Abstract
Purpose
The purpose of this study was to assess the impact of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on corneal stroma characteristics, ocular manifestations, and post-recovery refractive surgery outcomes after varying recovery durations.
Methods
Fresh corneal lenticules from patients with post-coronavirus disease 2019 (COVID-19; recovered within 135 days) and healthy controls (HCs) after small incision lenticule extraction (SMILE) surgery were obtained for experimental validation of SARS-CoV-2 susceptibility, morphological changes, and immune response of the corneal stroma. Corneal optical density (CD) was measured using the Pentacam HR. Corneal epithelium thickness (ET) and endothelium parameters were evaluated by wide-field optical coherence tomography (OCT) and non-contact specular microscopy (SP-1P), respectively. All the patients were assessed after SMILE surgery until 3 month of follow-up.
Results
The cornea was susceptible to SARS-CoV-2 with the presence of SARS-CoV-2 receptors (CD147 and ACE2) and spike protein remnants (4 out of 58) in post-recovery corneal lenticules. Moreover, SARS-CoV-2 infection triggered immune responses in the corneal stroma, with elevated IL-6 levels observed between 45 and 75 days post-recovery, which were then lower at around day 105. Concurrently, corneal mid-stromal nerve length and branching were initially higher in the 60D to 75D group and returned to control levels by day 135. A similar trend was observed in CD within zones 0 to 2 and 2 to 6 and in the hexagonal cells (HEX) ratio in endothelial cells, whereas ET remained consistent. Notably, these changes did not affect the efficacy, safety, or predictability of post-recovery SMILE surgery.
Conclusions
SARS-CoV-2 induces temporal alterations in corneal stromal morphology and function post-recovery. These findings provided a theoretical basis for corneal health and refractive surgery management in the post-COVID-19 milieu.
Keywords: post coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), corneal immune response, mid-stoma nerves, corneal optical density (CD), refractive surgery
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus, has developed into a worldwide pandemic.1 This RNA virus infects cells through interactions between its Spike protein and surface receptors, such as ACE2 and TMPRSS2, leading to pathophysiological changes in multiple tissues and organs.1 As increasing populations have infected and recovered, persistent symptoms even months following SARS-CoV-2 infection (also known as long COVID or post-COVID syndrome) have raised growing concerns.2,3 These symptoms encompass a spectrum from fatigue and dyspnea to cognitive challenges, implicating a gamut of bodily systems, such as respiratory, neurological, cardiac, gastrointestinal, and ophthalmological systems.4–8 However, the exact structural and pathological changes associated with post-COVID syndrome are not fully understood. Long-term tissue damage, viral reservoir, and pathological inflammation are likely to be key determinants in post-COVID syndrome.9
Particularly intriguing is the evidence that some organs serve as SARS-CoV-2 reservoirs, possibly driving post-COVID symptoms.10 Remarkably, persistent SARS-CoV-2 RNA was detected in the brain, ocular tissues, and multiple other tissues even after 230 days from the symptom onset.11 Moreover, reverse-transcribed SARS-CoV-2 RNA is capable of integrating into the DNA of infected human cells.12 More importantly, because the immune system has limited access to certain tissues known as immune-privileged tissues (such as the brain, testicles, retina, and corneal stroma),13–16 the clearance of SARS-CoV-2 viral components might be even harder. As a result, remaining SARS-CoV-2 components might continuously stimulate immunity, leading to excessive inflammatory response and consequent dysfunction of tissues and organs. As such, exploring the viral clearance of certain organs and its effects on physiological functions is vital for long-term health management in the post COVID-19 era.
Notably, ocular manifestations of COVID-19 are an important topic to be investigated.17,18 Among the anatomic sites of the eye, the cornea is a target of SARS-CoV-2.19 First, the classic SARS-CoV-2 receptors ACE2 and TMPRSS2 are expressed in human corneal epithelium and endothelium.20–22 Moreover, direct evidence showed that SARS-CoV-2 could infect human corneal epithelium ex vivo.19 Accordingly, SARS-CoV-2 RNA and Spike protein were detected in the corneal epithelium of donors with active COVID-19 infection at the time of death.19,23 Most importantly, as one of the immune-privileged sites, limited evidence showed that the corneal stroma might also be affected by SARS-CoV-2. For example, keratitis was reported as a manifestation of COVID-19,24,25 among which the stroma is affected. Moreover, SARS-CoV-2 infection can potentially cause immune privilege collapse of the cornea,17 which might account for acute corneal graft rejection.26,27 As such, SARS-CoV-2 could invade the corneal stroma and cause aberrant immune responses in the corneal stroma, which is supported by previous studies (Bitirgen et al.,28 Kolkedi et al.,29 and Gulfidan et al.28). Their studies reported significantly higher dendric cell (DC) density in the cornea of patients after SARS-CoV-2 infection via corneal confocal microscopy. In addition, corneal confocal microscopy also showed that the morphology of sub-basal corneal small fibers remained altered even months after recovery from SARS-CoV-2 infection.28,29 However, currently, there has not been a systematic study addressing how long SARS-CoV-2 could affect the corneal stroma, including the exact pathological and structural changes in corneal stroma after COVID-19 recovery. Moreover, it remains uncertain if corneal surgery outcomes differ among patients operated on at various intervals following COVID-19 recovery.
To address these problems, small incision lenticule extraction (SMILE) surgery provides an excellent model for both direct analysis of corneal morphology and immune status after COVID-19 recovery and thorough evaluation of visual functions. On one hand, fresh human corneal stroma tissues could be obtained in SMILE surgery. On the other hand, normal corneal stromal morphological and functional parameters are vital for corneal surgery outcomes such as keratorefractive surgeries.30,31 As such, we enrolled patients with COVID-19 who recovered after different recovered durations and healthy controls (HCs) undergoing SMILE surgery and evaluated the pathological and structural changes of the corneal stroma and their surgery outcomes after 3 months of follow-up. Such analysis could provide direct evidence for longer-term impacts of SARS-CoV-2 infection on corneal stroma and keratorefractive surgery in the post COVID-19 era.
Patients and Methods
Study Design and Subjects
This cross-sectional study included patients who had SMILE surgery from January 2023 to April 2023. The included patients are divided into two groups: patients who recovered from COVID-19 and HCs who were never infected with COVID-19. None of the HCs tested positive for COVID-19 via oropharyngeal swabs, rapid antigen detection (RAD) tests, or RT-PCR within the month before enrollment, and none exhibited suspicious symptoms. The study protocol was approved by the ethics board of the Zhongshan Ophthalmic Center of Sun Yat-Sen University (Identifier No. 2023KYPJ332). All participants gave their informed written consent before enrollment in the study. Inclusion criteria were as follows: eligible for SMILE treatments, previous diagnosis of COVID-19 infection by oropharyngeal swabs or RAD tests, able to provide a definitive date of COVID-19 infection, and date of recovery from the infection. Exclusion criteria comprised ineligible for SMILE surgery or a medical history of keratoconus or suspicious corneal topography and other ocular surgery, unable to provide a definitive date of COVID-19 infection, or with previous suspicious symptoms of COVID-19 infection. All patients had pre-operative examinations, including uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), cycloplegic and subjective refraction, non-contact intraocular pressure, slit lamp microscopy, and corneal topography via the Scheimpflug tomography system.
Corneal Endothelial Cell Density and Morphology
Corneal endothelium measurements were made using a Topcon SP-1P non-contact specular microscope (SP-1P; Topcon, Japan). Three images were obtained for each participant using specular microscopy when the reference line and circle displayed a sharp definition on the screen. Only images containing a minimum of 100 cells were included for subsequent statistical analysis. The measurement parameters include central corneal thickness (CCT), endothelial cell density (ECD; cell/mm2), coefficient of variation (CV) in the cell area, average area of corneal endothelial cells (AVG), and the percentage of hexagonal cells (HEX).
Corneal Densitometry
The assessment of the anterior segment was performed via the Pentacam Scheimpflug imaging system (Pentacam; Oculus Optikgeräte GmbH, Wetzlar, Germany). The examinations were performed under standard dim-light conditions by the same trained and experienced professionals. Both eyes underwent three measurements, and the one with the best alignment and fixation was included. When measuring, the cornea is automatically divided into three different layers: (1) the anterior layer (the anterior 120 µm), (2) the posterior layer (the posterior 60 µm), and (3) the central layer (between the anterior and posterior layers). The corneal apex is also automatically located, and a 12-mm diameter area around the apex is analyzed. The measured 12-mm diameter corneal area was divided into 4 concentric circles (central 0–2 mm, 2–6 mm, 6–10 mm, and 10–12 mm). The “cornea densito” maps display corneal optical density (CD) in grayscale units ranging from 0 to 100, where 0 signifies utmost transparency, and 100 signifies the highest level of opacity.
