Abstract
TT virus (TTV) is an unenveloped, circular, and single-stranded DNA virus commonly infecting human beings worldwide. TTV DNAs in paired serum and liver tissues from three viremic individuals were separated by gel electrophoresis and characterized biophysically. TTV DNAs in sera migrated in sizes ranging from 2.0 to 2.5 kb. TTV DNAs in liver tissues, however, migrated at 2.0 to 2.5 kb as well as at 3.5 to 6.1 kb. Both faster- and slower-migrating forms of TTV DNAs in the liver were found to be circular and of the full genomic length of 3.8 kb. TTV DNAs migrating at 2.0 to 2.5 kb, from either serum or liver tissues, were sensitive to S1 nuclease but resistant to restriction endonucleases, and therefore, they were single-stranded. By contrast, TTV DNAs in liver tissues that migrated at 3.5 to 6.1 kb were resistant to S1 nuclease. They migrated at 3.7 to 4.0 kb after digestion with EcoRI, which suggests that they represent circular, double-stranded replicative intermediates of TTV. When TTV DNAs were subjected to strand-specific primer extension and then amplified by PCR with internal primers, those in serum were found to be minus-stranded DNAs while those in liver tissues were found to be a mixture of plus- and minus-stranded DNAs. These results suggest that TTV replicates in the liver via a circular double-stranded DNA.
TT virus (TTV) is an unenveloped, single-stranded, and circular-DNA virus (12, 13, 22). It was originally recovered from a patient with posttransfusion hepatitis of unknown etiology (16, 21) and is very common in every country examined (3, 6, 14, 16, 21, 27, 33). TTV most closely resembles members of the Circoviridae family (9). It is transmitted parenterally through transfusions and blood products and can establish a persistent infection in hosts (16, 21, 27). It is excreted from the liver via the bile duct into feces with a buoyant density comparable to that in the circulation (19, 31), resulting in a possible fecal-oral transmission route. The presumed dual mode of transmission may help TTV penetrate deeply and broadly into the community. Species-specific TTVs are reported in high frequencies for chimpanzees and the other nonhuman primates (1, 8, 20, 32), as well as farm animals (8). Hence, TTV is a ubiquitous virus, being highly prevalent in many species of animals.
There has been limited information on the virological characteristics of TTV. It is not known in what organs TTV replicates, although TTV DNA is detected in liver tissues in levels 10 to 100 times higher than those in sera from the same infected individuals (21). TTV DNAs in paired serum and liver specimens from three infected individuals were fractionated by electrophoresis on agarose gel. Distinct molecular forms of TTV DNAs in serum and liver tissues were amplified by long-distance PCR for amplification of the full-length TTV DNA of 3.8 kb and by inverted PCR for demonstration of a circular genomic structure, and then they were examined for their polarity and single or double strandedness.
MATERIALS AND METHODS
Extraction of nucleic acids from serum and liver tissues.
Paired serum and liver specimens were obtained from three patients who were infected with TTV (Table 1). Serum (1 ml) was diluted twofold with Tris-HCl buffer (50 mM, pH 8.0) containing 150 mM NaCl and 1 mM EDTA and centrifuged in a TLA 100.3 rotor (Beckman Instruments, Inc., Palo Alto, Calif.) at 190,583 × g for 1 h. The pellet was suspended in Tris-HCl buffer (10 mM, pH 8.0) containing 5 mM EDTA and supplemented with 0.5 mg of proteinase K per ml as well as 0.5% (wt/vol) sodium dodecyl sulfate and incubated at 65°C for 2 h as described previously (25). Then, nucleic acids were extracted with phenol-chloroform, precipitated with ethanol, and dissolved in 100 μl of Tris-HCl buffer (10 mM, pH 8.0) containing 1 mM EDTA (TE buffer).
TABLE 1.
Characteristics of three patients with TTV infection whose paired serum and liver tissues were studied for characterization of TTV DNA
| Patient | Age (yr) | Sex | Clinical diagnosis | TTV DNA
|
||
|---|---|---|---|---|---|---|
| Genotype | Serum (copies/10 μl) | Livera (copies/10 mg) | ||||
| 1 | 20 | Male | Subarachnoid hemorrhage | 1a | 5 × 103 | 5 × 104 |
| 2 | 64 | Male | Chronic hepatitis of unknown etiology | 1a | 1 × 103 | 1 × 105 |
| 3 | 59 | Male | Alcoholic liver cirrhosis | 1b | 1 × 103 | 5 × 104 |
Liver tissues were obtained at necropsy from patient 1 and by needle biopsy from patients 2 and 3.
Liver tissues (10 or 100 mg) were homogenized and incubated in the presence of proteinase K-sodium dodecyl sulfate at 37°C for 14 h. Then, nucleic acids were extracted from them with phenol-chloroform and precipitated with ethanol. DNA species of chromosomal origin, which emerged as a cloudy precipitate immediately after addition of ethanol, were removed. The remaining nucleic acids were collected by centrifugation and dissolved in 200 μl of TE buffer.
Semiquantitation of TTV DNA.
Nucleic acids extracted from serum and liver were serially diluted 10-fold in TE buffer containing 20 μg of glycogen (Boehringer Mannheim, Mannheim, Germany) per ml, and the highest dilution in which TTV DNA was detectable by PCR with seminested primers was determined (19, 23). Based on the results, quantifications of TTV DNA in 10n copies per 10 μl in serum or per 10 mg of liver tissue were obtained.
Agarose gel electrophoresis and amplification of TTV DNA by PCR.
