Abstract
Background
Rates of prediabetes, which can lead to type 2 diabetes, are increasing worldwide. Interventions for prediabetes mainly focus on lifestyle changes to diet and exercise. While these interventions are effective, they are often delivered face-to-face, which may pose a barrier to those with limited access to healthcare. Given the evidence for digital interventions addressing other noncommunicable diseases, these may also be effective for prediabetes self-management. The aim of this scoping review was to assess the breadth of evidence around digital interventions for prediabetes self-management.
Methods
We developed a targeted search strategy and relevant studies were identified through searches conducted in four bibliographic databases (Medline, Embase, PsycInfo, and Scopus). Published studies were eligible if they included a digital intervention to support adults aged 18+ with prediabetes self-management. Titles and abstracts were first screened for relevance by one researcher. Full texts of selected records were assessed against the review criteria independently by two researchers for inclusion in the final analysis.
Results
Twenty-nine studies were included, of which nine were randomised controlled trials. Most efficacy studies reported significant changes in at least one primary and/or secondary outcome, including participants’ glycaemic control, weight loss and/or physical activity levels. About one-third of studies reported mixed outcomes or early significant outcomes that were not sustained at long-term follow-up. Interventions varied in length, digital modalities, and complexity. Delivery formats included text messages, mobile apps, virtually accessible dietitians/health coaches, online peer groups, and web-based platforms. Approximately half of studies assessed participant engagement/acceptability outcomes.
Conclusion
Whilst the evidence here suggests that digital interventions to support prediabetes self-management are acceptable and have the potential to reduce one’s risk of progression to type 2 diabetes, more research is needed to understand which interventions, and which components specifically, have the greatest reach to diverse populations, are most effective at promoting user engagement, and are most effective in the longer term.
Introduction
Prediabetes is a condition referring to the earliest identifiable stage of glucose dysregulation and is used in clinical settings as a mechanism to identify individuals at high risk for developing type 2 diabetes [1]. While diagnostic criteria and definitions for prediabetes may vary [1,2], evidence suggests that as many as 70% of prediabetes cases will progress to type 2 diabetes over the life span [2]. Type 2 diabetes is a progressive disease that is on the rise globally [3] and is characterised by the body’s loss of ability to process insulin. It is associated with a host of health complications, including heart attack, stroke, kidney disease, lower limb amputation, vision loss, nerve damage, and premature death [4]. However, prediabetes can itself be a risk factor for these complications [2]. Treatment of diabetes and its complications can pose a significant economic burden to families and health systems due to the high costs of medical care and loss of wages [4].
Numerous randomised controlled trials (RCTs) have demonstrated the effectiveness of various lifestyle and/or pharmacological methods to prevent or delay the onset of type 2 diabetes [5,6]. Lifestyle interventions have traditionally included dietary advice and group programs designed to increase individuals’ physical activity levels [5]. Some research looking at long-term outcomes has suggested that lifestyle changes can be equally effective as pharmacological treatments in reducing the development of diabetes in the longer term [7]. However, it has been noted that significant behaviour changes are required for diet and exercise interventions to be effective [6]. Similarly, recruitment and retention to in-person programs are limited by factors such as clinic and patient time and resources; patient transportation and access issues; and patient health literacy/language barriers [8,9].
Digital technology now has applications in every aspect of health and healthcare, including digitally mediated diagnostic tools, artificial intelligence, remote patient monitoring and consultation, and consumer-facing mobile health interventions [10]. Digital tools may offer a solution to barriers to in-person diabetes prevention interventions by providing remote education and support for self-management via text messages, applications, and websites [11] and have shown effectiveness in supporting self-management of type 2 diabetes [11–13]. However, there is less evidence regarding the outcomes of digital interventions targeting adults with prediabetes, and more clarity is needed about the specific mechanisms most effective in multi-faceted programs such as those with digital and non-digital components [11]. More evidence is also needed to understand which sub-groups of adults are more or less likely to see positive outcomes from participation in such interventions [11,14], particularly given that underlying disparities in access to care may persist in the digital health sphere [15]. This is especially important in a post-Covid-19 context in which a rapid and necessary transformation to digital health service provision has highlighted the need for more evidence about best practices to achieve value, equity, and quality of services in this sphere [16]. Furthermore, emerging evidence has found that infection with Covid-19 may aggravate metabolic conditions such as prediabetes and type 2 diabetes [17]. Providing all individuals at high risk for diabetes with efficient, effective support has never been more critical.
The aim of this scoping review was to assess the current breadth of evidence about digital interventions designed to promote self-management and improve health outcomes among adults with prediabetes.