Epithelial Thickness Measurement
Epithelial thickness (ET) maps with a 9-mm diameter region were acquired using the RTVueXR OCT system with a corneal adaptor module (RTVue-XR; Optovue Inc., USA). “PachymetryWide” scan mode was chosen, while participants were told to blink to lubricate their eyes before measuring the 9 mm diameter ET. Each participant was consecutively measured for 3 times; only measurements with a signal strength index of more than 30 were used for further statistical analysis. The “PachymetryWide” scan pattern displayed corneal thickness and ET maps over a 9-mm diameter area. The ET map is centered on the corneal apex and divided into 25 sectors by 8 meridians and 4 concentric circles. In this study, the 9-mm diameter area of the cornea was further split into 4 concentric zones, consisting of central (within 2 mm), paracentral (2–5 mm), mid-peripheral (5–7 mm), and peripheral (7–9 mm) zones. All the measurements were made at approximately the same time of the day, in the same equipment, by the same experienced investigator.
Management of Human Corneal Lenticules
Human corneal lenticules of patients with or without a COVID-19 history were collected from Zhongshan Ophthalmic Center from January 2023 to May 2023. The ethics board of the Zhongshan Ophthalmic Center of Sun Yat-Sen University approved the study (Identifier No. 2023KYPJ332). The study was carried out in compliance with the Declaration of Helsinki, and informed consent for the research was obtained. Patient information of age, sex, infection time and duration, ocular and systemic manifestations, and medicine intake were collected. The number of corneal lenticules used for each analysis and the correlated patient information is listed in Supplementary Tables S1 to S3. No statistically significant differences were found for age between the COVID-19 recovered and control groups (P > 0.05).
Immunofluorescence Assay
Fixed corneal lenticules were passed through a series of sucrose gradients (10%, 20%, and 30%), embedded in OCT compound, and frozen at −80°C. At least three sections for each tissue were examined (6 sections were examined for spike protein), maintaining a separation of eight slices between each. This approach allowed us to comprehensively cover both the central and peripheral regions of the corneal lenticules. Sections (9 µm thick) were immersed in 0.5% Triton X-100 for 10 minutes and blocked in blocking buffer with 10% goat serum at room temperature (RT) for 2 hours. After that, the slides were incubated with primary antibody (listed in Table 1), and diluted in 10% goat serum overnight at 4°C. After 3 washes, the slides were incubated with Alexa-Fluor 555 conjugated anti-rabbit antibody (1:500, 4413S; Cell Signaling Technology, USA) and/or Alexa-Fluor 488 conjugated anti-mouse antibody (1:500, 4408S; Cell Signaling Technology, USA), and diluted in 10% goat serum at RT for 1 hour. The slides were stained with DAPI, washed with PBS, and mounted with Anti-Fade Mounting Medium (Beyotime, Beijing, China). Slides stained without primary antibodies were used as negative controls. Images were captured using fluorescence microscopy (Axio Imager Z1; Carl Zeiss, Germany). Specifically, 3 to 5 images per section were captured using a 10 × lens for further analysis and spike examination, whereas higher magnification was utilized for detailed structural observation. The cell densities were normalized to DAPI+ cells instead of the area of tissue included.
Table 1.
Primary Antibodies Used in this Study
| Molecular Marker | Species | Company (Catalog #) | Working Dilution |
|---|---|---|---|
| ACE2 | Mouse | Santa Cruz (sc-390851) | 1:200 |
| EMMPRIN (CD147) | Mouse | Santa Cruz (sc-21746) | 1:300 |
| NRP1 | Mouse | Santa Cruz (sc-5307) | 1:100 |
| Spike | Rabbit | CST (99423S) | 1:100 |
| IL-6 | Rabbit | Proteintech (21865-1-AP) | 1:200 |
| NFkB-p65 | Rabbit | CST (8242T) | 1:200 |
| CD34 | Mouse | Proteintech (60180-1-IG) | 1:600 |
| CD45 | Mouse | Santa Cruz (sc-1178) | 1:200 |
Corneal Whole Mount Staining and Analysis of Corneal Mid-Stroma Nerves
Corneal lenticules were fixated with 4% paraformaldehyde (PFA) for 2 hours and washed with PBS for 3 times. Then, the corneal lenticules were blocked with 0.2% Triton X-100, 2% goat serum, and 1% BSA for 2 hours, and then incubated in the same buffer with neuronal class III β-tubulin mouse monoclonal antibody (Abcam, Waltham, MA, USA) at a concentration of 1:1000 overnight at 4°C. After washing with incubation buffer for 3 times, the tissues were incubated with Alexa-Fluor 555 conjugated anti-mouse antibody at 1:500 in incubation buffer for 2 hours at RT. After washing with PBS, the corneal lenticules were placed on a microscope slide. Four radial cuts were made, and the tissue was carefully covered with a cover slip mounted with antifade mounting medium (Beyotime, Beijing, China). Images were then taken with an Eclipse Ni-U fluorescence microscope. The mid-stroma nerves were then manually delineated with the Fiji plugin Neuroanatomy-SNT and skeletonized. The skeletonized images were then analyzed with Analyze-Skeleton to gain the information of total branch length, number of branches, and junctions of each case.
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD). The differences among mean values were evaluated with a two-tailed Student's t-test (for 2 groups) and analysis of variance (ANOVA; for > 2 groups). All calculations and statistical tests were performed with SPSS (IBM SPSS statistics 26). For all analyses, P < 0.05 was considered significant.
Results
Human Corneal Stroma is Susceptible to SARS-CoV-2
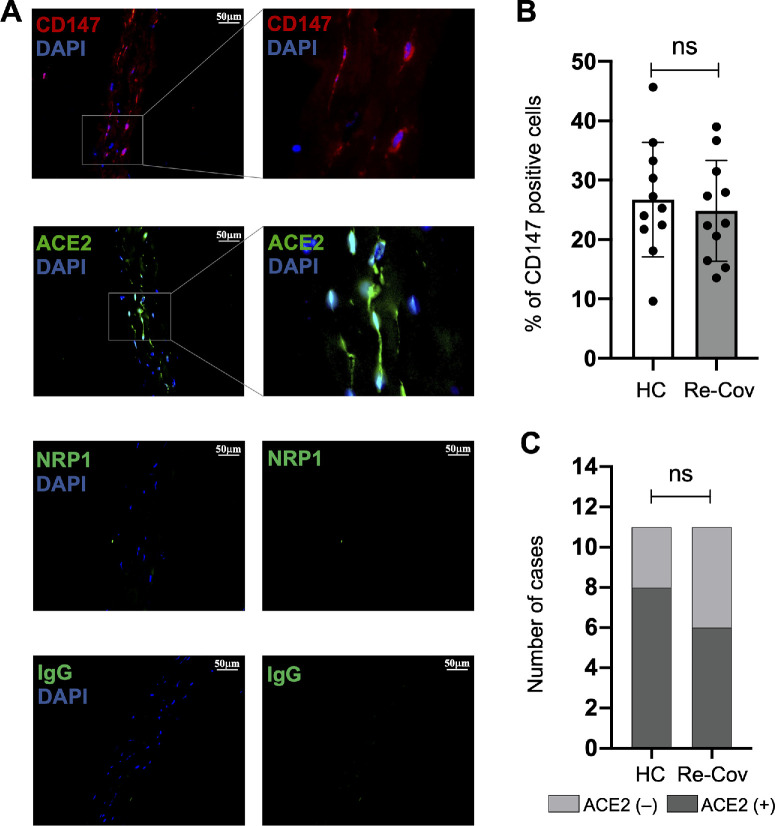
To explore the potential response of human corneal stroma to SARS-CoV-2, we evaluated the expression of SARS-CoV-2 specific receptors ACE2,32 NRP1,33 and CD14734 in the corneal lenticules of 22 individuals (11 patients recovered from COVID-19 and 11 HCs) who underwent SMILE surgery from January 12, 2023, to April 10, 2023. The detailed information of these patients is listed in Supplementary Table S1. Among these three SARS-CoV-2 receptors, CD147 was detected in all corneal lenticules, regardless of age or sex (Fig. 1A). The percentages of CD147-positive cells (%) in corneal lenticules of HCs and recovered patients with COVID-19 are 26.7 ± 9.6 and 24.9 ± 8.5, respectively, which are not significantly different (P = 0.95; Fig. 1B). Meanwhile, ACE2 was sporadically found in some of the corneal lenticules (see Fig. 1A). Among the specimens we measured, 8 from the HCs (72.7%) and 6 from the recovered patients with COVID-19 (54.5%) were ACE2-positive (Fig. 1C). The rates of ACE2-positive specimens are not significantly different between the two groups (P = 0.38). Last, however, NRP1 was not observed in the corneal lenticules (see Fig. 1A), which is supported by previous study.35 These results suggest that ACE2 and CD147 might enable SARS-CoV-2 infection in the corneal stroma.
Figure 1.
Expression of SARS-Cov2 receptors in the corneal stroma. (A) Immunofluorescent staining of human corneal lenticules depicts expression of CD147 (red) and ACE2 (green) receptors, while NRP1 (green) is absent. (B) The rates of CD147 positive cells are not significantly different between HC Re-Cov. (C) Similarly, the rates of ACE2 positive specimens between HC and Re-Cov are also not significantly different. HC: healthy control; Re-Cov: recovered patients with COVID-19.
