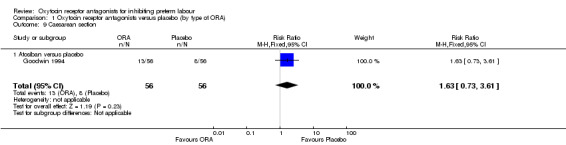
Comparison 1 Oxytocin receptor antagonists versus placebo (by type of ORA), Outcome 9 Caesarean section.
No difference was shown in caesarean section birth comparing atosiban with placebo (RR 1.63, 95% CI 0.73 to 3.61; one study with 112 women).
Comparison 2: Oxytocin receptor antagonists (ORA) compared with other tocolytic agents by type of other tocolytic agent
Eight studies (with 1402 women) are included in the comparison between ORA (atosiban only) and betamimetics (Cabar 2008; European 2001; French/Australian 2001; Goodwin 1996; Lin 2009; Moutquin 2000; Nonnenmacher 2009; Shim 2006). Two studies including 225 women are included in the comparison of ORA (atosiban only) and calcium channel blockers (CCB) (nifedipine only) (Kashanian 2005; Salim 2012). All studies used the ORA atosiban.
Primary outcomes
Birth less than 48 hours after trial entry (Analysis 2.1)
2.1. Analysis.
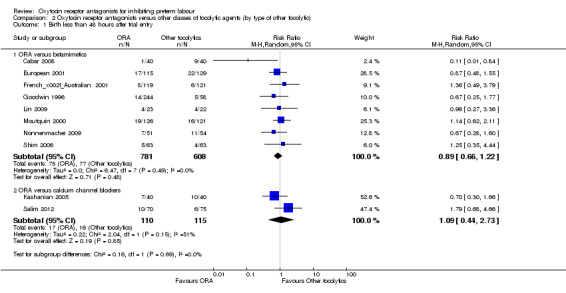
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 1 Birth less than 48 hours after trial entry.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 0.89, 95% CI 0.66 to 1.22 (eight studies with 1389 women).
ORA versus CCB: average: RR 1.09, 95% CI 0.44 to 2.73 (two studies with 225 women).
Moderate heterogeneity was present for the ORA versus CCB comparison (Tau² = 0.22; Chi² = 2.04, df = 1 (P = 0.15); I² = 51%); however, upon exploration of the possible reasons for heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
Very preterm birth (before completion of 34 weeks of gestation) (Analysis 2.3)
2.3. Analysis.
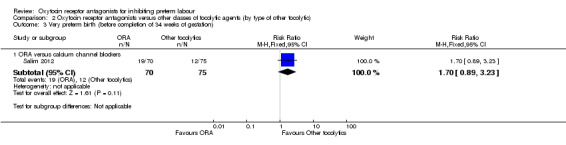
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 3 Very preterm birth (before completion of 34 weeks of gestation).
ORA versus betamimetics: no data were available.
ORA versus CCB: no statistically significant difference was shown (RR 1.70, 95% CI 0.89 to 3.23; one study with 145 women).
Perinatal mortality (Analysis 2.2), Stillbirth (Analysis 2.4) and Neonatal death (Analysis 2.5).
2.2. Analysis.
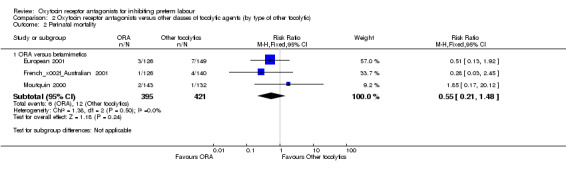
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 2 Perinatal mortality.
2.4. Analysis.
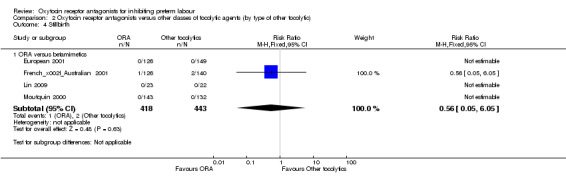
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 4 Stillbirth.
2.5. Analysis.
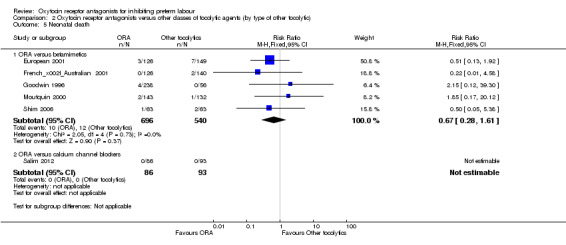
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 5 Neonatal death.
No statistically significant differences were shown within or across subgroups
ORA versus betamimetics: perinatal mortality (RR 0.55, 95% CI 0.21 to 1.48; three studies with 816 infants) (Analysis 2.2), stillbirth (RR 0.56, 95% CI 0.05 to 6.05; four studies with 861 infants) (Analysis 2.4) or neonatal death (RR 0.67, 95% CI 0.28 to 1.61; five studies with 1236 infants) (Analysis 2.5).
ORA versus CCB: the single trial in this comparison (Salim 2012, 179 infants), reported that no neonatal deaths occurred during the trial period. No data were available on stillbirth or perinatal mortality.
Serious maternal outcome (Analysis 2.6)
2.6. Analysis.
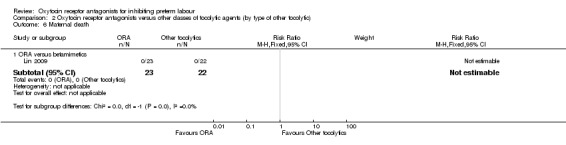
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 6 Maternal death.
ORA versus betamimetics: one study with 45 women (Lin 2009) reported that no maternal deaths occurred during the trial period.
ORA versus CCB: no data were available.
No other data were available on other serious maternal outcomes or long‐term outcomes for the child.
Secondary outcomes
For the infant/child
Interval between trial entry and birth (Analysis 2.10)
2.10. Analysis.
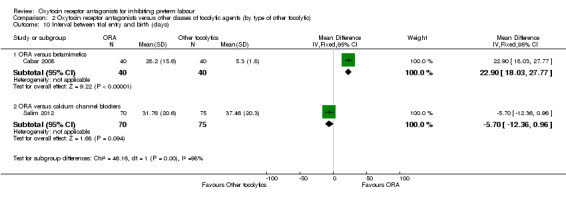
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 10 Interval between trial entry and birth (days).
ORA versus betamimetics: an increase in the Interval between trial entry and birth was shown with the use of ORA (MD 22.90 days, 95% CI 18.03 to 27.77; one study with 80 women) (Cabar 2008).
ORA versus CCB: no difference was shown in the interval between trial entry and birth (Salim 2012) (MD ‐5.70 days, 95% CI ‐12.36 to 0.96; one study with 145 women) (Salim 2012).
These results were statistically significantly different across subgroups; test for subgroup differences:Chi² = 46.16, df = 1 (P < 0.01), I² = 97.8%.
Preterm birth (before completion of 37 weeks of gestation) (Analysis 2.11)
2.11. Analysis.
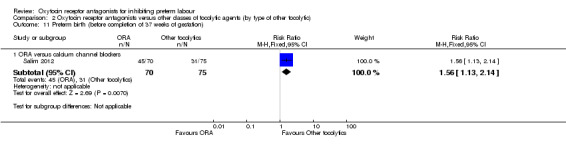
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 11 Preterm birth (before completion of 37 weeks of gestation).
ORA versus betamimetics: no data were available.
ORA versus CCB: in a single study (145 women) ORA (atosiban) resulted in significantly more preterm births compared with CCB (RR 1.56, 95% CI 1.13 to 2.14; NNTH 5, 95% CI 19 to 3).
Extremely preterm birth (before completion of 28 weeks of gestation) (Analysis 2.12)
2.12. Analysis.
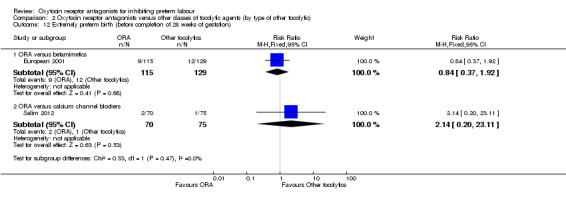
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 12 Extremely preterm birth (before completion of 28 weeks of gestation).
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 0.84, 95% CI 0.37 to 1.92 (one study with 244 women).
ORA versus CCB: RR 2.14, 95% CI 0.20 to 23.11 (one study with 145 women).
Gestational age at birth (Analysis 2.13)
2.13. Analysis.
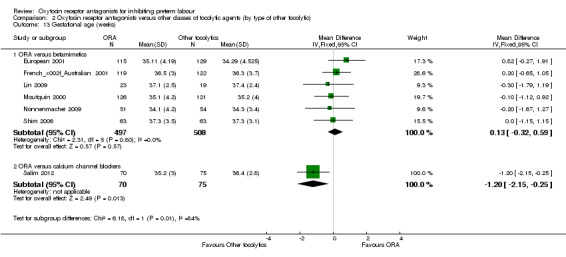
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 13 Gestational age (weeks).
ORA versus betamimetics: no difference was shown in gestational age at birth (MD 0.13 weeks, 95% CI ‐0.32 to 0.59; six studies with 1005 women).
ORA versus CCB: a lower mean gestational age for ORA group (atosiban) compared with CCB (MD ‐1.20 weeks, 95% CI ‐2.15 to ‐0.25; one study with 145 women).
These results were statistically significantly different across subgroups; test for subgroup differences: Chi² = 6.18, df = 1 (P = 0.01), I² = 83.8%.
Birthweight (Analysis 2.14)
2.14. Analysis.
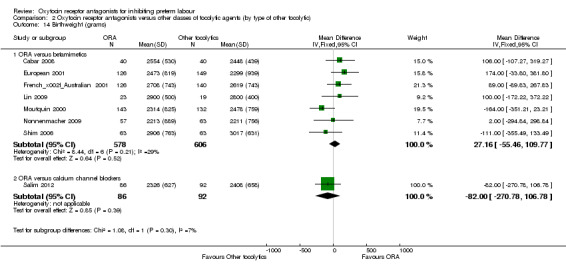
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 14 Birthweight (grams).
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: MD 27.16 g, 95% CI ‐55.46 to 109.77 (seven studies with 1184 infants).
ORA versus CCB: no difference was shown MD ‐82.00 weeks, 95% CI ‐270.78 to 106.78; (one study with 178 infants).
Apgar score less than seven at five minutes (Analysis 2.15)
2.15. Analysis.
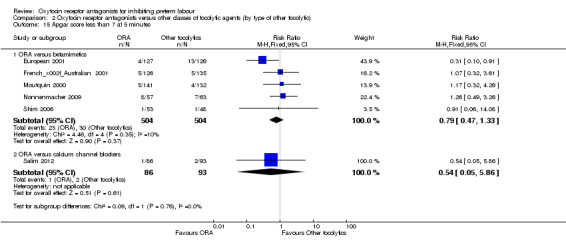
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 15 Apgar score less than 7 at 5 minutes.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 0.79, 95% CI 0.47 to 1.33 (five studies with 1008 infants).
ORA versus CCB: RR 0.54, 95% CI 0.05 to 5.86 (one study with 179 infants).
Respiratory distress syndrome (Analysis 2.16)
2.16. Analysis.
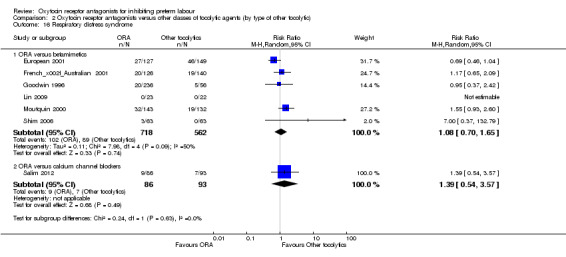
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 16 Respiratory distress syndrome.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: (average RR 1.08; 95% CI 0.70 to 1.65; random‐effects; six studies with 1280 infants). Moderate heterogeneity was evident (Tau² = 0.11, Chi² = 7.98, df = 4 (P = 0.09), I² = 50%) which was driven by one study (European 2001). However, as no clear reason for the heterogeneity could be identified, we considered an overall summary was meaningful using a random‐effects analysis.
ORA versus CCB: (RR 1.39, 95% CI 0.54 to 3.57; one study with 179 infants).
Use of mechanical ventilation (Analysis 2.17)
2.17. Analysis.

Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 17 Use of mechanical ventilation.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 3.00, 95% CI 0.63 to 14.30 (one study with 126 infants).
ORA versus CCB: RR 1.41, 95% CI 0.65 to 3.04 (one study with 179 infants).
Duration of mechanical ventilation (Analysis 2.18)
2.18. Analysis.
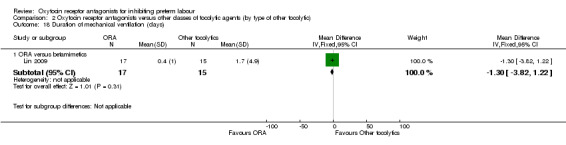
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 18 Duration of mechanical ventilation (days).
No statistically significant differences were shown.
ORA versus betamimetics: MD ‐1.30 days, 95% CI ‐3.82 to 1.22 (one study with 32 infants).
ORA versus CCB: no data were available.
Intraventricular haemorrhage (Analysis 2.19)
2.19. Analysis.
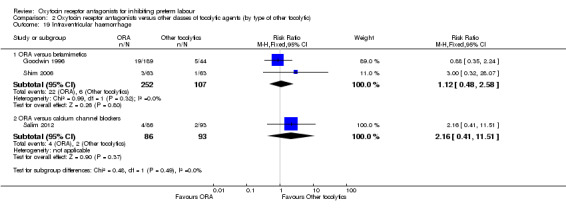
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 19 Intraventricular haemorrhage.
No statistically significant differences were shown.
ORA versus betamimetics: RR 1.12, 95% CI 0.48 to 2.58 (two studies with 359 infants).
ORA versus CCB: RR 2.16, 95% CI 0.41 to 11.51 (one study with 179 infants).
Necrotising enterocolitis (Analysis 2.20)
2.20. Analysis.
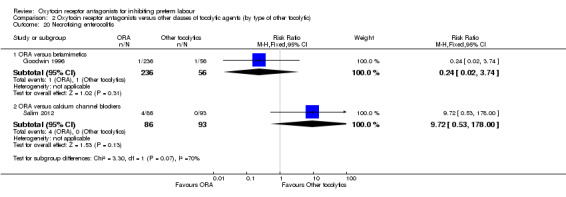
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 20 Necrotising enterocolitis.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 0.24, 95% CI 0.02 to 3.74 (one study with 292 infants).
ORA versus CCB: RR 9.72, 95% CI 0.53 to 178.00 (one study with 179 infants).
These results were statistically significantly different across subgroups; test for subgroup differences: Chi² = 3.30, df = 1 (P = 0.07), I² = 69.7%
Retinopathy of prematurity (Analysis 2.21)
2.21. Analysis.
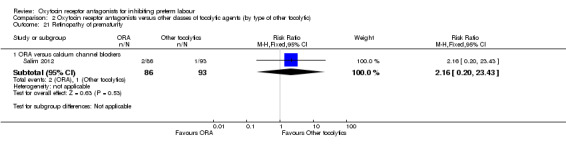
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 21 Retinopathy of prematurity.
No statistically significant differences were shown.
ORA versus betamimetics: no data were available.
ORA versus CCB: RR 2.16, 95% CI 0.20 to 23.43 (one study with 179 infants).
Neonatal sepsis (Analysis 2.22)
2.22. Analysis.
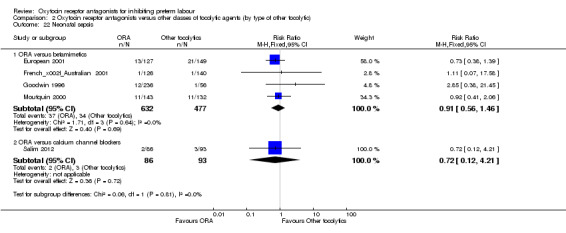
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 22 Neonatal sepsis.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: RR 0.91, 95% CI 0.56 to 1.46 (four studies with 1109 infants).
ORA versus CCB: RR 0.72, 95% CI 0.12 to 4.21 (one study with 179 infants).