The concentration of TTV in plasma is estimated to be 50 to 50,000 (geometric mean, 620) copies per ml (27). Since sera and liver tissues from infected individuals contained TTV DNA in titers too low to be detected by Southern hybridization, the length and strandedness of the TTV genome were determined by the following procedure.
DNAs (25 μl) extracted from sera and liver tissues from the three viremic patients (patients 1 to 3) were subjected to electrophoresis on 1% (wt/vol) agarose gel (SeaKem GTG agarose; FMC BioProducts, Rockland, Maine) in DNase-free electrophoresis buffer (pH 8.3) containing 40 mM Tris-acetate and 1 mM EDTA (1:10 dilution of 10× TAE buffer; Gibco-BRL, Grand Island, N.Y.). They were run horizontally for 115 mm in parallel with a size marker (500-bp DNA ladder; TaKaRa Shuzo Co. Ltd., Shiga, Japan) and 1 pg of the cloned linear and double-stranded DNA of hepatitis B virus (HBV) (24), which served as a control (Fig. 1A). DNAs (25 μl) from liver tissues from patients 1 to 3 were subjected to electrophoresis with or without prior digestion with 360 U of EcoRI (TaKaRa Shuzo) at 37°C for 12 h (Fig. 1B). After electrophoresis, the gel was stained with ethidium bromide.
FIG. 1.
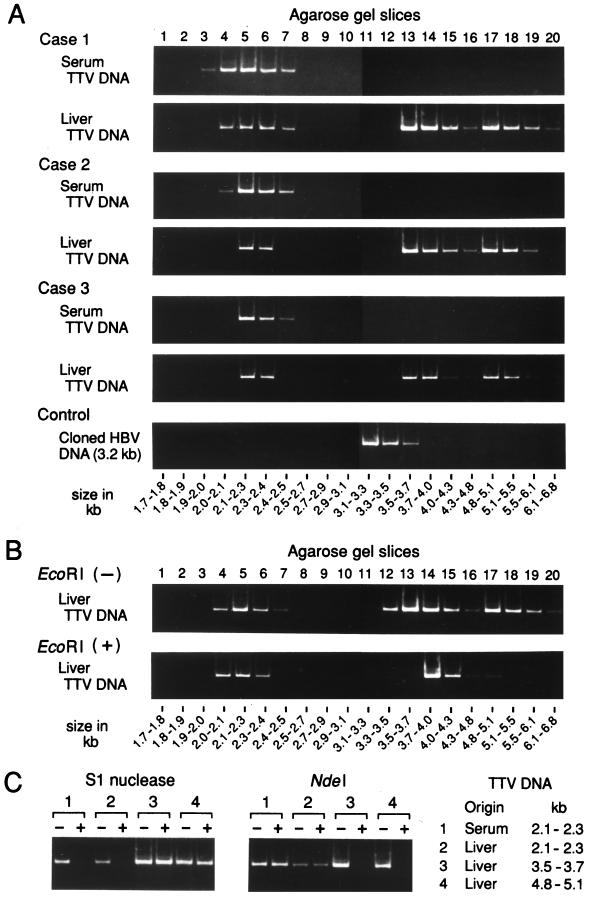
(A) Separation of TTV DNAs extracted from serum or liver tissues by gel electrophoresis. TTV DNA samples extracted from paired serum and liver tissues from three viremic patients (Cases 1 to 3) along with a molecular size marker (500-bp DNA ladder [TaKaRa Shuzo]) were subjected to electrophoresis, and areas corresponding to 1.7 to 6.8 kb were cut into 20 gel slices. HBV DNA run in parallel served as a control. DNA was recovered from each slice and amplified by PCR for TTV DNA or HBV DNA. The size of DNA was estimated by reference to slice numbers. (B) Separation of TTV DNAs extracted from liver tissues with or without prior digestion with EcoRI. TTV DNAs from liver tissues of patient 1 were digested with EcoRI and electrophoresed on agarose gel. (C) Susceptibility of TTV DNAs from serum or liver tissues of patient 1 to S1 nuclease or restriction endonuclease NdeI. DNAs extracted from agarose gel slices were digested with S1 nuclease or NdeI and then amplified by PCR for a sequence bearing the NdeI cutting site. PCR was also performed without digestion with S1 nuclease or NdeI. The products were subjected to electrophoresis, and signals were compared for intensity.
First, the full-length gel, spreading over 115 mm from the baseline to the bottom, was cut into 23 slices at 5-mm intervals with a razor, and DNAs were recovered from each gel slice in a volume of approximately 80 μl with a GenElute agarose spin column (Sigma Chemical Co., St. Louis, Mo.). A 4- to 15-μl portion thereof was subjected to amplification by PCR with the primers NG061 and NG063 (Table 2) for 35 cycles as described previously (23) to generate a fragment of 271 bp. The screening revealed TTV DNAs from patients 1 to 3 to be exclusively in the area of 1.7 to 6.8 kb in size. Based on these findings, the gel corresponding to this size was cut into 20 slices at 2.5-mm intervals. Thereafter, DNAs were recovered from each gel slice and subjected to amplification by PCR with NG061 and NG063 as described above.
TABLE 2.