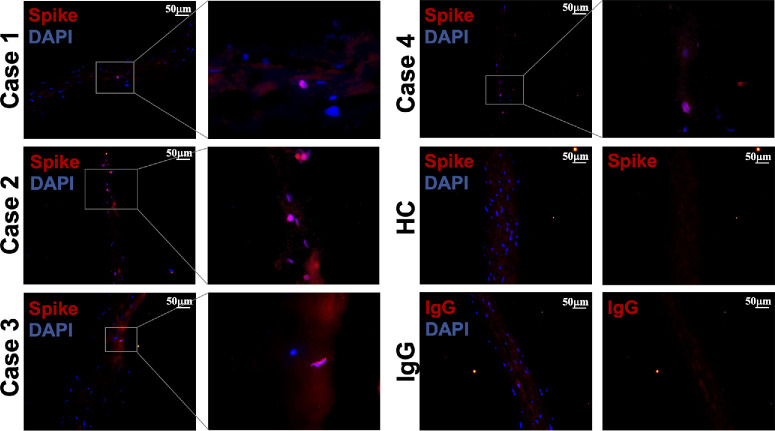
Because CD147 and ACE2 are positive in the corneal stroma, our next step was to evaluate whether SARS-CoV-2 could remain in the corneal stroma after COVID-19 recovery. We included 58 patients who recovered from COVID-19 and evaluated the existence of the SARS-CoV-2 spike protein in the corneal lenticules of these patients by immunofluorescent staining. Ten specimens from the HCs who were never infected with SARS-CoV-2 were included as negative controls. As a result, no signal was detected in the corneal lenticules of the HCs (Fig. 2). Importantly, sparse spike-positive cells were found in the corneal lenticules in 4 out of 58 COVID-19 recovered cases (6.89%). The detailed information on these 4 cases is listed in Table 2. Notably, the spike protein is detected in the corneal lenticules up to 53 days after COVID-19 recovery. Taken together, the SARS-CoV-2 spike protein could remain in the corneal lenticules after COVID-19 recovery, which might lead to pathophysiological changes in the corneal stroma.
Figure 2.
Residue of SARS-Cov2 spike protein in the corneal lenticules of recovered COVID-19 patients. Sparse spike-positive cells (red) were found in the corneal stroma of 4 out of 58 COVID-19 recovered cases (6.89%). The images were captured at 10 × and then magnified. No signals were observed in the HCs and the IgG controls. HC: healthy control.
Table 2.
Demographic and Clinical Characteristics of Spike-Positive Patients
| Patient ID | Recovered Days | Age | Sex | Primary Ocular Disease | Ocular Manifestation | Duration of Illness | Systematic Manifestation | Highest Temperature | COVID Vaccine | Last Vaccine | Medication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Re-CoV-S1 | 22 | 20 | Male | None | Eye pain | 10 | Fever, coughing, dryness of throat, muscle aching | 39 | 3 | 2021/12/20 | Acetaminophen |
| Re-CoV-S2 | 50 | 18 | Female | None | None | 6 | Fever, coughing | 37.6 | 2 | 2021/8/22 | None |
| Re-CoV-S3 | 53 | 32 | Female | None | None | 4 | Fever, coughing | 39 | 3 | 2021/12/17 | Antipyretic analgesic, details unknown |
| Re-CoV-S4 | 11 | 20 | Male | None | None | 8 | Fever | 38.2 | 4 | 2022/12/22 | Ibuprofen |
Duration of illness: days from the last positive test to the first negative test.
Inflammatory Response is Activated in the Corneal Stroma of COVID-19 Recovered Individuals With Elevation of Inflammatory Markers
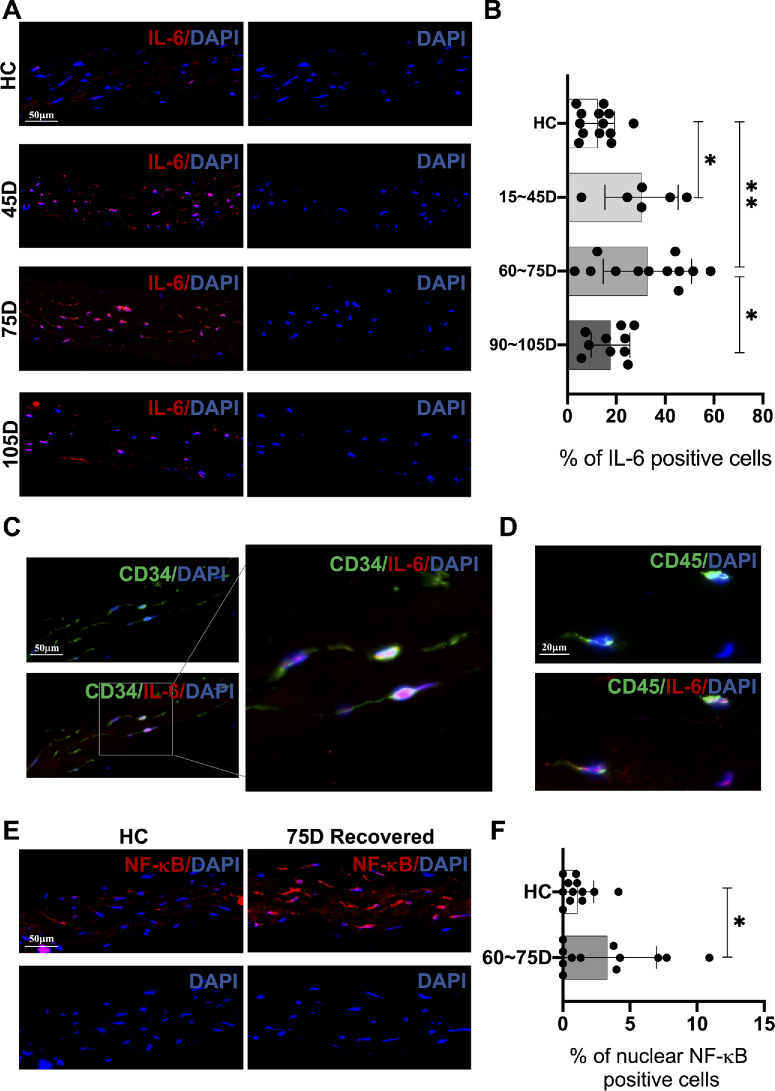
SARS-CoV-2 persistence has been connected to autoimmune responses and subsequent activated inflammation in various tissues.36 It was predicted that IL-6, an autoimmune-related inflammatory marker associated with COVID-19,37,38 is activated in several corneal cell types in patients with acute COVID-19.39 As such, we measured the expression levels of IL-6 in the corneal lenticules of HCs and patients with COVID-19 who recovered at different time points after recovery. The detailed information of these individuals is listed in Supplementary Table S2. Our data identified distinct IL-6 expression profiles in the corneal lenticules after different times of COVID-19 recovery. As shown in Figures 34A and 3B, the percentage of IL-6 positive cells was significantly higher in the 15 D to 45 D group after COVID-19 recovery compared to the HCs (HC = 12.4% ± 6.9%; approximately 15 to 45 D = 30.3% ± 15.0%, *P = 0.047). When it reached around 75 days after recovery, the rate of IL-6 positive cells in the corneal stroma was relatively high and also significantly elevated compared to the HCs (HC = 12.4% ± 6.9%; approximately 60 to 75 D = 33.8% ± 20.0%, **P = 0.001). However, the expression of IL-6 was significantly lower in the 105 D group compared to around 75 days (approximately 60 to 75 D = 33.8% ± 20.0%; approximately 90 to 105 D = 17.7% ± 7.9%, *P = 0.048), which was similar to the HCs (HC = 12.4% ± 6.9%; approximately 90 to 105 D = 17.7% ± 7.9%, P = 0.99). To further determine the cellularity of IL-6 expression, we carried out double staining of IL-6 and the keratocyte marker CD34, together with IL-6 and the leukocyte marker CD45 in IL-6-highly expressed tissues. As shown in Figures 3C and 3D, IL-6 was expressed in both CD45+ and CD34+ cells. However, given the limited presence of CD45+ cells within the corneal lenticules we examined, IL-6 expression was significantly more pronounced in the CD34+ keratocyte population.
Figure 3.
Changes of inflammatory markers in the corneal stroma at different time points after COVID-19 recovery. (A, B) Compared to HC, IL-6 positive cell rates peak between 45 and 75 days post-recovery, then converge to the HC levels around 105 days. (C) IL-6 is expressed in CD34+ cells. (D) CD45+ positive cells are observed in the corneal lenticular tissue, and IL-6 is also expressed in CD45+ cells. (E, F) Similarly, NF-κB positive cell rates increased approximately 75 days post recovery. HC: healthy control.