Admission to neonatal intensive care unit (Analysis 2.23)
2.23. Analysis.
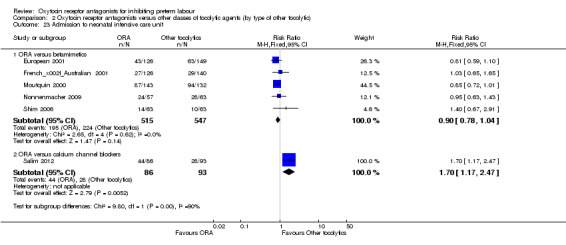
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 23 Admission to neonatal intensive care unit.
ORA versus betamimetics: no difference in admission to neonatal intensive care unit was shown (RR 0.90, 95% CI 0.78 to 1.04; five studies with 1062 infants).
ORA versus CCB: an increase in admission to neonatal intensive care unit was shown for the ORA (atosiban) group (RR 1.70, 95% CI 1.17 to 2.47; NNTH 5, 95% CI 3 to 19; one study with 179 infants).
Neonatal length of hospital stay (Analysis 2.24)
2.24. Analysis.
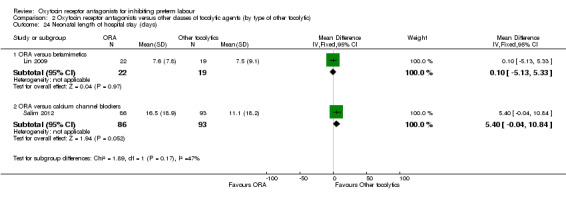
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 24 Neonatal length of hospital stay (days).
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetics: MD 0.10 days, 95% CI ‐5.13 to 5.33 (one study with 41 infants).
ORA versus CCB: MD 5.40 days, 95% CI ‐0.04 to 10.84 (one study with 179 infants).
For the woman
Maternal adverse effects (Analysis 2.7)
2.7. Analysis.
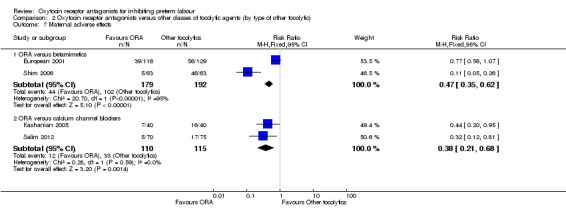
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 7 Maternal adverse effects.
ORA versus betamimetics: maternal adverse effects were reported by two studies comparing ORA (atosiban) with betamimetics. Due to substantial statistical heterogeneity when these data were combined (I² = 95%) results are reported separately. While both studies showed a reduction in these events, in one study comparing ORA (atosiban) with betamimetics (terbutaline) (European 2001), the reduction was not statistically significant (RR 0.77, 95% CI 0.56 to 1.07). In the other study comparing ORA (atosiban) with betamimetics (ritodrine) (Shim 2006), a statistically significant substantial reduction was shown for ORA (atosiban) (RR 0.11, 95% CI 0.05 to 0.26; NNTB 2, 95% CI 1 to 2).
ORA versus CCB: a reduction was shown in maternal adverse effects comparing ORA (atosiban) versus calcium channel blockers (RR 0.38, 95% CI 0.21 to 0.68; NNTB 6, 95% CI 5 to 11; two studies with 225 women).
Maternal adverse effects requiring cessation of treatment (Analysis 2.8)
2.8. Analysis.
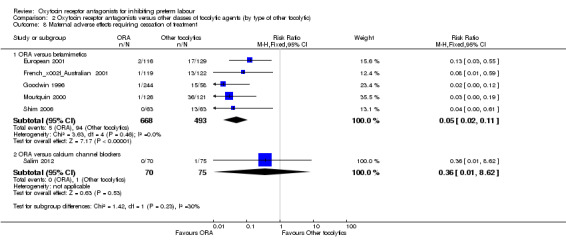
Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 8 Maternal adverse effects requiring cessation of treatment.
ORA versus betamimetics: a reduction in maternal adverse effects requiring cessation of treatment was shown for ORA (atosiban) compared with betamimetics (RR 0.05, 95% CI 0.02 to 0.11; NNTB 6, 95% CI 6 to 6; five studies with 1161 women).
ORA versus CCB: no difference in maternal adverse effects requiring cessation of treatment was shown for ORA (atosiban) compared with CCB (RR 0.36, 95% CI 0.01 to 8.62; one study with 145 women).
Caesarean section (Analysis 2.9)
2.9. Analysis.

Comparison 2 Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic), Outcome 9 Caesarean section.
No statistically significant differences were shown within or across subgroups.
ORA versus betamimetic: RR 0.87, 95% CI 0.50 to 1.52 (one study with 247 women).
ORA versus CCB: RR 1.34, 95% CI 0.37 to 4.79 (one study with 145 women).
Discussion
Summary of main results
Fourteen trials involving 2485 women contributed data to the review. We compared oxytocin receptor antagonists (ORA) with placebo (by type of ORA), with betamimetics and with calcium channel blockers (CCB) (all studies using nifedipine). Apart from one small study which used barusiban, all included studies used the ORA atosiban.
In this review, ORA (mainly atosiban) were not shown to prolong pregnancy or improve short‐term neonatal outcomes compared with either placebo or other tocolytics, i.e. betamimetic and CCB. The ORA atosiban was used in all placebo‐controlled trials apart from one small trial which used barusiban and the lack of data did not allow adequate examination of possible differential effects by type of ORA.
Sixteen per cent of women receiving ORA had an adverse effect requiring cessation of treatment compared with 4% of those who received placebo. ORA resulted in fewer maternal adverse effects than betamimetics or CCB.
Data from one study (Romero 2000), which compared ORA with placebo, showed an increase in births less than 28 weeks' gestation and deaths to 12 months of age (0.7% in placebo group versus 4.2% in the ORA group), indicating that on average one additional infant death would occur for every 29 women (and up to 380) receiving ORA. However, this finding may be confounded due to randomisation of more women with pregnancy less than 26 weeks' gestation to the atosiban group.
In one small study (145 infants) Salim 2012, which did not blind the intervention, an increase in the number of preterm births (before 37 weeks' gestation) (RR 1.56, 95% CI 1.13 to 2.14; NNTH 5, 95% CI 3 to 19), a lower gestational age at birth (MD ‐1.2 weeks, 95% CI ‐2.15 to ‐0.25) and an increase in admission to neonatal intensive care unit (RR 1.70, 95% CI 1.16 to 2.49; NNTH 5, 95% CI 3 to 20) was shown.
Overall completeness and applicability of evidence
Although ORA result in fewer maternal side effects than other tocolytics, there is no evidence of benefit for the infant when compared with other tocolytics or placebo. Limitations of the review findings include, minimal neonatal outcome data, no long‐term infant outcomes and overall suboptima trial quality.
Short‐term outcomes of birth within 48 hours, perinatal mortality, preterm birth (less ss than 37 weeks), respiratory distress syndrome, admission to neonatal care and maternal adverse drug reaction were the most frequently reported outcomes. Data on a number of clinically important neonatal outcomes were limited and the only included longer‐term outcome (reported by one study) was infant mortality.
An increase in births less than 28 weeks' gestation and deaths to 12 months of age was shown for ORA when compared with placebo. However, because the adverse outcomes are nearly entirely the result of one study (Romero 2000), and because of a paucity of further trials comparing atosiban with placebo, no definite conclusion can be drawn about whether atosiban is beneficial or harmful compared with placebo. Neurological follow‐up data from this study (although not included in this review) showed no difference between the atosiban and placebo groups at the age of six months, one year, and two years of age. However, this result should be interpreted with caution due to the potential for bias resulting from high loss to follow‐up.
A plausible explanation for the increase in adverse infant outcome in the atosiban group could be a chance imbalance in the allocation of women with threatened preterm labour in very early gestation (under 26 weeks) with significantly more women in this subgroup being allocated to the atosiban group. The mean gestational age on admission was statistically significantly greater for the placebo group than for the atosiban group, and there were nearly twice as many atosiban‐treated patients enrolled at < 26 weeks’ gestation. Among the women enrolled at less than 26 weeks' gestation, the number who had advanced cervical dilation was higher in the atosiban group compared with the placebo group. In the same study (Romero 2000), there were more births before 28 weeks' gestation in the atosiban group compared with placebo, which is not surprising given the short prolongation of pregnancy that most tocolytics can achieve. Another explanation, which was suggested by the US Food and Drug Administration (FDA), is that fetal vasopressin receptor blockade by atosiban could give changes in maternal amniotic fluid volume, with resultant alterations to fetal renal development, and secondary alterations to fetal lung development (FDA 1998a). A similar increase in adverse events among infants exposed to atosiban was reported to the FDA for one study of maintenance therapy (Valenzuela 2000) comparing placebo and atosiban (FDA 1998b).
The study comparing barusiban versus placebo (Thornton 2009) presented long‐term follow‐up data for mental development performance and psychomotor performance at one year of age (not included in this review). Although the findings were not significant, the results suggested mildly delayed psychomotor development in 4% in the barusiban group and 8% in the placebo group (Thornton 2009). None of the infants in the placebo group and 3% of infants in the barusiban group were classified as having significantly delayed psychomotor performance (Thornton 2009). In addition, there was mildly delayed mental performance in 8% of infants in the placebo group and 10% in the barusiban group (Thornton 2009). Based on the findings in this clinical trial (Thornton 2009), barusiban is currently not in use for inhibition of preterm labour.
It is also possible that rescue treatment confounded the estimation of the true effects of atosiban when compared with placebo. In the study by Romero et al (Romero 2000), rescue tocolysis was given in 43% of the atosiban group and in 51% of the placebo group. In one placebo‐controlled study (Goodwin 1994), rescue tocolysis was used in 20% and 32% of the participants in the atosiban and placebo groups respectively after a short infusion of study medication (two hours).
While data are limited on important clinical outcomes when comparing ORA with other tocolytics; eight studies (1402 women) comparing ORA (atosiban only) with betamimetics and two (225 women) comparing ORA (atosiban) with CCB did not show clear benefit other than reduced maternal adverse effects.
The difficulty in demonstrating that ORA is an effective tocolytic may relate to its mechanism of action (FDA 1998a). It interacts with oxytocic receptors in myometrial cells, the density of which is gestation dependent. It is possible that ORA is more effective for women in preterm labour at higher gestations, whereas the pressing clinical need is for tocolytics effective at lower gestations, where delay in delivery has greater potential to improve the outcomes for neonates.
Quality of the evidence
While three of the four placebo‐controlled trials were considered of good quality (allocation to treatment and intervention were blinded), four of the eight trials comparing ORA with betamimetic and both trials comparing ORA with CCB trials considered of low quality (no blinding of the intervention and often no blinding of allocation to treatment).
Sample attrition was not considered a serious source of bias as the majority (13 of the 14 included studies) had minimal or no attrition. In 12 of the 14 included studies, no reporting bias was evident and in the remaining studies it was unclear.
For the majority of trials no other sources of bias was evident. However, in four studies, the risk of other bias was considered to be unclear (Cabar 2008; Goodwin 1996; Kashanian 2005; Shim 2006) and in two studies, the risk of other bias was judged to be high (Goodwin 1994; Romero 2000). These latter two studies, both in the placebo comparison, included women of lower gestational age in the atosiban group compared with placebo group. One of these studies also recruited women from five different centres, and the inclusion criteria differed between the centres, which may have introduced bias. In the largest placebo‐controlled trial, while judged to be at low risk of bias for all other quality criteria, there were nearly twice as many atosiban‐treated patients enrolled at < 26 weeks’ gestation.
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and assessed risk of bias independently; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
We found that although ORA (mainly atosiban) resulted in fewer maternal side effects than other tocolytics; there is no clear evidence of benefit in terms of prolongation of pregnancy or important infant outcomes when compared with other tocolytics or placebo. A recent network meta‐analysis (Haas 2012) showed different results, reporting that atosiban was better than placebo in delaying delivery at 48 hours (odds ratio 2.02; 95% CI 1.1 to 3.8), although it also found that atosiban had a lower probability of being superior to other tocolytics. We did not show benefit for ORA in delaying birth for 48 hours when compared with placebo as in the Haas et al review (Haas 2012). However, due to different methodological approaches, findings are difficult to compare. Further, the review reported that nifedipine (the most commonly used CCB) is the preferred first‐line tocolytic (Haas 2012). Another review, using indirect comparison of randomised trials, of the ORA atosiban with nifedipine concluded that nifedipine was more effective than atosiban and lowered the incidence of respiratory distress syndrome (Coomarasamy 2003). In the Cochrane review on CCB for inhibiting preterm labour, CCB (mainly nifedipine) was shown to prolong pregnancy and reduce neonatal morbidity (update of King 2003 in progress). Similar to our review, this review found limited trial outcome data comparing ORA with CCB (including the same two small studies included in this review (Kashanian 2005; Salim 2012)). However, in our review, some indication for benefit of CCB (nifedipine) over the ORA atosiban was shown in the results of one small trial (145 women) (Salim 2012).
Consistent with our conclusions, Haas et al (Haas 2012) supported further well‐designed, randomised, placebo‐controlled trials to evaluate ORA in prolonging pregnancy for women in preterm labour. The CCB, nifedipine has the advantage of ease of oral administration and is very inexpensive compared with atosiban (Papatsonis 2004). However, more robust evidence from well‐designed, randomised trials with direct comparisons of nifedipine and ORA are required before strong recommendations for clinical practice can be made. One such study is ongoing (APOSTEL III).
Adequate comparison of ORAs with COX inhibitors (such as indomethacin) for preterm labour is also lacking. COX inhibitors have been the focus of a recent Cochrane review (Khanprakob 2012), which concluded that, due to insufficient evidence, further well‐designed, placebo‐controlled trials are needed and should include comparisons with other agents and incorporate long‐term follow‐up of infants.
Authors' conclusions
Implications for practice.
This review did not demonstrate superiority of oxytocin receptor antagonists (ORA) (largely atosiban) as a tocolytic agent compared with placebo, betamimetics or calcium channel blockers (CCB) (largely nifedipine) in terms of pregnancy prolongation or neonatal outcomes, although ORA was associated with fewer maternal adverse effects than treatment with the CCB or betamimetics. The finding of an increase in infant deaths and more births before completion of 28 weeks of gestation in one placebo‐controlled study warrants caution. However, due to limitations of the small numbers studied and methodological quality, there is currently insufficient evidence on the role of ORA in the management of women in preterm labour to inform clinical practice.
Implications for research.
Further well‐designed randomised controlled studies of tocolytic therapy are needed, in particular comparisons of ORA versus CCB (which have a better side‐effect profile than betamimetics), and further placebo‐controlled studies may also be warranted. All future studies with use of tocolytic agents should measure all important short‐ and long‐term outcomes for women and infants, and costs. Future studies should address the possibility that different tocolytic strategies are needed at different gestational ages in order to optimise safety and efficacy. At the time of writing this review, a randomised controlled trial comparing atosiban versus nifedipine in the management of preterm labour is ongoing in The Netherlands, (APOSTEL III) NTR2947 and may help to fill this gap in knowledge.
Feedback
Goodwin, September 2005
Summary
I have a number of concerns about this review. First, the inclusion of two reports (Goodwin 1994 and Goodwin 1996) is misleading, as neither study had delay in delivery or prolongation of pregnancy as endpoints. Information was collected on these endpoints, but the studies were not designed to demonstrate efficacy. In Goodwin 1994 the intervention was an intravenous infusion of atosiban for just 2 hours. This aim of this study was to describe the effect on uterine activity as measured by external tocodynamometry. Women were specifically chosen as having uterine activity, but unlikely to be in true preterm labor. Goodwin 1996 was a dose ranging study in which most women (3 of 4 arms) received doses far below what is currently recommended. Any effect of atosiban would therefore be underestimated. As these studies were not designed and executed as efficacy trials it seems inappropriate to use them in a discussion of efficacy.
Second, the excess perinatal mortality in Romero 2000 is overemphasized. This excess has not been seen in other trials, and had a plausible explanation based on study design. Although this is acknowledged in the review, the finding is given undo emphasis in the conclusions. The statement in the discussion that the excess mortality in Romero 2000 reaches statistical significance when the 2 infant deaths up to 12 months are included seems to rely on counting these 2 deaths between 28 days and 12 months twice.
Third, the concern about long term follow up of exposed infants, because of 45% being lost to follow up, is arbitrary. There are few precedents for attempting to follow up a cohort in which 90% of the children are well. The only other study to attempt follow up on this scale (the Canadian Preterm Labor Investigators Trial) simply avoided the problem by selecting an available portion for follow up.