Methods
We employed scoping review methods for this study, as they permit a broad mapping of existing evidence and identification of gaps in the research about a topic [18,19]. Our methods are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) S1 Checklist [20] and the protocol was not published.
Search strategy
A targeted bibliographic database search was developed with input from clinical and information specialists. Searches were conducted on 16 December 2022 using OVID Medline, Embase, PsycInfo, and Scopus. Searches were limited to papers published in English. Search strings were adapted to each database as appropriate; those applied to Medline can be found in S1 Table. Reference lists of relevant previous reviews and included studies were searched for additional papers. Results were managed using Endnote 20 [21].
Eligibility criteria
Studies were eligible if they included a digital intervention designed to promote self-management of prediabetes and targeted participants aged 18+ years meeting clinical criteria for prediabetes. As clinical diagnoses for prediabetes differ internationally [1,22], no standard definition was applied across the studies. We instead relied on each study’s definition of prediabetes. Studies involving participants with gestational, type 1 or 2 diabetes were included if results for participants with prediabetes were reported separately or were discernible in the results. All study designs were included with the exception of protocols and reviews. Studies published prior to December 2022 were eligible. There were no restrictions on comparator or outcome measures. Only full-text articles published in peer-reviewed journals were included; studies published only in the form of conference abstracts were excluded. Studies were excluded if published in languages other than English. S2 Table summarises the review criteria according to the PICOS framework.
Study selection
Records were managed using Rayyan, an online platform to facilitate screening for systematic reviews [23]. Titles and abstracts were first screened for relevance by K.G. Full texts of selected records were assessed against the review criteria independently by K.G. Ten percent of articles were screened independently by R.D. at each stage to test application of inclusion criteria; disagreements between reviewers were discussed and resolved.
Data extraction and synthesis
Relevant data were extracted intro a structured form including author(s), year, aims, country of publication, study design, participant/population characteristics, intervention/control descriptions, and key outcomes/findings. Results were compared and summarised using a descriptive narrative synthesis methodology.
Quality assessment
Consistent with scoping review methods, our aim was to provide an overview of the existing evidence rather than a critically appraised and synthesised summary [18,19]. A quality assessment was therefore not conducted in this study.
Results
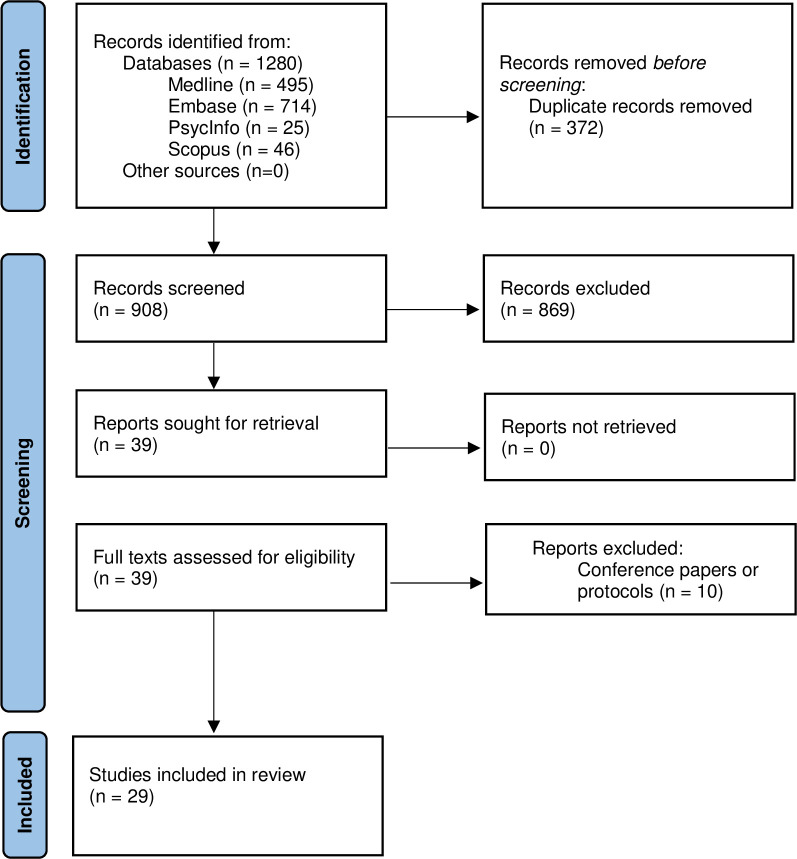
A total of 29 studies met our review criteria (Fig 1) [24–52]. S3 Table presents an overview of included studies; key characteristics are further summarised below.