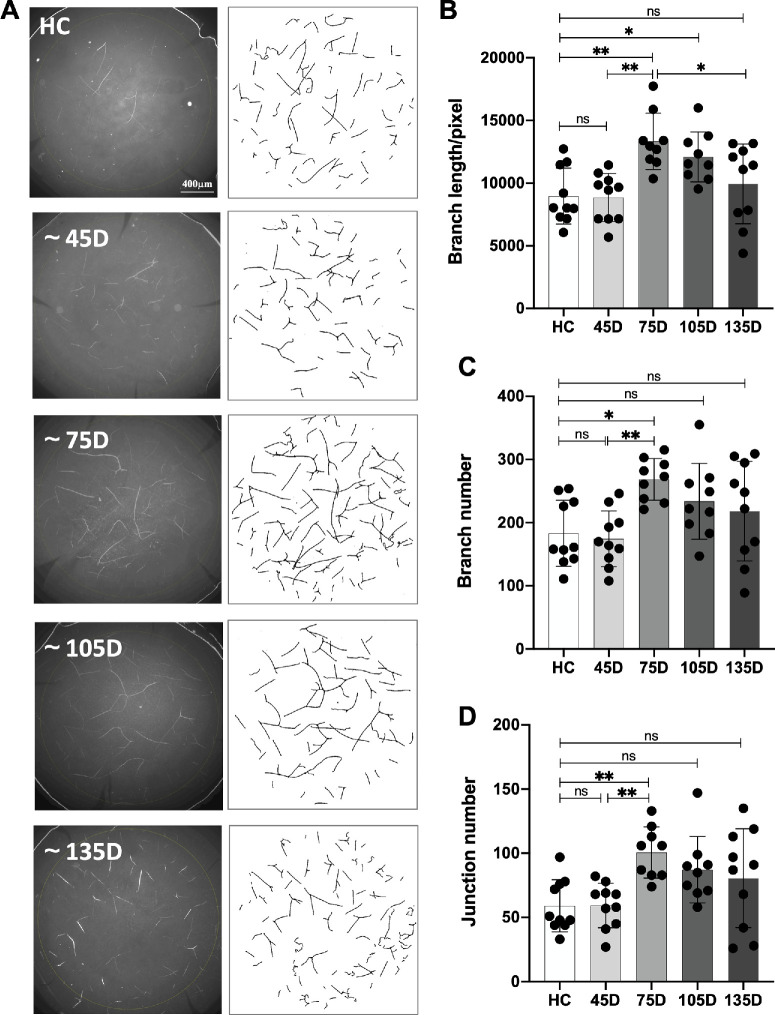
Figure 4.
Corneal mid-stroma nerve changes after different time of COVID-19 recovery. (A) Corneal mid-stroma nerves are identified using whole-mount staining of beta III tubulin and are manually traced using the Fiji SNT plugin. (B-D) Relative to HC, nerve branch length, numbers, and junctions matched HC at 45 days, were significantly higher at 75 days, and lower at around 105- and 135-days post-recovery.
In addition, because NF-κB can be activated by IL-639,40 and is tightly associated with viral infection in the corneal stroma,41 we then measured the activated status of NF-κB in the corneal lenticules of the HCs and recovered patients. Because the rate of NF-κB positive nucleus was relatively low in the human corneal fibroblasts,42 only the HCs and around 75 days of recovered patients were included. As a result, the percentage of NF-κB positive nucleus was elevated in the COVID-19 recovered group compared to the HCs (HC = 1.0% ± 1.1%; approximately 60 to 75 D, 3.3% ± 3.7%, *P = 0.04).
Taken together, these results suggested that after COVID-19 recovery, the inflammatory status was first stimulated in the corneal stroma compared to the HCs at around 45 to 75 days after recovery and became similar to the HCs after around 105 days.
Corneal Mid-Stoma Nerve Parameters are Altered in Patients at Different Time Points After COVID-19 Recovery
Dysregulation of the immune system due to COVID-19 is reported to affect the peripheral nervous system.43 As such, to evaluate the possible impact of COVID-19 on corneal nerves after recovery, we carried out whole-mount staining of corneal lenticules with anti-beta III tubulin. A total of 38 patients recovered from COVID-19 and 10 HCs who were never infected by SARs-Cov-2 were included. The COVID-19 recovered group was further divided into four groups according to the time duration from the first time they tested negative after SARS-CoV-2 infection to sampling. There was no significant difference among the age, sex, and myopic diopters between the groups (see Supplementary Table S3). As a result, the total length of corneal mid-stroma nerves was significantly higher at around 75 days after COVID-19 recovery, and was lower in the next 2 groups, until it became similar to the level of the HCs in the 135 D group post recovery (HC = 8967.4 ± 2121.9; approximately 45 D = 8858.5 ± 1820.2, P > 0.99; approximately 75 D = 13335.2 ± 2113.1, **P = 0.002; and approximately 105 D = 12095.3 ± 1881.8, *P = 0.047; approximately 135 D = 9946.3 ± 3016.7, P = 0.89) (Figs. 4A, 4B). Meanwhile, the number of branches share a similar changing pattern, where they are significantly higher at approximately 75 days compared to the HCs and lower in the approximately 105 D and approximately 135 D groups, until it reaches a plateau slightly higher than the HCs (HC = 183.2 ± 49.6; approximately 45 D = 174.5 ± 41.7, P > 0.99; approximately 75 D = 268.4 ± 31.2, *P = 0.02; approximately 105 D = 233.8 ± 56.7, P = 0.30; and approximately 135 D = 218.3 ± 74.7, P = 0.63) and the number of junctions (HC = 59.1 ± 19.2; approximately 45 D = 59.3 ± 16.4, P > 0.99; approximately 5 D = 100.7 ± 18.8, **P = 0.008; approximately 105 D = 87.2 ± 24.4, P = 0.14; and approximately 135 D = 80.6 ± 36.5, P = 0.35; see Figs. 4C, 4D). Taken together, the morphology of corneal mid-stroma nerves followed a specific changing pattern over time after COVID-19 recovery, which is in accordance with the changing pattern of immune response in the corneal stroma.
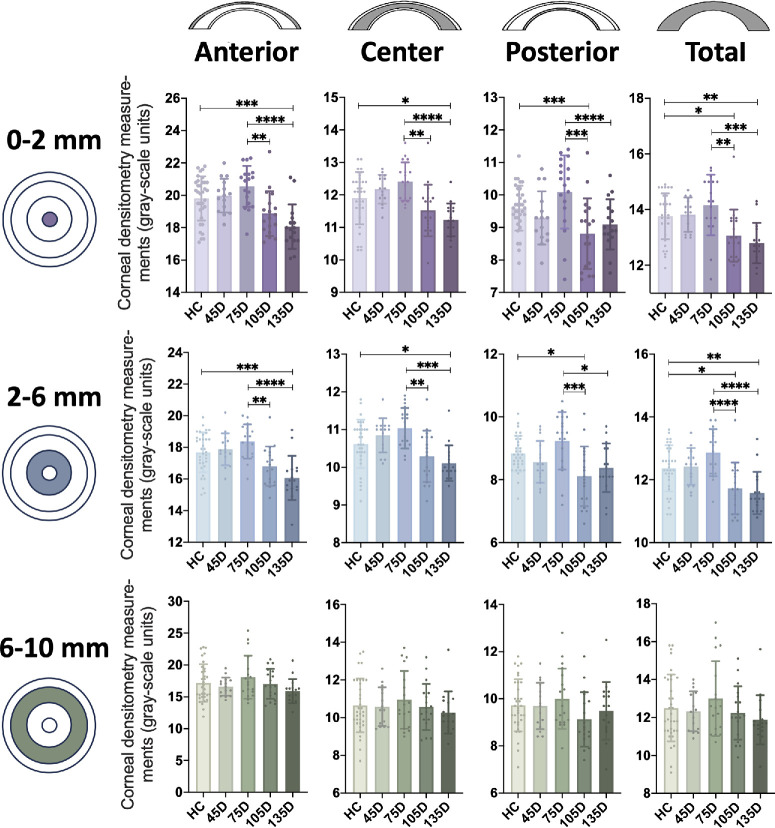
Corneal Optical Density Follows a Specific Changing Pattern After COVID-19 Recovery
With the corneal inflammation and corneal nerve alternations after COVID-19 recovery, we wonder whether these changes affect clinical practice after refractive surgeries. CD is highly associated with both corneal inflammation and refractive surgery evaluation.44 We first evaluated the changing pattern of CD at different time points after COVID-19 recovery. A total of 34 HCs and 67 recovered patients were included. The recovered patients are further divided into 4 groups according to the time duration from the first negative test after SARS-CoV-2 infection to the ocular examination. The detailed information of these individuals is listed in Supplementary Table S4. As shown in Figure 5 and Table 3, the CD values of anterior 0 to 2 zones were first mildly higher in the 45 D and the 75 D groups after COVID-19 recovery compared to the HCs, and then became similar to the HCs at around 105 D and even lower than the HCs at around 135 post recovery. Moreover, the CD values of central and total 0 to 2 zones followed a similar changing pattern. Furthermore, the CD values of the posterior 0 to 2 zones were first higher in the 75 D group post recovery, then became lower than the HCs in the 105 D group and were slightly higher at 135 D post recovery.
Figure 5.
Changes of corneal optical density (CD) after different time of COVID-19 recovery. CD was vertically measured across three different layers [anterior (the superficial 120-µm-thick area), central (between anterior and posterior layer), and posterior (the innermost 60-µm-thick area)] and horizontally across three different zones (diameters of 0 to 2, 2 to 6, 6 to 10, and 10 to 12 mm). The CD values of the anterior, central, posterior, and total 0-2 zones and 2-6 zones followed a similar changing pattern after different time duration of COVID-19 recovery, whereas the changes of anterior, central, posterior, and total 6 to 10 zones were not significantly different at different recovered duration. CD: corneal optical density.