Finally, the exclusion of Romero 2000 from any discussion of efficacy (and the inappropriate inclusion of other trials ‐ see above) is confusing. The pre‐specified endpoint of delay without requirement for an alternate tocolytic (approved by the US FDA) remains the only way to describe a placebo trial in the US. To simply say that it is not included oversimplifies the complexity of studying and understanding tocolytics. It is true to say there is insufficient evidence of a tocolytic benefit, but it is an overstatement to say that there is no such evidence.
One of the main reasons I wish to comment on the review is that the acknowledgement of my assistance may give the impression that I concur with the conclusions. While I was happy to help with gathering of some information, I feel that this analysis is flawed and not up to the high standards of Cochrane Reviews.
(Summary of comment from Murphy Goodwin, September 2005)
Reply
To respond to these comments in the order in which they are made:
First, our pre‐specified methods did not exclude studies based on either duration or dose of tocolysis. Both Goodwin 1994 and Goodwin 1996 where therefore eligible for inclusion. We disagree that trials should have been excluded if delay in delivery or prolongation of pregnancy were not primary endpoints. Also, both these studies enrolled women judged to be in preterm labour based on definitions that are consistent with those used in the other trials within the review, and that meet commonly used clinical criteria for tocolysis. Although Goodwin 1996 was a dose‐finding study, three of the four treatment arms used doses that are now considered reasonable clinical regimens.
Second, we disagree that the excess in perinatal and infant mortality in Romero 2000 is overemphasized. The explanation given by the trial authors for the excess perinatal mortality is that it may have been due to an unexpected imbalance in randomisation between the atosiban and placebo groups for women randomised before 26 weeks (13/255 versus 24/246). We are not aware they have published an analysis controlling for gestational age to support this explanation, however. Also, our attempts to obtain further information from the authors about possible reasons for this imbalance between the placebo and atosiban groups have been unsuccessful. In particular, it would be helpful to clarify whether this imbalance was likely to be due to chance alone, or whether there was any possibility of bias at trial entry, or an error in the randomisation sequence. We do not think we have double counted any infant deaths. Romero 2000 reported 10 infant deaths in the treatment group versus 2 in the placebo group, a difference which achieves statistical significance (RR 5.12, 95% CI 1.13‐23.17). Our understanding is that there were two additional deaths in the atosiban group. This is based on unpublished data in the document "Antocin Final Two‐Year Infant Safety report" (issued 15 July 1999) which was initially supplied by Ferring UK to the Royal College of Obstetricians and Gynaecologist in the UK, and which Ferring later kindly agreed to share with us. This document describes 12 infant deaths plus 3 fetal deaths (total 15 deaths) in the atosiban group and 2 infant deaths plus 3 fetal deaths (total 5 deaths) in the placebo group. Thus, for infant deaths this 12 versus 2. If there is any dispute about these data it would be helpful if the results of this two‐year follow‐up study could be presented for public scrutiny in a peer reviewed journal.
Third, high attrition is a common problem in long‐term follow. It is reassuring that the losses were similar between the groups and that the majority of children were neurologically normal. Nevertheless, in the absence of any explanation for the high loss to follow up, concerns remain about potential systematic differences between the groups amongst those who were not contacted and assessed. Once again, to help allay such concerns it would be helpful if this follow‐up study could be published.
Finally, the outcome measures in our review were all prespecified. Romero 2000 did not report outcome for all women who remained undelivered after 24 hours, 48 hours or 7 days. Data were only available for women who remained undelivered and did not require an additional (rescue) tocolytic drug. Data for the outcomes in our review have been requested from the trial authors, but to date have not been received.
It was not our intention to imply that Professor Goodwin agreed with our conclusions. We remain grateful for his help in supplying additional data, and agree that such assistance in no way implies concurrence with our conclusions.
(Summary of response from Dimitri Papatsonis, January 2006)
Contributors
Murphy Goodwin
Åkerlund et al, December 2005
Summary
The authors conclude that the review failed to demonstrate the superiority of atosiban over placebo or betamimetics in terms of either tocolytic efficacy or infant outcomes. We disagree with this conclusion for the following reasons:
For the comparison of atosiban with placebo
In Goodwin 1994 the decrease in uterine contractions was significantly greater with atosiban than placebo. There was a complete cessation of contractions for 25% of women receiving atosiban and 5% of women receiving placebo. Romero 2000 and Valenzuela 2000 followed different study protocols with different primary end‐points, and provide data for safety analyses only.
In Romero 2000 and Valenzuela 2000 women in the placebo group were treated with bed‐rest and hydration. Hydration reduces oxytocin secretion, which may have contributed to the decrease in contractions in the placebo group. Atosiban is also a vasopressin V1a receptor‐ inhibiting compound, more potent than an oxytocin antagonist. Vasopressin may well be involved in the aetiology of preterm labour, and its secretion may also be reduced by hydration.
For the comparison of atosiban with betamimetics
The reviewers state that atosiban is no better than other classes of drug in delaying preterm birth. However, in European 2001 atosiban was significantly better, in terms of fewer women undelivered and not requiring alternative tocolysis after 7 days of treatment, than either ritodrine, salbutamol or pooled data for three betamimetics.
For infant outcomes
In European 2001 1.2% of the infants died in the atosiban group versus 2.3% for betamimetics. There were no differences in infant deaths between atosiban and placebo in Goodwin 1994, Romero 2000 or Valenzuela 2000. The only exception to this was for infants of women randomised before 26 weeks gestation in Romero 2000.
Our main criticism of the review is that the rationale underlying the selection of trials for inclusion is unclear. For example, the reviewers included Valenzuela 2000, even though it was excluded from other perspectives. Furthermore, the studies were not designed to have infant follow up and so these data were incomplete.
Romero 2000 is included as a high‐quality trial, despite an imbalance between the atosiban and placebo groups for women randomised before 26 weeks gestation. To compare tocolytic efficacy and infant outcome of these data is not relevant. That was also the attitude of authorities when atosiban was registered in European countries.
Finally, the conclusions state that calcium channel blockers are superior to betamimetics, although this comparison is not part of the review. Meta‐analysis is an efficient way of providing the basis for evidence‐based medicine. However, the weaknesses of this method, such as selection bias and lack of quality weighting, are well recognized (Spector 1991). This Cochrane review exemplifies the drawbacks of meta‐analysis, and its limitation in yielding valid conclusions.
(Summary of comments from Mats Åkerlund, Karel Marsl, and Ingemar Ingemarsson, December 2005)
Reply
First, to clarify any misunderstanding about whether there was a rationale for the selection of trials to include in our review. The criteria used for selecting trials were described a priori in the protocol, published on The Cochrane Database of Systematic Reviews. These methods adhere to the rigorous process defined by the Cochrane Collaboration, and followed by the Cochane Pregnancy and Childbirth Group. Therefore, the threat to the internal validity of our review from bias in selection of which studies to include was minimised.
Quality weighting in a meta‐analysis has not be shown empirically to impact on reliability of the summary statistic, hence why it was not done within our review
For the comparison of atosiban with placebo
We agree that Goodwin 1994 reported a cessation of contractions for 25% of women receiving atosiban compared with 5% of those receiving placebo. As this was a small study, however, these data are based on just 14 women versus 5 women who ceased contractions. Also, a more clinically important measure of tocolytic efficacy is the proportion of women who delivered within 48 hours. This outcome was not statistically significant between the groups (relative risk 2.50, 95% CI 0.51 to 12.35).
Valenzuela 2000 was excluded from the review because it evaluated maintenance tocolysis, and so did not meet our inclusion criteria.
The impact of hydration in the placebo group on any decrease in contraction is likely to be limited. The effect of atosiban and hydration on vasopressin V1a receptors is known, although its impact, if any, on preterm labour is less clear. Our view is that the overall effect of hydration on the incidence of preterm labour is limited, although hydration could lead to some reduction in oxytocin release.
Atosiban is also a vasopressin receptor antagonist. The human placenta is permeable to atosiban and the fetus has functional vasopressin receptors in the third trimester. The exact effect of fetal vasopressin receptor blockade on the fetus, following administration of atosiban, is unclear. Also unclear is whether a decrease in vasopressin due to hydration has any effect on preterm labour. For Romero 2000 this is not an issue, however, as hydration seems to have been similar in both groups.
For the comparison of atosiban with betamimetics
The conclusion that atosiban is no better than betamimetics in delaying preterm birth is based on the lack of clear benefit for atosiban on the number of infants delivered after seven days of starting treatment, or any other prespecified outcome. We did not use the composite outcome "delay in delivery and no alternate tocolytic agent". This was because the decision to start an alternative tocolytic may have been biased by awareness of the study treatment allocation, due to maternal signs and symptoms such as palpitations, flushing and tachycardia associated with betamimetics. The potential benefit of atosiban on this composite outcome measure, reported by some trials in the review, is clearly questionable as this benefit did not translate into improved outcome for the infants.
For infant outcomes
The incidence of infant deaths was similar in the trials comparing atosiban and betamimetics.
Although Romero 2000 met the criteria for inclusion in the review, we agree there were methodological concerns and these are described under 'methodological quality of included studies'. There was an imbalance between the groups in women randomised before 26 weeks' gestation (24/246 [10%] atosiban versus 13/255 [5%] placebo), with fewer women randomised after 32 weeks allocated atosiban compared with placebo (96/246 [4%] versus 116/255 [5%]). The increase in fetal‐neonatal deaths in the atosiban group may, therefore, be explained by this imbalance. However, we are not aware of an analysis of infant outcome controlling for this imbalance. As the randomisation sequence was adequately concealed at trial entry, the risk of this imbalance having been due to bias seems to be low. While the reason for the imbalance remains unclear, and has not been provided by the principal investigators nor the pharmaceutical company sponsoring the trial, it is possible that it occurred by chance alone due to the fact that randomisation was not stratified by gestational age.
Although some of included trials were not designed for follow‐up, this is not a reason to exclude them from the analysis of short term outcomes.
When a new tocolytic drug is being developed, the question most parents are likely to want answered is whether there is any beneficial effect for the child. Trials of tocolytic drugs should be designed to establish any such effect. It is therefore surprising that the authorities in Europe did not ask for any evidence of tocolytic efficacy or benefit for the fetus before approving atosiban for use in Europe, in contrast to similar authorities in the USA who did require such evidence.
Finally, we stand by the conclusions of our systematic review that the superiority of atosiban over placebo or betamimetics was not been demonstrated, in terms of either delay in delivery or any short or longer term neonatal morbidity or mortality.
(Summary by Dimitri Papatsonis, on behalf of the review authors, May 2006)
Contributors
Mats Åkerlund, Karel Marsl, and Ingemar Ingemarsson
Thornton S et al, July 2006
Summary
We are concerned about the conclusions and implications for clinical practice in this review. In particular, (i) the trial methodology may not allow reliable evaluation of outcome; (ii) there seems undue importance attached to the risk of infant deaths in one study (Romero 2000) with imbalance at baseline, and (iii) the conclusion that calcium channel blockers are associated with a better neonatal outcome is not qualified.
First, the review acknowledges that some women in the trials of oxytocin receptor antagonists required rescue tocolysis. In practice, this means that women are randomised to treatment or comparator/placebo, and those who progress in labour are given an alternative tocolytic. This means that any women could be given an effective drug for rescue, which prevents direct comparison of outcome. It is therefore not possible to categorically say that one of the agents administered initially is superior, or inferior, to the other. The most reasonable inferences that can be drawn, in studies where co‐intervention is likely to have a substantial impact on outcome, concern the effects observed under treatment combinations. The effectiveness of initial tocolytic agents alone cannot be studied. What can be studied is the effect of initial plus rescue tocolysis allowed in the care protocol. Therefore it is acknowledged that in such trials direct comparison of many (including neonatal) outcomes is inappropriate (Romero 2000). For this reason the endpoint of delay in delivery without alternate tocolytic has been used in that study. Given that it is inappropriate to compare neonatal outcomes in such trials, it is disappointing that the outcomes are given such importance in the conclusion.
Second, it is also disappointing that the abstract states Atosiban is associated with an increase in infant deaths at 12 months of age compared with placebo. As`the trial randomised more women in the Atosiban arm at very early gestational ages, this would be expected to increase mortality. Randomisation (when methodologically sound) uses a chance procedure for group allocation, which may produce imbalances in important prognostic variables at baseline by chance alone. If such differences are observed, an appropriate analysis of the trial would include statistical corrections for baseline differences to have valid results. Moreover, it is not clear why it was felt that mortality at one year should be included in the analysis when outcomes up to two years were excluded. If Atosiban were associated with an increase in mortality risk for the child, it is likely that this would have been demonstrated in the numerous other large clinical trials. As there is no increase in mortality in other trials, it is a reasonable assumption that the excess mortality in the placebo controlled trial was due to the disproportionate allocation to Atosiban at early gestational ages.
Finally, the conclusion suggests that calcium channel blockers are associated with better neonatal outcome and fewer maternal side effects than betamimetics. Although it is stated that no trials have directly compared nifedipine with placebo, it is not acknowledged that the clinical studies on calcium channel blockers were not blinded, that comparison was often with an extremely high dose of ritodrine and that these studies also often included rescue tocolysis. The conclusions regarding the possible improvement in outcome with calcium channel blockers must therefore be taken in context.
(Summary of comments from Steve Thornton, Khalid S Khan, and Patrick FW Chien, July 2006)
Potential conflict of interest: Steve Thornton provides consultancy advice for the pharmaceutical industry. Khalid Khan has a UK NHS HTA research grant on prevention of preterm birth.
Reply
Responding to the comments in the order in which they were made:
First, we do not agree that the use of rescue tocolysis means direct comparison of outcome is inappropriate. We used the standard methods as described in the Cochrane Reviewers Handbook (Higgins 2011). The inclusion criteria, outcome measures and comparisons, as with all methods of this review, were pre‐specified on the basis of ensuring that a clinically relevant "real life" question was addressed. Rescue tocolysis is a real life situation and therefore was handled in a necessarily pragmatic approach in this review. Our pre‐specified methods did not exclude studies based on having an alternative (rescue) tocolytic agent. Romero 2000 did report that a substantial number of women received an alternate "rescue tocolytic agent" (42% in the atosiban arm versus 51% in the placebo). However, we remain convinced that this study, and its outcomes, should be included in our review as it fulfils the inclusion criteria. We remain convinced that the pre‐specified clinically important outcomes of all women undelivered after 24 hours, 48 hours or 7 days should remain. The outcome used in Romero 2000, of women who were undelivered and did not require an alternate tocolytic agent, does not reflect real life and is more susceptible to bias. Despite repeated requests to the authors of Romero 2000 for data on the outcome of delay in delivery, in a format which would enable inclusion in this review, no such data have been forthcoming.
Secondly, as discussed in an earlier response to a comment on this Cochrane review, we disagree that the excess in perinatal and neonatal mortality in Romero 2000 is overemphasized. Our view is that the data on outcome for this trial are presented and discussed appropriately. Although the trial authors stated that the excess mortality may have been attributable to an imbalance between the groups in women randomised before 26 weeks gestation. Without an analysis controlling for gestation this remains a tentative explanation. We are not aware that any such analysis has been undertaken. We have attempted on numerous occasions to obtain further information from the trial authors, but to date have been unsuccessful. As central computer randomisation was used, we concluded that the imbalance was most likely due to the lack of stratification by gestational age (random error) and not bias due to flaws in the allocation concealment, and have clearly stated this in the review.
Finally, whilst we agree with Prof Thornton that there were no placebo controlled trials in the Cochrane review on calcium channel blockers compared with betamimetics, we also agree with the conclusions of the relevant review about the superiority of calcium channel blockers in terms of safety and neonatal outcomes. Blinding of studies, when comparing calcium channel blockers with betamimetics, is almost impossible because of the cardiovascular side effects of betamimetics, such as palpitations and anxiety. In these studies the additional rescue tocolysis used was comparable for the different study arms.
We are aware that because there is only indirect comparison between atosiban and nifedipine as tocolytic agents (and therefore the evidence for which of two tocolytic agents is most effective and safe is inconclusive) both tocolytic agents are currently advocated by obstetricians across several countries. Cost and mode of administration are also important considerations in the choice of tocolytic agent. We therefore believe that a well designed trial comparing oxytocin receptor antagonists and calcium channel blockers for the management of preterm labour is important in the advancement of care for women in preterm labour.