Fig 1. Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Study methods
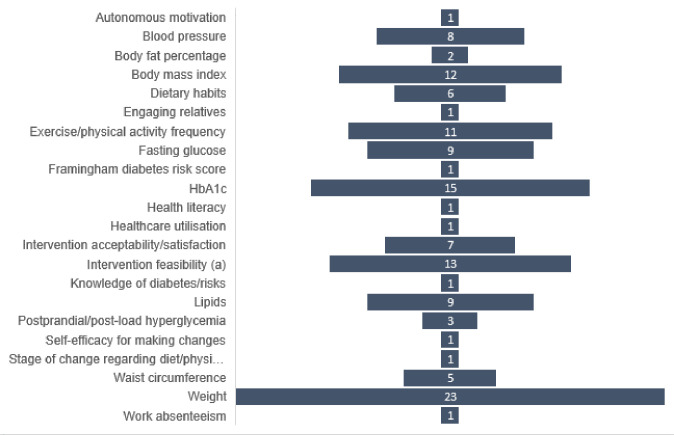
Of the studies included in this scoping review, nine were RCTs [27,28,34,38,40,42,43,49,51], six were pilot studies [29–31,37,41,52], nine were quasi-experimental studies [24,32,33,35,39,44–46,50], three were observational/secondary analysis studies [25,26,36], one was a process evaluation [47] and one was mixed-methods [48]. Sample sizes ranged from 18 [48] to 2062 [43]. Follow-up periods ranged from three months [30–32,37,46,48] to three years [45]. Some studies discussed elements of intervention co-design, such as seeking participant input on intervention modalities and content [28,33,34,39,43]. Frequency of primary and secondary outcomes specified in studies are presented in Fig 2. Participant changes in weight, HbA1c, physical activity level, and BMI were the most frequently reported outcomes to measure intervention efficacy.
Fig 2. Frequency of primary and secondary outcomes reported by studies.
(a) e.g. intervention uptake, retention, engagement, adherence, cost.
Participant characteristics
Approximately half the studies in our review were conducted with adult participants in the United States [27,31–39,41,44,45,51]. All studies were conducted to support individuals diagnosed with or meeting one or more clinical criterion for prediabetes. When specified, this was most frequently measured by haemoglobin A1c (HbA1c) [30,32–35,37–39,41–43,46,47,49], but also prediabetic fasting glucose [24,28–30,32,39–41,46,52], postprandial/glucose tolerance [30,32,39,40,46,52], elevated body mass index (BMI) [32,34,35,38–40,44–46,49], or prediabetes criteria published by national health associations/agencies [36,48,51]. All studies either explicitly required or assumed participant access to the technology featured in the intervention, such as internet, telephone, and/or smartphone, except for one of the interventions targeting healthcare practitioners [29].
Digital intervention characteristics
Table 1 presents a summary of the types of digital interventions reported. Some studies assessed multimodal interventions or standard care plus a digital intervention: for example, digitally delivered content plus in-person dietitian support [30,46], use of a digital bodyweight scale [25,32,36–40,44,45], a wearable activity tracker [25,26,31,37,38,41,44,45,49], or medication [46]. Some interventions utilised artificial intelligence [36], machine learning [32], or algorithms [27,37,40,50] to deliver tailored digital content to participants based on their progression toward the target outcomes. Pre-emptive tailoring of content to participants’ cultural, language, or literacy needs was also mentioned in some studies [33,34,39,43,50].
Table 1. Digital intervention delivery methods.
| Delivery method | Frequencya |
|---|---|
| Online / web-based program | 13 [27,29,38,39,41,42,44–50] |
| Virtually accessible, human dietitian/health coach | 13 [25,26,30,34,38–40,42,44–47,51] |
| Mobile app | 12 [24–27,30,32,36,37,40,42,47,51] |
| Social media / online group forums | 10 [24–26,38,39,42,44,47,50,51] |
| Text messages | 6 [28,33,34,43,49,52] |
| Interactive voice response (IVR) phone calls | 2 [27,31] |
| Electronic medical record | 1 [41] |
aNot mutually exclusive where multiple studies reported separate analyses on the same intervention or assessed multimodal interventions.
