ABSTRACT
Introduction:
Acute myeloid leukaemia (AML) is a tumour of hematopoietic progenitors caused by acquired oncogenic mutations that impede differentiation, leading to the accumulation of immature myeloid blasts in the marrow. Aberrant phenotype is a phenomenon in which lymphoid-associated and other myeloid lineage markers are expressed in myeloblasts or myeloid-associated markers are expressed in lymphoblasts.
Materials and Methods:
Diagnosed cases of AML were included in this study to study the aberrant expression using multiparametric flow cytometry.
Results:
Out of a sample size of 50, 30 cases expressed aberrant CD markers. Male: Female ratio was 0.76. Majority of cases belonged to the age group >60 years of age. CD 7 was overall the most common aberrant CD marker.
Conclusion:
Immunophenotyping has a significant role in diagnosis and predicting prognosis of hematopoietic malignancies in the absence of more advanced diagnostic tools like cytogenetics.
Keywords: Aberrant expression, acute myeloid leukaemia, flow cytometry, immunophenotyping
Introduction
Acute myeloid leukaemia (AML) is a tumour of hematopoietic progenitors caused by acquired oncogenic mutations that impede differentiation, leading to the accumulation of immature myeloid blasts in the marrow. The diagnosis of AML is based on the presence of at least 20% myeloid blasts in the bone marrow.[1] A major role is played by multiparameter immunophenotyping flow cytometry in diagnosing AML in peripheral blood samples and bone marrow aspirate.[2,3] Aberrant phenotype is a phenomenon in which lymphoid-associated and other myeloid lineage markers expressed in myeloblasts or myeloid-associated markers are expressed in lymphoblasts.[4] The immunophenotyping study with multiparameter flow cytometry gives an idea about the prognosis of the disease.
Materials and Methods
This study is a single centre-based prospective observational study consisting of 50 diagnosed cases of AML on bone marrow studies attending the Department of Pathology, RIMS, Ranchi from May 2021 to May 2022. This study was conducted after proper clearance from Institutional Ethical Committee, RIMS, Ranchi (Memo No. –185, Dated 03/04/21). Immunophenotyping was done on acute leukaemia panels on already diagnosed patients of AML in bone marrow studies. A sample comprising of peripheral blood or bone marrow aspirate was processed as per the protocol. Tube 1 (unstained), tube 2 (B tube), tube 3 (T tube), tube 4 (myeloid tube) and tube 5 (cytoplasmic tube) comprise of CD45 (pan leukocytes marker), B cell markers, T cell markers, myeloid markers and cytoplasmic markers, respectively, as per acute leukaemia panel [Table 1]. After processing, the sample was acquired in BD FACS 6 colour flow cytometer.
Table 1.
Acute leukaemia panel (original)
| FITC | PE | PerCP Cy5.5 | PE-Cy7 | APC | APC-H7 | |
|---|---|---|---|---|---|---|
| Tube 1 (unstained) | Blank | Blank | Blank | Blank | Blank | CD 45 (3 µL) |
| Tube 2 (B-Tube) | CD 20 (10 µL) | CD 10 (10 µL) | CD 38 (10 µL) | CD 19 (3 µL) | CD 34 (3 µL) | CD 45 (3 µL) |
| Tube 3 (T-Tube) | CD 8 (10 µL) | CD 5 (10 µL) | CD 3 (3 µL) | CD 4 (3 µL) | CD 7 (3 µL) | CD 45 (3 µL) |
| Tube 4 (M-Tube) | CD 64 (10 µL) | CD 33 (10 µL) | HLA-DR (10 µL) | CD 13 (3 µL) | CD 117 (3 µL) | CD 45 (3 µL) |
| Tube 5 (Cytoplasmic Tube) | cMPO (10 µL) (added later) | CD 79a (10 µL) (added later) | CD 3 (10 µL) (added later) | Blank | CD 34 (3 µL) | CD 45 (3 µL) |
Results
In this study, among 50 diagnosed cases of AML on microscopy, 30 cases (60%) showed aberrant expression. Aberrant expression was observed more in females 17 cases (54%) as compared to males 13 cases (46%) with M: F ratio of 0.76. Age distribution among the cases of aberrant expression is listed in Table 2. The mean age of cases of AML with aberrant expression was found to be 48.34.