(Reply from Dimitri Papatsonis, on behalf of the review authors, August 2007)
Contributors
Steve Thornton, Khalid S Khan, and Patrick FW Chien
Thornton J, July 2006
Summary
I am concerned that there is unintentional bias in favour of the use of calcium channel blockers and against oxytocin antagonists in two recent Cochrane reviews, this one and the review of calcium channel blocker trials (King 2003).
Objective judgement of trial quality
Four studies of oxytocin antagonists (European 2001; French/Australian 2001; Moutquin 2000; and Romero 2000) are recorded as 'Blinding outcome assessment: unknown' despite their using a double dummy technique with no mention that the blinding was broken. Another, Goodwin 1994, is classified as 'Blinding outcome assessment: no' despite the review authors correctly noting that a double dummy technique was used. The relevant section of the published paper reads as follows: "the pharmacist would open the envelope to reveal the patient's treatment assignment for the purpose of preparing the study drug infusion solution. The treatment assignment was not revealed to other persons, and the individual preparing the drug was not involved in patient care." Surely all five trials should be classified as 'Blinding outcome assessment: yes'.
Subjective judgement of trial quality
In the text of the calcium channel review (King 2003), the trials are classified as of reasonable quality and no statement is made about quality in the abstract.
In fact none were blinded; they were all relatively small (mean group size 43) and only four had performed a sample size calculation. The lack of blinding is particularly important since all the reported outcomes favouring calcium channel blockers are susceptible to biased ascertainment, and the only hard outcome, perinatal death, showed a trend against calcium channel blockers (see below).
In contrast the oxytocin antagonist reviewers classify Goodwin 1996 as 'not high quality' because it was unblinded.
Choice of outcomes to report in the abstract
The calcium channel review (King 2003) abstract finds space to report seven beneficial effects of calcium channel blockers on surrogate outcomes, either prolongation of labour or surrogate fetal outcomes, but fails to mention perinatal deaths which had a relative risk 1.65 (95% CI 0.74‐3.64) favouring other tocolytics. Nor are total pregnancy losses mentioned. These would include the four neonatal deaths reported by Koks 1998 in a ratio of 3:1 against calcium channel blockers.
In the oxytocin antagonist review abstract, five unfavourable conclusions against placebo are reported. Although all of them might be explained by the gestational age imbalance at trial entry in the relevant trial (Romero 2000), this qualification is only mentioned in relation to one, infant death, and is removed from the synopsis where the association is repeated. In the comparison with beta‐mimetics, the first outcome reported is birth weight under 1,500g, an outcome which was not pre‐specified in the review methods and which is the only statistically significant outcome out of 21 reported for this comparison. Only later is the reduction in adverse drug reactions compared to beta‐mimetics reported.
Choice of language
In the review of calcium channel blockers (King 2003), all of the seven sentences in the abstract conclusions and the plain language summary contain a favourable opinion of calcium channel blockers. The single exception is a call for research into the effect of different dosing regimes, with the implication that the primary effectiveness question has been answered.
The authors' conclude: "it is considered unlikely that [placebo controlled trials of calcium channel blockers] will be conducted given the unequivocal impact that this method of tocolysis has on short term postponement of delivery" This statement is much too strong. It is based entirely on unblended trials against other tocolytics. Two of the five relevant outcomes (birth prior to 37 weeks, and birth within 48 hours) showed only a non‐significant effect, two (birth prior to 34 weeks and within seven days) just reached the 0.05 level, and the final outcome (pregnancy prolongation in days), while statistically significant, shows significant heterogeneity between trials.
In neither the abstract nor the conclusion section of the calcium channel blocker review is it mentioned that there have been no placebo‐controlled trials of calcium channel blockers in preterm labour.
In contrast, instead of saying that oxytocin antagonists had shown equivalent efficacy to other tocolytics in four high quality trials, the authors phrase their summaries as either 'has failed to demonstrate superiority' or 'is no better than other drugs'. This seems gratuitous negativity.
Choice of outcomes to report
The outcomes selected for the oxytocin review differ significantly from those chosen for the calcium channel blocker review. The reason is not clear.
Finally, the oxytocin antagonist review claims to be going to look at predefined outcomes measured related to the prolongation of pregnancy. However the predefined outcomes for the two placebo‐controlled trials, namely 'time to delivery' or 'therapeutic failure' were not reported.
Authorship of the reviews
I note that both these reviews share an author, Dimitri Papatsonis, who is the first author of the largest trial of calcium channel blockers, upon which many of the favourable calcium channel blocker meta analyses depend.
I recognise that it is probably impossible to always avoid using trial authors to write systematic reviews, and that Dr Papatsonis acknowledges his possible conflict of interest. Nor do I accuse him, or any of the review authors, of any intentional bias. Nevertheless, I am concerned about possible unintentional bias against commercially developed pharmacological agents. This risks harming the future development of drugs for use in pregnancy, something which I am sure everyone would support.
Conflict of Interest
I have acted as advisor to Ferring and when I was editor of BJOG the journal received sponsorship from Ferring to publish supplements.
Jim Thornton, July 2006
Reply
On behalf of the review authors, we respond to Professor Thornton's comments about the review of calcium channel blockers (CCB) [King 2003] and the review of oxytocin receptor antagonists (ORA) [New Reference] for preventing preterm birth.
Judgement of trial quality
For the ORA review, blinding of the intervention is not synonymous with blinding of outcome assessment. Unless authors stated so in their original reports, or in response to further queries, we cannot presume that those assessing the outcome of interest were blinded to the allocated intervention. For example, in trials comparing betamimetics with atosiban, blinding of the intervention is difficult due to the maternal and fetal side effects of betamimetics, particularly tachycardia and maternal palpitations. Therefore, until further information is received from the trial authors, blinding of assessment of outcome is classified as "unknown" for these four trials (European 2001; French/Australian 2001; Moutquin 2000; and Romero 2000). We agree Goodwin 1994 should also be classified as "unknown", and this is now corrected.
We disagree that assessment of trial quality was subjective. The statements "reasonable quality" used in the calcium channel blocker (CCB) review and "not high quality" in the ORA review are intended to imply that the trials were neither poor quality nor high quality. Studies were judged to be of poor quality if no adequate method of allocation concealment was described, as this is one of the most important quality indicators regardless of whether the intervention was blinded. In accordance with Cochrane methodology, small numbers and lack of sample size calculations were not considered indicators of trial quality.
Choice of outcomes to report in the abstract
For the CCB review, we believe we have adequately acknowledged the potential for bias in the ascertainment of neonatal outcomes. We also note that the results were consistent across the included trials, but acknowledge that this does not rule out bias. A statement regarding trial quality will be included in the abstract for the next update of the CCB review
The outcome measures in the abstract of the CCB review were considered to be clinically important outcomes for this review. We will include the outcome of perinatal mortality in the abstract for the next update of the review.
In the ORA review abstract, the potential for bias due to the gestational age imbalance at trial entry in the Romero trial is acknowledged. We have made it clearer how this relates to the other data presented by stating at the start of this paragraph the number of trials and women in this comparison. We prespecified birthweight as a clinically important outcome measure for the review, and considered it reasonable to include the finding of birthweight <1500gms in the abstract. In the abstract results, the ordering of text on maternal drug reaction for the comparison of ORA with betamimetics provides consistency with the reporting of the outcomes for the comparison of atosiban versus placebo.
Choice of language
We appreciate that the upper confidence interval for a number of the statistically significant outcomes reported approached 1, no difference. However, based on the point estimates of the effects and the consistency in the findings across these outcomes, we believe that the conclusions of the CCB review and wording of the abstract accurately reflects the findings. The statistical heterogeneity found for the outcome of pregnancy prolongation we believe was appropriately managed in this review with the use of a random‐effects model for the meta‐analysis of this outcome.
While we feel the language used in the ORA review abstract accurately reflects the results, we will rephrase to take account of the perception we may have been too negative about atosiban.
Choice of outcomes to report
We accept the outcomes for the ORA and CCB reviews differ, as they do in other tocolysis reviews, and that this is not helpful for readers of the review. As there is overlap between the review teams for these two reviews, we will rectify this during the next update of these reviews.
Regarding the use of 'predefined' outcomes, this term relates to outcomes chosen by the reviewers as clinically meaningful and defined in the review protocol before the review begins. These outcomes may or may not match those reported for individual trials. If reported outcomes did not match those pre‐specified for the review, wherever possible, additional information was sought from authors. For the placebo controlled trials in the ORA review, data on pregnancy prolongation was not provided in a format to enable inclusion in the review; while additional data were sought from the authors, these were not forthcoming. The outcome of "therapeutic failure" was reported in the individual trials, but was not chosen as an outcome for either the ORA or CCB reviews as it was considered susceptible to bias.
Authorship of the reviews
Whilst it is appropriate and common practice for experts to undertake systematic reviews within their area of expertise, we agree that this carries with it the potential for bias. For Cochrane reviews, however (including the ORA and CCB reviews), a number of steps are in place to ensure that this risk is minimised. These steps include: transparency of the review process through publication of the protocol for the review prior to commencement, rigorous peer review (including an external referee) of the protocol and the review, multiple review authors aiming for a mix of expertise and experience, and a feedback system which allows anyone to comment on reviews and protocols. In addition, the regular updating of reviews means that any errors or misperceptions can be corrected. We think it unlikely therefore that any harm will come to future development of drugs for use in pregnancy due to bias, whether intentional or not, in our review.
(Summary of response, October 2007: Vicki Flenady, Dimitri Papatsonis, James King and Helen Liley on behalf of the authors for the ORA and CCB reviews.)
Contributors
Feedback: Jim Thornton Response: Vicki Flenady, Dimitri Papatsonis, James King and Helen Liley on behalf of the authors for the ORA and CCB reviews
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2013 | New citation required but conclusions have not changed | Review updated. |
| 1 December 2013 | New search has been performed | Search updated in December 2013. This updated review includes eight additional trials involving 790 women, giving a total of 14 trials involving 2485 women included in the review. We revised primary and secondary outcomes ‐ see Differences between protocol and review for details. We also aligned comparisons between oxytocin receptor antagonists and other tocolytic drugs with the Cochrane Pregnancy and Childcare Group consensus statement on tocolysis for inhibiting preterm labour. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 17 September 2009 | Amended | Search updated. Twenty‐one new reports added to 'Studies awaiting classification'. |
| 24 April 2008 | Amended | Converted to new review format. |
| 31 July 2006 | Feedback has been incorporated | Feedback from Steve Thornton, Khalid S Khan, and Patrick FW Chien added, with a reply from Dimitri Papatsonis on behalf of the review authors. Feedback from Jim Thornton added, with a reply from Vicki Flenady, Dimitri Papatsonis, James King and Helen Liley on behalf of the authors for the ORA and CCB reviews. |
| 1 December 2005 | Feedback has been incorporated | Feedback from Mats Åkerlund, Karel Marsl and Ingemar Ingemarsson added, with a reply from Dimitri Papatsonis on behalf of the review authors. |
| 1 September 2005 | Feedback has been incorporated | Feedback from Murphy Goodwin added, with a reply from Dimitri Papatsonis. |
Acknowledgements
We would like to acknowledge Drs K Marsal, and TM Goodwin for providing additional data for this review. We thank James King for assistance with the development of the protocol and Katie Welsh, Viviana Rodriguez and Aleena Wojcieszek for assistance with reference management.
We also acknowledge the guidance and support of Philippa Middleton in completion of this review, Sonja Henderson for support and advice regarding the Cochrane Pregnancy and Childbirth Group methods and procedures, and Lynn Hampson for her assistance with searching for potentially eligible trials.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Methods used to assess trials in the earlier version of this review
We used the standard methods of The Cochrane Collaboration as described in the Cochrane Reviewers' Handbook (Alderson 2004a). Two review authors (Vicki Flenady, Dimitri Papatsonis) considered trials for inclusion, evaluated methodological quality and extracted trial data independently. We resolved differences in interpretation by discussion. Where necessary, we contacted investigators of identified trials for additional information or data. We contacted the authors of seven trials for additional outcome data (Al‐Omari 2004; Anonymous 2004; European 2001; French/Australian 2001; Goodwin 1994; Renzo 2003; Romero 2000), and at the time of this review we received additional data for two trials (European 2001; Goodwin 1994). When there was consensus about the additional data received from the original authors, we included these data in the analysis; when there was no consensus among the review authors or the data were incomplete, we asked the original authors again for additional data or comments.
Quality assessment
We conducted quality assessment according to the methods described in Section six of the Cochrane Reviewers' Handbook (Alderson 2004b). We considered four major sources of potential bias and methods of avoidance of these biases when assessing trial quality: (1) selection bias ‐ blinding of randomisation; (2) performance bias ‐ blinding of intervention; (3) attrition bias ‐ complete follow up; (4) detection bias ‐ blinding of outcome assessment. We assigned a quality rating to each trial for the criterion of blinding of randomisation as follows: (A) adequate, (B) unclear, (C) inadequate, or (D) not used. We assigned a quality rating of (A) yes, (B) cannot tell, or (C) no, to the other quality components (blinding of intervention, completeness of follow up and blinding of outcome assessment). High‐quality trials were defined as those receiving an A rating for blinding of randomisation (central computerised randomisation service or sealed opaque envelopes) and for blinding of the intervention (use of a placebo). The quality assessment rating included in the table of 'Characteristics of Included Studies' refers to blinding of randomisation in the studies.
Data collection and analysis
We conducted data management and analysis using the Review Manager software (RevMan 2003) (method of data extraction is described above). For individual trials mean differences (and 95% confidence intervals), where possible, were reported for continuous variables. For continuous variables we have, where possible, reported mean differences (and 95% confidence intervals) for individual trials. For categorical outcomes, we reported the relative risk and risk difference (and 95% confidence intervals).
One trial (Goodwin 1996b) randomised women to one of five groups: four atosiban groups of different dosing regimens and one ritodrine group. For the meta‐analysis we combined the four atosiban treatment arms.
Where more than 20% of outcomes for participants were missing, data were not included in the review. This applied to the only trial which reported longer term infant outcomes (Romero 2000), where data on neurodevelopmental outcome at one and two years were excluded due to a 35% and 45% loss to follow‐up respectively. However, data on infant death (to 12 months of age) reported in this trial were included in the review, as the follow‐up appeared to be complete.
We conducted meta‐analysis using the fixed‐effect model. We assessed heterogeneity by visual inspection of the outcomes tables and by using two statistics (H and I² test) of heterogeneity (Higgins 2002).
Using a fixed‐effect model, statistical heterogeneity was evident for three non‐statistically significant outcomes in the comparison of atosiban versus betamimetics. These were birthweight, respiratory distress syndrome and admission to neonatal intensive care unit. Use of a random‐effects model for these outcomes did not alter our interpretation of the results. On visual inspection of the graphs and through sensitivity analyses, we identified one trial (Moutquin 2000) as an outlier for all of these outcomes. A possible explanation could be, as the authors of the trial stated themselves, that more women with a multiple pregnancy were randomised to the atosiban group. Although, there was no difference in the mean gestational age at entry into the trial or the mean gestational age at delivery between the two groups in this trial or when compared to the other trials, multiple gestation could have independently affected these outcomes. It is unclear why there was an imbalance in randomisation for multiple gestations in this study.
Due to insufficient data, planned subgroup analyses by population characteristics, tocolytic regimens that include use of maintenance therapy, and oxytocin receptor antagonist compared with calcium channel blockers were not undertaken.