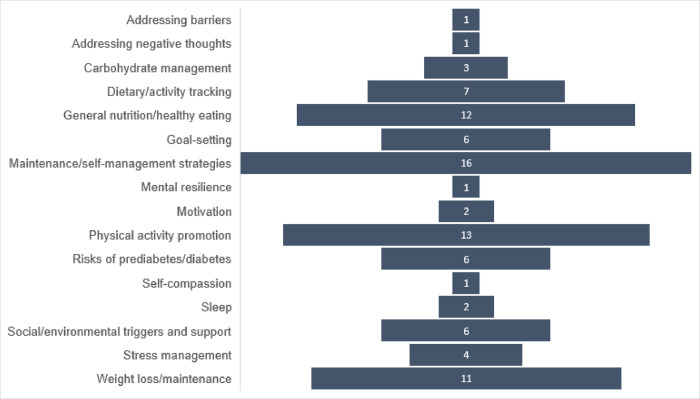
Frequency of topics mentioned by studies is summarised in Fig 3. Over one-third of studies [25–27,33–36,38,39,44,45,49,51] tested a digital version of the Centers for Disease Control and Prevention’s (CDC) Diabetes Prevention Program, which consists of a 16-week core curriculum focusing on topics such as healthy eating, physical activity, and social triggers, followed by eight months of maintenance programming [25]. Others described theories or strategies underpinning the digital interventions, including motivational interviewing [29,34,40,42,49,51]; theory of planned behaviour [27,49,52]; behavioural change theory [32,47]; Specific, Measurable, Achievable, Relevant and Time-bound (SMART) goal setting [41,48]; stages of change [41,43]; social cognitive theory [27,52]; behavioural economics [27]; cognitive restructuring [40]; and positive psychology [27].
Fig 3. Frequency of topics specified in studies.
Efficacy outcomes
Most efficacy studies in this review reported significant changes in at least one primary and/or secondary outcome. Among the outcomes most frequently reported, studies described significant intervention-related improvements to intervention participants’ weight [24–27,29,30,34,36,38,40,44,45,51], BMI [25,27,29,30,39,51,52], HbA1c [27,35,38,40,44,45,50], and physical activity level [25,26,28,30,41] at follow-up. About one-third of studies reported mixed outcomes or early significant outcomes that were not sustained at long-term follow-up [24,28,32,34,35,40,41,46,48,51,52]. Four studies reported no significant changes among intervention participants [33,42,43,49]. These studies had longer follow-up time points, ranging from six to 24 months.
Of the nine RCTs included in this review, two-thirds reported significant outcomes as a result of the intervention. Compared to control participants, intervention participants in these studies showed significant improvements in weight [27,34,38,40,51], BMI [27,51], HbA1c [27,38,40], and physical activity levels [28].
Feasibility and acceptability outcomes
Amongst the 15 studies assessing intervention feasibility and/or acceptability [29,32–35,37,39,40,42–48,51], methods to assess these varied and results were mixed. For instance, one study reported a retention rate of 91% after 12 months of a text messaging intervention (defined as those who did not explicitly drop out) [33], while another reported that one-quarter of enrolled participants in a multimodal online program did not engage at all during the 16-week intervention (based on program usage data) [47]. Some studies collecting qualitative feedback described barriers to engagement such as difficulties using a platform, lack of time, lack of interest, and lack of internet access [46]. Positive qualitative feedback from participants included the opportunity to interact with peers with similar experiences, lifestyle changes that felt feasible, and positive perceptions of intervention content [48].
Discussion
This research provides an overview of current evidence about digital interventions to promote health and support self-management among adults with prediabetes. This review is an important contribution to the literature as the majority of reviews in this area were published prior to the Covid-19 pandemic [11,53–55]; other recent work has had a narrower scope in terms of study population or design [14,56]. Furthermore, more than half of included studies in this review were published in 2020 or later, meaning that the evidence presented is current.
Most studies in this review reported significant positive results in participants’ weight/BMI, HbA1c, and/or physical activity levels, showing the potential of digital interventions for prediabetes. However, non-RCT study designs, intervention complexity, and follow-up time points varied and thus make it difficult to draw firm conclusions about the efficacy of particular digital interventions. For instance, most RCTs were multimodal and shared in common a mechanism to digitally connect participants with a human dietician/health coach [34,38,40,51] or algorithm-tailored content [27] and thus made it difficult to distinguish which elements (automated or human) were most effective. However, the ability to connect with human peers and coaches virtually appears to be an important component in many of these interventions; it is also possible that simply having multiple methods of engagement is sufficient to increase user engagement and satisfaction since it allows participants to engage in the ways most useful to them. The need for more evidence regarding multimodal digital health interventions and their mechanisms of impact has been highlighted by others [57–60]. Recent work assessing the (cost) effectiveness of digital interventions of different modalities to support type 2 diabetes self-management have demonstrated that while cost-effectiveness might be similar across some of the common modalities [61], text message and app-based interventions may be more effective overall and have greater reach than web-based interventions [62]. Future work should seek to address these questions for interventions targeting prediabetic populations.