Table 2.
Age distribution of aberrant cases (original)
| Age (years old) | No of aberrant cases | Percentage of aberrant cases |
|---|---|---|
| 19–40 | 10 | 33.33% |
| 41–60 | 8 | 26.66% |
| >60 | 12 | 40% |
The most common aberrant lymphoid antigen expressions observed were of T cell lineage (16 cases, 53.33%). Aberrant expressions of both T and B cell markers were seen in 6 cases (20%), and 8 cases (26.66%) showed only B cell markers.
Out of 30 cases, single aberrant lymphoid expression (ly +) was seen in 19 cases, double lymphoid antigen expression (ly ++) in 9 cases and triple lymphoid antigen expression (ly +++) in 2 cases. In T lymphoid lineage, CD7 was the most common aberrant antigen (7 cases, 23.33%), followed by CD3 (3 cases, 10%) and CD4 (2 cases, 6.66%). In B lymphoid lineage, CD19 (4 cases, 13.33%) was the most common.
Case distribution of aberrant expressions (single and co-expression) of different lymphoid markers is depicted in Tables 3-7.
Table 3.
Cases of aberrant expression of CD7 (single and co-expression) (original)
| Aberrant expression | Number of cases |
|---|---|
| CD7 | 7 |
| CD7 and CD3 | 1 |
| CD7 and CD4 | 1 |
| CD7 and CD19 | 1 |
| CD7 and CD10 | 1 |
| CD7, CD19 and CD10 | 1 |
| CD7, CD4 and cCD79a | 1 |
Table 7.
Cases of aberrant expression of CD10 (single and co-expression) (original)
| Aberrant expression | Number of cases |
|---|---|
| CD10 | 3 |
| CD10 and CD4 | 1 |
| CD10 and CD3 | 1 |
| CD10 and CD7 | 1 |
| CD10, CD19 and CD7 | 1 |
Table 4.
Cases of aberrant expression of CD3 (single and co-expression) (original)
| Aberrant expression | Number of cases |
|---|---|
| CD3 | 3 |
| CD3 and CD7 | 1 |
| CD3 and CD10 | 1 |
| CD3 and CD4 | 1 |
Table 5.
Cases of aberrant expression of CD4 (single and co-expression) (original)
| Aberrant expression | Number of cases |
|---|---|
| CD4 | 2 |
| CD4 and CD19 | 2 |
| CD4 and CD10 | 1 |
| CD4 and CD7 | 1 |
| CD4 and CD3 | 1 |
| CD4, cCD79a and CD7 | 1 |
Table 6.
Cases of aberrant expression of CD19 (single and co-expression) (original)
| Aberrant expression | Number of cases |
|---|---|
| CD19 | 4 |
| CD19 and CD4 | 2 |
| CD19 and CD7 | 1 |
| CD 19, CD7 and CD10 | 1 |
Haematological profiles of cases with aberrant expression are shown in Tables 8-10. Figure 1 shows CD7 positivity. Heterogeneous positivity for CD7 & CD3 is shown in Figure 2. Figure 3 depicts CD19 positivity. CD7 & CD4 heterogeneous positivity is presented in Figure 4. Positivity for CD10 is shown in Figure 5.
Table 8.
Haemoglobin levels in AML with aberrant expression (original)
| Haemoglobin (gm%) | AML with aberrant expression (n=30) |
|---|---|
| <5 | 6 (20%) |
| 5–8 | 19 (63.33%) |
| >8 | 5 (16.66%) |
Table 10.