Data and analyses
Comparison 1. Oxytocin receptor antagonists versus placebo (by type of ORA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth less than 48 hours after trial entry | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.43] |
| 1.1 Atosiban versus placebo | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.43] |
| 2 Perinatal mortality (stillbirth and neonatal death up to 28 days) | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.79, 6.38] |
| 2.1 Atosiban versus placebo | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.79, 6.38] |
| 2.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Stillbirth | 4 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.04, 4.47] |
| 3.1 Atosiban versus placebo | 3 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.04, 4.47] |
| 3.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal death | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [0.88, 19.07] |
| 4.1 Atosiban versus placebo | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [0.88, 19.07] |
| 4.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Infant death (up to 12 months) | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.13 [1.38, 27.13] |
| 5.1 Atosiban versus placebo | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.13 [1.38, 27.13] |
| 6 Maternal death | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Atosiban versus placebo | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal adverse effects | 2 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.02, 2.32] |
| 7.1 Atosiban versus placebo | 2 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.02, 2.32] |
| 8 Maternal adverse effects requiring cessation of treatment | 3 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [2.05, 7.85] |
| 8.1 Atosiban versus placebo | 2 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [2.05, 7.85] |
| 8.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Caesarean section | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.73, 3.61] |
| 9.1 Atosiban versus placebo | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.73, 3.61] |
| 10 Preterm birth (before completion of 37 weeks of gestation) | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.32] |
| 10.1 Atosiban versus placebo | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.99, 1.37] |
| 10.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.59, 1.51] |
| 11 Extremely preterm birth (before completion of 28 weeks of gestation) | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.02, 9.51] |
| 11.1 Atosiban versus placebo | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.02, 9.51] |
| 12 Gestational age (weeks) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.57, 0.57] |
| 12.1 Atosiban versus placebo | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.57, 0.57] |
| 13 Birthweight (grams) | 2 | 676 | Mean Difference (IV, Fixed, 95% CI) | ‐138.86 [‐250.53, ‐27.18] |
| 13.1 Atosiban versus placebo | 2 | 676 | Mean Difference (IV, Fixed, 95% CI) | ‐138.86 [‐250.53, ‐27.18] |
| 14 Respiratory distress syndrome | 3 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.82] |
| 14.1 Atosiban versus placebo | 2 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.93, 1.77] |
| 14.2 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.36, 20.06] |
| 15 Intraventricular haemorrhage | 1 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.62] |
| 15.1 Atosiban versus placebo | 1 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.62] |
| 16 Necrotising enterocolitis | 1 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.76] |
| 16.1 Atosiban versus placebo | 1 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.76] |
| 17 Neonatal jaundice | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.86, 1.99] |
| 17.1 Barusiban versus placebo | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.86, 1.99] |
| 18 Admission to neonatal intensive care unit | 1 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 18.1 Atosiban versus placebo | 1 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
Comparison 2. Oxytocin receptor antagonists versus other classes of tocolytic agents (by type of other tocolytic).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth less than 48 hours after trial entry | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ORA versus betamimetics | 8 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.22] |
| 1.2 ORA versus calcium channel blockers | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.44, 2.73] |
| 2 Perinatal mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 ORA versus betamimetics | 3 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.48] |
| 3 Very preterm birth (before completion of 34 weeks of gestation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ORA versus calcium channel blockers | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.89, 3.23] |
| 4 Stillbirth | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 ORA versus betamimetics | 4 | 861 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 6.05] |
| 5 Neonatal death | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 ORA versus betamimetics | 5 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.61] |
| 5.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 ORA versus betamimetics | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal adverse effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 ORA versus betamimetics | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.35, 0.62] |
| 7.2 ORA versus calcium channel blockers | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.68] |
| 8 Maternal adverse effects requiring cessation of treatment | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 ORA versus betamimetics | 5 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.02, 0.11] |
| 8.2 ORA versus calcium channel blockers | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.62] |
| 9 Caesarean section | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 ORA versus betamimetics | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.52] |
| 9.2 ORA versus calcium channel blockers | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.37, 4.79] |
| 10 Interval between trial entry and birth (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 ORA versus betamimetics | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 22.9 [18.03, 27.77] |
| 10.2 ORA versus calcium channel blockers | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐12.36, 0.96] |
| 11 Preterm birth (before completion of 37 weeks of gestation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 ORA versus calcium channel blockers | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.13, 2.14] |
| 12 Extremely preterm birth (before completion of 28 weeks of gestation) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 ORA versus betamimetics | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.92] |
| 12.2 ORA versus calcium channel blockers | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.20, 23.11] |
| 13 Gestational age (weeks) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 ORA versus betamimetics | 6 | 1005 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.32, 0.59] |
| 13.2 ORA versus calcium channel blockers | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.15, ‐0.25] |
| 14 Birthweight (grams) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 ORA versus betamimetics | 7 | 1184 | Mean Difference (IV, Fixed, 95% CI) | 27.16 [‐55.46, 109.77] |
| 14.2 ORA versus calcium channel blockers | 1 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐82.0 [‐270.78, 106.78] |
| 15 Apgar score less than 7 at 5 minutes | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 ORA versus betamimetics | 5 | 1008 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.33] |
| 15.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 5.86] |
| 16 Respiratory distress syndrome | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 ORA versus betamimetics | 6 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.65] |
| 16.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.54, 3.57] |
| 17 Use of mechanical ventilation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 ORA versus betamimetics | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.63, 14.30] |
| 17.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.65, 3.04] |
| 18 Duration of mechanical ventilation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 ORA versus betamimetics | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.82, 1.22] |
| 19 Intraventricular haemorrhage | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 ORA versus betamimetics | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.48, 2.58] |
| 19.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.41, 11.51] |
| 20 Necrotising enterocolitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 ORA versus betamimetics | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 3.74] |
| 20.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.72 [0.53, 178.00] |
| 21 Retinopathy of prematurity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.20, 23.43] |
| 22 Neonatal sepsis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 ORA versus betamimetics | 4 | 1109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.56, 1.46] |
| 22.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.12, 4.21] |
| 23 Admission to neonatal intensive care unit | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 ORA versus betamimetics | 5 | 1062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 23.2 ORA versus calcium channel blockers | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.17, 2.47] |
| 24 Neonatal length of hospital stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 ORA versus betamimetics | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐5.13, 5.33] |
| 24.2 ORA versus calcium channel blockers | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 5.4 [‐0.04, 10.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cabar 2008.
| Methods | Randomised trial. | |
| Participants | 80 women with singleton pregnancy at 23 to 33 completed weeks with intact membranes, uterine contractions every 5 min, cervical dilation 1‐3 cm, cervical effacement greater than 50%, amniotic fluid index: 5‐25. Exclusion criteria: maternal diseases (pre‐eclampsia, chronic hypertension, diabetes mellitus, congenital heart defects, hyperthyroidism, asthma, collagenosis, antiphospholipid syndrome, anaemia, allo immunity), fetal or placental diseases, fetal growth restriction (< 10th percentile), cervical incompetence, chorioamnionitis or temperature > 38ºC. |
|
| Interventions | ORA group: atosiban. Initial bolus dose of 6.75 mg (i.v.) followed by 300 µg/min for 3.5 h and 100 µg/min for 3.5 h. Thereafter, as required, 100 µg/min for 12.5 h repeated for up to 45 h. Control group: terbutaline. Administered as i.v. infusion of 2.5 mg in 500 mL 5% glucose at a rate of 20 mL/h. If uterine activity persisted, infusion rate was increased by 20 mL/h until contractions ceased and the dose was maintained for 24 h. |
|
| Outcomes | Evaluating the safety and maternal and fetal side effects of atosiban. | |
| Notes | Publication has been translated to English by the Cochrane Pregnancy and Childbirth Group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to determine how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not able to determine. |
European 2001.
| Methods | Multicentre, randomised, placebo‐controlled trial. | |
| Participants | 249 women with singleton pregnancies in preterm labour defined as regular uterine contractions of > 30 sec, duration ≤ 4/30 min. Cervical effacement > 50% and dilatation 0‐3 cm (nullipara), 1‐3 cm (primi‐ multiparas) and at 23‐33 completed weeks' gestation. Exclusion criteria: high‐order multiple pregnancy (≥ triplets), ruptured membranes, severe pre‐eclampsia/hypertension, use of NSAID therapy ≤ 12 h prior to randomisation, temperature > 37.5ºC (UK and Czech Republic) or 38ºC (Sweden and Denmark), alcohol or drug abuse, hypersensitivity to any components of study drugs, contraindications to the use of terbutaline, participation in clinical trial < 1 month, fetal/placental abnormalities (e.g. chorioamnionitis, abruptio placenta, placenta praevia, intrauterine growth retardation, fetal distress/death, major congenital anomaly, hydramnios, retained intrauterine device), serious maternal disease (e.g. cardiovascular disease, hyperthyroidism, uncontrolled diabetes mellitus, pheochromocytoma, asthma), study standard maternal or fetal contraindications to tocolysis. |
|
| Interventions | ORA group: atosiban. Bolus dose of 6.75 mg (i.v.) followed by infusion of 300 µg/min for 3 h, then 100 µg/min up to 18 h. Separate but simultaneous i.v. infusion of 5% dextrose given as placebo. Control group: terbutaline. Given in 5% dextrose at 10‐25 µg/min (i.v.). Simultaneous bolus dose (0.9 mL NaCl) injection followed by infusion of 5% dextrose (i.v.) were given as placebo. Both infusions were given up to 18 h. |
|
| Outcomes | Primary goals were the safety and effectiveness of atosiban versus terbutaline as tocolytic agents, assessed as women undelivered after 48 h and 7 days and maternal side effects. Secondary outcomes: contraction rate with time, gestational age at delivery, proportion of women re‐treated with study medication, proportion of infants born at differing gestational age and number of infants requiring neonatal intensive care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation lists for each center was used to allocate women to study treatment and women were stratified by gestational age at enrolment." The review authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | Prepared by Ferring Pharmaceuticals: computer‐generated randomisation stratified by GA and centre, supplied to pharmacists at each centre in pre‐randomised boxes labelled with country code and case number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Through the use of a double‐blind, double‐dummy technique, the utmost effort was made to keep the study blinded. […] the somewhat obvious side effects profile of terbutaline may have compromised the blinding." The review authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probably low risk as double‐placebo technique was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the atosiban group and 4 women in the terbutaline group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
French/Australian 2001.
| Methods | Multicentre, randomised, placebo‐controlled trial. | |
| Participants | 241 women with preterm labour, defined as duration of contractions ≥ 30 sec at a rate of ≥ 4/30 min, cervical dilation 0‐3 cm (nullipara) or 1‐3 cm (primi‐ or multipara) and effacement ≥ 50%, at 23‐33 completed weeks' gestation and ≥ 18 years of age. Exclusion criteria: multiple pregnancy (> twins), ruptured membranes, major vaginal bleeding, use of NSAID's within 12 h (Australia), ß‐agonists within 30 min and NSAID's or calcium channel blockers within 24 h (France), severe pre‐eclampsia or hypertension, temperature > 37.5ºC, urinary tract infection, fetal/placental abnormalities (e.g. chorioamnionitis, abruptio placenta, placenta praevia, intrauterine growth retardation, fetal distress/death, major congenital anomaly, hydramnios, retained intrauterine device), serious maternal disease (e.g. cardiovascular disease, hyperthyroidism, uncontrolled diabetes mellitus, pheochromocytoma, asthma), contraindication to salbutamol, alcohol or drug abuse, hypersensitivity to any components of the study drugs, participation in a clinical trial < 1 month, significant renal impairment (Australia). |
|
| Interventions | ORA group: atosiban. Bolus dose 6.75 mg (i.v.) followed by infusion at 300 µg/min for 3 h then 100 µg/min up to 48 h. A placebo i.v. infusion of 5% dextrose was given separately but simultaneously. Control group: salbutamol. Bolus injection of 0.9 mL NaCl followed by i.v. infusion of 5% dextrose. Separately but simultaneously salbutamol was given as an i.v. infusion in 5% dextrose at 5‐25 µg/min (France) or 2.5‐45 µg/min (Australia). Both infusions were continued for 48 h. |
|
| Outcomes | Primary goals were the safety and effectiveness of atosiban versus terbutaline as tocolytic agents, assessed as women undelivered after 48 h and 7 days and maternal side effects. Secondary outcomes: contraction rate with time, gestational age at delivery, proportion of women re‐treated with study medication, proportion of infants born at differing gestational age and number of infants requiring neonatal intensive care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation lists for each center was used to allocate women to study treatment and women were stratified by gestational age at enrolment." The review authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | Prepared by Ferring Pharmaceuticals: computer‐generated randomisation stratified by GA and centre, supplied to pharmacists at each centre in pre‐randomised boxes labelled with country code and case number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Through the use of a double‐blind, double‐dummy technique, the utmost effort was made to keep the study blinded. […] the somewhat obvious side effects profile of terbutaline may have compromised the blinding." The review authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probably low risk as double‐placebo technique was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the salbutamol group was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Goodwin 1994.
| Methods | Multicentre, randomised, placebo‐controlled trial. | |
| Participants | 120 women with preterm labour defined as number of contractions: ≥ 6/h (4 centres) and > 4/last 30 min (1 centre), cervical dilatation and effacement: < 2 cm/50% (1 centre), ≤ 3 cm/50% (1 centre), ≤ 3 cm (1 centre), < 2 cm/80% (1 centre), unspecified (1 centre) at gestational age: 20‐35 (1 centre), 20‐34 (1 centre), 25‐35 (1 centre), < 35 (1 centre) and 28‐37 (1 centre) weeks with no change in cervix during ≥ 1 h. Exclusion criteria: prior study participation, ruptured membranes, amnionitis, contraindications to tocolysis, abruptio placenta, fetal distress, lethal fetal anomaly, fetal death. |
|
| Interventions | ORA group: atosiban. Infusion rate 300 µg/min for 2 h (i.v.). Control group: placebo. Infusion for 2 h (i.v.). |
|
| Outcomes | Percentage change in contractions per h. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation schedule with a block size of four." The review authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | "the pharmacist would open the sequentially numbered, opaque, sealed envelope." The review authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The personnel preparing the drug was not involved in patient care, and the treatment allocation was not revealed to personnel involved with patient care. The study was placebo‐controlled. The review authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Intervention was blinded and risk of outcome assessment bias can be considered low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women in each treatment group were excluded from analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | High risk | The average gestational age in the atosiban group was lower than that of the placebo. The authors consider this to be high risk of introducing bias. Centres had different inclusion criteria which may introduce bias. |
Goodwin 1996.
| Methods | Multicentre, randomised trial. | |
| Participants | 302 women with preterm labour (1‐3 cm dilatation and 75% effacement or 3 cm dilatation and 50% effacement at contraction rate 4/30 min, with progressive cervical change, defined as 1 cm change or > 50% change in effacement) at 20‐35 weeks' gestation. Exclusion criteria: PROM , prior enrolment in the study, cervix dilation > 3 cm, multiple gestation, blood pressure of 150/100 mmHg or pre‐eclampsia, > 1 prior preterm labour, temperature > 100ºF, urinary tract infection, trauma, fetal anomaly, retained intrauterine device, hydramniosis, alcohol or drug abuse, prior tocolytic therapy within 72 h, serious maternal disease, contraindication to tocolysis. |
|
| Interventions | ORA group: atosiban. 4 dosing regimens; I. 6.5 mg bolus dose of atosiban followed by i.v. infusion of atosiban at 300 µg/min; II. Placebo bolus dose followed by i.v. infusion of atosiban at 300 µg/min; III. 2 mg bolus dose of atosiban followed by i.v. infusion of atosiban at 100 µg/min; IV. 0.6 mg bolus dose of atosiban followed by i.v. infusion of atosiban at 30 µg/min. Treatment was continued for 6 h after last contraction up to 12 h. Control group: ritodrine. Infusion (i.v.) started at 0.1 mg/min and increased up to 0.35 mg/min or until cessation of contractions. |
|
| Outcomes | To establish the minimal effective dose regimen for atosiban in the initial treatment of preterm labour and to evaluate the effect of a bolus atosiban. | |
| Notes | Treatment was discontinued if: cervical dilation ≥ 1 cm, uterine contractions > 6 h, or severe adverse side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned to one of five arms according to a computer‐generated randomisation schedule […]. The randomisation was stratified by institution." The review authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | "assignments were maintained in sealed, opaque envelopes in the pharmacy at each site." The review authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "double‐blind (between atosiban arms)." Participants and personnel were not blinded to type of treatment, only dosage of atosiban. The review authors consider this approach high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The non‐blinded approach of treatment allocation suggests non‐blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post‐randomisation exclusions or losses at follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Unclear risk | The distribution of women to the different treatments was unequal in regards to gestational age; fewer women at 25‐29 weeks' gestation were enrolled in the ritodrine arm which may introduce bias. Atosiban therapy was limited to 12 h whereas ritodrine was unlimited which may introduce bias. |
Kashanian 2005.