Half of studies in this review occurred in the United States and a high degree of content was based on the CDC Diabetes Prevention Program (DPP), making this review very US-focused. While most of these studies reported significant positive effects of the digital adaptations of the DPP, it is unclear whether the content would be generalizable to non-US contexts. Only six of the included studies took place outside Asia or North America and most discussed challenges with recruiting diverse samples, representing predominantly female [25,26,31,33,34,39,41,45,46,48,51] and/or racial majority [25,27,31,32,35,36,38,39,47,48,50] participant populations. Having access to the required technology was an explicit or implicit criterion for participation in all studies and most were only offered in one language. More research is therefore needed to understand the feasibility and efficacy of such interventions among more representative populations, where access to technology and cultural considerations may be more variable. A key aspect of digital health is in the potential to provide public health intervention with reach into populations where barriers to existing services exist. Without research studies specifically targeting these groups, arguably the full potential of digital health prediabetes interventions will not be achievable.
Only half of included studies reported engagement data, which is disappointing. Understanding how users engage with digital tools is essential to understanding the effective components. For those that did report on engagement, it was clear that not all participants engaged as expected, and in some cases, there were participants who did not engage at all. There are many barriers to engagement with digital tools, for example technical difficulties, lack of interest, and lack of internet access [45], and ensuring we design tools that minimise the likelihood of these is essential. Further work is also needed to understand how users engage with these tools in real-word settings outside of research trials. It is likely that engagement will be further reduced without controlled research study environments, thus making efforts to increase user engagement in the design even more important.
It was encouraging to see more than half of studies following up with participants at 12 months or later [26,33–36,38,39, 42–45,47,49–52], considering that prediabetes is a long-term condition and sustained behaviour change is unlikely with interventions of short duration. Digital interventions have an advantage over face-to-face interventions in their ability to reach individuals over longer periods of time in a cost-effective manner, thus it is important to continue evaluating the efficacy and sustainability of these programmes with long-term outcomes in mind.
This scoping review had several strengths and limitations. The methodology allowed for exploration of heterogeneous intervention modalities and enabled us to summarise research using a variety of study designs and methodologies, with a variety of outcomes reported. As a result, this scoping review provides a broad overview of the latest published evidence investigating the use of digital interventions in prediabetes. Further, the review appears timely, given the increasing number of studies published in the last few years. However, there are also limitations to scoping reviews. While this review provides a descriptive overview of findings, a full systematic review is a recommended next step to provide a thorough analysis and appraisal of the evidence. The heterogeneity across studies, in particular with respect to study designs, intervention modalities, and outcomes, is also a limitation as it prevented in-depth analysis and synthesis of the findings. Finally, our reliance on published data subjected the review to publication bias; it is possible that real-world trials of digital interventions may have been missed.
Conclusion
This scoping review has provided an overview of current existing evidence about digital interventions to support individuals in the self-management of prediabetes. Existing interventions, covering a breadth of study methods and design, suggest that digital interventions are acceptable and have the potential to reduce adults’ risk of progressing from prediabetes to type 2 diabetes. However, more research is needed to understand which interventions, and which components therein, have the greatest reach into diverse populations, are most effective at promoting user engagement, and are most effective in the longer term.
Supporting information
(PDF)
(DOCX)
(DOCX)
BMI indicates body mass index; HbA1c, haemoglobin A1c; RCT, randomised controlled trial; CDC, Centers for Disease Control and Prevention; SMART goal-setting, Specific, Measurable, Achievable, Relevant and Time-bound goal-setting.
(DOCX)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding for this project was received from a project grant from Precision Driven Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Melanie Stowell gratefully acknowledges financial support by the Fulbright U.S. Scholar Program, which is sponsored by the U.S. Department of State and New Zealand Fulbright Commission. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the Fulbright Program, the Government of the United States, or the New Zealand Fulbright Commission.