Platelets count in AML with aberrant expression (original)
| Platelets count (x10^5/mL) | AML with aberrant expression (n=30) |
|---|---|
| <0.5 | 20 |
| 0.5 to 1 | 7 |
| >1 | 3 |
Figure 1.
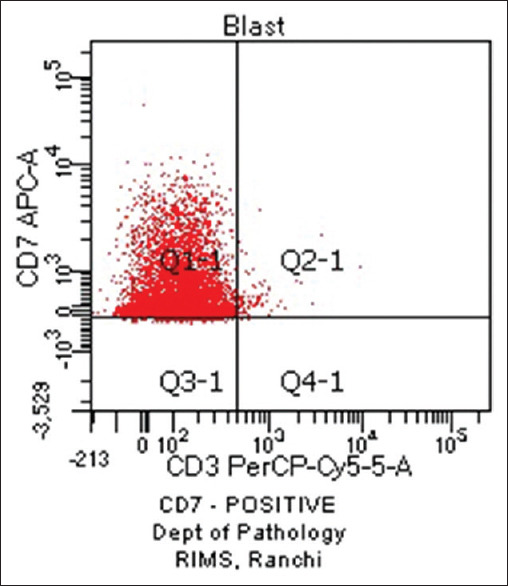
CD7 Positive (original)
Figure 2.
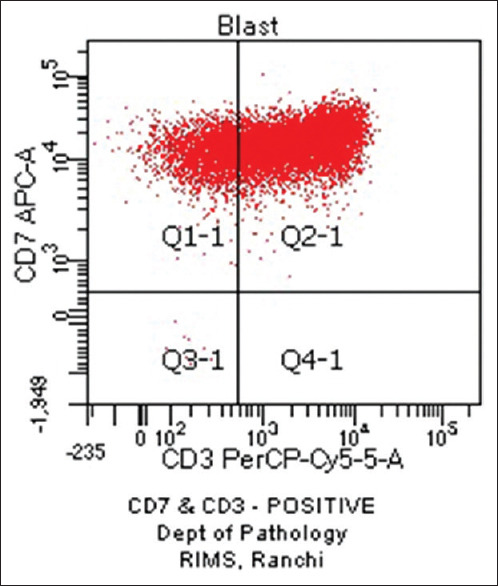
CD7 and CD3 Positive (original)
Figure 3.
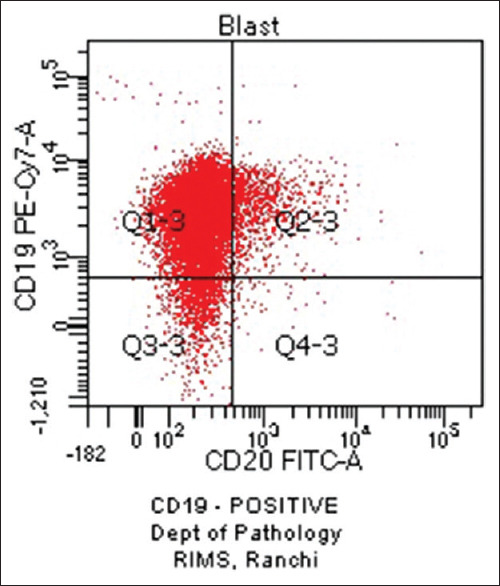
CD19 Positive (original)
Figure 4.
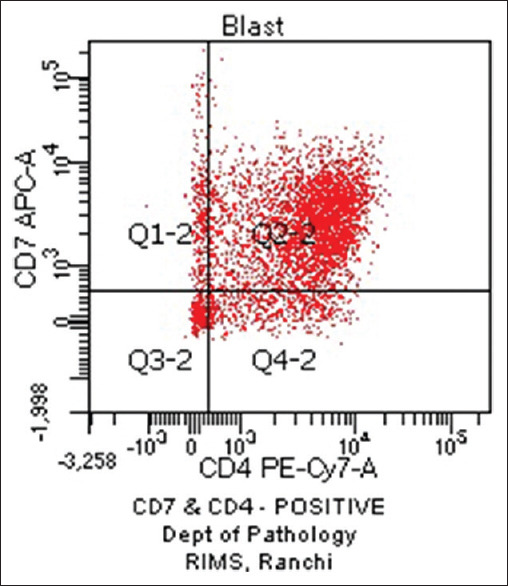
CD7 and CD4 Positive (original)
Figure 5.