| Methods | Randomised controlled trial. | |
| Participants | 80 women with preterm labour, defined by contraction frequency 4/20 min or 8/60 min and cervical dilation ≥ 1 cm and cervical effacement ≥ 50% at 26 to 34 weeks' gestation (confirmed by definite last menstrual period and first trimester sonography). Multiple pregnancy included. Exclusion criteria: PROM, vaginal bleeding, fetal death or fetal distress, IUGR, history of trauma, cervical dilatation > 3 cm, maternal systemic disorders, known uterine anomaly (by history or sonography), blood pressure < 90/50 mmHg. |
|
| Interventions | ORA group: atosiban. Administered at 300 µg/min (i.v.) up to 12 h, or up to 6 h after the contractions ceased. Control group: nifedipine. Initially 10 mg sublingually every 20 min for 4 doses. If contractions inhibited, nifedipine continued orally 20 mg every 6 h for 24 h, and then every 8 h for 24 h. |
|
| Outcomes | Maternal: delivery within 7 days; delivery within 48 h; maternal adverse drug reactions. No neonatal outcomes. |
|
| Notes | Drug side effect distributive ‐ some women experienced 2‐3 side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "4‐part, ABCD, block‐random allocation was used." The review authors consider this approach low risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | " because the two drugs are completely different in shape and form a blind study was not an option." Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All vaginal examinations and drug administration were performed by the same investigator." Because of the unblinded nature of this study, it is likely that outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post‐randomisation exclusions or losses at follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Minimal neonatal outcome data reported. |
| Other bias | Unclear risk | Not able to determine. |
Lin 2009.
| Methods | Randomised, unblinded, controlled trial. | |
| Participants | 45 women with spontaneous preterm labour (duration of contractions 30 sec or more at a rate of ≥ 4/min, cervical dilation of 0‐3 cm and cervical effacement of ≥ 50%) at 24‐33 weeks' gestation. Exclusion criteria: multiple pregnancy (> twins), ruptured membranes, major vaginal bleeding (continuous vaginal bleeding or bleeding volume > 100 mL); pre‐eclampsia or hypertension (blood pressure > 140/90 mmHg), temperature > 37.5°C, urinary tract infection, fetal placental/amniotic abnormalities (e.g. major fetal anomalies, chorioamnionitis, polyhydramnios, fetal growth restriction or placenta praevia), serious maternal disease (e.g. cardiovascular disease, hyperthyroidism, diabetes, pheochromocytoma or asthma), contraindications to the use of betamimetics, alcohol or drug abuse, exposure to NSAID tocolytic drugs < 12 hr of study entry, hypersensitivity to any component of the study drugs and participation in an clinical trial < 1 month. |
|
| Interventions | ORA group: atosiban. A bolus dose (6.75 mg in 0.9 mL saline, i.v.) followed by infusion at 18 mg/h (300 µg/min) for 3 h and then 6 mg/h (100 µg/min) for 15 h. Control group: ritodrine. Initial infusion of 20 mL/h (66.6 µg/min, i.v.) and increased by 10 mL/h (33.3 µg/min) every 10‐30 min, until the desirable uterine response (uterine quiescence < 4 contractions/h) was obtained. Both atosiban and ritodrine was administered for maximum 18 h during first treatment. |
|
| Outcomes | Compare the tocolytic efficacy and safety profile of atosiban and ritodrine in an Asian population. Monitoring of adverse events, particularly tachycardia. Primary endpoint: to compare the proportion of women who did not deliver and did not receive an alternative tocolytic therapy after 7 days of treatment. Secondary endpoints: proportion of women who did not deliver after 2 days of treatment and did not require an alternative tocolytic. Other parameter measured: frequency of uterine contractions, gestational age at delivery, birthweight, maternal and fetal deaths and early drug discontinuation with/without alternative tocolytic treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women were computer‐randomized into two groups." Unable to determine how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Moutquin 2000.
| Methods | Multicentre, randomised, placebo‐controlled trial. | |
| Participants | 252 women with preterm labour defined as contractions ≥ 30 sec in duration and ≥ 4/30 min, cervical dilation 0‐3 cm (nullipara) or 1‐3 cm (primi‐ or multipara) and effacement ≥ 50% at 23 and 33 completed weeks' gestation. Exclusion criteria: multiple pregnancy (≥ triplets), ruptured membranes, major vaginal bleeding, use of NSAID's within 12 h prior to study, severe pre‐eclampsia or hypertension, temperature > 37.5ºC, urinary tract infection, fetal or placental abnormalities (e.g. chorioamniotic, abruptio placenta, placenta praevia, IUGR, fetal distress/death, major congenital anomaly, hydramnios, incompetent cervix), serious maternal disease (e.g. cardiovascular disease, hyperthyroidism, diabetes mellitus, pheochromocytoma, asthma), contraindications to tocolysis, alcohol or drug abuse, hypersensitivity to components of study drugs, previous study participation < 1 month. |
|
| Interventions | ORA group: atosiban. Bolus dose 6.75 mg (i.v.) followed by infusion of 300 µg/min for 3 h, then 100 µg/min up to 18 h. A placebo infusion of 5% dextrose (i.v.) was given separately but simultaneously. Control group: ritodrine. Bolus placebo dose was administered followed by an i.v. 5% dextrose infusion. Separately but simultaneously ritodrine was given as an i.v. infusion in 5% dextrose at 0.10‐0.35 mg/min, by increments every 10 minutes as required until contractions ceased. Both infusions were continued for 18 h. |
|
| Outcomes | Primary outcomes: effectiveness and safety of atosiban versus ritodrine, assessed as women who remained undelivered at 48 h and 7 days and maternal side effects and neonatal morbidity. Secondary outcomes: change in uterine contraction rate over time, gestational age at delivery and number of infants requiring neonatal intensive care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated block randomisation […] stratified by gestational age." The authors consider this approach low risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was placebo controlled. The authors consider this technique to be low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is likely that the placebo‐controlled approach is low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were included in analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Nonnenmacher 2009.
| Methods | Prospective, randomised, controlled trial. | |
| Participants | 105 women > 18 years at 24‐33 completed weeks' gestation, ≥ 4 contractions every 30 min lasting ≥ 30 seconds, opening of the uterine orifice and/or an ultrasound‐verified residual cervix < 25 mm for single pregnancies and < 20 mm for multiple pregnancies. Exclusion criteria: heavy vaginal bleeding, severe pre‐eclampsia, extreme high blood pressure, temperature > 37.5ºC, fetal or placental abnormalities (e.g. chorioamnionitis, premature separation of the placenta, delayed intrauterine growth, fetus in a stressed state, intrauterine fetal death, severe dysplasia, severe maternal health problems, e.g. heart or circulatory disease, hyperthyroidism, alcohol or drug abuse and hypersensitivity to any component of the study drug. |
|
| Interventions | ORA group: atosiban. Bolus injection of 6.75 mg, then 18 mg/h for 3 h (i.v.) and thereafter 6 mg/h up to 45 h. Control group: fenoterol. 2 x 0.5 mg ampoules in 50 mL NaCl solution by pulsatile administration with an allowance of 1000 IU heparin. Initial dose 1.5 µg/min or 2.0 µg/min; if no improvement was seen after 30 min, the dose was incrementally increased to 3.5 µg/min. At cessation of labour the dose was reduced within 3‐6 h to 1 µg/min and 0.5 µg/min. |
|
| Outcomes | Percentage of women who experienced a prolongation of pregnancy. Primary endpoints: proportion of women who experienced a prolongation of pregnancy of 48 h and 7 days. Secondary endpoints: time to reduction of contractions to ≤ 1/30min, maternal and fetal side effects and neonatal outcomes. |
|
| Notes | Publication has been translated to English by the Cochrane Pregnancy and Childbirth Group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to determine how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Richter 2005.
| Methods | Prospective, randomised trial. | |
| Participants | 40 women with preterm labour (contraction duration > 30 sec, rate > 4/30 min, cervical effacement > 50%, cervical dilation of 0‐3 cm (nullipara) or 1‐3 cm (primi‐ and multipara) at 18‐24 weeks' gestation. Exclusion criteria: serious maternal disease, temperatures > 37.5ºC, PROM with anhydraemia, major vaginal bleeding, major congenital syndrome, growth restriction, chorioamnionitis, polyhydramnios, intrauterine fetal death, multiple pregnancy (≥ triplets), alcohol and drug abuse, hypersensitivity to atosiban and its components, previous study participation within the last 6 months. |
|
| Interventions | ORA group: atosiban. Administered in accordance with standard protocol: bolus injection (i.v., 6.75 mg in 0.9 mL NaCl), followed by 300 µg/min in 0.9% NaCl 3 h. Thereafter 100 µg/min (i.v.) for 45 h. Control group: saline solution. Administered as continuous infusion (i.v.). Both groups: antibiotic treatment was administered if vaginal infection was evident. If needed, vaginal pH was corrected. If inhibition of uterine contractions was achieved but with persistent cervical dilatation, women were offered cerclage and/or total occlusion of the cervix. |
|
| Outcomes | To assess the effectiveness and safety of atosiban. Effectiveness was assessed by: 1. Measuring the time to the onset of the tocolytic effect; 2. The interval until uterine contractions completely ceased; 3. The duration of complete absence of uterine contractions; and 4. The average prolongation of pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A prospective, randomised design was chosen." Unable to determine how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different administration regimens were sued for the atosiban and control groups: "In the treatment group atosiban was administered as follows in accordance with the standard protocol: initial i.v. bolus injection (approximately 1 min, 6.75 mg of atosiban in 0.9 mL of sodium chloride), followed immediately by high‐dosage saturation infusion with atosiban in 0.9% sodium chloride for 3 h (300 µg/min) followed by a low‐dosage continuous infusion with atosiban in 0.9% sodium chloride for up to 45 h (100 µg/min). In the control group saline solution was continuously given via i.v. infusion." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Romero 2000.
| Methods | Randomised, placebo‐controlled trial. | |
| Participants | 531 women with preterm labour assessed by ≥ 4 contractions over 30 min of ≥ 40 sec and cervical criteria: dilation 1‐3 cm/effacement > 50% or 1 cm increase in dilation or 50% increase in effacement. Exclusion criteria: fetal or placental abnormalities, fetal distress, chorioamnionitis, maternal indications for delivery, urinary tract infection, substance abuse. |
|
| Interventions | ORA group: atosiban. Bolus dose of 6.75 mg followed by an infusion of 300 µg/min for 3 h, then 100 µg/min up to 45 h. Control group: bolus dose of placebo followed by an infusion of placebo up to 48 h. Maintenance therapy with subcutaneous placebo or atosiban until 36 weeks. |
|
| Outcomes | Primary end‐point: time to delivery or therapeutic failure (progression of labour necessitating an alternate tocolytic agent). Secondary end‐points: proportion of women successfully treated up to 24 h, 48 h, and 7 days, and maternal‐fetal side effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation schedule according to permuted blocks of 6." The authors consider the approach low risk. |
| Allocation concealment (selection bias) | Low risk | "Prenumbered randomisation envelopes provided to the pharmacist at each study site center were to be opened in sequential order." The authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators, study personnel and monitors remained blinded throughout the study." The trial was also placebo controlled. The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators, study personnel and monitors remained blinded throughout the study." The authors consider this approach low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 women in each treatment group were excluded post‐randomisation. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | High risk | "The mean gestational age on admission was statistically significantly greater for the placebo group than for the atosiban group, and there were nearly twice as many atosiban‐treated patients enrolled at <26 weeks’ gestation. Among patients enrolled at <26 weeks, the percentage who had advanced cervical status (modified Bishop score ≥4) was greater for those allocated to the atosiban group." The authors consider these factors to be high risk. |
Salim 2012.
| Methods | Randomised controlled trial between January 2008 and December 2011. | |
| Participants | 149 women in preterm labour with intact membranes diagnosed between 24 to 33+6 weeks' gestation were included in the study. Preterm labour was identified as > 4 contractions lasting > 30 seconds within 30 min and confirmed by external topography, 50% cervical effacement and < 4 cm dilation (nullipara) or 1‐4 cm dilation (multipara). Singletons and twins included. Exclusion criteria: rupture of membranes, vaginal bleeding resulting from placenta praevia or placental abruption, temperature > 38ºC, severe pre‐eclampsia, maternal cardiovascular or liver diseases, systolic blood pressure < 90 mmHg, known uterine malformation, IUGR < 5th percentile, non reassuring fetal status, antepartum diagnosis of major fetal malformations, multiple gestations (≥ triplets) or fetal death. |
|
| Interventions | ORA group: atosiban. Given as a loading dose of 6.75 mg in 0.9% sodium chloride solution (i.v.), followed by i.v. infusion of 300 μg/min in 0.9% sodium chloride solution for the first 3 h and then 100 μg/min for another 45 hours. Control group: nifedipine. Given as a loading dose of 20 mg orally followed by 2 x 20 mg, 20‐30 minutes apart "as needed". Maintenance was started after 6 h with 20‐40 mg 4 times daily for 48 h. Both groups: rescue treatment with the alternative study drug was initiated if labour progressed between 1‐48 h or if adverse reactions occurred. If both drugs failed and gestational age was ≤ 28 weeks, indomethacin treatment was initiated. Drug treatment continued for 48 h. Corticosteroids and group B strep prophylactic treatment were administered according to standard protocol. |
|
| Outcomes | Tocolytic efficacy and tolerability profile by pregnancy prolongation for 48 h without the need for an alternate tocolytic, pregnancy prolongation for 7 days without the need for an alternate tocolytic, pregnancy prolongation for 48 h and 7 days, preterm birth, interval between enrolment and delivery, maternal adverse drug effects, gestational age at delivery, neonatal morbidity and mortality related to prematurity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of the groups was performed in blocks of 10 using a computer randomisation sequence generation program." Probably done. |
| Allocation concealment (selection bias) | Low risk | "…the randomisation results were kept in the labor and delivery ward in a closed study box. The sequence was concealed until intervention was assigned, i.e., just before administrating the tocolytic drug." Allocation concealment was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because the study drugs were administered by different roots, blinding of participants or care providers was not performed." Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women in each group were excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | The trial was registered in a publicly available trials registry and all outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Shim 2006.