Positions and nucleotide sequences of oligonucleotide primers used for PCR amplification and primer extension
| Primer | Polarity | Nt positiona | Nucleotide sequenceb |
|---|---|---|---|
| NG001 | Sense | 1862–1881 | 5′-CAC CAG GAG CAT ATA CAG AC-3′ |
| NG017 | Sense | 3547–3566 | 5′-ATG GTG GAC AAC ATC TTC CG-3′ |
| NG021 | Antisense | 3690–3709 | 5′-AAA GAG GAA GGA AGT CAG CC-3′ |
| NG055 | Sense | 601–623 | 5′-TGG TGG CGC CGA AGG AGA AGA CG-3′ |
| NG056 | Antisense | 736–759 | 5′-TCT CCT ATA TCT CCT CCT CCA CCT-3′ |
| NG057 | Sense | 621–642 | 5′-ACG GTG GCG CAG GTG GAG ACG C-3′ |
| NG058 | Antisense | 706–729 | 5′-TCT GCG GCG TCT CCT TAC GTT TCT-3′ |
| NG061 | Sense | 1915–1938 | 5′-GGC AAC ATG YTR TGG ATA GAC TGG-3′ |
| NG063 | Antisense | 2161–2185 | 5′-CTG GCA TTT TAC CAT TTC CAA AGT T-3′ |
| NG133 | Sense | 91–115 | 5′-GTA AGT GCA CTT CCG AAT GGC TGA G-3′ |
| NG147 | Antisense | 211–233 | 5′-GCC AGT CCC GAG CCC GAA TTG CC-3′ |
| NG212 | Sense | 211–233 | 5′-GGC AAT TCG GGC TCG GGA CTG GC-3′ |
| NG214 | Sense | 211–238 | 5′-GGC AAT TCG GGC TCG GGA CTG GCC GGG C-3′ |
| NG215 | Antisense | 185–210 | 5′-CCT TGA CTG CGG TGT GTA AAC TCA CC-3′ |
| NG222 | Antisense | 179–210 | 5′-CCT TGA CTG CGG TGT GTA AAC TCA CCT HCG GC-3′ |
| RD038 | Antisense | 2258–2277 | 5′-TGA CTG TGC TAA AGC CTC TA-3′ |
The amplification was performed with or without digestion of the recovered DNAs from patients 1 to 3 by the restriction endonuclease NdeI (TaKaRa Shuzo) under conditions described previously (21). In parallel, the effects of prior digestion with 10 U of S1 nuclease (TaKaRa Shuzo) at 37°C for 15 min were evaluated. The products were electrophoresed on a 2% NuSieve 3:1 agarose gel (FMC BioProducts), stained with ethidium bromide, and photographed under UV light. The strandedness of TTV DNAs was evaluated over an additional three genomic sequences. They spanned, respectively, nucleotides (nt) 91 to 233, amplifiable by PCR with NG133 and NG147 and having an AccII site; nt 621 to 729, amplifiable by PCR with NG057 and NG058 and carrying an NcoI site; and nt 3547 to 3709, amplifiable by PCR with NG017 and NG021 and bearing an SplI or AfaI site. HBV DNA was amplified by PCR by the method reported elsewhere (7), which generated a fragment of 233 bp.
Long-distance PCR for amplification of the full-length TTV genome.
The full-length genomic DNA of TTV was amplified by a long-distance PCR with nested primers having their 5′ ends back to back in the circular genomic DNA (Fig. 2A).
FIG. 2.
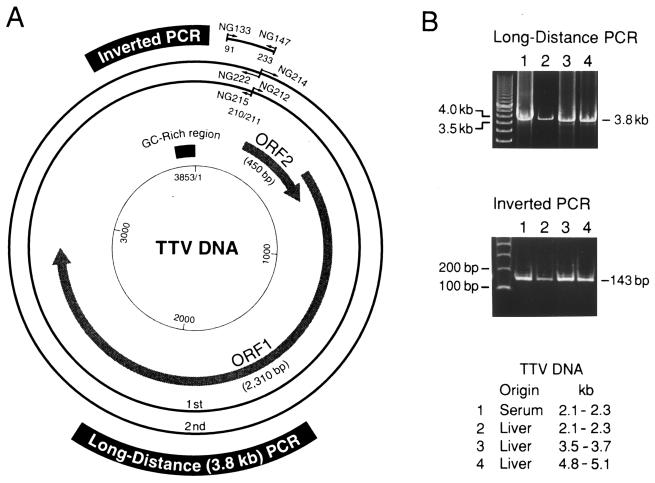
(A) Organization of the TTV genome and primers for long-distance or inverted PCR. The genomic structure of the prototype TTV isolate (TA278) (21, 22) is shown in the center. The products of long-distance PCR with nested primers for amplification of the entire genome and those of inverted PCR are shown in the periphery. The first round of long-distance PCR was performed with the primer pair NG212-NG215, and the second round of PCR was performed with NG214-NG222. Sense primers (NG212 and NG214) were designed to have their 5′ ends at nt 211, and antisense primers (NG215 and NG222) were designed to have theirs at nt 210. The inverted PCR was carried out with the primer pair NG133-NG147 to amplify a sequence covering both ends of the products of long-distance PCR. Primer NG147 for inverted PCR had a sequence complementary to that of NG212 for long-distance PCR and had its 3′ end at nt 211. ORF, open reading frame. (B; upper panel) Agarose gel electrophoresis of the amplification products of the long-distance PCR. TTV DNAs of various migrating sizes recovered from paired serum and liver tissues from patient 1 (Fig. 1A) were used as templates. The products of 3.8 kb (6 μl) were electrophoresed vertically on 1% (wt/vol) SeaKem GTG agarose gel (FMC BioProducts). The 500-bp DNA ladder (TaKaRa Shuzo) on the left served as a size marker. (Lower panel) TTV DNAs amplified by inverted PCR. The products (6 μl) of inverted PCR, on templates of TTV DNAs from serum or liver tissues of patient 1, were electrophoresed on 3% NuSieve 3:1 agarose gel (FMC BioProducts) for generating products of 143 bp. The 100-bp DNA ladder (Gibco-BRL) on the left was used as a size marker.