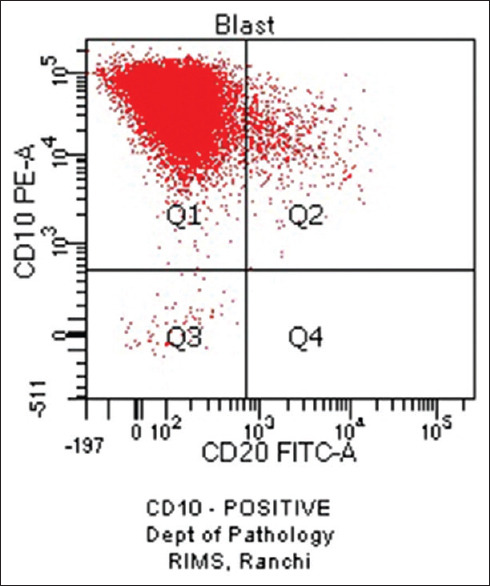
CD10 Positive (original)
Table 9.
Total leucocytic count (TLC) in AML with aberrant expression (original)
| TLC (X10^3/µL) | AML with aberrant expression (n=30) |
|---|---|
| <4 | 3 |
| 4 to 11 | 5 |
| 11 to 50 | 12 |
| >50 | 10 |
Figure 6.
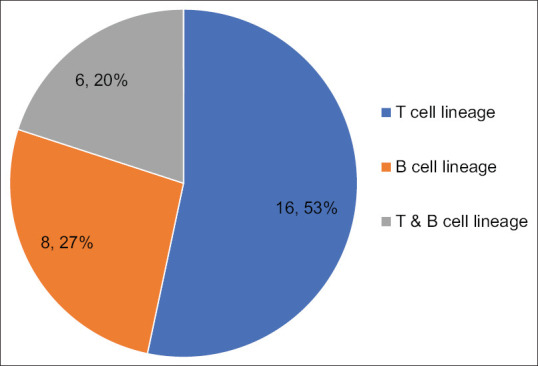
Distribution of aberrant lymphoid markers (original)
Discussion
There were a total of 50 cases of acute leukaemia during the study period. Among 50 cases of AML, 30 cases had an expression of aberrant antigens. There was a female preponderance (57%) against the male counterpart (43%) in aberrant cases. Majority of cases of AML with aberrant expression was observed in the age group of >60 years old. T cell lymphoid markers were more common in this study as compared to B cell markers. Among T cell markers, CD7 was the most common marker. CD19 was the most common B cell marker in this study. A comparative analysis of our study with other similar studies conducted all over the globe is represented in Table 11.
Table 11.