| Methods | Multicentre, randomised, placebo‐controlled trial. | |
| Participants | 128 women presenting with uterine contractions (≥ 30 sec in duration, rate 4/30 min or more, confirmed by minimum 1 h tocography), cervical dilation 0‐3 cm and cervical effacement of ≥ 50%. Gestational age between 24–33+6 weeks (confirmed by ultrasound < 20 weeks and/or reliable menstrual dates). Exclusion criteria: multiple pregnancy, ruptured membranes, major vaginal bleeding, severe pre‐eclampsia or hypertension, temperature > 37.5ºC, urinary tract infection, fetal/placental/amniotic fluid abnormalities (major fetal anomalies, chorioamnionitis, polyhydramnios, fetal growth restriction, placental previa), serious maternal disease (cardiovascular disease, hyperthyroidism, diabetes, pheochromocytoma, asthma), contraindication to beta‐agonists, alcohol or drug abuse, use of NSAID tocolytic drugs within 12 h prior to study entry, hypersensitivity to components of the study drugs and participation in clinical trials ≤ 1 month. |
|
| Interventions | ORA group: atosiban. Administered as a bolus dose (6.75 mg in 0.9 mL NaCl, i.v.), followed by 300 µg/min in 5% dextrose infusion for 3 h and 100 mg/min in 5% dextrose up to 48 h. Simultaneous placebo i.v. infusion of 5% dextrose was given, corresponding to ritodrine treatment. Control group: ritodrine. Administered in 5% dextrose (i.v.) at a rate of 0.1–0.35 mg/min up to 48 h, with 0.05 mg/min increments every 10 minutes as required, or until contractions ceased. After 12 h of continuous infusion at the effective dose or when contractions ceased, the dose was decreased every 30 min by 0.05‐mg/min. Simultaneous injection of placebo (i.v. bolus dose of 0.9 mL sodium chloride) followed by i.v. infusion of 5% dextrose was administered, corresponding to atosiban treatment. Both groups: corticosteroids were administered as required. |
|
| Outcomes | Comparison of tocolytic efficacy and safety of atosiban and ritodrine in the treatment of preterm labour in Korean women. Primary end‐points: proportion of women who were not delivered and did not need an alternative tocolytic therapy after 48 h and 7 days. Days to delivery, therapeutic failure maternal adverse events and neonatal morbidity were also assessed. Secondary end‐points: frequency of contractions with time, gestational age at birth, birthweight, duration of stay in neonatal intensive care unit, duration of ventilatory care, concomitant diseases in neonate and neonatal death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two computer‐generated randomisation lists were prepared and issued by." The authors consider the approach to be low risk. |
| Allocation concealment (selection bias) | Unclear risk | "…[the study drugs] were supplied in randomised boxes labelled with the centre code and case number." Allocation concealment is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All infusates were […] administered by a piggy‐back method." The authors consider this method to be low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The authors consider the risk to be low because of the administration method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women in the ritodrine group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Unclear risk | 8 women in the atosiban group and 1 woman in the ritodrine group were given tocolysis prior to randomisation, with a 6 h wash‐out period between dosing. |
Thornton 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 163 women with uterine contractions (duration ≥ 30 sec, rate ≥ 6/30 min) at gestational age between 34 + 0 and 35 + 6 inclusive. Cervical length ≤ 15 mm (by transvaginal ultrasound) and cervical dilatation > 1 cm and 4 cm (by vaginal examination). Exclusion criteria: contraindications to tocolysis, diabetes mellitus (existing or gestational), eclampsia or severe pre‐eclampsia, previous major uterine surgery, congenital uterine abnormality, large leiomyomas, retained intrauterine contraceptive device, ruptured membranes, placenta praevia, oligohydramnios or polyhydramnios (amniotic fluid index < 7.2 cm or > 27.8 cm), fetal weight outside 2 standard deviations for gestational age (based on ultrasound), cervical cerclage, multiple pregnancy, suspected abnormal karyotype or major malformations, abnormal fetal heart rate consistent with fetal distress, suspected or history of thromboembolic disorders, hypo coagulability or coagulation deficiency, known or suspected haemoglobinopathies, known or suspected or history (last 12 months) of alcohol or drug abuse, treatment with anticoagulants or fibrinolytics before screening with betamimetics, atosiban, or progesterone within 7 days before randomisation or with nifedipine, prostaglandin synthase inhibitors, magnesium sulphate, or any investigational drug during the current pregnancy. |
|
| Interventions | ORA group: barusiban. Administered as a single bolus dose (1 mL, i.v.) of 1 of the following treatments: 0.3 mg (n = 32), 1 mg (n = 31), 3 mg (n = 32) or 10 mg (n = 36) barusiban. Control group: placebo. Administered as a single bolus dose (1 mL, i.v.) of acetate buffer. |
|
| Outcomes | Primary end‐point: percentage of women who did not deliver within 48 h. Secondary end‐points: percentage of women who did not deliver within 12 and 48 h, time to delivery, percentage change from baseline in number of uterine contractions at each h during the initial 12 h and at each assessment time point during the 12‐48 h after dosing, incidence and severity of adverse maternal, fetal and neonatal outcomes, time from delivery to expulsion of the placenta, incidence and severity of postpartum haemorrhage, change in haemoglobin level from screening to 24‐48 h after delivery, time from delivery to established lactation, percentage of mothers lactating 5 days after delivery, pharmacokinetic parameters of barusiban; plasma concentration‐time curve AUC, Cmax and t1/2. |
|
| Notes | For this review, outcomes for all barusiban dosing regimens have been combined for the comparison between barusiban and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was computer generated for each participating site by an independent statistician [...] At each site, randomisation took place after the screening assessment and before any investigational medicinal product was administered." The authors consider this approach to be low risk. |
| Allocation concealment (selection bias) | Low risk | "Each site was given a coded envelope for randomization." The authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All participants and study personnel, including those assessing the outcomes, were blinded to treatment assignment for the duration of the study." The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As stated above: "...including those assessing the outcomes, were blinded [...] for the duration of the study." The authors consider this approach low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
GA: gestational age h: hour i.m.: intramuscular i.v.: intravenous IUGR: intrauterine growth restriction mg: milligram min: minutes NaCl: saline NSAID: nonsteroidal anti‐inflammatory drug ORA: oxytocin receptor antagonists PROM: premature rupture of membranes µg: microgram sec: seconds
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Omari 2006 | Quasi‐random allocation. |
| de Heus 2008 | A prospective randomised trial in term labour comparing the effects of atosiban with ritodrine for acute tocolysis or intrauterine resuscitation. |
| Gagnon 1998 | Trial of maintenance tocolysis. |
| Husslein 2006 | Comparison of 2 similar atosiban doses scheme where depending upon the criteria the atosiban was given early or standard. No comparison between 2 different tocolytic agents or 2 different doses scheme was performed. Data are from the treasure study group containing the treasure study. |
| Husslein 2007 | The control group was described as "usual care" and "included treatment with betamimetics, calcium channel blockers, magnesium sulphate, or any other tocolytic, alone or in combination, and/or bedrest". |
| Poppiti 2009 | The trial compares atosiban immediately versus atosiban + 2 weeks later repeat course atosiban treatment for women in preterm labour. |
| Steinwall 2005 | Study concerning an vasopressin receptor antagonist for inhibiting preterm labour. |
| Valenzuela 1997 | Trial was to measure oestradiol levels before and after treatment with atosiban. |
| Valenzuela 2000 | Trial of maintenance tocolysis which is not within the scope of this review. |
Characteristics of studies awaiting assessment [ordered by study ID]
de Heus 2009.
| Methods | Randomised controlled trial of fetal effects of tocolytic treatment. |
| Participants | Women between 25‐33 weeks' gestation with no previous tocolytic treatment. Exclusion criteria: multiple pregnancy, severe vaginal bleeding, fetal congenital anomaly and signs of intrauterine infection. |
| Interventions | ORA group: atosiban. Single i.v. dose 6.75 mg in 0.9 mL NaCl, followed by i.v. infusion 300 µg/min in 5% dextrose for 3 h, then 100 µg/min in 5% dextrose up to 48 h. Control group: nifedipine. 4 oral 10 mg capsules given 15 min apart, thereafter 30 mg slow‐release 8 h apart up to 48 h. Both groups: 2 doses of betamethasone 12 mg 24 h apart. |
| Outcomes | Primary outcomes: effects of tocolysis on fetal heart rate and its variation. Secondary endpoints: effects on fetal movement and blood flow parameters (umbilical artery and middle cerebral artery). |
| Notes | No data are available for the parameters of interest in this review ‐ awaiting response from authors request for additional data. |
Lenzen 2012.
| Methods | A randomised, prospective multicentre study. |
| Participants | Women between 24‐33 completed weeks' gestation. Exclusion criteria: multiple pregnancy, PPROM, vaginal bleeding |
| Interventions | ORA group: atosiban. Standard dose as per product information. Control group: fenoterol. 2 µg/min (i.v.) followed by 1.5‐3 µg/min. |
| Outcomes | Primary outcomes: the rate of the mother’s side effects and acceptance of treatment. Secondary outcomes: efficacy of tocolysis to prolong pregnancy for at least 48 h. |
| Notes | No data are available for the parameters of interest in this review ‐ awaiting response from authors to request for additional data. |
Neri 2009.
| Methods | Randomised trial. |
| Participants | 62 women with singleton pregnancies and preterm labour at 26‐33 weeks' gestation with intact membranes were enrolled. Preterm labour was defined as > 6 contractions in 60 min (confirmed by external tocography) and cervical dilatation and/or effacement. Exclusion criteria: cervical dilatation ≥ 3 cm, vaginal bleeding, pre‐eclampsia or gestational hypertension, severe maternal diseases, intrauterine growth restriction, oligohydramnios, impaired utero‐placental blood flow, or clinical suspicion of chorioamnionitis (white cells ≥ 15,000/mm3 µL, C‐reactive protein ≥1 mg/dL). |
| Interventions | ORA group: atosiban. Bolus‐dose 6.75 mg (i.v.) followed by infusion of 37.5 mg in 250 mL saline at 24 mL/h for 3 h and then 8 mL/h for 48 h. Control group: ritodrine. 150 mg in 500 mL saline infused at 100‐350 µg/min until uterine contractions ceased or until maternal heart rate reached 140 bpm. Both groups: corticosteroids (betamethasone, 12 mg 24 h apart, i.m.) were given to both groups. |
| Outcomes | To compare the fetal cardiovascular effects of ritodrine and atosiban treatment by analysing the computerised nonstress test. |
| Notes | Antenatal steroids administered at the same time, 12 mg i.m. 24 h apart, 2 doses. |
Renzo 2003.
| Methods | Clinical trial comparing atosiban with ritodrine. |
| Participants | 43 women with preterm labour (defined as uterine contractions > 30 sec in duration at a rate of > 4/30 min, cervical dilatation 0‐3 cm (nullipara) or 1‐3 cm (primi‐ or multipara) and effacement > 50%) between 23‐33 completed weeks' gestation. |
| Interventions | ORA group: atosiban. Bolus dose 6.75 mg in 0.9 mL NaCl followed by 300 µg/min in 5% dextrose (i.v.) for 3 h, then 100 µg/min in 5% dextrose up to 45 h. Control group: ritodrine. 0.1 up to 0.35 mg/min in 5% NaCl (i.v.) in increments every 10 min as required or until contractions ceased. |
| Outcomes | Tocolytic effectiveness assessed as total number of women who had not delivered at 48 h and 7 days after starting treatment. Safety assessed as maternal side effects. |
| Notes | No data are available for the parameters of interest in this review ‐ awaiting response from authors to additional data request. |
Snidow 2013.
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled phase 2 trial. |
| Participants | Women with singleton pregnancy between 30‐35 weeks' gestation with spontaneous preterm labour defined as contraction rate ≥ 4/30 min or ≥ 6/h and cervical dilatation between 1‐4 cm. |
| Interventions | Women were randomised to receive 48 h treatment with i.v. retosiban or placebo. |
| Outcomes | To determine the efficacy and safety of retosiban given to women in spontaneous preterm labour. |
| Notes | No data are available for the parameters of interest in this review ‐ awaiting response from authors to request for additional data. |
bpm: beats per minute h: hour i.v.: intravenous min: minutes ORA: oxytocin receptor antagonists PPROM: preterm premature rupture of membranes
Characteristics of ongoing studies [ordered by study ID]
APOSTEL III.
| Trial name or title | APOSTEL III |
| Methods | Randomised controlled trial. |
| Participants | Women ≥ 18 years old with a singleton pregnancy with a gestational age of 25‐34 weeks in threatened preterm labour, as defined by: uterine contractions, ≥ 3 contractions per 30 minutes, and 1 of the following: 1. cervical length of ≤ 10 mm or; 2. cervical length of 11‐30 mm and a positive Fibronectin test or; 3. ruptured amniotic membranes. |
| Interventions | ORA group: atosiban. Bolus dose 6.75 mg (i.v.), followed by 18 mg/h for 3 h (i.v.) then 6 mg/h for 45 h. Control group: nifedipine. Bolus dose of 20 mg orally, followed by 20 mg every 6 h for 47 h. |
| Outcomes | Primary outcome: composite poor neonatal outcome, including broncho pulmonary dysplasia (BPD), periventricular leucomalacia (PVL) > grade 1, intracerebral haemorrhage > grade 2, necrotising enterocolitis (NEC) > stage 1, proven sepsis and in‐hospital death. |
| Starting date | 21 June 2011 |
| Contact information | Dr K Heida Dept. of Obstetrics UMC Utrecht Utrecht The Netherlands |
| Notes | NTR:2947 |
h: hour i.v.: intravenous ORA: oxytocin receptor antagonists
Differences between protocol and review
We revised the primary and secondary outcomes to more clearly and comprehensively address important outcome measures and to enhance consistency with other Cochrane reviews of tocolytics for preterm birth.
Contributions of authors
In the first version of the is review Dimitri Papatsonis lead the review with Vicki Flenady working collaboratively in all aspects of the review including selection of studies and data extraction and quality assessment. Vicki Flenady lead this review update in collaboration with Dimitri Papatsonis. Eashan Tambimuttu assisted with data extraction for studies identified in this update of the review. Hanna Reinebrant undertook data extraction and quality assessment for all studies included in the review, undertook data entry and completion of the 'Risk of bias' tables. Helen Liley assisted with resolving differences in data extraction, revised the background for this update and assisted with editing throughout the review. All authors were involved in interpretation of the results of the review and final editing.
Sources of support
Internal sources
Centre for Clinical Studies ‐ Women's and Children's Health, Mater Hospital, South Brisbane, Queensland, Australia.
Division of Obstetrics and Gynaecology, John Hunter Hospital, New South Wales, Australia.
Department of Obstetrics and Gynaecology, Amphia Hospital Breda, Breda, Netherlands.
Department of Neonatology, Mater Mothers' Hospital, South Brisbane, Queensland, Australia.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra, Australia.
Declarations of interest
Dimitri Papatsonis is the first author on a completed multicentre trial of nifedipine tocolysis. Vicki Flenady and Dimitri Papatsonis are authors on a Cochrane review titled 'Calcium channel blockers for inhibiting preterm birth' (update of King 2003 in progress). Vicki Flenady is an author on a Cochrane review titled 'Cyclo‐oxygenase (COX) inhibitors for treating preterm labour' (King 2005).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cabar 2008 {published data only}
- Cabar FR, Bittar RE, Gomes CM, Zugab M. Atosiban as a tocolytic agent: a new proposal of a therapeutic approach. Revista Brasileira de Ginecologia y Obstetricia 2008;30(2):87‐92. [DOI] [PubMed] [Google Scholar]
European 2001 {published data only}
- The European Atosiban Study Group. The oxytocin antagonist atosiban versus the ß‐agonist terbutaline in the treatment of preterm labor: a randomized, double‐blind, controlled study. Acta Obstetricia et Gynecologica Scandinavica 2001;80:413‐22. [PubMed] [Google Scholar]
- The Worldwide Atosiban versus Beta‐antagonists Study Group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta‐adrenergic agonists in the treatment of preterm labour. BJOG 2001;108:133‐42. [PubMed] [Google Scholar]
French/Australian 2001 {published data only}
- French/Australian Atosiban Investigators Group. Treatment of preterm labor with the oxytocin antagonist atosiban: a double‐blind, randomized, controlled comparison with salbutamol. European Journal Obstetrics, Gynecology and Reproductive Biology 2001;98:177‐85. [DOI] [PubMed] [Google Scholar]
- The Worldwide Atosiban versus Beta‐antagonists Study Group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta‐adrenergic agonists in the treatment of preterm labour. BJOG 2001;108:133‐42. [PubMed] [Google Scholar]
Goodwin 1994 {published data only}
- Goodwin TM, Paul R, Silver H, Spellacy W, Parsons M, Chez R, et al. The effect of the oxytocin antagonist atosiban on preterm uterine activity in the human. American Journal of Obstetrics and Gynecology 1994;170:474‐8. [DOI] [PubMed] [Google Scholar]
- Goodwin TM, Paul RH, Silver H, Parsons M, Chez R, Spellacy W, et al. Safety and efficacy of the oxytocin antagonist atosiban in threatened preterm labor: initial US trial. American Journal of Obstetrics and Gynecology 1992;166:359. [DOI] [PubMed] [Google Scholar]
Goodwin 1996 {published data only}
- Goodwin TM, Valenzuela G, Silver H, Hayashi R, Creasy GW, Lane R. Treatment of preterm labor with the oxytocin antagonist atosiban. American Journal of Perinatology 1996;13(3):143‐6. [DOI] [PubMed] [Google Scholar]
- Goodwin TM, Valenzuela GJ, Silver H, Creasy G, Atosiban Study Group. Dose ranging study of the oxytocin antagonist atosiban in the treatment of preterm labor. Obstetrics & Gynecology 1996;88(3):331‐6. [DOI] [PubMed] [Google Scholar]
Kashanian 2005 {published data only}
- Kashanian M, Akbarian AR, Soltanzadeh M. Atosiban and nifedipin for the treatment of preterm labor. International Journal of Gynecology & Obstetrics 2005;91(1):10‐4. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Soltanzadeh M, Sheikh Ansari N. Atosiban and nifedipin for the treatment of preterm labor. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):69. [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin CH, Lin SY, Shyu MK, Chen SU, Lee CN. Randomized trial of oxytocin antagonist atosiban versus beta‐adrenergic agonists in the treatment of spontaneous preterm labor in Taiwanese women. Journal of the Formosan Medical Association = Taiwan Yi Zhi 2009;108(6):493‐501. [DOI] [PubMed] [Google Scholar]
Moutquin 2000 {published data only}
- Moutquin J, Rabinovici J. Comparison of atosiban versus ritodrine in the treatment of pre‐term labour. Acta Obstetrica et Gynecologica Scandinavica 1997;76(167):33. [Google Scholar]
- Moutquin JM, Sherman D, Cohen H, Mohide PT, Hochner‐Celnikier D, Fejgin M, et al. Double‐blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. American Journal of Obstetrics and Gynecology 2000;182(5):1191‐9. [DOI] [PubMed] [Google Scholar]
Nonnenmacher 2009 {published data only}
- Nonnenmacher A, Hopp H, Dudenhausen J. Effectiveness and safety of atosiban vs. pulsatile administration of fenoterol in the treatment of preterm labour. Zeitschrift fur Geburtshilfe und Neonatologie 2009;213(5):201‐6. [DOI] [PubMed] [Google Scholar]
Richter 2005 {published data only}
- Richter ON, Dorn C, Vondel P, Ulrich U, Schmolling J. Tocolysis with atosiban: experience in the management of premature labor before 24 weeks of pregnancy. Archives of Gynecology and Obstetrics 2005;272(1):26‐30. [DOI] [PubMed] [Google Scholar]
Romero 2000 {published data only}
- Goodwin TM. 1st International preterm labour congress. Strategies to prevent the morbidity and mortality associated with prematurity; 2002 June 27‐30. Le Montreux Palace, Montreux Switzerland. 2002. [PubMed]
- Goodwin TM. Long‐term safety with oxytocin antagonists. 4th World Congress on Controversies in Obstetrics, Gynecology and Infertility; 2003 April 24‐27; Berlin, Germany. 2003:291.