Table 3.
Corneal Densitometry in Different Corneal Circles and Depth in Myopic Refractive Surgery Candidates at Different Time Points After COVID-19 Recovery
| CD Values (Mean ± SD) | HC (n = 34) | ~45 D (n = 15) | ~75 D (n = 18) | ~105 D (n = 18) | ~135 D (n = 14) | P Value# | P Value^ | P Value† | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| 0 to 2 mm | |||||||||
| Anterior | 19.81 ± 1.37 | 19.98 ± 1.04 | 20.55 ± 1.20 | 18.88 ± 1.38 | 18.06 ± 1.38*** | 0.9934 | 0.306 | 0.1159 | 0.0003 |
| Central | 11.90 ± 0.80 | 12.17 ± 0.44 | 12.41 ± 0.59 | 11.52 ± 0.79 | 11.23 ± 0.50* | 0.6942 | 0.0827 | 0.3217 | 0.0138 |
| Posterior | 9.59 ± 0.69 | 9.29 ± 0.82 | 10.09 ± 1.12 | 8.81 ± 1.09† | 9.09 ± 0.77 | 0.8149 | 0.3007 | 0.0266 | 0.352 |
| Total | 13.76 ± 0.82 | 13.82 ± 0.62 | 14.17 ± 1.09 | 13.07 ± 0.93† | 12.80 ± 0.72** | 0.9995 | 0.4866 | 0.0493 | 0.0032 |
| 2 to 6 mm | |||||||||
| Anterior | 17.68 ± 1.27 | 17.87 ± 1.01 | 18.37 ± 1.08 | 16.81 ± 1.25 | 16.06 ± 1.39*** | 0.9858 | 0.3054 | 0.1081 | 0.0003 |
| Central | 10.62 ± 0.64 | 10.85 ± 0.45 | 11.03 ± 0.54 | 10.29 ± 0.68 | 10.11 ± 0.47* | 0.7142 | 0.1139 | 0.3064 | 0.0372 |
| Posterior | 8.83 ± 0.57 | 8.57 ± 0.67 | 9.24 ± 0.91 | 8.11 ± 0.95† | 8.38 ± 0.78 | 0.7919 | 0.3605 | 0.0134 | 0.2953 |
| Total | 12.36 ± 0.74 | 12.43 ± 0.59 | 12.87 ± 0.75 | 11.73 ± 0.83† | 11.58 ± 0.68** | 0.9987 | 0.1321 | 0.027 | 0.0051 |
| 6 to 10 mm | |||||||||
| Anterior | 17.17 ± 2.95 | 16.60 ± 1.44 | 18.07 ± 3.38 | 17.02 ± 2.35 | 15.90 ± 1.90 | 0.9554 | 0.7708 | 0.9997 | 0.5035 |
| Central | 10.65 ± 1.43 | 10.58 ± 1.03 | 10.94 ± 1.53 | 10.57 ± 1.22 | 10.27 ± 1.12 | 0.9998 | 0.9371 | 0.9996 | 0.8771 |
| Posterior | 9.72 ± 1.11 | 9.69 ± 0.98 | 10.00 ± 1.28 | 9.13 ± 1.15 | 9.49 ± 1.23 | 0.9999 | 0.9227 | 0.3941 | 0.9611 |
| Total | 12.50 ± 1.77 | 12.33 ± 1.05 | 13.00 ± 1.97 | 12.24 ± 1.41 | 11.88 ± 1.30 | 0.9965 | 0.8207 | 0.9792 | 0.6989 |
Compared 45 D with HCs.
Compared 75 D with HCs.
Compared 105 D with HCs
Compared 135 D with HCs.
Moreover, the CD values of anterior, central, posterior, and total 2 to 6 zones followed a similar changing pattern with corresponding portions in the 0 to 2 zones. The CD values of the anterior 2 to 6 zones were first mildly higher in the 45 D and the 75 D groups after COVID recovery compared to the HCs, and became lower at the 105 D and 135 D groups to a level lower than the HCs. Furthermore, the CD values of the central and the total 2 to 6 zones followed a similar changing pattern. Furthermore, the CD values of the posterior 2 to 6 zones were first higher in the 75 D group post recovery, then became lower than the HCs in the 105 D group and were slightly higher at 135 D post recovery.
However, although the CD values of the anterior, central, posterior, and total 6 to 10 zones followed a similar changing pattern with corresponding portions in the 0 to 2 and 2 to 6 zones, the changes were not significant.
Taken together, the CD values in the 0 to 2 and 2 to 6 zones follow a specific changing pattern at different time points post COVID-19 recovery, whereas they were first higher in the 45 D or 75 D groups and became lower in the 105 D and 135 D groups to a level lower than the HCs.
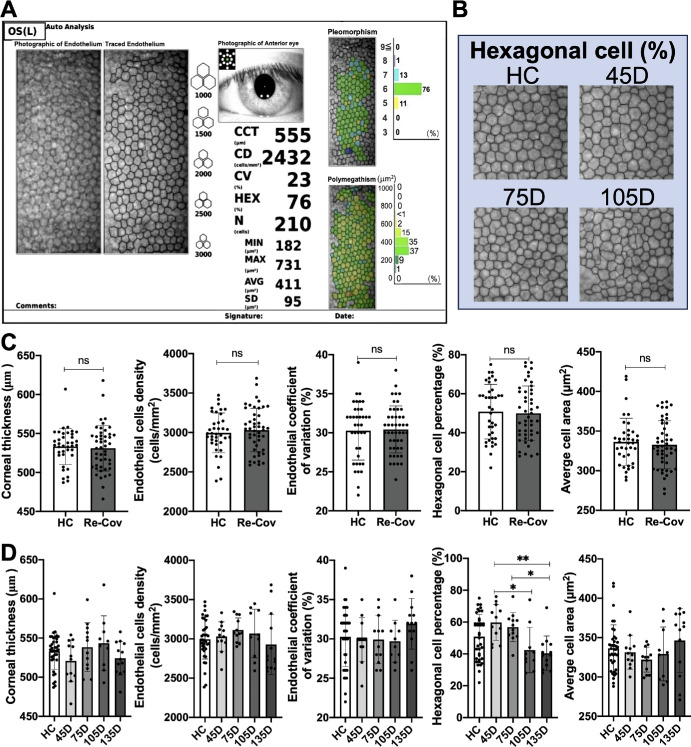
Endothelial HEX are Reduced in Patients Recovered From COVID-19 After Around 135 Days of Recovery
Because endothelial cells are essential for the maintenance of stromal uptake and corneal transparency, we then evaluated the parameters of endothelial cells by non-contact specular microscope (Topcon SP-1P; Fig. 6A). As a result, there were no significant differences in the CCT (µm), ECD (cells/mm2), endothelial CV (%), HEX percentage (%), and AVG (µm2) between the HCs and the recovered patients with COVID regardless of recovered time duration (Fig. 6C). Then, we further divided the COVID-19 recovered group into 4 subgroups based on the duration post their first negative test. As shown in Figures 6B and D, the percentage of HEX (%) was first slightly higher in the 45 D group post recovery compared to the HCs (P = 0.22) and was then significantly downregulated compared to 45 days at around 105 days (*P = 0.02) and 135 days (**P = 0.002). However, there were no significant differences in the other parameters across varying recovery spans.
Figure 6.
Evaluation of endothelial cell parameters after COVID-19 recovery. (A) Endothelial cells of the HCs and the patients with COVID-19 who recovered are measured by non-contact specular microscope Topcon SP-1P. (B) Endothelium images of the HCs and the patients with COVID-19 who recovered after different times of recovery are displayed. (C) Metrics including CCT (µm), CD (cells/mm2), CV (%), hexagonal cell percentage (%), and AVG (µm2) exhibit no significant variations between HCs and the patients with COVID who recovered. (D) Among the recovered groups, hexagonal cell percentages experience notable changes, peaking in the 45 D group and then progressively decreasing in the next 3 groups, with pronounced declines in the 105 D (*P = 0.02) and 135 D groups post-recovery (**P = 0.002). Nonetheless, CCT, ECD, CV, and AVG metrics remained consistent across HC and recovery subgroups. HC: healthy control; CCT: central corneal thickness; ECD: endothelial cell density; CV: endothelial coefficient of variation; AVG: average cell area.