On a template of DNAs extracted from gel slices from electrophoresis, for paired serum and liver tissues from patients 1 to 3, the first-round PCR was performed with NG212 and NG215 for 35 cycles (94°C for 45 s with an additional 3 min in the first cycle, 65°C for 45 s, and 72°C for 4 min with an additional 7 min in the last cycle) in the presence of TaKaRa LA Taq with GC buffer (TaKaRa Shuzo). On the products of the first-round PCR (5 μl), the second round was performed for 25 cycles under the same conditions as in the first round. PCR products were run by electrophoresis on 1% (wt/vol) SeaKem GTG agarose gel (FMC BioProducts) and examined for a band at 3.8 kb.
Inverted PCR for confirming the circular nature of TTV DNA.
An inverted PCR was performed, with the primers indicated in Fig. 2A and Table 2, for demonstrating the circular nature of TTV DNA by the connection between nt 210 and 211 in the target genome. On a template of DNAs extracted from gel slices, for paired serum and liver tissues, and in the presence of Perkin-Elmer AmpliTaq Gold (Roche Molecular Systems, Inc., Branchburg, N.J.), PCR was performed for 35 cycles with NG133 and NG147. The antisense primer NG147 had a sequence complementary to the sense primer NG212, which was used in the full-length PCR and possessed nt 211 at its 5′ end.
Sequence analysis of TTV DNA.
DNAs were extracted from the gel slices containing slower-migrating forms of TTV DNA in the liver (slice 13, corresponding to 3.5 to 3.7 kb, and slice 17, corresponding to 4.8 to 5.1 kb). Using the DNAs as templates, PCR was performed with four sets of primers (NG061 and NG063, NG133 and NG147, NG057 and NG058, and NG017 and NG021 [Table 2]). The products were subjected to reaction with a BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, Calif.) and then analyzed in an automatic DNA sequencer (ABI PRISM310 Genetic Analyzer; PE Applied Biosystems). The products obtained with the long-distance PCR for amplification of a full-length TTV genome were inserted into pT7Blue T-Vector (Novagen Inc., Madison, Wis.) to transform Escherichia coli. Nucleotide sequences representing TTV DNA in obtained clones were determined as indicated above.
Polarity of TTV DNA.
On a template of DNAs (5 μl) extracted from serum or liver tissues from three infected individuals, strand-specific primer extension was performed with either the sense or the antisense primer in the presence of Perkin-Elmer AmpliTaq Gold (Roche Molecular Systems) for 50 cycles (95°C for 30 s with an additional 9 min in the first cycle, 55°C for 30 s, and 72°C for 45 s with an additional 7 min in the last cycle) in a reaction volume of 50 μl. The extension was carried out for 50 additional cycles, with addition of the supplemental primer and enzyme in equal amounts to 50 μl of the buffer that was provided with the enzyme kit. From 100 μl of the extension products, 3 μl was separated and amplified with a pair of primers internal to the sense and antisense primers used for extension.
As controls, TTV DNA of 3,254 bp (nt 12 to 3265) was excised from the pTV-TRM1-1 clone (DDBJ/EMBL/GenBank accession no. AB038340 [23]) and inserted into the M13 phage vectors mp18 and mp19 (New England Biolabs, Inc., Beverly, Mass.), and single-stranded DNAs having a plus- or minus-stranded sequence of TTV [designated M13-TTV(+)-ssDNA and M13-TTV(−)-ssDNA] were obtained.
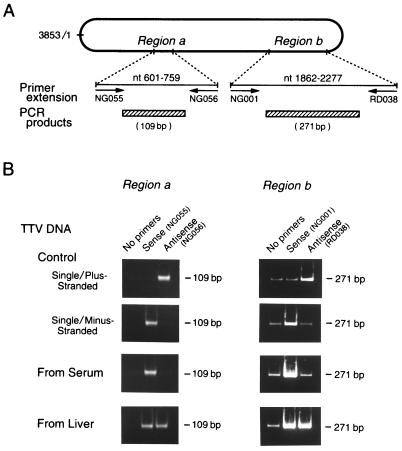
The polarity of two sequences was evaluated (see regions a and b in Fig. 3B). One sequence, spanning nt 601 to 759, was extended with the sense primer NG055 or the antisense primer NG056, and the products were amplified with a primer pair (NG057 and NG058), which gave rise to a fragment of 109 bp. The other sequence, stretching from nt 1862 to 2277, was extended with the sense primer NG001 or the antisense primer RD038, and the products were amplified with another primer pair (NG061 and NG063) to generate a fragment of 271 bp. The extension was also performed without primers to obtain mock products. An intensification of PCR signals, with prior extension by the used primer, verified the extension; it was attributed to the nature of the target strand that was complementary to the primer.
FIG. 3.