References
- 1.Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42(1):59–77. doi: 10.1146/annurev-publhealth-090419-102644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hostalek U. Global epidemiology of prediabetes—present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. doi: 10.1186/s40842-019-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):104–9. doi: 10.2174/1570161117666190405165911 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. Global report on diabetes. Geneva: World Health Organisation; 2016. [Google Scholar]
- 5.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. doi: 10.1136/bmj.39063.689375.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract. 2015;107(3):320–31. doi: 10.1016/j.diabres.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. doi: 10.1016/S2213-8587(15)00291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zare H, Delgado P, Spencer M, Thorpe RJ, Thomas L, Gaskin DJ, et al. Using community health workers to address barriers to participation and retention in Diabetes Prevention Program: a concept paper. J Prim Care Community Health. 2022;13:21501319221134563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams Baucom KJ, Pershing ML, Dwenger MK, Cohan JN, Ozanne EM. Barriers and facilitators to enrollment and retention in the National Diabetes Prevention Program: perspectives of women and clinicians within a health system. Womens Health Rep. 2021;2(1):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abernethy A, Adams L, Barrett M, Bechtel C, Brennan P, Butte A, et al. The promise of digital health: then, now, and the future. NAM Perspect. 2022. doi: 10.31478/202206e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rhoon L, Byrne M, Morrissey E, Murphy J, McSharry J. A systematic review of the behaviour change techniques and digital features in technology-driven type 2 diabetes prevention interventions. Digit Health. 2020;6:2055207620914427. doi: 10.1177/2055207620914427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin C, Courtney KL, Naylor P, E Rhodes R. Tailored mobile text messaging interventions targeting type 2 diabetes self-management: a systematic review and a meta-analysis. Digit Health. 2019;5:2055207619845279. doi: 10.1177/2055207619845279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagherazzi G, Ravaud P. Digital diabetes: Perspectives for diabetes prevention, management and research. Diabetes Metab. 2019;45(4):322–9. doi: 10.1016/j.diabet.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 14.Jeem YA, Andriani RN, Nabila R, Emelia DD, Lazuardi L, Koesnanto H. The use of mobile health interventions for outcomes among middle-aged and elderly patients with prediabetes: a systematic review. Int J Environ Res Public Health. 2022;19(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyles CR, Nguyen OK, Khoong EC, Aguilera A, Sarkar U. Multilevel determinants of digital health equity: a literature synthesis to advance the field. Annu Rev of Public Health. 2023;44(1):null. doi: 10.1146/annurev-publhealth-071521-023913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Anza B, Pronovost PJ. Digital health: unlocking value in a post-pandemic world. Popul Health Manag. 2022;25(1):11–22. doi: 10.1089/pop.2021.0031 [DOI] [PubMed] [Google Scholar]
- 17.Steenblock C, Hassanein M, Khan EG, Yaman M, Kamel M, Barbir M, et al. Diabetes and Covid-19: short- and long-term consequences. Horm and Metab Res. 2022;54(8):503–9. doi: 10.1055/a-1878-9566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J of Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 19.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6. doi: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 20.Tricco AC, Lillie E, Zarin W, et al. Prisma extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 21.The Endnote Team. Endnote. Philadelphia, PA: Clarivate; 2013. [Google Scholar]
- 22.Makaroff LE. The need for international consensus on prediabetes. Lancet Diabetes Endocrinol. 2017;5(1):5–7. doi: 10.1016/S2213-8587(16)30328-X [DOI] [PubMed] [Google Scholar]
- 23.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Hamdan R, Avery A, Al-Disi D, Sabico S, Al-Daghri NM, McCullough F. Efficacy of lifestyle intervention program for arab women with prediabetes using social media as an alternative platform of delivery. J Diabetes Investig. 2021;12(10):1872–80. doi: 10.1111/jdi.13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alwashmi MF, Mugford G, Abu-Ashour W, Nuccio M. A digital diabetes prevention program (Transform) for adults with prediabetes: secondary analysis. JMIR Diabetes. 2019;4(3):e13904. doi: 10.2196/13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batten R, Alwashmi MF, Mugford G, Nuccio M, Besner A, Gao Z. A 12-month follow-up of the effects of a digital diabetes prevention program (VP Transform for Prediabetes) on weight and physical activity among adults with prediabetes: secondary analysis. JMIR Diabetes. 2022;7(1):e23243. doi: 10.2196/23243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block G, Azar KM, Romanelli RJ, Block TJ, Hopkins D, Carpenter HA, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):No Pagination Specified. doi: 10.2196/jmir.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bootwong P, Intarut N. The effects of text messages for promoting physical activities in prediabetes: a randomized controlled trial. Telemed J E-Health. 2022;28(6):896–903. doi: 10.1089/tmj.2021.0303 [DOI] [PubMed] [Google Scholar]