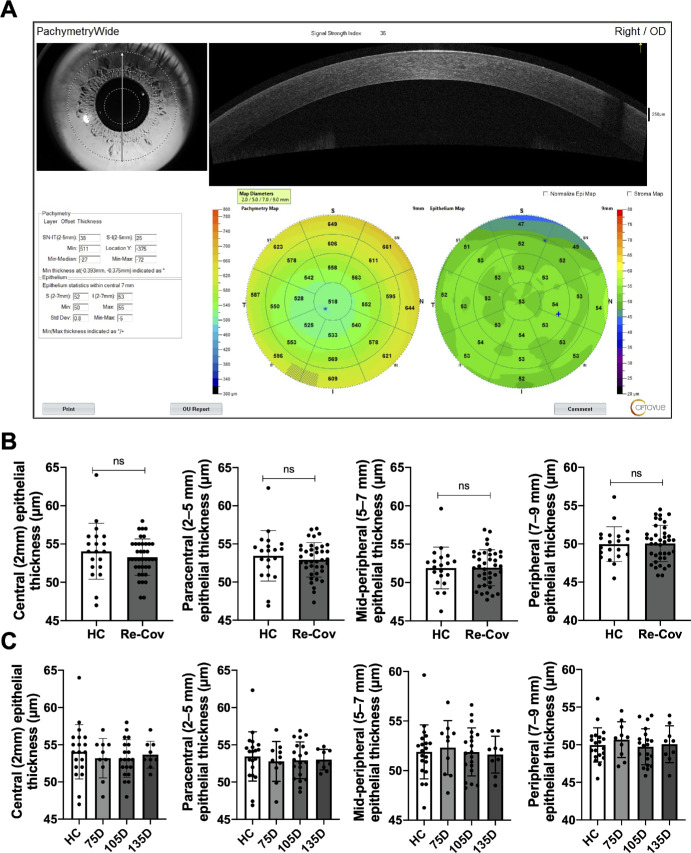
Epithelial Thickness is not Significantly Changed After COVID-19 Recovery
To explore possible changes in epithelial cells after COVID-19 recovery, we measured ET using wide-field OCT (Fig. 7A). As a result, the ET (µm) in the central (2 mm), paracentral (2–5 mm), mid-peripheral (5–7 mm), or peripheral (7–9 mm) zones were not significantly different between the HCs and recovered patients with COVID-19 regardless of recovered time duration (Fig. 7B). Then, we further divided the COVID-19 recovered group into 4 subgroups based on the duration post their first negative test. However, there were also no significant differences in the epithelial metrics after different recovered durations (Fig. 7C). Taken together, the epithelial cell thickness was not significantly altered in patients who recovered from COVID-19.
Figure 7.
Epithelial cell thickness is not significantly altered in COVID-19 recovered patients. ET (mm) of central (2 mm), paracentral (2–5 mm), mid-peripheral (5–7 mm), or peripheral (7–9 mm) zones was measured by wide-field OCT (A). No notable disparities in the ET were detected between HC and Re-Cov across recovery periods (B and C). ET: epithelial thickness; HC: healthy control; Re-Cov: recovered patients with COVID-19.
SARS-CoV-2 Infection Does Not Affect Long-Term Outcomes of Refractive Surgery After Recovery
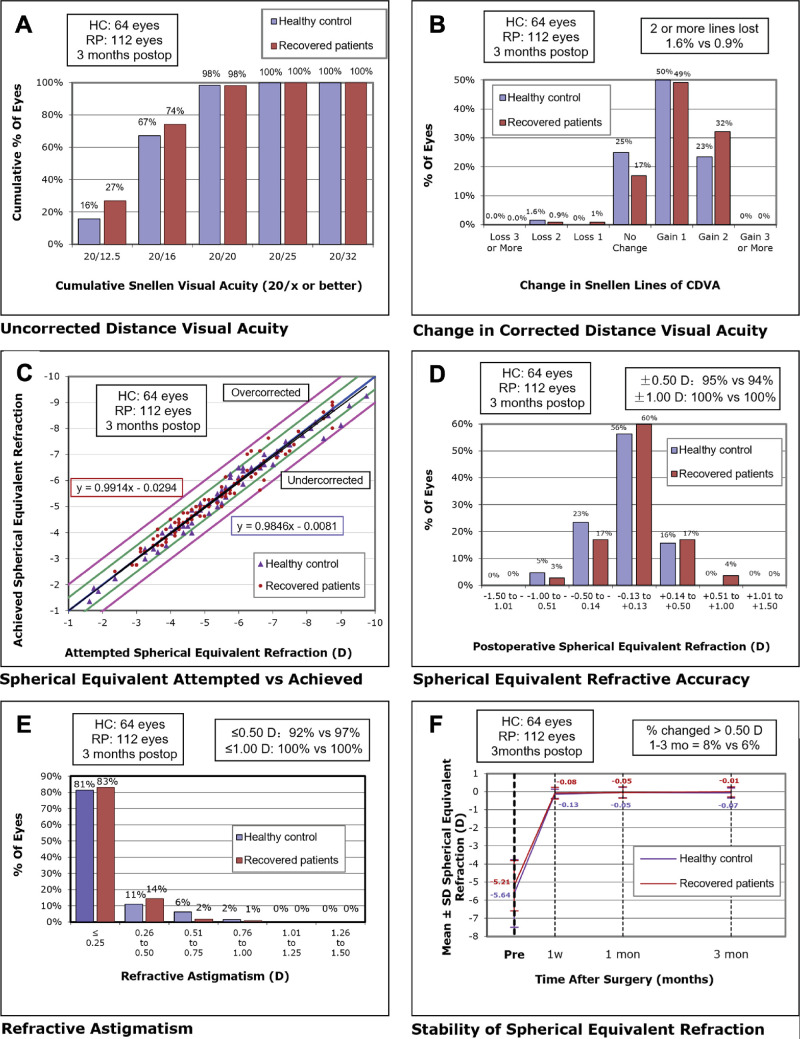
To assess whether the changes in the corneal stroma and endothelium would affect the surgery outcomes of SMILE, we recruited 39 HCs and 54 patients with COVID-19 who recovered and underwent SMILE surgery. The pre-operative demographic and clinical characteristics of the HCs and the patients who recovered from COVID-19 are not significantly different (Supplementary Table S5). All procedural interventions were completed uneventfully, without any intra-operative or postoperative complications. As a result, both groups of participants who underwent SMILE procedures attained favorable clinical outcomes after 3 months of follow-up. The postoperative visual acuity and refractive correction data are presented in Table 4 and Figure 8. As a result, the UDVA logMAR (P = 0.83) and CDVA logMAR (P = 0.77) at 3 months follow-up were not significantly different between the HCs and the patients who recovered from COVID-19. Specifically, 98% of the eyes in both the HC group and the COVID-19 recovered group attained a UDVA of 20/20 or better 3 months postoperatively (see Fig. 8A). Moreover, 98% of the eyes in both groups show an unchanged or better CDVA (Fig. 8B). Furthermore, the surgical predictability of both groups, which is reflected by the attempted and achieved spherical equivalent (SE) correction, is illustrated with a scatterplot in Figure 8C. Both cohorts displayed high precision, with 95% (HC) and 94% (recovered patients) achieving within ± 0.50 D. In addition, the SE of all eyes in both groups achieved ± 1.0 D at 3 months follow-up, as illustrated in Figure 8D. Concerning astigmatism correction, the postoperative astigmatism of 92% treated eyes in the HC group and 97% treated eyes in the recovered patients group were within ± 0.50 D cylinder, as depicted in Figure 8E. Additionally, 8% and 7% of eyes in the HCs and the patients who recovered from COVID-19, respectively, experienced a change of more than 0.50 D in SE from 1 to 3 months, as presented in Figure 8F. Taken together, there were no significant differences in the effectiveness, safety, and predictability of SMILE surgery between the HCs and the patients who recovered from COVID-19.
Table 4.
Post-SMILE Visual Outcomes in Healthy Controls and Recovered Patients at 3 Months Follow-Up
| Parameters (Mean ± SD) | Healthy Control (HC) (n = 32) | Recovered Patients (n = 56) | P Value |
|---|---|---|---|
| UDVA (logMAR) | −0.07 ± 0.06 (−0.18 to 0.00) | −0.08 ± 0.07 (−0.18 to 0.10) | 0.8293 |
| CDVA (logMAR) | −0.08 ± 0.07 (−0.18 to 0.00) | −0.09 ± 0.07 (−0.18 to 0.05) | 0.7697 |
| Sphere (D) | 0.08 ± 0.27 (−0.75 to 0.50) | 0.08 ± 0.28 (−0.75 to 0.75) | 0.9707 |
| Cylinder (D) | −0.26 ± 0.22 (−0.75 to 0.00) | −0.21 ± 0.24 (−1.00 to 0.00) | 0.5598 |
| SE (D) | −0.05 ± 0.28 (−0.88 to 0.50) | −0.03 ± 0.28 (−0.75 to 0.75) | 0.8287 |
Figure 8.
Visual outcomes after SMILE in HCs and recovered patients. (A) UDVA outcomes, (B) changes in CDVA, (C) distribution of achieved SE outcomes, (D) SE refractive accuracy, (E) refractive astigmatism, and (F) stability of SE refraction at 3 months postoperatively. SMILE: small incision lenticule extraction; HC: healthy controls; UDVA: uncorrected distance visual acuity; CDVA: corrected distance visual acuity; SE: spherical equivalent; D: diopters.
Then, because corneal morphology and function alternations followed specific changing patterns, we wonder whether patients who underwent surgery after different recovered durations would exhibit different surgery outcomes. As a result, however, there are no significant differences in the postoperative UDVA, CDVA, or achieved SE among the HC group and different groups of the patients with COVID-19 who recovered (underwent SMILE surgery at around 45 days, 75 days, and 105 days after COVID-19 recovery; see Table 5).
Table 5.