(A) Primer extension of TTV DNA sequence. Using DNAs extracted from serum or liver tissues as templates, strand-specific primer extension was performed on two genomic regions (a and b) with sense or antisense primers specific for TTV. Nucleotides were numbered from position 1 (nt 1) of the coding strand of the prototype TTV isolate of genotype 1a (TA278) (21, 22), complementary to the viral strand, because TTV was found to have a minus-stranded DNA in the circulating virion. Single-stranded DNAs from recombinant M13 phage, either plus-stranded DNA [M13-TTV(+)-ssDNA] or minus-stranded DNA [M13-TTV(−)-ssDNA], served as controls. (B) Amplification of extension products by PCR. Using extension products as templates, PCR was performed with primer pairs internal to those used for primer extension. The extension was performed without primers to obtain mock products. The amplification products were subjected to electrophoresis, and signals were compared between the products with and without prior extension.
Nucleotide sequence accession numbers.
The nucleotide sequence data in this report have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession no. AB040776 to AB040788.
RESULTS
Strandedness of TTV DNA in paired serum and liver tissues.
DNAs recovered from paired serum and liver tissues from the three viremic individuals (patients 1 to 3) were subjected to electrophoresis (Fig. 1A). TTV DNAs in serum migrated at sizes ranging from 2.0 to 2.5 kb, while those in liver tissues migrated at two positions of different sizes. One of them migrated at 2.0 to 2.5 kb, and the other migrated at 3.5 to 6.1 kb. DNA of HBV run in parallel, as a control, migrated at a peak size range of 3.1 to 3.3 kb, in agreement with the genome size of HBV at 3.2 kb.
TTV DNAs from liver tissues of patients 1 to 3 were digested with EcoRI. The positions of TTV DNAs, migrating at 2.0 to 2.5 kb, did not change after they had been digested with EcoRI. By contrast, TTV DNAs from patient 1 migrating at 3.5 to 6.1 kb, including those at 3.5 to 4.3 kb and 4.8 to 6.1 kb, shifted to the position of 3.7 to 4.3 kb after digestion with EcoRI (Fig. 1B); the same results were obtained for TTV DNAs recovered from patients 2 and 3. Hence, these slower-migrating forms of TTV DNAs in liver tissues are in double-stranded, replicative intermediate forms. They were linearized to have a genomic size of 3.8 kb after digestion with EcoRI; there is only one restriction site for this enzyme in the TTV DNA of genotype 1a or 1b (4, 13, 22).
The strandedness of TTV DNA in serum or liver tissues was determined in four different regions by digestion with S1 nuclease and four restriction endonucleases, followed by detection of TTV DNA by PCR with primers designed to amplify each of the four regions. Examples are shown for a target sequence (nt 1915 to 2185) bearing the NdeI site, amplifiable with primers NG061 and NG063 (Fig. 1C). When TTV DNAs were digested with S1 nuclease, those migrating at 2.1 to 2.3 kb, recovered from either serum or liver tissues, were no longer amplified while those that migrated at 3.5 to 3.7 kb and 4.8 to 5.1 kb stayed amplifiable. When digested with NdeI, however, TTV DNA molecules that migrated at 2.1 to 2.3 kb, recovered from either serum or liver tissues, remained amplifiable. By contrast, TTV DNAs migrating at 3.5 to 3.7 kb or 4.8 to 5.1 kb were no longer amplified after digestion with NdeI. The susceptibility of TTV DNAs of distinct migration positions to treatment with S1 nuclease and restriction endonucleases was evaluated with three other sequences of TTV DNA with reproducible results. They were a target sequence of PCR (nt 91 to 233) with an AccII site, a sequence (nt 621 to 729) with an NcoI site, and a sequence (nt 3547 to 3709) with an SplI site (data not shown). The results obtained with TTV DNAs from patient 1 were confirmed with those recovered from patients 2 and 3.
Slower-migrating DNAs recovered from liver tissues were confirmed to have the nucleotide sequences specific for TTV by the following procedure. Slower-migrating TTV DNAs in liver tissues from patients 1 to 3 were recovered from gel slices (slice 13, corresponding to 3.5 to 3.7 kb, and slice 17, corresponding to 4.8 to 5.1 kb). Using the DNAs as templates, PCR was performed with four primer sets (NG061-NG063, NG133-NG147, NG057-NG058, and NG017-NG021) as described above, and the products were sequenced. The slower-migrating DNA from patients 1 and 2 possessed sequences 96.8 to 100% similar to that of a TTV isolate of genotype 1a (TA278 [22]), while that from patient 3 had a sequence 97.6 to 98.9% similar to that of a TTV isolate of genotype 1b (JA20 [4]).
On the basis of these results, TTV DNAs in serum were single stranded while those in liver tissues existed in both single- and double-stranded forms. The double-stranded TTV DNAs in liver tissues represent replicative intermediates. These results were in agreement with the previous observation that TTV DNA in plasma is digested with mung bean nuclease that has a specificity for the single-stranded DNA (21).
Circular structure of TTV DNA in serum and liver tissues.
A two-stage, long-distance PCR method was performed on TTV DNAs from paired serum and liver tissues from patient 1. The PCR was designed to amplify the full-length TTV genome, using sense primers with the 5′ ends at nt 211 and antisense primers with the 5′ ends at nt 210 (Fig. 2A). A product of 3.8 kb in size, compatible with the full-length genome, was amplified on single-stranded TTV DNAs from either serum or liver tissues that migrated at 2.1 to 2.3 kb, as well as on double-stranded TTV DNAs from liver tissues that migrated at 3.5 to 3.7 kb or 4.8 to 5.1 kb (Fig. 2B). The products of 3.8 kb (Fig. 2B, lanes 3 and 4) were sequenced within the 5′-terminal 549 nt (nt 211 to 759) and the 3′-terminal 530 nt (nt 3534 to 3853 and nt 1 to 210). The sequences were 99.6% similar to that of the TA278 isolate, thereby confirming that the product of long-distance PCR contained TTV sequences.