- 29.Chen P, Chai J, Cheng J, Li K, Xie S, Liang H, et al. A smart web aid for preventing diabetes in rural China: preliminary findings and lessons. J Med Internet Res. 2014;16(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Su H, Kunii D, Kudou K, Zhang Y, Zhang D, et al. The effects of mobile-app-based low-carbohydrate dietary guidance on postprandial hyperglycemia in adults with prediabetes. Diabetes Ther. 2020;11(10):2341–55. doi: 10.1007/s13300-020-00906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Educ Counseling. 2008;72(1):34–41. [DOI] [PubMed] [Google Scholar]
- 32.Everett E, Kane B, Yoo A, Dobs A, Mathioudakis N. A novel approach for fully automated, personalized health coaching for adults with prediabetes: pilot clinical trial. J Med Internet Res. 2018;20(2):e72. doi: 10.2196/jmir.9723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer HH, Durfee MJ, Raghunath SG, Ritchie ND. Short message service text message support for weight loss in patients with prediabetes: pragmatic trial. JMIR Diabetes. 2019;4(2):e12985. doi: 10.2196/12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer HH, Fischer IP, Pereira RI, Furniss AL, Rozwadowski JM, Moore SL, et al. Text message support for weight loss in patients with prediabetes: a randomized clinical trial. Diabetes Care. 2016;39(8):1364–70. doi: 10.2337/dc15-2137 [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick SL, Mayhew M, Rawlings AM, Smith N, Nyongesa DB, Vollmer WM, et al. Evaluating the implementation of a digital diabetes prevention program in an integrated health care delivery system among older adults: results of a natural experiment. Clin Diabetes. 2022;40(3):345–53. doi: 10.2337/cd21-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham SA, Pitter V, Hori JH, Stein N, Branch OH. Weight loss in a digital app-based diabetes prevention program powered by artificial intelligence. Digit Health. 2022;8:20552076221130619. doi: 10.1177/20552076221130619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griauzde D, Kullgren JT, Liestenfeltz B, Ansari T, Johnson EH, Fedewa A, et al. A mobile phone-based program to promote healthy behaviors among adults with prediabetes who declined participation in free diabetes prevention programs: mixed-methods pilot randomized controlled trial. JMIR MHealth UHealth. 2019;7(1):e11267. doi: 10.2196/11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katula JA, Dressler EV, Kittel CA, Harvin LN, Almeida FA, Wilson KE, et al. Effects of a digital diabetes prevention program: an RCT. Am J of Prev Med. 2022;62(4):567–77. [DOI] [PubMed] [Google Scholar]
- 39.Kim SE, Castro Sweet CM, Cho E, Tsai J, Cousineau MR. Evaluation of a digital diabetes prevention program adapted for low-income patients, 2016–2018. Prev Chronic Dis. 2019;16:E155. doi: 10.5888/pcd16.190156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim SL, Ong KW, Johal J, Han CY, Yap QV, Chan YH, et al. A smartphone app-based lifestyle change program for prediabetes (D’LITE study) in a multiethnic Asian population: a randomized controlled trial. Front in Nutr. 2021;8:780567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann DM, Palmisano J, Lin JJ. A pilot randomized trial of technology-assisted goal setting to improve physical activity among primary care patients with prediabetes. Prev Med Rep. 2016;4:107–12. doi: 10.1016/j.pmedr.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLeod M, Stanley J, Signal V, Stairmand J, Thompson D, Henderson K, et al. Impact of a comprehensive digital health programme on HbA1c and weight after 12 months for people with diabetes and prediabetes: a randomised controlled trial. Diabetologia. 2020;63(12):2559–70. [DOI] [PubMed] [Google Scholar]
- 43.Nanditha A, Thomson H, Susairaj P, Srivanichakorn W, Oliver N, Godsland IF, et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in india and the UK: a randomised controlled trial. Diabetologia. 2020;63(3):486–96. doi: 10.1007/s00125-019-05061-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sepah S, Jiang L, Peters AL. Long-term outcomes of a web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17(4):No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sepah S, Jiang L, Ellis RJ, McDermott K, Peters AL. Engagement and outcomes in a digital diabetes prevention program: 3-year update. BMJ Open Diabetes Res Care. 2017;5(1):e000422. doi: 10.1136/bmjdrc-2017-000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sevilla-Gonzalez MDR, Bourguet-Ramirez B, Lazaro-Carrera LS, Martagon-Rosado AJ, Gomez-Velasco DV, Viveros-Ruiz TL. Evaluation of a web platform to record lifestyle habits in subjects at risk of developing type 2 diabetes in a middle-income population: prospective interventional study. JMIR Diabetes. 