Post-SMILE Visual Outcomes at 3 Months Follow-Up of Healthy Controls and Recovered Patients Underwent Surgery at Different Time of Recovery
| Days From COVID-19 Recovery (Tested Negative) to Sampling | |||||
|---|---|---|---|---|---|
| Parameter | Healthy Control (HC) (n = 32) | 10 ∼ 55 D (n = 16) | 55 ∼ 95 D (n = 20) | 95 ∼ 136 D (n = 20) | P Value |
| UDVA (logMAR) | −0.07 ± 0.06 (−0.18 to 0.00) | −0.08 ± 0.06 (−0.18 to 0.00) | −0.05 ± 0.07 (−0.18 to 0.10) | −0.09 ± 0.07 (−0.18 to 0.00) | 0.1328 |
| CDVA (logMAR) | −0.08 ± 0.07 (−0.18 to 0.00) | −0.10 ± 0.06 (−0.18 to 0.00) | −0.07 ± 0.07 (−0.18 to 0.05) | −0.10 ± 0.06 (−0.18 to 0.00) | 0.3936 |
| Sphere (D) | 0.08 ± 0.27 (−0.75 to 0.50) | 0.06 ± 0.23 (−0.50 to 0.50) | 0.11 ± 0.40 (−0.75 to 0.75) | 0.05 ± 0.13 (−0.25 to 0.25) | 0.9032 |
| Cylinder (D) | −0.26 ± 0.22 (−0.75 to 0.00) | −0.27 ± 0.23 (−0.75 to 0.00) | −0.24 ± 0.29 (−1.00 to 0.00) | −0.15 ± 0.17 (−0.50 to 0.00) | 0.3584 |
| SE (D) | −0.05 ± 0.28 (−0.88 to 0.50) | −0.07 ± 0.20 (−0.50 to 0.25) | −0.01 ± 0.40 (−0.75 to 0.75) | −0.03 ± 0.16 (−0.25 to 0.25) | 0.9006 |
Discussion
In the present study, we evaluated the susceptibility of corneal stroma to SARS-CoV-2 and comprehensively assessed the changes in immune response, nerve parameters, and function of the corneal stroma after COVID-19 recovery as well as the impact on SMILE surgery. To our knowledge, this study illustrated for the first time the detailed alternations of living tissue at different time points after COVID-19 recovery with direct evidence. Moreover, this study also provides a theoretical basis for corneal health and refractive surgery strategy in the post COVID-19 era.
We first addressed the susceptibility of corneal stroma to SARS-CoV-2 infection by assessing the expression of receptors ACE2 and CD147. Although previous studies have reported that CD147 is expressed in the corneal stroma,39,45 it remains controversial whether ACE2 is expressed in the corneal stroma. Heidi et al.46 and Kiyofumi et al.39 reported positive expression of ACE2 in the corneal stroma by immunofluorescence and single-cell sequencing, respectively. However, other studies showed negative results.20,22 For these contrary discoveries, we speculate that the discrepancy between studies might be due to a lack of sample size and distinct assessment methods, because according to our results, only 63.6% of samples are positive, and the rates of ACE-positive cells vary greatly in different individuals. In addition, with the verification of SARS-CoV-2 receptors, we identified spike protein residue in the corneal stroma, even 53 days after COVID-19 recovery, which indicates that SARS-CoV-2 might remain for a time. Previously, only one article reported SARS-CoV-2 RNA remanence in the corneal epithelium of a patient with COVID-19 who recovered with unilateral keratouveitis.47 However, no studies have reported SARS-CoV-2 remanence in human corneal stroma after COVID-19 recovery. We presume that the relatively low concentration of SARS-CoV-2 residue might account for the lack of discovery. Despite the low concentration of residue, the persistence of the SARS-CoV-2 component might partially account for disturbed immune response and aberrant morphology of the cornea stroma,48 which is then suggested by our results.
Moreover, the strength of our study is the demonstration of inflammatory response and mid-stroma nerve changes in the corneal stroma after COVID-19 recovery. Our data showed that the immune response was stimulated at around 15 days to 75 days post COVID-19 recovery and improved after around 105 days (see Fig. 3A). Although sustained corneal immune response after COVID-19 recovery was reported by several studies,28,29,49 their results only provided indirect evidence. For example, Zsofia et al. found elevated corneal DC density in patients who recovered for 13.9 ± 6.1 weeks in the cornea,29 and no differences were found in the DC densities later after 144.4 ± 104 days of recovery.50 These studies supported the time points of our findings. As reported by a recent study, keratocytes could express and secret IL-6 after sterile corneal injury, which is associated with corneal nerve loss.51 Accordingly, we found that similar to immune changes, the length, and the branches of corneal mid-stroma nerves were first higher in the 45 D and 75 D groups and returned to the level of the HCs at around 135 days post recovery (see Fig. 3). It can be inferred that the mid-stromal nerves initially exhibit a proliferative response in the presence of local inflammatory stimuli. It is postulated that the delayed peak in mid-stromal nerve parameters, observed approximately at 75 days, may be consequent to an underlying immune-mediated proliferative response. Subsequently, as the inflammation resolves, the density of these nerve fibers appears to regress toward baseline values. This discovery could be explained by the previous studies. Many studies indicated that there is a synergy between immune activation and corneal nerve changes.29,52–54 Some inflammatory molecules (including IL-6) could enhance neurite growth and axonal regeneration.55,56 Moreover, the nerve-growth potential stimulated by neuroinflammation was stronger in younger generations,56 whereas our study mainly focused on the mid-twenties (25.46 ± 5.28 years old). As such, increased corneal nerve branch density was observed in the corneas of patients after COVID-19 recovery. It is worth mentioning that because this is a cross-sectional study, the changing patterns we observed are based on various patients recovered from COVID-19 after different durations, instead of longitudinal observations of the same cohorts.
According to experimental results, we observed that CD was initially higher and then became lower after SARS-CoV-2 infection, which is highly similar to the pattern of changes in corneal inflammation post-infection.57 We hypothesize that SARS-CoV-2 might induce an inflammatory response in the corneal stroma through inflammatory factors and effector cells (such as DCs), leading to a decrease in corneal transparency and an increase in CD. Meanwhile, the late decrease in CD may represent a reparative response subsequent to inflammation.44 It is worth mentioning that previous studies have demonstrated a correlation between corneal optical density and a variety of visual parameters, including visual clarity, higher-order aberrations, and contrast sensitivity, depending on the extent of densitometry alterations.44,58,59 The findings from our current research indicate that post-COVID-19 changes in corneal densitometry do not detrimentally affect the visual acuity of patients who have undergone refractive surgery at 3 months of follow-up. However, further studies are needed to incorporate a more comprehensive array of visual quality evaluations.
Moreover, considering the higher expression of classical SARS-CoV-2 receptors on the corneal epithelium and endothelium, we also evaluated changes in these layers.46 The results indicate that there were no significant changes in corneal epithelium during the COVID-19 recovery period. Interestingly, the HEX proportion of the endothelial cells exhibited a late decrease, possibly attributed to transient enhancement in endothelial cell function due to inflammatory stimulation. Do these changes in corneal stroma and endothelium during the COVID-19 recovery period affect corneal refractive surgery? Through clinical follow-up observations, we have confirmed for the first time that the safety, efficacy, and stability of SMILE surgery are comparable between patients in the COVID-19 recovery period and patients with normal high myopia. Therefore, it is appropriate for patients with prior SARS-CoV-2 infection to undergo SMILE surgery, although some parameters changed. Our study provides important additional information regarding the clinical indications for SMILE surgery.
In general, we obtained fresh corneal stroma through SMILE surgery from patients with COVID-19 who recovered and uninfected individuals and provided direct experimental evidence of structural and functional changes in the cornea during the COVID-19 recovery period. Moreover, we confirmed the effectiveness and safety of performing SMILE surgery on patients who had previously recovered from COVID-19. The direct acquisition of fresh samples is a significant advantage of this study, which is highly beneficial for investigating the COVID-19 recovery period and also has great potential for application in research on other diseases, such as cognitive impairments and depression. This study has significant implications not only for the evaluation of corneal refractive surgery in the post-pandemic era but also for research in other fields.
Supplementary Material
Acknowledgments
Supported and funded by Science and Technology Planning Project of Guangzhou City (Grant No. 201803010091).
Author Contributions: Conception and design: Keming Yu and Jing Zhuang; Analysis and interpretation: Yuke Huang, Taiwei Chen, Xi Chen; Data collection: Linxi Wan, Jingyi Jiang, Xiangtao Hou, Jiejie Zhuang, Yan Li, Jin Qiu; Overall responsibility: Keming Yu and Jing Zhuang.