The circular nature of TTV DNAs from serum and liver tissues of patient 1 was confirmed by an inverted PCR with a primer pair (NG133-NG147) designed to amplify a sequence of 143 bp that includes nt 210 and 211; these primers represented both ends of the product of full-length PCR (Fig. 2B). The connection between nt 210 and 211 was confirmed by determination of the 143-bp sequence. These results were confirmed by similar analyses of TTV DNAs from sera and liver tissues of patients 2 and 3. Together, they indicate that a circular full-length TTV DNA of 3.8 kb was present both in serum and liver tissues.
Polarity of TTV DNA in serum and liver tissues.
The polarity of TTV DNA was evaluated under the assumption that the detection of TTV DNA by PCR would be enhanced by prior extension with primers having a complementary sequence. For verifying this notion, two single-stranded TTV DNAs of plus or minus polarity [M13-TTV(+)-ssDNA and M13-TTV(−)-ssDNA] were extended over two regions (a and b) for 100 cycles with appropriate sense or antisense primers and then the products were tested by PCR with the primers that were internal to both primers (Fig. 3A). With M13-TTV(+)-ssDNA as a template, the amplification signal was intensified only when it had been extended by the antisense primer; the reverse was the case for M13-TTV(−)-ssDNA. The same results were obtained over two distinct regions (a and b) of the TTV genome. A weak signal was detected by PCR with region b primers in lanes with no primers. Hence, weak signals observed for the extension products with the sense primer on the single- and minus-stranded TTV DNA, as well as those for the extension products observed with the antisense primers on the single- and plus-stranded TTV DNA, were attributed to the amplification of these regions on the template. The lower intensities of signals for region a than region b were due to the sizes of products of region a being less than half those of region b. The findings with single-stranded TTV DNA of plus or minus polarity attested to the credibility of extension procedures used to determine the polarity.
When TTV DNAs from the serum of patient 1 were extended by these procedures, the amplification signal was intensified remarkably by prior extension with sense, but not antisense, primers for the two regions examined (Fig. 3B). The extension was performed with TTV DNAs from sera of patients 2 and 3, and the same types of intensification were obtained. Based on these results, the TTV DNA in circulating virions was deduced to have a sequence complementary to the two sense primers and, therefore, to be minus stranded.
By contrast, the amplification of TTV DNAs in the liver tissues of patient 1 was enhanced markedly by prior extension with either sense primers or antisense primers for the two regions (Fig. 3B). The same results were obtained with TTV DNAs in the liver tissues of patients 2 and 3. On the basis of these results, there were two kinds of TTV DNA species in the liver, one of which was minus stranded and the other of which was plus stranded.
DISCUSSION
Although TTV DNA is detected in liver tissues (21, 26, 34), it has not yet been characterized. In the present study, a circular nature was demonstrated for TTV DNAs from the paired serum and liver tissues of three infected individuals (patients 1 to 3). Since TTV is a DNA virus, it should replicate in the nuclei of hepatocytes. The origin of TTV DNA in liver tissues has not been identified, however; it is not known whether it is in the episomes, in the nucleus, or both.
As an unenveloped, single-stranded, and circular DNA virus, TTV would be classified as belonging in the Circoviridae family (9, 12). There are, however, some characteristics of TTV distinct from those of known animal circoviruses. First, TTV does not share sequence similarity with any of the three animal circoviruses reported, i.e., chicken anemia virus, beak and feather disease virus of parrots, and porcine circovirus. Second, the genomic size of TTV is approximately twice as large as those of these three animal circoviruses, which possess 1,758 to 2,319 bases (11, 15, 17). Third, animal and plant circoviruses conserve stem-loop structures, with a 9-nt motif in the loop that is essential for the replication of viral DNA (2, 10). This motif, however, has not been identified in TTV.
TTV DNA of genotype 1 is detected in liver tissues in levels 10 to 100 times higher than those in corresponding sera (21). This was confirmed for three patients with TTV of genotype 1a or 1b in the present study (Table 1). A 10- to 100-fold difference in TTV DNA titers was observed also in individuals infected with TTV of genotype 2 or 3 (unpublished observations). Hence, the present results obtained with TTV of genotype 1 can be extrapolated to many other genotypes that have accumulated up to the present (13, 14, 16, 21, 23, 28, 29). TTV DNA titers in both serum and liver tissues, however, are too low to allow analysis by Southern hybridization. Therefore, TTV DNAs were separated by electrophoresis on agarose gel, and then those in gel slices were amplified by PCR to determine their sizes.
Viruses with a circular single-stranded DNA genome depend on a double-stranded DNA for the correct transcription and replication of the virus DNA (17). In the present study, double-stranded DNA of TTV was isolated from liver tissues from three infected patients. TTV DNAs from serum showed only one form, which migrated at 2.0 to 2.5 kb, while TTV DNAs from the liver showed, in addition, two slower-migrating forms at 3.5 to 4.3 kb and 4.8 to 6.1 kb; they both generated a product of 3.8 kb, in accord with the size of the full-length genome, by long-distance PCR. Slower-migrating TTV DNAs in liver tissues, when digested with EcoRI, generated fragments migrating at 3.7 to 4.0 kb, sizes compatible with the size of the full-length genome. Hence, size differences in the two species of slower-migrating TTV DNA were generated by conformation and not by differences in the genomic length; both are circular molecules. These findings suggest that two forms of circular, double-stranded species of TTV DNA exist in the liver. Of slower-migrating TTV DNAs, those that migrated at 3.5 to 4.3 kb represent a closed-circular duplex and those that migrated at 4.8 to 6.1 kb represent a relaxed- or open-circular duplex. Two such species of double-stranded viral DNAs have also been reported for cells infected with chicken anemia virus (11, 30). The sizes of TTV DNAs in paired serum and liver tissues were in accord among the three studied patients who were infected.