Comparison of results of our study with other studies (original)
| Various studies | Percentage of aberrant lymphoid expression in AML | Common aberrant markers |
|---|---|---|
| Our study | 30/50; 60% | CD 7 |
| Al-Anizi et al.[5] | 85/202; 42.07% | CD7 |
| Mudallal et al.[2] | 17/30; 56.6% | CD9> CD56. |
| Abdulateef et al.[6] | 27/40; 54.79% | CD56> CD7 |
| Jha et al.[7] | 35/100; 35% | CD7 |
| Sarma et al.[8] | 21/36; 58.3% | CD7 |
| Jahedi et al.[4] | 32/56; 57.1% | CD7 |
| Rodríguez et al.[9] | 55/208; 26.4% | CD 7 |
| Chughtai et al.[10] | 23/50; 46% | CD7 |
| Khurram et al.[11] | 15/27; 55.5% | CD 7 |
| Momani et al.[12] | 44/192; 22.91% | CD7 |
| El-Sissy et al.[13] | 16/34; 47% | CD9> CD7 |
Conclusion and Take-Home Message
Flow cytometry helps in the classification of leukaemias. Immunophenotyping has a significant role in diagnosis and predicting prognosis of hematopoietic malignancies in the absence of more advanced diagnostic tools like cytogenetics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kumar, Abbas, Aster . Pathologic basis of disease:Diseases of White Blood Cells, Lymph Nodes, Spleen, and Thymus;Acute Myeloid Leukemia. 10th edition. Vol. 1. New Delhi: Elsevier; 2021. pp. 617–620. [Google Scholar]
- 2.Al-Mudallal SS, Ali-Mamoori HS, Hassan AS. Evalution of CD9 and CD56 antigens expression in adult acute myeloid leukemia. Int J Adv Res. 2017;5:2138–50. [Google Scholar]
- 3.Osman IM, Humeida AA, Eltayeb O, Abdelrhman I, Elhadi TA. Flowcytometric Immunophenotypic characterization of acute myeloid leukemia (AML) in Sudan. Int J Hematol Dis. 2015;2:10–17. [Google Scholar]
- 4.Jahedi M, Shamsasenjan K, Sanaat Z, Aliparasti M, Almasi S, Mohamadian M, et al. Aberrant phenotype in Iranian patients with acute myeloid leukemia. Adv Pharm Bull. 2014;4:43–7. doi: 10.5681/apb.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Anizi WM, Al-Mashta MA. The frequency of aberrant lymphoid antigens expression in 202 Iraqi patients with de novo acute myeloid leukemia. Iraqi J Hematol. 2017;6:49–54. [Google Scholar]
- 6.Abdulateef NA, Ismail MM, Aljedani H. Clinical significance of co-expression of aberrant antigens in acute leukemia:A retrospective cohort study in Makah Al Mukaramah, Saudi Arabia. Asian Pac J Cancer Prev. 2014;15:221–7. doi: 10.7314/apjcp.2014.15.1.221. [DOI] [PubMed] [Google Scholar]
- 7.Jha R, Grover G, Bose P. Lymphoid associated antigen expression in new cases of acute myeloid leukemia. J Pathol Nepal. 2013;3:487–90. [Google Scholar]
- 8.Sarma A, Hazarika M, Das D, Kumar Rai A, Sharma JD, Bhuyan C, et al. Expression of aberrant CD markers in acute leukemia:A study of 100 cases with immunophenotyping by multiparameter flowcytometry. Cancer Biomark. 2015;15:501–5. doi: 10.3233/CBM-150482. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Rodríguez S, Pomerantz A, Demichelis-Gómez R, Barrera-Lumbreras G, Barrales-Benítez OV, Lopez-Karpovitch X, et al. Impact of aberrant antigens in the outcome of patients with acute leukemia at a referral institution in Mexico city. Rev Invest Clin. 2016;68:305–13. [PubMed] [Google Scholar]
- 10.Chughtai O, Chughtai AS. Aberrant expression of CD markers in acute leukemia. Ann Pak Inst Med Sci. 2013;9:99–102. [Google Scholar]
- 11.Khurram MM, Jafri SA, Mannan A, Nadeem A, Jamal A. Frequency of aberrant expression of CD markers in cases of acute leukemia. Med J Islamic World Acad Sci. 2010;18:55–60. [Google Scholar]
- 12.Momani A, Abbasi N, Alsokhni H, Habahbeh L, Khasawneh R, Kamal N. Aberrant antigen expression in patients with acute leukemias;experience of King Hussein Medical Center in Jordan. JRMS. 2016;23:59–67. [Google Scholar]
- 13.El-Sissy AH, El-Mashari MA, Bassuni WY, El-Swaayed AF. Aberrant lymphoid antigen expression in acute myeloid leukemia in Saudi Arabia. J Egypt Natl Canc Inst. 2006;18:244–9. [PubMed] [Google Scholar]