Ethics Approval and Consent to Participate: Human subjects were included in this study. The study protocol was approved by the ethics board of the Zhongshan Ophthalmic Center of Sun Yat-Sen University (Identifier No. 2023KYPJ332) and the study honored the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
Disclosure: Y. Huang, None; T. Chen, None; X. Chen, None; L. Wan, None; X. Hou, None; Jiejie Zhuang, None; J. Jiang, None; Y. Li, None; J. Qiu, None; K. Yu, None; Jing Zhuang, None
References
- 1. Hu B, Guo H, Zhou P, Shi ZL.. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021; 19(3): 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The L. Understanding long COVID: a modern medical challenge. Lancet. 2021; 398(10302): 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akbarialiabad H, Taghrir MH, Abdollahi A, et al.. Long COVID, a comprehensive systematic scoping review. Infection. 2021; 49(6): 1163–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woltsche JN, Horwath-Winter J, Dorn C, et al.. Neuropathic corneal pain as debilitating manifestation of long-COVID. Ocul Immunol Inflamm. 2022; 31(6): 1216–1218. [DOI] [PubMed] [Google Scholar]
- 5. Asadi-Pooya AA, Akbari A, Emami A, et al.. Long COVID syndrome-associated brain fog. J Med Virol. 2022; 94(3): 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Huang L, Wang Y, et al.. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021; 397(10270): 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wasfy T, Eldesouky MA, Serag Y, Elbedewy HA.. Concurrent and post COVID-19 ophthalmological implications. Clin Ophthalmol (Auckland, NZ). 2021; 15: 4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehandru S, Merad M.. Pathological sequelae of long-haul COVID. Nat Immunol. 2022; 23(2): 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021; 53(10): 737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Proal AD, VanElzakker MB.. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021; 12: 698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein SR, Ramelli SC, Grazioli A, et al.. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022; 612(7941): 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R.. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci USA. 2021; 118(21): e2105968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forrester JV, McMenamin PG, Dando SJ.. CNS infection and immune privilege. Nat Rev Neurosci. 2018; 19(11): 655–671. [DOI] [PubMed] [Google Scholar]
- 14. Fijak M, Meinhardt A.. The testis in immune privilege. Immunol Rev. 2006; 213: 66–81. [DOI] [PubMed] [Google Scholar]
- 15. Hori J, Yamaguchi T, Keino H, Hamrah P, Maruyama K.. Immune privilege in corneal transplantation. Prog Retin Eye Res. 2019; 72: 100758. [DOI] [PubMed] [Google Scholar]
- 16. Chen M, Luo C, Zhao J, Devarajan G, Xu H.. Immune regulation in the aging retina. Prog Retin Eye Res. 2019; 69: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin TPH, Ko CN, Zheng K, et al.. COVID-19: update on its ocular involvements, and complications from its treatments and vaccinations. Asia Pac J Ophthalmol (Phila). 2021; 10(6): 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong L, Collin J, Mostafa I, Queen R, Figueiredo FC, Lako M.. In the eye of the storm: SARS-CoV-2 infection and replication at the ocular surface? Stem Cells Transl Med. 2021; 10(7): 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maurin C, He Z, Mentek M, et al.. Exploration of the ocular surface infection by SARS-CoV-2 and implications for corneal donation: an ex vivo study. PLoS Med. 2022; 19(3): e1003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sungnak W, Huang N, Bécavin C, et al.. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020; 26(5): 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roehrich H, Yuan C, Hou JH.. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020; 39(12): 1556–1562. [DOI] [PubMed] [Google Scholar]
- 22. Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ.. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020; 18(4): 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawant OB, Singh S, Wright RE 3rd, et al.. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf. 2021; 19: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cano-Ortiz A, Leiva-Gea I, Ventosa ÁS, et al.. Stromal interstitial keratitis in a patient with COVID-19. J Fr Ophtalmol. 2022; 45(4): e175–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zuo DM, Xue LP, Fan H, et al.. COVID-19 infection with keratitis as the first clinical manifestation. Int J Ophthalmol. 2022; 15(9): 1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin SX, Juthani VV.. Acute corneal endothelial graft rejection with coinciding COVID-19 infection. Cornea. 2021; 40(1): 123–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh G, Mathur U.. Acute graft rejection in a COVID-19 patient: co-incidence or causal association? Indian J Ophthalmol. 2021; 69(4): 985–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bitirgen G, Korkmaz C, Zamani A, et al.. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. 2022; 106(12): 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolkedi Z, Csutak A, Szalai E.. Corneal cellular and neuroinflammatory changes after SARS-CoV-2 infection. Cornea. 2022; 41(7): 879–885. [DOI] [PubMed] [Google Scholar]
- 30. Yoo TK, Ryu IH, Lee G, et al.. Adopting machine learning to automatically identify candidate patients for corneal refractive surgery. NPJ Digit Med. 2019; 2(1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ambrósio R Jr., Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg. 2003; 19(1): 24–29. [DOI] [PubMed] [Google Scholar]
- 32. Beyerstedt S, Casaro EB, Rangel ÉB.. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021; 40(5): 905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al.. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020; 370(6518): 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang K, Chen W, Zhang Z, et al.. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020; 5(1): 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin G, Wolf J, Lapp T, et al.. Viral S protein histochemistry reveals few potential SARS-CoV-2 entry sites in human ocular tissues. Sci Rep. 2021; 11(1): 19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gusev E, Sarapultsev A, Solomatina L, Chereshnev V.. SARS-CoV-2-specific immune response and the pathogenesis of COVID-19. Int J Mol Sci. 2022; 23(3): 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aliyu M, Zohora FT, Anka AU, et al.. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol. 2022; 111: 109130. [DOI] [PubMed] [Google Scholar]
- 38. Anaya JM, Herrán M, Beltrán S, Rojas M.. Is post-COVID syndrome an autoimmune disease? Expert Rev Clin Immunol. 2022; 18(7): 653–666. [DOI] [PubMed] [Google Scholar]
- 39. Hamashima K, Gautam P, Lau KA, et al.. Potential modes of COVID-19 transmission from human eye revealed by single-cell atlas. bioRxiv. 2020:2020.05.09.085613, doi: 10.1101/2020.05.09.085613. [DOI]
- 40. Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010; 86(2): 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Orita T, Kimura K, Zhou HY, Nishida T.. Poly(I:C)-induced adhesion molecule expression mediated by NF-{kappa}B and phosphoinositide 3-kinase-Akt signaling pathways in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2010; 51(11): 5556–5560. [DOI] [PubMed] [Google Scholar]
- 42. Liu P, Liu Y, Zheng H, et al.. Sulforaphane suppresses polyinosinic‑polycytidylic acid‑stimulated release of cytokines, chemokines and MMPs by human corneal fibroblasts. Mol Med Rep. 2020; 22(6): 5463–5471. [DOI] [PubMed] [Google Scholar]
- 43. Andalib S, Biller J, Di Napoli M, et al.. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2021; 21: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He Y, Ma BS, Zeng JH, Ma DJ.. Corneal optical density: Structural basis, measurements, influencing factors, and roles in refractive surgery. Front Bioeng Biotechnol. 2023; 11: 1144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Määttä M, Tervahartiala T, Kaarniranta K, et al.. Immunolocalization of EMMPRIN (CD147) in the human eye and detection of soluble form of EMMPRIN in ocular fluids. Curr Eye Res. 2006; 31(11): 917–924. [DOI] [PubMed] [Google Scholar]
- 46. Roehrich H, Yuan C, Hou JH.. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020; 39(12): 1556–1562. [DOI] [PubMed] [Google Scholar]
- 47. Kuo IC, Mostafa HH.. Detection of SARS-CoV-2 RNA in the corneal epithelium of a patient after recovery from COVID-19. Am J Ophthalmol Case Rep. 2021; 22: 101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erren TC, Lewis P.. Secret hiding places in the eye and beyond: what about after SARS-CoV-2 infection? Graefes Arch Clin Exp Ophthalmol. 2021; 259(12): 3815–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gulfidan B, Celalettin K, Adil Z, et al.. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. 2022; 106(12): 1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Midena E, Cosmo E, Cattelan AM, et al.. Small fibre peripheral alterations following COVID-19 detected by corneal confocal microscopy. J Pers Med. 2022; 12(4): 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee HJ, Kim HJ, Ko JH, Oh JY.. Myeloid cells protect corneal nerves against sterile injury through negative-feedback regulation of TLR2-IL-6 axis. J Neuroinflammation. 2023; 20(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tepelus TC, Chiu GB, Huang J, et al.. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol. 2017; 255(9): 1771–1778. [DOI] [PubMed] [Google Scholar]
- 53. Hamrah P, Cruzat A, Dastjerdi MH, et al.. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010; 117(10): 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cruzat A, Witkin D, Baniasadi N, et al.. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011; 52(8): 5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chisholm SP, Cervi AL, Nagpal S, Lomax AE.. Interleukin-17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J Neurosci. Jan 25 2012; 32(4): 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L.. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediators Inflamm. 2017; 2017: 9478542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Orucoglu F, Talaz S, Aksu A, Muftuoglu O.. Corneal densitometry evaluation in archipelago keratitis. Int Ophthalmol. 2014; 34(1): 99–102. [DOI] [PubMed] [Google Scholar]
- 58. Tekin K, Kiziltoprak H, Koc M, Goker YS, Kocer AM, Yilmazbas P.. The effect of corneal infiltrates on densitometry and higher-order aberrations. Clin Exp Optom. 2019; 102(2): 140–146. [DOI] [PubMed] [Google Scholar]
- 59. Hou C, Li J, Li J, Peng H, Wang Q.. In vivo confocal microscopy of sub-basal corneal nerves and corneal densitometry after three kinds of refractive procedures for high myopia. Int Ophthalmol. 2023; 43(3): 925–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.