Circoviruses possess up to seven open reading frames, including the one coding for Rep protein, which is involved in rolling-circle replication (2, 15). The sequences corresponding to two of the four conserved Rep protein motifs are identified in the product of open reading frame 1 of TTV isolates (13), suggesting that TTV might replicate via circular double-stranded DNA intermediates, possibly by rolling-circle replication. However, the Rep protein motifs are not conserved in the majority of TTV sequences reported so far (4, 5).
By means of primer extension analysis, the polarity of TTV DNA in serum was determined to be negative in the present study. A minus-strandedness has been postulated for TTV DNA extracted from human plasma by a hybridization-nuclease protection assay (13). The minus polarity of TTV is consistent with that of chicken anemia virus and at variance with that of beak and feather disease virus of parrots and porcine circovirus, which have ambisense genomes (15). Circular double-stranded TTV DNAs in liver tissues most likely represent replicative intermediate forms of the TTV genome. The exact location for replication of TTV needs to be determined by PCR in situ hybridization, which allows for detection of limited viral copies (18), as well as immunological detection of virus-encoded proteins in the liver.
REFERENCES
- 1.Abe K, Inami T, Ishikawa K, Nakamura S, Goto S. TT virus infection in nonhuman primates and characterization of the viral genome: identification of simian TT virus isolates. J Virol. 2000;74:1549–1553. doi: 10.1128/jvi.74.3.1549-1553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassami M R, Berryman D, Wilcox G E, Raidal S R. Psittacine beak and feather disease virus nucleotide sequence analysis and its relationship to porcine circovirus, plant circoviruses, and chicken anaemia virus. Virology. 1998;249:453–459. doi: 10.1006/viro.1998.9324. [DOI] [PubMed] [Google Scholar]
- 3.Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998;28:839–842. doi: 10.1002/hep.510280335. [DOI] [PubMed] [Google Scholar]
- 4.Erker J C, Leary T P, Desai S M, Chalmers M L, Mushahwar I K. Analyses of TT virus full-length genomic sequences. J Gen Virol. 1999;80:1743–1750. doi: 10.1099/0022-1317-80-7-1743. [DOI] [PubMed] [Google Scholar]
- 5.Hijikata M, Takahashi K, Mishiro S. Complete circular DNA genome of a TT virus variant (isolate name SANBAN) and 44 partial ORF2 sequences implicating a great degree of diversity beyond genotypes. Virology. 1999;260:17–22. doi: 10.1006/viro.1999.9797. [DOI] [PubMed] [Google Scholar]
- 6.Hohne M, Berg T, Muller A R, Schreier E. Detection of sequences of TT virus, a novel DNA virus, in German patients. J Gen Virol. 1998;79:2761–2764. doi: 10.1099/0022-1317-79-11-2761. [DOI] [PubMed] [Google Scholar]
- 7.Iizuka H, Ohmura K, Ishijima A, Satoh K, Tanaka T, Tsuda F, Okamoto H, Miyakawa Y, Mayumi M. Correlation between anti-HBc titers and HBV DNA in blood units without detectable HBsAg. Vox Sang. 1992;63:107–111. doi: 10.1111/j.1423-0410.1992.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 8.Leary T P, Erker J C, Chalmers M L, Desai S M, Mushahwar I K. Improved detection systems for TT virus reveal high prevalence in humans, non-human primates and farm animals. J Gen Virol. 1999;80:2115–2120. doi: 10.1099/0022-1317-80-8-2115. [DOI] [PubMed] [Google Scholar]
- 9.Lukert P D, de Boer G F, Dale J L, Keese P, McNulty M S, Randers J W, Tisher I. Family Circoviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Classification and nomenclature of viruses. Sixth report of the International Committee on Taxonomy of Viruses. New York, N.Y: Springer-Verlag; 1995. pp. 166–168. [Google Scholar]
- 10.Mankertz A, Mankertz J, Wolf K, Buhk H J. Identification of a protein essential for replication of porcine circovirus. J Gen Virol. 1998;79:381–384. doi: 10.1099/0022-1317-79-2-381. [DOI] [PubMed] [Google Scholar]
- 11.Meehan B M, Todd D, Creelan J L, Earle J A P, Hoey E M, McNulty M S. Characterization of viral DNAs from cells infected with chicken anaemia agent: sequence analysis of the cloned replicative form and transfection capabilities of cloned genome fragments. Arch Virol. 1992;124:301–319. doi: 10.1007/BF01309811. [DOI] [PubMed] [Google Scholar]
- 12.Miyata H, Tsunoda H, Kazi A, Yamada A, Khan M A, Murakami J, Kamahora T, Shiraki K, Hino S. Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J Virol. 1999;73:3582–3586. doi: 10.1128/jvi.73.5.3582-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mushahwar I K, Erker J C, Muerhoff A S, Leary T P, Simons J N, Birkenmeyer L G, Chalmers M L, Pilot-Matias T J, Dexai S M. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA. 1999;96:3177–3182. doi: 10.1073/pnas.96.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naoumov N V, Petrova E P, Thomas M G, Williams R. Presence of a newly described human DNA virus (TTV) in patients with liver disease. Lancet. 1998;352:195–197. doi: 10.1016/S0140-6736(98)04069-0. [DOI] [PubMed] [Google Scholar]