- Goodwin TM, Randall H, Perry K, Menard MK, Bauer C, Shangold G, et al. A report on infant outcomes at 6 and 12 months after the use of atosiban in the management of preterm labor. 46th ACOG Annual Meeting; 1998 May 9‐13; New Orleans, Louisiana. 1998.
- Goodwin TM, Randall H, Perry K, Menard MK, Bauer C, Shangold G, et al. A report on infant outcomes up to 24 months after the use of atosiban in the management of preterm labor. 46th ACOG Annual Meeting; 1998 May 9‐13; New Orleans, Louisiana. 1998.
- Romero R, Sibai BM, Sanchez‐Ramos L, Valenzuela GJ, Veille JC, Tabor B, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double‐blind, placebo‐controlled trial with tocolytic rescue. American Journal of Obstetrics and Gynecology 2000;182(5):1173‐83. [DOI] [PubMed] [Google Scholar]
- Sibai B, Romero R, Sanchex‐Ramos L, Valenzuela G, Veille J, Tabor B, et al. A double‐blind placebo‐controlled trial of an oxytocin‐receptor antagonist (antocin) in the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S2. [DOI] [PubMed] [Google Scholar]
- Silver H, Valenzuela G, Sanchez‐Ramos L, Romero R, Sibai B, Goodwin T, et al. Maternal side effects and safety of the oxytocin receptor antagonist antocin. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S45. [Google Scholar]
Salim 2012 {published data only}
- Garmi G. Nifedipine compared to atosiban for treating preterm labor [ClinicalTrials.gov (http://clinicaltrials.gov/)]. accessed 9 April 2008.
- Salim R, Garmi G, Nachum Z, Zafran N, Baram S, Shalev E. Nifedipine compared with atosiban for treating preterm labor: A randomized controlled trial. Obstetrics and Gynecology 2012;120(6):1323‐31. [DOI] [PubMed] [Google Scholar]
Shim 2006 {published data only}
- Shim JY, Park YW, Yoon BH, Cho YK, Yang JH, Lee Y, et al. Multicentre, parallel group, randomised, single‐blind study of the safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in Korean women. BJOG 2006;113(11):1228‐34. [DOI] [PubMed] [Google Scholar]
Thornton 2009 {published data only}
- Arce JC. A proof of concept study assessing the effect of four different stage bolus intravenous doses of FE200440 and placebo on stopping preterm labor (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of a selective oxytocin antagonist (barusiban) in threatened preterm labour: a randomized, double‐blind, placebo‐controlled trial. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA. 2008:Abstract no: 129.
- Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of barusiban on plasma concentrations and uterine contractility in threatened preterm labour: a randomised, double‐blind, placebo‐controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa11‐Fa12. [Google Scholar]
- Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of barusiban, a selective oxytocin antagonist, in threatened preterm labor at late gestational age: a randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2009;200(6):627.e1‐627.e10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Omari 2006 {published data only}
- Al‐Omari WR, Al‐Shammaa HB, Al‐Tikriti EM, Ahmed KW. Atosiban and nifedipine in acute tocolysis: a comparative study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;128(1‐2):129‐34. [DOI] [PubMed] [Google Scholar]
- Al‐Omari WR, Al‐Tikri E, Al‐Shammaa H. Atosiban and nifedipine in acute tocolysis, comparative study. XVIIIth European Congress of Obstetrics and Gynaecology; May 12‐15; Athens, Greece. 2004:103.
de Heus 2008 {published data only}
- Heus R, Mulder EJ, Derks JB, Korver PH, Wolfswinkel L, Visser GH. A prospective randomized trial of acute tocolysis in term labour with atosiban or ritodrine. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;139(2):139‐45. [DOI] [PubMed] [Google Scholar]
Gagnon 1998 {published data only}
- Gagnon D, Martens L, Creasy G, Henke C. An economic analysis of atosiban maintenance therapy in preterm labor. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S181. [Google Scholar]
Husslein 2006 {published data only}
- Anonymous. TREASURE (Tractocile efficacy assessment survey in Europe). Trial to commence in July. http://www.ferring.com/ (accessed 24 May 2004).
- Husslein P, Roura LC, Dudenhausen J, Helmer H, Frydman R, Rizzo N, et al. Clinical practice evaluation of atosiban in preterm labour management in six European countries. BJOG: an international journal of obstetrics and gynaecology 2006;113(Suppl 3):105‐10. [DOI] [PubMed] [Google Scholar]
Husslein 2007 {published data only}
- Husslein P, Cabero Roura L, Dudenhausen JW, Helmer H, Frydman R, Rizzo N, et al. Atosiban versus usual care for the management of preterm labor. Journal of Perinatal Medicine 2007;35(4):305‐13. [DOI] [PubMed] [Google Scholar]
Poppiti 2009 {published data only}
- Poppiti R, Nazzaro G, Placido G, Palmieri T, Locci M. Prevention of preterm delivery in twin pregnancies with atosiban. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(Suppl 1):61. [Google Scholar]
Steinwall 2005 {published data only}
- Steinwall M, Bossmar T, Brouard R, Laudanski T, Olofsson P, Urban R, et al. The effect of relcovaptan (SR 49059), an orally active vasopressin V1a receptor antagonist, on uterine contractions in preterm labor. Gynecological Endocrinology 2005;20(2):104‐5. [DOI] [PubMed] [Google Scholar]
Valenzuela 1997 {published data only}
- Valenzuela G, Diaz A, Germain A, Foster T, Hayashi R, Seron‐Ferre M. Estradiol levels are increased before and after preterm labor treatment with an oxytocin (OT) antagonist: evidence for chronic activation of the mechanism of labor. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):88. [Google Scholar]
Valenzuela 2000 {published data only}
- Sanchez‐Ramos L, Valenzuela G, Romero R, Silver H, Koltun W, Millar L, et al. A double‐blind placebo‐controlled trial of oxytocin receptor antagonist (antocin) maintenance therapy in patients with preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S30. [Google Scholar]
- Valenzuela GJ, Sanchez‐Ramos L, Romero R, Silver HM, Koltun WD, Millar L, et al. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The atosiban ptl‐098 study group. American Journal of Obstetrics and Gynecology 2000;182(5):1184‐90. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
de Heus 2009 {published data only}
- Heus R, Mulder EJ, Derks JB, Visser GH. The effects of the tocolytics atosiban and nifedipine on fetal movements, heart rate and blood flow. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(6):485‐90. [DOI] [PubMed] [Google Scholar]
Lenzen 2012 {published data only}
- Lenzen V, Bartz C, Rath WH. Atosiban versus fenoterol treatment of pre‐term labour: randomised, prospective, multicentre study [Atosiban versus fenoterol zur behandlung vorzeitiger wehen: randomisierte, prospektive multizenterstudie]. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S197‐S198. [Google Scholar]
Neri 2009 {published data only}
- Neri I, Monari F, Valensise H, Facchinetti F, Bellafronte M, Volpe A. Computerized evaluation of fetal heart rate during tocolytic treatment: comparison between atosiban and ritodrine. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):22. [DOI] [PubMed] [Google Scholar]
- Neri I, Monari F, Valensise H, Vasapollo B, Facchinetti F, Volpe A. Computerized evaluation of fetal heart rate during tocolytic treatment: comparison between atosiban and ritodrine. American Journal of Perinatology 2009;26(4):259‐63. [DOI] [PubMed] [Google Scholar]
Renzo 2003 {published data only}
- Renzo DGC, Mignosa MM, Rosati A, Burnelli L, Epicoco G, Luzi G. Can we effectively arrest premature labour in women with multiple gestations or other high‐risk factors?. 4th World Congress on Controversies in Obstetrics, Gynecology & Infertility; 2003 April 24‐27; Berlin, Germany. 2003:286‐90.
Snidow 2013 {published data only}
- Anonymous. The safety, tolerability and metabolism of GSK221149A, in pregnant women (34‐36 weeks), in preterm labor. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 June 2007).
- Snidow J, Miller H, Valenzuela G, Thornton S, Stier B, Clayton L, et al. A multicenter, randomized, double‐blind, placebo‐controlled phase 2 trial of retosiban, a selective oxytocin receptor antagonist, for the management of preterm labor. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S155. [Google Scholar]
References to ongoing studies
APOSTEL III {published data only}
- Heida K. Nifedipine versus Atosiban in the treatment of threatened preterm labour: APOSTEL III. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2947 (accessed 6 January 2014). [DOI] [PMC free article] [PubMed]
Additional references
Akerlund 1999
- Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. British Journal of Obstetrics and Gynaecology 1999;106(10):1047‐53. [DOI] [PubMed] [Google Scholar]
Anotayanonth 2006
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012 Jun 9;379(9832):2162‐72. [DOI] [PubMed] [Google Scholar]
Borthwick 2013
- Borthwick AD, Liddle J. Retosiban and epelsiban: potent and selective orally available oxytocin antagonists. In: Dömling A editor(s). Protein‐Protein Interactions in Drug Discovery. KGaA, Weinheim, Germany: Wiley‐VCH Verlag GmbH & Co, 2013:225‐56. [Google Scholar]
Chang 2013
- Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns‐Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013;381(9862):223‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coomarasamy 2003
- Coomarasamy A, Knox EM, Gee H, Song F, Khan KS. Effectiveness of nifedipine versus atosiban for tocolysis in preterm labour: a meta‐analysis with an indirect comparison of randomised trials. BJOG: an international journal of obstetrics and gynaecology 2003;110:1045‐9. [PubMed] [Google Scholar]
Crowley 1996
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database of Systematic Reviews 1996, Issue 2. [DOI: 10.1002/14651858.CD000065.pub2] [DOI] [PubMed] [Google Scholar]
Crowther 2002
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Dorling 2008
- Dorling JS, Field DJ. Value and validity of neonatal disease severity scoring systems. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F80‐F82. [DOI] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duckitt 2002
- Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002860] [DOI] [PubMed] [Google Scholar]
FDA 1998a
- Advisory Committee for Reproductive Health Drugs. Issue‐NDA 20‐797 Antocin, (atosiban injection) for use in the management of preterm labor. Centre for Drug Evaluation and Research, Food and Drug Administration. Gaithersburg, Maryland April 20 1998:83‐5.
FDA 1998b
- Advisory Committee for Reproductive Health Drugs. Issue‐NDA 20‐797 Antocin, (atosiban injection) for use in the management of preterm labor. Centre for Drug Evaluation and Research, Food and Drug Administration. Gaithersburg, Maryland April 20 1998:81.
FDA 2011
- U.S. Food, Drug Administration. Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. http://www.fda.gov/Drugs/DrugSafety/ucm243539.htm.
Gates 2004
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG 2004;111(3):213‐9. [DOI] [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75‐84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodwin 1996b
- Goodwin TM, Valenzuela G, Silver H, Hayashi R, Creasy GW, Lane R. Treatment of preterm labor with the oxytocin antagonist atosiban. American Journal of Perinatology 1996;13(3):143‐6. [DOI] [PubMed] [Google Scholar]
Goodwin 1998a
- Goodwin TM, Randall H, Perry K, Menard MK, Bauer C, Shangold G, et al. A report on infant outcomes up to 24 months of age after the use of atosiban in the management of preterm labour. 46th ACOG annual meeting; 1998 May 9‐13; New Orleans, Louisiana. 1998.
Goodwin 1998b
- Goodwin TM, Zograbyan A. Oxytocin receptor antagonists. Update. Clinics in Perinatology 1998;25(4):859‐71. [PubMed] [Google Scholar]
Gyetvai 1999
- Gyetvai K, Hannah M, Hodnett E, Ohlsson A. Tocolysis for preterm labor: a systematic review. Obstetrics & Gynecology 1999;94(5):869‐77. [DOI] [PubMed] [Google Scholar]
Haas 2012
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thompson S. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21:1559‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, Version 5.1.0 [updated March 2011].. [Google Scholar]
Khanprakob 2012
- Khanprakob T, Laopaiboon M, Lumbiganon P, Sangkomkamhang US. Cyclo‐oxygenase (COX) inhibitors for preventing preterm labour. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007748.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 1988
- King JF, Grant A, Keirse MJNC, Chalmers I. Beta‐mimetics in preterm labour: an overview of the randomized controlled trials. British Journal of Obstetrics and Gynaecology 1988;95:211‐22. [DOI] [PubMed] [Google Scholar]
King 2003
- King JF, Flenady VJ, Papatsonis DNM, Dekker GA, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002255] [DOI] [PubMed] [Google Scholar]
King 2005
- King J, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
Koks 1998
- Koks CA, Brolmann HA, Kleine MJ, Manger PA. A randomized comparison of nifedipine and ritodrine for suppression of preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;77(2):171‐6. [DOI] [PubMed] [Google Scholar]
Lasswell 2010
- Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low‐birth‐weight and very preterm infants: a meta‐analysis. JAMA 2013;304(9):992‐1000. [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151‐61. [DOI] [PubMed] [Google Scholar]
Melin 1994
- Melin P. Development of an oxytocin antagonist atosiban. Research Clinical Forums 1994;16:155‐68. [Google Scholar]
Nilsson 2003
- Nilsson L, Reinheimer T, Steinwall M, Akerlund M. FE 200 440: a selective oxytocin antagonist on the term‐pregnant human uterus. BJOG 2003;110(11):1025‐8. [PubMed] [Google Scholar]
Papatsonis 2004
- Papatsonis DNM, Decker GA. Nifedipine in the management of preterm labour: evidence from the literature. In: Critchley H, Bennett P, Thornton S editor(s). Preterm Birth. London: RCOG Press, 2004:296‐307. [Google Scholar]
Petrou 2011
- Petrou S, Eddama O, Mangham L. A structured review of the recent literature on the economic consequences of preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F225‐F232. [DOI] [PubMed] [Google Scholar]
Plunkett 2008
- Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Annals of Medicine 2008;40:167‐95. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Schappin 2013
- Schappin R, Wijnroks L, Uniken Venema MMAT, Jongmans MJ. Rethinking stress in parents of preterm infants: a meta‐analysis. PLoS ONE 2013;8(2):e54992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spector 1991
- Spector TD, Thompson SG. The potential and limitations of meta‐analysis. Journal of Epidemiology and Community Health 1991;45:89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2010
- Su LL, Samuel M, Chong YS. Progestational agents for treating threatened or established preterm labour. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006770.pub2] [DOI] [PubMed] [Google Scholar]
Tsatsaris 2004
- Tsatsaris V, Carbonne B, Cabrol D. Atosiban for preterm labour. Drugs 2004;64:375‐82. [DOI] [PubMed] [Google Scholar]
Tucker 2004
- Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329(7467):675‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrachnis 2011
- Vrachnis N, Malamas FM, Sifakis S, Deligeorogluo E, Iliodromiti Z. The oxytocin‐oxytocin receptor system and its antagonists as tocolytic agents. International Journal of Endocrinology 2011;2011:350546. [DOI: 10.1155/2011/350546] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- Howson CP, Kinney MV, Lawn JE (editors). Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
Yelland 2011
- Yelland L, Salter A, Ryan P, Makrides M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account?. Paediatric and Perinatal Epidemiology 2011;25(3):283‐97. [DOI] [PubMed] [Google Scholar]
Zeitlin 2008
- Zeitlin J, Draper ES, Kollée L, Milligan D, Boerch K, Agostino R, et al. for the MOSAIC research group. Differences in rates and short‐term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics 2008;121(4):e936‐e944. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Papatsonis 2005
- Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004452.pub2] [DOI] [PubMed] [Google Scholar]