2022;7(1):e25105. doi: 10.2196/25105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Signal V, McLeod M, Stanley J, Stairmand J, Sukumaran N, Thompson D-M, et al. A mobile- and web-based health intervention program for diabetes and prediabetes self-management (BetaMe/Melon): process evaluation following a randomized controlled trial. J Med Internet Res. 2020;22(12):e19150. doi: 10.2196/19150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Signore AK, Jung ME, Semenchuk B, Kullman SM, Tefft O, Webber S, et al. A pilot and feasibility study of a randomized clinical trial testing a self-compassion intervention aimed to increase physical activity behaviour among people with prediabetes. Pilot Feasibility Stud. 2022;8(1):No pagination specified. doi: 10.1186/s40814-022-01072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staite E, Bayley A, Al-Ozairi E, Stewart K, Hopkins D, Rundle J, et al. A wearable technology delivering a web-based diabetes prevention program to people at high risk of type 2 diabetes: randomized controlled trial. JMIR MHealth UHealth. 2020;8(7):e15448. doi: 10.2196/15448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Summers C, Tobin S, Unwin D. Evaluation of the low carb program digital intervention for the self-management of type 2 diabetes and prediabetes in an NHS England general practice: single-arm prospective study. JMIR Diabetes. 2021;6(3):e25751. doi: 10.2196/25751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toro-Ramos T, Michaelides A, Anton M, Karim Z, Kang-Oh L, Argyrou C, et al. Mobile delivery of the diabetes prevention program in people with prediabetes: randomized controlled trial. JMIR mHealth uHealth. 2020;8(7): e17842. doi: 10.2196/17842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong CK, Fung CS, Siu SC, Lo YY, Wong KW, Fong DY, et al. A short message service (SMS) intervention to prevent diabetes in Chinese professional drivers with pre-diabetes: a pilot single-blinded randomized controlled trial. Diabetes Res Clin Pract. 2013;102(3):158–66. doi: 10.1016/j.diabres.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 53.Bian RR, Piatt GA, Sen A, Plegue MA, De Michele ML, Hafez D, et al. The effect of technology-mediated diabetes prevention interventions on weight: a meta-analysis. J Med Internet Res. 2017;19(3):e76. doi: 10.2196/jmir.4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta-analysis. Prev Med. 2017;100:194–207. doi: 10.1016/j.ypmed.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grock S, Ku J-h, Kim J, Moin T. A review of technology-assisted interventions for diabetes prevention. Curr Diab Rep. 2017;17(11):107. doi: 10.1007/s11892-017-0948-2 [DOI] [PubMed] [Google Scholar]
- 56.Barengo NC, Diaz Valencia PA, Apolina LM, Estrada Cruz NA, Fernandez Garate JE, Correa Gonzalez RA, et al. Mobile health technology in the primary prevention of type 2 diabetes: a systematic review. Curr Diab Rep. 2022;22(1). doi: 10.1007/s11892-021-01445-w [DOI] [PubMed] [Google Scholar]
- 57.Hewitt S, Sephton R, Yeowell G. The effectiveness of digital health interventions in the management of musculoskeletal conditions: systematic literature review. J Med Internet Res. 2020;22(6):e15617. doi: 10.2196/15617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcelle ET, Nolting L, Hinshaw SP, Aguilera A. Effectiveness of a multimodal digital psychotherapy platform for adult depression: a naturalistic feasibility study. JMIR Mhealth Uhealth. 2019;7(1):e10948. doi: 10.2196/10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avramovic P, Rietdijk R, Attard M, Kenny B, Power E, Togher L. Cognitive and behavioral digital health interventions for people with traumatic brain injury and their caregivers: a systematic review. J Neurotrauma. 2023;40(3–4):159–94. doi: 10.1089/neu.2021.0473 [DOI] [PubMed] [Google Scholar]
- 60.Siopis G, Moschonis G, Eweka E, Jung J, Kwasnicka D, Asare B, et al. Effectiveness, reach, uptake, and feasibility of digital health interventions for adults with hypertension: a systematic review and meta-analysis of randomised controlled trials. Lancet Digit. 2023;5(3):e144–e159. doi: 10.1016/S2589-7500(23)00002-X [DOI] [PubMed] [Google Scholar]
- 61.Willems R, Annemans L, Siopis G, Moschonis G, Vedanthan R, Jung J, et al. Cost effectiveness review of text messaging, smartphone application, and website interventions targeting T2DM or hypertension. NPJ Digit. Med. 2023;6:150. doi: 10.1038/s41746-023-00876-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moschonis G, Siopis G, Jung J, Eweka E, Willems R, Kwasnicka D, et al. Effectiveness, reach, uptake, and feasibility of digital health interventions for adults with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Lancet Digit. 2023;5(3):e125–2143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
BMI indicates body mass index; HbA1c, haemoglobin A1c; RCT, randomised controlled trial; CDC, Centers for Disease Control and Prevention; SMART goal-setting, Specific, Measurable, Achievable, Relevant and Time-bound goal-setting.
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.