- 15.Niagro F D, Forsthoefel A N, Lawther R P, Kamalanathan L, Ritchie B W, Latimer K S, Lukert P D. Beak and feather disease virus and porcine circovirus genomes: intermediates between the geminiviruses and plant circoviruses. Arch Virol. 1998;143:1723–1744. doi: 10.1007/s007050050412. [DOI] [PubMed] [Google Scholar]
- 16.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 17.Noteborn M H, de Boer G F, van Roozelaar D J, Karreman C, Kranenburg O, Vos J G, Jeurissen S H, Hoeben R C, Zantema A, Koch G, van Ormondt H, van der Eb A J. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol. 1991;65:3131–3139. doi: 10.1128/jvi.65.6.3131-3139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuovo G J. PCR in situ hybridization. Methods Mol Biol. 1994;33:223–241. doi: 10.1385/0-89603-280-9:223. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto H, Akahane Y, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, Mayumi M. Fecal excretion of a nonenveloped DNA virus (TTV) associated with posttransfusion non-A-G hepatitis. J Med Virol. 1998;56:128–132. [PubMed] [Google Scholar]
- 20.Okamoto H, Fukuda M, Tawara A, Nishizawa T, Itoh Y, Hayasaka I, Tsuda F, Tanaka T, Miyakawa Y, Mayumi M. Species-specific TT viruses and cross-species infection in nonhuman primates. J Virol. 2000;74:1132–1139. doi: 10.1128/jvi.74.3.1132-1139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M. Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res. 1998;10:1–16. [Google Scholar]
- 22.Okamoto H, Nishizawa T, Ukita M, Takahashi M, Fukuda M, Iizuka H, Miyakawa Y, Mayumi M. The entire nucleotide sequence of a TT virus isolate from the United States (TUS01): comparison with reported isolates and phylogenetic analysis. Virology. 1999;259:437–448. doi: 10.1006/viro.1999.9769. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto H, Takahashi M, Nishizawa T, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, Mayumi M. Marked genomic heterogeneity and frequent mixed infection of TT virus demonstrated by PCR with primers from coding and noncoding regions. Virology. 1999;259:428–436. doi: 10.1006/viro.1999.9770. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo R I, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto H, Yotsumoto S, Akahane Y, Yamanaka T, Miyazaki Y, Sugai Y, Tsuda F, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990;64:1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura A, Yoshioka M, Kubota M, Kikuta H, Ishiko H, Kobayashi K. Detection of a novel DNA virus (TTV) sequence in peripheral blood mononuclear cells. J Med Virol. 1999;58:174–177. doi: 10.1002/(sici)1096-9071(199906)58:2<174::aid-jmv12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Simmonds P, Davidson F, Lycett C, Prescott L E, MacDonald D M, Ellender J, Yap P L, Ludlam C A, Haydon G H, Gillon J, Jarvis L M. Detection of a novel DNA virus (TTV) in blood donors and blood products. Lancet. 1998;352:191–195. doi: 10.1016/s0140-6736(98)03056-6. [DOI] [PubMed] [Google Scholar]
- 28.Takayama S, Yamazaki S, Matsuo S, Sugii S. Multiple infection of TT virus (TTV) with different genotypes in Japanese hemophiliacs. Biochem Biophys Res Commun. 1999;256:208–211. doi: 10.1006/bbrc.1999.0270. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Mizokami M, Orito E, Ohno T, Nakano T, Kato T, Kato H, Mukaide M, Park Y M, Kim B S, Ueda R. New genotypes of TT virus (TTV) and a genotyping assay based on restriction fragment length polymorphism. FEBS Lett. 1998;437:201–206. doi: 10.1016/s0014-5793(98)01231-9. [DOI] [PubMed] [Google Scholar]
- 30.Todd D, Creelan J L, Meehan B M, McNulty M S. Investigation of the transfection capability of cloned tandemly-repeated chicken anaemia virus DNA fragments. Arch Virol. 1996;141:1523–1534. doi: 10.1007/BF01718252. [DOI] [PubMed] [Google Scholar]
- 31.Ukita M, Okamoto H, Kato N, Miyakawa Y, Mayumi M. Excretion into bile of a novel unenveloped DNA virus (TT virus) associated with acute and chronic non-A-G hepatitis. J Infect Dis. 1999;179:1245–1248. doi: 10.1086/314716. [DOI] [PubMed] [Google Scholar]
- 32.Verschoor E J, Langenhuijzen S, Heeney J L. TT viruses (TTV) of non-human primates and their relationship to the human TTV genotypes. J Gen Virol. 1999;80:2491–2499. doi: 10.1099/0022-1317-80-9-2491. [DOI] [PubMed] [Google Scholar]
- 33.Woodfield D G, Gane E, Okamoto H. Hepatitis TT virus is present in New Zealand. N Z J Med. 1998;111:195–196. [PubMed] [Google Scholar]
- 34.Yamamoto T, Kajino K, Ogawa M, Gotoh I, Matsuoka S, Suzuki K, Moriyama M, Okubo H, Kudo M, Arakawa Y, Hino O. Hepatocellular carcinomas infected with the novel TT DNA virus lack viral integration. Biochem Biophys Res Commun. 1998;251:339–343. doi: 10.1006/bbrc.1998.9420. [DOI] [PubMed] [Google Scholar]